Abstract
The present investigation aimed to improve callus biomass, polyphenolic content, biosynthesis of mangiferin and biological potential following application of different elicitor treatments for medicinally important Salacia chinensis L. The leaf-derived callus cultures were established on Murashige and Skoog’s (MS) medium supplemented with 2,4-dichlorophenoxyacetic acid (2,4-D: 2.0 mg/l) and 6-benzylaminopurine (BAP: 1.5 mg/l). These cultures were treated with different elicitors viz. jasmonic acid (JA), methyl jasmonate (MeJA) and yeast extracts (YE). The highest calli biomass (five-fold increase within 4 weeks) was achieved when callus was treated with JA (75 µM). The callus obtained on MS medium supplemented with 2,4-D (2.0 mg/l), BAP (1.5 mg/l) and treated with JA (75 µM) displayed augmented values for total phenolics, flavonoids and mangiferin contents. Besides, same treatment elicits the calli for antioxidant properties as evaluated by 2,2-diphenyl-2-picrylhydrazyl (DPPH), ferric-reducing antioxidant power (FRAP) and metal chelating assays. This is the first report on the elicitation study in genus Salacia and, therefore, the discoveries suggested that, S. chinensis calli might be a perfect source for large-scale production of industrially important secondary metabolites. Concurrently data provide accumulated information demonstrating its prominent antioxidant effect revealing its potential without disturbing natural resources.
Keywords: Antioxidant, Callus, Elicitation, Flavonoids, Mangiferin, Phenolics, Salacia chinensis
Introduction
Over-developing interest of bioactive compounds from natural resources has enforced the implication of diverse biotechnological tools for sustainable utilization of medicinally important plants (Salma et al. 2018). Plant tissue culture is among the attainable biotechnological strategies which offer enormous scope for production of plantlets, conservation, restoration as well as the use of in vitro cultures for production of bioactive metabolites under controlled conditions, independent of seasonal and topographical conditions (Smetanska 2008; Chavan et al. 2018). One of the important advantage of in vitro cultures is one can achieve the differential accumulation and biosynthesis of natural products using elicitors and precursors. For quite few decades, plant cell cultures were utilized for synthesis of a few industrially significant secondary metabolites (Rao and Ravishankar 2002; Espinosa-Leal et al. 2018). Additionally, exploiting cell culture elicitation offers a striking elective way to reinforce yield, defeating the constrained accessibility and scaling-up of biologically active and medicinally significant metabolites in several in vitro cultures (Zafar et al. 2017; Ahmad et al. 2019; Khan et al. 2019).
Genus Salacia (family—Celastraceae) comprises over 200 woody lianas, shrubs or small trees. The genus is having multi-potent species which are used in food and pharmaceutical industries. Salacia chinensis L. is commonly known as Saptarangi is among the foremost-investigated medicinal plants and convenient source of mangiferin, salacinol and kotalanol, which are known for their strong antidiabetic, anticancerous and anti-HIV properties (Yoshikawa et al. 2001; Thuan 2005; Silpraist et al. 2011; Chavan et al. 2015b). More recently, mangiferin has been effectively used for preventing neurodegeneration in Alzheimer's and Parkinson's disease (Feng et al. 2019). Besides, the plant is chief source of several number of metabolites belonging to diverse groups viz. polyphenols, alkaloids, glycosides, anthocyanidins, quinones, friedo-oleanones, terpenoids, coumarins, steroids, saponins, tannins, gums and mucilage (Majid et al. 2016a; Ghadage et al. 2017). The commercial utilization of Salacia species into Asian and European Countries as packing’s of root concentrates and tablets in the late 1990s provides the stage for its familiarization into the international pharmaceutical market. At present, the global exchange of Salacia species has developed into a multimillion-dollar industry (Dubey et al. 2011). Unavoidably, the developing worldwide interest for Salacia species (including S. chinensis) crude materials came about in over-abuse of its wild populaces in Asian Countries (Chavan et al. 2015a).
Despite the fact that, Salacia species contains several numbers of biologically active compounds, the utility of plant tissue culture techniques are restricted to the development of micropropagation conventions for their conservation and that excessively confined with S. chinensis, S. reticulata and S. oblonga (Dhanasri et al. 2013; Deepak et al. 2015; Majid et al. 2016b; Laxmi et al. 2018). However, in previous report (Chavan et al. 2015a), we have effectively developed an in vitro propagation system along with elevated accumulation of mangiferin during various regeneration stages of S. chinensis. More recently, Bagnazari et al. (2018) assessed the antioxidant and antidiabetic potential of aerial parts of in vitro regenerated plantlets of S. chinensis. Till date, no report has been published on elicitor-treated increase in biomass, industrially important biomolecules with in vitro pharmacological properties in callus cultures of genus Salacia.
Circumstantial literature motivate us to explore whether elicitor-treated undifferentiated callus cultures of S. chinensis can upgrade the production of biomass, metabolites and biological properties, hence following objectives were focused during the current investigation: (i) optimization of elicitor treatment for extensively growing callus cultures; (ii) appraisal of the total content of phenolics and flavonoids, (iii) evaluation of mangiferin accumulation using RP-HPLC and (iv) characterizing callus extract (CE) for its antioxidant capacity using 2,2-diphenyl-2-picrylhydrazyl, ferric-reducing antioxidant power and metal chelating assay.
Materials and methods
Plant material
Samples of S. chinensis were collected from Amboli locality (15° 58′ 05.6″ N; 73° 59′ 48.7″ E; altitude: 739 m) of the Northern Western Ghats, India. The authentically identified specimen was at Herbarium, Department of Botany, Shivaji University, Kolhapur (Voch. No. JC/SC/04).
Chemicals, reagent and analytical instruments
Phenolic acid (gallic acid), flavonoid (quercetin), 2,2,-diphenyl-1-picrylhydrazyl (DPPH), 2,4,6-tris (2-pyridyl)-s-triazine (TPTZ), mangiferin (99% pure), jasmonic acid (JA) and methyl jasmonate (MeJA) were procured from Sigma-Aldrich (St. Louis, MO, USA). Yeast extract were procured from Merck, India. N,N-Dimethyl formamide (DMF) and methanol (MeOH) were of HPLC grade (Spectrochem, India). The chemicals used for tissue culture experiments were from Himedia, India and Qualigens, India. The hot air oven utilized in this study was from Thermo Scientific, Germany. The optical density measurements for various experiments including polyphenolics and antioxidant capacity were recorded utilizing UV–visible spectrophotometer (Shimadzu UV1800, Japan). Waters chromatographic system (Model no. HPLC-W6590, Waters, Milford, USA) with dual UV absorbance detector (W-2487) was used for quantitation of mangiferin content.
Establishment and maintenance of callus cultures
The callus cultures were initiated by inoculating healthy leaf pieces of S. chinensis as portrayed by Chavan et al. (2015a). The leaves washed under running tap water were kept in labogent solution (0.5%, v/v) for 5 min, treated with HgCl2 (0.1%) for 3 min and washed thrice with sterile water. The surface disinfected leaf pieces were transferred aseptically on MS medium (Murashige and Skoog 1962) fortified with 2,4-D (2.0 mg/l) and BAP (1.5 mg/l). The media was enriched with sucrose (30 g/l), solidified with 2.0 mg/l ClariGel and autoclaved at 121 °C for 20 min after adjusting the pH to 5.8. The cultures were maintained at 25 ± 5 °C with provision of light irradiance (40 µmol/m/s) for 16 h photoperiod.
Preparation and treatment of elicitors
The stock solutions of elicitor viz. jasmonic acid and methyl jasmonate were prepared in 96% ethanol and filter-sterilized through 0.45 μM Millipore filter (Minisart, Germany). Various concentrations of JA (25–125 µM) and MeJA (50–250 µM) were added to MS medium after sterilization containing a mixture of 2,4-D (2.0 mg/l) and BAP (1.5 mg/l). Yeast extract was added to culture medium with concentration ranging from 100 to 500 mg/l. Range of the elicitor treatments were chosen based on the preliminary experiments.
Growth kinetics and biomass production
Callus from exponential developmental stage (20th day) was harvested and inoculated individually either on control medium [MS + 2,4D (2.0 mg/l) + BAP (1.5 mg/l)] or alongside elicitor treatments. The cultures were incubated at 25 ± 5 °C with provision of light irradiance (40 µmol/m/s) for 16 h photoperiod. Increase in callus biomass in response to varied elicitor treatments were measured as fresh (FW) and dry (DW) weights after 30 days of incubation. Calli were carefully taken out from culture vessels, washed gently with sterile water, pressed softly on paper to the excess water and weighed for fresh weight. The calli harvested were oven dried at 45 °C for 24 h to determine dry weight (DW). However, callus proliferation frequency (CPF) was calculated using following formula,
Extract preparation for polyphenols and antioxidant assays
Extract preparation was carried out using the previously described method for S. chinensis by Chavan et al. (2013) with little change. The calli harvested from 30 days of elicitor treatments were oven dried at 45 °C for 24 h were crushed to fine powder. One gram of dried callus powder was extracted with 100 ml of MeOH for 10 min. CE was prepared using steam bath-assisted extraction (SBAE) on stirred thermal water bath (Equitron, India) with consistent temperature (70 °C). The extracts were filtered through Whatman No. 1 filter paper and adjusted to 100 ml and were used for further analysis.
Determination of polyphenol content
Total phenolic content (TPC) was estimated spectrophotometrically using Folin–Ciocalteu method (Singleton and Rossi 1965). A mixture of CE (0.125 ml) and 1.8 ml of ten-fold diluted Folin–Ciocalteu reagent were permitted to react at 25 °C for 6 min. After addition of 15% Na2CO3 (1.2 ml), the reaction mixture was kept at room temperature for 90 min and the readings were recorded at 765 nm.
Total flavonoid content (TFC) was determined using aluminium chloride calorimetric method described by Chang et al. (2002) with little alteration. CE (0.5 ml), 10% AlCl3 (0.1 ml), MeOH (1.5 ml), 1 M potassium acetate (0.1 ml) and distilled water (2.8 ml) were vortex for 7 min. After 30 min incubation at room temperature, the absorbance of the mixture was measured at 416 nm. The results were compared with standard curves of gallic acid and quercetin for TPC and TFC, respectively, and were expressed as milligram equivalent per gram dry weight.
RP-HPLC analysis of mangiferin from elicitated callus cultures
Extraction and quantitative determination of mangiferin was carried out by following the previously described method for S. chinensis (Chavan et al. 2015b). One gram of callus powder was added to 100 ml of N,N-dimethyl formamide (DMF, 30%); suspension was mixed well and heated for 10 min of exposure period (SBAE). After cooling, final volume was adjusted to 100 ml with DMF. Extracts filtered through 0.45 mm nylon filter (Axiva) were transferred into an Agilent amber vial and stored at 4 °C until chromatographic analysis. 10 mg of standard mangiferin was dissolved in 50 ml of DMF (30%), warmed on steam bath for 10 min and cooled at room temperature. Final volume was adjusted to 100 ml with DMF (30%). The chromatographic system and conditions were maintained as per the previous report described for S. chinensis (Chavan et al. 2015b). The separation was completed on Waters C18 column (Princeton SPHER, 5 μ, 250 × 4.6 mm). Mobile phase comprising of “A” (0.2% triethylamine pH 4.0 with orthophosphoric acid) and “B” (acetonitrile) was used for separation with 89% “A” as to 11% “B” in an isocratic mode. The injection volume was 20 μl and the flow rate was maintained at 1 ml/min with detection wavelength of dual λ absorbance detector at 250 and 260 nm. The analysis was done for 10 min for standard as well as samples. The content of mangiferin was recorded as mg/g DW.
Determination of antioxidant potential of callus extracts
The antioxidant capacity of CE extract was evaluated using three different assays viz. 2,2-diphenyl-2-picrylhydrazyl (DPPH), ferric-reducing antioxidant power (FRAP) and metal chelating antioxidant power. The antioxidant capacity using DPPH assay of CE was measured using method developed by Brand-Williams et al. (1995). The stock solution of reagent prepared (24 mg of DPPH in 100 ml of MeOH) was stored at − 20 °C. 10 ml of reagent added to 45 ml of MeOH to obtain absorbance value of 1.1 ± 0.02 at 517 nm on UV–Vis Spectrophotometer. CE (100 µl) was mixed with DPPH solution (2.9 ml) and allowed to react for 30 min in dark at room temperature. The absorbance of resulting solution was recorded at 517 nm and the results were expressed as antioxidant capacity (%) using following formula,
where A is the absorbance.
The FRAP assay was performed according to the procedure described by Benzie and Strain (1996). The working FRAP reagent was formulated with a combination of 20 mM FeCl3·6H2O, 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) in 40 mM HCl and 300 mM acetate buffer (pH 3.6), in 1:1:10 ratio and mixture was heated in hot water bath for 10 min at 37 °C. CE (100 µl) was allowed to react with 2.9 ml of FRAP reagent in the 3.0 ml of reaction mixture. After 30 min incubation in dark, the absorbance of ferrous–tripyridyltriazine complex was measured at 593 nm and the FRAP values were determined as optical density readings.
Metal chelating antioxidant power assay was performed according to Dinis et al. (1994) with little modification. A reaction mixture was prepared with 2 mM FeCl3 (50 µl), deionized water (2750 µl) and CE (100 µl) and the reaction was initiated after addition of 5 mM ferrozine solution (100 µl). A mixture was allowed to stand for 20 min at room temperature, inhibition of ferrozine to Fe2+ complex was recorded at 562 nm and antioxidant potential was expressed using the formula,
where A is the absorbance.
Experimental design and data analysis
The callus culture experiments were conducted three times with 20 replicates per treatment and data were analyzed using one-way ANOVA. The significant differences among the means were assessed by Dunnett multiple comparison test. All phytochemical and biological potential determination experiments were repeated thrice. The data shown represents the mean ± standard error (SE) for three independent experiments. All statistical analysis was performed using GraphPad Instat (GraphPad Soft-ware, Inc, USA) and Microsoft Excel (Microsoft Co. ltd.)
Results and discussion
Callus induction and biomass accumulation
The well-growing callus cultures were achieved from young and healthy leaf explants (sterilized with 0.1% HgCl2 for 3 min) of S. chinensis on MS medium with 2,4-D (2.0 mg/l) and BAP (1.5 mg/l) as described by Chavan et al. (2015a). Noticeably, the calli showed three distinctive coloring patterns (pale-yellow–brown–black) all through the 30 days’ incubation time (Fig. 1A–C). As per the previous reports from same laboratory, 30 days’ incubation period was found optimal for obtaining higher values for calli biomass in S. chinensis (Chavan et al. 2015a). The blackening of calli might be due to the accumulation of polyphenols. However, blackening does not affect the callus proliferation rate. In contrast, Gao et al. (2020) reported heavy browning phenomenon hinders proliferation rate in callus cultures of Paeonia suffruticosa. However, they have identified browning linked genes through transcriptome sequencing which will help to reduce this phenomenon. In the present study, the calli harvested from 20th day of culture were transferred to different elicitor treatments by keeping steady hormonal supplementation. The biomass of callus was recorded as fresh and dry weight after a period of 30 days of cultures under the influence of various elicitor treatments (Table 1). The rate of callus growth and morphology was altered by the elicitor treatment. The calli pieces transferred on MS medium in presence of lower concentrations of JA was produced dark brown to blackish and friable calli; however, MeJA treatments yielded brown, compact and hard callus (Table 1). All calli lines were undifferentiated. The fresh and dry weight was significantly higher when calli were treated with JA followed by MeJA and YE. Among the treatments, the weights of the calli ranged from 1.92 ± 0.1 g to 8.10 ± 0.6 g FW and 0.13 ± 0.03 to 0.81 ± 0.03 g DW, respectively. This demonstrated the differential responses of calli of S. chinensis to various elicitor treatments. JA treatment (75 µM) heightened biomass accumulation (8.10 ± 0.6 g FW) in 95% of cultures with growing callus (Table 1, Fig. 2). The findings affirmed that, 4.97 and 4.7-fold enhancement in fresh and dry callus biomass, respectively, when calli were treated with JA (75 µM). Notably, comparable calli biomass production (3.95 ± 1.1 g FW) was observed when cultures were treated with MeJA (100 µM). The present outcomes confirmed the arousing proficiency of elicitors on triggering of cells to achieve higher growth than control cultures. Likewise, elicitor-dependent in vitro growth and development has been reported for medicinally important plants viz. Rosa hybrid (Ram et al. 2013) and Rauvolfia serpentine (Zafar et al. 2017).
Fig. 1.
Establishment of callus cultures of S. chinensis on MS medium fortified with 2,4,D (2.0 mg/l) and BAP (1.5 mg/l). A Pale-yellow callus, B brownish callus and C black-colored callus
Table 1.
Callus proliferation frequency (CPF) and biomass profile in response with elicitor treatments
| S. no. | Elicitor | Conc. | CPF (%) | Biomass (g) | Remark | |
|---|---|---|---|---|---|---|
| FW | DW | |||||
| 1 | Elicitor free | 90 | 1.63 ± 0.3 | 0.17 ± 0.01 | Light Brown | |
| 2 | Jasmonic acid (μM) | 25 | 90 | 3.30 ± 0.7* | 0.29 ± 0.03* | Dark brown, friable |
| 3 | 50 | 90 | 4.33 ± 1.0** | 0.38 ± 0.04** | Black, friable | |
| 4 | 75 | 95 | 8.10 ± 0.6** | 0.81 ± 0.03** | Black, semi-hard | |
| 5 | 100 | 90 | 5.03 ± 0.8** | 0.45 ± 0.07** | Black, semi-hard | |
| 6 | 125 | 85 | 2.81 ± 0.4* | 0.31 ± 0.03** | Black, compact | |
| 7 | Methyl jasmonate (μM) | 50 | 70 | 2.12 ± 0.8 ns | 0.28 ± 0.02* | Brown, compact |
| 8 | 100 | 70 | 3.95 ± 1.1** | 0.38 ± 0.03** | Brown, compact | |
| 9 | 150 | 75 | 3.29 ± 0.4* | 0.33 ± 0.02** | Brown, semi-hard | |
| 10 | 200 | 70 | 3.25 ± 0.4* | 0.21 ± 0.06ns | Brown, semi-hard | |
| 11 | 250 | 65 | 2.55 ± 0.3* | 0.13 ± 0.03ns | Brown, hard | |
| 12 | Yeast extract (mg/l) | 100 | 75 | 1.92 ± 0.1 ns | 0.24 ± 0.02ns | Yellowish, compact |
| 13 | 200 | 75 | 1.98 ± 0.9ns | 0.24 ± 0.05ns | Yellowish, watery | |
| 14 | 300 | 60 | 2.76 ± 0.5* | 0.29 ± 0.01* | Semi-green | |
| 15 | 400 | 65 | 2.06 ± 0.5ns | 0.25 ± 0.03* | Greenish, friable | |
| 16 | 500 | 65 | 2.30 ± 0.8* | 0.19 ± 0.07ns | Greenish, friable | |
Values are significantly different at ns—non significant, *P < 0.05 and **P < 0.01 level as compared by Dunnett multiple comparisons test
Fig. 2.
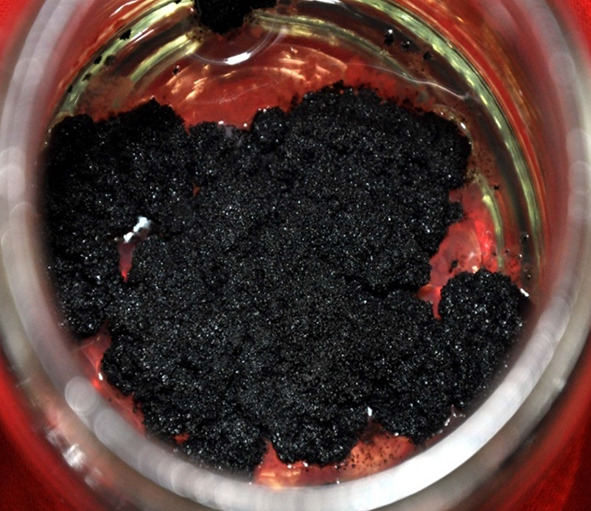
Growth and proliferation of callus on jasmonic acid (75 µM) treated cultures of S. chinensis
The callus cultured in presence of higher concentrations of MeJA (250 µM) demonstrated the reduced biomass productivity (FW: 2.55 ± 0.3 g, DW: 0.13 ± 0.03 g) in relation to the lower MeJA concentration. So also, among all the yeast extract treatments, no demonstrable decrease or increase in biomass has been observed as compared to control cultures (Table 1). Similarly, lower concentrations showed negligible response during callus induction and accumulation of secondary metabolites in Dregea volubilis (Yogananth et al. 2019). In contrast, Vijayalakshmi and Shourie (2019) recently reported the yeast extract triggered biomass accumulation in callus cultures of licorice. More recently, phytohormone regulated calli biomass production has been demonstrated for S. chinensis and S. macrosperma (Chavan et al. 2015a; Mahendra et al. 2020). However, the current investigation on elicitor treatment is crucial to meet fairly higher biomass production and therefore increment in accumulation of metabolites of interest in callus cultures of S. chinensis.
Polyphenolic profile
In the present study, the content for total phenolics and flavonoids was explored from dried callus samples treated with various elicitors for S. chinensis. The content of total phenolics and flavonoids ranged between 44.00 ± 1.34 to 68.49 ± 0.90 mg GAE/g DW and 8.89 ± 0.33 to 26.18 ± 0.35 mg QE/g DW, respectively (Table 2). The calli treated with JA (75 µM) demonstrated the most noteworthy content of phenolics (68.49 ± 0.90 mg GAE/g DW), which was 29% higher than the control (Table 2) and higher flavonoids content (26.18 ± 0.35 mg QE/g DW) in S. chinensis. Also, Astello-Garciaet et al. (2013) recommended the addition of jasmonic acid treatment for improved production of polyphenolic contents in callus cultures of Opuntia robusta. On contrary, yeast extract demonstrated the least impact on polyphenol accumulation in callus cultures of S. chinensis. The total contents of phenolics (44.00 ± 1.34 mg GAE/g DW) and flavonoids (8.89 ± 0.33 mg QE/g DW) were found minimal in calli treated with yeast extract (500 mg/l), which was beneath the levels recorded in control cultures (Table 2). The significant decrease in the flavonoid content was observed at the different concentrations of methyl jasmonate and yeast extract-treated callus culture extract. Similarly, the declined levels of flavonoid content in response to elicitor treatment were reported in cell suspension cultures of Phoenix dactylifera (Al-Khayri and Naik 2020). Literature survey suggests that there are various reports on elicitated production of polyphenolic compounds in callus cultures of medicinal plants (Khan et al. 2019); however, the present work is the first attempt on standardization of elicitor treatments for optimal metabolites production in callus cultures for genus Salacia.
Table 2.
Efficiency of elicitors on total phenolics and flavonoids content in callus cultures of S. chinensis
| S. no. | Elicitor | Conc. | TPC (mg GAE/g DW) | TFC (mg QE/g DW) |
|---|---|---|---|---|
| 1 | Elicitor free | 48.63 ± 1.80 | 20.18 ± 0.91 | |
| 2 | Jasmonic acid (μM) | 25 | 55.34 ± 0.72ns | 24.10 ± 1.00* |
| 3 | 50 | 62.00 ± 2.21* | 19.23 ± 0.30ns | |
| 4 | 75 | 68.49 ± 0.90** | 26.18 ± 0.35** | |
| 5 | 100 | 66.22 ± 1.11** | 20.13 ± 0.40ns | |
| 6 | 125 | 61.30 ± 0.90* | 16.76 ± 0.12ns | |
| 7 | Methyl jasmonate (μM) | 50 | 51.10 ± 0.94ns | 20.23 ± 1.20ns |
| 8 | 100 | 61.54 ± 0.34* | 19.68 ± 0.24ns | |
| 9 | 150 | 52.80 ± 0.12ns | 11.28 ± 1.10ns | |
| 10 | 200 | 52.11 ± 0.70ns | 18.36 ± 0.88ns | |
| 11 | 250 | 49.89 ± 1.00ns | 16.61 ± 0.60ns | |
| 12 | Yeast extract (mg/l) | 100 | 50.40 ± 1.88 ns | 12.35 ± 1.00ns |
| 13 | 200 | 51.96 ± 2.20ns | 18.90 ± 0.98ns | |
| 14 | 300 | 50.78 ± 2.00ns | 10.28 ± 0.44ns | |
| 15 | 400 | 44.23 ± 1.20ns | 12.30 ± 0.39ns | |
| 16 | 500 | 44.00 ± 1.34ns | 08.89 ± 0.33ns | |
Values are significantly different at ns—non significant, *P < 0.05 and **P < 0.01 level as compared by Dunnett multiple comparisons test
Mangiferin accumulation
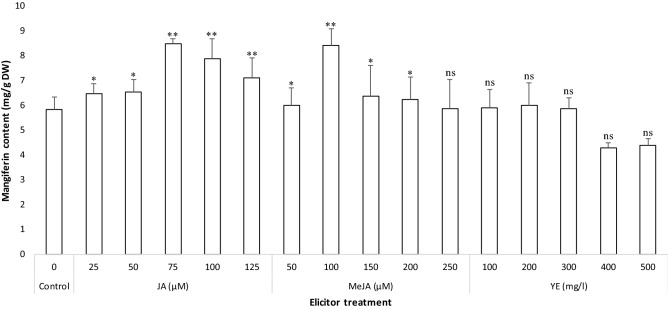
Mangiferin (a xanthone glucoside) is an active principal compound predominantly present in different organs of S. chinensis and known for broad spectrum of pharmacological properties including antidiabetic, anticancer and anti-HIV. In the current study, the quantification and accumulation of the mangiferin in the elicitor-treated callus extract is determined using RP-HPLC (Fig. 3A, B). Based on the RP-HPLC analysis, all samples were found to contain mangiferin; however, the change in the contents was observed according to the elicitor treatment (Fig. 4). The results of current study revealed that, JA (75 µM) supported the maximum calli biomass production along with elevated content of mangiferin. Similarly, Chavan et al. (2015a) reported the media composition which supports the higher biomass production also supported the higher accumulation of mangiferin in S. chinensis. MeJA also supported the mangiferin accumulation while the yeast extract was not effective in terms of increased mangiferin accumulation in callus cultures of S. chinensis. Eliciting the cultures with JA (75 µM) and MeJA (100 µM) triggered the mangiferin production with values of 8.493 ± 0.193 mg/g DW (Fig. 3B) and 8.439 ± 0.645 mg/g DW, respectively, which was in excess of about 1.5-fold than the un-elicited cultures (control). So also, various studies recommended the essentiality of adding elicitors in the callus cultures for the production of bioactive drugs (Sailo et al. 2018; Sarmadi et al. 2018; Ahmad et al. 2019; Rajan et al. 2020). The outcomes of current study showed that, the rise in biomass resulted in enhancing the biosynthesis and accumulation of mangiferin in callus cultures of S. chinensis. These results are in accordance with the biosynthesis of anticancer alkaloids (vincristine and vinblastine) in callus cultures of periwinkle (Mekky et al. 2018). The calli treated with yeast extract was able to produce minimal contents of mangiferin. Incidentally, these values were less than the amounts of mangiferin accumulated in un-elicited cultures (Fig. 4).
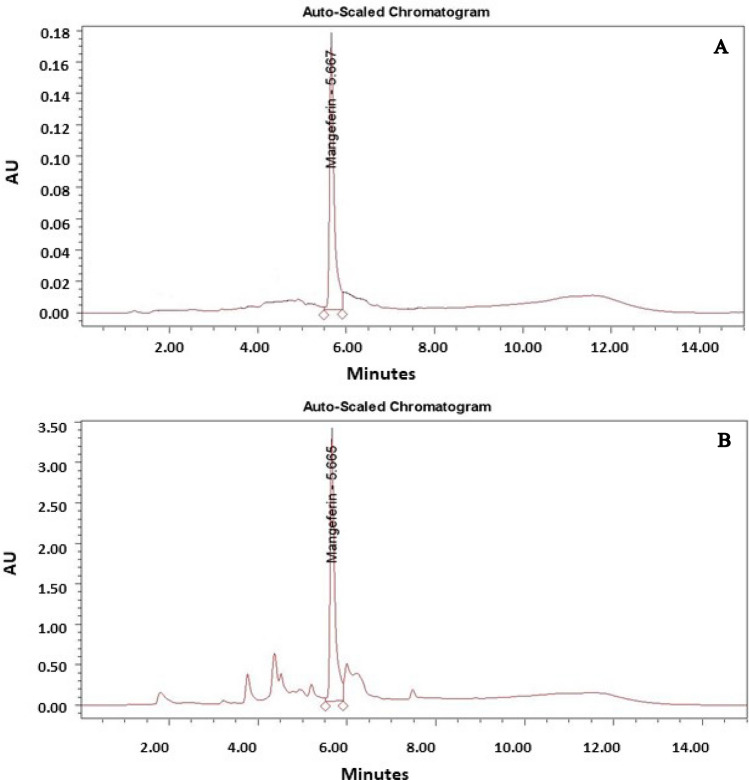
Fig. 3.
RP-HPLC chromatogram of mangiferin. A Standard mangiferin, B callus treated with JA (75 µM)
Fig. 4.
Mangiferin content estimated by RP-HPLC in jasmonic acid (75 µM) elicitated callus cultures of S. chinensis. Values are significantly different at ns—non significant, *P < 0.05 and **P < 0.01 level as compared by Dunnett multiple comparisons test
Antioxidant properties
In the current study, the existence of noteworthy amount of polyphenols and mangiferin recommend their conceivable contribution to antioxidant activity. However, it is complicated to accurately assess the antioxidant capacity of a system having various components using single test. As, each method changes as far as principle and environmental conditions; therefore, diverse reaction mechanisms are needed to authenticate antioxidant potential of the plant extracts (Jauhari et al. 2019). Hence, 2,2-diphenyl-2-picrylhydrazyl (DPPH), ferric-reducing antioxidant power (FRAP) and metal chelating mechanisms were used to assess the antioxidant potential of elicitor-treated calli extracts of S. chinensis. Antioxidant potential of all the elicitor-treated callus samples are given in Fig. 5A–C.
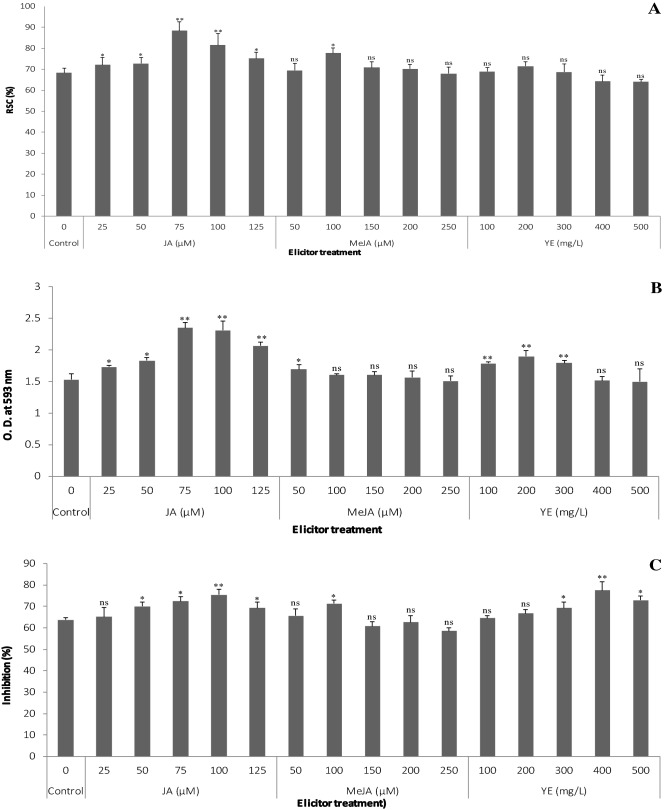
Fig. 5.
Antioxidant capacity of elicitor-treated callus cultures of S. chinensis. A DPPH assay, B FRAP assay and C metal chelating assay. Values are significantly different at ns—non significant, *P < 0.05 and **P < 0.01 level as compared by Dunnett multiple comparisons test
Among the elicitor treatments, JA (75 µM) exhibited the most elevated antioxidant capacity (88.3 ± 4.3%) in DPPH assay (Fig. 5A). The alterations in ability of extracts to reduce Fe3+ to Fe2+ radicals were seen in FRAP assay and a similar propensity was seen in the metal chelating assay supported the formation of ferrozine to Fe2+ complex. The maximum antioxidant capacity in FRAP (2.350 ± 0.08 OD) and metal chelating assays (77.4 ± 2.3%) recorded in calli treated with JA (75 µM) and YE (400 mg/l), respectively (Fig. 5B, C). Considering all three assays (DPPH, FRAP and metal chelating), JA-treated calli showed greater antioxidant potential when compared to other elicitor treatments and control. The least antioxidant activity values such as 64 ± 1.3% (in DPPH assay), 1.499 ± 0.2 OD (in FRAP assay) and 58.5 ± 1.5% (in metal chelating assay) were exhibited by the calli extract obtained from YE treatment (500 mg/l in DPPH and FRAP) and MeJA (250 µM), respectively (Fig. 5A–C). The findings of the present study revealed the chemical elicitation remarkably improved the antioxidant potential of callus cultures of S. chinensis. Recently, few researchers have assessed the impact of plant growth regulators on antioxidant capability of callus and aerial parts of micropropagated Salacia spp. (Chavan et al. 2015a; Bagnazari et al. 2018; Mahendra et al. 2020); however, the present study is the first and sole report describing elicitor-mediated increase in antioxidant potential of callus cultures of S. chinensis.
Conclusion
Our investigation demonstrated the heightened results when diverse elicitor treatments were applied for callogenesis, biomass accumulation, and production of active phytochemicals coupled with antioxidant potential in S. chinensis. This is the first report on elicitation studies in callus cultures for S. chinensis (perhaps for genus Salacia). Among various elicitors, JA (75 µM) enhanced the callus proliferation frequency with higher calli biomass accumulation. Moreover, same elicitor treatment resulted in increased polyphenol, mangiferin accumulation as well as antioxidant properties. Our findings provide evidence that, the use of elicitor treatment is promising approach for improved calli biomass production, secondary metabolites and antioxidant properties in S. chinensis. Various bioactive and industrially important compounds are found in S. chinensis; therefore, further study is necessary for optimization of efficiency of diverse class of elicitors, precursors, genetic engineering approach, and triggering the cultures within bioreactors would be advantageous for exploiting the full potential of this taxa.
Acknowledgements
Authors are grateful to Rashtriya Uchchtar Shiksha Abhiyan (RUSA), MHRD, Govt. of India, New Delhi for providing funds for creation of high-end research facility under component 8. We extend our sincere gratitude towards the Head, Department of Botany and the Director, Yashavantrao Chavan Institute of Science, Satara (Autonomous) for providing necessary laboratory facilities.
Abbreviations
- 2,4-D
2,4-Dichlorophenoxyacetic acid
- BAP
6-Benzylaminopurine
- CE
Callus extract
- CPF
Callus proliferation frequency
- DMF
N,N-Dimethyl formamide
- DPPH
2,2-Diphenyl-2-picrylhydrazyl
- DW
Dry weight
- FRAP
Ferric-reducing antioxidant power
- FW
Fresh weight
- GAE
Gallic acid equivalent
- JA
Jasmonic acid
- MeJA
Methyl jasmonate
- MeOH
Methanol
- MS
Murashige and Skoog’s medium
- QE
Quercetin equivalent
- RP-HPLC
Reversed-phase high-performance liquid chromatography
- SBAE
Steam bath-assisted extraction
- SE
Standard error
- TFC
Total flavonoid content
- TPC
Total phenolic content
- TPTZ
2,4,6-Tripyridyl-s-triazine
- YE
Yeast extract
Author contributions
JJC conceived, designed and performed the experiments, wrote the draft of manuscript. PRK, SGJ, VMN, STG performed the experiments, contributed to review and edit the manuscript. SRP contributed to resources and analyzed the data.
Funding
The present work was financially supported by Science and Engineering Research Board (SERB) of Department of Science and Technology (DST), Govt. of India, New Delhi (No. SB/FT/LS-259/2012) through Fast-Track Scheme for Young Scientist.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Ahmad Z, Shahzad A, Sharma S. Chitosan versus yeast extract driven elicitation for enhanced production of fragrant compound 2-hydroxy-4-methoxybenzaldehyde (2H4MB) in root tuber derived callus of Decalepis salicifolia (Bedd. ex Hook.f.) Venter. Plant Cell Tiss Org Cult. 2019;136(1):29–40. doi: 10.1007/s11240-018-1488-4. [DOI] [Google Scholar]
- Al-Khayri JM, Naik PM. Elicitor-induced production of biomass and pharmaceutical phenolic compounds in cell suspension culture of date palm (Phoenix dactylifera L.) Molecules. 2020;25:4669. doi: 10.3390/molecules25204669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astello-Garcia MG, Robles-Martinez M, Barba-de la Rosa AP, Santos-Diaz MS. Establishment of callus from Opuntia robusta Wendl., a wild and medicinal cactus, for phenolic compounds production. Afr J Biotechnol. 2013;12:3204–3207. [Google Scholar]
- Bagnazari M, Mahesh MG, Saidi M, Kini KR, Prakash HS, Geetha N. Evaluation of genetic stability using FRAPD markers as novel method along with antioxidant and antidiabetic properties of micropropagated Salacia chinensis L. Acta Physiol Plantarum. 2018;40:128. doi: 10.1007/s11738-018-2705-9. [DOI] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. Lebensm Wiss Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Chang C, Yang M, Wen H, Chen J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Chavan JJ, Jagtap UB, Gaikwad NB, Dixit GB, Bapat VA. Total phenolics, flavonoids and antioxidant activity of Saptarangi (Salacia chinensis L.) fruit pulp. J Plant Biochem Biotechnol. 2013;22(4):409–413. doi: 10.1007/s13562-012-0169-3. [DOI] [Google Scholar]
- Chavan JJ, Ghadage DM, Bhoite AS, Umdale SD. Micropropagation, molecular profiling and RP-HPLC determination of mangiferin across various regeneration stages of Saptarangi (Salacia chinensis L.) Ind Crop Prod. 2015;76:1123–1132. doi: 10.1016/j.indcrop.2015.08.028. [DOI] [Google Scholar]
- Chavan JJ, Ghadage DM, Kshirsagar PR, Kudale SS. Optimization of extraction techniques and RP-HPLC analysis of antidiabetic and anticancer drug mangiferin from roots of ‘Saptarangi’ (Salacia chinensis L.) J Liq Chromato Reld Technol. 2015;38:963–969. doi: 10.1080/10826076.2014.999199. [DOI] [Google Scholar]
- Chavan JJ, Gaikwad NB, Dixit GB, Yadav SR, Bapat VA. Biotechnological interventions for propagation, conservation and improvement of ‘Lantern Flowers’ (Ceropegia spp.) S Afr J Bot. 2018;114:192–216. doi: 10.1016/j.sajb.2017.10.021. [DOI] [Google Scholar]
- Deepak KGK, Suneetha G, Surekha C. In vitro clonal propagation of Salacia oblonga Wall. An endangered medicinal plant. Ann Phytomed. 2015;4(2):67–70. doi: 10.1007/s11418-015-0932-6. [DOI] [PubMed] [Google Scholar]
- Dhanasri G, Reddy M, Naresh B, Cherku D. Micropropagation of Salacia reticulate—an endangered medicinal plant. Plant Tiss Cult Biotechnol. 2013;23(2):221–229. doi: 10.3329/ptcb.v23i2.17523. [DOI] [Google Scholar]
- Dinis T, Madeira V, Almeida T. Action of phenolic derivatives (acetaminophen, salicylate and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–165. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- Dubey GP, Agarwal A, Vyas N, Rajamanickam VG (2011) Herbal formulation for the prevention and management of diabetes mellitus and diabetic micro-vascular complications (US20090214678A1)
- Espinosa-Leal CA, Puente-Garza CA, Garcia-Lara S. In vitro plant tissue culture: means for production of biological active compounds. Planta. 2018;248:1–18. doi: 10.1007/s00425-018-2910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng ST, Wang ZZ, Yuan YH, Sun HM, Chen NH, Zhang Y. Mangiferin: a multipotent natural product preventing neurodegeneration in Alzheimer’s and Parkinson’s disease models. Pharmacol Res. 2019;146:104336. doi: 10.1016/j.phrs.2019.104336. [DOI] [PubMed] [Google Scholar]
- Gao J, Xue J, Xue Y, LiuR RX, Wang S, Zhang X. Transcriptome sequencing and identification of key callus browning-related genes from petiole callus of Tree Peony (Paeonia suffruticosa cv. Kao) cultured on media with three browning inhibitors. Plant Physiol Biochem. 2020;149:36–49. doi: 10.1016/j.plaphy.2020.01.029. [DOI] [PubMed] [Google Scholar]
- Ghadage DM, Kshirsagar PR, Pai SR, Chavan JJ. Extraction efficiency, phytochemical profiles and antioxidative properties of different parts of Saptarangi (Salacia chinensis L.)—an important underutilized plant. Biochem Biophy Rep. 2017;12:79–90. doi: 10.1016/j.bbrep.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauhari N, Bharadwaj R, Sharma N, Bharadvaja N. Assessment of bacoside production, total phenol content and antioxidant potential of elicited and non-elicited shoot cultures of Bacopa monnieri (L.) Environ Sustain. 2019;2:441–453. doi: 10.1007/s42398-019-00071-3. [DOI] [Google Scholar]
- Khan T, Khan T, Hano C, Abbas BH. Effects of chitosan and salicylic acid on the production of pharmacologically attractive secondary metabolites in callus cultures of Fagonia indica. Ind Crop Prod. 2019;129:525–535. doi: 10.1016/j.indcrop.2018.12.048. [DOI] [Google Scholar]
- Laxmi M, Raviraja Shetty G, Souravi K, Rajasekharan PE. In vitro conservation studies in Salacia chinensis L. a threatened medicinal plant. J Pharmacog Phytochem. 2018;SP3:78–81. [Google Scholar]
- Mahendra C, Murali M, Manasa G, Sudarshana MS. Biopotentiality of leaf and leaf derived callus extracts of Salacia macrosperma Wight.—an endangered medicinal plant of Western Ghats. Ind Crop Prod. 2020;143:111921. doi: 10.1016/j.indcrop.2019.111921. [DOI] [Google Scholar]
- Majid BN, Kini KR, Prakash HS, Geetha N. Phytomorphology, phytochemistry and pharmacological activities of Salacia chinensis L., an endangered antidiabetic medicinal plant: a comprehensive review. Int J Agric Biosci. 2016;5(1):1–7. [Google Scholar]
- Majid BN, Sampath KK, Prakash HS, Geetha N. Rapid mass propagation of Salacia chinensis L., an endangered valuable medicinal plant through direct organogenesis. Ind J Sci Technol. 2016;9(4):1–8. [Google Scholar]
- Mekky H, Al-Sabahi J, Abdel-Kreem MFM. Potentiating biosynthesis of the anticancer alkaloids vincristine and vinblastine in callus cultures of Catharanthus roseus. S Afr J Bot. 2018;114:29–31. doi: 10.1016/j.sajb.2017.10.008. [DOI] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Rajan M, Feba KS, Chandran V, Shahena S, Mathew L. Enhancement of rhamnetin production in Vernonia anthelmintica (L.) Willd. cell suspension cultures by eliciting with methyl jasmonate and salicylic acid. Physiol Mol Biol Plants. 2020;26:1531–1539. doi: 10.1007/s12298-020-00829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram M, Prasad KV, Singh SK, Hada BS, Kumar S. Influence of salicylic acid and methyl jasmonate elicitation on anthocyanin production in callus cultures of Rosa hybrida L. Plant Cell Tiss Org Cult. 2013;113:459–467. doi: 10.1007/s11240-013-0287-1. [DOI] [Google Scholar]
- Rao SR, Ravishankar GA. Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv. 2002;20(2):101–153. doi: 10.1016/S0734-9750(02)00007-1. [DOI] [PubMed] [Google Scholar]
- Sailo L, Upadhya V, Naik PM, Desai N, Pai SR, Al-Khayri JM. Effect of chemical elicitors on pentacyclic triterpenoid production in in vitro cultures of Achyranthes aspera L. In: Kumar N, editor. Biotechnological approaches for medicinal and aromatic plants. Springer Nature Singapore Pte Ltd: Singapore; 2018. [Google Scholar]
- Salma U, Kundu S, Ali MN, Mandal N. Elicitor mediated enhancement of wedelolactone in cell suspension culture of Eclipta alba (L.) Hassk. Plant Cell Tiss Org Cult. 2018;134(3):409–421. doi: 10.1007/s11240-018-1431-8. [DOI] [Google Scholar]
- Sarmadi M, Karimi N, Palazon J, Ghassempour A, Mirjalili MH. The effects of salicylic acid and glucose on biochemical traits and taxane production in a Taxus baccata callus culture. Plant Physiol Biochem. 2018;132:271–280. doi: 10.1016/j.plaphy.2018.09.013. [DOI] [PubMed] [Google Scholar]
- Silpraist K, Seetaha S, Pongsanarakul P, Hannongbua S, Choowongkomon K. Anti-HIV-1reverse transcriptase activities of hexane extracts from Asian medicinal plants. J Med Plants Res. 2011;5(19):4899–4906. [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am J Enol Viticult. 1965;16:144–158. [Google Scholar]
- Smetanska I. Production of secondary metabolites using plant cell cultures. Adv Biochem Eng Biotechnol. 2008;111:187–228. doi: 10.1007/10_2008_103. [DOI] [PubMed] [Google Scholar]
- Thuan LC (2005) Chemistry of Southeast Asian plants. Ph.D. thesis, National University of Singapore
- Vijayalakshmi U, Shourie A. Yeast extract-mediated elicitation of anti-cancerous compounds licoisoflavone B, licochalcone A, and liquirtigenin in callus cultures of Glycyrrhiza glabra. Biotechnologia. 2019;100(4):441–451. doi: 10.5114/bta.2019.90245. [DOI] [Google Scholar]
- Yogananth N, Bhakyaraj R, Syed Ali M, Muthezhilan R. Effect of yeast elicitor on the enhancement of kaempferol from in vivo and in vitro callus cultures of Dregea volubilis Benth. Asian Journal of Biological Sciences. 2019;12:278–283. doi: 10.3923/ajbs.2019.278.283. [DOI] [Google Scholar]
- Yoshikawa M, Nishida N, Shimoda H, Takada M, Kawahara Y, Matsuda H. Polyphenol constituents from Salacia species: quantitative analysis of mangiferin with alpha-glucosidase and aldose reductase inhibitory activities. Yakugaku Zasshi. 2001;121:371–378. doi: 10.1248/yakushi.121.371. [DOI] [PubMed] [Google Scholar]
- Zafar N, Mujib A, Ali M, Tonk D, Gulzar B. Aluminum chloride elicitation (amendment) improves callus biomass growth and reserpine yield in Rauvolfia serpentine leaf callus. Plant Cell Tiss Org Cult. 2017;130(2):357–368. doi: 10.1007/s11240-017-1230-7. [DOI] [Google Scholar]