Abstract
Background
Many DNA methylation-based indicators have been developed as summary measures of epigenetic aging. We examine the associations between 13 epigenetic clocks, including 4 second generation clocks, as well as the links of the clocks to social, demographic, and behavioral factors known to be related to health outcomes: sex, race/ethnicity, socioeconomic status, obesity, and lifetime smoking pack-years.
Methods
The Health and Retirement Study is the data source which is a nationally representative sample of Americans over age 50. Assessment of DNA methylation was based on the EPIC chip and epigenetic clocks were developed based on existing literature.
Results
The clocks vary in the strength of their relationships with age, with each other and with independent variables. Second generation clocks trained on health-related characteristics tend to relate more strongly to the sociodemographic and health behaviors known to be associated with health outcomes in this age group.
Conclusions
Users of this publicly available data set should be aware that epigenetic clocks vary in their relationships to age and to variables known to be related to the process of health change with age.
Keywords: DNA methylation, DunedinPoAm38, Epigenetic age, GrimAge, PhenoAgeAcceleration
Studies of aging in large populations have increasingly included indicators of molecular and cellular aging in their collection efforts. These inclusions have been encouraged by developments in geroscience which have identified biological “hallmarks of aging” which population studies are beginning to incorporate (1–4). One hallmark of aging is epigenetic change or change in DNA methylation (DNAm) linked to age. In recent years, a number of epigenetic clock measures have been proposed for measuring an individual’s epigenetic age and by comparison with chronological age to indicate accelerated or decelerated aging (5). Epigenetic age is thought to have value as a composite measure of aging which can be measured across the life span, can be measured with 1 blood-based or saliva test, and which may prove to be a target of intervention for delaying aging (5–7). Several indicators have been described as “clocks” ticking away time or the years of aging. When compared with chronological age, these clocks lead to an estimate of the years of aging acceleration and deceleration for individuals.
Epigenetic change has been linked to age but also to many health outcomes associated with age including physiological dysregulation (6,8), physical and cognitive functioning (6,9), frailty (10), chronic disease (6,11), and mortality (11–13). Epigenetic age has also been associated with a number of indicators of life-span adversity which are, in turn, related to health outcomes of aging including adult socioeconomic status (14) and childhood adversity, both socioeconomic and emotional (6,15). Some measures have been shown to differ by sex (8,16) and race (17). Although most analyses have been done within European origin populations, results within African American samples also find associations of methylation measures with health outcomes (16,18). Accelerated epigenetic aging has also been related to health behaviors such as body mass index or obesity, physical activity, alcohol consumption, smoking, and diet (14,16,19). Thus, epigenetic aging may be a mechanism by which social and behavioral factors get under the skin to affect the rate of aging and the onset of downstream health problems. This does not imply that only social and behavioral factors affect methylation. Methylation as well as social factors may be affected by genetic and other environmental factors.
Multiple indicators of biological aging based on DNAm have been suggested and are used in the literature (20). Initially these measures were developed by associating age with indicators of DNAm (5,21); these are the clocks we term first generation clocks. Four of the more recently developed measures, known as second generation clocks are based on the links between methylation and health risks or outcomes (DunedinPoAm38 (6); PhenoAgeAccel (13); GrimAge (11); Zhang (22)). For instance, Zhang et al’s (22) score reflects disease-related genetic markers; Levine’s (13) clock was trained to measures of morbidity and mortality; the Horvath group’s GrimAge (11) incorporated multiple DNAm-based plasma protein estimates, a DNAm pack-years of smoking estimate, and age and sex, and mortality; the DunedinPoAm38 clock was trained on changes in a measure of the pace of aging between the ages of 26 and 38 in a single birth cohort. This measure included change over 12 years in 18 blood chemistry or organ system functioning indicators comprising the Pace of Aging measure (6). So this clock is based on health change with age and may actually be the first of a third generation of clocks.
Levine and colleagues have described the features of 11 of these clocks and indicated the variability in their molecular characteristics and their functional associations (23). They clarify that the clocks are based on the assessment of methylation at widely varying numbers of CpG sites and were developed in different tissues. While all the clocks are supposed to measure or be related to accelerated aging, they do not appear to all be highly related to each other (6,8,17). Not all of these epigenetic measures relate to outcomes in the same way (9); nor do they appear to relate to independent variables reflecting risk for poor health outcomes in the same way including sex, race and ethnicity, and measures of socioeconomic hardship, adversity, and health behaviors (8,14,17,19). Differences in the associations of the clocks with variables recognized as risk factors for age-related outcomes could lead to variability in findings that will make it difficult to generalize about the role of epigenetic change as a determinant of health outcomes. Understanding which risk factors relate to which clocks may help researchers synthesize existing research findings as well as shed light on the source of the differences in the epigenetic measures.
We examine 13 indicators of epigenetic age in the Health and Retirement Study (HRS), a nationally representative sample of Americans over the age of 50 with extensive data on both lifetime social and economic conditions as well as health outcomes. Our intent is to clarify how these epigenetic clocks are associated with basic demographic characteristics including age, sex, race/ethnicity, and education, a measure of lifetime socioeconomic status. In addition, we link the epigenetic clocks to 2 measures of health behaviors: current obesity and current and past smoking behavior. These clocks have been released by HRS in a public use file and the intent of the current paper is to provide researchers with descriptive comparisons of the clocks which will be useful to users of these data as well as those using data on clocks in general. The focus of our analysis is to see how the clocks relate to each other and how similarly the clocks relate to demographic and social variables in a relatively large and representative sample of older Americans. We also note how the second generation of clocks differs from the first generation of clocks in relationships.
Method
The HRS Methylation Sample
DNA methylation assays were done on a nonrandom subsample (N = 4018) of people who participated in the Health and Retirement 2016 Venous Blood Study (24). The sample used for regressions equations in 3966 because of missing data in the independent variables. The weighted sample is 54.3% female and has a median age of 66 years and ranges in age from 50 to 100. It has racial diversity: non-Hispanic White and others (81.1%), non-Hispanic Black (10.0%), and Hispanic (8.9%). The sample is also socioeconomically diverse as indicated by the educational distribution: less than high school (14.0%), high school/GED (29.9%), some college (25.8%), and college+ (30.3%). More than a third of the sample is obese (44.5%), 11.0% are current smokers, and 44.2% are former smokers. The sample is weighted to be representative of the U.S. population.
Methods for DNAm
DNA methylation data are based on assays using the Infinium Methylation EPIC BeadChip completed at the Advanced Research and Diagnostics Laboratory at the University of Minnesota. Samples were randomized across plates by key demographic variables (ie, age, cohort, sex, education, race/ethnicity) with 39 pairs of blinded duplicates. Analysis of duplicate samples showed a correlation >0.97 for all CpG sites. The minfi package in R software was used for data preprocessing, and quality control; 3.4% of the methylation probes (n = 29 431 out of 866 091) were removed from the final data set due to suboptimal performance (using a detection p-value threshold of 0.01). Analysis for detection p-value failed samples was done after removal of detection p-value failed probes. Using a 5% cutoff (minfi) we removed 58 samples. We also removed sex-mismatched samples and any controls (cell lines, blinded duplicates). High-quality methylation data are available for 97.9% of the samples (n = 4018).
DNAm Measures
Thirteen epigenetic clocks have been estimated from the HRS data; as indicated above 9 of these clocks are first generation and trained on age, while the 4 second generation clocks were trained on health-related outcomes (Zhang, PhenoAge, GrimAge, DunedinPoAm38). Eleven of these clocks were estimated independently by Morgan Levine as well as HRS staff in order to ensure reliability. GrimAge was estimated by HRS staff with assistance of Steve Horvath; and the 13th clock, DunedinPoAm38 was estimated by Eileen Crimmins and Thalida Arpawong with the assistance of Karen Sugden (6). Descriptive data on the 13 clocks are shown in Table 1. Most clocks are identified by the name associated with the seminal article introducing the clock; however, some clocks are identified in the literature by other names which are shown (eg, PhenoAge, GrimAge, DunedinPoAm38). Eight of the clocks are expressed in units of years (Table 1); DunedinPoAm38 is measured in years of accelerated aging per year of aging or “years of physiological decline occurring per 12 months of calendar time (6).”
Table 1.
Descriptive Measures for DNA Methylation Epigenetic Clocks and Pearson Correlation With Age: HRS (N = 4018)
| Mean | SD | Minimum | Maximum | Correlation With Age | |
|---|---|---|---|---|---|
| Clocks | |||||
| Horvath 1 (2013) | 65.01 | 9.31 | 23.31 | 114.52 | 0.73**** |
| Hannum (2013) | 53.83 | 8.84 | 25.06 | 107.79 | 0.82**** |
| Lin (2015) (25) | 57.59 | 10.62 | 1.91 | 133.27 | 0.69**** |
| Weidner (2014) | 66.78 | 11.46 | 25.22 | 148.87 | 0.40**** |
| VidalBralo (2016) (26) | 63.52 | 6.09 | 36.47 | 109.95 | 0.56**** |
| Horvath 2 (2018) (27) | 68.78 | 8.55 | 36.97 | 101.29 | 0.86**** |
| Yang (2016) (28) | 0.07 | 0.02 | 0.03 | 0.23 | 0.26**** |
| Bocklandt (2011) (29) | 0.39 | 0.08 | 0.10 | 0.89 | −0.39**** |
| Garagnani (2012) (30) | 0.71 | 0.07 | 0.43 | 0.99 | 0.67**** |
| Zhang (2017) | −1.10 | 0.46 | −2.53 | 0.60 | 0.31**** |
| Levine - PhenoAge (2018) | 56.58 | 9.89 | 26.72 | 101.68 | 0.72**** |
| Lu - GrimAge (2019) | 67.07 | 8.47 | 42.67 | 99.61 | 0.83**** |
| DunedinPoAm38 (2020) | 1.07 | 0.09 | 0.74 | 1.46 | 0.06*** |
| Accelerated aging† | |||||
| Horvath 1 AccelAge | 0.00 | 6.31 | −36.28 | 48.30 | 0.00 |
| Hannum AccelAge | 0.00 | 5.08 | −29.77 | 45.66 | 0.00 |
| Lin AccelAge | 0.00 | 7.67 | −54.63 | 58.54 | 0.00 |
| Weidner AccelAge | 0.00 | 10.51 | −35.00 | 71.45 | 0.00 |
| VidalBralo AccelAge | 0.00 | 5.04 | −31.35 | 40.27 | 0.00 |
| Horvath 2 AccelAge | 0.00 | 4.40 | −24.35 | 22.45 | 0.00 |
| Yang AccelAge | 0.00 | 0.02 | −0.04 | 0.16 | 0.00 |
| Bocklandt AccelAge | 0.00 | 0.07 | −0.31 | 0.53 | 0.00 |
| Garagnani AccelAge | 0.00 | 0.05 | −0.31 | 0.28 | 0.00 |
| Zhang AccelAge | 0.00 | 0.44 | −1.36 | 1.73 | 0.00 |
| Levine - PhenoAccelAge | 0.00 | 6.84 | −28.05 | 42.07 | 0.00 |
| Lu - GrimAge AccelAge | 0.00 | 4.75 | −16.70 | 22.66 | 0.00 |
| DunedinPoAm38 AccelAge | 0.00 | 0.09 | −0.33 | 0.39 | 0.00 |
Notes: HRS = Health and Retirement Study; SD = standard deviation.
†Accelerated aging is the residual from a regression of the clock on chronological age.
****p < .0001; ***p < .001.
The clocks have very different mean values, ranges, minimum and maximum ages (Table 1). Some of the clocks expressed in years have very high maximum ages (eg, Lin 133 and Weidner 148) and some very low minimum ages (eg, Lin 1.9). The DunedinPoAm38 clock as expected has a mean close to 1, as on average the pace of aging is 1 year of aging with a year of age, and a range of about three-quarters of a year to a year and a half (0.74–1.46). Age acceleration was calculated for each clock taking the residual of the clock values regressed on age, so they can all be compared without the effect of age. As expected, average age acceleration is 0 for all of the clocks; again, the lowest value indicating decelerated aging and the highest value indicating accelerated aging cover a large range (eg, the Lin clock −55 years to +59 years).
Analytic Approach
First we examine the correlations of the clocks with age; then we examine the correlations among the 13 clocks, and measures of accelerated aging, to see how they relate to each other. Next we perform ordinary least squares regressions of accelerated aging measures on sex, education, race/ethnicity, obesity I and obesity II, and being a former or current smoker to examine the independent association of each variable with each aging acceleration measure. We then rerun the regressions controlling for cell distribution using 4 different approaches to estimating cell composition (31).
Results
All clock values are related to age, but the Horvath 2, GrimAge and Hannum clocks most strongly (Pearson correlation coefficients of 0.86, 0.83, 0.82, respectively) (Table 1). The Horvath 2 and Hannum clocks are first generation clocks which we might expect to relate more strongly to chronological age; GrimAge, however, has a component of age in its estimation. The Yang, Zhang, and DunedinPoAm38 clocks are the least correlated with age; these are a mixture of first and second generation clocks and the DunedinPoAm38 clock would have been expected to have no correlation with age, has a small but significant correlation (0.06). The Bocklandt clock is negatively associated with age because it is a single CpG in which DNAm declines with age; all signs on this clock will thus be reversed in interpretation. By design, the measures of accelerated aging have no significant link to age.
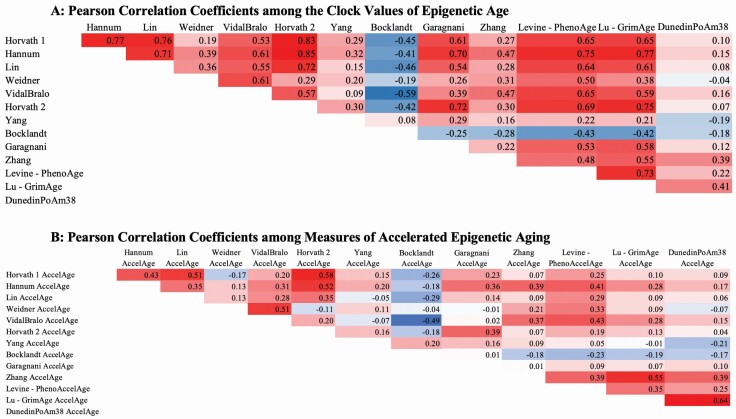
Correlations between clocks are shown in Figure 1A, which is presented as a heat map, so it is easy to see the patterns of relationships. The second generation clocks are the last 4 clocks in the matrix. The 2 Horvath clocks and the GrimAge clock are highly correlated and these 3 clocks are also relatively highly intercorrelated with Hannum, Levine, Lin, and VidalBralo. The Weidner, Yang, Zhang, Bocklandt, and DunedinPoAm38 clocks have lower associations with the other clocks.
Figure 1.
(A) Pearson correlation coefficients among the clock values of epigenetic age. Note: All coefficients significant at <.0001 except DunedinPoAm38 with Weidner (p = .0071). (B) Pearson correlation coefficients among measures of accelerated epigenetic aging. Note: All coefficients significant at <.0001; N = 4018. Except DunedinPoAm38 with Lin AccelAge (p = .0001), and with Horvath 2 AccelAge (p = .0140). Except Yang AccelAge with GrimAge (p = .5256), with Lin AccelAge (p = .0034), with Levine AccelAge (p = .0038). Except Bocklandt AccelAge with Weidner AccelAge (p = .0044). Except Garagnani AccelAge with Weidner AccelAge (p = .4693), with VidalBralo AccelAge (p = .2715), with Zhang AccelAge (p = .5220), with Bocklandt AccelAge (p = .4074).
Figure 1B shows the correlations among the measures of epigenetic age acceleration. Correlations are lower than among the epigenetic ages. The highest correlation is between two of the more recent measures, DunedinPoAm38 AcceleratedAge and GrimAgeAccel (0.64). The second generation measure, Levine PhenoAgeAccel, is not as strongly correlated with either GrimAge (0.35) or DunedinPoAm38 (0.25). The Zhang et al clock is only modestly related to the other clocks. Some of the earlier clocks had relatively high associations between their measures of acceleration, for example, Horvath 1, Horvath 2, and Hannum; these 3 measures have relatively weak correlations with GrimAgeAccel and DunedinPoAm38 and somewhat stronger correlations with PhenoAgeAccel.
Regression coefficients that show the associations of sex, race/ethnicity, education, obesity, and smoking with the accelerated age epigenetic measures are shown in Table 2. All measures are included in each equation, so the each effect is independent of the other variables. For the measures that are in years of age, the interpretation of the coefficients is the number of years of accelerated aging associated with the independent variable. Females have slower age acceleration as compared to men for most clocks; the largest estimate of slower female aging in years is from the GrimAge clock (−2.97 years). The sex difference in accelerated aging is insignificant in the Yang clock; the Garagnani clock is an exception showing faster aging among females.
Table 2.
Regression Coefficients From Measures of Accelerated Aging on Sex, Education, Race/Ethnicity, Obesity, and Smoking (N = 3966)
| Horvath 1 AccelAge | Hannum AccelAge | Lin AccelAge | Weidner AccelAge | VidalBralo AccelAge | Horvath 2 AccelAge | Yang AccelAge | |
|---|---|---|---|---|---|---|---|
| Female | −0.98**** | −1.80**** | −1.22**** | −0.84* | −1.51**** | −0.84**** | 0.00 |
| Education (16+ y as ref) | |||||||
| 13–15 y | −0.08 | −0.14 | −0.67* | −0.55 | 0.08 | 0.18 | 0.00 |
| 12 y | 0.18 | 0.31 | −0.83** | −0.89* | −0.20 | 0.32 | 0.00 |
| 0–11 y | −0.11 | 0.37 | −1.38** | −0.79 | −0.04 | 0.23 | 0.00**** |
| Race/ethnicity (White as ref) | |||||||
| Black | −0.25 | −1.97**** | −0.59 | −0.31 | −1.25**** | −0.48* | 0.01**** |
| Hispanic | −1.33*** | 0.15 | −1.54*** | 0.84 | −0.65* | −0.12 | 0.01**** |
| BMI (underweight/normal as ref) | |||||||
| Overweight | −0.21 | 0.10 | −0.21 | 0.02 | 0.08 | 0.17 | −0.00 |
| Obese Class I (BMI: 30–34.99) | 0.30 | 0.47* | 0.24 | 0.44 | 0.30 | 0.41 | −0.00 |
| Obese class II (BMI: 35+) | 0.85** | 0.89*** | 1.02** | 0.01 | 0.69** | 0.68** | −0.00 |
| Smoking (never smoked as ref) | |||||||
| Past smoker | 0.23 | 0.00 | 0.03 | −0.87* | −0.09 | 0.16 | −0.00 |
| Current smoker | 0.21 | 0.91*** | −0.80 | −1.64** | 0.15 | 0.36 | 0.00* |
| R 2 | 0.0120 | 0.0503 | 0.0182 | 0.0038 | 0.0294 | 0.0123 | 0.0611 |
| Bocklandt AccelAge | Garagnani AccelAge | Zhang AccelAge | Levine - PhenoAccelAge | Lu - GrimAge AccelAge | DunedinPoAm38 AccelAge | ||
| Female | 0.02**** | 0.01**** | −0.20**** | −1.11**** | −2.97**** | −0.01**** | |
| Education (16+ y as ref) | |||||||
| 13–15 y | −0.00 | 0.00 | 0.04* | 0.51 | 1.19**** | 0.01*** | |
| 12 y | 0.00 | 0.01** | 0.09**** | 0.66* | 1.61**** | 0.02**** | |
| 0–11 y | 0.01** | 0.00 | 0.15**** | 1.10** | 2.03**** | 0.03**** | |
| Race/ethnicity (White as ref) | |||||||
| Black | 0.04**** | −0.00 | −0.10**** | 0.57 | 1.24**** | 0.02**** | |
| Hispanic | 0.01** | 0.00 | −0.06* | −0.46 | −0.49* | −0.00 | |
| BMI (underweight/normal as ref) | |||||||
| Overweight | −0.00 | 0.00 | −0.02 | −0.05 | −0.02 | 0.00 | |
| Obese Class I (BMI: 30–34.99) | 0.00 | 0.00 | −0.01 | 0.34 | 0.30 | 0.01 | |
| Obese class II (BMI: 35+) | −0.01 | 0.01 | 0.08*** | 1.49**** | 1.13**** | 0.02**** | |
| Smoking (never smoked as ref) | |||||||
| Past smoker | −0.01* | 0.00 | 0.09**** | 0.42 | 1.94**** | 0.03**** | |
| Current smoker | −0.01*** | 0.01**** | 0.35**** | 1.19** | 7.29**** | 0.12**** | |
| R 2 | 0.048 | 0.0098 | 0.1404 | 0.0186 | 0.3871 | 0.1988 |
Notes: BMI = body mass index.
****p < .0001; ***p < .001; **p < .01; *p < .05.
Lower education is linked to faster age acceleration in 6 clocks—Zhang, PhenoAge, GrimAge, DunedinPoAm38, Yang, and Garagnani. However, lower education is linked to slower aging in the Lin clock. The relationship is very strong in the GrimAge clock with those in the lowest education group aging 2 years faster than those with 16 or more years of education. Generally, the expected gradient with education is found in the Zhang, PhenoAgeAceleration, GrimAge Acceleration, and DunedinPoAm38 clocks indicating the second generation of clocks comes closer to hypothesized relationships of a gradient with education.
The results on race/ethnicity are not as consistent. Three of the clocks indicate faster aging for African Americans compared to Whites and five (including Bocklandt) indicate slower aging for African Americans. Two of the second generation clocks, GrimAge, and DunedinPoAm38 are linked to faster aging in African Americans. Six clocks indicate slower aging among Hispanics and one indicates accelerated aging.
Nine clocks, and all of the second generation clocks, indicated accelerated aging among the Class II obese. Class I obesity was only related to accelerated aging in the Hannum clock. Eight clocks, including all 4 second generation clocks, link current smoking to faster aging. Only 1 clock indicates both past and current smoking is linked to slower aging (Weidner).
While many variables are significant in explaining the values of accelerated aging, the overall R2 is quite low in most of these equations with 9 clocks having less than 5% of variance explained. These demographic and behavioral variables explain the most variance in accelerated aging measured using GrimAge (39%); variance explained by these variables is also high for the Zhang (14%) and DunedinPoAm38 measures (20%). Because smoking-related methylation was included in the methylation measurement of GrimAge, we ran the equation without current and former smoking among the independent variables and the R2 was reduced to 19%, still much higher than all the other clocks, except the DunedinPoAm38 clock.
In Supplementary Table 1, we show the standardized regression coefficients which allow us to get a better sense of what variables are important in explaining the variability across the 13 measures. Three of the second generation clocks are strongly associated with gender, education, obesity, and smoking; two of these clocks also indicate faster aging among African Americans. The Hannum, Yang, and Bocklandt clocks are relatively strongly linked to race but the Hannum and Bocklandt clocks indicate slower aging among African Americans.
The above analysis does not control for cell composition which is known to be related to methylation outcomes as well as demographic and health measures (31). Health and Retirement Study has performed flow cytometry and directly estimated cell subsets which can be used to control for the individual heterogentity in cell composition which would affect observed relationships. In analysis presented in Supplementary Table 2a, we add the percentages of 6 cell subsets (CD8 naïve, CD4 total, CD8 total, monocytes, and B cells) to the regressions in Table 2 to see if results change. Changes could be due to confounding but they could also reflect changes that are part of the process that is producing differences in the epigenetic clocks. There is very little substantive change; 19 coefficients out of 143 change enough to either lose or gain significance. Persons who are overweight and or obese appear to age significantly faster on the Horvath2 clock, Bocklandt, Garagnani, GrimAge, and DunedonPoAm38 clocks.
We also controlled for 3 additional estimates of cell subsets that might be used by other researchers in other data sets without access to flow cytometry results: two based on algorithms of Houseman et al (32) and developed by Reinius et al (33) (Supplementary Table 2b) and Salas et al (34) (Supplementary Table 2c) and the third which is supplied as part of the GrimAge output (11) (Supplementary Table 2d). The cell estimates for the two based on Houseman et al were implemented using ewastools by Jonah Fisher of HRS. The use of all three of these approaches resulted in more changes in the significance of results from the original analysis presented in Table 2 than did the control based on observed cell subsets from the flow cytometry discussed above. The number of changed results were 29 Reinius, 35 Salas, and 15 GrimAge. In the case with the most change, the changes in significance occurred in all variables and the Weidner, Yang, and Phenotypic Age Acceleration measures had 6 or more changes each.
Discussion
Our intent was to clarify how 13 measures of epigenetic aging relate to known risk factors associated with poor health at older ages. The clocks and their associated measures of accelerated aging vary markedly in how they relate to these variables. First, the correlation with age varies from 0.2 to 0.9 indicating very different relationships with age and likely the health outcomes linked to age. We can make comparisons with the strength of age association with 3 clocks in the Understanding Society British study to see how associations of 3 epigenetic clock measures with age compare to HRS. Correlations with age are stronger in the British panel than in the HRS: Levine (0.88 vs 0.72), Hannum (0.92 vs 0.82), and Horvath 1 (0.91 vs 0.73). This could be differences in the age of the sample or could be due to differences in age-related methylation.
Most, but not all indicate of these epigenetic clocks indicate accelerated aging among men. A number of clocks link lower education and faster aging but the consistency of this association varies across the range of education. The majority of the clocks associate accelerated aging with obesity and lifetime smoking. On the other hand, there is a lot of variability in how accelerated aging estimated by the various clocks relates to race/ethnicity. The inconsistency of these results and the lack of expected effects of race and ethnicity have been clear indicators that not all clocks are the same in how they relate to human aging or aging within race/ethnic groups. This may reflect different CpG sites included in the clocks, small numbers of subjects in race/ethnic groups, different epigenetic profiles of race/ethnic groups that may be influenced by genetics, different interactions of genetics and socioeconomic and environmental factors. Detailed investigations of epigenetics in much larger samples of African Americans and Hispanics are called for in the future which could be done in the future with the HRS sample if DNAm was measured in more respondents from non-White race/ethnic groups.
The clocks most commonly used in the literature are the Horvath (1) clock and the Hannum clock and these are often compared to each other in analyses. One of the reasons these clocks engendered initial skepticism among those who study human health is that they were not related in expected ways to some of the known risk factors. For instance, in this sample they did not relate to education or race in expected ways. This lack of relationships is one of the factors leading to the second generation of clocks trained on a variety of health indicators rather than age alone: Zhang, PhenoAgeAcceleration, GrimAgeAcceleration, and DunedinPoAm38. These 4 clocks relate to the gender, education, race (but not for Zhang and Levine), obesity, and smoking in the expected direction leading to more confidence that this generation of clocks will improve ability to explain health outcomes. Our analysis would indicate that the second generation of clocks might be more useful in research on aging health. Some of the first generation clocks have little association even with age (Yang, Bocklandt, Weidner) and some of them estimate ranges of epigenetic age far outside the range of observed human life expectancy (Lin and Weidner).
A limitation of our analysis to this point is that we have not considered how the clocks vary in their relationship to health outcomes in this sample. This will aid in considering the value of the different clocks. Clocks that relate to both the factors thought to determine methylation change as well as the outcomes affected by methylation will be more useful to researchers. Additional work is also needed to understand the role of the distribution of cell subsets and their effect on methylation. In addition, the reliability of the underlying indicators of methylation requires additional assessment. It is likely that the development of clocks will continue in the future as science advances. Methods of measuring methylation and developing clocks as well as understanding their biological significance are likely to improve in the future. The availability of these numerous measures of DNAm in a publicly available data set should aide in scientific progress.
Supplementary Material
Funding
This work was supported by the National Institute on Aging (R01 AG AG060110). The Health and Retirement Study is supported by National Institute on Aging U01 AG009740.
Conflict of Interest
None declared.
References
- 1. Kennedy BK, Berger SL, Brunet A, et al. . Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levine ME. Assessment of epigenetic clocks as biomarkers of aging in basic and population research. J Gerontol A Biol Sci Med Sci. 2020;75:463–465. doi: 10.1093/gerona/glaa021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sierra F, Kohanski R. Geroscience and the trans-NIH Geroscience Interest Group, GSIG. Geroscience. 2017;39:1–5. doi: 10.1007/s11357-016-9954-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belsky DW, Caspi A, Arseneault L, et al. . Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. eLife. 2020;9:54870. doi: 10.7554/eLife.54870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19:371–384. doi: 10.1038/s41576-018-0004-3 [DOI] [PubMed] [Google Scholar]
- 8. McCrory C, Fiorito G, McLoughlin S, et al. . Epigenetic clocks and allostatic load reveal potential sex-specific drivers of biological aging. J Gerontol A Biol Sci Med Sci. 2020;75:495–503. doi: 10.1093/gerona/glz241 [DOI] [PubMed] [Google Scholar]
- 9. Maddock J, Castillo-Fernandez J, Wong A, et al. . DNA methylation age and physical and cognitive aging. J Gerontol A Biol Sci Med Sci. 2020;75:504–511. doi: 10.1093/gerona/glz246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Breitling LP, Saum KU, Perna L, Schöttker B, Holleczek B, Brenner H. Frailty is associated with the epigenetic clock but not with telomere length in a German cohort. Clin Epigenetics. 2016;8:21. doi: 10.1186/s13148-016-0186-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu AT, Quach A, Wilson JG, et al. . DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). 2019;11:303–327. doi: 10.18632/aging.101684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen BH, Marioni RE, Colicino E, et al. . DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY). 2016;8:1844–1865. doi: 10.18632/aging.101020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levine ME, Lu AT, Quach A, et al. . An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018;10:573–591. doi: 10.18632/aging.101414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Quach A, Levine ME, Tanaka T, et al. . Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging (Albany NY). 2017;9:419–446. doi: 10.18632/aging.101168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCrory C, Fiorito G, Ni Cheallaigh C, et al. . How does socio-economic position (SEP) get biologically embedded? A comparison of allostatic load and the epigenetic clock(s). Psychoneuroendocrinology. 2019;104:64–73. doi: 10.1016/j.psyneuen.2019.02.018 [DOI] [PubMed] [Google Scholar]
- 16. Zhao W, Ammous F, Ratliff S, et al. . Education and lifestyle factors are associated with DNA methylation clocks in older African Americans. Int J Environ Res Public Health. 2019;16:3141. doi: 10.3390/ijerph16173141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu Z, Chen BH, Assimes TL, Ferrucci L, Horvath S, Levine ME. The role of epigenetic aging in education and racial/ethnic mortality disparities among older U.S. women. Psychoneuroendocrinology. 2019;104:18–24. doi: 10.1016/j.psyneuen.2019.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bressler J, Marioni RE, Walker RM, et al. . Epigenetic age acceleration and cognitive function in African American adults in midlife: the atherosclerosis risk in communities study. J Gerontol A Biol Sci Med Sci. 2020;75:473–480. doi: 10.1093/gerona/glz245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ryan J, Wrigglesworth J, Loong J, Fransquet PD, Woods RL. A systematic review and meta-analysis of environmental, lifestyle, and health factors associated with DNA methylation age. J Gerontol A Biol Sci Med Sci. 2020;75:481–494. doi: 10.1093/gerona/glz099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Field AE, Robertson NA, Wang T, Havas A, Ideker T, Adams PD. DNA methylation clocks in aging: categories, causes, and consequences. Mol Cell. 2018;71:882–895. doi: 10.1016/j.molcel.2018.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hannum G, Guinney J, Zhao L, et al. . Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49:359–367. doi: 10.1016/j.molcel.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Y, Wilson R, Heiss J, et al. . DNA methylation signatures in peripheral blood strongly predict all-cause mortality. Nat Commun. 2017;8:14617. doi: 10.1038/ncomms14617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu Z, Leung D, Thrush K, et al. . Underlying features of epigenetic aging clocks in vivo and in vitro. Aging Cell. 2020;00:1–11. doi: 10.1111/acel.13229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crimmins EM, Faul J, Thyagarajan B, Weir D. 2017. Venous blood collection and assay protocol in the 2016 Health and Retirement Study 2016 Venous Blood Study (VBS). http://hrsonline.isr.umich.edu/modules/meta/vbs/2016/desc/HRS2016VBSDD.pdf.
- 25. Lin Q, Wagner W. Epigenetic aging signatures are coherently modified in cancer. PLoS Genet. 2015;11:e1005334. doi: 10.1371/journal.pgen.1005334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vidal-Bralo L, Lopez-Golan Y, Gonzalez A. Simplified assay for epigenetic age estimation in whole blood of adults. Front Genet. 2016;7:126. doi: 10.3389/fgene.2016.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Horvath S, Oshima J, Martin GM, et al. . Epigenetic clock for skin and blood cells applied to Hutchinson Gilford Progeria Syndrome and ex vivo studies. Aging (Albany NY). 2018;10:1758–1775. doi: 10.18632/aging.101508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang Z, Wong A, Kuh D, et al. . Correlation of an epigenetic mitotic clock with cancer risk. Genome Biol. 2016;17:205. doi: 10.1186/s13059-016-1064-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bocklandt S, Lin W, Sehl ME, et al. . Epigenetic predictor of age. PLoS One. 2011;6:e14821. doi: 10.1371/journal.pone.0014821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garagnani P, Bacalini MG, Pirazzini C, et al. . Methylation of ELOVL2 gene as a new epigenetic marker of age. Aging Cell. 2012;11:1132–1134. doi: 10.1111/acel.12005 [DOI] [PubMed] [Google Scholar]
- 31. Campbell KA, Colacino JA, Park SK, Bakulski KM. Cell types in environmental epigenetic studies: biological and epidemiological frameworks. Curr Environ Health Rep. 2020;7:185–197. doi: 10.1007/s40572-020-00287-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Houseman EA, Accomando WP, Koestler DC, et al. . DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics. 2012;13:86. doi: 10.1186/1471-2105-13-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reinius LE, Acevedo N, Joerink M, et al. . Differential DNA methylation in purified human blood cells: implications for cell lineage and studies on disease susceptibility. PLoS One. 2012;7:e41361. doi: 10.1371/journal.pone.0041361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Salas LA, Koestler DC, Butler RA, et al. . An optimized library for reference-based deconvolution of whole-blood biospecimens assayed using the Illumina HumanMethylationEPIC BeadArray. Genome Biol. 2018;19:64. doi: 10.1186/s13059-018-1448-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.