Abstract
Lead (Pb) not only negatively alters plant growth and yield but may also have potentially toxic risks to human health. Nevertheless, the interaction between rice (Oryza sativa L.) plants and the molecular cell dynamics induced by lead-methyl jasmonate (MJ) remains unknown. Here, plants were hydroponically exposed to Pb (150 and 300 µM) alone or in combination with 0.5 and 1 µM MJ. The application of MJ modulated the expression of the HMAs, PCS1, PCS2 and ABCC1 genes, thereby immobilizing the Pb in the roots and lessening its translocation to the aerial parts of the rice plant. The supplementation of MJ improved the growth and yield of Pb-stressed rice by adjusting the proline and chlorophyll metabolism, increasing the phytochelatins (PCs) accumulation and diminishing the accumulation of Pb in the shoots. the application of MJ alleviated the oxidative stress of rice plants exposed to Pb toxicity by enhancing the activity of antioxidant enzymes and enzymes of the glyoxalase system (glyoxalase I and II) and decreasing the endogenous levels of malondialdehyde (MDA), hydrogen peroxide (H2O2) and methylglyoxal (MG). Therefore, the results of the present study could provide a molecular insight and cellular interplay scheme for the development of a promising strategy in Pb-contaminated areas to produce healthy food.
Keywords: Lead toxicity, Rice, Methyl jasmonate, Oxidative stress, Phytochelatin, Vacuolar sequestration
Introduction
Due to various anthropogenic activities such as the burning of fossil fuel, paints, discharge from batteries, smelting of ores and automobile exhaust, lead (Pb) is found abundantly as one of the environmental pollutants in various ecosystems (Pourrut et al. 2011; Chenery et al. 2012). Pb toxicity has been shown to reduce growth and germination rates, respiration and photosynthesis processes, and changes in cell division and development of chloroplasts (Gupta et al. 2010; Bharwana 2013). Pb has been reported to inhibit the activity of enzymes involved in the biosynthesis of photosynthetic pigments, plastoquinone, and the Calvin cycle, and to disrupt the electron transfer chain, leading to the stomatal closure. Pb also induces oxidative stress and damages the biomolecules structures, resulting in metabolic dysfunction by increasing the production of reactive oxygen species (ROS) (Sharma and Dubey 2005; Singh et al. 2010).
Plants can counteract Pb toxicity in three ways: (a) inhibition of Pb uptake by roots (passive mechanisms), (b) induction of antioxidant network to counteract ROS, (c) excretion of Pb into the extracellular space or sequestration in vacuoles (inducible mechanisms) (Pourrut et al. 2011; Ashraf et al. 2015). The antioxidant network involved in scavenging the accumulated ROS under heavy metal toxicity includes enzymatic antioxidants such as peroxidase (POX), catalase (CAT), glutathione reductase (GR) and superoxide dismutase (SOD), and non-enzymatic ones such as tocopherols, ascorbic acid and glutathione (Mishra and Choudhary 1998; Mittler 2002). In addition to the network of antioxidants, the accumulation of osmolytes compounds such as proline improves plant tolerance under stressful conditions by maintaining osmotic potential and protecting cellular structures (Ali et al. 2014; Ghorbani et al. 2019b).
Recent studies have shown that P1B-type ATPases are significantly involved in the absorption of heavy metals and their transport to the shoots (Colangelo and Guerinot 2006; Kraemer et al. 2007). Nine and eight members of type P1B-ATPases were identified in Oryza sativa L. and Arabidopsis thaliana L., respectively, which are divided into two subgroups based on distinct metal selectivity: a lead (Pb)/cadmium (Cd)/cobalt (Co)/zinc (Zn) group (I) and a silver (Ag)/copper (Cu) group (II) (Axelsen and Palmgren 2001; Williams and Mills 2005; Takahashi et al. 2012). Heavy Metal-transporting P1B ATPases 1 (HMA1)-HMA3 and HMA4-HMA9 in rice and HMA1-HMA4 and HMA5-HMA8 in Arabidopsis belong to groups I and II, respectively (Takahashi et al. 2012). HMA2 and HMA3 have been shown to play a role in cadmium transport in rice (Shao et al. 2018; Tezuka et al. 2010; Ueno et al. 2010). It has been indicated that HMA1 and HMA9 are involved in the transport of zinc in rice (Suzuki et al. 2012; Lee et al. 2007). Previous reports have also suggested that OsHMA4 and OsHMA5 may be involved in copper loading and detoxification (Huang et al. 2016; Deng et al. 2013). However, the role of the HMAs transporter family in the transport and detoxification of Pb in rice is not well understood.
Phytohormones not only regulate plant growth and development but also play an essential role in coping with environmental stresses and improving plant adaptation under stressful conditions. Jasmonates, as biosynthesized compounds from the octadecanoic pathway, are one of the important phytohormones involved in plant adaptation to abiotic and biotic stresses (Wasternack 2014). Jasmonic acid is involved in the physiological processes of root growth, nutrient storage, tuber formation, seed germination, senescence and fruit ripening (Wasternack and Hause 2013; Wasternack 2014). Jasmonic acid, as a signaling molecule, has recently been shown to protect plants from salinity, desiccation, cold and heavy metals stress (Wasternack and Hause 2013; Piotrowska et al. 2009; Bali et al. 2018; Kamal and Komatsu 2016). Jasmonic acid has been reported to improve photosynthetic pigments and reduce the levels of malondialdehyde and hydrogen peroxide by improving the antioxidant network in Vicia faba under Cd stress (Ahmad et al. 2017) and Chlorella vulgaris under Pb stress (Piotrowska et al. 2009). Bali et al. (2018) indicated that jasmonic acid enhanced the tolerance of tomato under Pb phytotoxicity by decreasing Pb uptake, increasing metal-chelating compounds and osmolytes content, as well as modulating the ascorbate–glutathione cycle. Therefore, in the current study, the role of methyl jasmonate (MJ) in regulating the expression of genes involved in the heavy metal sequestration network (HMAs, ABCCs and PCSs) as a novel target of Pb toxicity response induced by phytohormone MJ in rice as a model crop was investigated. The results were coupled with antioxidant network response, proline and chlorophyll metabolism as well as rice yield, which could help to better understand the role of MJ in rice adaptation mechanism to Pb toxicity.
Material and methods
Plant material, treatments and growth conditions
Oryza sativa L. (variety Tarom hashemi) seeds were procured from the Iran Rice Research Institute (Amol), and after germination, 12-day-old seedlings were shifted to Hoagland pots. Hoagland solution (pH 6.0) was replaced every 5 days. Rice plants were grown under the controlled condition with 16 h photoperiod, 22/26 °C and 70–80% humidity. Appropriate concentrations of Pb (Pb(NO3)2, 0, 150 and 300 µM) and MJ (C13H20O3, 0, 0.5 and 1 µM) were added to Hoagland solution after 10 days of seedlings adaptation. Pb concentrations were standardized based on the results of preliminary experiments. The treatments applied were as follows: (1) Hoagland solution (control), (2) MJ 0.5 µM, (3) MJ 1 µM, (4) Pb 150 µM, (5) Pb 150 µM + MJ 0.5 µM, (6) Pb 150 µM + MJ 1 µM, (7) Pb 300 µM, (8) Pb 300 µM + MJ 0.5, (9) Pb 300 µM + MJ 1 µM. Sampling was done at 60 and 90 days after transplanting in panicle heading (biochemical and molecular attributes) and maturity (yield attributes) phases (Ghorbani et al. 2011), respectively.
Photosynthetic pigments and chlorophyll fluorescence
The carotenoids and chlorophyll a and b contents were assessed based on Wellburn (1994) method and recording the optical density at 470, 652 and 665 nm as mg/g fresh weight. Using a PAM 2500 fluorimeter (Walz), chlorophyll fluorescence yield (Fv/Fm) was determined after 20 min of dark adaptation.
Pb concentration
After drying the root and shoot tissues and digestion with HClO4:HNO3:H2SO4 mixture in 1:3:1 ratio, Pb concentrations were determined by ICP-MS (Agilent 7500 cx).
Proline content
Proline contents were measured by ninhydrin reagent (acetic acid + orthophosphoric acid (6 mM) + 125 g ninhydrin) according to Bates et al. (1973) method. After crushing the fresh leaves in sulfosalicylic acid and centrifuging at 10,000 ×g for 15 min, the supernatant was mixed with ninhydrin reagent and incubated at 100 °C (1 h). The samples were cooled and mixed with toluene and read spectrophotometrically at 520 nm.
Lipid peroxidation and H2O2 contents
The malondialdehyde (MDA) content of rice leaves was measured according to Heath and Packer (1968) method for estimating oxidized lipids. Fresh rice leaves were extracted by trichloroacetic acid (10%) and 2-thiobarbituric acid (0.65%). The absorbance of the supernatant after centrifugation at 12,000 ×g for 20 min was read spectrophotometrically at 532 and 600 nm.
For H2O2 estimation in rice leaves, fresh tissues were extracted using thiobarbituric acid (0.1%). After centrifugation of the extract at 10,000 ×g for 20 min, the supernatant was mixed with 1 M potassium iodide and 10 mM phosphate buffer (pH 7.0) and read spectrophotometrically at 390 nm (Velikova et al. 2000).
Enzyme extraction assays
Extraction buffer containing 100 mM potassium phosphate (pH 7.0), Triton X-100 (0.5%) and polyvinylpyrrolidone (1%) was used to evaluate the activity of enzymes in fresh leaves. To measure the activity of ascorbate peroxidase (APX), 2 mM ascorbate was added to the extract. The extract mixture was centrifuged at 12,000 ×g for 15 min at 4 °C and the supernatant was employed as an enzyme extract to measure enzyme activity and protein content.
The activity of catalase (CAT) enzyme was calculated according to Aebi (1984) and recording the photochemical reduction in the level of H2O2 at 240 nm for 2 min. The activity of the superoxide dismutase (SOD) enzyme was measured according to the method earlier published by Dhindsa and Matowe (1981) and determining the decline of nitroblue tetrazolium. Glutathione reductase (GR) activity was estimated by measuring the NADPH oxidation induced by oxidized glutathione as per Foyer and Halliwell (1976). The activity of ascorbate peroxidase (APX) was measured by monitoring H2O2-induced ascorbate oxidation at 290 nm for 2 min according to Nakano and Asada (1981).
The activity of Glyoxalase (Gly) I enzyme was calculated by measuring the photochemical rise in the absorbance of the reaction solution containing the protein extract, 15 mM magnesium sulfate, 1.7 mM GSH, 3.5 mM MG, potassium phosphate buffer (100 mM. pH 7.0) at 240 nm for 1 min (Hasanuzzaman et al. 2011). Gly II activity was calculated by determining the photochemical increase in the absorbance of the reaction solution containing the enzymatic extract, 5,5´-dithiobis (2-nitrobenzoic acid) (DTNB, 0.2 mM), Tris–HCl buffer (100 mM pH 7.2) and S-D-lactoylglutathione (1 mM) at 40 nm for 1 min as per Principato et al. (1987).
The activity of δ-aminolevulinic acid dehydratase enzyme was calculated by recording the difference in the level of porphobilinogen at 550 nm for 1 min as per Jain and Gadre (2004). The activities of chlorophyllase, Δ1-pyrroline-5-carboxylate synthetase and proline dehydrogenase enzymes were determined based on the methods previously documented by Costa et al. (2005), Sumithra et al. (2006) and Charest and Phan (1990), respectively. Using bovine serum albumin as standard, the protein contents were measured as per Bradford (1976).
Ascorbic acid, glutathione and phytochelatin contents
Fresh tissue of rice leaves was extracted with the extract mixture (metaphosphoric acid (5%) including 1 mM EDTA) and centrifuged at 10,000 ×g for 20 min. The supernatant was utilized to estimate the contents of ascorbic acid (AsA) and glutathione (GSH).
After neutralizing the supernatant with 0.5 mM potassium phosphate buffer (pH 7.0), dithiothreitol (0.1 M) was added to the solution to reduce oxidized AsA. Then, potassium phosphate buffer (100 mM, pH 7.5) and ascorbate oxidase (1 unit) were added to the solutions. The reduced and total AsA content were calculated by determining the spectrophotometric absorbance at 265 nm. The reduced AsA was measured as per Dutilleul et al. (2003) and using the standard curve of AsA. Oxidized AsA (DHA) was calculated by the following equation: DHA = total AsA − AsA.
The reaction solution containing the supernatant, glutathione reductase (10 U mL–1), 6 mM DTNB and 0.3 mM NADPH was used to estimate total GSH. For oxidized GSH (GSSG), the reaction solution containing the supernatant, 2-vinylpyridine and triethanolamine (50%, v/v) was incubated at 25 °C for 20 min and read spectrophotometrically at 412 nm. The reduced glutathione (GSH) was calculated by subtracting GSSG from the total glutathione as per Gill et al. (2015).
The content of phytochelatins (PCs) was determined as per De Vos et al. (1992) method and the extraction of non-protein thiols. First, fresh leaves and roots were extracted using the sulfosalicylic acid (3%) and centrifuged at 10,000 ×g for 20 min at 4 °C. The reaction mixture containing the supernatant, 120 mM phosphate buffer (pH 7.5), EDTA (5 mM) and 5,5′-dithiobis (2-nitrobenzoic acid) (0.6 mM) were used to measure PCs. After deducting glutathione from non-protein thiols read at 410 nm, the PCs content was calculated.
Gene expression pattern
Using TRIzol reagent (Invitrogen, USA), total RNA was extracted from the roots and leaves of rice according to the manufacturer’s protocol. cDNA was synthesized by reverse transcriptase, dNTPs (10 mM) and oligo (dT) primers (Thermo Scientific, Germany). Maxima SYBR Green/ROX qPCR Master Mix (Thermo Scientific) was employed to conduct qPCR of the target genes by the C1000TM Thermal Cycler device (BioRad). The 2−ΔΔCt method and Actin gene were employed for the data analysis and the normalization, respectively (Livak and Schmittgen 2001). The primers (Table 1) were designed by online software (Primer3) and checked by OLIGO5 analyzer software.
Table 1.
List of gene-specific primers used for RT-PCR analysis
| Gene name | Genbank accession | Forward primer (5′-3′) | Primer efficiency (%) |
|---|---|---|---|
| OsHMA2 | AP004278 | F: ACCAGCTGATTACAAACAAGCAT | 98.6 |
| R: CAGTCCTTTACTTCCTCGACCTC | |||
| OsHMA3 | AP005246 | F: CTTCTTTACTGGATTGCAAGCAT | 98.8 |
| R: GGTCAGCATAACCGACTTGATG | |||
| OsHMA4 | AP004184 | F: TGCTGGGGAAATATCTGGAG | 98.4 |
| R: CTTCTGAACTGGAGCCCTTG | |||
| ABCC1 | NM_001036039 | F: TCGAACTGTGGCAGTCTTTG | 98.4 |
| R: AGTCCATTCAATGCCTCACC | |||
| PCS1 | LC192429 | F: TCGCTTCAAATACCCTCCTC | 98.8 |
| R: TTTACTTGGGCTGGATCCTC | |||
| PCS2 | LC192431 | F: CAGGGGGTTCATGCTTATCT | 98.5 |
| R: GGCAGGAAGGGATTTCACTA | |||
| Actin | XM_015774830 | F: TCCTCCGTGGAGAAGAGCTA | 98.8 |
| R: GCAATGCCAGGGAACATAGT |
Yield and yield components
Rice panicles were separated manually after harvest and spikelet per panicle and the percentage of filled grain were determined. To estimate the 1000-grain weight, 1000 filled grains (5 samples per replicate) were randomly weighed (Ghorbani et al. 2009). The tillers containing panicles per hill were counted at maturity to determine the number of productive tillers. Harvest index was determined as: (grain yield/total dry weight) × 100.
Statistical analysis
Five biological replicates were employed to calculate the mean of morphological, biochemical and yield attributes, while for the gene expression, three biological replicates were used, each of which had three technical replications. Statistical analysis was done by SAS 9.1.3 software. the least significant difference (LSD) test was employed to determine the difference between the means (p < 0.05).
Results
Growth and photosynthetic pigments
The height of rice seedlings was found to be reduced by 17.3 and 34.2% under 150 and 300 μM Pb, respectively than the control ones. Treatment of rice seedlings with 0.5 and 1 μM MJ significantly improved plant height in Pb-stressed seedlings. Application of 0.5 and 1 μM MJ increased plant height by 5.4 and 12.6% under 150 μM Pb, and 16 and 33.1% under 300 μM Pb, respectively (Table 2). Pb treatments (150 and 300 μM) significantly declined the total dry weight the highest decline was recorded under high Pb level. However, the MJ addition improved the total dry weight of Pb-exposed seedlings (Table 2).
Table 2.
Effects of lead (Pb, 150 and 300 µM) alone or in combination with methyl jasmonate (MJ, 0.5 and 1 μM) on height, total dry weight, photosynthetic pigments and chlorophyll florescence in rice plants
| Treatments | Height (cm) | TDW (g) | Chlorophyll a (mg g−1 fw) |
Chlorophyll b | Carotenoids | Fv/Fm |
|---|---|---|---|---|---|---|
| Control | 45.18 ± 1.03a | 4.29 ± 014ab | 2.44 ± 0.12a | 1.83 ± 0.11bc | 0.272 ± 0.017abc | 0.656 ± 0.013a |
| 0.5 µM MJ | 43.95 ± 1.32ab | 4.40 ± 0.14ab | 2.42 ± 0.13a | 2.04 ± 0.08a | 0.293 ± 0.016ab | 0.646 ± 0.014a |
| 1 µM MJ | 44.84 ± 1.70a | 4.47 ± 0.22a | 2.39 ± 0.22a | 2.00 ± 0.14ab | 0.298 ± 0.017a | 0.655 ± 0.013a |
| 150 µM Pb | 37.37 ± 1.58d | 3.83 ± 0.08c | 1.74 ± 0.13d | 1.63 ± 0.10d | 0.223 ± 0.011d | 0.574 ± 0.014c |
| 150 µM Pb + 0.5 µM MJ | 39.37 ± 1.01cd | 4.19 ± 0.16b | 2.08 ± 0.11bc | 1.66 ± 0.14 cd | 0.263 ± 0.022c | 0.614 ± 0.009b |
| 150 µM Pb + 1 µM MJ | 42.06 ± 1.49b | 4.17 ± 0.12b | 2.26 ± 0.06ab | 1.91 ± 0.07ab | 0.268 ± 0.021bc | 0.619 ± 0.009b |
| 300 µM Pb | 29.71 ± 0.76f | 3.21 ± 0.11d | 1.25 ± 0.09e | 0.92 ± 0.10f | 0.165 ± 0.016e | 0.420 ± 0.010f |
| 300 µM Pb + 0.5 µM MJ | 34.45 ± 0.89e | 3.65 ± 0.13c | 1.84 ± 0.12d | 1.36 ± 0.11e | 0.225 ± 0.009d | 0.484 ± 0.013e |
| 300 µM Pb + 1 µM MJ | 39.55 ± 0.95c | 3.74 ± 0.15c | 1.90 ± 0.18 cd | 1.43 ± 0.11e | 0.256 ± 0.007c | 0.530 ± 0.013d |
Values (means ± SD, n = 5) followed by the same letter are not significantly different (P < 0.05; LSD test)
Photosynthetic pigments (chlorophyll a, b and carotenoids) were significantly lowered in rice seedlings exposed to Pb, with the largest decrease recorded at 300 μM Pb. At both Pb levels, MJ significantly restored the content of photosynthetic pigments and the highest increase was recorded under 1 μM MJ (Table 2). A 12.5 and 36% decline in chlorophyll fluorescence (Fv/Fm) was observed under 150 and 300 μM Pb treatments over to the control. However, MJ application raised Fv/Fm at both Pb levels compared to Pb exposure alone (Table 2).
Pb uptake and proline accumulation
The results showed that 150 and 300 μM Pb caused the accumulation of 1.76 and 2.81 µmol (g DW)−1 Pb in the roots and 1.44 and 2.46 µmol (g DW)−1 Pb in the shoots. In the roots, application of 0.5 and 1 μM MJ elevated Pb uptake by 18.8 and 33.5% under 150 μM Pb and 23 and 35% under 300 μM lead treatment, respectively compared to Pb treatments alone (Table 3). MJ supplementation lessened the accumulation of Pb in the shoots of Pb-treated plants (Table 3). Seedlings exposed to 150 and 300 μM Pb accumulated average 3.2 and 5.27 µmol (g DW)−1 Pb, respectively at the whole-plant level. However, MJ had no significant effect on Pb content in the whole plant (except for 1 μM MJ which reduced Pb accumulation in 300 μM Pb-exposed plants) (Table 3).
Table 3.
Effects of lead (Pb, 150 and 300 µM) alone or in combination with methyl jasmonate (MJ, 0.5 and 1 μM) on lead (Pb) accumulation and proline content in rice plants
| Treatments (mmol g−1dw) | Pb in root (µmol g−1dw) | Pb in shoot (µmol g−1dw) | Pb in whole plant (µmol g−1dw) | Proline |
|---|---|---|---|---|
| Control | n.d | n.d | n.d | 2.09 ± 0.15f |
| 0.5 µM MJ | n.d | n.d | n.d | 2.30 ± 0.17f |
| 1 µM MJ | n.d | n.d | n.d | 1.99 ± 0.16f |
| 150 µM Pb | 1.76 ± 0.12f | 1.44 ± 0.13c | 3.20 ± 0.25c | 8.28 ± 0.40e |
| 150 µM Pb + 0.5 µM MJ | 2.09 ± 0.13e | 0.97 ± 0.11d | 3.07 ± 0.10c | 9.75 ± 0.49d |
| 150 µM Pb + 1 µM MJ | 2.35 ± 0.14d | 0.64 ± 0.11e | 2.99 ± 0.04c | 9.19 ± 0.37d |
| 300 µM Pb | 2.81 ± 0.18c | 2.46 ± 0.16a | 5.27 ± 0.06a | 11.85 ± 0.62c |
| 300 µM Pb + 0.5 µM MJ | 3.28 ± 0.16b | 1.77 ± 0.12b | 5.05 ± 0.05a | 14.38 ± 0.69b |
| 300 µM Pb + 1 µM MJ | 3.52 ± 0.17a | 0.92 ± 0.17d | 4.44 ± 0.33b | 17.64 ± 0.76a |
Values (means ± SD, n = 5) followed by the same letter are not significantly different (P < 0.05; LSD test)
Proline content in leaves increased 4- and 5.7-fold in 150 and 300 μM Pb exposure, respectively over the control. However, MJ supplementation enhanced the proline content by 17.8 and 11% in 100 μM Pb-treated plants and 21.4 and 48.9% in 300 μM Pb-treated plants, respectively compared to Pb treatments alone (Table 3).
Proline and chlorophyll metabolism
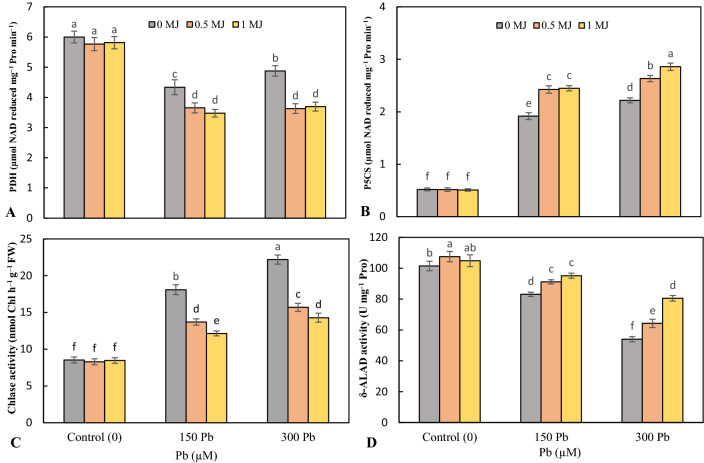
The data indicated that Pb phytotoxicity significantly lessened the activity of proline dehydrogenase (PDH) enzyme and the highest decrease was achieved in 150 μM Pb by 27.7% over the nontreated plants. In control treatment (non-Pb treatment), MJ had no significant effect on PDH activity, however, at both Pb levels, MJ application reduced PDH activity but there was no significant difference between MJ levels (Fig. 1a). 1-pyrroline-5-carboxylate synthetase (P5CS) activity significantly enhanced under 150 and 300 μM Pb toxicity and the highest increase was obtained at 300 μM Pb. However, MJ further elevated P5CS activity in Pb-stressed rice seedlings (Fig. 1b).
Fig. 1.
Effects of lead (Pb, 150 and 300 µM) alone or in combination with methyl jasmonate (MJ, 0.5 and 1 μM) on the activity of proline dehydrogenase (PDH, A), 1-pyrroline-5-carboxylate synthetase (P5CS, B), chlorophyllase (Chlase, C) and δ-aminolevulinic acid dehydratase (δ-ALAD, D) in rice leaves. Values (means ± SD, n = 5) followed by the same letter are not significantly different (P < 0.05; LSD test)
Pb in a concentration-dependent manner enhanced chlorophyllase (Chlase) activity over the non-treated plants. At both levels of Pb, the supplementation of MJ meaningfully declined Chlase activity and was most pronounced at 1 μM MJ (Fig. 1c). The addition of 150 and 300 μM Pb resulted in an 18.1% and 46.8% decline in δ-aminolevulinic acid dehydratase (δ-ALAD) activity, respectively over to the control. However, 0.5 and 1 μM MJ up-regulated δ-ALAD activity by 9.7 and 14.5% in 150 μM Pb-exposed plants and 18.9 and 49.1% in 300 μM Pb-exposed plants, respectively compared to Pb treatments alone (Fig. 1d).
ROS and MG metabolism
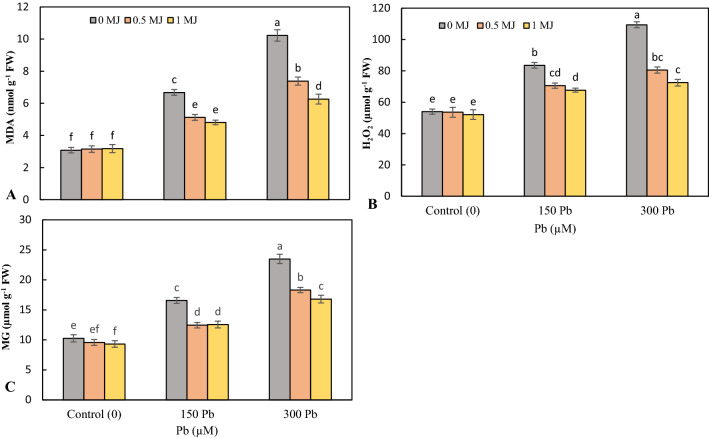
Application of Pb at concentrations of 150 and 300 μM induced oxidative stress and enhanced the contents of MDA, H2O2 and MG in rice seedling leaves, with the largest increase recorded at high concentrations of Pb. MJ supplementation in a dose-dependent manner significantly reduced the level of MDA, H2O2 and MG in both Pb levels over to Pb treatments alone (Fig. 2a–c).
Fig. 2.
Effects of lead (Pb, 150 and 300 µM) alone or in combination with methyl jasmonate (MJ, 0.5 and 1 μM) on of malondialdehyde (MDA, A), hydrogen peroxide (H2O2, B) and methylglyoxal (MG, C) in rice leaves. Values (means ± SD, n = 5) followed by the same letter are not significantly different (P < 0.05; LSD test)
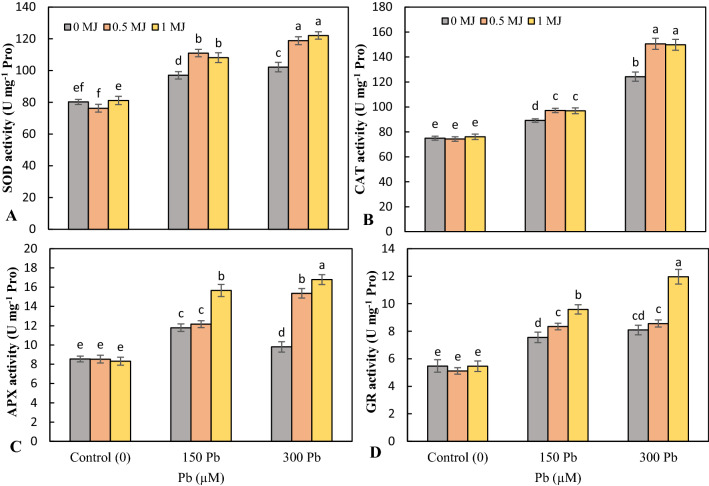
Pb toxicity upregulated the activity of SOD, CAT, APX and GR enzymes in the leaves than the untreated plants and the highest activity of antioxidant enzymes was observed at 300 μM Pb. Application of both concentrations of MJ (0.5 and 1 μM) caused a further rise in the activity of antioxidant enzymes in Pb-stress rice seedlings, the highest increase was related to the concentration of 1 μM MJ (Fig. 3).
Fig. 3.
Effects of lead (Pb, 150 and 300 µM) alone or in combination with methyl jasmonate (MJ, 0.5 and 1 μM) on the activity of superoxide dismutase (SOD, A), catalase (CAT, B), ascorbate peroxidase (APX, C) and glutathione reductase (GR, D) in rice leaves. Values (means ± SD, n = 5) followed by the same letter are not significantly different (P < 0.05; LSD test)
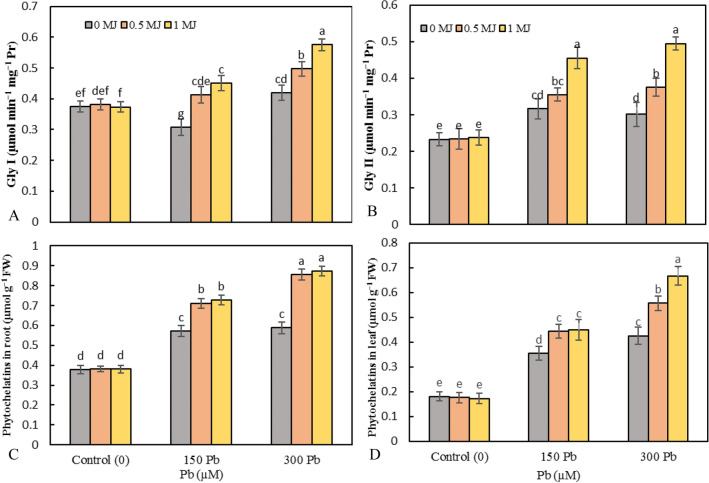
The activities of Gly I decreased by 18% under 150 μM Pb and increased by 11.8% under 300 μM Pb over untreated plants. At both levels of Pb toxicity, MJ application significantly up-regulated the Gly I activity in a concentration-dependent manner compared to Pb treatments alone (Fig. 4a). Toxicity of 150 and 300 μM Pb resulted in a 35.8 and 29.3% raise in Gly II activity, respectively over the control plants. However, at both Pb levels, the application of MJ significantly elevated the Gly II activity compared to the Pb treatments alone (Fig. 4b).
Fig. 4.
Effects of lead (Pb, 150 and 300 µM) alone or in combination with methyl jasmonate (MJ, 0.5 and 1 μM) on the activity of glyoxalase I (Gly I, A), glyoxalase II (Gly II, B), and the phytochelatins content in root (C) and leaf (D) in rice. Values (means ± SD, n = 5) followed by the same letter are not significantly different (P < 0.05; LSD test)
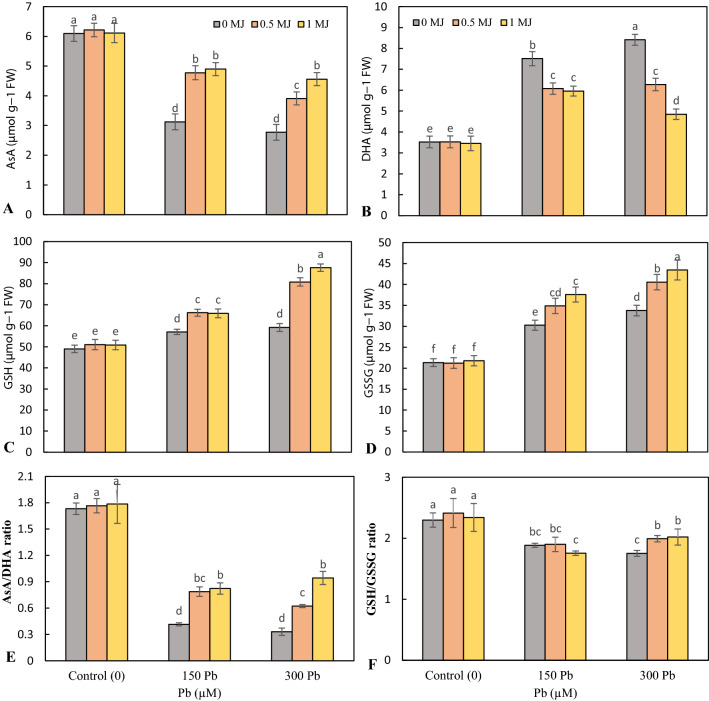
Phytochelatins and AsA-GSH cycle
The content of phytochelatins (PCs) in rice roots significantly enhanced in the presence of Pb (52.1 and 55.2% with 150 and 300 μM Pb, respectively compared to the control ones). Application of 0.5 and 1 μM MJ enhanced root PCs by 24.3 and 27.1% in 150 μM Pb-treated plants and 45.6 and 48.5% in 300 μM Pb-treated plants, respectively over the Pb treatments alone (Fig. 4c). Pb toxicity raised the accumulation of PCs in rice seedling leaves over the control and the highest accumulation of PCs was recorded in 300 μM Pb. However, further increase by 24.9 and 26.3% in 150 μM Pb-treated plants and 30.9 and 57% in 300 μM Pb-treated plants was recorded by the MJ supplementation (Fig. 4d).
Pb toxicity significantly declined AsA content in rice seedling leaves, however, the addition of MJ improved AsA content in both Pb levels (Fig. 5a). DHA content enhanced by 2.1- and 2.4-fold in 150 and 300 μM Pb-stressed seedlings, respectively over the control ones. MJ supplementation to Pb-stressed rice seedlings declined DHA content over the seedlings exposed with Pb alone (Fig. 5b). Concentrations of 150 and 300 μM Pb elevated the accumulation of GSH in the leaves. At both levels of Pb, MJ enhanced GSH accumulation further than seedlings treated with Pb alone (Fig. 5c). An increasing trend was observed with increasing Pb concentration in leaves GSH accumulation that supplementation of MJ to Pb-stressed plants caused a further enhancement in GSH content, which was the highest increase at 1 μM MJ (Fig. 5d). Pb toxicity significantly reduced the ratio of AsA/DHA compared to non-stress conditions; however, no significant difference was observed between 150 and 300 μM Pb. The use of MJ in a concentration-dependent manner improved the ratio of AsA/DHA in plants stressed with Pb (Fig. 5e). Adding 150 and 300 μM Pb reduced the GSH/GSSG ratio, although there was no significant difference between them. In non-treated (control) and 150 μM Pb-treated plants, the use of MJ had no significant effect on the GSH/GSSG ratio, but in 300 μM Pb toxicity, MJ significantly enhanced the GSH/GSSG ratio (Fig. 5f).
Fig. 5.
Effects of lead (Pb, 150 and 300 µM) alone or in combination with methyl jasmonate (MJ, 0.5 and 1 μM) on the contents of reduced ascorbic acid (AsA, A), oxidized ascorbic acid (DHA, B), reduced glutathione (GSH, C) and oxidized glutathione (GSSG, D) and the ratios of AsA/DHA (E) and GSH/GSSG (F) in rice leaves. Values (means ± SD, n = 5) followed by the same letter are not significantly different (P < 0.05; LSD test)
Expression of genes involved in Pb transport and sequestration
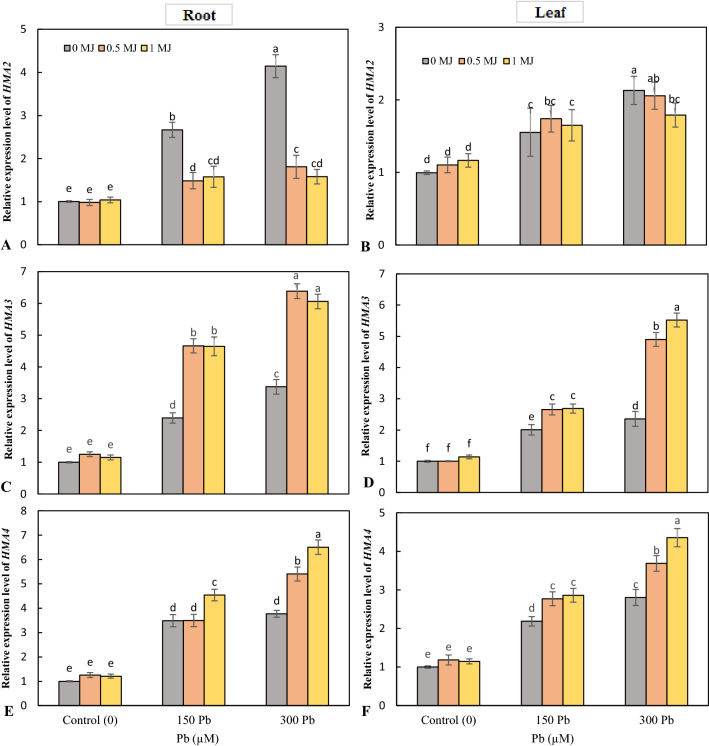
The results of gene expression showed that the Pb toxicity upregulated the expression of HMA2 gene in roots and leaves and the highest level of HMA2 expression was recorded under 300 μM Pb. At the root, exogenous application of MJ downregulated HMA2 expression at both Pb levels compared to Pb treatment alone, but there was no significant difference between the two MJ levels. In leaves, 0.5 μM MJ had no significant effect on HMA2 gene expression; however, 1 μM MJ downregulated HMA2 expression in Pb-stressed rice seedlings (Fig. 6a, b). Application of Pb in a concentration-dependent manner upregulated the expression of HMA3 and HMA4 in the roots and leaves. However, MJ supplementation further increased the expression of HMA3 and HMA4 in the roots and leaves of Pb-stressed rice (Fig. 6c–f).
Fig. 6.
Effects of lead (Pb, 150 and 300 µM) alone or in combination with methyl jasmonate (MJ, 0.5 and 1 μM) on the expression of HMA2 (A, B), HMA3 (C & D), HMA4 (E & F) genes in rice roots and leaves. Values (means ± SD, n = 5) followed by the same letter are not significantly different (P < 0.05; LSD test)
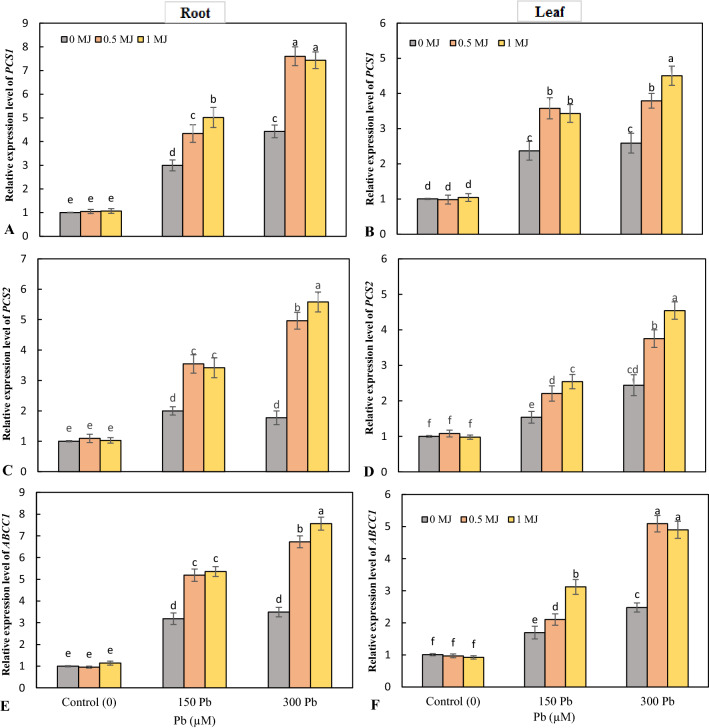
In roots and leaves, Pb toxicity upregulated the expression levels of the PCS1 and PCS2 over the control, with the highest increase observed at 300 μM Pb. At both 150 and 300 μM Pb stress, the application of MJ further increased the expression of PCS1 and PCS2 in the roots and leaves (Fig. 7a–d). Expression of ABCC1 was significantly enhanced in roots and leaves under Pb toxicity, with the highest increase recorded at high Pb level. In both root and leaf tissues, application of MJ in a dose-dependent manner upregulated the expression of ABCC1 gene in Pb-stressed seedlings (Fig. 7e, f).
Fig. 7.
Effects of lead (Pb, 150 and 300 µM) alone or in combination with methyl jasmonate (MJ, 0.5 and 1 μM) on the expression of PCS1 (A, B), PCS2 (C, D), ABCC1 (E,F) genes in rice roots and leaves. Values (means ± SD, n = 5) followed by the same letter are not significantly different (P < 0.05; LSD test)
Yield and yield components
Pb stress at a concentration of 300 μM resulted in a 15.8% decline in tiller than the control. The MJ application did not have a considerable effect on tiller in non-stressed and 150 μM Pb-stressed plants. However, 0.5 and 1 μM MJ treatments enhanced tiller by 9.4% and 8.1%, respectively in comparison to rice subjected to Pb alone (Table 4). Pb toxicity significantly reduced the grains per panicle and the percentage of filled grains in comparison to untreated plants. The highest decrease in the grains per panicle and the percentage of filled grains was related to the toxicity of 300 μM Pb. Exogenous application of MJ improved the filled grains percentage and grains per panicle at both levels of Pb toxicity compared to Pb treatment alone (Table 4). Compared to the control treatment, 150 μM Pb stress did not have a significant effect on 1000-grain weight, but 300 μM Pb stress remarkably declined 1000-seed weight by 9.3%. In 300 μM Pb-stressed plants, exogenous supplementation of 0.5 and 1 μM MJ improved 1000-grain weight by 4.5 and 5%, respectively, compared to 300 μM Pb treatment alone (Table 4). Pb toxicity significantly reduced the harvest index over the control. However, at both levels of Pb toxicity, MJ improved the harvest index in comparison to Pb stress alone (Table 4).
Table 4.
Effects of lead (Pb, 150 and 300 µM) alone or in combination with methyl jasmonate (MJ, 0.5 and 1 μM) on yield and yield-related attributes in rice plants
| Treatments | Tiller/hill | Grains/panicle | Filled grain percentage (%) | 1000-grain weight (g) | Harvest Index |
|---|---|---|---|---|---|
| Control | 10.33 ± 0.23a | 149.9 ± 6.15ab | 86.73 ± 2.96a | 25.87 ± 0.45ab | 73.18 ± 0.61ab |
| 0.5 µM MJ | 10.52 ± 0.18a | 147.8 ± 7.15ab | 84.72 ± 3.51a | 26.24 ± 0.28a | 73.95 ± 0.43a |
| 1 µM MJ | 10.55 ± 0.28a | 153.8 ± 7.05a | 85.47 ± 4.50a | 24.93 ± 0.72cde | 72.69 ± 0.64bc |
| 150 µM Pb | 10.65 ± 0.41a | 132.0 ± 6.66e | 73.63 ± 2.90c | 25.31 ± 0.46bcd | 69.39 ± 0.46e |
| 150 µM Pb + 0.5 µM MJ | 10.58 ± 0.34a | 143.9 ± 4.90bcd | 75.29 ± 2.51c | 25.61 ± 0.38abc | 70.91 ± 0.69d |
| 150 µM Pb + 1 µM MJ | 10.49 ± 0.35a | 146.2 ± 3.65abc | 81.72 ± 3.31ab | 25.51 ± 0.43abc | 71.97 ± 0.63 cd |
| 300 µM Pb | 8.70 ± 0.44c | 115.8 ± 5.93f | 54.95 ± 3.91d | 23.46 ± 0.33f | 56.25 ± 0.64 g |
| 300 µM Pb + 0.5 µM MJ | 9.52 ± 0.29b | 138.0 ± 4.08cde | 71.59 ± 3.46c | 24.51 ± 0.31e | 65.19 ± 0.66f |
| 300 µM Pb + 1 µM MJ | 9.40 ± 0.21b | 136.2 ± 4.56de | 76.04 ± 2.41bc | 24.63 ± 0.38de | 65.24 ± 1.02f |
Values (means ± SD, n = 5) followed by the same letter are not significantly different (P < 0.05; LSD test)
Discussion
Pb, as one of the most important heavy metals in polluting ecosystems, can have negative effects on essential plant processes such as water balance, respiration, nutrient uptake, photosynthesis, root growth and the activity of essential enzymes and thus inhibit plant growth (Cenkci et al. 2010; Pourrut et al. 2011). The results showed that Pb at concentrations of 150 and 300 μM reduced the height and total dry weight of rice and the highest growth reduction was recorded in seedlings subjected to 300 μM Pb. Similar findings of Pb phytotoxicity on Oryza sativa (Ashraf et al. 2017a, b), Brassica napus L. (Shakoor et al. 2014) and Lycopersicon esculentum (Bali et al. 2018) growth have been previously reported. Ali et al. (2014) revealed that Pb stress, by damaging the roots and interfering with nutrients uptake, disrupts metabolic processes and, as a result, reduces plant growth and biomass. Bali et al. (2018) showed that Pb toxicity negatively affected tomato plant growth by inducing oxidative stress and diminishing stomatal conductance and photosynthetic pigments. However, our findings showed that the application of MJ (0.5 and 1 μM) effectively improved the height and biomass production of Pb-stressed rice plants, which is in accordance with the reports of Piotrowska et al. (2009),Bali et al. (2018). Jasmonic acid has been reported to increase tomato tolerance under Pb toxicity by improving the AsA-GSH cycle and the activity of antioxidant enzymes, as well as increasing the content of metal chelating and osmolyte compounds (Bali et al. 2018). Improved plant growth by MJ application under arsenic (Farooq et al. 2016; Mousavi et al. 2020) and cadmium (Ali et al. 2018) toxicity has also been reported, which may indicate the role of this phytohormone in protecting plants against heavy metal toxicity.
Our findings showed that Pb stress declined the photosynthetic pigment and the δ-ALAD activity, and increased the Chlase activity. Decreased photosynthetic pigments and Fv/Fm ratio can be caused by disruption of photosynthetic apparatus, protein complex, and chloroplasts in plants stressed by Pb (Vassilev et al. 1995; Ali et al. 2013). Hegedus et al. (2001) reported that the decrease in chlorophyll content could be due to the induction of Chlase activity in heavy metals-stressed plants, which is consistent with the results of the current study. Therefore, the results indicated that Pb toxicity had a negative effect on the photosynthetic apparatus and, consequently, plant growth by altering chlorophyll metabolism and reducing photosynthetic pigments. However, the supplementation of MJ was found to restore chlorophyll content and Fv/Fm in Pb-stressed plants by increasing δ-ALAD activity and decreasing Chlase activity. Similar results of the effects of MJ on photosynthetic pigments under Pb (Piotrowska et al. 2009; Bali et al. 2018), As (Mousavi et al. 2020), Cd (Keramat et al. 2010) and salinity (Rezai et al. 2013) stress have been reported, indicating the protecting effects of MJ on photosynthetic pigments. MJ has been shown to upregulate the expression of genes that regulate chlorophyll biosynthesis by increasing 5-aminolevulinic acid production (Ueda and Saniewski 2006). Therefore, MJ increased the photosynthetic pigments and the efficiency of the photosynthetic apparatus by modulating the chlorophyll metabolism and, consequently, increased the tolerance of plants under Pb toxicity.
Proline under stressful conditions plays an important role in ROS scavenging, maintaining cellular osmolarity, improving redox homeostasis and protein functions (Ghorbani et al. 2018a; Ghasemi-Omran et al. 2021). Here, we found that the Pb toxicity downregulated the PDH activity and upregulated P5CS activity and consequently, enhanced proline levels in the leaves, which could be due to water stress induced by heavy metal toxicity (Ahmad et al. 2020). Increased proline accumulation in wheat (Lamhamdi et al. 2011) and tomato (Bali et al. 2018) under Pb toxicity had already been documented. Sharma and Dubey (2005) indicated that proline, as a protein stabilizer and metal chelator, improves plant tolerance under heavy metal toxicity. Therefore, increasing proline accumulation in Pb-stressed plants can indicate the induction of the plant defense mechanism to adapt to stressful conditions. However, by modulating the activity of enzymes involved in proline metabolism, MJ further increased proline in the leaves of Pb-stressed rice, which is similar to the results documented in Lycopersicon esculentum (Bali et al. 2018) and Solanum nigrum (Yan et al. 2015) under the toxicity of Pb and Cd, respectively. Therefore, our findings confirmed that MJ by modulating the activity of enzymes involved in proline metabolism increased proline accumulation, which can play an effective role in improving rice tolerance to Pb toxicity.
The results showed that although MJ had no significant effect on Pb uptake in the whole plant (except for 1 μM MJ in 300 μM Pb-stressed plants), MJ reduced the Pb accumulation in the shoots, indicates that MJ declined the Pb translocation to the above-ground organs by immobilizing the Pb in the root. Similar results of reduced uptake of Pb (Bali et al. 2018; Piotrowska et al. 2009), Cd (Ahmad et al. 2017) and As (Farooq et al. 2018; Mousavi et al. 2020) by MJ treatment have been previously reported. As a signaling molecule, MJ may downregulate the transcription level of heavy metal transporter genes or increase PCs biosynthesis, which in turn may reduce the uptake and transport of metal to shoots (Bali et al. 2018). Increased synthesis of PCs in roots and decreased translocation of heavy metal to shoots in plants treated with MJ by Maksymiec et al. (2007) and Bali et al. (2018) have also been reported. Therefore, MJ prevented damage to photosynthetic organs by accumulating Pb in the roots and reducing its translocation to the shoot.
Levels of H2O2, MG and MDA indicate oxidative stress-induced damage under stressful environmental conditions. (Ghorbani et al. 2018b). Our findings revealed that the Pb toxicity enhanced the accumulation of H2O2, MG and MDA in the leaves, indicating the induction of oxidative stress and damage to biological membranes. Similar results of MDA and H2O2 accumulation in Carthamus tinctorius (Namdjoyan et al. 2020) and Brassica napus (Shakoor et al. 2014) under Pb stress have also been documented. Accumulation of ROS induced by stressful conditions causes damages to cellular physiological processes that, by provoking the Haber–Weiss cycle, resulting in the peroxidation of lipids and pigments as well as the production of hydroxyl radicals, which impairs membrane permeability and function (Mittler et al. 2012; Ghorbani et al. 2019a). Correspondingly, MJ supplementation induced the activity of CAT, SOD, GR, APX, Gly I and Gly II, as a result, reduced MDA, Mg and H2O2 levels in the Pb-stressed rice, which is consistent with the results of Bali et al. (2018). MJ may protect against damage to cellular organelles such as chloroplasts by stimulating the antioxidant defense system and scavenging toxic free radicals. Similar findings of MJ-induced oxidative stress alleviation in Wolffia arrhiza and Lycopersicon esculentum under Pb toxicity have been previously shown (Piotrowska et al. 2009; Bali et al. 2018). Farooq et al. (2016) indicated that MJ enhanced the expression of antioxidant enzymes and, as a result, induced the activity of antioxidant enzymes, which effectively improved the tolerance of plant under heavy metal toxicity. Therefore, MJ may upregulate the de novo synthesis and activity of antioxidant enzymes by inducing alterations in the post-transcriptional and translational levels of antioxidant enzymes as well as enzymes included in the glyoxalase system, thereby improving the efficiency of the plant's defense system under Pb toxicity. Improving the activity of enzymes involved in the AsA-GSH cycle by MJ greatly helps to maintain redox homeostasis, H2O2 reduction and thus protect the photosynthetic apparatus (Mousavi et al. 2020). Therefore, balancing the GSH and AsA contents induced by modulating the GR and APX activity could improve cellular functioning under Pb stress by reducing the level of toxic hydroxyl radicals and protecting the function and structure of proteins (Ghorbani et al. 2020). The results showed that MJ ameliorated glutathione and ascorbate levels and the ratios AsA/DHA and GSH/GSSG in Pb-stressed seedlings, which is consistent with the results reported in tomato (Bali et al. 2018) and rice (Mousavi et al. 2020) grown under Pb and As toxicity, respectively. MJ has been shown to activate heavy metal-response signaling pathways that enhance the GSH content and induce the antioxidant machinery (Farooq et al. 2016). Therefore, MJ reduced ROS and lipid peroxidation by improving the redox status of the AsA-GSH cycle and the activity of antioxidant enzymes, thereby improving the rice growth under Pb toxicity.
PCs have an outstanding role in the reduction of the phytotoxicity induced by heavy metals by chelating the metal ions (Hall 2002). Our findings showed that Pb toxicity increased PCs accumulation in roots and leaves, which were further enhanced with MJ application. Bali et al. (2018) reported that the MJ application increased the tolerance of Pb-exposed tomato by regulating the contents of non-protein and protein thiol. By PCs complexation, plants can accumulate higher concentrations of heavy metals in the above-ground parts (Souri et al. 2017). Although MJ lessened the Pb accumulation in the leaves of Pb-exposed plants, however, the accumulation of Pb in Pb-exposed plants treated with MJ was higher than control plants. Therefore, MJ can effectively reduce the toxic effects of Pb accumulated in leaves by increasing PCs content. MJ here upregulated the PCS1 and PCS2 gene transcript abundances in the roots and leaves of the Pb-stressed rice, which correspond to the increase in PCs accumulation as expected. It has been suggested that upregulating the transcription of genes associated with heavy metal sequestration effectively increases the rapid tolerance of plants under heavy metal toxicity (Hasan et al. 2015). Two transporters of ABCC1 and ABCC2 were identified in Arabidopsis thaliana that have an outstanding role in the transfer of the heavy metal-PCs complexes into the vacuoles and, consequently, their sequestration and detoxification (Song et al. 2010). Song et al. (2014) showed that ABCC1 transporters in rice, which have a similar function to AtABCC1 and AtABCC2 transporters, play an important role in detoxifying heavy metals as well as reducing their translocation to the shoots, especially rice grains. In the present study, the MJ supplementation enhanced the transcription level of ABCC1 gene, especially in the roots of Pb-stressed plants, which could play an effective role in detoxification of Pb and improving plant tolerance. Upregulation ABCC1 gene expression is consistent with the upregulated expression of PCS1 and PCS2 and enhanced accumulation of PCs to form the PCs-heavy metal complex and vacuole sequestration. The results revealed that higher expression levels of PCS1, PCS2 and ABCC1 were observed in the roots than that of the leaves. Thus, MJ upregulated the expression of genes implicated in Pb sequestration (PCS1, PCS2 and ABCC1) and increased GSH and PCs levels, thereby immobilizing Pb at the roots and protecting the sensitive photosynthetic organs from Pb phytotoxicity.
It is accepted that HMAs transporters are implicated in the transport of heavy metals in various plant tissues. Takahashi et al. (2012) reported that OsHMA2 is implicated in the loading of heavy metals, especially Cd and Zn, to the xylem and their translocation to the shoot. The results showed that the expression of HMA2 gene in the roots displayed a significant upregulation in Pb-stressed plants that corresponds to the increase in Pb accumulation in the shoots. However, MJ downregulated HMA2 expression at both levels of Pb toxicity, which was consistent with reduced Pb translocation to the shoot, which may indicate the role of this transporter in Pb translocation. OsHMA3 and OsHMA4 transporters are other identified members of the HMAs family in rice plants that are implicated in the transport of Cd and Cu into the vacuole, respectively (Sasaki et al. 2014; Huang et al. 2016). The results revealed that although Pb toxicity upregulated the expression of HMA3 and HMA4 genes, however, MJ caused a further increase in the expression of these genes in Pb-stressed plants. The MJ-induced upregulation in the expression of HMA3 and HMA4 in the roots was greater than that in the leaves, which is consistent with increased root Pb accumulation and decreased translocation to the shoots. Therefore, MJ prevented Pb translocation to shoots and damage to photosynthetic organs by regulating the expression level of HMA3 and HMA4 genes and sequestering Pb into vacuoles in the roots.
The inhibitory effects induced by heavy metal toxicity on yield components may ultimately reduce plant yield. According to the results, Pb toxicity, especially 300 μM, reduced the yield components and yield of rice, which is according to the results documented by Ashraf et al. (2017a, b). Ashraf and Tang (2017) indicated that Pb toxicity negatively affected the growth and biomass of rice by reducing the contents of photosynthetic pigments and inducing oxidative stress, which in turn decreased rice yield. However, the application of MJ improved the rice yield under Pb stress, which is consistent with the results obtained by Mousavi et al. (2020) under stress. Mousavi et al. (2020) revealed that MJ restored the chlorophylls content and improved the rice yield under as phytotoxicity by reducing as uptake and improving Fe translocation to the shoots. Therefore, the supplementation of MJ enhanced the rice yield under Pb toxicity, which could be due to the immobilization of Pb in the roots and reduce its translocation to the shoots, as well as strengthening the antioxidant defense system in Pb-stressed plants.
Conclusion
In environments contaminated with toxic metals, the ability to sequester and/detoxify or heavy metals has played an outstanding role in plant survival. MJ revealed the complex and protective impacts on the proline and photosynthetic pigments metabolism, antioxidant redox status, MG and ROS scavenging, PCs biosynthesis and, as a result, Pb sequestration in the vacuoles. Furthermore, MJ protected the photosynthetic organs from toxic Pb by modulating the expression of genes implicated in Pb transport to shoots (HMA2) and sequestering Pb into vacuoles (HMA3, HMA4, PCS1, PCS2 and ABCC1), thereby improving the growth and yield of rice plants under Pb toxicity. Our findings could provide molecular insights and cellular interplay scheme to produce effective strategies for healthy food farming, particularly in Pb-contaminated environments. However, more research at the molecular levels is required to confirm the role of MJ in Pb detoxification as well as to recognize subsequent signaling cascades.
Author contributions
Conceptualization and Methodology, JS and HF; Validation and Investigation, YN; Analysis, DBR; Resources, JS; Writing original, HF and YN; Review and editing. All authors have read and agreed to the published version of the manuscript.
Declarations
Conflict of interest
The authors declare that they do not have any conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aebi H. Catalase in vitro. In: Colowick S, Kaplan N, editors. Methods in enzymology. Florida: Elsevier; 1984. pp. 121–126. [DOI] [PubMed] [Google Scholar]
- Ahmad P, Alyemeni MN, Wijaya L, Alam P, Ahanger MA, Alamri SA. Jasmonic acid alleviates negative impacts of cadmium stress by modifying osmolytes and antioxidants in faba bean (Vicia faba L.) Arch Agron Soil Sci. 2017;63:1889–1899. [Google Scholar]
- Ahmad P, Alam P, Balawi TH, Altalayan FH, Ahanger MA, Ashraf M. Sodium nitroprusside (SNP) improves tolerance to arsenic (As) toxicity in Vicia faba through the modifications of biochemical attributes, antioxidants, ascorbate-glutathione cycle and glyoxalase cycle. Chemosphere. 2020;244:125480. doi: 10.1016/j.chemosphere.2019.125480. [DOI] [PubMed] [Google Scholar]
- Ali B, Wang B, Ali S, Ghani MA, Hayat MT, Yang C, Xu L, Zhou WJ. 5-aminolevulinic acid ameliorates the growth, photosynthetic gas exchange capacity and ultrastructural changes under cadmium stress in Brassica napus L. J Plant Growth Regul. 2013;32:604–614. [Google Scholar]
- Ali B, Xu X, Gill RA, Yang S, Ali S, Tahir M, Zhou W. Promotive role of 5-aminolevulinic acid on mineral nutrients and antioxidative defense system under lead toxicity in Brassica napus. Ind Crop Prod. 2014;52:617–626. [Google Scholar]
- Ali E, Hussain N, Shamsi IH, Jabeen Z, Siddiqui MH, Jiang LX. Role of jasmonic acid in improving tolerance of rape-seed (Brassica napus L.) to Cd toxicity. J Zhejiang Univ Sci B. 2018;19(2):130–14. doi: 10.1631/jzus.B1700191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf U, Tang X. Yield and quality responses, plant metabolism and metal distribution pattern in aromatic rice under lead (Pb) toxicity. Chemosphere. 2017;176:141–155. doi: 10.1016/j.chemosphere.2017.02.103. [DOI] [PubMed] [Google Scholar]
- Ashraf U, Kanu AS, Mo ZW, Hussain S, Anjum SA, Khan I, Abbas RN, Tang X. Lead toxicity in rice; effects, mechanisms and mitigation strategies-a mini review. Environ Sci Pollut Res. 2015;22:18318–18332. doi: 10.1007/s11356-015-5463-x. [DOI] [PubMed] [Google Scholar]
- Ashraf U, Hussain S, Anjum SA, Abbas F, Tanveer M, Noor MA, Tang X. Alterations in growth, oxidative damage, and metal uptake of five aromatic rice cultivars under lead toxicity. Plant Physiol Biochem. 2017;115:461–471. doi: 10.1016/j.plaphy.2017.04.019. [DOI] [PubMed] [Google Scholar]
- Ashraf U, Kanu AS, Deng Q, Mo Z, Pan S, Tian H, Tang X. Lead (Pb) toxicity; physio-biochemical mechanisms, grain yield, quality, and Pb distribution proportions in scented rice. Front Plant Sci. 2017;8:259. doi: 10.3389/fpls.2017.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsen KB, Palmgren MG. Inventory of the superfamily of P-type ion pumps in Arabidopsis. Plant Physiol. 2001;126:696–706. doi: 10.1104/pp.126.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bali S, Kaur P, Kohli SK, Ohri P, Thukral AK, Bhardwaj R, Wijaya L, Alyemeni MN, Ahmad P. Jasmonic acid induced changes in physio-biochemical attributes and ascorbate-glutathione pathway in Lycopersicon esculentum under lead stress at different growth stages. Sci Total Environ. 2018;645:1344–1360. doi: 10.1016/j.scitotenv.2018.07.164. [DOI] [PubMed] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Bharwana S. Alleviation of lead toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes suppressed lead uptake and oxidative stress in cotton. J Bioremed Biodegr. 2013;4:4. doi: 10.1016/j.ecoenv.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cenkci S, Ciğerci İH, Yıldız M, Özay C, Bozdağ A, Terzi H. Lead contamination reduces chlorophyll biosynthesis and genomic template stability in Brassica rapa L. Environ Exp Bot. 2010;67:467–473. [Google Scholar]
- Charest C, Phan CT. Cold-acclimation of wheat (Triticum aestivum)—properties of enzymes involved in proline metabolism. Physiol Plantarum. 1990;80:159–168. [Google Scholar]
- Chenery SR, Izquierdo M, Marzouk E, Klinck B, Palumbo-Roe B, Tye AM. Soil–plant interactions and the uptake of Pb at abandoned mining sites in the Rookhope catchment of the N. Pennines, UK—a Pb isotope study. Sci Total Environ. 2012;433:547–560. doi: 10.1016/j.scitotenv.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Colangelo EP, Guerinot ML. Put the metal to the petal: metal uptake and transport throughout plants. Curr Opin Plant Biol. 2006;9:322–330. doi: 10.1016/j.pbi.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Costa ML, Civello PM, Chaves AR, Martínez GA. Effect of ethephon and 6–benzylaminopurine on chlorophyll degrading enzymes and a peroxidase–linked chlorophyll bleaching during post-harvest senescence of broccoli (Brassica oleracea L.) at 20 C. Postharvest Biol Technol. 2005;35(2):191–199. [Google Scholar]
- De Vos RCH, Vonk MJ, Vooijs R, Schat H. Glutathione depletion due to copper-induced phytochelatin synthesis causes oxidative stress in Silene cucubalus. Plant Physiol. 1992;98l:853–858. doi: 10.1104/pp.98.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng F, Yamaji N, Xia J, Ma JF. A member of the heavy metal P-type ATPase OsHMA5 is involved in xylem loading of copper in rice. Plant Physiol. 2013;163:1353–1362. doi: 10.1104/pp.113.226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhindsa RS, Matowe W. Drought tolerance in two mosses: correlated with enzymatic defence against lipid peroxidation. J Exp Bot. 1981;32:79–91. [Google Scholar]
- Dutilleul C, Driscoll S, Cornic G, De Paepe R, Foyer CH, Noctor G. Functional mitochondrial complex I is required by tobacco leaves for optimal photosynthetic performance in photorespiratory conditions and during transients. Plant Physiol. 2003;131:264–275. doi: 10.1104/pp.011155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq MA, Gill RA, Islam F, Ali B, Liu H, Xu J, He S, Zhou W. Methyl jasmonate regulates antioxidant defense and suppresses arsenic uptake in Brassica napus L. Front Plant Sci. 2016;7:468. doi: 10.3389/fpls.2016.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133(1):21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 2011;155:2–18. doi: 10.1104/pp.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi-Omran VO, Ghorbani A, Sajjadi-Otaghsara SA. Melatonin alleviates NaCl-induced damage by regulating ionic homeostasis, antioxidant system, redox homeostasis, and expression of steviol glycosides-related biosynthetic genes in in vitro cultured Stevia rebaudiana Bertoni. Vitro Cell Dev Biol-Plant. 2021 doi: 10.1007/s11627-021-10161-9. [DOI] [Google Scholar]
- Ghorbani A, Zarinkamar F, Fallah A. The effect of cold stress on the morphologic and physiologic characters of tow rice varieties in seedling stage. J Crop Breed. 2009;1:50–66. [Google Scholar]
- Ghorbani A, Zarinkamar F, Falah A. Effect of cold stress on the anatomy and morphology of the tolerant and sensitive cultivars of rice during germination. J Cell Tissue. 2011;2(3):235–244. [Google Scholar]
- Ghorbani A, Razavi SM, Ghasemi Omran VO, Pirdashti H. Piriformospora indica inoculation alleviates the adverse effect of NaCl stress on growth, gas exchange and chlorophyll fluorescence in tomato (Solanum lycopersicum L.) Plant Biol. 2018;20:729–736. doi: 10.1111/plb.12717. [DOI] [PubMed] [Google Scholar]
- Ghorbani A, Razavi SM, Ghasemi Omran VO, Pirdashti H. Piriformospora indica alleviates salinity by boosting redox poise and antioxidative potential of tomato. Russ J Plant Physiol. 2018;65:898–907. [Google Scholar]
- Ghorbani A, Ghasemi Omran VO, Razavi SM, Pirdashti H, Ranjbar M. Piriformospora indica confers salinity tolerance on tomato (Lycopersicon esculentum Mill.) through amelioration of nutrient accumulation, K+/Na+ homeostasis and water status. Plant Cell Rep. 2019;38:1151–1163. doi: 10.1007/s00299-019-02434-w. [DOI] [PubMed] [Google Scholar]
- Ghorbani A, Razavi SM, Ghasemi V, Pirdeshti H. Effects of endophyte fungi symbiosis on some physiological parameters of tomato plants under 10 day long salinity stress. J Plant Proc Func. 2019;7(27):193–208. [Google Scholar]
- Ghorbani A, Tafteh M, Roudbari N, Pishkar L, Zhang W, Wu C. Piriformospora indica augments arsenic tolerance in rice (Oryza sativa) by immobilizing arsenic in roots and improving iron translocation to shoots. Ecotoxicol Environ Saf. 2020;209:111793. doi: 10.1016/j.ecoenv.2020.111793. [DOI] [PubMed] [Google Scholar]
- Gill RA, Ali B, Islam F, Farooq MA, Gill MB, Mwamba TM, Zhou W. Physiological and molecular analyses of black and yellow seeded Brassica napus regulated by 5-aminolivulinic acid under chromium stress. Plant Physiol Biochem. 2015;94:130–143. doi: 10.1016/j.plaphy.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Gupta DK, Huang HG, Yang XE, Razafindrabe BHN, Inouhe M. The detoxification of lead in Sedum alfredii H. is not related to phytochelatins but the glutathione. J Hazard Mater. 2010;177:437–444. doi: 10.1016/j.jhazmat.2009.12.052. [DOI] [PubMed] [Google Scholar]
- Hall JL. Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot. 2002;53:1–11. [PubMed] [Google Scholar]
- Hasan MK, Ahammed GJ, Yin L, Shi K, Xia X, Zhou Y, Yu J, Zhou J. Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front Plant Sci. 2015;6:601. doi: 10.3389/fpls.2015.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M, Hossain MA, Fujita M. Nitric oxide modulates antioxidant defense and methylglyoxal detoxification system and reduces salinity induced damage in wheat seedling. Plant Biotecnol Rep. 2011;5:353–365. doi: 10.1007/s12011-011-8958-4. [DOI] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hegedus A, Erdel S, Horvath G. Comparative studies of H2O2 detoxifying enzymes in green and greening barley seedlings under Cd stress. Plant Sci. 2001;160:1085–1093. doi: 10.1016/s0168-9452(01)00330-2. [DOI] [PubMed] [Google Scholar]
- Huang XY, Deng F, Yamaji N, Pinson ARM, Fujii-Kashino M, Danku J, Douglas A, Guerinot ML, Salt DE, Ma JF. A heavy metal P-type ATPase OsHMA4 prevents copper accumulation in rice grain. Nat Commun. 2016;7:12138. doi: 10.1038/ncomms12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M, Gadre R. Inhibition of chlorophyll biosynthesis by mercury in excised etiolated maize leaf segments during greening: effect of 2-oxoglutarate. Indian J Exp Biol. 2004;42:419–423. [PubMed] [Google Scholar]
- Kamal AHM, Komatsu S. Jasmonic acid induced protein response to biophoton emissions and flooding stress in soybean. J Proteome. 2016;133:33–47. doi: 10.1016/j.jprot.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Keramat B, Kalantari KM, Arvin MJ. Effects of methyl jasmonate treatment on alleviation of cadmium damages in soybean. J Plant Nutr. 2010;33:1016–1025. [Google Scholar]
- Kraemer U, Talke IN, Hanikenne M. Transition metal transport. FEBS Lett. 2007;581:2263–2272. doi: 10.1016/j.febslet.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Lamhamdi M, Bakrim A, Aarab A, Lafont R, Sayah F. Effects of lead phytotoxicity on wheat (Triticum aestivum L.) seed germination and seedling growth. CR Biol. 2011;334:118–126. doi: 10.1016/j.crvi.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim Y, Lee Y, An G. Rice P1B-type heavy-metal ATPase, OsHMA9, is a metal efflux protein. Plant Physiol. 2007;145:831–842. doi: 10.1104/pp.107.102236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maksymiec W, Wojcik M, Krupa Z. Variation in oxidative stress and photochemical activity in Arabidopsis thaliana leaves subjected to cadmium and excess copper in the presence or absence of jasmonate and ascorbate. Chemosphere. 2007;66(3):421–427. doi: 10.1016/j.chemosphere.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Mishra A, Choudhary MA. Amelioration of lead and mercury effects on germination and rice seedling growth by antioxidants. Biol Plantarum. 1998;41:469–473. [Google Scholar]
- Mittler R. Oxidative stress, antioxidant and stress tolerance. Trends Plant Sci. 2002;7:841–851. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Mittler R, Finka A, Goloubinoff P. How do plants feel the heat? Trends Biochem Sci. 2012;37:118–125. doi: 10.1016/j.tibs.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Mousavi SR, Niknejad Y, Fallah H, Barari Tari D. Methyl jasmonate alleviates arsenic toxicity in rice. Plant Cell Rep. 2020;39:1041–1060. doi: 10.1007/s00299-020-02547-7. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Namdjoyan S, Soorki AA, Elyasi N, Kazemi N, Simaei M. Melatonin alleviates lead-induced oxidative damage in safflower (Carthamus tinctorius L.) seedlings. Ecotoxicology. 2020;29(1):108–118. doi: 10.1007/s10646-019-02136-9. [DOI] [PubMed] [Google Scholar]
- Piotrowska A, Bajguz A, Godlewska-Żyłkiewicz B, Czerpak R, Kamińska M. Jasmonic acid as modulator of lead toxicity in aquatic plant Wolffia arrhiza (Lemnaceae) Environ Exp Bot. 2009;66:507–513. [Google Scholar]
- Pourrut B, Shahid M, Camille D, Peter W, Eric P. Lead uptake, toxicity, and detoxification in plants. Rev Environ Contam Toxicol. 2011;213:113–136. doi: 10.1007/978-1-4419-9860-6_4. [DOI] [PubMed] [Google Scholar]
- Principato GB, Rosi G, Talesa V, Govannini E, Uolila L. Purification and characterization of two forms of glyoxalase II from rat liver and brain of Wistar rats. Biochem Biophys Acta. 1987;911:349–355. doi: 10.1016/0167-4838(87)90076-8. [DOI] [PubMed] [Google Scholar]
- Rezai S, Orojloo M, Bidabadi SS, Soleimanzadeh M. Possible role of methyl jasmonate in protection to NaCl-induced salt stress in pepper cv “Green Hashemi”. Int J Agric Crop Sci. 2013;6:1235. [Google Scholar]
- Sasaki A, Yamaji N, Ma JF. Overexpression of OsHMA3 enhances Cd tolerance and expression of Zn transporter genes in rice. J Exp Bot. 2014;65(20):6013–6021. doi: 10.1093/jxb/eru340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoor MB, Ali S, Hameed A, Farid M, Hussain S, Yasmeen T, Najeeb U, Bharwana SA, Abbasi GH. Citric acid improves lead (pb) phytoextraction in Brassica napus L. by mitigating pb-induced morphological and biochemical damages. Ecotoxicol Environ Saf. 2014;109:38–47. doi: 10.1016/j.ecoenv.2014.07.033. [DOI] [PubMed] [Google Scholar]
- Shao JF, Xia J, Yamaji N, Shen RF, Ma JF. Effective reduction of cadmium accumulation in rice grain by expressing OsHMA3 under the control of the OsHMA2 promote. J Exp Bot. 2018;69:2743–2752. doi: 10.1093/jxb/ery107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Dubey RS. Lead toxicity in plants. Braz J Plant Physiol. 2005;17:35–52. [Google Scholar]
- Singh R, Tripathi R, Dwivedi S, Kumar A, Trivedi P, Chakrabarty D. Lead bioaccumulation potential of an aquatic macrophyte Najas indica are related to antioxidant system. Bioresour Technol. 2010;101:3025–3032. doi: 10.1016/j.biortech.2009.12.031. [DOI] [PubMed] [Google Scholar]
- Song WY, Park J, Mendoza-Cózatl DG, Suter-Grotemeyer M, Shim D, Hörtensteiner S, Geisler M, Weder B, Rea PA, Rentsch D, Schroeder JI, Lee Y, Martinoia E, Koornneef M. Arsenic tolerance in arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc Natl Acad Sci USA. 2010;107(49):21187–21192. doi: 10.1073/pnas.1013964107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WY, Yamaki T, Yamaji N, Ko D, Jung KH, Fujii-Kashino M, An G, Martinoiaa E, Lee Y, Ma JF. A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc Natl Acad Sci USA. 2014;111:15699–15704. doi: 10.1073/pnas.1414968111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souri Z, Karimi N, Sandalio LM. Arsenic hyperaccumulation strategies: an overview. Front Cell Dev Biol. 2017;5:67. doi: 10.3389/fcell.2017.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumithra K, Jutur PP, Carmel BD, Reddy AR. Salinity-induced changes in two cultivars of Vigna radiata: responses of antioxidative and proline metabolism. Plant Growth Regul. 2006;50:11–22. [Google Scholar]
- Suzuki M, Bashir K, Inoue H, Takahashi M, Nakanishi H, Nishizawa NK. Accumulation of starch in Zn-deficient rice. Rice. 2012;5:9. doi: 10.1186/1939-8433-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi R, Ishimaru Y, Shimo H, Ogo Y, Senoura T, Nishizawa NK, Nakanishi H. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and cd in rice. Plant Cell Environ. 2012;35:1948–1957. doi: 10.1111/j.1365-3040.2012.02527.x. [DOI] [PubMed] [Google Scholar]
- Tezuka K, Miyaadte H, Katou K, Kodama I, Matsumoto S, Kawamoto T, Masaki S, Satoh H, Yamaguchi M, Sakurai K, Takahashi H, Satoh-Nagasawa N, Watanabe A, Fujimura T, Akagi H. A single recessive gene controls cadmium translocation in the cadmium hyperaccumulating rice cultivar Cho-Ko-Koku. Theor Appli Genet. 2010;120:1175–1182. doi: 10.1007/s00122-009-1244-6. [DOI] [PubMed] [Google Scholar]
- Ueda J, Saniewski M. Methyl jasmonate–induced stimulation of chlorophyll formation in the basal part of tulip bulbs kept under natural light conditions. J Fruit Ornam Plant Res. 2006;14:199–210. [Google Scholar]
- Ueno D, Yamaji N, Kono I, Huang CF, Ando T, Yano M, Ma JF. Gene limiting cadmium accumulation in rice. Proc Natl Acad Sci. 2010;107:16500–16505. doi: 10.1073/pnas.1005396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev A, Iordanov I, Chakalova E, Kerin V. Effect of cadmium stress on growth and photosynthesis of young barley (H. vulgare L.) plants. 2. Structural and functional changes in the photosynthetic apparatus. Bulg J Plant Physiol. 1995;21:12–21. [Google Scholar]
- Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants—protective role of exogenous polyamines. Plant Sci. 2000;151:59–66. [Google Scholar]
- Wasternack C. Action of jasmonates in plant stress responses and development—applied aspects. Biotechnol Adv. 2014;32:31–39. doi: 10.1016/j.biotechadv.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Wasternack C, Hause B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. Ann Bot. 2013;111:1021–1058. doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellburn AR. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol. 1994;144:307–313. [Google Scholar]
- Williams LE, Mills RF. P1B-ATPases-an ancient family of transition metal pumps with diverse functions in plants. Trends Plant Sci. 2005;10:491–502. doi: 10.1016/j.tplants.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Yan Z, Zhang W, Chen J, Li X. Methyl jasmonate alleviates cadmium toxicity in Solanum nigrum by regulating metal uptake and antioxidative capacity. Biol Plant. 2015;59:373–381. [Google Scholar]