Highlights
-
•
The social patterning of the 5 inflammatory markers was heterogeneous.
-
•
Educational attainment was most strongly related to CRP and fibrinogen.
-
•
The social gradient for IL-6 was weaker compared to CRP and fibrinogen.
-
•
TNF-α and IL-1β were not socially patterned by educational attainment.
-
•
The social patterning of inflammatory biomarkers was variable by country.
Keywords: Inflammation, Cohort studies, Educational level, Social inequalities in health
Abstract
Background
Evidence suggests that the inflammatory reaction, an adaptive response triggered by a variety of harmful stimuli and conditions involved in the risk and development of many chronic diseases, is a potential pathway through which the socioeconomic environment is biologically embedded. Difficulty in interpreting the role of the inflammatory system in the embodiment dynamic arises because of heterogeneity across studies that use a limited but varied number of inflammatory markers. There is no consensus in the literature as to which inflammatory markers beyond the C-reactive protein and to a lesser extent interleukin 6 are related to the social environment. Accordingly, we aimed to investigate the association between educational attainment, and several markers of inflammation – C-reactive protein, fibrinogen, interleukin 6, interleukin 1β and tumor necrosis factor α– in 6 European cohort studies.
Methods
Up to 17,470 participants from six European cohort studies with data on educational attainment, health behaviors and lifestyle factors, and at least two different inflammatory markers. Four sub-datasets were drawn with varying numbers of participants to allow pairwise comparison of the social patterning of C-reactive protein and any other inflammatory markers. To evaluate within each sub-dataset the importance of the context and cohort specificities, linear regression-based analyses were performed separately for each cohort and combined in a random effect meta-analysis to determine the relationship between educational attainment and inflammation.
Results
We found that the magnitude of the relationship between educational attainment and five inflammatory biomarkers (C-reactive protein, fibrinogen, interleukin 6 and 1β and tumor necrosis factor α) was variable. By far the most socially patterned biomarker was C-reactive protein, followed by fibrinogen and to lesser extent interleukin 6, where a low educational attainment was associated with higher inflammation even after adjusting for health behaviours and body mass index. No association was found with interleukin 1β and tumor necrosis factor α.
Conclusions
Our study suggests different educational patterning of inflammatory biomarkers. Further large-scale research is needed to explore social differences in the inflammatory cascade in greater detail and the extent to which these differences contribute to social inequalities in health.
1. Introduction
Inflammation is an important and necessary component of the immune system broadly defined as the body’s protective reaction in response to infection or injury. A successful acute inflammatory response results in the elimination of the inciting stimulus followed by a resolution and repair phase in order to restore homeostasis (Medzhitov, 2008). Much less is known about the causes and mechanisms of chronic systemic inflammation, it could occur when the organism has to adapt when confronted with homeostatic imbalances (Medzhitov, 2008). Chronic systemic inflammation in response to persistent threats could contribute to disease progression through dysregulation of homeostatic functions, control of the metabolism, tissue repair, and of the hypothalamus-pituitary axis (Straub, 2017, Bennett et al., 2018, Franceschi et al., 2018). As such, chronic inflammation provides a unifying pathophysiological mechanism involved in many chronic diseases, including diabetes and obesity, cardiovascular disease, certain cancers and inflammatory bowel diseases, arthritis, and osteoporosis (Bennett et al., 2018, Furman et al., 2019).
Because the inflammatory response may be influenced by a large range of environmental challenges, an overall increase in systemic inflammation has emerged as one possible physiological pathway through which the social environment ‘gets under the skin’ to affect biological processes and confers a risk for negative health outcomes (Miller et al., 2009). This refers to a dynamic process, namely the biological embodiment of the social environment, or the social-to-biological transition (Blane et al., 2013). First, socially disadvantaged groups are more likely to live in areas where they are disproportionately exposed to harm, such as air-pollution and damp housing, overcrowded conditions, poor housing quality, or insufficient access to sanitation, that are likely to increase systemic inflammation (Gares et al., 2017). Second, adverse health behaviours, including smoking, alcohol consumption and nutrition are more prevalent among socially disadvantaged groups (Petrovic et al., 2018) and are known to influence inflammation. Third, since socially disadvantaged groups are more likely to be exposed to a higher burden of exposures in different domains of everyday life including stressful life situations, negative life events, family stress or adversities (Fagundes et al., 2013), social disadvantage across the life course may result in an over-solicited stress response involving the inflammatory system. This nexus of dynamic processes may contribute to heightening the level of basal inflammation. Therefore chronic inflammation may be crucial in understanding social inequalities in health by affecting levels of inflammatory cytokines and acute-phase proteins (Kivimäki and Steptoe, 2018).
Numerous studies have examined the association between various indicators of socioeconomic position (SEP), such as education, occupation and income, and markers of systemic inflammation. Most research has focused on C reactive protein (CRP), an acute-phase protein released by the liver in response to interleukin 1, 6 and 7 (IL-1, IL-6 and IL-7) (Eklund, 2009). In 2007, a systematic review of 25 population-based studies reported an inverse relationship between adult SEP and CRP across countries independently of age, sex, body mass index (BMI), and smoking, that where controlled for in most studies (Nazmi and Victora, 2007). More recently, a meta-analysis of 43 North American studies including sub-sets of investigations on IL-6 (N = 18 studies) and CRP (N = 35 studies) found a higher level of CRP and IL-6 among socially disadvantaged participants compared to their advantaged counterparts (measured by educational attainment or household income). The association with CRP was partly explained by obesity and smoking while the association with IL-6 weakened with age but was not affected by BMI and smoking (Muscatell et al., 2018). Among the other biomarkers of inflammation, fibrinogen, an acute-phase protein also released by the liver, is less frequently used. Panagiotakos et al., 2004, Pollitt et al., 2008, Steinvil et al., 2008, Fraga et al., 2015 found a higher level of fibrinogen in participants with lower education. While this relationship was attenuated by health behaviours and cardiovascular risk factors, evidence of the association remained after adjustment (Pollitt et al., 2008, Steinvil et al., 2008). A limited number of studies have examined another inflammatory biomarker, tumour necrosis factor α, TNF-α (Steptoe et al., 2002, Koster et al., 2006, Fraga et al., 2015), an important immune mediator secreted by macrophages and monocytes released after a stimulus (i.e. in response to IL-1). For this inflammatory marker the results are not conclusive (Steptoe et al. (2002) and Koster et al., 2006, Fraga et al., 2015.
There is no consensus in the literature about the most relevant inflammatory markers in terms of social patterning nor standard biomarkers that would be relevant for indicating the presence of health-damaging chronic inflammation (Furman et al., 2019). A difficulty in interpreting the role of the inflammatory system in the embodiment of SEP arises because of the heterogeneity of studies that use a limited but varied number of inflammatory markers. Furthermore, cross-country comparison is limited by the variety of SEP indicators used, their meaning in different national contexts, and the lack of harmonisation of both SEP and inflammatory markers across studies. Additionally, evidence on the pathways relating SEP to inflammatory markers across countries is limited due to the fact that behavioural and personal characteristics that may mediate the association between SEP and inflammation are inconsistently taken into account. It is therefore unclear whether the association between lower SEP and chronic inflammation is a stable phenomenon across countries, persisting even after adjusting for health behaviours, and whether this relationship depends on the biomarkers used to measure inflammation.
We previously reported a consistent inverse association between SEP, (using father’s occupation, education, and last occupation) and CRP using data from six European cohort studies from three countries (Berger et al., 2019). In that study, educational attainment was the SEP marker most strongly related to CRP level in adulthood, after adjusting for health behaviours, body mass index and later-in-life SEP. Educational attainment captures components of the social environment from earlier in life, and is associated with later occupational social class and income (d’Errico et al 2017). It is also a useful variable when comparing SEP across countries or cohorts because it is often relatively consistently collected, is usually available and can be harmonised across contexts. As with all SEP proxy variables, it must be used while remaining aware of local time, place and cohort differences. Based on these findings and to capture the complex regulatory cascades involved in inflammation, the present study aims to describe in detail the educational patterning of several different inflammatory markers. To achieve this, we assessed the relationship between educational attainment and CRP, fibrinogen, IL-6, IL-1β and TNF-α – using harmonised data across six European cohort studies within the Lifepath consortium. We also described these associations adjusted for behavioural variables to examine the patterns of adjusted associations between education and each inflammatory biomarker.
2. Methods
2.1. Study population
This study is part of a Horizon 2020 Research and Innovation Program European funded project, the Lifepath project (http://www.lifepathproject.eu/), which includes a consortium of eighteen cohort studies across different countries and time period, with demographic, clinical, biological and socioeconomic data, with the aim to investigate biological pathways underlying social differences in healthy ageing. Data harmonization were performed in order to pool and analyse the cohorts together (Vineis et al., 2017). We selected the six cohort studies for which data on at least two different measures of inflammatory markers including CRP, educational attainment, smoking, alcohol consumption, physical activity and BMI were available. This selection allowed for sets of comparative analyses wherein one consistent inflammatory biomarker (CRP) was common across them all. The selected cohorts were:
-one cohort from Finland: the Young Finns study: YFS (Raitakari et al., 2008)
-two cohorts from Switzerland: SKIPOGH (Alwan et al., 2014) and CoLaus (Firmann et al., 2008)
-three British cohorts: Whitehall II (Marmot et al., 1991), the National Child Development Study, NCDS (Power and Elliott, 2006) and the English Longitudinal Study of Ageing, ELSA (Steptoe et al., 2013). A detailed description of all cohorts included in the Lifepath consortium has been given elsewhere (Vineis et al., 2017).
2.2. Educational attainment
We used the harmonised definition of educational attainment across cohort study described in details by d’Errico et al. (d’Errico et al., 2017). Briefly, participant’s educational attainment was categorised in three groups (i) ‘low’ (no school, primary or lower secondary school: from 7 to 9 years after kindergarten with a basic curriculum in languages, mathematics and other subjects), (ii) ‘medium’ (higher secondary school: around 4–5 years more, high school diploma level) and (iii) ‘high’ educational level (tertiary education: any degree after high school, such as BSc, MSc, and further education).
2.3. Inflammatory markers
We used five biomarkers to measure inflammation: CRP, fibrinogen, IL-6, IL-1β and TNF-α; selecting the first available wave in which CRP and any other inflammatory markers had been measured. In Colaus and Skipogh, CRP, IL-6, IL-1β and TNF-α were collected at baseline while in ELSA, both CRP and fibrinogen were collected in the second wave and in Whitehall, CRP, fibrinogen and IL-6 were measured in the third data of collection wave. CRP and fibrinogen were measured when participants were 45 years old in NCDS, with the exception of YFS for which we selected measures at the last available wave in order to obtain an age range among participants comparable to the age of participants in the other cohort studies. For each cohort study, a detailed description of sampling methods and laboratory analyses is given in Supplementary Table 1. Given their skewed nature, CRP, fibrinogen, IL-6 and TNF-α values were natural log-transformed to normalise distributions and because of both the skewness of the distribution and the high number of 0 values (41.3%), a constant (1) was added to IL-1 β values prior to the log transformation.
While the association between social measures and CRP is widely described, examining other components of the inflammatory system using different inflammatory biomarkers would further our understanding of potential social-to-biological processes. The inflammatory biomarkers we selected for this analysis were available across some of the cohorts, but they also represent different stages of inflammatory responses.
IL-6, IL-1β and TNF-α are all pro-inflammatory cytokines produced within adipose tissue (Coppack, 2001), their synthesis being related to adiposity and fatty acid metabolism. TNF-α also down-regulates insulin-stimulated glucose uptake, all three appear to act upon adipose tissue regulation to different extents (Coppack 2001). These markers are upstream of CRP, namely, IL-6 is the primary cytokine leading to hepatic CRP production (Ridker 2016). These biomarkers are indeed related, however are also implicated in different inflammatory mechanisms. The importance of the IL-6, IL-1β and TNF-α cytokines in adipose tissue metabolism may be an important consideration for social-to-biological questions, given the well-established socioeconomic patterns in overweight and obesity prevalence.
Fibrinogen however is produced in the liver, forming part of the clotting cascade. It represents a link between inflammation, thrombosis and cardiovascular risk, and is up-regulated during the acute phase inflammatory response driven upstream by cytokines such as IL-6 (Rooney et al 2011). While fibrinogen and CRP have similarities in terms of their synthesis and place in the cascade, fibrinogen also acts as a marker for haemostatic processes. The role of fibrinogen may be interesting as a potential social-to-biological mediator given the socioeconomic pattern of cardiovascular disease.
2.4. Covariates
Pre-defined, harmonised covariates included potential confounders, such as age, sex and potential mediators were alcohol consumption, physical activity, smoking and body mass index (BMI). These variables were selected from the closest data collection wave to that of the inflammatory marker measurements.
Alcohol consumption was harmonised in units of pure alcohol per week and we categorised participants as abstainers (0 units per week), moderate consumers (men ≤ 21 alcohol units per week; women ≤ 14 alcohol units per week) and high consumers (men > 21 alcohol units per week; women > 14 alcohol units per week). Because the physical activity was measured with different questions in each study, sedentariness was derived from leisure physical activity and dichotomised into sedentary yes/no, except in the NCDS cohort in which this variable was not available. Self-reported smoking was categorised into never, current and former smoker, except in YFS in which a binary smoking status indicator (never versus ever smoker) was available. BMI was calculated from height and weight and categorised into the WHO BMI groups using standard cut-offs: underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2) and obese (>30 kg/m2). If data were missing at the same wave, we used data from the next available data wave (for CoLaus: n = 12, 0.2%; ELSA: n = 66, 1.7%; Skipogh: n = 266, 28.4%; Whitehall: n = 374, 5.7%).
2.5. Ethics statement
Each study was approved by the relevant local or national ethics committees/board and all participants gave informed consent to participate.
2.6. Statistical analysis
In total, four sub-datasets (one for each CRP plus one other inflammatory marker-pair[either fibrinogen, IL-6 TNF-α or IL-1β]) with varying numbers of participants were created: sub-dataset 1 included cohorts that measured inflammation using CRP and fibrinogen (3 studies); sub-dataset 2 included cohorts that measured inflammation using CRP and IL-6 (4 studies); sub-dataset 3 included cohorts that measured inflammation using CRP and TNF-α (3 studies); and sub-dataset 4 included cohorts that measured inflammation by CRP and IL-1β (2 studies).
For the bivariate analysis in each sub-dataset and cohort, means and frequencies were reported for all continuous and categorical characteristics by educational attainment. We used Chi-squared test or Fisher exact test for the categorical variables and T-test or Wilcoxon rank test for continuous variables to estimate bivariate associations.
For the multivariate analysis in each cohort, linear regression models were used to assess the relationship between educational attainment and each inflammatory marker. In a basic statistical model, effect estimates were adjusted for age (continuous) and sex (for NCDS, sex only as participants are the same age) (Model 1). To examine whether the association between educational attainment and inflammation was mediated by alcohol consumption, smoking status, sedentary behaviour and BMI, we defined a second model controlling for all potential intermediate factors (Model 2). Sedentary behaviour was not available in the NCDS, therefore Model 2 for this cohort adjusted only for alcohol consumption, smoking status and BMI. The high educational level group was used as the reference category; therefore a positive regression coefficient indicates an increased level of inflammatory marker in participants with lower educational attainment.
As proposed elsewhere (Berger et al., 2019), we used a meta-analysis in each sub-dataset, based on random effects modelling with a restricted maximum likelihood estimator using the metafor R package to combine the results from each cohort within each sub-dataset. The combined effect represents the mean of the population of ‘true effects’. Test of heterogeneity among studies was provided by the Q-statistic and I2 measurement was calculated representing the proportion of total variability that can be attributed to heterogeneity (a higher value indicates a greater degree of heterogeneity).
In sensitivity analyses, we examined the SEP- inflammatory marker association separately in men and women. We also used an alternative statistical transformation for the values of each inflammatory marker. First, each inflammatory marker was categorised into quartiles. Then we defined a binary indicator ‘high’ vs ‘low’ concentration based on the highest quartiles (4th quartile) vs. the three lower quartiles (1st – 3rd quartiles). To evaluate the association between SEP and the inflammatory markers (high concentration = 1 and low concentration = 0) we used a logistic regression and the results were expressed as odds ratios (OR) and 95% confidence intervals (95% CI). We performed additional sensitivity analyses restricting our study population to never smoked participants or participants with a normal BMI [18.5–24.9 kg/m2].
All statistical analyses were performed using R (R Core Team, 2014) version 3.1.2 within the Rstudio environment (RStudio Team, 2016).
3. Results
3.1. Study population
Table 1 presents a description of the variables for each sub-dataset. Sub-dataset 1 only included participants from the three British cohort studies (37.4%, 21.7%, 40.9% for Whitehall, ELSA and NCDS respectively) while sub-dataset 2 included participants from 4 out of the 6 cohorts (43.5%,38.7%;11.5%,6.2% for Whitehall, Colaus, YFS and Skipogh respectively). Sub-dataset 3 and 4 were mostly constituted of Swiss participants (79.6% and 100% respectively) with some participants from YFS in dataset 3. Comparisons between each sub-dataset are given in Supplementary Table 2 and show small to moderate differences of the participants’ key characteristics: the proportion of participants with a lower educational attainment ranged from 59.1% in sub-dataset 1 to 45.8% in sub-dataset 2. Irrespective of the sub-dataset considered, participants with a low educational attainment were more likely to smoke and to be overweight/obese and sedentary compared to the participants with a higher educational attainment.
Table 1.
Summary characteristics of the participants for each sub-dataset µ Smoking status in YFS was categorised as ever vs never, *sedentary not available in NCDS.
| Sub-dataset 1 (N = 17 470) CRP-Fibrinogen |
Sub-dataset 2 (N = 15 019) CRP-IL6 |
Sub-dataset 3 (N = 8 485) CRP-TNFA |
Sub-dataset 4 (N = 6751) CRP-IL1B |
||
|---|---|---|---|---|---|
| Mean (SD) or N(%) | Mean (SD) or N(%) | Mean (SD) or N(%) | Mean (SD) or N(%) | ||
| Cohorts | Whitehall, NCDS, ELSA | Whitehall, Colaus, YFS, Skipogh | Colaus, YFS, Skipogh | Colaus, Skipogh | |
| Demographics | |||||
| Age | 51.3 (8.8) | 49.6 (10.2) | 49.3 (12.5) | 52.3 (12.1) | |
| Sex | Men | 9875 (56.53) | 8549 (56.92) | 4012 (47.28) | 3244 (48.05) |
| Women | 7595 (42.47) | 6470 (43.08) | 4473 (52.72) | 3507 (51.95) | |
| Inflammatory level | |||||
| CRP (mg/L) | 2.4 (5.0) | 2.2 (3.9) | 2.4 (3.7) | 2.5 (3.8) | |
| Fibrinogen (g/L) | 2.8 (0.7) | ||||
| IL6 (pg/mL) | 5.6 (35) | ||||
| TNFA (pg/mL) | 16.4 (45.9) | ||||
| IL1B (pg/mL) | 4.3 (16.3) | ||||
| Socioeconomic position | |||||
| Educational attainment | High | 4174 (23.89) | 5260 (35.02) | 2980 (35.12) | 1900 (28.14) |
| Middle | 2979 (17.05) | 2885 (19.21) | 1132 (13.34) | 1077 (15.95) | |
| Low | 10,317 (59.06) | 6874 (45.77) | 4373 (51.54) | 3774 (55.9) | |
| Intermediate factors | |||||
| Alcohol consumption | Abstainer | 4316 (24.71) | 3748 (24.96) | 2541 (29.95) | 1862 (27.58) |
| Low | 9214 (52.74) | 8914 (59.35) | 4624 (54.5) | 4227 (62.61) | |
| High | 3940 (22.55) | 2357 (15.69) | 1320 (15.56) | 662 (9.81) | |
| Smoking statusµ | Never | 8076 (46.23) | 6848 (45.60) | 3616 (42.62) | 2741 (40.6) |
| Former | 6171 (35.32) | 4592 (30.57) | 2185 (25.75) | 2185 (32.37) | |
| Current | 3223 (18.45) | 2720 (18.11) | 1825 (21.51) | 1825 (27.03) | |
| Ever | 859 (5.72) | 859 (10.12) | |||
| BMI | Underweight | 75 (0.43) | 129 (0.86) | 94 (1.11) | 80 (1.19) |
| Normal weight | 7888 (45.15) | 7416 (49.38) | 4007 (47.22) | 3175 (47.03) | |
| Overweight | 6825 (39.07) | 5527 (36.8) | 3060 (36.06) | 2446 (36.23) | |
| Obese | 2682 (15.35) | 1947 (12.96) | 1324 (15.6) | 1050 (15.55) | |
| Sedentary | No | 8106 (46.40) | 11,089 (73.83) | 5862 (69.09) | 4339 (64.27) |
| Yes | 2215 (12.68) | 3930 (26.17) | 2623 (30.91) | 2412 (35.73) | |
| Missing | 7149 (40.92) | ||||
Description of participants’ characteristics by cohort is provided in Supplementary Table 3 and 4. A total of 25 955 participants were included (53.5% of men). YFS was the youngest cohort with a mean age of 37.7 (SD 5.0), while ELSA was the oldest with a mean age of 64.0 (SD 8.5). Whitehall had the smallest proportion of women (30.6%) compared to other cohort studies. The percentage of participants with a low educational attainment ranged from 34.5% (YFS) to 76.9% (NCDS). YFS had the largest proportion of participants (37.9%) that reported drinking more than 21 or 14 alcoholic units per week respectively for men and women. The proportion of participants who had never smoked varied from 39.9% (CoLaus and ELSA) to 50.5% (YFS). There was 28.2% of obese in ELSA and 39.4% of Skipogh participants were sedentary.
3.2. Association between educational attainment and each inflammatory marker in each sub-dataset
Table 2 and Fig. 1 present the linear regression results from the random effects meta-analyses of educational attainment and each inflammatory marker in each sub-dataset. Despite the moderate to high degree of heterogeneity in study-specific estimates, we observed an association between educational attainment and CRP in Model 1 in all sub-datasets: the difference in the log of CRP concentration between the lowest and the highest educational group was 40% [24%; 60%] in sub-dataset 1; 27% [18%; 36%] in sub-dataset 2; 27% [14%; 42%] in sub-dataset 3 and 35% [27%; 43%] in sub-dataset 4. After additional adjustment for alcohol consumption, smoking status, sedentary behaviour and BMI (Model 2), these observed associations were weakened: 19% [9%; 30%]in sub-dataset 1; 11% [7%; 15%] in sub-dataset 2; 11% [6%; 17%] in sub-dataset 3 and 12% [6%; 19%]in sub-dataset 4. Except in sub-dataset 3, we observed a weaker association between a medium level of education and CRP compared to those with a low level of education and CRP (Model 1) and, except in the sub-dataset 1, the association weakened further after controlling for the four intermediate factors (Model 2).
Table 2.
Results from the meta-analyses of the association between educational attainment and each inflammatory markers in each sub-dataset.
| Sub-dataset 1 (N = 17 470) | CRP |
Fibrinogen |
|||
|---|---|---|---|---|---|
| Ref.: High | Medium | Low | Medium | Low | |
| Model 1¤ | % Change in β (95% CI) | 21% (9%; 35%) | 40% (24%; 60%) | 2% (0%; 4%) | 5% (3%; 6%) |
| P-value | <0.001 | <0.001 | 0.063 | <0.001 | |
| I2 | 65.738 | 87.492 | 70.244 | 65.555 | |
| PH | 0.043 | <0.001 | 0.034 | 0.043 | |
| Model 2ϒ | % Change in β (95% CI) | 10% (3%; 18%) | 19% (9%; 30%) | 1% (−1%; 2%) | 2% (1%; 3%) |
| P-value | 0.005 | <0.001 | 0.466 | <0.001 | |
| I2 | 30.685 | 75.076 | 43.945 | 0.000 | |
| PH | 0.275 | 0.011 | 0.164 | 0.534 | |
| Sub-dataset 2 (N = 15 019) | CRP |
IL-6 |
|||
| Medium | Low | Medium | Low | ||
| Model 1¤ | % Change in β (95% CI) | 9% (3%; 15%) | 27% (18%; 36%) | 4% (−2%; 11%) | 7% (−1%; 16%) |
| P-value | 0.002 | <0.001 | 0.207 | 0.091 | |
| I2 | 0.080 | 58.901 | 52.308 | 88.360 | |
| PH | 0.214 | 0.067 | 0.109 | <0.001 | |
| Model 2 ϒ | % Change in β (95% CI) | 4% (−2%; 9%) | 11% (7%; 15%) | 3% (−3%; 9%) | 2% (−1%; 6%) |
| P-value | 0.177 | <0.001 | 0.414 | 0.241 | |
| I2 | 0.013 | 0.000 | 47.679 | 39.609 | |
| PH | 0.353 | 0.945 | 0.126 | 0.137 | |
| Sub-dataset 3 (N = 8 485) | CRP |
TNF-α |
|||
| Medium | Low | Medium | Low | ||
| Model 1¤ | % Change in β (95% CI) | 6% (−5%; 18%) | 27% (14%; 42%) | 2% (−7%; 11%) | 0% (−5%; 6%) |
| P-value | 0.284 | <0.001 | 0.740 | 0.981 | |
| I2 | 23.749 | 63.734 | 51.580 | 58.491 | |
| PH | 0.151 | 0.052 | 0.126 | 0.088 | |
| Model 2 ϒ | β (95% CI) | 1% (−6%; 9%) | 11% (6%; 17%) | 1% (−7%; 10%) | −1% (−5%; 4%) |
| P-value | 0.743 | <0.001 | 0.741 | 0.730 | |
| I2 | 0.023 | 0.000 | 47.237 | 33.705 | |
| PH | 0.267 | 0.834 | 0.156 | 0.279 | |
| Sub-dataset 4 (N = 6 751) | CRP |
IL-1β |
|||
| Medium | Low | Medium | Low | ||
| Model 1¤ | % Change in β (95% CI) | 9% (−1%; 20%) | 35% (27%; 43%) | 6% (−1%; 15%) | 2% (−3%; 8%) |
| P-value | 0.086 | <0.001 | 0.105 | 0.391 | |
| I2 | 13.953 | 0.000 | 0.000 | 0.000 | |
| PH | 0.281 | 0.637 | 0.746 | 0.968 | |
| Model 2 ϒ | % Change in β (95% CI) | 3% (−6%; 14%) | 12% (6%; 19%) | 7% (−1%; 15%) | 4% (−2%; 10%) |
| P-value | 0.511 | <0.001 | 0.081 | 0.204 | |
| I2 | 20.499 | 0.000 | 0.000 | 0.000 | |
| PH | 0.262 | 0.959 | 0.697 | 0.819 | |
Abb.: CI confidence interval, I2 percentage of between study heterogeneity, PH P-value of heterogeneity test.
Model 1 adjusted for age and sex.
Model 2 controlled for age, sex and additionally alcohol, smoking, body mass index and sedentary.
Fig. 1.
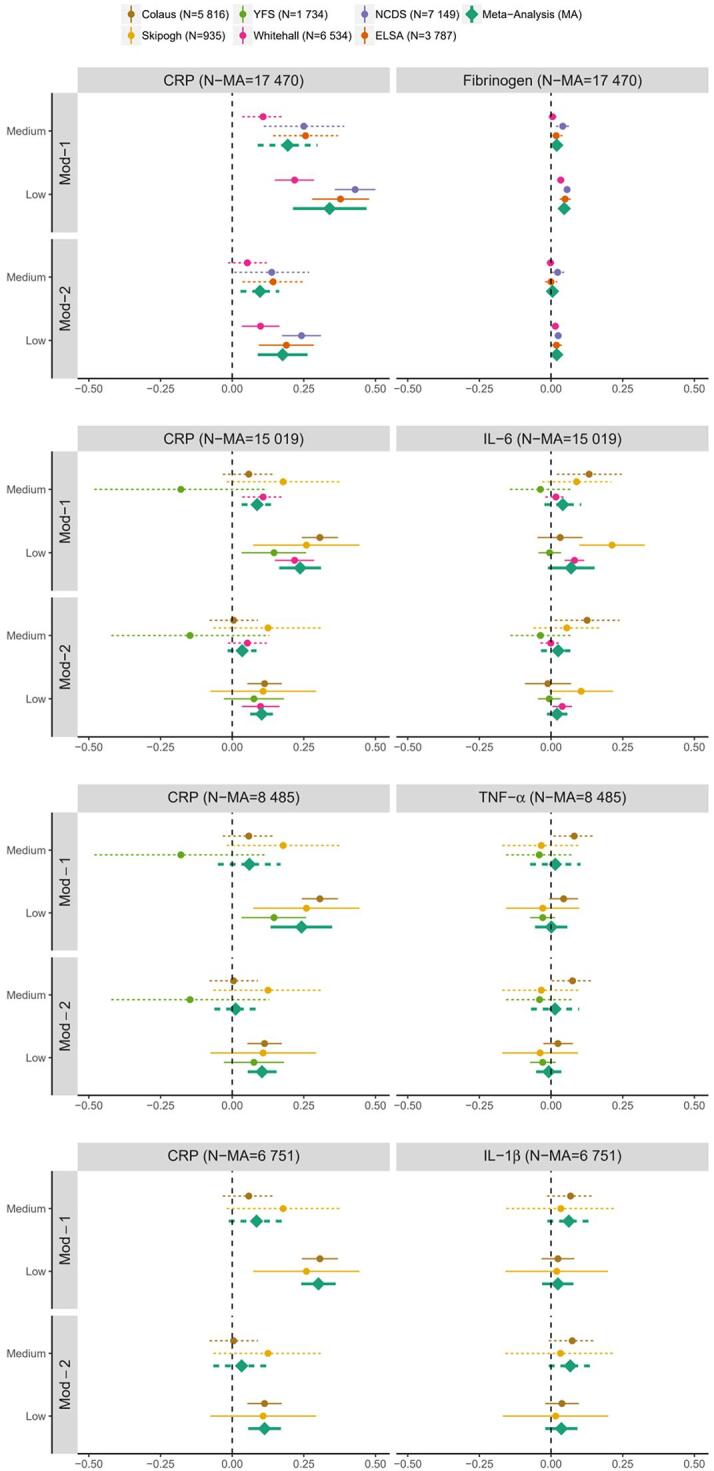
Forest plot of regression coefficients [95% confidence interval] for the association between participant’s educational attainment and inflammatory markers concentration by cohort and in random effect meta-analysis framework for each sub-dataset for Model 1 (Mod-1), after adjustment for each intermediate factor (Mod-2). The high SEP group was used as reference, dotted lines represent the medium SEP group and solid lines the low SEP group.
In the sub-dataset 1 including CRP and fibrinogen, we also observed an association between educational attainment and fibrinogen: participants with a low level of education had higher levels of fibrinogen compared to those with a high level of education, the difference was 5% [3%; 6%]. This association was attenuated after adjustment for the four intermediate factors in Model 2 (2% [1%; 3%]). We observed a weaker association between a medium level of education and fibrinogen (Model 1,2%[0%; 4%]) which weakened further in Model 2 (1% [-1%; 2%]).
In sub-dataset 2 including CRP and IL6, results for IL-6 were weaker but directionally consistent: a higher level of IL-6 was found in participants with a lower educational attainment (Model 1,7% [−1%; 16%]) mostly explained by the intermediate factors (Model 2,2% [−1%; 6%]).
There was weak evidence of an association between education and TNF-α and IL-1β in sub-datasets 3 and 4 respectively.
3.3. Pattern of inflammatory markers by educational attainment in each cohort study
Association results by cohort study are detailed in Supplementary Table 5 and presented in Fig. 1. We observed an inverse association of educational attainment with CRP, fibrinogen and IL-6 in Whitehall, where participants with a low level of education were more likely to have higher levels of all three inflammatory markers compared to those with a high level of education (Model 1: β [95%CI] = 0.22 [0.15, 0.29] for CRP, β[95%CI] = 0.03 [0.02, 0.05] for fibrinogen, β[95%CI] = 0.08 [0.05, 0.12] for IL-6). These associations were attenuated after adjustment for intermediate factors (β[95%CI] = 0.10 [0.03, 0.17] for CRP, β[95%CI] = 0.02 [0.002, 0.03] for fibrinogen and β[95%CI] = 0.04 [0.01; 0.08] for IL-6).
Participants with a low level of education had a higher level of both CRP and fibrinogen compared to those with a high level of education in NCDS (β[95%CI] = 0.056 [0.044, 0.068]) and in ELSA (β[95%CI] = 0.049 [0.03, 0.069]). These associations were attenuated after additional adjustment for intermediate factors (β[95%CI] = 0.025 [0.013, 0.037]) and in ELSA (β[95%CI] = 0.019 [−0.001, 0.038].
In Skipogh in which CRP, IL-6, TNF-α and IL-1β were measured, only CRP and IL-6 were associated with educational attainment (Model 1: β [95%CI] = 0.259 [0.073, 0.444] for CRP and β [95%CI] = 0.213 [0.099; 0.327] for IL-6). These associations were attenuated upon adjustment for intermediate factors for IL-6 (Model 2: β [95%CI] = 0.108 [-0.076, 0.293] for CRP and β [95%CI] = 0.105 [−0.007, 0.217] for IL-6).
In both Colaus and YFS, only the level of CRP was patterned by educational attainment whereas no associations between educational attainment and IL-6, TNF- α and IL1- β were observed in Colaus. Similarly in YFS, no associations were observed for the other inflammatory markers (IL-6 and TNF- α).
3.4. Sensitivity analyses
Sex-stratified results from random-effects meta-analysis are provided in Supplementary Table 6. In sub-dataset 1, the association between educational attainment and CRP was attenuated upon adjustment for the four intermediate factors in men (Model 2, β [95%CI] = 0.177 [0.102; 0.252]) but not in women (Model 2, β [95%CI] = 0.14 [−0.026; 0.306]). An association between educational attainment and CRP after adjustment on the four intermediate factors was observed in men and women in the other three sub-datasets.
In sub-dataset 1, we also observed an association between educational attainment and fibrinogen in both men (β [95%CI] = 0.044 [0.026, 0.062]) and women (β [95%CI] = 0.049 [0.036, 0.062]). Evidence of these associations, though attenuated, remained after adjustment for the four intermediate factors in Model 2 (β [95%CI] = 0.022 [0.011, 0.033] in men and β [95%CI] = 0.014 [0.001, 0.026] in women).
In both men and women, there was no association between educational attainment and IL-6, TNF-α and IL-1β (P > 0.05 in all settings) in sub-datasets 2, 3 and 4 respectively.
Our main results were robust when using an alternative transformation of each inflammatory marker (see Methods, Supplementary Fig. 1 and Supplementary Table 7). In additional sensitivity analyses, restricting our study population to ‘never smoked’ participants or participants with a normal BMI size reduced statistical power and measures of association were subsequently weakened but did not materially affect our main results and conclusion (Supplementary Table 8 and 9).
4. Discussion
Our study aimed to capture the association between education, and the complex regulatory cascades involved in inflammation. We found that the magnitude of the relationship between educational attainment and five inflammatory biomarkers (CRP, fibrinogen, IL-6, IL-1β and TNF-α) was variable, and by far the most socially patterned biomarker was CRP, followed by fibrinogen and to lesser extent IL-6, where low educational attainment was associated with higher inflammation. Results from the meta-analysis for TNF-α and IL-1β were null and thus not consistent with the other inflammatory biomarkers. Country specific results suggest that the overall direction of the association was broadly similar across available biomarkers (Whitehall, ELSA and NCDS), although some heterogeneity in results was found between cohorts (Colaus, Skipogh and YFS).
The association between educational attainment and CRP or fibrinogen in adulthood was only partly attenuated after accounting for behavioural factors (smoking status, alcohol consumption and sedentary) and BMI suggesting that (i) educational attainment may be a consistent and important upstream independent variable for elevated inflammation and that (ii) educational attainment may also operate through intermediate pathways other than health behaviours which deserve attention in future analyses. These could for include, for example, psychosocial pathways such as adverse childhood experiences (Lacey et al., 2020), loneliness (Vingeliene et al., 2019, Smith et al., 2020), and social relationships (Yang et al., 2014, Smith et al., 2020) might play a role in the relationship between education and inflammation. Our aim in this paper was to describe the association between education and inflammation using different inflammatory biomarkers. Our rationale for adjusting the models was to describe and compare the patterns of adjustment, providing additional descriptive information about associations between education and inflammation. The covariates may be on the causal pathway between education and inflammation, however, to establish mechanisms and pathways further analyses using causal inference approaches is required.
4.1. Prior literature
Our findings are in agreement with data-driven analyses on diseases across all organ systems that have linked low SEP to increased risk of health conditions characterized by elevated systemic inflammation, such as diseases of the pancreas, liver, kidney, vascular and respiratory system, lung cancer, and dementia (Kivimäki et al., 2020). In line with a number of studies on inflammatory markers, our results support studies reporting an association between disadvantaged SEP and high CRP levels (Nazmi and Victora, 2007, Muscatell et al., 2018) and further add to the empirical evidence supportive of the social patterning of fibrinogen (Davillas et al., 2017, Panagiotakos et al., 2004, Pollitt et al., 2008, Steinvil et al., 2008). The weaker association observed between educational attainment and IL-6 is in contrast with another recently published meta-analysis that reported a social gradient for IL-6 (Muscatell et al., 2018). It is noteworthy that their work relied mainly on North American studies, whereas only European studies were included in our study. Little is known about the association between SEP and the other inflammatory markers addressed in our work. The present results indicated no association between low educational attainment and higher level of IL-1β or TNF-α in line with the somewhat conflicting results from previous research work (Fraga et al., 2015, Koster et al., 2006, Steptoe et al., 2002). Future research including other novel biomarkers of inflammation such as Glyc-A would also make important contributions to understanding the relationship between social factors and the inflammatory system.
4.2. Cohort specificities
Because educational attainment reflects the broad socio-economic, cultural and historical context in which individuals live and grow, we also described the educational patterning of inflammation in each cohort study from the UK, Switzerland and Finland covering different contextual times and place. Our findings were directionally consistent across country for both CRP and fibrinogen and to lesser extent for IL-6 suggesting some shared social to biological processes across these European populations. The discrepancies between cohorts observed for IL-1β and TNF-α suggests that some more context specific mechanisms may be at play in the biological embodiment of the social environment. Alternatively, these discrepancies may reflect differences in study protocols and methodology between the cohort studies.
There is no consensus in the literature about the most relevant inflammatory markers to use for analysing the social patterning of inflammation. Our study shows that CRP and fibrinogen are consistently socially patterned suggesting that these two biomarkers have special status in the physiological cascade linking SEP with health. However, the fact that the relationship between SEP and inflammation varies according to biomarkers used deserves to be further studied.
4.3. Mechanisms
CRP and fibrinogen, both synthesised by the liver and non-specific, were found to be socially patterned in our study. These inflammatory markers do not provide information about the type of the inflammatory response, nor the initial trigger of the inflammatory cascade. However, in this framework it may be justified to hypothesise that CRP and fibrinogen are maintenance proteins with both immune and non-immune functions suggesting that elevated CRP may favour the organism’s homeostasis and its somatic maintenance (Del Giudice and Gangestad, 2018). When inflammation is triggered, IL-6, TNF-α and IL-1β are released into circulation (by macrophages and other cells) to exert their specific and various roles in inflammation. The different social patterning observed for these three inflammatory markers may reflect not only different functions but also their different sources of production. Our work suggests that the inflammatory response of the social environment may be a demonstration of one out of a variety of embodiment processes, in line with the general notion that humans respond also at a biological level to their social environment. However, this may explain only a proportion of the educational patterning in poor health outcomes, an issue that warrants further research.
4.4. Weaknesses and strengths
The main limitation of this study was that we did not have exactly the same set of inflammatory markers across the 6 cohort studies which constrained our methodological choices: the use of 4 sub-datasets made the comparisons of findings across the sub-dataset non-optimal. The six cohorts we used may not be representative of the general population and are susceptible to attrition leading to a potential under-representation of the most extreme socio-economic groups. Additionally, in each cohort study, health behaviours and educational attainment were self-reported which may result in misclassification. The variables were then harmonised which may indicate that some cohort specificities are attenuated or lost, leading to a certain degree of residual confounding. We cannot rule out the possibility that other factors may contribute to the mechanisms linking SEP and inflammation. These factors may include psychological factors, chemical exposures or infectious diseases that are related to both SEP and inflammation. Indeed, environmental pollutants as well as infectious and chronic disease prevalence across the lifecourse and other exposures may also influence the association between education and inflammation with country/ cohort variability. From a social perspective information on race/ ethnicity or migration status which may be confounding factors in an association between education and inflammation were not available and may contribute to unmeasured confounding.
Regarding the imputation of missing data, the last observation carried forward (LOCF) approach reduces within-individual variability which leads to underestimating error. This can result in falsely finding 'statistically significant' associations. Given the relatively low proportion of missing values (around or below 5% in all studies except SKIPOGH), the influence of missing data or the imputation method we used on the associations we found in our work is probably weak.
Important strengths include the opportunity of using harmonised data from 6 well-characterised cohort studies on participants from three different European countries. Four sub-datasets were derived from these 6 cohort studies allowing the pairwise comparison of the social patterning of CRP and any other inflammatory markers; fibrinogen in sub-dataset 1, IL-6 in sub-dataset 2, TNF-α in sub-dataset 3 and IL-1β in sub-dataset 4. The availability of these data allows us to begin elucidating the complex association between the social environment and other inflammatory-related biomarkers beyond CRP from the complex regulatory cascades involved in inflammation. These relationships were modelled separately for each cohort study and examined jointly using a random effect meta-analysis. The stability of our results was further confirmed by the multiple sensitivity analyses carried out.
5. Conclusion
In this multi-cohort study, we provide a thorough examination of SEP inequalities in five inflammatory markers (CRP, fibrinogen, IL-6, IL-1β and TNF-α) using data from several European countries. This work contributes to a better understanding of the socioeconomic patterning of the inflammatory response and suggests that CRP and fibrinogen may be particularly influenced by SEP. The finding that the relationship between educational attainment and each of five inflammatory biomarkers investigated was variable suggests that the way SEP is embodied through inflammation may involve complex processes. These results point to several promising directions to explore chronic inflammation, including the investigation among the full human set of inflammatory-related molecules and events to identify which ones are activated in response to the social environment, at which life stages and to which extent they contribute to explain social inequalities in inflammatory related-diseases. This study calls for further research to better understand how the inflammatory cascade, in response to the social environment, might alter health outcomes.
Funding sources
This study was supported by the European Commission Horizon 2020 grant number [633666] to Paolo Vineis. Mika Kivimäki was supported by NordForsk, the UK Medical Research Council [MRC S011676], the US National Institute on Aging [R01AG056477], the Academy of Finland [311492], and Helsinki Institute of Life Science during the conduct of the study. Cathal McCrory is supported by the Health Research Board (HRB) of Ireland under an Emerging Investigator Award (EIA-2017-012).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful to The Centre for Longitudinal Studies, UCL Institute of Education for the use of these data and to the UK Data Archive and UK Data Service for making them available.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2020.09.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Alwan H., Pruijm M., Ponte B., Ackermann D., Guessous I., Ehret G., Staessen J.A., Asayama K., Vuistiner P., Younes S.E., Paccaud F., Wuerzner G., Pechere-Bertschi A., Mohaupt M., Vogt B., Martin P.-Y., Burnier M., Bochud M. Epidemiology of masked and white-coat hypertension: the family-based SKIPOGH study. PloS One. 2014;9 doi: 10.1371/journal.pone.0092522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J.M., Reeves G., Billman G.E., Sturmberg J.P. Inflammation-nature’s way to efficiently respond to all types of challenges: implications for understanding and managing “the epidemic” of chronic diseases. Front. Med. 2018;5:316. doi: 10.3389/fmed.2018.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E., Castagné R., Chadeau-Hyam M., Bochud M., d’Errico A., Gandini M., Karimi M., Kivimäki M., Krogh V., Marmot M., Panico S., Preisig M., Ricceri F., Sacerdote C., Steptoe A., Stringhini S., Tumino R., Vineis P., Delpierre C., Kelly-Irving M. Multi-cohort study identifies social determinants of systemic inflammation over the life course. Nat. Commun. 2019;10:773. doi: 10.1038/s41467-019-08732-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blane, D., Kelly-Irving, M., d’Errico, A., Bartley, M., Montgomery, S., 2013. Social-biological transitions: how does the social become biological? Longitud. Life Course Stud. 4, 136–146. doi: 10.14301/llcs.v4i2.236.
- d’Errico, A., Ricceri, F., Stringhini, S., Carmeli, C., Kivimaki, M., Bartley, M., McCrory, C., Bochud, M., Vollenweider, P., Tumino, R., Goldberg, M., Zins, M., Barros, H., Giles, G., Severi, G., Costa, G., Vineis, P., Lifepath Consortium, 2017. Socioeconomic indicators in epidemiologic research: a practical example from the LIFEPATH study. PLOS ONE 12, e0178071. doi: 10.1371/journal.pone.0178071. [DOI] [PMC free article] [PubMed]
- Davillas A., Benzeval M., Kumari M. Socio-economic inequalities in C-reactive protein and fibrinogen across the adult age span: findings from Understanding Society. Sci. Rep. 2017;7:2641. doi: 10.1038/s41598-017-02888-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M., Gangestad S.W. Rethinking IL-6 and CRP: why they are more than inflammatory biomarkers, and why it matters. Brain. Behav. Immun. 2018;70:61–75. doi: 10.1016/j.bbi.2018.02.013. [DOI] [PubMed] [Google Scholar]
- Eklund C.M. Proinflammatory cytokines in CRP baseline regulation. Adv. Clin. Chem. 2009;48:111–136. doi: 10.1016/s0065-2423(09)48005-3. [DOI] [PubMed] [Google Scholar]
- Fagundes C.P., Glaser R., Kiecolt-Glaser J.K. Stressful early life experiences and immune dysregulation across the lifespan. Brain. Behav. Immun. 2013;27:8–12. doi: 10.1016/j.bbi.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firmann M., Mayor V., Vidal P.M., Bochud M., Pécoud A., Hayoz D., Paccaud F., Preisig M., Song K.S., Yuan X., Danoff T.M., Stirnadel H.A., Waterworth D., Mooser V., Waeber G., Vollenweider P. The CoLaus study: a population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc. Disord. 2008;8:6. doi: 10.1186/1471-2261-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga S., Marques-Vidal P., Vollenweider P., Waeber G., Guessous I., Paccaud F., Barros H., Stringhini S. Association of socioeconomic status with inflammatory markers: a two cohort comparison. Prev. Med. 2015;71:12–19. doi: 10.1016/j.ypmed.2014.11.031. [DOI] [PubMed] [Google Scholar]
- Franceschi C., Garagnani P., Parini P., Giuliani C., Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018;14:576–590. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- Furman D., Campisi J., Verdin E., Carrera-Bastos P., Targ S., Franceschi C., Ferrucci L., Gilroy D.W., Fasano A., Miller G.W., Miller A.H., Mantovani A., Weyand C.M., Barzilai N., Goronzy J.J., Rando T.A., Effros R.B., Lucia A., Kleinstreuer N., Slavich G.M. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gares, V., Panico, L., Castagne, R., Delpierre, C., Kelly-Irving, M., Lifepath Consortium, 2017. The role of the early social environment on Epstein Barr virus infection: a prospective observational design using the Millennium Cohort Study. Epidemiol. Infect. 145, 3405–3412. doi: 10.1017/S0950268817002515. [DOI] [PMC free article] [PubMed]
- Kivimäki M., Batty G.D., Pentti J., Shipley M.J., Sipilä P.N., Nyberg S.T., Suominen S.B., Oksanen T., Stenholm S., Virtanen M., Marmot M.G., Singh-Manoux A., Brunner E.J., Lindbohm J.V., Ferrie J.E., Vahtera J. Association between socioeconomic status and the development of mental and physical health conditions in adulthood: a multi-cohort study. Lancet Public Health. 2020;5:e140–e149. doi: 10.1016/S2468-2667(19)30248-8. [DOI] [PubMed] [Google Scholar]
- Kivimäki M., Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nat. Rev. Cardiol. 2018;15:215–229. doi: 10.1038/nrcardio.2017.189. [DOI] [PubMed] [Google Scholar]
- Koster, A., Bosma, H., Penninx, B.W.J.H., Newman, A.B., Harris, T.B., van Eijk, J.T.M., Kempen, G.I.J.M., Simonsick, E.M., Johnson, K.C., Rooks, R.N., Ayonayon, H.N., Rubin, S.M., Kritchevsky, S.B., Health ABC Study, 2006. Association of inflammatory markers with socioeconomic status. J. Gerontol. A. Biol. Sci. Med. Sci. 61, 284–290. [DOI] [PubMed]
- Lacey R.E., Pinto Pereira S.M., Li L., Danese A. Adverse childhood experiences and adult inflammation: single adversity, cumulative risk and latent class approaches. Brain. Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot M.G., Smith G.D., Stansfeld S., Patel C., North F., Head J., White I., Brunner E., Feeney A. Health inequalities among British civil servants: the Whitehall II study. Lancet Lond. Engl. 1991;337:1387–1393. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Miller G., Chen E., Cole S.W. Health psychology: developing biologically plausible models linking the social world and physical health. Annu. Rev. Psychol. 2009;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Muscatell K.A., Brosso S.N., Humphreys K.L. Socioeconomic status and inflammation: a meta-analysis. Mol. Psychiatry. 2018;1 doi: 10.1038/s41380-018-0259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazmi A., Victora C.G. Socioeconomic and racial/ethnic differentials of C-reactive protein levels: a systematic review of population-based studies. BMC Public Health. 2007;7:212. doi: 10.1186/1471-2458-7-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotakos D.B., Pitsavos C.E., Chrysohoou C.A., Skoumas J., Toutouza M., Belegrinos D., Toutouzas P.K., Stefanadis C. The association between educational status and risk factors related to cardiovascular disease in healthy individuals: The ATTICA study. Ann. Epidemiol. 2004;14:188–194. doi: 10.1016/S1047-2797(03)00117-0. [DOI] [PubMed] [Google Scholar]
- Petrovic D., de Mestral C., Bochud M., Bartley M., Kivimäki M., Vineis P., Mackenbach J., Stringhini S. The contribution of health behaviors to socioeconomic inequalities in health: a systematic review. Prev. Med. 2018;113:15–31. doi: 10.1016/j.ypmed.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Pollitt R.A., Kaufman J.S., Rose K.M., Diez-Roux A.V., Zeng D., Heiss G. Cumulative life course and adult socioeconomic status and markers of inflammation in adulthood. J. Epidemiol. Community Health. 2008;62:484–491. doi: 10.1136/jech.2006.054106. [DOI] [PubMed] [Google Scholar]
- Power C., Elliott J. Cohort profile: 1958 British birth cohort (National Child Development Study) Int. J. Epidemiol. 2006;35:34–41. doi: 10.1093/ije/dyi183. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2014. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing 73, 3–36.
- Raitakari O.T., Juonala M., Rönnemaa T., Keltikangas-Järvinen L., Räsänen L., Pietikäinen M., Hutri-Kähönen N., Taittonen L., Jokinen E., Marniemi J., Jula A., Telama R., Kähönen M., Lehtimäki T., Akerblom H.K., Viikari J.S.A. Cohort profile: the cardiovascular risk in Young Finns Study. Int. J. Epidemiol. 2008;37:1220–1226. doi: 10.1093/ije/dym225. [DOI] [PubMed] [Google Scholar]
- RStudio Team, 2016. RStudio: Integrated Development Environment for R.
- Smith, K.J., Gavey, S., RIddell, N.E., Kontari, P., Victor, C., 2020. The association between loneliness, social isolation and inflammation: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 112, 519–541. doi: 10.1016/j.neubiorev.2020.02.002. [DOI] [PubMed]
- Steinvil A., Shirom A., Melamed S., Toker S., Justo D., Saar N., Shapira I., Berliner S., Rogowski O. Relation of educational level to inflammation-sensitive biomarker level. Am. J. Cardiol. 2008;102:1034–1039. doi: 10.1016/j.amjcard.2008.05.055. [DOI] [PubMed] [Google Scholar]
- Steptoe A., Breeze E., Banks J., Nazroo J. Cohort profile: the English longitudinal study of ageing. Int. J. Epidemiol. 2013;42:1640–1648. doi: 10.1093/ije/dys168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A., Owen N., Kunz-Ebrecht S., Mohamed-Ali V. Inflammatory cytokines, socioeconomic status, and acute stress responsivity. Brain. Behav. Immun. 2002;16:774–784. doi: 10.1016/s0889-1591(02)00030-2. [DOI] [PubMed] [Google Scholar]
- Straub R.H. The brain and immune system prompt energy shortage in chronic inflammation and ageing. Nat. Rev. Rheumatol. 2017;13:743–751. doi: 10.1038/nrrheum.2017.172. [DOI] [PubMed] [Google Scholar]
- Vineis, P., Avendano-Pabon, M., Barros, H., Chadeau-Hyam, M., Costa, G., Dijmarescu, M., Delpierre, C., D' Errico, A., Fraga, S., Giles, G., Goldberg, M., Zins, M., Kelly-Irving, M., Kivimaki, M., Lang, T., Layte, R., Mackenbach, J.P., Marmot, M., McCrory, C., Carmeli, C., Milne, R.L., Muennig, P., Nusselder, W., Polidoro, S., Ricceri, F., Robinson, O., Stringhini, S., 2017. The biology of inequalities in health: the LIFEPATH project. Longitud. Life Course Stud. 8, 33. doi: 10.14301/llcs.v8i4.448.
- Vingeliene S., Hiyoshi A., Lentjes M., Fall K., Montgomery S. Longitudinal analysis of loneliness and inflammation at older ages: english longitudinal study of ageing. Psychoneuroendocrinology. 2019;110 doi: 10.1016/j.psyneuen.2019.104421. [DOI] [PubMed] [Google Scholar]
- Yang Y.C., Schorpp K., Harris K.M. Social support, social strain and inflammation: evidence from a national longitudinal study of U.S. adults. Soc. Sci. Med. 2014;107:124–135. doi: 10.1016/j.socscimed.2014.02.013. 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


