Abstract
Purpose
We investigated whether response classification after total thyroidectomy and radioactive iodine (RAI) therapy could be affected by serum levels of recombinant human thyrotropin (rhTSH)-stimulated thyroglobulin (Tg) measured at different time points in a follow-up of patients with papillary thyroid carcinoma (PTC).
Methods
A total of 147 PTC patients underwent serum Tg measurement for response assessment 6 to 24 months after the first RAI therapy. Serum Tg levels were measured at 24 h (D1Tg) and 48–72 h (D2-3Tg) after the 2nd injection of rhTSH. Responses were classified into three categories based on serum Tg corresponding to the excellent response (ER-Tg), indeterminate response (IR-Tg), and biochemical incomplete response (BIR-Tg). The distribution pattern of response classification based on serum Tg at different time points (D1Tg vs. D2-3Tg) was compared.
Results
Serum D2-3Tg level was higher than D1Tg level (0.339 ng/mL vs. 0.239 ng/mL, P < 0.001). The distribution of response categories was not significantly different between D1Tg-based and D2-3Tg-based classification. However, 8 of 103 (7.8%) patients and 3 of 40 (7.5%) patients initially categorized as ER-Tg and IR-Tg based on D1Tg, respectively, were reclassified to IR-Tg and BIR-Tg based on D2-3Tg, respectively. The optimal cutoff values of D1Tg for the change of response categories were 0.557 ng/mL (from ER-Tg to IR-Tg) and 6.845 ng/mL (from IR-Tg to BIR-Tg).
Conclusion
D1Tg measurement was sufficient to assess the therapeutic response in most patients with low level of D1Tg. Nevertheless, D2-3Tg measurement was still necessary in the patients with D1Tg higher than a certain level as response classification based on D2-3Tg could change.
Keywords: Recombinant human thyrotropin, Thyroglobulin, Papillary thyroid carcinoma, Response classification, Radioactive iodine
Introduction
Therapeutic response is usually evaluated about 6–24 months after surgery (total or near-total thyroidectomy) followed by radioactive iodine (RAI) therapy in patients with differentiated thyroid carcinoma (DTC) [1]. The assessment of therapeutic response is usually performed using imaging studies (such as neck ultrasound and diagnostic iodine scan) and serum thyroglobulin (Tg) under TSH stimulation. TSH can be elevated by two different methods: recombinant human thyrotropin (rhTSH) injection and thyroid hormone withdrawal (THW). rhTSH is used to stimulate TSH exogenously for the preparation for RAI therapy and follow-up study in patients with DTC [2–5]. It serves as a safe and effective alternative to THW-aided stimulation, avoiding signs and symptoms of hypothyroidism after THW [6, 7].
Serum Tg level usually reaches its peak concentration 3 days after the 2nd injection of rhTSH in DTC patients who underwent initial therapy (surgery and RAI therapy) [8]. It has an implication that response assessment based on rhTSH-stimulated serum Tg could be different depending on the time point of Tg measurement after rhTSH-aided stimulation. There is no definite consensus about rhTSH-stimulated Tg criteria for response evaluation after initial therapy in DTC patients.
In this study, we investigated whether response classification after surgery and RAI therapy could be affected by serum levels of rhTSH-stimulated Tg measured at different time points in a follow-up of patients with papillary thyroid carcinoma (PTC).
Materials and Methods
Patients
Patients who underwent the first RAI therapy after total or near-total thyroidectomy from January 2014 to October 2019 were initially enrolled. Among these patients, those with distant metastases (n = 1), serum anti-Tg antibody (TgAb) > 100 IU/mL (n = 9) [9, 10], THW in preparation for follow-up study (n = 5), serum Tg measured only once either at 24 h or 48–72 h after the 2nd injection of rhTSH (n = 4), and serum Tg level measured by different assay methods on different time point (n = 20) were excluded. Finally, a total of 147 patients were analyzed in this study.
Follow-up Study
The follow-up study was performed about 6–24 months after RAI therapy. It consisted of diagnostic whole-body scan (DxWBS) and serum Tg measurement. DxWBS was taken 1 day after I-123 or 2 days after I-131 administration. For TSH stimulation, all the enrolled patients were injected 2 consecutive daily doses of 0.9 mg rhTSH (Thyrogen, Sanofi Genzyme, Cambridge, MA, USA) intramuscularly. I-123 (0.185 GBq) or I-131 (0.111 GBq) was administered orally 24 hours after the second injection of rhTSH. Serum Tg levels in each patient were measured at 24 h (D1Tg) and 48 or 72 h (D2-3Tg) after the 2nd injection of rhTSH.
Study Design
In order to figure out the effect of rhTSH-stimulated Tg values measured at different time points on response classification, imaging findings including ultrasonography and DxWBS were excluded, and therapeutic responses were classified into three categories based on stimulated Tg criteria corresponding to the excellent response (ER), indeterminate response (IR), and biochemical incomplete response (BIR) suggested in the 2015 ATA guidelines [1]: (1) serum Tg < 1 ng/mL (ER-Tg), (2) 1 ≤ serum Tg < 10 ng/mL (IR-Tg), and (3) serum Tg ≥ 10 ng/mL (BIR-Tg). We evaluated whether there was difference in the distribution pattern of response classification based on serum Tg levels measured at different time points (D1Tg vs. D2-3Tg). The optimal cutoff value of D1Tg for the change of response classification was determined using receiver operating characteristics (ROC) curve analysis. Tumors and lymph node metastases were analyzed according to the staging system of the Union for International Cancer Control (UICC) and the 8th edition of the American Joint Committee on Cancer (AJCC) staging manual.
Serum Thyroglobulin, Anti-thyroglobulin Antibody, and TSH Measurement
Serum Tg levels were measured using an electrochemiluminescent immunoassay (ECLIA) kit (COBAS, Roche Diagnostics GmbH, Mannheim, Germany) with a lower detection limit of 0.1 ng/mL. Serum TgAb was measured either by using radioimmunoassay (RIA anti-Tgn, BRAHMS GmbH, Hennigsdorf, Germany) or by using the ECLIA kit (COBAS, Roche Diagnostics GmbH, Mannheim, Germany) with a lower detection limit of 20 IU/mL for both. Serum TSH was determined either by using IRMA (TSH-CTK-3, DiaSorin, Saluggia, Italy) or by using ECLIA kit (COBAS, Roche Diagnostics GmbH, Mannheim, Germany) with a lower detection limit of 0.07 μIU/mL and 0.4 μIU/mL, respectively.
Statistical Analysis
Continuous data are presented as mean ± standard deviation (SD) or median with range, where indicated. Categorical data are presented as percentage. To determine the differences in variables between two groups, Wilcoxon signed-rank test was used for continuous variables. Cohen’s Kappa test was used to assess the consistency in response classification evaluated based on either D1Tg or D2-3Tg. ROC curve analysis was used to define the optimal cutoff value of D1Tg. P value lower than 0.05 was considered statistically significant. The statistical analysis was conducted using IBM SPSS for Windows®, version 21.0 (IBM Corp., Armonk, NY, USA).
Results
Patient Characteristics
Table 1 lists the summary of patient characteristics. The study population comprised of more females (71.4%) than males (28.6%). Their mean age was 47.7 ± 12.8 years (range, 15–72 years). All of the patients were diagnosed with papillary thyroid carcinoma in the postoperative pathological evaluation. The most common pathologic categories were T1 (57.8%) and N1a (54.4%). One hundred twenty-two (83.0%) patients were in the intermediate risk category at the time of RAI therapy according to the ATA risk stratification system. The first follow-up was done 385.0 ± 38.9 days (range, 331–741 days) after RAI therapy. One hundred and seven (72.8%) patients were administered I-123 (serum Tg checked at D1 and D2), and the rest I-131 (serum Tg checked at D1 and D3), for follow-up iodine scan, respectively.
Table 1.
Patient characteristics (n = 147)
| Parameters | Values (range or %) |
|---|---|
| Age (year) | 47.7 ± 12.8 (15–72) |
| Sex | |
| Male | 42 (28.6%) |
| Female | 105 (71.4%) |
| T category | |
| T1 | 85 (57.8%) |
| T2 | 14 (9.5%) |
| T3 | 34 (23.2%) |
| T4 | 14 (9.5%) |
| N category | |
| N0a or N0b | 22 (15.0%) |
| N1a | 80 (54.4%) |
| N1b | 45 (30.6%) |
| Histology | |
| Papillary | 147 (100%) |
| ATA initial risk | |
| Low | 11 (7.5%) |
| Intermediate | 122 (83.0%) |
| High | 14 (9.5%) |
| RAI dose | |
| 1.30 GBq (35 mCi) | 17 (11.6%) |
| 1.48 GBq (40 mCi) | 11 (7.4%) |
| 1.85 GBq (50 mCi) | 62 (42.2%) |
| 2.96 GBq (80 mCi) | 30 (20.4%) |
| 3.70 GBq (100 mCi) | 26 (17.7%) |
| 5.55 GBq (150 mCi) | 1 (0.7%) |
| Follow-up study | |
| Diagnostic scan | |
| I-123 (D1Tg and D2Tg) | 107 (72.8%) |
| I-131 (D1Tg and D3Tg) | 40 (27.2%) |
| Days between RAI therapy and follow-up | 385.0 ± 38.9 (331–741) |
RAI, radioactive iodine; Tg, thyroglobulin; D1Tg, D2Tg, and D3Tg, serum Tg measured 24 h, 48 h, and 72 h after the 2nd rhTSH injection
Comparison of Serum Thyroglobulin Levels Measured at Different Time Points
Serum D2-3Tg level was significantly higher than D1Tg level in all enrolled patients [0.339 ng/mL (0–87.240 ng/mL) vs. 0.239 ng/mL (0–55.070 ng/mL), P < 0.001] (Fig. 1a). In selected patients with serum Tg level 0.1 ng/mL or more, serum D2-3Tg level was also higher than D1Tg level [1.330 ng/mL (0.109–87.240 ng/mL) vs. 0.994 ng/mL (0.107–55.070 ng/mL), P < 0.001] (Fig. 1b). In addition, serum D2Tg and D3Tg level did not show significant difference [0.247 ng/mL (0–22.000 ng/mL) vs. 0.984 ng/mL (0–87.240 ng/mL), P = 0.093] although the comparison between D2Tg and D3Tg was performed in different patient groups.
Fig. 1.
Comparison of serum thyroglobulin (Tg) levels between D1 and D2-3. a Analysis with all the serum Tg values included (n = 147). D1Tg 0.239 ng/mL (0–55.070 ng/mL) and D2-3Tg 0.339 ng/mL (0–87.240 ng/mL), expressed as median (range). b Analysis with serum Tg values more than 0.1 ng/mL (n = 89). D1Tg 0.994 ng/mL (0.107–55.070 ng/mL) and D2-3Tg 1.330 ng/mL (0.109–87.240 ng/mL), expressed as median (range). Y-axis of the graph is on square root–transformed scale. Statistical significance was evaluated by Wilcoxon signed-rank test
Change of Response Classification
Therapeutic responses were classified based on serum Tg level measured at either D1 or D2-3 (Table 2). The overall distribution of ER-Tg, IR-Tg, and BIR-Tg was not significantly different between D1Tg-based (70.1%, 27.2%, and 2.7%, respectively) and D2-3Tg-based (65.3%, 29.9%, and 4.8%, respectively) analyses by Cohen’s Kappa test (P < 0.001) (Table 2). There was no patient classified to the structural incomplete response (SIR) group regardless of imaging findings.
Table 2.
Change in the distribution of response classification based on serum D1Tg or D2-3Tg
| Number of patients based on D2-3Tg | Kappa | P-value | |||||
|---|---|---|---|---|---|---|---|
| ER-Tg | IR-Tg | BIR-Tg | Total | ||||
| Number of patients based on D1Tg | ER-Tg | 95 | 8 | 0 | 103 (70.1%) | 0.822 | < 0.001 |
| IR-Tg | 1 | 36 | 3 | 40 (27.2%) | |||
| BIR-Tg | 0 | 0 | 4 | 4 (2.7%) | |||
| Total | 96 (65.3%) | 44 (29.9%) | 7 (4.8%) | 147 | |||
ER-Tg, IR-Tg, and BIR-Tg, serum thyroglobulin corresponding to the excellent response, indeterminate response, and biochemical incomplete response; D1Tg, D2Tg, and D3Tg, serum Tg measured 24 h, 48 h, and 72 h after the 2nd rhTSH injection
*Statistical significance was evaluated by Cohen’s Kappa test
Twelve patients demonstrated a change in response classification according to the time point of Tg measurements. Eight of 103 (7.8%) patients in the ER-Tg category based on D1Tg were reclassified to the IR-Tg category based on D2-3Tg evaluation. Three of 40 (7.5%) patients in the IR-Tg category based on D1Tg were reclassified to the BIR-Tg category based on D2-3Tg evaluation. Interestingly, one patient in the IR-Tg category based on D1Tg was reallocated to the ER-Tg category based on D2-3Tg evaluation (Table 2).
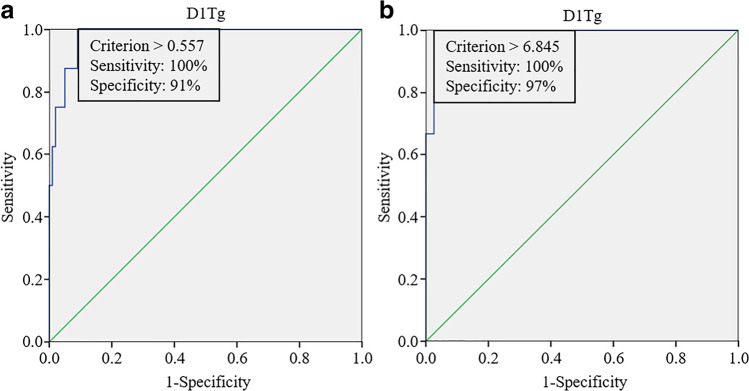
The optimal cutoff values of D1Tg were 0.557 ng/mL for change from the ER-Tg category to the IR-Tg category (Fig. 2a) and 6.845 ng/mL for change from the IR-Tg category to the BIR-Tg category (Fig. 2b), respectively. Representative cases are shown in Fig. 3.
Fig. 2.
Receiver operating characteristic (ROC) curve of D1-thyroglobulin (D1Tg) for the prediction of change in response classification. a The optimal cutoff value of D1Tg was 0.557 ng/mL for the change of Tg category corresponding to excellent response (ER-Tg) to Tg category corresponding to indeterminate response (IR-Tg) with sensitivity and specificity of 100% and 91%, respectively. b The optimal cutoff value of D1Tg was 6.845 ng/mL for the change from the IR-Tg to Tg category corresponding to biochemical incomplete response (BIR-Tg) category with sensitivity and specificity of 100% and 97%, respectively
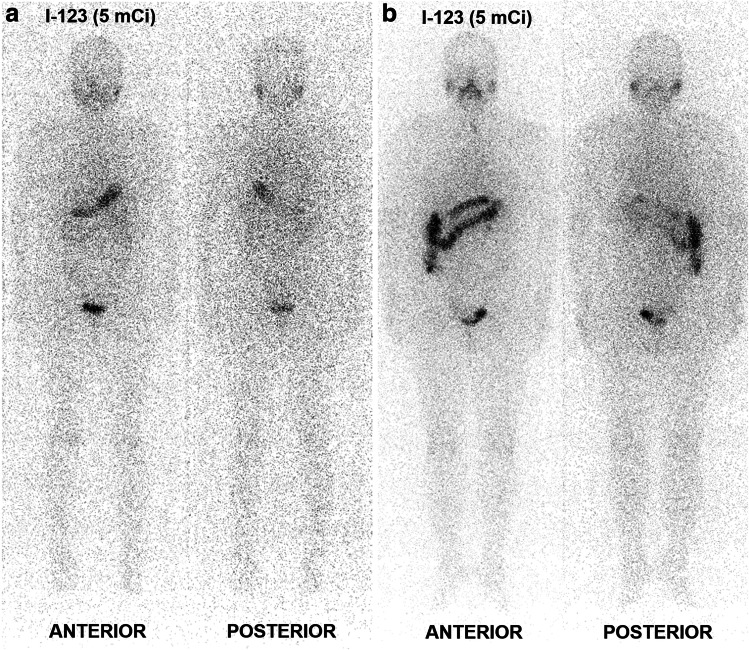
Fig. 3.
Representative cases showing the change in response classification. a A patient with D1-thyroglobulin (D1Tg) of 0.732 ng/mL was initially classified into Tg category corresponding to the excellent response (ER-Tg) but was shifted to Tg category corresponding to the indeterminate response (IR-Tg) based on D2Tg of 1.24 ng/mL. There was no significant finding on I-123 diagnostic whole-body scan (DxWBS). b A patient initially allocated to the IR-Tg with D1Tg of 9.07 ng/mL was reallocated to Tg category corresponding to the biochemical incomplete response (BIR-Tg) based on D2Tg of 16.87 ng/mL. There were non-specific findings on DxWBS, which did not affect response classification
Discussion
Our data demonstrated that the overall distribution of response-to-therapy classification was not statistically different regardless of the time point of rhTSH-stimulated Tg measurements (D1Tg or D2-3Tg), even though D2-3Tg values were higher than D1Tg values. However, the response classification changed in 12 patients. Moreover, 11 out of 12 patients were allocated to an escalated response category when reclassified using D2-3Tg.
Serum Tg level increases in response to rhTSH stimulation, usually reaching its peak concentration more than 2 days after the second rhTSH injection [8]. This time lapse in the serum Tg to reach the highest level after TSH stimulation is due to complex processes of iodination and hormone formation of Tg and its slow movement through the follicular colloid [11]. Consequently, serum Tg values differ depending on the time point of measurement after rhTSH stimulation, which could affect the distribution of response classification. In our study, serum D2-3Tg level increased significantly compared to D1Tg level (Fig. 1). Although the distribution of response-to-therapy classification based on D1Tg was not significantly different from that based on D2-3Tg, the response classification changed in 11 patients with D1Tg higher than a certain level determined by ROC curve. Especially, patients with serum D1Tg level above 0.557 ng/mL or 6.845 ng/mL showed high probability of the change in response classification from the ER-Tg to IR-Tg or from the IR-Tg to BIR-Tg categories, respectively (Fig. 2).
Our study evaluated response-to-therapy classification based on only stimulated Tg criteria suggested in the 2015 ATA guidelines, excluding imaging findings (ultrasonography and DxWBS) in order to figure out the effect of rhTSH-stimulated Tg values measured at different time points on response classification. Although there was no patient classified into structural incomplete response on DxWBS, 34 patients showed faint iodine uptakes on the anterior neck. However, even when DxWBS findings were included, there was no significant difference in the overall distribution of therapeutic responses (ER, IR, and BIR) regardless of different time points of serum Tg measurement (55.1%, 42.2%, and 2.7% based on D1Tg and DxWBS vs. 52.4%, 42.9%, and 4.7% based on D2-3Tg and DxWBS, κ = 0.910, P < 0.001). Nevertheless, 7 patients (4 in the ER group and 3 in the IR group based on serum D1Tg level) were reallocated to escalated response categories based on D2-3Tg, showing the requirement of additional D2-3Tg measurement for better response evaluation.
The stimulated Tg criteria of response-to-therapy classification in the 2015 ATA guidelines are based on serum Tg values obtained only after THW-aided stimulation [12, 13]. Several studies demonstrated that the serum Tg level obtained after rhTSH stimulation is significantly lower compared to that obtained after THW in the same patients [14, 15]. Kowalska et al. [16] reported that THW-stimulated Tg is 3–5 times higher than rhTSH-stimulated Tg and that the same cutoff of serum Tg level should not be applied in patients with different methods of TSH stimulation. As seen in recommendation 80 of the 2015 ATA guidelines, different cutoff values of serum Tg are suggested according to the TSH stimulation method to determine empiric RAI therapy in patients with high level of serum Tg and negative DxWBS [1]. It has an implication that the same level of serum Tg has different prognostic values depending on the method of TSH stimulation.
The cutoff values of Tg corresponding to each response category could be different according to the method of TSH stimulation, although the patients in our study were classified into response categories suggested in the 2015 ATA guidelines. Further investigation is necessary to reestablish the rhTSH-stimulated Tg criteria for response classification through long-term follow-up. Mutsuddy et al. [17] showed that the cutoff value of serum Tg for the prediction of prognosis differed when measured 7 days before, on the day of, and 2 days after RAI therapy aided by rhTSH stimulation. If an optimal cutoff of serum Tg for the evaluation of prognosis could be determined at each time point after rhTSH stimulation, a delayed test such as D2-3Tg might not be necessary.
Our study has several limitations. First, as with all retrospective studies, a selection bias was inevitable. Especially, our follow-up protocol changed based on the availability of diagnostic radioiodine. D1Tg and D2Tg or D1Tg and D3Tg were measured when I-123 or I-131 was used, respectively. D2Tg has been measured instead of D3Tg when I-123 was used because otherwise the patient would have had to visit the hospital for an entire week even on Friday for D3Tg check-up. However, D2Tg and D3Tg did not show significant differences; thus, we could use D2-3Tg as one group for analysis throughout the study. In addition, TgAb or TSH measurements were performed using two different assay kits, although it might not have affected the study results significantly. Second, there were a large number of patients with either D1Tg or D2-3Tg level less than 0.1 ng/mL (39.5% of total patients), which could affect the distribution of therapeutic response and statistical analyses. More patients with variable ranges of serum Tg should be enrolled in order to examine the effect of rhTSH-stimulated Tg values on response classification. Third, the median follow-up period of the enrolled patients was too short to evaluate the prognosis. Studies with longer follow-up period are required to determine rhTSH-stimulated Tg criteria for the evaluation of prognosis.
Conclusion
The distribution of therapeutic responses was not significantly different regardless of rhTSH-stimulated serum Tg level at different time points, which had an implication that D1Tg measurement was sufficient to assess the therapeutic response in most patients with low level of D1Tg. Nevertheless, delayed serum Tg (D2-3Tg) measurement was still necessary in patients with D1Tg higher than a certain level because response classification can change with the elevation of serum Tg.
Acknowledgements
The authors thank Ms. Cho Hee Hwang, Chonnam National University Hospital Biomedical Research Institute, for her assistance of statistical analysis.
Author Contribution
Conceptualization, JBM, SYK; data curation, JBM, SJ; formal analysis, JBM; methodology, JBM, SYK, SJ, KSP, SWY, SRK, and SGC; project administration, SYK; supervision: SYK, CL, HCS, JJM, and HSB; validation, KSP, SWY, SRK, SGC, JK, CL, and HCS; Writing—original draft, JBM; Writing—review and editing, JBM, SYK, JK, CL, HCS, JJM, and HSB. All authors have read and agreed to the published version of the manuscript.
Data Availability
Data will not be shared.
Declarations
Conflict of Interest
Jang Bae Moon, Subin Jeon, Ki Seong Park, Su Woong Yoo, Sae-Ryung Kang, Sang-Geon Cho, Jahae Kim, Changho Lee, Ho-Chun Song, Jung-Joon Min, Hee-Seung Bom, Seong Young Kwon declare no conflict of interest.
Ethical Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki declaration as revised in 2013 and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Consent for Publication
The institutional review board of our institute approved this retrospective study, and the requirement to obtain informed consent was waived.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giovanella L, Duntas LH. Management of endocrine disease: the role of rhTSH in the management of differentiated thyroid cancer: pros and cons. Eur J Endocrinol. 2019;181:R133–R145. doi: 10.1530/EJE-19-0149. [DOI] [PubMed] [Google Scholar]
- 3.Torlontano M, Crocetti U, D’Aloiso L, Bonfitto N, Di Giorgio A, Modoni S, et al. Serum thyroglobulin and 131I whole body scan after recombinant human TSH stimulation in the follow-up of low-risk patients with differentiated thyroid cancer. Eur J Endocrinol. 2003;148:19–24. doi: 10.1530/eje.0.1480019. [DOI] [PubMed] [Google Scholar]
- 4.Bombardieri E, Seregni E, Villano C, Aliberti G, Mattavelli F. Recombinant human thyrotropin (rhTSH) in the follow-up and treatment of patients with thyroid cancer. Tumori. 2003;89:533–536. doi: 10.1177/030089160308900515. [DOI] [PubMed] [Google Scholar]
- 5.Mazzaferri EL, Kloos RT. Using recombinant human TSH in the management of well-differentiated thyroid cancer: current strategies and future directions. Thyroid. 2000;10:767–778. doi: 10.1089/thy.2000.10.767. [DOI] [PubMed] [Google Scholar]
- 6.Pacini F, Ladenson PW, Schlumberger M, Driedger A, Luster M, Kloos RT, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J Clin Endocrinol Metab. 2006;91:926–932. doi: 10.1210/jc.2005-1651. [DOI] [PubMed] [Google Scholar]
- 7.Dow KH, Ferrell BR, Anello C. Quality-of-life changes in patients with thyroid cancer after withdrawal of thyroid hormone therapy. Thyroid. 1997;7:613–619. doi: 10.1089/thy.1997.7.613. [DOI] [PubMed] [Google Scholar]
- 8.Weiss R, Magner J. Serial measurements of serum thyroglobulin in response to recombinant human thyrotropin stimulation. Thyroid. 2015;25:708–710. doi: 10.1089/thy.2014.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn BC, Lee WK, Jeong SY, Lee SW, Lee J. Estimation of true serum thyroglobulin concentration using simultaneous measurement of serum antithyroglobulin antibody. Int J Endocrinol. 2013;2013:210639. doi: 10.1155/2013/210639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim TY, Kim WB, Kim ES, Ryu JS, Yeo JS, Kim SC, et al. Serum thyroglobulin levels at the time of 131I remnant ablation just after thyroidectomy are useful for early prediction of clinical recurrence in low-risk patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2005;90:1440–1445. doi: 10.1210/jc.2004-1771. [DOI] [PubMed] [Google Scholar]
- 11.Di Jeso B, Arvan P. Thyroglobulin from molecular and cellular biology to clinical endocrinology. Endocr Rev. 2016;37:2–36. doi: 10.1210/er.2015-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaisman F, Momesso D, Bulzico DA, Pessoa CH, Dias F, Corbo R, et al. Spontaneous remission in thyroid cancer patients after biochemical incomplete response to initial therapy. Clin Endocrinol (Oxf) 2012;77:132–138. doi: 10.1111/j.1365-2265.2012.04342.x. [DOI] [PubMed] [Google Scholar]
- 13.Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010;20:1341–1349. doi: 10.1089/thy.2010.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pacini F, Molinaro E, Lippi F, Castagna MG, Agate L, Ceccarelli C, et al. Prediction of disease status by recombinant human TSH-stimulated serum Tg in the postsurgical follow-up of differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2001;86:5686–5690. doi: 10.1210/jcem.86.12.8065. [DOI] [PubMed] [Google Scholar]
- 15.Haugen BR, Pacini F, Reiners C, Schlumberger M, Ladenson PW, Sherman SI, et al. A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant or cancer. J Clin Endocrinol Metab. 1999;84:3877–3885. doi: 10.1210/jcem.84.11.6094. [DOI] [PubMed] [Google Scholar]
- 16.Kowalska A, Palyga I, Gasior-Perczak D, Walczyk A, Trybek T, Sluszniak A, et al. The cut-off level of recombinant human TSH-stimulated thyroglobulin in the follow-up of patients with differentiated thyroid cancer. PLoS ONE. 2015;10:e0133852. doi: 10.1371/journal.pone.0133852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mutsuddy P, Jeon S, Yoo SW, Zhang Y, Chowdhury MSA, Kim J, et al. Optimization of serum thyroglobulin measured at different time points for prognostic evaluation in differentiated thyroid carcinoma patients. Medicine (Baltimore) 2020;99:e19652. doi: 10.1097/MD.0000000000019652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will not be shared.