Abstract
Stage I seminoma is the most frequent tumour in young men. It has a very good prognosis thanks to the use of a multidisciplinary therapeutic approach including surgery, radiotherapy and systemic chemotherapy. Late (after 2 years) and very late (after 5 years) relapses are uncommon, but not impossible, even if standardized follow-up for testicular tumours lasts up to 5 years after the diagnosis. We report a case of a 67-year-old Caucasian man with metachronous bilateral testicular seminoma who developed a retroperitoneal relapse of testicular seminoma 23 years after the first orchiectomy. Based on histological confirmation of testicular relapse, the patient underwent four cycles of systemic chemotherapy with bleomycin, etoposide and cisplatin (PEB), with no adverse reactions. He subsequently achieved complete radiological response at restaging computed tomography imaging, confirmed by the absence of glucose metabolism on positron emission tomography. In conclusion, this case report suggests the importance of longer standardized follow-up for patients treated for testicular tumours in order to detect earlier recurrence, which can be successfully treated.
Keywords: Late relapse, Retroperitoneum, Stage I seminoma
Key Summary Points
| Stage I seminoma has a very good prognosis, with a survival rate about 99%. |
| Standardized seminoma follow-up, including laboratory and radiological exams, lasts up to 5 years after diagnosis. |
| Late (> 2 years) and very late (> 5 years) relapses of testicular cancer are rare, but not impossible, especially for seminoma. |
| We reported a case of a Caucasian man with metachronous bilateral testicular seminoma that developed retroperitoneal seminoma relapse 23 years after first surgery, successfully treated with chemotherapy. |
| We suggest the necessity to personalise standardized follow-up for testicular cancers, according to risk factors (primary tumour size and rete testis invasion). |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13603853.
Introduction
Testicular cancers are divided into seminomatous and non-seminomatous germ cells tumours, characterized by different therapeutic strategies [1]. Stage I seminoma represents 40–45% of germ cell cancers diagnosed [2], and overall survival (OS) after a successful multidisciplinary approach is close to 99% [3]. However, 4–15% of these patients experience a recurrence after the initial treatment [4]. Seventy-five percent of tumour recurrence occurs within the first 2 years after surgery (early relapse), while late relapse (> 2 years) and very late relapse (> 5 years) are uncommon, even if the extent and pattern are similar between settings [5]. We report a case of a Caucasian man who developed retroperitoneal relapse of testicular seminoma 23 years after surgery.
Case Presentation
We present the case of a 67-year-old man with a history of right orchiectomy in January 1992 for a typical seminoma limited to the testicle (stage I), with intratubular malignant germ cells (in situ seminoma) in the near parenchyma (Fig. 1). The spermatic cord was free of neoplasia. A biopsy of the contralateral testicle showed in situ seminoma which, probably due to the young age, was monitored by regular sonography instead of removal by contralateral orchiectomy. From February to March 1992, the patient underwent radiation therapy on the inguinal and iliac bilateral lymph nodes (2540 cGy) and lumbo-aortic lymph nodes (2680 cGy), according to the guidelines of the time. In 1997, the patient underwent left orchiectomy for a palpable nodule in the left testicle, and the histological examination revealed typical testicle seminoma with infiltration of the albuginea, rete testis and spermatic cord (Fig. 2). Clinical and imaging surveillance were performed, according to international guidelines. In January 2020, the patient presented to our hospital with lumbar pain irradiating to the left leg, increasing in the last month. Nuclear magnetic resonance imagining (MRI) of the lumbosacral spine was performed and showed a large anterolateral left para-vertebral mass (9.9 × 8 cm), extending from L1 to L3–L4, which encased the abdominal aorta and the inferior vena cava. A staging chest and abdomen computed tomography (CT) scan confirmed the left para-aortic solid expansive mass (10 × 7 × 9 cm) (Fig. 3). Blood tests showed normal levels of alpha-fetoprotein (AFP) (2.4 ng/mL) and beta human chorionic gonadotropin (beta-hCG) (3 mIU/mL) and elevated levels of lactate dehydrogenase (LDH) (257 U/L). A CT scan-guided biopsy of the left retroperitoneal lesion was performed; the histological examination revealed fibro-adipose muscular striated tissue infiltrated by germinal neoplasia, type seminoma (SALL4+, OCT4+, PLAP−, CK AE1/AE3−, CD30−) (Figs. 4, 5, 6, 7). In addition, the patient underwent electrocardiography and cardiac ultrasound, which showed no alterations, and spirometry, which showed moderate restrictive respiratory failure. Therefore, after multidisciplinary discussion, chemotherapy regimens including TIP (paclitaxel, ifosfamide and cisplatin) or PEI (modified cisplatin, etoposide and ifosfamide) were proposed to the patient, who refused these options because of the hospitalization required. For this reason he was treated with four cycles of chemotherapy with bleomycin, etoposide and cisplatin (PEB) with a modified schedule of bleomycin (only first day of treatment) due to the respiratory failure, and with the support of granulocyte colony-stimulating factor (pegfilgrastim). Chemotherapy was administered without any complications except for mild dyspnoea, occasional hiccups and dry eye sensation. There was a progressive normalisation of LDH levels, whereas AFP and beta-hCG remained within the normal range. A chest and abdominal CT scan performed after the fourth cycle of chemotherapy showed a significant reduction in the left retroperitoneal para-aortic mass, with no further extension between left renal vessels. The residual tissue remained indistinguishable from the left psoas muscle and from the longitudinal vertebral anterior ligament (Fig. 8). Fluorodeoxyglucose positron emission tomography (FDG CT-PET) was performed and showed a minimal increase in glucose metabolism (standardized uptake value: max 2.8) of the left retroperitoneal para-aortic solid residue tissue, indicating a nearly complete response to the chemotherapy. Approximately 6 weeks later, a second FDG CT-PET confirmed complete response to treatment, and the patient was started on clinical and imaging surveillance. Written informed consent was obtained for publication of the patient’s clinical details.
Fig. 1.
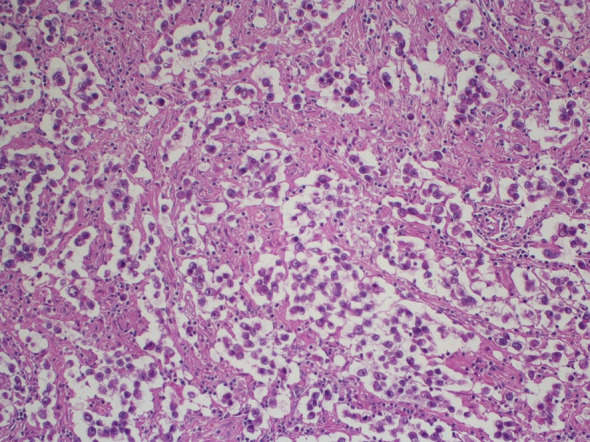
Hematoxylin and eosin staining of the orchiectomy specimen in 1992
Fig. 2.
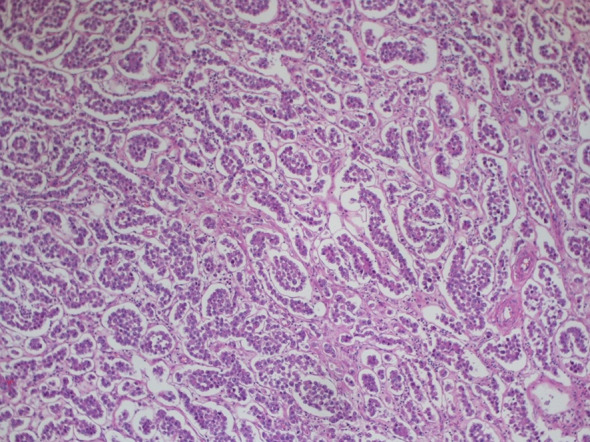
Hematoxylin and eosin staining of the orchiectomy specimen in 1997
Fig. 3.
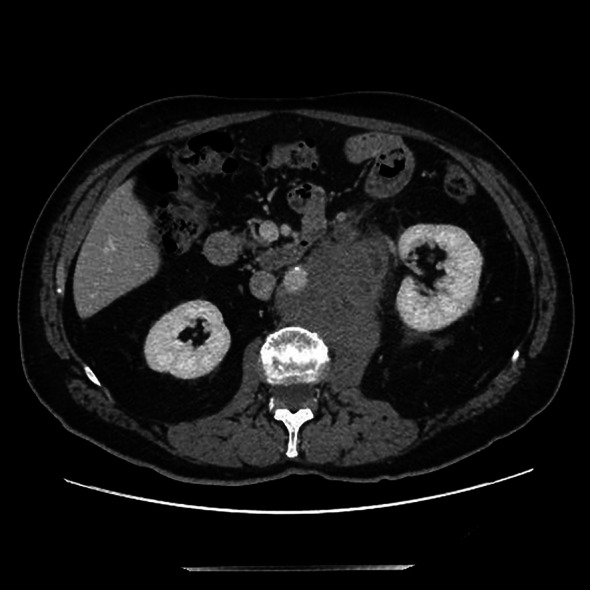
CT scan of retroperitoneal left mass at diagnosis
Fig. 4.
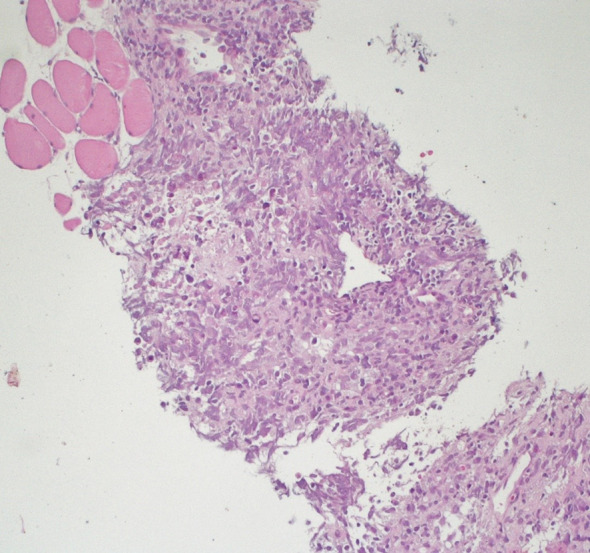
Hematoxylin and eosin staining of the retroperitoneal dissected specimen
Fig. 5.
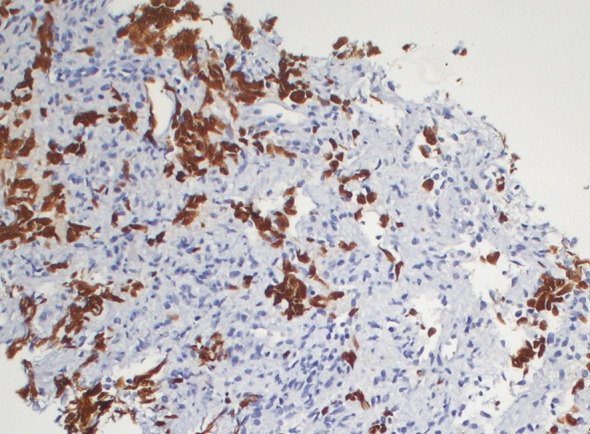
Immunohistochemical staining for SALL4 of the retroperitoneal dissected specimen
Fig. 6.
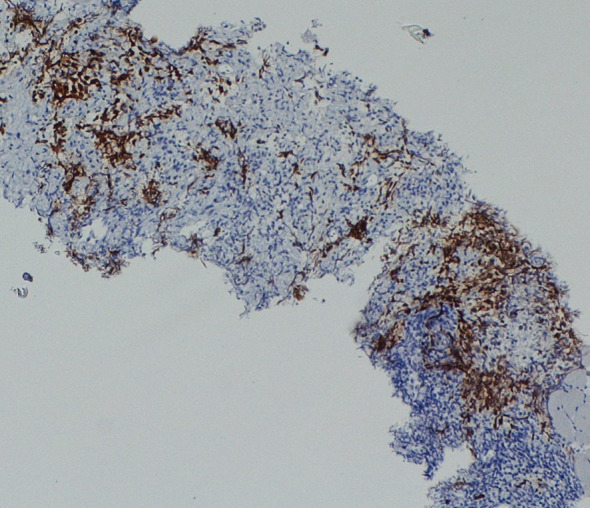
Immunohistochemical staining for OCT4 of the retroperitoneal dissected specimen
Fig. 7.
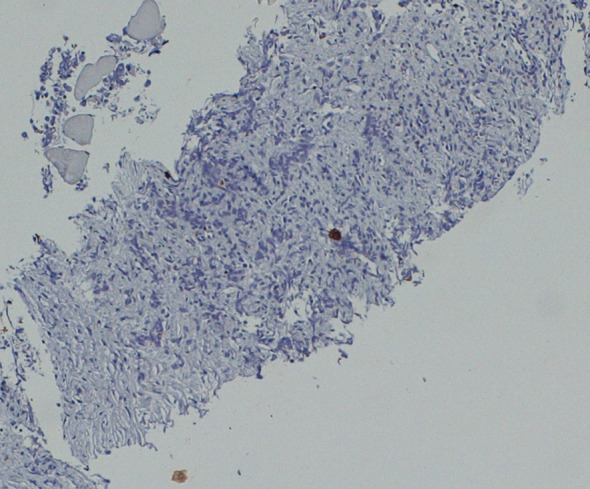
Immunohistochemical staining for CKAE1/AE3 of the retroperitoneal dissected specimen
Fig. 8.
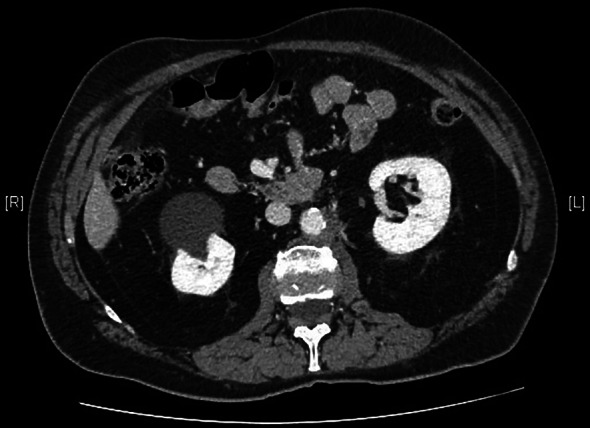
CT scan of retroperitoneal left mass after four cycles of chemotherapy
Discussion
Patients with stage I seminoma achieve a survival rate of about 99%, regardless of therapeutic strategy including adjuvant radiotherapy, adjuvant chemotherapy with one cycle of carboplatin, or surveillance [6]. Good prognosis post-orchiectomy has been confirmed in several trials: Kamba et al. performed a Japanese multi-institutional retrospective observational study on 425 patients, showing 10-year OS of 100%, 100% and 99% in the surveillance, chemotherapy and radiotherapy groups, respectively. Conversely, the relapse-free survival rates at 10 years were 79%, 94% and 94%, respectively, in the same groups [7]. The risk of relapse is highest during the first 2 years after treatment (early relapses); the annual risk rate is > 5% for surveillance and > 1% for radiotherapy or chemotherapy. However, late relapses are very uncommon and occur more frequently in the retroperitoneal space, followed by the chest or neck [8]. They are generally detected by symptoms because they occur after the conclusion of the standard follow-up, and up to half can present with elevation of tumour markers [9, 10]. The treatment of stage I seminoma late relapse is based on the previous therapy, with good prognosis. In particular, after radiation, late relapses occur mainly outside the prior radiation field, most commonly in the pelvis, with a recurrence rate of 3–4%, and are usually responsive to cisplatin-based chemotherapy. Otherwise, late relapses after single-agent carboplatin are mostly salvaged by cisplatin chemotherapy or by radiotherapy [8, 11].
Primary tumour size greater than 4 cm and rete testis invasion represent the major prognostic factors for relapse [12]. For example, Chung et al. showed an increase in the risk of relapse at 3 years from 2% for tumour size equal to or less than 1 cm, to 25% for tumour size of 6 cm or greater [13]. AFP and beta-hCG represent the common biomarkers used in the follow-up of germ cell tumours, even if the increase in AFP is typical in late-relapsing of non-seminoma neoplasms, with levels greater than 100 U/L appearing to indicate poor prognosis [14]. On the contrary, in relapses of seminoma, the level of LDH increases according to the volume of disease, especially in late relapses [15], as we found in our patient’s blood test. Several studies have evaluated differences between early and late relapses of germ cell tumours. Fedyanin et al. showed that patients with late relapse were usually older and had more frequent pure seminoma in primary tumours. Both groups showed similar prognostic factors at the time of relapse, even though patients with late relapse showed a larger size of disease, especially in seminoma recurrence. Regarding the outcome, subjects with late relapse achieved a lower response rate after chemotherapy, 3-year progression-free survival (PFS) and 3-year OS, and prognosis seems even worse in the case of pure seminoma [15]. On the contrary, Mortensen et al. analysed 2000 cases of seminoma and revealed no significant differences in disease-specific or overall survival between early, late and very late groups, although increased time to relapse was negatively associated with survival [16]. The role of the time to relapse is not clear, and the literature data are conflicting, due to the rarity of testicular disease. Regarding advanced disease, the prognosis of patients relapsing after cisplatin-based first-line treatment is variable, with 2-year survival rates ranging from 75% (very low risk) to 6% (very high risk). In these cases, salvage treatment is based on high-dose chemotherapy or conventional-dose chemotherapy, without significant differences in OS or PFS. Considering the variability in chemotherapy response, surgery should be conducted whenever possible, especially in localized relapses and in chemotherapy-refractory patients [17, 18].
Finally, our case report underlines the importance of an individualised approach to prolong duration of follow-up in patients treated for testicular cancer, considering the patient’s age and the primary risk factors (rete testis invasion and tumour size), due to the possibility of disease relapse even after many years from the initial treatment. Moreover, these patients have an increased risk for developing secondary malignant neoplasms following radiation exposure from diagnostic imaging and due to radiotherapy, which is correlated with increased risk of developing both solid tumours, leukaemia and cardiovascular diseases [19, 20].
Conclusion
We reported a case of very late retroperitoneal relapse of pure seminoma successfully treated with chemotherapy. Even if there is not a standardized long follow-up for testicular cancers, our case suggests that, according to risk factors such as size of primary tumour and rete testis invasion, patients treated for testicular cancers need to be monitored longer to detect earlier recurrence and/or secondary malignant neoplasms.
Acknowledgements
We thank the participant of the study.
Funding
No funding or sponsorship was received for publication of this case report.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Sonia Crocetti, Laura Tassone, Mariangela Torniai, Chiara Pierantoni, Luciano Burattini, Alessandra Mandolesi, Maika Di Benedetto, Giovanna Mantello and Marina Scarpelli have nothing to disclose. Rossana Berardi is an Editor-in-Chief of Oncology and Therapy but has nothing else to disclose.
Compliance with Ethics Guidelines
Written informed consent was obtained for publication of the patient’s clinical details.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analysed during the current study.
Footnotes
Sonia Crocetti and Laura Tassone contributed equally to this case report.
References
- 1.Honecker F, Aparicio J, Berney D, et al. ESMO consensus conference on testicular germ cell cancer diagnosis, treatment and follow-up. Ann Oncol. 2018;29(8):1658–1686. doi: 10.1093/annonc/mdy217. [DOI] [PubMed] [Google Scholar]
- 2.Mead GM, Fossa SD, Oliver RT, et al. Randomized trials in 2466 patients with stage I seminoma: patterns of relapse and follow-up. J Natl Cancer Inst. 2011;103(3):241–249. doi: 10.1093/jnci/djq525. [DOI] [PubMed] [Google Scholar]
- 3.Chung P, Warde P. Stage I seminoma: Adjuvant treatment is effective but is it necessary? J Natl Cancer Inst. 2011;103:194–196. doi: 10.1093/jnci/djq535. [DOI] [PubMed] [Google Scholar]
- 4.Warde P, Specht L, Horwich A, et al. Prognostic factors for relapse in stage I seminoma managed by surveillance: a pooled analysis. J ClinOncol. 2002;20(22):4448–4452. doi: 10.1200/JCO.2002.01.038. [DOI] [PubMed] [Google Scholar]
- 5.Hosni A, Warde P, Jewett M, et al. Clinical characteristics and outcomes of late relapse in stage I testicular seminoma. ClinOncol (R CollRadiol) 2016;28(10):648–654. doi: 10.1016/j.clon.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 6.De Wit R, Fizazi K. Controversies in the management of clinical stage I testis cancer. J ClinOncol. 2006;24:5482–5492. doi: 10.1200/JCO.2006.07.9434. [DOI] [PubMed] [Google Scholar]
- 7.Kamba T, Kamoto T, Okubo K, et al. Outcome of different post-orchiectomy management for stage I seminoma: Japanese multi-institutional study including 425 patients. Int J Urol. 2010;17(12):980–987. doi: 10.1111/j.1442-2042.2010.02645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oldenburg J, Wahlqvist R, Fosså SD. Late relapse of germ cell tumors. World J Urol. 2009;27(4):493–500. doi: 10.1007/s00345-009-0411-3. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Daneshmand S. Modern management of testicular cancer. Cancer Treat Res. 2018;175:273–308. doi: 10.1007/978-3-319-93339-9_13. [DOI] [PubMed] [Google Scholar]
- 10.Shahidi M, Norm AR, Dearnaley DP, et al. Late recurrence in 1263 men with testicular germ cell tumors. Multivariate analysis of risk factors and implications for management. Cancer. 2002;95(3):520–530. doi: 10.1002/cncr.10691. [DOI] [PubMed] [Google Scholar]
- 11.Beck SDW. Optimal management of testicular cancer: from self-examination to treatment of advanced disease. Open Access J Urol. 2010;2:143–154. doi: 10.2147/OAJU.S7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel MI, Motzer RJ, Sheinfeld J. Management of recurrence and follow-up strategies for patients with seminoma and selected high-risk groups. UrolClin North Am. 2003;30(4):803–817. doi: 10.1016/S0094-0143(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 13.Chung P, Daugaard G, Tyldesley S, et al. Evaluation of a prognostic model for risk of relapse in stage I seminoma surveillance. Cancer Med. 2015;4(1):155–160. doi: 10.1002/cam4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dieckmann KP, Albers P, Classen P, et al. Late relapse of testicular germ cell neoplasms: a descriptive analysis of 122 cases. J Urol. 2005;173(3):824–829. doi: 10.1097/01.ju.0000154013.96349.36. [DOI] [PubMed] [Google Scholar]
- 15.Fedyanin M, Tryakin A, Kanagavel D, et al. Late relapses (>2 years) in patients with stage I testicular germ cell tumors: predictive factors and survival. UrolOncol. 2013;31(4):499–504. doi: 10.1016/j.urolonc.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Mortensen MS, Lauritsen J, Kier MG, et al. Late relapses in stage I testicular cancer patients on surveillance. EurUrol. 2016;70(2):365–371. doi: 10.1016/j.eururo.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Oldenburg J, Fossa SD, Nuver J, et al. Testicular seminoma and non-seminoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(6):125–132. doi: 10.1093/annonc/mdt304. [DOI] [PubMed] [Google Scholar]
- 18.Margolin K. Management of advanced germ cells cancer in patients with unfavorable prognosis. J NatlComprCancNetw. 2005;3(1):77–83. doi: 10.6004/jnccn.2005.0004. [DOI] [PubMed] [Google Scholar]
- 19.Curreri SA, Fung C, Beard CJ. Secondary malignant neoplasms in testicular cancer survivors. UrolOncol. 2015;33(9):392–398. doi: 10.1016/j.urolonc.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Vossen CY, Horwich A, Daugaard G, et al. Patterns of care in the management of seminoma stage I: results from a European survey. BJU Int. 2012;110(4):524–531. doi: 10.1111/j.1464-410X.2011.10887.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analysed during the current study.


