Abstract
Rationale:
The activation of the glucagon-like peptide-1 receptor (GLP-1R) has been purported to have antidepressant-like and cognitive-enhancing effects. Many people suffering from major depressive disorder (MDD) also experience deficits in cognition. While currently approved antidepressant pharmacotherapies can alleviate the mood symptoms in some patients, they do not treat the cognitive ones.
Objectives:
We tested whether systemic administration of a GLP-1R agonist would alter location discrimination, a cognitive task that is diminished in humans with MDD.
Methods:
Male and female laboratory mice (6–8 weeks old, N=6–14/sex) were trained in a touchscreen operant task of location discrimination. Upon reaching baseline criterion, mice were administered vehicle or a GLP-1R agonist, Exendin-4, systemically prior to testing in probe trials of varying difficulty.
Results:
Following GLP-1R activation, males showed modest yet non-significant performance in the location discrimination task. Females, however, showed enhanced performance during the most difficult probe tests following Exendin-4 administration.
Conclusions:
GLP-1R activation appears to enhance overall performance in the location discrimination task and does so in a sex- and difficulty-dependent manner. These preliminary yet impactful data indicate that GLP-1R agonists may be useful as an adjunctive pharmacotherapy to treat cognitive deficits associated with MDD and/or multiple neurological disorders.
Keywords: GLP-1, cognition, location discrimination, depression
INTRODUCTION
Drugs targeting the glucagon-like peptide-1 receptor (GLP-1R) are integral in treating type II diabetes and obesity due to its long-lasting effects on both peripheral and central actions on the receptor. GLP-1R activation decreases food [1] and drug [2,3] reward as well as enhances cognitive function. For instance, Grieg and colleagues have shown that GLP-1R agonists attenuate traumatic brain injury-induced memory deficits [4], while systemic activation or hippocampal overexpression of GLP-1R enhances learning and memory [5]. Chronic GLP-1R agonist administration improves reference memory [6] and attenuates spatial memory deficits induced by intrahippocampal lipopolysaccharide injections [7].
Cognitive dysfunction, while debilitating in and of itself, is often concomitantly manifested with other diseases, such as major depressive disorder (MDD) and multiple neurological conditions. Depression-induced cognitive decline or dysfunction is a serious health problem, affecting 35–45% of patients diagnosed with MDD [8]. Pharmacotherapies are only moderately effective for the mood components, with only one-third of patients undergoing remission after one round of antidepressant treatment. Another form of therapy, cognitive behavioral therapy (CBT), has been found to be even more effective in some patients [9]. The efficacy of CBT is dependent upon cognitive function [10]. However, as MDD is often concomitant with deficits in cognitive function, the likelihood of success with CBT is diminished, and some data suggest that neither antidepressant treatment nor disease remission improve this memory loss [8,11].
Furthermore, (sub)chronic administration of GLP-1R agonists decreased immobility time in the forced swim test (FST)—a possible indicator of antidepressant potential—in rodents [6],,. Similar to antidepressant drugs, GLP-1R activation also stimulates neurogenic marker expression [4] and neurogenesis [12] within the dentate gyrus (DG) [13]. On the contrary, GLP-1R activation can be anxiogenic [14]. It is hypothesized that this is a consequence of GLP-1-mediated HPA axis dysfunction at the level of the central nucleus of the amygdala [14]. Given that cognitive function worsens concomitantly with HPA axis dysfunction [15], the specific neuronal subsets of GLP-1R affected by a pharmacological treatment must be considered. To that end, GLP-1R modulation is an intriguing and novel target for both rapid and chronic therapeutic efficacy in mood disorders and cognitive function [16].
The deficits in patients with MDD suffer from are particularly focused on episodic memory [10,17], a domain that is highly dependent upon the hippocampus [18]. Pattern separation is one key feature of episodic memory. Pattern separation is a process in which the hippocampus must form a distinct output (memory) from inputs that are very similar [19,20]. At its core, pattern separation occurs when the output firing patterns of a network are less similar to one another than the input firing patterns. The dorsal DG of the hippocampus, specifically, is required for spatial pattern separation to occur [19]. Interestingly, patients with MDD show significant deficits in pattern separation tests and DG activity, with depression severity negatively correlated to the ability to distinguish between similar patterns [21]. Moreover, cells and circuits within the hippocampus highly express GLP-1Rs [22]. In rodents, specific touchscreen-based operant tasks have been developed to allow assessment of pattern separation, also referred to as location discrimination, by comparing performance in a dissimilar condition (large physical separation of images) with that in a similar condition (small physical separation of images) [23]. Using this location discrimination task, we hypothesized that systemic administration of a GLP-1R agonist would enhance performance in mice.
METHODS
Animals:
Male and female C57Bl/6J (6–8 weeks, Jackson Laboratories, Bar Harbor, ME) were housed 2–5/cage when possible and were provided with rodent chow and tap water ad libitum. Mice were housed in a temperature- and humidity-controlled AAALAC-approved facility that is maintained on a 12:12 h light:dark cycle. Following at least a week of habituation to the facility, mice were individually housed and food deprived. Mice were weighed daily and given gradually decreasing amounts of regular chow (4, 3.5, 2.5, 2 g) until mice were 85–90% of their starting weight. The initial body weights were 24.6 g±0.5 g for males and 19.5 g±0.3 g for females. As mice at this age are still growing, we reassessed weight goals weekly using growth curves for C57Bl/6J mice by adding the expected weight gain to the weight goal each week (Jackson Laboratories). During testing, mice were fed after the completion of the day’s testing. All protocols were approved by the local Institutional Animal Care and Use Committee, and all studies were performed in accordance with the recommendations in the National Institute of Health’s Guide for the Care and Use of Laboratory Animals.
Location Discrimination Task:
Upon reaching the goal weight, mice were trained and tested in the location discrimination task in Bussey/Saksida operant chambers with some modifications (Lafayette Instruments, Lafayette, IN) [23]. Testing occurred 5–7 days per week during the animals’ light cycle (usually between 10 AM-2 PM) with approximately 24 hrs between each session. On days in which mice were not tested, they were fed the appropriate amount of chow to maintain body weight. The chambers are trapezoidal in shape with a touchscreen on one end and a liquid reward dispenser on the other. The screen was covered by an insert with two horizontal rows of 6 squares each; only the bottom row was used here and is referred to as squares 1–6. Mice were trained in the apparatus through a series of progressively more difficult stages in order to receive a vanilla milkshake reward (33% Ensure™). Mice were given access to Vanilla Ensure™ for the last 2 days of food restriction prior to testing. Mice performed one session per day. Mice are first habituated to the chamber, whereby 1500 μL of diluted Ensure was delivered upon entry, and then 30 μL (the standard amount delivered for the remainder of the experiment) was delivered every 10 sec once the mouse inserted its head into the lit reward tray. Upon completing 30 trials over 20 min, mice progressed through several stages of training, attaining a set criterion before moving to the next, more difficult stage. In Stage 1, Initial Touch, one of the six squares on the touchscreen was lit randomly, and a reward (along with a tone and illumination of the reward tray light) was delivered every 10 sec. If the mouse touched the lit square, the milkshake reward was tripled. Next, they entered Stage 2, Must Touch, whereby the same procedure was followed as in Initial Touch, only the reward was not delivered until the mouse pressed the lit square. Stage 3, Must Initiate, was similar to Stage 2 except mice must start the trial by nosepoking the reward delivery tray (signaled by a light within the tray) before a random square was lit on the screen. Following the same procedure as the preceding stage, Stage 4, Punish Incorrect, incorporated a 5 sec time out along with illumination of the overhead house light for blank screen touches (i.e., touching a non-lit square). In Stage 5, One Choice Reversal, two boxes of intermediate distance (boxes 2 and 5) were illuminated. Mice were randomly assigned a default “correct” side (left or right, counterbalanced across groups). Mice must press the “correct” side/box before receiving the milkshake reward. However, after correct touches on 7 of 8 consecutive trials, the “correct” side was reversed. Mice were evaluated by the number of reversals per session. Stage 5, One Choice Reversal, was the baseline session, and mice were maintained at this level until the Probe Trials, which varied in difficulty (Stages 6 and 7). At the start of the Probe Trials, mice were tested in the Easy Probe Trial (Stage 6), which is identical to Stage 5, One Choice Reversal, except there are large separations between the lit squares (boxes 1 and 6). This was followed by Stage 7 (Hard Probe Trial), which is the exact same procedure as Stage 6, Easy Probe Trial, except boxes 3 and 4 (small separation) were lit. These probe trials were 120 min in length and included reversals (i.e., correct side alternated every 7 of 8 correct trials). The number of reversals per probe test were assessed.
About 30 min prior to each probe trial, mice were administered (ip) saline or 30 μg/kg Exendin-4 (Ex-4; Bachem Americas Inc., Torrance, CA), a GLP-1R agonist. Mice were treatment-naïve until this point, and received only one dose of either Ex-4 or saline prior to each probe test. Treatments were counterbalanced evenly across the sexes. Mice were first tested with the easy probe trial. Following a minimum 5 day washout, mice were retested in the more difficult probe trial but administered the other drug. For our initial cohort, male and female mice were run through each probe trial twice such that the easy and difficult probe trials were repeated using the opposite drug as the previous sessions after a 10 day washout. Our final cohort (all females) were tested in each probe trial once. The baseline session (Stage 5, One Choice Reversal) was performed periodically between probe sessions to assess performance. To account for crossover effects due to retesting as well as to normalize the data from different cohorts, data from each probe trial were normalized to that of the corresponding saline × sex group. In total, 6 males and 14 females were used.
Statistical Analysis:
Data were analyzed using GraphPad Prism 8 (GraphPad Software, San Diego, CA) and presented as means ± standard error of the means (SEM). Statistical significance was set at p<0.05. Student’s t-test and analysis of variances (1- and 2-way ANOVAs) were utilized where applicable and subjected to Sidak’s post hoc analyses. Main effect variables included Sex, Treatment, and Difficulty level (easy vs. hard probes), where applicable.
RESULTS
Task Acquisition:
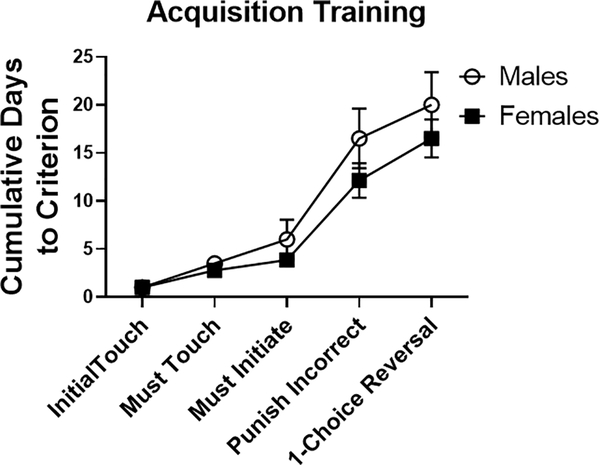
Male and female mice acquired the location discrimination task similarly in the initial stages. However, females took fewer days to learn compared to males in the later training phases (Fig. 1; main effect of Sex, F(1, 90)=4.128). Due to this significant main effect of Sex, probe test analyses were performed separately in males and females.
Fig. 1.
Females took significantly less time overall than males to complete training in the location discrimination task (p<0.05). Data are shown as the cumulative number of days to achieve criterion for each stage. N=6–14/group
Probe Testing:
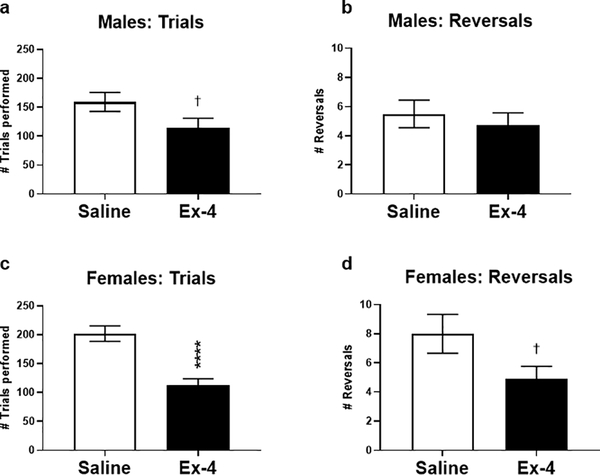
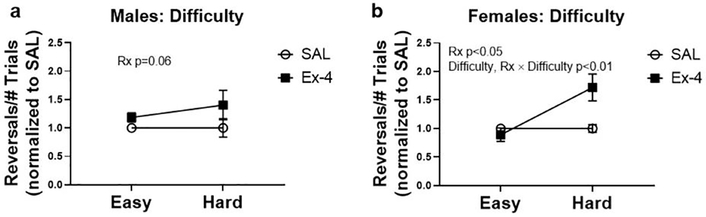
Once mice maintained baseline criterion at the One Choice Reversal stage, they were subdivided into treatment groups (saline or Ex-4) and tested in the easy then more difficult probe tests. It was found that males and females administered Ex-4 exhibited hypoactivity (Fig. 2), which was especially pronounced in females. As a result, Ex-4-treated mice performed fewer trials and reversals per session compared to vehicle-treated mice (Fig. 3, data combined across Difficulty level as no significant differences were found). In fact, two mice (one male and one female) treated with Ex-4 during the difficult probe test did not perform any reversals at all due to inactivity and were removed from analyses. To eliminate this potential bias, we compared the number of reversals relative to the number of trials performed (reversals/total trials) as the primary output, followed by normalization to the respective sex × saline group to account for cohort effects (i.e., saline values should approximately equal 1). It should be noted that despite the differences in locomotor activity between the treatments, there was no significant difference in activity when comparing the difficulty levels within sexes (Easy vs. Hard; males: t(21)=0.8907, p=0.3832; females: t(37)=0.1557, p=0.8748; data not shown). Furthermore, when comparing locomotor activity for the Treatment × Difficulty groups within sex (e.g., SAL × Easy vs. SAL × Hard and Ex-4 × Easy vs. Ex-4 × Hard groups), post hoc analyses revealed no significant differences between these groups (data not shown). Males administered Ex-4 systemically had performed better than controls during the probe trials, although this value did not reach statistical significance (Fig. 4a; Treatment: F(1, 19)=3.962, p=0.0611). On the contrary, there were significant main effects of Difficulty (F(1, 35)=9.563, p<0.01) and Treatment (F(1, 35) =5.194, p<0.05) in the females (Fig. 4b). A significant interaction of Difficulty × Treatment (F(1, 35)=9.563, p<0.01) revealed that Ex-4-treated females outperformed controls in the more difficult probe trial (Fig. 4b, p<0.01).
Fig. 2.
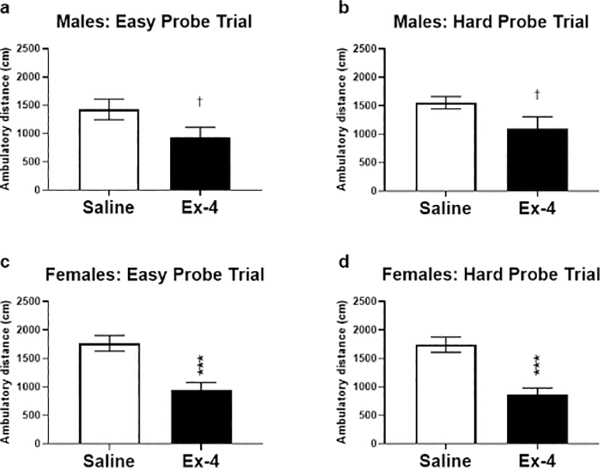
Ex-4 treatment resulted in a hypoactive phenotype during probe testing. Control males ambulated more than Ex-4-treated males in the easy (a; t(10)=1.925, p=0.0831) and difficult (b; t(9)=2.079, p=0.0677) probes, although these did not reach the level of significance. Females administered Ex-4 moved significantly less than saline-treated females in both the easy (c; t(18)=4.292, p<0.001) and difficult (d; t(17)=4.878, p<0.001) probe trials. † p<0.1; *** p<0.001
Fig. 3.
Males (a; t(21)=1.970, p=0.0698) and females (c; t(37)=5.074, p<0.0001) administered Ex-4 during probe trials completed fewer trials than their respective controls, although this reached significance only in females. The GLP-1R agonist did not alter the total number of reversals in probe trials in males (b; t(21)=0.6041, p=0.5522) but did non-significantly decrease the number of reversals in females (d; t(37)=1.939, p=0.0603). † p<0.1; **** p<0.0001
Fig. 4.
During probe testing, Ex-4 treatment in males (a) enhanced performance overall but not to a level of significance (p=0.06). Ex-4 treatment did significantly enhance task execution, specifically in the more difficult trial, in females (b). N=5–10/group
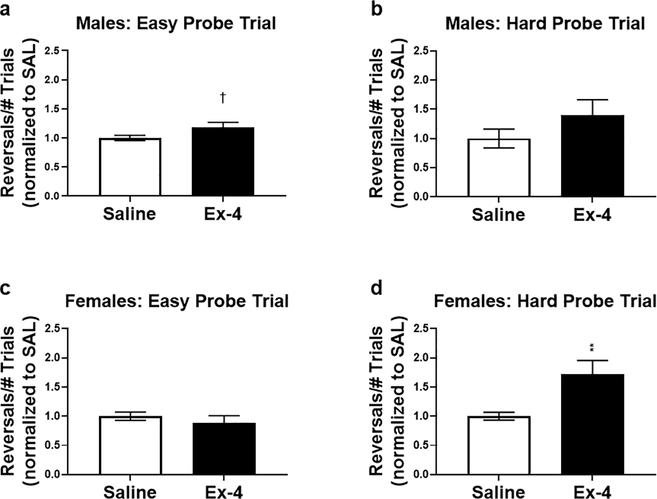
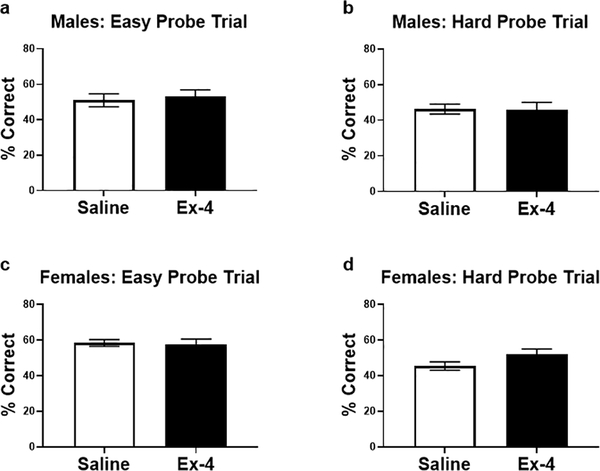
Examining each probe trial separately, Ex-4-treated males performed better, albeit non-significantly, than controls in the easy probe trial (Fig. 5a; t(10)=2.003, p=0.0729). There was no difference in performance in the more difficult trial (Fig. 5b; t(9)=1.372, p=0.2034). On the contrary, females, regardless of treatment, performed similarly in the easy trial (Fig. 5c; t(18)=0.7892, p=0.4403). However, Ex-4 administration resulted in enhanced performance during the difficult probe trial for females (Fig. 5d; t(17)=3.080, p<0.01). Interestingly, Ex-4-treated males and females were equally as accurate as their control counterparts in both types of probe tests, as indicated by the correct percentage (Fig. 6).
Fig. 5.
Following normalization, males treated with Ex-4 performed better than controls in the easy probe (a), but this did not reach statistical significance (p=0.07). Ex-4 did not alter performance for males in the difficult trial (b). On the contrary, treatment did not alter task execution for females in the easy trial (c), but Ex-4 improved performance in the difficult phase. † p<0.1; ** p<0.01
Fig. 6.
Ex-4 administration did not alter the accuracy of performance in either sex, regardless of the difficulty level of the probe trial. N=5–10/group
DISCUSSION
Patients with MDD often suffer from other ailments related to the primary diagnosis. Cognitive impairment is one such condition and is in fact one of the nine criteria for diagnosing MDD in the current Diagnostic and Statistical Manual of Mental Disorders (DSM-5). In some studies, up to half of all participants with MDD suffer from significant cognitive deficits [8,24], and no FDA-approved pharmacotherapies are available to specifically treat MDD-related cognitive decline [25]. Even after the mood symptoms remit, many patients still suffer from the cognitive deficits [8], and this can impede a patient’s recovery. A recent longitudinal study using 3 different monoamine reuptake inhibitors demonstrated no improvement in cognitive impairment in patients after remission of mood symptoms [11], indicating the necessity for treatment options that target cognitive impairment in MDD. Because one of the more effective non-pharmacological treatments, CBT, is dependent upon cognitive integrity, its usefulness is minimized in affected patients [10]. Thus, adjunctive therapies that do not hinder the mood-enhancing effects of current MDD pharmacotherapies yet also boost cognitive health are needed. Here, we tested whether a GLP-1R agonist, Ex-4, could improve performance in a location discrimination task, which tests a cognitive domain negatively affected by MDD in humans.
Our data indicate that acute administration of Ex-4 enhances execution of the LD task in a sex-dependent manner, with females benefitting most from the Ex-4 treatment. GLP-1R activation increased the number of reversals during the easy task in males following normalization, although this value did not reach significance. While performance in females did not differ between groups in the easier probe task, Ex-4 administration significantly improved performance in the more difficult task in females, indicating that GLP-1R activation affects cognitive function in a sex-dependent manner. Based on these results, this effect may be contingent upon cognitive load. Many studies have noted that there are significant sex differences in learning and memory as well as learning strategies [for an in-depth review, see [26]]. Generally, males tend to outperform females in hippocampus-dependent tests [27], including the more difficult phases of pattern separation [28]. There is, however, some evidence that females outperform males in some cognitive tasks (object-location binding memory test) when the cognitive load or difficulty increases [29]. These GLP-1R-specific sex-dependent effects warrant additional examination with larger groups sizes and dose-response studies, but the current data indicate a potentially impactful and novel mechanism to treat MDD-related cognitive impairment.
One implication is that sex hormones contribute to these differences, as has been suggested previously [26]. In rats, neurogenesis within the DG is greater in females undergoing proestrous, when estrogen levels peak, compared to males or females in the estrous or diestrous stages [30]. However, some data indicate that there is no significant sex difference in neurogenesis in mice [31], while others demonstrate that males have increased neurogenic response, both basally and following stress, compared to females [32]. These findings are key, as ablation of hippocampal neurogenesis in female mice diminishes performance in the difficult probe test in location discrimination task [19]; it is not clear how this manipulation alters performance in males. Moreover, there is evidence to suggest that chronic stress is more detrimental to cognitive processes in males than females, who tend to perform better following stress [for review see [33]]. Whether the moderate yet chronic food restriction is stressful has yet to be determined, but it is unlikely that this would improve performance in females, as food restriction may worsen learning and memory in females rodents [34]. Unfortunately, we did not assess the estrous cycles of the females during testing, nor did we measure cortisol or hormone concentrations afterwards. In the future, we will assess these outcomes as well as measure neurogenic markers in the brain following testing.
One of the caveats of this study was that systemic GLP-1R activation induced hypoactivity, particularly in the females. Similarly, Ex-4-treated mice performed a fewer number of trials based on the raw data. There was not, however, a statistically significant decrease in the number of reversals, despite a slight downward trend in females, nor did it alter performance accuracy. Furthermore, there were no differences in activity levels between hard vs. easy probe trials within each treatment group, indicating that despite the increased distance between targets during the easy trials, locomotor activity did not contribute to the overall changes in performance. These data indicate that despite decreased activity levels, systemic GLP-1R activation does not overtly diminish performance or produce the sex-dependent effects at the two difficulty levels. Others have shown that GLP-1R agonist administration decreases locomotor activity in rodents [1,35] while antagonizing the receptor induces a hyperactive phenotype [36]. However, two of the more well-known outcomes of GLP-1R activation are satiety and decreased food intake, which could be related to decreased food reward [1], outcomes which has been exploited by pharmaceutical companies to treat obesity [37]. It is also feasible that the decreased number of trials due to GLP-1R agonist administration may be due to satiety and/or decreased food-motivated behavior. To reconcile this caveat, determining which specific subsets of the GLP-1R modulate this cognitive enhancement yet does not interfere with its role in food intake and satiety is crucial. This study used systemic administration, potentially activating both central and peripheral GLP-1Rs. Location discrimination, or pattern separation, is associated with the hippocampal DG, specifically the dorsal subsection [19,20]. GLP-1R is expressed in this region [22]. Interestingly, GLP-1R activation within the ventral hippocampus does indeed alter food intake in rodents by modulation of NMDA receptors within the medial prefrontal cortex [38]. As the downstream circuits of the dorsal and ventral hippocampus are generally segregated [39], it is not known whether injection into the dorsal region will alter food intake yet simultaneously enhance task execution. While these data indicate a role for GLP-1R activation in this task, it is unclear to what extent DG GLP-1Rs plays in location discrimination, if these effects are due to directly GLP-1R stimulation or upstream effects, or if these alterations are due to off-target effects entirely. Current research is underway to examine the specific role of this GLP-1R-associated subset in location discrimination and the circuitry associated with it. Likewise, future studies utilizing a range of doses to further implicate GLP-1R’s role in this cognitive domain as well as to elucidate the potential sex differences are necessary to draw further conclusions on the current data set.
Additional therapeutic targets that alleviate both the mood and cognitive aspects of MDD are greatly needed. GLP-1R activation has been shown to have an antidepressant-like effect in rodents [6], and these current data indicate that a GLP-1R agonist enhances MDD-related cognitive measures as well. As more women are affected by MDD than men [40], these findings indicate a potential sex-specific therapeutic target for treatment. Because GLP-1R ligands are currently approved for treatment of diabetes and obesity, these preliminary yet impactful findings could quickly translate into new clinical applications for MDD-related cognitive deficits.
Acknowledgements:
We would like to thank Matt Croxall (Lafayette Instruments) for technical advice.
Funding: This work was supported by National Institutes of Health grant R03MH110749 (DLG), the Florida State University Council on Research & Creativity (DLG), and the Florida State University College of Medicine.
Footnotes
Conflict of Interest/Competing Interests: The authors declare no conflict of interest nor any competing financial or commercial interests.
DECLARATIONS
Ethics Approval: All protocols were approved by the local Institutional Animal Care and Use Committee, and all studies were performed in accordance with the recommendations in the National Institute of Health’s Guide for the Care and Use of Laboratory Animals.
Consent to Participate: N/A
Consent for Publication: All authors have read and approved of the manuscript and its publication.
Availability of Data and Material: The authors confirm that the data supporting the findings of this study are available within the article as well as from the corresponding author upon reasonable request.
Code Availability: N/A
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP (2012) The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci 32 (14):4812–4820. doi:32/14/4812 [pii] 10.1523/JNEUROSCI.6326-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egecioglu E, Steensland P, Fredriksson I, Feltmann K, Engel JA, Jerlhag E (2013) The glucagon-like peptide 1 analogue Exendin-4 attenuates alcohol mediated behaviors in rodents. Psychoneuroendocrinology 38 (8):1259–1270. doi: 10.1016/j.psyneuen.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 3.Graham DL, Erreger K, Galli A, Stanwood GD (2013) GLP-1 analog attenuates cocaine reward. Mol Psychiatry 18 (9):961–962. doi:mp2012141 [pii] 10.1038/mp.2012.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tweedie D, Rachmany L, Rubovitch V, Lehrmann E, Zhang Y, Becker KG, Perez E, Miller J, Hoffer BJ, Greig NH, Pick CG (2013) Exendin-4, a glucagon-like peptide-1 receptor agonist prevents mTBI-induced changes in hippocampus gene expression and memory deficits in mice. Exp Neurol 239:170–182. doi: 10.1016/j.expneurol.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, Haile CN (2003) Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med 9 (9):1173–1179. doi: 10.1038/nm919 [DOI] [PubMed] [Google Scholar]
- 6.Isacson R, Nielsen E, Dannaeus K, Bertilsson G, Patrone C, Zachrisson O, Wikstrom L (2011) The glucagon-like peptide 1 receptor agonist exendin-4 improves reference memory performance and decreases immobility in the forced swim test. Eur J Pharmacol 650 (1):249–255. doi: 10.1016/j.ejphar.2010.10.008 [DOI] [PubMed] [Google Scholar]
- 7.Huang HJ, Chen YH, Liang KC, Jheng YS, Jhao JJ, Su MT, Lee-Chen GJ, Hsieh-Li HM (2012) Exendin-4 protected against cognitive dysfunction in hyperglycemic mice receiving an intrahippocampal lipopolysaccharide injection. PLoS One 7 (7):e39656. doi: 10.1371/journal.pone.0039656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhalla RK, Butters MA, Mulsant BH, Begley AE, Zmuda MD, Schoderbek B, Pollock BG, Reynolds CF 3rd, Becker JT (2006) Persistence of neuropsychologic deficits in the remitted state of late-life depression. Am J Geriatr Psychiatry 14 (5):419–427. doi: 10.1097/01.JGP.0000203130.45421.69 [DOI] [PubMed] [Google Scholar]
- 9.Weitz ES, Hollon SD, Twisk J, van Straten A, Huibers MJ, David D, DeRubeis RJ, Dimidjian S, Dunlop BW, Cristea IA, Faramarzi M, Hegerl U, Jarrett RB, Kheirkhah F, Kennedy SH, Mergl R, Miranda J, Mohr DC, Rush AJ, Segal ZV, Siddique J, Simons AD, Vittengl JR, Cuijpers P (2015) Baseline Depression Severity as Moderator of Depression Outcomes Between Cognitive Behavioral Therapy vs Pharmacotherapy: An Individual Patient Data Meta-analysis. JAMA Psychiatry 72 (11):1102–1109. doi: 10.1001/jamapsychiatry.2015.1516 [DOI] [PubMed] [Google Scholar]
- 10.McDermott LM, Ebmeier KP (2009) A meta-analysis of depression severity and cognitive function. J Affect Disord 119 (1–3):1–8. doi: 10.1016/j.jad.2009.04.022 [DOI] [PubMed] [Google Scholar]
- 11.Shilyansky C, Williams LM, Gyurak A, Harris A, Usherwood T, Etkin A (2016) Effect of antidepressant treatment on cognitive impairments associated with depression: a randomised longitudinal study. Lancet Psychiatry 3 (5):425–435. doi: 10.1016/S2215-0366(16)00012-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, Kortesmaa J, Mercer A, Nielsen E, Ronnholm H, Wikstrom L (2008) Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J Neurosci Res 86 (2):326–338. doi: 10.1002/jnr.21483 [DOI] [PubMed] [Google Scholar]
- 13.Hunter K, Holscher C (2012) Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci 13:33. doi: 10.1186/1471-2202-13-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moller C, Sommer W, Thorsell A, Rimondini R, Heilig M (2002) Anxiogenic-like action of centrally administered glucagon-like peptide-1 in a punished drinking test. Prog Neuropsychopharmacol Biol Psychiatry 26 (1):119–122 [DOI] [PubMed] [Google Scholar]
- 15.Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10 (6):434–445. doi: 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- 16.Chaves Filho AJM, Cunha NL, de Souza AG, Soares MV, Juca PM, de Queiroz T, Oliveira JVS, Valvassori SS, Barichello T, Quevedo J, de Lucena D, Macedo DS (2020) The GLP-1 receptor agonist liraglutide reverses mania-like alterations and memory deficits induced by D-amphetamine and augments lithium effects in mice: Relevance for bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 99:109872. doi: 10.1016/j.pnpbp.2020.109872 [DOI] [PubMed] [Google Scholar]
- 17.Bierman EJ, Comijs HC, Jonker C, Beekman AT (2005) Effects of anxiety versus depression on cognition in later life. Am J Geriatr Psychiatry 13 (8):686–693. doi: 10.1176/appi.ajgp.13.8.686 [DOI] [PubMed] [Google Scholar]
- 18.Greenberg DL, Rice HJ, Cooper JJ, Cabeza R, Rubin DC, Labar KS (2005) Co-activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia 43 (5):659–674. doi: 10.1016/j.neuropsychologia.2004.09.002 [DOI] [PubMed] [Google Scholar]
- 19.Clelland CD, Choi M, Romberg C, Clemenson GD Jr., Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ (2009) A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325 (5937):210–213. doi: 10.1126/science.1173215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yassa MA, Stark CE (2011) Pattern separation in the hippocampus. Trends Neurosci 34 (10):515–525. doi: 10.1016/j.tins.2011.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujii T, Saito DN, Yanaka HT, Kosaka H, Okazawa H (2014) Depressive mood modulates the anterior lateral CA1 and DG/CA3 during a pattern separation task in cognitively intact individuals: a functional MRI study. Hippocampus 24 (2):214–224. doi: 10.1002/hipo.22216 [DOI] [PubMed] [Google Scholar]
- 22.Graham DL, Durai HH, Trammell T, Noble BL, Mortlock DP, Galli A, Stanwood GD (2020) A novel mouse model of glucagon-like peptide-1 receptor expression: a look at the brain. J Comp Neurol. doi: 10.1002/cne.24905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oomen CA, Hvoslef-Eide M, Heath CJ, Mar AC, Horner AE, Bussey TJ, Saksida LM (2013) The touchscreen operant platform for testing working memory and pattern separation in rats and mice. Nat Protoc 8 (10):2006–2021. doi: 10.1038/nprot.2013.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rock PL, Roiser JP, Riedel WJ, Blackwell AD (2014) Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med 44 (10):2029–2040. doi: 10.1017/S0033291713002535 [DOI] [PubMed] [Google Scholar]
- 25.Ledford H (2016) Drugmakers target depression’s cognitive fog. Nature 530 (7588):17. doi: 10.1038/530017a [DOI] [PubMed] [Google Scholar]
- 26.Yagi S, Galea LAM (2019) Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology 44 (1):200–213. doi: 10.1038/s41386-018-0208-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonasson Z (2005) Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neurosci Biobehav Rev 28 (8):811–825. doi: 10.1016/j.neubiorev.2004.10.006 [DOI] [PubMed] [Google Scholar]
- 28.Yagi S, Chow C, Lieblich SE, Galea LA (2016) Sex and strategy use matters for pattern separation, adult neurogenesis, and immediate early gene expression in the hippocampus. Hippocampus 26 (1):87–101. doi: 10.1002/hipo.22493 [DOI] [PubMed] [Google Scholar]
- 29.Park J, Shin GI, Park YM, Kim IY, Jang DP (2017) Sex differences of cognitive load effects on object-location binding memory. Biomed Eng Lett 7 (4):305–309. doi: 10.1007/s13534-017-0038-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanapat P, Hastings NB, Reeves AJ, Gould E (1999) Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci 19 (14):5792–5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lagace DC, Fischer SJ, Eisch AJ (2007) Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus 17 (3):175–180. doi: 10.1002/hipo.20265 [DOI] [PubMed] [Google Scholar]
- 32.Tzeng WY, Chen LH, Cherng CG, Tsai YN, Yu L (2014) Sex differences and the modulating effects of gonadal hormones on basal and the stressor-decreased newly proliferative cells and neuroblasts in dentate gyrus. Psychoneuroendocrinology 42:24–37. doi: 10.1016/j.psyneuen.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 33.Bangasser DA, Eck SR, Telenson AM, Salvatore M (2018) Sex differences in stress regulation of arousal and cognition. Physiol Behav 187:42–50. doi: 10.1016/j.physbeh.2017.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajab E, Alqanbar B, Naiser MJ, Abdulla HA, Al-Momen MM, Kamal A (2014) Sex differences in learning and memory following short-term dietary restriction in the rat. Int J Dev Neurosci 36:74–80. doi: 10.1016/j.ijdevneu.2014.05.011 [DOI] [PubMed] [Google Scholar]
- 35.Erreger K, Davis AR, Poe AM, Greig NH, Stanwood GD, Galli A (2012) Exendin-4 decreases amphetamine-induced locomotor activity. Physiol Behav 106 (4):574–578. doi:S0031-9384(12)00119-9 [pii] 10.1016/j.physbeh.2012.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knauf C, Cani PD, Ait-Belgnaoui A, Benani A, Dray C, Cabou C, Colom A, Uldry M, Rastrelli S, Sabatier E, Godet N, Waget A, Penicaud L, Valet P, Burcelin R (2008) Brain glucagon-like peptide 1 signaling controls the onset of high-fat diet-induced insulin resistance and reduces energy expenditure. Endocrinology 149 (10):4768–4777. doi: 10.1210/en.2008-0180 [DOI] [PubMed] [Google Scholar]
- 37.Brown E, Cuthbertson DJ, Wilding JP (2018) Newer GLP-1 receptor agonists and obesity-diabetes. Peptides 100:61–67. doi: 10.1016/j.peptides.2017.12.009 [DOI] [PubMed] [Google Scholar]
- 38.Hsu TM, Noble EE, Liu CM, Cortella AM, Konanur VR, Suarez AN, Reiner DJ, Hahn JD, Hayes MR, Kanoski SE (2017) A hippocampus to prefrontal cortex neural pathway inhibits food motivation through glucagon-like peptide-1 signaling. Mol Psychiatry. doi: 10.1038/mp.2017.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moser MB, Moser EI (1998) Functional differentiation in the hippocampus. Hippocampus 8 (6):608–619. doi: [DOI] [PubMed] [Google Scholar]
- 40.Substance Abuse and Mental Health Services (2013) Results from the 2012 National Survey on Drug Use and Health: Mental Health Findings. NSDUH Series H-47, HHS Publication No. (SMA) 13–4805. Rockville, MD [Google Scholar]