Abstract
Background:
COVID-19 ongoing pandemic has proved beyond doubt that all countries in the world from high income to low- and middle-income countries were unprepared with under-diagnosed and underreported losses of precious human lives on already overstretched healthcare delivery infrastructure. Thus, the urgent need of the hour is to understand and identify the operational issues and challenges encountered in the sample collection process and also at the testing labs in order to respond at the earliest. This early and effective response will help not only to address the identified issues in the whole chain of sample collecting to test result communication but also it will help to improve the functioning of the entire system involved in this process.
Objectives:
The present study was undertaken to identify the issues faced during various steps involved in laboratory testing as part of the COVID-19 control activities in selected remote districts of North East and East India. Further, perceived adequacy of human resources, equipment, diagnostic kits, and other essential consumables including PPEs vis-a-vis the load of samples received from the catchment areas of the testing laboratories were also explored.
Methods:
The study was a qualitative research using in-depth interview method to collect and collate the data from the chain of personnel involved in sample collection, storage, transportation, and testing by recorded telephonic interview by state-level collaborators as per the study protocol. The respondents were recruited from randomly selected sites of remote districts for sample collection, storage, transportation, and dedicated testing labs in six states of North East and Eastern India. The study findings were analyzed by two-dimensional scaling and hierarchical cluster analysis to get the collective picture involving transcription, preliminary data scrutiny, content analysis, and interpretation of the verbal IDI; classified and summarized by triangulation; free listing and pile sorting of suggestions.
Results:
The entire laboratory testing related human resources has been working on war-footing round-the-clock to fulfil the expectation of the stakeholders and maintaining high quality despite the ever-increasing load of sample testing in both the public and private sectors. The findings indicated that the healthcare workers from all levels of laboratory diagnosis have taken it as a challenge to control the pandemic even with limitations of logistics to capacity building. Positive suggestions to improve laboratory services were to increase human resources, infrastructure, IT with the robust mechanism of monitoring and supervision.
Conclusions:
Upgradation of laboratory capacities and expertise in public health has become one of the points of concern to contain the COVID-19 pandemic of the new millennium.
Keywords: Collection, COVID-19, laboratory testing, storage, transportation
Introduction
The COVID-19 was declared as a global pandemic by the World Health Organization on March 11 2020.[1] Globally it is affecting 216 countries with approximately 100 million confirmed COVID 19 cases leading to more than 2 million deaths reported to WHO till last week of January 2021.[2] COVID-19 cases present with a variety of symptoms from asymptomatic carrier to symptoms viz. fever, cough, myalgia, dry cough, headache, hemoptysis, diarrhoea, dyspnoea, and in very severe cases, acute respiratory distress syndrome.[3] The role of quality and quantity of the laboratory tests in early diagnosis and capacity building for prompt treatment come into play to halt this dreaded pandemic.[4] All these should strictly follow the national and international precautions with technical and safety specifications.[5] COVID-19 ongoing pandemic has proved beyond doubt that all countries in the world from high income to low- and middle-income countries were unprepared with under-diagnosed and underreported losses of precious human lives on already overstretched healthcare delivery infrastructure[6]
In the scenario mentioned above, the current study was undertaken to understand the various challenges faced by the healthcare workers (HCW) during sequential steps involved in the testing of the samples. It was including the activities involved in the collection, storage, transportation to the testing of COVID-19 samples in selected remote districts of EAG states of North East and Eastern India. Also, the study tried to explore the adequacy of resources, equipment, kits, and other essential consumables by in-depth interview (IDI) of personnel working in this laboratory chain of functioning.
Methods
In this COVID-19 pandemic, the present qualitative research study was conducted to explore operational challenges faced by the HCWs including doctors at the healthcare delivery sites where samples are collected, stored, transported, and at designated testing laboratories where samples are tested. The data was collected by IDI undertaken using structured guidelines to explore the operational issues faced by HCWs from the following sites:
District-level sample collection sites for COVID-19 suspects
District-level sample storage and transportation facilities to the designated laboratories
Laboratory test of sample, document, and intimate test results to sample collection network
Overall perspectives of the operational issue from District Program Managers (DPM)
Experience on COVID-19 sample collection, storage, transportation, and testing by Polio programme infrastructure with the help of the Polio Surveillance Medical Officer.
STUDY PROTOCOL: The project protocol had clear guidance for the interview process for investigators/collaborators (as the interviewer) and participants/respondent (as interviewee); including “Audio-Visual Verbal consent.” Ethical clearance was obtained from the Institutional Ethics Committee of SMIMS, Gangtok. Structured guideline prepared for IDI attempted to assess ground level problems and practical difficulties regarding sample collection, storage, transportation, and testing. “IDI” was recorded over electronic devices in a real-time manner and submitted to Principal Investigator for further analysis.
SAMPLE SIZE AND SAMPLING METHOD: Study sites were randomly selected from remote districts [156 districts] in EAG states of Assam and Sikkim in North East India and Bihar, Odisha, Chhattisgarh, and Jharkhand in Eastern India where samples were collected, stored, transported. Respondents were HCWs engaged in sample collection and transportation (One each) from one site of the selected district, District Program Manager (DPM), Health personnel involved in Polio surveillance like Surveillance Medical Officer (SMO)/District Immunization Officer (DIO), In-charge of the designated Government or private COVID-19 testing laboratory receiving samples from the selected district sites as per approved protocol.
DATA COLLECTION: All respondents were digitally communicated with self-explanatory “Subject Information sheet” along with “Informed consent form” were informed that recording of the whole telephonic interview would be done starting with the “Verbal Consent.” After the formal introduction of the interviewer with credentials, the IDI was done by virtual digital platform from the selected HCWs. Adequate information was furnished in a self-contained manner to enable the investigator to assess the project by five sets of structured interview guidelines for HCWs from Laboratory technician, In-charge of store and transportation, DPM, Polio workers/officers, and In-charge of the testing Labs.
Data analysis
All the 28 telephonic IDIs collected as recorded audio were transcribed and translated from local language (if any) into English; close to verbatim; transcripts were manually coded, and any discrepancies in the coding were sorted out after discussion. Statements in italics indicate respondent verbatim. Overall qualitative data were analyzed by two-dimensional scaling and hierarchical cluster analysis to get the collective picture. Finally, a free listing exercise using Smith's Salience value and pile sorting exercise of suggestions given by respondents were conducted using Visual Anthropac, version 1.0 software [ANTHROPAC: Analytic Technologies]. Thus, emerging themes were identified for suggestions. Lastly, debriefing of the findings of the free list, pile sorting, and IDI to the participants was done to increase the credibility of the results.
Results
Overall 28 telephonic IDIs explored the operational issues faced by HCWs involved in different activities as well as different tiers of the health system; six laboratory technicians (LT) involved in sample collection; six involved in storage/transportation of sample; six DPMs; six laboratory in-charge from designated Govt. laboratories while two from private labs; two WHO–SMO involved in Polio surveillance. A triangulation of qualitative methods like IDI of key respondents, free list, pile sort exercise were undertaken to increase the validity of results. The findings have been discussed in five parts for the results concerned with the operational issues faced by HCWs involved in different sample related activities viz. A. Collection, B) Storage and transportation, C) Lab-in-charge of designated laboratory, D) DPM, E) SMO. Major problems highlighted by the respondents were summarized in search of “How it could have been better?”
A) COVID-19 sample collection
There was an adequate supply of the forms for the sample collection in all the selected sites; some were directly entering data in the online form. There was a shortage of PPE in the initial days, however, supply for decent quality PPE in adequate amount were ensured later. Currently, no such issue was observed anywhere. Virus transport media (VTM) and Nylon swabs for sample collection were adequate in all the facilities at present. The respondent from Chhattisgarh faced problem because of lack of personal identity-related documents of COVID-19 suspects initially; matters were resolved immediately. Respondents vowed cooperation from the patients mostly during sample collection; some were agitated because of delay or left the place without giving a sample—in such cases HCW had to contact again. Initially, in some spots, designated collection site was lacking that caused problem of the collection; patient cooperation gradually increased over time. The human resource was initially major issues for collection; resolved presently in the majority of the sites. The respondent from Assam, said, “at the beginning, adequate manpower was there; some were deputed at Guwahati currently leading to more workload for us.” Adequate instruments, logistics were available in most of the places; yet sterilization was an issue. Some proactive collectors carried sterilization equipment to the field with reported lack of appropriate logistics like sodium hypochlorite solution. The respondent from Assam was having few dissatisfactions regarding the sterilization of working space in initial days coordination with the hospital administration was good in all the sites; poor internet network connection, and landslide/transport issue sometimes hampered the coordination in Sikkim.
Most of the respondents faced lesser stigma after initial resistance; instead, people seem to encourage and praise HCWs who were motivated enough with the pride of doing something for the national interest. However, the respondent from Sikkim reported, “Seeing some agitated people without any mask in containment zone during sample collection, I thought that I might contract infection this time. I didn't go home voluntarily as I thought I might transmit the infection to others.”
Adequate training for donning and doffing had been imparted to the HCWs as reported by most of the respondents. “More training would be helpful,” respondent from Bihar remarked.
Designated area for sample collection was available in most of the places and through glass kiosk in few sites. Accredited Social Health Activist (ASHA), Auxially Nurse Midwife (ANM), Anganwadi Worker (AWW) helped in arranging area and mobilizing people for outreach collection. Separate room for sample collection was required in selected facility in Chhattisgarh.
HCWs asked for contact details, symptoms, travel history, contact history, and then counselled and briefed the subject and obtained informed consent before the procedure. All respondents reported that they are adequately trained for collecting both nasopharyngeal and oropharyngeal swabs. Some difficulties in sample collection were encountered by them in cases when sometimes blood comes while collecting the sample or subject may have cough/sneezing or the sample provider has a deviated nasal septum or rhinitis.
Clear cut SOP was available regarding filling up of forms, registration, sample collection, testing, reporting, contact tracing, etc., in all of the facilities. Biomedical wastes generated during sample collection have been disposed of properly. The respondent from Sikkim said that “we are using sodium hypochlorite solution after Rapid Antigen Test (RAT) disposing of PPE in red bag and masks in the yellow bag.”
SUGGESTIONS TO IMPROVE SAMPLE COLLECTION
Reverse isolation, that is, isolation of negative persons may be applied if the positivity rate is very high in any area;
More human resource will be required if the caseload increases beyond capacities;
The report should be available early to control the infection as well as to address repeated query from subjects;
Improvement of sample collection area ensuring cleanliness, continuous electric supply, etc., Designated glass shield kiosk to be constructed in every site. Separate room for registration, collection and donning-doffing required everywhere;
The crowd should be managed efficiently so that they do not get restless or angry.
B) Storage and transportation of sample
One Jharkhand respondent stated - no dedicated refrigerator to store samples and hence storing in vaccine carrier and transporting within 4 h to the testing lab. The facility from Bihar lacked dedicated refrigerator for storage; sending 100 samples daily to the testing site for RT-PCR and on rest of the samples performed RAT in-house. A respondent from Sikkim said, “We are storing sample for maximum one night in the normal refrigerator, sending samples next day morning to the testing lab.” Selected Chhattisgarh district in-charge told the same, they were storing sample overnight in vaccine carrier with an icepack. Assam and Odishahad a good deep freezer with 24 h of electricity, inverter and generator facility for storage.
Coordination between hospital administration and sample collection team was excellent; others coordinated among themselves using the WhatsApp group. The respondent from Bihar told shortage of Lab Technician (LT) and Doctor. Shortage of PPE was there in earlier days, but now most of the facilities are having adequate numbers of PPE. Perception regarding the quality of PPE was mixed, ranging from bad to very good. Adequate number of container/forms/cold chain maintenance equipment was available in all selected districts. Shortage of vaccine carrier and icepacks were reported by the respondent from Assam when caseload started to increase, but now it is resolved. All the selected districts have dedicated vehicle for transportation along with required human resources. Human resource was a major issue reported by most respondents; rapid increase in sample load requires more human resources; HCWs were being deputed in some sites from other departments or programs (e.g. RNTCP, Malaria) to solve this crisis. Staffs related to storage and transportation received training from an appropriate authority like District Epidemiologist, WHO–SMO, trained Microbiologist, etc., Virtual training like video conference had been arranged in Assam. All the selected facilities were following guidelines and standard operating procedures (SOPs) regarding sanitization, donning–doffing, VTM and label management, BMW management, etc., as circulated by their state or district IDSP office though written SOPs were absent in some of the areas. Stigma was still prevailing in the community as well as among health staffs according to the respondents. Respondent from Sikkim told that “some of the hospital staffs itself were scared of me as I am in COVID team.” Some of the respondents faced discrimination from the community initially. Personal apprehensions like transmitting the infection to family members also were there among the respondents. Respondents from Odisha said that “family members of positive patient think others will discriminate them from society, even after recovery.”
Few respondents reported that initial stage of management they face problem because of the frequent change of form and guideline. The respondent from Odisha told that “earlier protocol had asked that every positive patient should be kept in institutional quarantine, but now guideline say asymptomatic cases can be kept in home isolation- causes the problem and community residents become agitated and angry to us.”
However, the respondent from Jharkhand supported the change as it is for betterment of service.
SUGGESTIONS TO IMPROVE:
Need a dedicated team in each district to manage such pandemic in future;
Train and counsel HCWs to disseminate health education and awareness to the society;
Require more human resources with the gradually increasing caseload. Existing workforce is overburden and not getting a break in between.
C) Designated laboratory in-charge (GOVERNMENT and PRIVATE LAB)
Designated laboratories were not available in beginning in few states, for instance in Sikkim, sample were being referred to Central Laboratory of North Bengal Medical College in West Bengal, as Sikkim got its designated COVID lab in July 2020; designated laboratory of Kishanganj district of Bihar was initially Central Laboratory of Patna Medical College but during the study period, it got changed to Darbhanga Medical College.
There is no problem of availability of kits at most sites; however, initially, there was the problem of RNA extraction kit in most of the areas. Two laboratory in-charges reported problem on the supply of PPE, gloves, other consumables. Lab in-charge of a private hospital in Jharkhand said: “we are lacking kit for automated extractor despite having the instrument” [Table 1].
Table 1.
Catchment area, burden of work and average reporting time of selected Lab
| States | Catchment area | Burden of load (samples/day) | Average reporting time |
|---|---|---|---|
| Bihar | Kishanganj, Purnia and Darbhanga district | 700; capacity 300 | Within same day of sample collection |
| Jharkhand (Govt.) | Varible; mainly Ranchi, Dumka, Palamu, Koderma, Hazaribag, Deoghar etc. | 400; even 100-1400 | -do- require more time if sample load is more |
| Jharkhand (Private) | Entire Jamshedpur | 250-300 | -do- |
| Odisha | Mainly Cuttack district; from rest of the districts if required | 900-1200; target 1800-2000 | 3.5 h |
| Sikkim | Entire Sikkim | 300; even upto 2000 | 12-48 h |
| Chhattisgarh | Institute itself and four other districts | 800-900; but now decreasing due to more pool positivity | 24-48 h; in emergency case within 12 h |
| Assam (Govt.) | Seven districts | 700 | 6-7 h |
| Assam (Private) | Kamrup, Barpeta, Nalbari | 75-100 | 4 h |
Most of the labs were not using thermocyclers and gel electrophoresis equipment. The regular supply of good quality PPE for HCWs involved in the sample collection process was available in adequate number in most of the facilities; quality improved since April. Issues cropped upon the availability of gloves in Chhattisgarh; only 6.5 size gloves were available [Table 2].
Table 2.
Availability, functionality and adequacy of other related equipments in selected Lab
| States | Instruments available | Remarks |
|---|---|---|
| Bihar | 5 RT-PCR, 4 centrifuges and vortex mixture, 3 BSC, 2 deep freezer, 7 freezer, generator | Functional and adequate |
| Jharkhand (Private) | 1 RT-PCR, 2 UPS, 5 centrifuges, 2 vortex mixture, 2 BSC, 1 deep freezer, 1 freezer, others also O.K. | Mostly functional and adequate; availability issue of pipette and PCR Tubes; 1 BSC procured- yet to install |
| Jharkhand (Govt.) | 3 RT-PCR, 3 UPS, 2 centrifuges, 3 vortex mixture, 3 BSC, 1 deep freezer, 6 freezer, others available | All are functional. Require more centrifugers and BSC |
| Odisha | 2 RT-PCR, 2 centrifuges, others also available | Functional and adequate |
| Sikkim | 2 BSC, others available | Functional and adequate. Require 1 more BSC. |
| Chhattisgarh | 3 RT-PCR, 4 centrifuges, 3 BSC, UPS, vortex mixture, gel electrophoresis, deep freezer, freezer, consumables also available | All centrifuge functional except 1; Need more freezer and deep freezer. Shortage of plastic ware- instead of automated, using manual extractor; procurement of these is a challenge - current funds are insufficient. |
| Assam | 3 RT-PCR, central UPS, 3 centrifuges, 3 vortex mixture, 4 BSC, 4 deep freezer, others O.K | 1 RT-PCR is non-functional; shortage of pipette tips and PCR plate. |
| Assam (Private) | 1 RT-PCR, central UPS, 2 centrifuges, 1 vortex mixture, 2 BSC, 1 deep freezer, 8 freezer, others also available | Functional and adequate |
RT-PCR=Reverse transcription polymerase chain reaction; BSC=Biological Safety Cabinets; UPS: Uninterruptible power supply
Responses were mixed on human resource—satisfactory to vowing for more. Respondent from Bihar and Odisha: “require more as existing workers doing job since March without holiday.” Shortages of data entry operator (DEO) lead to huge backlog in some areas. Lab-in-charge of Bihar said “sometimes one sample collection centre collect sample for 3 days without sending and at one go sent all samples. I doubt whether they store samples properly.” Otherwise no issues with regard to coordination with sample collection. Most respondents had good coordination with state health as well as ICMR. Chhattisgarh respondent: “Sometimes target for district from State is very high. Clinicians from hospital sometimes sending sample for follow up testing without following the guideline leading to backlog of samples.” Slow internet speed hampered coordination with State Health and ICMR in some cases. Govt. lab in-charge from Assam asked fund from ICMR for internet connection, purchase of consumables, etc. Reportedly, ICMR portal sometimes ran very slow leading to backlog of uploading data. Poor network connectivity and scarcity of DEO added to data backlog; private labs were only uploading test results in ICMR portal. Initially due to lockdown, procurement process of kits and consumables was an issue everywhere, later on streamlined; yet minor issues existed. Chhattisgarh lab in-charge reported procurement delay through hospital supply chain. In Sikkim, laboratory has to procure consumables from Kolkata by them without much coordination with State government.
One respondent reported fear of psychosis to be still very high even among senior faculty members. Few LT were asked to stay in separate accommodation by their neighbors in Odisha. Relatives and friend asked one laboratoryin-charge “don't come close.” Respondent from Chhattisgarh was apprehensive: “my son play with friends, their parents may object.”
Laboratory personnel of all the testing sites had received adequate training for the conduct of tests. Manufacturing company personnel also guided them regarding technical operation. Clear SOPs had been developed for different activities viz. what to wear, kit criteria, extraction, processing, Bio Medical Waste (BMW) management, etc. Frequent change of guideline was a hindrance for this SOP development. Regular record maintenance was an important component, and all the testing sites were performing to their best. District-wise data was being kept both in soft and hard copy formats in Bihar. The respondent reported a massive backlog of adaptation of data in ICMR portal from Sikkim because of lack of DEO. The respondent from Odisha said, “after doing the test, we wait for 1-1.5 hour for data to be updated with single computer and single DEO.”
Biomedical wastes were disposed of by respective hospital authority following the guideline after segregation though they had asked for more ICMR support on this. Autoclaving and incineration were being done wherever the facility is available. A large weighing machine was urgently required for disposal of wastes in the selected government laboratory in Assam.
SUGGESTIONS TO IMPROVE:
a. Concerned authority (District, State, ICMR, etc.) to be made aware of the capacity of the testing laboratory and must be considerate to provide feasible target; otherwise with a huge backlog, the sample has to be stored leading to degradation; also this hindered sample collection and documentation; b. Laboratory infrastructure development with more space, more LT, DEO, sanitation workers, more equipment and consumables, computer with high speed internet facility required for smooth functioning; c. Targeted testing required as lot of resources got wasted because of asymptomatic and VIPs/referred by VIPs cases; d. Fast and streamlined purchase of consumables was the need of the hour; e. Regular payment for the concerned staffs must be ensured to keep them motivated. In new recruitment, hike of salary for the staffs might be considered; f. Extensive counseling and IEC was needed to reduce stigma; g. COVID-19 testing sites must be BSL-3 category like Swine flu as chance of infection to HCWs more in BSL-2 lab; h. Periodic inspection and feedback by competent authority to different laboratorywill motivate and encourage the whole team.
D) District program managers (DPM)
Initially, logistics viz. PPE, N95 mask, sanitizer, VTM kit, and trained manpower with available designated COVID hospital was issued. A DPM reported “we had faced lack of co-operation from the villagers in some places during contact tracing,” however, later on it became streamlined and they shared good coordination with hospitals, testing sites, State health authorities, ICMR, etc. DPM from Chhattisgarh raised an issue of delay in reporting for last 1 month although testing laboratory was just 90 km away. Some of DPMs narrated that they were lacking adequate number of DEO which was posing hindrance to daily online updatation. DEO got infected in one district of Odisha causing problems in documentation. Rest of the sites had no issues with record maintenance till now.
Majority of the interviewees were comfortable with the reporting in online portal. In Bihar, DPM told that earlier they had to enter data on ICMR and State Govt. portals which was cumbersome. Nevertheless, now for the last 2 weeks, both portal have been merged making it easier for the DEO. DPM of one of the remotest district in Odisha reported that samples took 14–16 h to reach ICMR laboratory because of adverse road condition; they also added that non-availability of continuous internet connection is posing as a barrier because of extreme climatic situation. DPM in Sikkim told “DEO of IDSP is the only person doing the job but as she has to enter data for all other program, she is over burdened.”
Procurement and disbursement of PPEs, VTM kits, other essential consumables were a problem initially but later on it was regularized everywhere. In Chhattisgarh, there are some shortage of gloves and TrueNAT kit in a few selected districts. However, PPEs were of acceptable quality.
Samples from the inmates of quarantine centers were collected with by a team comprising of LT, DEO, sanitary worker, etc. In some of the areas migrant workers initially refused to give sample. According to one interviewee, “Good interpersonal communication over telephone, prior motivation and counseling to the inmates of quarantine centre made the process smooth.” In few areas, DPM faced problem in transportation for sample collection or referral of positive patients as private car owners refused to send vehicle. Lack of trained manpower was also an issue earlier in several states. Staff in one of the districts made innovation by creating a hole over the glass of the vehicle for hassle-free and safer sample collection. Biomedical waste generated was either outsourced by dedicated authorized team after collection of samples at the quarantine centers or disposed in safety pit after disinfecting with sodium hypochlorite. In this regard, none of the districts reportedly faced any difficulties. For reuse of PPE kits, the states were not provided with any specific guidelines initially. It was observed that all the centers were discarding the kits after using that for 6–7 h. Staff members in Sikkim were trained on donning/doffing of PPE though a video conference from AIIMS, New Delhi. DPM of Chhattisgarh told, “We have guideline from state on reusing of goggles and face shield but not PPE.” In Assam, one district nodal officer informed that they are instructing health personnel to reuse N95 mask after 72 h.
District task force conducted sample collection drive twice to achieve 97% target in Jharkhand. Rapid Antigen testing (RAT) was done on symptomatic patients in Odisha; if positive, patients were asked to remain at home or institutional isolation depending upon their condition. Negative RAT sample were further tested by RT-PCR and positive reports were managed as per guideline. Contact tracing and testing was done following stipulated guideline. Regular training of HCW was conducted in Jharkhand and Odisha since COVID-19 pandemic was declared. Authorized Medical officer provided training to ANM, ASHA, Staff nurse, Pharmacist, AYUSH doctors whenever required. In Bihar and Chhattisgarh, online training was given to concerned personnel on various activities. Faculty from different medical colleges were designated as resource persons for training in Assam scheduled for 7 days. Initially, the lockdown hampered training in Sikkim but the challenge was overcome by conducting telephonic training. Everyone opined that after initial training the workers had learnt by doing the actual activity and by experience.
Clear SOPs were available from ICMR and respective state to the concerned district mentioning sampling, collection, testing, biomedical waste management protocols. DPM in Odisha even told that they had developed SOPs for future plasma collection. Each selected district was getting regular feedback from higher authorities via WhatsApp and they were cooperating in identifying problem if any and immediate measures were taken to sort them out. For instance, a few selected districts of Chhattisgarh were unable toachieve the target of RAT as per DHS, but, once they received the feedback from the concerned authorities they worked on increasing the testing drive and were able to achieve the testing targets.
SUGGESTIONS TO IMPROVE:
a. More human resource recruitment at grassroots, LT, and DEO is needed; b. In one district, doctors were being asked to remain in quarantine for 7 days after doing duty for 7 days despite negative confirmed reports within 2 days of duty completion. This issue needed to be addressed to resolve manpower issue; c. merging of all online portals will make the data entry process much smoother; d. Some DPMs urged for more supply of kits for RT-PCR, TrueNAT, RAT to perform more test, this demand may be considered for achieving the testing target within a specific timeframe; e. Reduction of delay in reporting can be helpful to control the pandemic; f. Proper infrastructure and system development to be done before converting any hospital to Pre-COVID/COVID hospital; g. Improvement of commutation especially with respect to transportation of samples from remote areas may be regulated in lockdown and unlock situations, so that the samples reach the testing sites early and do not degenerate; h. Stigma was still prevailing in the community specially in remote areas; so extensive IEC along with health education and training of ASHA, ANM, AWW, School teachers and even cured patients might be very helpful in this regard; i. Regular incentives for all types of workers must be ensured as there were reports that the contractual workers in some places did not receive their salary; j. Increasing the financial aid allocated for diet of positive patients from existing Rs. 60/- has been requested by one of the selected DPM.
E) WHO – SMO involved in polio surveillance
AFP and MR surveillance activities were very much affected in this pandemic. Total number of stool and serum sample collection has drastically gone down compared to pre-COVID-19. Major difficulties faced were: a. Stigma – in spite of sufficient manpower, lack of motivation among HCWs was barrier to sample collection. One respondent said “if the person for sample collection is unavailable due to any reason, others were not motivated enough to collect sample; people used to run away”; b. Transport problem - streamlining collection of sample, processing, transportation were major challenges due to lockdown as movements were restricted. Even sample couldn't be sent to laboratory for 2–3 months because of lack of transportation facility in one state. Fortunately, everything is back to normalcy currently; c. Technical difficulty—sometimes sample collector were not sound enough to collecttechnical things viz. name, address, sample id, etc., causing reporting problems; d. No issues were reportedwith testing laboratoryas they were WHO accredited with standard protocol including strict maintenance of cold chain. Once the sample reached the lab, there was no delay in reporting even in this pandemic situation.
SUGGESTIONS TO IMPROVE:
a. Decentralized approach: This was very helpful as concerned block was intimated and pursued to send vehicle on the day of data collection. Laboratory technician went to the field in that vehicle and came back after collecting sample. Thus, sample collection and transport issues were resolved; b. Government ownership: AFP and MR surveillance activities along with CoVID-19 management may be prioritized by the concerned authorities, as sustaining good quality of surveillance activity will prove to be beneficial in control and eradication of these diseases; c. Standardized sample collection form/sheet will smooth the process; d. Continuous and regular supply of vaccine carrier, icepack and other equipment must be ensured as they need to be discarded after single use after carrying COVID samples; e. Financial incentive or certification from state or central health authorities recognizing the contribution of the HCWs might be a motivating factor for them which will encourage and boost their morale.
The two-dimensional scaling and hierarchical clustering showed one independent and three subgroups of problems. Independent factor was stigma [Figure 1].
Figure 1.
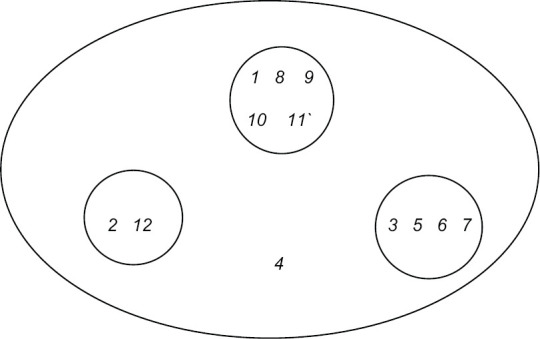
Cognitive Map with Two dimensional scaling and hierarchical cluster analysis of problems faced by Key respondents. 1. Lack of sample collection kiosk, 2.Less manpower, 3. Irregular sanitation, 4. Stigma, 5. Poor network connection, 6. Transportation problem, 7. Lack of dedicated refrigerator, 8. Average quality PPE, 9. Frequent change in guideline, forms, portal, 10. Irregular supply of consumables, 11. BMW disposal, 12. Intermittent salary
Summary of free listing using Smith's Salience value and pile sorting exercise was reported in five major themes, which subsequently emerged from the pile sort data, viz. Infrastructural and Resource related, monitoring and supervision, IT related, testing, policy decision [Tables 3 and 4].
Table 3.
Relative ranking of suggestions given by Key Respondents
| Item | Smith’s Salience Score |
|---|---|
| More HR | 0.738 |
| Feasible target by district | 0.348 |
| More IEC | 0.333 |
| High speed internet | 0.333 |
| Reduce delay in reporting | 0.310 |
| Merging of all portal | 0.300 |
| Regular supply of consumables | 0.267 |
| Improve infrastructure | 0.200 |
| specific quarantine strategy | 0.200 |
| Regular salary | 0.200 |
| Periodic inspection | 0.119 |
| Upgrade to BSL3 lab | 0.067 |
| Targeted testing approach | 0.067 |
| Dedicated RRT | 0.057 |
| Improve sample collection area | 0.033 |
| Isolation of negative ones | 0.029 |
Table 4.
Pile sorting of suggestions given by Key respondents into themes with reasons
| Pile number | Theme | Suggestions | Reason for grouping |
|---|---|---|---|
| 1 | Infrastructural and resource related | More Human Resource | Directly related to manpower, money, material resources and infrastructural issues |
| Fast purchasing and regular supply of consumables | |||
| Regular salary of staffs | |||
| Upgradation of Laboratoryto BSL-3 level | |||
| Infrastructure improvement of Pre-CoVID/CoVID Hospital | |||
| 2 | Monitoring and Supervision | District target should be feasible | All are directly or indirectly related with monitoring of different activities as well as supervision. |
| Reduce delay in reporting | |||
| Periodic inspection and feedback by competent authority may | |||
| encourage staff | |||
| More IEC to reduce stigma | |||
| Dedicated RRT in district to combat pandemic | |||
| 3 | IT related | High speed internet connection | Related to technology |
| Merging of all portal into one | |||
| 4 | Testing | Improvement of sample collection area | Attributes to testing strategy |
| Targeted testing approach | |||
| 5 | Policy decisions | Isolation of negative persons | Related to isolation and quarantine |
| Specific quarantine strategy for HCW |
BSL-3=Biosafety level 3, IEC=Information Education Communication, RRT=Rapid response team, IT=Information technology
Discussions
The present study was undertaken to identify various issues confronted by the healthcare workers of North Eastern and Eastern India. The study participants with whom the qualitative research was conducted included all the front line health workers (commonly termed COVID worriers) viz, lab technicians and primary care physicians as sample collectors & in-charge for sample storage with transportation. Many of the primary care physicians were directly or indirectly involved in the different activities of COVID pandemic as overall in-charge of sample collection, storage, and transportation to the designated lab. Thus, the findings of this paper will help not only for policy decisions at the highest level but also for rectifications and for providing effective solutions to the problems in sample collection, storage, and transportation at the local level.
Overall impression of this qualitative in-depth study revealed the urgent need in the present context of pandemic to understand the bottlenecks of the operational issues and subsequently design a plan to address such challenges at the earliest in the best possible ethical means in letter and spirit. COVID-19 has become global public health problem and there is a dire need to provide baseline data to frame out foundation of Clinical Practice Guidelines/SOP from confirmation of diagnosis to individual case management to predict prognosis. Research groups have been working everywhere on the operational issues to improve quality and quantity of the laboratory confirmation of COVID-19 received from the catchment areas of the testing laboratories. Further, solving the challenges faced by the laboratory personnel has become a point of discourse among the global stakeholders to become the key issue to contain the unmitigated pandemic of the new millennium.[7,8]
Needless to mention that laboratory diagnosis is the mainstay in the COVID-19 pandemic as novel lifetime experience for the HCWs and research groups are working in search of newer diagnostic techniques for precise and rapid diagnosis. The natural history of COVID-19 is grossly debated even after initial cases were reported nearly a year back. Literature supports RT-PCR as “Gold standard” with alternatives tests. However, research groups also endeavor to classify severity cases by correlating with other variables and parameters.[9,10]
The sequence of events in the COVID labs from collection of samples to identification of RNA polymerase gene by RT-PCR and other tests with comparable sensitivity and specificity remains the indispensable means for precise diagnosis of COVID-19 symptomatic cases. The issues of manpower and logistics related to testing of samples become more pronounced in both asymptomatic and symptomatic cases with co-morbidities needing increased efficiency of the overall precise laboratory testing mechanism to prevent community transmission.[11,12]
To increase competency of laboratory medicine personnel in search of higher precision of the molecular diagnosis biosafety issues are cropping up as critical issues in different set ups even in developed countries that can save valuable manpower. Collection, storage, and transportation of SARS-COV-2 body secretions with highest probabilities of virus loads, from the most high risk sources should follow Clinical and Laboratory Standards Institute guidelines. Rapid laboratory preparedness may focus highly on safety at workplace, defining turnaround time, using specialized kits to prevent spread of virus among all “frontline warriors.” CLSI also expects optimum stress management of laboratory related HCWs. The American Association for Clinical Chemistry precisely stress on logistics for the biosafety of manpower in work schedule and emotional quotient.[13,14]
Good Laboratory Practices and Universal precautions have been emphasized during this pandemic from sample collection, storage, transport, testing to disposal with regular disinfection of all levels with Sodium Hypochlorite and Glutaraldehyde. Further, dedicated areas for storage, centrifugation, donning, and doffing can only minimize COVID-19 related health hazards and should be the incorporated in the whole laboratory system.[15]
What is to be done!
In the international and national levels, all the professionals related with the healthcare have raised pertinent issues on the mitigation and preparedness of this unprecedented SARS-COV-2 pandemic as none of us has professional experience of passing through any pandemic earlier.[9,16,17] The laboratory investigation is the most important aspect of early diagnosis for prompt intervention to interrupt the chain of transmission. We have to upgrade both logistics and biosafety measures for healthcare providers “with a human face” to halt this global disaster. Laboratory related HCWs deserve equivalence in acknowledgment with their peers in clinical care of COVID-19 cases as they are also indirectly involved from bench to the bedside.[10,18]
This ongoing pandemic made situation knotty on already overburdened healthcare delivery infrastructure especially in low and middle income countries with reported deficit of healthcare essentials, instruments, and infrastructure on one side, and the acute shortage of competent laboratory personnel—which has amplified in cases when they are infected and quarantined.[6]
It was generally reported that the creative mindset of the HCWs have been using vaccine carrier and icepacks, previously supplied for national immunization programme, for storage and transport of the COVID-19 samples from collection site to the testing places. The investigators of this research group have a hunch that, once the pandemic is over, we need to be very careful to universally dispose off all these vaccine carrier and icepacks so that they may not become a propagated source of infection in the long run. WHO provided precautions regarding testing on clinical specimens and meeting the suspect cases should be performed in appropriately equipped laboratories by staff trained in the relevant technical and safety procedures.[5]
STRENGTHS OF THE STUDY:
The need of the hour in this pandemic was to urgently understand the problems and challenges faced firstly at the peripheral and remote places of the healthcare delivery system where samples are collected and secondly at the testing laboratories in order to respond at the earliest. Since Government of India has declared COVID-19 as natural disaster, we planned to collect feedback information from all levels of dedicated HCWs while discharging of COVID-19 related duties and responsibilities to the most sincere ways. Attempts was also done to find out perceived adequacy of human resources, equipment, diagnostic kits, and other essential consumables including PPEs vis-a-vis the load of samples from cases and contacts. The feedback of this early and effective response will help to address the problems in the whole chain of collection of samples to communication of results by the assorted list of queries sent earlier for warming up to initiate discussions for better data collections in tailor-made suggestions to streamline functional aspects of the system.
LIMITATIONS OF THE STUDY:
We could have collected data from one district of each state that may be insufficient to draw conclusion with limited external validity.
FUTURE DIRECTIONS OF THE STUDY:
The learning from our study can also be replicated to similar other situations in the current pandemic and also in our routine disease control measures which involve samples to be tested at laboratories away from the site of collection. This research will give a learning experience for all other public health emergencies in future and can streamline the functioning of the entire system involved in sample collection, transportation and testing and mitigation in the days to come as follows:
-
(a)
Short term: This research might help in increasing the efficiency of the overall testing mechanism which is vital to detect cases for control and prevention of any pandemic as it would be able to manage the lacunae and challenges faced during various data collection procedures with appropriate measures.
-
(b)
Long term: These sites can become incubatory sites for deep learning of the problem faced and management issues learnt with local procurements, further they can become learning for health system by enlarge. The observations from our study will also help to understand the bottlenecks in our healthcare delivery system and make it for a larger good not only in COVID-19 but as a whole with all other infectious disease control programme.
On the basis of various findings of the study and conclusions, following summary is given and recommendations suggested which may be adopted –
More and more human resource is required along with fast purchasing and regular supply of consumables needs to be ensured. Salary of staffs engaged in different tiers should be taken care of.
Infrastructural improvement like upgradation of testing lab to BSL-3 level, facilities, and service of Pre-COVID/COVID Hospital, dedicated sample collection kiosk, adequate storage facility, regular sanitation, and proper streamlined BMW disposal, high speed internet connection, etc. should be ensured at all level.
Concerned authority (District, State, ICMR, etc.) should be aware of the capacity of each testing lab and should give feasible target. Otherwise lab would face huge backlog and sample have to be stored for long leading to destruction of it. Excess target was also hampering the sample collection and documentation process.
Reverse isolation, that is, isolation of negative persons may be helpful in the areas where positivity rate is very high.
Stigma is still prevailing in the community specially in remote areas; so extensive IEC along with health education and training of ASHA, ANM, AWW, School teachers even cured patients might be very helpful in this regard.
Targeted testing is required as lot of resources was getting wasted due to testing of asymptomatic and VIPs/referred by VIPs cases.
Merging of all online portals to a single one will make the whole process smoother.
Some of the district program manager urged for more supply of kits for RT-PCR, True NAT, RAT so that they can perform more tests.
Periodic inspection and feedback by competent authority may act as motivation to already overburden staffs. Financial incentive or certification from ICMR in this regard will encourage them.
There should be dedicated team in each district so that they can manage such type of pandemic in future.
Conclusions
Thus this research group feels that basic delivery services need to be geared up, with mitigation strategies for regular upgradation of laboratory medicine to avert and combat any upcoming pandemics in the days to come regarding the current pandemic situation. Overall, 28 telephonic IDIs explored operational concerns faced by HCWs of COVID-19 laboratory testing activities of the sites in six districts in the healthcare delivery system. It is a well-known fact that control and prevention strategies for COVID-19 is directly or indirectly dependent on detecting active cases which in turn is dependent on sample collection, storage, and transportation of the samples to the testing laboratory at correct place, in a correct infrastructure, by performing correct methods at correct time by correctly trained person. These known variables together with uncountable confounders assume more significance in already overburdened infrastructure in terms of deprived healthcare delivery system in remote areas of North East and Eastern regions of India. This research conducted rapid assessment and provided feedback to the regulatory agencies and stakeholders as they felt this as an urgent need to halt progress of the COVID-19 pandemic regarding operational issues and subsequent plan to improve laboratory testing in the remote areas in this part of the country.
Financial support and sponsorship
Indian Council of Medical Research (ICMR) has funded the study.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The researchers are indebted with participants of the study without whose active support the study could not have been completed.
References
- 1.Arnaldez FI, O’Day SJ, Drake CG, Fox BA, Fu B, Urba WJ, et al. The Society for Immunotherapy of Cancer perspective on regulation of interleukin-6 signaling in COVID-19-related systemic inflammatory response. J Immunother Cancer. 2020;8:e000930. doi: 10.1136/jitc-2020-000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronavirus Disease (COVID-19) Dashboard. Data last updated: 2021/1/25, 10:26am CET. Geneva: World Health Organization; Online. Retrieved from: https://covid19.who.int/ [Google Scholar]
- 3.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pierce JD, McCabe S, White N, Clancy RL. Biomarkers: An important clinical assessment tool. Am J Nurs. 2012;112:52–8. doi: 10.1097/01.NAJ.0000418926.83718.28. [DOI] [PubMed] [Google Scholar]
- 5.Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases. World Health Organization Geneva; Online. cited 2020 May 21. Retrieved from: who.int . [Google Scholar]
- 6.Behnam M, Dey A, Gambell T, Talwar V. COVID-19: Overcoming supply shortages for diagnostic testing. July 15, 2020 | Article. [Online] cited 2020 Aug 25. Retrieved from: https://www.mckinsey.com/industries/pharmaceuticalsand-medical-products/our-insights/covid-19-overcomingsupply-shortages-for-diagnostic-testing# .
- 7.Pocius DM. In the Field, Clinical Laboratory Specimen Transportation is Being Complicated by the COVID-19 Pandemic. [online] cited 2020 Jun 10. Retrieved from: https://www.darkdaily.com/in-the-field-clinical-laboratoryspecimen-transportation-is-being-complicated-by-thecovid-19-pandemic/
- 8.GAO Report Highlights Testing Data Challenges During COVID-19 Pandemic. [online] cited 2020 Jun 25. Retrieved from: https://www. 360dx.com/clinical-lab-management/gao-report-highlights-testing-data-challenges-during-covid-19-pandemic#.X1ua_GgzbIU .
- 9.Mitra P, Misra S, Sharma P. COVID-19 Pandemic in India: What lies ahead. Indian J Clin Biochem. 2020;35:257–9. doi: 10.1007/s12291-020-00886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahirwar AK, Asia P, Sakarde A, Bhardwaj S. COVID 19 outbreak: Potential of biochemistry speciality. Indian J Clin Biochem. 2020;18:1–2. doi: 10.1007/s12291-020-00885-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lippi G, Plebani M. The critical role of laboratory medicine during coronavirus disease 2019 (COVID-19) and other viral outbreaks. Clinical Chemistry and Laboratory Medicine (CCLM) [Internet] 2020. Mar 19, cited 2020 May 15. Available from: https://www.degruyter.com/view/journals/cclm/ahead-of-print/article-10.1515-cclm-2020-0240/article-10.1515-cclm-2020-0240.xml . [DOI] [PubMed]
- 12.kja-20182.pdf [Internet] cited 2020 May 21. Available from: https://ekja.org/upload/pdf/kja-20182.pdf .
- 13.Hoyne J. Lab Preparedness During the COVID-19 Pandemic. [online] cited 08.06.2020. Retrieved from: https://www.aacc.org/publications/cln/articles/2020/may/lab-preparedness-during-the-covid-19-pandemic .
- 14.Laboratories in the Age of the Pandemic. We must work together to fight the outbreak of COVID-19. [online] cited 08.06.2020. Retrieved from: https://thepathologist.com/outside-the-lab/laboratories-in-the-age-of-the-pandemic .
- 15.Odega KI, Ehijie I, Ibadin EE, Idomeh FA, Odega DE. Safe laboratory practices in the light of covid-19 pandemic: Way forward in a resource limited setting. [online] cited 08.06.2020. doi: 10.20944/preprints202004.0103.v1. [Google Scholar]
- 16.Raina SK, Kumar R, Galwankar S, Garg S, Bhatt R, Dhariwal AC, et al. Are we prepared? Lessons from Covid-19 and OMAG position paper on epidemic preparedness. J Family Med Prim Care. 2020;9:2161–6. doi: 10.4103/jfmpc.jfmpc_384_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pal R. COVID: We need citizen's charter for common folks. J Family Med Prim Care. 2020;9:5083–4. doi: 10.4103/jfmpc.jfmpc_1125_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh A. COVID: Lab medicine expect due respect. J Family Med Prim Care. 2020;9:5437–8. doi: 10.4103/jfmpc.jfmpc_1164_20. [DOI] [PMC free article] [PubMed] [Google Scholar]


