Abstract
Background
It remains controversial whether high protein diets improve cardiometabolic profile. We investigated whether increasing protein intake to 1.3 g/kg/day in functionally limited older adults with usual protein intake ≤RDA (0.8 g/kg/day) improves visceral fat accumulation and serum cardiovascular risk markers more than the recommended daily allowance (RDA).
Methods
The Optimizing Protein Intake in Older Men Trial was a placebo-controlled, randomized trial in which 92 functionally limited men, ≥65 years, with usual protein intake ≤RDA were randomized for 6 months to: 0.8 g/kg/day protein plus placebo; 1.3 g/kg/day protein plus placebo; 0.8 g/kg/day protein plus testosterone enanthate 100 mg weekly; or 1.3 g/kg/day protein plus testosterone enanthate 100 mg weekly. In this substudy, metabolic and inflammatory serum markers were measured in 77 men, and visceral adipose tissue (VAT) was assessed using dual-energy x-ray absorptiometry in 56 men.
Results
Treatment groups were similar in their baseline characteristics. Randomization to 1.3 g/kg/day protein group was associated with greater reduction in VAT compared to 0.8 g/kg/day group (between-group difference: −17.3 cm2, 95% confidence interval [CI]: −29.7 to −4.8 cm2, p = .008), regardless of whether they received testosterone or placebo. Changes in fasting glucose, fasting insulin, HOMA-IR, leptin, adiponectin, IL-6, and hs-CRP did not differ between the 0.8 versus 1.3 g/kg/day protein groups regardless of testosterone use.
Conclusions
Protein intake >RDA decreased VAT in functionally limited older men but did not improve cardiovascular disease risk markers.
Clinical Trials Registration Number
Keywords: Insulin resistance, Metabolic, Protein intake, Testosterone, Visceral fat
Aging is associated with loss of muscle mass and strength, and impairment in physical function (1,2). Both high protein diets and function-promoting anabolic agents, such as testosterone and selective androgen receptor modulators, are being investigated to reverse or prevent the loss of muscle mass and physical function associated with aging (3,4). The relationship between dietary protein intake and cardiometabolic health has received much attention recently. High protein diets have been proposed to improve body composition and other metabolic parameters by several mechanisms which include alterations in satiety and orexigenic hormones, and upregulation of thermogenesis, gluconeogenesis, and muscle protein synthesis (5). High dietary protein has been demonstrated to improve adiposity in the setting of energy-restricted diets and exercise training in overweight/obese adults (6,7). However, population studies and randomized trials have yielded conflicting results on the effect of dietary protein on cardiovascular disease risk (8–10). Concerns have also been raised about the safety of high protein diets given the association of branched-chain amino acids (a significant component of dietary protein) with insulin resistance and diabetes (11). Thus, the benefits and risks of high protein diets on cardiometabolic health remain unclear. The inconsistencies between studies may be due to differences in study populations, intervention duration, sources of protein (animal vs vegetable), and differences in definition of high versus low protein diets. Furthermore, some studies were conducted using hypocaloric diets, while others provided an isocaloric diet.
Interventional trials in hypogonadal and eugonadal older men have consistently demonstrated favorable body composition changes with testosterone administration, including increased total lean body mass and decreased total fat mass (12). A large body of preclinical evidence supports the view that testosterone plays an important role in regulating glucose metabolism. Testosterone may exert direct effects on insulin sensitivity by increasing the expression of insulin receptors as well as potentiating insulin signaling, resulting in enhanced glucose uptake into muscle and adipose tissue (13). However, the metabolic effects of high protein diets alone or in combination with testosterone supplementation in older men with mobility limitation have not been studied.
In the Optimizing Protein Intake in Older Men Trial (OPTIMEN), we demonstrated that increasing protein intake to a level (1.3 g/kg/day) above the recommended daily allowance (RDA) did not result in greater gains in lean body mass, muscle performance, or physical function compared with daily protein intake of 0.8 g/kg/day (14). We also found that intake of 1.3 g/kg/day protein was associated with greater reduction in whole body fat mass, compared to the RDA regardless of testosterone administration. In this substudy of the OPTIMEN Trial, we sought to determine whether high protein intake above the RDA, when administered in conjunction with a muscle anabolic agent, reduces visceral fat accumulation and improves cardiometabolic parameters in older men with physical limitations.
Method
Study Design
The OPTIMen Trial was a parallel group, double-blind randomized trial, approved by the institutional review boards at Boston Medical Center and Brigham and Women’s Hospital. The design and primary findings of the OPTIMen Trial are published (14) and briefly described here. All participants provided written informed consent.
Eligibility
The participants were community-dwelling men, aged ≥65 years, with moderate physical function limitation, whose average daily protein intake was ≤0.8 g/kg/day (RDA). We excluded men with prostate cancer, severe lower urinary tract symptoms, untreated sleep apnea, myocardial infarction, or stroke within 6 months, or erythrocytosis (14). Men with diabetes requiring insulin or A1c > 8%, were excluded.
Randomization and Study Intervention
Eligible participants entered a run-in period, during which they were asked to eat a custom diet containing 0.8 g/kg/day protein for 10–16 days. Participants, who consumed <75% of provided meals or supplements, were excluded.
Participants were randomized to receive placebo injections intramuscularly weekly plus 0.8 g/kg/day protein; placebo plus 1.3 g/kg/day protein; testosterone enanthate 100 mg weekly plus 0.8 g/kg/day protein; or testosterone enanthate 100 mg weekly plus 1.3 g/kg/day protein.
Dietary Intervention
Daily energy and protein intakes were apportioned between prepackaged meals, protein or carbohydrate (placebo) supplements, and discretionary food allowances (15). Packaged meals with individualized protein and energy contents were supplied by Personal Chef to Go, Mechanicsville, VA, and provided 0.7 g/kg/day protein and 80% of daily energy requirements. Discretionary foods (fruits/vegetables, coffee/tea, alcoholic beverages, and other foods) provided an additional 0.1 g/kg/day of protein and 15% of energy. The difference between the prescribed protein and energy intakes and protein and energy contents of packaged meals was made up by protein or carbohydrate supplements. Participants received their daily protein allotment through packaged meals (0.7 g/kg/day), supplement (0 g/kg/day for control and 0.5 g/kg/day for higher protein group), and discretionary foods (0.1 g/kg/day). Participants in the 1.3 g/kg/day group received a supplement containing 0.5 g/kg/day of casein and whey protein mix, bringing their protein intake to 1.3 g/kg/day; the control group received a supplement containing 0.5 g/kg/day of carbohydrate powder. Twenty percent of daily energy intake was provided as breakfast, 40% as lunch, and 40% as dinner. Energy and protein contents of each participant’s individualized diet were standardized by providing packaged meals and supplements. Daily energy requirement was calculated using Dietary Reference Intake equation plus an activity factor (16). A 7-day supply of packaged food was picked up by participants or was home delivered. Participants received 1260 mg calcium, 1000-IU vitamin D3, and a multivitamin daily.
Adherence
Every week, a nutritionist reviewed either 24-hour food recalls or Dietary Compliance Checklists to reinforce dietary instructions. Dietary Compliance checklists involved participants checking off food items, discretionary food, and portions consumed (15).
Abdominal Visceral Fat
Abdominal visceral adipose tissue (VAT) was measured by dual-energy x-ray absorptiometry (DXA) calibrated using a soft tissue phantom. DXA fat depots were measured in a 5-cm wide region placed across the abdomen just above the iliac crest at a level that coincides with the fourth lumbar vertebra (L4). The software locates the outer and inner margins of the abdominal wall based on fat and lean mass profiles across the abdomen at L4 level. The amount of subcutaneous adipose tissue in the region of interest is estimated by measuring the subcutaneous fat between the skin line and outer abdominal wall, and is subtracted from the total abdominal fat mass measured within the region of interest to yield DXA VAT (17). The VAT area measured using the DXA has been shown to correlate strongly with VAT area measured by computed tomography at the L4-L5 level (18).
Metabolic Outcomes
Plasma glucose was quantified using Beckman Glucose Analyzer 2 (Beckman Instruments, California). Insulin, leptin and high-sensitivity C-reactive protein (hs-CRP) were measured using high-sensitivity sandwich ELISA (Alpco Diagnostics, Salem, NH; Millipore, Billerica, MA). Total adiponectin was measured using a radio-immunoassay (Millipore). Interleukin (IL)-6 was measured by electrochemiluminescence immunoassay (Meso Scale Diagnostics, Rockville, MD). Insulin resistance was calculated using the homeostatic model assessment (HOMA) index (19).
Statistical Analyses
For this substudy, a subset of 77 men from the original trial with available blood samples at baseline and at least one postrandomization visit (3 months and/or 6 months) were evaluated. A 2 × 2 factorial design was applied with the model including interactions between visits, testosterone, and protein interventions. A mixed-effects regression model was used to assess 3-month and 6-month outcomes simultaneously, controlling for baseline values and diabetes status and allowing for unstructured correlation between participants’ serial measurements. The mean between-group differences in postrandomization change in outcomes, for the effect of protein intake, were extracted from a mixed-model framework (ie, between the groups receiving 0.8 vs 1.3 kg/day of protein) using treatment contrasts and associated 95% confidence intervals (CIs). In a similar fashion, the effects of testosterone intervention (testosterone enanthate 100 mg weekly vs placebo) were calculated from the model. Two-sided type 1 error was set at 0.05 for testing of all hypotheses. Statistical analyses were conducted using SAS 9.3 (SAS Institute, Inc, Cary, NC) and R software version 3.2.5 (R Foundation).
Results
Flow of Participants
Of the 14 276 individuals who underwent screening, 154 met eligibility criteria and entered a diet run-in period; 95 met dietary adherence requirements; and 92 were randomized. Eighty-one completed 3 months and 78 completed 6 months of intervention; 77 had a metabolic assessment at baseline and at least one postrandomization visit, and constituted the analytic sample.
Baseline Characteristics
Baseline characteristics were similar among the 4 treatment groups (Table 1). Mean age of the participants was 73.3 ± 6.1 years; 14.3% had diabetes. The adherence with dietary prescription (81%–86%) and testosterone/placebo injections (>99%) was high (14).
Table 1.
Baseline Characteristics of the Study Population by Treatment Group
| Treatment Group | |||||
|---|---|---|---|---|---|
| Variable | 0.8 g/kg/d Protein +Placebo (n = 21) |
0.8 g/kg/d Protein +Testosterone (n = 17) |
1.3 g/kg/d Protein +Placebo (n = 20) |
1.3 g/kg/d Protein +Testosterone (n = 19) |
p Value |
| Age, years | 74.0 ± 4.9 | 74.2 ± 7.0 | 72.0 ± 5.9 | 73.5 ± 6.9 | .24 |
| Body weight, kg | 94.9 ± 12.5 | 83.1 ± 13.9 | 95.4 ± 16.8 | 88.7 ± 16.4 | .38 |
| Body mass index, kg/m2 | 30.5 ± 4.2 | 28.3 ± 4.6 | 31.9 ± 5.6 | 29.5 ± 4.8 | .47 |
| Fasting Glucose, mg/dL | 103.4 ± 27.5 | 101.8 ± 13.5 | 98.8 ± 20.2 | 101.1 ± 14.6 | .91 |
| Fasting Insulin, uIU/mL | 9.0 ± 6.5 | 7.3 ± 4.9 | 6.9 ± 4.0 | 5.5 ± 2.8 | .15 |
| HOMA-IR | 2.5 ± 2.5 | 1.9 ± 1.4 | 1.7 ± 1.1 | 1.4 ± 0.9 | .18 |
| IL-6, pg/mL | 2.8 ± 1.3 | 3.4 ± 2.3 | 2.7 ± 1.2 | 3.1 ± 1.1 | .51 |
| Leptin, ng/mL | 47.1 ± 22.3 | 38.1 ± 27.0 | 36.6 ± 23.4 | 33.7 ± 21.7 | .31 |
| Adiponectin, ug/mL | 5.7 ± 2.0 | 5.6 ± 2.0 | 5.7 ± 3.8 | 7.0 ± 3.1 | .37 |
| hs-CRP, mg/dL | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.2 ± 0.3 | 0.2 ± 0.2 | .55 |
| *VAT area, cm2 | 246.7 ± 59.5 (15) | 214.8 ± 59.4 (13) | 187.1 ± 67.0 (16) | 214.2 ± 67.0 (12) | .09 |
Notes: Values are means ± SD. p-values for the test of any difference between groups extracted from ANOVA model. CRP = C-reactive protein; VAT = Visceral adipose tissue.
*There were no significant differences between baseline characteristics for the subset of participants with VAT area records available at the start of the study (N = 56).
Metabolic Outcomes
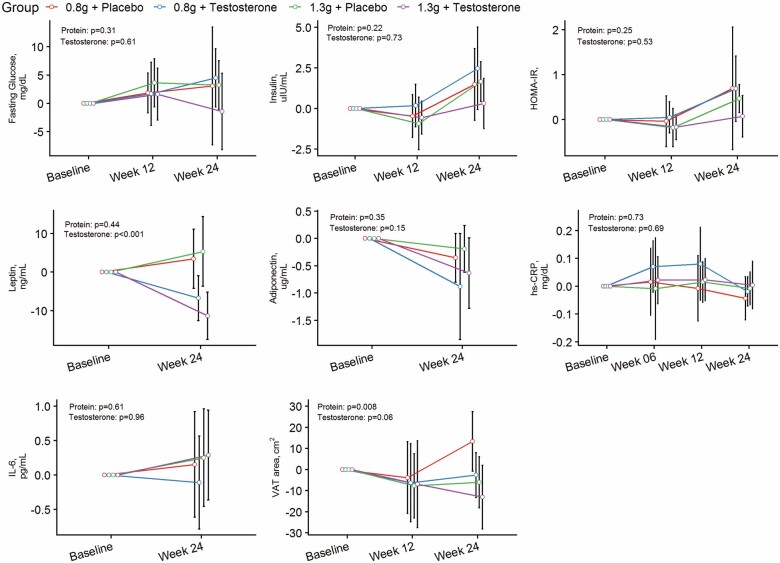
Changes in abdominal VAT area and metabolic parameters from baseline to 6 months postintervention are depicted by treatment arm in Figure 1 and Table 2.
Figure 1.
Estimated changes from baseline and 95% confidence intervals in abdominal visceral adipose tissue area and metabolic parameters over 6-month intervention by treatment arm.
Table 2.
Point Estimates and 95% Confidence Intervals for Mean Change From Baseline in Outcomes for Protein Intake and Testosterone Effects
| Variables | *Comparison Between Protein Groups | *Comparison between Testosterone Groups | ||||||
|---|---|---|---|---|---|---|---|---|
| Protein 0.8 g | Protein 1.3 g | Difference | p Value | Placebo | Testosterone | Difference | p Value | |
| Fasting Glucose, mg/dL | 3.85 (−0.96, 8.65) | 0.42 (−4.22, 5.06) | −3.42 (−10.1, 3.27) | .31 | 3.00 (−1.55, 7.55) | 1.27 (−3.61, 6.14) | −1.73 (−8.40, 4.94) | .61 |
| Insulin, uIU/mL | 2.07 (0.74, 3.41) | 0.90 (−0.40, 2.21) | −1.17 (−3.05, 0.71) | .22 | 1.65 (0.37, 2.93) | 1.33 (−0.02, 2.68) | −0.32 (−2.19, 1.54) | .73 |
| HOMA-IR | 0.72 (0.13, 1.31) | 0.24 (−0.33, 0.81) | −0.48 (−1.30, 0.34) | .25 | 0.61 (0.05, 1.16) | 0.35 (−0.25, 0.95) | −0.26 (−1.07, 0.56) | .53 |
| hs-CRP, mg/dL | −0.02 (−0.07, 0.02) | −0.01 (−0.06, 0.03) | 0.01 (−0.05, 0.08) | .73 | −0.02 (−0.07, 0.02) | −0.01 (−0.06, 0.04) | 0.01 (−0.05, 0.08) | .69 |
| Leptin, ng/mL | −1.02 (−5.92, 3.88) | −3.74 (−8.56, 1.08) | −2.72 (−9.66, 4.22) | .44 | 4.99 (0.31, 9.67) | −9.75 (−14.8, −4.75) | −14.7 (−21.6, −7.9) | <.001 |
| Adiponectin, µg/mL | −0.65 (−1.08, −0.23) | −0.38 (−0.78, 0.03) | 0.28 (−0.31, 0.87) | .35 | −0.30 (−0.70, 0.10) | −0.73 (−1.16, −0.30) | −0.43 (−1.02, 0.16) | .15 |
| IL-6, pg/mL | 0.07 (−0.41, 0.54) | 0.24 (−0.21, 0.68) | 0.17 (−0.48, 0.82) | .61 | 0.14 (−0.30, 0.59) | 0.16 (−0.31, 0.63) | 0.02 (−0.64, 0.67) | .96 |
| VAT area, cm2 | 6.51 (−2.16, 15.2) | −10.7 (−19.5, −2.00) | −17.3 (−29.7, −4.8) | .008 | 3.66 (−4.44, 11.8) | −7.89 (−16.9, 1.13) | −11.6 (−0.58, 23.7) | .06 |
Note: CRP = C-reactive protein; VAT = Visceral adipose tissue.
*Point estimates, 95% confidence intervals and p-values extracted from 2 × 2 factorial design using mixed model framework. Models were adjusted for baseline values and diabetes status.
There was a significant effect of protein level on VAT area over 6 months. Men randomized to 1.3 g/kg/day protein had a significantly greater decrease from baseline in VAT area than those randomized to 0.8 g/kg/day protein (between-group difference: −17.3 cm2, 95% CI, −29.7 to −4.8 cm2, p = .008). There was no significant effect of protein level on fasting insulin, fasting glucose or HOMA-IR, leptin, adiponectin, IL-6, and hs-CRP over 6 months. The changes from baseline in these biomarkers did not differ among men assigned to 0.8 or 1.3 g/kg/day protein groups (Table 2), regardless of whether they received testosterone or placebo.
Testosterone administration was associated with a decrease in VAT area compared to placebo, regardless of protein intake; however, the between-group difference did not reach statistical significance (between-group difference: −11.6 cm2, 95% CI: −0.58 to 23.7 cm2, p = .06). Men randomized to testosterone had greater reductions in leptin levels than those randomized to placebo (between-group difference: −9.75 ng/mL, 95% CI: −14.8 to −4.75 ng/mL, p < .001), regardless of protein intake. There was no significant change from baseline in fasting insulin and glucose, HOMA-IR, adiponectin, hs-CRP, and IL-6 levels in men randomized to testosterone compared to placebo, regardless of protein intake.
Results were mostly unchanged in sensitivity analyses that excluded subjects with diabetes (Supplementary Table 1). However, there was a nonsignificant trend in glucose reduction in men randomized to 1.3 g/kg/day protein than those randomized to 0.8 g/kg/day protein (between-group difference: −6.20 mg/dL, 95% CI: −12.9, 0.49, p = .07), regardless of whether they received testosterone or placebo.
Exploratory Analyses
Among participants receiving high protein diet, the decrease from baseline in VAT area was not associated with changes in metabolic parameters (data not shown). We had insufficient power to test for interaction; however, among the 2 high protein groups, the decreases in VAT area from baseline were larger in those receiving testosterone compared to placebo (Supplementary Table 2).
Discussion
In this randomized-controlled trial of mobility-limited older men, daily protein intake higher than the RDA was associated with significantly greater reduction in abdominal VAT compared with protein intake equaling the RDA. However, higher protein intake did not improve glucose metabolism or the circulating levels of adipokines and inflammatory markers, or plasma lipids (reported previously (14)), regardless of whether participants received testosterone or placebo. Furthermore, decreases in VAT observed in the participants receiving protein intake >RDA were not associated with changes in metabolic and inflammatory serum markers. Thus, the reduction in VAT associated with higher protein intake did not translate into beneficial effects on serum cardiometabolic profile.
High protein diets have gained increasing popularity as a promising strategy for weight loss by improving satiety and reducing fat mass. The mechanisms by which increased protein intake decreases fat mass are not well understood but may include increased energy expenditure due to the thermic effect of protein and changes in appetite regulating hormones (5). In a cross-sectional study of older adults, protein intake equal to the RDA was associated with lower fat mass than protein intake <RDA (49). We previously reported that protein intake >RDA attenuated the gains in whole body fat mass relative to the RDA (14). These findings are consistent with weight loss trials showing greater loss of fat mass in subjects consuming higher protein in settings of energy-restricted diets and/or exercise training (6,7). However, few studies have investigated the effect of high protein diets on both subcutaneous and visceral abdominal tissue compartments in older adults. In one study of 65 overweight or obese subjects randomized to 2 energy-restricted diets with different protein and carbohydrate contents for 6 months, intrabdominal adipose tissue area decreased by 33 cm2 and 16.7 cm2 in high (25% total energy content) versus low protein (12% total energy content) groups, respectively (20). In a study of 215 overweight/obese subjects randomized to one of 2 hypo-caloric diets for 12 weeks, greater reductions in total and abdominal fat mass were seen in subjects consuming high protein (27% total energy content) diet compared to standard protein (16% total energy content) diet (10). In our current controlled feeding study of older men using isocaloric diets for 24 weeks, we show that protein intake >RDA resulted in greater loss of VAT compared to protein intake equal to the RDA. Although both visceral and subcutaneous adipose tissue are correlated with metabolic risk, visceral fat remains more strongly associated with an adverse metabolic profile (21). Our study adds to the scarce literature demonstrating that abdominal VAT may be an important pathogenic fat depot that is responsive to a high protein diet.
Previous studies have reported inconsistent or even conflicting results on the effect of high versus low protein diets on metabolic health. Some short-term studies have reported beneficial effects of higher versus lower protein diets on metabolic parameters (20,22,23) and insulin secretion (24). There also has been concerns of harmful effects of long-term high protein intake, particularly branched-chain amino acid rich diets, given their association with insulin resistance and type 2 diabetes (11). In a systematic review of 15 RCTs of ≥12 weeks duration in persons without diabetes, high protein diets showed neither improvement nor worsening of fasting glucose, HbA1c, insulin levels, lipids, and CRP, compared to low protein diets (8). These findings were corroborated by another meta-analysis of 74 RCTs demonstrating no significant changes in LDL-cholesterol, hsCRP, HbA1c, and fasting glucose following high protein compared to low protein diets (25). Another meta-analysis of studies in patients with type 2 diabetes showed that higher protein diets improved lipids and HOMA-IR, but did not significantly improve fasting glucose, insulin, or HbA1c (26). We show here that a higher level of protein intake (>RDA) for 24 weeks exerted neither beneficial nor harmful effects on markers of glucose metabolism and inflammatory markers regardless of diabetes status. Although we found significant reductions in VAT with higher protein intake, this loss of VAT did not correlate with changes in cardiovascular disease risk markers. Thus, the clinical significance of the observed reduction in VAT with higher dietary protein is unclear and requires further study.
Testosterone therapy in hypogonadal and eugonadal men has been associated with improvement in body fat composition (12), including a reduction in whole body and visceral fat (27). It is unclear if the beneficial effects of testosterone replacement on body composition in these men might be enhanced by higher protein supplementation. Consistent with other studies, testosterone administration was associated with an appreciable reduction in VAT area compared to placebo, regardless of protein intake; although this did not quite reach statistical significance due to our limited sample size. Although the study had insufficient power to statistically test for interaction of both protein and testosterone effects, our data suggest that protein intake >RDA may enhance the VAT reductions induced by testosterone administration.
Our study has notable strengths and some limitations. The trial had concealed randomization, a placebo control, parallel group design, blinding, and oversight by an independent DSMB. We recruited men with physical limitations whose protein intake was ≤RDA, a group most likely to benefit from higher protein intake. This is the longest and largest randomized controlled-feeding study of protein intervention conducted in older adults. The 24-week duration of this study should be long enough to demonstrate any significant change in these cardiovascular risk markers as a result of loss in VAT. However, measurement of cardiovascular disease risk markers was not the primary outcome of the trial. The majority of subjects in each group at study entry were either overweight or slightly obese typical of the middle-aged and older participants in our randomized trials in Boston. However, controlling for baseline BMI did not alter the results.
In conclusion, protein intake >RDA reduced abdominal VAT but had no significant effects on metabolic and inflammatory biomarkers in functionally limited older men. Our findings do not support the hypothesis that protein intake >RDA improves cardiometabolic health with or without concomitant use of a muscle anabolic, such as testosterone. Future studies are needed to clarify the long-term effects of high protein diets on major adverse cardiovascular events in older men.
Supplementary Material
Acknowledgments
Data and Safety Monitoring Board: The National Institute on Aging appointed a Data and Safety Monitoring Board to oversee the trial’s progress and participant safety. The DSMB met through conference calls twice a year throughout the duration of the trial to review study’s progress and the safety data.
DSMB Chair: Dr. Richard Grimm served as the DSMB chair from the trial’s inception until June 2015, when Dr. Connie Bales assumed this position until the end of the trial. Other members of the DSMB included Drs. Stephanie Studenski (2009–2014), Peter Peduzzi (2009 to 2014); Anne Kenny; Philip Miller (2015–2017); and Dennis Villareal (2013–2017).
Funding
This study was funded primarily by NIH grant R01AG037547 from the National Institute on Aging. Additional support was provided by the infrastructural resources of the Boston Claude D. Pepper Older Americans Independence Center for Function Promoting Therapies, which is supported by grant P30AG031679 from the National Institute on Aging. This study was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through BU-CTSI Grant Number 1UL1TR001430 and the Boston Nutrition and Obesity Research Center, through P30DK046200. Additional support was also provided by the National Heart, Lung, and Blood Institute (Grant Number K08HL132122-02). Dietary supplements were provided by Abbott Laboratories, Bariatrix Nutrition Inc., and the National Dairy Council. Endo Pharmaceuticals provided testosterone enanthate for this trial. We thank the staff of the Brigham and Women’s Hospital’s Center for Clinical Investigation, which is supported in part from Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The funding agencies played no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. We are grateful to the volunteers who participated in this trial and gave generously of their time and commitment.
Conflict of Interest
S.B. has received research grants from the National Institute on Aging, the National Institute of Nursing Research, the National Institute of Child Health and Human Development, the Patient Centered Outcomes Research Institute, AbbVie, Metro International Biotechnology, Alivegen, and Abbott Pharmaceuticals outside of this work; these grants are managed by the Brigham and Women’s Hospital. He has equity interest in FPT, LLC, and has consulted for AbbVie. These conflicts are managed according to the rules and regulations of the Partners Healthcare System. C.M.A. reports grants from National Institutes of Health, nonfinancial support from Abbott Laboratories, nonfinancial support from Bariatrix Nutrition Inc., nonfinancial support from National Dairy Council, during the conduct of the study; personal fees from Nutrisystem, personal fees from Zafgen, personal fees from Sanofi-Aventis, grants and personal fees from Orexigen, personal fees from NovoNordisk, grants from Aspire Bariatrics, grants and personal fees from GI Dynamics, grants from Myos, grants and personal fees from Takeda, personal fees from Scientific Intake, grants and personal fees from Gelesis, other from Science-Smart LLC, personal fees from Merck, personal fees from Johnson and Johnson, grants from Vela Foundation, grants from Dr. Robert C. and Veronica Atkins Foundation, grants from Coherence Lab, grants from Energesis, grants from PCORI, outside the submitted work.
References
- 1. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 2. Bhasin S, Tenover JS. Age-associated sarcopenia–issues in the use of testosterone as an anabolic agent in older men. J Clin Endocrinol Metab. 1997;82:1659–1660. doi: 10.1210/jcem.82.6.4061 [DOI] [PubMed] [Google Scholar]
- 3. Bhasin S, Jasuja R. Selective androgen receptor modulators as function promoting therapies. Curr Opin Clin Nutr Metab Care. 2009;12:232–240. doi: 10.1097/MCO.0b013e32832a3d79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deutz NE, Bauer JM, Barazzoni R, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33:929–936. doi: 10.1016/j.clnu.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pesta DH, Samuel VT. A high-protein diet for reducing body fat: mechanisms and possible caveats. Nutr Metab (Lond). 2014;11:53. doi: 10.1186/1743-7075-11-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim JE, O’Connor LE, Sands LP, Slebodnik MB, Campbell WW. Effects of dietary protein intake on body composition changes after weight loss in older adults: a systematic review and meta-analysis. Nutr Rev. 2016;74:210–224. doi: 10.1093/nutrit/nuv065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campbell WW, Kim JE, Amankwaah AF, Gordon SL, Weinheimer-Haus EM. Higher total protein intake and change in total protein intake affect body composition but not metabolic syndrome indexes in middle-aged overweight and obese adults who perform resistance and aerobic exercise for 36 weeks. J Nutr. 2015;145:2076–2083. doi: 10.3945/jn.115.213595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schwingshackl L, Hoffmann G. Long-term effects of low-fat diets either low or high in protein on cardiovascular and metabolic risk factors: a systematic review and meta-analysis. Nutr J. 2013;12:48. doi: 10.1186/1475-2891-12-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haring B, von Ballmoos MC, Appel LJ, Sacks FM. Healthy dietary interventions and lipoprotein (a) plasma levels: results from the Omni Heart Trial. PLoS One. 2014;9:e114859. doi: 10.1371/journal.pone.0114859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clifton PM, Bastiaans K, Keogh JB. High protein diets decrease total and abdominal fat and improve CVD risk profile in overweight and obese men and women with elevated triacylglycerol. Nutr Metab Cardiovasc Dis. 2009;19:548–554. doi: 10.1016/j.numecd.2008.10.006 [DOI] [PubMed] [Google Scholar]
- 11. Rietman A, Schwarz J, Tomé D, Kok FJ, Mensink M. High dietary protein intake, reducing or eliciting insulin resistance? Eur J Clin Nutr. 2014;68:973–979. doi: 10.1038/ejcn.2014.123 [DOI] [PubMed] [Google Scholar]
- 12. Bhasin S, Woodhouse L, Casaburi R, et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281:E1172–E1181. doi: 10.1152/ajpendo.2001.281.6.E1172 [DOI] [PubMed] [Google Scholar]
- 13. Parthasarathy C, Renuka VN, Balasubramanian K. Sex steroids enhance insulin receptors and glucose oxidation in Chang liver cells. Clin Chim Acta. 2009;399:49–53. doi: 10.1016/j.cca.2008.09.011 [DOI] [PubMed] [Google Scholar]
- 14. Bhasin S, Apovian CM, Travison TG, et al. Effect of protein intake on lean body mass in functionally limited older men: a randomized clinical trial. JAMA Intern Med. 2018;178:530–541. doi: 10.1001/jamainternmed.2018.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Apovian CM, Singer MR, Campbell WW, et al. Development of a novel six-month nutrition intervention for a randomized trial in older men with mobility limitations. J Nutr Health Aging. 2017;21:1081–1088. doi: 10.1007/s12603-017-0990-4 [DOI] [PubMed] [Google Scholar]
- 16. Wheeler ML. Nutrient database for the 2003 exchange lists for meal planning. J Am Diet Assoc. 2003;103:894–920. doi: 10.1016/s0002-8223(03)00376-6 [DOI] [PubMed] [Google Scholar]
- 17. Goldberg EK, Fung EB. Precision of the hologic DXA in the assessment of visceral adipose tissue. J Clin Densitom. 2020;23:664–672. doi: 10.1016/j.jocd.2019.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kelly T. Visceral Fat Evaluation and Clinical Significance.www.hologic.com. 2012. Hologic, Inc. [Google Scholar]
- 19. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 20. Skov AR, Toubro S, Rønn B, Holm L, Astrup A. Randomized trial on protein vs carbohydrate in ad libitum fat reduced diet for the treatment of obesity. Int J Obes Relat Metab Disord. 1999;23(5):528–36. [DOI] [PubMed] [Google Scholar]
- 21. Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355 [DOI] [PubMed] [Google Scholar]
- 22. Clifton PM, Keogh J. Metabolic effects of high-protein diets. Curr Atheroscler Rep. 2007;9:472–478. doi: 10.1007/s11883-007-0063-y [DOI] [PubMed] [Google Scholar]
- 23. Krieger JW, Sitren HS, Daniels MJ, Langkamp-Henken B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: a meta-regression 1. Am J Clin Nutr. 2006;83:260–274. doi: 10.1093/ajcn/83.2.260 [DOI] [PubMed] [Google Scholar]
- 24. El Khoury D, Hwalla N. Metabolic and appetite hormone responses of hyperinsulinemic normoglycemic males to meals with varied macronutrient compositions. Ann Nutr Metab. 2010;57:59–67. doi: 10.1159/000317343 [DOI] [PubMed] [Google Scholar]
- 25. Santesso N, Akl EA, Bianchi M, et al. Effects of higher- versus lower-protein diets on health outcomes: a systematic review and meta-analysis. Eur J Clin Nutr. 2012;66:780–788. doi: 10.1038/ejcn.2012.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu Z, Nan F, Wang LY, Jiang H, Chen W, Jiang Y. Effects of high-protein diet on glycemic control, insulin resistance and blood pressure in type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2020;39:1724–1734. doi: 10.1016/j.clnu.2019.08.008 [DOI] [PubMed] [Google Scholar]
- 27. Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154:899–906. doi: 10.1530/eje.1.02166 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.