Abstract
Introduction
A post hoc analysis of a double-blind (DB) active control trial and an open-label extension (OLE) study was conducted to evaluate the long-term effects of lurasidone in patients with schizophrenia.
Methods
In the DB trial, patients were randomised to receive lurasidone or risperidone for 12 months. In OLE, all patients received lurasidone for an additional 6 months. Treatment-emergent adverse events (TEAEs) were evaluated. Efficacy assessments included relapse rate (DB trial only), and Positive and Negative Syndrome Scale, Clinical Global Impression–Severity scale, and Montgomery–Åsberg Depression Rating Scale.
Results
In the DB trial, patients with schizophrenia were randomised to lurasidone (n = 399) and risperidone (n = 190), of whom 129 and 84 continued into OLE, respectively. During the DB trial, incidence of TEAEs was similar for lurasidone (84.1%) and risperidone (84.2%). Lurasidone was associated with minimal changes in metabolic variables and prolactin levels, whereas risperidone was associated with clinically significant increases in prolactin and fasting glucose levels. The proportion of patients with metabolic syndrome was significantly lower in patients treated with lurasidone versus risperidone at the end of the DB trial (25.5% vs 40.4%; p = 0.0177). During OLE, patients switching from risperidone to lurasidone experienced a reduction in weight and prolactin levels; those continuing treatment with lurasidone experienced minimal changes in metabolic variables and prolactin. At the end of OLE, the proportion of patients with metabolic syndrome was no longer significantly different between groups (23.5% vs 31.5%; p = not significant). Efficacy outcomes were generally similar between groups during the DB trial, and were maintained during OLE.
Conclusion
Lurasidone was generally well tolerated and effective in clinically stable schizophrenia patients over the long term. Lurasidone was also generally well tolerated and maintained effectiveness over 6 months in patients switching from risperidone. Patients switching from risperidone experienced improvements in metabolic parameters and prolactin levels. These findings confirm lurasidone’s long-term effectiveness and favourable metabolic profile in patients with schizophrenia.
Trial Registration
ClinicalTrials.gov identifier NCT00641745.
Keywords: Atypical antipsychotic, Cardiometabolic, Lurasidone, Metabolic syndrome, Prolactin, Risperidone, Schizophrenia, Switch
Key Summary Points
| People with schizophrenia are at higher risk than the general population of cardiometabolic diseases, the risk of which is further increased by some antipsychotics. |
| This analysis evaluated the long-term effects of lurasidone in patients with schizophrenia, who received lurasidone during a double-blind trial and its open-label extension study, or who received risperidone during the double-blind trial but then switched to lurasidone during the open-label study. |
| Lurasidone demonstrated sustained long-term efficacy and was associated with minimal changes in metabolic variables and prolactin levels. |
| Patients switching from risperidone to lurasidone experienced improvements in metabolic parameters and prolactin levels. |
| These findings confirm lurasidone’s long-term efficacy and favourable metabolic safety profile in patients with schizophrenia. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13084943.
Introduction
Schizophrenia is a severe and chronic mental illness that affects approximately 20 million people worldwide [1]. People with schizophrenia are 2–3 times as likely to die early as the general population, resulting in a reduced life expectancy of 10–20 years [2, 3]. This reduction in life expectancy largely results from an increased likelihood of cardiovascular disease, diabetes mellitus, and other physical conditions, exacerbated by insufficient prevention of modifiable risk factors [3, 4]. Cardiovascular disease is the leading cause of death in schizophrenia, individuals with the disorder having significantly higher risks of metabolic syndrome, abdominal obesity, dyslipidaemia, and hypertension than the general population [5–8]. Moreover, metabolic disturbances in those with schizophrenia increase with disease duration and age [5]. Although cardiometabolic disturbances appear to be an intrinsic part of schizophrenia itself, the risk of such disturbances is frequently increased by antipsychotic treatment, particularly treatment with atypical antipsychotics [3, 5]. With the exception of clozapine, the efficacy of atypical antipsychotics is largely similar, but the agents vary greatly in terms of safety profiles, most notably with regard to cardiometabolic risk [9, 10].
Lurasidone is a once-daily second-generation antipsychotic that is widely approved for the treatment of schizophrenia, including in Europe and the USA [11, 12]. Similar to most other atypical antipsychotics, lurasidone has high binding affinity for dopamine D2 and serotonin 5-HT2A receptors and moderate affinity for D3 and 5-HT1A receptors, but it differs from other atypical agents in being an antagonist with high affinity for 5-HT7 receptors and having negligible affinity for histamine H1 and muscarinic M1 receptors [11, 13]. Lurasidone has a relatively benign cardiometabolic profile when compared with most other atypical antipsychotics, being associated with minimal weight gain and no clinically meaningful alterations in lipid, glucose, and prolactin levels or the electrocardiogram (ECG) QT interval [9, 10, 14].
The long-term safety, tolerability, and efficacy of lurasidone were assessed in a 12-month, international, double-blind (DB), active-controlled trial [Study 237], in which patients were randomised to receive treatment with either lurasidone or risperidone [15]. This was followed by a 6-month open-label extension (OLE) study (Study 237-EXT), in which all patients received treatment with lurasidone (those having received risperidone during the initial DB trial switching to lurasidone) [16]. These studies included patients with a primary diagnosis of schizophrenia or schizoaffective disorder, as established by a structured diagnostic interview and application of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria [6]. Patients with schizoaffective disorder experience the psychotic symptoms of schizophrenia (e.g. delusions, hallucinations, disorganised thinking, flat affect), along with symptoms of a mood disorder, such as depression and/or mania [17], but tend to have more favourable outcomes than those with schizophrenia [18]. There are currently no specific treatment guidelines for schizoaffective disorder, due to a lack of evidence in patients with the disorder [19], and there may be differences in sensitivity to antipsychotics in term of efficacy and safety between patients with schizophrenia and schizoaffective disorder.
Given the differences in diagnostic criteria and outcomes for schizophrenia and schizoaffective disorder, post hoc analyses of Studies 237 and 237-EXT were conducted in order to assess the safety, tolerability, and efficacy of lurasidone versus risperidone over 12 months, specifically in patients with schizophrenia, and to further assess the long-term safety, tolerability, and efficacy of lurasidone over an additional 6 months in patients with schizophrenia treated with lurasidone in Study 237 and in those who switched from risperidone to lurasidone at the start of Study 237-EXT.
Methods
The methodologies of Studies 237 (DB trial) and 237-EXT (OLE study) were published previously, and the studies are registered on ClinicalTrials.gov (NCT00641745) [15, 16]. Both were conducted in accordance with the Good Clinical Practice Guidelines of the International Conference on Harmonisation and with the ethical principles of the Declaration of Helsinki. The studies were reviewed and approved by an independent ethics committee or institutional review board at each study centre, and all patients provided written informed consent prior to participation [15, 16].
Study Population
Key inclusion criteria for schizophrenia patients in the initial DB trial were: age 18–75 years; primary diagnosis of schizophrenia (DSM-IV criteria) of at least 1-year duration; ‘clinically stable’ (non-acute phase of illness) for ≥ 8 weeks before baseline; no change in antipsychotic medications for ≥ 6 weeks before screening; no hospitalisation for psychiatric illness for ≥ 8 weeks before screening; and moderate or lower (≤ 4) severity rating on the Positive and Negative Syndrome Scale (PANSS) items of delusions, conceptual disorganisation, hallucinations, and unusual thought content. Key exclusion criteria included: current clinically significant somatic disorders or abnormal laboratory testing; clinically significant suicidal ideation, suicidal behaviour, or violent behaviour in the past 6 months; a history of a poor/inadequate response or intolerability to risperidone; and body mass index (BMI) < 18.5 or > 40 kg/m2 [15]. Patients who completed the initial DB trial were eligible to continue into the OLE study [16].
Study Design
In the initial DB trial, patients were randomised in a 2:1 ratio to receive lurasidone (flexibly dosed, 37–111 mg/day) or risperidone (flexibly dosed, 2–6 mg/day) for 12 months [15]. In the OLE study, all patients were treated with lurasidone. To maintain the DB in the initial trial, all patients entering the OLE study received 3 days of single-blind placebo washout followed by 7 days of lurasidone 80 mg/day, after which lurasidone dosing could be adjusted, based on the judgment of the investigator, within a dose range of 37–111 mg/day over a treatment period of 6 months [16].
Study Assessments
In the DB trial, patients were monitored for safety, tolerability, and efficacy every 1–3 weeks for the first 12 weeks and monthly thereafter [15]. In the OLE study, assessments were conducted at OLE baseline and monthly thereafter [16]. Assessments were the same during the DB trial and OLE study, with the exception of relapse rate, which was only measured during the DB trial [15, 16].
Safety was assessed by evaluation of treatment-emergent adverse events (TEAEs), serious TEAEs, TEAEs leading to discontinuation, extrapyramidal symptom (EPS)-related TEAEs, and metabolic-related TEAEs, and by monitoring of metabolic variables (total cholesterol, low-density lipoprotein [LDL] cholesterol, high-density lipoprotein [HDL] cholesterol, triglycerides, glucose, glycated haemoglobin [HbA1c], and insulin), prolactin, weight, BMI, waist circumference, and ECG parameters. TEAEs presented for the OLE study were those recorded during the period from the baseline of the OLE study to the end of the OLE study. EPS-related and metabolic-related TEAEs were determined by medical review of preferred terms prior to unblinding in the DB trial. EPS-related TEAEs comprised bradykinesia, cogwheel rigidity, drooling, dystonia, muscle rigidity, oculogyric crisis, oromandibular dystonia, parkinsonism, psychomotor retardation, torticollis, tremor, and trismus. Metabolic-related TEAEs included increased blood glucose, increased blood triglycerides, diabetes mellitus, increased HbA1c, hyperglycaemia, hyperlipidaemia, hypertriglyceridaemia, metabolic syndrome, overweight, type 2 diabetes mellitus, and weight increase. The proportion of patients with metabolic syndrome was assessed at baseline, month 6, and month 12. Patients were classified as having metabolic syndrome based on the 2005 revision of the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria [20] when any three of the following five criteria were met: large waist circumference (≥ 102 cm for men; ≥ 88 cm for women [US criteria]), elevated triglycerides (≥ 150 mg/dL), low HDL cholesterol (< 40 mg/dL in men; < 50 mg/dL in women), elevated blood pressure (systolic ≥ 130 mmHg or diastolic ≥ 85 mmHg), or elevated fasting glucose (≥ 100 mg/dL). In the current analysis, an NCEP ATP III criterion was not considered to be met if a patient had normal values for triglycerides, blood pressure, HDL cholesterol, and/or glucose while receiving drug treatment for one or more of these parameters. Movement disorders were evaluated using the Barnes Akathisia Scale (BAS) [21], Simpson-Angus Scale (SAS) [22], and Abnormal Involuntary Movement Scale (AIMS) [23].
Efficacy assessments included relapse rate (DB trial only), PANSS [24], Clinical Global Impression–Severity scale (CGI-S) [23], and Montgomery–Åsberg Depression Rating Scale (MADRS) [25]. Relapse was defined as the earliest occurrence of: worsening of the PANSS total score by 30% from baseline and CGI-S > 3; rehospitalisation for worsening of psychosis; or emergence of suicidal ideation, homicidal ideation, and/or risk of harm to self or others [15].
Statistical Analysis
Full details of the statistical methodologies employed have been published previously [15, 16]. The safety population was defined as all randomised patients who received at least one dose of study medication, and the intent-to-treat (ITT) population was defined as all randomised patients who received at least one dose of study medication and had a baseline and at least one post-baseline efficacy assessment for PANSS or CGI-S.
In the DB trial, the changes in continuous variables from baseline were evaluated and compared between treatment groups using a non-parametric rank analysis of covariance at month-12 last observation carried forward (LOCF) endpoint. For some variables, shifts from baseline to LOCF endpoint were additionally assessed as the percentage of patients with values below, within, and above the normal range. Categorical safety outcomes were further evaluated using the number needed to harm (NNH). The 95% confidence interval (CI) for NNH was calculated based on the Wald method [26, 27]. Time to relapse was analysed using the Kaplan–Meier method and Cox progression hazards model. PANSS, CGI-S, and MADRS scores were analysed using a mixed model for repeated measurement [15].
In the OLE study, changes from baseline were calculated from DB baseline to OLE LOCF endpoint, and from OLE baseline to OLE LOCF endpoint, comparing the groups of patients who initially received lurasidone in the DB trial (‘lurasidone–lurasidone’ group) with those who initially received risperidone and switched to lurasidone at the start of the OLE study (‘risperidone–lurasidone’ group). Observed cases and LOCF analyses were performed [16].
Results
Patient Disposition
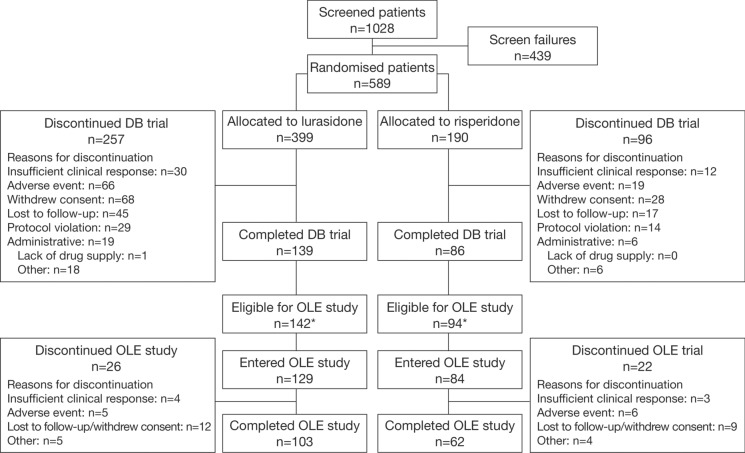
The randomised population of the initial DB trial included 629 patients [15], of whom 589 had schizophrenia and 40 had schizoaffective disorder. Of the 589 patients with schizophrenia who were included in the current study, 399 and 190 were randomised to receive treatment with lurasidone and risperidone, respectively (Fig. 1). Overall, 139/399 (34.8%) patients treated with lurasidone and 86/190 (45.3%) patients treated with risperidone completed the 1-year DB trial. The most common reasons for discontinuation (lurasidone vs risperidone) were withdrawal of consent (17.0% vs 14.7%), adverse events (16.5% vs 10.0%), loss to follow-up (11.3% vs 8.9%), and insufficient clinical response (7.5% vs 6.3%). A total of 129 patients treated with lurasidone and 84 patients treated with risperidone continued into the OLE study, of whom 103/129 (79.8%) and 62/84 (73.8%) completed the OLE study, respectively (Fig. 1). The most common reason for discontinuation during the OLE study (lurasidone vs risperidone) was loss to follow-up/withdrawal of consent (9.3% vs 10.7%).
Fig. 1.
Disposition of patients with schizophrenia. *Due to a lack of drug supply at study centres in Argentina and Brazil, patients who had not completed the DB trial had the option of enrolling in the OLE study or discontinuing the study. DB double-blind, OLE open-label extension
Patient Characteristics
Demographic and clinical characteristics were generally well balanced between treatment groups at baseline in both the DB trial and the OLE study (Table 1). The mean (standard deviation [SD]) age in the lurasidone versus risperidone groups was 41.9 (11.3) versus 41.1 (11.3) years at baseline in the DB trial, and 44.2 (10.8) versus 42.5 (10.8) years at baseline in the OLE study. A slightly higher proportion of lurasidone versus risperidone patients were male (74.2% vs 63.7% at DB baseline; 75.2% vs 66.7% at OLE baseline). The PANSS total, CGI-S total, and MADRS total scores were similar between treatment groups at baseline in both the DB trial and OLE study.
Table 1.
Demographic and baseline characteristics in (A) the DB trial and (B) the OLE study (safety population)
| Characteristic | (A) DB trial (Study 237) | (B) OLE study (Study 237-EXT) | ||
|---|---|---|---|---|
| Lurasidone N = 391 |
Risperidone N = 190 |
Lurasidone–lurasidone N = 129 |
Risperidone–lurasidone N = 84 |
|
| Gender, n (%) | ||||
| Male | 290 (74.2) | 121 (63.7) | 97 (75.2) | 56 (66.7) |
| Female | 101 (25.8) | 69 (36.3) | 32 (24.8) | 28 (33.3) |
| Age, years | ||||
| Mean (SD) | 41.9 (11.3) | 41.1 (11.3) | 44.2 (10.8) | 42.5 (10.8) |
| Median (range) | 43.0 (18–73) | 43.0 (18–65) | 46.0 (19–70) | 46.0 (20–62) |
| Race, n (%) | ||||
| Black/African American | 208 (53.2) | 95 (50.0) | 64 (49.6) | 38 (45.2) |
| White | 136 (34.8) | 80 (42.1) | 46 (35.7) | 38 (45.2) |
| Asian | 17 (4.3) | 3 (1.6) | 6 (4.7) | 1 (1.2) |
| Other | 30 (7.7) | 12 (6.3) | 13 (10.1) | 7 (8.3) |
| Ethnicity, n (%) | ||||
| Not Hispanic or Latino | 312 (79.8) | 148 (77.9) | 95 (73.6) | 60 (71.4) |
| Hispanic or Latino | 79 (20.2) | 42 (22.1) | 34 (26.4) | 24 (28.6) |
| Duration of illness, years | ||||
| Mean (SD) | 3.8 (6.2)a,b | 3.5 (5.0)a | 17.1 (10.8) | 17.5 (11.8) |
| Median (range) | 1.0 (0–34)a,b | 2.0 (0–34)a | 16.0 (1–47) | 16.0 (1–42) |
| Number of prior hospitalisations, n (%) | ||||
| 0 | 83 (21.2) | 37 (19.5) | 35 (27.1) | 15 (17.9) |
| 1 | 67 (17.1) | 35 (18.4) | 24 (18.6) | 17 (20.2) |
| 2 | 57 (14.6) | 30 (15.8) | 24 (18.6) | 17 (20.2) |
| 3 | 54 (13.8) | 21 (11.1) | 18 (14.0) | 10 (11.9) |
| ≥ 4 | 130 (33.2) | 67 (35.3) | 28 (21.7) | 25 (29.8) |
| PANSS total score at DB baseline | ||||
| Mean (SD) | 65.0 (12.5) | 65.7 (12.2) | 63.8 (13.2) | 64.2 (12.7) |
| Median (range) | 65.0 (34–98) | 66.0 (34–103) | 63.0 (34–95) | 65.0 (34–103) |
| CGI-S total score at DB baseline | ||||
| Mean (SD) | 3.4 (0.6) | 3.5 (0.6) | 3.4 (0.7) | 3.5 (0.6) |
| Median (range) | 3.0 (1.0–5.0) | 4.0 (2.0–4.0) | 3.0 (1–4) | 4.0 (2–4) |
| MADRS total score at DB baseline | ||||
| Mean (SD) | 7.2 (6.8) | 8.1 (7.4) | 6.2 (6.3) | 7.0 (6.4) |
| Median (range) | 6.0 (0–34) | 6.0 (0–34) | 5.0 (0–32) | 6.0 (0–34) |
| PANSS total score at OLE baseline | ||||
| Mean (SD) | NA | NA | 55.1 (13.8) | 55.8 (11.2) |
| Median (range) | 56.0 (30–103) | 56.5 (30–78) | ||
| CGI-S total score at OLE baseline | ||||
| Mean (SD) | NA | NA | 2.8 (0.8) | 2.9 (0.8) |
| Median (range) | 3.0 (1–4) | 3.0 (1–5) | ||
| MADRS total score at OLE baseline | ||||
| Mean (SD) | NA | NA | 4.8 (5.4) | 4.4 (4.4) |
| Median (range) | 4.0 (0–33) | 4.0 (0–29) | ||
aLast acute episode to randomisation
bN = 389
CGI-S Clinical Global Impression–Severity scale, DB double-blind, MADRS Montgomery–Åsberg Depression Rating Scale, NA not applicable, OLE open-label extension, PANSS Positive and Negative Syndrome Scale, SD standard deviation
Antipsychotic Treatment
The majority of patients had been treated with antipsychotic medication before enrolment (lurasidone, 96.2%; risperidone, 96.3%), and the mean (SD) duration of prior exposure was 198.7 (147.4) days in the lurasidone group (median, 196.0; range 1–391) and 225.9 (154.0) days in the risperidone group (median, 321.0; range, 1–397). The mean (SD) daily doses at DB baseline were 78.6 (20.2) mg/day for lurasidone (median, 74.0; range 37.6–110.4) and 4.3 (1.0) mg/day for risperidone (median, 4.0; range, 2.0–6.0). The modal daily doses were 37 (13.3%), 74 (60.1%), and 111 (26.6%) mg/day for lurasidone, and 2 (10.5%), 4 (61.1%), and 6 (28.4%) mg/day for risperidone.
During the OLE study, mean (SD) exposure to lurasidone was 168.5 (49.3) days in the lurasidone–lurasidone group (median, 186.0; range, 1–215) and 158.9 (55.6) days in the risperidone–lurasidone group (median, 182.5; range, 4–208). The mean (SD) dose of lurasidone during the OLE study was 74.4 (11.7) mg/day in the lurasidone–lurasidone group (median, 74.0; range, 39–105) and 77.3 (13.1) mg/day in the risperidone–lurasidone group (median, 74.0; range, 39–108). Modal daily doses were 37 (6.2%), 74 (86.8%), and 111 (7.0%) in the lurasidone–lurasidone group, and 37 (3.6%), 74 (81.0%), and 111 (15.5%) in the risperidone–lurasidone group. The total exposure to lurasidone was 60 patient-years for the lurasidone–lurasidone group and 37 patient-years for the risperidone–lurasidone group.
Safety and Tolerability
Study 237
TEAEs
The proportion of patients with TEAEs was similar between the lurasidone and risperidone groups (84.1% vs 84.2%) (Table 2a). The incidence of serious TEAEs was 10.7% in the lurasidone group and 8.9% in the risperidone group. The most frequently reported serious TEAEs (≥ 2% of patients) in the lurasidone versus risperidone groups were psychotic disorder (2.6% vs 4.2%) and schizophrenia (2.0% vs 1.1%). Suicidal ideation was reported as a serious TEAE in two patients (0.5%) treated with lurasidone and two patients (1.1%) treated with risperidone. A greater proportion of patients treated with lurasidone versus risperidone discontinued due to TEAEs (21.0% vs 14.2%), with an NNH of 15 (95% CI, 8–276). The rate of discontinuation due to individual TEAEs did not differ between groups by more than approximately 1%, and most TEAEs led to discontinuation in < 1% of patients in either group.
Table 2.
Summary of TEAEs during (A) the DB trial and (B) the OLE study (safety population)
| (A) DB trial (Study 237) | |||
|---|---|---|---|
| TEAE category | Number of patients (%) | NNH (95% CI)a | |
| Lurasidone N = 391 |
Risperidone N = 190 |
||
| Any TEAE | 329 (84.1) | 160 (84.2) | |
| Most frequently reported TEAEsb | |||
| Insomnia | 60 (15.3) | 24 (12.6) | 37 (NS) |
| Nausea | 60 (15.3) | 20 (10.5) | 21 (NS) |
| Sedation | 54 (13.8) | 27 (14.2) | −251 (NS) |
| Akathisia | 53 (13.6) | 14 (7.4) | 17 (9, 87) |
| Somnolence | 53 (13.6) | 33 (17.4) | −27 (NS) |
| Headache | 38 (9.7) | 28 (14.7) | −20 (NS) |
| Weight increase | 38 (9.7) | 38 (20.0) | −10 (−26, −6) |
| Vomiting | 37 (9.5) | 7 (3.7) | 18 (11, 55) |
| Anxiety | 35 (9.0) | 16 (8.4) | 189 (NS) |
| Weight decrease | 29 (7.4) | 9 (4.7) | 38 (NS) |
| Dizziness | 24 (6.1) | 6 (3.2) | 34 (NS) |
| Nasopharyngitis | 21 (5.4) | 12 (6.3) | −106 (NS) |
| Psychotic disorder | 19 (4.9) | 13 (6.8) | −51 (NS) |
| Parkinsonism | 17 (4.3) | 10 (5.3) | −110 (NS) |
| Dystonia | 13 (3.3) | 12 (6.3) | −34 (NS) |
| Constipation | 7 (1.8) | 11 (5.8) | −26 (−234, −14) |
| Any EPS-related TEAEc | 48 (12.3) | 36 (18.9) | −15 (−457, −8) |
| Any metabolic-related TEAEd | 52 (13.3) | 43 (22.6) | −11 (−41, −7) |
| Any serious TEAE | 42 (10.7) | 17 (8.9) | 56 (NS) |
| Most frequently reported serious TEAEse | |||
| Psychotic disorder | 10 (2.6) | 8 (4.2) | −61 (NS) |
| Schizophrenia | 8 (2.0) | 2 (1.1) | 101 (NS) |
| Suicidal ideation | 2 (0.5) | 2 (1.1) | −185 (NS) |
| Any TEAE leading to discontinuation | 82 (21.0) | 27 (14.2) | 15 (8, 276) |
| Most frequently reported TEAEs leading to discontinuatione | |||
| Psychotic disorder | 13 (3.3) | 8 (4.2) | −113 (NS) |
| Schizophrenia | 12 (3.1) | 4 (2.1) | 104 (NS) |
| Suicidal ideation | 4 (1.0) | 2 (1.1) | −3377 (NS) |
| Akathisia | 4 (1.0) | 2 (1.1) | −3377 (NS) |
| Hallucination, auditory | 4 (1.0) | 0 | 98 (50, 3906) |
| Vomiting | 4 (1.0) | 0 | 98 (50, 3906) |
| Electrocardiogram QT prolonged | 0 | 2 (1.1) | −96 (NS) |
| (B) OLE study (Study 237-EXT) | ||
|---|---|---|
| TEAE category | Number of patients (%) | |
| Lurasidone–lurasidone N = 129 |
Risperidone–lurasidone N = 84 |
|
| Any TEAE | 76 (58.9) | 49 (58.3) |
| Most frequently reported TEAEsb | ||
| Headache | 6 (4.7) | 7 (8.3) |
| Psychotic disorder | 6 (4.7) | 6 (7.1) |
| Parkinsonism | 5 (3.9) | 5 (6.0) |
| Insomnia | 3 (2.3) | 5 (6.0) |
| Anxiety | 2 (1.6) | 6 (7.1) |
| Any EPS-related TEAEc | 11 (8.5) | 6 (7.1) |
| Any metabolic-related TEAEd | 4 (3.1) | 5 (6.0) |
| Any serious TEAE | 7 (5.4) | 3 (3.6) |
| Types of serious TEAE | ||
| Psychotic disorder | 2 (1.6) | 1 (1.2) |
| Schizophrenia | 1 (0.8) | 1 (1.2) |
| Completed suicide | 1 (0.8) | 0 |
| Ankle fracture | 1 (0.8) | 0 |
| Non-small cell lung cancer | 1 (0.8) | 0 |
| Convulsion | 1 (0.8) | 0 |
| Carbon monoxide poisoning | 0 | 1 (1.2) |
| Any TEAE leading to discontinuation | 7 (5.4) | 6 (7.1) |
| Most frequently reported TEAEs leading to discontinuatione | ||
| Psychotic disorder | 1 (0.8) | 2 (2.4) |
| Nausea | 0 | 1 (1.2) |
| Hepatitis C | 0 | 1 (1.2) |
| Anxiety | 0 | 1 (1.2) |
| Schizophrenia | 0 | 1 (1.2) |
aLurasidone versus risperidone. NNH is provided only for comparisons in which the 95% CI did not include infinity, denoting statistical significance at the p ≤ 0.05 threshold. NNH = 1/(rate with lurasidone − rate with risperidone) and rounded up. A negative NNH denotes an advantage for lurasidone relative to risperidone and can be expressed as a positive number if the comparison is risperidone vs lurasidone instead of lurasidone vs risperidone
b ≥ 5% of patients in either group
cEPS-related TEAEs were determined by medical review of preferred terms prior to unblinding in the DB trial and comprised: bradykinesia, cogwheel rigidity, drooling, dystonia, muscle rigidity, oculogyric crisis, oromandibular dystonia, parkinsonism, psychomotor retardation, torticollis, tremor, and trismus
dMetabolic-related TEAEs were determined by medical review of preferred terms prior to unblinding in the DB trial and comprised: increased blood glucose, increased blood triglycerides, diabetes mellitus, increased glycosylated haemoglobin, hyperglycaemia, hyperlipidaemia, hypertriglyceridaemia, metabolic syndrome, overweight, type 2 diabetes mellitus, and weight increase
e ≥ 1% of patients in either group
CI confidence interval, EPS extrapyramidal symptoms, NNH number needed to harm, NS not significant (the 95% CI contains infinity), TEAE treatment-emergent adverse event
EPS-related TEAEs were reported less frequently in patients treated with lurasidone versus risperidone (12.3% vs 18.9%), with an NNH of −15 (95% CI, −457 to −8). The most frequently reported EPS-related TEAEs (≥ 3% of patients in either group) were parkinsonism (lurasidone, 4.3%; risperidone, 5.3%), dystonia (3.3% vs 6.3%), and tremor (3.1% vs 3.2%).
Metabolic-related TEAEs were reported less frequently in patients treated with lurasidone versus risperidone (13.3% vs 22.6%), with an NNH of −11 (95% CI, −41 to −7). This difference was driven primarily by the lower incidence of increased weight with lurasidone versus risperidone (9.7% vs 20.0%), and this was the only metabolic-related TEAE reported by > 1% of patients in either group.
The most frequently reported TEAEs (≥ 15% of patients in either group) were insomnia (lurasidone, 15.3%; risperidone, 12.6%), nausea (15.3% vs 10.5%), somnolence (13.6% vs 17.4%), and increased weight (9.7% vs 20.0%). A higher proportion of lurasidone versus risperidone patients experienced akathisia (13.6% vs 7.4%; NNH, 17 [95% CI, 9–87]) and vomiting (9.5% vs 3.7%; NNH, 18 (95% CI, 11–55), whereas a lower proportion of lurasidone versus risperidone patients experienced increased weight (9.7% vs 20.0%; NNH, −10 [95% CI, −26 to −6]) and constipation (1.8% vs 5.8%; NNH, −26 [95% CI, −234 to −14]).
There were nine reports of suicidal ideation, six (1.5%) in the lurasidone group and three (1.6%) in the risperidone group.
Laboratory Parameters
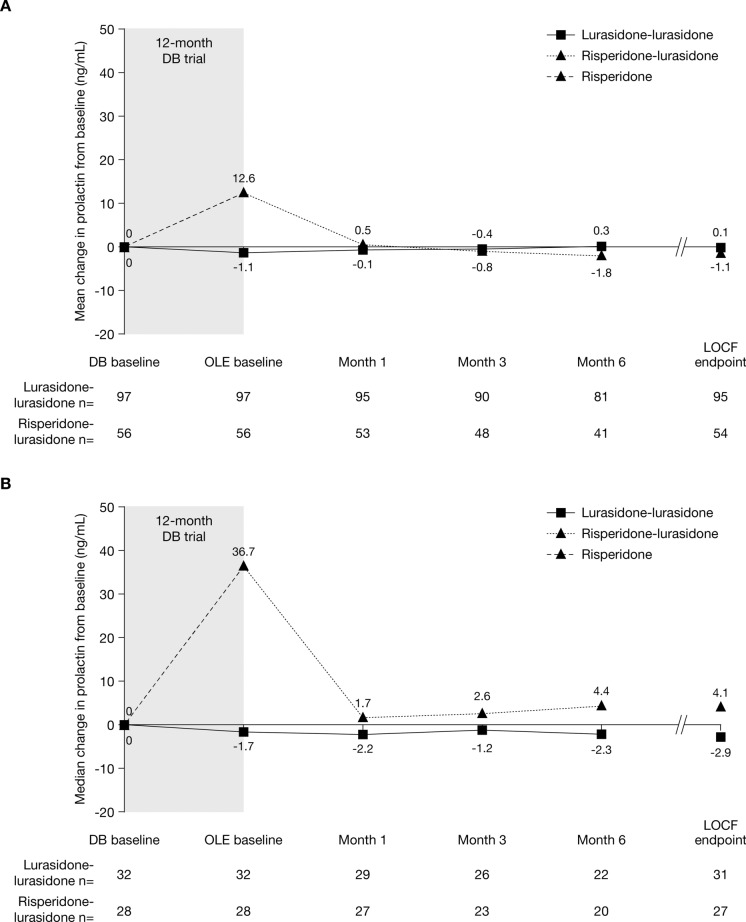
Table 3 summarises changes from baseline to LOCF endpoint and the proportion of patients shifting from low or normal values to high or abnormal values for metabolic variables and for prolactin levels. The median fasting level of HDL cholesterol remained stable in patients treated with lurasidone but decreased in patients treated with risperidone (median change, 0 vs −2.0 mg/dL; p = 0.042). The proportion of patients whose HDL cholesterol level shifted from high/normal to low was 8.4% for lurasidone versus 17.3% for risperidone (NNH, −12 [−67 to −7]). Median fasting levels of glucose remained stable in patients treated with lurasidone but increased in patients treated with risperidone (median change, 0 vs 2.0 mg/dL; p = 0.034), and the proportion of patients whose glucose level shifted from low/normal to high was 21.9% for lurasidone versus 33.3% for risperidone (NNH, −9 [−131 to −5]). Median fasting levels of insulin decreased slightly with lurasidone but increased with risperidone (median change, −0.3 vs 1.0 mU/L; p = 0.007), although the proportion of patients whose insulin level shifted from low/normal to high was similar between groups (8.6% for lurasidone vs 11.7% for risperidone; NNH, −32 [95% CI, not significant; contains infinity]). The greatest difference between groups was observed for prolactin levels, which increased substantially in male and female patients treated with risperidone, but only marginally in patients treated with lurasidone (Table 3; Fig. 2). This was also reflected in the proportion of patients who shifted from low/normal to high prolactin levels, which was substantially lower for lurasidone than for risperidone in both male patients (14.2% vs 40.9%; NNH, −4 [−7 to −3]) and female patients (13.6% vs 58.0%; NNH, −3 [−4 to −2]).
Table 3.
Summary of changes in metabolic variables and prolactin during (A) the DB trial and (B) the OLE study (safety population)
| (A) DB trial (Study 237) | ||||||
|---|---|---|---|---|---|---|
| Variable | Median change; mean change (SD); n | p value | Proportion shift low/normal to higha | NNH (95% CI)b | ||
| Lurasidone N = 391 |
Risperidone N = 190 |
Lurasidone N = 391 |
Risperidone N = 190 |
|||
| Total cholesterol, mg/dL | −2.0; −3.1 (29.0); 295 | −5.0; −2.7 (36.7); 143 | NS | 29/181 (16.0%) | 10/82 (12.2%) | 27 (NSc) |
| HDL cholesterol, mg/dL | 0; −0.3 (8.7); 295 | −2.0; −2.0 (9.4); 143 | 0.042 | 22/261 (8.4%) | 22/127 (17.3%) | −12 (−67, −7) |
| LDL cholesterol, mg/dL | −1.0; −1.1 (25.2); 295 | −3.0; −3.3 (27.5); 143 | NS | 30/209 (14.4%)d | 8/100 (8.0%)d | 16 (NSc) |
| Triglycerides, mg/dL | −4.0; −7.3 (75.2); 295 | −4.0; 9.7 (106.2); 143 | NS | 17/255 (6.7%) | 9/123 (7.3%) | −154 (NSc) |
| Glucose, mg/dL | 0; 2.1 (24.5); 293 | 2.0; 4.4 (19.0); 144 | 0.034 | 44/201 (21.9%) | 35/105 (33.3%) | −9 (−131, −5) |
| HbA1c, % | 0; 0.0 (0.3); 329 | 0; 0.1 (0.3); 158 | NS | 17/265 (6.4%) | 5/137 (3.6%) | 37 (NSc) |
| Insulin, mU/L | −0.3; 0.7 (26.6); 322 | 1.0; 6.0 (27.4); 151 | 0.007 | 25/292 (8.6%) | 17/145 (11.7%) | −32 (NSc) |
| Prolactin—male, ng/mL | 0; 2.4 (13.5); 258 | 7.3; 9.4 (14.3); 107 | < 0.001 | 33/232 (14.2%) | 38/93 (40.9%) | −4 (−7, −3) |
| Prolactin—female, ng/mL | 0.5; 4.2 (35.2); 95 | 25.7; 34.1 (55.4); 59 | < 0.001 | 11/81 (13.6%) | 29/50 (58.0%) | −3 (−4, −2) |
| Weight, kg | −0.3; −1.0 (5.1); 384 | 1.1; 1.5 (5.1); 185 | < 0.001 | |||
| ≥ 7% increase | 29/384 (7.6%) | 26/185 (14.1%) | −16 (−120, −9) | |||
| ≥ 7% decrease | 50/384 (13.0%) | 11/185 (5.9%) | 15 (9, 44) | |||
| Body mass index, kg/m2 | −0.1; −0.3 (1.7); 384 | 0.4; 0.6 (1.8); 185 | < 0.001 | 18/384 (4.7%)e | 17/185 (9.1%)e | −23 (NSc) |
| Waist circumference, cm | 0; −0.5 (6.0); 288 | 1.0; 1.7 (6.0); 148 | < 0.001 | – | – | – |
| (B) OLE study (Study 237-EXT) | ||
|---|---|---|
| Variable | Lurasidone–lurasidone N = 129 |
Risperidone–lurasidone N = 84 |
| Total cholesterol, mg/dL | ||
| n | 118 | 75 |
|
DB baseline, mean (SD) Median change from DB baseline to OLE LOCF endpoint |
198.2 (46.6) −11.0 |
187.3 (49.5) −3.0 |
| Median change from OLE baseline to OLE LOCF endpoint | −4.0 | 4.0 |
| HDL cholesterol, mg/dL | ||
| n | 118 | 75 |
| DB baseline, mean (SD) | 47.6 (13.9) | 46.8 (13.3) |
| Median change from DB baseline to OLE LOCF endpoint | 0 | −1.0 |
| Median change from OLE baseline to OLE LOCF endpoint | 0 | 3.0 |
| LDL cholesterol, mg/dL | ||
| n | 118 | 75 |
| DB baseline, mean (SD) | 120.7 (36.9) | 111.0 (37.5) |
| Median change from DB baseline to OLE LOCF endpoint | −6.5 | 1.0 |
| Median change from OLE baseline to OLE LOCF endpoint | −2.5 | 8.0 |
| Triglycerides, mg/dL | ||
| n | 118 | 75 |
| DB baseline, mean (SD) | 129.2 (64.5) | 135.3 (91.5) |
| Median change from DB baseline to OLE LOCF endpoint | −11.0 | −11.0 |
| Median change from OLE baseline to OLE LOCF endpoint | −3.5 | −4.0 |
| Glucose, mg/dL | ||
| n | 118 | 75 |
| DB baseline, mean (SD) | 95.3 (13.7) | 93.8 (12.0) |
| Median change from DB baseline to OLE LOCF endpoint | −1.0 | 2.0 |
| Median change from OLE baseline to OLE LOCF endpoint | 0 | −1.0 |
| HbA1c, % | ||
| n | 121 | 75 |
| DB baseline, mean (SD) | 5.7 (0.4) | 5.6 (0.4) |
| Median change from DB baseline to OLE LOCF endpoint | 0 | 0.1 |
| Median change from OLE baseline to OLE LOCF endpoint | 0 | 0 |
| Insulin, mU/L | ||
| n | 124 | 79 |
| DB baseline, mean (SD) | 13.2 (19.4) | 11.5 (15.4) |
| Median change from DB baseline to OLE LOCF endpoint | −0.9 | −0.2 |
| Median change from OLE baseline to OLE LOCF endpoint | 0.1 | −0.6 |
| Prolactin—male, ng/mL | ||
| n | 95 | 54 |
| DB baseline, mean (SD) | 8.0 (6.9) | 10.6 (9.8) |
| Median change from DB baseline to OLE LOCF endpoint | 0.1 | −1.1 |
| Median change from OLE baseline to OLE LOCF endpoint | 0.1 | −10.5 |
| Prolactin—female, ng/mL | ||
| n | 31 | 27 |
| DB baseline, mean (SD) | 21.4 (25.4) | 16.4 (39.8) |
| Median change from DB baseline to OLE LOCF endpoint | −2.9 | 4.1 |
| Median change from OLE baseline to OLE LOCF endpoint | 0 | −29.7 |
| Weight, kg | ||
| n | 127 | 81 |
| DB baseline, mean (SD) | 79.4 (18.3) | 81.7 (18.3) |
| Median change from DB baseline to OLE LOCF endpoint | −0.6 | 0.6 |
| Median change from OLE baseline to OLE LOCF endpoint | −0.5 | −1.5 |
| Body mass index, kg/m2 | ||
| n | 127 | 81 |
| DB baseline, mean (SD) | 27.2 (5.3) | 28.1 (5.6) |
| Median change from DB baseline to OLE LOCF endpoint | −0.2 | 0.2 |
| Median change from OLE baseline to OLE LOCF endpoint | −0.1 | −0.5 |
| Waist circumference, cm | ||
| n | 108 | 66 |
| DB baseline, mean (SD) | 93.4 (13.9) | 96.3 (14.7) |
| Median change from DB baseline to OLE LOCF endpoint | −0.5 | 0 |
| Median change from OLE baseline to OLE LOCF endpoint | 0 | −1.0 |
All variables measured under fasting conditions except HbA1c and prolactin
aNormal ranges: HDL cholesterol, > 35 mg/dL; glucose, 59–99 mg/dL; insulin, 3–28 mU/L; prolactin, 2.1–17.7 ng/mL (men) and 2.8–29.2 ng/mL (women)
bLurasidone versus risperidone. NNH is provided only for comparisons in which the 95% CI did not include infinity, denoting statistical significance at the p ≤ 0.05 threshold. NNH = 1/(rate with lurasidone − rate with risperidone) and rounded up. A negative NNH denotes an advantage for lurasidone relative to risperidone and can be expressed as a positive number if the comparison is risperidone vs lurasidone instead of lurasidone vs risperidone
c95% CI contains infinity
dFor HDL cholesterol, the shift measured was from high/normal to low
eFor body mass index, the shift measured was any upward shift (i.e. from underweight to normal or higher, normal to overweight or obese, and overweight to obese)
CI confidence interval, DB double-blind, HbA1c glycosylated haemoglobin, HDL high-density lipoprotein, LDL low-density lipoprotein, LOCF last observation carried forward, NNH number needed to harm, NS not significant, OLE open-label extension, SD standard deviation
Fig. 2.
Change in prolactin over time from DB baseline to OLE LOCF endpoint, by treatment assignment in DB trial, for (a) males and (b) females. DB double-blind, LOCF last observation carried forward, OLE open-label extension
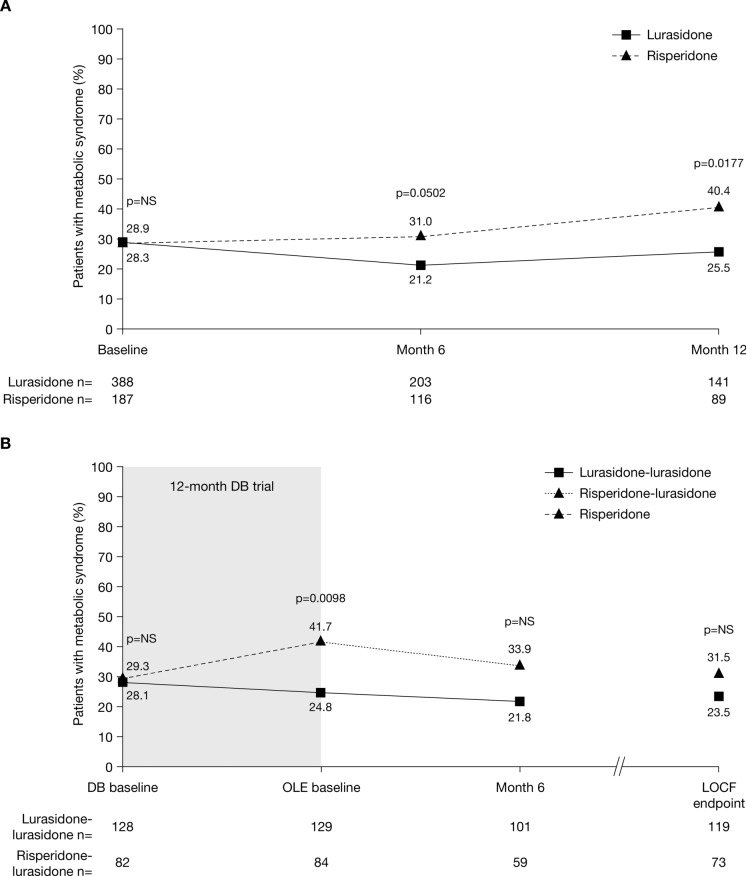
During the DB trial, the proportion of patients with metabolic syndrome decreased slightly following treatment with lurasidone (from 28.9% at baseline to 25.5% at month 12), but increased following treatment with risperidone (from 28.3% at baseline to 40.4% at month 12), the between-group difference being significant at month 12 (p = 0.0177; Fig. 3a).
Fig. 3.
Change in percentage of patients with metabolic syndrome (a) from DB baseline to month 12 and (b) from DB baseline to OLE LOCF endpoint, by treatment assignment in Study 237 (safety population). DB double-blind, LOCF last observation carried forward, NS not significant, OLE open-label extension
Electrocardiography
There were no clinically relevant ECG changes from baseline to LOCF endpoint in either treatment group, and no patients had a Fridericia’s corrected QT interval of > 500 ms or an increase of ≥ 60 ms at any time during the study.
Weight, BMI, and Waist Circumference
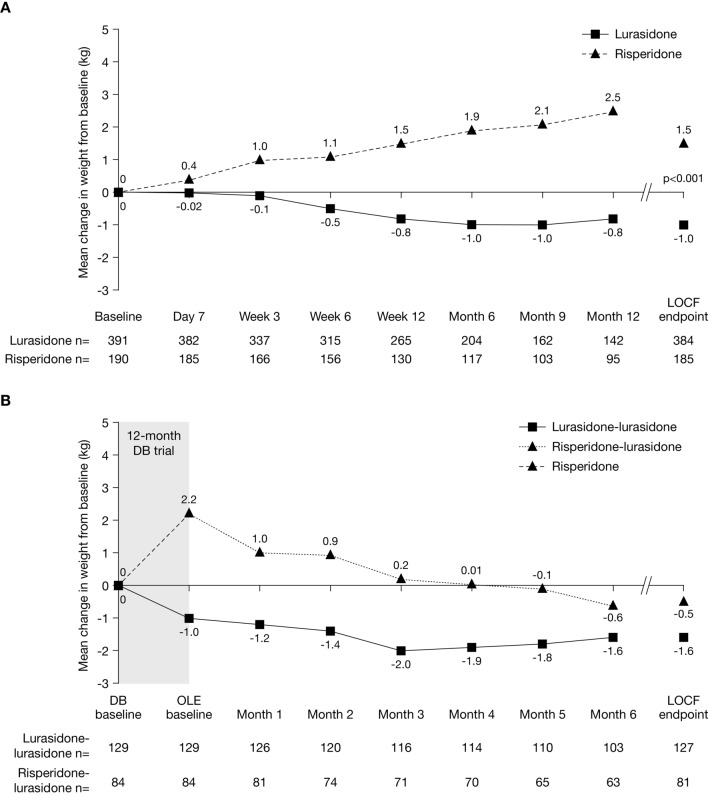
Changes from baseline in weight, BMI, and waist circumference differed significantly between groups (Table 3; Fig. 4a). The median change in weight during the DB trial was −0.3 kg for lurasidone versus +1.1 kg for risperidone (p < 0.001). The proportion of patients experiencing ≥ 7% increase in weight was lower for lurasidone than for risperidone (7.6% vs 14.1%; NNH, −16 [−120 to −9]), and the proportion of patients who experienced ≥ 7% decrease in weight was significantly higher for lurasidone than for risperidone (13.0% vs 5.9%; NNH, 15 [9–44]). The median change in BMI during the DB trial was −0.1 kg/m2 for lurasidone versus +0.4 kg/m2 for risperidone (p < 0.001). The proportion of patients who experienced an upward shift in BMI category (i.e. from underweight to normal or higher, normal to overweight or obese, or overweight to obese) was lower with lurasidone than with risperidone, although this difference was not significant (4.7% vs 9.1%; NNH, −23 [95% CI, not significant; contains infinity]). Median and mean increases in waist circumference were experienced by patients treated with risperidone (median, 1 cm; mean, 17 cm) but not by those treated with lurasidone (mean, 0 cm; median, −0.5 cm) (p < 0.001).
Fig. 4.
Change in weight over time (a) from DB baseline to DB LOCF endpoint and (b) from DB baseline to OLE LOCF endpoint, by treatment assignment in DB trial. DB double-blind, LOCF last observation carried forward, OLE open-label extension
Movement Rating Scales
The mean (SD) baseline BAS total scores were 0.3 (0.9) and 0.2 (0.7) in the lurasidone and risperidone groups, respectively. There was a small but statistically significant increase from baseline to LOCF endpoint in the mean (standard error [SE]) BAS total score in patients treated with lurasidone (0.14 [0.04]; p = 0.002), but there was no significant change in the risperidone group (−0.10 [0.06]; p = 0.110). The between-group treatment difference at LOCF endpoint was 0.2 (SE, 0.07; p = 0.001). Mean (SD) baseline AIMS total scores were 0.6 (1.6) and 0.5 (1.4) in the lurasidone and risperidone groups, respectively. These scores did not change significantly from baseline to the LOCF endpoint, and the between-group treatment difference was non-significant. Mean (SD) baseline SAS 10-item scores were 0.1 (0.2) and 0.1 (0.3) in the lurasidone and risperidone groups, respectively. As with AIMS, these scores did not change significantly from baseline to the LOCF endpoint, and the between-group treatment difference was non-significant.
Study 237-EXT
TEAEs
The proportion of patients with TEAEs was similar between the lurasidone–lurasidone and risperidone–lurasidone groups (58.9% vs 58.3%), as were the proportions of patients with EPS-related TEAEs (8.5% vs 7.1%) and serious TEAEs (5.4% vs 3.6%) (Table 2b). The most frequently reported TEAEs (≥ 5% of patients in the overall population) were headache (lurasidone–lurasidone, 4.7%; risperidone–lurasidone, 8.3%) and psychotic disorder (4.7% vs 7.1%).
The most frequently reported EPS-related TEAEs were parkinsonism (lurasidone–lurasidone, 3.9%; risperidone–lurasidone, 6.0%) and dystonia (1.6% vs 1.2%). All other EPS-related TEAEs occurred in no more than one patient in both groups combined. Akathisia was reported as a TEAE for 3.1% of patients in the lurasidone–lurasidone group and 2.4% of patients in the risperidone–lurasidone group.
The most frequently reported metabolic-related TEAEs were weight increase (lurasidone–lurasidone, 0.8%; risperidone–lurasidone, 2.4%) and increased blood triglycerides (1.6% vs 0%). All other metabolic-related TEAEs occurred in no more than one patient in both groups combined.
Serious TEAEs occurred in 10 patients (4.7%) overall. Serious TEAEs occurring in more than one patient were psychotic disorder (lurasidone–lurasidone, 1.6%; risperidone–lurasidone, 1.2%) and schizophrenia (0.8% vs 1.2%). There was one completed suicide in the lurasidone–lurasidone group, and suicidal depression was reported for one patient in the risperidone–lurasidone group. TEAEs leading to discontinuation occurred in 5.4% and 7.1% of patients in the lurasidone–lurasidone and risperidone–lurasidone groups, respectively. The only TEAE that led to discontinuation of more than one patient was psychotic disorder (lurasidone–lurasidone, n = 1 [0.8%]; risperidone–lurasidone, n = 2 [2.4%]).
Laboratory Parameters
In the lurasidone–lurasidone group, there was a slight decrease from OLE baseline to OLE LOCF endpoint in median total cholesterol and triglyceride levels, with minimal or no changes in other metabolic variables (Table 3b). In the risperidone–lurasidone group, there was an increases from OLE baseline to OLE LOCF endpoint in LDL cholesterol, HDL cholesterol, and total cholesterol levels, and a decrease in triglyceride and insulin levels, with minimal or no changes in other metabolic variables. Prolactin levels remained stable in male and female patients in the lurasidone–lurasidone group, but there was a marked reduction in prolactin levels in male and female patients in the risperidone–lurasidone group (Table 3b; Fig. 2).
At OLE baseline, the proportion of patients with metabolic syndrome was significantly lower in the lurasidone–lurasidone group than the risperidone–lurasidone group (24.8% vs 41.7%; p = 0.0098) (Fig. 3b). The proportion of patients with metabolic syndrome decreased in both groups during the OLE, but to a greater extent in the risperidone–lurasidone group than the lurasidone–lurasidone group, and the between-group difference for lurasidone–lurasidone versus risperidone–lurasidone was no longer significantly different at month 6 (21.8% vs 33.9%) or LOCF endpoint (23.5% vs 31.5%).
Electrocardiography
There were no clinically meaningful changes in mean ECG parameters during the OLE study.
Weight, BMI, and Waist Circumference
There was a slight decrease in mean weight in the lurasidone–lurasidone group from OLE baseline to OLE LOCF endpoint, with minimal changes in median BMI and waist circumference (Table 3b; Fig. 4b). In the risperidone–lurasidone group, mean weight decreased by approximately 2.5 kg from OLE baseline to OLE LOCF endpoint, resulting in a slight decrease in mean weight from DB baseline (Fig. 4b), and a slight decrease was also observed in median BMI and waist circumference (Table 3b). At OLE LOCF endpoint, the proportion of patients who experienced ≥ 7% increase in weight from OLE baseline was 3.1% in the lurasidone–lurasidone group and 2.5% in the risperidone–lurasidone group, and the proportion of patients who experienced ≥ 7% decrease in weight was 6.3% and 16.0%, respectively.
Movement Rating Scales
In the lurasidone–lurasidone and risperidone–lurasidone groups, the mean change in BAS total score from OLE baseline to OLE LOCF endpoint was 0.0 in both groups (median changes also 0.0), the mean change in SAS was −0.01 and 0.00 (median, 0.0 and 0.0), respectively, and the mean change in AIMS total score was 0.3 and 0.3 (median, 0.0 and 0.0), respectively.
Efficacy
Study 237
Relapse Rates
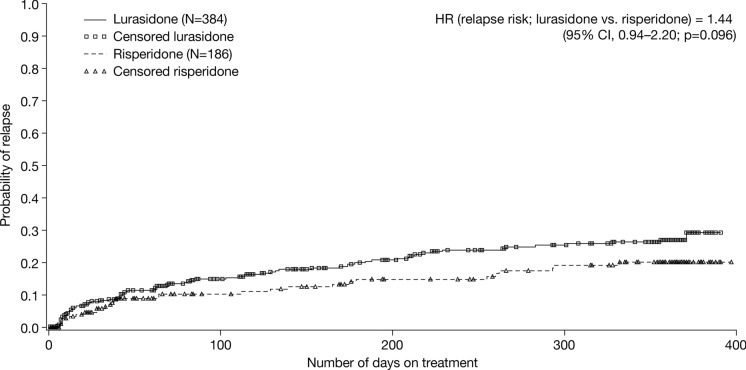
In total, 79/384 (20.6%) patients treated with lurasidone and 29/186 (15.6%) patients treated with risperidone experienced relapse during the DB trial (ITT population) (Fig. 5). The Kaplan–Meier estimate for probability of relapse ranged from 10.2% at week 6 to 27.0% at month 12 in patients treated with lurasidone, and from 8.9% at week 6 to 20.1% at month 12 in patients treated with risperidone. Since the estimates at month 12 were < 50% for both groups, the median survival times to relapse could not be calculated. The relapse hazard ratio for lurasidone versus risperidone was 1.44 (95% CI, 0.94–2.20; p = 0.096).
Fig. 5.
Kaplan–Meier estimate of the probability of relapse in the DB trial (Study 237) (ITT population). CI confidence interval, DB double-blind, HR hazard ratio, ITT intent-to treat
Positive and Negative Syndrome Scale
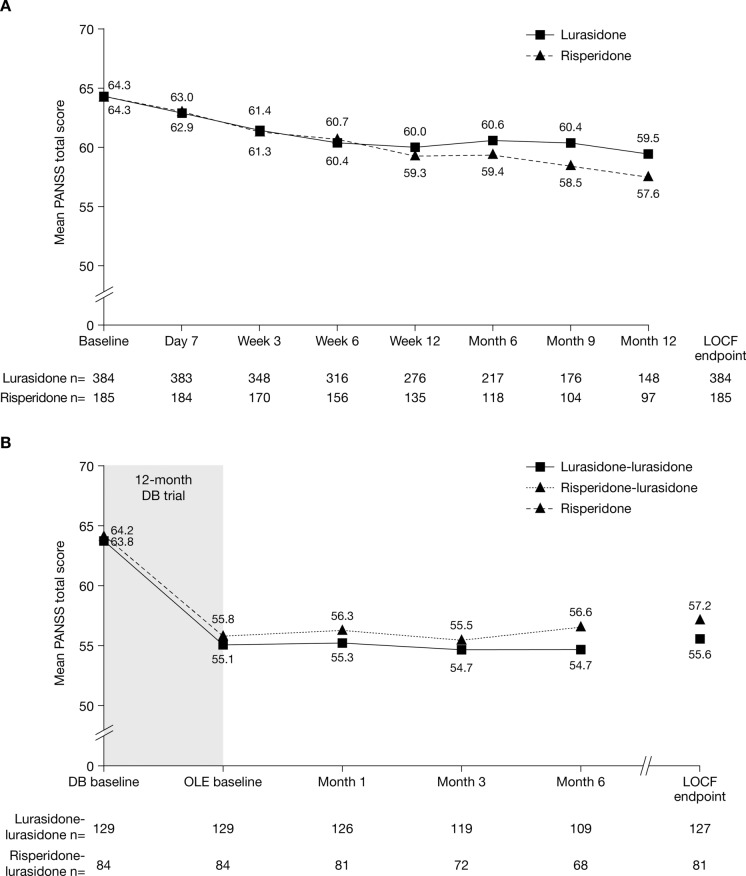
The mean PANSS total score decreased from baseline during the 12-month DB trial in both the lurasidone and risperidone groups (mean [95% CI] change, −4.8 [−6.5, −3.0] and −6.6 [−8.9, −4.4], respectively), with no significant differences between groups at any time point (Fig. 6A).
Fig. 6.
Change in PANSS total score over time (a) from DB baseline to DB LOCF endpoint and (b) from DB baseline to OLE LOCF endpoint, by treatment assignment in Study 237 (ITT population). DB double-blind, ITT intent-to treat, LOCF last observation carried forward, OLE open-label extension, PANSS Positive and Negative Syndrome Scale
Clinical Global Impression–Severity
The mean CGI-S score decreased from DB baseline to month 12 in both the lurasidone and risperidone groups (mean [95% CI] change, −0.4 [−0.5, −0.3] and −0.4 [−0.5, −0.2], respectively), with no significant differences between groups at any time point.
Montgomery–Åsberg Depression Rating Scale
The mean MADRS total score decreased from DB baseline to month 12 in both the lurasidone and risperidone groups (mean [95% CI] change, −0.8 [−1.6, 0.0] and −2.3 [−3.2, −1.3], respectively). The difference between groups was statistically significant at month 12 (p = 0.013) but not at earlier time points.
Study 237-EXT
Positive and Negative Syndrome Scale
The improvement in PANSS total score observed during the DB trial was maintained during the OLE study in both the lurasidone–lurasidone and risperidone–lurasidone groups (mean [95% CI] change from OLE baseline to OLE LOCF endpoint, 0.6 [−0.9, 2.1] and 1.3 [−0.6, 3.1], respectively) (Fig. 6b).
Clinical Global Impression–Severity
The improvement in CGI-S score observed during the DB trial was maintained during the OLE study in both treatment groups (mean [95% CI] change from OLE baseline to OLE LOCF endpoint: lurasidone–lurasidone, 0.0 [−0.1, 0.2]; risperidone–lurasidone, 0.0 [−0.1, 0.2]).
Montgomery–Åsberg Depression Rating Scale
The improvement in MADRS total score observed during the DB trial was maintained during the OLE study in both treatment groups (mean [95% CI] change from OLE baseline to OLE LOCF endpoint: lurasidone–lurasidone, 0.1 [−0.7, 0.9]; risperidone–lurasidone, 1.1 [0.1, 2.1]).
Discussion
This post hoc analysis of a 12-month, DB, active-controlled trial and 6-month OLE study demonstrated that lurasidone was generally well tolerated and effective in treating clinically stable patients with schizophrenia over the long term. It also demonstrated that lurasidone was generally well tolerated and maintained effectiveness in patients with schizophrenia who switched to lurasidone having been treated with risperidone for 12 months in the DB trial. These findings are consistent with those of the original DB trial [15] and OLE study [16], which included patients with schizoaffective disorder as well as schizophrenia, but confirm lurasidone’s tolerability and effectiveness specifically in patients with schizophrenia, who have been shown to have less favourable outcomes than those with schizoaffective disorder [18, 28].
During the DB trial, the proportions of patients with TEAEs and serious TEAEs were similar in the lurasidone and risperidone groups. Although a greater proportion of patients in the lurasidone versus risperidone group discontinued due to TEAEs (NNH: 15), the rate of discontinuation due to individual TEAEs did not differ between groups by more than approximately 1%. It is also noteworthy that a key exclusion criterion for participation in the trial was a history of a poor or an inadequate response or intolerability to risperidone, meaning that the population may have been enriched in terms of tolerability and response to risperidone. A higher proportion of patients treated with lurasidone versus risperidone experienced akathisia (NNH: 17), and there was a small but statistically significant increase from baseline in BAS score with lurasidone but not with risperidone. By contrast, EPS-related TEAEs were reported less frequently with lurasidone than with risperidone (NNH: −15). Metabolic-related TEAEs were also reported less frequently with lurasidone versus risperidone (NNH: −11), primarily due to the lower incidence of weight gain with lurasidone in comparison with risperidone (NNH: −10).
Consistent with the findings for metabolic-related TEAEs, lurasidone demonstrated a more benign profile than risperidone in terms of metabolic variables, prolactin, weight, BMI, and waist circumference. The greatest difference between groups was observed for prolactin levels, the proportion of patients shifting from low/normal to high prolactin levels being substantially lower for lurasidone versus risperidone, in both male and female patients (NNH: −4 [male]; −3 [female]). Lurasidone treatment was associated with a negligible impact on fasting glucose levels, whereas risperidone treatment resulted in a median increase of 2.0 mg/dL over the 12 months of the trial [NNH: −9]. The proportion of patients experiencing a clinically significant weight increase (≥ 7% increase) was approximately two-fold higher for risperidone versus lurasidone (NNH: −16), whereas the proportion who experienced a clinically significant weight decrease (≥ 7% decrease) was approximately two-fold higher for lurasidone versus risperidone (NNH: 15). Differences in the metabolic impact of lurasidone and risperidone were also reflected in the proportions of patients with NCEP ATP III-defined metabolic syndrome, which were similar between groups at baseline but significantly higher for risperidone versus lurasidone at the end of the DB trial. Indeed, the proportion of patients with metabolic syndrome decreased slightly following lurasidone treatment.
During the OLE study, the proportions of patients experiencing TEAEs, EPS-related TEAEs, serious TEAEs, and TEAEs leading to discontinuation were generally comparable between patients who received lurasidone throughout the DB trial and OLE study (lurasidone–lurasidone group) and those who switched from risperidone to lurasidone at the start of the OLE study (risperidone–lurasidone group). Psychotic disorder was the only serious TEAE reported by more than one patient in either group (lurasidone–lurasidone, n = 2; risperidone–lurasidone, n = 1) and the only TEAE leading to discontinuation of more than one patient in either group (risperidone–lurasidone, n = 2; lurasidone–lurasidone, n = 1). Changes in movement rating scales (BAS, SAS, and AIMS) were minimal and similar between groups during the OLE study.
The proportion of patients with metabolic-related TEAEs was lower in the lurasidone–lurasidone than in the risperidone–lurasidone group, although the incidence was low in both groups, and most individual metabolic-related TEAEs occurred in no more than one patient in both groups combined. Patients in the lurasidone–lurasidone group experienced a slight decrease in the median levels of total cholesterol (−4.0 mg/dL) and triglycerides (−3.5 mg/dL) from OLE baseline to OLE endpoint, and minimal or no change in other metabolic variables. In the risperidone–lurasidone group, there was an increase from OLE baseline to OLE endpoint in the median levels of LDL cholesterol (+8 mg/dL), HDL cholesterol (+3.0 mg/dL), and total cholesterol (+4.0 mg/dL), and a decrease in the median levels of triglycerides (−4.0 mg/dL) and insulin (−0.6 mU/L), with minimal or no changes in other metabolic variables. As in the DB trial, the greatest change was observed for prolactin levels, which remained stable in the lurasidone–lurasidone group but decreased substantially in both male and female patients in the risperidone–lurasidone group (median changes: −10.5 ng/mL [male] and −29.7 ng/mL [female]). Median body weight decreased during the OLE study in both groups, but the decrease was greater in patients in the risperidone–lurasidone group (−1.5 kg) than in the lurasidone–lurasidone group (−0.5 kg). The mean weight decrease in the risperidone–lurasidone group during the OLE study was approximately 2.5 kg, resulting in a slight decrease in weight from DB baseline. Similar patterns were observed for BMI and waist circumference. During the OLE study, the proportion of patients who experienced clinically significant weight gain was low in both groups. However, the proportion of patients who experienced a clinically significant decrease in weight was substantially higher in the risperidone–lurasidone than in the lurasidone–lurasidone group (16.0% vs 6.3%). The proportion of patients with metabolic syndrome was significantly higher in the risperidone–lurasidone versus lurasidone–lurasidone group at OLE baseline, but decreased in both groups during the OLE study, particularly in the risperidone–lurasidone group, and the between-group difference was no longer significant by the end of the OLE trial.
The safety/tolerability findings observed in the current study are consistent with the known safety profiles of lurasidone [11] and risperidone [29]. The rate of akathisia observed with lurasidone during the DB trial (13.6%) was similar to that reported in short-term placebo-controlled trials (12.9%) [11]. However, the rate of discontinuation due to akathisia in the lurasidone group was low (1.0%), and akathisia was not reported as a common TEAE in the OLE study in either group. Risperidone is commonly associated with prolactin elevation and weight gain [29], as observed in the current study. The findings of this study are also consistent with those from other long-term studies; for example, in a pooled analysis of six studies (including two with the active comparators risperidone and quetiapine XR) which assessed the effect of 12 months of lurasidone treatment on weight in patients with schizophrenia, the mean change in weight from baseline to month 12 was −0.4 kg with lurasidone, versus +2.6 kg with risperidone and +1.2 kg with quetiapine XR [30]. Moreover, several meta-analyses of available evidence for atypical antipsychotics have demonstrated that lurasidone has a relatively benign cardiometabolic profile in comparison with other agents, whereas risperidone is associated with a moderate risk of weight gain and high risk of prolactin elevation [9, 10, 14].
An important finding of the current study was that patients who switched from risperidone to lurasidone at the start of the OLE phase experienced a marked decrease in weight and prolactin levels, which had increased during 12 months of treatment with risperidone in the DB trial. These findings are consistent with previous findings [31–33]. In a 6-month OLE study of a 6-week trial during which patients with acute exacerbation of schizophrenia were treated with lurasidone or olanzapine, patients who had gained weight while being treated with olanzapine experienced decreased weight and improved lipid levels after switching to lurasidone in the OLE study, whereas those treated with lurasidone during the initial trial and OLE study experienced minimal changes in weight and lipid parameters [31]. Similarly, prolactin elevation that occurred during olanzapine treatment in the initial trial decreased following the switch to lurasidone in the OLE study [31]. In another study, in which patients with schizophrenia or schizoaffective disorder were switched to lurasidone after being stable on treatment with a range of antipsychotics (most commonly quetiapine, risperidone, and aripiprazole), improvements in body weight and lipid levels were observed following 6 weeks of treatment with lurasidone [32]. During the subsequent OLE study, in which all patients continued to be treated with lurasidone, there were no clinically relevant adverse changes in body weight, lipids, glucose, insulin, or prolactin [33].
Efficacy was assessed as a secondary objective of the current study. During the DB trial, patients treated with lurasidone and risperidone experienced improvements in PANSS total score, CGI-S score, and MADRS total score. There were no significant differences between treatment groups at any time point, with the exception of the MADRS total score, which was decreased (improved) to a significantly greater extent in the risperidone versus lurasidone group at month 12, but not at earlier time points. A higher proportion of patients in the lurasidone versus risperidone group experienced relapse during the DB trial, the relapse hazard ratio for lurasidone versus risperidone being 1.44 (p = 0.096). Once again, it should be pointed out that patients who previously showed a poor or inadequate response to risperidone were excluded from participation in the trial, which may have enriched the population in terms of response to risperidone. During the OLE study, improvements in PANSS total score, CGI-S score, and MADRS total score observed during the DB trial were maintained in both the lurasidone–lurasidone group and the risperidone–lurasidone group.
There is increasing recognition of the importance of addressing the physical as well as the mental health of patients with conditions such as schizophrenia. Indeed, the Lancet Psychiatry Commission has recently published a ‘blueprint’ outlining strategies for protecting the physical health of people with mental illness, which highlights that protecting the physical health of people receiving treatment for mental illness should be regarded as within the scope of clinical duty of care [34]. Individuals with schizophrenia have a significantly higher risk of cardiometabolic complications than the general population (5–8-fold), which is often exacerbated by the effects of antipsychotic therapy, especially treatment with atypical antipsychotics [3, 5]. Treatment guidelines therefore advocate screening patients for cardiometabolic risk, and intervening where necessary to improve their physical health, not only through lifestyle interventions (such as diet, exercise, and smoking) and by actively treating cardiometabolic conditions (such as hypertension and dyslipidaemia), but also by choosing and adapting antipsychotic treatment in order to minimise the likelihood of long-term adverse physical sequelae [34-37]. Since atypical antipsychotics vary greatly in terms of their safety profiles, particularly with regard to cardiometabolic risk [9, 10], the choice of antipsychotic treatment is particularly relevant, and guidelines highlight the importance of choosing an antipsychotic at the outset of treatment that will minimise the risk of developing or exacerbating cardiometabolic complications, and of switching antipsychotic treatment where necessary in order to reverse or minimise the development and impact of such complications [34-37]. Within this context, the findings of the current study are encouraging, not only in confirming that lurasidone is associated with minimal changes in cardiometabolic parameters over the long term, but also in demonstrating that patients who have developed weight gain, other metabolic disturbances (e.g. raised glucose levels), or prolactin elevation while being treated with risperidone can experience improvements in these parameters after switching to lurasidone.
As previously noted, a limitation of the current study is that it excluded patients with a history of a poor or inadequate response or intolerability to risperidone. This may have introduced bias by enriching the study population for patients who were responsive to risperidone and who had previously demonstrated tolerability to the agent, which could have affected both the safety/tolerability and effectiveness outcomes in favour of risperidone. The study was also limited in that it was a post hoc subgroup analysis, and the OLE phase was limited by its open-label design and the lack of a control arm. Since this study was conducted in patients with clinically stable schizophrenia, its findings cannot be extrapolated to those with acute exacerbation of schizophrenia.
Conclusion
In summary, the findings from this DB trial and OLE study confirm that lurasidone is generally well tolerated and effective in treating patients with clinically stable schizophrenia over the long term (up to 18 months). Lurasidone was also generally well tolerated and maintained effectiveness over 6 months in patients with schizophrenia who switched to lurasidone having previously been treated with risperidone for 12 months. Long-term lurasidone treatment was associated with minimal changes in metabolic variables and prolactin levels, and patients who switched from risperidone to lurasidone experienced improvements in prolactin levels, weight and other metabolic parameters. These findings support the use of lurasidone within the context of addressing the physical as well as mental health of patients with schizophrenia.
Acknowledgements
Funding
The study was funded by Sunovion Pharmaceuticals Europe Ltd. The journal’s Rapid Service Fees were also funded by Sunovion Pharmaceuticals Europe Ltd.
Medical Writing, Editorial, and Other Assistance
Editorial support for the preparation of this manuscript was provided by John Scopes of mXm Medical Communications and funded by Sunovion Pharmaceuticals Europe Ltd.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Preeya J. Patel, Christian Weidenfeller and Andrew P. Jones are employees of Sunovion Pharmaceuticals Europe Ltd. Jens Nilsson was an employee of Sunovion Pharmaceuticals Europe Ltd at the time of this study but has been a full-time employee of Vifor Pharma Nordiska since September 2020. Jay Hsu is an employee of Sunovion Pharmaceuticals Inc.
Compliance with Ethics Guidelines
Studies 237 and 237-EXT (registered on ClinicalTrials.gov; NCT00641745) were both conducted in accordance with the Good Clinical Practice Guidelines of the International Conference on Harmonisation and with the ethical principles of the Declaration of Helsinki. The studies were reviewed and approved by an Independent Ethics Committee or Institutional Review Board at each study centre and all patients provided written informed consent prior to participation.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392(10159):1789–858. 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed]
- 2.Laursen TM, Nordentoft M, Mortensen PB. Excess early mortality in schizophrenia. Annu Rev Clin Psychol. 2014;10:425–448. doi: 10.1146/annurev-clinpsy-032813-153657. [DOI] [PubMed] [Google Scholar]
- 3.Grajales D, Ferreira V, Valverde ÁM. Second-generation antipsychotics and dysregulation of glucose metabolism: beyond weight gain. Cells. 2019;8(11):E1336. doi: 10.3390/cells8111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Šimunović Filipčić I, Filipčić I. Schizophrenia and physical comorbidity. Psychiatr Danub. 2018;30(Suppl 4):152–157. [PubMed] [Google Scholar]
- 5.Penninx BWJH, Lange SMM. Metabolic syndrome in psychiatric patients: overview, mechanisms, and implications. Dialogues Clin Neurosci. 2018;20(1):63–73. doi: 10.31887/DCNS.2018.20.1/bpenninx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vancampfort D, Wampers M, Mitchell AJ, Correll CU, De Herdt A, Probst M, et al. A meta-analysis of cardio-metabolic abnormalities in drug naïve, first-episode and multi-episode patients with schizophrenia versus general population controls. World Psychiatry. 2013;12(3):240–250. doi: 10.1002/wps.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vancampfort D, Stubbs B, Mitchell AJ, De Hert M, Wampers M, Ward PB, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry. 2015;14(3):339–347. doi: 10.1002/wps.20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Correll CU, Solmi M, Veronese N, Bortolato B, Rosson S, Santonastaso P, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16(2):163–180. doi: 10.1002/wps.20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leucht S, Leucht C, Huhn M, Chaimani A, Mavridis D, Helfer B, et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review, Bayesian meta-analysis, and meta-regression of efficacy predictors. Am J Psychiatry. 2017;174(10):927–942. doi: 10.1176/appi.ajp.2017.16121358. [DOI] [PubMed] [Google Scholar]
- 10.Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–951. doi: 10.1016/S0140-6736(19)31135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aziende Chimiche Riunite Angelini Francesco–A.C.R.A.F. S.p.A. Latuda® Summary of Product Characteristics. 2020. https://www.ema.europa.eu/en/documents/product-information/latuda-epar-product-information_en.pdf. Accessed 7 Oct 2020.
- 12.Sunovion Pharmaceuticals Inc. Latuda® Prescribing Information. 2019. https://www.latuda.com/LatudaPrescribingInformation.pdf. Accessed 7 Oct 2020.
- 13.Greenberg WM, Citrome L. Pharmacokinetics and pharmacodynamics of lurasidone hydrochloride, a second-generation antipsychotic: a systematic review of the published literature. Clin Pharmacokinet. 2017;56(5):493–503. doi: 10.1007/s40262-016-0465-5. [DOI] [PubMed] [Google Scholar]
- 14.Kim DH, Maneen MJ, Stahl SM. Building a better antipsychotic: receptor targets for the treatment of multiple symptom dimensions of schizophrenia. Neurotherapeutics. 2009;6(1):78–85. doi: 10.1016/j.nurt.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Citrome L, Cucchiaro J, Sarma K, Phillips D, Silva R, Tsuchiya S, et al. Long-term safety and tolerability of lurasidone in schizophrenia: a 12-month, double-blind, active-controlled study. Int Clin Psychopharmacol. 2012;27(3):165–176. doi: 10.1097/YIC.0b013e32835281ef. [DOI] [PubMed] [Google Scholar]
- 16.Mattingly GW, Haddad PM, Tocco M, Xu J, Phillips D, Pikalov A, et al. Switching to lurasidone following 12 months of treatment with risperidone: results of a 6-month, open-label study. BMC Psychiatry. 2020;20(1):199. doi: 10.1186/s12888-020-02523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wy TJP, Saadabadi A. Schizoaffective disorder. 2020. https://www.ncbi.nlm.nih.gov/books/NBK541012/. Accessed 7 Oct 2020.
- 18.Harrow M, Grossman LS, Herbener ES, Davies EW. Ten-year outcome: patients with schizoaffective disorders, schizophrenia, affective disorders and mood-incongruent psychotic symptoms. Br J Psychiatry. 2000;177:421–426. doi: 10.1192/bjp.177.5.421. [DOI] [PubMed] [Google Scholar]
- 19.Muñoz-Negro JE, Cuadrado L, Cervilla JA. Current evidences on psychopharmacology of schizoaffective disorder. Actas Esp Psiquiatr. 2019;47(5):190–201. [PubMed] [Google Scholar]
- 20.De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114–126. doi: 10.1038/nrendo.2011.156. [DOI] [PubMed] [Google Scholar]
- 21.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 22.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 23.Guy W. ECDEU assessment manual for psychopharmacology, revised. DHEW Publication No. (ADM) 76–338. Rockville, MD: National Institute of Mental Health; 1976.
- 24.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 25.Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 26.Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317(7168):1309–1312. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bender R. Calculating confidence intervals for the number needed to treat. Control Clin Trials. 2001;22(2):102–110. doi: 10.1016/s0197-2456(00)00134-3. [DOI] [PubMed] [Google Scholar]
- 28.Grossman LS, Harrow M, Goldberg JF, Fichtner CG. Outcome of schizoaffective disorder at two long-term follow-ups: comparisons with outcome of schizophrenia and affective disorders. Am J Psychiatry. 1991;148(10):1359–1365. doi: 10.1176/ajp.148.10.1359. [DOI] [PubMed] [Google Scholar]
- 29.Janssen-Cilag Ltd. Risperdal® Summary of Product Characteristics. 2008. https://www.ema.europa.eu/en/documents/referral/risperdal-article-30-referral-annex-i-ii-iii-iv_en-0.pdf. Accessed 7 Oct 2020.
- 30.Meyer JM, Mao Y, Pikalov A, Cucchiaro J, Loebel A. Weight change during long-term treatment with lurasidone: pooled analysis of studies in patients with schizophrenia. Int Clin Psychopharmacol. 2015;30(6):342–350. doi: 10.1097/YIC.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stahl SM, Cucchiaro J, Simonelli D, Hsu J, Pikalov A, Loebel A. Effectiveness of lurasidone for patients with schizophrenia following 6 weeks of acute treatment with lurasidone, olanzapine, or placebo: a 6-month, open-label, extension study. J Clin Psychiatry. 2013;74(5):507–515. doi: 10.4088/JCP.12m08084. [DOI] [PubMed] [Google Scholar]
- 32.McEvoy JP, Citrome L, Hernandez D, Cucchiaro J, Hsu J, Pikalov A, et al. Effectiveness of lurasidone in patients with schizophrenia or schizoaffective disorder switched from other antipsychotics: a randomized, 6-week, open-label study. J Clin Psychiatry. 2013;74(2):170–179. doi: 10.4088/JCP.12m07992. [DOI] [PubMed] [Google Scholar]
- 33.Citrome L, Weiden PJ, McEvoy JP, Correll CU, Cucchiaro J, Hsu J, et al. Effectiveness of lurasidone in schizophrenia or schizoaffective patients switched from other antipsychotics: a 6-month, open-label, extension study. CNS Spectr. 2014;19(4):330–339. doi: 10.1017/S109285291300093X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Firth J, Siddiqi N, Koyanagi A, Siskind D, Rosenbaum S, Galletly C, et al. The Lancet Psychiatry Commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry. 2019;6(8):675–712. doi: 10.1016/S2215-0366(19)30132-4. [DOI] [PubMed] [Google Scholar]
- 35.Cooper SJ, Reynolds GP; With expert co-authors (in alphabetical order), Barnes T, England E, Haddad PM, Heald A, et al. BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. J Psychopharmacol. 2016; 30(8):717–48. 10.1177/0269881116645254. [DOI] [PubMed]
- 36.Taylor D, Barnes T, Young A. The Maudsley prescribing guidelines in psychiatry. 13. Hoboken: Wiley-Blackwell; 2018. [Google Scholar]
- 37.Leucht S, Arango C, Fleischhacker WW, Kapur S, Stroup S, van Os J et al. CINP Schizophrenia Guideline. 2020. https://www.cinp.org/resources/Documents/CINP-schizophrenia-guideline-24.5.2013-A-C-method.pdf. Accessed 7 Oct 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.