Abstract
Study aim:
Melanomas arising in acral sites are associated with a poorer prognosis than other melanoma subtypes. The aim of this study was to evaluate clinical-pathological and genetic characteristics as well as therapeutic responses of a larger cohort of patients with melanomas arising in acral sites.
Methods:
Clinical data of 134 patients with melanomas arising in acral sites from the Dept. of Dermatology Essen was collected and analyzed with regard to clinic-pathological characteristics and treatment responses. Genetic analysis with targeted next-generation-sequencing was done on 50 samples.
Results:
In our cohort, BRAF (30%), NRAS (28%), TERT promoter (26%), NF1 (14%), and KIT (6%) were frequently identified mutations. Comparing tumors situated on palms and soles with melanomas arising on dorsal acral sites, a higher frequency of NRAS (39.1% vs. 25%) and NF1 (17.3% vs. 0%) and lower frequencies of BRAF (21.7% vs. 75%) and TERT promoter (8.6% vs. 50%) mutations were observed. MAPK activating mutations were identified in 64% of tumors. Overall survival (OS) was longer in patients treated with immune checkpoint inhibitors as first line treatment than in patients receiving other systemic therapies (i.e. BRAF/MEK inhibitors and chemotherapy).
Conclusion:
Our data suggest that the genetics of melanomas arising in acral sites varies by tumor location and may influence biological behavior.
Keywords: acral melanoma, melanomas arising in acral sites, melanoma treatment, Next-Generation Sequencing, TERT promotor mutation, immune checkpoint inhibitor treatment
Introduction
Melanomas arising in acral locations are generally identified at a later stage resulting in a poorer prognosis and different clinico-pathological and genetic characteristics [1–3] compared with other cutaneous melanoma (CM) subtypes [2]. While some authors define acral melanomas as tumors diagnosed histologically as acral lentiginous melanomas (ALM) arising on the palms, soles, and nail bed [4], other studies also include melanomas arising on dorsal acral sites [1, 2, 5].
Surgical excision with clear margins is of paramount importance in the treatment of early-stage melanoma. However, in melanomas arising in acral sites, the recommended surgical margins are not always achievable due to constraints imposed by anatomic location and a desire to preserve function [4]. Systemic therapy for advanced disease is also challenging in this melanoma subgroup. BRAF mutations are the most common activating mutation in CM and represent an important therapeutic option in advanced disease. In acral melanomas (AM), BRAF mutations are less frequent [6, 7] and patient responses to BRAF-inhibitors are modest, with reported progression-free survival (PFS) and overall survival (OS) of 3.6 and 6.2 months, respectively [8]. Activating mutations and copy number gains of KIT are present in AM [9], but targeted therapies with inhibitors such as imatinib generally demonstrate poor or non-durable responses [10]. Immune checkpoint blockade therapies, in particular antibodies targeting PD-1, have shown great potential in CM [11, 12]. PD-L1 expression in the tumor microenvironment has been associated with improved objective response rates and progression-free survival following anti-PD-1/PD-L1 monotherapy [13–15]. PD-L1 expression was observed in only 31% of AMs compared to 62% in non-acral chronic-sun-damaged (CSD) melanomas [16]. This may be one factor why although AM respond to anti-PD-1/PD-L1 monotherapy, the response rate is lower than in non-acral CM [17].
AM represents an aggressive melanoma subtype rarely harboring currently known targetable mutations. A better understanding of this tumor subgroup will be critical to improve patient outcome. In this study, we evaluated the clinico-pathological and genetic characteristics as well as therapeutic responses in a cohort of patients with melanomas arising in acral locations in order to better understand the pathogenesis and biological behavior of these tumors.
MATERIALS AND METHODS
Data collection and sample selection
Patient medical history and data was retrieved from the medical databases/documentation system of the University Hospital Essen for a time period of 10 years (Januar 2009 – February 2019). Included were all melanomas arising in acral sites (volar, dorsal, interdigital, subungual, periungual location). Fifty samples were retrieved from the biobank of the Department of Dermatology, Essen, Germany, for further genetic analysis. The study was performed after approval of the institutional ethical committee of the medical faculty of the University Duisburg-Essen (IRB protocol number 17–7904-Bo).
DNA isolation and targeted sequencing
Genetic analysis was performed in the subgroup of patients, of whom tumor tissue was available, hereafter referred to as the “mutational analysis subgroup”. DNA isolation, targeted sequencing, and data analysis was performed as previously described [18], applying a custom designed amplicon-based sequencing panel covering the TERT promoter and the coding regions of 29 genes that are recurrently mutated in cutaneous and uveal melanoma (Supplemental Table 1).
Statistical analysis
Statistical analyses were conducted using SPSS 23.0 (IBM., Armonk NY, USA). Associations between covariates were investigated using chi-squared tests and Fisheŕs exact tests as appropriate. Univariate survival data was generated using the Kaplan Meier method with log-rank tests. OS was determined from time of initial diagnosis to last follow-up or death, whichever occurred first. P-values of p ≤0.05 were interpreted as being statistically significant.
RESULTS
Clinical characteristics and therapeutic treatment of melanomas arising in acral sites
We identified 134 patients with melanomas arising in acral sites, 74 (55.2%) female and 60 (44.8%) male (Table 1). Median age at diagnosis was 67 years (range 19 – 93 years). Location of the primary tumor was lower extremity/foot in 125 (93.3%) patients and upper extremity/hand in 9 (6.7%) patients. The histological subtypes and the tumor location (volar, dorsal, other) are shown in Table 1. At the time of initial diagnosis, 20 patients (14.9%) were stage IA, 6 (4.5%) IB, 14 (10.4%) IIA and IIB, respectively, 18 (13.4%) IIC, 10 (7.5%) IIIA, 14 (10.4%) IIIB, 23 (17.2%) IIIC, 2 (1.5%) IIID and 5 (3.7%) stage IV. Stage at diagnosis was unknown for 3 patients (2.2%); 5 patients (3.7%) had a melanoma in situ (Table 1).
Table 1.
Characteristics of the patients in the full analysis set and mutational analysis subgroup
| Variable | All patients (N=134) | Mutational analysis subgroup (N=50) | ||
|---|---|---|---|---|
| Gender | n | % | n | % |
| Female | 74 | 55.2 | 24 | 48 |
| Male | 60 | 44.8 | 26 | 52 |
| Age, years | ||||
| <=67 | 68 | 50.7 | 27 | 54 |
| >67 | 66 | 49.2 | 23 | 46 |
| Histological subtype | ||||
| ALM | 65 | 48.5 | 23 | 46 |
| SSM | 9 | 6.7 | 4 | 8 |
| NM | 19 | 14.2 | 8 | 16 |
| U | 36 | 26.8 | 15 | 30 |
| Mis | 5 | 3.7 | 0 | 0 |
| Stage at initial diagnosis | ||||
| IA | 20 | 14.9 | 3 | 6 |
| IB | 6 | 4.5 | 0 | 0 |
| IIA | 14 | 10.4 | 9 | 18 |
| IIB | 14 | 10.4 | 5 | 10 |
| IIC | 18 | 13.4 | 9 | 18 |
| IIIA | 10 | 7.5 | 5 | 5 |
| IIIB | 14 | 10.4 | 3 | 6 |
| IIIC | 23 | 17.2 | 13 | 26 |
| IIID | 2 | 1.5 | 2 | 4 |
| IV | 5 | 3.7 | 0 | 0 |
| U | 3 | 2.2 | 1 | 2 |
| Mis | 5 | 3.7 | 0 | 0 |
| Tumor location | ||||
| upper extremity | 9 | 6.7 | 4 | 8 |
| lower extremity | 125 | 93.3 | 46 | 92 |
| Anatomic location | ||||
| dorsal | 12 | 9 | 4 | 8 |
| volar | 54 | 40.3 | 23 | 46 |
| other | 55 | 41 | 18 | 36 |
| U | 13 | 9.7 | 5 | 10 |
| Adjuvant Treatment | ||||
| Yes | 41 | 30.6 | 15 | 30 |
| No | 93 | 69.4 | 34 | 68 |
| Systemic Treatment | ||||
| Yes | 55 | 41.8 | 30 | 60 |
| No | 79 | 58.2 | 20 | 40 |
| Treatment | ||||
| < 2 treatment options | 98 | 73.1 | 39 | 78 |
| >=2 treatment options | 34 | 25.4 | 11 | 22 |
| U | 2 | 1.5 | 0 | 0 |
| Targeted Therapy | ||||
| Yes | 17 | 12.7 | 7 | 14 |
| No | 113 | 84.3 | 43 | 86 |
| U | 4 | 3.0 | 0 | 0 |
| Immune checkpoint Inhibitors | ||||
| Yes | 37 | 27.6 | 25 | 50 |
| No | 97 | 72.4 | 25 | 50 |
| U | 0 | 0 | 0 | 0 |
| Chemotherapy | ||||
| Yes | 25 | 18.7 | 9 | 18 |
| No | 109 | 81.3 | 41 | 82 |
| U | 0 | 0 | 0 | 0 |
Abbreviations: ALM, acral lentiginous melanoma; Mis, melanoma in situ; NM, nodular melanoma; SSM, superficial spreading melanoma; U, unknown.
Systemic therapy
Adjuvant treatment was received by 41 (30.6%) patients, 55 (41.0%) patients received systemic treatment for advanced metastatic disease (23 of which had previously received adjuvant treatment) (Table 2). Of these 55 patients, 8 (14.6%) were stage IIIB or IIIC at start of first systemic treatment, 44 (80%) patients were in stage IV at start of therapy and in 3 patients the stage was unknown. Immunotherapeutic agents included: PD-1 inhibitors (n=29), CTLA4-inhibitors (n=19), a combination of PD-1 and CTLA-4 inhibitors (n=3), or more than 1 line of immunotherapy (n=9). A total of 17 patients received targeted therapy consisting of BRAF-inhibitor alone (n=4), MEK inhibitor alone (n=7), and MEK-inhibitor in combination with a BRAF-inhibitor (n=6). Single or multi-agent cytotoxic chemotherapy was given to 25 patients (Table 1). A total of 34 (25.4%) patients received 2 or more treatment options. Therapy sequences included: (1) chemotherapy followed by immunotherapy (n=4), targeted therapy (n=1), or other chemotherapies (n=6), (2) immunotherapy followed by targeted therapy (n=1), other immunotherapy (7), chemotherapy (n=1), or therapy within a clinical trial/others (n=4), (3) targeted therapy followed by other targeted therapies (n=3), immunotherapies (n=1), or therapy within a clinical trial (n=1). In 5 patients the first line treatment was within a clinical trial followed by targeted therapy (n=1), immunotherapies (n=2), or other therapies (n=2). Patients in stage III received PD-1 (n=1) or CTLA-4- inhibitors (n=1), BRAF (n=1) or MEK-inhibitors (n=1), chemotherapy (n=1) or treatment within a clinical trial (n=3).
Table 2.
Therapy sequences received
| Adjuvant treatment | Systemic treatment | |||
|---|---|---|---|---|
|
Yes Total N=41 - low-dose IFN N= 23 - high-dose IFN N=10 (1 switch to low-dose) - others N=6 - unknown N=2 |
First-line therapy | Second-line therapy | Third-line therapy | Further therapies |
| PD-1 N=6 |
CTLA-4 N=2 |
PD-1 N=1 |
||
| Others N=1 |
PD-1 N=1 |
|||
| CTLA-4 N=3 |
PD-1 N=2 |
Chemo N=1 |
Chemo N=1, then PD-1+CTLA-4 |
|
| Others N=1 |
||||
| BRAF N=3 |
MEK N=1 (additional) |
|||
| MEK N=2 |
CTLA-4 N=1 |
Chemo N=1 |
||
| Others N=1 |
||||
| Chemo N=7 |
MEK N=1 |
|||
| CTLA-4 N=1 | ||||
| Chemo N=2 |
Chemo N=2 |
Chemo N=1, then PD-1 |
||
| Others N=1 |
BRAF N=1 |
CTLA-4 N=1 |
||
| No Total N=93 | PD-1 N=7 |
BRAF+MEK N=1 |
BRAF+MEK N=1 |
|
| CTLA-4 N=1 |
||||
| Chemo N=1 |
Others N=1 |
PD-1 + CTLA-4 N=1 |
||
| Others N=1 |
Others N=1 |
|||
| CTLA-4 N=2 |
PD-1 N=2 |
|||
| PD-1+CTLA-4 N=1 |
PD-1+MEK N=1 |
|||
| BRAF N=4 |
MEK N=1 |
|||
| BRAF+MEK N=1 |
CTLA-4 N=1 |
PD-1 N=1 |
||
| BRAF+MEK N=1 |
||||
| MEK N=2 |
||||
| Chemo N=11 |
CTLA-4 N=3 |
PD-1 N=1 Others N=1 |
||
| Chemo N=4 |
CTLA-4 N=2 |
|||
| Others N=4 |
PD-1 N=1 |
|||
| PD-1 + CTLA-4 N=1 |
Others N=1 |
PD-1 N=1, then PD-1 + CTLA-4 |
||
| Others N=2 |
PD-1 N=1 Others N=1 |
|||
Follow-up data of patients with melanomas arising in acral sites
At the time of analysis, 57/134 (42.5%) patients were alive, 53/134 (39.6%) had died and in 24/134 (17.9%) the status was unknown. Cause of death was documented as melanoma-related in 31/53 (58.5%), non-melanoma related in 2/53 (3.8%) and unknown in the remaining cases. In total 44/134 (32.8%) patients had reported locoregional or distant recurrent disease, 34/134 (25.4%) had no reported recurrence, leaving the remaining cases with unknown recurrence status.
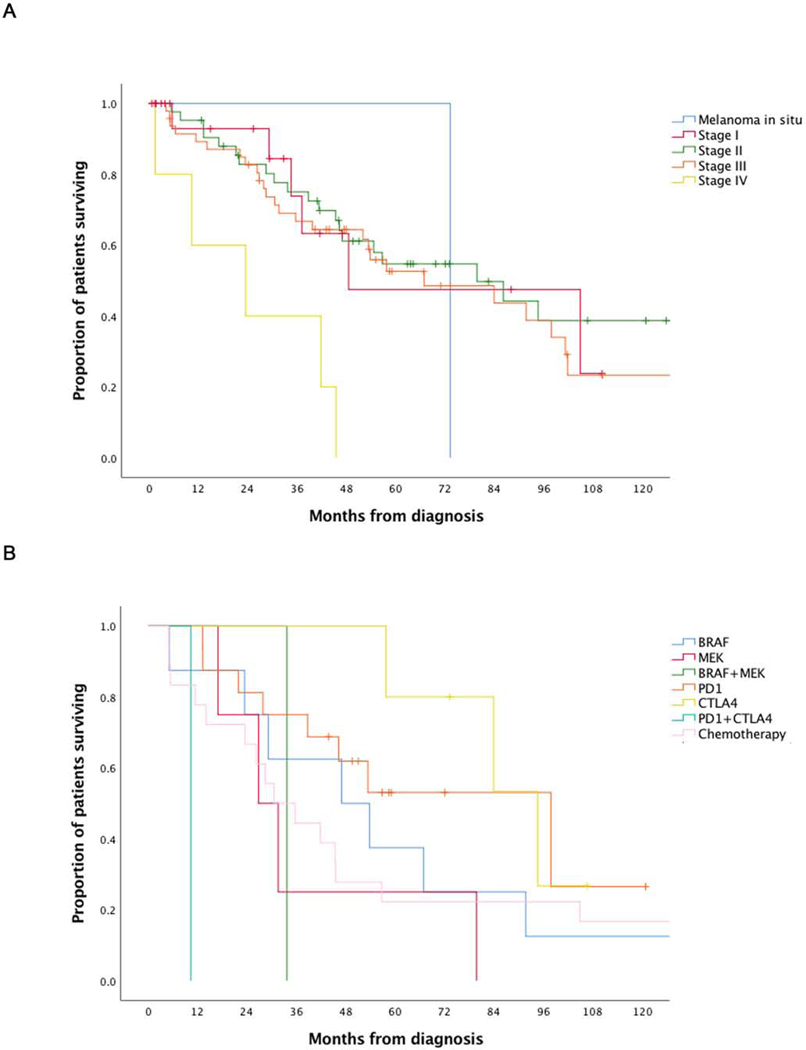
Statistical significant differences in OS were observed regarding stage at initial diagnosis and first line of treatment for stage IV melanoma (Table 3, Figure 1). A median OS survival of 49 months was seen for stage I melanoma, 80 months for stage II, 67 months for stage III and 23 months for stage IV melanoma, p=0.009. Patients receiving anti-PD-1/PDL1 (n=16) or anti-CTLA-4 (n=5) checkpoint inhibitors as first line treatment had an OS of 98 months and 95 months respectively, which was higher compared to other first line therapies (p=0.02) (Table1). Associations of other clinical and pathological covariates (age, gender, tumor location, histologic subtype, tumor thickness, ulceration of the primary tumor) with survival were not statistically significant. The median follow-up was 41.5 (0.1 – 207.4) months.
Table 3.
Characteristics of the patients in the full analysis set and overall survival
| Variable | N | OS | p-value | |
|---|---|---|---|---|
| Median (range) | ||||
| Age | <= 67 years > 67 years |
61 57 |
84 (52–116) 53 (41–66) |
0.10 |
| Sex | Male Female |
56 62 |
80 (42–118) 57 (32–82) |
0.54 |
| Tumor location |
Dorsal Volar |
12 51 |
54 (17–91) 80 (50–110) |
0.85 |
| Other | 47 | 95 (39–150) | ||
| Stage at diagnosis | Melanoma in situ Stage I Stage II Stage III Stage IV |
1 20 42 47 5 |
73 (NA) 49 (0–108) 80 (40–120) 67 (27–107) 23 (0–52) |
0.009 |
| Histologic subtype | ALM SSM NM |
54 9 20 |
84 (50–118) 31 (0–73) 46 (18–73) |
0.26 |
| First systemic treatment | BRAF - inhibitor MEK - inhibitor BRAF + MEK - inhibitor PD1 - inhibitor CTLA4 - inhibitor PD1 + CTLA4 - inhibitor Chemotherapy |
8 4 1 16 5 1 18 |
47 (13–81) 27 (12–41) 34 (NA) 98 (41–155) 95 (64–125) 10 (NA) 31 (16–45) |
0.02 |
Abbreviations: ALM, acral lentiginous melanoma; NA, not applicable; NM, nodular melanoma; OS, overall survival; SSM, superficial spreading melanoma.
The p-values in stage at diagnosis and first systemic treatment might be substantially influenced by the groups Melanoma in situ, first line combination treatment of BRAF and MEK inhibitors, and first line combination treatment of PD-1 and CTLA-4 inhibitors with n=1.
Figure 1. Survival analysis of the full sample set.
(A) Kaplan-Meier (KM) plot of overall survival (OS) by stage: melanoma in situ – 73 months (n=1), stage I melanoma – 49 months (n=20), stage II melanoma – 80 months (n=42), stage III melanoma – 67 months (n=47), stage IV melanoma – 23 months (n=5).(B) Kaplan-Meier (KM) plot of OS by first line treatment: BRAF inhibitors – 47 months (n=1), MEK inhibitors – 27 months (n=4), BRAF and MEK inhibitors – 34 months (n=1), PD-1/PD-L1 inhibitor – 98 months (n=16), CTLA-4 inhibitor – 95 months (n=5), chemotherapy – 31 months (n=18). The median follow-up was 41.5 (0.1–207.4) months. The p-values in stage at diagnosis and first systemic treatment might be substantially influenced by the groups Melanoma in situ, first line combination treatment of BRAF and MEK inhibitors, and first line combination treatment of PD-1 and CTLA-4 inhibitors with n=1.
Mutational analysis
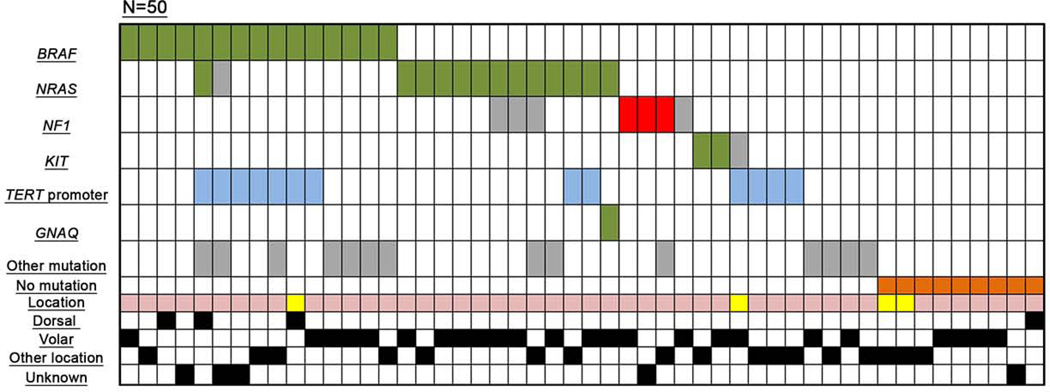
Tumor samples from 50 melanomas arising in acral sites patients were analyzed with a target sequencing panel. Mutations in at least one of 29 tested genes were identified in 41 (82%) samples. In 9 (18%) cases no mutations were identified and tumors were classified as wild-type (Table 4, Figure 2).
Table 4.
Mutational analysis of the subgroup
| Nr. | Age | Sex | Histological subtype | Location | Sample type | BRAF | NRAS | NF1 | KIT | TERT Promoter | other mutations |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45 | F | LE - ALM | volar | U | V600E | |||||
| 2 | 56 | F | LE - U | other | U | V600E | |||||
| 3 | 21 | M | LE - SSM | dorsal | M | V600E | |||||
| 4 | 46 | M | LE - NM | unknown | M | V600E | |||||
| 5 | 35 | M | LE - NM | dorsal | U | V600E | Q61K | C250T | ARID1A G1375S | ||
| 6 | 58 | M | LE - ALM | unknown | P | V600E | A155V | C250T | EZH2 D185H | ||
| 7 | 61 | F | LE - U | unknown | P | V600E | CC242–3TT | ||||
| 8 | 54 | F | LE - U | other | U | V600E | C250T | ||||
| 9 | 66 | M | LE - ALM | other | U | V600E | CC228–9TT C250T | SMARCA4 F1142S | |||
| 10 | 90 | F | UE - NM | dorsal | U | V600E | C225T | ||||
| 11 | 72 | F | LE - ALM | volar | U | V600E | CC228–9TT CC242–3TT |
||||
| 12 | 66 | M | LE - ALM | volar | U | V600E | TERT R1086H, ARID1A N906K | ||||
| 13 | 39 | F | LE - U | volar | M | V600E | PIK3CA I391M | ||||
| 14 | 71 | M | LE - ALM | volar | U | V600E | SMARCA4 G101E, SMARCA4 R1640W | ||||
| 15 | 69 | M | LE - SSM | other | U | V600E | ARID1A P1710S, ARID2 Q397L, CDKN2A R10G CDKN2A I11fs IDH1 A141fs IDH1 Y183C | ||||
| 16 | 80 | F | LE - NM | volar | U | Q61R | |||||
| 17 | 79 | F | LE - U | other | M | Q61H | |||||
| 18 | 76 | F | LE - U | volar | M | Q61K | |||||
| 19 | 76 | F | LE - SSM | volar | U | Q61R | |||||
| 20 | 67 | F | LE - ALM | volar | U | G12S | |||||
| 21 | 60 | F | LE - ALM | volar | P | Q61R | M1461E | ||||
| 22 | 75 | M | LE - NM | volar | P | Q61R | V1308L | ||||
| 23 | 75 | F | LE - ALM | other | P | G12D | N2128K | ARID1A R1202Q ARID2 M428I PTEN Y318D | |||
| 24* | 69 | M | LE - ALM | volar | U | Q61R | BAP1 S473M ARID1A R1551C ARID1A P1568S TERT P313L | ||||
| 25 | 75 | F | LE - ALM | other | P | G12D | CC228–9TT | ||||
| 26 | 64 | M | LE - U | volar | M | Q61K | C250T | ||||
| 27 | 84 | M | LE - NM | volar | P | Q61R | GNAQ R183G | ||||
| 28 | 72 | F | LE - ALM | volar | U | L1109* | |||||
| 29 | 74 | M | LE - ALM | unknown | U | T1565fs | |||||
| 30 | 68 | M | LE - U | other | R | C152fs | ARID1A R1202Q | ||||
| 31 | 62 | M | LE - ALM | volar | P | R1337W | |||||
| 32 | 71 | M | LE - U | other | P | L576 P | |||||
| 33 | 82 | F | LE - SSM | volar | P | K642 E | |||||
| 34 | 57 | M | UE - U | other | U | A829 P | C250T | ||||
| 35 | 48 | F | LE - NM | other | U | CC242–3TT | |||||
| 36 | 64 | M | LE - ALM | other | P | C250T | |||||
| 37 | 63 | M | LE - ALM | other | P | CC242–3TT | |||||
| 38 | 74 | M | LE - U | volar | P | WT1 G317E | |||||
| 39 | 28 | F | LE - ALM | other | U | IDH1 R222S | |||||
| 40 | 72 | M | LE - NM | volar | P | TERT S803R | |||||
| 41 | 57 | M | LE - U | other | M | MAP2K2 G286R WT1T221I SMARCA4 R842Q TP53 K120R | |||||
| 42 | 55 | F | UE - ALM | other | U | ||||||
| 43 | 34 | M | LE - ALM | volar | P | ||||||
| 44 | 75 | M | LE - ALM | unknown | U | ||||||
| 45 | 79 | F | LE - U | volar | P | ||||||
| 46 | 61 | F | LE - ALM | volar | U | ||||||
| 47 | 57 | M | LE - U | dorsal | M | ||||||
| 48 | 69 | M | LE - ALM | volar | M | ||||||
| 49 | 55 | F | UE - ALM | other | U | ||||||
| 50 | 61 | F | LE - U | other | P |
Only mutations with a frequency >20% are displayed due to the high number of identified mutations
Abbreviations: ALM, acral lentiginous melanoma; F, female; LE, lower extremity; M, male; Met, metastasis; NM, nodular melanoma, P, primary tumour; R, recurrence; SSM; superficial spreading melanoma; U, unknown; UE, upper extremity.
Figure 2. Oncoprint – Mutations identified by targeted next-generation sequencing in the mutational analysis subgroup.
Green: mutations known or assumed to be activating; red: loss of function mutations; blue: mutations in the TERT promoter region; grey: missense mutation (frequently with unknown functional consequences); orange: wild-type samples (showing no mutation in the analyzed gene panel). Tumor location: yellow – upper extremity, light pink – lower extremity.
Mutations were identified in BRAF in 15 (30%) samples, NRAS in 14 (28%) samples, TERT promoter mutations in 13 (26%) samples, NF1 mutations in 7 (14%) samples (3 of which were clearly inactivating mutations) and KIT mutations in 3 (6%) samples (two being known activating mutations). One sample presented concurrent activating BRAF and NRAS mutations. Of 13 samples with TERT promoter mutations, seven (54%) also harbored BRAF mutations. Mutation distribution with regard to tumor location (dorsal, volar, other) is shown in Table 5a. Out of the 4 melanomas arising on dorsal acral sites, 3 exhibited a BRAF mutation, 2 TERT promotor mutations, one an NRAS mutation, and one sample was wild-type. A significant difference was observed for TERT promotor mutations being more frequent in melanomas arising on dorsal acral sites and other acral locations compared to volar location, 2/4 and 7/18 versus 2/23 (p= 0.0381), respectively. No other statistically significant associations were observed between tumor site (dorsal, volar, other), gender and BRAF, RAS, and NF1 mutational status (Table 5b).
Table 5.
Clinical, pathological and genetic characterization according to tumor location in the mutational analysis subgroup
| a. |
| Dorsal | Volar | Other | Unknown | Total | |
|---|---|---|---|---|---|
| Variable | (N=4) | (N=23) | (N=18) | (N=5) | (N=50) |
| Gender, no (%) | |||||
| Female | 1 (25) | 12 (52.1) | 10 (55.5) | 1 (20) | 24 (48) |
| Male | 3 (75) | 11 (47.8) | 8 (44.4) | 4 (80) | 26 (52) |
| Mutational status, no (%) | |||||
| BRAF | 3 (75) | 5 (21.7) | 4 (22.2) | 3 (60) | 15 (30) |
| NRAS | 1 (25) | 9 (39.1) | 3 (16.6) | 1 (20) | 14 (28) |
| NF1 | 0 | 4 (17.3) | 2 (11.1) | 1 (20) | 7 (14) |
| KIT | 0 | 1 (4.3) | 2 (11.1) | 0 | 3 (6) |
| TERT Promotor | 2 (50) | 2 (8.6) | 7 (38.8) | 2 (40) | 13 (26) |
| No mutation | 1 (25) | 4 (17.3) | 3 (16.6) | 1 (20) | 9 (18) |
| Histological subtype, no (%) | |||||
| ALM | 0 | 12 (52.1) | 8 (44.4) | 3 (60) | 23 (46) |
| SSM | 1 (25) | 2 (8.6) | 1 (5.5) | 0 | 4 (8) |
| NM | 2 (25) | 4 (17.3) | 1 (5.5) | 1 (20) | 8 (16) |
| U | 1 (25) | 5 (21.7) | 8 (44.4) | 1 (20) | 15 (30) |
| b. |
| Tumor site | Sex | Mutation status | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | p-value | BRAF WT | p-value | RAS | WT | p-value | NF1 | WT | p-value | TERT promoter | WT | p-value | ||
| Dorsal | 1 | 3 | 0.53 | 3 1 | 0.072 | 1 | 3 | 0.547 | 0 | 4 | 0.600 | 2 | 2 | 0.038 | |
| Volar | 12 | 11 | 5 18 | 9 | 14 | 4 | 19 | 2 | 21 | ||||||
| other | 10 | 8 | 4 14 | 3 | 2 | 2 | 16 | 7 | 11 | ||||||
Abbreviations: ALM, acral lentiginous melanoma; NM, nodular melanoma; SSM, superficial spreading melanoma; U, unknown.
Other rare mutations were found in the following genes: GNAQ, ARID1A, ARID2, IDH, PTEN, PIK3CA, SMARCA4, EZH2, BAP1, WT1, MAP2K2, and TP53 (Table 4).
Clinical findings in the mutational analysis subgroup
In 46 (92%) cases, the primary tumor was localized on the foot and in 4 (8%) cases on the hand (Table 4). The different histological subtypes as well as the anatomic distribution volar, dorsal or other acral sites are demonstrated in Table 5a. Other acral sites comprise interdigital, digital, edge of the foot, periungual or subungual location. Histologically, melanomas arising on dorsal acral sites showed one SSM, two NM, and in one case the histological subtype was unknown (Table 5).
Adjuvant treatment was given to 15 (30%) patients, consisting of interferon (14/16 cases) and treatment within a clinical trial (1/16). Systemic treatment for advanced disease was given to 30 (60%) patients (Table 1).
Statistical analysis between patients without mutational analysis and the mutational analysis subgroup
The two patient groups were statistically analyzed regarding age, gender, primary tumor location, stage at initial diagnosis, histological subtype (ALM, SSM and NM), adjuvant treatment, systemic treatment for advanced metastatic disease, and different treatment regimens for advanced metastatic disease. The only statistically significant difference identified regarded treatment; more patients in the mutational analysis subgroup received systemic treatment for stage IV melanoma (30/50, 60%) compared to the non-mutational analysis group (27/84, 32.1%; p=0.002). More patients from the mutational analysis subgroup received treatment with anti-CTLA4 and anti-PD-1/PD-L1 inhibitors than in the non-mutational analysis group, 11/50 vs. 8/76 (p=0.045) and 22/50 vs. 9/95 (p<0.001) respectively (Supplementary Table 2).
DISSCUSSION
ALM presents the most frequent, yet not the only histopathological type of melanoma in acral sites [5]. Interestingly, Carrera et al. could show that SSM subtypes located in acral sites were associated with red hair color variants, thus indicating that pigmentation might have an impact even in melanomas arising on non-sun-unexposed body sites, although 50% of SSM were located on the dorsum/ankle/wrist [5]. Additionally, Nagore et al. showed that patients with ALM were older, did not remember severe sunburn at a higher frequency, had a lower number of common melanocytic nevi, and had family histories of other non-cutaneous cancers compared to patients with SSM, NM, or LMM [19]. Furthermore, it was shown that melanoma from dorsal acral sites more frequently were SSM (16/21 cases) compared to subungual and interdigital sites (0/13) or volar acral sites (3/9) [1]. Again, patients with melanomas arising on dorsal distal extremities were younger, more often Caucasian, and more frequently harbored BRAF-mutations compared to those from other acral sites (volar, subungual/interdigital), supporting that melanomas arising on dorsal acral sites are distinct from AM from other sites [1]. This goes along with our findings and suggests that melanomas arising on dorsal acral sites and especially SSM should be considered differently in the evaluation of acral melanoma.
Comparing our single center study of a large cohort of 134 melanomas arising in acral sites with existing data sets, the male to female relation of 55.2% to 44.8% is comparable to the 59.6% to 40.4% distribution in the dataset of 2050 ALM patients from the Central Malignant Melanoma Registry (CMMR) of the German Dermatologic Society [2]. Our study found primary tumor location on the lower extremity/foot in 93.3% and in 6.7% of cases on the upper extremity/hand, comparable to 82% and 18%, respectively in the CMMR dataset [2]. This data, with other studies [20], confirms that the majority of melanomas arising in acral sites occur on the lower extremity.
One factor responsible for poor prognosis of melanomas arising in acral sites may be high recurrence rate. We documented locoregional and distant disease recurrence in 32.8% (44/134) of patients. Similar frequencies (35% [5] and 29% [2]) for relapse and local recurrence, respectively, were reported in other studies, consistent with melanomas arising in acral sites having a recurrence rate nearly twice as high as locoregional relapses of other melanoma subgroups, reported with 14.3% for non-ALM [2].
Due to the relative rarity of AM, data is scarce concerning response to therapy. In our cohort OS was significantly lower for patients receiving targeted therapy as either monotherapy or combination therapy, and chemotherapy as first line treatment compared to patients who received immunotherapies (p=0.02).
In our analysis OS for patients with in situ and stage I melanoma was lower than for patients with stage II and III melanoma (73 and 49 months vs. 80 and 67 months, respectively). This finding is a result of sampling bias, as patients with melanoma in situ, stage I, and some stage II melanomas are not followed-up in our skin cancer units but by primary care dermatologists; therefore follow-up data from most of these patients is not available for inclusion in our analysis. Only if progression occurs are patients re-referred to our skin cancer unit, allowing documentation of follow-up data. Thus, sampling bias represents a limitation of our study. In our cohort, 91/134 patients (67.9%) either underwent SLN biopsy or had evidence of lymph nodedistant metastases at initial diagnosis. In 48 (52.7%) patients SLN biopsy was performed. The SLN was reported negative in 37/48 (77%) cases.
Some stage III patients enrolled in our study received systemic treatment with either a PD-1-inhibitor (n=1), a CTLA-4-inhibitor (n=1), a BRAF- or MEK-inhibitor (each n=1), or chemotherapy (n=1). Due to small sample size, a possible influence on therapy sequence could not be further investigated.
The gene mutation profile of melanomas arising in acral sites differs from non-acral CM [9, 21]. Melanomas situated on dorsal acral sites (n=4) had a mutation profile reminiscent of CM with intermittent sun exposure, with 75% BRAF, 25% NRAS, and 50% TERT promotor mutations. A similar distribution of 67% BRAF and 19% NRAS mutations in melanomas arising in acral sites was recently reported by Haugh et al. [1]. The number of NRAS mutations identified in volar AM (39.1%) is slightly higher than previously in literature, 33% (3/9) [1] and 27.9% [7]. The frequency of BRAF (21.7%) and NF1 (17.3%) mutations was similar to the 21.3% and 14.8%, respectively, published by Yeh et al. [7]. Melanomas located on other acral sites harbored 22.2% BRAF, 16.6% NRAS and 38.8% TERT promotor mutations. The 6% KIT mutations (n=3, 2 of which being known activating mutations) is lower than previously reported [7, 9, 22]. The 18% (9/50) of tumors where no mutation was identified is slightly lower than reported in the Korean cohort, 25% (16/64) [22].
In 9 samples TERT promotor mutation coexisted with BRAF (7) or NRAS (2) mutations. TERT promotor mutations were significantly less frequent on volar sites compared to melanomas arising on dorsal and other acral sites. Half of melanomas arising on dorsal acral sites (2/4, 50%) had a TERT promotor mutation, a pattern reminiscent of non-acral CM [23, 24]. An interesting finding was the high TERT promotor mutation frequency (38.8%) of melanomas arising in other locations such as periungual, interdigital, digital, or subungual.
One sample harbored an activating BRAF as well as an NRAS mutation. The latter is a well-documented resistance mechanism and may have emerged as a result of the patients BRAF-inhibitor treatment [25].
Melanomas arising in acral sites are not only distinct from non-acral CM in terms of clinical and pathological characteristics but also genetically. The frequency of mutations identified in BRAF, NRAS and KIT is less than that described in some previous studies [1, 9, 21]. However, the overall presence of MAPK-activating mutations (i.e. mutations in NF1, RAS, KIT, BRAF) in 64% of tumors in our cohort highlights the central role of the MAPK pathway in the pathogenesis of melanomas arising in acral sites.
Our findings suggest that melanomas arising on dorsal acral sites genetically resemble non-acral CM and should be distinguished from acral melanoma arising on volar sites and other acral localizations such as subungual or interdigital sites. The varying genetic pathogenesis based on tumor location likely influences biological behavior and therapeutic response.
Supplementary Material
Highlights.
Mutations in the TERT promoter were observed in 26% of tumor samples
MAPK activating mutations were identified in 64% of tumors
Tumors situated on palms and soles presented higher frequencies of NRAS mutations and lower frequencies of BRAF and TERT promoter mutations than tumors on dorsal sites
Patients who received first line treatment with immune checkpoint inhibitors (anti-PD-1, anti-PD-L1, anti-CTLA-4) had a statistically significant longer OS compared to other systemic treatments
ACKNOWLEDGMENTS
We thank Nadine Stadler and Julia Kretz (Department of Dermatology, University Hospital University Hospital Essen) for excellent technical assistance.
This work was supported in part by the Hiege-Stiftung gegen Hautkrebs.
Role of the Funding Source
Funding from the Hiege-Stiftung gegen Hautkrebs was used for targeted sequencing.
FUNDING
This work was supported in part by the Hiege-Stiftung gegen Hautkrebs.
Elisabeth Livingstone has had intermittent advisory board relationships with Roche, BMS, Novartis and Actelion and has received travel grants and honoraria from Roche, BMS, MSD, Amgen, Novartis, Boehringer-Ingelheim, medac. Eva Hadaschik received travel support and honoraria from Essex, Abbott, MSD, Janssen-Cilag, Novartis, Roche, BMS and La Roche Posay. Alexander Roesch received travel grants and honoraria from Roche, TEVA, Bristol-Myers Squibb, MSD, Amgen, and Novartis, and research grants from Novartis, BMS and Adtec. Selma Ugurel declares research support from BMS, medac, and Merck Serono, speakers and advisory board honoraria from BMS, MSD, Novartis, Roche, and Merck Serono, and travel support from BMS, medac, MSD, and Roche. Cindy Franklin received honoraria from BMS and Novartis and travel support from BMS. Alexander Roesch received travel grants and honoraria from Roche, TEVA, Bristol-Myers Squibb, MSD, Amgen and Novartis, and research grants from Novartis, BMS and Adtec. Dirk Schadendorf reports grants, personal fees and non-financial support from BMS, personal fees and non-financial support from Roche, grants, personal fees and non-financial support from Novartis, non-financial support from Regeneron, personal fees from Sanofi, personal fees and non-financial support from MSD/Merck, personal fees and non-financial support from Amgen, personal fees and non-financial support from 4SC, personal fees and non-financial support from Merck-EMD, personal fees from Array, personal fees and non-financial support from Pierre Fabre, personal fees and non-financial support from Philiogen, personal fees and non-financial support from Incyte, personal fees from Pfizer, outside the submitted work. Ioana Cosgarea has received travel grants from Roche, GSK, BMS, TEVA and Novartis. Anne Zaremba has received travel grants from Novartis and BMS.
Footnotes
CONFLICT OF INTEREST
All other authors have nothing to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Haugh AM, Zhang B, Quan VL, Garfield EM, Bubley JA, Kudalkar E, et al. Distinct Patterns of Acral Melanoma Based on Site and Relative Sun Exposure. J Invest Dermatol. 2018;138:384–93. [DOI] [PubMed] [Google Scholar]
- [2].Teramoto Y, Keim U, Gesierich A, Schuler G, Fiedler E, Tuting T, et al. Acral lentiginous melanoma: a skin cancer with unfavourable prognostic features. A study of the German central malignant melanoma registry (CMMR) in 2050 patients. Br J Dermatol. 2018;178:443–51. [DOI] [PubMed] [Google Scholar]
- [3].Sondermann W, Zimmer L, Schadendorf D, Roesch A, Klode J, Dissemond J. Initial misdiagnosis of melanoma located on the foot is associated with poorer prognosis. Medicine (Baltimore). 2016;95:e4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Goydos JS, Shoen SL. Acral Lentiginous Melanoma. Cancer Treat Res. 2016;167:321–9. [DOI] [PubMed] [Google Scholar]
- [5].Carrera C, Gual A, Diaz A, Puig-Butille JA, Nogues S, Vilalta A, et al. Prognostic role of the histological subtype of melanoma on the hands and feet in Caucasians. Melanoma Res. 2017;27:315–20. [DOI] [PubMed] [Google Scholar]
- [6].Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545:175–80. [DOI] [PubMed] [Google Scholar]
- [7].Yeh I, Jorgenson E, Shen L, Xu M, North JP, Shain AH, et al. Targeted genomic profiling of acral melanoma. J Natl Cancer Inst. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bai X, Mao LL, Chi ZH, Sheng XN, Cui CL, Kong Y, et al. BRAF inhibitors: efficacious and tolerable in BRAF-mutant acral and mucosal melanoma. Neoplasma. 2017;64:626–32. [DOI] [PubMed] [Google Scholar]
- [9].Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24:4340–6. [DOI] [PubMed] [Google Scholar]
- [10].Guo J, Si L, Kong Y, Flaherty KT, Xu X, Zhu Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:2904–9. [DOI] [PubMed] [Google Scholar]
- [11].Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521–32. [DOI] [PubMed] [Google Scholar]
- [13].Daud AI, Wolchok JD, Robert C, Hwu WJ, Weber JS, Ribas A, et al. Programmed Death-Ligand 1 Expression and Response to the Anti-Programmed Death 1 Antibody Pembrolizumab in Melanoma. J Clin Oncol. 2016;34:4102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Madore J, Vilain RE, Menzies AM, Kakavand H, Wilmott JS, Hyman J, et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res. 2015;28:245–53. [DOI] [PubMed] [Google Scholar]
- [15].Obeid JM, Erdag G, Smolkin ME, Deacon DH, Patterson JW, Chen L, et al. PD-L1, PD-L2 and PD-1 expression in metastatic melanoma: Correlation with tumor-infiltrating immune cells and clinical outcome. Oncoimmunology. 2016;5:e1235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kaunitz GJ, Cottrell TR, Lilo M, Muthappan V, Esandrio J, Berry S, et al. Melanoma subtypes demonstrate distinct PD-L1 expression profiles. Lab Invest. 2017;97:1063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shoushtari AN, Munhoz RR, Kuk D, Ott PA, Johnson DB, Tsai KK, et al. The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer. 2016;122:3354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cosgarea I, Ugurel S, Sucker A, Livingstone E, Zimmer L, Ziemer M, et al. Targeted next generation sequencing of mucosal melanomas identifies frequent NF1 and RAS mutations. Oncotarget. 2017;8:40683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nagore E, Pereda C, Botella-Estrada R, Requena C, Guillen C. Acral lentiginous melanoma presents distinct clinical profile with high cancer susceptibility. Cancer Causes Control. 2009;20:115–9. [DOI] [PubMed] [Google Scholar]
- [20].Rex J, Paradelo C, Mangas C, Hilari JM, Fernandez-Figueras MT, Ferrandiz C. Management of primary cutaneous melanoma of the hands and feet: a clinicoprognostic study. Dermatol Surg. 2009;35:1505–13. [DOI] [PubMed] [Google Scholar]
- [21].Liang WS, Hendricks W, Kiefer J, Schmidt J, Sekar S, Carpten J, et al. Integrated genomic analyses reveal frequent TERT aberrations in acral melanoma. Genome Res. 2017;27:524–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Moon KR, Choi YD, Kim JM, Jin S, Shin MH, Shim HJ, et al. Genetic Alterations in Primary Acral Melanoma and Acral Melanocytic Nevus in Korea: Common Mutated Genes Show Distinct Cytomorphological Features. J Invest Dermatol. 2017. [DOI] [PubMed] [Google Scholar]
- [23].Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–61. [DOI] [PubMed] [Google Scholar]
- [24].Cancer Genome Atlas N. Genomic Classification of Cutaneous Melanoma. Cell. 2015;161:1681–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Van Allen EM, Wagle N, Sucker A, Treacy DJ, Johannessen CM, Goetz EM, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014;4:94–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.