Abstract
Human cytomegalovirus (HCMV) infection is associated with neuropathology in patients with impaired immunity and/or inflammatory diseases. However, the association between gray matter volume (GMV) and HCMV has never been examined in major depressive disorder (MDD) despite the presence of inflammation and impaired viral immunity in a subset of patients.
We tested this relationship in two independent samples consisting of 179 individuals with MDD and 41 healthy controls (HC) (sample 1) and 124 MDD participants and 148 HCs (sample 2). HCMV positive (HCMV+) and HCMV negative (HCMV−) groups within each sample were balanced on up to eleven different clinical/demographic variables using inverse probability of treatment weighting. GMV of 87 regions was measured with FreeSurfer. There was a main effect of HCMV serostatus but not diagnosis that replicated across samples. Relative to HCMV− subjects, HCMV+ subjects in sample 1 showed a significant reduction of volume in six regions (puncorrected<0.05). The reductions in GMV of the right supramarginal gyrus (standardized beta coefficient (SBC)=−0.26) and left fusiform gyrus (SBC=−0.25) in sample 1 were replicated in sample 2: right supramarginal gyrus (puncorrected <0.05, SBC=−0.32), left fusiform gyrus (PFDR<0.01, SBC=−0.51). Posthoc tests revealed that the effect of HCMV was driven by differences between the HCMV+ and HCMV− MDD subgroups. HCMV IgG level, a surrogate marker of viral activity, was correlated with GMV in the left fusiform gyrus (r=−0.19, Puncorrected=0.049) and right supramarginal gyrus (r=−0.19, puncorrected=0.043) in the HCMV+ group of sample 1. Conceivably, HCMV infection may be a treatable source of neuropathology in vulnerable MDD patients.
Keywords: Cytomegalovirus, Depression, Inflammation, Magnetic Resonance Imaging, Gray Matter Volume
INTRODUCTION
Approximately 50% of the US population is infected with human cytomegalovirus (HCMV). HCMV is not cleared after initial infection but establishes life-long latent infections, persisting in myeloid lineage cells, vascular pericytes, endothelial cells of the blood-brain barrier as well as glia, neurons, and neural precursor cells1–3. The capacity of HCMV to infect the brain may explain why it is a well-established cause of CNS damage4. This neuropathology is traditionally thought to occur only within limited clinical contexts such as in immunologically-naïve or immune-compromised patients (congenital infection, AIDS patients, transplant recipients) in whom the once dormant virus becomes reactivated. However, even in individuals who are not immunosuppressed, reactivation of HCMV occurs periodically when anti-viral immunity is weakened, i.e. during times of stress, including depression, as well as in the context of inflammation and cellular damage5–8. This raises the possibility that HCMV may also be a source of neuropathology in these populations.
HCMV IgG titer is a surrogate marker of viral activity with higher levels indicative of an active infection9. Neuro-immune stress pathways promote reactivation of HCMV via adrenergic signaling10. Consistent with this mechanism, HCMV IgG titer was found to be higher during exams in medical students6, 11; caregivers to disabled children had elevated IgG titers5, and viral DNA shedding was higher in astronauts directly before and after space travel12, 13. Similarly, associations between psychological stressors and herpesviruses (including HCMV) levels are commonly reported13–17. Reactivation of HCMV is also induced by inflammatory mediators such as TNF7 and inflammatory diseases such as sepsis are known drivers of HCMV reactivation, a risk factor for adverse outcomes in these patients8.
Among patients with inflammatory disorders, including autoimmune disorders18 and HIV19, there is evidence that HCMV infection is a major driver of pathology. For example, HCMV positive (HCMV+) multiple sclerosis patients showed greater brain atrophy over time than HCMV negative (HCMV−) patients20 and higher antibody levels during a first demyelinating event predicted greater loss of gray matter volume (GMV) over time20. Brain tissue donated by clergy showed that higher lifetime anti-HCMV IgG titers were associated with the presence of neurofibrillary tangles21. Further, the CD4+ response to the HCMV pp65 antigen was associated with a pathological diagnosis of Alzheimer’s disease (AD)21. In depression, inflammation is present22–24 but it is not known whether HCMV is a source of neuropathology in these patients.
Although the relationship between structural brain abnormalities and HCMV has not to our knowledge been studied in major depressive disorder (MDD), HCMV has been linked with depression in at least 13 studies25–38. This may be because depression is associated with impaired anti-viral immunity, rendering individuals vulnerable to both initial infection and subsequent reactivation of HCMV. Evidence for impaired adaptive immunity in depression takes the form of a decreased proliferative response of lymphocytes to mitogens, decreased natural killer cell function and lymphopenia in vitro39, upregulated expression of inflammation-related genes together with down-regulated expression of antiviral genes40, 41, poorer control of chronic viral infections42, impairment of vaccine-induced immunity to the varicella-zoster virus43, reduced vaccine-induced antibody response to hepatitis B44, and a loss of childhood vaccine-induced immunity to measles45. Of note, loss of immunity to measles has been shown to occur in contexts where HCMV is known to cause disease, i.e. in patients undergoing chemotherapy46, organ transplantion47 or with HIV48. Further, experimental studies show that subjects exposed to rhinovirus or influenza are more likely to become infected and show clinical symptoms if they endorse recent stress49, 50.
Taken together, there is mounting evidence that: (1) HCMV infection can damage the brain in immunosuppressed populations; (2) HCMV is a source of pathology in non-immune-suppressed populations with inflammatory diseases; (3) HCMV is reactivated by stress and inflammation; (4) a biotype of MDD is characterized by inflammation and impaired viral immunity; and (5) these depressed individuals may be vulnerable to reactivation of HCMV. We therefore hypothesized that HCMV may contribute to structural brain abnormalities in MDD. This is not just a question of theoretical interest but has treatment implications given the existence of well-tolerated anti-HCMV medications and the ongoing development of HCMV vaccines51, 52.
METHODS
Participants
Approval for the study was obtained from the Western Institutional Review Board and written informed consent was obtained from all participants. Two independent groups of participants were included in the study, 303 participants in sample 1, and 462 in sample 2.
Sample 1.
Participants were aged 18–55 years and either had no personal history of psychiatric illness (healthy controls, HC) or received a DSM-V diagnosis of MDD (with or without comorbid anxiety) based on the Mini International Neuropsychiatric Inventory (MINI). Data were collected between January 2015 and February 2017. Subjects completed PROMIS scales for depression and anxiety and the childhood trauma questionnaire (CTQ) for early-life stress measurement. Lifetime alcohol use was measured by the Customary Drinking and Drug use Record (CDDR) interview. Exclusion criteria included: comorbid psychiatric disorders (except anxiety disorders), neurological disorders, unstable medical conditions, a history of moderate-to-severe traumatic brain injury, a positive urine drug screen, and general MRI exclusion criteria (details in53).
Sample 2.
Participants (aged 18–55 years) met DSM-IV-TR criteria for MDD based on the Structured Clinical Interview for the DSM-IV-TR and unstructured psychiatric interviews. Data was collected between October 2010 and November 2016. Depressive symptoms were measured with the Montgomery-Asberg Depression Rating Scale (MADRS). Exclusion criteria included medical conditions or medications likely to influence CNS or immunological function, a history of drug or alcohol abuse within 6 months or a history of drug or alcohol dependence within 1 year (DSM-IV-TR criteria), and general MRI exclusion criteria. The same exclusion criteria applied to HCs (details in54, 55).
Anti-CMV IgG Antibodies and C-Reactive Protein
Morning blood samples were collected and frozen at −80 °C. Blood samples were processed using standard laboratory procedures. Thawed plasma samples were tested blind to diagnosis for IgG antibodies using a solid-phase ELISA (IBL America, catalog #EI2570–9601G). A sample was considered seropositive if it had an optical density value of >0.5, which is equivalent to approximately ten international units of antibody. Intra-and inter-assay coefficients of variation were 5% and 10%, respectively. Due to the non-normal distribution, the density value was converted to a z score.
For the sample 1, serum concentrations of c-reactive protein (CRP) were analyzed with the V-PLEX Neuroinflammation Panel-1 Human Kit (Meso Scale Diagnostics) with a lowest level of quantification (LLOQ) of 0.027 mg/L and intra- and inter-assay coefficients of variation of 2% and 10%, respectively. For sample 2, hs-CRP was measured immunoturbidimetrically with the Kamiya Biomedical K-Assay in a hospital laboratory with an LLOQ of 0.1 mg/L.
Image acquisition
For both samples T1-weighted anatomical images were acquired on two identical 3T scanners (GE Discovery MR750) using an MPRAGE sequence with following parameters: FOV=240mm, 186 slices, slice thickness=0.9mm, voxel dimensions=0.938×0.938×0.9mm3, image matrix=256×256, TR/TE=5/2.012 ms, acceleration factor R=2 in the phase encoding direction, flip angle=8 degrees.
Image preprocessing
Cortical reconstruction and volumetric segmentation were performed using FreeSurfer version 6.0.056. Whole-brain GMVs were estimated from individual anatomical images including 68 cortical regions (34 regions per hemisphere) using the Desikan-Killiany atlas and 19 subcortical regions using FreeSurfer standard subcortical segmentation57 (ventricles, cerebrospinal fluid, and white matter regions were excluded). Visual inspection of all cortical segmentation was performed before analysis for quality assurance purpose. FreeSurfer has been validated against histological measurements58 and demonstrates good test-retest reliability59.
Statistical analysis
Statistical analyses were performed using RStudio V1.1.463 and R version 3.5.3.
Confounding is a major concern in observational studies. Although multivariate regression is commonly used to control for potential confounds, it can be highly sensitive to the form of the model and interaction terms. Additionally, if the groups differ greatly in the distribution of covariates, regression can lead to biased estimation. In contrast, the propensity score approach, i.e. the inverse probability of treatment weighting (IPTW)60, 61, offers a transparent and effective analytical tool for adjusting confounding factors. The method creates a weighted population that has similar baseline characteristics between groups which then only differ on the independent variable of interest, in this case, HCMV status.
Following standard guidelines62, several steps were carried out to implement the IPTW: First, a propensity score was used to combine the information from the covariates into a single variable. The propensity score was defined as the likelihood of being HCMV+ and was determined from a multivariate logistic regression model containing the following independent variables for sample 1: age, sex, BMI, education, income, PROMIS depression score, PROMIS anxiety score, medication status (yes/no), early-life stress (CTQ score), number of depressive episodes (MINI interview), and lifetime alcohol use (CDDR interview). The propensity score for sample 2 was obtained from the same variables except that depression was measured with the MADRS and anxiety scores, income, number of depressive episodes, and lifetime alcohol use were not available. Standardized mean differences were calculated to examine covariate balance before and after IPTW. Second, the stabilized weights were calculated from the propensity score using the ‘ipw’ package. By down-weighting the characteristics of subjects who were over-represented and up-weighting subjects who were under-represented in the samples, this procedure is thought to allow the weighted samples to estimate the characteristics of samples derived from randomized experiments63. Finally, to estimate the main effect of HCMV on GMV, the main effect of diagnosis, and their interaction while accounting for the weights and estimating robust standard error, weighted generalised linear regression models from the ‘Survey’ package were used at each of the regions. The total intracranial volume (TIV) was added into the model as a covariate to adjust for individual differences in overall brain size. Posthoc tests were performed to estimate the effect of HCMV in the MDD and HC groups, separately.
We used a discovery/replication design64 and set an uncorrected p<0.05 (two-tailed) as the threshold. The regions showing significant differences in sample 1 were selected as ROIs to be tested in sample 2. The ROIs that were significantly associated with HCMV status in both sample 1 and sample 2, and were in the same direction in both samples were considered statistically significant. The false discovery rate (FDR) was used to control for multiple testing. To assess the sensitivity of results to the selection of balancing covariates, a general linear model without-adjustment for any covariates except TIV was also performed. To evaluate the robustness of the results to potential unmeasured confounders, we calculated the E-value using the ‘EValue’ package65. The E-value estimates the minimum effect an unmeasured confounder would need to have in order to be able to explain away an observed association with the outcome of interest. To test for associations between HCMV IgG level, CRP concentrations, and GMVs, CRP was log-transformed and then correlation analyses were performed within the combined MDD/HC HCMV+ groups.
Data availability
The full analysis code, datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Results
Study population and covariate balance
Out of a total of 765 subjects, we excluded 83 subjects from sample 1 and 190 subjects from sample 2, leaving a total of 492 subjects (Figure 1). Demographic characteristics before applying IPTW are summarized in Supplementary Table 1. After applying IPTW, the differences between subgroups diminished substantially. The plot of weights and propensity score distributions in Supplementary Figure 1 demonstrated that no extreme weights were present and the propensity weighting achieved balance between the HCMV+ and HCMV− groups. In the sample 1 HC subgroup, standardized mean differences (SMD range from 0.22–0.99) remained between the HCMV+ and HCMV− samples that could not be corrected for by IPTW due to the small sample size. In the sample 1 MDD subgroup and the sample 2 MDD and HC groups, standardized mean differences for all of the covariates were less than 0.1, indicative of well-balanced samples. Other than education in the sample 1 HC subgroup, there were no statistically significant group differences in any of the measured covariates between HCMV+ and HCMV− subgroups in both sets of MDD and HC samples (Table 1).
Figure 1.
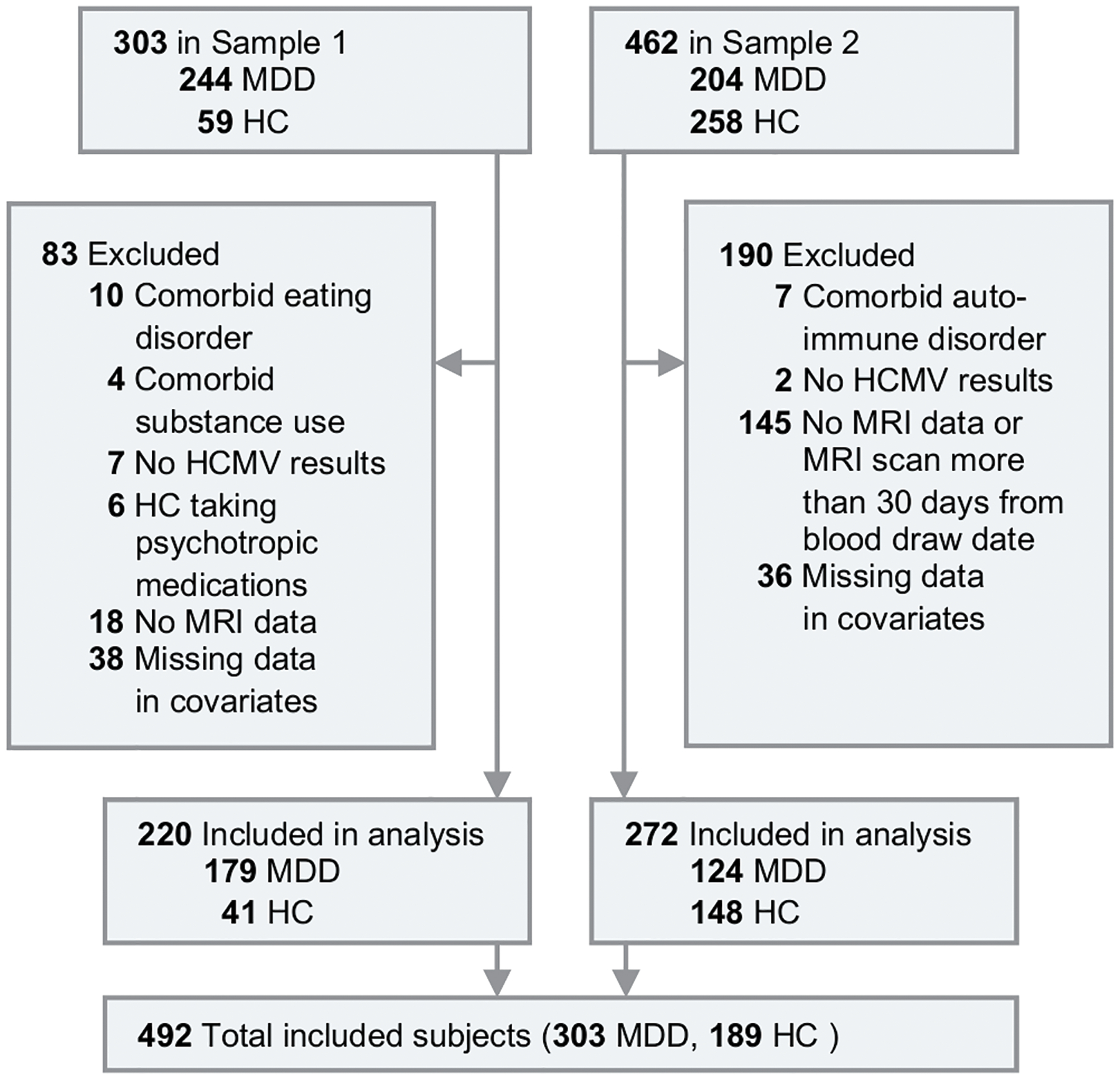
Flow Diagram of Selection of Participants.
Table 1.
Demographic Characteristics of Study Participants After Application of Applied Inverse Probability of Treatment Weighting
| MDD | HC | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample 1 | HCMV− | HCMV+ | P a | SMDb | HCMV− | HCMV+ | P a | SMDb |
| n (weighted population) | 78 (75.17) | 101 (101.84) | 26 (20.67) | 15 (9.70) | ||||
| Age (mean (SD)) | 35.40 (11.08) | 36.03 (11.02) | 0.73 | 0.06 | 33.11 (11.35) | 30.46 (10.47) | 0.55 | 0.24 |
| Sex (Male (%)) | 23.9 (31.8) | 31.7 (31.1) | 0.93 | 0.02 | 9.7 (47.0) | 4.8 (49.5) | 0.90 | 0.05 |
| BMI (mean (SD)) | 28.89 (5.57) | 29.01 (5.37) | 0.89 | 0.02 | 26.76 (6.07) | 28.82 (4.69) | 0.27 | 0.38 |
| Education (mean (SD))c | 6.53 (1.44) | 6.45 (1.75) | 0.76 | 0.05 | 7.02 (1.48) | 5.77 (1.01) | <0.01 | 0.99 |
| Income (mean (SD))d | $50,330 ($67,542) | $45,371 ($39,029) | 0.51 | 0.09 | $58,016 ($35,822) | $58,486 ($36,757) | 0.97 | 0.01 |
| Depression severity (mean (SD))e | 61.03 (7.10) | 61.00 (7.52) | 0.98 | 0.00 | 44.22 (6.11) | 41.31 (6.76) | 0.28 | 0.45 |
| Anxiety severity (mean (SD))f | 62.64 (6.90) | 62.59 (6.31) | 0.96 | 0.01 | 46.48 (8.16) | 44.01 (6.80) | 0.38 | 0.33 |
| Number of episodes (mean (SD))g | 4.27 (3.48) | 4.39 (3.49) | 0.83 | 0.04 | 0.00 (0.00) | 0.00 (0.00) | NA | 0.00 |
| Un-medicated (%)h | 21.7 (28.8) | 27.7 (27.2) | 0.83 | 0.04 | 20.7 (100.0) | 9.7 (100.0) | NA | 0.00 |
| CTQ (mean (SD))i | 47.95 (17.06) | 49.25 (21.62) | 0.67 | 0.07 | 31.54 (6.96) | 33.68 (11.79) | 0.50 | 0.22 |
| Alcohol use (mean (SD))j | 5.22 (2.35) | 5.22 (2.62) | 1.00 | 0.00 | 4.30 (2.82) | 3.61 (2.78) | 0.57 | 0.25 |
| HCMV IgG level (mean (SD))k | 0.97 (0.15) | 2.74 (0.61) | <0.001 | 3.96 | 0.97 (0.17) | 2.44 (0.68) | <0.001 | 2.99 |
| CRP (mean (SD))l | 0.53 (1.42) | 0.74 (1.45) | 0.35 | 0.15 | −0.34 (1.25) | −0.05 (1.17) | 0.46 | 0.24 |
| Sample 2 | ||||||||
| n (weighted population) | 67 (66.6) | 57 (57.1) | 79 (79.21) | 69 (68.47) | ||||
| Age (mean (SD)) | 36.04 (11.09) | 35.36 (10.59) | 0.76 | 0.06 | 31.76 (10.69) | 32.06 (9.66) | 0.86 | 0.03 |
| Sex (Male (%)) | 15.3(23.0) | 13.3(23.3) | 0.98 | 0.01 | 23.0(29.0) | 19.3(28.2) | 0.92 | 0.02 |
| BMI (mean (SD)) | 27.77 (6.09) | 28.25 (6.57) | 0.72 | 0.08 | 26.95 (6.06) | 26.90 (5.72) | 0.97 | 0.01 |
| Education (mean (SD))b | 5.34 (0.89) | 5.32 (0.85) | 0.89 | 0.03 | 5.36 (0.81) | 5.37 (0.87) | 0.93 | 0.02 |
| Depression severity (mean (SD))m | 17.94 (11.98) | 18.06 (11.88) | 0.96 | 0.01 | 1.53 (2.11) | 1.36 (2.01) | 0.67 | 0.09 |
| Un-medicated (%)h | 44.4 (66.7) | 49.1 (69.2) | 0.78 | 0.05 | 79.2 (100.0) | 68.5 (100.0) | NA | 0.00 |
| CTQ (mean (SD))i | 51.19 (23.38) | 49.05 (20.84) | 0.69 | 0.10 | 32.83 (11.06) | 33.54 (13.66) | 0.74 | 0.06 |
| HCMV IgG level (mean (SD))k | 1.24 (0.15) | 2.95 (0.68) | <0.001 | 3.47 | 1.27 (0.16) | 2.73 (0.78) | <0.001 | 2.60 |
| CRP (mean (SD))l | 1.86 (0.85) | 1.95 (1.04) | 0.62 | 0.10 | 1.99 (1.07) | 1.89 (0.87) | 0.59 | 0.10 |
Abbreviations: MDD, major depressive disorder; HC, healthy control; HCMV, human cytomegalovirus; HCMV−, human cytomegalovirus seronegative; HCMV+, human cytomegalovirus seropositive; SMD, standardized mean difference; BMI, body mass index; CTQ, childhood trauma questionnaire; CRP, C-reactive protein.
Calculated using X2 test for categorical variables and 2-tailed t-test for continuous variables.
The standardized mean differences less than 0.1 reveals a negligible imbalance.
Measured by an ordered categories. Full categories sees Supplementary Table 4.
Annual household income.
PROMIS depression T score was used.
PROMI anxiety T score was used.
Measured by MINI interview. Subjects had over 10 episodes were treated as had 10 episodes.
Un-medicated defined as not taking antipsychotic medication.
Childhood trauma questionnaire total score was used.
Log-transformed lifetime alcohol usage were used. Data obtained from CDDR interview.
HCMV IgG level z score was used.
CRP concentration log transferred.
Montgomery-Asberg Depression Rating Scale (MADRS) score was used.
Main effect of HCMV
Relative to HCMV− subjects, HCMV+ subjects in sample 1 showed a reduction of GMV in 67 out of 87 cortical and subcortical ROIs although only six of these regions were statistically significant (puncorrected<0.05), i.e. the left pars orbitalis gyrus (standardized beta coefficient (SBC) =−0.38, [95%CI, −0.68 to −0.07]), left fusiform gyrus (SBC=−0.25, [95%CI, −0.49 to −0.01]), left inferior parietal lobule (SBC=−0.25, [95%CI, −0.49 to −0.01]), right lateral orbitofrontal gyrus (SBC=−0.23, [95%CI, −0.46 to −0.01]), right parahippocampal gyrus (SBC)=−0.32, [95%CI, −0.62 to −0.03]), and right supramarginal gyrus (SBC=−0.26, [95%CI, −0.51 to −0.01]). Note that an SBC of −1 indicates that seropositive HCMV individuals show a one standard deviation smaller volume than seronegative HCMV individuals. Relative to HCMV− subjects, HCMV+ subjects in sample 2 had reduced GMV in 73 out of 87 regions with seven regions significant (p<0.05, uncorrected). No regions were significantly larger in the HCMV+ versus the HCMV− groups in either of the samples (Supplementary Table 2).
Two out of the six regions that were significantly reduced in sample 1 also showed a significant reduction of GMV in sample 2, indicating a replication of the HCMV main effect for the left fusiform gyrus (SBC=−0.51, [95%CI, −0.80 to −0.23]) and the right supramarginal gyrus (SBC=−0.32, [95%CI, −0.64 to −0.01]) (Table 2, Figure 2). Posthoc analyses revealed a similar effect of HCMV in the MDD subgroups, but not in the HC subgroups, in both samples, suggesting that the effects of HCMV were mainly driven by the MDD participants (Table 2, Figure 2.C).
Table 2.
Regional Effect of HCMV Infection on Gray Matter Volume
| IPTW adjusted | Sample 1 | Sample 2 | ||||
|---|---|---|---|---|---|---|
| ROI | SBCa | 95%CIb | p | SBC | 95%CI | p |
| Main effect of HCMV | ||||||
| L pars orbitalis gyrus | −0.38 | −0.68 ~ −0.07 | 0.02 | −0.15 | −0.56 ~ 0.25 | 0.47 |
| L fusiform gyrus | −0.25 | −0.49 ~ −0.01 | 0.04 | −0.51 | −0.80 ~ −0.23 | <0.001 *** |
| L inferior parietal lobule | −0.25 | −0.49 ~ −0.01 | 0.05 | −0.06 | −0.51 ~ 0.39 | 0.79 |
| R lateral orbitofrontal gyrus | −0.23 | −0.46 ~ −0.01 | 0.04 | −0.21 | −0.63 ~ 0.22 | 0.34 |
| R parahippocampal gyrus | −0.32 | −0.62 ~ −0.03 | 0.03 | 0.25 | −0.06 ~ 0.57 | 0.12 |
| R supramarginal gyrus | −0.26 | −0.51 ~ −0.01 | 0.04 | −0.32 | −0.64 ~ −0.01 | <0.05 * |
| HCMV effect in MDD | ||||||
| L pars orbitalis gyrus | −0.37 | −0.68 ~ −0.07 | 0.02 | −0.16 | −0.57 ~ 0.25 | 0.44 |
| L fusiform gyrus | −0.25 | −0.49 ~ −0.02 | 0.04 | −0.50 | −0.77 ~ −0.22 | <0.001 *** |
| L inferior parietal lobule | −0.25 | −0.50 ~ −0.01 | 0.05 | −0.07 | −0.50 ~ 0.36 | 0.75 |
| R lateral orbitofrontal gyrus | −0.24 | −0.47 ~ −0.01 | 0.04 | −0.22 | −0.65 ~ 0.21 | 0.31 |
| R parahippocampal gyrus | −0.33 | −0.63 ~ −0.04 | 0.03 | 0.29 | −0.06 ~ 0.64 | 0.10 |
| R supramarginal gyrus | −0.27 | −0.53 ~ −0.02 | 0.04 | −0.34 | −0.67 ~ −0.01 | <0.05 * |
| HCMV effect in HC | ||||||
| L pars orbitalis gyrus | 0.05 | −0.51 ~ 0.63 | 0.86 | −0.04 | −0.34 ~ 0.27 | 0.82 |
| L fusiform gyrus | −0.05 | −0.52 ~ 0.43 | 0.84 | −0.09 | −0.35 ~ 0.18 | 0.52 |
| L inferior parietal lobule | −0.25 | −0.81 ~ 0.31 | 0.38 | −0.02 | −0.36 ~ 0.33 | 0.93 |
| R lateral orbitofrontal gyrus | 0.11 | −0.32 ~ 0.54 | 0.61 | −0.32 | −0.61 ~ −0.03 | 0.03 |
| R parahippocampal gyrus | −0.11 | −0.78 ~ 0.55 | 0.75 | −0.29 | −0.62 ~ 0.04 | 0.08 |
| R supramarginal gyrus | −0.16 | −0.79 ~ 0.47 | 0.62 | −0.23 | −0.55 ~ 0.09 | 0.17 |
Abbreviation: ROI, region of interest; R, right; L, left;
Standardized beta coefficient. SBC of 1 indicates that the mean gray matter volume of the HCMV+ subgroup is 1 standard deviation different from the HCMV− subgroup. A negative value indicates HCMV+ < HCMV−, and a positive value indicates HCMV+ > HCMV−.
95%CI, 95% confidence interval, robust standard errors was used to calculate 95%CI.
Puncorrected less than 0.05 in both samples and in the same direction.
PFDR < 0.01
Figure 2.
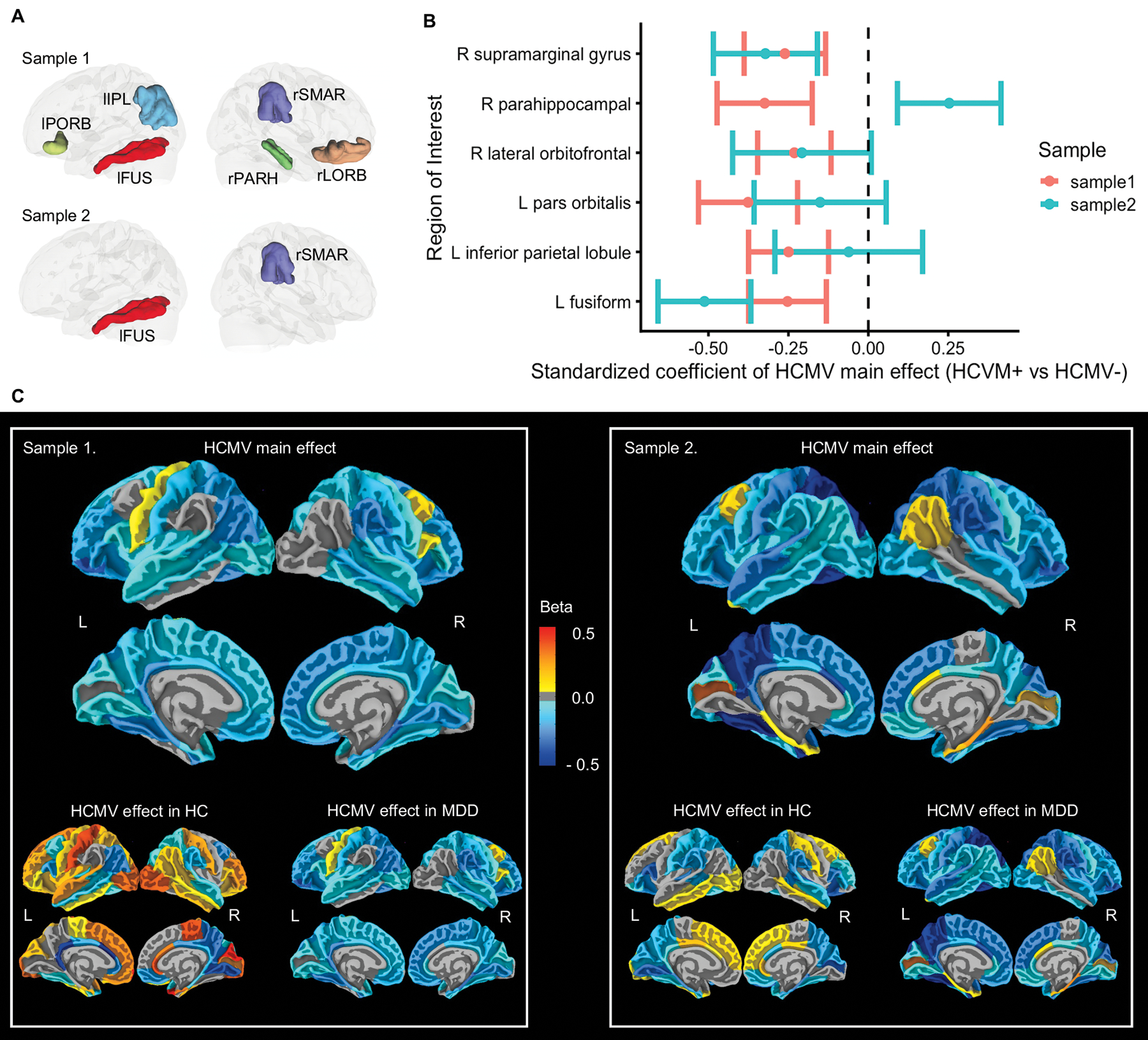
Regional Effect of HCMV infection on gray matter volumes. (A) Illustration of regions that showed main effect of HCMV. Six regions were significantly smaller in HCMV+ versus HCMV− subjects in sample 1 at puncorrected < 0.05. Two out of these six regions were also significant decreased in sample 2 at puncorrected < 0.05. (B) Standardized beta coefficient as effect size with robust standard error as error bar, estimated from IPTW adjusted regression model. (C) Mapping of HCMV main effect, HCMV effect in HC, and the HCMV effect in MDD at all the cortical regions without thresholding. Colors represent the standardized beta coefficients, estimated from IPTW adjusted regression model. They range from −0.5 to 0.5, which means the mean gray matter volume of HCMV+ subgroup in given region increased or decreased by 0.5 standard deviations from HCMV− subgroup. Blue colors represent smaller gray matter volumes in HCMV+ groups, whereas yellow-red colors represent larger gray matter volumes in HCMV+ groups. Relative to HCMV− subjects, HCMV+ subjects showed smaller gray matter volumes across cortical regions in both samples, most prominently in orbitofrontal, temporal and parietal regions. The observed effects of HCMV were mainly driven by the MDD groups. Within the HCs there were less consistent differences between HCMV+ and HCMV− subgroups and the effect sizes were generally smaller.
Abbreviations: rSMAR, right supramarginal gyrus; rPARH, right parahippocampal gyrus; rLORB, right lateral orbitofrontal gyrus; lPORB, left pars orbitalis gyrus; lIPL, left inferior parietal lobule; lFUS, left fusiform gyrus.
The unadjusted model showed similar results to the IPTW model (Supplementary Table 3). Additional sensitivity analyses using the E-value suggested that the observed associations were at least moderately robust to potential unmeasured confounding. For instance, the estimated E-value of 1.82 for the left fusiform gyrus in sample 1 indicates that in order to explain away the observed effect of HCMV with effect size of SBC=−0.25, a putative unmeasured confounder would need to increase the probability of a subject being HCMV+ and having a smaller fusiform gyrus by 1.82-times each. The E-values estimated for the left fusiform gyrus in sample 2, and the right supramarginal gyri in samples 1 and 2 were 2.56, 1.85, and 2.01 respectively (Supplementary Figure 2).
Main effect of diagnosis and interaction effect
There were four subcortical regions (bilateral caudate and bilateral nucleus accumbens) that significantly differed in MDD versus HC subjects in sample 1. However, none of these regions replicated in sample 2. Conversely, there were three cortical regions (right parahippocampal gyrus, and right pericalcarine cortex) that significantly differed in MDD versus HC subjects in sample 2, but the results did not replicate in sample 1 (Supplementary Figure 3). There was no significant diagnosis-by-HCMV interaction effect in sample 1 although two regions (the right parahippocampal gyrus and the left fusiform gyrus) showed a significant interaction effect in sample 2 (Supplementary Figure 4).
Correlations between HCMV level and CRP
Correlation analyses were performed in the combined MDD/HC HCMV+ samples for the two regions that replicated across sample 1 and sample 2. HCMV IgG level was inversely correlated with GMV in the left fusiform gyrus (r=−0.19, puncorrected=0.049) and the right supramarginal gyrus (r=−0.19, puncorrected=0.043) in sample 1 but not in sample 2 (Figure 3). Sample 1 results were similar in the MDD group alone: left fusiform gyrus (r=−0.22, puncorrected=0.029); right supramarginal gyrus (r=−0.16, puncorrected=0.122). There were no significant correlations between CRP and HCMV level or CRP and GMVs in either sample.
Figure 3.
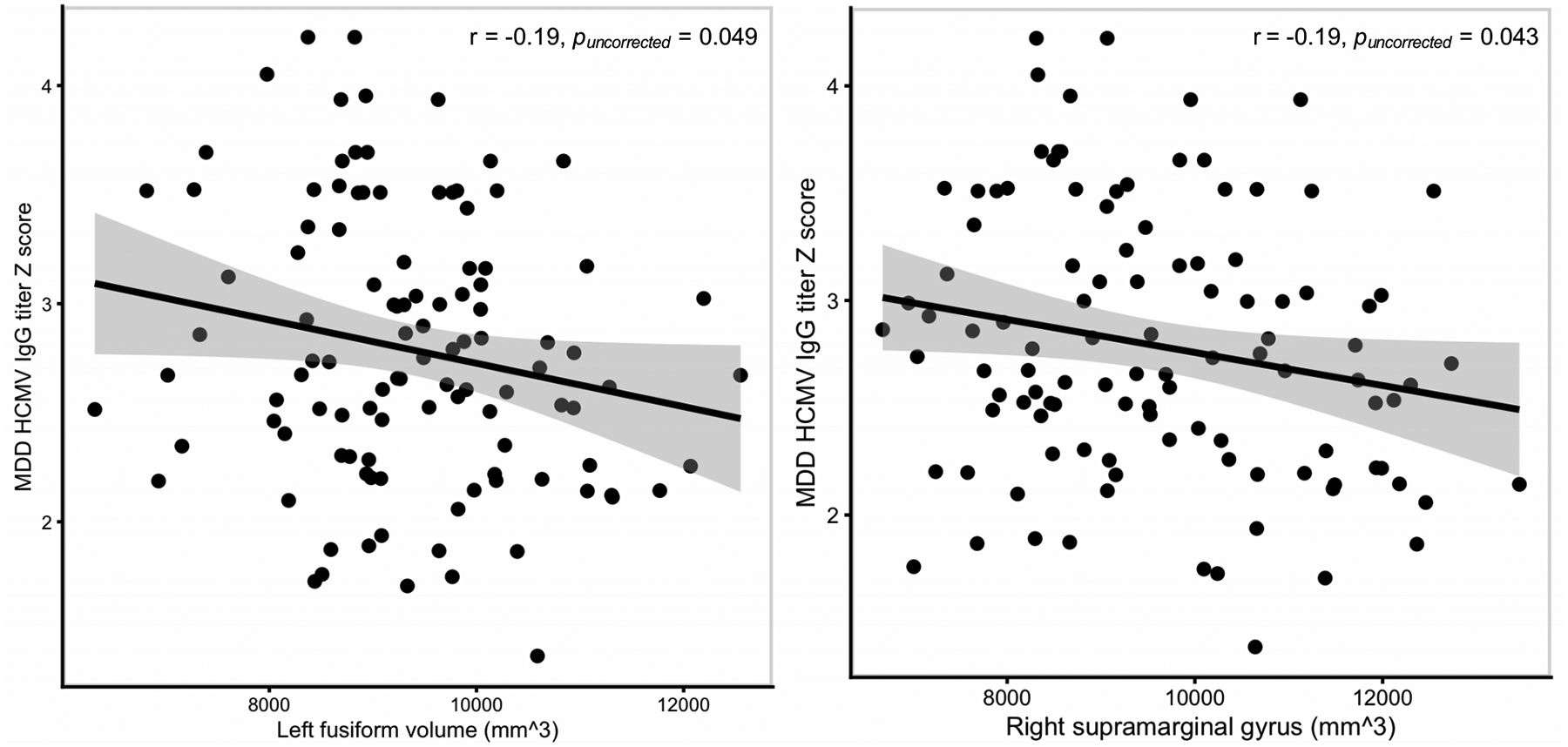
HCMV IgG level is inversely correlated with gray matter volume in the combined MDD/HC HCMV+ group of sample 1.
DISCUSSION
In two independent samples with different inclusion/exclusion criteria we observed a significant main effect of HCMV serostatus: HCMV+ subjects had smaller left fusiform gyri and right supramarginal gyri than HCMV− subjects matched on up to 11 different potential confounding variables. Multiple additional regions were reduced in volume but did not replicate across samples. Although we did not detect a significant interaction between HCMV serostatus and diagnosis, it is clear from Table 2 and Figure 2.C that the significant main effect of HCMV was driven by differences between the HCMV+ and the HCMV− MDD subgroups rather than the HCMV+ and the HCMV− HC groups. This is consistent with our hypothesis that control of HCMV infection is diminished in MDD because of inflammation and impaired viral immunity. Nevertheless, HCs may also experience periods of stress which potentially predispose to viral reactivation, and therefore putative HCMV−mediated changes in brain volume. Thus, because the direction (but not magnitude) of the effect of HCMV on GMV is likely similar in both MDD and HC populations, sample sizes greater than 90 in each subgroup will likely be required to detect significant interaction effects based on the interaction effect size observed in sample 2. Future studies with larger samples in HC subjects are warranted to comfirm the HCMV effect in HC as well as the interaction effect between HCMV and diagnosis.
It is unclear whether the supramarginal gyrus and fusiform gyrus are more vulnerable to HCMV infection than other brain regions. Congenital HCMV infection appears to cause gross structural abnormalities leading to mental retardation, cerebral palsy, and sensorineural hearing loss4 while HCMV encephalitis can result in destruction of the periventricular regions in HIV patients66. While not classically associated with MDD, reductions in thickness of the supramarginal and fusiform gyri have been reported in recent ENIGMA consortium MDD samples67, 68. In addition, structural abnormalities of these regions have been observed in the context of viral infection (HIV69, 70) and autoimmunity (systemic lupus erythematosus71, 72; multiple sclerosis73, 74). Further, the supramarginal gyrus has been hypothesized to be particularly vulnerable to neuropathological processes associated with Alzheimer’s disease because it is part of the heteromodal cortex that undergoes significant myelination during development75. Additional studies are needed to clarify the neuroanatomical specificity of any HCMV−mediated effects. Nevertheless, our results raise the possibility that an attenuated form of HCMV−induced neuropathology may occur in some HCMV+ MDD patients with a mild impairment of anti-viral immunity. This hypothesis receives support from the only other neuroimaging study in a psychiatric population which reported that HCMV+ patients with bipolar disorder and schizophrenia had smaller right hippocampal volumes than HCMV− patients76.
The mechanisms through which HCMV infection putatively leads to reductions in GMV are unclear but there are at least two possibilities. First, HCMV may directly damage the brain or elicit a microglia-mediated anti-viral immune response. Second, HCMV may contribute to systemic inflammation via several mechanisms including the long-term accumulation of cytotoxic CD28− T-cells18. Thus, even if HCMV does not always infect the brain, peripheral sites of inflammation could exert systemic effects, impacting GMV. Several studies have reported correlations between circulating inflammatory mediators and reductions in GMV54, 77. In this regard, the absence of a significant association between CRP and IgG level is unclear. It is plausible, and perhaps even likely, that the single time point at which CRP was measured did not always overlap with viral shedding since the two studies were not designed to evaluate participants with an active HCMV infection. It is also possible that specific markers of viral infection such as IP-10/CXCL10 or macrophage activation such as sCD1478 would be more sensitive to HCMV reactivation than CRP.
There was a significant inverse correlation between HCMV IgG level and volume of the left fusiform gyrus and right supramarginal gyrus although these relationships were modest and only present in sample 1. Our results are partly consistent with a previous report of an inverse association between HCMV IgG level and right hippocampal volumes in patients with schizophrenia and bipolar disorder76. However, IgG level only provides an approximate measure of HCMV shedding since they have a half-life of <30 days and are also influenced by host factors. Thus, the signal-to-noise ratio of these correlational analyses are likely low.
Given the cross-sectional design, we cannot definitively conclude that HCMV is the cause of the reduction in GMV since an unknown causal factor may be correlated with HCMV serostatus. Nevertheless, we minimized this possibility through careful balancing of potential confounding variables in the HCMV+ and HCMV− groups. In particular, when computing propensity scores, we included childhood trauma which we previously demonstrated to be more prevalent in HCMV+ MDD subjects79 as well as education level and household income, a surrogate marker for childhood socioeconomic status80. Childhood trauma and socioeconomic status have previously been associated with reductions in GMV81, 82. Second, we cannot differentiate between the acute and cumulative effects of HCMV on GMV. Longitudinal studies and/or quantification of HCMV−specific T-cell populations are required to address this question. Third, we cannot exclude the possibility that other viral infections that co-occur with HCMV accounted for the reductions in GMV. Nevertheless, HCMV is more strongly linked with congenital brain abnormalities than other herpesviruses and recurrent HCMV reactivation disrupts the balance of the immune system to a greater extent than other herpesviruses83.
In conclusion, after careful balancing of HCMV+ and HCMV− groups for up to eleven baseline demographic and clinical variables, we found suggestive evidence for an HCMV−associated reduction in GMV in two independent samples. While causal conclusions cannot be drawn, the results raise the possibility that HCMV infection may be a treatable source of structural brain abnormalities in a subset of depressed patients.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank all the research participants and wish to acknowledge the contributions of Brenda Davis, Debbie Neal, Chibing Tan, and Ashlee Taylor from the laboratory of TKT towards the transport, processing, and handling of all blood samples.
This work was supported by The William K. Warren Foundation, the National Institute of Mental Health (K01MH096077 and R21MH11387 to JS; R01MH098099 to JB), and the National Institute of General Medical Sciences (P20GM121312 to MPP). The funding sources had no role in the design and conduct of the study: collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
CONFLICT OF INTEREST
Dr Hunt reported receiving support from Merck (donated drug) for a clinical trial of letermovir. All other authors have no conflicts of interest to report.
Supplementary information is available at MP’s website
REFERENCES
- 1.Tsutsui Y, Kosugi I, Kawasaki H. Neuropathogenesis in cytomegalovirus infection: indication of the mechanisms using mouse models. Rev Med Virol 2005; 15(5): 327–345. [DOI] [PubMed] [Google Scholar]
- 2.Poland SD, Costello P, Dekaban GA, Rice GP. Cytomegalovirus in the brain: in vitro infection of human brain-derived cells. J Infect Dis 1990; 162(6): 1252–1262. [DOI] [PubMed] [Google Scholar]
- 3.Alcendor DJ, Charest AM, Zhu WQ, Vigil HE, Knobel SM. Infection and upregulation of proinflammatory cytokines in human brain vascular pericytes by human cytomegalovirus. J Neuroinflammation 2012; 9: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev 2013; 26(1): 86–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pariante CM, Carpiniello B, Orru MG, Sitzia R, Piras A, Farci AM et al. Chronic caregiving stress alters peripheral blood immune parameters: the role of age and severity of stress. Psychother Psychosom 1997; 66(4): 199–207. [DOI] [PubMed] [Google Scholar]
- 6.Glaser R, Kiecolt-Glaser JK, Speicher CE, Holliday JE. Stress, loneliness, and changes in herpesvirus latency. J Behav Med 1985; 8(3): 249–260. [DOI] [PubMed] [Google Scholar]
- 7.Docke WD, Prosch S, Fietze E, Kimel V, Zuckermann H, Klug C et al. Cytomegalovirus reactivation and tumour necrosis factor. Lancet 1994; 343(8892): 268–269. [DOI] [PubMed] [Google Scholar]
- 8.Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, Neff MJ et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. Jama 2008; 300(4): 413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iglesias-Escudero M, Moro-Garcia MA, Marcos-Fernandez R, Garcia-Torre A, Alvarez-Arguelles ME, Suarez-Fernandez ML et al. Levels of anti-CMV antibodies are modulated by the frequency and intensity of virus reactivations in kidney transplant patients. PLoS One 2018; 13(4): e0194789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prosch S, Wendt CE, Reinke P, Priemer C, Oppert M, Kruger DH et al. A novel link between stress and human cytomegalovirus (HCMV) infection: sympathetic hyperactivity stimulates HCMV activation. Virology 2000; 272(2): 357–365. [DOI] [PubMed] [Google Scholar]
- 11.Sarid O, Anson O, Yaari A, Margalith M. Academic stress, immunological reaction, and academic performance among students of nursing and physiotherapy. Res Nurs Health 2004; 27(5): 370–377. [DOI] [PubMed] [Google Scholar]
- 12.Mehta SK, Stowe RP, Feiveson AH, Tyring SK, Pierson DL. Reactivation and shedding of cytomegalovirus in astronauts during spaceflight. J Infect Dis 2000; 182(6): 1761–1764. [DOI] [PubMed] [Google Scholar]
- 13.Mehta SK, Laudenslager ML, Stowe RP, Crucian BE, Sams CF, Pierson DL. Multiple latent viruses reactivate in astronauts during Space Shuttle missions. Brain Behav Immun 2014; 41: 210–217. [DOI] [PubMed] [Google Scholar]
- 14.Janicki-Deverts D, Cohen S, Doyle WJ, Marsland AL, Bosch J. Childhood environments and cytomegalovirus serostatus and reactivation in adults. Brain Behav Immun 2014; 40: 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fagundes CP, Glaser R, Malarkey WB, Kiecolt-Glaser JK. Childhood adversity and herpesvirus latency in breast cancer survivors. Health Psychol 2013; 32(3): 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirtcliff EA, Coe CL, Pollak SD. Early childhood stress is associated with elevated antibody levels to herpes simplex virus type 1. Proc Natl Acad Sci U S A 2009; 106(8): 2963–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rooney BV, Crucian BE, Pierson DL, Laudenslager ML, Mehta SK. Herpes Virus Reactivation in Astronauts During Spaceflight and Its Application on Earth. Front Microbiol 2019; 10: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bano A, Pera A, Almoukayed A, Clarke THS, Kirmani S, Davies KA et al. CD28 (null) CD4 T-cell expansions in autoimmune disease suggest a link with cytomegalovirus infection. F1000Res 2019; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis 2011; 203(10): 1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zivadinov R, Chin J, Horakova D, Bergsland N, Weinstock-Guttman B, Tamano-Blanco M et al. Humoral responses to herpesviruses are associated with neurodegeneration after a demyelinating event: results from the multi-center set study. J Neuroimmunol 2014; 273(1–2): 58–64. [DOI] [PubMed] [Google Scholar]
- 21.Lurain NS, Hanson BA, Martinson J, Leurgans SE, Landay AL, Bennett DA et al. Virological and immunological characteristics of human cytomegalovirus infection associated with Alzheimer disease. J Infect Dis 2013; 208(4): 564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dantzer R Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiol Rev 2018; 98(1): 477–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 2016; 16(1): 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mechawar N, Savitz J. Neuropathology of mood disorders: do we see the stigmata of inflammation? Transl Psychiatry 2016; 6(11): e946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Appels A, Bar FW, Bar J, Bruggeman C, de Baets M. Inflammation, depressive symptomtology, and coronary artery disease. Psychosom Med 2000; 62(5): 601–605. [DOI] [PubMed] [Google Scholar]
- 26.Rector JL, Dowd JB, Loerbroks A, Burns VE, Moss PA, Jarczok MN et al. Consistent associations between measures of psychological stress and CMV antibody levels in a large occupational sample. Brain Behav Immun 2014; 38: 133–141. [DOI] [PubMed] [Google Scholar]
- 27.Miller GE, Freedland KE, Duntley S, Carney RM. Relation of depressive symptoms to C-reactive protein and pathogen burden (cytomegalovirus, herpes simplex virus, Epstein-Barr virus) in patients with earlier acute coronary syndromes. The American journal of cardiology 2005; 95(3): 317–321. [DOI] [PubMed] [Google Scholar]
- 28.Dickerson F, Wilcox HC, Adamos M, Katsafanas E, Khushalani S, Origoni A et al. Suicide attempts and markers of immune response in individuals with serious mental illness. J Psychiatr Res 2017; 87: 37–43. [DOI] [PubMed] [Google Scholar]
- 29.Simanek AM, Cheng C, Yolken R, Uddin M, Galea S, Aiello AE. Herpesviruses, inflammatory markers and incident depression in a longitudinal study of Detroit residents. Psychoneuroendocrinology 2014; 50: 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgdorf KS, Trabjerg BB, Pedersen MG, Nissen J, Banasik K, Pedersen OB et al. Large-scale study of Toxoplasma and Cytomegalovirus shows an association between infection and serious psychiatric disorders. Brain Behav Immun 2019. [DOI] [PubMed] [Google Scholar]
- 31.Frye MA, Coombes BJ, McElroy SL, Jones-Brando L, Bond DJ, Veldic M et al. Association of Cytomegalovirus and Toxoplasma gondii Antibody Titers With Bipolar Disorder. JAMA Psychiatry 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simanek AM, Zheng C, Yolken R, Haan M, Aiello AE. A Longitudinal Study of the Association Between Persistent Pathogens and Incident Depression Among Older U.S. Latinos. J Gerontol A Biol Sci Med Sci 2019; 74(5): 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dickerson F, Origoni A, Schweinfurth LAB, Stallings C, Savage CLG, Sweeney K et al. Clinical and Serological Predictors of Suicide in Schizophrenia and Major Mood Disorders. J Nerv Ment Dis 2018; 206(3): 173–178. [DOI] [PubMed] [Google Scholar]
- 34.Jaremka LM, Fagundes CP, Glaser R, Bennett JM, Malarkey WB, Kiecolt-Glaser JK. Loneliness predicts pain, depression, and fatigue: understanding the role of immune dysregulation. Psychoneuroendocrinology 2013; 38(8): 1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prossin AR, Yolken RH, Kamali M, Heitzeg MM, Kaplow JB, Coryell WH et al. Cytomegalovirus Antibody Elevation in Bipolar Disorder: Relation to Elevated Mood States. Neural plasticity 2015; 2015: 939780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips AC, Carroll D, Khan N, Moss P. Cytomegalovirus is associated with depression and anxiety in older adults. Brain Behav Immun 2008; 22(1): 52–55. [DOI] [PubMed] [Google Scholar]
- 37.Trzonkowski P, Mysliwska J, Godlewska B, Szmit E, Lukaszuk K, Wieckiewicz J et al. Immune consequences of the spontaneous pro-inflammatory status in depressed elderly patients. Brain Behav Immun 2004; 18(2): 135–148. [DOI] [PubMed] [Google Scholar]
- 38.Coryell W, Wilcox H, Evans SJ, Pandey GN, Jones-Brando L, Dickerson F et al. Latent infection, inflammatory markers and suicide attempt history in depressive disorders. J Affect Disord 2020; 270: 97–101. [DOI] [PubMed] [Google Scholar]
- 39.Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain, behavior, and immunity 2001; 15(3): 199–226. [DOI] [PubMed] [Google Scholar]
- 40.Cole SW. Human social genomics. PLoS genetics 2014; 10(8): e1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leday GGR, Vertes PE, Richardson S, Greene JR, Regan T, Khan S et al. Replicable and Coupled Changes in Innate and Adaptive Immune Gene Expression in Two Case-Control Studies of Blood Microarrays in Major Depressive Disorder. Biol Psychiatry 2018; 83(1): 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans DL, Ten Have TR, Douglas SD, Gettes DR, Morrison M, Chiappini MS et al. Association of depression with viral load, CD8 T lymphocytes, and natural killer cells in women with HIV infection. Am J Psychiatry 2002; 159(10): 1752–1759. [DOI] [PubMed] [Google Scholar]
- 43.Irwin MR, Levin MJ, Laudenslager ML, Olmstead R, Lucko A, Lang N et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2013; 56(8): 1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Afsar B, Elsurer R, Eyileten T, Yilmaz MI, Caglar K. Antibody response following hepatitis B vaccination in dialysis patients: does depression and life quality matter? Vaccine 2009; 27(42): 5865–5869. [DOI] [PubMed] [Google Scholar]
- 45.Ford BN, Yolken RH, Dickerson FB, Teague TK, Irwin MR, Paulus MP et al. Reduced immunity to measles in adults with major depressive disorder. Psychol Med 2018: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bochennek K, Allwinn R, Langer R, Becker M, Keppler OT, Klingebiel T et al. Differential loss of humoral immunity against measles, mumps, rubella and varicella-zoster virus in children treated for cancer. Vaccine 2014; 32(27): 3357–3361. [DOI] [PubMed] [Google Scholar]
- 47.Rocca S, Santilli V, Cotugno N, Concato C, Manno EC, Nocentini G et al. Waning of vaccine-induced immunity to measles in kidney transplanted children. Medicine (Baltimore) 2016; 95(37): e4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Titanji K, De Milito A, Cagigi A, Thorstensson R, Grutzmeier S, Atlas A et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood 2006; 108(5): 1580–1587. [DOI] [PubMed] [Google Scholar]
- 49.Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A 2012; 109(16): 5995–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marsland AL, Bachen EA, Cohen S, Rabin B, Manuck SB. Stress, immune reactivity and susceptibility to infectious disease. Physiol Behav 2002; 77(4–5): 711–716. [DOI] [PubMed] [Google Scholar]
- 51.Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N Engl J Med 2017; 377(25): 2433–2444. [DOI] [PubMed] [Google Scholar]
- 52.Diamond DJ, La Rosa C, Chiuppesi F, Contreras H, Dadwal S, Wussow F et al. A fifty-year odyssey: prospects for a cytomegalovirus vaccine in transplant and congenital infection. Expert Rev Vaccines 2018; 17(10): 889–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Victor TA, Khalsa SS, Simmons WK, Feinstein JS, Savitz J, Aupperle RL et al. Tulsa 1000: a naturalistic study protocol for multilevel assessment and outcome prediction in a large psychiatric sample. BMJ Open 2018; 8(1): e016620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Savitz J, Drevets WC, Smith CM, Victor TA, Wurfel BE, Bellgowan PS et al. Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology 2015; 40(2): 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ford BN, Yolken RH, Dickerson FB, Teague TK, Irwin MR, Paulus MP et al. Reduced immunity to measles in adults with major depressive disorder. Psychol Med 2019; 49(2): 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999; 9(2): 179–194. [DOI] [PubMed] [Google Scholar]
- 57.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33(3): 341–355. [DOI] [PubMed] [Google Scholar]
- 58.Cardinale F, Chinnici G, Bramerio M, Mai R, Sartori I, Cossu M et al. Validation of FreeSurfer-estimated brain cortical thickness: comparison with histologic measurements. Neuroinformatics 2014; 12(4): 535–542. [DOI] [PubMed] [Google Scholar]
- 59.Iscan Z, Jin TB, Kendrick A, Szeglin B, Lu H, Trivedi M et al. Test-retest reliability of freesurfer measurements within and between sites: Effects of visual approval process. Hum Brain Mapp 2015; 36(9): 3472–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011; 46(3): 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Little RJ, Rubin DB. Causal effects in clinical and epidemiological studies via potential outcomes: concepts and analytical approaches. Annu Rev Public Health 2000; 21: 121–145. [DOI] [PubMed] [Google Scholar]
- 62.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015; 34(28): 3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thomas L, Li F, Pencina M. Using Propensity Score Methods to Create Target Populations in Observational Clinical Research. Jama 2020. [DOI] [PubMed] [Google Scholar]
- 64.Huffman JE. Examining the current standards for genetic discovery and replication in the era of mega-biobanks. Nature communications 2018; 9(1): 5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med 2017; 167(4): 268–274. [DOI] [PubMed] [Google Scholar]
- 66.Kalayjian RC, Cohen ML, Bonomo RA, Flanigan TP. Cytomegalovirus ventriculoencephalitis in AIDS. A syndrome with distinct clinical and pathologic features. Medicine (Baltimore) 1993; 72(2): 67–77. [PubMed] [Google Scholar]
- 67.Tozzi L, Garczarek L, Janowitz D, Stein DJ, Wittfeld K, Dobrowolny H et al. Interactive impact of childhood maltreatment, depression, and age on cortical brain structure: mega-analytic findings from a large multi-site cohort. Psychol Med 2020; 50(6): 1020–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmaal L, Hibar DP, Samann PG, Hall GB, Baune BT, Jahanshad N et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry 2017; 22(6): 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.MacDuffie KE, Brown GG, McKenna BS, Liu TT, Meloy MJ, Tawa B et al. Effects of HIV Infection, methamphetamine dependence and age on cortical thickness, area and volume. Neuroimage Clin 2018; 20: 1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kallianpur KJ, Kirk GR, Sailasuta N, Valcour V, Shiramizu B, Nakamoto BK et al. Regional cortical thinning associated with detectable levels of HIV DNA. Cereb Cortex 2012; 22(9): 2065–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jung RE, Segall JM, Grazioplene RG, Qualls C, Sibbitt WL, Roldan CA. Cortical thickness and subcortical gray matter reductions in neuropsychiatric systemic lupus erythematosus. PLoS One 2010; 5(3): e9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Niu C, Tan X, Liu X, Han K, Niu M, Xu J et al. Cortical thickness reductions associate with abnormal resting-state functional connectivity in non-neuropsychiatric systemic lupus erythematosus. Brain imaging and behavior 2018; 12(3): 674–684. [DOI] [PubMed] [Google Scholar]
- 73.Steenwijk MD, Geurts JJ, Daams M, Tijms BM, Wink AM, Balk LJ et al. Cortical atrophy patterns in multiple sclerosis are non-random and clinically relevant. Brain 2016; 139(Pt 1): 115–126. [DOI] [PubMed] [Google Scholar]
- 74.Tsagkas C, Chakravarty MM, Gaetano L, Naegelin Y, Amann M, Parmar K et al. Longitudinal patterns of cortical thinning in multiple sclerosis. Hum Brain Mapp 2020; 41(8): 2198–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jacobs HI, Van Boxtel MP, Jolles J, Verhey FR, Uylings HB. Parietal cortex matters in Alzheimer’s disease: an overview of structural, functional and metabolic findings. Neurosci Biobehav Rev 2012; 36(1): 297–309. [DOI] [PubMed] [Google Scholar]
- 76.Houenou J, d’Albis MA, Daban C, Hamdani N, Delavest M, Lepine JP et al. Cytomegalovirus seropositivity and serointensity are associated with hippocampal volume and verbal memory in schizophrenia and bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 2014; 48: 142–148. [DOI] [PubMed] [Google Scholar]
- 77.Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry 2008; 64(6): 484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gianella S, Moser C, Vitomirov A, McKhann A, Layman L, Scott B et al. Presence of asymptomatic cytomegalovirus and Epstein--Barr virus DNA in blood of persons with HIV starting antiretroviral therapy is associated with non-AIDS clinical events. AIDS 2020; 34(6): 849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ford BN, Yolken RH, Aupperle RL, Teague TK, Irwin MR, Paulus MP et al. Association of Early-Life Stress With Cytomegalovirus Infection in Adults With Major Depressive Disorder. JAMA Psychiatry 2019; 76(5): 545–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dodgeon B, Patalay P, Ploubidis GB, Wiggins RD. Exploring the role of early-life circumstances, abilities and achievements on well-being at age 50 years: evidence from the 1958 British birth cohort study. BMJ Open 2020; 10(2): e031416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mackes NK, Golm D, Sarkar S, Kumsta R, Rutter M, Fairchild G et al. Early childhood deprivation is associated with alterations in adult brain structure despite subsequent environmental enrichment. Proc Natl Acad Sci U S A 2020; 117(1): 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yaple ZA, Yu R. Functional and Structural Brain Correlates of Socioeconomic Status. Cereb Cortex 2020; 30(1): 181–196. [DOI] [PubMed] [Google Scholar]
- 83.Klenerman P, Oxenius A. T cell responses to cytomegalovirus. Nat Rev Immunol 2016; 16(6): 367–377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The full analysis code, datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.


