Abstract
The cholinergic nervous system has been implicated in mood disorders, evident in the fast-onset antidepressant effects of scopolamine, a potent muscarinic antagonist, in clinical studies. One prominent disadvantage of the use of scopolamine in the treatment of depression is its detrimental effects on cognition, especially as such effects might aggravate cognitive deficits that occur with depression itself. Thus, the identification of antimuscarinic drugs that are free of such detrimental effects may provide an important avenue for the development of novel therapeutics for the management of depression. The present data in rats indicate that a historical muscarinic antagonist, L-687,306, and a muscarinic antagonist of our own design, CJ2100, were as or more effective than scopolamine in antagonizing both the bradycardic effects of the muscarinic agonist arecoline in cardiovascular studies and its discriminative stimulus and rate-decreasing effects in behavioral studies. Additionally, both novel muscarinic antagonists were as effective as scopolamine in decreasing immobility in the forced swim test, a preclinical indicator of potential antidepressant activity. However, at equieffective or even larger doses, they were considerably less disruptive than scopolamine in assays of cognition-related behavior. All three drugs displayed high specificity for the mAChRs with few off-target binding sites, and CJ2100 showed modest affinity across the mAChRs when compared with L-687,306 and scopolamine. These data emphasize the dissimilar pharmacological profiles that are evident across antimuscarinic compounds and the potential utility of novel antagonists for the improved treatment of depression.
SIGNIFICANCE STATEMENT
Some clinical studies with the muscarinic antagonist scopolamine document its ability to produce antidepressant effects in patients with mood disorders; however, scopolamine also has well known adverse effects on both autonomic and centrally mediated physiological functions that limit its therapeutic use. This study characterizes the cardiovascular and discriminative stimulus effects of two novel muscarinic antagonists, L-687,306 and CJ2100, that produce antidepressant-like effects in a rodent model (forced swim test) without affecting touchscreen-based cognitive performance (titrating psychomotor vigilance and delayed matching-to-position).
Introduction
Major depressive disorder (MDD) affects approximately 16% of Americans (Kessler et al., 2006) and, notwithstanding some recent advances, an urgent need remains for antidepressant drugs that, unlike tricyclic antidepressants and selective serotonin reuptake inhibitors, have immediate beneficial effects. Furthermore, many currently available antidepressant medications impair cognition and produce other adverse effects that limit their widespread application (Blair and Dauner, 1992; Sayyah et al., 2016). Improvement in side effect liability, as well as faster onset and better clinical efficacy, are major therapeutic goals in the development of novel antidepressants (O’Leary et al., 2015; Wang et al., 2015).
It is widely accepted that the cholinergic nervous system plays a prominent role in learning and memory. It is becoming better known that this system is also implicated in the control of mood states (Deutsch, 1971; Leaderbrand et al., 2016; Dulawa and Janowsky, 2019; Drevets et al., 2020). For example, clinical data show that the muscarinic acetylcholine receptor (mAChR) antagonist scopolamine produces antidepressant effects in patients with either MDD or bipolar disorder (Drevets and Furey, 2010; Drevets et al., 2013; Furey et al., 2006; Drevets et al., 2020; Witkin et al., 2020). Despite such intriguing and hopeful clinical findings, the therapeutic value of scopolamine for treating MDD is limited by its well documented adverse effects, which include disturbances that are both autonomic (blurred vision, tachycardia) and centrally mediated (drowsiness, light-headedness) (Drevets et al., 2020). Large doses, potentially above those required for antidepressant activity, can result in mental confusion or delirium, and memory loss (Furey et al., 2008; Klinkenberg and Blokland, 2010; Lakstygal et al., 2019; Drevets et al., 2020). Since MDD is also associated with deficits in cognition (Porter et al., 2003; Clark et al., 2009), the use of scopolamine has the potential to exacerbate this problem. Nevertheless, the favorable clinical data obtained with scopolamine have led to the question of whether it may be possible to develop antimuscarinic drugs that act via anticholinergic mechanisms to relieve depression, yet have fewer effects on learning, memory, and attention.
One compound that has spurred interest in its further development as an antidepressant medication is L-687,306. This novel muscarinic ligand, developed as a possible treatment of Alzheimer disease in the early 1990s (Freedman et al., 1992, 1993; Baker et al., 1992), was shown to have good affinity with limited or no efficacy at the M1, M2, and M3 receptor subtypes and, in vivo, a reduced side effect profile compared with conventional antimuscarinics like scopolamine. L-687,306 was shown to attenuate the brief but profound bradycardia produced by the muscarinic agonist arecoline (Winger et al., 2020), indicative of its scopolamine-like antagonist actions in the autonomic nervous system. L-687,306 was able to antagonize both the discriminative stimulus and rate-suppressing effects of arecoline far better than scopolamine (Winger et al., 2020), confirming that it has central anticholinergic activity with apparently less rate-suppressing, possibly sedative, effects. In conjunction, these findings encourage the view that it is possible to develop novel antimuscarinics that may be therapeutically effective and produce fewer side effects than the conventional antimuscarinic scopolamine. Notwithstanding these intriguing findings with L-687,306, its complicated stereoselective synthesis has made it a difficult compound to study. To address this limitation, we have recently advanced a cyclopropyl analog of arecoline, CJ2100. Of importance to our efforts, CJ2100, unlike L-687,306, can be produced in only a few synthetic steps with readily available starting materials.
The purpose of the present studies was to compare the activity of L-687,306 and CJ2100 with that of scopolamine in vitro and across several in vivo assays of autonomic and central activity. The autonomic effects of the three antimuscarinics (scopolamine, L-687,306, and CJ2100) were interrogated by examining their ability to antagonize the effects of arecoline on heart rate. The centrally mediated effects of these antimuscarinics were studied by determining their ability to 1) antagonize the discriminative stimulus and rate-suppressing effects of arecoline; 2) decrease measures of immobility in the forced swim test (FST) as a first-pass indication of potential antidepressant action; and 3) produce a direct disruption of cognition-related behavior. The outcome of these studies shows that we have identified molecules that bind with high specificity to the mAChRs with established antimuscarinic actions and scopolamine-like activity in the FST, but with few other direct behavioral effects. These findings show that disruption of cognition-related behavior is not an inevitable result of blockade of muscarinic receptors and, thus, support the further development of antidepressant drugs with an antimuscarinic basis of action and reduced side effect liability.
Materials and Methods
Animals.
Adult male Sprague-Dawley rats weighing approximately 275–375 g purchased from Envigo RMS (Indianapolis, IN) were used in the cardiovascular, arecoline discrimination, and FST studies. Because of the superior visual abilities in rat strains with pigmented eyes (Jacobs et al., 2001), 12 adult Long-Evans rats (six male and six female) weighing approximately 275–375 g purchased from Charles River Laboratories (Wilmington, MA) were used in the touchscreen-based cognition studies. Previous work demonstrated that there were few, if any, behavioral differences or effects of drugs in the FST between Sprague-Dawley and Long-Evan rats (Jutkiewicz et al., 2003). All rats were housed in climate-controlled vivaria with a 12-hour light/dark cycle with lights on at 7:00 AM. Subjects in the cardiovascular assay and FST had ad libitum access to food and water. All other animals were maintained at approximately 85% of their free-feeding weight via postsession portions of ∼10–15 g of rodent chow and had unrestricted access to water in their home cage. Experimental sessions were conducted 5 days a week (Monday through Friday). All experimental protocols were approved by the Institutional Animal Care and Use Committees at the University of Texas Health San Antonio, the University of Michigan, and McLean Hospital, and conformed to the guidelines from the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animals Resources, Commission on Life Sciences (National Research Council, 2011).
In Vitro Assays.
Primary and secondary binding assays were performed by the National Institute of Mental Health’s Psychoactive Drug Screening Program. The National Institute of Mental Health utilizes an integrated sample storage, retrieval, and liquid handling robotic system to prepare assay plates. Using stable CHO lines expressing one of M1-M5 mAChRs, primary radioligand binding assays are performed using a 96-well drug plate containing multiple test compounds (each run in quadruplicate), one reference compound, or buffer only ([3H]QNB only to determine total binding) with 25 μl per well at 50 μM.
For primary assays, compounds showing a minimum of 50% inhibition at 10 μM were evaluated in secondary radioligand binding assays to determine binding affinities at equilibrium for specific targets of interest. Compounds are tested at 11 concentrations in triplicate with the hot ligand at concentrations close to its Kd. Readers are referred to the procedures found on the PDSP website for specific experimental details: https://pdsp.unc.edu/ims/investigator/web/
Cardiovascular Assay.
Rats were prepared with indwelling transmitters to obtain heart rate data. Under ketamine (90 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) anesthesia, a telemetric transmitter (TA11PA-C40 or TL11M2-C50-PXT, Data Sciences International; Transoma Medical Inc., St. Paul, MN) was surgically inserted into a subcutaneous pocket on the rat’s side. Transmitters conveyed heart rate data that was collected and stored digitally on a computer using Physiotel receivers and the Data Sciences International’s Data Exchange Matrix (Data Sciences International; Transoma Medical Inc.) and compiled with the Dataquest A.R.T. system and its Gold Analysis 3.01 software. After surgery, rats were singly housed and allowed to recover for at least 7 days before initiating studies. The procedures are described in detail in Jutkiewicz et al. (2013), and followed the description of cardiovascular assays by Delaunois et al. (2009). At the start of an experimental session, rats in their home cages were placed on top of the receivers; heart rate data were collected for at least 1 hour from undisturbed animals. Each rat was then given a saline injection to provide data on the response to injection and handling procedures. Thirty minutes later, a second injection was given: either a second saline injection, 0.3 mg/kg CJ2100, or 1 mg/kg CJ2100. After 15 minutes, 10 mg/kg arecoline was given as the final injection. Heart rate measures were taken during the light cycle, between 10:00 AM and 3:00 PM. Data were collected for at least 2 hours after the last injection. All injections were administered subcutaneously in a volume of 1 ml/kg.
This same procedure was used to evaluate the effects of a range of doses of L-687,306 and scopolamine on arecoline-induced bradycardia. Those data are published in Winger et al. (2020), and the most effective doses of these antagonists are included in the Results section for comparison with CJ2100.
Arecoline Discrimination.
Drug discrimination procedures were performed in standard 2-lever operant conditioning chambers (30.5 × 24.1 × 21.0 cm) with stainless steel grid floors and contained within ventilated, sound-attenuating boxes (Med Associates, St. Albans, VT). Subjects (male Sprague-Dawley rats) were initially trained to respond under a fixed-ratio (FR) 10 schedule of reinforcement on either lever. Completion of the schedule requirements resulted in delivery of a 45 mg sucrose pellet. Subsequently, on alternating days, saline or arecoline (1 mg/kg) was given subcutaneously just before the rat was placed in the chamber. The session began 5 minutes later with illumination of the house light and stimulus lights and presentation of both levers. Responses on the left lever were designated correct after drug administration; completing the FR requirement on that lever resulted in retraction of both levers and delivery of a sucrose pellet. When saline rather than arecoline was injected before a session, completing the FR requirement on the right lever resulted in retraction of the levers and reinforcer delivery. Completing the FR requirement on the incorrect lever caused the levers to be retracted and the stimulus lights to be turned off for 10 seconds with no sucrose pellet delivery. Testing began when the following criteria were met on two consecutive training days: 1) responding on the first FR of the session was completed on the injection-appropriate lever, and 2) > 85% of total session responses were made on the injection-appropriate lever. During test sessions, completed FRs on either lever were reinforced with sucrose pellet delivery. For evaluation of antagonist effects, a dose of the selected antagonist was administered 15 minutes before the start of the session, and animals were returned to their home cages. Arecoline was given immediately before the rats were placed in the response chambers, as indicated above. Rats were tested no more than twice weekly.
Forced Swim Test.
The FST used in the current study has been described previously (Jutkiewicz et al., 2003, 2004) and consists of a 1-day, 15-minute swim only. Previous studies demonstrated that known, clinically-used antidepressants produce similar effects in a 1-day FST to those observed in the 2-day Porsolt swim test (Broom et al., 2002). Removing the first preexposure swim may eliminate some concerns about rats “learning” to become immobile and the potential effects of evaluating drugs that alter learning and memory (e.g., scopolamine). Although the FST lacks face and construct validity for modeling depression in rats or mice, this assay is useful for identifying drugs that have antidepressant actions in clinical populations (for reviews see West, 1990; Lucki, 1997; Borsini and Meli, 1988; Petit-Demouliere et al., 2005; Abelaira et al., 2013; Commons et al., 2017; Unal and Canbeyli, 2019).
In the current study, rats (male Sprague-Dawley) were placed in a cylindrical container (46 cm tall × 20 cm diameter) that contained 25°C water to a depth of 30 cm. Test compounds were given by subcutaneous injection 15 minutes prior to the swim period (n = 6 rats per dose per drug). Rats swam for 15 minutes, and behavior was videotaped from above the swim tank for the entire swim duration. The videotapes were scored for swimming (moving limbs underwater in an active manner), climbing (active forepaw movement out of the water, usually against the side of the chamber), and immobility (floating with sufficient movement only to keep the head above water) by a trained observer who was blinded to the testing condition (Broom et al., 2002). Behavior was recorded every 5 seconds over the 15-minute swim period. After the swim session, rats were removed from the water, towel-dried, and placed in a warmed cage for 10–15 minutes.
Locomotor Activity.
Locomotor activity was measured in a square, acrylic arena (14″ × 14″ × 8″) with infrared beams spaced 2 inches apart. Infrared detectors were placed along both sides of the arena (x- and y-axes), 1 inch off the arena floor to capture ambulation and, along one side of the arena (x-axis), 6 inches off the arena floor to capture rearing behaviors (Opto-M3 Activity Monitor). Beam breaks were quantified using the Multi-Device Interface Software (Columbus Instruments, Columbus, OH). Male Sprague-Dawley rats (n = 8 per condition) were habituated to the arena for at least 1 hour and then received a subcutaneous injection of saline. Approximately 30 minutes later, rats received a subcutaneous injection of saline, 0.3 mg/kg scopolamine, 3 mg/kg L-687,306, or 3 mg/kg CJ2100, and beam breaks were recorded for at least 3 hours.
Titrating Delay Matching-to-Position.
Studies of cognition-related behavior were conducted in touch-sensitive experimental chambers custom designed for rats (Kangas and Bergman, 2017). A titrating delay matching-to-position task [modified for rats from Kangas et al. (2010)] was used to examine the effects of scopolamine and other muscarinic antagonists on short-term spatial memory. Each trial began with presentation of a 5 × 5 cm sample stimulus (green box) on the 17″ touchscreen (black background) in one of two screen locations (to the far left or far right of center). The subjects (male and female Long-Evans rats) were required to touch the stimulus three times. After completion of this response requirement, the retention interval began. The stimulus disappeared and was replaced by a 5 × 5 cm purple circle that was centered near the top of the touchscreen, requiring the subject to rear upward to respond. Requiring a response to this center stimulus positioned the subject in the center of the chamber during the retention interval. The first response to the center stimulus after the retention interval elapsed resulted in the removal of the center stimulus and presentation of both the left and right comparison stimuli. A response to the comparison stimulus on the side that matched the previously presented sample stimulus resulted in a 0.1 ml milk reward paired with an 880 millisecond 440 Hz tone and yellow screen flash that was followed by a 10-second blackout intertrial interval. An incorrect response resulted in a 20-second blackout intertrial interval. The initial retention interval was 0 seconds during the first trial of each 48-trial session. If the subject correctly matched the previously presented stimulus position on two consecutive trials, the retention interval on the following trial increased by 1 second; each error decreased the retention interval by 1 second. These titrating contingencies were designed to capture the subject’s short-term spatial memory across 48-trial training sessions. Steady-state performance in individual subjects was obtained prior to drug testing and was defined as five consecutive session means within ±20% of the average mean of the five sessions. Subjects were given doses of scopolamine (0.01–3.2 mg/kg), L-687,306 (0.1–10 mg/kg), CJ2100 (0.1–10 mg/kg), or saline 15 minutes prior to select test sessions with at least three intervening training sessions.
Psychomotor Vigilance Task.
A psychomotor vigilance task [modified for rats from Mackworth (1948); Weed and Gold (1998); Kangas et al. (2016)] was used to examine the effects of scopolamine and other muscarinic antagonists on attentional processes. Each trial began with presentation of a 7 × 7 cm stimulus (pink square) on the screen (blue background) in one of six locations (evenly spaced 3 × 2 matrix). The intermittency of stimulus presentation (after a 15-, 30-, or 45-second intertrial interval) as well as the stimulus location were randomized across trials. The duration of stimulus presentation was 2 seconds on the first trial. If the subject successfully responded by rearing and touching the stimulus with its paw within the 2-second duration, a 0.1-ml milk reward was delivered paired with an 880 millisecond 440 Hz tone and yellow screen flash, and the stimulus duration on the following trial was decreased by 0.25 seconds. If the subject failed to respond to the stimulus before the duration had elapsed, the duration of the stimulus increased by 0.25 seconds on the following trial. These titrating contingencies were designed to capture the subject’s reaction time and ability to maintain task performance across a 96-trial training session (i.e., focused vigilance). Steady-state performance in individual subjects was obtained prior to drug testing and was defined as five consecutive session means within ±20% of the average mean of the five sessions. Subjects were given scopolamine (0.01–3.2 mg/kg), L-687,306 (0.1–10 mg/kg), CJ2100 (0.1–10 mg/kg), or saline 15 minutes prior to select test sessions with at least three intervening training sessions.
Data Analysis
Cardiovascular Assay.
The analysis program calculated an average heart rate every 10 seconds. These 10-second epochs were averaged over 1 minute per rat, and data from four to eight rats were averaged for each treatment group with the standard error of the mean (SEM) as a measure of variability. The graphs show data for 20 minutes after administration of arecoline. Data were fitted with a linear mixed model (jamovi version 1.2) with P < 0.001 as criteria for significance.
Arecoline Discrimination.
The completion of at least one FR was required for discrimination analysis. Full or complete generalization to a discriminative cue was defined as > 85% of responding on the drug-associated aperture. Response rate was calculated as number of responses made on either lever during the period when the stimulus light was illuminated.
Forced Swim Test.
The total counts for each behavior were averaged across rats within each treatment group. Data were analyzed by one-way ANOVA. When appropriate, ANOVAs were followed by a Dunnett’s post hoc analysis. The criterion for significance was set at P < 0.05. All statistical analyses were conducted using GraphPad Prism 8 Software (San Diego, CA).
Locomotor Activity.
The number of consecutive beam breaks in the X-Y plane and the Z plane were recorded every minute, added together, and then summed into 10-minute bins. Beam breaks were averaged within a treatment group to generate a time course of activity. Data were analyzed by repeated measure two-way ANOVA with time (repeated measure, within subject) and dose (between subject) as independent variables. Since a significant interaction was not expected, main effects between treatments were compared with Tukey’s multiple comparisons post hoc analyses. The criterion for significance was set at P < 0.05. All statistical analyses were conducted using GraphPad Prism 8 Software.
Titrating Delay Matching-to-Position Task.
The primary dependent measure under training conditions was mean titrated retention interval across the 48 trials.
Psychomotor Vigilance Task.
The primary dependent measure under training conditions was mean titrated duration value across the 96 trials.
Data from the FST, titrating delay matching-to-position task, and psychomotor vigilance tasks were analyzed by one-way ANOVA. When appropriate, ANOVAs were followed by a Dunnett’s post hoc analysis. The criterion for significance was set at P < 0.05. All statistical analyses were conducted using GraphPad Prism 8 Software.
Drugs
Scopolamine hydrobromide trihydrate was purchased from Sigma Aldrich (St. Louis, MO). L-687,306 and CJ2100 were synthesized for these studies by the present author (CRJ) in the Department of Pharmaceutical Sciences, University of Maryland School of Pharmacy.
Synthesis of L-687,306.
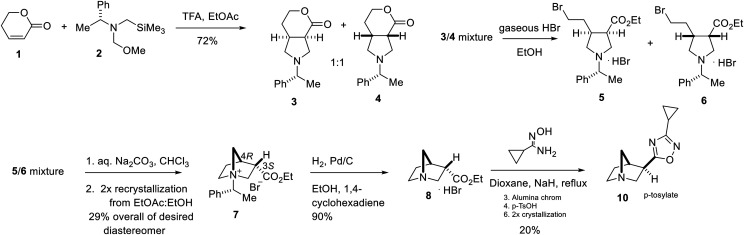
The procedure of (Cottrell et al., 1991) was modified for the synthesis of L-687,306 (Fig. 1). An acid catalyzed [2 + 3] cycloaddition with azomethine ylide precursor (2) and 5,6-dihydropyranone (1) afforded the pyrrolidine ring system as a 1:1 mixture of diastereomers that was purified via the formation of the maleate salt. The mixture (free-base) was then saturated with HBr (acetyl bromide/ethanol), which resulted in the fracture of the pyranone ring to yield (5) and (6), which were neutralized with sodium carbonate to afford a mixture of 3S,4R and 3R,4S quaternary salts. Recrystallization in acetone/diethyl ether afforded the pure (R) diastereomer (7). Transfer hydrogenation with 1,4-cyclohexadiene to remove the chiral auxiliary afforded (10), which was recrystallized in acetone/diethyl ether to provide the hydrobromide salt. After free-basing via standard protocol, oxadiazole formation with N′-hydroxycyclopropanecarboximidamide with sodium hydride under reflux yielded the desired compound. This method was preferred to that over previous work by Street et al. (1990) as it allowed scalable access to the desired compound in enantiomerically pure form.
Fig. 1.
Synthesis of L-687,306. TFA=trifluoroacetic acid
5-((1R,3R,4R)-1-azabicyclo[2.2.1]heptan-3-yl)-3-Cyclopropyl-1,2,4-Oxadiazole.
The p-toluenesulfonic acid salt was generated according to literature procedures (Houghton et al., 1993). m/z [M + H]+: C11H15N3O, 206.1. Optical rotation was measured of intermediate (8) as detailed in Cottrell et al., (1991): [α]D +29.7° (c = 0.5 in EtOH).
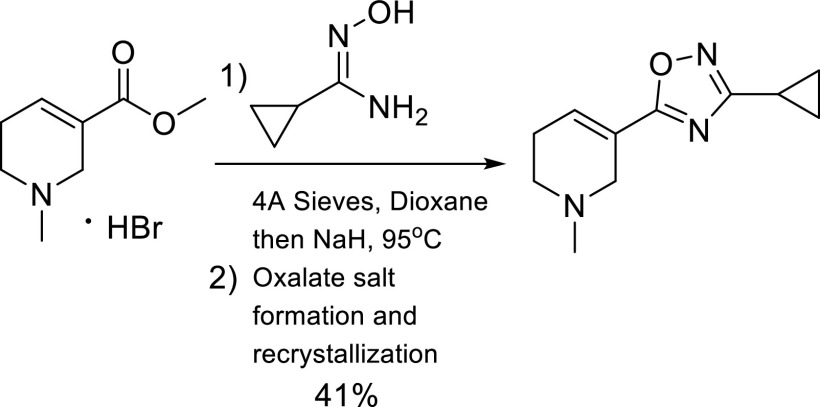
Synthesis of CJ2100.
The procedure of (Street et al., 1990; Showell et al., 1991) was modified for the synthesis of CJ2100 (Fig. 2). Arecoline was free based and reacted with cyclopropyl oxime under basic conditions to afford the oxime in moderate yield. Subsequent oxalate salt formation followed by recrystallization (EtOH/diethyl ether) afforded CJ2100 as a white solid.
Fig. 2.
Synthesis of CJ2100.
3-Cyclopropyl-5-(1-Methyl-1,2,5,6-Tetrahydropyridin-3-yl)-1,2,4-Oxadiazole Oxalate.
1H NMR (CD3OD, 400 MHz, parts per million): δ 7.07–7.05 (m, 1H), 3.33–3.30 (m, 2H), 3.18–3.17 (m, 2H), 2.89 (s, 3H), 2.65–2.63 (m, 2H), 2.00–1.96 (m, 1H), 0.97–0.88 (m, 2H), 0.87–0.85 (m, 2H). 13C NMR (CD3OD, 100 MHz, parts per million): δ 171.02, 170.18, 132.58, 116.35, 48.47, 47.62, 40.19, 21.19, 5.05, 4.31. m/z [M + H]+: C11H15N3O, 206.1 .
Results
In Vitro Binding Assays.
Table 1 shows the binding profile of L-687,306, CJ2100, and scopolamine. As shown in Table 1, L-687,306, CJ2100, and scopolamine have a high specificity for the mAChRs, with little off-target binding. L-687,306 displays 2- to 5-fold higher affinity for the individual mAChR subtypes than CJ2100, but still 10- to 100-fold lower affinity compared with scopolamine. This correlates well with in vivo doses between the two compounds as shown in the following figures. The only notable off-target binding site is CJ2100 at 5HT2B, although follow-up efficacy screens are necessary to determine whether this is pharmacologically significant.
TABLE 1.
In vitro binding profiles of L-687–306, CJ2100, and scopolamine
Full binding profiles were performed through the Psychoactive Drug Screening Program. For primary binding assays, compounds showing a minimum of 50% inhibition at 10 μM were screened for secondary binding assays to determine Ki values (11 concentrations in triplicate). 5HT3=5-hydroxytryptamine receptor or serotonin receptor; D3=Dopamine D3 receptor; MOR=Mu opioid receptor
| Ki(nm) | M1 | M2 | M3 | M4 | M5 | Off-Target |
|---|---|---|---|---|---|---|
| L-687,306 | 70.13 | 77.47 | 15.31 | 44.68 | 14.57 | 5HT3: 1591.12 |
| D3: 4083.48 | ||||||
| CJ2100 | 353.83 | 182.91 | 71.21 | 283.77 | 159.96 | 5HT2B: 1670.66 |
| D1: >10,000 | ||||||
| MOR: >10,000 | ||||||
| Scopolamine | 2.16 | 8.20 | 0.19 | 0.80 | 0.91 |
Heart Rate.
Figure 3 shows the dramatic and short-lived effect of subcutaneous 10 mg/kg arecoline on heart rate. A dose of 0.3 mg/kg CJ2100 produced some attenuation of the arecoline-induced bradycardia, and a dose of 1.0 mg/kg CJ2100 was completely effective in blocking this effect of arecoline. Figure 3 includes data on the smallest doses of L-687,306 and scopolamine that prevented arecoline’s agonist effects on heart rate. Complete dose-effect curves using these two muscarinic antagonists are shown in Winger et al. (2020); these data are included in the figure for comparison with CJ2100. The effects of treatment [F (4180) = 57.80], time [F (9216) = 20.1], and the treatment × time interaction [F (36,216) = 8.11] were each statistically significant (P < 0.001). Bonferroni-corrected multiple comparisons among the cells means that the results obtained with arecoline were significantly different from saline at 2–5 minutes only (P < 0.001). Each of the three muscarinic antagonists given 15 minutes earlier largely prevented this arecoline-induced bradycardia.
Fig. 3.
Antagonism by 0.3 mg/kg CJ2100 (closed triangles), and 1.0 mg/kg CJ2100 (open squares) of the brief, profound bradycardia produced by subcutaneous administration of 10 mg/kg arecoline (closed circles). Saline control data and the effects of the smallest, most effective doses of L-687,306 and scopolamine are shown in the dashed and dotted lines for comparison with CJ2100. The arecoline and saline data were from eight rats (n=8), and the effects of the antagonists came from four rats each (n=4). Data were fitted (R2 = 0.77) with a linear mixed model using treatment and time as fixed factors and subject (n = 12) as random factor (Jamovi version 1.2). The effects of treatment [F (4180) = 57.80], time [F (9216) = 20.1], and the treatment × time interaction [F (36,216) = 8.11] were each statistically significant (P < 0.001). Bonferroni-corrected multiple comparisons among the cells means that the results obtained with arecoline were significantly different from saline at 2–5 minutes only (P < 0.001). At 2–4 minutes, the results obtained in all three pretreatment conditions were significantly different from arecoline (P < 0.001) and not from saline. Note that the initial bradycardia is an artifact of handling and injecting the rats and occurs too rapidly to be a drug effect. HR=heart rate; Scopol=Scopolamine
These data indicate that these doses of the antimuscarinic compounds are equally effective in antagonizing arecoline-induced bradycardia. In conjunction with previous data on scopolamine and L-687,306 under identical conditions (Winger et al., 2020), these findings indicate that scopolamine is 10 times more potent than L-687,306, and CJ2100 is likely to be at least equipotent with L-687,306 in this measure of antimuscarinic action.
Arecoline Discrimination.
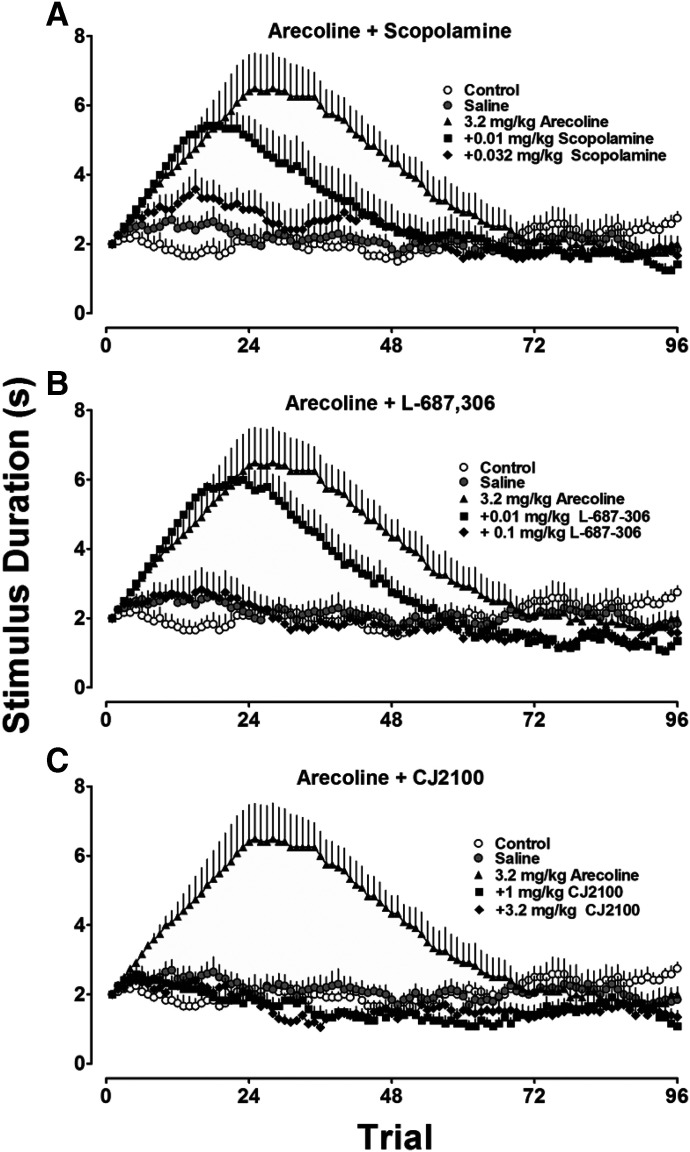
As shown in Fig. 4A, scopolamine was unable to greatly modify the discriminative stimulus effects of arecoline (top) at doses that, in combination with arecoline, produced profound decreases in rates of responding (bottom). L-687,306 (Fig. 4B), on the other hand, produced a dose-related reduction in the potency of arecoline as a discriminative stimulus (top). L-687,306 also markedly decreased the ability of arecoline to suppress rates of responding (bottom). CJ2100 (Fig. 4C), similar to L-687,306, attenuated both the discriminative stimulus effects of arecoline (top) and the rate-suppressing effects of arecoline (bottom), although this effect was not as dose-related or as profound as that shown with L-687,306.
Fig. 4.
Top: the interaction between scopolamine (A), L-687,306 (B), and CJ2100 (C) on the discriminative stimulus effects of arecoline (closed circles). Bottom: simultaneous measures of the interactions of these drugs on the rates at which the rats carried out the discrimination task. The effect of saline on arecoline discrimination and rates of responding are shown as open circles. n = 6-8. Scopol=Scopolamine; Sal=Saline; Sec=Second
Forced Swim Test.
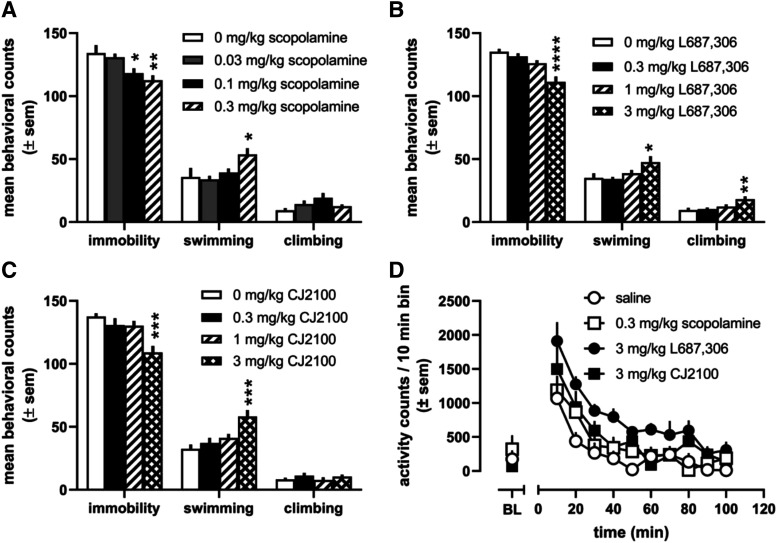
As shown in Fig. 5A, scopolamine produced a dose-dependent decrease in immobility [F (3,20) = 5.5, P = 0.007] after administration of 0.1 mg/kg (P < 0.05) and 0.3 mg/kg (P < 0.01). Also, scopolamine increased swimming behaviors [F (3,20) = 3.5, P = 0.04] with a significant increase observed after administration of 0.3 mg/kg (P < 0.05). Although scopolamine slightly increased climbing behavior at 0.1 mg/kg, these effects were not statistically significant [F (3,20) = 2.6, P = 0.08]. L-687,306 dose-dependently decreased immobility [F (3,20) = 12.5, P < 0.0001], increased swimming [F (3,20) = 3.4, P = 0.04], and increased climbing behaviors [F (3,20) = 4.7, P = 0.01], but all of these effects were statistically significant only at the 3 mg/kg dose (Fig. 5B). CJ2100 decreased immobility [F (3,20) = 7.9, P = 0.001] and increased swimming behaviors [F (3,20) = 7.6, P = 0.001] at the largest dose tested (3 mg/kg). However, CJ2100 did not alter climbing behaviors at any dose tested (Fig. 5C).
Fig. 5.
Effects of several doses of scopolamine (A), L-687,306 (B), and CJ2100 (C) on counts of immobility, swimming, and climbing in the forced swim test. n = 6 rats per dose per drug; *P < 0.05; **P < 0.01; ***P < 0.001. Locomotor activity was evaluated at doses that produced significant decreases in immobility in the FST (D), which demonstrate significant main effects of treatment [F (328) = 6.6, P = 0.002] and time [F (10,280) = 31.6, P < 0.0001]. BL=Baseline
Locomotor Activity.
The effects of scopolamine (0.3 mg/kg), L-687,306 (3 mg/kg), and CJ2100 (3 mg/kg) on locomotor activity were evaluated at doses that produced significant decreases in immobility in the FST (Fig. 5D). Although there was no significant interaction of treatment and time as expected, there were significant main effects of treatment [F (3,28) = 6.6, P = 0.002] and time [F (10,280) = 31.6, P < 0.0001]. Injection, handling, and saline injection increased locomotor activity initially that returned to baseline levels within 20–30 minutes. Similar to the saline condition, injection of scopolamine, L-687,306, and CJ2100 all increased locomotor activity immediately after injection, but only L-687,306 significantly increased locomotor activity as compared with the saline injection (Tukey’s post hoc analysis of main effects of treatment, P = 0.001). The doses of scopolamine and CJ2100 tested did not significantly alter locomotor activity in this assay.
Psychomotor Vigilance Task.
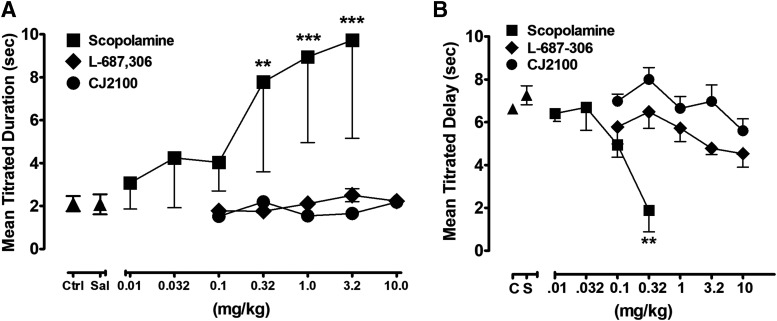
As shown in Fig. 6A, under control and saline conditions, all male and female rats consistently responded to the presented stimulus within the 2-second duration of its presentation. Scopolamine produced significant dose-related adverse effects on mean titrated duration relative to performance after saline administration [F (5) = 9.53, P < 0.0001]. Small doses of scopolamine (0.01–0.1 mg/kg) somewhat increased the variability of the responding, but did not significantly increase the mean titrated duration of the stimulus. Larger doses of scopolamine, however, significantly increased the mean titrated duration of the stimulus after administration of 0.32 (P < 0.01), 0.1 (P < 0.001), and 3.2 mg/kg (P < 0.001). Administration of L-687,306 failed to impair performance under this task across a range of doses (0.1–10 mg/kg). Similarly, a wide range of doses of CJ2100 (0.1–10 mg/kg) did not modify the ability of the rats to respond in this task; mean titrated duration of the stimulus remained at approximately 2 seconds with very little variability.
Fig. 6.
(A) Dose-related effects of scopolamine (closed squares), L-687,306 (closed diamonds), and CJ2100 (closed circles) on mean titrated duration in second in the psychomotor vigilance task n = 6. (B) Dose-related effects of the three muscarinic antagonists on the mean titrated delay in the titrating delay matching-to-position task. n = 6; **P < 0.01; ***P < 0.001.Ctrl and C=Control; Sal and S=Saline
Titrating Delay Matching-to-Position.
As shown in Fig. 6B, under control conditions and after saline administration, titrating delay matching-to-position performance consistently produced mean titrated delay values approximating 7 seconds with low levels of variability among the male and female rats. Scopolamine treatment was associated with significant dose-related impairment of spatial memory relative to performance after saline treatment [F (5) = 7.52, P = 0.0007], which is reflected by decreases in accuracy resulting in lower mean titrated duration values, most evident after administration of 0.1 and 0.32 mg/kg (P < 0.001). Unlike scopolamine, however, both L-687,306 [F (5) = 2.47, P = 0.06] and CJ2100 [F (5) = 2.14, P = 0.09] failed to significantly impair spatial memory up to doses of 10 mg/kg.
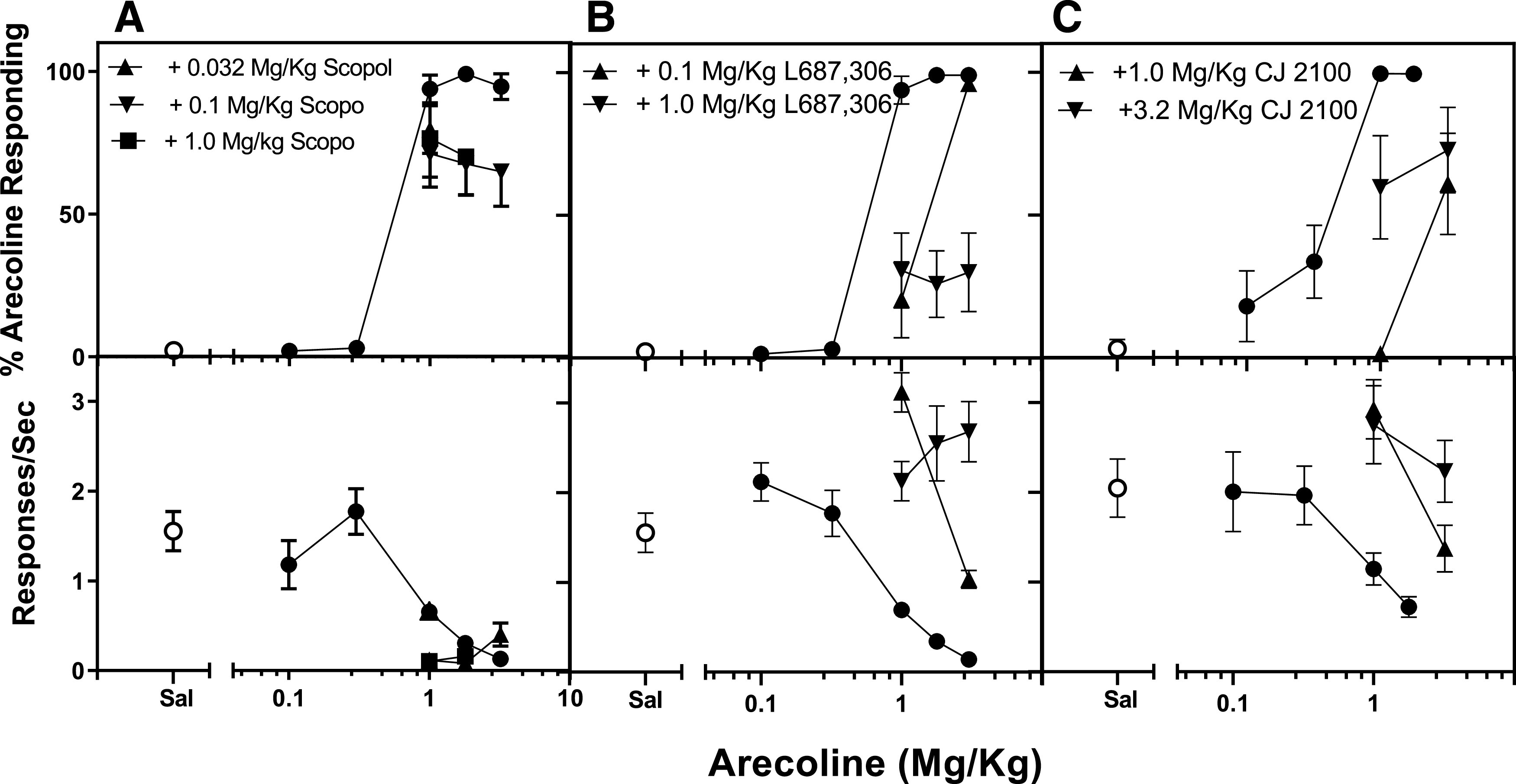
The muscarinic agonist arecoline also disrupted responding on the psychomotor vigilance task. The pattern of disruption after arecoline administration was distinct from that after administration of scopolamine. Arecoline had a fast onset of effect and completely eliminated responding. After an interval of time that was related to the dose of arecoline, responding returned to control levels. The action of 3.2 mg/kg arecoline likely was through a muscarinic mechanism, since this disruption was reversed in a dose-dependent manner by scopolamine, L-687,306, and CJ2100 (Fig. 7). Note that doses of each antagonist that blocked the effects of arecoline in this vigilance task were smaller than those required to affect performance in this task, indicating that the antimuscarinic properties of these drugs were present at doses below those that impaired vigilance. In addition, each of the antagonists produced complete or nearly complete reversal of the effects of 3.2 mg/kg arecoline in this task at doses that were a log-unit smaller than those that prevented the effects of 10 mg/kg arecoline on heart rate (Fig. 3).
Fig. 7.
Antagonism of the effects of 3.2 mg/kg arecoline on the psychomotor vigilance task by a range of doses of scopolamine (A), L-687,306 (B), and CJ2100 (C). n = 6.
Discussion
The discovery that the muscarinic antagonist scopolamine can rapidly and persistently reverse symptoms of depression (Furey and Drevets, 2006; Drevets and Furey, 2010; Drevets et al., 2013, 2020; Witkin et al., 2020) raised the possibility that anticholinergics might prove to be clinically useful antidepressant drugs. However, the direct adverse side effects of short- and long-term administration of scopolamine and other conventional centrally active antimuscarinic drugs and, in particular, their sedative effects and potential impact on cognitive function has limited enthusiasm for this line of medication development.
Interestingly, another rapid-onset drug with antidepressant potential, ketamine (Corriger and Pickering 2019), also has disruptive effects on learning and memory, likely through its block of NMDA receptors that mediate long-term potentiation in the hippocampus (Lüscher and Malenka, 2012). The possible interaction between NMDA and cholinergic antagonism and their potentially common influence on both mood and memory through potentiation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor function has been discussed (Witkin et al., 2019). Less often raised is the question of whether cognitive impairment and memory deficits produced by the anticholinergic scopolamine and the NMDA antagonist ketamine can be meaningfully dissociated from beneficial antidepressant effects. Directly related to this, a relatively recent selective serotonin reuptake inhibitor with mixed serotonergic activity, vortioxetine, actually improves cognitive performance in patients that are depressed (Frampton, 2016), indicating that antidepressant action is not inextricably linked with memory disturbance. The present data further support the separation of cognitive impairment and antidepressant activity by demonstrating that two novel muscarinic antagonists, L-687,306 and CJ2100, at doses that are equieffective with scopolamine in a preclinical assay of potential antidepressant effectiveness, do not impair cognitive functions in rodent models of memory and attention. Although no direct comparison of the three compounds was made on measures of sedation (e.g., sleep-wake cycles), it was found that a dose of 3 mg/kg of either L-687,306 or CJ2100 did not suppress ongoing rates of food-maintained behavior (data not shown). Scopolamine, on the other hand, completely suppressed these rates at a dose of 0.3 mg/kg (data not shown). It is also worthwhile to note that Freedman et al. (1993) stated that, at doses up to 30 mg/kg, L-687,306 was unique among a group of muscarinic antagonists in that “no salivation, diarrhea, tremor, hypothermia, or other side effects normally associated with muscarinic stimulation in vivo” (page 493) were observed.
Like scopolamine, L-687,306 and CJ2100 are effective muscarinic antagonists in that they each completely antagonized the disruptive effects of the muscarinic agonist arecoline on heart rate, an autonomically mediated effect, and on psychomotor vigilance, a centrally mediated effect. Interestingly, the three drugs did not display the same rank order potency as antagonists in these two tasks. For example, L-687,306 and CJ2100 were equipotent in attenuating arecoline-induced bradycardia, whereas scopolamine was approximately 10 times more potent than either drug in this assay. In the psychomotor vigilance assay, scopolamine was equipotent with L-687,306 as an arecoline antagonist, and approximately 10 times more potent than CJ2100. This difference in rank order potency suggests that the antagonists are acting through distinct receptors or sets of receptors to block the effects of arecoline in the two assays. The bradycardia produced by arecoline on heart rate likely is mediated through the M2 receptor (Harvey 2019), whereas the type of muscarinic receptors mediating the central effects of arecoline are unknown.
Each of the three antimuscarinic compounds also had similar direct effects in the FST, decreasing immobility and increasing either swimming or climbing. Importantly, scopolamine and CJ2100 decreased immobility in the FST without significantly altering locomotor activity at the same doses, suggesting that these effects in the FST were independent of general activity-stimulating properties. L-687,306 significantly increased locomotor activity; however, these effects were less robust than that observed with psychomotor stimulants in the same apparatus (Altshuler et al., 2019). These data highlight that locomotor-stimulating properties do not necessarily predict behaviors in the FST. Further, the FST has been validated as an animal assay of antidepressant activity (Krishnan and Nestler, 2011; Yankelevitch-Yahav et al., 2015) and, consistent with its effects in this assay, scopolamine has been found to have a robust, fast-acting antidepressant action in some clinical studies (Furey and Drevets, 2006; Drevets and Furey, 2010; Drevets et al., 2013). The present findings raise the possibility that L-687,306 and CJ2100 also might be clinically effective antidepressants.
Despite the finding that all three antagonists could reverse the effects of arecoline in an autonomic assay, and that all three decreased immobility in the FST, scopolamine differed qualitatively from either L-687,306 or CJ2100 in drug discrimination and cognition assays. As reported previously, behaviorally active doses of scopolamine did not consistently block the discriminative stimulus effects of arecoline. It failed to attenuate or even potentiated the rate-decreasing effects of arecoline, other mAChR agonists, and acetylcholinesterase inhibitors (Jung et al., 1987; Yamamoto et al., 1993; Winger et al., 2020). On the other hand, L-687,306 and CJ2100 produced rightward displacements in the discriminative stimulus and rate-suppressing effects of arecoline, indicative of muscarinic antagonism. Scopolamine’s effects also differed from those of the other two muscarinic antagonists in vigilance and delayed matching-to-position studies. In both these measures of cognitive function, L-687,306 or CJ2100 had little effect over a wide range of doses that were beyond the effective dose in the FST assay. In contrast, the dose of scopolamine that was similarly effective in the FST assay (0.32 mg/kg) produced clear disruptions in performance of both tasks. The data revealed at least a 3-fold separation in the potency with which L-687,306 and CJ2100 produced desirable (decreased immobility in FST) and undesirable (cognitive impairment) effects. In sharp contrast, scopolamine displayed no separation between desirable and undesirable effects. With regard to the potential clinical application of novel antimuscarinics such as L-687,306 or CJ2100, e.g., in the treatment of depression, these results indicate that the projected therapeutic window may markedly improve over that of conventional antagonists like scopolamine.
Results of the present studies illustrate clear differences in the direct effects of antimuscarinics. In previous studies, Witkin et al. (1987) also noted that the behavioral effects of antimuscarinics can differ among ligands. For example, they found that schedule-controlled responding in rats was decreased by four of seven antimuscarinic compounds and initially increased by the remaining three compounds. In conjunction with such observations, the present findings emphasize the point that differences in the behavioral profiles of antimuscarinic drugs perhaps can be exploited to identify clinically valuable therapeutics. Thus, all antimuscarinic compounds can block at least some effects of muscarinic agonists like arecoline, but not all produce the degree of cognitive impairment that is often associated with scopolamine.
The differences among the behavioral effects of antimuscarinic compounds may reflect differences in their relative affinity and efficacy at the five subtypes of muscarinic receptors. Witkin et al. (2014) reported that M1 and M2 null mice were not responsive to the effects of scopolamine in a forced swim assay, whereas M3, M4, and M5 null mice continued to respond normally to scopolamine. This implicates M1 and M2 receptor subtypes in the antidepressant actions of scopolamine. The antagonism by all three of the compounds described in this manuscript on the bradycardia produced by arecoline indicates activity at the M2 receptor. However, the lack of mAChR subtype specificity of L-687,306 and CJ2100, as shown in the Table, does not provide any additional information on what receptor subtype might mediate the cognitive deficits produced by some anticholinergic compounds.
Studies with compounds that have greater specificity for the M1 receptor such as biperidine, pirenzepine, and VU0255035 reviewed in Witkin et al. (2020) indicate that they have antidepressant-like activity in rodent assays, supporting a role for M1 receptors in mediating the antidepressant effects of cholinergic antagonists. Likewise, SCH226206, an M2 subtype-selective compound had antidepressant-like actions in mice [Witkin et al., 2014; reviewed in Witkin et al. (2020)]. Therefore, there is consistent support for the importance of the M1 and M2 receptor subtypes in mediating antidepressant activity. Whether the cognitive impairment produced by scopolamine is due to additional action at the other three subtypes, and why L-687,306 and CJ2100 generally lack these side effects remains to be determined.
There are a few caveats regarding the present studies that warrant consideration. First, with the exception of the cognition studies, only male rats were examined. Diagnoses of MDD are more prevalent in women (Hyde and Mezulis, 2020) and, therefore, in future preclinical efforts in muscarinic drug development with L-687,306 and CJ2100, it will be important to determine whether these drugs produce similar discriminative stimulus and FST effects in female rats (Kokras et al., 2015). Second, the FST is an assay often used to rapidly screen compounds for potential antidepressant-like activity but is thought to have little face validity for modeling depression or depressive symptoms in patients. Although there is much debate in the field whether any animal model can adequately model human depression, future studies should aim to evaluate the consistency of these muscarinic antagonists across the many assays used to evaluate novel antidepressant compounds. Finally, although it remains important to evaluate the binding characteristics of promising compounds, it must be recognized that unique blends of activity—either combined or cooperative (Witkin et al., 1987; Leaderbrand et al., 2016)—at more than one receptor may be necessary to produce some of the distinctive behavioral effects of muscarinic antagonists (e.g., functional selectivity). Under such circumstances, the role of in vivo assays that are well validated and highly translatable to clinical conditions becomes more prominent. The assessment here of the effects of novel muscarinic antagonists on different types of cognitive function serves as an example of the value of this approach.
Acknowledgments
The authors would like to thank Aaron Ajlen, Oanh Luc, Yong Gong Shi, and Lisa Wooldridge for their technical assistance conducting these studies. Ki determinations and receptor binding profiles were generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program, Contract # HHSN-271–2018-00023-C (NIMH PDSP). The NIMH PDSP is directed by Bryan L. Roth at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscoll at NIMH, Bethesda.
Abbreviations
- CJ2100
3-cyclopropyl-5-(1-methyl-1,2,5,6-tetrahydropyridin-3-yl)-1,2,4-oxadiazole oxalate
- EtOH
ethanol
- FR
fixed-ratio
- FST
forced swim test
- L-687,306
5-((1R,3R,4R)-1-azabicyclo[2.2.1]heptan-3-yl)-3-cyclopropyl-1,2,4-oxadiazole
- mAChR
muscarinic acetylcholine receptor
- MDD
major depressive disorder
- NMDA
N-methyl d-aspartate
Authorship Contributions
Participated in research design: Johnson, Kangas, Jutkiewicz, Winger, Bergman, Coop, Woods.
Conducted experiments: Johnson, Kangas, Jutkiewicz, Winger.
Performed data analysis: Johnson, Kangas, Jutkiewicz, Winger.
Wrote or contributed to the writing of the manuscript: Johnson, Kangas, Jutkiewicz, Winger, Bergman, Coop, Woods.
Footnotes
Funding for the research was provided by National Institutes of Health National Institute of Mental Health [Grant R01-MH107499].
The authors are coholders of a pending United States patent on CJ2100 (PCT/US2020/026802) and declare no other conflicts of interest.
References
- Abelaira HM, Réus GZ, Quevedo J (2013) Animal models as tools to study the pathophysiology of depression. Br J Psychiatry 35 (Suppl 2):S112–S120. [DOI] [PubMed] [Google Scholar]
- Altshuler RD, Carpenter CA, Franke TJ, Gnegy ME, Jutkiewicz EM (2019) The protein kinase Cβ-selective inhibitor, enzastaurin, attenuates amphetamine-stimulated locomotor activity and self-administration behaviors in rats. Psychopharmacology (Berl) 236:3231–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R, Showell GA, Street LJ, Saunders J, Hoogsteen K, Freedman SB, Hargreaves RJ (1992) Synthesis, physicochemical and conformational properties of (3R, 4R)-3-(3-cyclopropyl-1,2,4-oxadiazol-5-yl)-1- azabicyclo[2.2.1]heptane, a novel M1 selective muscarinic partial agonist. J Chem Soc Chem Commun 11:817–819. [Google Scholar]
- Blair DT, Dauner A (1992) Extrapyramidal symptoms are serious side-effects of antipsychotic and other drugs. Nurse Pract 17:56–62–64, 67. [DOI] [PubMed] [Google Scholar]
- Borsini F, Meli A (1988) Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology (Berl) 94:147–160. [DOI] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH (2002) Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology 26:744–755. [DOI] [PubMed] [Google Scholar]
- Clark L, Chamberlain SR, Sahakian BJ (2009) Neurocognitive mechanisms in depression: implications for treatment. Annu Rev Neurosci 32:57–74. [DOI] [PubMed] [Google Scholar]
- Commons KG, Cholanians AB, Babb JA, Ehlinger DG (2017) The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chem Neurosci 8:955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriger A, Pickering G (2019) Ketamine and depression: a narrative review. Drug Des Devel Ther 13:3051–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell IF, Hands D, Kennedy DJ, Paul KJ, Wright SHB, Hoogsteen K (1991) A synthesis of 1-azabicyclo[2.2.1]heptane-3-carboxylic acid esters in enantiomerically pure form. J Chem Soc Perkin Trans 1 5:1091–1097. [Google Scholar]
- Delaunois A, Dedoncker P, Hanon E, Guyaux M (2009) Repeated assessment of cardiovascular and respiratory functions using combined telemetry and whole-body plethysmography in the rat. J Pharmacol Toxicol Methods 60:117–129. [DOI] [PubMed] [Google Scholar]
- Deutsch JA (1971) The cholinergic synapse and the site of memory. Science 174:788–794. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Bhattacharya A, Furey ML (2020) The antidepressant efficacy of the muscarinic antagonist scopolamine: past findings and future directions. Adv Pharmacol 89:357–386. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Furey ML (2010) Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry 67:432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Zarate CA Jr, Furey ML (2013) Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol Psychiatry 73:1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Janowsky DS (2019) Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol Psychiatry 24:694–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton JE (2016) Vortioxetine: a review in cognitive dysfunction in depression. Drugs 76:1675–1682. [DOI] [PubMed] [Google Scholar]
- Freedman SB, Dawson GR, Iversen LL, Baker R, Hargreaves RJ (1993) The design of novel muscarinic partial agonists that have functional selectivity in pharmacological preparations in vitro and reduced side-effect profile in vivo. Life Sci 52:489–495. [DOI] [PubMed] [Google Scholar]
- Freedman SB, Patel S, Harley EA, Iversen LL, Baker R, Showell GA, Saunders J, McKnight A, Newberry N, Scholey K, et al. (1992) L-687,306: a functionally selective and potent muscarinic M1 receptor agonist. Eur J Pharmacol 215:135–136. [DOI] [PubMed] [Google Scholar]
- Furey ML, Drevets WC (2006) Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry 63:1121–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Haxby JV, Drevets WC (2008) Selective effects of cholinergic modulation on task performance during selective attention. Neuropsychopharmacology 33:913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RD (2019) Muscarinic receptor agonists and antagonists: effects on cardiovascular function, in Muscarinic Receptors (Fryer A, Christopoulos A, Nathanson NM eds) vol 208, pp 299–316, Springer-Verlag Berlin Heidelberg, Heidelberg, Germany. [DOI] [PubMed] [Google Scholar]
- Houghton PG, Humphrey GR, Kennedy DJ, Roberts DC, Wright SHB (1993) Enantiospecific synthesis of the (4R)-1-azabicyclo[2.2.1]heptane ring system. J Chem Perkin Trans 1:1421–1424. [Google Scholar]
- Hyde JS, Mezulis AH (2020) Gender differences in depression: biological, affective, cognitive and sociocultural factors. Harv Rev Psychiatry 28:4–13. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Fenwick JA, Williams GA (2001) Cone-based vision of rats for ultraviolet and visible lights. J Exp Biol 204:2439–2446. [DOI] [PubMed] [Google Scholar]
- Jung M, Costa L, Shearman GT, Kelly PH (1987) Discriminative stimulus properties of muscarinic agonists. Psychopharmacology (Berl) 93:139–145. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Eller EB, Folk JE, Rice KC, Traynor JR, Woods JH (2004) Delta-opioid agonists: differential efficacy and potency of SNC80, its 3-OH (SNC86) and 3-desoxy (SNC162) derivatives in Sprague-Dawley rats. J Pharmacol Exp Ther 309:173–181. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Rice KC, Carroll FI, Woods JH (2013) Patterns of nicotinic receptor antagonism II: cardiovascular effects in rats. Drug Alcohol Depend 131:284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Rice KC, Woods JH, Winsauer PJ (2003) Effects of the delta-opioid receptor agonist SNC80 on learning relative to its antidepressant-like effects in rats. Behav Pharmacol 14:509–516. [DOI] [PubMed] [Google Scholar]
- Kangas BD, Bergman J (2017) Touchscreen technology in the study of cognition-related behavior. Behav Pharmacol 28:623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Leonard MZ, Shukla VG, Alapafuja SO, Nikas SP, Makriyannis A, Bergman J (2016) Comparisons of Δ9-tetrahydrocannabinol and anandamide on a battery of cognition-related behavior in nonhuman primates. J Pharmacol Exp Ther 357:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Vaidya M, Branch MN (2010) Titrating-delay matching-to-sample in the pigeon. J Exp Anal Behav 94:69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Akiskal HS, Ames M, Birnbaum H, Greenberg P, Hirschfeld RM, Jin R, Merikangas KR, Simon GE, Wang PS (2006) Prevalence and effects of mood disorders on work performance in a nationally representative sample of U.S. workers. Am J Psychiatry 163:1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkenberg I, Blokland A (2010) The validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studies. Neurosci Biobehav Rev 34:1307–1350. [DOI] [PubMed] [Google Scholar]
- Kokras N, Antoniou K, Mikail HG, Kafetzopoulos V, Papadopoulou-Daifoti Z, Dalla C (2015) Forced swim test: what about females? Neuropharmacology 99:408–421. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ (2011) Animal models of depression: molecular perspectives. Curr Top Behav Neurosci 7:121–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakstygal AM, Kolesnikova TO, Khatsko SL, Zabegalov KN, Volgin AD, Demin KA, Shevyrin VA, Wappler-Guzzetta EA, Kalueff AV (2019) Dark classics in chemical neuroscience: atropine, scopolamine, and other anticholinergic deliriant hallucinogens. ACS Chem Neurosci 10:2144–2159. [DOI] [PubMed] [Google Scholar]
- Leaderbrand K, Chen HJ, Corcoran KA, Guedea AL, Jovasevic V, Wess J, Radulovic J (2016) Muscarinic acetylcholine receptors act in synergy to facilitate learning and memory. Learn Mem 23:631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I (1997) The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol 8:523–532. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC (2012) NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb Perspect Biol Jun 1;4:a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackworth NH (1948) The breakdown of vigilance during prolonged visual search. Quat J Exper Psych 1:6–21. [Google Scholar]
- National Research Council (2011) Guidelines for the care and use of laboratory animals, 8th ed, National Academies Press, Washington, DC. [Google Scholar]
- O’Leary OF, Dinan TG, Cryan JF (2015) Faster, better, stronger: towards new antidepressant therapeutic strategies. Eur J Pharmacol 753:32–50. [DOI] [PubMed] [Google Scholar]
- Petit-Demouliere B, Chenu F, Bourin M (2005) Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl) 177:245–255. [DOI] [PubMed] [Google Scholar]
- Porter RJ, Gallagher P, Thompson JM, Young AH (2003) Neurocognitive impairment in drug-free patients with major depressive disorder. Br J Psychiatry 182:214–220. [DOI] [PubMed] [Google Scholar]
- Sayyah M, Eslami K, AlaiShehni S, Kouti L (2016) AlaiShehni S, and Kouti, L (2016) Cognitive function before and during treatment with selective serotonin reuptake inhibitors in patients with depression or obsessive-compulsive disorder. Psychiatry J 2016:5480391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showell GA, Gibbons TL, Kneen CO, MacLeod AM, Merchant K, Saunders J, Freedman SB, Patel S, Baker R (1991) Tetrahydropyridyloxadiazoles: semirigid muscarinic ligands. J Med Chem 34:1086–1094. [DOI] [PubMed] [Google Scholar]
- Street LJ, Baker R, Book T, Kneen CO, MacLeod AM, Merchant KJ, Showell GA, Saunders J, Herbert RH, Freedman SB, et al. (1990) Synthesis and biological activity of 1,2,4-oxadiazole derivatives: highly potent and efficacious agonists for cortical muscarinic receptors. J Med Chem 33:2690–2697. [DOI] [PubMed] [Google Scholar]
- Unal G, Canbeyli R (2019) Psychomotor retardation in depression: a critical measure of the forced swim test. Behav Brain Res 372:112047. [DOI] [PubMed] [Google Scholar]
- Wang SM, Han C, Lee SJ, Patkar AA, Masand PS, Pae CU (2015) Five potential therapeutic agents as antidepressants: a brief review and future directions. Expert Rev Neurother 15:1015–1029. [DOI] [PubMed] [Google Scholar]
- Weed MR, Gold LH (1998) The effects of dopaminergic agents on reaction time in rhesus monkeys. Psychopharmacology (Berl) 137:33–42. [DOI] [PubMed] [Google Scholar]
- West AP (1990) Neurobehavioral studies of forced swimming: the role of learning and memory in the forced swim test. Prog Neuropsychopharmacol Biol Psychiatry 14:863–877. [DOI] [PubMed] [Google Scholar]
- Winger G, Jutkiewicz EM, Woods JH (2020) Comparison of the muscarinic antagonist effects of scopolamine and L-687,306. Behav Pharmacol 31:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Gordon RK, Chiang PK (1987) Comparison of in vitro actions with behavioral effects of antimuscarinic agents. J Pharmacol Exp Ther 242:796–803. [PubMed] [Google Scholar]
- Witkin JM, Overshiner C, Li X, Catlow JT, Wishart GN, Schober DA, Heinz BA, Nikolayev A, Tolstikov VV, Anderson WH, et al. (2014) M1 and m2 muscarinic receptor subtypes regulate antidepressant-like effects of the rapidly acting antidepressant scopolamine. J Pharmacol Exp Ther 351:448–456. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Martin AE, Golani LK, Xu NZ, Smith JL (2019) Rapid-acting antidepressants. Adv Pharmacol 86:47–96. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Smith JL, Golani LK, Brooks EA, Martin AE (2020) Involvement of muscarinic receptor mechanisms in antidepressant drug action. Adv Pharmacol 89:311–356. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Ohno M, Sugimachi K, Ueki S (1993) Discriminative stimulus properties of NIK-247 and tetrahydroaminoacridine, centrally active cholinesterase inhibitors, in rats. Pharmacol Biochem Behav 44:769–775. [DOI] [PubMed] [Google Scholar]
- Yankelevitch-Yahav R, Franko M, Huly A, Doron R (2015) The forced swim test as a model of depressive-like behavior. J Vis Exp 97:52587. [DOI] [PMC free article] [PubMed] [Google Scholar]