Abstract
Fosfomycin (FOS) administered intravenously has been recently rediscovered for the treatment of systemic infections due to multidrug-resistant bacteria. Its pharmacokinetic properties suggest a time-dependent dosing schedule with more clinical benefits from prolonged (PI) or continuous infusion (CI) than from intermittent infusion. We revised literature concerning PI and CI FOS to identify the best dosing regimen based on current evidence. We performed a MEDLINE/PubMed search. Ninety-one studies and their pertinent references were screened. Seventeen studies were included in the present review. The activity of FOS against Gram-negative and Gram-positive bacteria was evaluated in fourteen and five studies, respectively. Six studies evaluated FOS activity in combination with another antibiotic. Daily dosing of 12, 16, 18 or 24 g, administered with different schedules, were investigated. These regimens resulted active against the tested isolates in most cases. Emergence of resistant isolates has been shown to be preventable through the coadministration of another active antibiotic. FOS is a promising option to treat systemic infections caused by multidrug-resistant bacteria. Coadministration with another active molecule is required to prevent the emergence of resistant bacterial strains. The results of our review suggest that a therapeutic regimen including a loading dose of FOS 8 g followed by a daily dose of 16 g or 24 g CI could be the best therapeutic approach for patients with normal renal function. The dosing regimens in patients with renal insufficiency and CI or PI superiority compared with intermittent infusion in clinical settings should be further investigated.
Keywords: Fosfomycin, Continuous infusion, Prolonged infusion, Pharmacokinetics, Pharmacodynamics, Infection
Introduction
The worrying increase of antimicrobial resistance, both in inpatients and outpatients, prompts clinicians to find new therapeutic options. Fosfomycin (FOS), administered intravenously, has been recently re-evaluated for the treatment of systemic infections caused by multidrug-resistant (MDR) bacteria. FOS acts with a unique mechanism of action on the bacterial wall. It is active against many aerobic Gram-negative and -positive bacterial strains (Table 1) [2], and it should be administered with (at least) another active drug to prevent the emergence of resistance [3, 4].
Table 1.
Aerobic Gram-positive and Gram-negative strains susceptible to fosfomycin [1].
| Aerobic gram-positive cocci | Aerobic GNB (Enterobacterales) | Aerobic GNB—selected non-fermentative |
|---|---|---|
| Enterococcus spp. (also VRE) | E. coli (+ ESBL and KPC* producers) | P. aeruginosa* |
| S. aureus (also MRSA) | Klebsiella spp. (+ ESBL and KPC* producers) | |
| Staphylococcus spp. coagulase-negative | Citrobacter spp. | |
| S. lugdunensis | Enterobacter spp. | |
| S. saprophyticus* | P. vulgarisa | |
| Serratia spp. |
*Weak activity
GNB, Gram-negative bacilli; VRE, vancomycin-resistant enterococci; MRSA, methicillin-resistant S. aureus; KPC, K. pneumoniae carbapenemase; ESBL, extended-spectrum beta-lactamase
FOS is marketed both as oral (fosfomycin trometamol, fosfomycin calcium) and intravenous (fosfomycin disodium) formulation. Both time- and concentration-dependent activity have been suggested according to the bacteria evaluated, but due to its short half-life and rapid bactericidal action a time-dependent approach is more often employed [3, 5, 6]. FOS serum half-life is 4–5.7 h for oral formulation [7, 8] and approximately halved when administered intravenously [8, 9]. Although literature data on FOS volume of distribution are controversial (ranging from 40 to 136 L [6, 10]), an excellent tissue penetration is reported, including the central nervous system, soft tissues and bone tissues [6] (AUC0–4 ratio for muscle over plasma was 0.71 for patients with soft tissue infections [11]). FOS is an appealing therapeutic option also for lower respiratory tract infections, biliary tract infections and abscesses [12–15]. FOS is cleared non-metabolized by the kidney and reaches in urine concentrations higher than the minimum inhibitory concentrations (MICs) [8, 16, 17]. Urinary concentrations are higher when FOS is administered intravenously [8]. Its pharmacokinetic properties suggest a time-dependent dosing schedule, with potential clinical benefits deriving from prolonged (PI) or continuous infusion (CI) compared with intermittent infusion (II), the dosing schedule most frequently used to-date. Despite this, guidelines on the best dosing regimen for FOS are lacking. Therefore, we revised literature concerning FOS CI or PI to hypothesize the best dosing regimen based on the actual evidence.
Materials and methods
We performed a MEDLINE/PubMed search and the complete search string was as follows: “(fosfomycin[Text Word]) AND (continuous[Text Word] OR prolonged[Text Word] OR extended[Text Word]) AND (infusion[Text Word] OR intravenous[Text Word] OR pharmacodynamics[Text Word]OR pharmacokinetics[Text Word] OR “opat”[Text Word] OR outpatient[Text Word] OR elastomeric[Text Word] OR pump[Text Word])”. Ninety-one papers from inception to 4 November 2020 were identified and underwent title, abstract and full text screening. Papers written in languages other than English were excluded. Seventy-six papers were excluded for the aforementioned reasons. In addition, pertinent references of included papers and abstracts from international congresses (from 2016 to 2020) were reviewed and discussed. A total of seventeen papers were included in the present review.
Results
Seventeen papers (14 original articles, 4 of which clinical trials, 2 abstracts from international congress and 1 review) were reviewed and discussed. Preclinical and clinical studies evaluated in the present review are briefly listed in Table 2 and Table 3, respectively.
Table 2.
Review of literature concerning FOS in continuous or prolonged infusion (preclinical studies).
| Author, country and year | Type of paper | Methods | Bacteria (number) | Combination or comparison with | FOS dosing regimens | Comments |
|---|---|---|---|---|---|---|
| Guggenbichler et al. 1992 [18] | Original article | Catheter infection model; catheter sepsis (5) | S. aureus (1), S. epidermidis (1) | - | 24-, 48- or 96-hr CI at a concentration of 100 μg/mL (flow rate 20 mL/hr) | Combination of FOS CI and imipenem/cilastatin resulted in microbiological and clinical success in 5 out of 5 episodes (S. epidermidis). |
| Chavanet et al. 1995 [19] | Original article | Fibrin clots infection model | S. pneumoniae (1) | Cefotaxime |
FOS monotherapy 6-hr CI including a 25 mg/kg LD followed by 75 mg/kg FOS + CTX FOS 6-hr CI including a 25 mg/kg LD followed by 75 mg/kg + CTX 25 mg/kg LD followed by 75 mg/kg |
The authors also evaluated single-dose FOS and CTX, alone and in combination, and this resulted in a higher AUC compared with CI. |
| Xiong et al. 1995 [20] | Original article | Rabbit endocarditis model | P. aeruginosa (2) | Ciprofloxacin, pefloxacin |
FOS + CIP FOS 300 mg/kg 24-hr CI + CIP 64 mg/kg 24-hr CI FOS + PEF FOS 300 mg/kg 24-hr CI + PEF 64 mg/kg 24-hr CI |
Additive and synergistic effect was observed for the combinations FOS + CIP and FOS + PEF, respectively. FOS + CIP lead to a greater reduction in the number of CFU per gram of vegetations. |
| Bugnon et al. 1997 [21] | Original article | Rabbit endocarditis model | P. aeruginosa (2) | - | 300 mg/kg/day CI | Compared with pefloxacin, FOS had a greater and more constant bactericidal effect. |
| Docobo-Pérez et al. 2015 [22] | Original article | Hollow fibre infection model | ESBL-producing E. coli (3) | Meropenem |
FOS MIC ≤ 1 mg/L FOS monotherapy 12 g CI FOS + MEM Not evaluated as continuous infusion. FOS 4 g q8hr + MEM 1 g q8hr |
For one isolate even higher dosages of FOS monotherapy (24g q24hr; 36 g q24hr) were ineffective due to selection of resistant mutants. |
| Asuphon et al. 2016 [23] | Original article | Monte Carlo simulation | P. aeruginosa (120) | Carbapenems |
Non-MDR isolates FOS monotherapy 4 g q8hr PI (above 90% PTA) FOS + carbapenems FOS 16 g CI + MEM 1–2 g q8hr PI (80% PTA) FOS 16 g CI + DOM 1 g q8hr PI (80% PTA) MDR isolates FOS monotherapy 4 g q4hr PI (above 90% PTA) FOS + carbapenems All combinations could not achieve the PK/PD targets against MDR PA. FOS 8 g q8hr PI + DOM 1 g q8hr PI achieved the target against CRPA. |
Prolonged and continuous infusions improved PK/PD exposure compared with dosage regimens using traditional 30-min infusions. |
| Albiero et al. 2016 [24] | Original article | Monte Carlo simulation | KPC-producing K. pneumoniae (18) | Meropenem |
FOS monotherapy - FOS + MEM FOS 6 g q6hr PI + MEM 1.5 g q6hr PI (80–90% PTA) FOS 8 g q8hr PI + MEM 1.5 g q6hr PI (80–90% PTA) |
Data were simulated for patients with normal renal function. For patients with renal impairment, percentages of PTA are higher (FOS monotherapy 6 g q6hr PI or 8 g q8hr PI above 90%). In case of MEM MICs ≥ 32 mg/L, none of the dosing regimens of MEM reached 90% PTA. |
| Bhavnani et al. 2017 [25] | Abstract | PK model simulation | Enterobacterales (considered for their representative MICs) | - |
FOS MIC ≤ 64 mg/L, ClCr ≥ 50 mL/min/1.73m2 6 g q8hr PI (> 90% PTA) |
- |
| Louie et al. 2018 [4] | Original article | Hollow fibre infection model | P. aeruginosa (1) | - | 12 g CI or 18 g CI (see Comments). | All FOS regimens rapidly selected for resistant isolates, irrespective of the dose or fractionation schedule. With CI (12 g CI or 18 g CI) regimens, resistance emerged later (6 hr vs. 4 hr). |
| Diep et al. 2018 [26] | Original article | Hollow fibre infection model | K. pneumoniae KPC-producing (2) | Polymyxin B |
FOS monotherapy 6 g q6hr PI (1-hr or 3-hr infusion) Rapid bactericidal effect, followed by regrowth after 24 hr FOS + PMB FOS 6 g q6hr PI (1-hr or 3-hr infusion) + PMB 2.5-mg/kg LD (2-hr infusion) followed by 1.5 mg/kg q12hr (1-hr infusion) |
The combination of FOS and PMB had a synergistic effect with sustained bactericidal effect. |
| Rodrìguez-Gascón et al. 2019 [27] | Review | Revision of literature | Comparison with MICs for Enterobacterales, P. aeruginosa and Staphylococcus spp. | - |
FOS MIC ≤ 64 mg/L 6 g q 6hr PI (30 min) 8 g q 8hr PI (30 min or 6 hr) |
FOS monotherapy was not able to achieve PK/PD targets for strains of MIC ≥ 128 mg/L. |
| Leelawattanachai et al. 2020 [28] | Original article | Monte Carlo simulation | Carbapenem-resistant Enterobacterales: 116 K. pneumoniae, 12 E. coli, 1 E. cloacae | - |
FOS MIC ≤ 128 mg/L, weight 50 kg, ClCr ≥ 80 mL/min/1.73m2 8 g q8hr PI (90% PTA) 8 g q12hr PI (90% PTA) 4 g q6hr PI (90% PTA) |
- |
FOS, fosfomycin; CTX, cefotaxime; CIP, ciprofloxacin; PEF, pefloxacin; MEM, meropenem; DOM, doripenem; PMB, polymyxin B; CI, continuous infusion; PI, prolonged infusion; LD, loading dose; MIC, minimum inhibitory concentration; MDR, multidrug-resistant; PK, pharmacokinetics; PD, pharmacodynamics; PTA, probability of target attainment; AUC, area under the curve; ClCr, creatinine clearance; CRPA, carbapenem-resistant P. aeruginosa; ESBL, extended-spectrum beta-lactamase; KPC, K. pneumoniae carbapenemase
Table 3.
Review of literature concerning FOS in continuous or prolonged infusion (clinical studies).
| Author, country and year | Type of paper | Methods | Bacteria (number) | Combination or comparison with | FOS dosing regimens | Comments |
|---|---|---|---|---|---|---|
| Merino-Bohórquez et al. 2018 [29] | Original article (clinical trial) | Bacteraemic UTI; Monte Carlo simulation | MDR E. coli (16) | - | 4 g q6hr PI (non-superior: 8 g q8hr PI) | Decrease 1-log bacterial burden in 89–96% (EUCAST breakpoints) and 33–54% (CLSI breakpoints) of patients. |
| Matzneller et al. 2019 [30] | Abstract | Clinical (healthy volunteers) | P. aeruginosa* | - | 1 g/hr CI preceded by a LD of 8 g over 30 min | CI resulted in 100% PTA for MICs up to 128 mg/L. Intermittent intravenous infusion resulted in markedly lower % PTA. |
|
Eckburg et al. 2017 [31] Kaye et al. 2019 [32] |
Original article (clinical trial) | 184 hospitalized patients with complicated UTI or acute pyelonephritis (+ 231 treated with piperacillin-tazobactam) | Enterobacterales, P. aeruginosa, A. baumannii-calcoaceticus complex, E. faecalis, S. aureus, S. saprophyticus | - |
ClCr ≥ 20 mL/min/1.73 m2 6 g q8hr PI |
FOS resulted as non-inferior to piperacillin-tazobactam. FOS resulted in overall success rate of 64.7% (119/184 patients). PIP/TAZ resulted in overall success rate of 54.5% (97/178 patients). |
| Al Jalali et al. 2020 [33] | Original article (clinical trial) | Randomized crossover study in 8 healthy volunteers |
- (PK/PD study) |
- | 8 g over 30 min LD + 1 g/hr CI | Comparison with intermittent infusion 8 g over 30 min every 8 hr showed better PK/PD parameters in volunteers who received CI. |
*The study was conducted on healthy volunteers and data obtained were compared with representative MICs of P. aeruginosa isolates
FOS, fosfomycin; PIP/TAZ, piperacillin/tazobactam; CI, continuous infusion; PI, prolonged infusion; LD, loading dose; MIC, minimum inhibitory concentration; MDR, multidrug-resistant; PK, pharmacokinetics; PD, pharmacodynamics; ClCr, creatinine clearance; UTI, urinary tract infection
Fourteen studies investigated FOS dosing regimens in the setting of Gram-negative bacteria (2 in vivo studies, 8 simulation studies, 4 clinical trials, 1 review [4, 20–32]), while FOS dosing regimens against Gram-positive bacteria were evaluated in 5 studies (2 in vitro studies, 2 clinical trials, 1 review [18, 19, 27, 31, 32]). One study [33] did not evaluate the activity of FOS administered in CI since its objective was to report PK/PD parameters in healthy volunteers. Six studies [19, 20, 22–24, 26] evaluated FOS in combination with cefotaxime, ciprofloxacin, pefloxacin, meropenem, doripenem and polymyxin B.
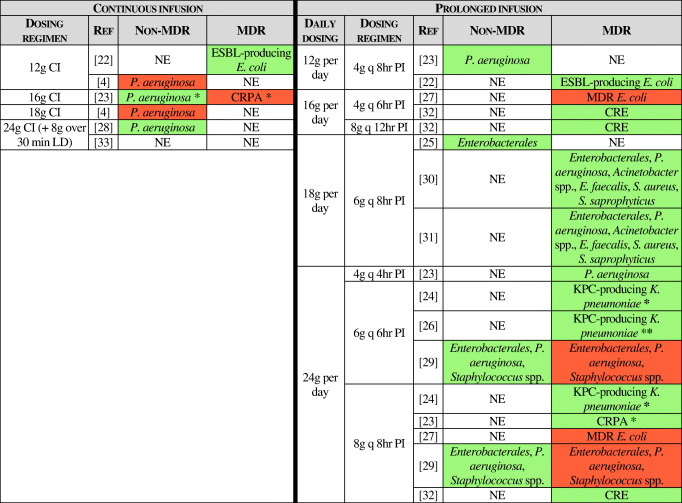
With regard to CI, the daily dosing regimens in the setting of FOS monotherapy were 12 g [4, 22], 18 g [4] and 24 g [30, 33], while FOS in combination with carbapenems was evaluated at daily dose of 16 g [23], resulting active against Pseudomonas aeruginosa in two studies [23, 30] and Escherichia coli extended-spectrum beta-lactamase (ESBL)-producing, but not against carbapenem-resistant P. aeruginosa.
With regard to PI, seven different dosing regimens were evaluated. A schedule of 12 g per day (4g q8hr PI) was evaluated in two studies against non-MDR isolates, administered as monotherapy [23] or combination therapy [22]. FOS monotherapy 16 g per day, administered either as 4 g q6hr PI [28, 29] or 8 g q12hr PI [28], resulted active against non-MDR isolates in two studies. Administration of 18 g per day (6 g q8hr PI) was evaluated in a PK model simulation [25] and in the ZEUS trial [31, 32]. Finally, dosing regimens of 24 g per day, either as 4 g q4hr PI [23], 6 g q6hr PI [24, 26, 27], or 8 g q8hr PI [24, 27–29], resulted active also against MDR isolates.
When FOS given as monotherapy did not result to be active, this was due to the emergence of resistant strains [4, 22, 26]. FOS resistance occurred later when FOS was administered in CI compared with intermittent infusion [23, 26]. The administration of FOS with another active antibiotic was able to overcome resistance in most cases obtaining sustained bactericidal effect [23, 26].
PI resulted in 80–90% probability of target attainment (PTA) in studies simulating the efficacy of FOS against both P. aeruginosa and Enterobacterales [23–25, 28]. FOS administered in CI showed even better results, reaching 100% PTA against P. aeruginosa isolates in the study by Matzneller et al. [30].
Table 4 sums up the investigated dosing regimens and their effectiveness against the tested isolates.
Table 4.
FOS administered as continuous or prolonged infusion: dosing regimens evaluated in the reviewed studies. Dosing regimens active against the tested isolates are highlighted in green, while ineffective regimens are presented in red. NE, not evaluated; CI, continuous infusion; PI, prolonged infusion; LD, loading dose; REF, reference; ESBL, extended-spectrum beta-lactamase; KPC, K. pneumoniae carbapenemase; CRPA, carbapenem-resistant P. aeruginosa; CRE, carbapenem-resistant Enterobacterales. *In combination with carbapenems. **In combination with polymyxin B
Discussion and conclusion
This is the first systematic review evaluating FOS administered as CI or PI. Actual guidelines or expert opinions indicate slightly different dosages for the administration of FOS in CI [34, 35].
Our revision suggests that FOS 8 g loading dose followed by a daily dose of 16 g or up to 24 g CI is the best approach for patients with normal renal function. This dosage should be tailored considering the site of infection and the FOS MIC of the bacteria responsible of the infection. A critical evaluation of different dosing regimens should always be performed. For instance, evaluation of FOS penetration in abscesses reported a long half-life of the molecule (32 ± 39 h) in the pus, suggesting that FOS CI would not add any advantage compared with II in this scenario [36]. This is due to the fact that CI leads to higher AUC but reduced Cmax compared with II [30, 33].
FOS administered according to dosing regimens CI or PI is an option to keep in mind to treat systemic infections caused by MDR bacteria. Although FOS turned out to be well tolerated, thrombophlebitis and circumscribed paresthesia were reported to occur especially when the antibiotic is administered according to the CI or PI regimens [30, 33]. Administration of IV Ringer’s lactate simultaneously with FOS reduced the risk of thrombophlebitis in one study [33].
Dose adjustment according to renal function is required to keep the good safety profile of the drug, as acute or chronic kidney injury can cause a reduction in the glomerular filtration and therefore in the drug elimination [37, 38].
The emergence of resistant bacterial strains resulted in a weak activity of FOS in some series [4, 22, 26]. About this critical issue, CI delayed the development of resistance to FOS compared with II [4]. FOS has excellent synergistic properties [39] and these can lead to a long-lasting bactericidal effect [23, 26]. Furthermore, taking the advantages obtained by the synergism of FOS with other antibiotics, FOS can be considered for the combination treatment of some isolates intrinsically resistant to FOS or against which FOS has only a weak activity, i.e., P. aeruginosa or Acinetobacter spp. [23, 40–43]. Indeed, FOS represents a good option for combination therapies with antibiotics active against such bacteria.
Another advantage of PI or CI is the potential decrease of electrolyte imbalance if compared with rapid infusion [44]. In fact, the intravenous formulation contains 13.5 mEq/g of sodium; therefore, caution is needed to avoid hyopokalemia, especially in patients with heart insufficiency or who are undergoing dialysis [34].
Although few clinical studies evaluating FOS in CI or PI against Gram-positive bacteria are available to-date, this review suggests potential benefits from the use of this antibiotic in this setting [18, 19, 27, 31, 32]. This is interesting if we take into consideration the anti-biofilm properties of FOS, against both Gram-positive and Gram-negative bacterial strains [45, 46].
To the best of our knowledge, no study evaluated the efficacy of FOS prescribed as CI in outpatients through elastomeric pumps. Due to its long-term stability, intravenous FOS CI might be an option also for the outpatient parenteral antimicrobial therapy (OPAT), thus shortening hospitalization and its related risks and costs.
In summary, this systematic review suggests that FOS 8 g loading dose followed by a daily dose of 16 g or up to 24 g CI is a promising therapeutic regimen in the treatment of systemic infections including those due to MDR organisms. Future studies on FOS administered according to the CI regimen should include the evaluation of dosing regimens in patients with chronic renal failure and in haemodialysed patients. The efficacy of FOS according to the site of infection requires further investigation and expert advice should always be sought. Furthermore, as evaluation of PK/PD parameters on healthy volunteers after CI showed better results compared with II [30, 33], clinical trials comparing the superiority of CI or PI to II in different settings are desirable.
Acknowledgments
Availability of data and material
As described in the Methods.
Code availability
Not applicable.
Authors’ contribution
Conception and design: R.M.A., S.D.B., A.E.M., R.L.; Analysis and interpretation of data: R.M.A, S.D.B.; Drafting the article: R.M.A.; Revising the article for critically important intellectual content and final approval of the version to be published: S.D.B., A.E.M., R.L.
Funding
Open access funding provided by Università degli Studi di Trieste within the CRUI-CARE Agreement.
Declarations
Ethical approval
Not required for this kind of publication. Not applicable.
Consent to participate
As this is a systematic review of literature, consent to participate was not necessary. Not applicable.
Consent for publication
All authors agree for publication in European Journal of Clinical Microbiology and Infectious Diseases.
Conflict of interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Roberta Maria Antonello, Email: rma.roby@gmail.com.
Stefano Di Bella, Email: stefano932@gmail.com.
Alberto Enrico Maraolo, Email: albertomaraolo@mail.com.
Roberto Luzzati, Email: roberto.luzzati@asugi.sanita.fvg.it.
References
- 1.Sanford guide to antimicrobial therapy 2020 - pocket edition [Internet]. [cited 2020 21];https://store.sanfordguide.com/antimicrobial-therapy-2020-pocket-edition-4375-x-65-p151.aspx
- 2.Falagas ME, Kastoris AC, Kapaskelis AM, Karageorgopoulos DE. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum beta-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet. Infect. Dis. 2010;10:43–50. doi: 10.1016/S1473-3099(09)70325-1. [DOI] [PubMed] [Google Scholar]
- 3.Fransen F, Hermans K, Melchers MJB, Lagarde CCM, Meletiadis J, Mouton JW. Pharmacodynamics of fosfomycin against ESBL- and/or carbapenemase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2017;72:3374–3381. doi: 10.1093/jac/dkx328. [DOI] [PubMed] [Google Scholar]
- 4.Louie A, Maynard M, Duncanson B, Nole J, Vicchiarelli M, Drusano GL. Determination of the dynamically linked indices of fosfomycin for Pseudomonas aeruginosa in the hollow fiber infection model. Antimicrob. Agents Chemother. 2018;1:62. doi: 10.1128/AAC.02627-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roussos N, Karageorgopoulos DE, Samonis G, Falagas ME. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int. J. Antimicrob. Agents. 2009;34:506–515. doi: 10.1016/j.ijantimicag.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Falagas ME, Vouloumanou EK, Samonis G, Vardakas KZ. Fosfomycin. Clin. Microbiol. Rev. 2016;29:321–347. doi: 10.1128/CMR.00068-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel SS, Balfour JA, Bryson HM. Fosfomycin tromethamine. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single-dose oral treatment for acute uncomplicated lower urinary tract infections. Drugs. 1997;53:637–656. doi: 10.2165/00003495-199753040-00007. [DOI] [PubMed] [Google Scholar]
- 8.Bergan T, Thorsteinsson SB, Albini E. Pharmacokinetic profile of fosfomycin trometamol. Chemotherapy. 1993;39:297–301. doi: 10.1159/000239140. [DOI] [PubMed] [Google Scholar]
- 9.Soraci AL, Perez DS, Martinez G, Dieguez S, Tapia MO, Amanto F, et al. Disodium-fosfomycin pharmacokinetics and bioavailability in post weaning piglets. Res. Vet. Sci. [Internet] 2011 [cited 2020 9];90:498–502. https://pubmed.ncbi.nlm.nih.gov/20696447/: 10.1016/j.rvsc.2010.07.011 [DOI] [PubMed]
- 10.Rizek C, Ferraz JR, van der Heijden IM, Giudice M, Mostachio AK, Paez J, et al. In vitro activity of potential old and new drugs against multidrug-resistant gram-negatives. J. Infect. Chemother. [Internet] 2015 1 [cited 2020 9];21:114–7. https://pubmed.ncbi.nlm.nih.gov/25456893: 10.1016/j.jiac.2014.10.009 [DOI] [PubMed]
- 11.Joukhadar C, Klein N, Dittrich P, Zeitlinger M, Geppert A, Skhirtladze K, et al. Target site penetration of fosfomycin in critically ill patients. J. Antimicrob. Chemother. [Internet] 2003 1 [cited 2020 9];51:1247–52. https://pubmed.ncbi.nlm.nih.gov/12668580/: 10.1093/jac/dkg187 [DOI] [PubMed]
- 12.Matzi V, Lindenmann J, Porubsky C, Kugler SA, Maier A, Dittrich P, et al. Extracellular concentrations of fosfomycin in lung tissue of septic patients. J. Antimicrob. Chemother. [Internet] 2010 12 [cited 2020 9];65:995–8. https://pubmed.ncbi.nlm.nih.gov/20228081/: 10.1093/jac/dkq070 [DOI] [PubMed]
- 13.Lastra CF, Mariño EL, Barrueco M, Gervós MS, Gil AD. Disposition of phosphomycin in patients with pleural effusion. Antimicrob. Agents Chemother. [Internet] 1984 [cited 2020 9];25:458–62. https://pubmed.ncbi.nlm.nih.gov/6732214/doi: 10.1128/AAC.25.4.458 [DOI] [PMC free article] [PubMed]
- 14.Müller O, Rückert U, Walter W, Haag R, Sauer W. Fosfomycin concentrations in serum and bile. Infection [Internet] 1982 [cited 2020 9];10:18–20. https://pubmed.ncbi.nlm.nih.gov/7068230/doi: 10.1007/BF01640831 [DOI] [PubMed]
- 15.Dijkmans AC, Zacarías NVO, Burggraaf J, Mouton JW, Wilms EB, van Nieuwkoop C, et al. Fosfomycin: pharmacological, clinical and future perspectives [Internet]. Antibiotics 2017 1 [cited 2020 9];6. /pmc/articles/PMC5745467/?report=abstract: 10.3390/antibiotics6040024 [DOI] [PMC free article] [PubMed]
- 16.Loose M, Naber KG, Hu Y, Coates A, Wagenlehner FME. Urinary bactericidal activity of colistin and azidothymidine combinations against mcr-1-positive colistin-resistant Escherichia coli. Int. J. Antimicrob. Agents [Internet] 2019 1 [cited 2020 23];54:55–61. http://www.ncbi.nlm.nih.gov/pubmed/31034939doi: 10.1016/j.ijantimicag.2019.04.011 [DOI] [PubMed]
- 17.Michalopoulos AS, Livaditis IG, Gougoutas V. The revival of fosfomycin [Internet]. Int. J. Infect. Dis. 2011 [cited 2020 9];15. https://pubmed.ncbi.nlm.nih.gov/21945848/doi: 10.1016/j.ijid.2011.07.007 [DOI] [PubMed]
- 18.Guggenbichler JP, Berchtold D, Allerberger F, Bonatti H, Hager J, Pfaller W, et al. In vitro and in vivo effect of antibiotics on catheters colonized by staphylococci. Eur. J. Clin. Microbiol. Infect. Dis. [Internet] 1992 [cited 2020 18];11:408–15. http://link.springer.com/10.1007/BF01961855doi: 10.1007/BF01961855 [DOI] [PubMed]
- 19.Chavanet P, Beloeil H, Pechinot A, Duigou F, Buisson JC, Duong M, et al. In vivo activity and pharmacodynamics of cefotaxime or ceftriaxone in combination with fosfomycin in fibrin clots infected with highly penicillin- resistant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 1995;39:1736–1743. doi: 10.1128/AAC.39.8.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong YQ, Potel G, Caillon J, Stephant G, Jehl F, Bugnon D, et al. Comparative efficacies of ciprofloxacin and pefloxacin alone or in combination with fosfomycin in experimental endocarditis induced by multidrug-susceptible and -resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1995;39:496–499. doi: 10.1128/AAC.39.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bugnon D, Potel G, Xiong YQ, Caillon J, Navas D, Gras C, et al. Bactericidal effect of pefloxacin and fosfomycin against Pseudomonas aeruginosa in a rabbit endocarditis model with pharmacokinetics of pefloxacin in humans simulated in vivo. Eur. J. Clin. Microbiol. Infect. Dis. [Internet] 1997 [cited 2020 24];16:575–80. http://link.springer.com/10.1007/BF02447919doi: 10.1007/BF02447919 [DOI] [PubMed]
- 22.Docobo-Pérez F, Drusano GL, Johnson A, Goodwin J, Whalley S, Ramos-Martín V, et al. Pharmacodynamics of fosfomycin: insights into clinical use for antimicrobial resistance. Antimicrob. Agents Chemother. [Internet] 2015 1 [cited 2020 11];59:5602–10. https://pubmed.ncbi.nlm.nih.gov/26124169/doi: 10.1128/AAC.00752-15 [DOI] [PMC free article] [PubMed]
- 23.Asuphon O, Montakantikul P, Houngsaitong J, Kiratisin P, Sonthisombat P. Optimizing intravenous fosfomycin dosing in combination with carbapenems for treatment of Pseudomonas aeruginosa infections in critically ill patients based on pharmacokinetic/pharmacodynamic (PK/PD) simulation. Int. J. Infect. Dis. 2016 1;50:23–9. 10.1016/j.ijid.2016.06.017 [DOI] [PubMed]
- 24.Albiero J, Sy SKB, Mazucheli J, Caparroz-Assef SM, Costa BB, Alves JLB, et al. Pharmacodynamic evaluation of the potential clinical utility of fosfomycin and meropenem in combination therapy against KPC-2-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. [Internet] 2016 1 [cited 2020 27];60:4128–39. https://pubmed.ncbi.nlm.nih.gov/27139468/doi: 10.1128/AAC.03099-15 [DOI] [PMC free article] [PubMed]
- 25.Bhavnani SM, Trang M, Rubino CM, Lepak AJ, Andes DR, Flamm RK, et al. Pharmacokinetics-pharmacodynamics (PK-PD) target attainment analyses to support ZTI-01 (fosfomycin for injection) dose selection for patients with complicated urinary tract infections (cUTI).
- 26.Diep JK, Sharma R, Ellis-Grosse EJ, Abboud CS, Rao GG. Evaluation of activity and emergence of resistance of polymyxin B and ZTI-01 (fosfomycin for injection) against KPC-producing Klebsiella pneumoniae. 2018 [cited 2020 24];10.1128/AAC.01815-17.doi:10.1128/AAC.01815-17 [DOI] [PMC free article] [PubMed]
- 27.Rodríguez-Gascón A, Canut-Blasco A. Deciphering pharmacokinetics and pharmacodynamics of fosfomycin [Internet]. Rev. Esp. Quimioter. 2019 1 [cited 2020 1];32:19–24. /pmc/articles/PMC6555163/?report=abstract [PMC free article] [PubMed]
- 28.Leelawattanachai P, Wattanavijitkul T, Paiboonvong T, Plongla R, Chatsuwan T, Usayaporn S, et al. Evaluation of intravenous fosfomycin disodium dosing regimens in critically ill patients for treatment of carbapenem-resistant Enterobacterales infections using Monte Carlo simulation. Antibiotics [Internet] 2020 18 [cited 2020 26];9:615. https://www.mdpi.com/2079-6382/9/9/615doi: 10.3390/antibiotics9090615 [DOI] [PMC free article] [PubMed]
- 29.Merino-Bohórquez V, Docobo-Pérez F, Sojo J, Morales I, Lupión C, Martín D, et al. Population pharmacokinetics and pharmacodynamics of fosfomycin in non-critically ill patients with bacteremic urinary infection caused by multidrug-resistant Escherichia coli. Clin. Microbiol. Infect. [Internet] 2018 1 [cited 2020 1];24:1177–83. https://pubmed.ncbi.nlm.nih.gov/29649596/doi: 10.1016/j.cmi.2018.02.005 [DOI] [PubMed]
- 30.Peter M, al Jalali V, Beatrix W, Koch Birgit CP, Scharon C, Johan M, et al. Continuous infusion of fosfomycin in healthy volunteers. 2019. [Google Scholar]
- 31.Eckburg PB, Skarinsky D, Das A, Ellis-Grosse EJ. Phenotypic antibiotic resistance in ZEUS: a multi-center, randomized, double-blind phase 2/3 study of ZTI-01 vs. piperacillin-tazobactam (P-T) in the treatment of patients with complicated urinary tract infections (cUTI) including acute pyelonephritis (AP). Open Forum Infect. Dis. [Internet] 2017 1 [cited 2020 1];4:S522–S522. https://academic.oup.com/ofid/article/4/suppl_1/S522/4295818doi: 10.1093/ofid/ofx163.1360
- 32.Kaye KS, Rice LB, Dane AL, Stus V, Sagan O, Fedosiuk E, et al. Fosfomycin for injection (ZTI-01) versus piperacillin-tazobactam for the treatment of complicated urinary tract infection including acute pyelonephritis: ZEUS, a phase 2/3 randomized trial. Clin. Infect. Dis. [Internet] 2019 27 [cited 2020 1];69:2045–56. https://pubmed.ncbi.nlm.nih.gov/30861061/doi: 10.1093/cid/ciz181 [DOI] [PMC free article] [PubMed]
- 33.al Jalali V, Matzneller P, Wulkersdorfer B, Chou S, Bahmany S, Koch B C P, et al. Clinical pharmacokinetics of fosfomycin after continuous infusion compared with intermittent infusion: a randomized crossover study in healthy volunteers. Antimicrob. Agents Chemother. [Internet] 2020 26 [cited 2020 4];http://aac.asm.org/lookup/doi/10.1128/AAC.01375-20doi: 10.1128/AAC.01375-20 [DOI] [PMC free article] [PubMed]
- 34.Mensa J, Barberán J, Soriano A, Llinares P, Marco F, Cantón R, et al. Antibiotic selection in the treatment of acute invasive infections by Pseudomonas aeruginosa: Guidelines by the Spanish society of chemotherapy. Rev. Esp. Quimioter. [Internet] 2018 1 [cited 2020 26];31:78–100. /pmc/articles/PMC6159363/?report=abstract [PMC free article] [PubMed]
- 35.Candel FJ, Matesanz David M, Barberán J. New perspectives for reassessing fosfomycin: applicability in current clinical practice [Internet]. Rev. Esp. Quimioter. 2019 1 [cited 2020 14];32:1–7. /pmc/articles/PMC6555164/?report=abstract [PMC free article] [PubMed]
- 36.Sauermann R, Karch R, Langenberger H, Kettenbach J, Mayer-Helm B, Petsch M, et al. Antibiotic abscess penetration: fosfomycin levels measured in pus and simulated concentration-time profiles. Antimicrob. Agents Chemother. [Internet] 2005 [cited 2020 26];49:4448–54. http://aac.asm.org/doi: 10.1128/AAC.49.11.4448-4454.2005 [DOI] [PMC free article] [PubMed]
- 37.ten Doesschate T, van Haren E, Wijma RA, Koch BCP, Bonten MJM, van Werkhoven CH. The effectiveness of nitrofurantoin, fosfomycin and trimethoprim for the treatment of cystitis in relation to renal function. Clin. Microbiol. Infect. [Internet] 2020 1 [cited 2020 11];26:1355–60. https://pubmed.ncbi.nlm.nih.gov/32165321/doi: 10.1016/j.cmi.2020.03.001 [DOI] [PubMed]
- 38.Parker S, Lipman J, Koulenti D, Dimopoulos G, Roberts JA. What is the relevance of fosfomycin pharmacokinetics in the treatment of serious infections in critically ill patients? A systematic review [Internet]. Int. J. Antimicrob. Agents 2013 [cited 2020 11];42:289–93. https://pubmed.ncbi.nlm.nih.gov/23880170/doi: 10.1016/j.ijantimicag.2013.05.018 [DOI] [PubMed]
- 39.Antonello RM, Principe L, Maraolo AE, Viaggi V, Pol R, Fabbiani M, et al. Fosfomycin as partner drug for systemic infection management: a systematic review of its synergistic properties from in vitro and in vivo studies [Internet]. Antibiotics 2020 1 [cited 2020 11];9:1–74. https://pubmed.ncbi.nlm.nih.gov/32785114/doi: 10.3390/antibiotics9080500 [DOI] [PMC free article] [PubMed]
- 40.Ku NS, Lee SH, Lim Y soun, Choi H, Ahn JY, Jeong SJ, et al. In vivo efficacy of combination of colistin with fosfomycin or minocycline in a mouse model of multidrug-resistant Acinetobacter baumannii pneumonia. Sci. Rep. [Internet] 2019 1 [cited 2020 12];9:17127. http://www.ncbi.nlm.nih.gov/pubmed/31748527doi: 10.1038/s41598-019-53714-0 [DOI] [PMC free article] [PubMed]
- 41.Flamm RK, Rhomberg PR, Lindley JM, Sweeney K, Ellis-Grosse EJ, Shortridge D. Evaluation of the bactericidal activity of fosfomycin in combination with selected antimicrobial comparison agents tested against Gram-negative bacterial strains by using time-kill curves. Antimicrob. Agents Chemother. [Internet] 2019 1 [cited 2020 12];63. http://www.ncbi.nlm.nih.gov/pubmed/30858207doi: 10.1128/AAC.02549-18 [DOI] [PMC free article] [PubMed]
- 42.Zhu W, Wang Y, Cao W, Cao S, Zhang J. In vitro evaluation of antimicrobial combinations against imipenem-resistant Acinetobacter baumannii of different MICs. J. Infect. Public Health [Internet] 2018 1 [cited 2020 12];11:856–60. http://www.ncbi.nlm.nih.gov/pubmed/30057349doi: 10.1016/j.jiph.2018.07.006 [DOI] [PubMed]
- 43.Cuba G, Rocha-Santos G, Cayô R, Streling A, Nodari C, Gales A, et al. In vitro synergy of ceftolozane/tazobactam in combination with fosfomycin or aztreonam against MDR Pseudomonas aeruginosa. J. Antimicrob. Chemother. [Internet] 2020 [cited 2020 20];https://pubmed.ncbi.nlm.nih.gov/32240299/doi: 10.1007/s40265-013-0168-2 [DOI] [PubMed]
- 44.Florent A, Chichmanian RM, Cua E, Pulcini C. Adverse events associated with intravenous fosfomycin [Internet]. Int. J. Antimicrob. Agents 2011 [cited 2020 11];37:82–3. https://pubmed.ncbi.nlm.nih.gov/21074377/doi: 10.1016/j.ijantimicag.2010.09.002 [DOI] [PubMed]
- 45.Yu W, Zhang J, Tong J, Zhang L, Zhan Y, Huang Y, et al. In vitro antimicrobial activity of fosfomycin, vancomycin and daptomycin alone, and in combination, against linezolid-resistant Enterococcus faecalis. Infect. Dis. Ther. [Internet] 2020 [cited 2020 14];https://pubmed.ncbi.nlm.nih.gov/32964392/doi: 10.1007/s40121-020-00342-1 [DOI] [PMC free article] [PubMed]
- 46.Wang L, Di Luca M, Tkhilaishvili T, Trampuz A, Gonzalez Moreno M. Synergistic activity of fosfomycin, ciprofloxacin, and gentamicin against Escherichia coli and Pseudomonas aeruginosa biofilms. Front. Microbiol. [Internet] 2019 6 [cited 2020 12];10:2522. http://www.ncbi.nlm.nih.gov/pubmed/31781056doi: 10.3389/fmicb.2019.02522 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As described in the Methods.