Abstract
Salinity is a significant constraint for plant survival and productivity. Therefore, an immediate solution to this problem is sought to meet the human population's food demands. Recently, Menadione sodium bisulphite (MSB) has emerged as a significant regulator of plant defense response under abiotic stress. Studies on MSB are scarce, and a few reports on salinity (Arabidopsis and okra) and cadmium stress (okra) are present in the literature. However, these studies did not include the impact of MSB on physiological and plant water relation attributes, critical mediators of plant survival, and yield production under stress. Our results studied the impact of MSB on wheat administered to NaCl salinity in hydroponics medium. We used two wheat cultivars (salt-sensitive MH-97 and salt-tolerant Millat-2011, based on our pre-experimental studies). Seeds were primed in different MSB doses [control (unprimed), hydroprimed, 5, 10, 20, and 30 mM]. Salinity significantly diminished growth, chlorophyll molecules, photosynthesis, total free amino acids, water and turgor potentials, K, Ca, and P contents of wheat when administered NaCl salinity in the nutrient solution. Besides, a noteworthy accretion was present in oxidative stress markers [hydrogen peroxide & malondialdehyde], proline, ascorbic acid, antioxidant enzyme activities, and Na+ accumulation under salinity. Moreover, MSB noticeably enhanced chlorophyll molecules, proline, and oxidative defense to improve photosynthesis, plant water relations, and diminish specific ions toxicity. Our results manifested better defense regulation in salt-administered plants primed with 5 and 10 mM MSB. Our findings strongly advocated the use of MSB in improving plant salinity tolerance, particularly in wheat.
Keywords: MSB, Photosynthesis, Water relation, Secondary metabolites, Nutrient uptake, Specific ion toxicity, Antioxidant compounds
Introduction
Salinity has emerged as a significant environmental constraint that inhibits plant growth and yield production worldwide. The increase in soil salinization is massive over the last decade due to anthropogenic activities (Kumar Arora et al. 2020). The higher electrical conductivity of soil due to toxic salts accumulation decreases soil water potential. Consequently, plants and other life forms could merely survive in soils with low water potential (Mishra et al. 2018; Egamberdieva et al. 2019). Approximately 831 million hectares of arable land throughout the world are affected by salinity and sodicity. According to Food and Agriculture Organization (FAO), saline soils cover 397 million hectares of the world's arable land, while 434 million hectares of land are affected by soil sodicity (Sunita et al. 2020). The arid and semi-arid regions of the world are more prone to soil salinity due to minimal precipitation and high evapotranspiration (Zörb et al. 2019). Besides, excessive application of pesticides and fertilizers alongside climate change are also significant contributors to increasing soil salinity worldwide (Sunita et al. 2020). According to some alarming reports, salinity degrades nearly 1–2% of fertile soil each year around the globe (Etesami and Beattie 2018; Kumar Arora et al. 2020). It is expected that nearly 50% of fertile soil could be affected to various degrees by salinity in the next 35 years (Thiem et al. 2018). The annual losses incurred due to soil salinization during the last decade were around 27.3 billion US'$' (Qadir et al. 2014).
The growth retardation in plants under saline soils is due to salt-induced ionic imbalance, limited photosynthesis, enhanced reactive oxygen species (ROS) production, oxidative damage, hormonal imbalance, specific ion toxicity, and osmotic stress (Adhikari et al. 2020; Kumar et al. 2020). Osmotic adjustment is an essential defense mechanism adopted by plants to combat saline conditions. It involves organic compound accumulation, including proteins, soluble sugars, glycine betaine, and proline (García-Caparrós et al. 2020). Phenolics, anthocyanins, flavonoids, and ascorbate are important antioxidant compounds with significant ROS neutralization capacity (Petropoulos et al. 2017; Moradbeygi et al. 2020; Yildiztugay et al. 2020; Mahmood et al. 2020). Antioxidant enzymes also significantly detoxify ROS, such as superoxide radical (O2·−), hydroxyl radical (OH·), singlet oxygen (1O2), and hydrogen peroxide (H2O2) (Hasanuzzaman et al. 2018). Salinity significantly increases Na+ concentration in soil that subsequently reduces the uptake and accumulation of other essential nutrients such as K+, Ca2+, P, Fe2+, and Mg2+. In this regard, K+/Na+ and Ca2+/Na+ become critical for plant survival under saline conditions (Mahajan et al. 2020; Kamiab 2020).
Several approaches are used to improve plant salinity tolerance (Jhonson and Puthur 2021); some strategies, such as conventional breeding, are not achievable due to their execution time. Some other strategies, such as genetic modifications, are not presently accepted in many countries worldwide (Savvides et al. 2016). Seed priming has emerged as a better replacement approach to enhance plant salinity tolerance (Kahveci et al. 2021). Jhonson and Puthur (2021) reported that seed priming keeps plants in an alert state; hence plants react better when confronted with environmental restraints. Seed priming initiates priming memory that leads to transgenerational memory. Plants in a primed state respond better to stress signal perception by promptly activating the protection mechanism (Ellouzi et al. 2013). Plants can also achieve a primed state due to chemical treatment involving exposure to chemical priming agents (synthetic or natural). Chemical priming provides better opportunities to use plant priming in crop stress management and plant physiology studies (Savvides et al. 2016). Exogenous application of organic and inorganic chemicals is also suggested as an effective strategy to circumvent salinity effects on plant productivity (Akram et al. 2020). Menadione sodium bisulphite (MSB) is a water-soluble vitamin k derivative. MSB has been reported to improve plant tolerance against abiotic stresses (Rasheed et al. 2018; Ashraf et al. 2019; Jiménez-Arias et al. 2019). However, these reports on MSB-mediated plant defense response did not reveal changes in physiological attributes of plants under salinity. MSB was thought of as a synthetic compound previously; however, Binder et al. (1989) isolated MSB from phanerogams. MSB is a redox active compound that can act as either oxidant (attract electrons) or reducing agent (donate electrons). The redox properties of MSB are highly dependent on its concentration, making it physiologically active in plants (Askari et al. 2021). MSB is reported to initiate slight oxidative injury in plants, resulting in ROS scavenging proteins' accumulation (Ashraf et al. 2020). MSB could be converted to vitamin k upon metabolism (Manzotti et al. 2008). Mostly vitamin k is detected as a carrier inside thylakoid membrane, where it is an integral compound of photosystem I redox chain. MSB maintains an appropriate redox state of some plasma membrane-embedded proteins mediating plant stress response. Wheat is an important cereal crop that suffers significant yield losses due to salinity (Feghhenabi et al. 2020). To best of our knowledge, this is the first report that describes the impact of MSB on physiology and biochemistry of wheat under salinity. The main objectives of the study were to (1) investigate the impact of MSB on the morphology of wheat under salinity, (2) evaluate the MSB-mediated regulation of photosynthesis and water relation in wheat under salinity (3) examine MSB-induced strengthening of oxidative defense and changes in secondary metabolites accumulation, (4) assess the regulation of nutrient uptake by MSB under salinity, (5) find out whether or not MSB-mediated changes in biochemical indices neutralize salinity effects on wheat physiology.
Materials and methods
Experimental conditions and treatment details
Seeds of salt-tolerant (Millat-2011) and salt-sensitive (MH-97) wheat cultivars were used in the present study. We screened out 10 wheat cultivars in our pre-experimental studies to select tolerant and sensitive wheat cultivars (Figs. 1S, 2S & 3S). Seeds of two wheat cultivars were given different priming treatments for 12 h in distilled water and different MSB concentrations (5, 10, 20, and 30 mM MSB). Screening of MSB doses was done in our pre-experimental studies (Figs. 4S, 5S, 6S & 7S). Seeds were sown in plastic pots filled with thoroughly washed sand. Plants were transferred to hydroponics medium 16 days after germination. Seedlings were allowed to establish in hydroponics medium for 20 days. Later, NaCl salinity (50, 100, and 150 mM) was added to the nutrient solution. The nutrient solution was replaced after three days interval, and the solution was continuously aerated with an electric pump. We worked with a slightly modified composition of Hoagland's nutrient solution used by Habiba et al. (2015). The composition of hydroponics media was as follows 3500 μmol L−1 KNO3; 2500 μmol L−1 Ca(NO3)2; 150 μmol L−1 KH2PO4; 1000 μmol L−1 MgSO4; 60 μmol L−1 H3BO3; 0.07 μmol L−1 MnCl2.4H2O; 0.9 ZnSO4.7H2O; 0.4 μmol L−1 CuSO4.5H2O; 0.14 μmol L−1 H2MO4.H2O and 16 μmol L−1 Fe-EDTA. Since this was the first report on MSB-mediated modifications in physiological and biochemical responses of wheat under salinity, therefore, we used a hydroponics medium to nullify the effect of any edaphic factor. The detailed breakup of treatments is, (1) unprimed (no soaking) + non-saline hydroponics culture, (2) hydroprimed (water soaking) + non saline hydroponics culture, (3) 5 mM MSB priming + non-saline hydroponics culture, (4) 10 mM MSB priming + non-saline hydroponics culture, (5) 20 mM MSB priming + non-saline hydroponics culture, (6) 30 mM MSB priming + non-saline hydroponics culture, (7) unprimed (water soaking) + saline hydroponics culture, (8) hydroprimed (water soaking) + saline hydroponics culture, (9) 5 mM MSB priming + saline hydroponics culture, (10) 10 mM MSB priming + saline hydroponics culture, (11) 20 mM MSB priming + saline hydroponics culture, (12) 30 mM MSB priming + saline hydroponics culture. Data for different growth, physiological and biochemical indices were collected at the boot stage (Feeke: 10; Zadoks: 45). Data was taken 34 days after the transfer of plants to the hydroponics medium. Data was recorded at the boot stage because wheat is more sensitive to salinity effects at the boot stage (Saqib et al. 2013). The experimental details are further explained as a flow diagram in Fig. 1. During the experiment, the weather conditions were 987 μmol m−2 s−1 photosynthetically active radiation (PAR), 8.58 ± 2.85 mm rainfall, day 35 ± 2.3% and night 77 ± 2.0% relative humidity, day 25 ± 3.54 °C and night 15 ± 2.5 °C temperature, respectively. These are mean values for different variables of weather conditions. The weather conditions were taken from the meteorological Department of Ayub Agricultural Research Institute Faisalabad, Pakistan. The climate conditions were measured with the help of a maximum thermometer (China), minimum thermometer (G.H. Zeal), wet bulb thermometer (G.H. Zeal), and rain gauge (G.H. Zeal). There were total 96 pots, and these pots were further split into four sets of 24 pots each. Each pot was split into two halves containing 14 plants of each cultivar (Fig. 9S). There were four replicates of each treatment. Three factor-factorial experiment was performed in a completely randomized design (CRD) with four replications of each treatment. The difference among treatment means was evaluated with a post-hoc test LSD in IBM SPSS v26 statistical software. Principal component analysis (PCA) and corrplot were drawn using rstudio.
Fig. 1.
Scheme of different treatments applied to study the effect of MSB on wheat grown in salinized hydroponics medium
Agronomic features
Plants were harvested at the boot stage (Feeke: 10; Zadoks: 45) and important agronomic characters such as shoot length, fresh and dry biomass of shoot and roots, number of tillers, and leaf area were measured. Gradner et al. (1985) formula was applied to calculate the leaf area.
Chlorophyll pigments and gas exchange parameters
Chlorophyll pigments (a and b) and carotenoids were determined by the method of Arnon (1949). 0.5 g of the fresh leaf was homogenized in 10 mL of 80% acetone at 4 °C. Samples were centrifuged at 12,000 g for 5 min. The OD was taken at 480, 645, and 663 nm with a spectrophotometer. Photosynthesis rate, stomatal conductance, and transpiration rate were determined with an infra-red gas analyzer machine. The specifications of the instrument were as follows; photosynthetically active radiation (PAR) was 1084 μmol m−2 s−1, leaf temperature 29 to 33 °C and leaf chamber volume gas flow rate 295 mL/min, leaf chamber molar gas flow rate (U) 402 μmol s−1 and ambient pressure 97.95 kPa. (LI-COR Lincoln, NE, USA).
Water relation attributes
Water potential of leaf was measured by the method of Scholander et al. (1965). Leaf was excised to measure water potential with pressure chamber. Later, the leaf samples were frozen at − 40 °C for one week. Then leaf samples were thawed to take out the sap for measurement of leaf osmotic potential with an osmometer (Vapro, 5520). Turgor potential was obtained as the difference of water and osmotic potentials by the following formula;
where Ψp is leaf turgor potential, Ψs leaf osmotic potential and Ψw is leaf water potential.
Measurement of malondialdehyde (MDA) and hydrogen peroxide (H2O2)
Fresh leaf (0.25 g) was powdered with liquid nitrogen. Later, ground material was homogenized in 5 mL of 6% trichloroacetic acid. The homogenate was centrifuged at 10,000 g for 10 min to collect the supernatant. The supernatant (0.5 mL) was reacted with 5% thiobarbituric acid (TBA). The reaction solution was heated in a water bath at 95 °C for 45 min. The reaction solution's OD was read at advised wavelengths (532 and 600 nm) using a spectrophotometer (Cakmak and Horst 1991). H2O2 content in leaf was measured by the method by Velikova et al. (2000). For the measurement of H2O2, 0.5 mL of the supernatant used for MDA was reacted with 1 M KI in the presence of potassium phosphate buffer (50 mM, pH 7.5). The reaction solution was given an incubation of 50 min before reading OD at 390 nm on a spectrophotometer.
Enzyme assays
Fresh leaf material (0.5 g) was ground in liquid nitrogen. Ten mL potassium phosphate buffer (0.5 M, pH 7.5) was added to homogenize the powdered leaf material. The supernatant was collected after centrifugation at 10,000 g for 20 min at 4 °C. The supernatant was immediately stored at -80 °C for enzyme assays. This supernatant served as an enzyme extract. APX activity was determined by measuring the reduction in absorption at 290 nm for 120 s (Nakano and Asada 1981). SOD activity was measured by following the photochemical inhibition of nitroblue tetrazolium chloride in the enzyme's presence. The OD was taken at 560 nm (Giannopolitis and Ries 1977). Peroxidase activity was measured following the method by Polle et al. (1994). Briefly, 3 mL reaction solution contained guaiacol (20 mM), H2O2 (10 mM) and enzyme extract (100 uL). The increase in absorbance was read at 470 nm for 3 min. Catalase activity was measured by the method of Chance and Maehly (1955). The reaction solution contained 0.5 M phosphate buffer of pH 7.0, 20 mM H2O2, and 0.1 mL enzyme extract. The decrease in OD was monitored for 3 min at 240 nm. The activities of all antioxidant enzymes were expressed based on enzyme units per mg proteins.
Non-enzymatic antioxidants
Phenolics were determined following the method of Wolfe et al. (2003). 0.5 g fresh leaf was crushed in liquid nitrogen and homogenized in 10 mL 80% methanol. The homogenate was centrifuged at 12,000 g for 10 min at 4 °C. Supernatant was then treated with folin-ciocalteu reagent in the presence of 20% Na2CO3 and absorbance was taken at 750 nm. Flavonoids were determined spectrophotometrically by the method of Zhishen et al. (1999). Ascorbic acid was measured following the method by Mukherjee and Choudhuri (1983). Fresh leaf material (0.5 g) was powdered with liquid nitrogen and the subsequent powder was homogenized in 10 mL 6% TCA. The homogenate was filtered and the filtrate was reacted with 2% dinitrophenylhydrazine followed by the addition of one drop thiourea (10%). The reaction mixture was incubated at 95 °C for 40 min. Afterward, 80% sulfuric acid was added to the mixture. The OD of the mixture was read at 530 nm. Anthocyanin contents were determined from the leaf tissue (0.5 g) extracted in methanol containing 1% HCl. The OD was taken at 530 and 657 nm (Mita et al. 1997).
Total soluble protein (TSP)
TSP was determined by Bradford (1976) method. For this purpose, supernatant (used in antioxidant enzyme assays) was treated with Bradford reagent and absorbance was read at 595 nm.
Total soluble sugar and reducing sugar
Total soluble sugar was determined, followed by the method of Dubois et al. (1956). Potassium phosphate buffer samples were reacted with anthrone reagent and absorbance was taken at 490 nm. Futher, the reducing sugars were determined following the protocol of Henson and Stone (1988). The non-reducing sugar was estimated by taking difference of total soluble sugar and reducing sugar.
Total free amino acid (TFAA)
Total free amino acids were determined by the method of Hamilton and Van Slyke (1943). One mL of the extract used for antioxidant enzyme assay was reacted with 1 mL of 10% pyridine and 1 mL of 2% ninhydrin. The mixture was heated at 95 °C for 35 min before taking OD at 570 nm.
Proline content
Leaf proline was determined following Bates et al. (1973). Fresh leaf samples were homogenized in 3% sulfosalicylic acid. The homogenate was filtered and equal volume of the filtrate was reacted with equal volume of ninhydrin reagent. Toluene (5 mL) was added to the mixture after incubation at 95 °C for 45 min. The absorbance of reaction solution was taken at 520 nm.
Menadione sodium bisulphite (MSB) determination
We followed the protocol of Sidhom and El-Kommos (1982) with some modifications. This protocol was initially used to determine MSB from pharmaceutical products. However, we are reporting MSB determination from wheat leaves using thiosemicarbazide compound. Thiosemicarbazide solution was prepared by dissolving 0.5 g thiosemicarbazide in 10 mL water and diluted with n-propanol to 50 mL. Samples were ground in n-propanol. The supernatant (1 mL) was reacted with 0.2 mL thiosemicarbazide solution and 0.1 mL 0.1 N NaOH. The volume was maintained to 2.5 mL with n-propanol. The reaction mixture was incubated at room temperature, and OD was read at 540 nm. The standard curve of MSB solution with known concentrations was used to determine MSB from samples (Fig. 8S).
Determination of Na+, K+ and Ca+2 ions
Ions were determined following the protocol of Allen et al. (1976). Plant dry material (0.1 g) was acid digested with concentrated H2SO4. 2 mL acid was added to dry material in the digestion flask. The digestion mixture was incubated for 24 h. Later, the mixture was heated at 150 °C followed by the addition of 35% H2O2. This step was repeated until the appearance of transparent solution. The digested solution was used to determine K+, Na+ and Ca2+ using flame photometer (Sherwood, Model 360). Phosphorus was determined from the digested solution following the method of Jackson (1962).
Results
Agronomic characteristics
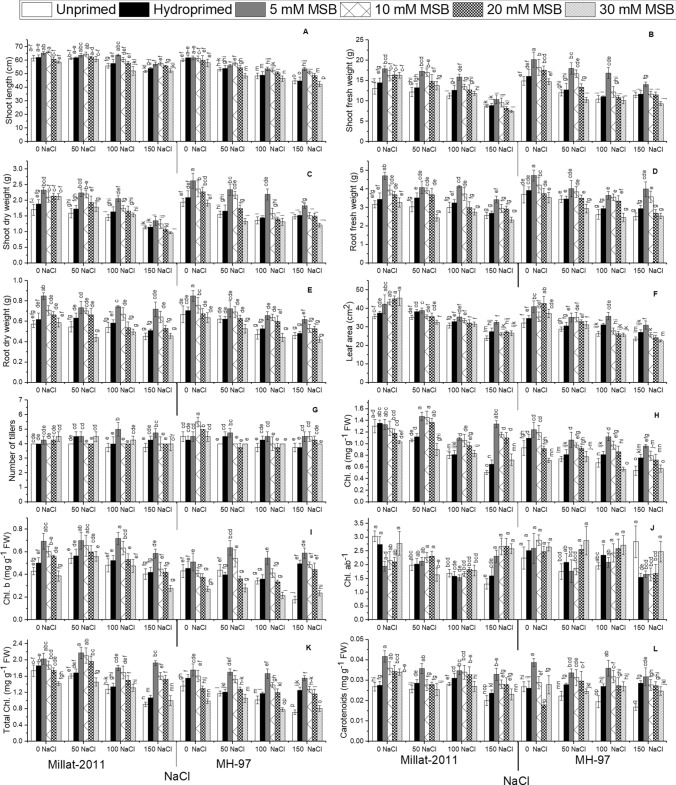
Salinity significantly (P ≤ 0.001) decreased shoot and root fresh and dry weights in wheat cultivars. Salinity induced decrease in different growth characteristics was dose dependent. For example, 150 mM NaCl caused maximal reduction in growth attributes. Further, MSB notably enhanced (P ≤ 0.001) these growth characteristics in wheat plants. Priming with different doses of MSB (5, 10, 20 and 30 mM) improved growth under salinity. Plants primed with 5 and 10 mM MSB had higher biomass under saline conditions. In contrast, plants treated with 30 mM MSB showed further growth reduction under salinity. However, wheat cultivars showed significant differences (P ≤ 0.05) for shoot fresh and dry weights. In contrast root fresh and dry weights did not differ between the cultivars. Decrease in growth attributes was more prominent in MH-97 than in Millat-2011. Likewise, our results display a noteworthy depression (P ≤ 0.001) in shoot length and leaf area of wheat plants. The number of tillers showed reduction in wheat plants under salinity (Table 1S, Fig. 2A–F).
Fig. 2.
Menadione sodium bisulphite-induced changes in growth and photosynthetic pigments in two genetically different wheat cultivars under salinity. (n = 4; means ± S.E.). Chl chlorophyll, T.Chl total chlorophyll
Chlorophyll contents
Salinity produced remarkable (P ≤ 0.001) decrease in chlorophyll molecules (Chl. a and Chl. b and total Chl.) and carotenoids in wheat plants. Higher salinity level (150 mM) caused drastic reduction in chlorophyll molecules. MSB doses significantly (P ≤ 0.001) improved chlorophyll contents. Seeds primed with 5 and 10 mM MSB showed more significant improvements in all chlorophyll molecules. MSB priming with higher doses (20 and 30 mM) was not effective concerning these variables. A similar trend was present for Chl. ab−1 in wheat cultivars under salinity. Millat-2011 showed higher chlorophyll contents as compared with MH-97 under salinity (Table 1S, Fig. 2H–L).
Gas exchange attributes
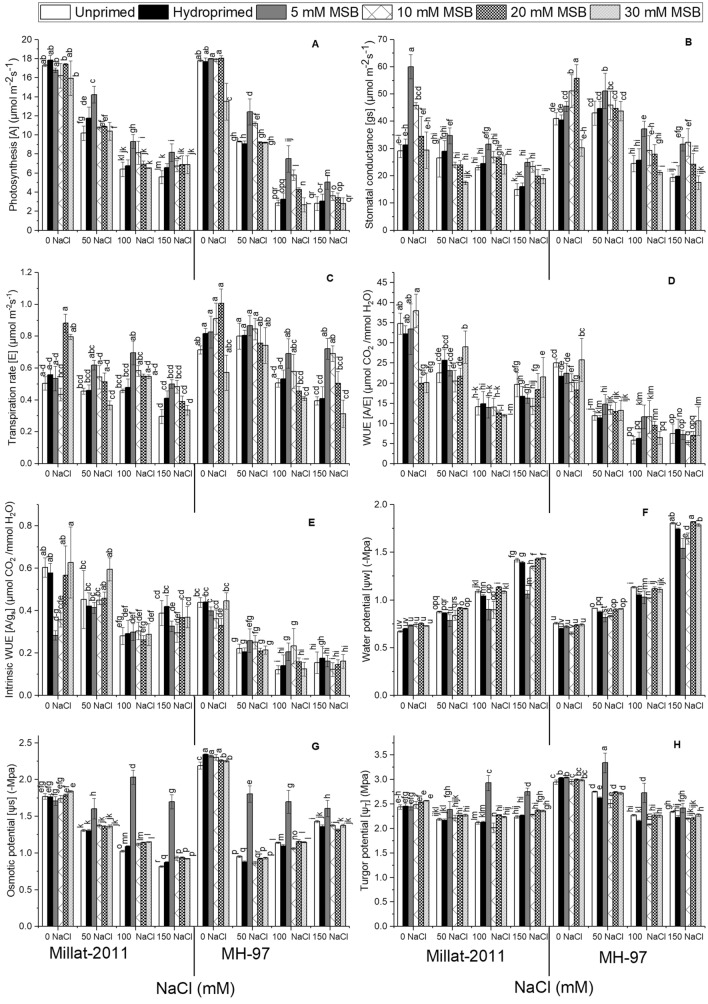
The data presented in Fig. 3A and Table 1S indicated the drastic effect (P ≤ 0.001) of salinity on photosynthesis in two wheat cultivars. The results manifested a maximal reduction in photosynthesis in plants under (150 mM) NaCl salinity. Exogenous MSB (5, 10, 20, and 30 mM) showed a more significant influence (P ≤ 0.001) on this variable in wheat plants grown in salinized hydroponics culture. Plants raised from 5 and 10 mM MSB priming had more photosynthetic rates than other doses of MSB. Minimal values for photosynthesis were evident in plants treated with 30 mM MSB under saline conditions. The cultivars (P ≤ 0.001) differed notably for this variable under salinity. The cultivar Millat-2011 displayed higher photosynthesis under salinity than cv. MH-97.
Fig. 3.
Menadione sodium bisulphite-induced changes in gas exchange and water relation attributes in two genetically different wheat cultivars under salinity. (n = 4; means ± S.E.)
Salinity resulted in a more significant drop (P ≤ 0.001) in stomatal conductance in wheat plants. This variable was different remarkably (P ≤ 0.001) between the cultivars. Plants grown in nutrient solution with (150 mM) NaCl salinity manifested a noteworthy depression in stomatal conductance. MSB priming (5, 10, 20, and 30 mM) showed a pronounced impact (P ≤ 0.001) on stomatal conductance. Plants treated with 5 and 10 mM MSB as seed priming had a noteworthy accretion in this variable under saline conditions. Plants primed with 30 mM MSB exhibited a further decline in stomatal conductance under salinity. The cultivar MH-97 had greater stomatal conductance compared with cv. Millat-2011 under salinity (Fig. 3B; Table 1S).
Transpiration rate declined more significantly (P ≤ 0.001) in two wheat cultivars due to NaCl salinity in the nutrient solution. The results showed a noticeable (P ≤ 0.001) variation in transpiration rate between the two cultivars. A higher transpiration rate was evident in cv. MH-97, whereas cv. Millat-2011 was inferior in this context. Seed priming with different MSB doses (5, 10, 20, and 30 mM) had a substantial effect (P ≤ 0.001) on transpiration rate in wheat plants under salinity. Plants raised from 5 and 10 mM seed priming had maximal values for transpiration rate in two wheat cultivars under salinity (Fig. 2; Table 1S). Likewise, the results also manifested significant (P ≤ 0.001) variation between the cultivars for water use efficiency and intrinsic water use efficiency under salinity. These variables declined remarkably (P ≤ 0.001) in plants grown in salinized hydroponics culture. Further, 150 mM NaCl salinity produced maximal reduction in water use efficiency and intrinsic water use efficiency in cv. MH-97. MSB priming did not influence these variables under salinity (Fig. 3C, D, and E; Table 1S).
Plant water relations
Salinity induced a noteworthy (P ≤ 0.001) decline in water potential in wheat cultivars. The results displayed a noticeable (P ≤ 0.001) difference between the cultivars for water potential under salinity. A more drastic reduction in water potential was seen in cv. MH-97, whereas cv. Millat-2011 was superior in this regard. Seed priming with different MSB doses (5, 10, 20, and 30 mM) profoundly (P ≤ 0.001) impacted this variable. For instance, plants treated with 5 mM MSB produced many folds increase in water potential in two wheat cultivars under salinity (Fig. 3F; Table 1S).
The results exhibited a noteworthy (P ≤ 0.001) decrease in osmotic potential in wheat cultivars. The cultivars differed significantly (P ≤ 0.001) for osmotic potential under salinity. In cv. MH-97, after an initial decline in osmotic potential due to salinity, we found a consistent rise in this variable under salinity. Contrary, cv. Millat-2011 showed a concentration-dependent drop in osmotic potential under salinity. Exogenous MSB treatments (5, 10, 20 and 30 mM) noticeably (P ≤ 0.001) affected osmotic potential of wheat plants exposed to salinity in the nutrient solution. MSB priming with 5 mM displayed several folds accretion in osmotic potential than other MSB doses under salinity (Fig. 3G; Table 1S).
Turgor potential decreased more significantly (P ≤ 0.001) in cv. MH-97 compared with cv. Millat-2011 under salinity. The difference between cultivars for turgor potential was also significant (P ≤ 0.001). A higher reduction in turgor potential was recorded in plants exposed to 150 mM NaCl salinity in nutrient solution. Exogenous MSB application significantly (P ≤ 0.001) enhanced turgor potential of plants under salinity. MSB-mediated improvement in turgor potential was evident in plants treated with 5 mM level under saline conditions (Fig. 3H; Table 1S).
Flavonoids
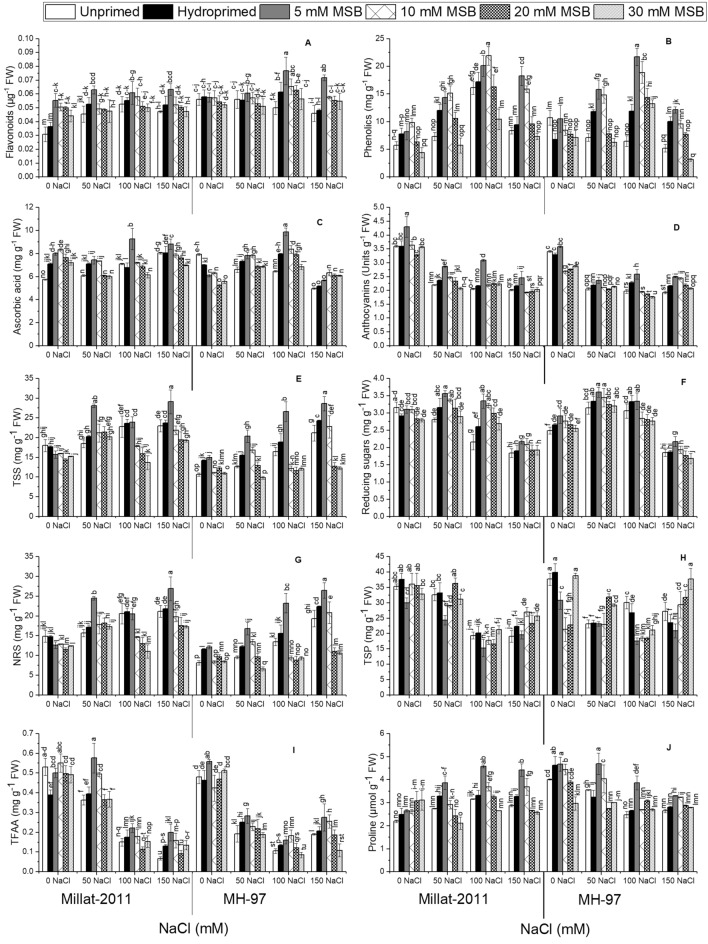
The analysis of variance exhibited that salinity substantially affected (P ≤ 0.001) flavonoids in wheat cultivars. The flavonoid levels were significantly variable (P ≤ 0.001) in two wheat cultivars under salinity. The cultivar MH-97 displayed a noteworthy decline in flavonoids at higher salinity levels (100 and 150 mM), whereas cv. Millat-2011 had a slight decline in flavonoids at 150 mM salinity. The results substantiated a strong effect (P ≤ 0.001) of MSB on this variable. MSB priming with 5 and 10 mM persuaded a noticeable buildup in flavonoids under salinity (Fig. 4A; Table 1S).
Fig. 4.
Menadione sodium bisulphite-induced changes in biochemical attributes of two genetically different wheat cultivars. (n = 4; means ± S.E.). TSS total soluble sugars, RS reducing sugars, NRS non-reducing sugars, TSP total soluble proteins, TFAA total free amino acids
Phenolics
Plants administered NaCl salinity in the nutrient solution manifested significant changes (P ≤ 0.001) in phenolics. The phenolics levels were different (P ≤ 0.001) in two wheat cultivars under saline conditions. Salinity produced a substantial increase in phenolics in cv. Millat-2011, whereas cv. MH-97 had a reduction in this variable due to salinity. Plants supplemented with MSB as seed priming exhibited a significant increase in phenolics under salinity. However, a higher MSB level (30 mM) had a profound negative impact (P ≤ 0.001) on phenolics under saline or non-saline conditions. Besides, MSB priming with 5 and 10 mM resulted in maximum phenolics production in plants grown in salinized hydroponics medium (Fig. 4B; Table 1S).
Ascorbic acid
Ascorbic acid content showed profound alteration (P ≤ 0.001) due to salinity in wheat cultivars. The accumulation of ascorbic acid was variable (P ≤ 0.001) in two wheat cultivars. The tolerant wheat cultivar Millat-2011 showed a conspicuous increase in ascorbic acid content due to salinity. Contrary, salt-sensitive MH-97 cultivar exhibited many folds drop in ascorbic acid content at 150 mM NaCl salinity. Plant administered MSB had more significant (P ≤ 0.001) ascorbic acid contents under salinity. Further, plants from seeds primed with 5 and 10 mM MSB manifested several folds raise in ascorbic acid accumulation (Fig. 4C; Table 1S).
Anthocyanins
The decrease in anthocyanins due to salinity was significant (P ≤ 0.001) in wheat plants. Salinity produced a dose-dependent drop in this variable as minimal values were seen in plants administered 150 mM salinity in the hydroponics culture. Two wheat cultivars revealed apparent variation (P ≤ 0.001) for anthocyanins accumulation under salinity. Seed priming with MSB produced a profound increasing effect (P ≤ 0.001) on this variable in plants supplied with salinity, especially in plants treated with 5 mM MSB (Fig. 4D; Table 1S).
Soluble sugars
Our results revealed more significant accretion (P ≤ 0.001) in total soluble sugars (TSS) in wheat plants administered to saline conditions. The accumulation of total soluble proteins was different (P ≤ 0.001) between the cultivars. In this context, we recorded higher TSS in cv. Millat-2011, while cv. MH-97 was inferior in this regard. TSS levels were higher (P ≤ 0.001) in plants primed with 5 mM MSB. Seed priming with 30 mM MSB profoundly diminished TSS contents under salinity (Fig. 4E; Table 1S).
We found a noteworthy drop (P ≤ 0.001) in reducing sugars in cv. Millat-2011 under salinity, while cv. MH-97 displayed a decline in this variable at 150 mM salinity. MSB priming significantly impacted (P ≤ 0.001) reducing sugars under salinity. In this regard, we found higher reducing sugars in plants treated with 5 and 10 mM MSB under salinity. The other MSB doses were not effective for this variable in wheat plants subjected to salinity (Fig. 4F; Table 1S).
The results manifested a significant rise (P ≤ 0.001) in non-reducing sugars (NRS) in wheat plants under salinity. The difference between the cultivars for NRS was not the same under salinity. In this context, cv. Millat-2011 showed profoundly higher (P ≤ 0.001) NRS levels than cv. MH-97 under salinity. MSB priming, especially with 5 mM, significantly improved (P ≤ 0.001) NRS in plants subjected to salinity. Besides, priming with 30 mM MSB depicted a substantial drop in this variable (Fig. 4G; Table 1S).
Total soluble proteins
Total soluble proteins (TSP) dropped significantly (P ≤ 0.001) in wheat cultivars under salinity. A higher salinity level (150 mM) produced a maximal reduction in TSP in cv. Millat-2011 than cv. MH-97. MSB priming with higher doses (20 and 30 mM) manifested a significant increase (P ≤ 0.001) in TSP under salinity (Fig. 4H; Table 1S).
Total free amino acids
Total free amino acids (TFAA) displayed a noteworthy depression (P ≤ 0.001) in wheat plants due to salinity. The decline in TFAA due to salinity was dose-dependent as we found maximal reduction at 150 mM salinity. Our results revealed higher TFAA (P ≤ 0.001) in cv. Millat-2011 than cv. MH-97 under salinity. MSB priming with 5 and 10 mM profoundly enhanced (P ≤ 0.001) this variable under salinity (Fig. 4I; Table 1S).
Proline
Salinity significantly impacted (P ≤ 0.01) the proline contents in two wheat cultivars. In cv. Millat-2011, a substantial increase due to salinity was seen, while cv. MH-97 showed a decline in this variable under salinity. Priming with MSB remarkably influenced (P ≤ 0.001) this variable under salinity. Further, plants supplied with 5 and 10 mM had greater values for proline under salinity (Fig. 4J; Table 1S).
Menadione sodium bisulphite (MSB)
Salinity stress produced a notable (P ≤ 0.001) upsurge in MSB levels of wheat plants. The rise in MSB contents was concentration-dependent as maximal MSB contents were evident in plants grown at 150 mM NaCl salinity. Exogenous MSB produced a further rise (P ≤ 0.001) in this variable under saline or non-saline conditions. Salinity sensitive cv. MH-97 manifested maximal values for MSB compared with salinity tolerant cv. Millat-2011 (Fig. 8S; Table 1S).
Oxidative stress markers
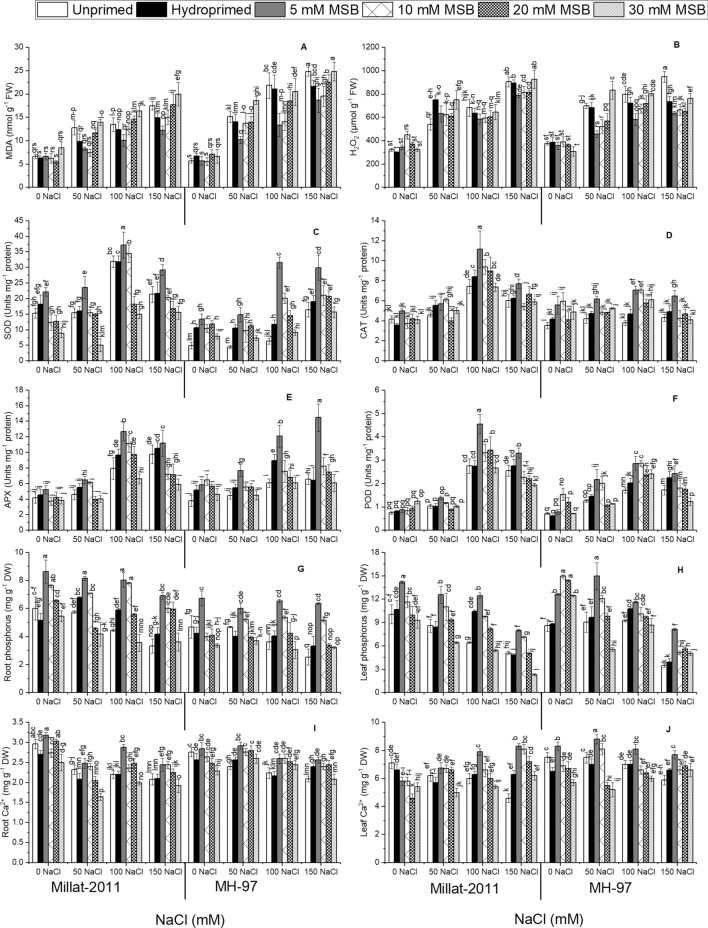
Oxidative damage due to salinity was measured in the form of MDA and H2O2 accumulation. Wheat plants administered to salinity revealed a noteworthy increase (P ≤ 0.001) in MDA and H2O2 levels. We did not see any statistical difference between the cultivars for H2O2, while a significant variation (P ≤ 0.001) for MDA was evident in plants under salinity. MSB priming significantly diminished MDA and H2O2 levels, especially in plants with 5 and 10 mM MSB priming (Fig. 5A and B; Table 1S).
Fig. 5.
Menadione sodium bisulphite-induced changes in oxidative stress, antioxidant enzyme activities and elemental uptake in two genetically different wheat cultivars. (n = 4; means ± S.E.)
Enzymatic antioxidants
Antioxidant enzyme activities (SOD, POD, CAT, APX) substantially improved (P ≤ 0.001) in wheat plants supplied with NaCl salinity in the nutrient solution. MSB priming gave noteworthy improvements (P ≤ 0.001) in their activities under salinity. Plants primed with 5 mM significantly strengthened antioxidant enzyme activities. Higher antioxidant enzyme activities (P ≤ 0.001) were seen in cv. Millat-2011 than cv. MH-97 under salinity. Besides, APX activities did not vary between the cultivar under salinity (Fig. 5C–F; Table 1S).
Nutrient uptake
Salinity produced a substantial drop (P ≤ 0.001) in root and leaf phosphorus (P) content in wheat cultivars. Salinity-mediated decrease in P content was more drastic (P ≤ 0.001) in salinity sensitive cv. MH-97 than salt-tolerant cv. Millat-2011. Priming with different MSB doses also significantly impacted (P ≤ 0.001) plant P level under salinity. Further, plants from seeds primed with 5 and 10 mM salinity exhibited more significant improvement in P contents under salinity (Fig. 5G and H; Table 1S).
Likewise, calcium (Ca2+) contents also decreased (P ≤ 0.001 for root Ca2+; P ≤ 0.05 for leaf Ca2+) significantly in the roots and leaves of wheat plants administered to NaCl salinity in the nutrient solution. Ca2+ accumulation was more significant (P ≤ 0.001) in cv. Millat-2011 than cv. MH-97, especially under salinity. MSB priming with 5 and 10 mM profoundly enhanced (P ≤ 0.001) plant Ca2+ contents under salinity (Fig. 5I and J; Table 1S).
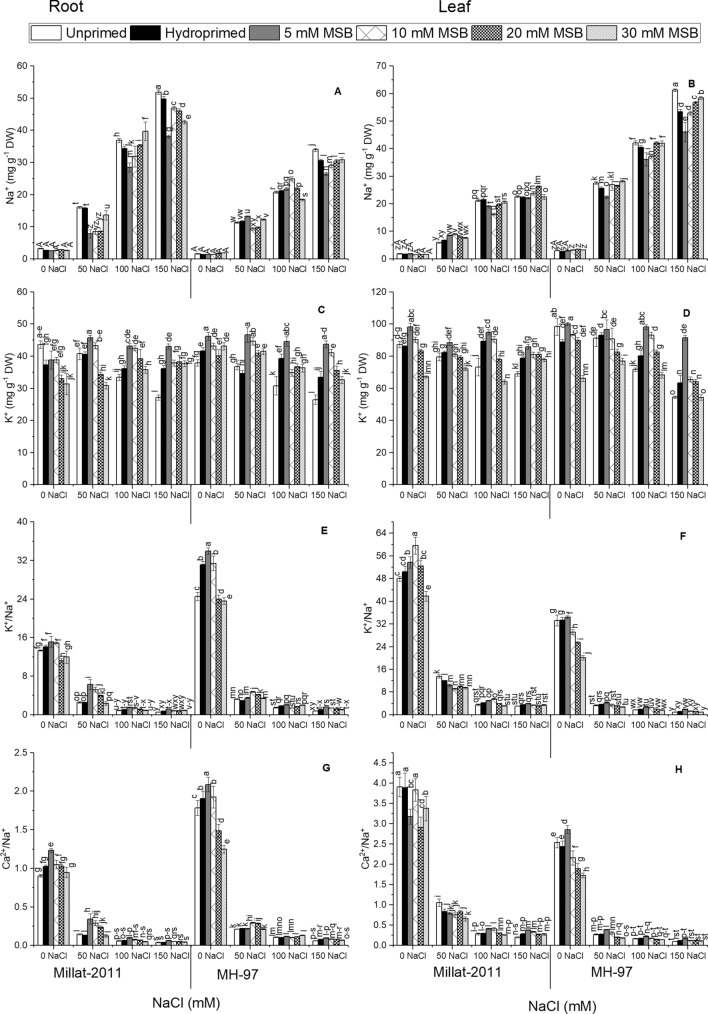
An increase in sodium (Na+) levels was substantial (P ≤ 0.001) in wheat cultivars administered salinity in hydroponics medium. The pattern of Na+ accumulation was variable (P ≤ 0.001) between the cultivars. For instance, salt-tolerant cv. Millat-2011 manifested more significant Na+ accumulation in root than leaf, while salt-sensitive cv. MH-97 displayed more Na+ in leaf than root. MSB priming, notably with 5 mM displayed minimal values (P ≤ 0.001) for Na+ contents in plants under salinity (Fig. 6A and B; Table 1S).
Fig. 6.
Menadione sodium bisulphite-induced changes in elemental uptake in two genetically different wheat cultivars. (n = 4; means ± S.E.)
Potassium (K+) contents decreased notably (P ≤ 0.001) in roots and leaves of wheat plants supplemented with NaCl salinity in hydroponics medium. The cultivars varied significantly (P ≤ 0.01) for root K+, while non-significant difference was seen for leaf K+ in two wheat cultivars. MH-97 displayed more root K+ contents. Priming treatments noticeably (P ≤ 0.001) with 5 and 10 mM increased plant K+ contents by several folds in wheat plants under salinity (Fig. 6C and D; Table 1S).
Salinity produced a noteworthy depression (P ≤ 0.001) in root and leaf K+/Na+ and Ca2+/Na+ ratios in hydroponically grown wheat plants. K+/Na+ and Ca2+/Na+ ratios were higher in the roots of salt-sensitive wheat cultivar MH-97, while salt-tolerant cv. Millat-2011 depicted higher K+/Na+ and Ca2+/Na+ in leaf under salinity. MSB priming notably with 5 and 10 mM resulted in profound accretion (P ≤ 0.001) in these variables under salinity (Fig. 6E–H; Table 1S).
Discussion
The outcomes of the present research exhibited a significant drop in plant growth and chlorophyll under saline conditions (Ashraf et al. 2019). Plant growth and chlorophyll contents diminished considerably under salinity due to ROS-induced oxidative injury, osmotic stress, ion imbalance, accumulation of Na+ ions at toxic level and reduction in the uptake of mineral ions Ca+2 and K+ (Farouk and Arafa 2018; Sofy et al. 2020). Our results displayed a negative association of antioxidant compounds and antioxidant enzymes with H2O2 and MDA contents (Fig. 8). MSB significantly enhanced plant growth under salinity by reducing oxidative injury (Fig. 5A–F). MSB produced better osmotic adjustment due to higher proline and soluble sugar accumulation (Fig. 4E and J). The degradation of chlorophyll molecules was also minimal due to MSB-mediated reduction in ROS generation (Fig. 2H, I and K). MSB enhanced the uptake of P, K+ and Ca2+ alongside the concomitant drop in Na+ uptake. MSB maintained higher K+/Na+ and Ca2+/Na+ in plants under salinity (Fig. 5G and H; Fig. 6A–H). Our results also indicated a strong negative correlation of photosynthetic pigments and antioxidant system with oxidative stress markers (Figs. 7 and 8). Consequently, MSB restored growth by improving cell turgor and photosynthesis under salinity (Fig. 9).
Fig. 8.
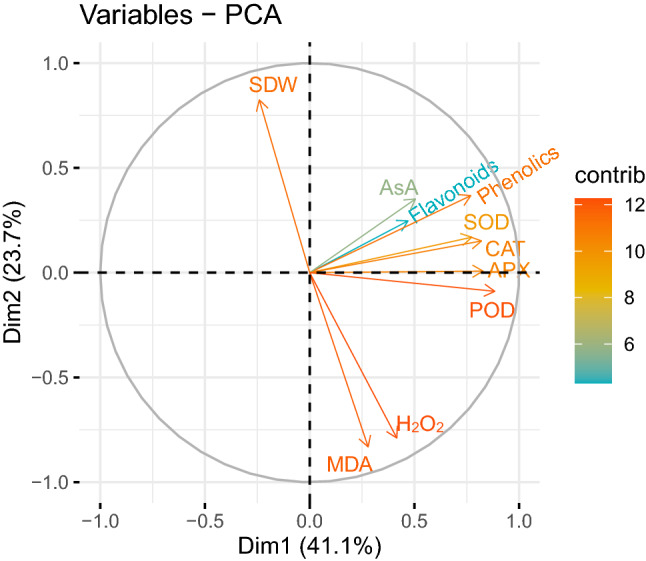
Principal component analysis showing association among growth, oxidative defense and oxidative stress injury in salinity stressed wheat plants treated with menadione sodium bisulphite. Abbreviations: SDW, shoot dry weight; MDA, malondialdehyde; H2O2, hydrogen peroxide; SOD, superoxide dismutase; CAT, catalase; POD, peroxidase; APX, ascorbate peroxidase
Fig. 7.
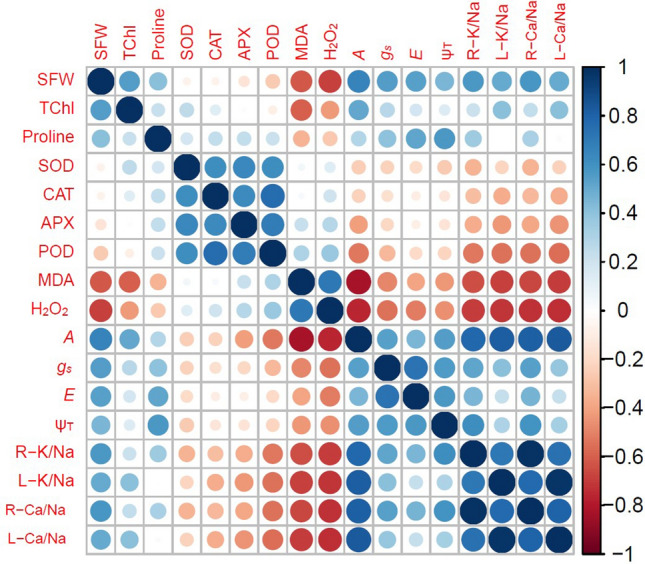
Pearson correlation among the growth and physiochemical attributes of wheat treated with exogenous menadione sodium bisulphite under salinity. SFW shoot fresh weight, TChl total chlorophyll, MDA malondialdehyde, H2O2 hydrogen peroxide; A, net photosynthesis rate; E, transpiration rate; gs, stomatal conductance; ψT, turgor potential; R-K/Na, root K/Na; L-K/Na, leaf K/Na; R-Ca/Na, root Ca/Na; L-Ca/Na, leaf Ca/Na
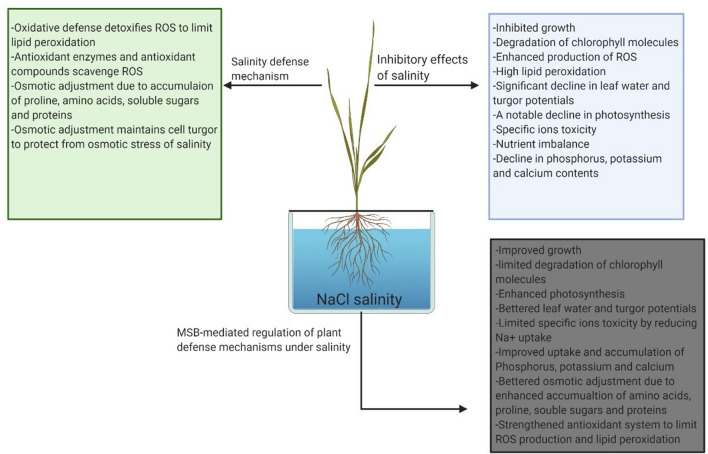
Fig. 9.
MSB-induced alterations in physiological and biochemical stress tolerance mechanisms to improve salinity tolerance in wheat
NaCl salinity induced a drastic decline in photosynthesis, stomatal conductance, and transpiration rate (Kwon et al. 2019). In glycophytes like wheat, the decline in photosynthesis could be due to both stomatal and non-stomatal factors (Kwon et al. 2019). Likewise, we found a significant decline in stomatal conductance and transpiration rate in wheat plants under salinity (Fig. 3B and C). Besides, some non-stomatal factors also seemed to be causing a decline in photosynthesis. For instance, the photosynthesis rate displayed a strong positive correlation with K+/Na+ and Ca2+/Na+ ratios. Further, MDA and H2O2 had a negative impact on photosynthesis. Proline was also found to have a positive correlation with photosynthesis under salinity (Fig. 7). Additionally, the salinity-mediated decline in photosynthesis was due to a reduction in sink activity and an increase in the degradation of chlorophyll molecules (Vasantha et al. 2010). Salinity caused a decrease in photosynthesis in several plant species such as wheat (Ashraf and Parveen 2002), tomato (Romero-Aranda et al. 2001), Linum usitatissimum (Khan et al. 2007), and cotton (Meloni et al. 2003). Salt-induced drop in stomatal conductance and transpiration rate has also been reported earlier in wheat (Ashraf and Shahbaz 2003), cotton (Meloni et al. 2003), and rice (Moradi and Ismail 2007). Salinity reduced water potential that declined stomatal conductance and transpiration rate in wheat (James et al. 2002). MSB significantly improved photosynthesis in wheat under salinity. This could have been due to MSB-mediated higher K+/Na+ and Ca2+/Na+ alongside improved osmotic adjustment in wheat plants under salinity (Fig. 6 E–H). Kwon et al. (2019) reported that accumulation of toxic Na+ and salinity-induced degradation of chlorophyll molecules reduced photosynthesis in Dianthus caryophyllus. In the present study, MSB significantly reduced Na+ accumulation and prevented degradation of chlorophyll molecules to increase photosynthesis under salinity (Figs. 2K, 6A, and B).
Secondary metabolites are among diverse biologically active compounds that regulate growth and plant defense responses under biotic and abiotic stresses (Khademian et al. 2019). Secondary metabolites accumulation is determined by environmental factors such as drought or salinity (Akula and Ravishankar 2011). The accumulation of secondary metabolites under stress conditions indicates their involvement in mediating plant responses to the stress (Khademian et al. 2019). Among secondary metabolites, phenolics and flavonoids are widely studied and reported to have a meaningful impact on plants under stress (Hoang et al. 2020). Phenolics and flavonoids protect plants from salt-induced oxidative damage by neutralizing ROS (Hasanuzzaman et al. 2020). Ghorbani et al. (2018) reported an increase in phenolics and flavonoids accumulation that protected tomato plants from oxidative injury by removing free radicals under salinity. Hoang et al. (2020) found a salt-induced increase in phenolics and flavonoids in Amaranthus tricolor L. The present investigation results also confirmed a salinity-mediated increase in these secondary metabolites (Fig. 4A and B). MSB priming with 5 and 10 mM produced a notable rise in secondary metabolites accumulation. H2O2 and MDA levels are also minimal in plants treated with 5 and 10 mM MSB under salinity (Fig. 5A and B).
The accumulation of organic compounds and inorganic ions is many folds higher in plants undergoing osmotic adjustment to regulate plant water status (Sharma et al. 2019). Soluble sugars, proteins, total free amino acids and proline are essential osmolytes maintaining plant turgor (Latef et al. 2017). MSB significantly improved leaf turgor potential by promoting the accumulation of these osmolytes. This increase was evident in plants pretreated with 5 and 10 mM MSB (Fig. 4E, I and J). On the contrary, higher MSB doses (20 and 30 mM) induced maximal rise in proteins (Fig. 4H). We found a significant positive correlation of proline with leaf turgor potential (Fig. 7). El Moukhtari et al. (2020) reported that proline improved leaf water contents, leaf turgor potentials and restored water use efficiency in plants under salinity. MSB-mediated increase in plant-water relation in our study could be ascribed to improvement in proline and soluble sugars (Figs. 3F and H, 4E and J).
The antioxidant enzyme activities such as SOD, POD, CAT, and APX were notably higher in plants under salinity. Exogenous MSB, especially 5 and 10 mM, produced a noteworthy accretion in antioxidant enzyme activities and antioxidant compounds such as phenolics, ascorbic acid, anthocyanins, flavonoids, and proline (Figs. 4A–D and J; Fig. 5 C–F). Since MSB significantly strengthened the antioxidant system; therefore, we recorded minimal MDA and H2O2 values in plants treated with 5 and 10 mM MSB under salinity. The correlation of antioxidant enzymes and antioxidant compounds demonstrated a robust negative association with H2O2 and MDA. ROS scavenging by the antioxidant system was significant that ultimately decreased oxidative injury mirrored as H2O2 and MDA levels in plants under salinity (Fig. 8). Khan et al. (2020) reported higher antioxidant enzyme activities (POD, CAT, SOD, and GR) and high levels of antioxidant compounds (phenolics and flavonoids) that protected pearl millet plants from oxidative damage under salinity. Gou et al. (2020) reported that higher antioxidant enzyme activities neutralized oxidative damage. Abdelaal et al. (2020) reported that the negative association of H2O2 and MDA with antioxidant enzyme activities protected pepper plants from oxidative injury. Besides, MSB (5 and 10 mM) reduced Na+ accumulation in the leaf that could have also contributed to minimal oxidative injury. Khan et al. (2020) also found similar findings in pearl millet under salinity.
Salinity-mediated increase in Na+ ions was reported in various plant species, including maize (Ashraf et al. 2018), canola (Tunçtürk et al. 2011), and wheat (Mohsin et al. 2020). Salinity caused several folds increase in Na+ accumulation alongside the accompanying drop in the accumulation of K+ and Ca2+ (Mohsin et al. 2020). Plants accumulate toxic ions due to disturbance in ions homeostasis under salinity (Sofy et al. 2020). In Ocimum basilicum L., Farouk et al. (2020) reported a conspicuous increase in Na+ accumulation that, in turn, decreased K+, significantly dropping K+/Na+ under salinity. K+/Na+ is a potential determinant for salinity tolerance ability of plants (Sofy et al. 2020). The nutritional imbalance created by salinity due to greater Na+ accumulation is mirrored as a significant decline in K+, Ca2+ and P contents (Kaya and Ashraf 2020). MSB priming with 5 and 10 mM restored salinity-mediated nutrient imbalance and thereby significantly improved K+, Ca2+, P and K+/Na+ and Ca2+/Na+ ratios in wheat under salinity (Figs. 5G–J, 6).
Conclusion
Salinity significantly decreased growth due to enhanced degradation of chlorophyll molecules with a concurrent drop in photosynthesis, plant turgor potential, and uptake of essential nutrients (K+, P, and Ca2+). Additionally, oxidative injury due to enhanced lipid peroxidation and H2O2 accumulation also contributed remarkably to suppress plant growth under salinity. Our results suggested the involvement of both stomatal and non-stomatal factors in the reduction of photosynthesis under salinity. Further, specific ions toxicity due to Na+ accumulation decreased photosynthesis, produced oxidative injury, and reduced K+/Na+ and Ca2+/Na+ ratios under salinity. Salinity effects were more apparent in sensitive cultivar MH-97 reflected in growth reduction, chlorophyll degradation, loss of cell turgidity, the decline in photosynthesis, and more severe oxidative damage than salt-tolerant wheat cultivar Millat-2011. MSB significantly promoted oxidative defense, osmotic adjustment, and regulated ions homeostasis to restore plant growth under salinity. MSB improved photosynthesis and maintained cell turgor in wheat under saline conditions. The results of the present study could pave a way out for improving salinity tolerance in other cereals. However, further studies are recommended, particularly MSB mediated changes in stress-related gene expression, to get further insights into MSB-induced salinity tolerance in wheat.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Data presented in the manuscript is the part of PhD research work of Mr. Ali Akbar, a PhD student (2012-GCUF-06707) at the Department of Botany, Government College University Faisalabad, Pakistan. The data is taken from PhD thesis of Mr. Ali Akbar.
Author contribution
Ali Akbar conducted the experiment and lab analysis. Muhammad Arslan Ashraf conceived the idea and supervised the research work. Shafaqat Ali helped in ions analysis. Muhammad Rizwan performed the statistical analysis and correlation analysis. Rizwan Rasheed provided technical help during physiological analysis and manuscript write up.
Funding
Higher Education Commission Islamabad, Pakistan (HEC) provided funds for the present research work under project No. 8345/Punjab/NRPU/R&D/HEC/2017.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-021-01001-6.
References
- Abdelaal KA, El-Maghraby LM, Elansary H, Hafez YM, Ibrahim EI, El-Banna M, El-Esawi M, Elkelish A. Treatment of sweet pepper with stress tolerance-inducing compounds alleviates salinity stress oxidative damage by mediating the physio-biochemical activities and antioxidant systems. Agronomy. 2020;10(1):26. [Google Scholar]
- Adhikari B, Dhungana SK, Kim ID, Shin DH. Effect of foliar application of potassium fertilizers on soybean plants under salinity stress. J Saudi Soc Agric Sci. 2020;19(4):261–269. doi: 10.1016/j.jssas.2019.02.001. [DOI] [Google Scholar]
- Akram NA, Hafeez N, Farid-ul-Haq M, Ahmad A, Sadiq M, Ashraf M. Foliage application and seed priming with nitric oxide causes mitigation of salinity-induced metabolic adversaries in broccoli (Brassica oleracea L.) plants. Acta Physiol Plant. 2020;42(10):155. doi: 10.1007/s11738-020-03140-x. [DOI] [Google Scholar]
- Akula R, Ravishankar GA. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011;6(11):1720–1731. doi: 10.4161/psb.6.11.17613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S, Grimshow H, Parkinson J, Quarmby C, Roberts J. Chemical analysis in methods in plant ecology by Chapman. London: Black Well; 1976. [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24(1):1. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M, Parveen N. Photosynthetic parameters at the vegetative stage and during grain development of two hexaploid wheat cultivars differing in salt tolerance. Biol Plant. 2002;45(3):401–407. [Google Scholar]
- Ashraf M, Shahbaz M. Assessment of genotypic variation in salt tolerance of early CIMMYT hexaploid wheat germplasm using photosynthetic capacity and water relations as selection criteria. Photosynthetica. 2003;41(2):273–280. [Google Scholar]
- Ashraf MA, Akbar A, Parveen A, Rasheed R, Hussain I, Iqbal M. Phenological application of selenium differentially improves growth, oxidative defense and ion homeostasis in maize under salinity stress. Plant Physiol Biochem. 2018;123:268–280. doi: 10.1016/j.plaphy.2017.12.023. [DOI] [PubMed] [Google Scholar]
- Ashraf MA, Asma HF, Iqbal M. Exogenous menadione sodium bisulfite mitigates specific ion toxicity and oxidative damage in salinity-stressed okra (Abelmoschus esculentus Moench) Acta Physiol Plant. 2019;41(12):187. doi: 10.1007/s11738-019-2978-7. [DOI] [Google Scholar]
- Ashraf MA, Rasheed R, Zafar S, Iqbal M, Saqib ZA. Menadione sodium bisulfite neutralizes chromium phytotoxic effects in okra by regulating cytosolutes, lipid peroxidation, antioxidant system and metal uptake. Int J Phytoremed. 2020;3:1–11. doi: 10.1080/15226514.2020.1854171. [DOI] [PubMed] [Google Scholar]
- Askari SH, Ashraf MA, Ali S, Rizwan M, Rasheed R. Menadione sodium bisulfite alleviated chromium effects on wheat by regulating oxidative defense, chromium speciation, and ion homeostasis. Environ Sci Pollut Res. 2021;3:1–21. doi: 10.1007/s11356-021-13221-0. [DOI] [PubMed] [Google Scholar]
- Bates LS, Waldren RP, Teare I. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–207. [Google Scholar]
- Binder RG, Benson ME, Flath RA. Eight 1,4-naphthoquinones from Juglans. Phytochemistry. 1989;28:2799–2801. doi: 10.1016/S0031-9422(00)98092-0. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cakmak I, Horst WJ. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max) Physiol Plant. 1991;83(3):463–468. [Google Scholar]
- Chance B, Maehly A. [136] Assay of catalases and peroxidases. Meth Enzymol. 1955;2:764–775. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350–356. [Google Scholar]
- Egamberdieva D, Wirth S, Bellingrath-Kimura SD, Mishra J, Arora NK. Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front Microbiol. 2019;10:2791–2791. doi: 10.3389/fmicb.2019.02791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellouzi H, Hamed KB, Asensi-Fabado MA, Müller M, Abdelly C, Munné-Bosch S. Drought and cadmium may be as effective as salinity in conferring subsequent salt stress tolerance in Cakile maritima. Planta. 2013;237(5):1311–1323. doi: 10.1007/s00425-013-1847-7. [DOI] [PubMed] [Google Scholar]
- El-Moukhtari A, Cabassa-Hourton C, Farissi M, Savouré A. How does proline treatment promote salt stress tolerance during crop plant development? Front Plant Sci. 2020;11:1127. doi: 10.3389/fpls.2020.01127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etesami H, Beattie GA. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front Microbiol. 2018;9:148. doi: 10.3389/fmicb.2018.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farouk S, Arafa SA. Mitigation of salinity stress in canola plants by sodium nitroprusside application. Span J Agric Res. 2018;16(3):16. [Google Scholar]
- Farouk S, Elhindi KM, Alotaibi MA. Silicon supplementation mitigates salinity stress on Ocimum basilicum L. via improving water balance, ion homeostasis, and antioxidant defense system. Ecotoxicol Environ Saf. 2020;206:111396. doi: 10.1016/j.ecoenv.2020.111396. [DOI] [PubMed] [Google Scholar]
- Feghhenabi F, Hadi H, Khodaverdiloo H, van Genuchten MT. Seed priming alleviated salinity stress during germination and emergence of wheat (Triticum aestivum L.) Agric Water Manag. 2020;231:106022. doi: 10.1016/j.agwat.2020.106022. [DOI] [Google Scholar]
- García-Caparrós P, Llanderal A, Hegarat E, Jiménez-Lao M, Lao MT. Effects of exogenous application of osmotic adjustment substances on growth, pigment concentration, and physiological parameters of Dracaena sanderiana sander under different levels of salinity. Agronomy. 2020;10(1):125. [Google Scholar]
- Gardner F, Pearce R, Mitchell R. Physiology of crop plants. Ames: Iowa State University Press; 1985. p. 327. [Google Scholar]
- Ghorbani A, Razavi SM, Omran VOG, Pirdashti H. Piriformospora indica alleviates salinity by boosting redox poise and antioxidative potential of tomato. Russ J Plant Physiol. 2018;65(6):898–907. doi: 10.1134/S1021443718060079. [DOI] [Google Scholar]
- Giannopolitis CN, Ries SK. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977;59(2):309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou T, Chen X, Han R, Liu J, Zhu Y, Gong H. Silicon can improve seed germination and ameliorate oxidative damage of bud seedlings in cucumber under salt stress. Acta Physiol Plant. 2020;42(1):12. doi: 10.1007/s11738-019-3007-6. [DOI] [Google Scholar]
- Habiba U, Ali S, Farid M, Shakoor MB, Rizwan M, Ibrahim M, Abbasi GH, Hayat T, Ali B. EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Environ Sci Pollut Res. 2015;22(2):1534–1544. doi: 10.1007/s11356-014-3431-5. [DOI] [PubMed] [Google Scholar]
- Hamilton P, Van Slyke D. Amino acid determination and metal accumulation by Brassica juncea L. Int J Plant Prod. 1943;3(1):1735–8043. [Google Scholar]
- Hasanuzzaman M, Bhuyan M, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants. 2020;9(8):681. doi: 10.3390/antiox9080681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M, Nahar K, Alam MM, Bhuyan MHMB, Oku H, Fujita M. Exogenous nitric oxide pretreatment protects Brassica napus L. seedlings from paraquat toxicity through the modulation of antioxidant defense and glyoxalase systems. Plant Physiol Biochem. 2018;126:173–186. doi: 10.1016/j.plaphy.2018.02.021. [DOI] [PubMed] [Google Scholar]
- Henson C, Stone J. Variation in α-amylase and α-amylase inhibitor activities in barley malts. J Cereal Sci. 1988;8(1):39–46. [Google Scholar]
- Hoang HL, de Guzman CC, Cadiz NM, Hoang TTH, Tran DH, Rehman H. Salicylic acid and calcium signaling induce physiological and phytochemical changes to improve salinity tolerance in red amaranth (Amaranthus tricolor L.) J Soil Sci Plant Nutr. 2020;20:1–11. [Google Scholar]
- Jackson ML. Soil chemical analysis. Englewood Cliffs: Prentice-Hall; 1962. pp. 214–221. [Google Scholar]
- James RA, Rivelli AR, Munns R, von Caemmerer S. Factors affecting CO2 assimilation, leaf injury and growth in salt-stressed durum wheat. Funct Plant Biol. 2002;29(12):1393–1403. doi: 10.1071/FP02069. [DOI] [PubMed] [Google Scholar]
- Jiménez-Arias D, García-Machado FJ, Morales-Sierra S, Suárez E, Pérez JA, Luis JC, Garrido-Orduña C, Herrera AJ, Valdés F, Sandalio LM, Borges AA. Menadione sodium bisulphite (MSB): Beyond seed-soaking. Root pretreatment with MSB primes salt stress tolerance in tomato plants. Environ Exp Bot. 2019;157:161–170. doi: 10.1016/j.envexpbot.2018.10.009. [DOI] [Google Scholar]
- Johnson R, Puthur JT. Seed priming as a cost effective technique for developing plants with cross tolerance to salinity stress. Plant Physiol Biochem. 2021;162:247–257. doi: 10.1016/j.plaphy.2021.02.034. [DOI] [PubMed] [Google Scholar]
- Kahveci H, Bilginer N, Diraz-Yildirim E, Kulak M, Yazar E, Kocacinar F, Karaman S. Priming with salicylic acid, β-carotene and tryptophan modulates growth, phenolics and essential oil components of Ocimum basilicum L. grown under salinity. Sci Hortic. 2021;281:109964. [Google Scholar]
- Kamiab F. Exogenous melatonin mitigates the salinity damages and improves the growth of pistachio under salinity stress. J Plant Nutr. 2020;43(10):1468–1484. doi: 10.1080/01904167.2020.1730898. [DOI] [Google Scholar]
- Kaya C, Ashraf M. The endogenous L-cysteine desulfhydrase and hydrogen sulfide participate in supplemented phosphorus-induced tolerance to salinity stress in maize (Zea mays) plants. Turk J Bot. 2020;44(1):36–46. [Google Scholar]
- Khademian R, Asghari B, Sedaghati B, Yaghoubian Y. Plant beneficial rhizospheric microorganisms (PBRMs) mitigate deleterious effects of salinity in sesame (Sesamum indicum L.): Physio-biochemical properties, fatty acids composition and secondary metabolites content. Ind Crops Prod. 2019;136:129–139. doi: 10.1016/j.indcrop.2019.05.002. [DOI] [Google Scholar]
- Khan I, Raza MA, Awan SA, Shah GA, Rizwan M, Ali B, Tariq R, Hassan MJ, Alyemeni MN, Brestic M, Zhang X, Ali S, Huang L. Amelioration of salt induced toxicity in pearl millet by seed priming with silver nanoparticles (AgNPs): the oxidative damage, antioxidant enzymes and ions uptake are major determinants of salt tolerant capacity. Plant Physiol Biochem. 2020;156:221–232. doi: 10.1016/j.plaphy.2020.09.018. [DOI] [PubMed] [Google Scholar]
- Khan MN, Siddiqui MH, Mohammad F, Khan M, Naeem M. Salinity induced changes in growth, enzyme activities, photosynthesis, proline accumulation and yield in linseed genotypes. World J Agric Sci. 2007;3(5):685–695. [Google Scholar]
- Kumar Arora N, Fatima T, Mishra J, Mishra I, Verma S, Verma R, Verma M, Bhattacharya A, Verma P, Mishra P, Bharti C. Halo-tolerant plant growth promoting rhizobacteria for improving productivity and remediation of saline soils. J Adv Res. 2020;26:69–82. doi: 10.1016/j.jare.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SS, Gaurav AK, Srivastava S, Verma JP. Plant growth-promoting bacteria: biological tools for the mitigation of salinity stress in plants. Front Microbiol. 2020;11:1216–1216. doi: 10.3389/fmicb.2020.01216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OK, Mekapogu M, Kim KS. Effect of salinity stress on photosynthesis and related physiological responses in carnation (Dianthus caryophyllus) Hortic Environ Biotechnol. 2019;60(6):831–839. doi: 10.1007/s13580-019-00189-7. [DOI] [Google Scholar]
- Latef AAHA, Alhmad MFA, Abdelfattah KE. The possible roles of priming with ZnO nanoparticles in mitigation of salinity stress in lupine (Lupinus termis) plants. J Plant Growth Regul. 2017;36(1):60–70. [Google Scholar]
- Mahajan M, Sharma S, Kumar P, Pal PK. Foliar application of KNO3 modulates the biomass yield, nutrient uptake and accumulation of secondary metabolites of Stevia rebaudiana under saline conditions. Ind Crops Prod. 2020;145:112102. doi: 10.1016/j.indcrop.2020.112102. [DOI] [Google Scholar]
- Mahmood N, Hameed A, Hussain T. Vitamin E and selenium treatment alleviates saline environment-induced oxidative stress through enhanced antioxidants and growth performance in suckling kids of beetal goats. Oxid Med Cell Longev. 2020 doi: 10.1155/2020/4960507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzotti P, De Nisi P, Zocchi G. Vitamin K in plants. Funct Plant Sci Biotech. 2008;2:29–35. [Google Scholar]
- Meloni DA, Oliva MA, Martinez CA, Cambraia J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot. 2003;49(1):69–76. [Google Scholar]
- Mishra J, Fatima T, Arora NK. Plant microbiome: stress response. Berlin: Springer; 2018. Role of secondary metabolites from plant growth-promoting rhizobacteria in combating salinity stress; pp. 127–163. [Google Scholar]
- Mita S, Murano N, Akaike M, Nakamura K. Mutants of Arabidopsis thaliana with pleiotropic effects on the expression of the gene for β-amylase and on the accumulation of anthocyanin that are inducible by sugars. Plant J. 1997;11(4):841–851. doi: 10.1046/j.1365-313x.1997.11040841.x. [DOI] [PubMed] [Google Scholar]
- Mohsin SM, Hasanuzzaman M, Parvin K, Fujita M. Pretreatment of wheat (Triticum aestivum L.) seedlings with 2,4-D improves tolerance to salinity-induced oxidative stress and methylglyoxal toxicity by modulating ion homeostasis, antioxidant defenses, and glyoxalase systems. Plant Physiol Biochem. 2020;152:221–231. doi: 10.1016/j.plaphy.2020.04.035. [DOI] [PubMed] [Google Scholar]
- Moradbeygi H, Jamei R, Heidari R, Darvishzadeh R. Investigating the enzymatic and non-enzymatic antioxidant defense by applying iron oxide nanoparticles in Dracocephalum moldavica L. plant under salinity stress. Sci Hortic. 2020;272:109537. doi: 10.1016/j.scienta.2020.109537. [DOI] [Google Scholar]
- Moradi F, Ismail AM. Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann Bot. 2007;99(6):1161–1173. doi: 10.1093/aob/mcm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee SP, Choudhuri MA. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant. 1983;58(2):166–170. doi: 10.1111/j.1399-3054.1983.tb04162.x. [DOI] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22(5):867–880. [Google Scholar]
- Petropoulos SA, Levizou E, Ntatsi G, Fernandes Â, Petrotos K, Akoumianakis K, Barros L, Ferreira ICFR. Salinity effect on nutritional value, chemical composition and bioactive compounds content of Cichorium spinosum L. Food Chem. 2017;214:129–136. doi: 10.1016/j.foodchem.2016.07.080. [DOI] [PubMed] [Google Scholar]
- Polle A, Otter T, Seifert F. Apoplastic peroxidases and lignification in needles of norway spruce (Picea abies L.) Plant Physiol. 1994;106(1):53–60. doi: 10.1104/pp.106.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadir M, Quillérou E, Nangia V, Murtaza G, Singh M, Thomas RJ, Drechsel P, Noble AD. Economics of salt-induced land degradation and restoration. Nat Resour Forum. 2014;38(4):282–295. doi: 10.1111/1477-8947.12054. [DOI] [Google Scholar]
- Rasheed R, Ashraf MA, Kamran S, Iqbal M, Hussain I. Menadione sodium bisulphite mediated growth, secondary metabolism, nutrient uptake and oxidative defense in okra (Abelmoschus esculentus Moench) under cadmium stress. J Hazard Mater. 2018;360:604–614. doi: 10.1016/j.jhazmat.2018.08.043. [DOI] [PubMed] [Google Scholar]
- Romero-Aranda R, Soria T, Cuartero J. Tomato plant-water uptake and plant-water relationships under saline growth conditions. Plant Sci. 2001;160(2):265–272. doi: 10.1016/s0168-9452(00)00388-5. [DOI] [PubMed] [Google Scholar]
- Saqib M, Akhtar J, Abbas G, Nasim M. Salinity and drought interaction in wheat (Triticum aestivum L.) is affected by the genotype and plant growth stage. Acta Physiol Plant. 2013;35(9):2761–2768. [Google Scholar]
- Savvides A, Ali S, Tester M, Fotopoulos V. Chemical priming of plants against multiple abiotic stresses: mission possible? Trends Plant Sci. 2016;21(4):329–340. doi: 10.1016/j.tplants.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Scholander PF, Bradstreet ED, Hemmingsen E, Hammel H. Sap pressure in vascular plants: negative hydrostatic pressure can be measured in plants. Science. 1965;148(3668):339–346. doi: 10.1126/science.148.3668.339. [DOI] [PubMed] [Google Scholar]
- Sharma A, Shahzad B, Kumar V, Kohli SK, Sidhu GPS, Bali AS, Handa N, Kapoor D, Bhardwaj R, Zheng B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules. 2019;9(7):285. doi: 10.3390/biom9070285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhom MB, El-Kommos ME. Spectrophotometric determination of menadione and menadione sodium bisulfite. J Assoc off Anal Chem. 1982;65(1):141–143. [Google Scholar]
- Sofy MR, Elhawat N, Tarek A. Glycine betaine counters salinity stress by maintaining high K+/Na+ ratio and antioxidant defense via limiting Na+ uptake in common bean (Phaseolus vulgaris L.) Ecotoxicol Environ Saf. 2020;200:110732. doi: 10.1016/j.ecoenv.2020.110732. [DOI] [PubMed] [Google Scholar]
- Sunita K, Mishra I, Mishra J, Prakash J, Arora NK. Secondary metabolites from halotolerant plant growth promoting rhizobacteria for ameliorating salinity stress in plants. Front Microbiol. 2020;11:2619. doi: 10.3389/fmicb.2020.567768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiem D, Gołębiewski M, Hulisz P, Piernik A, Hrynkiewicz K. How does salinity shape bacterial and fungal microbiomes of alnus glutinosa roots? Front Microbiol. 2018;9:651. doi: 10.3389/fmicb.2018.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunçtürk M, Tunçtürk R, Yildirim B, Çiftçi V. Effect of salinity stress on plant fresh weight and nutrient composition of some canola (Brassica napus L.) cultivars. Afr J Biotechnol. 2011;10(10):1827–1832. [Google Scholar]
- Vasantha S, Venkataramana S, Rao PG, Gomathi R. Long term salinity effect on growth, photosynthesis and osmotic characteristics in sugarcane. Sugar Technol. 2010;12(1):5–8. [Google Scholar]
- Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 2000;151(1):59–66. [Google Scholar]
- Wolfe K, Wu X, Liu RH. Antioxidant activity of apple peels. J Agric Food Chem. 2003;51(3):609–614. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- Yildiztugay E, Ozfidan-Konakci C, Kucukoduk M, Turkan I. Flavonoid naringenin alleviates short-term osmotic and salinity stresses through regulating photosynthetic machinery and chloroplastic antioxidant metabolism in Phaseolus vulgaris. Front Plant Sci. 2020;11:682. doi: 10.3389/fpls.2020.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555–559. [Google Scholar]
- Zörb C, Geilfus CM, Dietz KJ. Salinity and crop yield. Plant Biol. 2019;21:31–38. doi: 10.1111/plb.12884. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.