Abstract
Glutaredoxins (Grxs) are short, cysteine-rich glutathione (GSH)-mediated oxidoreductases. In this study, a chickpea (Cicer arietinum L.) glutaredoxin [LOC101493651 (CaGrx)] gene has been selected based on screening experiments with two contrasting varieties of chickpea, PUSA-362 (drought-tolerant) and ICC-1882 (drought-sensitive) under drought and salinity. The tolerant variety showed higher CaGrx gene expression, as compared to less in the sensitive variety, under both the stresses. The CaGrx gene was then over-expressed in Arabidopsis thaliana and were exposed to drought and salinity. The over-expression of CaGrx elevated the activity of glutaredoxin, which induced antioxidant enzymes (glutathione reductase; GR, glutathione peroxidase; GPX, catalase; CAT, ascorbate peroxidase; APX, glutathione-S-transferase; GST, superoxide dismutase; SOD, monodehydroascorbate reductase; MDHAR, and dehydroascorbate reductase; DHAR), antioxidants (GSH and ascorbate) and stress-responsive amino acids (cysteine and proline). Enhancement in the antioxidant defense system possibly administered tolerance in transgenics against both stresses. CaGrx reduced stress markers (H2O2, TBARS, and electrolyte leakage) and enhanced root growth, seed germination, and survival against both stresses. The physiological parameters (net photosynthesis; PN, water use efficiency; WUE, stomatal conductance; gs, transpiration; E, electron transport rate; ETR, and photochemical quenching; qP), chlorophylls and carotenoids, were improved in the transgenics during both stresses, that maintained the photosynthetic apparatus and protected the plants from damage. The enhanced activity of the cysteine biosynthesis enzyme, o-acetylserine (thiol) lyase (OAS-TL), increased the cysteine level in the transgenics, which elevated glutathione biosynthesis to maintain the ascorbate–glutathione cycle under both stresses. This investigation verified that the CaGrx gene provides tolerance against salinity and drought, maintaining physiological and morphological performances, and could be exploited for genetic engineering approaches to overcome both the stresses in various crops.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-021-00999-z.
Keywords: Antioxidant, Drought, Salinity, Glutaredoxin, Glutathione, ROS
Introduction
Various abiotic environmental stresses like salinity, drought, cold, abnormal temperatures and heavy metals adversely influence plant growth and crop productivity. Among them, both drought and salinity are critical limitations, affecting agronomical aspects in many regions of the world (Leng and Hall 2019). Yeo (1998) revealed that almost 20% of irrigated and 25% of the total land in the entire world is influenced by salinity. Drought and salinity are the substantial yield-restricting factors in crops that prompt changes in the plant's physiological and biochemical performances (Cruz de Carvalho 2008; Hussain et al. 2019). Abiotic stresses like drought and salinity incite the accumulation of reactive oxygen species (ROS) like singlet oxygen (1O2), hydrogen peroxide (H2O2), superoxide anion (O2·−), and hydroxyl radical (·OH), in the different cellular compartments principally, mitochondria, chloroplasts, as well as peroxisomes (Van Breusegem and Dat 2006; Das and Roychoudhury 2014). Salt stress reduces the osmotic potential of soil that causes water deficit conditions in plants and finally produces oxidative stress in plants by the generation of ROS (Cheeseman 1988). Although ROS is continuously being generated at basal levels under favorable conditions and cannot cause damage due to the action of different antioxidant mechanisms present in the plant (Foyer and Noctor 2005). Some studies explored that ROS work as signal molecules for several metabolic activities and regulates plant development; however, severe abiotic stress induces the higher accumulation of ROS, which can rattle the natural signaling in plants by destructing the essential macromolecules and disturb the cellular redox equilibrium (Gilroy et al. 2016). Excess ROS also causes chlorophyll degradation and alters membrane fluidity through membrane lipid peroxidation (Verma and Mishra 2005). The protective system in plants such as ROS scavengers, various osmoprotectants, antioxidant enzymes, antioxidant molecules (glutathione and ascorbate), and various oxidoreductases (thioredoxins, peroxiredoxins, and glutaredoxins) control excessive ROS and protect plants from damage (Rouhier et al. 2008b).
Glutaredoxins (Grxs) are heat stable and cysteine-rich proteins of nearly a hundred amino acid residues (10–15 kDa) that adjust the cellular redox and redox-mediated signaling. Glutaredoxins are glutathione-mediated oxidoreductases that affect protein function through reversible glutathionylation, with the assistance of NADPH and GR, as a response to oxidative stress (Rouhier et al. 2008a). The cysteine residues of Grx protect the proteins from irreversible oxidation by reversible post-translational alteration of thiol (-SH) groups with the inclusion of GSH, also called S-glutathionylation (Gallogly and Mieyal 2007). Glutaredoxins are involved in ROS reduction as well as redox signaling by activating peroxiredoxins, antioxidant enzymes that reduce H2O2 (Rouhier et al. 2008a).
The comparative genomic analysis has revealed several Grxs in various species, in light of conserved sequences of amino acid as well as cysteine alignment in the active-site (CxxS or CxxC) motifs and distributed into three extensive classes; CGFS, CPYC, and CC-type class (Couturier et al. 2009). The CC-type class is well-known exclusively in terrestrial plants, while CPYC and CGFS are available in all living beings. In Arabidopsis thaliana, almost 31 glutaredoxin genes (17 monocysteinic and 14 bicysteinic) are reported (Rouhier et al. 2004). Several investigations have revealed the physiological, biochemical as well as molecular roles of Grx in plants. Arabidopsis Grx, AtGRXcp, identified by Cheng et al. (2006), enhanced tolerance in yeast grx5 cells under H2O2 as well as during protein’s oxidative stress. Another CGFS type Grx from Arabidopsis, AtGRX4, also effectively took part in plant protection under stress environments (Cheng 2008). Arabidopsis GRXS14 (chloroplast) and GRXS15 (mitochondria) were both implied for protection under oxidative stress induced by H2O2 (Cheng et al. 2006; Bandyopadhyay et al. 2008). A Grx from Pteris vittata, PvGRX5, was reported for its role in plant tolerance under various abiotic stresses such as heavy metal (arsenic), high temperature, and oxidative burst (Sundaram et al. 2009). OsGRX8 from rice, a CC-type Grx gene, increased tolerance under oxidative stress, salinity, and osmotic stress in Arabidopsis (Sharma et al. 2013). A rice CYPC-type Grx gene OsGRX20 enhanced tolerance against salinity, bacterial blight, and methyl viologen (Ning et al. 2018). Wu et al. (2017) revealed that the over-expression of Arabidopsis AtGRXS17 elevated drought tolerance in tomato (Solanum lycopersicum L.). Ding et al. (2019) identified the maize CC-type Grx (ZmGRXCC) associated with drought response. Two rice (Oryza sativa) Grx genes (LOC_Os01g27140 and LOC_Os02g40500) enhanced tolerance in Arabidopsis under drought by inducing the antioxidant defense system and diminished oxidative stress (Kumar et al. 2020b).
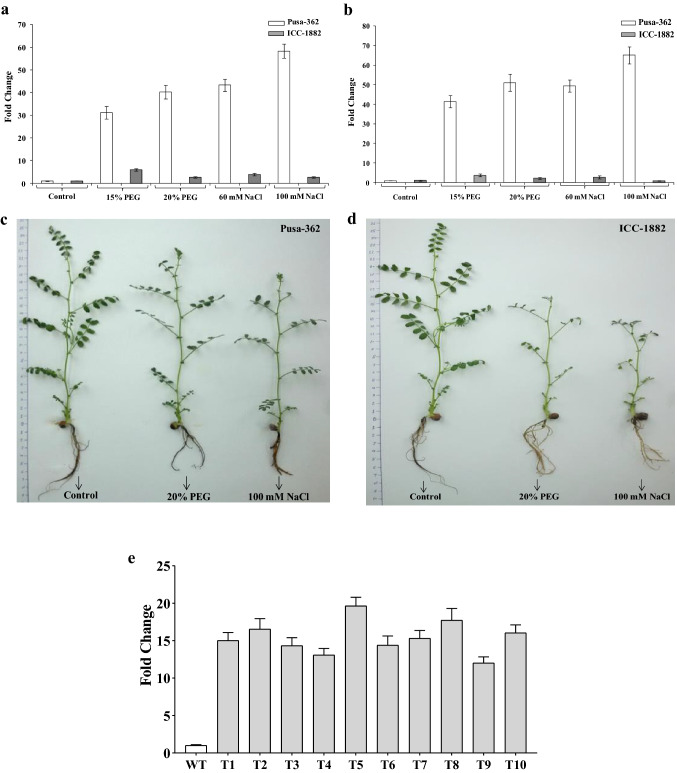
In an earlier study, we have explored the protective role of CaGrx in oxidative stress caused by various heavy metals (Kumar et al. 2020a). In this study, the chickpea Grx gene [LOC101493651 (CaGrx)] has been selected based on its higher expression against drought and salt stress, during the process of screening (Fig. 1a–d), in two different chickpea varieties, PUSA-362 (drought-tolerant) and ICC-1882 (drought-sensitive). The up-regulation of CaGrx gene was observed in the tolerant variety compared to less in the sensitive variety, under both stresses. Higher up-regulation in tolerant variety displays the protective aspect of CaGrx in oxidative stress that arises under both types of stresses. To reveal the probable function of CaGrx in drought and salinity, this CaGrx gene was over-expressed in Arabidopsis thaliana, and over-expressing lines were exposed to drought and salinity. After subjecting to both the stresses, multiple stress-amenable biochemical, physiological, as well as morphological parameters were assessed to explore the possible role of CaGrx against both stresses.
Fig. 1.
qRT-PCR analysis showing the total transcript level of the CaGrx gene. Expression of CaGrx gene in a leaves and b roots of two different varieties of chickpea (Cicer arietinum L.), PUSA-362 (drought-tolerant), and ICC-1882 (drought-sensitive), hydroponically grown under different PEG (15% and 20%) and salt (60 mM and 100 mM NaCl) treatment for one week. Higher up-regulation in the relative expression of the CaGrx gene in roots and leaves of PUSA-362 compared to less in ICC-1882 variety suggests the possible protective role of the CaGrx gene under salt and drought stress. Morphological changes in both varieties of chickpea after one week of treatment (20% PEG and 100 mM NaCl) showed a higher reduction in growth in the sensitive variety, ICC-1882 (c), as compared to the tolerant variety, PUSA-362 (d), under both (salt and drought) the treatments. e The relative expression of CaGrx gene in over-expressing lines (T3 generation) Arabidopsis thaliana driven by CaMV35S promoter. All given values are means of a minimum of three replicates ± SD
Materials and methods
Plant material and growth conditions
Two different varieties of chickpea (Cicer arietinum L.), ICC-1882 (drought-sensitive), and PUSA-362 (drought-tolerant) were used for screening the expression of the CaGrx gene in drought and salt stress. The seeds of both varieties were germinated and grown hydroponically in the tray having perforated cups and supplemented with Hoagland’s medium. Each germination tray had plantlets of both the varieties, tolerant and sensitive, and kept at 24 ± 1 °C in a culture room with the photoperiod (16 h light and 8 h dark). After two weeks, the plantlets were exposed to different PEG (0%, 15%, and 20%) and NaCl (0 mM, 60 mM, and 100 mM) treatments separately in the hydroponic medium for one week, while the control plantlets were grown up in Hoagland’s medium only. Total RNA from the leaves and roots of PEG and NaCl treated chickpea was isolated, and cDNA synthesis was performed to analyze the CaGrx gene expression using gene-specific primers. The relative gene expression data were analyzed using the actin gene of chickpea as an internal control (Table S1).
Arabidopsis thaliana plants (Columbia ecotype) were grown in cups having sterilized soilrite mixture and irrigated with water and nutrient (Hoagland) solution alternatively after 3–4 days. The pots were kept at 22 °C in a growth chamber (Conviron, Canada) with the photoperiod (16 h light and 8 h dark) and light (150 μmol m−2 s−1). The Agrobacterium-mediated transformation was performed at the flowering stage following the procedure of Clough and Bent (1998).
Plasmid construction, plant transformation, and selection of transgenics
The coding region (CDS) of the chickpea Grx [LOC101493651 (CaGrx)] consisting of an ORF of 378 bp was taken from NCBI (NCBI ref seq NC_021164.1). The arcelin 5-I UTR (3′ UTR-137 bp and 5′ UTR-13 bp) from Phaseolus vulgaris (L.) was additionally added in ORF to enhance the mRNA stability as well as translational efficiency (Mishra et al. 2013). After the modification, the artificial synthesis of CDS (558 bp) was performed and cloned at the Bam HI and Sac I restriction site in the cloning vector, pUC 57. The pUC 57 vector containing the CaGrx gene was transformed into E.coli (DH5α strain). After that, the restriction digestion of plasmid was performed, and the digested fragment of CaGrx was ligated with NBRI1.2 (plant expression vector), a customized form of pBI121 containing two scaffold attachment regions (SAR1) from C. arietinum (L.) that cloned at the ends of T-DNA region to increase expression of the transgene (Singh et al. 2016) (Fig. S1). The construct (NBRI1.2-CaGrx) was finally electroporation-mediated transformed into GV3101 (A. tumefaciens strain) in the electroporation system (Gene Pulser, Bio-Rad, USA), and fully grown Arabidopsis (at the flowering stage) plants were transformed using the floral dip procedure given by Clough and Bent (1998). Transformed plants were placed under dark for 24 h and, after that, kept in a plant growth chamber, under controlled conditions (16 h light & 8 h dark photoperiod), at 22 °C and relative humidity (85–90%).
Putatively transformed seeds (T1) were selected on ½ MS + kanamycin (50 mg L−1) plate. Positively screened transformants were grown, and genomic DNA was extracted from leaves using the GenElute™ plant DNA miniprep kit (Sigma, USA) to perform PCR amplification (Table S1) with the help of specific primers that confirmed gene insertion within the transformants (Fig. S2). CaGrx over-expressing plants were fully grown up to the third-generation (T3) for achieving homozygosity. The analysis was accomplished within the ten (T1 to T10) over-expressing lines of the T3 generation and also with untreated control (C), treated wild-type (WT), and transgenic control (TC) plants. The control Col-0 (C) and transgenic control (TC) plants were used under controlled conditions (well-watered) during the whole experiment.
Expression study of CaGrx by qRT-PCR
Total RNA from leaves of transgenic (T3 generation), as well as wild-type (WT) control, were isolated with the help of Spectrum plant RNA miniprep kit (Sigma, USA) and evaluated the expression of CaGrx gene driven by CaMV35S promoter. cDNA was prepared using the cDNA synthesis kit (Sigma, USA), and cDNA was used in qRT-PCR to quantify the gene expression by using the SYBR Green qPCR Master Mix (Applied Biosystems, USA). As an internal control, the Arabidopsis β-actin gene was used. The qRT-PCR was conducted in StepOne qRT-PCR machine (Applied Biosystems, USA). The CaGrx gene expression was evaluated according to the 2−^^CT methodology (Livak and Schmittgen 2001). The primers for each gene are given in Table S1.
Assessment of germination and growth tolerance under drought and salinity stress
The CaGrx gene's role in drought and salt tolerance was observed by assessing the germination efficacy of the seeds of transgenic and control either in ½ MS medium or ½ MS supplemented with 300 mM mannitol and 200 mM NaCl separately. The seeds were disinfected with 70% (v/v) ethanol, treated by 2% (v/v) sodium hypochlorite, finally, with 5% (v/v) labolene detergent, and washed several times with autoclaved water. After sterilization, the seeds were plated on ½ MS plate and initially incubated at 4 °C for 2–3 days, and then in a growth chamber set at 22 °C for five days. After five days, the germination efficacy was recorded in transgenic seeds along with (Col-0) control under drought and salinity treatments, respectively.
To measure the impact of drought on root growth, 5 days old plantlets of control (Col-0) and transgenic were transferred separately on ½ MS agar plate enriched with 300 mM mannitol and 200 mM NaCl, and root growth was monitored after 5 days. Five days old Arabidopsis plantlets (transgenic and control) were shifted from ½ MS plate to cups having inert soilrite potting mixture and allowed to grow for 2 weeks in well-watered conditions. The plantlets were irrigated with water and nutrient solution (Hoagland) alternately after 3 days with 50 ml of water (water holding capacity of the cup's soilrite mix is 55 ml). After the growth of plantlets (2 weeks old), transgenic and WT were subjected to drought by withholding water for 10 days. For salt stress, 50 ml nutrient media enriched with 200 mM NaCl for each cup was provided to the transgenic as well as WT plants repeatedly after three days. Well-watered and treated (drought and salt) sampling was done after ten days during the entire study.
Estimation of biochemical parameters under drought and salinity stress
The total protein from transgenic as well as control plant leaves was separated by utilizing the Protein isolation Kit (Merck, Germany) and spectrophotometrically estimated at 595 nm following the methodology of Bradford (1976).
The ratio between reduced and oxidized glutathione (GSH: GSSG) was analyzed according to Rahman et al. (2006). The level of ascorbate (Asc), as well as dehydro-ascorbate (DHA), was estimated at 525 nm following the procedure of Kampfenkel et al. (1995).
The estimation of TBARS (thiobarbituric acid reactive substances) was performed following Hodges et al. (1999) and quantified by deducting the turbidity measured at 600 nm from that at 532 nm. H2O2 quantification was performed following the method of Sergiev et al. (1997), and the absorbance was measured at 390 nm.
For the enzymatic analysis, about 300 mg of fresh leaves were crushed in liquid nitrogen, and the extract was made in 3 ml of phosphate (100 mM, pH 7.5) buffer along with EDTA (1 mM) and 1% PVP (polyvinylpyrrolidone) followed by centrifugation for 15 min at 12,000 rpm at 4 °C.
The activity of glutaredoxin was estimated using HED (2-hydroxyethyl disulfide), and absorbance was recorded at 340 nm (Holmgren and Aslund 1995). The glutathione reductase (GR; EC 1.6.4.2) activity was quantified, following the methodology of Smith et al. (1988), by estimating the transformation of 1 mM of GSSG into GSH per min. The glutathione peroxidase (GPX; EC 1.11.1.9) activity was quantified by the procedure of Takeda et al. (1993) and quantified at 340 nm. The glutathione S-transferase (GST; EC 2.5.1.13) activity was quantified by the coupling of CDNB (1-chloro, 2, 4-dinitrobenzene) with glutathione (GSH) at 340 nm (Habig et al. 1974). Superoxide dismutase (SOD; EC 1.15.1.1) activity was estimated following Beauchamp and Fridovich (1971), and the absorbance was recorded at 560 nm. Catalase (CAT; EC 1.11.1.6) activity was quantified according to Chandlee and Scandalios (1984) and observed at 240 nm. Ascorbate peroxidase (APX; EC 1.11.1.11) activity was quantified following Nakano and Asada (1981) procedure, and absorbance was measured at 290 nm. The guaiacol peroxidase (POD; EC 1.11.1.7) activity was estimated using a method of Hemeda and Klein (1990) at 470 nm.
Monodehydroascorbate reductase (MDHAR; EC 1.6.5.4) activity was analyzed following the methodology of Vanacker et al. (1998), and the absorbance was measured at 340 nm. The dehydroascorbate reductase activity (DHAR; EC 1.8.5.1) was quantified by the methodology of Doulis et al. (1997) and recorded at 265 nm.
The biochemical parameters methods have been described in detail in our earlier publication (Kumar et al. 2020a).
Estimation of drought-amenable amino acids
The estimation of cysteine was accomplished by the method of Gaitonde (1967). The reaction composition was 0.5% pre-chilled trichloroacetic acid (TCA), glacial acetic acid, plant extract, acid ninhydrin reagent, and the absorbance was taken at 560 nm. Proline estimation was performed following the methodology of Bates et al. (1973). The reaction composition was ninhydrin, glacial acetic acid, and plant extract in equal ratio and incubated for one h at 100 °C. Thereafter, 4 ml toluene was mixed, and absorbance was recorded at 520 nm.
Estimation of cysteine biosynthesis catalyzing enzyme o-acetylserine (thiol) lyase (OAS-TL)
The activity of OAS-TL (o-acetylserine (thiol) lyase) was estimated following the procedure of Gaitonde (1967). The reaction was performed at room temperature by preparing a reaction mixture containing HEPES (100 mM; pH 7.5), Na2S (10 mM), OAS (10 mM), DTT (5 mM), and isolated protein sample and represented as the amount of the enzyme which catalyzes the formation of 1 µmol cysteine per min.
Estimation of chlorophyll and carotenoids levels
Chlorophyll (Chl a and Chl b) and total carotenoid (Cx+c) content were measured from control and treated (drought and salinity) Arabidopsis leaves. Initially, at least five leaves (approximately 20 mg) were harvested from each of the well-watered as well as treated plants, and the extract was prepared in 80% acetone. Chlorophyll and total carotenoids were estimated according to Wellburn (1994) by using equations which are as follows:
Measurement of physiological performance
The physiological parameters were measured in over-expressing lines, as well as control plants with or without treatment. The water use efficiency (WUE), net photosynthetic rate (PN), stomatal conductance (gs), transpiration (E), Fv/Fm (variable to maximum fluorescence) proportion, qP (photochemical quenching), NPQ (non-photochemical quenching), and ETR (electron transport rate) were recorded in entirely open leaves with a photosynthetic system, Li-6400 (LI-COR, USA). The level of CO2 in the leaf chamber was retained at 400 µmol (CO2) mol−1 air. The photosynthetic photon flux density (PPFD) was retained at 300 μmol (photons) m−2 s−1. The level of VPD (vapor pressure deficit) was lower than 2 kPa. The leaf temperature was at 25 °C, and RH (relative humidity) was 55–60%. All the physiological parameters were recorded between 07:00 to 10:00 h.
Evaluation of RWC (relative water content)
The fresh weight (FW) of 4–5 fresh leaves was recorded, and then they were incubated in water at room temperature for 4–6 h to become fully turgid, and then the turgid weight (TW) was measured. The fully turgid leaves were dried entirely for 48–72 h at 70 °C, and their dry weight (DW) was measured (Lafitte 2002). The given equation was performed to measure the relative water content:
Estimation of electrolyte leakage (relative electrolyte conductivity)
The relative electrolyte conductivity was measured following the method of Bandurska (2000). Approximately 100 mg leaves were cut into pieces and incubated in autoclaved water at 25 °C for three hours, and conductivity was recorded by an Electrolyte Conductivity (EC) meter (Eutech PC700, Thermo Scientific™). After that, leaves were boiled for 20 min at 70 °C and cooled at room temperature. After cooling, conductivity was re-recorded. The following formula estimated the percentage injury index (I):
where C1—conductivity of samples before boiling, C2—conductivity of samples after boiling.
Data analysis
All reported result quantities are the means of a minimum of three individual replicates. The standard error was estimated from the mean of the three replicates. The estimated data were applying to one-way ANOVA through DMRT (Duncan’s Multiple Range Test) to the estimation of the significant difference among the means (p < 0.05).
Results
Expression profile of CaGrx
The higher expression of the CaGrx gene was found during screening in two different chickpea varieties, PUSA-362 (drought-tolerant) and ICC-1882 (drought-sensitive), against drought and salt stress. The tolerant variety showed higher expression, compared to less in the sensitive variety, under both the stresses (Fig. 1a, b). Tolerant variety showed morphologically better than sensitive variety under both stresses (Fig. 1c, d). Further CaGrx gene was transformed in Arabidopsis thaliana, and expression of the CaGrx in transgenics was estimated by qRT-PCR analysis. Arabidopsis actin gene was used as an internal control. The results obtained showed the enhanced transcript level of CaGrx, ranging from 12 (T-9 line) to 19 (T-5 line) fold change (Fig. 1e).
Over-expression of CaGrx enhanced plant growth against drought and salinity
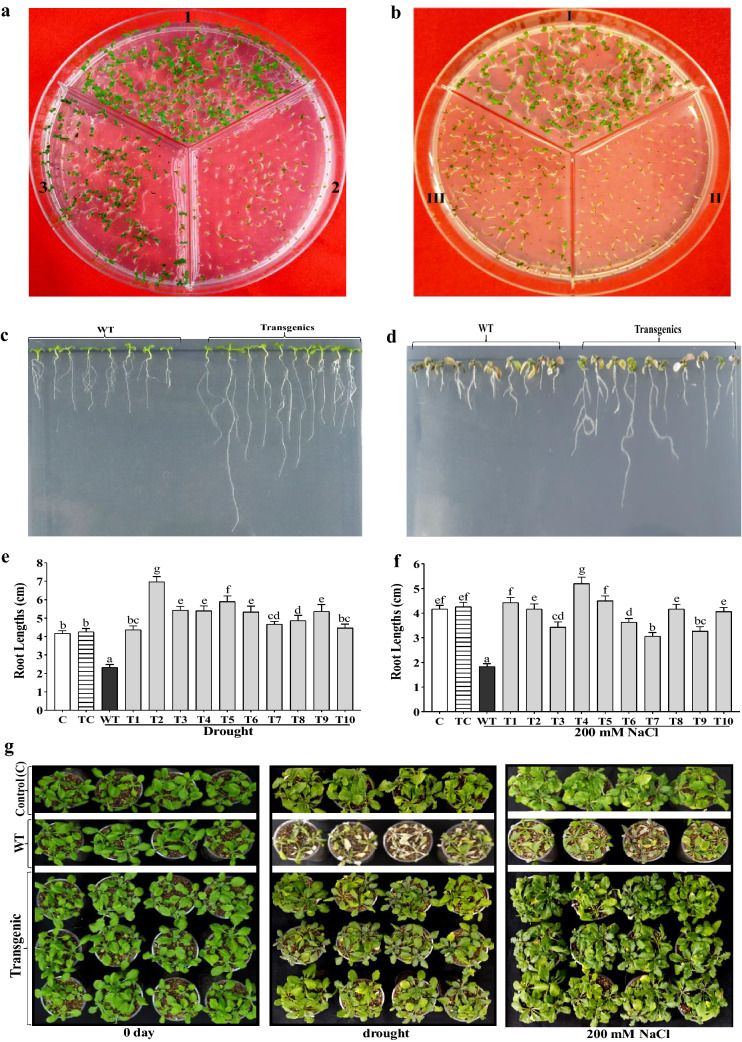
The survival adequacy of transgenic seeds was elevated considerably during germination on 300 mM mannitol and 200 mM NaCl supplemented ½ MS plate separately in comparison to inconsequential in non-transformed control (Col-0) seeds (Fig. 2a, b). Root advancement under five days of drought on 300 mM mannitol and five days salt salinity on 200 mM NaCl supplemented ½ MS plates showed higher root growth in CaGrx transgenics in comparison with their WT control plants (Fig. 2c–f). Drought and salt tolerance were found in transgenic plantlets of the CaGrx gene following ten days of drought and salt stress separately compared to hindered growth in their WT plants (Fig. 2g).
Fig. 2.
The over-expression of the CaGrx gene increase drought and salinity tolerance in Arabidopsis. a The germination efficacy of Arabidopsis seeds of LOC101493651 together with control (Col-0) in ½ MS plate augmented with 300 mM mannitol. (1) Col-0 seeds inoculated in ½ MS medium, (2) Col-0 seeds in ½ MS + 300 mM mannitol, and (3) transgenic seeds inoculated in ½ MS + 300 mM mannitol. b The germination efficacy of Arabidopsis seeds of LOC101493651 with control (Col-0) in ½ MS plate augmented with 200 mM NaCl. (I) Col-0 seeds inoculated in ½ MS medium, (II) Col-0 seeds in ½ MS + 200 mM NaCl, and (III) transgenic seeds inoculated in ½ MS + 200 mM NaCl. Morphological growth of root, as well as the measurement of root length in transgenic Arabidopsis of LOC101493651 in ½ MS plate, augmented with 300 mM mannitol (c, e) and ½ MS plate, augmented with 200 mM NaCl (d, f) after five days. g After ten days of drought by with-holding water and salinity by 200 mM NaCl, transgenic Arabidopsis plants of LOC101493651 showed enhanced growth in comparison to their wild-type (WT) plants. Control (C) plants were maintained in well-watered controlled conditions throughout experiments. The standard error was estimated from the mean of three replicates. The estimated data were applying to one way ANOVA through DMRT (Duncan’s Multiple Range Test) to the estimation of the significant difference among the means (p < 0.05)
Impact of CaGrx over-expression on the stress markers and antioxidants level under salinity and drought stress
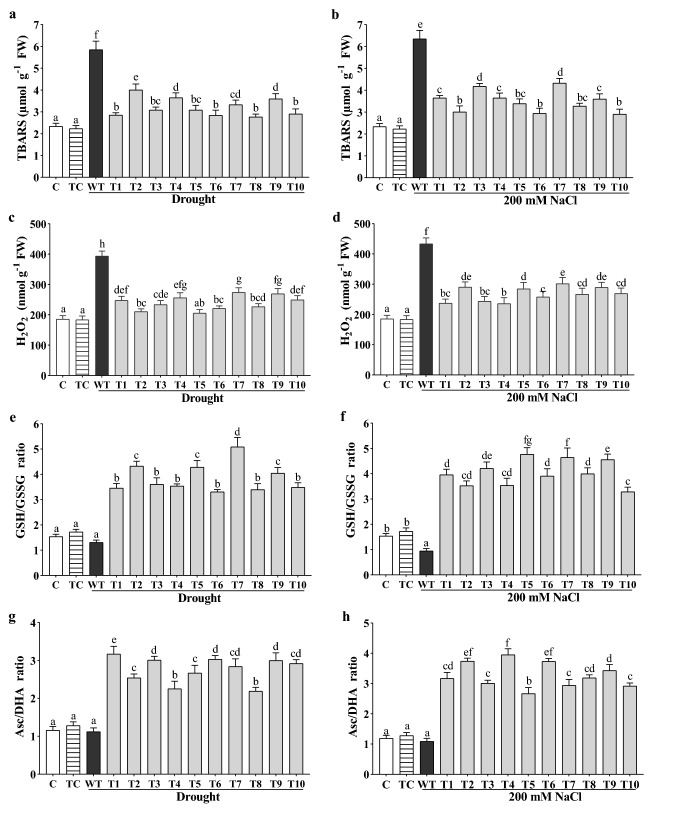
Numerous changes were observed in the biochemical parameters under drought and salt stress. TBARS (Thiobarbituric acid reactive substances) functions as a marker, which shows the stress levels in plants, synthesized during lipid peroxidation, and its quantity reveals the stress level of plants. Under drought, the highest reduction by CaGrx was up to 51% (T-8 line), average 43%, and under salt stress, the highest reduction by CaGrx was up to 55% (T-10 line), average 47%, compared to their WT controls, which showed that the transgenic lines managed a less stressful environment than WT control plants (Fig. 3a, b). The H2O2 level likewise was decreased in all transgenic lines (Fig. 3c, d) against both stresses. The highest reduction for CaGrx under drought was up to 48% (T-5 line), average 40%, and up to 45% (T-4 line), average 39%, under salinity in comparison to their WT control plants. Transgenics showed slightly higher levels of TBARS and H2O2 under both stresses than non-stressed controls.
Fig. 3.
Over-expression of CaGrx affects stress markers (H2O2 and TBARS) and antioxidant molecules (GSH/GSSG and Asc/DHA ratio) under drought and salinity. The levels of stress markers a, b TBARS and c, d H2O2 were reduced in all CaGrx overexpressing lines in comparison to wild-type (WT) plants under salinity and drought, respectively. The levels of antioxidant molecules, e, f GSH/GSSG ratio, g, h Asc/DHA ratio in Arabidopsis of LOC101493651 were increased in comparison to wild-type (WT) against both stresses. Col-0 control (C) and transgenic control (TC) in well-watered conditions also have been evaluated. The standard error was estimated from the mean of three replicates. The estimated data were applying to one way ANOVA through DMRT (Duncan’s multiple range test) to the estimation of the significant difference among the means (p < 0.05)
Both drought and salt induce ROS accumulation, which causes changes in the cellular environment from reducing to oxidizing. The antioxidant systems trigger and enhance reductants' synthesis like glutathione (GSH) to maintain the cell's reducing environment against ROS. In the present study, the proportion of GSH/GSSG was expanded in all transgenic lines under stress. The maximum increased proportion for transgenics was found to be 290% (T-7 line), average 195%, under drought, and 392% (T-5 line), average 328%, during salt stress, in comparison to their WT plants (Fig. 3e, f). Essentially, the proportion of Asc/DHA was expanded in transgenic lines under both stresses in contrast to their WT plants. The maximum increased proportion for transgenics was found to be 154% (T-1 line), average 146%, under drought, and 262% (T-4 line), average 192%, under salt stress (Fig. 3g, h). Transgenics also showed the higher proportion of GSH/GSSG and Asc/DHA under both stresses than non-stressed controls.
CaGrx induced the accumulation of drought-amenable amino acids
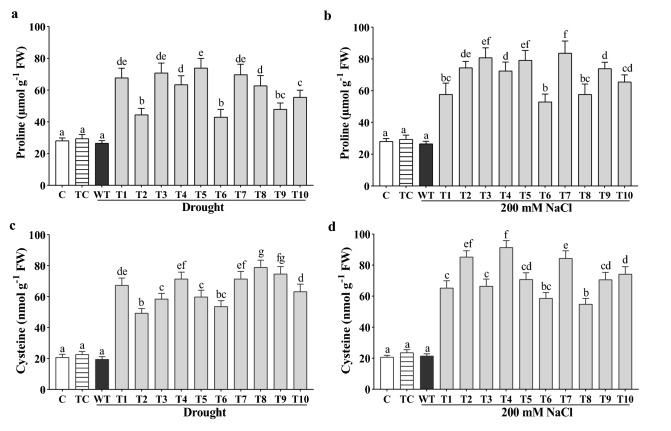
Both amino acids, proline as well as cysteine, give a response under drought and salinity stress. In this study, the proline and cysteine concentration was elevated in all the transgenic lines compared to WT plants and non-stressed controls. The highest concentration of proline was found ~ threefold (T-5 line), average 2.3 fold, under drought as well as also ~ threefold (T-7 line), average 2.7 fold, under salinity (Fig. 4a, b). The highest enhancement of cysteine was found ~ fourfold (T-8 line), average 3.3 fold, under drought, and ~ fivefold (T-4 line), average 3.8 fold, under salt stress (Fig. 4c, d).
Fig. 4.
Increased content of stress-amenable amino acids under drought and salinity in transgenic Arabidopsis transformed with the CaGrx gene. a, b Proline and c, d cysteine contents were elevated in the over-expressing lines of LOC101493651 in comparison to wild-type (WT) plants against both stresses. Col-0 control (C) and transgenic control (TC) in well-watered controlled conditions also have been evaluated. The standard error was estimated from the mean of three replicates. The estimated data were applying to one way ANOVA through DMRT (Duncan’s Multiple Range Test) to the estimation of the significant difference among the means (p < 0.05)
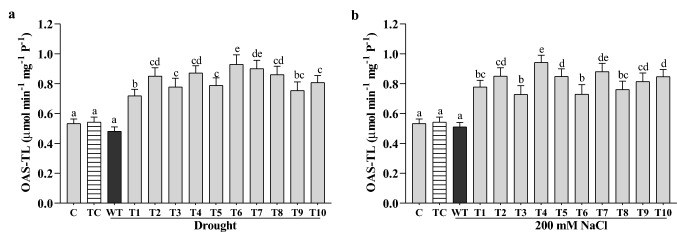
CaGrx enhanced the activity of cysteine biosynthesis catalyzing enzyme (OAS-TL) under drought and salt stress
OAS-TL (O-acetylserine (thiol) lyase) catalyzes the biosynthesis of cysteine, which is essential in glutathione (GSH) biosynthesis and stress response. In our investigation, the OAS-TL activity was elevated in all the CaGrx transgenic lines under drought and salinity compared to their WT plants. The highest increased activity was found to be 90% (T-6 line), average 70%, under drought and 84% (T-4 line), average 60%, under salinity in comparison to their WT plants (Fig. 5a, b).
Fig. 5.
Over-expression of CaGrx enhanced the activity of cysteine biosynthesis catalyzing enzyme, o-acetyl serine (thiol) lyase (OAS-TL; μmol min−1 mg−1 P−1) under (a) drought and (b) salinity. Col-0 control (C) and transgenic control (TC) in well-watered controlled conditions also have been evaluated. The standard error was estimated from the mean of three replicates. The estimated data were applying to one way ANOVA through DMRT (Duncan’s Multiple Range Test) to the estimation of the significant difference among the means (p < 0.05)
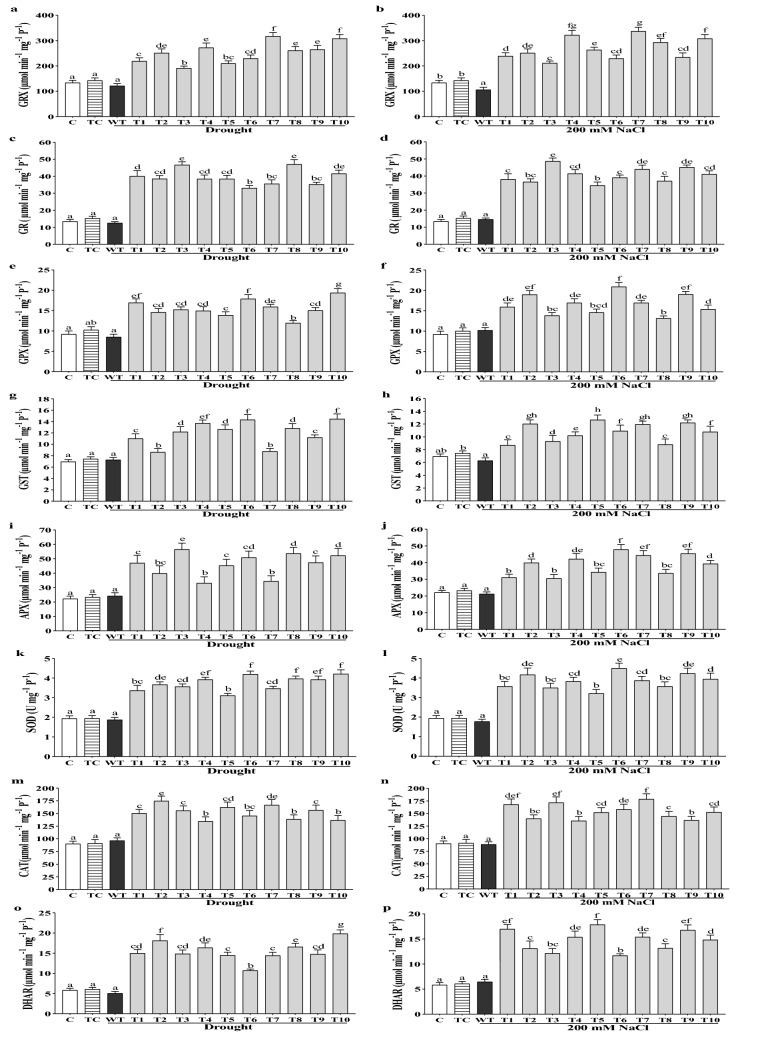
Over-expression of CaGrx raised the activity of antioxidant enzymes under drought and salinity stress
Glutaredoxins are known as redox enzymes, that function as an antioxidant to combat oxidative stress. In this study, Grx activity was elevated considerably in all the transgenic lines under drought and salinity than their WT plants and non-stressed controls. The maximum increased activity of Grx was found to be 163% (T-7 line), average 110%, under drought, and 212% (T-7 line), average 155%, under salinity, in comparison to their WT plants (Fig. 6a, b).
Fig. 6.
Over-expressing lines of CaGrx showed increased activities of antioxidant enzymes under salinity and drought. Antioxidant enzyme assays of the over-expressing lines of LOC101493651 with wild-type (WT) control. a, b Grx (μmol min−1 mg−1 P−1), c, d GR (μmol min−1 mg−1 P−1), e, f GPX (μmol min−1 mg−1 P−1), g, h GST (μmol min−1 mg−1 P−1), i, j APX (μmol min−1 mg−1 P−1), k, l SOD (U mg−1 P−1), m, n CAT (μmol min−1 mg−1 P−1), o, p DHAR (μmol min−1 mg−1 P−1). Col-0 control (C) and transgenic control (TC) in well-watered controlled conditions also have been evaluated. The standard error was estimated from the mean of three replicates. The estimated data were applying to one way ANOVA through DMRT (Duncan’s Multiple Range Test) to the estimation of the significant difference among the means (p < 0.05)
Glutathione reductase (GR) activity was elevated in all the transgenic lines under both stresses in comparison to their WT plants and non-stressed controls. The highest increase in activity was found to be 261% (T-8 line), average 215%, under drought stress, and 231% (T-3 line), average 180%, under salt stress (Fig. 6c, d).
Glutathione peroxidase (GPX) activity was raised in all the transgenic lines under both stresses compared to their WT plants and non-stressed controls. The maximum increased activity was 127% (T-10 line), average 84%, under drought and 103% (T-6 line), average 62%, under salt stress (Fig. 6e, f).
GST's activity (glutathione S-transferase) was seen as expanded in all the transgenic lines under both stresses in comparison to their WT plants and non-stressed controls. The maximum increase in activity was 98% (T-10 line), average 66%, under drought and 102% (T-5 line), average 72%, under salt stress (Fig. 6g, h).
APX (ascorbate peroxidase) actively performs the conversion of H2O2 into H2O by using ascorbate (Asc) as an electron donor. In this study, the APX activity was enhanced in all the transgenic lines under drought and salinity in comparison to their WT plants and non-stressed controls. The highest increased activity was obtained 133% (T-3 line), average 92%, under drought and 123% (T-6 line), average 81%, under salinity (Fig. 6i, j). The activity of SOD (Fig. 6k, l), catalase (Fig. 6m, n), and DHAR (Fig. 6o, p) were also enhanced in all the transgenic lines under drought and salinity in comparison to their WT plants as well as non-stressed controls.
Most of the CaGrx over-expressing lines were observed to show the enhanced activity of POD and MDHAR under drought and salinity in comparison to their WT plants and non-stressed controls (Supplementary Fig. S3). The obtained results supported the hypothesis that the over-expression of CaGrx enhanced the activities of antioxidant enzymes and protected the plants under drought as well as salt stress.
Over-expression of CaGrx maintained the chlorophyll and carotenoid contents under drought and salinity
Chlorophyll helps plants to absorb energy from light and maintain the activity of photosystems during photosynthesis. Under drought and salinity, chlorophyll content is reduced and results in the impairment of photosynthesis. In our study, Chl a and Chl b were observed to be elevated in all the transgenic lines under both stresses in comparison to their WT plants and nearer to the non-stressed controls. The maximum enhancement in Chl a was found to be 130% (T-7 line), average 91%, under drought, and 103% (T-5 line), average 85%, under salinity compared to WT plants (Table 1). Similarly, The maximum enhancement in Chl b was observed to be 86% (T-9 line), average 56%, under drought, and 65% (T-9 line), average 40%, under salt stress (Table 1). The total chlorophyll (Chl a + Chl b) content and the ratio between Chl a/Chl b were higher in transgenic lines under both the stresses than their WT plants (Table 1).
Table 1.
a and b The levels of chlorophyll and carotenoid content under drought and salinity
| Arabidopsis plants | Chl a (mg g−1 FW) | Chl b (mg g−1 FW) | Chl a + b (mg g−1 FW) | Chl a/b ratio | Total carotenoids (mg g−1 FW) |
|---|---|---|---|---|---|
| (a) | |||||
| C | 1.17 ± 0.10f | 0.79 ± 0.05gh | 1.97 ± 0.17e | 1.47 ± 0.35bc | 0.18 ± 0.01a |
| TC | 1.21 ± 0.09f | 0.83 ± 0.06h | 2.04 ± 0.16e | 1.44 ± 0.65bc | 0.19 ± 0.01a |
| WT | 0.49 ± 0.02a | 0.38 ± 0.02a | 0.88 ± 0.05a | 1.27 ± 0.09a | 0.21 ± 0.01a |
| T-1 | 1.06 ± 0.08b | 0.65 ± 0.04ef | 1.71 ± 0.11d | 1.63 ± 0.28cd | 0.31 ± 0.02bc |
| T-2 | 0.86 ± 0.05de | 0.45 ± 0.02b | 1.32 ± 0.09b | 1.91 ± 0.11e | 0.32 ± 0.03bc |
| T-3 | 1.11 ± 0.09bc | 0.58 ± 0.02de | 1.70 ± 0.10cd | 1.89 ± 0.24e | 0.24 ± 0.01ab |
| T-4 | 0.82 ± 0.08c | 0.57 ± 0.03de | 1.40 ± 0.11bc | 1.43 ± 0.23bc | 0.37 ± 0.03cd |
| T-5 | 0.93 ± 0.08e | 0.60 ± 0.08e | 1.54 ± 0.14c | 1.54 ± 0.43c | 0.25 ± 0.01ab |
| T-6 | 0.87 ± 0.09c | 0.63 ± 0.04ef | 1.51 ± 0.11c | 1.37 ± 0.31b | 0.43 ± 0.03d |
| T-7 | 1.13 ± 0.10de | 0.69 ± 0.04fg | 1.82 ± 0.16de | 1.63 ± 0.62cd | 0.32 ± 0.02bc |
| T-8 | 0.83 ± 0.06d | 0.48 ± 0.03bc | 1.31 ± 0.12b | 1.74 ± 0.83d | 0.30 ± 0.02bc |
| T-9 | 0.92 ± 0.07d | 0.71 ± 0.05fg | 1.63 ± 0.14cd | 1.29 ± 1.82a | 0.41 ± 0.03d |
| T-10 | 0.89 ± 0.06d | 0.54 ± 0.03cd | 1.43 ± 0.13bc | 1.65 ± 1.82cd | 0.35 ± 0.02bcd |
| (b) | |||||
| C | 1.17 ± 0.10f | 0.79 ± 0.05f | 1.97 ± 0.17g | 1.47 ± 0.12b | 0.18 ± 0.01b |
| TC | 1.21 ± 0.09f | 0.83 ± 0.06f | 2.04 ± 0.16g | 1.44 ± 0.09b | 0.19 ± 0.01b |
| WT | 0.54 ± 0.03a | 0.40 ± 0.04a | 0.95 ± 0.04a | 1.34 ± 0.09a | 0.17 ± 0.01a |
| T-1 | 0.88 ± 0.04b | 0.57 ± 0.03cd | 1.45 ± 0.12b | 1.54 ± 0.45bc | 0.26 ± 0.01c |
| T-2 | 1.09 ± 0.07de | 0.45 ± 0.03b | 1.55 ± 0.10c | 2.39 ± 0.15h | 0.31 ± 0.02d |
| T-3 | 0.92 ± 0.06bc | 0.52 ± 0.02cd | 1.45 ± 0.11b | 1.78 ± 0.09e | 0.28 ± 0.01cd |
| T-4 | 0.95 ± 0.06c | 0.48 ± 0.03bc | 1.43 ± 0.08b | 1.97 ± 0.13f | 0.32 ± 0.02de |
| T-5 | 1.10 ± 0.08e | 0.64 ± 0.04e | 1.74 ± 0.05f | 1.68 ± 0.09d | 0.27 ± 0.02cd |
| T-6 | 0.94 ± 0.05c | 0.53 ± 0.05c | 1.47 ± 0.09b | 1.76 ± 0.14e | 0.31 ± 0.03d |
| T-7 | 1.08 ± 0.09de | 0.47 ± 0.04bc | 1.55 ± 0.10c | 2.28 ± 0.16g | 0.30 ± 0.02d |
| T-8 | 1.05 ± 0.07d | 0.53 ± 0.05c | 1.59 ± 0.12cd | 1.96 ± 0.12f | 0.24 ± 0.01c |
| T-9 | 1.02 ± 0.10d | 0.65 ± 0.03e | 1.67 ± 0.10e | 1.55 ± 0.13bc | 0.27 ± 0.02cd |
| T-10 | 1.00 ± 0.09d | 0.55 ± 0.04cde | 1.55 ± 0.09c | 1.81 ± 0.10ef | 0.33 ± 0.01e |
Chlorophyll (Chl a, Chl b, Chl a + b, Chl a/b) and total carotenoid (Cx+c) content of over-expressing lines of CaGrx under drought (a) and salinity (b) respectively in comparison to wild-type (WT) plants. Col-0 control (C) and transgenic control (TC) in well-watered controlled conditions also have been evaluated. The standard error was estimated from the mean of the three replicates. The estimated data were applying to one way ANOVA through DMRT (Duncan’s Multiple Range Test) to the estimation of the significant difference among the means (p < 0.05)
Carotenoids are light-harvesting pigments and play a photoprotective role by quenching the triplet chlorophyll molecules, and scavenging ROS formed within the chloroplast. Drought, as well as salt stress, reduced the level of carotenoids and damaged the photosynthetic systems. In this study, the carotenoid levels were observed to increase in all the transgenic lines under drought and salinity compared with their WT plants and non-stressed controls. The maximum enhancement in carotenoid content was found to be 104% (T-6 line), average 60%, under drought, and 94% (T-10 line), average 70%, under salt stress (Table 1).
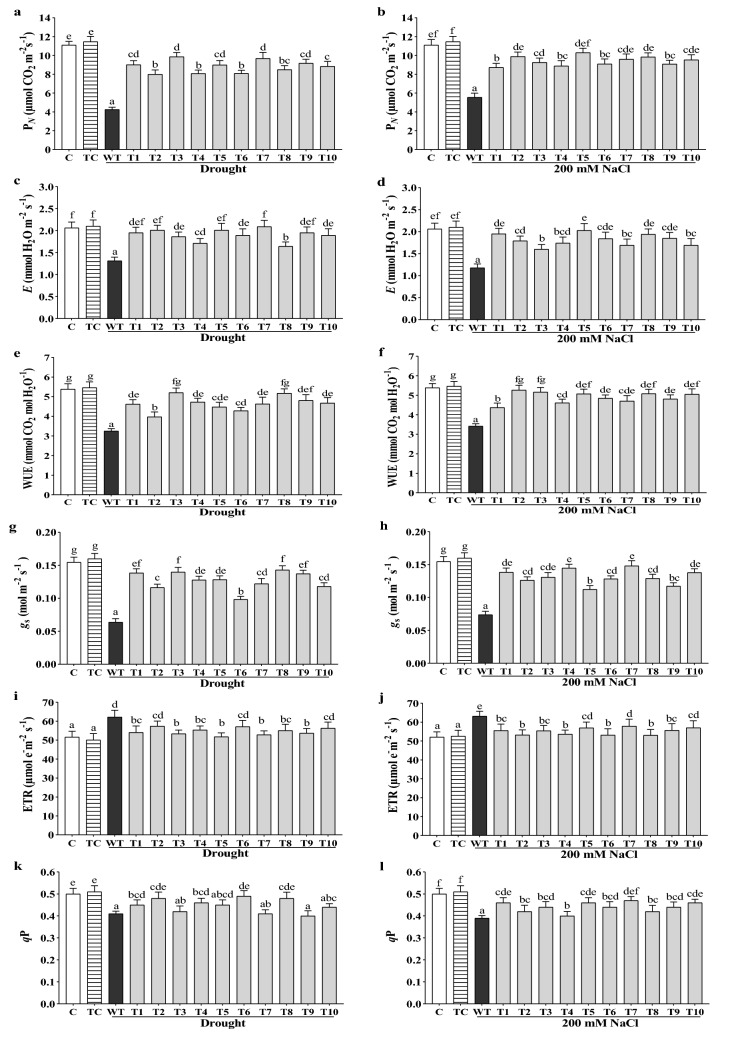
Over-expression of CaGrx maintained the physiological parameters under drought and salinity
Different physiological parameters were explored during drought and salt stress, and they were improved in transgenic lines of CaGrx compared to WT plants but slightly lesser than non-stressed controls. Net photosynthesis (PN) was considerably increased in all the transgenic lines under drought and salinity in comparison to their WT plants. The maximum enhancement was observed to be 132% (T-3 line), average 107%, under drought and 85% (T-5 line), average 70%, under salinity (Fig. 7a, b).
Fig. 7.
Over-expression of CaGrx maintains the physiological performance of Arabidopsis against drought and salinity. a, b Net photosynthesis (PN; μmol CO2 m−2 s−1), c, d transpiration (E; mmol H2O m−2 s−1), e, f WUE (mmol CO2 mol H2O−1), g, h stomatal conductance (gs; mol m−2 s−1), i, j ETR (µmol e− m−2 s−1), k, l PHOTOCHEMICAL quenching (qP). Col-0 control (C) and transgenic control (TC) in well-watered controlled conditions also have been evaluated. The standard error was estimated from the mean of three replicates. The estimated data were applying to one way ANOVA through DMRT (Duncan’s Multiple Range Test) to the estimation of the significant difference among the means (p < 0.05)
CaGrx gene elevates carbon assimilation by expanding stomatal conductance and the transpirational rate under both stresses compared to their WT plants. The maximum enhancement in transpiration rate (E) was found to be 59% (T-7 line), average 46%, under drought, and 72% (T-5 line), average 53%, under salt stress (Fig. 7c, d).
During prolonged water deficit conditions, the Water-Use Efficiency (WUE) enhancement is a response reaction in plants to provide tolerance. Salt stress also reduces WUE in plants. In this study, WUE was heightened in all the transgenic lines under drought and salinity stress compared to their WT plants. The maximum enhancement in WUE was observed to be 62% (T-3 line), average 44%, under drought and 54% (T-2 line), average 43%, under salinity (Fig. 7e, f).
Stomatal conductance (gs) was also found to be the higher all the transgenic lines under both stresses in comparison with their WT control plants. The maximum enhancement in stomatal conductance was observed to be 112% (T-8 line), average 98%, and 102% (T-7 line), average 80%, under salt stress (Fig. 7g, h).
The electron transport rate (ETR) increases when photosystem II is over-excited; this condition force heat consumption by NPQ (non-photochemical quenching), and finally, photosynthesis decreases. In this study, ETR values of transgenic lines under drought and salinity were lesser than their WT plants but slightly higher than non-stressed controls (Fig. 7i, j). The photochemical quenching (qP) was observed to be heightened in all the transgenic lines under drought as well as salt stress in comparison to their WT plants (Fig. 7k, l). A slightly higher Fv/Fm values and lesser activity of non-photochemical quenching (NPQ) in the transgenics of CaGrx under both stresses than their WT control were also observed (Supplementary Fig. S4).
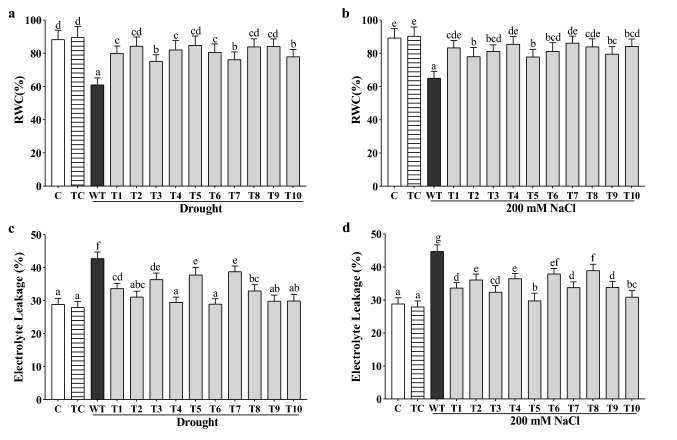
Over-expression of CaGrx maintained the relative water content (RWC) and reduced electrolyte leakage against drought and salinity
The relative water content (RWC) was maintained nearly to the non-stressed controls in all transgenic lines under drought and salinity in comparison to less in their WT plants. The maximum RWC was observed to be 84.84% (T-5 line), an average 81%, under drought and 86.19% (T-7 line), an average 82%, under salinity (Fig. 8a, b).
Fig. 8.
Enhanced relative water content (RWC) and reduced electrolyte leakage (%) were found in the CaGrx transgenic lines under drought and salinity. a, b RWC of CaGrx over-expressed plants was higher compared to wild-type (WT) under both stresses. c, d Electrolyte leakage (%) decreased in transgenics of CaGrx in comparison to highest in wild-type (WT) under both stresses. Col-0 control (C) and transgenic control (TC) in well-watered controlled conditions also have been evaluated. The standard error was estimated from the mean of three replicates. The estimated data were applying to one way ANOVA through DMRT (Duncan’s Multiple Range Test) to the estimation of the significant difference among the means (p < 0.05)
Water scarcity and salinity in plants lead to cell membrane damage, enhancing the ion's permeability, and is evaluated by estimating the electrolyte leakage (Relative Conductivity). In this study, under both stresses, WT plants showed the highest percentage of electrolyte leakage in drought (43%) and salinity (45%) in comparison to less in transgenic lines. Transgenics showed electrolyte leakage nearly to the non-stressed controls under both stresses. The rate of electrolyte leakage (EL) ranges from 27% (T-6 line) to 38% (T-7 line), average 32%, under drought, and 27% (T-6 line) to 38% (T-7 line), average 34%, under salinity, for transgenics displayed less cell membrane injury (Fig. 8c, d).
Discussion
Past investigations uncovered the vital role of glutaredoxins in abiotic stress tolerance like oxidative stress (Fernandes and Holmgren 2004; Laporte et al. 2011) and metals (Sundaram et al. 2009), but in salinity and drought has not been extensively investigated. In our study, chickpea (Cicer arietinum L.) Grx [LOC101493651 (CaGrx)] gene, in light of their higher expression against drought and salinity during our primary screening, has been used to reveal possible functions in salinity and drought in Arabidopsis thaliana. This CaGrx gene was over-expressed in Arabidopsis thaliana and evaluated for several stress-related biochemical and physiological performances.
Results showed that CaGrx increased plant tolerance under drought and salinity by positively regulating the antioxidant defense system and various stress-related parameters. The enhanced expression of CaGrx strengthens the plant's biochemical and physiological performances under oxidative stress, caused by salinity and drought, by activating the antioxidant defense system. Previous studies have reported that the over-expression of different glutaredoxin genes overcomes the stresses emerging from salinity (Sharma et al. 2013; Ning et al. 2018) and drought (Guo et al. 2010; Kumar et al. 2020b). Salt stress constrains root growth by inhibiting cell elongation (Van Zelm et al. 2020). Enhanced efficacy in seed germination and elongated root lengths were observed in the CaGrx over-expressing lines against drought and salinity, in comparison to WT plants, demonstrates that the CaGrx gene provides morphological and physiological tolerance under drought and salinity stress, and this is supported by a study of Guo et al. (2010) that displayed a similar trend in Arabidopsis thaliana. Several glutaredoxins have been explored to be responsible for mitigating oxidative stress and plant development (Xing and Zachgo 2008; Laporte et al. 2011).
Both proline and cysteine amino acids are assumed to have critical functions in plants against different stresses and have been analyzed in several investigations (Good and Zaplachinski 1994; Yang et al. 2000). Cysteine served as a sulfur donor to synthesize sulfur-containing antioxidant molecules like glutathione (GSH), enhancing tolerance during different stresses. Cysteine is known as the rate-limiting precursor of GSH biosynthesis, and GSH-mediated antioxidant activity depends upon the availability of cysteine (Romero et al. 2014). OAS-TL (O-acetylserine (thiol) lyase) enzyme, also known as cysteine synthase, catalyzed the biosynthesis of cysteine (Heeg et al. 2008). In this study, the transgenic lines showed enhanced OAS-TL activity than, which supports higher cysteine content in CaGrx transgenic lines under salinity and drought.
While proline acts as an osmolyte, an antioxidative molecule, as well as a signaling molecule under drought and salinity, which maintains membrane integrity under stress conditions (Ahmad and Sharma 2010). Under stress conditions, proline also works as a molecular chaperone, stabilizing the protein structure, enzyme activities, and its accumulation maintains cellular pH as well as cellular redox (Hoque et al. 2008). In the present investigation, the increased proline and cysteine levels in the transgenic lines in comparison to WT and non-stressed control plants display their protective function in drought and salinity stress.
Drought and salinity elevate the cellular accumulation of ROS like O2− and H2O2; these are highly reactive molecules that degrade DNA, lipids, and proteins (O’Kane et al. 1996; Van Breusegem and Dat 2006). H2O2 acts as a stress indicator was lowered in all the transgenic lines than WT plants but slightly higher than non-stressed control plants. The reduced level of ROS in over-expressing lines under salinity and drought might be due to the over-expression of CaGrx which elevates the APX (Ascorbate peroxidase) and catalase activity, that reduces H2O2 and DHA directly (Zaffagnini et al. 2008; Sousa et al. 2018). The elevated values of ROS in transgenics than in control (C) plants are due to the signaling function of H2O2, that enhanced several biochemical developmental and physiological processes under stress (García-Mata and Lamattina 2013).
The second stress-amenable molecule, TBARS, is synthesized during lipid peroxidation, which occurs under stress conditions that cause cellular toxicity (Taulavuori et al. 2001). Under both the types of stresses, decreased values of TBARS in the transgenics, in comparison to the maximum in their WT plants, showed less toxicity in plants. Less oxidative injury in transgenics might be due to the stimulation of the antioxidant system and the accumulation of protective osmolytes like proline by the over-expression of the CaGrx gene.
Numerous antioxidant enzymes and molecules play an essential function in salinity and drought to diminish ROS's impact in cellular redox (Ahmad et al. 2010; Laxa et al. 2019). Some previous studies have revealed that ascorbic acid is a crucial antioxidant that reduces H2O2 along with O2·−, ·OH, and lipid hydroperoxides and enhances plant tolerance against abiotic stresses like salinity and drought (Reddy et al. 2004; Ahmad et al. 2010). Under both stresses, a higher Asc/DHA ratio in transgenic lines than WT plants and non-stressed controls shows higher ascorbate production in the cell, which scavenges ROS directly and provides abiotic stress tolerance in transgenic lines (Akram et al. 2017). Glutathione (GSH) plays a critical role in oxidative stress arising from several biotic and abiotic stresses and protects the plants from damage (Yousuf et al. 2012). Some previous studies also revealed the protective role of GSH under salt stress in plants (Ruiz and Blumwald 2002; Mullineaux and Rausch 2005). The GSH/GSSG ratio was also elevated in transgenics of CaGrx than WT plants and non-stressed controls under drought and salt stress. Ascorbate (Asc) and glutathione (GSH) both retain cellular redox under oxidative stress (Foyer and Noctor 2005; Nahar et al. 2015). The CaGrx gene upholds cellular redox either by elevating glutathione (GSH) production or by recycling.
Antioxidant enzymes' activity was considerably increased under abiotic stress, signifying their active involvement, and protects against stresses. Our investigation showed that all the transgenic lines showed enhanced antioxidant enzyme activities compared to WT plants and non-stressed controls, thereby providing tolerance during drought and salinity. Increased activities of APX and catalase (CAT) in transgenic lines enhanced tolerance against ROS, like H2O2, either by reduction or direct scavenging (Zaffagnini et al. 2008). Some previous studies have also reported that the activity of various antioxidant enzymes like APX, SOD, CAT, GR, and DHAR was elevated during salt stress (Azooz et al. 2011; Koyro et al. 2012). Similarly, studies by Yang et al. (2009) have revealed that APX, POD, and CAT activities were elevated in transgenic rice under drought. SOD enzyme is assumed to be the first line of defense under ROS, the leading scavenger of superoxide (O2·−) anions (Almoguera et al. 1995). The activity of SOD under drought and salinity was also increased in all transgenic lines, while less in their WT control plants.
Glutaredoxin retains cellular redox buffer with the assistance of GR, NADPH, and GSH (Fernandes and Holmgren 2004). Grx enzyme mediates the reversible glutathionylation to protect proteins from ROS damage produced in various stresses (Klatt and Lamas 2000). Grx assay displayed the up-regulated activity of the Grx enzyme in transgenic lines in comparison to WT plants and enhanced tolerance under salinity and drought stress. The reversal of GSSG (oxidized glutathione) to the GSH (reduced glutathione) is mediated by glutathione reductase (GR) with the assistance of NADPH that combats oxidative stress for maintaining the redox status of cells. GR plays a critical role in retaining the pool of glutathione (GSH) and ascorbate (Asc) in the ROS scavenging pathway in the chloroplast (Yousuf et al. 2012). In this study, the GR activity under drought and salinity stress was also found to be enhanced in the transgenic lines compared with their WT control plants, which was also supported by the increased proportion of GSH/GSSG observed in the over-expressing plants. Hernandez et al. (2000) reported that the GR activity was enhanced in NaCl-tolerant pea plants. A study by Dubey et al. (2019) has also reported similar responses under drought stress in Arabidopsis thaliana.
Glutathione peroxidase (GPX) activity was enhanced in all transgenic lines in comparison to WT plants under both stresses, which protects the plant from oxidative stress with the help of GSH to reduce lipid hydroperoxides as well as H2O2 (Noctor et al. 2002).
GST (Glutathione-S-transferase) stimulates the coupling of glutathione (GSH) to xenobiotic compounds and detoxification at the cost of GSH (Edwards et al. 2000). The enhancement in GST activity was also reported in transgenics that showed tolerance towards drought and salinity.
The DHAR and MDHAR activities were observed to be elevated in all transgenic lines under drought and salinity in comparison to their WT plants. Both (DHAR and MDHAR) are responsible for enhancing the accumulation of ascorbate (Asc) under oxidative stress, which is involved in the detoxification of H2O2 (Eltayeb et al. 2006, 2007).
Electrolyte leakage (EL) is defined as the leakage or loss of ions from the cell membrane during stresses due to enhanced cellular lipid peroxidation (Bajji et al. 2002; Blokhina et al. 2003). In this study, the over-expression of the CaGrx gene reduces electrolyte conductivity by enhancing the proline concentration in cells that stabilizes membrane integrity by maintaining cell turgor (osmotic balance) in comparison to higher leakage in WT plants because of less proline content (Hayat et al. 2012).
Drought and salinity disturb plants' physiological performance by altering the leaf water potential, water use efficiency, net photosynthesis, cell elongation and expansion, stomatal closure, turgor pressure, and electron transport rate (Mingchi et al. 2010; Farooq et al. 2012). Stomatal closure occurs under stress, which reduces CO2 concentration in the leaves that limit carbon fixation and unveil the chloroplasts under extreme excitation energy, which might elevate ROS production (Cruz de Carvalho 2008). Oxidative stress arising due to drought and salinity causes chlorophyll degradation and finally alters the photosystems' activity (Verma and Mishra 2005).
In this study, the net photosynthesis (PN) was observed to be heightened in all the transgenic lines in comparison to WT plants and nearly to the non-stressed controls, which might be due to the over-expression of CaGrx against drought and salinity, which protect the chlorophyll from degradation by ROS. The enhanced levels of chlorophyll support this in the transgenic lines under both stresses. Some earlier studies showed that drought and salinity decrease photosynthesis in plants due to the reduction in relative water content (RWC) and leaf water potential (Lawlor and Cornic 2002; Polash et al. 2018). Some previous studies reported that the relative water content (RWC) could be expanded from 80 to 90% and declined up to 40% in critical water deficit conditions (Kaydan and Yagmur 2008). In this study, RWC was estimated to be increased in all the transgenic lines of CaGrx, nearly to the non-stressed controls, while less in WT plants, which shows the over-expression of the CaGrx gene enhanced the water content in Arabidopsis plants under salinity and drought. The enhanced proline content in transgenic lines of CaGrx might be involved in maintained plant-water relations by maintaining the cells' turgidity under stress, which sustained the rate of photosynthesis (Hayat et al. 2012; Polash et al. 2018). Carotenoids play a crucial role in photosynthesis as well as participate in defense mechanisms during oxidative stress (Gill and Tuteja 2010). In our investigation, the elevated level of carotenoids in all transgenic lines under drought and salinity suggested the proper functioning of photosystems in comparison to their altered function in their WT plants.
The term water-use efficiency (WUE) demonstrates the water consumption and stress compatibility of plants (Martin et al. 1999). Enhancement in WUE may be the strategy of plants adapted to water deficit conditions (Jaleel et al. 2008). WUE was maintained by transpiration (E) as well as stomatal conductance (gs) of the plants under salinity and drought (Gholipoor et al. 2002; Polley 2002). The non-photochemical quenching (NPQ) is a defensive mechanism during oxidative stress (Zlatev 2009). In this study, NPQ was observed to be higher than control (C) but fewer than WT plants under both stresses. The photochemical quenching (qP) was seen to be enhanced in all the transgenic lines. The values of qP and NPQ indicate the increased efficacy of the photosystem II (PSII) reaction center of transgenic lines compared to WT plants under drought and salinity. In our investigation, over-expression of CaGrx considerably elevates chlorophyll as well as carotenoid contents, stomatal conductance (gs), water use efficiency (WUE), and transpiration (E) nearly to the non-stressed controls, resulting in enhanced photosynthesis (PN) that provide tolerance of transgenics under salinity and drought.
Although the definite mechanism of CaGrx in biochemical as well as physiological responses against drought and salinity is not well known but based on the outcome of this study, we can hypothesize the putative functions of CaGrx. As a possible protective mechanism, CaGrx restricts the overproduction of ROS, participates in redox signaling, and enhanced direct or indirect antioxidant defense systems. CaGrx enhanced the level of stress-amenable amino acids, cysteine as well as proline. Proline works as an osmoprotectant and a radical scavenger, while cysteine functions as a rate-limiting precursor of (GSH) glutathione biosynthesis act as an antioxidant molecule. Glutaredoxins are glutathione-dependent oxidoreductases in which GSH acts as a cofactor. CaGrx might up-regulate the cellular accumulation of glutathione (GSH) and ascorbate (Asc) by activating the ascorbate–glutathione pathway (Foyer-Halliwell-Asada cycle) by increasing the antioxidant enzymes activities participating in this pathway and participated in ROS scavenging to provides stress tolerance. The higher proportion of glutathione (GSH) and ascorbate (Asc) in this study also supports the defensive role of CaGrx.
Conclusions
This present study explored the defensive role of CaGrx under salinity and drought, which has not been analyzed in detail regarding its physiological as well as biochemical aspects. This investigation uncovers the fact that the over-expressing lines of CaGrx overcome oxidative stress produced due to salinity and drought by provoking the defense system, such as stress-amenable amino acids (proline and cysteine), antioxidant enzymes, and molecules (Asc and GSH). The OAS-TL enzyme's enhanced activity elevated the production of cysteine, which enhanced cellular GSH production. The depletion in stress markers (TBARS, H2O2), as well as electrolyte leakage, enhancement in water use efficiency (WUE), and relative water content (RWC), maintained the physiological performance in transgenics of CaGrx under salinity and drought. The increased levels of chlorophyll, as well as carotenoids in the over-expressing lines, maintained the physiological performance under drought and salinity. The results inferred from the investigation of the various biochemical and physiological parameters validate that the over-expression of CaGrx confers tolerance in the over-expressing Arabidopsis compared to WT plants against drought and salinity. The CaGrx gene's over-expression can be exploited to develop crops tolerant to salinity and drought from the sensitive ones.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to the Director, CSIR-NBRI, Lucknow, for infrastructural support. We express gratitude towards C.S.I.R., New Delhi, for funds under the Project BSC 0204. The authors are also thankful to C.S.I.R., New Delhi (AK) U.G.C., New Delhi (VK, SN, SK), and D.S.T., New Delhi (M) for providing research fellowships. The Institutional manuscript number is CSIR-NBRI_MS/2021/03/06.
Author’s contribution
Conceptualization: AK & AKD, Formal analysis: AK, VK, SN & MAA, Funding acquisition: IS & Vivek Pandey, Investigation: AK, VK, Meenakshi & SK, Methodology: AKD & AK, Project administration: IS, Resources: VK, Supervision: IS & Veena Pande, validation: AK, roles/writing—original draft: AK, writing—review & editing: IS and AK.
Declaration
Conflict of interest
All the authors declare that they have not any conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmad P, Sharma S. Physio-biochemical attributes in two cultivars of mulberry (Morus alba L.) under NaHCO3 stress. Int J Plant Prod. 2010;4:79–86. doi: 10.22069/IJPP.2012.685. [DOI] [Google Scholar]
- Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S. Roles of enzymatic and non-enzymatic antioxidants in plants during abiotic stress. Crit Rev Biotech. 2010;30:161–175. doi: 10.3109/07388550903524243. [DOI] [PubMed] [Google Scholar]
- Akram NA, Shafiq F, Ashraf M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front Plant Sci. 2017;8:613. doi: 10.3389/fpls.2017.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almoguera C, Coca MA, Jordano J. Differential accumulation of sunflower tetraubiquitin mRNAs during zygotic embryogenesis and developmental regulation of their heat-shock response. Plant Physiol. 1995;107:765–773. doi: 10.1104/pp.107.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azooz MM, Youssef AM, Ahmad P. Evaluation of salicylic acid (SA) application on growth, osmotic solutes and antioxidant enzyme activities on broad bean seedlings grown under diluted seawater. Int J Plant Physiol Biochem. 2011;3:253–264. doi: 10.5897/IJPPB11.052. [DOI] [Google Scholar]
- Bajji M, Kinet JM, Lutts S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002;36:61–70. doi: 10.1023/A:1014732714549. [DOI] [Google Scholar]
- Bandurska H. Does proline accumulated in leaves of water deficit stressed barley plants confine cell membrane injury? I. Free proline accumulation and membrane injury index in drought and osmotically stressed plants. Acta Physiol Plant. 2000;22:409–415. doi: 10.1007/s11738-000-0081-7. [DOI] [Google Scholar]
- Bandyopadhyay S, Gama F, Molina Navarro MM, Gualberto JM, Claxton R, Naik SG, Huynh BH, Herrero E, Jacquot JP, Johnson MK, Rouhier N. Chloroplast monothiol glutaredoxins as scaffold proteins for the assembly and delivery of [2Fe–2S] clusters. EMBO J. 2008;27:1122–1133. doi: 10.1038/emboj.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chandlee JM, Scandalios JG. Analysis of variants affecting the catalase developmental program in maize scutellum. Theor Appl Genet. 1984;69:71–77. doi: 10.1007/BF00262543. [DOI] [PubMed] [Google Scholar]
- Cheeseman JM. Mechanisms of salinity tolerance in plants. Plant Physiol. 1988;87:547–550. doi: 10.1104/pp.87.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NH. AtGRX4, an Arabidopsis chloroplastic monothiol glutaredoxin, is able to suppress yeast grx5 mutant phenotypes and respond to oxidative stress. FEBS Lett. 2008;582:848–854. doi: 10.1016/j.febslet.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Cheng NH, Liu JZ, Brock A, Nelson RS, Hirschi KD. AtGRXcp, an Arabidopsis chloroplastic glutaredoxin, is critical for protection against protein oxidative damage. J Biol Chem. 2006;281:26280–26288. doi: 10.1074/jbc.M601354200. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Couturier J, Jacquot JP, Rouhier N. Evolution and diversity of glutaredoxins in photosynthetic organisms. Cell Mol Life Sci. 2009;66:2539–2557. doi: 10.1007/s00018-009-0054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz de Carvalho MH. Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signal Behav. 2008;3:156–165. doi: 10.4161/psb.3.3.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K, Roychoudhury A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci. 2014;2:53. doi: 10.3389/fenvs.2014.00053. [DOI] [Google Scholar]
- Ding S, He F, Tang W, Du H, Wang H. Identification of maize CC-type glutaredoxins that are associated with response to drought stress. Gene. 2019 doi: 10.3390/genes10080610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doulis AG, Debian N, Kingston-Smith AH, Foyer CH. Differential localization of antioxidants in maize leaves. Plant Physiol. 1997;114:1031–1037. doi: 10.1104/pp.114.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey AK, Kumar N, Kumar A, Ansari MA, Ranjan R, Gautam A, Meenakshi SN, Pandey V, Behera SK, Mallick S, Pande V, Sanyal I. Over-expression of CarMT gene modulates the physiological performance and antioxidant defense system to provide tolerance against drought stress in Arabidopsis thaliana L. Ecotoxicol Environ Saf. 2019;171:54–65. doi: 10.1016/j.ecoenv.2018.12.050. [DOI] [PubMed] [Google Scholar]
- Edwards R, Dixon DP, Walbot V. Plant glutathione S-transferases: enzymes with multiple functions in sickness and in health. Trends Plant Sci. 2000;5:193–198. doi: 10.1016/S1360-1385(00)01601-0. [DOI] [PubMed] [Google Scholar]
- Eltayeb AE, Kawano N, Badawi GH, Kaminaka H, Sanekata T, Morishima I, Shibahara T, Inanaga S, Tanaka K. Enhanced tolerance to ozone and drought stresses in transgenic tobacco over-expressing dehydroascorbate reductase in cytosol. Physiol Plant. 2006;127:57–65. doi: 10.1111/j.1399-3054.2006.00624.x. [DOI] [Google Scholar]
- Eltayeb AE, Kawano N, Badawi GH, Kaminaka H, Sanekata T, Shibahara T, Inanaga S, Tanaka K. Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta. 2007;225:1255–1264. doi: 10.1007/s00425-006-0417-7. [DOI] [PubMed] [Google Scholar]
- Farooq M, Hussain M, Wahid A, Siddique KHM. Drought stress in plants: an overview. Plant Responses Drought Stress. 2012 doi: 10.1007/978-3-642-32653-0_1. [DOI] [Google Scholar]
- Fernandes AP, Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17:1866–1875. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitonde MK. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J. 1967;104:627. doi: 10.1042/bj1040627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallogly MM, Mieyal JJ. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr Opin Pharmacol. 2007;7:381–391. doi: 10.1016/j.coph.2007.06.003. [DOI] [PubMed] [Google Scholar]
- García-Mata C, Lamattina L. Gasotransmitters are emerging as new guard cell signaling molecules and regulators of leaf gas exchange. Plant Sci. 2013;201:66–73. doi: 10.1016/j.plantsci.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Gholipoor M, Soltani A, Shekari F, Shekari FB. Effects of salinity on water use efficiency and its components in chickpea (Cicer arietinum L.) Acta Agro Hung. 2002;50:127–134. doi: 10.1556/AAgr.50.2002.2.2. [DOI] [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Gilroy S, Białasek M, Suzuki N, Górecka M, Devireddy AR, Karpiński S, Mittler R. ROS, calcium, and electric signals: key mediators of rapid systemic signaling in plants. Plant Physiol. 2016;171:1606–1615. doi: 10.1104/pp.16.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good AG, Zaplachinski ST. The effects of drought stress on free amino acid accumulation and protein synthesis in Brassica napus. Physiol Plant. 1994;90:9–14. doi: 10.1111/j.1399-3054.1994.tb02185.x. [DOI] [Google Scholar]
- Guo Y, Huang C, Xie Y, Song F, Zhou X. A tomato glutaredoxin gene SlGRX1 regulates plant responses to oxidative, drought and salt stresses. Planta. 2010;232:1499–1509. doi: 10.1007/s00425-010-1271-1. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. doi: 10.1016/S0021-9258(19)42083-8. [DOI] [PubMed] [Google Scholar]
- Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A. Role of proline under changing environments: a review. Plant Signal Behav. 2012;7:1456–1466. doi: 10.4161/psb.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeg C, Kruse C, Jost R, Gutensohn M, Ruppert T, Wirtz M, Hell R. Analysis of the Arabidopsis O-acetylserine (thiol) lyase gene family demonstrates compartment-specific differences in the regulation of cysteine synthesis. Plant Cell. 2008;20:168–185. doi: 10.1105/tpc.107.056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemeda HM, Klein BP. Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J Food Sci. 1990;55:184–185. doi: 10.1111/j.1365-2621.1990.tb06048.x. [DOI] [Google Scholar]
- Hernandez JA, Jiménez A, Mullineaux P, Sevilia F. Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defences. Plant Cell Environ. 2000;23:853–862. doi: 10.1046/j.1365-3040.2000.00602.x. [DOI] [Google Scholar]
- Hodges DM, DeLong JM, Forney CF, Prange RK. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207:604–611. doi: 10.1007/s004250050524. [DOI] [PubMed] [Google Scholar]
- Holmgren A, Aslund F (1995) Glutaredoxin. In: Methods in enzymology. Academic Press, vol, 252, pp 283–292. 10.1016/0076-6879(95)52031-7 [DOI] [PubMed]
- Hoque MA, Banu MNA, Nakamura Y, Shimoishi Y, Murata Y. Proline and glycinebetaine enhance antioxidant defense and methylglyoxal detoxification systems and reduce NaCl-induced damage in cultured tobacco cells. J Plant Physiol. 2008;165:813–824. doi: 10.1016/j.jplph.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Hussain S, Shaukat M, Ashraf M, Zhu C, Jin Q, Zhang J. Salinity stress in arid and semi-arid climates: effects and management in field crops. Clim Change Agric. 2019 doi: 10.5772/intechopen.87982. [DOI] [Google Scholar]
- Jaleel CA, Gopi R, Sankar B, Gomathinayagam M, Panneerselvam R. Differential responses in water use efficiency in two varieties of Catharanthus roseus under drought stress. CR Biol. 2008;331:42–47. doi: 10.1016/j.crvi.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Kampfenkel K, Van Montagu M, Inze D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem. 1995;225:165–167. doi: 10.1006/abio.1995.1127. [DOI] [PubMed] [Google Scholar]
- Kaydan D, Yagmur M. Germination, seedling growth and relative water content of shoot in different seed sizes of triticale under osmotic stress of water and NaCl. Afr J Biotech. 2008;7:2862–2868. doi: 10.5897/AJB08.512. [DOI] [Google Scholar]
- Klatt P, Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem. 2000;26:4928–4944. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- Koyro HW, Ahmad P, Geissler N (2012) Abiotic stress responses in plants: an overview. In: Environmental adaptations and stress tolerance of plants in the era of climate change. Springer, New York, NY, pp 1–28. 10.1007/978-1-4614-0815-4_1
- Kumar A, Dubey AK, Kumar V, Ansari MA, Narayan S, Meenakshi KS, Pandey V, Shirke PA, Pande V, Sanyal I. Over-expression of chickpea glutaredoxin (CaGrx) provides tolerance to heavy metals by reducing metal accumulation and improved physiological and antioxidant defence system. Ecotoxicol Environ Saf. 2020;192:110252. doi: 10.1016/j.ecoenv.2020.110252. [DOI] [PubMed] [Google Scholar]
- Kumar A, Dubey AK, Kumar V, Ansari MA, Narayan S, Meenakshi KS, Pandey V, Shirke PA, Pande V, Sanyal I. Over-expression of rice glutaredoxin genes LOC_Os02g40500 and LOC_Os01g27140 regulate plant responses to drought stress. Ecotoxicol Environ Saf. 2020;200:110721. doi: 10.1016/j.ecoenv.2020.110721. [DOI] [PubMed] [Google Scholar]
- Lafitte R. Relationship between leaf relative water content during reproductive stage water deficit and grain formation in rice. Field Crops Res. 2002;76:165–174. doi: 10.1016/S0378-4290(02)00037-0. [DOI] [Google Scholar]
- Laporte D, Olate E, Salinas P, Salazar M, Jordana X, Holuigue L. Glutaredoxin GRXS13 plays a key role in protection against photo-oxidative stress in Arabidopsis. J Exp Bot. 2011;63:503–515. doi: 10.1093/jxb/err301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DW, Cornic G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002;25:275–294. doi: 10.1046/j.0016-8025.2001.00814.x. [DOI] [PubMed] [Google Scholar]
- Laxa M, Liebthal M, Telman W, Chibani K, Dietz KJ. The role of the plant antioxidant system in drought tolerance. Antioxidants. 2019;8:94. doi: 10.3390/antiox8040094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Hall J. Crop yield sensitivity of global major agricultural countries to droughts and the projected changes in the future. Sci Total Environ. 2019;654:811–821. doi: 10.1016/j.scitotenv.2018.10.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Martin B, Tauer CG, Lin RK. Carbon isotope discrimination as a tool to improve water-use efficiency in tomato. Crop Sci. 1999;39:1775–1783. doi: 10.2135/cropsci1999.3961775x. [DOI] [Google Scholar]
- Mingchi L, Xiangli L, Jing H, Lihong G. Effect of simulated drought stress on plant growth, yield and fruit properties of tomato. Acta Hort. 2010;856:193–202. doi: 10.17660/ActaHortic.2010.856.26. [DOI] [Google Scholar]
- Mishra S, Jha S, Singh R, Chaudhary S, Sanyal I, Amla DV. Transgenic chickpea expressing a recombinant human α1-proteinase inhibitor (α1-PI) driven by a seed-specific promoter from the common bean Phaseolus vulgaris (L.) Plant Cell Tiss Org Cult. 2013;115:23–33. doi: 10.1007/s11240-013-0336-9. [DOI] [Google Scholar]
- Mullineaux PM, Rausch T. Glutathione, photosynthesis and the redox regulation of stress-responsive gene expression. Photo Res. 2005;86:459–474. doi: 10.1007/s11120-005-8811-8. [DOI] [PubMed] [Google Scholar]
- Nahar K, Hasanuzzaman M, Alam MM, Fujita M. Exogenous glutathione confers high temperature stress tolerance in mung bean (Vigna radiata L.) by modulating antioxidant defense and methylglyoxal detoxification system. Environ Exp Bot. 2015;112:44–54. doi: 10.1016/j.envexpbot.2014.12.001. [DOI] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. doi: 10.1093/oxfordjournals.pcp.a076232. [DOI] [Google Scholar]
- Ning X, Sun Y, Wang C, Zhang W, Sun M, Hu H, Liu J, Yang L. A rice CPYC-type glutaredoxin OsGRX20 in protection against bacterial blight, methyl viologen and salt stresses. Front Plant Sci. 2018;9:111. doi: 10.3389/fpls.2018.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Gomez L, Vanacker H, Foyer CH. Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J Exp Bot. 2002;53:1283–1304. doi: 10.1093/jexbot/53.372.1283. [DOI] [PubMed] [Google Scholar]
- O'Kane D, Gill V, Boyd P, Burdon R. Chilling, oxidative stress and antioxidant responses in Arabidopsis thaliana callus. Planta. 1996;198:371–377. doi: 10.1007/BF00620053. [DOI] [PubMed] [Google Scholar]
- Polash MAS, Sakil MA, Tahjib-Ul-Arif M, Hossain MA. Effect of salinity on osmolytes and relative water content of selected rice genotypes. Trop Plant Res. 2018;5:227–232. doi: 10.22271/tpr.2018.v5.i2.029. [DOI] [Google Scholar]
- Polley HW. Implications of atmospheric and climatic change for crop yield and water use efficiency. Crop Sci. 2002;42:131–140. doi: 10.2135/cropsci2002.1310. [DOI] [PubMed] [Google Scholar]
- Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- Reddy AR, Chaitanya KV, Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol. 2004;161:1189–1202. doi: 10.1016/j.jplph.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Romero LC, Aroca MÁ, Laureano-Marín AM, Moreno I, García I, Gotor C. Cysteine and cysteine-related signaling pathways in Arabidopsis thaliana. Mol Plant. 2014;7:264–276. doi: 10.1093/mp/sst168. [DOI] [PubMed] [Google Scholar]
- Rouhier N, Gelhaye E, Jacquot JP. Plant glutaredoxins: still mysterious reducing systems. Cell Mol Life Sci. 2004;61:1266–1277. doi: 10.1007/s00018-004-3410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhier N, San Koh C, Gelhaye E, Corbier C, Favier F, Didierjean C, Jacquot JP. Redox based anti-oxidant systems in plants: biochemical and structural analyses. Biochim Biophys Acta. 2008;1780:1249–1260. doi: 10.1016/j.bbagen.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Rouhier N, Lemaire SD, Jacquot JP. The role of glutathione in photosynthetic organisms: emerging functions for glutaredoxins and glutathionylation. Annu Rev Plant Biol. 2008;59:143–166. doi: 10.1146/annurev.arplant.59.032607.092811. [DOI] [PubMed] [Google Scholar]
- Ruiz J, Blumwald E. Salinity-induced glutathione synthesis in Brassica napus. Planta. 2002;214:965–969. doi: 10.1007/s00425-002-0748-y. [DOI] [PubMed] [Google Scholar]
- Sergiev I, Alexieva V, Karanov E. Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. C R Acad Bulg Sci. 1997;51:121–124. [Google Scholar]
- Sharma R, Chahar OP, Bhatnagar M, Bhatnagar A. Impact of osmotic stress and temperature on pigments and proteins of Anabaena strains. J Environ Biol. 2013;34:941–943. [PubMed] [Google Scholar]
- Singh R, Yadav R, Amla DV, Sanyal I. Characterization and functional validation of two scaffold attachment regions (SARs) from Cicer arietinum (L.) Plant Cell Tissue Org Cult. 2016;125:135–148. doi: 10.1007/s11240-015-0935-8. [DOI] [Google Scholar]
- Smith IK, Vierheller TL, Thorne CA. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis (2-nitrobenzoic acid) Anal Biochem. 1988;175:408–413. doi: 10.1016/0003-2697(88)90564-7. [DOI] [PubMed] [Google Scholar]
- Sousa RH, Carvalho FE, Lima-Melo Y, Alencar VT, Daloso DM, Margis-Pinheiro M, Komatsu S, Silveira JA. Impairment of peroxisomal APX and CAT activities increases protection of photosynthesis under oxidative stress. J Exp Bot. 2018;70:627–639. doi: 10.1093/jxb/ery354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram S, Wu S, Ma LQ, Rathinasabapathi B. Expression of a Pteris vittata glutaredoxin PvGRX5 in transgenic Arabidopsis thaliana increases plant arsenic tolerance and decreases arsenic accumulation in the leaves. Plant Cell Environ. 2009;32:851–858. doi: 10.1111/j.1365-3040.2009.01963.x. [DOI] [PubMed] [Google Scholar]
- Takeda T, Nakano Y, Shigeoka S. Effects of selenite, CO2 and illumination on the induction of selenium-dependent glutathione peroxidase in Chlamydomonas reinhardtii. Plant Sci. 1993;94:81–88. doi: 10.1016/0168-9452(93)90009-O. [DOI] [Google Scholar]
- Taulavuori E, Hellström EK, Taulavuori K, Laine K. Comparison of two methods used to analyse lipid peroxidation from Vaccinium myrtillus (L.) during snow removal, reacclimation and cold acclimation. J Exp Bot. 2001;52:2375–2380. doi: 10.1093/jexbot/52.365.2375. [DOI] [PubMed] [Google Scholar]
- Van Breusegem F, Dat JF. Reactive oxygen species in plant cell death. Plant Physiol. 2006;141:384–390. doi: 10.1104/pp.106.078295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zelm E, Zhang Y, Testerink C. Salt tolerance mechanisms of plants. Annu Rev Plant Biol. 2020 doi: 10.1146/annurev-arplant-050718-100005. [DOI] [PubMed] [Google Scholar]
- Vanacker H, Harbinson J, Ruisch J, Carver TLW, Foyer CH. Antioxidant defences of the apoplast. Protoplasma. 1998;205:129–140. doi: 10.1007/BF01279303. [DOI] [Google Scholar]
- Verma S, Mishra SN. Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense system. J Plant Physiol. 2005;162:669–677. doi: 10.1016/j.jplph.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Wellburn RW. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol. 1994;144:307–313. doi: 10.1016/S0176-1617(11)81192-2. [DOI] [Google Scholar]
- Wu Q, Hu Y, Sprague SA, Kakeshpour T, Park J, Nakata PA, Cheng N, Hirschi KD, White FF, Park S. Expression of a monothiol glutaredoxin, AtGRXS17, in tomato (Solanum lycopersicum) enhances drought tolerance. Biochem Biophys Res Comm. 2017;491:1034–1039. doi: 10.1016/j.bbrc.2017.08.006. [DOI] [PubMed] [Google Scholar]
- Xing S, Zachgo S. ROXY1 & ROXY2, two Arabidopsis glutaredoxin genes, are required for anther development. Plant J. 2008;53:790–801. doi: 10.1111/j.1365-313X.2007.03375.x. [DOI] [PubMed] [Google Scholar]
- Yang CW, Lin CC, Kao CH. Proline, ornithine, arginine and glutamic acid contents in detached rice leaves. Biol Plant. 2000;43:305–307. doi: 10.1023/A:1002733117506. [DOI] [Google Scholar]
- Yang Z, Wu Y, Li Y, Ling HQ, Chu C. OsMT1a, a type 1 metallothionein, plays the pivotal role in zinc homeostasis and drought tolerance in rice. Plant Mol Biol. 2009;70:219–229. doi: 10.1007/s11103-009-9466-1. [DOI] [PubMed] [Google Scholar]
- Yeo A. Predicting the interaction between the effects of salinity and climate change on crop plants. Sci Hortic. 1998;78:159–174. doi: 10.1016/S0304-4238(98)00193-9. [DOI] [Google Scholar]
- Yousuf PY, Hakeem KUR, Chandna R, Ahmad P (2012) Role of glutathione reductase in plant abiotic stress. In: Abiotic stress responses in plants. Springer, New York, NY, pp 149–158. 10.1007/978-1-4614-0634-1_8
- Zaffagnini M, Michelet L, Massot V, Trost P, Lemaire SD. Biochemical characterization of glutaredoxins from Chlamydomonas reinhardtii reveals the unique properties of a chloroplastic CGFS-type glutaredoxin. J Biol Chem. 2008;283:8868–8876. doi: 10.1074/jbc.M709567200. [DOI] [PubMed] [Google Scholar]
- Zlatev Z. Drought-induced changes in chlorophyll fluorescence of young wheat plants. Biotech Biotechnol Equip. 2009;23:438–441. doi: 10.1080/13102818.2009.10818458. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.