Abstract
Hydroxytyrosol (HT) from olives and polyphenols from almond skin (ASPs) possess cardioprotective properties. This pilot study evaluates the effect of supplementation with a combination of olive fruit and almond skin extracts on low-density lipoprotein (LDL) cholesterol oxidation, lipid homeostasis, and inflammatory parameters in adults with moderate hypercholesterolemia. A randomized, parallel, double-blind, placebo-controlled pilot study of 8 weeks was performed. The extract group (EG) received the supplement with 7.5 mg HT +210 mg ASPs, and the control group (CG) received a placebo composed of maltodextrin. Oxidized LDL (oxLDL) levels and the oxLDL/LDL ratio were lower in the EG than in the CG after 8 weeks of treatment (18.76 ± 3.91 vs. 10.34 ± 4.22, P < .001 and 0.151 ± 0.025 vs. 0.08 ± 0.023, P < .001, respectively). Interleukin-1β levels were significantly higher in the CG than in the EG at week 4 (P = .004), IL-6 was significantly higher in the CG than in the EG at week 4 (P = .049), and IL-10 was significantly increased at week 4 in both groups (P = .002 for CG and P = .001 for EG). In conclusion, daily consumption of a combination of an olive fruit extract and an almond skin extract for 8 weeks seems to protect LDL from oxidation and to prevent inflammatory status in moderately hypercholesterolemic subjects.
Keywords: almond skin polyphenols, atherosclerosis, hydroxytyrosol, inflammation, oxidized low-density lipoproteins
INTRODUCTION
Cardiovascular diseases (CVD) still are the leading cause of mortality worldwide, being responsible for an estimated 17.8 million deaths in 2017,1 which is supposed to be a 12.5% mortality increment since the past decade.2
Atherosclerotic vascular disease or atherosclerosis is one of the most important causes of CVD.3 This is a chronic vascular inflammatory disease associated to oxidative stress and endothelial dysfunction,4 in which oxidized low-density lipoproteins (oxLDLs) have been suggested to play an important role.5 OxLDL binds with higher affinity than native LDL to several receptors, so its uptake by macrophages is more rapid, leading to cholesterol accumulation and the formation of atherosclerotic plaque.6 Moreover, oxLDL promotes the activation and dysfunction of endothelial cells, the migration and proliferation of vascular smooth muscle cells, and the platelet activation, causing inflammatory response, endothelial dysfunction, and worsening the atherogenic process.7 Therefore, circulating level of oxLDL is considered as one of the most important biomarkers in the progression of atherosclerosis.6
Nevertheless, it is well known that most of CVD can be prevented by the promotion of a healthy lifestyle,8 and several dietary strategies to reduce cardiovascular risk have been developed.9 In this regard, the use of polyphenols as nutraceutical agents is spreading,10,11 and among those, hydroxytyrosol (HT) from olives and polyphenols from almond skin (ASPs) have been attributed as presenting cardioprotective properties.12–15 HT is a phenolic compound present both in the fruit and leaf of the olive (Olea europaea L.).16 There are several evidences about its anti-inflammatory and anti-atherogenic functions, such as the inhibition of LDL oxidation, platelet aggregation, and other factors involved in the development of atherosclerosis.17 Regarding ASPs, the most abundant were proanthocyanidins, hydrolysable tannins, and flavonoids.18 Several studies suggested that, beyond the antioxidant and anti-inflammatory properties of these polyphenols, they also improved lipid homeostasis.13 Therefore, the combined intake of both bioactive compounds may help to reduce some risk factors associated with CVD.
The aim of this pilot study is to evaluate the effect of supplementation with a combination of an olive fruit extract (standardized in HT) and an almond skin extract (standardized in total polyphenols) on LDL-cholesterol (LDL-c) oxidation in adults with moderately hypercholesterolemia. As secondary outcomes, lipid homeostasis and inflammatory parameters are also evaluated.
MATERIALS AND METHODS
Study subjects
Volunteers were recruited from the Endocrinology Service of Hospital San Cecilio in Granada (Spain) and from the database of the Clinical Studies Unit of Biosearch Life. Study participants met the following inclusion criteria: men and women from 18 to 65 years old that have moderately hypercholesterolemia (LDL-c >100 mg/dL and/or total cholesterol (TC) >200 mg/dL) and that are not receiving medical treatment for this condition. Exclusion criteria included pregnancy, to have a major medical problem, diabetes and/or neurovascular disease, and to present allergy to antibiotics or to some of the ingredients of the study. Subjects were also excluded if they were on diet or they were taking food supplements during the study. The study was conducted according to the Declaration of Helsinki, and the protocol was approved by the Regional Ethics Committee (Granada, Spain). Informed consent was obtained from all subjects. The trial was registered in the U.S. Library of Medicine (www.clinicaltrial.gov) as NCT04029727.
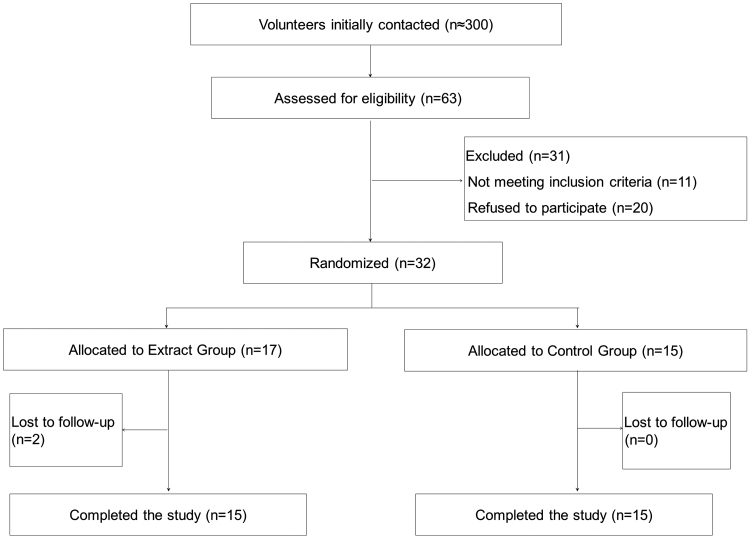
Approximately 300 subjects were contacted and invited to participate in the study, and 63 were assessed for eligibility. Out of them, 31 were excluded (11 did not meet the inclusion criteria, and the rest refused to participate). Finally, a total of 32 subjects agreed to participate in the intervention and were randomized (Fig. 1).
FIG. 1.
Diagram of the consolidated standards of reporting trials.
Study design
This was a randomized, parallel, double-blind, placebo-controlled pilot study of 8 weeks of duration. The study was performed between May and July 2019. A group of 32 subjects were enrolled and randomly assigned into two different groups according to a randomization scheme generated by a computer program (SIGESMU®). One group (extract group [EG]) received an oral supplementation with a combination of olive fruit extract and almond skin extract (7.5 mg HT +210 mg ASPs +33 mg maltodextrin per day), and the other (control group [CG]) received a placebo (800 mg maltodextrin per day). Treatments were given to subjects for 8 weeks, and they consumed two capsules at a time per day (consuming the extract supplement or the placebo) at lunch time. Participating subjects were instructed not to deviate from their regular habits and to maintain their normal diet and exercise level during the 8 weeks. Neither the researchers nor the subjects knew which treatment sequence the subjects had been assigned to; the researchers were unblinded only at the end of the study.
Study products
The daily dose of the study product consisted on a mix of 67 mg of olive fruit (Olea europaea L.) extract standardized to 11% HT and 700 mg of almond skin (Prunus dulcis (Mill.)) extract standardized to 30% total polyphenols. Therefore, volunteers consumed 7.5 mg HT +210 mg ASPs per day. Dose of HT was based on European Food Safety Authority (EFSA) claim for HT consumption and LDL protection from oxidation19 and the differences observed in HT bioavailability among several matrices.20 Dose of ASPs was selected for being the most effective in avoiding the degradation of the HT during digestion.21 Since volunteers consumed two capsules per day, each extract capsule contained 3.75 mg of HT from 33.5 mg of the standardized olive fruit extract, 105 mg of ASPs from 350 mg of the standardized almond skin extract, and 16.5 mg of maltodextrin. Each placebo capsule contained 400 mg of maltodextrin. Both extracts were obtained in the production plant of Biosearch Life in Talayuela, Cáceres (Spain), and the combination of the extracts was performed in the R&D facilities of Biosearch Life in Granada (Spain). Extracts were obtained from the fruit of O. europaea and the skin of P. dulcis through standardized extraction processes described elsewhere.21 The content of HT was analyzed by high-performance liquid chromatography with a diode-array detector (HPLC-DAD). HT maximum peak was detected at 280 nm with a retention time of 10.5 min.21 The content of total ASPs was determined by the Folin–Ciocalteu method.21 Capsules were prepared in the Department of Pharmaceutical Technology of the Faculty of Pharmacy of the University of Granada (Spain). The extracts and the placebo were provided in identical gelatin capsules packaged in identical plastic containers with a code number that referred to the volunteer code according to the randomization.
Study outcomes and data collection
The primary outcome of the study was the determination of oxLDL levels. Secondary outcomes included oxLDL/LDL-c ratio, TC, LDL-c levels, high-density lipoprotein-cholesterol (HDL-c) and serum triglycerides (TG) levels, and serum levels of the cytokines interleukin-1β (IL-1β), IL-6, and IL-10.
Subjects attended to Biosearch Life facilities in Granada at baseline, 4 weeks, and 8 weeks. Blood samples were collected after an overnight fast lasting at least 10 hours at baseline and at every follow-up visit, using Vacutainer® SST™ II Advance Tubes (BD, NJ) containing a thixotropic gel. Serum was obtained after centrifugation at 1000 g for 15 minutes and stored at −80°C.
Anthropometric measures were taken at every visit using standardized methods. Weight was determined using the Tanita BC-418 Body Composition Analyzer (Tanita, Tokyo, Japan). Height was determined using a height meter with an accuracy of 1 mm (range, 80–200 cm). Body mass index (BMI) was calculated as weight/height squared (kg/m2). All measurements were made by trained personnel.
Each subject's dietary intake was evaluated at baseline and at the end of the study to control any possible changes in their habitual habits. Subjects completed a 72 hours detailed dietary intake report, specifying the types of food consumed and serving weights. Daily food, energy intake, nutrient intake, and energy provided by macronutrients were calculated by the computer application Nutriber (Funiber, Barcelona, Spain). Subjects were also asked about their physical activity habits (type of activity and time performing per week) at baseline and at the end of the study.
Compliance was assessed at the end of the intervention by comparing the number of capsules provided and the number returned. Adverse events, defined as any unfavorable unintended effect, were recorded on the follow-up visits (at 4 and 8 weeks).
Biochemical parameter analysis
Lipid parameter levels (TC, LDL-c, HDL-c, and TG) were analyzed by an external laboratory (Reference Laboratory S.A, Barcelona, Spain) by standardized methods. Determination of oxLDL concentration in serum was done by ELISA Kit that recognizes natural and recombinant human oxLDL (Elabscience Biotechnology). The concentration of OxLDL (pg/mL) of each sample was read from the standard curve and finally expressed in ng/dl. Then the oxLDL/LDL-c ratio was calculated in ng/mg. Serum levels of IL-1β, IL-6, and IL-10 were analyzed by human uncoated ELISA Kits (Thermo Fisher Scientific).
Statistical analysis
Normal distribution was tested for all measured variables by normal probability plots and the Shapiro–Wilk test. Data are presented as mean (standard deviation) for continuous variables and as n (%) for categorical variables.
For comparisons between groups at the beginning of the study (extract vs. control), the continuous variables were analyzed with the Student t-test or the nonparametric method of Kruskal–Wallis, as appropriate, and categorical variables were analyzed with the chi-square tests.
All parameters will be compared between the groups, and the change with respect to the initial values per group will also be analyzed. For this purpose, bivariate analyses will be carried out, as well as the adjusted test based on the mixed regression model. The comparison between the means of the experimental group and the CG shall be carried out by means of the t-test when normality can be assumed. When non-normality can be assumed, nonparametric Mann–Whitney U test was performed. In addition, the mixed linear regression model that represents repeated measurements throughout the study (intrasubject-random effect) will be adjusted to the responses to test for change over time and between groups, as well as to find significant factors associated with the responses.
A general alpha level of 0.05 will be used as the cutoff point for statistical significance. Statistical analysis was carried out using SPSS software version 26.0 for Windows (SPSS, Chicago, IL).
RESULTS
A total of 30 subjects completed the study, since two subjects in the EG refused to participate before attending the baseline visit (Fig. 1). Compliance rate was confirmed to be very high (∼100%). No adverse events resulting from the intake of either type of treatment capsule were reported.
Of the 30 volunteers that finished the study, 13 were men (43.43%) and 17 were women (56.7%). Mean age of the subjects was 48.13 ± 12.9 years old (age range 20–64), and they presented a mean BMI of 25.38 ± 3.33 kg/m2. No significant differences were detected in the baseline characteristics of the volunteers except in the levels of cytokines IL-6, which was significantly higher in the CG (Table 1).
Table 1.
Baseline Characteristics of the Subjects Participating in the Study
| CG (n = 15) | EG (n = 15) | P-between groups | |
|---|---|---|---|
| Age (years) | 50.26 ± 13.29 | 46 ± 12.67 | .376 |
| Sex | .713 | ||
| Males | 6 (40) | 7 (46.7) | |
| Females | 9 (60) | 8 (53.3) | |
| BMI (kg/m2) | 25.57 ± 3.69 | 25.20 ± 3.05 | .769 |
| Obesity or overweight (>25 kg/m2) | 9 (60) | 8 (53.3) | .713 |
| Smokers | 2 (13.3) | 2 (13.3) | 1.000 |
| Practice physical activity regularly | 10 (66.7) | 8 (53.3) | .456 |
| Total-cholesterol (mg/dL) | 228.26 ± 28.09 | 222.93 ± 22.33 | .570 |
| LDL-c (mg/dL) | 120.26 ± 24.04 | 124.33 ± 20.34 | .621 |
| HDL-c (mg/dL) | 65.8 ± 12.49 | 60.05 ± 16.87 | .298 |
| Serum triglycerides (mg/dL) | 95 ± 34.98 | 101.93 ± 44 | .637 |
| Oxidized-LDL (ng/dL) | 12.34 ± 6.13 | 12.01 ± 6.38 | .887 |
| Oxidized-LDL/LDL-c ratio (ng/mg) | 0.104 ± 0.055 | 0.103 ± 0.058 | .952 |
| IL-1β (pg/mL) | 0.55 ± 0.25 | 0.32 ± 0.37 | .063 |
| IL-6 (pg/mL) | 0.58 ± 0.24 | 0.33 ± 0.18 | .004 |
| IL-10 (pg/mL) | 0.26 ± 0.33 | 0.06 ± 0.32 | .096 |
Values are mean ± SD for continuous variables and n (%) for categorical variables. P indicates differences between groups.
BMI, body mass index; CG, control group; EG, extract group; HDL-c, high-density lipoprotein-cholesterol; IL-1β, interleukin-1β; LDL, low-density lipoprotein; LDL-c, LDL-cholesterol; SD, standard deviation.
Energy and nutrient intakes at baseline and week 8 are shown in Table 2. There were no significant differences at baseline between the intervention groups nor between baseline and the end of the intervention in each group (Extract or Control). There were also no changes during the study in physical activity performance (data not shown), and subjects in both groups neither significantly changed their BMI between baseline and week 8 (Table 2).
Table 2.
Dietary and Anthropometric Parameters of the Subjects Consuming the Placebo or the Extract During 8 Weeks
| CG (n = 15) |
EG (n = 15) |
|||||
|---|---|---|---|---|---|---|
| Baseline | 8 weeks | P-time | Baseline | 8 weeks | P-time | |
| Energy (kcal) | 1737.86 ± 277.79 | 1687.4 ± 281.05 | .096 | 1708.28 ± 471.5 | 1683.32 ± 386.93 | .703 |
| Total fat (%En) | 38.53 ± 5.38 | 35.38 ± 8.81 | .287 | 34.92 ± 5.42 | 35 ± 5.49 | .960 |
| Saturated fat (%En) | 8.73 ± 3.27 | 7.37 ± 2.74 | .224 | 8.93 ± 1.72 | 8.8 ± 1.84 | .863 |
| Monosaturated fat (%En) | 18.78 ± 3.77 | 16.59 ± 4.79 | .181 | 15.94 ± 4.33 | 16.48 ± 4.13 | .637 |
| Polysaturated fat (%En) | 5.83 ± 2.59 | 5.41 ± 2.68 | .469 | 4.35 ± 1.36 | 4.3 ± 1.63 | .878 |
| Dietary cholesterol (mg/day) | 218.4 ± 85.44 | 204.38 ± 88.04 | .559 | 234.72 ± 99 | 222.76 ± 87.24 | .712 |
| Protein (%En) | 18.15 ± 2.35 | 18.55 ± 2.17 | .589 | 20.52 ± 2.47 | 18.93 ± 5.22 | .231 |
| Carbohydrates (%En) | 44.19 ± 7.7 | 45.2 ± 8.46 | .726 | 44.99 ± 7.38 | 45.47 ± 6.59 | .815 |
| Fiber (g/day) | 19.45 ± 6.45 | 16.46 ± 5.94 | .073 | 16.51 ± 5.33 | 14.73 ± 5.82 | .339 |
| BMI (kg/m2) | 25.57 ± 3.69 | 25.70 ± 3.87 | .524 | 25.20 ± 3.05 | 25.31 ± 2.96 | .359 |
Values are mean ± SD. P indicates differences between baseline and 8 weeks in each treatment group.
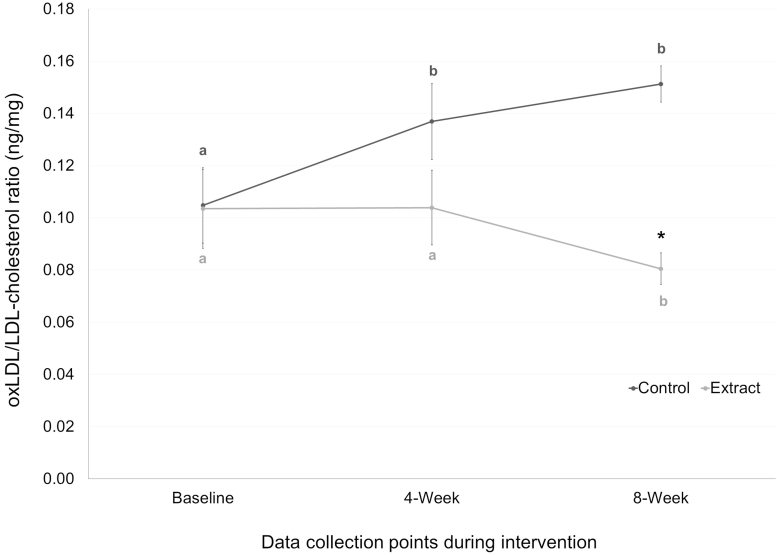
Table 3 shows the values for the lipid parameters and the oxidized lipid variables evaluated during the study. No significant changes were observed between baseline and the end of the intervention in TC, LDL-c, HDL-c, or serum TG in any group. Regarding lipid oxidative status, oxLDL levels significantly increased from baseline in the CG at week 4 (P = .01) and at week 8 (P = .03), whereas tended to decrease in the EG, being significantly lower in the EG than in the CG after 8 weeks of treatment (P < .001). When we analyzed the oxLDL/LDL-c ratio, an accurate estimation of in vivo LDL oxidation,22 we observed a significant increase of the oxLDL/LDL-c ratio from baseline in the CG at week 4 (P = .001) and at week 8 (P = .002) and a significant decrease of this ratio in the EG at week 8 (P = .047), being also significantly lower the oxLDL/LDL-c ratio levels in the EG than in the CG after 8 weeks of treatment (P < .001) (Table 3 and Fig. 2).
Table 3.
Lipid Parameters and Oxidized Low-Density Lipoprotein of the Subjects Consuming the Placebo or the Extract at Baseline, 4 Weeks, and 8 Weeks
| CG | EG | P-between groups | |
|---|---|---|---|
| Total cholesterol (mg/dL) | |||
| Baseline | 228.26 ± 28.09 | 222.93 ± 22.33 | .570 |
| 4 weeks | 232.6 ± 36.97 | 236.06 ± 39.77 | .807 |
| 8 weeks | 232.46 ± 48.39 | 233.13 ± 26.07 | .963 |
| LDL-c (mg/dL) | |||
| Baseline | 120.26 ± 24.04 | 124.33 ± 20.34 | .621 |
| 4 weeks | 128.4 ± 30.85 | 131.4 ± 27.72 | .781 |
| 8 weeks | 125.46 ± 35.61 | 131.86 ± 26.23 | .580 |
| HDL-c (mg/dL) | |||
| Baseline | 65.8 ± 12.49 | 60.05 ± 16.87 | .298 |
| 4 weeks | 66.39 ± 9.24 | 57.6 ± 12.87 | .073 |
| 8 weeks | 65.78 ± 14.62 | 57.53 ± 11.24 | .094 |
| Serum triglycerides (mg/dL) | |||
| Baseline | 95 ± 34.98 | 101.93 ± 44 | .637 |
| 4 weeks | 102.6 ± 41.2 | 106.33 ± 53.43 | .832 |
| 8 weeks | 101.13 ± 39.46 | 111.4 ± 54.49 | .559 |
| OxLDL (ng/dL) | |||
| Baseline | 12.34 ± 6.13 | 12.01 ± 6.38 | .887 |
| 4 weeks | 17.14 ± 6.83* | 13.24 ± 7.04 | .135 |
| 8 weeks | 18.76 ± 3.91* | 10.34 ± 4.22 | <.001 |
| OxLDL/LDL-c ratio (ng/mg) | |||
| Baseline | 0.104 ± 0.055 | 0.103 ± 0.058 | .952 |
| 4 weeks | 0.136 ± 0.056* | 0.103 ± 0.055 | .117 |
| 8 weeks | 0.151 ± 0.025* | 0.08 ± 0.023* | <.001 |
Values are mean ± SD adjusted by sex, age, obesity or overweight, smoking, and physical activity habits. P indicates differences between groups at each time point. Asterisks (*) indicate P < .05 between baseline and 4 weeks and 8 weeks.
OxLDL, oxidized low-density lipoprotein.
FIG. 2.
OxLDL/LDL-cholesterol ratio (ng/mg) of the subjects consuming the placebo or the extract at baseline, 4 weeks, and 8 weeks. Mean values are represented with points (dark gray for control group and light gray for extract group), whereas SE is represented by vertical bars. Different letters mean significant differences between time points in each group. Asterisk (*) indicates P < .05 between groups at each time point. OxLDL, oxidized low-density lipoprotein; SE, standard error.
Levels of serum cytokines evaluated in this study are shown in Table 4. Levels of IL-1β tended to decrease in both groups at the end of the intervention, although only reached significance at week 8 in the CG (P = .014). However, IL-1β levels were significantly higher in the CG than in the EG at week 4 (P = .004). Regarding levels of IL-6, they significantly increased at week 4 (P = .001) and week 8 (P = .002) in the CG, being significantly higher in the CG than in the EG at week 4 (P = .049). Levels of IL-10 significantly increased at week 4 in both groups (P = .002 for CG and P = .001 for EG) and then significantly decreased only in the CG at week 8 (P = .026).
Table 4.
Cytokine Levels of the Subjects Consuming the Placebo or the Extract at Baseline, 4 Weeks, and 8 Weeks
| CG | EG | P-between groups | |
|---|---|---|---|
| IL-1β (pg/mL) | |||
| Baseline | 0.55 ± 0.25 | 0.32 ± 0.37 | .063 |
| 4 weeks | 0.58 ± 0.24 | 0.33 ± 0.18 | .004 |
| 8 weeks | 0.26 ± 0.33* | 0.06 ± 0.32 | .096 |
| IL-6 (pg/mL) | |||
| Baseline | 0.23 ± 0.29 | 0.67 ± 0.51 | .007 |
| 4 weeks | 3.3 ± 2.67* | 1.56 ± 1.91 | .049 |
| 8 weeks | 3.03 ± 2.7* | 1.26 ± 2.35 | .067 |
| IL-10 (pg/mL) | |||
| Baseline | 1.44 ± 1.18 | 1.54 ± 1.04 | .815 |
| 4 weeks | 2.84 ± 0.7* | 2.65 ± 1.02* | .565 |
| 8 weeks | 1.31 ± 0.82 | 0.88 ± 0.73* | .137 |
Values are mean ± SD adjusted by sex, age, obesity or overweight, smoking, and physical activity habits. P indicates differences between groups at each time point.
Asterisks (*) indicate P < .05 between baseline and 4 weeks and 8 weeks.
DISCUSSION
The present study shows that the daily consumption of 7.5 mg of HT plus 210 mg of ASPs through an oral supplement seems to protect LDL from oxidation and to prevent inflammatory status in subjects with moderately hypercholesterolemia. Several studies have shown the beneficial cardiovascular effect of HT17 and ASPs,18 but, to our knowledge, this is the first study that evaluated the combined effect of both compounds on cardiovascular risk factor, such as lipid oxidation and inflammation parameters.
There are several clinical trials that reported a decrease in oxLDL levels after the intake of foods rich in HT.23–25 Indeed, the EFSA reported the claim that the daily consumption of 5 mg of HT (or its derivatives) from olive protects LDL from oxidative damage.19 This polyphenol seems to exert its antioxidant effect by the modulation of different pathways,26 such as the activation of multiple genes encoding antioxidant response elements in vascular endothelial cells, the stimulation of mitochondrial biogenesis by increasing PPARGC1α, and by the modulation of the expression of miRNAs that may affect the expression of genes involved in atherogenesis.27 Regarding ASPs, it has been observed in vitro and in animal studies that ASPs may protect LDL against oxidation by acting as ROS scavenger, inducing quinine reductase, and by the stabilization of the LDL conformation.28–30 Moreover, a recent randomized acute clinical study reported that milk enriched with ASPs increased the resistance of LDL to oxidation compared to skim milk.31 The results obtained in this study support the observed effect of HT and ASPs in protecting LDL from oxidation and, therefore, their role as cardioprotective compounds. Moreover, the combined intake of both ingredients may suppose a synergic action. An in vitro study performed by our research group suggests that the addition of ASPs may increase HT bioavailability,21 which has been observed to depend on the vehicle used, being more effective when ingested within the olive oil than as an extract.20 However, further studies will be needed to test these hypotheses.
Consumption of almonds is proved to have positive effects on lipoprotein profile, due to their favorable fat composition and fiber content.32 However, to our knowledge, no study has tested the effect of the almond skin extract on lipoprotein concentration. In addition, the effect of HT on lipid parameter levels remains unclear. Some animal studies showed that HT enriched extracts have reduced plasmatic levels of TC and lipids.33,34 However, results in clinical trials are controversial. Covas et al., in a crossover study with healthy men, found no changes in total-cholesterol or in LDL after 3 weeks of intervention with olive oil enriched in HT, but a significant increment of HDL levels.24 On the contrary, another study with the same product found no differences in lipid parameter concentration.25 Main difference between clinical trial and animal studies is that animals were fed with high fat or high cholesterol diets, and HT attenuated cardiovascular changes associated to these unhealthy diets. Although our volunteers are moderately hypercholesterolemic, they followed a relative healthy and nonchanging diet during the study, so this may be the reason we found no changes in lipid parameters after the intervention.
The anti-inflammatory properties of HT and ASPs have been reported in animal and in vitro studies.35,36 Moreover, the administration of olive oil enriched in HT decreased inflammatory parameters such as cytokines IL-6 and IL-1β in postprandial37,38 and long-term clinical studies.39,40 The effect on anti-inflammatory parameters such as the cytokine IL-10 is less clear, although polyphenols from olive may activate this cytokine in carotid plaques from hypertensive patients.41 Several authors claimed that oxidative stress and inflammation are inter-related processes, and therefore, the anti-inflammatory effect associated to HT and ASP may be related to the free radical scavenging capacity of both compounds.42 Indeed, a postprandial study performed in obese subjects showed that the reduction of the postprandial inflammatory response has attributed to the inhibition of nuclear factor kappa B, which is an important link between oxidation and inflammation in the postprandial state.38 In the present study, the intake of the HT+ ASPs supplement avoided the increase of IL-6 compared to the CG and tended to decrease IL-1β values, whereas it activated IL-10 at the midterm of the intervention. These results are in line with those obtained in LDL oxidation levels, taking into account the relationship between oxidation and inflammation. Therefore, through the protection of LDL from oxidative damage, the combined intake of HT and ASPs may prevent the inflammatory status associated to the oxidation process.
One strength of the present study was the design as a double-blind randomized controlled trial controlled by placebo. This methodology allowed to avoid bias related to confounding factors (through a CG), selection bias (through randomization), and interpretation bias (through double blinding). However, a limitation of the study was the low sample size, limiting by the fact that this is a pilot study. In addition, because of the fact that we only recruited moderately hypercholesterolemic subjects, the generalizability of the results cannot be assumed.
A recent study in Spanish population showed that one of the most related traits to the 10-year predicted risk of suffering a coronary event (Framingham-REGICOR score) was oxLDL levels, whereas LDL-c concentration per se was not related.43 Therefore, therapeutic measures to protect LDL from oxidative damage should be taken, to reduce cardiovascular risk. Our results support the use of the polyphenols HT and ASPs as effective and cardioprotective nutraceutical agents and demonstrated, for the first time, the efficacy of the combined intake of both compounds in protecting LDL from oxidation and preventing inflammation.
In conclusion, the daily consumption of a combination of an olive fruit extract and an almond skin extract (containing 7.5 mg of HT and 210 mg of ASPs) for 8 weeks seems to protect LDL from oxidation and to prevent inflammatory status in subjects with moderately hypercholesterolemia. This, together with the fact that we are using ingredients from foods, and the study subjects not having suffered from adverse effects make the use of this combination of extracts from olive fruit and almond skin, rich in HT and ASPs, a suitable and secure oral supplement that helps to reduce cardiovascular risk in moderately hypercholesterolemic subjects.
AUTHORS' CONTRIBUTIONS
J.L.L-L., M.O., and R.B-R. were responsible for study concept and design, J.F., J.A.M.-L., R.L., C.R., and O.B were responsible for carrying out the trial and data handling/validation. R.B-R. analyzed and interpreted the data and drafted the article, and M.O. reviewed the article and supervised the project. All authors have read and agreed to the published version of the article.
ETHICAL APPROVAL
This study was designed and conducted in accordance with the ethical standards as put forth in the Declaration of Helsinki 1975, as revised in 2008. The study protocol, informed consent forms, and associated documents were reviewed and approved by the Regional Ethics Committee (Granada, Spain). The trial was registered in the U.S. Library of Medicine (www.clinicaltrial.gov) as NCT04029727.
AUTHOR DISCLOSURE STATEMENT
J.A.M-L, R.L., C.R., O.B., J.L.L.-L., M.O., and R.B.-R. are employees of Biosearch Life, owner of the patent PCT/ES2020/070147, which is associated with the study product. J.F. declares that he has no competing interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the article; or in the decision to publish the results.
FUNDING INFORMATION
This research was funded by the Centre for the Development of Industrial Technology (CDTI) and cofinanced by European Regional Development Fund (ERDF) through Operational Program for Spain 2014 to 2020 (Project no. IDI_20190733).
REFERENCES
- 1. Kaptoge S, Pennells L, De Bacquer D, et al. : World Health Organization cardiovascular disease risk charts: Revised models to estimate risk in 21 global regions. Lancet Glob Health 2019;7:e1332–e1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Joseph P, Leong D, McKee M, et al. : Reducing the global burden of cardiovascular disease, part 1: The epidemiology and risk factors. Circ Res 2017;121:677–694 [DOI] [PubMed] [Google Scholar]
- 3. Frostegård J: Immunity, atherosclerosis and cardiovascular disease. BMC Med 2013;11:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marchio P, Guerra-Ojeda S, Vila JM, Aldasoro M, Victor VM, Mauricio MD: Targeting early atherosclerosis: A focus on oxidative stress and inflammation. Oxid Med Cell Longev 2019;1:8563845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arsenault BJ, Bourgeois R, Mathieu P: Do Oxidized lipoproteins cause atherosclerotic cardiovascular diseases? Can J Cardiol 2017;33:1513–1516 [DOI] [PubMed] [Google Scholar]
- 6. Trpkovic A, Resanovic I, Stanimirovic J, et al. : Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit Rev Clin Lab Sci 2015;52:70–85 [DOI] [PubMed] [Google Scholar]
- 7. Pirillo A, Norata GD, Catapano: AL. LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm 2013;2013:152786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arnett DK, Blumenthal RS, Albert MA, et al.: 2019. ACC/AHA guideline on the primary prevention of cardiovascular disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines J Am Coll Cardiol 2019;74:1376–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hunter PM, Hegele RA: Functional foods and dietary supplements for the management of dyslipidaemia. Nat Rev Endocrinol 2017;13:278–288 [DOI] [PubMed] [Google Scholar]
- 10. Szulińska M, Skrypnik D, Michałowska J, Bogdański P: Non-pharmacological modification of endothelial function: An important lesson for clinical practice. Adv Hyg Exp Med 2018;72:89–100 [Google Scholar]
- 11. Fraga CG, Croft KD, Kennedy DO, Tomás-Barberán FA: The effects of polyphenols and other bioactives on human health. Food Funct 2019;10:514–528 [DOI] [PubMed] [Google Scholar]
- 12. Kim Y, Keogh JB, Clifton PM: Benefits of nut consumption on insulin resistance and cardiovascular risk factors: Multiple potential mechanisms of actions. Nutrients 2017;9:1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bladé C, Arola L, Salvadó MJ: Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol Nutr Food Res 2010;54:37–59 [DOI] [PubMed] [Google Scholar]
- 14. Tejada S, Pinya S, Del Mar Bibiloni M, et al. : Cardioprotective effects of the polyphenol hydroxytyrosol from olive oil. Curr Drug Targets 2017;18:1477–1486 [DOI] [PubMed] [Google Scholar]
- 15. de Las Hazas MCL, Rubio L, Macia A, Motilva MJ: Hydroxytyrosol: Emerging trends in potential therapeutic applications. Curr Pharm Des 2018;24:2157–2179 [DOI] [PubMed] [Google Scholar]
- 16. Omar SH, Kerr PG, Scott CJ, Hamlin AS, Obied HK: Olive (Olea europaea L.) biophenols: A nutriceutical against oxidative stress in SH-SY5Y cells. Molecules 2017;22:E1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vilaplana-Pérez C, Auñón D, García-Flores LA, Gil-Izquierdo A: Hydroxytyrosol and potential uses in cardiovascular diseases, cancer, and AIDS. Front Nutr 2014;1:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bolling BW: Almond polyphenols: Methods of analysis, contribution to food quality, and health promotion. Compr Rev Food Sci Food Saf 2017;16:346–368 [DOI] [PubMed] [Google Scholar]
- 19. EFSA: Scientific opinion on the substantiation of health claims related to quercetin and protection of DNA, proteins and lipids from oxidative damage. EFSA J 2011;9:1–25 [Google Scholar]
- 20. Robles-Almazan M, Pulido-Moran M, Moreno-Fernandez J, et al. : Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res Int 2018;105:654–667 [DOI] [PubMed] [Google Scholar]
- 21. Bañuelos Hortigüela O, Blanco Rojo R, Maldonado Lobón JA, Pérez Martínez L, López Larramendi JL, Olivares Martín MM: Uses and compositions based on polyphenols to improve the oral bioavailability of hydroxytyrosol. OEPM (Spain) PCT/ES2020/070147 February 28, 2020 (applied) [Google Scholar]
- 22. Scheffer PG, Bos G, Volwater HGFM, Dekker JM, Heine RJ, Teerlink T: Associations of LDL size with in vitro oxidizability and plasma levels of in vivo oxidized LDL in Type 2 diabetic patients. Diabet Med 2003;20:563–567 [DOI] [PubMed] [Google Scholar]
- 23. Mateos R, Martínez-López S, Baeza Arévalo G, Amigo-Benavent M, Sarriá B, Bravo-Clemente L: Hydroxytyrosol in functional hydroxytyrosol-enriched biscuits is highly bioavailable and decreases oxidised low density lipoprotein levels in humans. Food Chem 2016;205:248–256 [DOI] [PubMed] [Google Scholar]
- 24. Covas M-I, Nyyssönen K, Poulsen HE, et al. : The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Ann Intern Med 2006;145:333–341 [DOI] [PubMed] [Google Scholar]
- 25. de la Torre-Carbot K, Chávez-Servín JL, Jaúregui O, et al. : Elevated circulating LDL phenol levels in men who consumed virgin rather than refined olive oil are associated with less oxidation of plasma LDL. J Nutr 2010;140:501–508 [DOI] [PubMed] [Google Scholar]
- 26. Marrugat J, Covas M-I, Fitó M, et al. : Effects of differing phenolic content in dietary olive oils on lipids and LDL oxidation—A randomized controlled trial. Eur J Nutr 2004;43:140–147 [DOI] [PubMed] [Google Scholar]
- 27. Tomé-Carneiro J, Crespo MC, Iglesias-Gutierrez E, et al. : Hydroxytyrosol supplementation modulates the expression of miRNAs in rodents and in humans. J Nutr Biochem 2016;34:146–155 [DOI] [PubMed] [Google Scholar]
- 28. Chen C-YO, Blumberg JB: In vitro activity of almond skin polyphenols for scavenging free radicals and inducing quinone reductase. J Agric Food Chem 2008;56:4427–4434 [DOI] [PubMed] [Google Scholar]
- 29. Chen C-Y, Milbury PE, Chung S-K, Blumberg J: Effect of almond skin polyphenolics and quercetin on human LDL and apolipoprotein B-100 oxidation and conformation. J Nutr Biochem 2007;18:785–794 [DOI] [PubMed] [Google Scholar]
- 30. Chen C-Y, Milbury PE, Lapsley K, Blumberg JB: Flavonoids from almond skins are bioavailable and act synergistically with vitamins C and E to enhance hamster and human LDL resistance to oxidation. J Nutr 2005;135:1366–1373 [DOI] [PubMed] [Google Scholar]
- 31. Chen C-YO, Milbury PE, Blumberg JB: Polyphenols in almond skins after blanching modulate plasma biomarkers of oxidative stress in healthy humans. Antioxidants 2019;8:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kamil A, Chen C-YO: Health benefits of almonds beyond cholesterol reduction. J Agric Food Chem 2012;60:6694–6702 [DOI] [PubMed] [Google Scholar]
- 33. Jemai H, Fki I, Bouaziz M, et al. : Lipid-lowering and antioxidant effects of hydroxytyrosol and its triacetylated derivative recovered from olive tree leaves in cholesterol-fed rats. J Agric Food Chem 2008;56:2630–2636 [DOI] [PubMed] [Google Scholar]
- 34. Poudyal H, Campbell F, Brown L: Olive leaf extract attenuates cardiac, hepatic, and metabolic changes in high carbohydrate–, high fat–fed rats. J Nutr 2010;140:946–953 [DOI] [PubMed] [Google Scholar]
- 35. Tabernero M, Sarriá B, Largo C, et al. : Comparative evaluation of the metabolic effects of hydroxytyrosol and its lipophilic derivatives (hydroxytyrosyl acetate and ethyl hydroxytyrosyl ether) in hypercholesterolemic rats. Food Funct 2014;5:1556–1563 [DOI] [PubMed] [Google Scholar]
- 36. Huang W-C, Chen C-Y, Wu S-J. Almond skin polyphenol extract inhibits inflammation and promotes lipolysis in differentiated 3T3-L1 adipocytes. J Med Food 2017;20:103–109 [DOI] [PubMed] [Google Scholar]
- 37. Camargo A, Rangel-Zuñiga OA, Haro C, et al. : Olive oil phenolic compounds decrease the postprandial inflammatory response by reducing postprandial plasma lipopolysaccharide levels. Food Chem 2014;162:161–171 [DOI] [PubMed] [Google Scholar]
- 38. Perez-Herrera A, Delgado-Lista J, Torres-Sanchez LA, et al. : The postprandial inflammatory response after ingestion of heated oils in obese persons is reduced by the presence of phenol compounds. Mol Nutr Food Res 2012;56:510–514 [DOI] [PubMed] [Google Scholar]
- 39. Fitó M, Cladellas M, de la Torre R, et al. : Anti-inflammatory effect of virgin olive oil in stable coronary disease patients: A randomized, crossover, controlled trial. Eur J Clin Nutr 2008;62:570–574 [DOI] [PubMed] [Google Scholar]
- 40. Casas R, Urpi-Sardà M, Sacanella E, et al.: Anti-inflammatory effects of the mediterranean diet in the early and late stages of atheroma plaque development. Mediators Inflamm 2017;2017:3674390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Filipek A, Czerwińska ME, Kiss AK, Polański JA, Naruszewicz M: Oleacein may inhibit destabilization of carotid plaques from hypertensive patients. Impact on high mobility group protein-1. Phytomedicine 2017;32:68–73 [DOI] [PubMed] [Google Scholar]
- 42. de Souza PAL, Marcadenti A, Portal VL: Effects of olive oil phenolic compounds on inflammation in the prevention and treatment of coronary artery disease. Nutrients 2017;9:1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hernáez Á, Soria-Florido MT, Schröder H, et al. : Role of HDL function and LDL atherogenicity on cardiovascular risk: A comprehensive examination. PLoS One 2019;14:e0218533. [DOI] [PMC free article] [PubMed] [Google Scholar]