Abstract
Objective
This study evaluated work and activity impairment in patients with multiple sclerosis (MS) treated with ocrelizumab (OCR) versus other disease-modifying therapies (DMTs).
Methods
Data were obtained from the Adelphi Real World Disease Specific Programme for Multiple Sclerosis. Patients with relapsing–remitting or secondary progressive MS who completed surveys in 2018 and 2019 and received ≥ 6 months of an eligible therapy, including OCR, injectable therapy, and oral therapy, were included. Outcomes were assessed using the patient-reported Work Productivity and Activity Impairment questionnaire. Doubly robust estimation, which combined propensity score weighting and regression modeling, was used to compare treatments, controlling for baseline clinical and demographic characteristics.
Results
This study included 630 patients (OCR, n = 90; injectable DMT, n = 224; oral DMT, n = 316) with a mean (standard deviation) age of 42 (11) years. A greater proportion of OCR-treated patients had an Expanded Disability Status Scale score of ≥ 3 at treatment initiation compared with those receiving oral and injectable DMTs (51 vs. 15% and 15%, respectively), and a smaller proportion of OCR-treated patients received treatment for ≥ 1 year (43 vs. 90% and 92%, respectively). OCR-treated patients had higher odds of employment [odds ratio (95% confidence interval) 3.4 (1.5–7.7) vs. oral DMT, 5.6 (2.6–12.0) vs. injectable DMT], lower overall work productivity loss [difference (95% confidence interval) − 10.0% (− 6.1 to − 15.0%) vs. oral DMT, − 13.0% (− 8.5 to − 17.0%) vs. injectable DMT] and lower activity impairment [difference (95% confidence interval) − 11% (− 7.1 to − 16.0%) vs. oral DMT, − 9.7% (− 5.0 to − 14.0%) vs. injectable DMT].
Conclusion
This real-world evidence suggests that patients with MS treated with OCR experience lower work and activity impairment than patients treated with other DMTs.
Electronic supplementary material
The online version of this article (10.1007/s40120-020-00224-1) contains supplementary material, which is available to authorized users.
Keywords: Disease-modifying therapies, Employment, Multiple sclerosis, Ocrelizumab, Work productivity
Plain Language Summary
Multiple sclerosis (MS) is the most common progressive neurological disease in young adults. It typically starts between the ages of 20 and 40 years—arguably some of the most productive years of an individual’s life—and it has a large impact on many aspects of everyday life for the rest of a person’s life. The reduction in the ability to do routine activities, including working, results in a large economic burden. Disease-modifying treatments (DMTs) available for MS, particularly high-efficacy DMTs, have been shown to improve work productivity. This study looked at work and activity impairment using the Work Productivity and Activity Impairment Questionnaire in patients with MS who were treated with ocrelizumab (OCR) or other DMTs for ≥ 6 months. A total of 630 patients with relapsing–remitting MS (RRMS) or secondary progressive MS (SPMS) from the Adelphi Real World Disease Specific Programme for Multiple Sclerosis were included in the study, including 90, 316 and 224 patients who completed ≥ 6 months of treatment with OCR, oral or injectable therapy. Compared with patients receiving oral or injectable DMTs, those receiving OCR had higher odds of employment [odds ratio (OR) vs. oral DMT 3.4; OR vs. injectable DMT 5.6], lower overall work productivity impairment (difference vs. oral DMT − 10%; difference vs. injectable DMT − 13%) and lower activity impairment (difference vs. oral DMT − 11%; difference vs. injectable DMT − 9.7%). These findings in patients with RRMS or SPMS being treated in the real world suggest that OCR may reduce the impact of MS disease on work productivity more than other DMTs.
Electronic supplementary material
The online version of this article (10.1007/s40120-020-00224-1) contains supplementary material, which is available to authorized users.
Key Summary Points
| Although ocrelizumab-treated patients with multiple sclerosis (MS) were generally sicker, after adjusting for baseline characteristics, they were more likely to be employed and experienced less impact on work productivity than those treated with oral (teriflunomide, fingolimod or dimethyl fumarate) or injectable (interferon β-1a, interferon β-1b, glatiramer acetate or peginterferon β-1a) therapies. |
| Reductions in work productivity impairment may also lessen indirect costs to both patients with MS (through lost work) and their employers (through maintaining work productivity levels). |
| The association of work productivity with ocrelizumab therapy should be taken into consideration when choosing therapy for patients, particularly since the majority of patients with MS are younger adults of working age. |
Digital Features
This article is published with digital features, including a summary slide and plain language summary, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13182734
Introduction
Multiple sclerosis (MS) is a chronic, immune-mediated disease of the central nervous system affecting an estimated 1 million people in the USA [1]. Regarded as the most common neurodegenerative disease in young adults [2], MS is typically diagnosed between the ages of 20 and 40 years and persists for the remainder of life [3–5]. The disease trajectory is highly heterogenous, with potential effects on mobility, balance, vision, cognition/behavior and other functional domains [6, 7]. All of these symptoms may impact the ability to work, leading to high rates of unemployment and impaired work productivity among people with MS [8, 9]. For example, people with MS have cited fatigue and lower limb disabilities as primary reasons for stopping employment [9]. Reduced cognitive processing speed, increased disability and depression are collectively predictive of employment status [8]. In addition to unemployment, presenteeism (being present at work but not fully functioning) is particularly high among people with MS [10], likely due to reduced cognitive processing speed, fatigue, depression and anxiety [11]. The effects of MS on work productivity may be evident years before a diagnosis. A large retrospective study found high levels of absenteeism (sick leave) up to 15 years prior to diagnosis in people with MS, which was more pronounced in those with progressive forms of the disease [12, 13].
The economic burden associated with the inability to work among people with MS is substantial for patients, employers and society in general [14]. One systematic review reported that annual indirect costs of MS per patient can range from US$2000 (when including sick leave, disability and workers’ compensation) to US$20,000 (when including costs of work time missed, underemployment and episodes of unemployment due to disability) [15]. Even individuals who experience only mild physical disability from MS have substantial costs associated with sick leave, work absenteeism and premature retirement [16]. Individuals with MS experience financial strain from reduced income due to missed work or early retirement. Employers are burdened by reduced productivity and workers’ compensation payments, while the economy suffers from labor force reductions and bears the costs of paying out disability benefits [12, 15, 16].
Aside from the economic implications, decreased work productivity has been associated with increased comorbidity rates and reduced quality of life in people with MS [14]. People with MS who work less are more likely to suffer from depression, anxiety and sleep issues [14, 17]. Indeed, one study found that early retirement due to MS predicted future decreases in quality of life [18]. Conversely, preservation of employment status and work productivity may confer psychological benefit, particularly if the individuals feel that they are in a supportive work environment [19, 20]. Employed individuals with MS report higher quality of life and lower depression compared with unemployed individuals with MS, suggesting that being employed may be part of a positive feedback loop that contributes to overall well-being [21]. Disease-modifying therapies (DMTs) that are capable of helping patients maintain the ability to work, therefore, may be particularly desirable in MS.
The US Food and Drug Administration has approved numerous DMTs for MS with diverse mechanisms of action, dosing schedules and routes of administration, providing a wide variety of choices for patients and their healthcare providers [22]. Ocrelizumab (OCR), an anti-CD20 humanized monoclonal antibody, was approved in 2017 and is the first and only therapy approved to treat both relapsing MS and primary progressive MS. While the clinical benefits of OCR therapy have been demonstrated in pivotal phase III trials [23, 24], the potential effects on economic outcomes, such as employment, work productivity and activity impairment, have not been established. The objective of this study was to evaluate the impact of OCR compared with other DMTs on these outcomes to inform patients with MS, payers and employer groups.
Methods
Study Population
Data were obtained from the Adelphi Real World Disease Specific Programme (DSP) for MS Waves 7 and 8 (2018–2019). The Adelphi DSPs are real-world, cross-sectional surveys administered to physicians, patients and caregivers to obtain a representative depiction of current clinical practice for various disease states [25]. Sample selection for the Adelphi DSPs has been described in detail elsewhere [25]. Briefly, physicians consenting to participate are asked to randomly identify 12–15 patients from their practice for enrollment in the survey collection and to complete physician-completed patient record forms (PRFs) on these patients. Patients then consent to participate and may complete patient self-completion questionnaires (PSCs). To enable a large enough sample of patients newly initiating OCR, physicians were asked to identify additional OCR patients for enrollment in the 2019 survey. A total of 304 OCR patients were identified in the DSPs, with an additional 352 patients enrolled as part of this oversampling strategy. We used data from PRFs regarding patient clinical characteristics linked to PSCs from patients.
This analysis did not require ethics committee approval, as confirmed by the data provider (Adelphi Real World, Macclesfield, UK) and Western Institutional Review Board (Puyallup, WA, USA), as it was based on previously conducted studies, data were de-identified, and it did not involve any new studies of human or animal subjects performed by any of the authors. The research was conducted in compliance with the US Health Insurance Portability and Accountability Act 1996.
Participants
Patients included in this analysis were required to live in the USA, be ≥ 18 years of age, have a diagnosis of relapsing–remitting MS (RRMS) or secondary progressive MS (SPMS) and be on eligible therapy [OCR or oral (teriflunomide, fingolimod or dimethyl fumarate) or subcutaneous/intramuscular injectable (interferon β-1a, interferon β-1b, glatiramer acetate or peginterferon β-1a)] for ≥ 6 months at the time of survey administration. To be included in the analysis, patients were required to have completed the Work Productivity and Activity Impairment questionnaire (WPAI). Of the 3389 patients included in the Adelphi Real World DSP for MS Waves 7 and 8, 1435 met the inclusion criteria. Of these patients, 630 had completed the WPAI, constituting the full cohort for the outcomes of employment and activity impairment. Of patients who completed the WPAI, 413 were employed and thus able to answer questions regarding absenteeism, presenteeism and overall work productivity loss. Of the 352 patients in the OCR oversample, 137 met the inclusion criteria, 71 completed the WPAI and 47 were employed.
Outcomes
The WPAI is a widely validated instrument measuring work productivity associated with general health and a variety of specific health problems [26–30]. It consists of six items regarding employment (yes/no) and four domains measuring level of impairment due to MS (range 0–100%), with higher scores indicating greater productivity impairment. The four domains, assessed over a 7-day period before the respondent completed the survey, include absenteeism (work time missed), presenteeism (impairment at work), work productivity loss (absenteeism + presenteeism) and activity impairment (impairment in performing regular daily activities other than work at a job) [26]. The WPAI was included in the PSCs and assessed for all patients meeting inclusion criteria. Employment and activity impairment outcomes were assessed in all patients, while the remaining domains were assessed only in patients who were employed. Absenteeism was dichotomized into having missed any work due to MS over the previous 7 days versus not in order to maximize power.
Clinical and demographic information was collected from PRFs and PSCs and included disease duration, line of therapy, disease subtype, duration of current therapy, age, sex and education. We also included the Expanded Disability Status Scale (EDSS) score—a commonly used measure to quantify disability in patients with MS—reported by physicians at therapy initiation [31].
Statistical Analysis
Chi-square and analysis of variance tests were used to compare categorical and continuous measures, respectively, across the OCR, injectable and oral treatment groups.
Separate analyses compared WPAI outcomes between (1) OCR and oral therapies and (2) OCR and injectable therapies. We used doubly robust estimation by combining inverse probability of treatment weighting (IPTW) with outcomes regression modeling. The IPTW method weights observations in the sample according to their propensity scores to create a “pseudo-population,” wherein treatment assignment is independent of the measured baseline characteristics. Two propensity score models were fit, one for each comparison (OCR vs. injectables; OCR vs. orals) in each population (full cohort and employed subgroup). These models included disease duration, EDSS score at therapy initiation, line of therapy, disease subtype, duration of current therapy, age and sex as predictors of treatment selection. Outcomes regression modeling was performed in the pseudo-population to ensure that the estimation of average treatment effect was robust to misspecification of either the propensity score model or the outcomes regression model. Logistic regression models were fit for employment and absenteeism outcomes, while linear regression models were fit for outcomes of activity impairment, presenteeism and overall work impairment. All regression models were adjusted for covariates used to develop the propensity scores. Employment models were additionally adjusted for education.
Sensitivity analyses included estimation of average treatment effect using IPTW or outcomes regression modeling alone and standardization and trimming of weights following IPTW to evaluate the effects of extreme observations on the results. Additionally, we conducted a subgroup analysis among patients who were on current therapy for ≤ 2 years. All tests for statistical significance were performed at α = 0.05. All analyses were performed using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA) [32, 33].
Results
Population
Baseline demographic and clinical characteristics of the full cohort and the employed subgroup are shown in Table 1 and Electronic Supplementary Material (ESM) Table S1, respectively. Overall, participants were predominantly female (62%), with a mean (standard deviation [SD]) age of 42 (11) years. The majority of patients had the RRMS subtype (93%), 20% had an EDSS score of ≥ 3, the average time living with an MS diagnosis was almost 6 years and 84% of patients had been on their current DMT for ≥ 1 year (Table 1). The employed subgroup (n = 413) was very similar but was slightly younger [mean (SD) age 40 (10) years] and a smaller proportion had an EDSS score of ≥ 3 (15%) (ESM Table S1).
Table 1.
Baseline demographic and clinical characteristics of the full Work Productivity and Activity Impairment questionnaire-respondent cohort
| Baseline demographic and clinical characteristics | Ocrelizumab (n = 90) | Oral DMTs (n = 316) | Injectable DMTs (n = 224) | p value |
|---|---|---|---|---|
| Age, years (mean ± SD) | 44 ± 9.6 | 41 ± 11 | 43 ± 12 | 0.02 |
| Female sex, n (%) | 61 (68) | 191 (60) | 141 (63) | 0.44 |
| Education, n (%) | 0.57 | |||
| Greater than high school | 76 (84) | 253 (80) | 185 (83) | |
| High school or less | 14 (16) | 63 (20) | 39 (17) | |
| Line of therapy, n (%) | < 0.01 | |||
| ≥ 2 | 78 (87) | 139 (44) | 60 (27) | |
| 0–1 | 12 (13) | 177 (56) | 164 (73) | |
| Disease subtype, n (%) | < 0.01 | |||
| RRMS | 73 (81) | 303 (96) | 211 (94) | |
| SPMS | 17 (19) | 13 (4) | 13 (6) | |
| EDSS score at treatment initiation, n (%) | < 0.01 | |||
| ≥ 3 | 46 (51) | 47 (15) | 33 (15) | |
| 0–2 | 44 (49) | 269 (85) | 191 (85) | |
| Years since diagnosis (mean ± SD) | 6.1 ± 5.3 | 4.9 ± 5.3 | 6.5 ± 6.4 | < 0.01 |
| Years on current therapy, n (%) | < 0.01 | |||
| ≥ 1 | 39 (43) | 283 (90) | 205 (92) | |
| < 1 | 51 (57) | 33 (10) | 19 (8) |
DMTs Disease-modifying therapies, EDSS Expanded Disability Status Scale, RRMS relapsing–remitting multiple sclerosis, SD standard deviation, SPMS secondary progressive multiple sclerosis
Substantial differences in characteristics were observed between patients receiving different regimens. Notably, OCR-treated patients tended to have more advanced disease than oral- and injectable-treated patients, with statistically significantly higher proportions of patients on later lines of therapy and having the secondary progressive subtype and an EDSS score of ≥ 3 at treatment initiation. OCR-treated patients also had a shorter duration of current therapy and were slightly older than oral- and injectable-treated patients. Significant differences in sex and education were not observed between groups (Table 1).
IPTW reduced differences between groups. Baseline characteristics of the propensity-weighted full WPAI-respondent and employed subgroup pseudo-populations are shown in Table 2 and ESM Table S2, respectively.
Table 2.
Baseline demographic and clinical characteristics of the propensity-weighted full Work Productivity and Activity Impairment questionnaire-respondent pseudo-population
| Baseline demographic and clinical characteristics | Ocrelizumab (n = 445) | Oral DMTs (n = 394) | p value | Ocrelizumab (n = 356) | Injectable DMTs (n = 282) | p value |
|---|---|---|---|---|---|---|
| Age, years (mean ± SD) | 41 ± 25 | 41 ± 12 | 0.87 | 42 ± 23 | 43 ± 13 | 0.35 |
| Female sex, n (%) | 267 (60) | 244 (62) | 0.86 | 206 (58) | 176 (62) | 0.73 |
| Education, n (%) | 0.52 | 0.86 | ||||
| Greater than high school | 375 (84) | 312 (79) | 296 (83) | 230 (81) | ||
| High school or less | 70 (16) | 82 (21) | 60 (17) | 52 (19) | ||
| Line of therapy, n (%) | 0.77 | 0.87 | ||||
| ≥ 2 | 218 (49) | 205 (52) | 139 (39) | 105 (37) | ||
| 0–1 | 227 (51) | 189 (48) | 217 (61) | 177 (63) | ||
| Disease subtype, n (%) | 0.89 | 0.78 | ||||
| RRMS | 420 (94) | 374 (95) | 334 (94) | 262 (93) | ||
| SPMS | 25 (6) | 20 (5) | 22 (6) | 20 (7) | ||
| EDSS score at treatment initiation, n (%) | 0.79 | 0.84 | ||||
| ≥ 3 | 83 (19) | 79 (20) | 73 (20) | 54 (19) | ||
| 0–2 | 362 (81) | 315 (80) | 283 (80) | 228 (81) | ||
| Years since diagnosis (mean ± SD) | 4.4 ± 11 | 5.1 ± 5.8 | 0.25 | 4.3 ± 12 | 6.4 ± 6.8 | < 0.01 |
| Years on current therapy, n (%) | 0.96 | 0.39 | ||||
| ≥ 1 | 365 (82) | 322 (82) | 292 (82) | 246 (87) | ||
| < 1 | 80 (18) | 72 (18) | 64 (18) | 26 (13) |
EDSS Expanded Disability Status Scale, RRMS relapsing–remitting multiple sclerosis, SPMS secondary progressive multiple sclerosis
WPAI Subscales
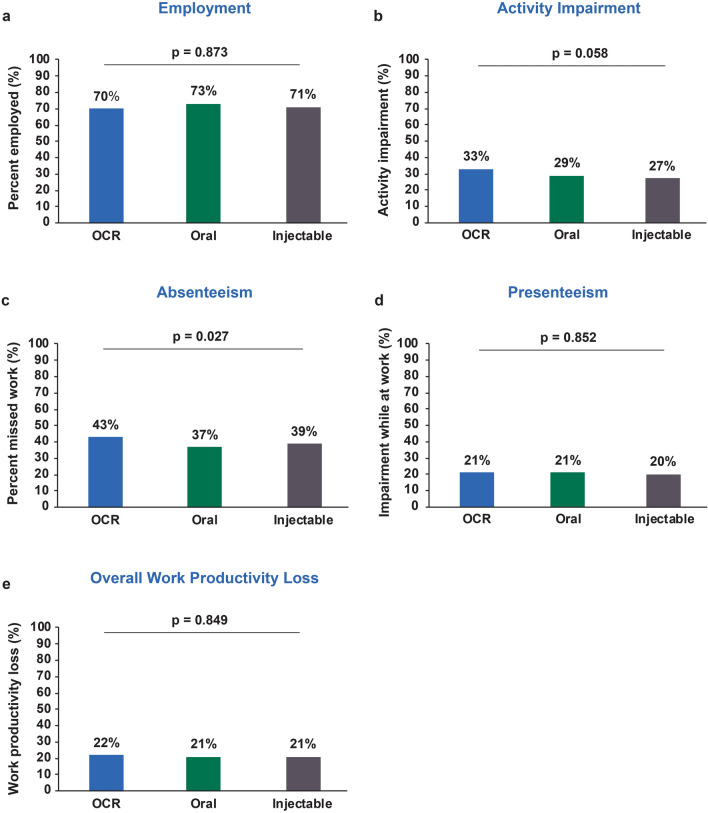
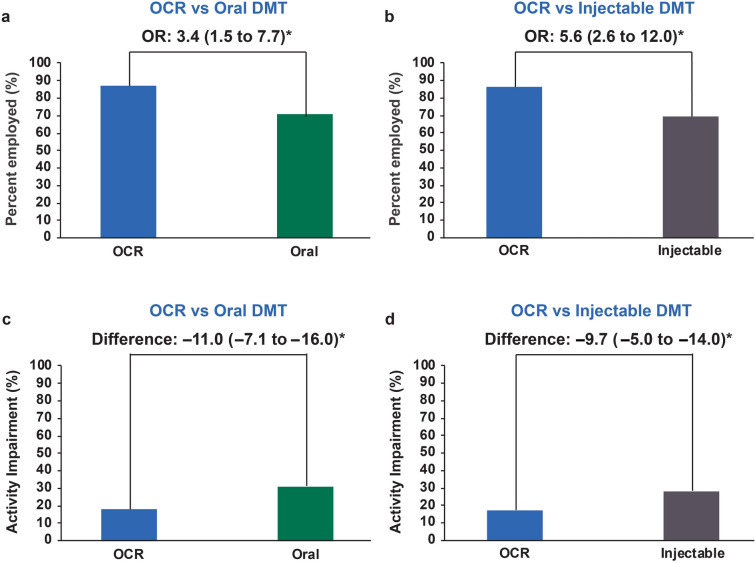
Unadjusted outcomes are shown in Fig. 1. Following multivariate adjustment, compared with oral- and injectable-treated patients, OCR-treated patients in the propensity-weighted full population had significantly higher odds of employment {odds ratio (OR) vs. oral DMT 3.4 [95% confidence interval (CI) 1.5–7.7]; OR vs. injectable DMT 5.6 (95% CI 2.6–12.0)} and lower activity impairment scores [difference vs. oral DMT − 11.0% (95% CI − 7.1 to − 16.0%); difference vs. injectable DMT − 9.7% (95% CI − 5.0 to − 14.0%)] (Fig. 2).
Fig. 1.
Unadjusted outcome data (employment and Work Productivity and Activity Impairment questionnaire domains) for the full population (a, b) and employed subgroup (c–e). OCR Ocrelizumab
Fig. 2.
Full Work Productivity and Activity Impairment questionnaire-respondent cohort results. Bars represent propensity-weighted population proportions (employment) and means (activity impairment). a, b Employment results with OCR vs. oral DMT (a) and OCR vs. injectable DMT (b). c, d Activity impairment results with OCR vs. oral DMT (c) and OCR vs. injectable DMT (d). Data are presented with 95% confidence intervals in parentheses. Asterisk indicates statistically significant difference at α = 0.05. DMT Disease-modifying therapy, OCR ocrelizumab, OR odds ratio
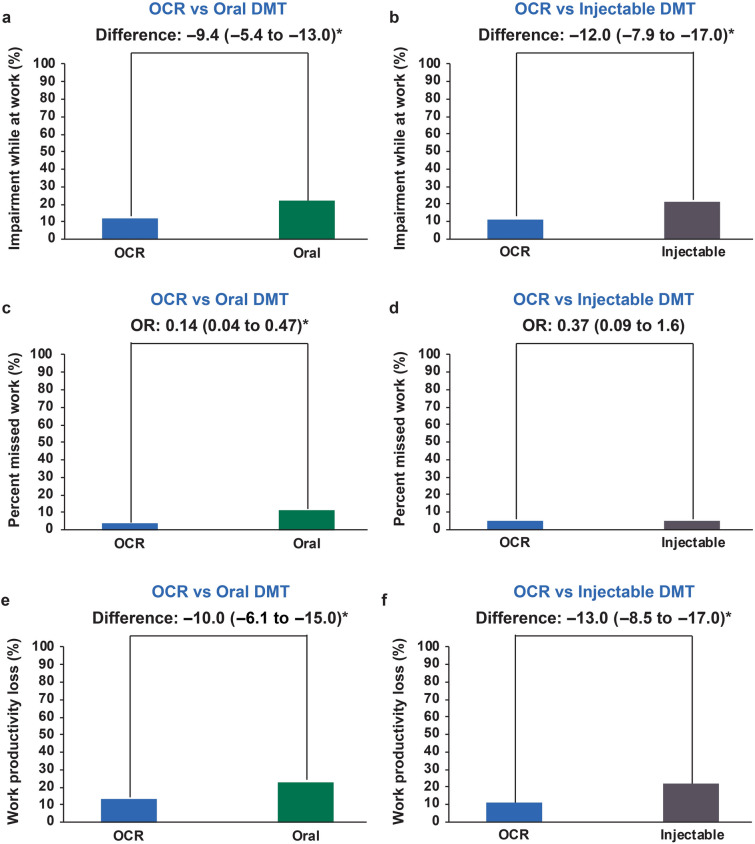
In the propensity-weighted employed subgroup, OCR-treated patients had significantly lower presenteeism scores [difference vs. oral − 9.4% (95% CI − 5.4 to − 13.0%); difference vs. injectable − 12.0% (95% CI − 7.9 to − 17.0%)] and overall work productivity loss scores [difference vs. oral − 10.0% (95% CI − 6.1 to − 15.0%); difference vs. injectable − 13.0% (95% CI − 8.5 to − 17.0%)] than oral- and injectable-treated patients (Fig. 3).
Fig. 3.
Employed subgroup results. Bars represent propensity-weighted population proportions (absenteeism) and means (presenteeism, overall work productivity loss). a, b Presenteeism results with OCR vs. oral DMT (a) and OCR vs. injectable DMT (b). c, d Absenteeism results with OCR vs. oral DMT (c) and OCR vs. injectable DMT (d). e, f Overall work productivity loss results with OCR vs. oral. DMT disease-modifying therapy, OCR ocrelizumab, OR odds ratio
OCR-treated patients in the propensity-weighted employed subgroup also had lower odds of missing any work [OR vs. oral DMT 0.14 (95% CI 0.04–0.47); OR vs. injectable DMT 0.37 (95% CI 0.09–1.6)] than oral- and injectable-treated patients; however, the comparison with injectable-treated patients was not statistically significant (Fig. 3). Similar results were observed in all sensitivity analyses (data not shown). Results of the subgroup analysis conducted on patients who were on therapy for ≤ 2 years are displayed in ESM Table S3.
Discussion
We found that OCR treatment was associated with improved scores across all domains of the WPAI instrument in a real-world setting. OCR-treated patients had higher odds of being employed and lower levels of activity impairment. Among employed patients in our sample, OCR-treated patients had lower overall work productivity loss, impairment at work (presenteeism) and absence from work (absenteeism), although the difference in absenteeism between OCR- and injectable-treated patients was not statistically significant.
This is the first evaluation of work productivity outcomes in OCR-treated patients. However, previous analyses also found that higher-efficacy DMTs improved work productivity outcomes compared with lower-efficacy DMTs. One longitudinal observational study in 874 patients in Australia found that those who used fingolimod and natalizumab were two- to threefold more likely to report improvements in amount of work, work attendance and work productivity than patients who used injectable DMTs (β-interferons and glatiramer acetate) [34]. Another analysis used data from 160 Adelphi Real World DSP for MS Wave 4 (2015) patients treated with dimethyl fumarate or injectable therapy to assess WPAI outcomes. In this analysis, patients receiving dimethyl fumarate had a mean difference in activity impairment, presenteeism and overall work impairment of − 17% (95% CI − 11 to − 24%), − 13% (95% CI − 7 to − 19%) and − 14% (95% CI − 8 to − 20%), respectively, compared with patients treated with injectable therapy [33]. We found similar results in comparing OCR-treated patients with injectable-treated patients; however, the average treatment effects identified in our analysis are not directly comparable with those of these previous studies as we used slightly different models. Additionally, our analysis included more recently approved injectable therapies as well as patients with SPMS.
Notably, prior to multivariate adjustment, statistically significant differences in outcomes were not observed between cohorts [with the exception of absenteeism where 43, 37 and 39% (p = 0.027) of OCR-, oral- and injectable-treated patients had missed any work time due to their MS, respectively]. OCR is a high-efficacy therapy that has been approved more recently than the DMTs analyzed in the comparator groups; therefore, sicker patients, or those not reaching treatment goals, and their providers may have anticipated its approval to switch to the newer DMT. We observed this in the data, finding that OCR-treated patients were slightly older and had more advanced disease, later lines of therapy and shorter duration of current therapy compared with the comparator cohorts. In our models, we chose to control for EDSS score at treatment initiation rather than current EDSS score. Although current EDSS score may be associated with current work productivity outcomes, treatment selection would be expected to affect current EDSS score. Therefore, adjusting for current EDSS score would preclude quantifying the association between treatment selection and our outcomes. After adjusting for key differences between the groups, we observed important benefits associated with OCR therapy.
Duration of therapy has implications not only for the effectiveness of treatment, but also the time since EDSS evaluation at treatment initiation. Due to the large discrepancy in this variable, wherein OCR-treated patients had a shorter duration of therapy than the patient cohorts treated with oral and injectable DMTs, respectively, in addition to adjusting for this variable in our models, we conducted a subgroup analysis among patients who were on therapy for between 6 months and 2 years. Results from the subgroup analysis on the 48% of patients who were on therapy for ≤ 2 years (OCR, n = 84; oral DMT, n = 144; injectable DMT, n = 72) were consistent in terms of directional effect and statistical significance comparing OCR with oral and injectable therapies. This result suggests that our base case analysis was not heavily biased by cohort differences in duration of therapy.
Indirect work productivity costs represent a substantial burden to patients with MS and their employers. In a systematic review, Kigozi and colleagues found that presenteeism costs comprised an average of 52% (range 19–85%) of total costs for interventions or disease conditions among a wide range of diseases [35]. The results from our analysis indicate that patients with RRMS or SPMS may have lower presenteeism with OCR therapy, which could therefore be associated with reduced indirect costs and may be a particularly important outcome for patients eligible for employment.
In addition to indirect costs, work impairment has previously been demonstrated to be associated with a variety of negative outcomes. Nicholas and colleagues found that among patients with RRMS, greater work impairment was associated with a higher comorbidity burden and poorer quality of life as measured by the Short Form-36 Physical and Mental Component Summaries and the EuroQol EQ-5D [14]. In addition, Lee et al. found that improvements in activity impairment and work productivity were associated with improved health-related quality of life as measured by two independently validated instruments (EQ-5D and the Hamburg Quality of Life Questionnaire in Multiple Sclerosis) [33].
Some strengths of our analysis included our ability to incorporate information from our full eligible population due to the statistical method used, robustness to sensitivity analyses and the up-to-date, real-world and representative nature of the data; however, there were limitations. First, the data we used were cross-sectional survey data, which can introduce recall or nonresponse bias in the overall population; however, we would expect this to be non-differential between groups, and we would not expect recall bias to be substantial over the short 7-day lookback period. Because the data were cross-sectional, we were also not able to evaluate longitudinal outcomes, such as changes in work productivity over time, and the results of this analysis may be better explained in the context of association, rather than causality. Second, WPAI outcomes for each patient were assessed over a specific 7-day period, which may not represent a patient’s average level of impairment since initiating treatment. Additionally, although we did require a minimum 6 months of current therapy, it is not clear how much time would be sufficient to appreciably impact activity- and employment-related outcomes. A longer minimum duration of therapy could have a greater impact on these outcomes, and future analyses should investigate this possibility. Third, some variables that may affect outcomes, such as income and insurance type, were not sufficiently captured for all patients in the database, potentially resulting in unmeasured confounding. However, many DMTs are associated with high product costs, and it is not clear how these variables would impact treatment selection, as DMTs are widely covered by insurance. Additionally, geographic variables, such as region or population density, were not available; these variables could be important considerations for treatment patterns or employment as well as provide information regarding the generalizability of the sample. Finally, due to the limited number of DMT-infused patients in the database, we were not able to include patients receiving other infused DMTs as a comparator group.
Conclusion
In conclusion, our analysis demonstrates that patients on OCR treatment for ≥ 6 months experienced lower impacts on their employment, activity and work productivity outcomes compared with those on oral or injectable therapy. Our analysis of patients with RMS or SPMS, which is representative of current clinical practice in a real-world setting, suggests that OCR could be considered as a therapy option to minimize productivity burden and work force impact, particularly in light of the significant impact that MS has on young adult patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This research and Rapid Service Fee were funded by Genentech, Inc., South San Francisco, CA, USA.
Medical Writing and/or Editorial assistance
Editorial assistance for this manuscript was provided by Liz LaFlamme, PhD, of Health Interactions, USA, and funded by Genentech, Inc., South San Francisco, CA, USA.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by all authors. The first draft of the manuscript was written by Edward E. Neuberger and Natalie J. Engmann, and all authors provided feedback on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Prior Presentation
This manuscript includes some findings that were previously presented at the International Society for Pharmacoeconomics and Outcomes Research Virtual 2020 meeting (Abstract PND122).
Disclosures
Edward E. Neuberger, Natalie J. Engmann, and Ibrahim M. Abbass are employees and shareholders of Genentech, Inc. Eddie Jones is an employee of Adelphi Real World.
Compliance with Ethics Guidelines
This analysis did not require ethics committee approval, as confirmed by the data provider (Adelphi Real World) and Western Institutional Review Board, as it was based on previously conducted studies, data were de-identified, and it did not involve any new studies of human or animal subjects performed by any of the authors. The research was conducted in compliance with the US Health Insurance Portability and Accountability Act 1996.
Data Availability
The data that support the findings of this study are available from Adelphi Real World but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. However, data are available from the authors upon reasonable request and with permission of Adelphi Real World.
References
- 1.Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029–e1040. doi: 10.1212/WNL.0000000000007035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filippi M, Bar-Or A, Piehl F, et al. Multiple sclerosis. Nat Rev Dis Primers. 2018;4(1):43. doi: 10.1038/s41572-018-0041-4. [DOI] [PubMed] [Google Scholar]
- 3.Johansson S, Ytterberg C, Claesson IM, et al. High concurrent presence of disability in multiple sclerosis. Associations with perceived health. J Neurol. 2007;254(6):767–773. doi: 10.1007/s00415-006-0431-5. [DOI] [PubMed] [Google Scholar]
- 4.Hirst C, Ingram G, Pickersgill T, Swingler R, Compston DA, Robertson NP. Increasing prevalence and incidence of multiple sclerosis in South East Wales. J Neurol Neurosurg Psychiatry. 2009;80(4):386–391. doi: 10.1136/jnnp.2008.144667. [DOI] [PubMed] [Google Scholar]
- 5.Conradsson D, Ytterberg C, von Koch L, Johansson S. Changes in disability in people with multiple sclerosis: a 10-year prospective study. J Neurol. 2018;265(1):119–126. doi: 10.1007/s00415-017-8676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brownlee WJ, Hardy TA, Fazekas F, Miller DH. Diagnosis of multiple sclerosis: progress and challenges. Lancet. 2017;389(10076):1336–1346. doi: 10.1016/S0140-6736(16)30959-X. [DOI] [PubMed] [Google Scholar]
- 8.Povolo CA, Blair M, Mehta S, Rosehart H, Morrow SA. Predictors of vocational status among persons with multiple sclerosis. Mult Scler Relat Disord. 2019;36:101411. doi: 10.1016/j.msard.2019.101411. [DOI] [PubMed] [Google Scholar]
- 9.Simmons RD, Tribe KL, McDonald EA. Living with multiple sclerosis: longitudinal changes in employment and the importance of symptom management. J Neurol. 2010;257(6):926–936. doi: 10.1007/s00415-009-5441-7. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Taylor B, Palmer AJ, et al. Estimating MS-related work productivity loss and factors associated with work productivity loss in a representative Australian sample of people with multiple sclerosis. Mult Scler. 2019;25(7):994–1004. doi: 10.1177/1352458518781971. [DOI] [PubMed] [Google Scholar]
- 11.Glanz BI, Dégano IR, Rintell DJ, Chitnis T, Weiner HL, Healy BC. Work productivity in relapsing multiple sclerosis: associations with disability, depression, fatigue, anxiety, cognition, and health-related quality of life. Value Health. 2012;15(8):1029–1035. doi: 10.1016/j.jval.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Landfeldt E, Castelo-Branco A, Svedbom A, Löfroth E, Kavaliunas A, Hillert J. Sick leave and disability pension before and after diagnosis of multiple sclerosis. Mult Scler. 2016;22(14):1859–1866. doi: 10.1177/1352458516667567. [DOI] [PubMed] [Google Scholar]
- 13.Castelo-Branco A, Landfeldt E, Svedbom A, Löfroth E, Kavaliunas A, Hillert J. Clinical course of multiple sclerosis and labour-force absenteeism: a longitudinal population-based study. Eur J Neurol. 2019;26(4):603–609. doi: 10.1111/ene.13863. [DOI] [PubMed] [Google Scholar]
- 14.Nicholas JA, Electricwala B, Lee LK, Johnson KM. Burden of relapsing-remitting multiple sclerosis on workers in the US: a cross-sectional analysis of survey data. BMC Neurol. 2019;19(1):258. doi: 10.1186/s12883-019-1495-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adelman G, Rane SG, Villa KF. The cost burden of multiple sclerosis in the United States: a systematic review of the literature. J Med Econ. 2013;16(5):639–647. doi: 10.3111/13696998.2013.778268. [DOI] [PubMed] [Google Scholar]
- 16.García-Domínguez JM, Maurino J, Martínez-Ginés ML, et al. Economic burden of multiple sclerosis in a population with low physical disability. BMC Public Health. 2019;19(1):609. doi: 10.1186/s12889-019-6907-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renner A, Baetge SJ, Filser M, Penner IK. Working ability in individuals with different disease courses of multiple sclerosis: factors beyond physical impairment. Mult Scler Relat Disord. 2020;46:102559. doi: 10.1016/j.msard.2020.102559. [DOI] [PubMed] [Google Scholar]
- 18.Marck CH, Aitken Z, Simpson S, Jr., Weiland TJ, Kavanagh A, Jelinek GA. Predictors of change in employment status and associations with quality of life: a prospective international study of people with multiple sclerosis. J Occup Rehabil. 2020;30(1):105–114. doi: 10.1007/s10926-019-09850-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorstyn DS, Roberts RM, Murphy G, Haub R. Employment and multiple sclerosis: a meta-analytic review of psychological correlates. J Health Psychol. 2019;24(1):38–51. doi: 10.1177/1359105317691587. [DOI] [PubMed] [Google Scholar]
- 20.Johnson KL, Yorkston KM, Klasner ER, Kuehn CM, Johnson E, Amtmann D. The cost and benefits of employment: a qualitative study of experiences of persons with multiple sclerosis. Arch Phys Med Rehabil. 2004;85(2):201–209. doi: 10.1016/S0003-9993(03)00614-2. [DOI] [PubMed] [Google Scholar]
- 21.Rumrill P, Li J, Strauser D, et al. Personal, health and function, and career maintenance factors as determinants of quality of life among employed people with multiple sclerosis. Work. 2020;67(1):81–94. doi: 10.3233/WOR-203254. [DOI] [PubMed] [Google Scholar]
- 22.National MS Society. Medications. https://www.nationalmssociety.org/Treating-MS/Medications. Accessed 23 June 2020.
- 23.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 24.Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- 25.Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real-world physician and patient behaviour across countries: disease-specific programmes—a means to understand. Curr Med Res Opin. 2008;24(11):3063–3072. doi: 10.1185/03007990802457040. [DOI] [PubMed] [Google Scholar]
- 26.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 27.Reilly MC, Bracco A, Ricci JF, Santoro J, Stevens T. The validity and accuracy of the Work Productivity and Activity Impairment questionnaire–irritable bowel syndrome version (WPAI: IBS) Aliment Pharmacol Ther. 2004;20(4):459–467. doi: 10.1111/j.1365-2036.2004.02091.x. [DOI] [PubMed] [Google Scholar]
- 28.Reilly MC, Tanner A, Meltzer EO. Work, classroom and activity impairment instruments. Clin Drug Investig. 1996;11(5):278–288. doi: 10.2165/00044011-199611050-00004. [DOI] [Google Scholar]
- 29.Reilly MC, Lavin PT, Kahler KH, Pariser DM. Validation of the Dermatology Life Quality Index and the Work Productivity and Activity Impairment–Chronic Hand Dermatitis questionnaire in chronic hand dermatitis. J Am Acad Dermatol. 2003;48(1):128–130. doi: 10.1067/mjd.2003.128. [DOI] [PubMed] [Google Scholar]
- 30.Wahlqvist P, Carlsson J, Stålhammar NO, Wiklund I. Validity of a Work Productivity and Activity Impairment questionnaire for patients with symptoms of gastro-esophageal reflux disease (WPAI-GERD)—results from a cross-sectional study. Value Health. 2002;5(2):106–113. doi: 10.1046/j.1524-4733.2002.52101.x. [DOI] [PubMed] [Google Scholar]
- 31.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS) Neurology. 1983;33(11):1444–1452. doi: 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 32.Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23(19):2937–2960. doi: 10.1002/sim.1903. [DOI] [PubMed] [Google Scholar]
- 33.Lee A, Pike J, Edwards MR, Petrillo J, Waller J, Jones E. Quantifying the benefits of dimethyl fumarate over β interferon and glatiramer acetate therapies on work productivity outcomes in MS patients. Neurol Ther. 2017;6(1):79–90. doi: 10.1007/s40120-016-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Taylor BV, Blizzard L, Simpson S, Jr, Palmer AJ, van der Mei IAF. Effects of multiple sclerosis disease-modifying therapies on employment measures using patient-reported data. J Neurol Neurosurg Psychiatry. 2018;89(11):1200–1207. doi: 10.1136/jnnp-2018-318228. [DOI] [PubMed] [Google Scholar]
- 35.Kigozi J, Jowett S, Lewis M, Barton P, Coast J. The estimation and inclusion of presenteeism costs in applied economic evaluation: a systematic review. Value Health. 2017;20(3):496–506. doi: 10.1016/j.jval.2016.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from Adelphi Real World but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. However, data are available from the authors upon reasonable request and with permission of Adelphi Real World.