Abstract
Several plant species synthesize biologically active secondary metabolites. Pyrrolizidine alkaloids are a large group of biotoxins produced by thousands of plant species to protect against the attack of insects and herbivores, but they are highly toxic for humans and animals. In this study, extracts from the aerial part of Senecio brasiliensis were obtained using different technologies: ultrasound-assisted extraction (UAE), pressurized liquid extraction (PLE), and microwave hydrodiffusion and gravity (MHG). The study aimed to evaluate the effectiveness of these technologies for the extraction of chemical compounds found in this plant, focusing on two pyrrolizidine alkaloids: integerrimine and senecionine. Influential parameters on yield and chemical composition were also evaluated: for UAE and MHG, temperature and pressure; for PLE, temperature, and percentage of ethanol. All the extraction techniques were efficient for the extraction of integerrimine and senecionine. The UAE and PLE stood out for the higher yields and number of compounds. The PLE presented a maximum yield of 18.63% for the matrix leaf and the UAE a maximum yield of 11.82% for the same matrix. These two techniques also stood out in terms of the number of compounds, once 36 different compounds were found via PLE and 17 via UAE.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-02845-1.
Keywords: Genus senecio, Innovative extraction methodologies, Phytochemicals, Alkaloids rich extracts
Introduction
Plants synthesize a wide range of secondary metabolites, most of which have evolved to promote the survival of plants protecting from external factors, such as insects, herbivores, predators, and pathogens (Macedo et al. 2017; Thakur et al. 2019). The secondary metabolites were conventionally divided into three categories: alkaloids, terpenoids, and phenolics (Zaynab et al. 2018). The pyrrolizidine alkaloids are among the most hepatotoxic and are widely distributed across the world (Xu et al. 2019). They are found in all the plant parts or specific tissues, like roots and seeds. Its ecologically important functions include the plant protection against pests and herbivores, the attraction of insects for pollination, and the composition of important compounds from the pharmacologic point of view (Takshak and Agrawal 2019); however, due to its toxicity, they also affect humans and animals (Wiedenfeld and Edgar 2011; Kaczyński and Łozowicka 2020).
They are mostly found in plants of the Asteraceae, Boraginaceae, Fabaceae, and Orchidaceae families (Ratmanova et al. 2020). Within the Asteraceae family, the genus Senecio is highlighted, which has toxic active compounds, such as the pyrrolizidine alkaloids. Special attention is paid to the S. brasiliensis species, which presents integerrimine and senecionine as the main pyrrolizidine alkaloids (Sandini et al. 2013). The highest contents of alkaloids are found when the plant is in the flowering period, with considerable variation in the amount of the toxic active ingredient in different parts of the plant (Karam et al. 2004). Senecio brasiliensis is a native and non-endemic plant species popularly known as “Flor-das-Almas”, “Margaridinha”, or “Maria mole” (De Souza et al. 2015; Macedo et al. 2017). It is usually present in the South, Southeast, and Midwest Brazilian regions, also occurring in Uruguay, Paraguay, and Argentina (Pilati and Barros 2007; Sandini et al. 2013).
Integerrimine and senecionine are compounds considered to be pyrrolizidine alkaloids or natural phytotoxins commonly found in plants of the genus Senecio (Trigo et al. 2003; Toma et al. 2004; Elias et al. 2011; De Souza et al. 2015). Plants with pyrrolizidine alkaloids are considered common causes of natural intoxications affecting livestock, wild animals, and humans (Elias et al. 2011; Sandini et al. 2013). In studies with different doses of pyrrolizidine alkaloids during pregnancy in animals, Guo et al. (2013) verified post-implant loss, fetal mortality, abortive, and fetotoxic effects. Sandini et al. (2013) indicate that prenatal exposure to S. brasiliensis integerrimine N-oxide induces toxicity and affects the postnatal development of rat offspring.
To obtain alkaloids with such activities from S. brasiliensis, some extraction methods can be used. The extractive method to be applied is an important consideration in the extraction of bioactive compounds from plants (Goltz et al. 2018). Emerging extraction technologies are being used successfully to achieve a “green” and sustainable extraction (Soquetta et al. 2018). Among them are ultrasound-assisted extraction (UAE) (Wei et al. 2019), microwave (MW), and pressurized liquids extraction (PLE) (Castejón et al. 2017; Santos et al. 2019b). These techniques have been considered capable of solving problems associated with conventional extraction methods. UAE is a process that simplifies handling and processing conditions, provides higher purity of the final product, reduces the amount of solvent used as well as the energy required when compared with conventional methods, by working at the lowest temperatures or avoiding expensive solvent elimination (Chemat et al. 2011).
PLE presents several characteristics that make it an excellent substitute for conventional extraction methods. The use of high temperature and pressure during the extraction not only enhances the extraction yield, but also reduces the time and solvent consumption (Mustafa and Turner 2011). The MW extraction has gained also enormous popularity as an environment-friendly process for secondary metabolites extraction (Yu et al. 2013; Filly et al. 2014), increasing the extract amount, reducing the extraction time, lowering the cost, and consumption of energy. Within the context of the use of MW, the extraction technique known as Microwave Hydrodiffusion and Gravity (MHG) (Chemat et al. 2017) leveraged the limits of microwave-assisted extraction to an innovative, fast, efficient, and ecological process, without any kind of degradation in the quality of the extract (González et al. 2013; López-Hortas et al. 2016; Benmoussa et al. 2018).
Considering that the literature reports for S. brasiliensis point out the presence of pyrrolizidine alkaloids, there was an interest in investigating this plant species. Also, there is a limited number of studies about the chemical composition and extraction yield of aerial parts of such plants (flowers, leaves, and stalk), and so far no references in the main scientific databases have been found using UAE, PLE, and MHG as extraction techniques. Thus, in this study different extraction methodologies were evaluated on the following responses: yield of extracts and chemical composition of the extracts, with special attention on pyrrolizidine alkaloids from the different plant matrices of Senecio brasiliensis.
Materials and methods
Samples preparation
The plant material of S. brasiliensis (flowers, leaves, and stalks) was collected in a pasture area with compacted soil in the north of the state of Rio Grande do Sul (S: 27° 55′ 39.43/W: 52° 7′ 37.14). The samples were dried at 40 ºC until constant mass and maintained at – 4 ºC until extractions.
Ultrasound-assisted extraction
The experimental apparatus (high-intensity ultrasound processor of 400 W and frequency of 24 kHz—Hielscher, Model UP 400S, Germany, titanium probe Model H22, Tip 22) used was described by Sallet et al. (2019). Each sample (2.5 g) and 100 mL of hydroalcoholic solution (60 mL of ethanol and 40 mL of water) were used for the extractions. All extractions were carried out at 40 ºC ± 2 °C for 30 min (defined based on the preliminary tests). The experiments were planned according to a Central Composite Rotatable Design (CCRD), where the variables analyzed were power intensity (maximum of 85 W/cm2) and duty cycle (maximum value of 1.0 s/s). After extraction, the samples were centrifuged at 10,000 rpm for 5 min. The liquid phase was carefully collected and the solvents were evaporated under vacuum at 40 °C. The control experiment was carried out with conventional maceration at the same temperature and solvent ratio (v/v), without sonication, but with a time larger than 24 h.
Pressurized liquids extraction
For pressurized liquid extraction, the dynamic extraction method was applied. Ethanol and/or water were used as solvents. For extraction procedures, approximately 10 g of sample (dried and grounded) were loaded into the extraction cell. Then, the temperature and pressure conditions were established and the solvent was pumped from the reservoir to the extraction cell by an HPLC pump. This method consists of a continuous solvent flow rate. The system was kept static for 20 min and the dynamic extraction started through the micrometric valve opening.
The experiments were based on a CCRD. The evaluated variables were temperature and percentage of ethanol in the solvent. The operational conditions were based on the work of Viganó et al. (2016). The temperatures used were 30, 45, and 60 °C and the ethanol percentages in the solvent were 70, 85, and 100% (v/v). The pressure was maintained at 10 MPa. The solvent flow rates were 3.0, 2.8, and 2.7 mL/min for ethanol percentages of 100, 85, and 70% respectively, to keep constant the solvent mass flow rate (2.4 g/min). The extraction time was 30 min. The obtained extracts were collected in glass flasks and stored at − 18 °C in absence of light for further analysis.
| 1 |
The global yield of extraction was calculated according to the following equation:
Microwave hydrodiffusion and gravity extraction
The MHG extraction was performed in a 2.45 GHz microwave equipment (NEOS-GR multimode Milestone, Bergamo, Italy). The operational conditions were established based on the work of Ferreira et al. (Ferreira et al. 2020), under atmospheric pressure (0.1 MPa) and 400 W of power for 20 min. Complementary details about the equipment and procedure have already been described by Ferreira et al. (2020). The samples (100 g for each plant matrix) were humidified before extraction, by soaking the plant in the solvent for 1 h, enabling the complete absorption of the liquid. Extractions soaking the matrices only in water or in a hydroalcoholic solution (60 mL of ethanol and 40 mL of water) were evaluated. After the extraction, the extract was collected in a vessel outside the microwave irradiation cavity and the solvent was evaporated for further chromatographic analysis.
Chemical composition
The samples were analyzed in a GC-Q/MS system. Procedures and details of the equipment were previously described by Confortin et al. (2019). Minor modifications were made to the program and the injector temperature was kept at 320 °C. 1 μL of each sample was injected with a separation ratio of 1:40. The oven temperature program used was 5 °C/min from 80 to 300 °C (15 min wait). The interface temperature was kept at 320 °C and the ion source temperature at 260 °C. The mass spectra were recorded over 35–500 amu, at 3.3 scan/s with ionization energy of 70 eV. The individual components identification was made using their relative retention indices, according to the Wiley Registry of Mass Spectral Data (Palisade Corporation, Newfield, NY).
Scanning electron microscopy
The sample morphology was evaluated using a scanning electron microscope (SEM) (Tescan, VEGA-3G, Czech Republic) coupled to a secondary electron detector to obtain the images. To proceed with this analysis, the samples were covered with gold (spray metallization process, using an electric current of 20 mA for 90 s).
Results and discussion
Extraction yields
Ultrasound-assisted extraction
Table 1 presents the variables (intensity and cycle) and the responses of the CCRD. The plant matrixes presented similar behaviors, with the highest yields obtained in assay 4 with high values of power intensity (75.11 W/cm2) and cycle (0.93), while the lowest yields were obtained in assay 5 with the lowest power intensity (17 W/cm2) and cycle (0.75). The weight yields varied from 7.14 to 3.56% for the flower, from 11.82 to 6.13% for the leaf, and from 5.69 to 2.85% for the stalk.
Table 1.
Yields of matrices flower, leaf, and stalk of S. brasiliensis obtained by ultrasound-assisted extraction using a CCRD
| Assay | Power intensity (W/cm2) | Duty cycle (s/s) | Yield (wt%) | ||
|---|---|---|---|---|---|
| Flower | Leaf | Stalk | |||
| 1 | 26.89 (− 1) | 0.57 (− 1) | 4.32 | 6.31 | 3.24 |
| 2 | 75.11 (1) | 0.57 (− 1) | 6.41 | 10.15 | 4.55 |
| 3 | 26.89 (− 1) | 0.93 (1) | 5.00 | 7.37 | 3.55 |
| 4 | 75.11 (1) | 0.93 (1) | 7.14 | 11.82 | 5.69 |
| 5 | 17 (− 1.41) | 0.75 (0) | 3.56 | 6.13 | 2.85 |
| 6 | 85 (1.41) | 0.75 (0) | 6.52 | 10.68 | 5.57 |
| 7 | 51 (0) | 0.50 (− 1.41) | 5.02 | 7.62 | 4.23 |
| 8 | 51 (0) | 1.0 (1.41) | 5.82 | 8.92 | 4.51 |
| 9 | 51 (0) | 0.75 (0) | 5.32 | 8.66 | 4.45 |
| 10 | 51 (0) | 0.75 (0) | 5.31 | 8.65 | 4.49 |
| 11 | 51 (0) | 0.75 (0) | 5.30 | 8.67 | 4.48 |
| 12 | 0 | 0 | 4.17 | 6.25 | 3.2 |
When comparing the conventional extraction (assay 12) with the UAE of higher yield (assay 4), significant yield increases of 60, 50, and 60% for flower, leaf, and stalk, respectively, were observed. The extraction time must also be taken into consideration. For conventional extraction, the time was 24 h while for UAE it was 30 min. These yield increases can be explained by the phenomenon called acoustic cavitation generated by the ultrasonic probe, which influences the fluid around the solid particles and causes changes in the internal structure of the plant material (affecting the cell wall and/or increasing its pores) (Trojanowska et al. 2019).
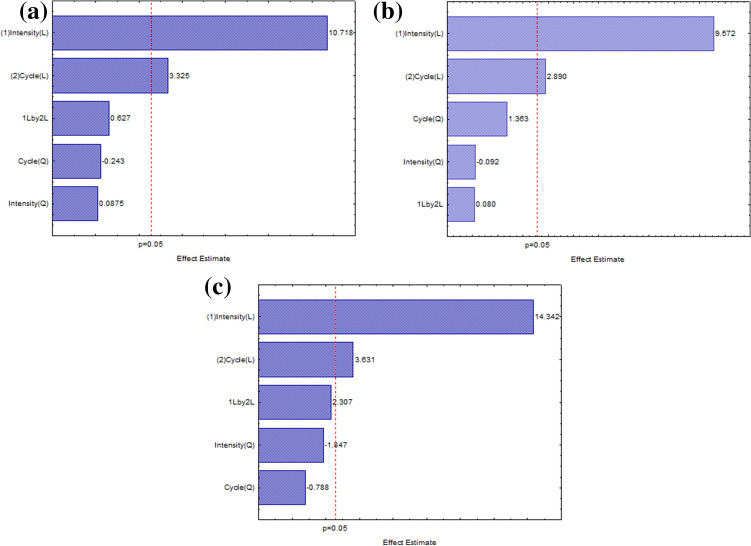
The results showed in Table 1 were used to estimate the linear, quadratic, and interaction terms of the studied variables over the responses. The effects of each variable over the responses were expressed in form of a Pareto chart (Fig. 1). The linear terms for power intensity and duty cycle of ultrasound were statistically significant (p < 0.05) for all the plant matrices studied (flower, leaves, and stalks), presenting a positive effect, which means that an increase in the values of the variables leads to the highest yields.
Fig. 1.
Pareto’s chart of process variables effects on the extraction yield of Senecio brasiliensis using UAE for a leaf, b flower, and c stalk
The response surface graphs illustrating the influence of the experimental variables (power intensity and duty cycle) over the extraction yield is summarized in Fig. S1 in the Supplementary data. The UAE resulted in maximal yield for the leaf when the power intensity varied from 75 to 85 W/cm2 and the duty cycle varied from 0.60 to 1.0. For the flower, the maximal yield was obtained in the same range of power intensity as for the leaf, while the duty cycle ranged from 0.75 to 1.0. Finally, for the stalk, the best range of power intensity was the same as the other matrices and the duty cycle ranged from 0.70 to 1.0. The results indicate that an increase in the values of the variables enhances the extraction of the compounds from the matrices evaluated in this work. These data are corroborated by Sharayei et al. (2019), which obtained the highest yields for extraction from Punica granatum at high power intensity and duty cycles. Increasing the power intensity of the ultrasound, the authors attribute the enhancement of extraction yield to the cavitation phenomenon generated by the sonication, which increases the plant tissue permeability. Sallet et al. (2019) also obtained highest yields of oil from Mortierella isabelina when using highest values for power intensity and duty cycle.
Ethanolic extractions
The yields for the different plant matrices studied are presented in Table 2. The behaviors of all plant matrices of S. brasiliensis studied were similar. Assay 3 (60 °C and 70% v/v ethanol) was the one that resulted in the highest yields, while the lowest yields were attained in assays with lowest temperature (30 °C) and highest ethanol amount (100% v/v). Notably, the yields using pressurized liquids were higher than when using the US.
Table 2.
Yields of matrices flower, leaf, and stalk of S. brasiliensis obtained by pressurized liquid extraction using a CCRD
| Assay | T (ºC) | Ethanol (% v/v) | Yield (wt%) | ||
|---|---|---|---|---|---|
| Leaf | Flower | Stalk | |||
| 1 | (− 1) 30 | (− 1) 70 | 11.99 | 7.72 | 8.65 |
| 2 | (− 1) 30 | (+ 1) 100 | 2.32 | 1.25 | 1.52 |
| 3 | (+ 1) 60 | (− 1) 70 | 18.63 | 12.96 | 10.65 |
| 4 | (+ 1) 60 | (+ 1) 100 | 4.06 | 3.68 | 3.03 |
| 5 | (0) 45 | (0) 85 | 9.29 | 5.09 | 6.27 |
| 6 | (0) 45 | (0) 85 | 9.39 | 5.09 | 6.85 |
| 7 | (0) 45 | (0) 85 | 9.24 | 5.89 | 6.10 |
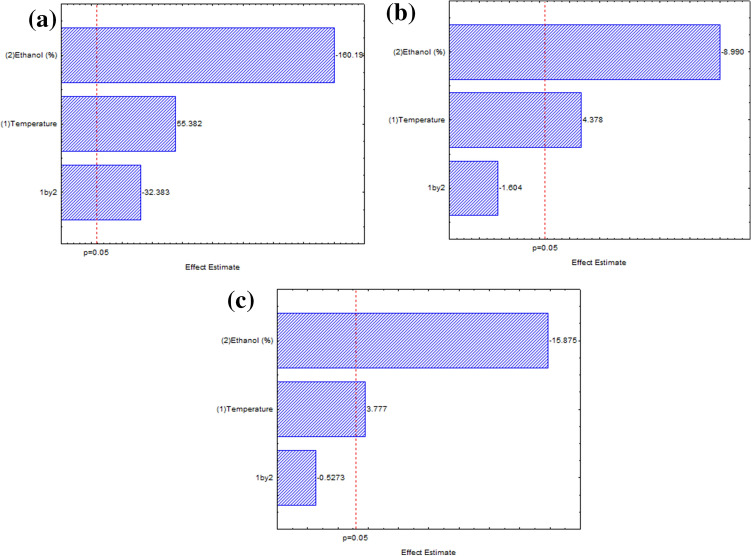
The results shown in Table 2 were used to estimate the linear, quadratic, and interaction terms of the studied variables over the responses. The effects of each variable over the responses were expressed in form of a Pareto chart (Fig. 2). The linear effects of temperature and ethanol amount were statistically significant (p < 0.05) for all the plant matrices of S. brasiliensis. The temperature presented a positive effect, which means that an increase in this variable leads to highest yields. When comparing assays 1 and 3, it can be seen that the highest yields were attained with the highest temperature, while the ethanol percentage was kept constant, that is, the increase in temperature leads to highest yields for all the ethanol percentages studied. Otherwise, ethanol percentage presented a negative effect, which means that the highest the ethanol percentage in the solvent, the lowest the yield.
Fig. 2.
Pareto’s chart of process variables effects on the extraction yield of Senecio brasiliensis using PLE for a leaf, b flower, and c stalk
The temperature in extractions with pressurized liquids plays an important role in the yield of obtained extracts (Carabias-Martínez et al. 2005). As the temperature increases, the viscosity, and surface tension of the solvents decrease and the diffusivity increases, thus increasing the solvents ability to penetrate the matrix and accelerating the dissolution of the compounds of interest in the extract (Hossain et al. 2011; Cheong et al. 2013; Viganó et al. 2016). Thus, the mass transfer rate increases and, consequently, highest global yields can be attained (Machado et al. 2015).
As for the influence of the percentage of ethanol, the highest yield of extraction was found with 70% v/v ethanol. According to Mustafa and Turner (2011), the use of solvent mixtures increases extraction yields, thus improving solubility and increasing the interaction between the target components and the extraction solvent. The use of binary mixtures can have a synergistic effect: while one solvent increases the solubility of target compounds, the other can favor its desorption, increasing extraction yields (Pereira et al. 2019). Therefore, water is generally important to help break the matrix and solute-matrix bonds (Viganó et al. 2016).
The results found in this work are following Zaibunnisa et al. (2009), where the oleoresin yield obtained from turmeric leaves increased significantly with increasing temperature. Viganó et al. (2016), when evaluating the yield of bioactive compounds in the passion fruit peel, reported an increase in yield when using highest temperatures and solvent mixture. Setyaningsih et al. (2016) reported the use of solvents mixture and elevated temperatures as an optimum extraction condition for phenolic compounds extraction from rice grain. For Barrales et al. (2018), the increase in temperature had a positive effect on the overall yield in all evaluated ethanol concentrations and with the composition of the solvent. The addition of water to ethanol increased the overall yield of the orange peel extracts.
Microwave hydrodiffusion and gravity extraction
The yield data are presented in Table 3. Only the use of different solvents for plant humidification was evaluated. The power output was 400 W and the time was 20 min, which was enough for complete plant extraction. The yields obtained for plant humidification with water and with the hydroalcoholic solution were very similar; however, the result using a hydroalcoholic solution presented a slight increase. The plant matrix that resulted in the best yield was the flower with 0.68%, followed by leaves with 0.64% and finally, stalk with 0.26% (Table 3).
Table 3.
Yields of matrices flower, leaf, and stalk of S. brasiliensis obtained by microwave hydrodiffusion and gravity extraction
| Assay | Microwave power (W) | Solvent | Yield (wt%) | ||
|---|---|---|---|---|---|
| Flower | Leaf | Stalk | |||
| 1 | 450 | Water | 0.68 | 0.64 | 0.26 |
| 2 | 450 | Hydroalcoholic solution | 0.64 | 0.63 | 0.27 |
There are no reports in the literature describing the use of MHG for Senecio brasiliensis. The yields achieved with this technique are similar to those obtained by Confortin et al. (2019) using supercritical carbon dioxide. It can be said that this extraction technique, when compared with the UAE and PLE obtained lowest yields. However, the MHG technique showed the capacity to extract the extracts from the plant matrices completely within 15 min. The yields obtained in this work using MHG for extraction were lower than the yield of essential cumin oil (1.57%) obtained by Benmoussa et al. (2018) in 16 min with a power of 200 W. Ferreira et al. (2020) achieved a yield of 2.32% in the extraction of Rosmarinus officinalis essential oil under the same conditions used in this work (power of 400 W and 20 min). The yields obtained in this work were higher than when compared with Bousbia et al. (2009) in Rosmarinus officinalis leaves extraction, where the yield was 0.33% with conditions of 15 min and 1000 W.
Low yields can be explained by the excessive use of microwave irradiation power. It is also important to mention that the MHG technique has a much lowest volume of solvent/mass of plant ratio and this can be an influentional factor, once less solvent is used in the process and, consequently, the efficiency is lowest. The usage of low extraction time was not enough to recover the totality of S. brasiliensis extract. Cui et al. (2017) describe that a highest irradiation power can cause a decrease in yield because the pyrolysis of some volatile components may occur. To accelerate the mass transfer rate and improve the extraction yield, best extraction conditions should be investigated for future works. However, it is important to mention that after MHG extraction the plant material is dried. Therefore, MHG can be used for dehydration and simultaneous obtaining of extract as recently proposed by Farias et al. (2021). Moreover, the dried plant can be used in combination with other extraction method for effective extraction.
Chemical composition by GC–MS
As can be seen in Tables 4, 5, 6, 7, 8, 9, and 10, the techniques used in secondary metabolite extraction were similar and the extracts contain several phytochemical constituents. Many of these compounds are described in the literature for presenting biological activity, such as 5H-1-pyridine, phenol, hexadecanoic acid, guanosine, octadecadienoic acid, 2-Methoxy-4-vinyl phenol, propanetriol, and cinerin. Some of the compounds found in the plant matrices and their bioactivities are described below:
2-Methoxy-4-vinyl phenol: antimicrobial, antioxidant, anti-inflammatory, and analgesic (Chhouk et al. 2018);
Octadienic acid: anti-inflammatory, nematicide, insecticide (Chhouk et al. 2018);
Hexadecanoic acid: antioxidant, nematicide, pesticide (Chhouk et al. 2018);
Phenol: antimicrobial, anti-inflammatory, antioxidant (Barretto and Vootla 2018; Chhouk et al. 2018);
Propanetriol: antimicrobial, anti-inflammatory, antifungal (Foo et al. 2015; Casuga et al. 2016);
Guanosine—anticancer (Lee et al. 2007);
D-Allose: antioxidant activity (Chhouk et al. 2018);
Cinerin II—Phyretrin: pesticide (Gallo et al. 2017; Chen et al. 2018);
Celidoniol: antibacterial (Bülent Köse et al. 2016), anti-inflammatory (Zakaria et al. 2014; Barretto and Vootla 2018).
Table 4.
Chemical compounds obtained using UAE for the leaf of Senecio brasiliensis
| Assasy | Relative area (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Leaf | ||||||||||
| Ultrasound | Maceration | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9/10/11 | 12 | |
| Compounds | ||||||||||
| Propanetriol | 3.26 | 3.52 | 3.32 | 1.0 | 3.86 | 3.63 | 3.27 | 3.96 | 4.12 | 2.10 |
| 5H-1-Pyrindine | 7.83 | 8.59 | 6.11 | 10.46 | 7.66 | 10.37 | 5.12 | 9.10 | 7.17 | 5.10 |
| Guanosine | 2.20 | 3.50 | 2.10 | 3.56 | 1.36 | 12.08 | – | 2.96 | 5.55 | – |
| d-Allose | 1.34 | – | 1.50 | 2.89 | 1.17 | 2.03 | 2.10 | 2.08 | 4.21 | – |
| alpha-d-Galactopyranoside | 1.28 | – | – | 1.65 | – | 1.25 | – | – | – | – |
| Phenol | 1.50 | 2.79 | 2.41 | 4.47 | 1.18 | 3.73 | 1.25 | 2.0 | 2.43 | 2.41 |
| Octadecadienoic acid | 1.84 | 0.68 | 2.10 | 1.66 | – | 1.40 | 1.36 | 3.12 | 5.63 | 1.20 |
| Hexadecanoic acid | 2.27 | 1.61 | 2.88 | 2.93 | 0.88 | – | 0.98 | 1.88 | 1.26 | 1.36 |
| Ethyl Linoleolate | 1.46 | 1.12 | 0.85 | 1.32 | – | 1.10 | – | – | – | – |
| Integerrimine | 67.25 | 67.18 | 71.56 | 60.26 | 71.98 | 54.18 | 75.91 | 59.02 | 55.47 | 73.91 |
| Tricosanoic acid | 3.01 | 1.24 | 2.36 | 0.21 | 2.36 | – | 2.36 | 8.25 | 7.52 | 2.23 |
| 2-Methoxy-4-vinylphenol | – | 2.79 | – | 3.70 | – | 2.76 | 1.85 | 1.10 | 1.82 | – |
| Cinerin II | – | 1.66 | 2.31 | 1.36 | 1.58 | 1.26 | 1.74 | 1.15 | 1.08 | 2.36 |
| Pentadecanoic acid | – | 0.63 | 0.25 | 0.25 | – | – | 0.92 | – | – | 4.21 |
| Senecionine | 6.20 | 1.19 | 1.23 | 3.19 | 6.92 | 4.11 | 1.36 | 2.78 | 2.25 | 5.12 |
| Germacrene D | 0.56 | 3.50 | 1.02 | 1.09 | – | 2.10 | 1.78 | 1.75 | 1.26 | – |
| Butanoic acid | – | – | – | – | 1.05 | – | – | 0.85 | 0.23 | – |
Table 5.
Chemical compounds obtained using UAE for the flower of Senecio brasiliensis
| Assay | Relative area (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Flower | ||||||||||
| Ultrasound | Maceration | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9/10/11 | 12 | |
| Compounds | ||||||||||
| Propanetriol | 3.73 | – | 3.72 | – | 4.42 | 0.63 | 4.62 | 0.80 | 1.15 | 3.68 |
| 5H-1-Pyrindine | 9.21 | 10.09 | 9.51 | 10.12 | 9.87 | 11.74 | 9.04 | 8.26 | 8.59 | 7.52 |
| Hexadecanoic acid | 2.36 | 3.08 | 3.24 | 4.12 | 2.01 | 3.12 | 2.05 | 1.65 | 1.23 | 0.96 |
| Octadecadienoic acid | 1.89 | 1.96 | 1.79 | 2.10 | 1.61 | 1.50 | 1.89 | 1.14 | 1.26 | – |
| Octadecanoic acid | 1.12 | 0.98 | 3.36 | 0.21 | 2.40 | 0.36 | 2.36 | 0.36 | 0.96 | 2.31 |
| Cinerin II | 1.38 | 0.35 | 1.99 | 1.36 | – | 0.69 | – | – | – | 2.96 |
| Integerrimine | 66.58 | 66.14 | 64.33 | 63.57 | 65.48 | 62.65 | 66.87 | 83.14 | 76.55 | 72.52 |
| 2-Methoxy-4-vinylphenol | 1.99 | 2.38 | 2.07 | 2.20 | 1.63 | 2.66 | 1.07 | 1.57 | 1.02 | – |
| Guanosine | 2.36 | 3.65 | 3.98 | 4.21 | 2.07 | 3.91 | 3.77 | 1.03 | 1.45 | – |
| Celidoniol | 1.96 | 2.34 | 1.36 | 3.20 | 2.01 | 2.83 | 1.39 | 1.75 | 1.63 | 1.34 |
| Phenol | 3.21 | 3.69 | 1.97 | 4.12 | 2.27 | 3.33 | 2.29 | 0.30 | 2.69 | – |
| Pyrrolidine | – | 2.36 | – | 1.10 | – | 3.37 | 0.89 | – | 1.40 | 2.34 |
| Senecionine | 4.21 | 2.98 | 2.68 | 3.69 | 6.23 | 3.21 | 3.76 | – | 2.07 | 6.37 |
Table 6.
Chemical compounds obtained using UAE for the stalk of Senecio brasiliensis
| Assay | Relative area (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stalk | ||||||||||
| Ultrasound | Maceration | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9/10/11 | 12 | |
| Compounds | ||||||||||
| Propanetriol | 2.18 | 1.95 | 1.25 | 1.46 | 1.06 | 1.93 | 1.82 | 1.34 | 3.95 | 2.23 |
| 5H-1-Pyrindine | 7.69 | 9.00 | 8.98 | 10.30 | 6.35 | 9.79 | 6.95 | 8.04 | 8.08 | 5.26 |
| 2-Methoxy-4-vinylphenol | – | 2.76 | – | 3.14 | – | 2.31 | 3.37 | 2.33 | 2.43 | – |
| d-Allose | 2.85 | 2.79 | 2.69 | 3.56 | 1.48 | 2.57 | 2.57 | 3.18 | – | 2.36 |
| Phenol | 4.31 | 5.04 | 4.58 | 6.61 | 4.14 | 6.37 | 5.17 | 5.48 | 5.45 | – |
| Benzenedicarboxylic acid | 2.41 | 2.28 | 2.44 | 2.10 | 2.07 | 2.27 | 1.59 | 1.23 | 2.36 | 2.10 |
| Octadecadienoic acid | 2.57 | – | – | 1.36 | 2.26 | 1.23 | – | – | – | 1.25 |
| Hexadecanoic acid | 4.05 | 5.59 | 3.69 | 5.47 | 3.59 | 4.38 | 4.23 | 4.56 | 4.20 | 3.15 |
| Eicosadienoic acid | 1.89 | – | – | 0.95 | 3.47 | 1.23 | – | – | – | 2.10 |
| Integerrimine | 58.11 | 55.66 | 62.26 | 50.40 | 63.83 | 50.02 | 69.58 | 61.65 | 61.35 | 79.45 |
| Tricosanoic acid | 2.24 | 2.10 | 2.54 | 2.96 | 2.08 | 2.85 | – | 1.39 | 1.02 | – |
| Guanosine | 4.69 | 5.21 | 4.69 | 5.31 | 4.89 | 7.45 | – | 4.25 | 4.27 | – |
| beta.-d-Glucopyranose | 2.45 | 2.36 | 2.54 | 2.09 | 1.03 | 2.62 | 1.15 | 2.36 | 2.53 | – |
| Cinerin II | 4.56 | 5.26 | 4.34 | 4.29 | 3.75 | 4.98 | 3.57 | 4.19 | 4.36 | 2.10 |
Table 7.
Chemical compounds obtained using PLE for the leaf of Senecio brasiliensis
| Assay | Relative area (%) | ||||
|---|---|---|---|---|---|
| Leaf | |||||
| 1 | 2 | 3 | 4 | 5/6/7 | |
| Compounds | |||||
| 1,2,3-propanetriol | – | 2.57 | 4.04 | 4.93 | – |
| 5 h-1-pyrindine | 6.26 | 4.45 | 5.71 | 6.41 | |
| Neophytadiene | – | – | 1.20 | 6.04 | 6.80 |
| Spathulenol | 3.80 | 2.58 | 1.49 | – | 1.47 |
| Caryophyllene oxide | – | – | 15.65 | – | – |
| 5,6-hydroxytryptamine | – | – | – | – | 2.68 |
| 1H-Indene, 5-butyl-6-hexyloctahydro- | – | – | – | – | 2.64 |
| Copper, bis(4-chloro-3,5-cyclohexadiene-1,2-dione 2-oximato-N2,O1)-(CAS) | – | – | – | – | 3.42 |
| 3,3-Dimethyl-4-(3,3,4,4-tetramethyloxetan-2-ylidene)butan-2-one | 4.06 | – | 1.02 | – | – |
| Androstan-17-one, 3-ethyl-3-hydroxy-, (5.alpha.)-(CAS) | 1.95 | – | 0.36 | – | – |
| 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester (CAS) | 3.31 | – | 5.23 | – | – |
| Benzoic acid, 2-hydroxy-, phenylmethyl ester (CAS) | 2.30 | – | 1.39 | – | – |
| 2-Hexadecen-1-ol | 1.03 | 16.89 | 2.65 | 5.60 | 6.45 |
| Eicosane ou eicosanol | 12.0 | 2.01 | 1.63 | – | 1.82 |
| 1,5,9,13-tetradecatetraene | 1.34 | – | – | – | – |
| N-Isobutyl-tetradeca-2,4-dienamide | 0.83 | – | – | – | – |
| Integerrimine | 35.44 | 32.46 | 36.27 | 10.54 | 24.35 |
| Tricosane | – | – | – | – | |
| Pentacosane | 8.29 | 2.86 | – | – | – |
| 2-Pentadecanone | – | 1.96 | 1.64 | – | – |
| Tetratetracontane | 7.49 | – | – | 1.84 | – |
| Hentriacontane | 4.67 | – | – | – | – |
| Tetracontane | – | – | – | 1.45 | – |
| Stigmast-5-en-3-ol | 2.32 | – | 4.68 | 9.33 | – |
| Methyl commate d | 4.91 | 2.90 | 11.09 | 56.88 | 41.09 |
| Hexadecanoic acid | – | – | 2.36 | – | – |
| Lupeyl acetate | – | 27.35 | – | – | – |
| Senecionine | – | 3.97 | 2.36 | – | 2.87 |
| Celidoniol | – | – | 1.23 | 3.39 | – |
Table 8.
Chemical compounds obtained using PLE for the matrix flower of Senecio brasiliensis
| Assay | Relative area (%) | ||||
|---|---|---|---|---|---|
| Flower | |||||
| 1 | 2 | 3 | 4 | 5/6/7 | |
| Compounds | |||||
| Dl-Glyceraldehyde | 3.84 | – | – | – | – |
| 2-Propanone, 1,3-dihydroxy | 2.59 | 3.52 | – | 1.44 | – |
| 5 h-1-pyrindine | 2.16 | 10.87 | 5.48 | 4.47 | 4.83 |
| Spathulenol | 11.00 | 2.92 | 2.33 | – | 1.85 |
| Androstan-17-one, 3-ethyl-3-hydroxy-, (5.alpha.) | 4.48 | – | – | – | – |
| Phenol | – | – | 1.45 | – | – |
| Octanal | 2.18 | – | – | – | – |
| Phosphonic acid, dioctadecyl ester | 1.63 | – | – | – | – |
| Neophytadiene | 5.24 | – | – | – | – |
| 2-pentadecanone | 3.51 | – | – | – | – |
| 1,2-Benzenedicarboxylic acid | 4.99 | 0.83 | 1.04 | 1.91 | 1.27 |
| 4-Benzyloxybenzoic acid | 8.08 | – | 0.46 | 1.27 | – |
| 2-Hexadecen-1-ol | 4.98 | – | 1.91 | – | 0.75 |
| 1,5,9,13-tetradecatetraene | 2.80 | – | – | – | – |
| Integerrimine | 9.94 | 2.97 | 46.24 | 1.65 | 17.31 |
| 1-eicosanol | 3.74 | – | – | – | |
| Hexacosane | 5.30 | – | – | – | 1.47 |
| Stigmast-5-en-3-ol | 23.54 | 3.65 | 6.30 | 1.91 | 4.07 |
| Hexadecanoic acid | – | 3.25 | 2.47 | – | 0.42 |
| Senecionine | – | 60.35 | 10.25 | 24.89 | 28.97 |
| 9-octadecenamide | – | 1.15 | – | – | – |
| Methyl commate d | – | 8.14 | 7.96 | 2.92 | 5.54 |
| Hentriacontane | – | 2.35 | – | 17.87 | – |
| Eicosane | – | – | – | 0.74 | – |
| Pentacosane | – | – | – | 12.78 | 0.53 |
| Tetratetracontane | – | – | – | 13.09 | 1.31 |
| Celidoniol, deoxy- | – | – | 0.69 | 12.71 | 22.47 |
| 14,16-hentriacontanedione | – | – | – | 2.35 | – |
| Andrographolide | – | – | 0.83 | – | – |
| Ethyl linoleate | – | – | 1.64 | – | – |
| N-Isobutyl-tetradeca-2,4-dienamide | – | – | 1.64 | – | 0.74 |
| N-Terpinenyl ester of n-pentanoic acid | – | – | 1.76 | – | – |
| 1-pentacontanol | – | – | 2.11 | – | – |
| Methyl commate c | – | – | 3.27 | – | 3.76 |
| Patchoulane | – | – | – | – | 1.02 |
| Usaramine | – | – | 2.17 | – | 0.83 |
Table 9.
Chemical compounds obtained using PLE for the stalk of Senecio brasiliensis
| Assay | Relative area (%) | ||||
|---|---|---|---|---|---|
| Stalk | |||||
| Compounds | 1 | 2 | 3 | 4 | 5/6/7 |
| 2-Propanone, 1,3-dihydroxy- (CAS) | 4.09 | 6.86 | 4.97 | 6.21 | 7.67 |
| Propanoic acid, 2-oxo-, methyl ester | 5.07 | – | 1.07 | – | |
| Dl-Glyceraldehyde | 9.05 | – | 3.90 | – | 5.40 |
| 2-furancarboxaldehyde, 5-(hydroxymethyl) | – | – | 1.80 | – | – |
| Spathulenol | 7.74 | – | 7.43 | – | 5.49 |
| 3.36 Caryophyllene oxide | – | 3.36 | |||
|
Androstan-17-one, 3-ethyl-3-hydroxy-, (5.alpha.)- (CAS) |
5.83 | – | – | – | – |
| 4.24 Phenol, 4-(3-hydroxy-1-propenyl)-2-methoxy- (CAS) | 4.24 | – | 1.04 | 14.20 | 5.30 |
| 1.34 2-Pentadecanone, 6,10,14-trimethyl- (CAS) | 1.34 | – | 2.47 | 5.25 | 3.02 |
| 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) | – | – | 3.88 | 2.36 | 1.73 |
| 4-Benzyloxybenzoic acid | 2.74 | ||||
| Heptadecanoic acid, ethyl ester (CAS) | 7.46 | – | 1.32 | 6.19 | 2.37 |
| Neophytadiene | – | – | 4.34 | – | 1.98 |
| 1-Heneicosyl formate | – | 7.33 | |||
| N-Methyl-l-prolinol | – | 19.10 | – | – | |
| 6-(2-methoxy-phenyl)-5,7-diphenyl-2,3,6,7-tetrahydro-1 h-pyrrolo[3,4-e][1,4]diazepin-8-one | – | 5.37 | – | – | |
| Quinic acid | – | 9.46 | – | – | |
| 1-eicosanol | – | – | 7.64 | – | 2.44 |
| Hexadecanoic acid, ethyl ester (CAS) | 8.27 | 16.72 | 1.82 | – | 12.93 |
| Cholesta-4,6-dien-3-ol, (3.beta.)- (CAS) | – | 4.56 | 2.14 | 8.85 | 4.49 |
| Stigmast-5-en-3-ol, (3.beta.)- (CAS) | 14.98 | 30.0 | 12.39 | 11.80 | 18.18 |
| Stigmast-4-en-3-one | 14.65 | – | 7.71 | 10.0 | 5.77 |
| Integerrimine | 17.28 | 7.93 | 24.32 | 23.61 | 9.36 |
| Senecionine | – | – | 3.36 | 4.20 | 0.79 |
| 5 h-1-pyrindine | – | – | 2.30 | – | 2.24 |
| 9-octadecenamide | – | 10.84 | |||
Table 10.
Chemical compounds obtained using MHG for the leaf, flower, and stalk of Senecio brasiliensis
| Assay | Relative area (%) | |||||
|---|---|---|---|---|---|---|
| Leaf | Flower | Stalk | ||||
| Water | Water/Ethanol | Water | Water/Ethanol | Water | Water/Ethanol | |
| Compounds | ||||||
| 2-Hexadecen-1-ol, 3,7,11,15-tetramethyl-, [R-[R*,R*-(E)]]- (CAS) | 23.49 | – | – | – | – | 8.45 |
| 9-Octadecenamide, (Z)- (CAS) | 20.0 | – | – | – | 17.02 | – |
| 5H-1-Pyrindine | 5.01 | 5.78 | 6.17 | 5.78 | 9.09 | |
| GERMACRENE-D | – | – | 9.98 | – | 12.21 | 24.29 |
| Senecionine | 1.36 | – | 6.83 | – | 2.28 | – |
| Integerrimine | 50.14 | 94.22 | 77.02 | 79.23 | 51.37 | 58.17 |
| 2,3-Butanediol | – | – | – | 14.99 | – | – |
| Bicyclo[2.2.1]heptane, 2-cyclopropylidene-1,7,7-trimethyl | – | – | – | – | 17.12 | – |
The extracts obtained by UAE from the S. brasiliensis leaves presented 17 different constituents, the flower extracts presented 13 constituents, and the stalk extracts 14 constituents as shown in Tables 4, 5, and 6, respectively. The UAE method was very efficient for pyrrolizidine alkaloids extraction. The results showed that integerrimine was the major compound, representing approximately 50% of the total peak area. Confortin et al. (2019) corroborate with the findings of this work, once using supercritical carbon dioxide, they obtained high levels of pyrrolizidine alkaloids such as integerrimine as a major compound in flowers and leaves of Senecio brasiliensis. It is worth mentioning that the pyrrolizidine alkaloid senecionine was also extracted from the matrices leaf and flower in this work.
Different compounds were found in different conditions of power intensity and duty cycle of the US (Tables 4, 5, and 6) for the three matrices tested. The highest amount of compounds was found at a power intensity of 85 W/cm2 and a duty cycle of 0.75 s/s. High values of power intensity and duty cycle were also responsible for highest yields of extraction, which means that the highest the US variables, the highest the extraction of the bioactive compounds and the highest the yields. The violent bubble collapses generated by the ultrasound cavitational phenomenon, which causes the destruction of cell walls and, consequently, favors the solvent access to the analytes that can explain this behavior (Goltz et al. 2018; Santos et al. 2019a). When comparing the maceration techniques and UAE in terms of the number of compounds extracted, it is evident that the UAE surpassed the conventional method of extraction. This result is corroborated by Das and Eun (2018), who compared the UAE technique with conventional extraction and obtained a significantly highest amount of bioactive metabolites from green tea extract using UAE.
In the extracts obtained by PLE, 29 compounds were identified for matrix leaf, 37 for flower, and 26 for stalk matrices (Tables 7, 8, and 9). The extracts from all the three matrices presented pyrrolizidine alkaloids in their constitution. When comparing the peak areas of the pyrrolizidine alkaloids obtained by PLE and UAE, PLE extracts presented lowest percentages of peak areas. Those findings can be explained by the fact that the PLE technique extracted a larger amount of bioactive compounds than the UAE technique, thus reducing the concentration of pyrrolizidine alkaloids in the extract. The compounds were extracted in a largest amount when using 60 °C and 70% (v/v) ethanol for all the matrices. These results can be explained by the characteristics of the solvent, once hydroalcoholic solutions solubilize hydrophilic and lipophilic compounds (Hirondart et al. 2020). Water and ethanol mixture is capable of dissolving moderate to high polarity compounds and that is appreciable when it is expected to extract a large variety of compounds (Barrales et al. 2018).
It should be noted that integerrimine was found in highest percentages in the mentioned condition (60 °C and 70% (v/v) ethanol). This technique was efficient for integerrimine extraction, once for the leaf it represented 35.27% of the total peak area, for the flower it was 46.24% and 24.32% for the stalk. The results obtained in the PLE are corroborated by Viganó et al. (2016), which also obtained better results in the condition of 60 °C and 70% ethanol when extracting bioactive compounds from passion fruit peel. Kopp et al. (2020) state that PLE is an efficient method for PA extraction and it can lead to considerably enhanced recovery rates when compared with conventional techniques, being an improved extraction strategy.
For the extractions carried out using MHG, eight different compounds were obtained and identified (Table 10). Despite the smallest number of extracted compounds when compared with the other extraction methods, MHG extraction was efficient and selective for integerrimine extraction, presenting for all the matrices more than 50% of the relative peak area for such compounds. It is important to say that one of MHG benefits is its speed, once 20 min was enough to extract a high percentage of the target compound. This result is corroborated by Benmoussa et al. (2018), who reported that in 16 min of MHG extraction satisfactory levels of cumin essential oil were obtained. When using hydroalcoholic solution, the percentages of integerrimine are more pronounced than when using only water, reaching values of 94.22% for leaf, 79.23% for flower, and 58.17% for the stalk. This is a promising result, once both solvents used are considered eco-friendly and it contributes to the use of more sustainable solutions (Prat et al. 2015).
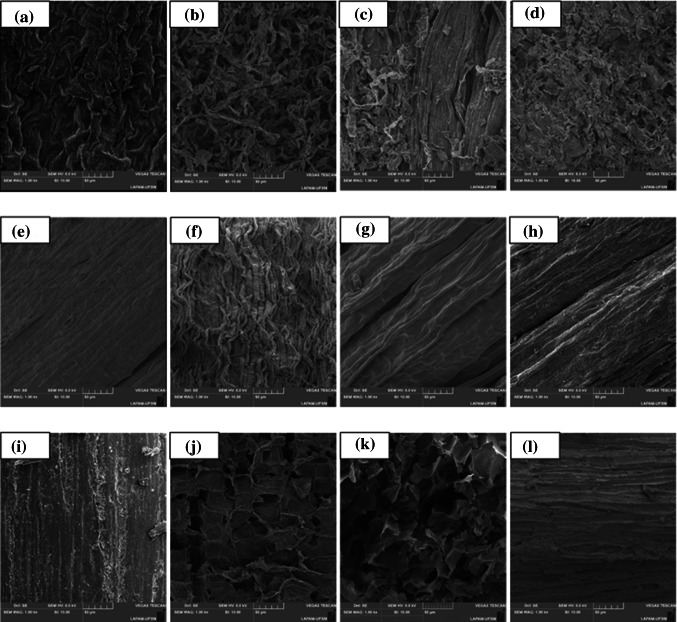
To have a best understanding of the results, the matrices were submitted to SEM analysis. Notably, structural changes occurred in the matrices when comparing before and after extraction through the three techniques used (Fig. 3). The surfaces of the matrices after UAE and PLE showed more visible changes when compared with the samples before extraction. It is clear that the cells are more damaged after UAE and PLE. The wall presents notable breaks, with the appearance of cracks. This may explain the highest yields and number of compounds obtained by these two techniques, as the ruptures facilitate the release of the compounds. When analyzing the MHG samples, a more integrity of the matrices cell wall can be observed after the extraction, but when comparing with the samples before extraction, slight cell disruption is noted. This explain the lowest yield and number of compounds when comparing to the other extraction techniques, once there was not an easy way of water release, which acts as compounds transporter.
Fig. 3.
Scanning electron microscopy of Senecio brasiliensis matrices before and after extractions: A before extraction (fresh raw material); B UAE; C PLE; D MHG (1) leaf; (2) flower; (3) stalk
Conclusion
The results obtained in this research reveal that plant Senecio brasiliensis has pyrrolizidine alkaloids in its constitution. Considering this is the first report to use UAE, PLE, and MHG for obtaining integerrimine rich extracts, it can be concluded that the techniques were efficient, being considered fast and eco-friendly. The UAE and PLE techniques were preferable, once they enabled higher yields and a larger number of extracted compounds. In addition to the pyrrolizidine compounds, many others were found, which encourages further studies regarding the biological activity.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Council for Scientific and Technological Development (CNPq) under Grant [number 308936/2017-5; 428180/2018-3; 306241/2020-0]; Coordination for the Improvement of Higher Education Personnel (CAPES) under Grant [number 001]; and Research Support Foundation of the State of Rio Grande do Sul (FAPERGS) under Grant [number 16/2551-0000522-2].
Declarations
Conflict of interest
The authors confirm that there are no conflicts of interest regarding this work.
References
- Barrales FM, Silveira P, de Barbosa PPM, et al. Recovery of phenolic compounds from citrus by-products using pressurized liquids—an application to orange peel. Food Bioprod Process. 2018;112:9–21. [Google Scholar]
- Barretto D, Vootla S. Gc-Ms analysis of bioactive compounds and antimicrobial activity of Cryptococcus rajasthanensis Ky627764 isolated from bombyx mori gut microflora. Int J Adv Res. 2018;6:525–538. [Google Scholar]
- Benmoussa H, Elfalleh W, He S, et al. Microwave hydrodiffusion and gravity for rapid extraction of essential oil from Tunisian cumin (Cuminum cyminum L.) seeds: optimization by response surface methodology. Ind Crops Prod. 2018;124:633–642. [Google Scholar]
- Bousbia N, Abert M, Ferhat MA, et al. Comparison of two isolation methods for essential oil from rosemary leaves: hydrodistillation and microwave hydrodiffusion and gravity. Food Chem. 2009;114:355–362. [Google Scholar]
- Bülent Köse Y, Iscan G, Demirci B. Antimicrobial activity of the essential oils obtained from flowering aerial parts of Centaurea lycopifolia Boiss. et Kotschy and Centaurea cheirolopha (Fenzl) Wagenitz from Turkey. J Essent Oil Bear Plants. 2016;19:762–768. [Google Scholar]
- Carabias-Martínez R, Rodríguez-Gonzalo E, Revilla-Ruiz P, Hernández-Méndez J. Pressurized liquid extraction in the analysis of food and biological samples. J Chromatogr A. 2005;1089:1–17. doi: 10.1016/j.chroma.2005.06.072. [DOI] [PubMed] [Google Scholar]
- Castejón N, Luna P, Señorans FJ. Ultrasonic removal of mucilage for pressurized liquid extraction of omega-3 rich oil from chia seeds (Salvia hispanica L.) J Agric Food Chem. 2017;65:2572–2579. doi: 10.1021/acs.jafc.6b05726. [DOI] [PubMed] [Google Scholar]
- Casuga FP, Castillo AL, Corpuz MJAT. GC–MS analysis of bioactive compounds present in different extracts of an endemic plant Broussonetia luzonica (Blanco) (Moraceae) leaves. Asian Pac J Trop Biomed. 2016;6:957–961. [Google Scholar]
- Chemat F, Zill-e-Huma, Khan MK. Applications of ultrasound in food technology: processing, preservation and extraction. Ultrason Sonochem. 2011;18:813–835. doi: 10.1016/j.ultsonch.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Chemat F, Rombaut N, Meullemiestre A, et al. Review of green food processing techniques. Preservation, transformation, and extraction. Innov Food Sci Emerg Technol. 2017;41:357–377. [Google Scholar]
- Chen M, Du Y, Zhu G, et al. Action of six pyrethrins purified from the botanical insecticide pyrethrum on cockroach sodium channels expressed in Xenopus oocytes. Pestic Biochem Physiol. 2018;151:82–89. doi: 10.1016/j.pestbp.2018.05.002. [DOI] [PubMed] [Google Scholar]
- Cheong MW, Tan AAA, Liu SQ, et al. Pressurised liquid extraction of volatile compounds in coffee bean. Talanta. 2013;115:300–307. doi: 10.1016/j.talanta.2013.04.034. [DOI] [PubMed] [Google Scholar]
- Chhouk K, Wahyudiono KH, Goto M. Efficacy of supercritical carbon dioxide integrated hydrothermal extraction of Khmer medicinal plants with potential pharmaceutical activity. J Environ Chem Eng. 2018;6:2944–2956. [Google Scholar]
- Confortin TC, Todero I, Canabarro NI, et al. Supercritical CO2 extraction of compounds from different aerial parts of Senecio brasiliensis: mathematical modeling and effects of parameters on extract quality. J Supercrit Fluids. 2019;153:104589. [Google Scholar]
- Cui Q, Wang LT, Liu JZ, et al. Rapid extraction of Amomum tsao-ko essential oil and determination of its chemical composition, antioxidant and antimicrobial activities. J Chromatogr B Anal Technol Biomed Life Sci. 2017;1061–1062:364–371. doi: 10.1016/j.jchromb.2017.08.001. [DOI] [PubMed] [Google Scholar]
- Das PR, Eun JB. A comparative study of ultra-sonication and agitation extraction techniques on bioactive metabolites of green tea extract. Food Chem. 2018;253:22–29. doi: 10.1016/j.foodchem.2018.01.080. [DOI] [PubMed] [Google Scholar]
- De Souza RR, Bretanha LC, Dalmarco EM, et al. Modulatory effect of Senecio brasiliensis (Spreng) Less. in a murine model of inflammation induced by carrageenan into the pleural cavity. J Ethnopharmacol. 2015;168:373–379. doi: 10.1016/j.jep.2015.03.032. [DOI] [PubMed] [Google Scholar]
- Elias F, Latorre AO, Pípole F, et al. Haematological and immunological effects of repeated dose exposure of rats to integerrimine N-oxide from Senecio brasiliensis. Food Chem Toxicol. 2011;49:2313–2319. doi: 10.1016/j.fct.2011.06.032. [DOI] [PubMed] [Google Scholar]
- Farias CAA, Moraes DP, Lazzaretti M, et al. Microwave hydrodiffusion and gravity as pretreatment for grape dehydration with simultaneous obtaining of high phenolic grape extract. Food Chem. 2021;337:127723. doi: 10.1016/j.foodchem.2020.127723. [DOI] [PubMed] [Google Scholar]
- Ferreira DF, Lucas BN, Voss M, et al. Solvent-free simultaneous extraction of volatile and non-volatile antioxidants from rosemary (Rosmarinus officinalis L.) by microwave hydrodiffusion and gravity. Ind Crops Prod. 2020;145:112094. [Google Scholar]
- Filly A, Fernandez X, Minuti M, et al. Solvent-free microwave extraction of essential oil from aromatic herbs: from laboratory to pilot and industrial scale. Food Chem. 2014;150:193–198. doi: 10.1016/j.foodchem.2013.10.139. [DOI] [PubMed] [Google Scholar]
- Foo LW, Salleh E, Nur S, Mamat H. P-53: extraction and qualitative analysis of piper betle leaves for antimicrobial activities. Int J Eng Technol Sci Res. 2015;2:1–8. [Google Scholar]
- Gallo M, Formato A, Ianniello D, et al. Supercritical fluid extraction of pyrethrins from pyrethrum flowers (Chrysanthemum cinerariifolium) compared to traditional maceration and cyclic pressurization extraction. J Supercrit Fluids. 2017;119:104–112. [Google Scholar]
- Goltz C, Ávila S, Barbieri JB, et al. Ultrasound-assisted extraction of phenolic compounds from Macela (Achyrolcine satureioides) extracts. J Supercrit Fluids. 2018;115:253–262. [Google Scholar]
- Gonzalez CP, Vega RS, González-Chávez M, et al. Anti-inflammatory activity and composition of Senecio salignus Kunth. Biomed Res Int. 2013;2013:814693. doi: 10.1155/2013/814693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Ma Z, Kou H, et al. Synergistic effects of pyrrolizidine alkaloids and lipopolysaccharide on preterm delivery and intrauterine fetal death in mice. Toxicol Lett. 2013;221:212–218. doi: 10.1016/j.toxlet.2013.06.238. [DOI] [PubMed] [Google Scholar]
- Hirondart M, Rombaut N, Fabiano-Tixier AS, et al. Comparison between pressurized liquid extraction and conventional Soxhlet extraction for rosemary antioxidants, yield, composition, and environmental footprint. Foods. 2020;9:584. doi: 10.3390/foods9050584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MB, Barry-Ryan C, Martin-Diana AB, Brunton NP. Optimisation of accelerated solvent extraction of antioxidant compounds from rosemary (Rosmarinus officinalis L.), marjoram (Origanum majorana L.) and oregano (Origanum vulgare L.) using response surface methodology. Food Chem. 2011;126:339–346. [Google Scholar]
- Kaczyński P, Łozowicka B. A novel approach for fast and simple determination pyrrolizidine alkaloids in herbs by ultrasound-assisted dispersive solid phase extraction method coupled to liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2020;187:113351. doi: 10.1016/j.jpba.2020.113351. [DOI] [PubMed] [Google Scholar]
- Karam FSC, Soares MP, Haraguchi M, et al. Aspectos epidemiológicos da seneciose na região sul do Rio Grande do Sul. Pesqui Vet Bras. 2004;24:191–198. [Google Scholar]
- Kopp T, Salzer L, Abdel-Tawab M, Mizaikoff B. Efficient extraction of pyrrolizidine alkaloids from plants by pressurised liquid extraction—a preliminary study. Planta Med. 2020;86:85–90. doi: 10.1055/a-1023-7419. [DOI] [PubMed] [Google Scholar]
- Lee PS, Shin DH, Lee KM, et al. Effects of guanosine on the pharmacokinetics of acriflavine in rats following the administration of a 1:1 mixture of acriflavine and guanosine, a potential antitumor agent. Arch Pharm Res. 2007;30:372–380. doi: 10.1007/BF02977621. [DOI] [PubMed] [Google Scholar]
- López-Hortas L, Conde E, Falqué E, Domínguez H. Flowers of Ulex europaeus L.—comparing two extraction techniques (MHG and distillation) Comptes Rendus Chim. 2016;19:718–725. [Google Scholar]
- Macedo GE, Gomes KK, Rodrigues NR, et al. Senecio brasiliensis impairs eclosion rate and induces apoptotic cell death in larvae of Drosophila melanogaster. Comp Biochem Physiol Part C Toxicol Pharmacol. 2017;198:45–57. doi: 10.1016/j.cbpc.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Machado APDF, Pasquel-Reátegui JL, Barbero GF, Martínez J. Pressurized liquid extraction of bioactive compounds from blackberry (Rubus fruticosus L.) residues: a comparison with conventional methods. Food Res Int. 2015;77:675–683. [Google Scholar]
- Mustafa A, Turner C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: a review. Anal Chim Acta. 2011;703:8–18. doi: 10.1016/j.aca.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Pereira DTV, Tarone AG, Cazarin CBB, et al. Pressurized liquid extraction of bioactive compounds from grape marc. J Food Eng. 2019;240:105–113. [Google Scholar]
- Pilati C, Barros CSL. Intoxicação experimental por Senecio brasiliensis (Asteraceae) em eqüinos. Pesqui Vet Bras. 2007;27:287–296. [Google Scholar]
- Prat D, Wells A, Hayler J, et al. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2015;18:288–296. [Google Scholar]
- Ratmanova NK, Andreev IA, Leontiev AV, et al. Strategic approaches to the synthesis of pyrrolizidine and indolizidine alkaloids. Tetrahedron. 2020;76:131031. [Google Scholar]
- Sallet D, Souza PO, Fischer LT, et al. Ultrasound-assisted extraction of lipids from Mortierella isabellina. J Food Eng. 2019;242:1–7. [Google Scholar]
- Sandini TM, Udo MSB, Spinosa HDS. Senecio brasiliensis e alcaloides pirrolizidínicos: toxicidade em animais e na saúde humana. Biotemas. 2013;26:83–92. [Google Scholar]
- Santos KA, Gonçalves JE, Cardozo-Filho L, da Silva EA. Pressurized liquid and ultrasound-assisted extraction of α-bisabolol from candeia (Eremanthus erythropappus) wood. Ind Crops Prod. 2019;130:428–435. [Google Scholar]
- Santos KA, Klein EJ, da Silva C, et al. Extraction of vetiver (Chrysopogon zizanioides) root oil by supercritical CO2, pressurized-liquid, and ultrasound-assisted methods and modeling of supercritical extraction kinetics. J Supercrit Fluids. 2019;150:30–39. [Google Scholar]
- Setyaningsih W, Saputro IE, Palma M, Barroso CG. Pressurized liquid extraction of phenolic compounds from rice (Oryza sativa) grains. Food Chem. 2016;192:452–459. doi: 10.1016/j.foodchem.2015.06.102. [DOI] [PubMed] [Google Scholar]
- Sharayei P, Azarpazhooh E, Zomorodi S, Ramaswamy HS. Ultrasound assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peel. Lwt. 2019;101:342–350. [Google Scholar]
- Soquetta MB, de Terra LM, Bastos CP. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CYTA J Food. 2018;16:400–412. [Google Scholar]
- Takshak S, Agrawal SB. Defense potential of secondary metabolites in medicinal plants under UV-B stress. J Photochem Photobiol B Biol. 2019;193:51–88. doi: 10.1016/j.jphotobiol.2019.02.002. [DOI] [PubMed] [Google Scholar]
- Thakur M, Bhattacharya S, Khosla PK, Puri S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J Appl Res Med Aromat Plants. 2019;12:1–12. [Google Scholar]
- Toma W, Trigo JR, Bensuaski de Paula AC, Monteiro Souza Brito AR. Modulation of gastrin and epidermal growth factor by pyrrolizidine alkaloids obtained from Senecio brasiliensis in acute and chronic induced gastric ulcers. Can J Physiol Pharmacol. 2004;82:319–325. doi: 10.1139/y04-023. [DOI] [PubMed] [Google Scholar]
- Trigo JR, Leal IR, Matzenbacher NI, Lewinsohn TM. Chemotaxonomic value of pyrrolizidine alkaloids in southern Brazil Senecio (Senecioneae: Asteraceae) Biochem Syst Ecol. 2003;31:1011–1022. [Google Scholar]
- Trojanowska A, Tsibranska I, Dzhonova D, et al. Ultrasound-assisted extraction of biologically active compounds and their successive concentration by using membrane processes. Chem Eng Res Des. 2019 doi: 10.1016/j.cherd.2019.05.018. [DOI] [Google Scholar]
- Viganó J, Brumer IZ, de Braga PAC, et al. Pressurized liquids extraction as an alternative process to readily obtain bioactive compounds from passion fruit rinds. Food Bioprod Process. 2016;100:382–390. [Google Scholar]
- Wei YQ, Sun MM, Fang HY. Dienzyme-assisted salting-out extraction of flavonoids from the seeds of Cuscuta chinensis Lam. Ind Crops Prod. 2019;127(232):236. [Google Scholar]
- Wiedenfeld H, Edgar J. Toxicity of pyrrolizidine alkaloids to humans and ruminants. Phytochem Rev. 2011;10:137–151. [Google Scholar]
- Xu J, Wang W, Yang X, et al. Pyrrolizidine alkaloids: an update on their metabolism and hepatotoxicity mechanism. Liver Res. 2019;3:176–184. [Google Scholar]
- Yu HB, Ding LF, Wang Z, Shi LX. Study on extraction of polyphenol from grape peel microwave-assisted activity. Adv Mater Res. 2013;864–867:520–525. [Google Scholar]
- Zaibunnisa AH, Norashikin S, Mamot S, Osman H. An experimental design approach for the extraction of volatile compounds from turmeric leaves (Curcuma domestica) using pressurised liquid extraction (PLE) LWT Food Sci Technol. 2009;42:233–238. [Google Scholar]
- Zakaria MB, Vijayasekaran K, Ilham Z, Muhamad NA. Anti-inflammatory activity of Calophyllum inophyllum fruits extracts. Procedia Chem. 2014;13:218–220. [Google Scholar]
- Zaynab M, Fatima M, Abbas S, et al. Role of secondary metabolites in plant defense against pathogens. Microb Pathog. 2018;124:198–202. doi: 10.1016/j.micpath.2018.08.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.