Abstract
Objectives:
To determine the incidence, prognosis, and predictors of major Valve Academic Research Consortium (VARC-2) vascular complications (VCs) and percutaneous vascular closure device failure (PCDF) following contemporary percutaneous transfemoral transcatheter aortic valve replacement (TF-TAVR).
Background:
Limited data exists on the incidence and predictors of VCs and PCDFs following percutaneous TF-TAVR using contemporary 14–16 French (F) sheaths.
Methods:
We recorded clinical and procedural characteristics, computer tomography (CT) angiographic data, 30-day VCs, mortality, and length of stay (LOS) in all consecutive percutaneous TF-TAVRs at a single center from June 2016 to October 2018. CT measures included common femoral artery (CFA) and external iliac artery (EIA) diameters, sheath to CFA and EIA ratios (SFAR and SEIAR), depth of CFA, extent and location of CFA calcification and pelvic vessel tortuosity (2 bends ≥90°). Multivariable regression was used to predict major VCs and percutaneous closure device failure (PCDF), respectively.
Results:
The final sample consisted of 303 percutaneous TF-TAVRs. Median age was 80 years, 51% were male, 86% Caucasian, 33% had diabetes mellitus (DM) and mean STS score was 5.8 ± 3.8%. Baseline characteristics were similar in patients with vs. without VCs, other than coronary artery disease (CAD) (69% vs. 54%, respectively; p = 0.029) and DM (21% vs. 36%, respectively; p = 0.02). There were 65 (21%) vascular complications: 19 major VCs [6.3%], 29 minor [VCs 9.6%] and 17 PCDFs [5.6%]. Overall, 30-day mortality was low (2.6%). Major VCs were associated with higher mortality (42% vs. 0%, p b 0.0001) while minor VCs (3% vs. 0%, mortality p = 0.99) and PCDFs (3% vs. 0% mortality, p = 0.99) were not. PCDFs were associated with a longer median LOS (4 vs. 3 days, p = 0.02). The independent predictors of major VCs were pelvic vessel tortuosity (OR 3.1; 95% CI 1.1–9.2) and presence of CAD (OR 8.2; 95% CI 1.8–37). Female gender showed a strong trend toward increased risk (OR 3.4; CI 0.84–14; p = 0.086). There were no independent predictors of PCDF.
Conclusion:
Contemporary percutaneous TF-TAVR is associated with a low risk of mortality, major VCs and PCDFs. Major VCs confer increased mortality and PCDFs prolong LOS. Pelvic vessel tortuosity and a history of CAD predict major VCs; there were no predictors of PCDFs. These results provide a contemporary update on the incidence and implications of these important vascular complications in the current era of percutaneous TF-TAVR using 14–16F vascular sheaths.
Keywords: Transcatheter aortic valve replacement, TAVR, Vascular complications
1. Introduction
Over the past decade, transcatheter aortic valve replacement (TAVR) has emerged as the preferred treatment of severe symptomatic aortic stenosis [1–5]. TAVR improves functional status and reduces mortality compared with balloon valvuloplasty and medical therapy [1,2] and reduces the risk of major bleeding and acute kidney injury compared to SAVR [4,6]. As transfemoral TAVR (TF-TAVR) enhances safety, speeds recovery and improves outcomes compared to transapical TAVR, it has emerged as the preferred approach in patients with adequate vascular access [7–9]. During the early TF-TAVR experience, femoral access was generally achieved using a surgical cutdown technique in a hybrid operating room. However, reduced sheath sizes (14–16 Fr for currently commercially available valve platforms) and increased operator familiarity with large bore percutaneous vascular closure techniques has led many operators to adopt a fully percutaneous “minimalist approach” that can be performed in a cardiac catheterization laboratory (percutaneousTF-TAVR) [10]. Consistent with this trend, in the PARTNER continued access registry, surgical cut-down use decreased from two-thirds of cases enrolled early to only one third of cases enrolled later in the study [11]. Although small observational studies suggest that percutaneous TF-TAVR may be associated with more residual stenosis and dissections than TF-TAVRs performed with surgical cut-down technique, the less invasive nature of the former facilitates conscious sedation and has been associated with less bleeding, lower rates of infection, earlier recovery, lower procedural costs and shorter hospital lengths of stay (LOS) [10,12,13]. Despite this, vascular complications (VCs) and percutaneous vascular closure device failure (PCDF) continue to limit the overall safety of percutanous TF-TAVR.
The second Valve Academic Research Consortium (VARC-2) criteria have been adopted as the favored method for defining adverse vascular events following TAVR [14,15] (Appendix 1). VARC-2 complications have been associated with prolonged hospitalization and increased 30-day mortality [15]. In prior studies with larger sheaths, female sex, larger sheath size, operator experience, femoral artery calcification and increased sheath to femoral artery ratio (SFAR > 1.05) have been identified as independent predictors of VCs [15–18]. However, these studies predate the advent of newer TAVR valve systems that may be delivered through 14–16F sheaths using ultrasound-guided micropuncture vascular access techniques. Furthermore, it is unclear whether computed tomography (CT) variables such as the depth of the common femoral artery (CFA) and extent and pattern/location of its calcification help predict the risk of VCs with percutaneous TF-TAVR. The primary aim of this study was to determine the incidence, prognosis and clinical and CT angiographic predictors of major VCs in the setting of percutaneous TF-TAVR.
2. Materials and methods
2.1. Subjects and study design
The study protocol was approved by the Tallahassee Memorial Hospital’s institutional review board. The sample was drawn from consecutive patients who underwent TAVR at a single experienced center from June 2016 to October 2018. Over this time, our TAVR program adopted a default strategy of percutaneous TF-TAVR performed in the cardiac catherization laboratory with ultrasound guided micropuncture vascular arterial access, conscious sedation, percutaneous vascular closure and a protocol for same day ambulation and early discharge (b72 h) [10]. All subjects presented with symptomatic severe aortic stenosis (aortic valve area of ≤1.0 cm2 and/or mean transvalvular gradient of ≥40 mmHg) and were deemed by our multidisciplinary heart valve team to be at intermediate or high risk for SAVR according to a Society of Thoracic Surgery (STS) estimated mortality of ≥3%, severe comorbidities and/or marked frailty. Patients who had no contraindication to intravenous contrast underwent pre-TAVR CT angiography (CTA) of the chest, abdomen and pelvis for preoperative assessment. Over this timeframe, 380 consecutive patients were treated, of which 77 were excluded, leaving a final analyzable study sample of 303 patients. Reasons for study exclusion included the CTA not being suitable for analysis of all vascular measurements, the use of femoral surgical cutdown techniques and other non-transfemoral TAVRs. The study flow is depicted in Fig. 1.
Fig. 1.
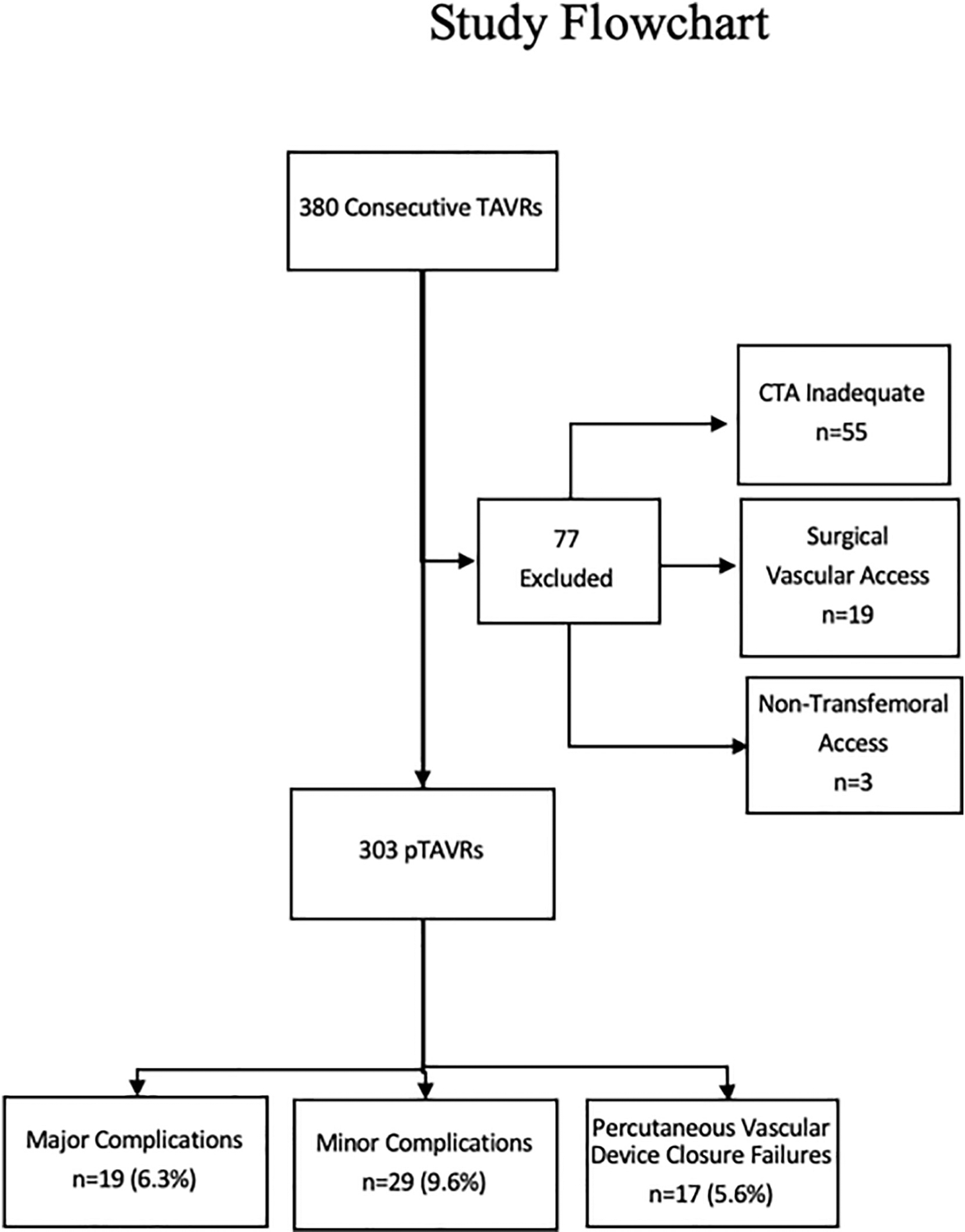
Study flowchart.
Percutaneous TF-TAVR was performed with local standard techniques by 3 experienced operators, each of whom had already performed over 75 TAVRs as a primary operator. Conscious sedation was administered (most often in the form of intravenous midazolam and fentanyl) and monitored by a cardiac anesthesiologist. Percutaneous CFA access was achieved with a micropuncture technique using direct ultrasound visualization to optimize puncture site location. Bilateral ipsilateral selective femoral arteriography was performed with a hand injection of ~5 cc of contrast to confirm appropriate access into each of the common femoral arteries prior to upsizing the sheaths. Vascular sheaths (7F) were placed in the right and left CFAs and in the right femoral vein (for temporary pacing). Intravenous heparin was administered to keep the activated clotting (ACT) over 300 s. Prior to upsizing the sheath to 14–16Fr, the femoral access site selected for percutaneous valve delivery was “preclosed” with 2 Perclose ProGlide® 6F sutures using established techniques [19,20]. Following successful valve implantation, the large sheath was removed and the 2 Perclose sutures fastened to achieve hemostasis [19]. The contralateral 7F femoral access site was routinely closed with an Angio-Seal™ VIP vascular closure device. Prior to vascular closure, a post TAVR digitally subtracted abdominal aortogram was performed and selective femoral arteriography repeated after the above closure technique to evaluate for vascular complications and the adequacy of vascular closure.
Baseline clinical and procedural characteristics were prospectively collected, and patients followed closely during their hospitalization for all complications, including VCs. Thirty day major and minor complications were defined using established VARC-2 criteria (Appendix 1). All VCs were confirmed by two independent reviewers (WB and KP) and the hospital record was cross-referenced with our site’s TVT registry data for ensure that VCs were recorded accurately. Concordance by both reviewers was necessary in order to classify each VARC-2 VC.
2.2. CT-angiographic measures
A single trained physician who was blinded to TAVR outcomes retrospectively reviewed the pre-TAVR CTAs and performed the following measurements: [1] CFA and external iliac artery (EIA) diameter, [2] depth of the CFA (measured as the distance from the skin of the abdominal wall to the anterior wall of the CFA, 1 mm proximal to its bifurcation), [3] maximum arc of CFA calcification (maximum circumferential degrees of calcification noted on the non-contrast CT axial images) and [4] location of CFA calcification (anterior, medial, lateral or posterior). The sheath to CFA and EIA ratios were calculated by dividing the outer diameter of the sheath (mm) by the corresponding minimal vessel diameter (mm). Pelvic vessel tortuosity was defined as at least 2 bends ≥90°. Post procedure percent (%) CFA stenosis was estimated visually from the post TAVR selective angiogram and the number of Perclose and Angioseal devices deployed was recorded. Representative CTA measurements are shown in Fig. 2.
Fig. 2.
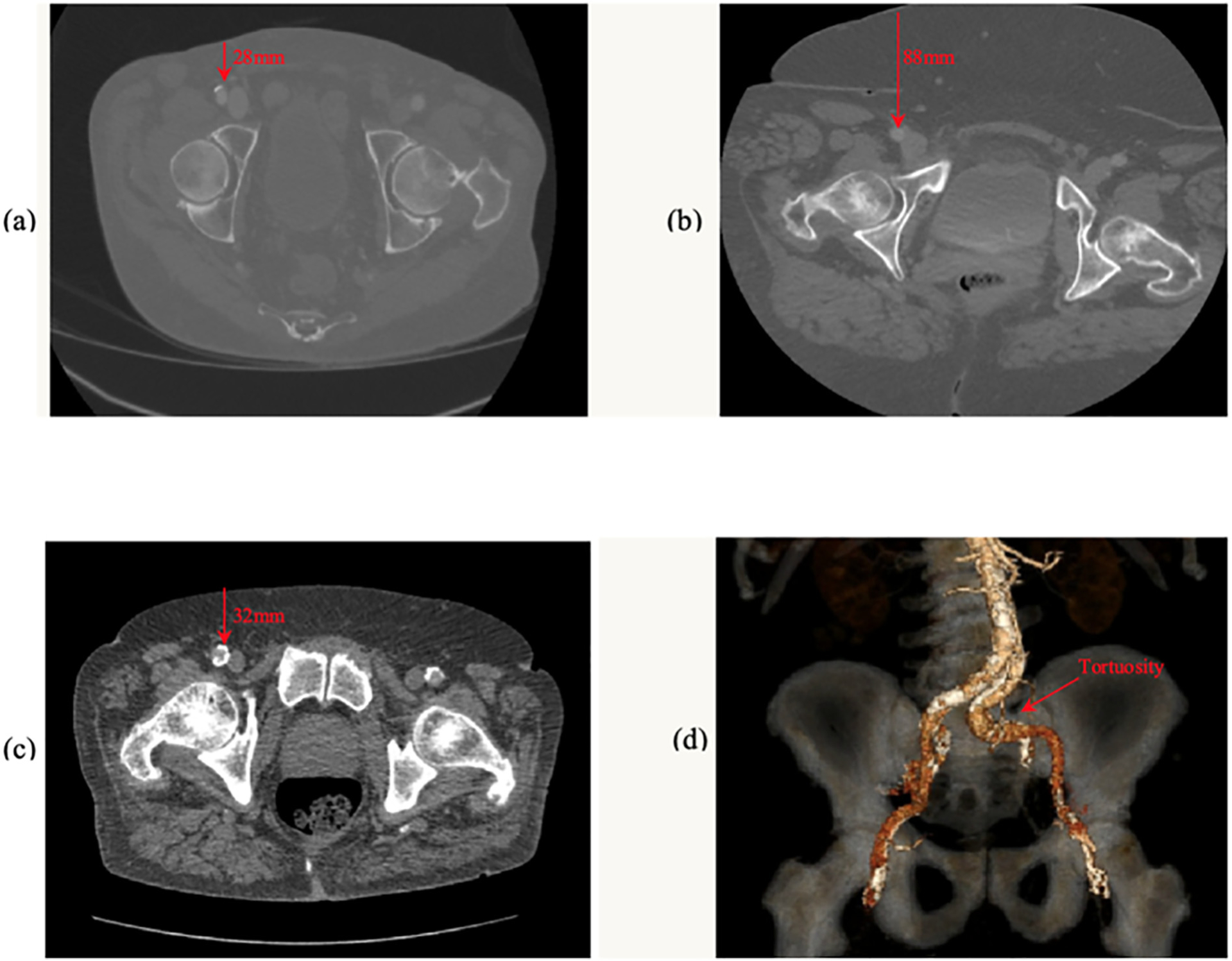
Computed tomography images of (a) non-contrast axial image of a shallow (28 mm depth) right CFA with anterior calcification, (b) a deep (88 mm) right CFA without calcification, (c) a relatively shallow right CFA with near complete circumferential calcification and (d) a 3-dimensional reconstructed view demonstrating marked tortuosity of the left common iliac artery. The red arrows in the figures indicate the depth (mm) and location of the corresponding CFA.
2.3. Treatment of vascular complications
The treatment of VCs was left to the discretion of the operating physicians. Interventions ranged from manual pressure to balloon angioplasty, stenting and surgical repair. In cases of residual bleeding from the access site, additional Perclose devices or Angioseals were deployed at the discretion of the operators. For cases in which additional closure devices were not adequate or bleeding was extensive and not amenable to manual pressure or percutaneous techniques, open surgical repair was performed.
2.4. Statistical analysis
Frequencies and percentages were used to summarize baseline data for categorical variables and means with standard deviations (SD) for normally distributed continuous variables. Medians and interquartile ranges were used to describe non-parametric data. Two-sided Student t-tests were used to compare continuous variables and chi-square or Fisher exact tests for discrete variables. A backward “stepwise” multivariable regression technique was used to determine the independent clinical, procedural and angiographic predictors of major VCs. Variables showing a significant multivariable association with VCs (p < 0.05) were kept in the final model. All statistical analyses were performed using SAS System software, version 9.2 or later (SAS Institute Inc., Cary, NC USA). Receiver operator curves were generated to identify the best cut-off for SEIAR and SFAR.
3. Results
3.1. Baseline clinical characteristics
Patient baseline characteristics are depicted in Table 1 for the entire study sample, patients with VC and patients without VC. The overall mean age was 80 years, 51% of patients were male and 86% white. Hypertension was present in the vast majority of patients. Age, gender, race, statin use, BMI, creatinine clearance, history of CAD, history of prior MI, history of PVD, prior CABG, prior CVA and COPD were equally prevalent in patients with vs. without VCs. Diabetes was present in a third of patients overall, with a higher prevalence in patients without vs. with VCs (37%vs 22%, p = 0.023). The mean STS score was 5.8 ± 3.8% and mean LV ejection fraction normal (55 ± 13%) and comparable between groups. Baseline procedural and CT-angiographic characteristics are also depicted in Table 1. Patients with VCs had a higher SEIAR (0.76 vs. 0.68, p = 0.0003), higher SFAR (0.74 vs. 0.72, p = 0.029), higher mean post-procedural CFA stenosis (22% vs. 5%, p = 0.0001) and a greater mean number of Perclose devices used for vascular closure (2.7 vs. 2.1, p = 0.0001). There was also a trend toward a smaller mean CFA diameter (7.3 mm vs. 7.6 mm, p = 0.09) in patients with VC vs. those without. The maximum arc of CFA calcification and its location were similar between those with and without VC. Median sheath size was 16 F vs. 14 F in patients with vs. without VC, however, this did not reach statistical significance (p = 0.17).
Table 1.
Baseline patient characteristics.
| Characteristic | Total (N = 303) | With VC (n = 65) | Without VC (n = 238) | P-value* |
|---|---|---|---|---|
| Clinical | ||||
| Age (years) | 80 ± 8.8 | 80 ± 7.7 | 80 ± 9.0 | 0.76 |
| Female [n (%)] | 127 (49) | 28 (57) | 111 (47) | 0.14 |
| White Race [n (%)] | 206 (86) | 57 (88) | 206 (86) | 0.84 |
| Diabetic [n (%)] | 101 (33) | 14 (21) | 87 (37) | 0.02 |
| On a Statin [n (%)] | 189 (62) | 44 (68) | 145 (60) | 0.52 |
| Tortuosity Present [n (%)] | 124 (41) | 28 (43) | 96 (40) | 0.69 |
| Femoral Calcification [n (%)] | 150 (50) | 38 (58) | 112 (47) | 0.10 |
| BMI (kg/m2) | 29 ± 6.9 | 29 ± 7.2 | 29 ± 6.8 | 0.48 |
| Creatinine Clearance (ml/min) | 42 ± 17 | 42 ± 16 | 41 ± 17 | 0.73 |
| History of CAD [n (%)] | 174 (57) | 45 (69) | 129 (54) | 0.02 |
| Prior MI [n (%)] | 103 (34) | 28 (43) | 75 (31) | 0.08 |
| History of PVD [n (%)] | 86 (28) | 20 (31) | 66 (28) | 0.63 |
| Prior CABG [n (%)] | 56 (18) | 14 (21) | 56 (18) | 0.47 |
| Prior CVA [n (%)] | 46 (15) | 12 (18) | 34 (14) | 0.40 |
| Hypertension [n (%)] | 301 (99) | 64 (98) | 237 (99) | 0.38 |
| History of COPD [n (%)] | 61 (20) | 14 (21) | 47 (20) | 0.75 |
| Average LVEF | 55 ± 13 | 55 ± 12 | 55 ± 13 | 0.90 |
| Median LOS (days) | 3 (Q1: 3, Q3: 5) | 4 (Q1: 3, Q3: 5) | 3 (Q1: 3, Q3: 4) | 0.09 |
| STS score (%) | 5.8 ± 3.8 | 5.6 ± 4.2 | 5.9 ± 3.6 | 0.61 |
| In-Hospital Mortality [n (%)] | 8 (2.6) | 8 (12) | 0 (0.0) | 0.0001 |
| Procedural/CT angiographic | ||||
| Valve Type [n (%)] | ||||
| Edwards | 136 (45) | 31 (48) | 105 (44) | – |
| Medtronic | 163 (54) | 34 (52) | 129 (54) | – |
| Other | 4 (1.3) | 0 (0.00) | 4 (1.7) | 0.53 |
| Sheath size (French) | 14 (14, 16) | 16 (14, 18) | 14 (14, 16) | 0.17 |
| CFA MLD (mm) | 7.6 (7.5, 8) | 7.3 (7.1, 8) | 7.6 (7.5, 8) | 0.09 |
| EIA MLD (mm) | 7.6 ± 1.3 | 7.2 ± 1.5 | 7.7 ± 1.2 | 0.01 |
| SEIAR | 0.70 ± 0.14 | 0.76 ± 0.16 | 0.68 ± 0.13 | 0.0003 |
| SFAR | 0.72 ± 0.45 | 0.74 ± 0.15 | 0.72 ± 0.50 | 0.03 |
| CFA calcification [n (%)] | 50% | 58% | 47% | 0.10 |
| Tortuosity present [n (%)] | 41% | 43% | 40% | 0.69 |
| Depth of CFA (mm) | 46 ± 20 | 45 ± 21 | 46 ± 20 | 0.31 |
| Number of percloses | 2.2 ± 0.69 | 2.7 ± 1.1 | 2.1 ± 0.40 | 0.0001 |
| Post procedure % stenosis | 8.9% | 34% | 2.1% | 0.0001 |
| CFA calcification (degree and location) | ||||
| Max Arc [n (%)] | n = 143 | n = 38 | n = 112 | |
| 30° | 43 (29) | 12 (32) | 31 (28) | – |
| 60° | 21 (14) | 8 (21) | 13 (12) | – |
| 90° | 40 (27) | 6 (16) | 34 (30) | – |
| 180° | 39 (26) | 11 (29) | 28 (25) | – |
| 200° | 1 (0.67) | 0 (0.00) | 1 (0.89) | – |
| 270° | 6 (4.0) | 1 (2.6) | 5 (4.5) | 0.4010 |
| Location [n (%)] | n = 150 | n = 39 | n = 112 | |
| Anteriomedial | 2 (1.3) | 2 (5.1) | 0 (0.00) | – |
| Anterior | 5 (3.3) | 1 (2.6) | 4 (3.6) | – |
| Lateral | 14 (9.3) | 2 (5.1) | 12 (11) | – |
| Posterolateral | 3 (2.0) | 1 (2.6) | 2 (1.8) | – |
| Medial | 70 (47) | 19 (49) | 51 (46) | – |
| Posteromedial | 1 (1.0) | 0 (0.0) | 1 (1.0) | – |
| Posterior | 55 (37) | 13 (33) | 42 (38) | 0.2755 |
Abbreviations: VC: Vascular complication; BMI: body mass index; CAD: coronary artery disease; MI: myocardial infarction; PVD: peripheral vascular disease; CABG: coronary artery bypass surgery; CVA: cerebrovascular accident; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; LOS: length of hospital stay; STS: Society of Thoracic Surgery; CFA: common femoral artery; EIA: external iliac artery; SEIAR: sheath to external iliac artery ratio; SFAR: sheath to femoral artery ratio.
P value for comparisons between patients with any vascular complication and those without.
3.2. VARC-2 complication rates, mortality and length of stay
The distribution of VARC-2 complications is shown in Fig. 3. There were 65 (21%) VCs overall, including 19 (6.3%) major VCs (2.6% fatal and 3.6% nonfatal), 29 (9.6%) minor VCs and 17 (5.6%) PCDFs. The 19 major VCs included 13 cases of access site related vascular injury leading to major bleeding or death (perforation, stenosis or dissection), 2 PCDFs resulting in severe bleeding and requiring blood transfusions, 2 episodes of severe ipsilateral lower extremity ischemia and 2 life threatening pericardial effusions. All but one of these 19 VCs were ipsilateral to the large bore femoral access site used to deliver the TAVR valve. The treatment of these 19 major VCs included percutaneous revascularization in 11 cases (angioplasty and/or stenting), surgical revascularization in 3 cases, pericardiocentesis in 2 cases and prolonged manual pressure in 3 cases. Of the 29 minor VCs, there were 25 access site related vascular injuries (stenosis, dissection and/or hematomas not meeting criteria for severe VC) and 4 unplanned vascular repairs not leading to death, life-threatening or major bleeding.
Fig. 3.
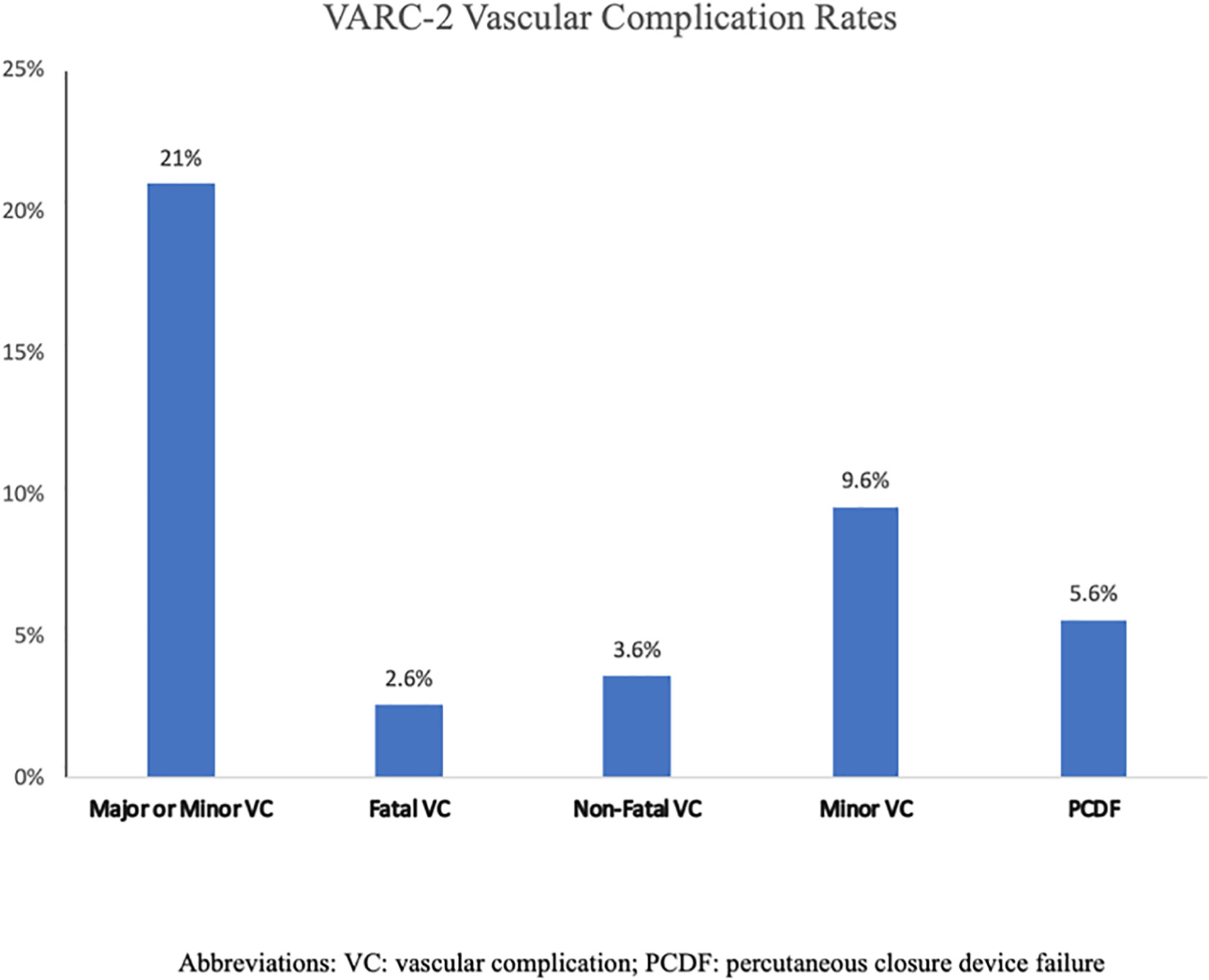
Graph depicting the incidence of VARC-2 vascular complications.
The mortality rates corresponding with each type of VARC-2 complication are shown in Fig. 4. Overall, there were 8 deaths (2.6%) within 30 days. All deaths occurred in patients who suffered from a VC, such that the mortality rate was 12% with VCs vs. 0% without (p = 0.0001). Major VCs were associated with a high 30-day mortality rate compared with no VC (42% vs. 0%; p < 0.0001). Mortality rates with minor VCs and PCDFs were not significantly different from those noted in patients without VCs (3% vs. 0%; p = 0.99 and 3% vs. 0%; p = 0.99, respectively). Median hospital LOS was 1 day longer in patients who experienced a PCDF (4 vs. 3 days, p < 0.02), but unaffected by minor VCs (3 vs 3 days, p = 0.59). Although major VCs were also associated with numerically longer median LOS, this did not reach statistical significance (4 vs. 3 days, p = 0.37).
Fig. 4.
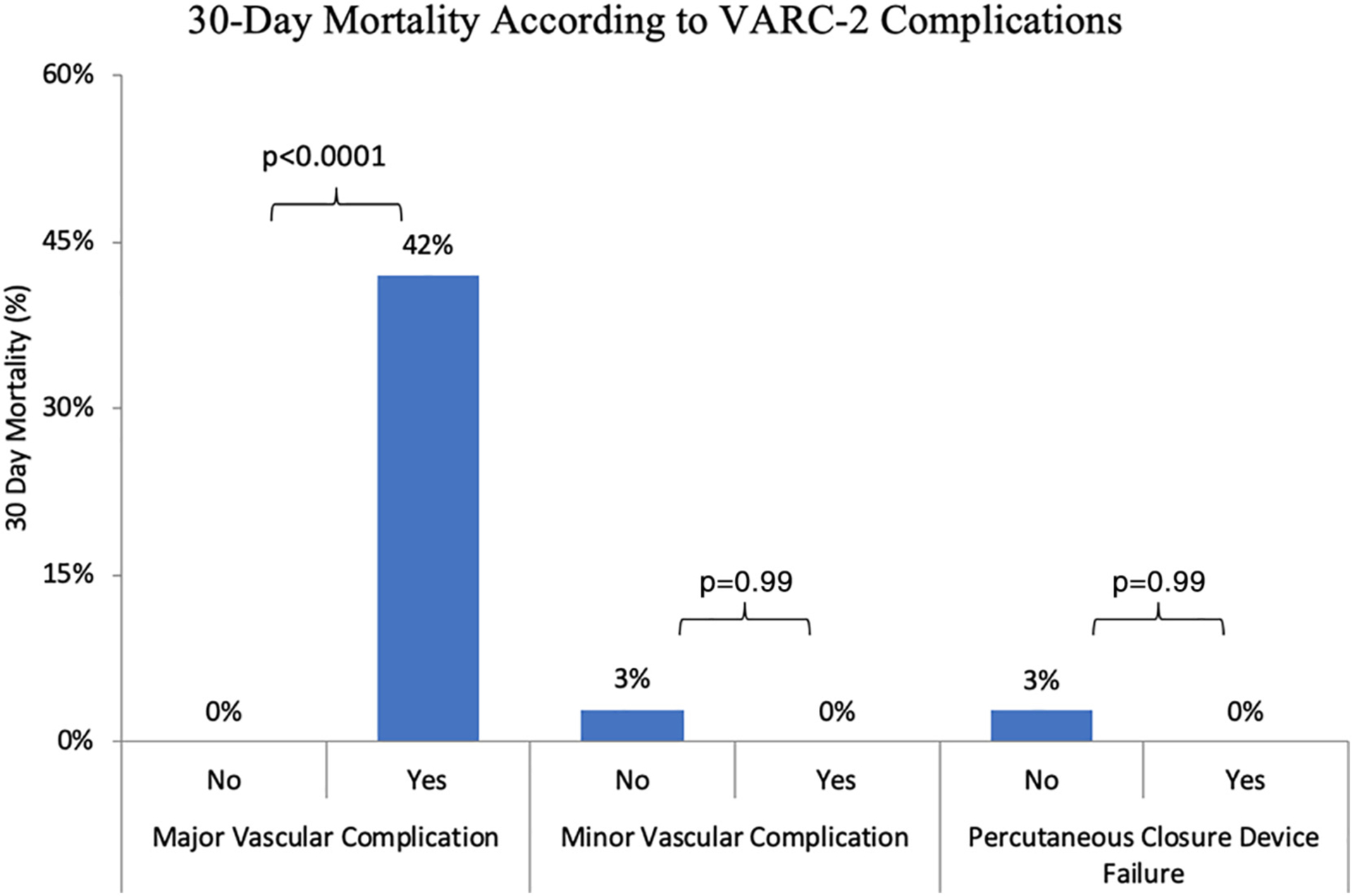
Graph depicting 30-day mortality according to various forms of VARC-2 vascular complications.
3.3. Clinical and angiographic predictors of vascular complications
Univariate and multivariate predictors of major VCs are shown in Table 2. Univariate predictors included female sex, CAD, CFA diameter, SEIAR and SFAR > 0.75 and EIA diameter. The SEIAR and SFAR variables showed collinearity, therefore only SFAR was included in the final multivariable model. The SFAR cut-off with the best discrimination was determined to be an SFAR > 0.75. With multivariable regression, the independent predictors of major VCs were pelvic vessel tortuosity (OR 3.1; CI 1.1–9.2, p = 0.043) and CAD (OR 8.2; CI 1.8–37, p = 0.0063). Female gender showed a strong trend toward increased risk (OR 3.4; CI 0.84–14, p = 0.086). The receiver operator curves (ROC) for the overall model and SEIAR > 0.75, SFAR > 0.75 are shown in Fig. 5. The C statistic for the final multivariable model was 0.84, indicating strong discriminative power (i.e. the ability of the model to separate individuals who develop VCs from those who do not). There were no independent predictors of PCDF.
Table 2.
Univariate and multivariate predictors of major vascular complications.
| Parameter | Univariate model | Multivariate modela | ||||
|---|---|---|---|---|---|---|
| SFAR cutpoint >0.75 | ||||||
| OR (95% CI) | p-value | Youden index | Cutpoint | OR (95% CI) | p-value | |
| Age, per 5y | 1.02 (0.78–1.3) | 0.89 | – | – | 0.98 (0.72–1.4) | 0.91 |
| Female | 3.13 (1.1–8.9) | 0.03 | – | – | 3.4 (0.84–14) | 0.08 |
| Diabetes | 0.51 (0.17–1.6) | 0.24 | – | – | 0.40 (0.12–1.4) | 0.15 |
| Tortuosity | 2.3 (0.81–5.3) | 0.12 | – | – | 3.1 (1.1–9.2) | 0.04 |
| CFA calcification | 1.1 (0.45–2.9) | 0.77 | – | – | 1.40 (0.48–4.1) | 0.53 |
| BMI | 0.95 (0.87–1.0) | 0.17 | – | – | 0.96 (0.86–1.1) | 0.46 |
| Creatinine clearance | 0.99 (0.96–1.0) | 0.41 | – | – | 1.0 (0.97–1.0) | 0.83 |
| PVD | 1.18 (0.43–3.2) | 0.74 | – | – | 0.74 (0.23–2.4) | 0.62 |
| CAD | 4.3 (1.2–15) | 0.02 | – | – | 8.2 (1.8–37) | 0.00 |
| Prior CVA | 1.05 (0.29–3.8) | 0.93 | – | – | 1.5 (0.36–6.1) | 0.59 |
| Prior MI | 1.45 (0.56–3.7) | 0.44 | – | – | 0.48 (0.14–1.6) | 0.24 |
| CFA diameter, mm | 0.65 (0.43–0.99) | 0.04 | – | – | 1.2 (0.51–3.1) | 0.63 |
| SEIAR | 33 (1.5–794) | 0.02 | 0.310 | 0.75 | 0.12 (0.00–459) | 0.61 |
| SEIAR > 0.8 | 2.3 (0.86–6.0) | 0.09 | – | – | – | – |
| SFAR | 1.2 (0.56–2.3) | 0.70 | 0.311 | 0.75 | – | – |
| SFAR > 0.8 | 2.34 (0.89–6.31) | 0.08 | – | – | – | – |
| SFAR > 0.72 | 2.5 (0.94–6.4) | 0.06 | – | – | – | – |
| SFAR > 0.75 | 3.1 (1.2–8.0) | 0.01 | – | – | 3.6 (0.62–21) | |
| EIA MLD, mm | 0.63 (0.42–0.93) | 0.02 | – | – | 0.63 (0.21–1.9) | 0.42 |
| CFA depth, mm | 0.99 (0.97–1.0) | 0.53 | – | – | 1.0 (0.97–1.0) | 0.98 |
| CFA Calcification | ||||||
| Maximum Arc | 0.24 (0.03–1.9) | 0.17 | – | – | – | – |
| Anterior location | 2.5 (0.27–23) | 0.42 | – | – | – | – |
Youden Index is sensitivity + specificity − 1.
Abbreviations: CAD: coronary artery disease; MI: myocardial infarction; PVD: peripheral vascular disease; CVA: cerebrovascular accident; SEIAR: sheath to external iliac artery ratio; SFAR: sheath to femoral artery ratio; EIA: external iliac artery; CFA: common femoral artery.
SEIAR > 0.75, SFAR > 0.75, Max Arc 91–360° and CFA calcification location are not included in MV model.
Fig. 5.
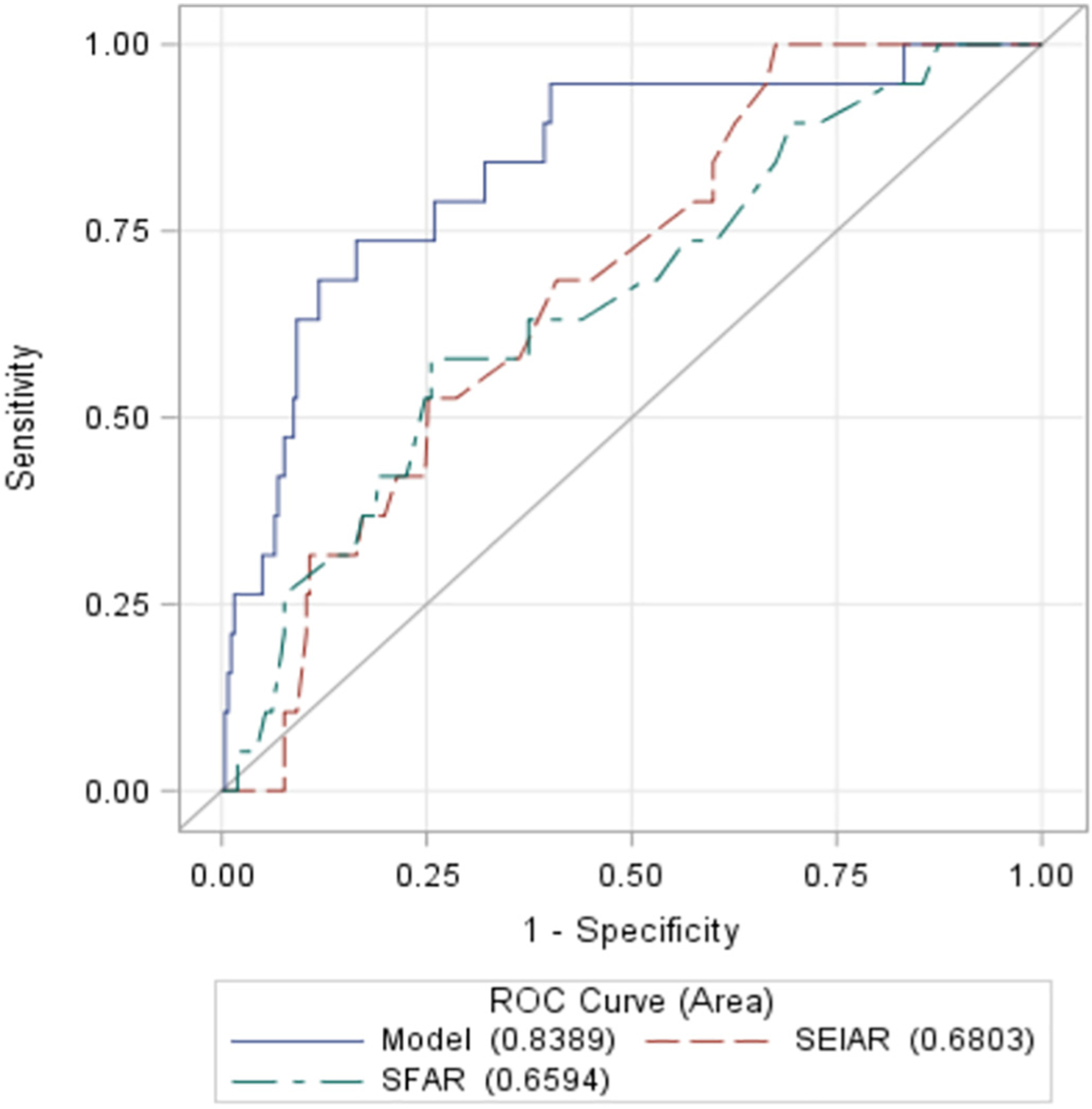
Receiver operator curves using SEIAR > 0.75, SFAR > 0.75 and the multivariable model as predictors of major VARC-2 vascular complications.
4. Discussion
We performed a comprehensive single center study of the incidence, prognosis and clinical and CT-angiographic predictors of VARC-2 VCs, including PCDFs following percutaneous TF-TAVR in 303 consecutive patients. To our knowledge, this is the largest study to report on the incidence and clinical relevance of these complications associated with contemporary percutaneous TF-TAVR performed with predominantly 14–16F sheaths. Using ultrasound-guided percutaneous micropuncture vascular access techniques, we noted the following 30-day rates of VARC-2 complications: 6.3% major VCs, 9.6% minor VCs, and 5.6% PCDFs. Although 30-day mortality was low overall (2.6%), all 8 deaths occurred in patients who had a major VC. Only major VCs, but not minor VCs or PCDFs, were associated with increased mortality. PCDFs were associated with a longer median LOS. After performing a detailed analysis of the pre-TAVR CT-angiographic data, we noted that female gender, CAD, CFA and EIA diameter, SEIAR, SFAR >0.75 each showed a univariate association with major VCs. However, only pelvic vessel tortuosity (2 bends ≥90°) and CAD emerged as independent predictors, with female gender showing a strong trend toward increased risk.
Completely percutaneous TF-TAVR is an important advance in technique. The advantages of this minimally invasive technique include quicker recovery, shorter length of stay, lower procedural costs and lower rates of wound infection compared to TAVR performed with femoral artery surgical cutdown techniques [10,12]. Four retrospective studies and a small randomized trial have demonstrated similar rates of VCs and clinical outcomes following completely percutaneous TF-TAVR compared to femoral surgical cutdown techniques [10,12,21–23]. The rate of major VCs with percutaneousTF-TAVR observed in our study (6%) was lower than that noted from several prior studies in which rates with both percutaneous and surgical cutdown TF-TAVR have ranged from 9 to 18% [12,15,16,18,24,21,22]. We believe that this is due to the smaller sheath sizes (14–16F) used in our study and advances in vascular access techniques, including ultrasound-guided micropuncture vascular access, which better reflect contemporary techniques. In the only randomized trial comparing percutaneous TF-TAVR to TAVR performed with surgical vascular access, Holper et al. reported an overall major VC rate of 13%, with similar rates noted between the 2 approaches [23]. Female gender and increased femoral arterial doppler velocity on preprocedural vascular ultrasound were the only predictors of VCs in that small pilot trial [23]. In another small retrospective cohort study, Babaliaros et al. reported comparable mid-term survival and rates of stroke, bleeding complications, permanent pacemaker and paravalvular aortic regurgitation following 70 p-TF TAVRs compared to 72 surgical access TAVRs [10]. In that study, percutaneous TF-TAVR was associated with shorter LOS and lower procedural costs. Major VARC-2 complication rates were rare (1% vs. 3%, respectively). However, with only 70 patients studied, the confidence intervals around these low VC rates are wide, therefore, we believe that our larger study might provide a more precise estimate of major VC risk.
Prior studies using larger vascular sheaths have revealed the following independent predictors of major VCs: SFAR of >1.05, renal failure, peripheral vascular disease, CFA calcification and larger sheath sizes (>19F) [15,18,17,25]. We specifically sought to determine if any other baseline CT-angiographic measures predicted major VCs. Accordingly, we collected several measurements from the pre-TAVR CT angiograms (CFA diameter, depth and location and severity of calcification) to determine if there was any relationship between these and major VCs. We were surprised that neither CFA calcification (severity and location) nor depth or size of the CFA emerged as important predictors. Although CFA diameter, SEIAR and SFAR > 0.75 each showed a univariate association with major VCs, none of these CT-derived measures were independent predictors in the multivariate model. Again, we believe that this is likely due to the use of smaller sheathes (and therefore lower SFARs) which weakens the relationship between SFAR and VCs, since the vast majority of CFA diameters (median 7.6 mm, IQR 7.5–8.0) can readily accommodate 14–16F sheaths (4.6–5.3 mm). This is consistent with a recent study by Kadakia et al. which showed that major VCs varied markedly (5-fold) according to tercile of SFAR, with the lowest tercile having a major VC rate of only 5% vs. 26% in the highest [22]. Several studies have also shown operator experience to be a predictor of major VCs [26,27]. Since all of our operators were already experienced, we did not observe a difference among implanting physicians and do not believe that this significantly influenced the risk of major VCs. Our study showed CAD to be an independent predictor of major VCs. This was an unexpected finding. We hypothesize that this relationship could be due to the fact that patients with CAD may have a greater burden of atherosclerosis and/or may be less able to compensate for VCs, leading to a higher proportion of VCs turning into major VCs. Interestingly, one of the most consistent independent predictors of major VCs following TAVR in the past has been female gender [18,21–22]. In our study, female gender was a univariate predictor and showed a strong trend toward increased risk in the multivariable model, however, this did not achieve statistical significance (OR 3.4; CI 0.84–14, p = 0.086). We suspect that this may be due to inadequate statistical power in our multivariable model to fully appreciate this independent relationship. Finally, it is important to note that the valve system selected, itself, did not influence outcomes. The 2 predominant TAVR valve systems (Edwards balloon-expandable vs. Medtronic self-expanding platforms) were associated with similar baseline vascular CTA parameters and comparable rates of major VCs. This is likely due to the fact that in our practice, these 2 valve systems were used with similar frequency (45 vs. 54%, respectively) rather than selecting any particular valve system solely for difficult vascular anatomy.
Despite the low rates of VARC-2 complications and mortality in our study, major VCs still carried high prognostic significance. Our low mortality rate (2.6%) was consistent with trends noted from more recent TAVR studies [10,11,28,5,29]. We attribute this to advances in technology, increased operator experience and a lower risk case-mix resulting from the approval of TAVR for intermediate risk patients (median STS score of ~6% in our study cohort). However, even with this low mortality rate and the low corresponding rate of VCs, major VCs still carried significant prognostic impact in our study. In fact, all 8 deaths that occurred within 30 days were seen in patients who had some form of major VC. However, it is also important to note that minor VCs and PCDFs did not confer incremental mortality risk. These findings are in line with prior studies and suggest that even though the risk of major VCs has diminished over time, their clinical impact remains high, while minor VCs are of much less consequence [4,24,16]. Accordingly, aggressive measures to reduce the risk of major VCs continue to be warranted. Only PCDFs prolonged LOS in our study. Although major VCs were associated with a 1 day increase in LOS, this did not reach statistical significance. We believe that this is due to the small number of events observed and also the fact that some patients with major VCs died early following TAVR, thereby paradoxically reducing LOS. Few studies, other than ours, have evaluated the clinical relevance of PCDFs in the setting of contemporary percutaneous TF-TAVR using 14–16F sheaths.
4.1. Limitations
Although, to our knowledge, this is the largest study of this kind to date it was still a relatively small, single-centered study that was not statistically powered for extensive analysis of clinical outcomes. The relatively low rate of VCs also limits our ability to identify all independent predictors of major VCs using multivariable regression. As a retrospective study, there is potential for known or unknown biases. We must also recognize that since the reported incidence of VCs is dependent on the methods used to detect them, rates may be underestimated. However, we believe that our method of searching both the TVT registry, reviewing every patient’s electronic health record for VCs and requiring the agreement of 2 adjudicators helped to improve accuracy and minimize underestimation. Finally, the routine performance of selective aorto-iliac and selective femoral angiography prior to and following percutaneous vascular closure should have also served to minimize under-detection of early vascular complications, at least within the pelvic and femoral vasculature.
5. Conclusions
In this study, contemporary percutaneous TF-TAVR was associated with low rates of VCs, PCDFs and 30-day mortality. However, when major VCs did occur, they were associated with markedly increased mortality. Minor VCs and PCDFs were generally well tolerated and did not appear to increase mortality, although PCDFs prolonged LOS. Pelvic vessel tortuosity (at least 2 bends ≥90°) and CAD were independent predictors of VC. We revealed no independent predictors of PCDFs. Common femoral artery depth and its degree and location of calcification failed to predict VC or PCDFs. These results provide a contemporary update on the incidence and implications of these important vascular complications in the current era of percutaneous TF-TAVR. Continued efforts to improve upon our existing percutaneous vascular access techniques are necessary to further minimize the occurrence of these infrequently encountered, but prognostically important complications.
Acknowledgements
The authors would like to acknowledge the Dudley Family for their continued contributions and support of the INOVA Dudley Family Center for Cardiovascular Innovation.
Abbreviations:
- TF-TAVR
transfemoral transcatheter aortic valve replacement
- VARC-2
Second Valve Academic Research Consortium
- PCDF
percutaneous closure device failure
- VC
Vascular complication
- CFA
common femoral artery
- EIA
external iliac artery
- LOS
length of stay in hospital
- SFAR
sheath to femoral artery ratio
- SEIAR
sheath to external iliac artery ratio
- CAD
coronary artery disease
- PVD
peripheral vascular disease
- COPD
chronic obstructive pulmonary disease
- CVA
cerebrovascular accident
- CABG
coronary artery bypass graft surgery
Appendix 1. Definitions of VARC-2 Complications
| 1. Major vascular complications |
| Any aortic dissection, aortic rupture, annulus rupture, left ventricle perforation, or new apical aneurysm/pseudo-aneurysm OR |
| Access site or access-related vascular injury (dissection, stenosis, perforation, rupture, arterio-venous fistula, pseudoaneurysm, hematoma, irreversible nerve injury, compartment syndrome, percutaneous closure device failure) leading to death, life-threatening or major bleeding*, visceral ischemia, or neurological impairment OR |
| Distal embolization (non-cerebral) from a vascular source requiring surgery or resulting in amputation or irreversible end-organ damage OR |
| The use of unplanned endovascular or surgical intervention associated with death, major bleeding, visceral ischemia or neurological impairment OR |
| Any new ipsilateral lower extremity ischemia documented by patient symptoms, physical exam, and/or decreased or absent blood flow on lower extremity angiogram OR |
| Surgery for access site-related nerve injury OR |
| Permanent access site-related nerve injury |
| 2. Minor vascular complications |
| Access site or access-related vascular injury (dissection, stenosis, perforation, rupture, arterio-venous fistula, pseudoaneuysms, hematomas, percutaneous closure device failure) not leading to death, life-threatening or major bleeding*, visceral ischemia, or neurological impairment OR |
| Distal embolization treated with embolectomy and/or thrombectomy and not resulting in amputation or irreversible end-organ damage OR |
| Any unplanned endovascular stenting or unplanned surgical intervention not meeting the criteria for a major vascular complication OR |
| Vascular repair or the need for vascular repair (via surgery, ultrasound-guided compression, transcatheter embolization, or stent-graft) |
| 3. Percutaneous closure device failure (PCDF) |
| Failure of a closure device to achieve hemostasis at the arteriotomy site leading to alternative treatment (other than manual compression or adjunctive endovascular ballooning) |
References
- [1].Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597–607. 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- [2].Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–98. 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- [3].Carnero-Alcázar M, Maroto LC, Cobiella-Carnicer J, Vilacosta I, Nombela-Franco L, Alswies A, et al. Transcatheter versus surgical aortic valve replacement in moderate and high-risk patients: a meta-analysis. Eur J Cardio-thoracic Surg 2017. 10.1093/ejcts/ezw388. [DOI] [PubMed] [Google Scholar]
- [4].Généreux P, Head SJ, Van Mieghem NM, Kodali S, Kirtane AJ, Xu K, et al. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: a weighted meta-analysis of 3,519 patients from 16 studies. J Am Coll Cardiol 2012;59:2317–26. 10.1016/J.JACC.2012.02.022. [DOI] [PubMed] [Google Scholar]
- [5].Thourani VH, Kodali S, Makkar RR, Herrmann V, Williams M, Babaliaros RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016. 10.1016/S0140-6736(16)30073-3. [DOI] [PubMed] [Google Scholar]
- [6].Carnero-Alc Azar M, Maroto LC, Cobiella-Carnicer J, Vilacosta I, Nombela-Franco i, Alswies A, et al. Transcatheter versus surgical aortic valve replacement in moderate and high-risk patients: a meta-analysis. doi: 10.1093/ejcts/ezw388. [DOI] [PubMed] [Google Scholar]
- [7].Greason KL, Suri RM, Nkomo VT, Rihal CS, Holmes DR, Mathew V. Beyond the learning curve: transapical versus transfemoral transcatheter aortic valve replacement in the treatment of severe aortic valve stenosis. doi: 10.1111/jocs.12323. [DOI] [PubMed] [Google Scholar]
- [8].Gaasch WH, D’Agostino RS. Transcatheter aortic valve implantation: the transfemoral versus the transapical approach. Ann Cardiothorac Surg 2012;1: 200–5. 10.3978/789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li X, Kong M, Jiang D, Dong A. Comparison 30-day clinical complications between transfemoral versus transapical aortic valve replacement for aortic stenosis: a meta-analysis review. J Cardiothorac Surg 2013;8:168. 10.1186/1749-8090-8-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Babaliaros V, Devireddy C, Lerakis S, Leonardi R, Iturra SA, Mavromatis K, et al. Comparison of transfemoral transcatheter aortic valve replacement performed in the catheterization laboratory (minimalist approach) versus hybrid operating room (standard approach): outcomes and cost analysis. JACC Cardiovasc Interv 2014:898–904. 10.1016/j.jcin.2014.04.005. [DOI] [PubMed] [Google Scholar]
- [11].Beohar N, Kirtane AJ, Blackstone E, Waksman R, Holmes D Jr, Minha S, et al. Trends in complications and outcomes of patients undergoing transfemoral transcatheter aortic valve replacement: experience from the PARTNER continued access registry. JACC Cardiovasc Interv 2016;9:355–63. 10.1016/j.jcin.2015.10.050. [DOI] [PubMed] [Google Scholar]
- [12].Nakamura M, Chakravarty T, Jilaihawi H, Doctor N, Dohad S, Fontana G, et al. Complete percutaneous approach for arterial access in transfemoral transcatheter aortic valve replacement: a comparison with surgical cut-down and closure. Catheter Cardiovasc Interv 2014;84:293–300. 10.1002/ccd.25130. [DOI] [PubMed] [Google Scholar]
- [13].Hernández-Enriquez M, Andrea R, Brugaletta S, Jiménez-Quevedo R, Hernández-García JM, Trillo S, et al. Puncture versus surgical cutdown complications of transfemoral aortic valve implantation (from the Spanish TAVI registry). Am J Cardiol 2016;118:578–84. 10.1016/j.amjcard.2016.05.054. [DOI] [PubMed] [Google Scholar]
- [14].Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation. J Am Coll Cardiol 2012. 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- [15].Hayashida K, Lefèvre T, Chevalier B, Hovasse T, Romano M, Garot P, et al. Transfemoral aortic valve implantation: new criteria to predict vascular complications. JACC Cardiovasc Interv 2011;4:851–8. 10.1016/J.JCIN.2011.03.019. [DOI] [PubMed] [Google Scholar]
- [16].Satler LF, Dvir D. Vascular complications during transcatheter aortic valve replacement. Catheter Cardiovasc Interv 2013. 10.1002/ccd.24833. [DOI] [PubMed] [Google Scholar]
- [17].Van Mieghem NM, Tchetche D, Chieffo A, Dumonteil N, Messika-Zeitoun D, van der Boon RMA, et al. Incidence, predictors, and implications of access site complications with transfemoral transcatheter aortic valve implantation. Am J Cardiol 2012. 10.1016/j.amjcard.2012.06.042. [DOI] [PubMed] [Google Scholar]
- [18].Généreux P, Webb JG, Svensson LG, Kodali SK, Satler LF, Fearon WF, et al. Vascular complications after transcatheter aortic valve replacement: insights from the PARTNER (placement of AoRTic TraNscathetER valve) trial. J Am Coll Cardiol 2012; 60(12):1043–52. 10.1016/J.JACC.2012.07.003. [DOI] [PubMed] [Google Scholar]
- [19].Perclose ProGlide®. Suture-Mediated Closure (SMC) system instructions for use. http://eifu.abbottvascular.com/content/dam/av/eifu-us/EL2105174Artwork.pdf.
- [20].Lee WA, Brown MP, Nelson PR, Huber TS. Total percutaneous access for endovascular aortic aneurysm repair (“Preclose” technique). J Vasc Surg 2007. 10.1016/j.jvs.2007.01.050. [DOI] [PubMed] [Google Scholar]
- [21].Bernardi FLM, Gomes WF, de Brito FSJ, Mangione JA, Sarmento-Leite R, Siqueira D, et al. Surgical cutdown versus percutaneous access in transfemoral transcatheter aortic valve implantation: insights from the Brazilian TAVI registry. Catheter Cardiovasc Interv 2015;86:501–5. 10.1002/ccd.25820. [DOI] [PubMed] [Google Scholar]
- [22].Kadakia MB, Herrmann HC, Desai ND, Fox Z, Ogbara J, Anwaruddin S, et al. Factors associated with vascular complications in patients undergoing balloon-expandable transfemoral transcatheter aortic valve replacement via open versus percutaneous approaches. Circ Cardiovasc Interv 2014;7:570–6. 10.1161/CIRCINTERVENTIONS.113.001030. [DOI] [PubMed] [Google Scholar]
- [23].Holper EM, Kim RJ, Mack M, Brown D, Brinkman W, Herbert M, et al. Randomized trial of surgical cutdown versus percutaneous access in transfemoral TAVR. Catheter Cardiovasc Interv 2014;83:457–64. 10.1002/ccd.25002. [DOI] [PubMed] [Google Scholar]
- [24].Chaudhry MA, Sardar MR. Vascular complications of transcatheter aortic valve replacement: a concise literature review. World J Cardiol 2017;9(7):574–82. 10.4330/wjc.v9.i7.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sari C, Ayhan H, Aslan A, Keleş T, Baştuğ S, Bayram NA, et al. Predictors and incidence of access site complications in transcatheter aortic valve implantation with the use of new delivery systems. Perfusion 2015;30:666–74. 10.1177/0267659115578002. [DOI] [PubMed] [Google Scholar]
- [26].Toggweiler S, Gurvitch R, Leipsic J, Wood DA, Willson AB, Binder RK, et al. Percutaneous aortic valve replacement: vascular outcomes with a fully percutaneous procedure. J Am Coll Cardiol 2012;59:113–8. 10.1016/j.jacc.2011.08.069. [DOI] [PubMed] [Google Scholar]
- [27].Van Mieghem NM, Chieffo A, Dumonteil N, Tchetche D, van der Boon RMA, Buchanan GL, et al. Trends in outcome after transfemoral transcatheter aortic valve implantation. Am Heart J 2013;165:183–92. 10.1016/j.ahj.2012.11.002. [DOI] [PubMed] [Google Scholar]
- [28].Mack MJ, Leon MB, Thourani VH, Makkar R, ,Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695–705. 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- [29].Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016. 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]


