Abstract
Testosterone (T) reduces male fat mass, but the underlying mechanisms remain elusive, limiting its clinical relevance in hypogonadism-associated obesity. Here, we subjected chemically castrated high-fat diet–induced adult obese male mice to supplementation with T or the nonaromatizable androgen dihydrotestosterone (DHT) for 20 weeks. Both hormones increased lean mass, thereby indirectly increasing oxygen consumption and energy expenditure. In addition, T but not DHT decreased fat mass and increased ambulatory activity, indicating a role for aromatization into estrogens. Investigation of the pattern of aromatase expression in various murine tissues revealed the absence of Cyp19a1 expression in adipose tissue while high levels were observed in brain and gonads. In obese hypogonadal male mice with extrahypothalamic neuronal estrogen receptor alpha deletion (N-ERαKO), T still increased lean mass but was unable to decrease fat mass. The stimulatory effect of T on ambulatory activity was also abolished in N-ERαKO males. In conclusion, our work demonstrates that the fat-burning action of T is dependent on aromatization into estrogens and is at least partially mediated by the stimulation of physical activity via extrahypothalamic ERα signaling. In contrast, the increase in lean mass upon T supplementation is mediated through the androgen receptor and indirectly leads to an increase in energy expenditure, which might also contribute to the fat-burning effects of T.
Keywords: sex steroids, testosterone, estradiol, hypogonadism, fat mass, physical activity
Obesity increases the risk for various chronic diseases, including depression, type 2 diabetes, and cardiovascular disease, as well as certain cancers and has therefore become a major global health concern (1–3). Thus, there is an urgent need to understand the mechanisms underlying obesity and its complications.
Testosterone (T) is a well-known regulator of male body fat and metabolism. Indeed, androgen deprivation therapy in prostate cancer patients increased the risk to develop obesity and type 2 diabetes (4, 5). On the other hand, T treatment reduced visceral fat mass along with improvements in metabolic parameters including insulin sensitivity and plasma triglyceride concentrations in men with age-related abdominal obesity (6). Meta-analyses confirmed the T-mediated decrease in male fat mass (7, 8). However, the most recent guidelines recommend that T alone should not be considered as an antiobesity drug in hypogonadal men, since randomized controlled trials specifically designed to study the effects of T therapy on body composition are not available and the impact on cardiovascular health is still debated (9).
Testosterone can exert its effects directly through activation of the androgen receptor (AR) or through the estrogen receptors (ERα/β) after aromatization into estradiol (E2) (10–12). Interestingly, in men with hypogonadism induced by a gonadotropin-releasing hormone analogue, the decrease in fat mass observed in the T-treated group was abolished in the presence of an aromatase inhibitor (13), indicating a potential role for aromatization of T in its fat-burning action. However, the precise mechanisms underlying the effects of T on male fat metabolism, be it through E2, are unclear. Apart from a direct effect on adipocyte differentiation (14, 15), potential indirect mechanisms might include action on energy homeostasis and/or physical activity, as obesity results from an imbalance between energy intake and energy expenditure (16). Short-term T treatment in men did not affect indirect calorimetric parameters including resting energy expenditure and respiratory exchange ratio, whereas prolonged treatment appeared to stimulate resting energy expenditure and fat oxidation (17, 18). However, whether the changes in energy expenditure upon T treatment contribute to the changes in body composition remain underexplored, since measuring energy intake (eg, assessing food consumption) and expenditure (eg, assessing physical activity) accurately in humans remains challenging (17). For this reason, preclinical investigation using animal models is needed.
Similar to the clinical data, in rodents, the T effects on male fat mass seem to be partly estrogen-mediated, since T but not the nonaromatizable androgen dihydrotestosterone (DHT) restored fat mass to sham levels in castrated male mice with diet-induced obesity (19). Regarding the underlying mechanisms of T action on fat mass, effects on energy balance and physical activity might be involved. Indeed, T administration to castrated rats showed increased energy expenditure (20), while orchidectomy of male mice decreased home-cage activity (19). In line with this, diminished ambulatory as well as wheel running activity was also observed in male global AR knockout (ARKO) mice (21–23), an effect which was mainly due to the concomitant estrogen deficiency (24). However, a thorough analysis of the T effects, directly or through aromatization, on energy balance and physical activity in hypogonadal obese male mice is still lacking. The abovementioned T effects on these parameters might be exerted through the central nervous system, since both AR and ERα are present in hypothalamic and extrahypothalamic neurons of the brain (25–27). Central ERα signaling is a well-known regulator of body fat through the effects on food intake, energy homeostasis, and activity in female mice, but studies in male mice are limited (28). While ERα deletion in hypothalamic brain regions did not affect male fat mass (29), the absence of ERα in the medial amygdala induced late-onset obesity in male mice as a result of reduced energy expenditure and physical activity (30). However, the contribution of extrahypothalamic ERα signaling to the fat-burning effects of T in males has not been elucidated yet.
Therefore, in this study, we hypothesized that T decreases body fat mass by regulating energy balance and/or physical activity in obese hypogonadal male mice, in part through ERα signaling in extrahypothalamic brain regions. To this end, we first investigated the role of T on body fat mass, energy balance, and physical activity in chemically castrated high-fat diet (HFD)-induced obese male mice and the potential involvement of aromatization in these effects. Next, using extrahypothalamic neuron-specific estrogen receptor alpha knockout (N-ERαKO) mice (31), we investigated whether extrahypothalamic ERα signaling might contribute to the T-induced changes in body fat mass, energy balance, and physical activity in obese hypogonadal male mice.
Materials and Methods
Animals
Thirty six 16-week-old male wild type (WT) mice (C57BL/6J genetic background) were purchased from the Charles River Laboratories (Brussels, Belgium) and subjected to the insertion of implants, as described below. Tamoxifen-inducible N-ERαKO male mice (C57BL/6J genetic background) were generated as previously described (31). Briefly, heterozygous female mice carrying ERα with a floxed exon 3 were mated with male mice expressing CreERT2 under the control of a modified Thy1 promoter in successive generations to achieve ERαfl/fl; Thy-CREert2+/- mice. At 6 weeks of age, male N-ERαKO mice (ERαfl/fl; Thy-CreERT2+/-) and control littermates (ERαfl/fl; Thy-CreERT2-/-, ERαwt/wt; Thy-CreERT2+/-, ERαwt/wt; Thy-CreERT2-/-) were orally gavaged with tamoxifen (190 mg/kg body weight; Sigma-Aldrich, St. Louis, MO, USA) once daily for 2 consecutive days. At 16 weeks of age, 14 N-ERαKO male mice were subjected to the surgical procedure described below. An additional group of 6 male WT mice (C57BL/6J) obtained from the KU Leuven animal facility were used to measure aromatase expression in several tissues at 8 weeks of age. Mice were group-housed (3–5 animals/cage) in conventional facilities at 20°C with a 12-hour light/dark cycle and ad libitum access to food and water. The animal experiments were conducted in accordance with the KU Leuven guidelines for animal experimentation and approved by the KU Leuven ethical committee (P192/2016).
Insertion of implants and experimental design
At 16 weeks of age, both WT and N-ERαKO male mice were subjected to chemical castration by administration of Degarelix (Ferring Pharmaceuticals, Saint-Prex, Switzerland; subcutaneous injection of 25 mg/kg every 4 weeks) as previously described (32). Under isoflurane anesthesia, mice were randomly assigned to receive either empty silastic implants (Silclear Tubing; Degania Silicone, Jordan Valley, Israel) or filled with T or DHT (Sigma-Aldrich, St. Louis, MO, USA) in the dorsal region, followed by meloxicam analgesia (Boehringer Ingelheim; Ingelheim am Rhein, Germany; subcutaneous injection of 5 mg/kg). The daily release of T and DHT from the implants is 23 and 45 µg, respectively (33, 34), which is slightly supraphysiological (19, 35). From 16 weeks of age, mice were fed with an HFD composed of 20 kcal% protein, 35 kcal% carbohydrate, and 45 kcal% fat (6 kcal% from soybean oil and 39% kcal from lard) (D12451; Research diet, New Brunswick, NJ, USA). Body weight was recorded weekly and body composition was determined every 2 weeks using EchoMRI (EchoMRI -100H Analyzer; Echo Medical Systems LLC, Houston, TX, USA). At 32–40 weeks of age, energy balance was assessed by indirect calorimetry and mice were euthanized by pentobarbital anesthesia followed by cardiac puncture. The sacrifice order of the animals was randomized to avoid potential confounders. Dissected tissue wet weights were measured.
EchoMRI
Lean and fat body mass were measured by quantitative magnetic resonance (EchoMRI -100H Analyzer; Echo Medical Systems LLC, Houston, TX, USA) according to the manufacturer’s instructions.
Indirect calorimetry
Mice were acclimatized to the individual housing condition and special drinking/feeding baskets for 7 days prior to recording the energy balance and ambulatory activity (TSE Phenomaster Calocages, Bad Homburg, Germany). Subsequently, mice were placed in single-housed automated cages for 72 hours at 20°C, with a 12-hour light/dark cycle and ad libitum access to food and water. Recorded data for food intake, oxygen consumption, heat production, respiratory exchange ratio, and ambulatory activity from the last 48 hours were analyzed as previously described (36). To determine whether changes in these parameters were due to androgen supplementation and/or changes in body composition, analysis of covariance (ANCOVA) was performed as described in (37).
Real time reverse transcription-polymerase chain reaction
Total ribonucleic acid (RNA) was extracted from the tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Copy DNA was synthesized from 1 µg RNA using FastGene Scriptase II kit (NIPPON Genetics Europe, Dueren, Germany) and random hexamer primers. The primer sequences are described in Supplemental Table S1 (38). The polymerase chain reactions were performed using Fast SYBR Green Master Mix or TaqMan Fast Advanced Master Mix (Applied Biosystems, Foster City, CA, USA). For quantification of gene expression, Applied Biosystems StepOnePlus Real-Time PCR system was used. The relative expression levels of the target genes were calculated as a ratio to the hypoxanthine guanine phosphoribosyl transferase (Hprt) gene.
Statistics
Statistical analysis was performed using GraphPad Prism v7.04 (GraphPad, La Jolla, CA, USA) and R (version 3.6.1, R Core Team). Unpaired two-tailed student’s t-test and one-way ANOVA with Bonferroni post hoc test were used to analyze differences between 2 or more groups, respectively. Two-way ANOVA with Bonferroni post hoc test was used in experiments with more than one independent variable. ANCOVA was used to analyze indirect calorimetry data. Data are represented as mean ± standard error of the mean, and P < 0.05 was considered statistically significant.
Results
Testosterone but not dihydrotestosterone decreases fat mass in obese hypogonadal male mice
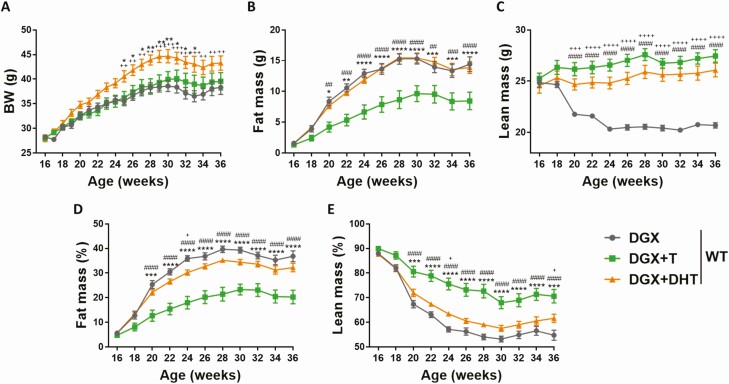
In order to investigate the effects of T and of the nonaromatizable androgen DHT on body composition in obese hypogonadal mice, 16-week-old male WT mice were chemically castrated with the gonadotropin-releasing hormone antagonist Degarelix (32), supplemented with placebo (DGX), T (DGX + T), or DHT (DGX + DHT), and subjected to HFD for 20 weeks. Body weight was comparable between DGX and DGX + T groups, while DGX + DHT animals showed a significant increase in body weight compared with the other 2 groups at most of the time points during the experiment (Fig. 1A). Testosterone reduced fat mass in obese hypogonadal mice, while lean mass was increased compared with DGX animals (Fig. 1B–1E). In contrast, DHT was not able to induce changes in fat mass (Fig 1B). The higher body weight in the DGX + DHT group was due to an increase in lean mass (Fig. 1C).
Figure 1.
Effect of T and DHT supplementation on body weight and body composition of HFD-fed chemically castrated male WT mice. Male WT mice were chemically castrated with degarelix and supplemented with placebo (DGX), T (DGX + T), or DHT (DGX + DHT) at 16 weeks of age. From 16 to 36 weeks of age, all animals were subjected to HFD (n = 12/group). Body weight (A) and body composition (B–C) of 16- to 36-week-old mice. Fat (D) and lean (E) body mass were normalized to body weight. Data were analyzed with two-way ANOVA with Bonferroni post hoc test. ####P < 0.0001 comparison between DGX and DGX + T. +P < 0.05, ++P < 0.01, +++P < 0.001, ++++P < 0.0001 comparison between DGX and DGX + DHT. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 comparison between DGX + T and DGX + DHT. Data are represented as mean ± SEM. Abbreviations: ANOVA, analysis of variance;BW, body weight; DGX, degarelix; DHT, dihydrotestosterone; HFD, high fat diet; SEM, standard error of the mean; T, testosterone; WT, wild type.
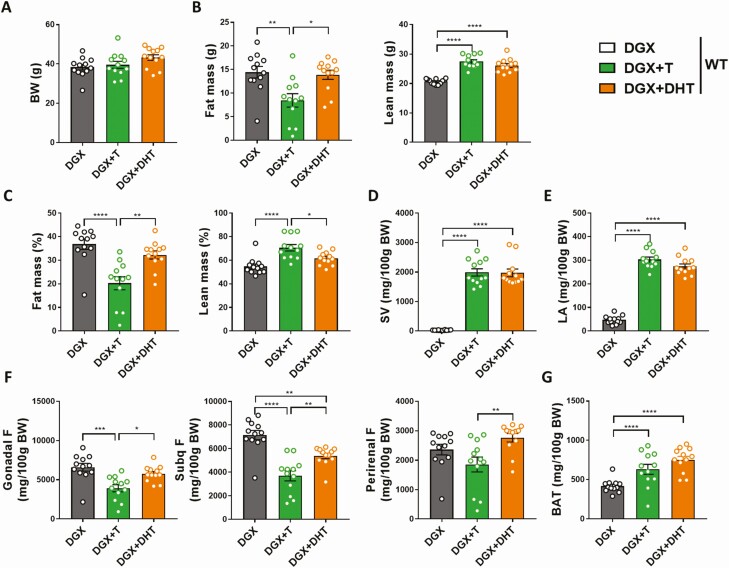
The effects of both hormones on body composition were confirmed at the termination point. Indeed, at 36 weeks of age, both the DGX + T and DGX + DHT groups showed increased lean mass compared with DGX animals, while T but not DHT was able to reduce fat mass (Fig. 2A–2C). In addition, both the DGX + T and DGX + DHT groups exhibited a significant increase in the weight of androgen-sensitive tissues, namely seminal vesicles and musculus levator ani, compared with the DGX group, suggesting that T and DHT replacement were effective (Fig. 2D and 2E). In particular, the effect of T in reducing fat mass was most pronounced in gonadal and subcutaneous fat pads (Fig. 2F), while interscapular brown adipose tissue weight was increased in the presence of both T and DHT (Fig. 2G).
Figure 2.
Effect of T and DHT supplementation on the weight of androgen-sensitive tissues and fat pads of HFD-fed chemically castrated male WT mice. Male WT mice were chemically castrated with degarelix and supplemented with placebo (DGX), T (DGX + T), or DHT (DGX + DHT) at 16 weeks of age. From 16 to 36 weeks of age, all animals were subjected to HFD (n = 12/group). Body weight (A) and body composition (B) of 36-week-old mice. C: Fat and lean body mass of 36-week-old mice were normalized to body weight. Weight of seminal vesicles (D) and levator ani muscle (E) of 36-week-old mice. Weight of various fat pads of white adipose tissue (F) and brown adipose tissue (G) of 36-week-old mice. Data were analyzed with one-way ANOVA with Bonferroni post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Data are represented as mean ± SEM. Abbreviations: ANOVA, analysis of variance;BAT, brown adipose tissue; BW, body weight; DGX, degarelix; DHT, dihydrotestosterone; F, fat; HFD, high fat diet; LA, levator ani; SEM, standard error of the mean; Subq, subcutaneous; SV, seminal vesicles; T, testosterone; WT, wild type.
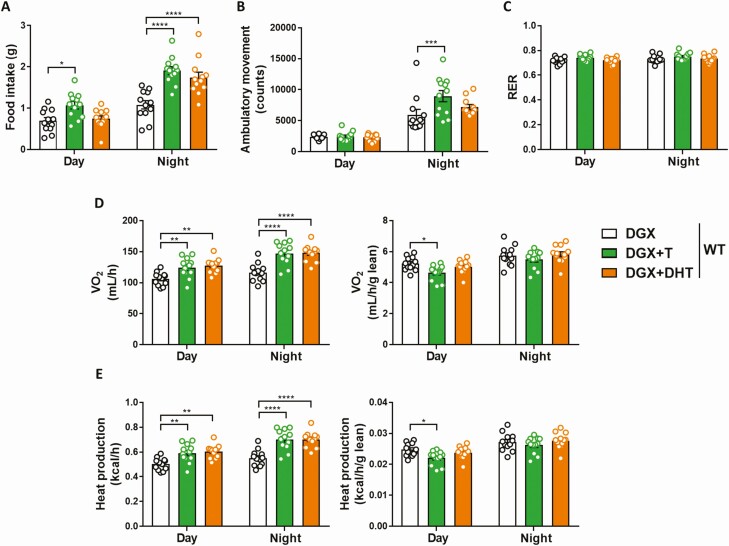
Testosterone but not dihydrotestosterone stimulates ambulatory activity while both hormones indirectly increase energy expenditure in obese hypogonadal male mice
To determine how T regulates fat mass in obese hypogonadal male mice, energy balance was assessed by indirect calorimetry. While both hormones increased food intake, supplementation with T but not DHT stimulated ambulatory activity (Fig. 3A and 3B). There were no differences among the DGX, DGX + T, and DGX + DHT groups in respiratory exchange ratio (Fig. 3C). Both DGX + T and DGX + DHT animals displayed a significant increase in oxygen consumption and heat production when compared with the DGX group, and was most pronounced during the night cycle (Fig. 3D and 3E, left panels). However, these effects were abolished when normalized to lean mass (Fig. 3D and 3E, right panels), suggesting they were secondary to the increase in lean mass observed for both hormones (Fig. 2B). ANCOVA confirmed that the changes in energy expenditure were driven indirectly by the T/DHT effect on lean mass rather than by the hormone supplementation itself (Supplemental Fig. S1 and Supplemental Table S2) (38, 39). In contrast, only T but not DHT reduced fat mass (Fig. 2B), pointing towards aromatization as a mediator of the fat-burning effects of T.
Figure 3.
Effect of T and DHT supplementation on physical activity and energy balance of HFD-fed chemically castrated male WT mice. Food intake (A), ambulatory movement (B), respiratory exchange ratio (C), oxygen consumption (D), and heat production (E) as measured by indirect calorimetry in 32- to 36-week-old male WT mice injected with degarelix, supplemented with placebo (DGX), T (DGX + T), or dihydrotestosterone (DGX + DHT) and subjected to HFD from 16 weeks of age (n = 12/group). D, E (right panels): Oxygen consumption and heat production were normalized to lean body mass. Data were analyzed with two-way ANOVA with Bonferroni post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Data are represented as mean ± SEM. Abbreviations: ANOVA; analysis of variance;DGX, degarelix; DHT, dihydrotestosterone; HFD, high fat diet; RER, respiratory exchange ratio; SEM, standard error of the mean; T, testosterone; VO2, oxygen consumption; WT, wild type.
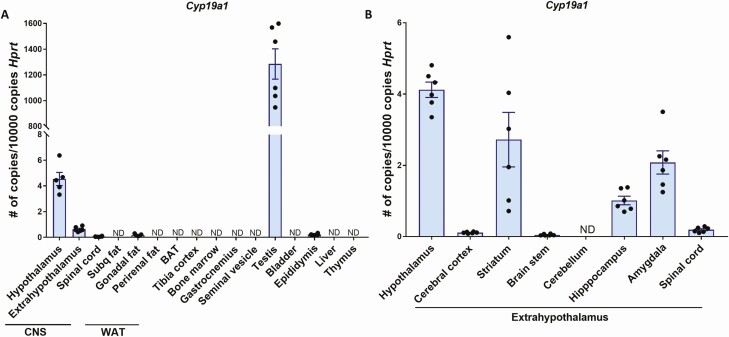
Aromatase is primarily expressed in brain and gonads of male mice
In contrast to humans, male mice have low or undetectable circulating estrogens. Therefore, E2 in male mice derives from local aromatization within target tissues and exerts its effects in a paracrine manner (40). In order to investigate which tissues may mediate T effects on body fat in male mice, we analyzed RNA transcript levels of Cyp19a1, which encodes the aromatase enzyme responsible for the conversion of T into E2. The highest expression was found in the brain and the gonads, whereas the majority of the other tissues including white adipose tissue fat pads, bone, muscle, and the liver showed no detectable aromatase expression (Fig. 4A). In particular, not only the hypothalamus, but also extrahypothalamic regions such as striatum, amygdala, and hippocampus displayed considerable aromatase expression in the male mouse brain (Fig. 4B).
Figure 4.
Aromatase expression in various tissues from male WT mice. Quantitative real-time PCR analysis of Cyp19a1 mRNA in various tissues (A) and the brain (B) of 8-week-old male WT mice (n = 5–6). Data are represented as mean ± SEM. Abbreviations: CNS, central nervous system; mRNA, messenger RNA; ND, not detected; PCR, polymerase chain reaction; SEM, standard error of the mean; WAT, white adipose tissue; WT, wild type.
Effect of testosterone on body fat mass is at least partially mediated by extrahypothalamic ERα signaling
Based on the finding that aromatase is highly expressed in extrahypothalamic brain regions in male mice (Fig. 4B), we hypothesized that central ERα signaling in extrahypothalamic areas might contribute to the fat-burning effects of T in obese hypogonadal males. To address this question, we subjected 16-week-old male mice with ERα deletion in N-ERαKO (31) to chemical castration and T supplementation followed by HFD for 20 weeks.
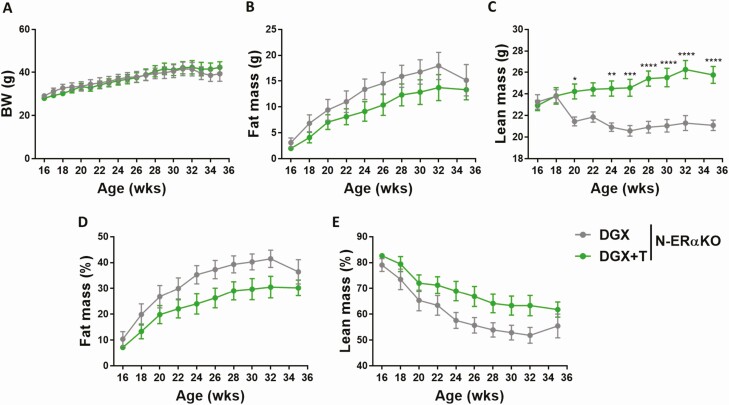
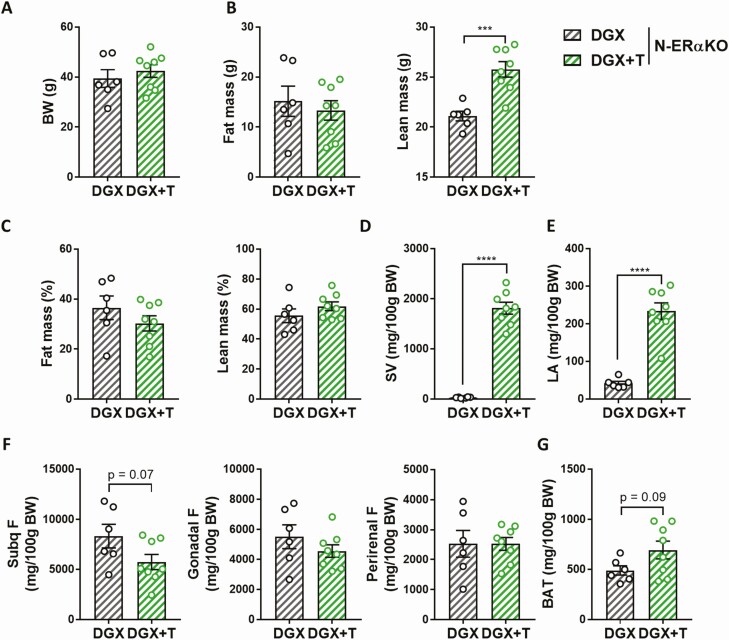
Body weight was comparable between DGX and DGX + T N-ERαKO animals (Fig. 5A). While T was still able to increase lean mass in obese hypogonadal N-ERαKO males, the fat-burning effect of T was, however, abolished in the absence of extrahypothalamic ERα (Fig. 5B–5E and Fig. 6A–6C). Testosterone supplementation was effective, as DGX + T N-ERαKO mice showed significantly increased androgen-sensitive tissue weights compared with DGX N-ERαKO mice (Fig. 6D and 6E). In line with the absence of T effect on whole body fat in N-ERαKO, the weight of individual fat pads were similar between N-ERαKO DGX and N-ERαKO DGX + T animals (Fig. 6F and 6G).
Figure 5.
Effects of T supplementation on body weight and body composition of HFD-fed chemically castrated male N-ERαKO mice. Male N-ERαKO mice were injected with degarelix and supplemented with placebo (DGX, n = 6) or T (DGX + T, n = 8) at 16 weeks of age and subsequently fed with HFD for 20 weeks. A: Body weight and body composition of 16 to 36-week-old mice (B–C). Fat (D) and lean (E) body mass were normalized to body weight. Data were analyzed with two-way ANOVA with Bonferroni post-hoc test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Data are represented as mean ± SEM. Abbreviations: ANOVA, analysis of variance; BW, body weight; DGX, degarelix; DHT, dihydrotestosterone; HFD, high fat diet; N-ErαKO, extrahypothalamice neuron-specific estrogen receptor α knockout; SEM, standard of error; T, testosterone.
Figure 6.
Effects of T supplementation on the weight of androgen-sensitive tissues and fat pads of HFD-fed chemically castrated male N-ERαKO mice. Male N-ERαKO mice were injected with degarelix and supplemented with placebo (DGX, n = 6) or T (DGX + T, n = 8) at 16 weeks of age and subsequently fed with HFD for 20 weeks. Body weight (A) and body composition (B) of 36-week-old mice. C: Fat and lean body mass of 36-week-old mice were normalized to body weight. Weight of seminal vesicles (D) and levator ani (E) of 36-week-old mice. Weight of various fat pads of white adipose tissue (F) and brown adipose tissue (G) of 36-week-old mice. Data were analyzed with unpaired two-tailed t-test. ****P < 0.0001. Data are represented as mean ± SEM. Abbreviations: BAT, brown adipose tissue; BW, body weight; DGX, degarelix; F, fat; HFD, high fat diet; LA, levator ani; N-ErαKO, extrahypothalamic neuron-specific estrogen receptor α knockout; SEM, standard error of the mean; Subq, subcutaneous; SV, seminal vesicles; T, testosterone.
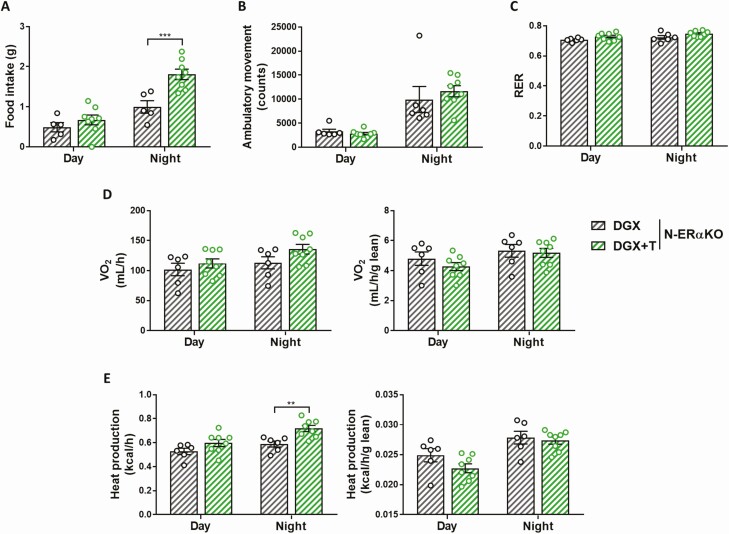
Testosterone is unable to stimulate ambulatory activity in N-ERαKO male mice
Food intake was significantly increased in DGX + T N-ERαKO mice compared with DGX N-ERαKO mice (Fig. 7A). However, in contrast to its effect in WT animals (Fig. 3B), T was unable to stimulate ambulatory activity in N-ERαKO male mice (Fig. 7B). Respiratory exchange ratio and oxygen consumption were similar between DGX and DGX + T N-ERαKO males (Fig. 6C and 6D). Heat production during the night cycle was increased upon T administration to N-ERαKO males, but this effect was abolished upon normalization to lean mass (Fig. 6E). ANCOVA confirmed that this change in energy expenditure was driven indirectly by the T effect on lean mass rather than by T itself (Supplemental Fig. S2 and Supplemental Table S3) (38, 39).
Figure 7.
Effects of T supplementation on physical activity and energy balance of HFD-fed chemically castrated male N-ERαKO mice. Food intake (A), ambulatory movement (B), respiratory exchange ratio (C), oxygen consumption (D), and heat production (E) as measured by indirect calorimetry in 36-week-old N-ERαKO male mice injected with degarelix and supplemented with placebo (DGX, n = 6) or T (DGX + T, n = 8) at 16 weeks of age and subsequently fed with HFD for 20 weeks. D, E (right panels): Oxygen consumption and heat production were normalized to lean body mass. Data were analyzed with two-way ANOVA with Bonferroni post hoc test. **P < 0.01, ***P < 0.001. Data are represented as mean ± SEM. Abbreviations: ANOVA, analysis of variance; DGX, degarelix; HFD, high fat diet; N-ERαKO = extrahypothalamice neuron specific estrogen receptor α knockout; RER, respiratory exchange ratio; SEM, standard error of the mean; T, testosterone; VO2, oxygen consumption.
Discussion
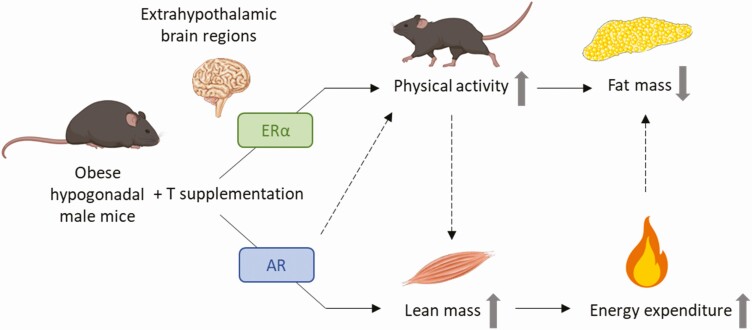
Testosterone is a well-known regulator of male body fat but the underlying mechanisms remain elusive. Here, we show for the first time that the T-associated reduction of fat mass in obese hypogonadal male mice depends on aromatization into estrogens and is at least partially mediated by stimulation of ambulatory activity via extrahypothalamic ERα signaling. The increase in lean mass upon T supplementation, in contrast, is mediated through the AR and indirectly leads to an increase in energy expenditure, which might also contribute to the fat-burning effects of T (Fig. 8).
Figure 8.
Proposed model for the fat-burning effects of T in obese hypogonadal male mice. Abbreviation: T, testosterone.
The concomitant changes in lean and fat mass upon T administration have been attributed to the effects of T on pluripotent mesenchymal precursor cell differentiation (ie, stimulation of their commitment into the myogenic lineage and inhibition of their differentiation into the adipogenic lineage) (15, 41). Our study, however, clearly demonstrates that distinct mechanisms mediate T effects on lean and fat mass. Indeed, while both T and DHT were able to increase lean mass indicating an AR-mediated effect, a decrease in fat mass was only observed upon supplementation with T but not DHT, indicating a role for aromatization into estrogens. These observations are in line with clinical data reporting the abolishment of T effects on fat but not lean mass in the presence of an aromatase inhibitor (13). Of note, preclinical research demonstrated that E2 effects on fat mass are mediated primarily through ERα, as disrupting ERα signaling causes excess fat gain whereas disrupting only ERβ signaling does not (42, 43). On the other hand, while DHT was unable to decrease whole body fat mass or visceral fat, its administration led to a small but significant decrease in subcutaneous fat (Fig. 2F), an observation which is in line with a recent study pointing towards the differential role of T metabolites in the regulation of depot-specific adipose tissue mass (44).
The effects on energy balance have been proposed to underly the fat-burning action of T in men (17), but assessment of these parameters in humans is challenging and hence preclinical investigation is needed. Our finding that the increase in energy expenditure upon T or DHT supplementation is abolished when normalized to lean mass points towards an indirect mechanism, where the increases in oxygen consumption and energy expenditure would result from the increased lean mass rather than being direct effects of the androgens. These data are in contrast with a recent report showing increased lean mass–normalized energy expenditure upon T supplementation of castrated rats (20). The reason for this discrepancy is unclear but may arise from species-specific effects or be related to differences in experimental design.
In contrast to humans, male rodents lack sex hormone binding globulin resulting in undetectable circulating E2. Therefore, estrogenic action is dependent on local aromatization within the target tissues (40). Noteworthy, the precise pattern of Cyp19a1 expression in murine tissues remains a matter of debate. Indeed, while some studies report aromatase expression in male fat (45), we found Cyp19a1 messenger RNA to be undetectable in subcutaneous, perirenal, and nuchal fat, while being close to zero in gonadal fat. In contrast, we observed high aromatase expression in gonads as well as in the brain. Aromatization in the testes could not contribute to the fat-burning effects of T in our model, as DGX administration leads to complete suppression of testicular T production (32). The brain is therefore the most plausible candidate to mediate T effects on male fat mass. Male mice with ERα deletion in hypothalamic brain regions did not show alterations in body weight or composition (29). In contrast, absence of ERα in the medial amygdala induced late-onset obesity in male mice (30). In line with these findings, our extrahypothalamic N-ERαKO model also developed obesity and increased adiposity at an advanced age (Supplemental Fig. S3) (39). Altogether, these findings suggest that central ERα signaling in extrahypothalamic areas might contribute to the fat-burning effects of T in male mice. Indeed, while T administration was still able to increase lean mass, we demonstrate that its fat-burning effect is abolished in the absence of extrahypothalamic ERα signaling.
In our model, T but not DHT increased ambulatory activity, in line with previous findings revealing an essential role for aromatization in this process (24). Importantly, T was unable to stimulate activity in N-ERαKO males, indicating the involvement of extrahypothalamic ERα signaling. This observation is in line with the crucial role for amygdala ERα in regulating physical activity (28, 30), as well as with earlier work demonstrating that striatal dopaminergic signaling at least partly mediates T stimulatory action on male physical activity after central aromatization (24, 46).
The main limitation of this study are the species differences in estrogen physiology between men and male mice (47), which might limit the clinical translation of our findings. Indeed, male mice have low or undetectable circulating estrogens. Therefore, E2 in male mice derives from local aromatization within target tissues and exerts its effects in a paracrine manner. In this study we show that, in contrast to humans, male mice do not express aromatase in fat, suggesting the effects of E2 on fat mass are indirect. Hence, these differences are also a strength. Indeed, since estrogens are neither circulating nor locally produced in murine adipose tissue, the mouse is an ideal model to study the role of central aromatization of T in the regulation of male adiposity.
In summary, we demonstrate that the increase in lean mass upon T supplementation of obese hypogonadal male mice relies on AR activation and indirectly increases energy expenditure. In addition, we show that the T-induced decrease in fat mass depends on aromatization into estrogens and at least partially relies on stimulation of physical activity through activation of extrahypothalamic ERα signaling. These findings increase our mechanistic insights into the fat-burning effects of T and might in the long run impact clinical strategies for the management of hypogonadism-associated obesity.
Acknowledgments
The authors thank Geert Carmeliet for the helpful discussions and Erik Van Herck for technical assistance. Jan-Åke Gustafsson thanks the Robert A. Welch Foundation for support. Degarelix was a kind gift from Ferring Pharmaceuticals.
Financial Support: This work was funded by a research grant from the Flemish Fund for Scientific Research (FWO; G0D2217N). Karel David received a personal grant from the FWO (119650N).
Author Contributions: V.D., F.C., B.D., and D.V. conceptualized and designed the study. N.R.K., K.C., L.D., and D.C. performed the experiment. N.R.K. collected and analyzed most of the data. N.R.K., K.D., R.V., B.V., B.D., F.C., D.V., and V.D. contributed to data interpretation. N.R.K., and V.D. wrote and drafted the manuscript. All authors participated critical revisions of the manuscript for important intellectual content and approved final version of the manuscript.
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Bhaskaran K, dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3.6 million adults in the UK. Lancet Diabetes Endocrinol. Published online October 30, 2018. doi: 10.1016/S2213-8587(18)30288-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Malik VS, Willet WC, Hu FB. Nearly a decade on — trends, risk factors and policy implications in global obesity. Nat Rev Endocrinol. 2020;16(11):615-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. Published online August 27, 2011. doi: 10.1016/S0140-6736(11)60814-3 [DOI] [PubMed] [Google Scholar]
- 4. Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. Published online September 21, 2016. doi: 10.1200/JCO.2006.06.2497 [DOI] [PubMed] [Google Scholar]
- 5. Zitzmann M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat Rev Endocrinol. Published online October 27, 2009. doi: 10.1038/nrendo.2009.212 [DOI] [PubMed] [Google Scholar]
- 6. Wang C, Nieschlag E, Swerdloff R, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males. Int J Androl. Published online September 16, 2008. doi: 10.1111/j.1365-2605.2008.00924.x [DOI] [PubMed] [Google Scholar]
- 7. Guo C, Gu W, Liu M, et al. Efficacy and safety of testosterone replacement therapy in men with hypogonadism: A meta-analysis study of placebo-controlled trials. Exp Ther Med. Published online December 23, 2015. doi: 10.3892/etm.2015.2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corona G, Giagulli VA, Maseroli E, et al. Testosterone supplementation and body composition: results from a meta-analysis of observational studies. J Endocrinol Invest. Published online May 30, 2016. doi: 10.1007/s40618-016-0480-2 [DOI] [PubMed] [Google Scholar]
- 9. Corona G, Goulis DG, Huhtaniemi I, et al. European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males: Endorsing organization: European Society of Endocrinology. Andrology. Published online February 5, 2020. doi: 10.1111/andr.12770 [DOI] [PubMed] [Google Scholar]
- 10. Helsen C, Kerkhofs S, Clinckemalie L, et al. Structural basis for nuclear hormone receptor DNA binding. Mol Cell Endocrinol. Published online January 30, 2012. doi: 10.1016/j.mce.2011.07.025 [DOI] [PubMed] [Google Scholar]
- 11. Claessens F, Denayer S, Van Tilborgh N, Kerkhofs S, Helsen C, Haelens A. Diverse roles of androgen receptor (AR) domains in AR-mediated signaling. Nucl Recept Signal. 2008;6(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naftolin F, Ryan KJ, Davies IJ. The formation of estrogens by central neuroendocrine tissues. Recent Prog Horm Res. Published online October 21, 2013. doi: 10.1016/b978-0-12-571131-9.50012-8 [DOI] [PubMed] [Google Scholar]
- 13. Finkelstein JS, Lee H, Burnett-Bowie SAM, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. Published online September 12, 2013. doi: 10.1056/NEJMoa1206168 [DOI] [PubMed] [Google Scholar]
- 14. Blouin K, Nadeau M, Perreault M, et al. Effects of androgens on adipocyte differentiation and adipose tissue explant metabolism in men and women. Clin Endocrinol (Oxf). Published online January 19, 2010. doi: 10.1111/j.1365-2265.2009.03645.x [DOI] [PubMed] [Google Scholar]
- 15. Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. Published online November 1, 2003. doi: 10.1210/en.2003-0741 [DOI] [PubMed] [Google Scholar]
- 16. Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. Published online February 23, 2001. doi: 10.1016/S0092-8674(01)00240-9 [DOI] [PubMed] [Google Scholar]
- 17. Santosa S, Khosla S, McCready LK, Jensen MD. Effects of estrogen and testosterone on resting energy expenditure in older men. Obesity. Published online May 6, 2010. doi: 10.1038/oby.2010.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gibney J, Wolthers T, Johannsson G, Umpleby AM, Ho KKY. Growth hormone and testosterone interact positively to enhance protein and energy metabolism in hypopituitary men. Am J Physiol. Published online February 22, 2005. doi: 10.1152/ajpendo.00483.2004 [DOI] [PubMed] [Google Scholar]
- 19. Dubois V, Laurent MR, Jardi F, et al. Androgen deficiency exacerbates high-fat diet-induced metabolic alterations in male mice. Endocrinology. Published online February 1, 2016. doi: 10.1210/en.2015-1713 [DOI] [PubMed] [Google Scholar]
- 20. Ali Abulmeaty MM, Almajwal AM, ElSadek MF, Berika MY, Razak S. Metabolic effects of testosterone hormone therapy in normal and orchiectomized male rats: from indirect calorimetry to lipolytic enzymes. Int J Endocrinol. 2019;2019:7546385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ophoff J, Callewaert F, Venken K, et al. Physical activity in the androgen receptor knockout mouse: Evidence for reversal of androgen deficiency on cancellous bone. Biochem Biophys Res Commun. Published online November 12, 2008. doi: 10.1016/j.bbrc.2008.11.016 [DOI] [PubMed] [Google Scholar]
- 22. Fan WQ, Yanase T, Nomura M, et al. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes. Published online April, 2005. doi: 10.2337/diabetes.54.4.1000 [DOI] [PubMed] [Google Scholar]
- 23. Rana K, Fam BC, Clarke MPV, Pang TPS, Zajac JD, MacLean HE. Increased adiposity in DNA binding-dependent androgen receptor knockout male mice associated with decreased voluntary activity and not insulin resistance. Am J Physiol. Published online January 28, 2011. doi: 10.1152/ajpendo.00584.2010 [DOI] [PubMed] [Google Scholar]
- 24. Jardí F, Laurent MR, Kim N, et al. Testosterone boosts physical activity in male mice via dopaminergic pathways. Sci Rep. Published online January 17, 2018. doi: 10.1038/s41598-017-19104-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gofflot F, Chartoire N, Vasseur L, et al. Systematic gene expression mapping clusters nuclear receptors according to their function in the Brain. Cell. Published online October 19, 2007. doi: 10.1016/j.cell.2007.09.012 [DOI] [PubMed] [Google Scholar]
- 26. Lein ES, Hawrylycz MJ, Ao N, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. Published online December 6, 2006. doi: 10.1038/nature05453 [DOI] [PubMed] [Google Scholar]
- 27. Mahfouz A, Lelieveldt BPF, Grefhorst A, et al. Genome-wide coexpression of steroid receptors in the mouse brain: Identifying signaling pathways and functionally coordinated regions. Proc Natl Acad Sci U S A. Published online January 25, 2016. doi: 10.1073/pnas.1520376113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu Y, O’Malley BW, Elmquist JK. Brain nuclear receptors and body weight regulation. J Clin Invest. Published online February 20, 2017. doi: 10.1172/JCI88891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu Y, Nedungadi TP, Zhu L, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. Published online October 5, 2011. doi: 10.1016/j.cmet.2011.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu P, Cao X, He Y, et al. Estrogen receptor-α in medial amygdala neurons regulates body weight. J Clin Invest. Published online January 22, 2015. doi: 10.1172/JCI80941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim NR, Jardí F, Khalil R, et al. Estrogen receptor alpha signaling in extrahypothalamic neurons during late puberty decreases bone size and strength in female but not in male mice. FASEB J. Published online April 2, 2020. doi: 10.1096/fj.202000272R [DOI] [PubMed] [Google Scholar]
- 32. Kim NR, Khalil R, David K, et al. Novel model to study the physiological effects of temporary or prolonged sex steroid deficiency in male mice. Am J Physiol Metab. Published online December 14, 2020. doi: 10.1152/ajpendo.00401.2020 [DOI] [PubMed] [Google Scholar]
- 33. Vanderschueren D, Van Herck E, Suiker AM, Visser WJ, Schot LP, Bouillon R. Bone and mineral metabolism in aged male rats: short and long term effects of androgen deficiency. Endocrinology. 1992;130(5):2906-2916. [DOI] [PubMed] [Google Scholar]
- 34. Vanderschueren D, Vandenput L, Boonen S, Van Herck E, Swinnen JV, Bouillon R. An aged rat model of partial androgen deficiency: Prevention of both loss of bone and lean body mass by low-dose androgen replacement. Endocrinology. Published online May 1, 2000. doi: 10.1210/endo.141.5.7472. [DOI] [PubMed] [Google Scholar]
- 35. Venken K, Movérare-Skrtic S, Kopchick JJ, et al. Impact of androgens, growth hormone, and IGF-I on bone and muscle in male mice during puberty. J Bone Miner Res. Published online December 4, 2009. doi: 10.1359/jbmr.060911 [DOI] [PubMed] [Google Scholar]
- 36. Ferrannini E. The theoretical bases of indirect calorimetry: A review. Metabolism. Published online April 2, 2004. doi: 10.1016/0026-0495(88)90110-2 [DOI] [PubMed] [Google Scholar]
- 37. Tschöp MH, Speakman JR, Arch JRS, et al. A guide to analysis of mouse energy metabolism. Nat Methods. Published online December 28, 2011. Doi: 10.1038/nmeth.1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim NR, David K, Corbeels K, et al. Supplemental Tables. Published online January 18, 2021. doi: 10.6084/m9.figshare.13603166.v1 [DOI]
- 39. Kim NR, David K, Corbeels K, et al. Supplemental Figures. Published online January 18, 2021. doi: 10.6084/m9.figshare.13603043.v1 [DOI]
- 40. Laurent MR, Hammond GL, Blokland M, et al. Sex hormone-binding globulin regulation of androgen bioactivity in vivo: validation of the free hormone hypothesis. Sci Rep. 2016;6:35539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Herbst KL, Bhasin S. Testosterone action on skeletal muscle. Curr Opin Clin Nutr Metab Care. Published online May, 2004. doi: 10.1097/00075197-200405000-00006 [DOI] [PubMed] [Google Scholar]
- 42. Lindberg MK, Weihua Z, Andersson N, et al. Estrogen receptor specificity for the effects of estrogen in ovariectomized mice. J Endocrinol. Published online August 1, 2002. doi: 10.1677/joe.0.1740167 [DOI] [PubMed] [Google Scholar]
- 43. Van Pelt RE, Gavin KM, Kohrt WM. Regulation of Body Composition and Bioenergetics by Estrogens. Endocrinol Metab Clin North Am. Published online June 20, 2015. doi: 10.1016/j.ecl.2015.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sebo ZL, Rodeheffer MS. Testosterone metabolites differentially regulate obesogenesis and fat distribution. Mol Metab. Published online December 9, 2020;44:101141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhao H, Innes J, Brooks DC, et al. A novel promoter controls Cyp19al gene expression in mouse adipose tissue. Reprod Biol Endocrinol. Published online April 24, 2009. doi: 10.1186/1477-7827-7-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jardí F, Laurent MR, Dubois V, et al. Androgen and estrogen actions on male physical activity: A story beyond muscle. J Endocrinol. Published online May 9, 2018. doi: 10.1530/JOE-18-0125 [DOI] [PubMed] [Google Scholar]
- 47. Almeida M, Laurent MR, Dubois V, et al. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev. Published online November 2, 2016. doi: 10.1152/physrev.00033.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.