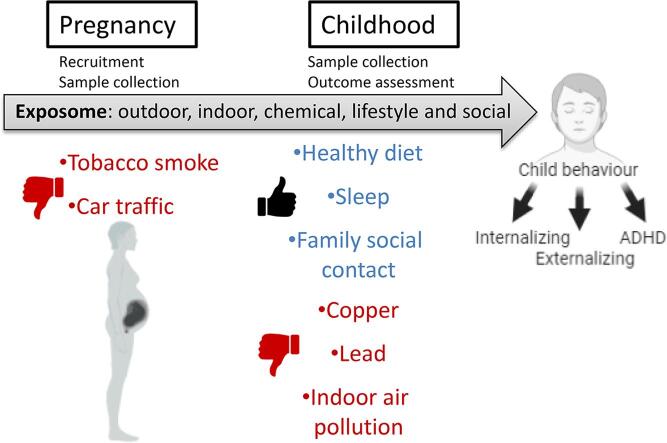
Graphical abstract
Keywords: Exposome, ADHD, Behavioural problems, CBCL, Chemicals, Children, Cohort, Environment, Epidemiology, Pollutants
Highlights
-
•
A wide range of pre- and postnatal environmental exposures affects child behavior.
-
•
The exposome includes outdoor, indoor, chemical, lifestyle and social exposures.
-
•
Maternal tobacco smoke and car traffic increased behavioural problems in children.
-
•
Child sleep, healthy diet and higher family social capital reduced symptoms.
-
•
Child exposure to lead, copper, indoor air pollution, increased symptoms.
Abstract
Background
Environmental exposures in early life influence the development of behavioral outcomes in children, but research has not considered multiple exposures. We therefore aimed to investigate the impact of a broad spectrum of pre- and postnatal environmental exposures on child behavior.
Methods and findings
We used data from the HELIX (Human Early Life Exposome) project, which was based on six longitudinal population-based birth cohorts in Europe. At 6–11 years, children underwent a follow-up to characterize their exposures and assess behavioral problems. We measured 88 prenatal and 123 childhood environmental factors, including outdoor, indoor, chemical, lifestyle and social exposures. Parent-reported behavioral problems included (1) internalizing, (2) externalizing scores, using the child behavior checklist (CBCL), and (3) the Conner’s Attention Deficit Hyperactivity Disorder (ADHD) index, all outcomes being discrete raw counts. We applied LASSO penalized negative binomial regression models to identify which exposures were associated with the outcomes, while adjusting for co-exposures. In the 1287 children (mean age 8.0 years), 7.3% had a neuropsychiatric medical diagnosis according to parent’s reports. During pregnancy, smoking and car traffic showing the strongest associations (e.g. smoking with ADHD index, aMR:1.31 [1.09; 1.59]) among the 13 exposures selected by LASSO, for at least one of the outcomes. During childhood, longer sleep duration, healthy diet and higher family social capital were associated with reduced scores whereas higher exposure to lead, copper, indoor air pollution, unhealthy diet were associated with increased scores. Unexpected decreases in behavioral scores were found with polychlorinated biphenyls (PCBs) and organophosphate (OP) pesticides.
Conclusions
Our systematic exposome approach identified several environmental contaminants and healthy lifestyle habits that may influence behavioral problems in children. Modifying environmental exposures early in life may limit lifetime mental health risk.
1. Introduction
Childhood is a critical stage for the mental health and well-being of individuals with half of all adult neuropsychiatric problems starting by 14 years of age (World health organization, 2019). Yet there are large gaps in what we know about the causes of behaviour problems. Common genetic variants across the genome account for only about 5–25% of behavioral disorder risk in the general population (Uher and Zwicker, 2017) with small effects from thousands of genetic variants that are contingent on complex environmental interaction. Research into the environmental factors for behavioral disorders has found a complex picture of multiple social and physical exposures occurring at different stages of life, in particular during sensitive prenatal and early childhood periods when brain development accelerates (van Os et al., 2010, Rapoport et al., 2012). These risk factors include unfavourable urban environment (Vassos et al., 2016), immigration and ethnic minority (Bourque, 2011); obstetric and pregnancy complications (Cannon et al., 2002), and exposure to industrial chemicals such as lead and mercury (Grandjean and Landrigan, 2014) but also protective factors such as stimulating home environments (Bradley et al., 1989) and healthy diets (Cohen et al., 2016) that may benefit brain development and child behavior. However, these factors have often been studied independently and cross-sectionally while the aetiology of behavioral disorders is known to consist of networks involving many elements. These elements are complex to measure since they belong to different domains such as psycho-social, physical and chemical environments, and their effect is often depend on their timing (e.g. pregnancy vs. adulthood), duration, and to which extent they are repeated exposures over time (Guloksuz et al., 2018).
There is a large body of epidemiological literature on the effects of early-life exposures on behavioural problems in children, but few studies have investigated several families of exposure, or applied an exposome approach—i.e., one encompassing all environmental exposures from conception onwards. Populations are simultaneously exposed to a wide range of environmental factors, some of which are suspected to affect child behaviour, especially during early life. Even in its partial forms, the exposome provides a useful framework to systematically evaluate many associations (Wild, 2005), and may be used to avoid problems of selective reporting, publication bias of only positive results, and, to some extent, confounding by co-exposures, ingrained in the typical one-by-one reporting of associations. Consequently, the exposome may help both in discovery and in setting priorities for prevention.
Recent examples of exposome, or multiple exposure, approaches, in child behavior research, particularly focused on pregnancy exposure to specific exposure families such as indoor air pollutants (Gonzalez-Casanova et al., 2018), persistent organic pollutants (“POPs”, e.g. polychlorinated biphenyls (PCBs), organochlorine (OC) pesticides, polybrominated diphenyl ethers (PBDEs), and perfluoroalkyl substances (PFAS) (Lenters et al., 2019) have applied a wider-exposure selection based on available cohort questionnaire-data (Steer et al., 2015). None of the previous studies combined chemical and non-chemical environmental stressors. A recent scoping review on human exposome studies identified mental health to be understudied in exposome research despite its crucial role in the fields of public health, health care, and treatment (Haddad et al., 2019).
Therefore, this study aims to evaluate the association between a wide array of pregnancy and childhood environmental exposures related to chemical and non-chemical environmental stressors and link this to a range of child behavioral symptoms in the large European Human Early-Life Exposome (HELIX) cohort (Vrijheid et al., 2014, Maitre et al., 2018).
2. Method
2.1. Study population
The study population is based on the HELIX (Human Early-Life Exposome) project, which pooled data on existing six population-based birth cohorts (BiB in United Kingdom, EDEN in France, KANC in Lithuania, INMA in Spain, MoBa in Norway, and RHEA in Greece) where large sets of previously collected longitudinal data from early pregnancy to childhood were available. Background information on the HELIX cohorts and the full HELIX protocols are described elsewhere (Haug et al., 2018, Maitre et al., 2018, Robinson et al., 2018, Tamayo-Uria et al., 2019). The HELIX subcohort consists of 1301 mother child-pairs that were followed-up at 6–11 years of age using common protocols for all the cohorts (between 12/2013 and 02/2016) including biological samples collection, face-to-face questionnaire, health examination and additional characterization for a wide range of exposures (Vrijheid et al., 2014). The study was approved by the individual national ethics committees for cohort recruitment, follow-up visits and secondary use of pre-existing data for HELIX. Written informed consent was obtained from all HELIX subcohort participants.
2.2. Child emotional and behavioral problems
Parents completed questionnaires related to child’s behavior, including the Conner rating scale’s (N = 1287) and child behavior checklist (CBCL, N = 1298), within a week before the follow-up visit at 6–11 years of age. The 99-item CBCL/6–18 version for school children was used to obtain standardized parent reports of children's problem behaviours, translated and validated in each native language of the participating six cohort populations (Achenbach and Rescorla, 2001). The parents responded along a 3-point scale with the code of 0 if the item is not true of the child, 1 for sometimes true, and 2 for often true. The internalizing score includes the subscales of emotionally reactive and anxious/depressed symptoms, as well as somatic complaints and symptoms of being withdrawn. The externalizing score includes attention problems and aggressive behaviors. In addition, an ADHD index based on the short form of the Conners’ rating scales of 27 items provided information on inattention and hyperactivity symptoms (Conners, 1997). The internal consistency (Cronbach's alpha) of each of the study scales was >0.80. All outcomes were analyzed as raw count scores.
2.3. Characterisation of the exposome
A wide range of exposures were evaluated during pregnancy and childhood (6–11 years) as presented in Table 1. This set of exposures was selected at the start of the HELIX project, because they were of concern for more than one of the health outcomes under study and because population exposure was widespread. We assessed five domains of the exposome: the outdoor, indoor, chemical, lifestyle and social domains, representing a total of 88 pregnancy exposures and 123 childhood exposures (Haug et al., 2018, Tamayo-Uria et al., 2019). An extended description on all exposure assessments and methods are provided in Annex 1 and described elsewhere in previous publications regarding the chemical assessment (Haug et al., 2018), questionnaires and general cohort description (Maitre et al., 2018) and correlations between exposures (Tamayo-Uria et al., 2019).
Table 1.
List of prenatal and childhood exposures assessed in this study.
| Exposure Group | List of variables | Preg. | Child | Where(buffer) - Matrix | Sampling pregnancy period | Sampling childhood period | Methoda | ||
|---|---|---|---|---|---|---|---|---|---|
| External general environment | Atmospheric pollutants | NO2, PM2.5, PM10, PMabs | 4 | 4 | Home | Pregnancy | Annual average | LUR model | |
| UV | Ambient ultraviolet radiation (UVR) levels | 0 | 1 | Home(300 m) | Pregnancy | Month | Average of satelite images | ||
| Surrounding natural spaces | Presence of a major green or blue space in a distance of 300 m, NDVI | 3 | 6 | Home/ School(green and blue 300 m; NDVI 100 m) | Pregnancy | Childhood | Spatial analysis of GIS layers | ||
| Meteorology | Temperature, humidity, pressure | 3 | 2 | Home | Pregnancy | Month | Weather stations | ||
| Built environment | Population and building density, street connectivity, accessibility and facility richnessb, walkability | 9 | 15 | Home/ School(300 m) | Pregnancy | Childhood | Spatial analysis of GIS layers | ||
| Road traffic | Traffic density and inverse distances to nearest roads | 3 | 5 | Home/ School(100 m) | Pregnancy | Childhood | LUR model | ||
| Road traffic noise | 24 h and evening time road noise levels | 1 | 3 | Home/ School | Pregnancy | Childhood | Local noise map + LUR model | ||
| External individual environment (contaminants) | OCs | DDE,DDT,HCB,PCB(118,138,153,170,180) | 8 | 9 | Serum/Plasma | Trim 1 | Childhood | GC–MS/MS | |
| PBDE | PBDE(47,153) | 2 | 2 | Serum/Plasma | Trim 1 | Childhood | GC–MS/MS | ||
| PFAS | PFOA, PFNA,PFUNDA,PFHxS,PFOS | 5 | 5 | Serum/Plasma/Blood | Trim 1 | Childhood | LC-MS/SPE-UHPLC-MS/MS | ||
| Metals and elements | As,Cd,Co,Cs,Cu,Hg,Mn,Pb | 10 | 10 | Whole and cord blood | Trim 1 and 3 | Childhood | Q-ICP-MS,AAS | ||
| Phthalates | MEP,MiBP,MnBP,MBzP,MEHP,MEHHP,MEOHP,MECPP,OHMiNP,OXOMiNP | 10 | 11 | Urinec | Trim 1 and 3 | Childhood | HPLC-MS | ||
| Phenols | MEPA,ETPA,BPA,PRPA,BUPA,OXBE,TRCS | 7 | 7 | Urinec | Pregnancy | Childhood | HPLC-MS | ||
| OPs | DMP,DMTP,DMDTP,DEP,DETP,DEDTP | 4 | 5 | Urinec | Pregnancy | Childhood | UPLC-MS | ||
| Tobacco smoking | Cotinine, questionaire on active and passive | 4 | 3 | Urinec, questionaire | Pregnancy | Childhood | Immulite® 2000 Nicotine | ||
| Water DBPs | THM, Choloroform, Brominated THMs | 3 | 0 | Home | Pregnancy | – | Multivariate + Spatial model | ||
| Indoor AP | NO2, TEX, Benzene, PM2.5, PMabs | 0 | 5 | Home | – | 1 week × 2 24 h × 2 | Passive samples + Multivariate model | ||
| Other | Lifestyle | Diet, physical activity - Only in pregnancy: alcohol - Only in childhood: allergy, sleep |
12 | 26 | Questionaires | Pregnancy | Childhood | – | |
| Social-economic capital | Family affluence, social contact, house crowding | 0 | 4 | ||||||
| Total | 211 | 88 | 123 | ||||||
Abbreviations used: NO2: nitrogen dioxide; PM2.5: particulate matter with an aerodynamic diameter of<2.5 μm; PM10: particulate matter with an aerodynamic diameter of<10 μm; PM2.5abs: absorbance of PM2.5 filters; TEX: toluene, ethylbenzene, xylene; DDE: 4,4′dichlorodiphenyl dichloroethylene; DDT: 4,4′dichlorodiphenyltrichloroethane; HCB: hexachlorobenzene; PCB: polychlorinated biphenyl – 118, 138, 153, 170, 180; PBDE 47, 2,2′,4,4′-tetra-bromodiphenyl ether, PBDE 153: 2,2′,4,4′,5,5′-hexa-bromodiphenyl ether; PFOA: perfluorooctanoate; PFNA: perfluorononanoate; PFUNDA: perfluoroundecanoate; PFHxS: perfluorohexane sulfonate; PFOS: perfluorooctane sulfonate; As: arsenic; Cd: cadmium; Co: cobalt; Cs: ceasium; Cu: copper; Hg: mercury; Mn: manganese; Mo: molybdenum; Pb: lead; Tl: thallium; MEP: monoethyl phthalate; MiBP: mono-iso-butyl phthalate; MnBP: mono-n-butyl phthalate; MBzP: mono benzyl phthalate; MEHP: mono-2-ethylhexyl phthalate; MEHHP: mono-2-ethyl-5-hydroxyhexyl phthalate; MEOHP: mono-2-ethyl-5-oxohexyl phthalate; MECPP: mono-2-ethyl 5-carboxypentyl phthalate; OHMiNP: mono-4-methyl-7-hydroxyoctyl phthalate; OXOMiNP: mono-4-methyl-7-oxooctyl phthalate; MEPA: methyl paraben; ETPA: ethyl paraben; BPA: bisphenol A; PRPA: propyl paraben; BUPA: N-butyl paraben; OXBE: oxybenzone; TRCS: triclosan; DMP: dimethyl phosphate: DMTP: dimethyl thiophosphate; DMDTP: dimethyl dithiophosphate; DEP: diethyl phosphate; DETP: diethyl thiophosphate; DEDTP: diethyl dithiophosphate; THMs: trihalomethanes.
Exposure assessment methods for each variable are described in Annex 1.
Excluded from childhood exposome due to very high correlation with facility density (r > 0.9).
During childhood, the urine sample analysed was a pool of equal amounts of two spot urine samples collected at bed time the day before and in the morning the day of the clinical examination.
In summary, outdoor exposures were assessed using remote sensing and geo-spatial methods at the home address for the pregnancy period and at the home and school addresses for the childhood period. Outdoor exposures included air pollution, measures of the built environment, meteorological conditions, natural spaces (green and blue spaces), road traffic, and traffic noise. Additional exposures assessed at the home address by predictive modeling include water disinfection by-products (for pregnancy only) and indoor pollutants (for childhood period only, based on questionnaire and in-house sensor data). Exposure to chemical contaminants was assessed through determination of concentrations in biological samples from the mother during pregnancy and the child. The chemical list was based on concern for child health and included organochlorine compounds (PCBs and OC pesticides), PBDEs, PFAS, metals and essential elements, phthalate metabolites, phenols (triclosan, oxybenzone, parabens and bisphenol A), organophosphate (OP) metabolites, and cotinine. When appropriate, the concentrations were adjusted for lipids or creatinine. Lifestyle factors were collected by questionnaire and included smoking habits, diet, physical activity, allergens, and sleep. During childhood, questionnaire information was collected on socio-economic capital of the family based on the Family Affluence Score (FAS, Boyce et al., 2006) and through summary variables for social participation, social contact and house crowding.
2.4. Statistical analysis
Exposure variables were transformed to achieve normality; when normality could not be achieved with a transformation, the variable was categorized, and then standardized by the inter-quartile range (IQR) (Hernandez-Ferrer et al., 2019). Missing exposure and covariate data were imputed using chained equations (White et al., 2011) with the mice package in R (van Buuren and Groothuis-Oudshoorn, 2011). Each imputation models included between 5 and 25 variables selected based on their correlation to the variable to impute, their proportion of missing and the main health outcomes. (20 imputed datasets were created, see more details in Annex 2). Missing values, ranged from no missing values for some child phthalate metabolites to 65% for fast-food intake during pregnancy (Table A2.1 in Tamayo-Uria et al., 2019). The mean percentage of missing values per exposure was 12% (first quartile 0.9% and third quartile 16.8%). None of the participants had complete data on all exposures. Yet, for 98% of individuals < 30% of exposure variables had missing values. Results of the complete case analysis are also available in Tables A4.2 and A4.3 with the number of complete cases for each exposure before imputation. In all subsequent regression models, Rubin’s rules were used to combine the results from the 20 imputed datasets, which allows the quantification of the uncertainty in results associated with imputation in the final standard errors, confidence intervals and p-values (White et al., 2011).
All analyses were based on negative binomial regression with adjustment for cohort, maternal age, child age, child gender, season of conception and maternal education. To explore separately the associations between the prenatal and childhood exposomes with externalizing, internalizing behavior scores and ADHD index we applied two methods. Our primary analysis relied on a maximum likelihood function plus a penalty, the least absolute shrinkage and selection operator (LASSO) to account for the complex correlation pattern of the exposome and to mitigate potential estimation problems due to collinearity (multi-exposure models, see Annex 2 for more details). We determined the overall penalty parameter, lambda, by maximizing the prediction log-likelihood using 10-fold cross-validation and providing the maximum mean cross-validated log-likelihood values. This type of variable selection was shown to be more robust than the traditional stepwise variable selection and also well adapted to count data with overdispersion as observed with CBCL and ADHD index data (Wang et al., 2016). For descriptive and comparative purposes, we also estimated associations by modeling one exposure at the time (single-exposure model, also called exposome-wide association study (ExWAS) analysis). To account for multiple comparisons in the single exposure models, a family-wise error rate was calculated to provide an indicative p-value threshold (5% divided by the effective number of tests, based on Li et al. (Li et al., 2012)).
The results are presented as adjusted mean ratios (aMR) with corresponding 95% confidence intervals (CIs). In all of these models, the exposure estimate was reported for one interquartile range (IQR) increase in exposure levels—for example, a ratio of mean score of 1.25 for an IQR increase in the exposure meant that those with an exposure at the 75th percentile had a 25% higher mean score, compared with those with an exposure at the 25th percentile.
2.5. Sensitivity analyses
Three sets of sensitivity analyses were performed to address the robustness of our results for the multi-exposure models: 1) further adjustment for child standardized body mass index (BMI), breastfeeding duration, prenatal smoking (only postnatal models) and maternal perceived stress (Cohen et al., 1988) (although some of these factors could act as mediators in the association between the exposure and child behavior); 2) evaluating the impact of outliers in exposure values and the linearity of the association between exposure and outcome were checked using generalized additive models (GAMs); 3) stratifying the models by cohort and testing for heterogeneity.
3. Results
3.1. Study population
At the time of delivery, pregnant women were aged 31 years on average, mostly highly educated (52%) and were a native of the cohort country (84%) (Table 2). Children were 45% girls and 55% boys. At the time of behavior assessment, children were on average 8 years old (range: 6.5 to 11 years), 3.9% regularly visited the psychologist and 7.3% had a neuropsychiatric diagnosis by the time of visit (according to parent’s reports, besides the CBCL screening). Boxplots with exposure level distribution (untransformed) by cohort are available online (Tamayo-Uria et al., 2019 Fig. A2.1 and Fig. A2.2).
Table 2.
Description of the Study Population.
| Parental characteristics | N (%) or median (quartile range) |
|---|---|
| Cohort of inclusion | |
| BIB, United Kingdom | 205 (15.9) |
| EDEN, France | 197 (15.3) |
| INMA, Spain | 218 (17) |
| KANC, Lithuania | 202 (15.7) |
| MoBa, Norway | 272 (21.2) |
| Rhea, Greece | 192 (14.9) |
| Family native from the country of the cohort | |
| Both native parents | 1056 (84) |
| 1 native parent | 62 (4.93) |
| No native parent | 139 (11.1) |
| Maternal age at inclusion, yrs | 31 (27.2–34) |
| Maternal education | |
| Primary | 171 (13.8) |
| Secondary | 429 (34.5) |
| Higher | 643 (51.7) |
| Paternal education | |
| Primary | 207 (17.3) |
| Secondary | 465 (38.8) |
| Higher | 526 (43.9) |
| Marital status | |
| Cohabitant | 183 (14.2%) |
| Divorced/separated | 81 (6.3%) |
| Married | 958 (74.5%) |
| Single | 54 (4.2%) |
| Other | 8 (0.6%) |
| Trimester of conception | |
| Jan-March | 409 (32.1) |
| April-June | 257 (20.2) |
| July-Sept | 275 (21.6) |
| Oct-Dec | 332 (26.1) |
| Who completed the behavioural questionnaires? | |
| Father | 63 (4.9%) |
| Mother | 1215 (94.5%) |
| Child Characteristics | |
| Child's ethnicity | |
| Caucasian | 1155 (89.9) |
| Non-Caucasian | 130 (10.1) |
| Child's BMI category* | |
| Thinness | 100 (7.8) |
| Normal wt | 929 (72.7) |
| Overweight | 180 (14.1) |
| Obese | 69 (5.4) |
| Sex | |
| Female | 580 (45.1) |
| Male | 706 (54.9) |
| Age (years) | 8.05 (6.4–8.9) |
| Visit psychologist regularly | |
| No | 1235 (96.0%) |
| Yes | 50 (3.9%) |
| Any previous neuropsychological diagnosis | |
| No | 1191 (92.6%) |
| Yes | 94 (7.3%) |
| Internalizing scales | 5 (2–9) |
| Externalizing scales | 5 (2–10) |
| ADHD Index (Conners) | 6 (2–11) |
BIB = Born in Bradford; EDEN = Étude des Déterminants pré et postnatals du développement et de la santé de l’Enfant; INMA = INfancia y Medio Ambiente; KANC = Kaunas Cohort; MoBa = Norwegian Mother, Father and Child Cohort Study; Rhea = Mother-Child Cohort in Crete.
According to the WHO growth reference for children age between 5 and 19 years. World Health Organization. Growth reference 5–19 years. Available at: http://www.who.int/growthref/who2007_bmi_for_age/en/. Accessed August 2, 2019.
3.2. Are pregnancy exposures associated with children behavioral symptoms?
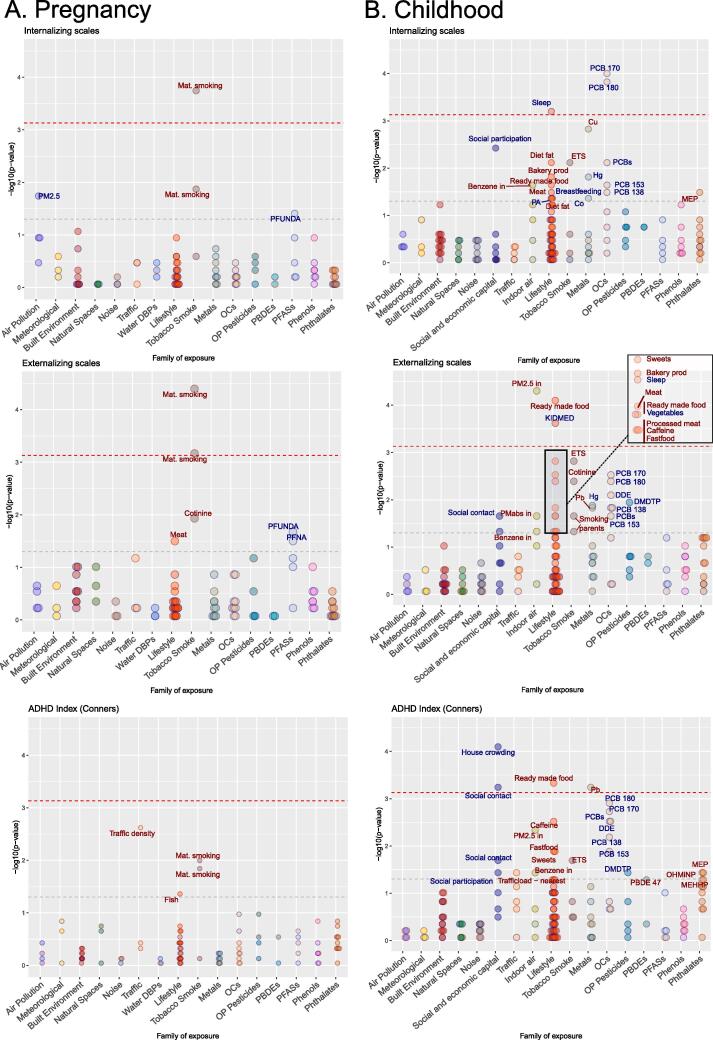
Out of the 88 pregnancy exposures, few were associated with behavioral symptoms in the main models adjusting for LASSO selected co-exposures (Table 3) or in the single exposure models as illustrated in Fig. 1 (eTable 3.4). In our main multiple exposure models, pregnancy tobacco exposure was associated with increased externalizing symptoms (Table 3); compared to non-smoking, passive smoking was associated with a 25% increase (adjusted mean ration, aMR = 1.25[1.09; 1.44]) and active smoking with a 31% increase (aMR = 1.31[1.09; 1.59]) in externalizing symptoms. Results were similar for internalizing symptoms. Residential car traffic density on nearest road was associated with increased externalizing symptoms (aMR = 1.07 [1.00–1.14]) and ADHD index (aMR = 1.10 [1.03; 1.17]). The presence of green space near the home address (within 300 m) was associated with externalizing symptoms only (aMR = 1.15 [1.01–1.31]; Table 3) and not significant in the single-exposure model (aMR = 1.12 [0.98; 1.28]).
Table 3.
Associations between pregnancy exposures and change in behavioral problems, showing exposures selected into the multiple-exposure modelsa (out of 88 candidate exposures, N = 1287).
|
Internalizing scale |
Externalizing scale |
ADHD index |
|||||
|---|---|---|---|---|---|---|---|
| Exposure (IQR or reference category) | Exposure family | aMR [95%CI]b | p-value | aMR [95%CI]b | p-value | aMR [95%CI]b | p-value |
| Traffic density on nearest road (3598 vehicles/day) | Road traffic | 1.07 [1.00; 1.14] | 0.050 | 1.10 [1.03; 1.17] | 0.004 | ||
| Green spaces (300 m) | Natural spaces | 1.15 [1.01; 1.31] | 0.034 | ||||
| Meat intake | Lifestyle/Diet | ||||||
| Medium (vs. low) | 0.96 [0.84; 1.10] | 0.578 | 1.02 [0.87; 1.20] | 0.808 | |||
| High (vs. low) | 1.11 [0.97; 1.27] | 0.131 | 1.16 [0.99; 1.36] | 0.060 | |||
| Fish and seafood | Lifestyle/Diet | ||||||
| Medium (vs. low) | 1.15 [0.99; 1.33] | 0.064 | |||||
| High (vs. low) | 1.12 [0.95; 1.32] | 0.191 | |||||
| Fastfood intake | Lifestyle/Diet | ||||||
| Medium (vs. low) | 0.84 [0.67; 1.04] | 0.113 | |||||
| High (vs. low) | 0.93 [0.75; 1.14] | 0.467 | |||||
| Moderate physical activity, t3 | Lifestyle/Physical activity | ||||||
| Often (vs. none or sometimes) | 1.10 [0.94; 1.29] | 0.244 | 1.09 [0.91; 1.29] | 0.347 | |||
| Very often (vs. none or sometimes) | 0.91 [0.78; 1.07] | 0.253 | 0.93 [0.78; 1.11] | 0.406 | |||
| Smoking in pregnancy | Tobacco smoking | ||||||
| Second-hand smoking (vs. none) | 1.28 [1.12; 1.45] | <0.001 | 1.25 [1.09; 1.44] | 0.002 | |||
| Active smoking (vs. none) | 1.20 [1.03; 1.39] | 0.020 | 1.31 [1.09; 1.59] | 0.005 | |||
| Cotinine (µg/L) | Tobacco smoking | ||||||
| 18.4–50 (vs. < 18.4) | 1.05 [0.96; 1.15] | 0.262 | |||||
| >50 (vs. < 18.4) | |||||||
| Bisphenol-A (4.96 µg/g) | Phenol | 1.06 [0.98; 1.15] | 0.152 | ||||
| Caesium (0.97 µg/L) | Metal | 0.94 [0.83; 1.06] | 0.281 | ||||
| Dimethyl phosphate (11.37 µg/g) | Organophosphate pesticides | 1.07 [1.00; 1.15] | 0.067 | ||||
| Perfluorooctanoate (1.96 µg/L) | Perfluoroalkyl substances (PFAS) | 0.95 [0.86; 1.05] | 0.301 | ||||
| Perfluoroundecanoate (0.18 µg/L) | Perfluoroalkyl substances (PFAS) | 0.93 [0.84; 1.03] | 0.162 | ||||
The selection of exposures was determined by the least absolute shrinkage and selection operator (LASSO) algorithm applying log link function.
The change in behavioral problems was modelled with negative binomial regression which model the ratio of the mean score for one unit change in the exposure (the coefficients obtained needed to be exponentiated). The results are presented as adjusted mean ratios (aMR) with corresponding 95% confidence intervals (CIs). In all of these models, the exposure estimate was reported for one interquartile range (IQR) increase in exposure levels—for example, a ratio of mean score of 1.25 for an IQR increase in the exposure meant that those with an exposure at the 75th percentile had a 25% higher mean score, compared with those with an exposure at the 25th percentile. These coefficients are adjusted for the other exposures and for cohort, maternal age, maternal education level, maternal pre-pregnancy body mass index, parity, parental country of birth, child age, child sex, and child height. Abbreviations: CI = confidence interval; IQR, interquartile range; cat., categories.
Fig. 1.
Exposome-wide associations (ExWAS) with child internalizing and externalizing scales (single-exposure models). On the left panel are the results as dot plots for the pregnancy exposome and the right panel the cross-sectional childhood exposome. The direction of the association is indicated by the color of the text label and the dot contour (blue, negative and red, positive associations). The horizontal red line across the plots represents the multiple testing threshold correction (based on effective number of tests). Any exposures above this line are considered significant. All the exposures with a p-value below 0.05 are labeled. The 0.05p-value threshold is also represented by a grey line across the plots. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Other exposures that were selected by LASSO, but with confidence intervals including 1 (Table 3), were meat intake (increased internalizing and externalizing symptoms), bisphenol A and DMP exposures, an OP pesticide metabolite (increased externalizing symptoms). Lower ADHD scores were observed in children whose mothers had low fish intake during pregnancy (<2 times a week) compared to 2–4 times a week.
3.3. Are childhood exposures associated with children behavioral symptoms?
Out of 123 childhood exposures, 11 exposures were selected in multi-pollutant penalized models for internalizing symptoms, 9 for externalizing symptoms, and 17 for the ADHD index (Table 4). In the single exposure models, as illustrated in Fig. 1 (eTable 3.4), only few exposures passed multiple correction testing. Exposures that were selected by LASSO and with a confidence interval not including 1 (Table 3) were: indoor air PM2.5 and high ready-made food intake, associated with increased externalizing symptoms; second-hand smoking, intake of bakery products, and copper exposure, associated with increased internalizing symptoms; and second-hand smoking, ready-made food, sweets, caffeinated drinks and lead exposure, associated with an increased ADHD index. A few chemical exposures were also associated with decreased symptoms (Table 4): the sum of PCBs was associated with all outcomes (e.g. for internalizing 0.84 [0.77–0.93]) and DMTP with ADHD index (0.92 [0.86–1.00]). Among exposures associated with reduced behavioral symptoms, sleep duration was associated with all the outcomes. Children sleeping one hour more per night (IQR: 0.93 h) had 11% lower internalizing symptoms score. Family social participation to organizations was associated with reduced internalizing symptoms, contact with family and friends (daily (almost) vs. less than once a week) and house crowding, i.e. number of persons living in the household, were associated with lower ADHD mean scores. The healthy diet score (KIDMED), was associated with decreased externalizing scores (Table 4).
Table 4.
Associations between childhood exposures and change in behavioral problems, showing exposures selected into the multiple-exposure modelsa (out of 123 candidate exposures, N = 1287).
|
Internalizing scale |
Externalizing scale |
ADHD index |
|||||
|---|---|---|---|---|---|---|---|
| Childhood exposure (IQR or reference category) | Exposure family | aMR [95%CI]b | p-value | aMR [95%CI]b | p-value | aMR [95%CI]b | p-value |
| Road traffic load (100 m) (1138814 vehicules/day m) |
Road traffic | 1.08 [0.98; 1.18] | 0.123 | ||||
| Indoor benzene (0.99 µg/m3) | Indoor air | 1.06 [0.99; 1.13] | 0.121 | ||||
| Indoor PM2.5 (6.5 µg/m3) | Indoor air | 1.09 [1.03; 1.16] | 0.005 | ||||
| Second-hand smoking | Tobacco smoking | 1.13 [1.01; 1.26] | 0.034 | 1.08 [0.95; 1.23] | 0.217 | 1.05 [0.94; 1.18] | 0.395 |
| Cotinine | Tobacco smoking | 1.09 [0.93; 1.28] | 0.297 | ||||
| Total hours of sleep | Lifestyle | 0.89 [0.83; 0.96] | 0.001 | 0.91 [0.85; 0.99] | 0.027 | 0.94 [0.87; 1.01] | 0.083 |
| Bakery products | Lifestyle/Diet | ||||||
| Medium (vs. low) | 1.00 [0.88; 1.13] | 0.997 | 1.03 [0.90; 1.18] | 0.649 | |||
| High (vs. low) | 1.18 [1.03; 1.36] | 0.017 | 1.15 [0.98; 1.34] | 0.081 | |||
| Dairy products | Lifestyle/Diet | ||||||
| Medium (vs. low) | 0.89 [0.79; 1.01] | 0.060 | |||||
| High (vs. low) | 0.98 [0.87; 1.12] | 0.806 | |||||
| Meat intake | Lifestyle/Diet | ||||||
| Medium (vs. low) | 0.99 [0.87; 1.11] | 0.818 | |||||
| High (vs. low) | 1.11 [0.99; 1.25] | 0.067 | |||||
| Ready made food | Lifestyle/Diet | ||||||
| Medium (vs. low) | 1.13 [0.99; 1.29] | 0.073 | 1.10 [0.97; 1.25] | 0.147 | |||
| High (vs. low) | 1.25 [1.09; 1.44] | 0.001 | 1.20 [1.05; 1.37] | 0.008 | |||
| Sweets | Lifestyle/Diet | ||||||
| Medium (vs. low) | 1.03 [0.90; 1.17] | 0.690 | 1.04 [0.92; 1.17] | 0.543 | |||
| High (vs. low) | 1.14 [1.00; 1.31] | 0.058 | 1.14 [1.01; 1.30] | 0.041 | |||
| Fastfood | Lifestyle/Diet | ||||||
| Medium (vs. low) | 1.03 [0.91; 1.15] | 0.668 | |||||
| High (vs. low) | 1.12 [0.93; 1.34] | 0.247 | |||||
| Caffeinated drinks | Lifestyle/Diet | ||||||
| Medium (vs. low) | 1.00 [0.86; 1.16] | 0.974 | |||||
| High (vs. low) | 1.14 [1.01; 1.30] | 0.037 | |||||
| Fish and seafood | Lifestyle/Diet | ||||||
| Medium (vs. low) | 0.90 [0.80; 1.03] | 0.116 | |||||
| High (vs. low) | 0.99 [0.86; 1.14] | 0.910 | |||||
| Healthy diet (KIDMED score) | Lifestyle/Diet | 0.93 [0.87; 0.99] | 0.034 | ||||
| Lead (5.91 µg/L) | Metal | 1.06 [1.00; 1.13] | 0.061 | 1.12 [1.05; 1.19] | 2.4E-4 | ||
| Copper (350 µg/L) | Metal | 1.10 [1.03; 1.17] | 0.002 | ||||
| Dimethylthiophosphate (4.89 µg/g) | OP pesticides | 0.91 [0.85; 0.97] | 0.007 | 0.92 [0.86; 0.98] | 0.013 | ||
| Sum of polychlorinated biphenyls (27.6 ng/g lipids) | OC compounds | 0.89 [0.82; 0.98] | 0.016 | 0.93 [0.84; 1.02] | 0.131 | 0.93 [0.84; 1.05] | 0.259 |
| Dichlorodiphenyldichloroethylene, DDE (34.0 ng/g lipids) | OC compounds | 0.95 [0.86; 1.04] | 0.242 | ||||
| Polybrominated diphenyl ether 47 (0.21 ng/g lipids) | OC compounds | 1.04 [1.00; 1.09] | 0.050 | ||||
| Monoethyl phthalate (59.84 µg/g) | Phtalates | 1.06 [0.98; 1.15] | 0.119 | ||||
| Social participation | Social capital | ||||||
| 1 organisation (vs none) | 0.98 [0.88; 1.10] | 0.775 | |||||
| >1 organisations (vs none) | 0.83 [0.71; 0.97] | 0.016 | |||||
| Contact with family and friends | Social capital | ||||||
| Once a week (vs. less than once a week) | 0.79 [0.63; 1.00] | 0.051 | |||||
| Almost daily (vs. less than once a week) | 0.69 [0.55; 0.87] | 0.002 | |||||
| House crowding | Social capital | 0.89 [0.85; 0.94] | <0.001 | ||||
Abbreviations: CI = confidence interval; IQR, interquartile range; cat., categories.
The selection of exposures was determined by the least absolute shrinkage and selection operator (LASSO) algorithm applying log link function.
The change in behavioral problems was modelled with negative binomial regression which model the ratio of the mean score for one unit change in the exposure (the coefficients obtained needed to be exponentiated). The results are presented as adjusted mean ratios (aMR) with corresponding 95% confidence intervals (CIs). In all of these models, the exposure estimate was reported for one interquartile range (IQR) increase in exposure levels—for example, a ratio of mean score of 1.25 for an IQR increase in the exposure meant that those with an exposure at the 75th percentile had a 25% higher mean score, compared with those with an exposure at the 25th percentile. These coefficients are adjusted for the other exposures and for cohort, maternal age, maternal education level, maternal pre-pregnancy body mass index, parity, parental country of birth, child age, child sex, and child height.
3.4. Sensitivity analyses
We observed similar findings after adjusting childhood models for breastfeeding duration, child’s BMI, perceived stress score of the mother at the time of child behavioral rating and pregnancy tobacco exposure (only tested for childhood exposome models) as presented in eTable 4.1. Adjustment for perceived maternal stress slightly attenuated the association between pregnancy tobacco smoking exposure with internalizing and externalizing symptoms (e.g. 1.31 [1.09; 1.59] vs. 1.19 [0.99;1.44] for active tobacco exposure with externalizing scale).
Most of the associations were consistent across cohorts, but some heterogeneity can be highlighted (i.e. I2 > 60%, eTable 4.2): 1) the association between pregnancy copper was driven by the BIB and KANC cohorts; 2) the association between child caffeinated drink consumption and ADHD was driven by the EDEN cohort (eFig. 4.1).
Linearity of the associations was observed for all exposures except for pregnancy levels of bisphenol A, PFUnDA, PFOA with externalizing scale (non monotonic inverted U-shaped curves), for traffic density on nearest road with ADHD index (eFig. 4.3) and for child levels of PCB 180 with internalizing scale and ADHD (monotonic multiphasic curve, eFig. 4.2) and for the KIDMED score with externalizing scale (non monotonic U-shaped curve).
4. Discussion
Our study is the first to comprehensively and systematically evaluate a wide range of exposures associated with behavioral problems, covering social, physical and chemical exposures occurring at different stages of development. Our results confirm the important harmful role of maternal smoking during pregnancy in multiple behavior symptoms in children, but also highlight the potential protective role of a healthy family lifestyle during childhood (diet, sleep, regular social contact). We report new evidence about the association between car traffic density, indoor air pollution and copper with increased behavioral problems in children.
Maternal tobacco smoke exposure during pregnancy was the most important prenatal exposure related to behavioral and emotional problems in children. The adverse effects of active maternal smoking during pregnancy are well established, including adverse birth outcomes and increased respiratory illness in children (Kramer, 1987, Burke et al., 2012). However, reports on adverse behavioral outcomes are inconsistent (Rice et al., 2018). Previous studies demonstrated that maternal smoking exposure is closely linked to other co-exposures such as parental psychopathology symptoms, socio-economic factors, father smoking habits and the home environment (quality of attachment, support, and stimulation that a child is exposed to at home) which may account for a large part of the effect of maternal smoking during pregnancy on child behavior (Roza et al., 2009, Hopson et al., 2016). In our study, we found that maternal perceived stress at the time of rating explained some of the association between pregnancy tobacco exposure and child behavior, and we cannot entirely rule out a role of other, unmeasured confounders.
We also found that increased residential car traffic density on the nearest road during pregnancy, was associated with higher externalizing and ADHD scores. This exposure might represent an umbrella of other factors based on its correlation with other exposures in our dataset such as outdoor air pollutants (PM absorbance: r(spearman) = 0.4) and traffic related noise level (r = 0.17). Previous studies have demonstrated that traffic-related air pollution was associated with child neurobehavior such as inattention and higher prevalence of ADHD and Autism Spectrum Disorder (Suades-González et al., 2015). Traffic-related airborne particulate matter (PM) is a complex and heterogeneous mixture that includes residues from fossil fuel combustion, organic chemicals, trace metals, nitrate, and sulphate. During gestation, PM adverse effects may be provoked in the fetus through various mechanisms including both indirect (e.g., maternal systemic and intrauterine inflammation) and/or direct (e.g., PM placenta translocation) manners, with recent evidence for translocation of black-carbon PM in human placenta (Bové et al., 2019). Therefore, an etiological role for traffic-related pollution during pregnancy in child behavioural disorders is biological plausible, although exact mechanisms in humans remains elusive. Finally, the presence of green space (300 m to residence) during pregnancy was associated with increased externalizing scale. This result is not in line with current studies which tend to find that greater access to or quantity of neighbourhood nature or public open space were associated with better mental health (Alderton et al., 2019). In our study, there may be some residual negative confounding effect in relation to different geo-localization patterns according to socio-economic position amongst the European countries, for example, MoBa sub-cohort sample showed higher greenness degree in lower social classes, an opposite pattern to other HELIX sub-cohorts (Robinson et al., 2018).
Postnatal tobacco exposure and car traffic density, were not as strongly associated with child behavior as pregnancy measurements, although the probability to be exposed at both periods for a child was high. Our results may indicate that the pregnancy period is most sensitive to the harmful effects of these exposures which is in line with the very rapid development of the nervous system during this time window but also the different ways individuals are exposed, for example in utero and/or passively to tobacco smoke (Peterson et al., 2015, Allen et al., 2017). In line with our results, childhood second-hand smoke (SHS) was not related to blood methylation in HELIX children (biomarkers of past exposure), indicating much weaker effects of recent SHS with respect to active maternal smoking in pregnancy (Vives-Usano et al., 2020).
Among childhood associations, we observed that healthy dietary habits (Mediterranean diet adherence score) and longer hours of sleep had protective effects on internalizing problems. Similarly, an unhealthy diet (readymade food, sweets, and caffeinated drinks) was associated with higher risk of ADHD symptoms. These results and previous studies support the hypothesis that an inadequate micronutrient intake, which may result from a Western dietary pattern, could cause suboptimal brain function in children (Howard et al., 2011). Impulsivity traits in children with ADHD may also lead to poor dietary choices and emotional eating. As previously found, young adults who displayed greater impulsivity were found to be more likely to choose snack food when hungry, and hence consume more calories, than were less impulsive participants (Nederkoorn et al., 2009). However, diet modification, e.g. reducing carbohydrate intake and as a result obesity, has been demonstrated to improve symptoms of ADHD patients (Pelsser et al., 2017). Furthermore, one of the strongest association with ADHD was in relation to the social capital of the parent (mostly of the mother), indeed children whose parent had contact with family or friends less than once a week had a 31% greater probability to have children with ADHD symptoms than average. This is an interesting novel finding in the literature among healthy children, in which the ‘psychosocial exposome’ is important in order to improve child behavior. When parents have strong ties with other families or friends, parents may receive helpful social support and feel less isolated and stressed. In addition, other adults provide additional social control and collective socialization, resulting in children less likely to develop behavioral problems (Bussing et al., 2015). Another possible explanation is that parents' ratings of their child's social skills is often positively associated with ratings of their own social skills (Mikami et al., 2010) and ADHD is generally considered a highly hereditable trait (~70–80%) (Faraone and Larsson, 2019).
We also confirmed established associations such as with lead. Since the removal of lead from fuel, a sharp decrease in blood lead levels world-wide has been observed (Landrigan, 2002), however, lead effects may occur at very low dose levels (Needleman and Bellinger, 1991) and potential new sources of exposures need to be considered, such as lead recycling activities (World Health Organization (WHO), 2017, The toxic truth, 2021). In a previous exposome study, lead was the only exposure adversely influencing children's language and cognition among many other metal and lifestyle exposures, and so consistently across three European countries (Calamandrei et al., 2020). Passive smoking and home indoor air pollution (PM2.5) exposures were also associated with increased internalizing and externalizing scales, respectively. These exposures are known as potential hazardous agents affecting neurodevelopment and cognitive function through enhancing pro-inflammatory reactions in the brain (Morales et al., 2009). Finally, child copper (Cu) exposure was associated with higher internalizing symptoms. Cu is an essential trace element required for numerous biological processes, including the proper development and functioning of the central nervous system (De Bie et al., 2007). Among the body organs, the brain is the most copper-rich. In line with the numerous Cu biological functions, we found in HELIX that child Cu was associated with multiple child health outcomes (Agier et al., 2019, Vrijheid et al., 2020, Cadiou et al., 2020, Warembourg et al., 2019) and with molecular omics signatures associated with immune response, lipid storage and sequestering of metal ions (Maitre et al. in preparation). Cu (and zinc) has been a candidate biomarker in mood disorders because of its fundamental role in the oxidative and nitrosative stress and consequent activation of the inflammatory response and acute phase proteins such as ceruloplasmin (a key protein involved in the storage of copper) (Styczeń et al., 2016).
Some childhood exposures, such as PCBs and OP pesticides, were unexpectedly associated with better child behavior. These results highlight a more complex exposure system than expected. Child DMTP was associated with decreased externalizing scale and ADHD score in the childhood exposome but pregnancy DMP was associated with increased externalizing scale (1.07 [1.00; 1.15]). Both DMTP and DMP are OP dialkylphosphate (DAP) metabolites that arise from exposure to OP pesticides, a class of insecticides widely used throughout the world, still applied in agriculture for insect control on food crops and mainly found in the general population through ingestion of residues on food products. Intake of fruits and vegetables is a main determinant of OP urinary metabolites in children and pregnant women, as demonstrated in the HELIX cohorts (Papadopoulou et al., 2019). This suggests that the observed association between cross-sectional child DMTP level and ADHD is likely to arise from a higher fresh fruit and vegetable intake which is beneficial for child mental health (Cohen et al., 2016), but also likely to represent a general healthier lifestyle (less readymade meals and fastfood) and more generally a better home environment. Additionally, the cross-sectional nature of the measurements might further explain this association because child behaviour can influence child eating habits (e.g. ADHD and sweet intake). However, we also observed a trend for an association between pregnancy DMP and child externalizing scale. This association is in line with previous studies indicating the detrimental role of OP pesticides on brain development, when the exposure occurs in utero (Philippat et al., 2018). Another unexpected association was between PCB 170 and 180 during childhood and a decrease in all behavioural outcomes, suggesting a protective effect on behaviour. PCBs, among other persistent organic pollutants (POPs), are considered developmental neurotoxicants, mainly based on studies looking at the association between in utero exposure and neuropsychological development (Grandjean et al., 2014). Results with postnatal exposures (in 0–2 years old exposed through breast milk) have been inconsistent (Lenters et al., 2019, Forns et al., 2016) and no studies were found on school-age PCB exposure for comparison. In our data, when stratifying the population by child zBMI categories, the protective effect of PCB 180 with externalizing scale was mainly seen among obese children (0.92 [0.82;1.03]), but opposite in underweight and normal weight children (1.11 [0.88;1.41]). PCBs are highly lipophilic (they store in fat tissue), and the amount of body fat and growth trajectories in children are expected to affect the toxicokinetics of POPs (Vrijheid et al., 2020). Therefore, residual confounding in PCB-behaviour associations may be present and these results should be interpreted with caution.
4.1. Strengths and limitations
This study used standardized outcome and exposure measurement methods across six different countries with a wide range of exposure measurement techniques such as geographic information systems and satellite data for collecting outdoor exposure (road traffic, meteorological factors, natural spaces) and sensors (indoor air pollution). These exposure assessments were completed by more traditional methods such as standardized questionnaires for lifestyle and social capital variables and targeted biomonitoring for selected known (e.g. heavy metals and OP pesticides) and less known neurotoxic chemicals (e.g. phthalates and parabens). In addition, this prospective birth cohort design allowed the collection of objective prenatal exposure measures free of recall and response biases.
Limitations of this study include exposure misclassification, which is expected to be differential across exposures (e.g., stronger for the least persistent chemicals and for exposures based on modelling of outdoor levels). The cross-sectional aspect of the childhood exposures particularly limited our interpretation of results for lifestyle (sleep, diet) because they may suffer from reverse causation bias, whereby child behavioural symptoms would influence sleep duration and dietary choices, instead of vice versa. For other cross-sectionally assessed exposures found to be associated with child behaviour in our study (childhood exposure to indoor air pollutants, lead and childhood passive smoke exposure), reverse causality bias is less of a concern, because it is unlikely that behavioural symptoms of the child influenced these exposures. Still, a clear temporal sequence between exposure and health outcome would strengthen any causal interpretation of associations. Finally, we adjusted for many potential confounders in our models but lacked information about general home environment and parenting styles that may influence exposure distribution and parent rating.
The exposome approach we adopted on the one hand systematically published all exposure-outcome associations correcting for multiple testing, and on the other hand adopted a penalized approach to develop multi-exposure models that address confounding by other exposures. The two approaches retained have each their advantages and drawbacks and both are complementary. ExWAS allows for screening all individual exposures, but does not take into account potential confounding between them and is at higher risk of false positive selection with our data structure (Agier et al., 2016). On the other hand, LASSO - a variable selection method which aims to optimally predict the outcome – has the advantage that it mutually adjusts for the effect of other exposures, but will tend to select only one predictor in the case that a true predictor is highly correlated with other exposures (e.g., within an exposure family) (Agier et al., 2016). Overall the estimates obtained in the multiple exposure models were similar to the ones obtained in the single-exposure models (i.e., ExWAS). However, changes in estimates (>5%) were observed in the multiple-exposure model for the following pregnancy exposures: active smoking (aMR single- vs. multi-exposure models = 1.21 vs. 1.31, externalizing scale) and fish consumption low vs medium intake (0.98 vs. 1.15), and for the following childhood exposures: bakery products (0.88 vs. 0.93), readymade food (1.18 vs. 1.25), childhood caffeinated drinks (1.21 vs. 1.14), fastfood (1.26 vs. 1.11), DDE (0.89 vs. 0.95) and PCB 180 (0.85 vs. 0.91) with ADHD index, possibly due to residual confounding in the single-exposure models. Correlations between exposures are known to present a challenge for exposome research. Statistical methods similar to the penalized models we used have been shown to provide a good compromise between minimizing false positives and maximizing the selection of truly associated exposures in comparison with other approaches, although in a context of many correlated exposures all methods have a limited ability to efficiently differentiate true predictors from correlated covariates (Agier et al., 2016).
5. Conclusion
In this first study of many environmental risk factors, child behavior problems were influenced by an array of early life environmental exposures in particular prenatal tobacco smoke and car traffic density. During childhood, a healthy lifestyle including healthy dietary habits, longer hours of sleep and a rich social capital of the family were protective, whereas indoor air pollution, copper and blood lead levels were associated with increased symptoms. Promoting early on healthy family habits at individual level and regulating air quality, lead exposure at population level could help improve people’s exposome and prevent the future development of mental health disorders.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
ISGlobal acknowledges support from the Spanish Ministry of Science and Innovation through the “Centro de Excelencia Severo Ochoa 2019-2023” Program (CEX2018-000806-S), and support from the Generalitat de Catalunya through the CERCA Program. We are grateful to all the participating children, parents, practitioners and researchers in the six countries who took part in this study. We further thank Muireann Coen, Sonia Brishoual, Angelique Serre, Michele Grosdenier, Prof Frederic Millot, Elodie Migault, Manuela Boue, Sandy Bertin, Veronique Ferrand-Rigalleau, Céline Leger, Noella Gorry, Silvia Fochs, Nuria Pey, Cecilia Persavente, Susana Gros, Georgia Chalkiadaki, Danai Feida, Eirini Michalaki, Mariza Kampouri, Anny Kyriklaki, Minas Iakovidis, Maria Fasoulaki, Ingvild Essén, Heidi Marie Nordheim, and the Yorkshire Water.
Funding/support
The LIFE-CYCLE project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 733206. This study has been funded by Instituto de Salud Carlos III through the projects “CP14/00108 & PI16/00261” (Co-funded by European Regional Development Fund “A way to make Europe”). LM is funded by a Juan de la Cierva-Incorporación fellowship (IJC2018-035394-I) awarded by the Spanish Ministerio de Economía, Industria y Competitividad. JJ hold a Miguel Servet contract (MS14/00108) awarded by the Spanish Institute of Health Carlos III (Ministry of Economy and Competitiveness). The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7/2007-206) under grant agreement no 308333 – the HELIX project. CW received funding from the Fondation de France (00069251, France). MC received funding from Instituto de Salud Carlos III (Ministry of Economy and Competitiveness) (MS16/00128). INMA data collections were supported by grants from the Instituto de Salud Carlos III, CIBERESP, and the Generalitat de Catalunya- CIRIT (Spain). KANC was funded by the grant of the Lithuanian Agency for Science Innovation and Technology (6-04-2014_31V-66). The Norwegian Mother, Father and Child Cohort Study is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research. The Rhea project was financially supported by European projects, and the Greek Ministry of Health (Program of Prevention of obesity and neurodevelopmental disorders in preschool children, in Heraklion district, Crete, Greece: 2011- 2014; “Rhea Plus”: Primary Prevention Program of Environmental Risk Factors for Reproductive Health, and Child Health: 2012-15). The work was also supported by MICINN [MTM2015-68140-R] and Centro Nacional de Genotipado- CEGEN- PRB2- ISCIII (Spain). This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Collaboration for Applied Health Research and Care (CLAHRC) for Yorkshire and Humber (UK). Core support for Born in Bradford is also provided by the Wellcome Trust (WT101597MA, UK). Project “PI16/00118”, funded by Instituto de Salud Carlos III and co-funded by European Union (ERDF) “A way to make Europe”. This study has been funded by Instituto de Salud Carlos III through the projects “CP14/00108 and PI16/00261” (Co-funded by European Regional Development Fund “A way to make Europe”).
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Handling Editor: Shoji F. Nakayama
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2021.106523.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Achenbach T., Rescorla L. VT: University of Vermont, Research Center for Children, Youth and Families; Burlington: 2001. Manual for the ASEBA School-Age Forms and Profiles. An Integrated System of Multi-informant Assessment. [Google Scholar]
- Agier, L., Basagaña, X., Maitre, L., Granum, B., Bird, P.K., Casas, M., et al., 2019. Early-life exposome and lung function in children in Europe: an analysis of data from the longitudinal, population-based HELIX cohort. Lancet Planet Heal [Internet]. Feb 1 [cited 2019 Apr 11];3(2):e81–92. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30737192. [DOI] [PubMed]
- Agier L., Portengen L., Chadeau-Hyam M., Basagaña X., Giorgis-Allemand L., Siroux V. A systematic comparison of linear regression-based statistical methods to assess exposome-health associations. Environ. Health Perspect. [Internet] 2016 doi: 10.1289/EHP172. May 24 [cited 2018 Mar 12];124(12). Available from: http://ehp.niehs.nih.gov/EHP172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderton A., Villanueva K., O’Connor M., Boulangé C., Badland H. Reducing inequities in early childhood mental health: how might the neighborhood built environment help close the gap? A systematic search and critical review. Int. J. Environ. Res. Public Health [Internet]. 2019 doi: 10.3390/ijerph16091516. [cited 2019 Aug 27];16(9). Available from: http://www.ncbi.nlm.nih.gov/pubmed/31035699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J.L., Oberdorster G., Morris-Schaffer K., Wong C., Klocke C., Sobolewski M. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: Parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology [Internet] 2017 doi: 10.1016/j.neuro.2015.12.014. Mar [cited 2019 Aug 27];59:140–54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26721665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque F, Ven E van der, medicine AM-P, 2011 undefined. A meta-analysis of the risk for psychotic disorders among first-and second-generation immigrants. cambridge.org [Internet]. [cited 2019 Oct 3]; Available from: https://www.cambridge.org/core/journals/psychological-medicine/article/metaanalysis-of-the-risk-for-psychotic-disorders-among-first-and-secondgeneration-immigrants/7427585927F99E8EF88C4F1AA6546C02. [DOI] [PubMed]
- Bové, H., Bongaerts, E., Slenders, E., Bijnens, E.M., Saenen, N.D., Gyselaers, W., et al., 2019. Ambient black carbon particles reach the fetal side of human placenta. Nat. Commun. [Internet]. Dec 1 [cited 2020 Aug 28];10(1):1–7. Doi: 10.1038/s41467-019-11654-3. [DOI] [PMC free article] [PubMed]
- Boyce, W., Torsheim, T., Currie, C., Zambon, A., 2006. The family affluence scale as a measure of national wealth: validation of an adolescent self-report measure. Soc. Indic. Res. [Internet]. Sep 20 [cited 2017 Sep 18];78(3):473–87. Available from: http://link.springer.com/10.1007/s11205-005-1607-6.
- Bradley Robert H., Caldwell Bettye M., Rock Stephen L., Ramey Craig T., et al Home environment and cognitive development in the first 3 years of life: A collaborative study involving six sites and three ethnic groups in North America. Dev. Psychol. 1989;25(2):217–235. doi: 10.1037/0012-1649.25.2.217. [DOI] [Google Scholar]
- Burke, H., Leonardi-Bee, J., Hashim, A., Pine-Abata, H., Chen, Y., Cook, D.G., et al., 2012. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics [Internet]. Apr [cited 2019 Sep 13];129(4):735–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22430451. [DOI] [PubMed]
- Bussing, R., Meyer, J., Zima, B.T., Mason, D.M., Gary, F.A., Garvan, C.W., 2015. Childhood ADHD symptoms: association with parental social networks and mental health service use during adolescence. Int. J. Environ. Res. Public Health [Internet]. Sep 22 [cited 2019 Aug 9];12(9):11893–909. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26402692. [DOI] [PMC free article] [PubMed]
- Cadiou, S., Bustamante, M., Agier, L., Andrusaityte, S., Basagaña, X., Carracedo, A., et al., 2020. Using methylome data to inform exposome-health association studies: An application to the identification of environmental drivers of child body mass index. Environ. Int. [Internet]. 138:105622. Available from: http://www.sciencedirect.com/science/article/pii/S0160412019333112. [DOI] [PMC free article] [PubMed]
- Calamandrei G., Ricceri L., Meccia E., Tartaglione A.M., Horvat M., Tratnik J.S. Pregnancy exposome and child psychomotor development in three European birth cohorts. Environ. Res. 2020 Feb;1(181) doi: 10.1016/j.envres.2019.108856. [DOI] [PubMed] [Google Scholar]
- Cannon Mary, Jones Peter B., Murray Robin M. Obstetric complications and schizophrenia: historical and meta-analytic review. AJP. 2002;159(7):1080–1092. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- Cohen, J.F.W., Gorski, M.T., Gruber, S.A., Kurdziel, L.B.F., Rimm, E.B., 2016. The effect of healthy dietary consumption on executive cognitive functioning in children and adolescents: a systematic review. Br. J. Nutr. [Internet]. Sep 28 [cited 2019 Sep 17];116(6):989–1000. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27487986. [DOI] [PubMed]
- Cohen J.F.W., Gorski M.T., Gruber S.A., Kurdziel L.B.F., Rimm E.B. The effect of healthy dietary consumption on executive cognitive functioning in children and adolescents: a systematic review. Br. J. Nutr. 2016;116(6):989–1000. doi: 10.1017/S0007114516002877. [DOI] [PubMed] [Google Scholar]
- Cohen S., Williamson G., Spacapam S., Oskamp S., Williamson G. Perceived stress in a probability sample of the United States. Soc. Psychol. Heal Claremont Symp. Appl. Soc. Psychol. 1988 Jan 1. [Google Scholar]
- Conners C. Conners’ Rating Scales - Revised. User’s manual. Conners 3r. Multi Health Systems (MHS Inc.), editor. North Tonawanda, New York; 1997.
- De Bie P., Muller P., Wijmenga C., Klomp L.W.J. Molecular pathogenesis of Wilson and Menkes disease: Correlation of mutations with molecular defects and disease phenotypes. J. Med. Genet. 2007 doi: 10.1136/jmg.2007.052746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone, S.V., Larsson, H., 2019. Genetics of attention deficit hyperactivity disorder [Internet]. Vol. 24, Molecular Psychiatry. Nature Publishing Group; [cited 2021 Jan 12]. p. 562–75. Doi: 10.1038/s41380-018-0070-0. [DOI] [PMC free article] [PubMed]
- Forns J., Mandal S., Iszatt N., Polder A., Thomsen C., Lyche J.L. Novel application of statistical methods for analysis of multiple toxicants identifies DDT as a risk factor for early child behavioral problems. Environ. Res. [Internet] 2016 doi: 10.1016/j.envres.2016.07.014. Nov 1 [cited 2021 Jan 13];151:91–100. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0013935116302973. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Casanova, I., Stein, A.D., Barraza-Villarreal, A., Feregrino, R.G., DiGirolamo, A., Hernandez-Cadena, L., et al., Prenatal exposure to environmental pollutants and child development trajectories through 7 years. Int. J. Hyg. Environ. Health [Internet]. 2018 May 1 [cited 2019 Aug 29];221(4):616–22. Available from: https://www.sciencedirect.com/science/article/pii/S1438463917307617?via%3Dihub. [DOI] [PMC free article] [PubMed]
- Grandjean, P., Landrigan, P.J., Bloom, B., Cohen, R., Freeman, G., Landrigan, P., et al., 2014. Neurobehavioural effects of developmental toxicity. Lancet Neurol. [Internet]. Mar [cited 2016 Dec 12];13(3):330–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24556010. [DOI] [PMC free article] [PubMed]
- Grandjean P., Landrigan P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014 Mar;13(3):330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guloksuz S., Rutten B.P.F., Pries L.-K., ten Have M., de Graaf R., van Dorsselaer S. The complexities of evaluating the exposome in psychiatry: a data-driven illustration of challenges and some propositions for amendments. Schizophr Bull. [Internet] 2018 doi: 10.1093/schbul/sby118. 2018 Aug 30 [cited 2018 Sep 4]; Available from: https://academic.oup.com/schizophreniabulletin/advance-article/doi/10.1093/schbul/sby118/5086671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad, N., Andrianou, X.D., Makris, K.C., 2019. A scoping review on the characteristics of human exposome studies [internet]. Vol. 5, Current Pollution Reports. Springer; [cited 2020 Oct 14]. p. 378–93. Doi: 10.1007/s40726-019-00130-7.
- Haug L.S., Sakhi A.K., Cequier E., Casas M., Maitre L., Basagana X. In-utero and childhood chemical exposome in six European mother-child cohorts. Environ. Int. [Internet] 2018 doi: 10.1016/j.envint.2018.09.056. Dec 1 [cited 2018 Nov 20];121:751–63. Available from: https://www.sciencedirect.com/science/article/pii/S016041201831225X?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- Hernandez-Ferrer, C., Wellenius, G.A., Tamayo, I., Basagaña, X., Sunyer, J., Vrijheid, M., et al., 2019. Comprehensive study of the exposome and omic data using rexposome Bioconductor packages. Kelso J, editor. Bioinformatics [Internet]. Jun 27 [cited 2019 Sep 13]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/31243429. [DOI] [PubMed]
- Hopson, M.B., Margolis, A., Rauh, V., Herbstman, J., 2016. Impact of the home environment on the relationship between prenatal exposure to environmental tobacco smoke and child behavior. Int. J. Child Health Hum. Dev. [Internet]. [cited 2019 Aug 29];9(4):453–64. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28845210. [PMC free article] [PubMed]
- Howard, A.L., Robinson, M., Smith, G.J., Ambrosini, G.L., Piek, J.P., Oddy, W.H., 2011. ADHD is associated with a “western” dietary pattern in adolescents. J. Atten. Disord. [Internet]. Jul 14 [cited 2020 Jun 12];15(5):403–11. Available from: http://journals.sagepub.com/doi/10.1177/1087054710365990. [DOI] [PubMed]
- Kramer M.S. Determinants of low birth weight: methodological assessment and meta-analysis. Bull. World Health Organ. 1987;65(5):663–737. [PMC free article] [PubMed] [Google Scholar]
- Landrigan P.J. The worldwide problem of lead in petrol. Bull. World Health Organ. 2002 [PMC free article] [PubMed] [Google Scholar]
- Lenters V., Iszatt N., Forns J., Čechová E., Kočan A., Legler J. Early-life exposure to persistent organic pollutants (OCPs, PBDEs, PCBs, PFASs) and attention-deficit/hyperactivity disorder: A multi-pollutant analysis of a Norwegian birth cohort. Environ. Int. [Internet] 2019 doi: 10.1016/j.envint.2019.01.020. Apr [cited 2019 Aug 1];125:33–42. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0160412018306810. [DOI] [PubMed] [Google Scholar]
- Li, M.-X., Yeung, J.M.Y., Cherny, S.S., Sham, P.C., 2012. Evaluating the effective numbers of independent tests and significant p-value thresholds in commercial genotyping arrays and public imputation reference datasets. Hum. Genet. [Internet]. May [cited 2017 Mar 15];131(5):747–56. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22143225. [DOI] [PMC free article] [PubMed]
- Maitre L., de Bont J., Casas M., Robinson O., Aasvang G.M., Agier L. Human Early Life Exposome (HELIX) study: a European population-based exposome cohort. BMJ Open [Internet] 2018 doi: 10.1136/bmjopen-2017-021311. Sep 10 [cited 2019 Apr 16];8(9):e021311. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30206078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitre L., de Bont J., Casas M., Robinson O., Aasvang G.M., Agier L. Human Early Life Exposome (HELIX) study: a European population-based exposome cohort. BMJ Open. 2018 Sep;8(9) doi: 10.1136/bmjopen-2017-021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami, A.Y., Jack, A., Emeh, C.C., Stephens, H.F., 2010. Parental influence on children with attention-deficit/hyperactivity disorder: I. Relationships between parent behaviors and child peer status. J. Abnorm. Child Psychol. [Internet]. Aug [cited 2019 Aug 9];38(6):721–36. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20339912. [DOI] [PMC free article] [PubMed]
- Morales, E., Julvez, J., Torrent, M., de Cid, R., Guxens, M., Bustamante, M., et al., 2009. Association of early-life exposure to household gas appliances and indoor nitrogen dioxide with cognition and attention behavior in preschoolers. Am. J. Epidemiol. [Internet]. Jun 1 [cited 2019 Aug 27];169(11):1327–36. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19395695. [DOI] [PubMed]
- Nederkoorn, C., Guerrieri, R., Havermans, R.C., Roefs, A., Jansen, A., 2009. The interactive effect of hunger and impulsivity on food intake and purchase in a virtual supermarket. Int. J. Obes. [Internet]. Aug [cited 2020 Sep 1];33(8):905–12. Available from: https://pubmed.ncbi.nlm.nih.gov/19546869/. [DOI] [PubMed]
- Needleman H.L., Bellinger D. The health effects of low level exposure to lead. Annu. Rev. Public Health [Internet] 1991 doi: 10.1146/annurev.pu.12.050191.000551. [cited 2019 Oct 21];12:111–40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1828669. [DOI] [PubMed] [Google Scholar]
- Papadopoulou E., Haug L.S., Sakhi A.K., Andrusaityte S., Basagaña X., Brantsaeter A.L. Diet as a source of exposure to environmental contaminants for pregnant women and children from six European countries. Environ. Health Perspect. [Internet] 2019 doi: 10.1289/EHP5324. Oct [cited 2019 Oct 22];127(10):107005. Available from: https://ehp.niehs.nih.gov/doi/10.1289/EHP5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelsser, L.M., Frankena, K., Toorman, J., Pereira, R.R., 2017. Diet and ADHD, reviewing the evidence: A systematic review of meta-analyses of double-blind placebo-controlled trials evaluating the efficacy of diet interventions on the behavior of children with ADHD [Internet]. Vol. 12, PLoS ONE. Public Library of Science; [cited 2021 Jan 12]. p. e0169277. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0169277. [DOI] [PMC free article] [PubMed]
- Peterson B.S., Rauh V.A., Bansal R., Hao X., Toth Z., Nati G. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry [Internet] 2015 doi: 10.1001/jamapsychiatry.2015.57. Jun 1 [cited 2019 Aug 27];72(6):531. Available from: http://archpsyc.jamanetwork.com/article.aspx?doi=10.1001/jamapsychiatry.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat, C., Barkoski, J., Tancredi, D.J., Elms, B., Barr, D.B., Ozonoff, S., et al., 2018. Prenatal exposure to organophosphate pesticides and risk of autism spectrum disorders and other non-typical development at 3 years in a high-risk cohort. Int. J. Hyg. Environ. Health [Internet]. [cited 2019 Jul 31];221(3):548–55. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29478806. [DOI] [PMC free article] [PubMed]
- Rapoport J.L., Giedd J.N., Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol. Psychiatry. 2012;17(12):1228–1238. doi: 10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice F., Langley K., Woodford C., Davey Smith G., Thapar A. Identifying the contribution of prenatal risk factors to offspring development and psychopathology: What designs to use and a critique of literature on maternal smoking and stress in pregnancy. Dev. Psychopathol. 2018 Aug 1;30(3):1107–1128. doi: 10.1017/S0954579418000421. [DOI] [PubMed] [Google Scholar]
- Robinson O., Tamayo I., de Castro M., Valentin A., Giorgis-Allemand L., Krog N.H. The urban exposome during pregnancy and its socioeconomic determinants. Environ. Health Perspect. 2018;126(7):77005. doi: 10.1289/EHP2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson O., Tamayo I., de Castro M., Valentin A., Giorgis-Allemand L., Hjertager Krog N. The urban exposome during pregnancy and its socioeconomic determinants. Environ. Health Perspect. [Internet] 2018 doi: 10.1289/EHP2862. Jul [cited 2019 Apr 11];126(7):077005. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30024382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roza, S.J., Verhulst, F.C., Jaddoe, V.W., Steegers, E.A., Mackenbach, J.P., Hofman, A., et al., 2009. Maternal smoking during pregnancy and child behaviour problems: the Generation R Study. Int. J. Epidemiol. [Internet]. Jun 1 [cited 2018 Feb 21];38(3):680–9. Available from: https://academic.oup.com/ije/article-lookup/doi/10.1093/ije/dyn163. [DOI] [PubMed]
- Steer C.D., Bolton P., Golding J. Preconception and prenatal environmental factors associated with communication impairments in 9 year old children using an exposome-wide approach. Liu H, editor. PLoS One [Internet] 2015 doi: 10.1371/journal.pone.0118701. Mar 4 [cited 2019 Aug 1];10(3):e0118701. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25739097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styczeń, K., Sowa-Kućma, M., Siwek, M., Dudek, D., Reczyński, W., Misztak, P., et al., 2016. Study of the serum copper levels in patients with major depressive disorder. Biol. Trace Elem. Res. [Internet]. Dec 1 [cited 2021 Jan 12];174(2):287–93. Available from: https://link.springer.com/article/10.1007/s12011-016-0720-5. [DOI] [PMC free article] [PubMed]
- Suades-González, E., Gascon, M., Guxens, M., Sunyer, J., 2019. Air pollution and neuropsychological development: a review of the latest evidence. Endocrinology [Internet]. Oct [cited 2019 Aug 27];156(10):3473–82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26241071. [DOI] [PMC free article] [PubMed]
- Tamayo-Uria I., Maitre L., Thomsen C., Nieuwenhuijsen M.J., Chatzi L., Siroux V. The early-life exposome: description and patterns in six European countries. Environ. Int. [Internet] 2019 doi: 10.1016/j.envint.2018.11.067. Feb 6 [cited 2019 Jan 14];123:189–200. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30530161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The toxic truth | UNICEF [Internet]. [cited 2021 Jan 12]. Available from: https://www.unicef.org/reports/toxic-truth-childrens-exposure-to-lead-pollution-2020.
- Uher Rudolf, Zwicker Alyson. Etiology in psychiatry: embracing the reality of poly-gene-environmental causation of mental illness. World Psychiatry. 2017;16(2):121–129. doi: 10.1002/wps.v16.210.1002/wps.20436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren S., Groothuis-Oudshoorn K. mice : Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011 Dec;45(3):1–67. [Google Scholar]
- van Os J., Kenis G., Rutten B.P.F. The environment and schizophrenia. Nature. 2010 Nov;468(7321):203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- Vassos E., Agerbo E., Mors O., Pedersen C.B. Urban–rural differences in incidence rates of psychiatric disorders in Denmark. Br. J. Psychiatry. 2016 May;208(05):435–440. doi: 10.1192/bjp.bp.114.161091. [DOI] [PubMed] [Google Scholar]
- Vives-Usano M., Hernandez-Ferrer C., Maitre L., Ruiz-Arenas C., Andrusaityte S., Borràs E. In utero and childhood exposure to tobacco smoke and multi-layer molecular signatures in children. BMC Med. [Internet] 2020;18(1):243. doi: 10.1186/s12916-020-01686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijheid M., Slama R., Robinson O., Chatzi L., Coen M., van den Hazel P. The human early-life exposome (HELIX): Project rationale and design. Environ. Health Perspect. [Internet] 2014 doi: 10.1289/ehp.1307204. Mar 7 [cited 2018 Mar 12]; Available from: http://ehp.niehs.nih.gov/1307204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijheid M., Fossati S., Maitre L., Márquez S., Roumeliotaki T., Agier L. Early-life environmental exposures and childhood obesity: an exposome-wide approach. Environ. Health Perspect. 2020;128(6):67009. doi: 10.1289/EHP5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z., Ma, S., Zappitelli, M., Parikh, C., Wang, C.-Y., Devarajan, P., 2016. Penalized count data regression with application to hospital stay after pediatric cardiac surgery. Stat. Methods Med. Res. [Internet]. Dec 30 [cited 2018 Apr 30];25(6):2685–703. Available from: http://journals.sagepub.com/doi/10.1177/0962280214530608. [DOI] [PMC free article] [PubMed]
- Warembourg C., Maitre L., Tamayo-Uria I., Fossati S., Roumeliotaki T., Aasvang G.M. Early-life environmental exposures and blood pressure in children. J. Am. Coll. Cardiol. 2019 Sep 10;74(10):1317–1328. doi: 10.1016/j.jacc.2019.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, I.R., Royston, P., Wood, A.M., 2011. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. [Internet]. Feb 20 [cited 2017 Aug 23];30(4):377–99. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21225900. [DOI] [PubMed]
- Wild C.P. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomarkers Prev. 2005 Aug;14(8):1847–1850. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO). WHO | Recycling used lead-acid batteries: [Internet]. WHO. World Health Organization; 2017 [cited 2019 Aug 27]. Available from: https://www.who.int/ipcs/publications/ulab/en/.
- World health organization. Fact sheet: Adolescent mental health [Internet]. [cited 2019 Oct 3]. Available from: https://www.who.int/news-room/fact-sheets/detail/adolescent-mental-health.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.