Abstract
Interoceptive awareness (IA), or the awareness of internal body states, is known to be impaired in individuals with eating disorders (EDs); however, little is understood about how IA and ED symptoms are connected. Network analysis is a statistical approach useful for examining how symptoms interrelate and how comorbidities may be maintained. The present study used network analysis to: (1) test central symptoms within an IA-ED network, (2) identify symptoms that may bridge the association between IA and ED symptoms, and (3) explore whether central and bridge symptoms predict ED remission at discharge from intensive treatment. A regularized partial correlation network was estimated in a sample of 428 adolescent (n=187) and adult (n=241) ED patients in a partial hospital program. IA was assessed using items from the Multidimensional Assessment of Interoceptive Awareness, and ED symptoms were assessed using items from the Eating Disorder Examination-Questionnaire. Central symptoms within the network were strong desire to lose weight, feeling guilty, and listening for information from the body about emotional state. The most central symptom bridging IA and ED symptoms was (not) feeling safe in one’s body. Of the central symptoms, greater desire to lose weight predicted lower likelihood of remission at treatment discharge. Bridge symptoms did not significantly predict remission. Body mistrust may be a mechanism by which associations between IA and EDs are maintained. Findings suggest targeting central and bridge symptoms may be helpful to improve IA and ED symptoms.
Keywords: network analysis, interoceptive awareness, eating disorder, comorbidity
General Scientific Summary:
This study suggests that desire to lose weight is central to eating disorder psychopathology and is associated with lower likelihood of remission. Feeling unsafe in one’s body connects eating disorder symptoms with body awareness.
Deficits in interoceptive awareness (IA), the ability to recognize and process internal body states (e.g., hunger/satiety, taste, touch, pain, heartrate, breathlessness, arousal), have been implicated in the etiology and maintenance of mental health disorders including panic disorder, depression, somatic disorders, substance use disorders, posttraumatic stress, generalized anxiety disorder, autism spectrum disorder, and eating disorders (EDs; Khalsa et al., 2017). Indeed, deficits in IA have been implicated in the etiology of EDs since their initial conceptualization (Bruch, 1962), and these deficits are posited to contribute to ED symptoms including body image disturbance and inappropriate response to hunger/satiety (Kaye et al., 2009). Although IA deficits are not required for ED diagnoses, self-report, behavioral, and neurobiological studies have found deficits in IA in individuals with EDs compared to controls, and a recent meta-analysis of this literature reported large IA deficits (Jenkinson, Taylor, & Laws, 2018). Empirical evidence of altered activity in brain regions involved in perception of body states among individuals with EDs further supports the relevance of disturbed interoceptive processes in this population (Kaye, Fudge, & Paulus, 2009), as do findings showing that greater IA deficits are associated with worse ED treatment outcome (Bizeul, Sadowsky, & Rigaud, 2001; Carter, Blackmore, Sutandar-Pinnock, & Woodside, 2004).
Recent research outside the ED field has conceptualized IA as a multidimensional construct comprised of several facets including abilities to notice body sensations, attend to body sensations without distraction or worry, regulate attention and emotions by attending to body signals, be aware of the link between body signals and emotions, and listen to and trust in body sensations (Mehling et al., 2012). However, the majority of studies examining the connections between IA and EDs have neglected this multidimensional nature. A better understanding of which IA dimensions are most strongly linked with specific ED symptoms will provide insights necessary to inform the development of more effective treatments to target the co-occurrence of disturbances in IA dimensions and ED symptoms (see McNally, 2016).
Network analysis represents a useful analytic approach to characterize the complex links between IA dimensions and ED symptoms. This statistical method is rooted in the network theory of mental disorders, which proposes that disorders arise from and are defined by dynamic relations among symptoms, which may reinforce one another over time (Borsboom, 2017; Cramer, Waldorp, van der Maas, & Borsboom, 2010). Network theory contrasts with the traditional view of mental disorders as a set of symptoms arising from a latent syndrome. Network analytic studies of psychopathology allow for identification of central symptoms (or “nodes”) that are core to and most influential within symptom networks (Borsboom & Cramer, 2013; McNally, 2016) which can inform targeted intervention selection (McNally, 2016). Supporting this concept, recent research has demonstrated that central nodes predict clinical outcomes (Elliot, Jones, & Schmidt, 2018; Olatunji, Christian, Brosof, Tolin, & Levinson, 2019; Olatunji, Levinson, & Calebs, 2018; Rodebaugh et al., 2018). Further, beyond deriving networks based on a single cluster of symptoms (e.g., ED symptoms), network analytic techniques can also be used to investigate relationships among interrelated or co-occurring symptom clusters (e.g., ED symptoms and IA dimensions). This is accomplished through the identification of bridge pathways, or symptoms in one cluster that are linked to symptoms in another (Borsboom & Cramer, 2013), which can provide insights into pathways underlying the co-occurrence of distinct symptom clusters. Of note, bridge symptoms are those that link the networks of distinct symptom clusters, whereas central symptoms are those that are at the core of the network. According to network theory, central symptoms should demonstrate predictive validity for disorder outcomes, while bridge symptoms should predict comorbidities between networks, rather than disorder outcomes (Borsboom & Cramer, 2013; Borsboom, 2017).
A number of studies have applied network analytic methods to EDs (DuBois, Rodgers, Franko, Eddy, & Thomas, 2017; Forbush, Siew, & Vitevitch, 2016; Forrest, Jones, Ortiz, & Smith, 2018; Forrest, Sarfan, Ortiz, Brown, & Smith, 2019; Levinson, Brosof, et al., 2018; Levinson, Vanzhula, & Brosof, 2018; Levinson et al., 2017; Vanzhula, Calebs, Fewell, & Levinson, 2019). Notably, few network studies in the ED field have examined whether central symptoms predict outcomes longitudinally. For instance, in one study of anorexia nervosa (AN), higher levels of weight- and shape-related central symptoms predicted failure to recover and greater impairment at 12-month follow-up (Elliot, Jones, & Schmidt, 2018). Research examing whether central ED symptoms predict longitudinal outcomes is critical to both test the prognostic utility of networks and to develop more targeted treatments (Rodebaugh et al., 2018). In the only study to our knowledge that has used network analysis to examine IA within an ED symptom network, findings revealed that interoceptive deficits were highly central to ED symptom networks at both admission and discharge in a large ED residential treatment sample (Olatunji, Levinson, & Calebs, 2018). However, the study utilized the Interoceptive Deficits subscale of the Eating Disorder Inventory-2 (EDI-2; Garner, 1991), conceptualized IA symptoms as part of the ED symptom network, rather than a separate network that may importantly connect to ED symptoms, and did not examine item level data. The EDI scale assesses both body- and emotion-related awareness and may conflate IA with alexithymia, a related, yet distinct construct focused on awareness of and reactivity to one’s emotions (Merwin, Zucker, Lacy, & Elliott, 2010). Further, the EDI assumes IA is unidimensional. Given recent research suggesting that IA includes multiple dimensions or subscales (Mehling et al., 2012), investigating pathways that connect specific IA dimensions and ED symptoms may help inform interventions targeting the co-occurrence of IA deficits and EDs. Network theory posits that disrupting bridge pathways should theoretically help to break the connection between symptom networks (e.g., disturbed IA and ED symptoms). Therefore, these targeted treatments may focus particularly on IA dimensions that connect most strongly to ED symptoms. For instance, the choice of a clinical approach may differ if the identified bridge symptom was emotion-related versus body-related awareness. Given that initial data suggest that greater IA deficits, as measured by the EDI, are associated with poorer ED treatment outcome (Bizeul, Sadowsky, & Rigaud, 2001; Carter, Blackmore, Sutandar-Pinnock, & Woodside, 2004), it is plausible that intervening on the connections between specific IA and ED symptoms may have positive downstream effects on longer-term ED outcomes.
The Multidimensional Assessment of Interoceptive Awareness (MAIA; Mehling et al., 2012) is a self-report measure of eight specific IA dimensions and is well-suited to probe associations between IA dimensions and ED symptoms. The measure was originally developed and evaluated in a body-aware population (e.g., yoga experts) to characterize adaptive and maladaptive IA dimensions. The MAIA differentiates between ability to: 1) notice body sensations (“Noticing” subscale), 2) attend to body sensations without distraction (“Not Distracting”) or 3) worry (“Not Worrying”), 4) control attention to body signals (“Attention Regulation”), 5) be aware of the link between body signals and emotions (“Emotional Awareness”), 6) regulate distress by attending to body signals (“Self-Regulation”), 7) listen to the body for insight (“Body Listening”) and 8) trust in body sensations (“Trusting”). A recent study from our group provided support for the reliability and validity of the MAIA in a clinical sample of adolescents and adults with EDs (Brown et al., 2017), with results also showing that several MAIA subscales were associated with specific symptoms of ED psychopathology. Specifically, lower ability to maintain awareness of body sensations without distraction (i.e., “Not Distracting”) was associated with higher restraint, eating concern, and weight and shape concern; lower abilities to regulate distress by attending to body sensations (i.e., “Self-Regulation”) and listen to the body for insight (i.e., “Body Listening”) was related to higher eating concerns; and lower trust in one’s body signals (i.e., “Trusting”) was associated with higher restraint, eating concern, weight and shape concern, and binge/purge symptoms (Brown et al., 2017). These findings tentatively suggest that that these IA dimensions, especially attending to body sensation without distraction and body trust, may have particular relevance to global ED psychopathology.
The present study used network analysis to test links between IA dimensions and specific ED symptoms in a sample of patients seeking ED treatment at the partial hospitalization level of care. Our first aim was to identify core symptoms within an IA and ED symptom network. Consistent with previous research (DuBois et al., 2017; Forbush et al., 2016; Forrest et al., 2018; Forrest et al., 2019; Levinson et al., 2017; Vanzhula et al., 2019), we hypothesized that symptoms related to weight/shape concerns would be central to the network. Secondly, we aimed to identify bridge pathways between IA and ED symptoms. Based on previous findings from our group described above, we hypothesized that items from the MAIA Not Distracting subscale and the Trusting subscale would serve as bridges to the ED symptom network. Finally, given the limited ED research examining the predictive validity of networks, we tested whether central and bridge symptoms within the network predicted likelihood of remission at treatment discharge. Given previous research and presuppositions from network theory, bridge symptoms were tested to demonstrate the predictive sensitivity of central symptoms. Thus, we hypothesized that central, but not bridge, symptoms would predict lower likelihood of remission.
Method
Participants and Procedure
Participants were 428 adult and adolescent patients (n = 241 and n = 187, respectively) admitted to the UCSD Eating Disorders Center for Treatment and Research between December 2013 and November 2018 who completed IA and ED measures. Participants completed IA and ED assessments within 14 days of treatment admission and 14 days of discharge. Discharge data to determine remission status were available for 71.3% (n = 305) of the sample. Diagnoses prior to August 2016 were assigned using an unstandardized semi-structured interview by staff psychiatrists, based on 2010 draft criteria for the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; APA, 2013). Diagnoses after August 2016 were determined using the Structured Clinical Interview for DSM-5 Disorders (SCID-5; First, Williams, & Spitzer, 2015) administered by trained, bachelor’s-level research assistants. Table 1 displays descriptive data, including overall levels of ED symptoms1. Adults and adolescents did not differ on sex, race, or ethnicity. Adolescents had a lower admission body mass index (BMI), shorter length of stay and length of illness, were less likely to be on medication, and more likely to be diagnosed with AN-restricting type (AN-R) compared to adults. Adolescents reported lower overall eating pathology. The Institutional Review Board approved all study procedures.
Table 1.
Demographic Characteristics of the Sample
| Total | Adolescents | Adults | |||
|---|---|---|---|---|---|
| Variable | M(SD)/n(%) | M(SD)/n(%) | M(SD)/n(%) | F/X2 | p |
| Age | 21.70 (8.78) | 15.69 (1.70) | 26.36 (9.22) | 243.97 | <.001 |
| BMI at admission | 20.41 (5.06) | 18.97 (3.96) | 21.54 (5.52) | 29.00 | <.001 |
| Length of Illness | 6.69 (8.06) | 2.26 (1.80) | 10.29 (9.29) | 134.97 | <.001 |
| Female | 406 (94.86) | 178 (95.19) | 228 (94.61) | 0.73 | .79 |
| Race | 5.53 | .35 | |||
| Caucasian | 317 (74.59) | 130 (69.89) | 187 (78.24) | ||
| Asian | 26 (6.12) | 13 (6.99) | 13 (5.44) | ||
| African American | 2 (0.47) | 1 (0.54) | 1 (0.42) | ||
| Native Hawaiian/Pacific Islander | 1 (0.24) | 0 (0.00) | 1 (0.42) | ||
| Native American/Alaskan Native | 3 (0.71) | 1 (0.54) | 2 (0.84) | ||
| Other | 76 (17.88) | 41 (22.04) | 35 (14.64) | ||
| Ethnicity | 2.27 | .13 | |||
| Hispanic | 70 (16.87) | 25 (13.74) | 45 (19.31) | ||
| Non-Hispanic | 345 (83.13) | 157 (86.26) | 188 (80.69) | ||
| Antidepressant Medication | 234 (59.85) | 62 (39.74) | 172 (73.19) | 43.65 | <.001 |
| Atypical Antipsychotics | 69 (17.65) | 20 (12.82) | 49 (20.85) | 4.16 | .04 |
| Mood Stabilizer | 72 (18.41) | 7 (4.49) | 65 (27.66) | 33.51 | <.001 |
| Anxiolytic | 36 (9.21) | 8 (5.12) | 28 (11.91) | 5.17 | .02 |
| ED Diagnosis | 59.89 | <.001 | |||
| Anorexia Nervosa – Restricting | 191 (44.73) | 121 (65.05) | 70 (29.04) | ||
| Anorexia Nervosa – Binge/Purge | 64 (14.99) | 24 (12.90) | 40 (16.60) | ||
| Bulimia Nervosa | 103 (24.12) | 28 (15.05) | 75 (31.12) | ||
| Binge Eating Disorder | 13 (3.04) | 3 (1.61) | 10 (4.15) | ||
| Other Specified Feeding or Eating Disorder | 56 (13.11) | 10 (5.38) | 46 (19.09) | ||
| Met Remission Criteria at Discharge | 110 (33.44) | 50 (33.3) | 60 (33.5) | .001 | .97 |
| Admission EDE-Q Global | 3.58 (1.64) | 3.30 (1.73) | 3.80 (1.53) | 9.79 | .002 |
Note. BMI = body mass index; ED = eating disorder. ED means reflect full-scale scores.
Measures
ED Symptoms.
The Eating Disorder Examination–Questionnaire (EDE-Q version 6.0; Fairburn & Beglin, 1994) was used to assess ED symptoms (see Figure 1 for item descriptions) during the previous 28 days. Consistent with prior research applying network analyses with ED samples (Levinson, Brosof, et al., 2018; Levinson et al., 2017), the present study used the continuously rated (non-frequency count) items (#s:1–12,19–28), which are all rated on a 0–6 scale, with higher values indicating more severe ED symptoms. The EDE-Q has demonstrated good internal consistency, construct validity, and 2-week test-retest reliability (Berg, Peterson, Frazier, & Crow, 2011). Internal consistency across items in the present study was α =.96 for adolescents and α = .95 for adults.
Figure 1.
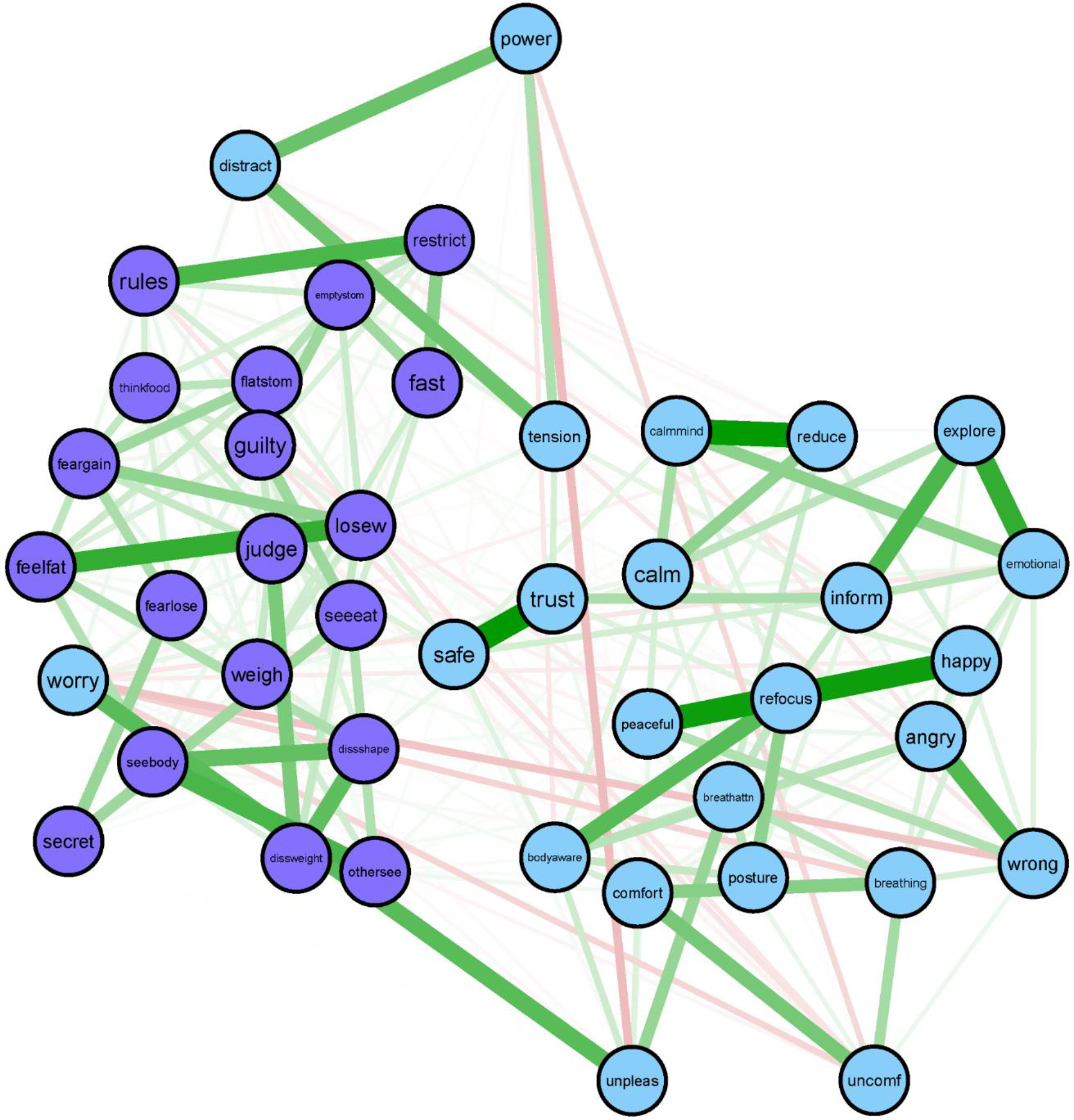
Interoceptive awareness (IA) and eating disorder (ED) symptom network.
IA symptoms (top of figure) are in light blue (light gray without color) and ED symptoms (bottom) are in purple (dark gray). Thicker lines represent stronger relationships.
IA labels: Angry = noticing body changes when angry; Bodyaware = maintain awareness of whole body when distressed; Breathattn = pay attention to breathe without being distracted; Breathing = notice changes in breathe; Calm = ability to calm mind by focusing on body/breathing; Calmmind = can calm mind by focusing on body/breathing; Comfort = notice where comfortable in the body; Emotional = listening to the body for information about emotional state; Explore = exploring how body feels when upset; Happy = noticing body changes when happy; Inform = listening to the body to inform action; Peaceful = noticing body differences when peaceful; Posture = pay attention to posture; Power = “power through” pain; Reduce = can use breathe to reduce tension; Refocus = refocus attention from thinking to sensing; Safe = feeling body is a safe place; Tension = Ignore physical tension/discomfort until severe; Trust = trusting body sensations; Unconf = notice body discomfort; Unpleas = notice unpleasant body sensations without worrying; Worry = worry something is wrong if uncomfortable; Wrong = feeling body changes when something is wrong.
ED labels: Dissshape = shape dissatisfaction; Dissweight = weight dissatisfaction; Emptystom = desire to have empty stomach; Fast = long time without eating; Feargain = fear of gaining weight; Fearlose = fear of losing control; Feelfat = feeling fat; Flatstom = desire to have flat stomach; Guilty = guilt after eating; Judge = overvaluation of weight; Losew = desire to lose weight; Othersee = uncomfortable with others seeing shape; Restrict = limiting amount of food; Rules = following rules regarding eating; Secret = eating in secret; Seeeat = concern about others observing eating; Seebody = discomfort seeing body; Thinkfood = concentration problems regarding eating; Weigh = distress about weighing.
Interoceptive Awareness.
As previously described, the MAIA (Mehling et al., 2012) is a 32-item self-report measure of eight IA dimensions: 1) Noticing (“I notice where in my body I am comfortable”), 2) Not-Distracting (“I distract myself from sensations of discomfort”), 3) Not-Worrying (“I can notice an unpleasant sensation without worrying”), 4) Attention Regulation (“I am able to consciously focus on my body as a whole”), 5) Emotional Awareness (“”When something is wrong in my life, I can feel it in my body”), 6) Self-Regulation (“When I am caught in my thoughts, I can calm my mind by focusing on my body/breathing”), 7) Body Listening (“I listen to my body to inform me about what to do”), and 8) Trusting (“I trust my body sensations”) (see Figure 1 for item descriptions). Items are rated on a 0 (never) to 5 (always) scale, with higher scores indicating better IA; however, to facilitate interpretation in the present study, items were recoded consistent with the EDE-Q so that higher scores were more pathological (i.e., indicated poorer IA). The MAIA subscales have demonstrated acceptable psychometric properties in ED (Brown et al., 2017) and non-ED populations (Machorrinho, Veiga, Fernandes, Mehling, & Marmeleira, 2019; Mehling et al., 2012). Internal consistency across the items used in the present study was α =.87 for adolescents and α = .89 for adults.
Remission Status.
Consistent with previous ED research (Bardone-Cone et al., 2010), individuals were considered remitted if they met the following criteria at treatment discharge: (1) BMI > 18.5; (2) no fasting, binge eating, or purging in the previous 28 days on the EDE-Q, and (3) EDE-Q Global scores within 1 SD of community means (Mond, Hay, Rodgers, & Owen, 2006).
Data Analyses
Missing Data.
One percent of the data at admission were missing. Little’s MCAR test was statistically significant (X2(2038) = 2268.2, p < 0.001), indicating data were not missing completely at random (MCAR). Thus, missing data were handled using multiple imputation, conducted using the mice package in R (Groothuis-Oudshoorn & Van Buuren, 2011). Data for determining remission status at discharge were not imputed.
Item Selection.
An empirical approach (Levinson et al., 2018) was used to select items for inclusion in the network, using the goldbricker function in R package networktools (Jones, 2017), which wraps the R package cocor (Diedenhofen & Musch, 2015). Goldbricker identifies potential issues with item redundancy by comparing every possible combination of correlations within the network. This function determines whether correlations between each node in a pair to each other node in the network are significantly different from one another; if only a small proportion of correlations are different, that suggests that the pair of nodes are redundant. Once the redundant pairs are identified, the best_goldbricker function suggests which nodes to remove. All MAIA items and all continuously rated items of the EDE-Q were initially retained; after applying goldbricker, EDE-Q items 3, 8, and 23 and MAIA items 1, 8, 12, 14, 17, 21, 23, and 30 were removed. This approach resulted in a final network with 45 nodes (26 MAIA, 19 EDE-Q).
Network Estimation.
Networks were estimated using a partial correlation graphical least absolute shrinkage and selection operator (GLASSO) estimator using bootnet. Regularized partial correlation networks (GLASSO networks) estimate edges (i.e., the partial correlation between two nodes) that are likely to be spurious as zero, leading to a more parsimonious and theoretically more accurate network (Epskamp & Fried, 2018). Correlations between nodes in GLASSO networks represent unique partial relationships among symptoms while accounting for all symptoms in the network. The initial networks were estimated using polychoric correlations. The resulting network was dense and had several unexpected negative edges. Thus, per recommendation of Epskamp and Fried (2018), we used Spearman correlations in our final analysis. Multidimensional scaling for visualization (Jones, Mair, & McNally, 2018) was used to allow for meaningful interpretation of distances between nodes.
Centrality Indices.
We identified central nodes by calculating strength centrality and strength expected influence (EI). Strength centrality is defined as the sum of the absolute values of the connections between one node and all other nodes (McNally, 2016). Similarly, EI is the sum of connections between one node and all other nodes, but it accounts for both positive and negative connections. EI may be a more accurate centrality measure in networks with negative edges (e.g., negative associations among nodes; McNally, 2016). We identified bridge nodes between MAIA and EDE-Q symptom clusters by calculating bridge strength centrality and bridge expected influence. Bridge strength is defined as the sum of the absolute values of the connections that exist between a node and all nodes that are not in the same cluster (IA items were designated as one cluster and ED symptoms as another). Bridge EI is a similar bridge centrality estimate that accounts for both positive and negative connections. Strength centrality and EI were calculated using the centralityPlot and centralityTable functions in the qgraph package in R (Epskamp, Cramer, Waldorp, Schmittmann, & Borsboom, 2012). We used the bridge function of the networktools package (Jones, 2017) to identify bridge symptoms between IA and ED symptom items. We also performed both node centrality and bridge centrality difference tests to determine whether nodes with higher centrality statistics were significantly different from nodes with lower values (Epskamp, Borsboom, & Fried, 2018).
Network Stability.
The R package bootnet (Epskamp et al., 2018) was used to assess stability of the networks. We computed five stability parameters (a strength centrality stability coefficient (CS-coefficient), an EI stability coefficient (EI-coefficient), an edge stability coefficient (ES-coefficient), a bridge strength stability coefficient (BS-coefficient), and a bridge EI stability coefficient (BEI-coefficient)), which reflect the maximum proportion of cases that can be dropped such that the correlation between original centrality indices and the reduced sample is at least .70. Coefficients between .20 and .50 are acceptable, above .50 and below .70 are good, and above .70 are excellent (Epskamp et al., 2018).
Network Comparison.
We conducted the Network Comparison Test (NCT) to test for differences in strength centrality between adult and adolescent networks using the NetworkComparisonTest package in R (van Borkulo et al., 2015). The NCT can determine if there are differences in network structure (i.e., node connectivity across samples), and/or global strength (i.e., the sum of the strengths of all edges in the network) differs across samples (van Borkulo et al., 2015).
Logistic Regression.
Finally, two separate logistic regression models were run to examine whether (1) central and (2) bridge symptoms predicted likelihood of remission at treatment discharge in the subset of individuals with discharge data (n = 304). In each model, the central or bridge symptoms with the highest centrality, as noted in the results, were included as predictors.
Results
Central Symptoms
Network stability was good (CS-coefficient = .67, EI-coefficient = .67; ES-coefficient = .67). Figure 1 presents the IA and ED symptom network and Figure 2 presents the centrality plots. Because our network had several negative edges, we chose to report both strength centrality and EI results. When negative edges are present, strength and EI values may provide unique information, although in our case they were highly correlated (r = .70, p < .001). The symptoms with the highest strength centrality were: having a strong desire to lose weight (losew; strength = 1.58), feeling guilty (guilty; strength = 1.45), and listening for information from the body about emotional state (emotional; strength = 1.35). Having a strong desire to lose weight had significantly higher strength centrality than 70% of other nodes, feeling guilty had significantly higher strength centrality than 53% of other nodes, and listening for information from the body about emotional state had significantly higher strength centrality than only 51% of the nodes. See Figure 3 for the centrality difference graph. The symptoms with the highest EI were: having a strong desire to lose weight (losew; EI = 1.42), (mis)trust in body sensations (trust; EI = .95), and restricting food intake (restrict; EI = .91). Having a strong desire to lose weight had significantly higher EI than 79% of other nodes, (mis)trust in body sensations and restricting food intake had significantly higher EI than only 40% of other nodes. Because nodes guilty, emotional, trust, and restrict had inconsistent strength and EI and were only significantly different from about half of other nodes, we chose not to interpret them in the discussion.
Figure 2.
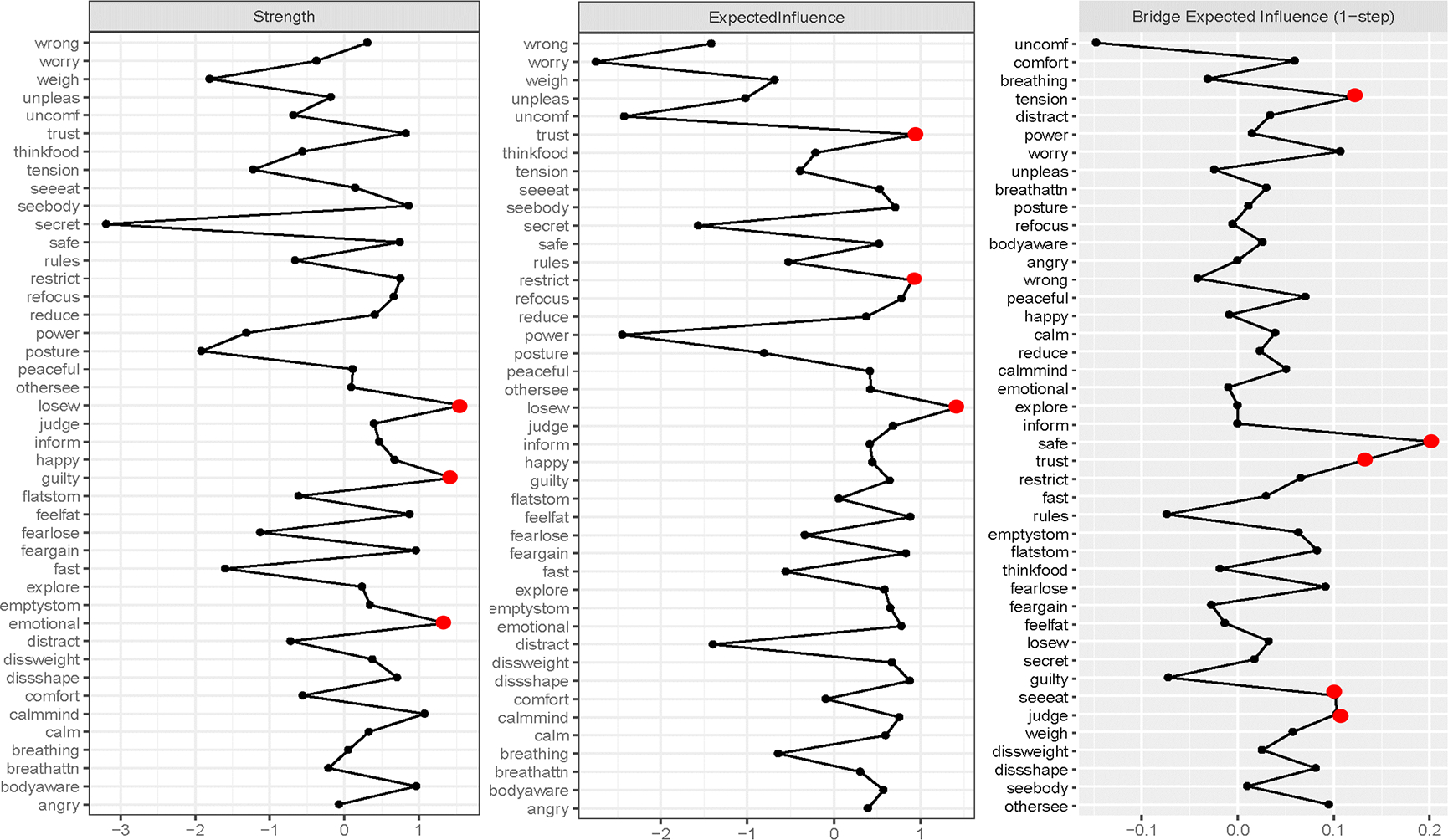
Network strength and expected influence and bridge expected influence plots for the interoceptive awareness (IA) and eating disorder (ED) symptom network.
The strongest central and bridge symptoms are highlighted with a red (dark gray) dot.
IA labels: Angry = noticing body changes when angry; Bodyaware = maintain awareness of whole body when distressed; Breathattn = pay attention to breathe without being distracted; Breathing = notice changes in breathe; Calm = ability to calm mind by focusing on body/breathing; Calmmind = can calm mind by focusing on body/breathing; Comfort = notice where comfortable in the body; Emotional = listening to the body for information about emotional state; Explore = exploring how body feels when upset; Happy = noticing body changes when happy; Inform = listening to the body to inform action; Peaceful = noticing body differences when peaceful; Posture = pay attention to posture; Power = “power through” pain; Reduce = can use breathe to reduce tension; Refocus = refocus attention from thinking to sensing; Safe = feeling body is a safe place; Tension = Ignore physical tension/discomfort until severe; Trust = trusting body sensations; Unconf = notice body discomfort; Unpleas = notice unpleasant body sensations without worrying; Worry = worry something is wrong if uncomfortable; Wrong = feeling body changes when something is wrong.
ED labels: Dissshape = shape dissatisfaction; Dissweight = weight dissatisfaction; Emptystom = desire to have empty stomach; Fast = long time without eating; Feargain = fear of gaining weight; Fearlose = fear of losing control; Feelfat = feeling fat; Flatstom = desire to have flat stomach; Guilty = guilt after eating; Judge = overvaluation of weight; Losew = desire to lose weight; Othersee = uncomfortable with others seeing shape; Restrict = limiting amount of food; Rules = following rules regarding eating; Secret = eating in secret; Seeeat = concern about others observing eating; Seebody = discomfort seeing body; Thinkfood = concentration problems regarding eating; Weigh = distress about weighing.
Figure 3.
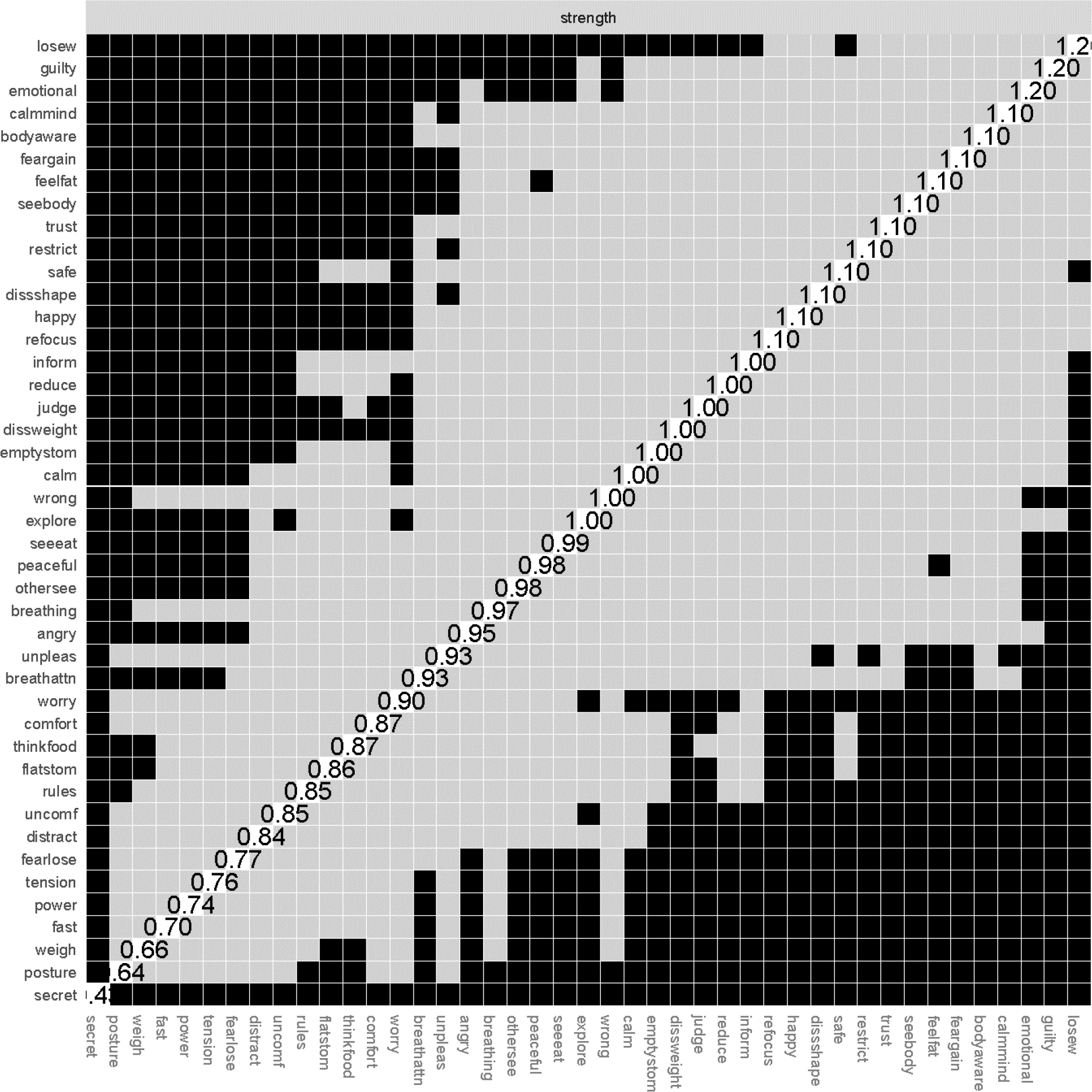
Strength centrality difference graph.
Symptoms are presented in descending order of strength. Values on the diagonal indicate unstandardized strength values. Black squares indicate statistically significant difference between nodes at p < .05.
Bridge Symptoms
Bridge strength stability was acceptable, but low (BS-coefficient = .36). Bridge EI was good (BEI-coefficient = .52). Due to low bridge strength stability, we chose to report and interpret bridge EI. The nodes with the highest bridge EI were all IA symptoms. The node with the highest bridge EI was the IA item, (not) feeling that one’s body is a safe place (safe, IA symptom, bridge EI = .20). It was most strongly associated with the following ED symptoms: overvaluation of weight (judge; part r = .06), and desire for a flat stomach (flatstom; part r = .06). Additional IA nodes with the highest bridge EI were (mis)trust in body sensations (trust; bridge EI = 13) and ignoring physical tension/discomfort until it becomes severe (tension; bridge EI = 12.). (Mis)trust in body sensations was most strongly associated with the following ED symptoms: shape dissatisfaction (dissshape; part r = .04) and distress about weighing (weigh; part r = .04). Ignoring physical tension/discomfort until it becomes severe was most strongly associated with the following ED symptoms: desire for an empty stomach (emptystom; part r = .05) and fear of losing control over eating (fearlose; part r = .04). These three top IA bridge symptoms were not significantly different from each other (ps > .05). Feeling that one’s body is a safe place had significantly higher bridge EI than 91% of other bridge nodes. (Mis)trust in body sensations had higher bridge EI than 47% of nodes, and ignoring physical tension/discomfort had higher bridge IE than 42% of nodes. See Figure 4 for the bridge EI centrality difference graph.
Figure 4.
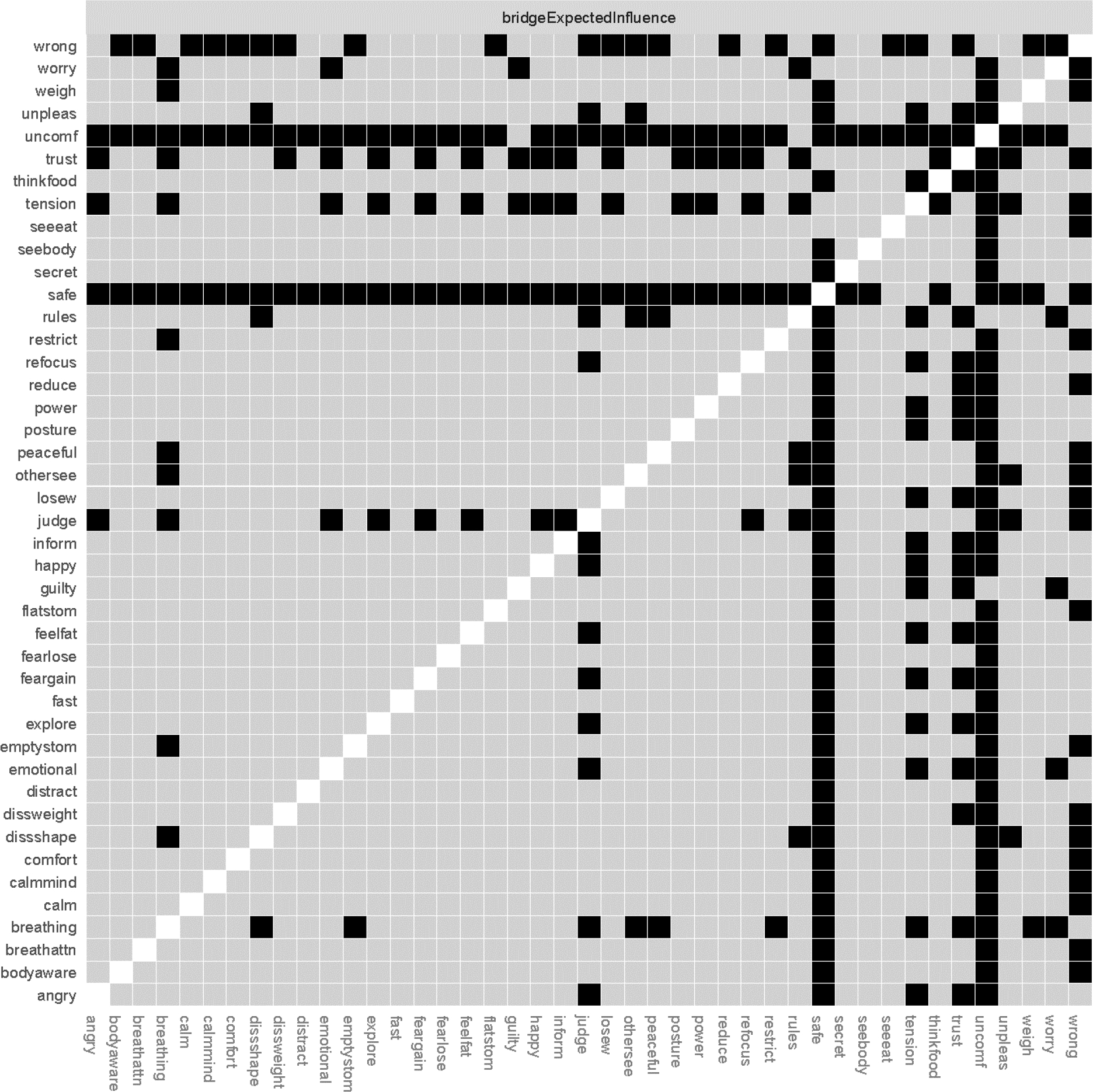
Bridge expected influence difference graph.
Black squares indicate statistically significant difference between nodes at p < .05.
The ED symptoms that were most strongly connected to the IA cluster were overvaluation of weight (judge, bridge EI = .10) and others seeing one’s shape (othersee; bridge EI = .10) Notably, these ED bridge symptoms were not significantly different from any other bridge symptoms, thus we did not interpret these bridge symptoms.
The NCT showed that the adult and adolescent networks were not different in overall structure (p = .32) or global strength (p = .81).
Predictive Validity of Central and Bridge Symptoms
Around one third of the sample (35.1%; n=110/313) met criteria for remission at discharge. Results from the logistic regression model of central symptoms (predictors: losew, guilty, emotional, trust, restrict) showed that stronger desire to lose weight at admission was associated with lower likelihood of achieving remission at discharge; no other central symptoms significantly predicted remission (see Table 2). Regarding the model of bridge symptoms (predictors: safe, trust, tension, judge, othersee), no symptoms predicted remission (see Table 2).
Table 2.
Central and Bridge Symptoms as Predictors of Remission Status at Treatment Discharge
| Remission Status at Discharge | ||||
|---|---|---|---|---|
| Model of Central Symptoms Predicting Discharge Remission Status | ||||
| Variable | OR | Wald X2 | p | 95% CI OR |
| Losew | 0.80 | 8.24 | .004 | [0.69, 0.99] |
| Guilty | 1.03 | 0.12 | .73 | [0.87, 1.22] |
| Emotional | 1.02 | 0.04 | .84 | [0.84, 1.13] |
| Trust | 1.03 | 0.11 | .74 | [0.86, 1.24] |
| Model of Bridge Symptoms Predicting Discharge Remission Status | ||||
| Variable | OR | Wald X2 | p | 95% CI OR |
| Safe | 1.04 | 0.11 | .74 | [0.81, 1.34] |
| Trust | 0.99 | 0.01 | .95 | [0.78, 1.27] |
| Tension | 0.95 | 0.46 | .50 | [0.81, 1.11] |
| Judge | 0.95 | 0.38 | .54 | [0.82, 1.11] |
| Othersee | 0.91 | 1.64 | .20 | [0.79, 1.05] |
Note. OR = Odds Ratio; Losew = strong desire to lose weight; Guilty = guilt after eating; Emotional= listening for information from the body about emotional state; Trust = trusting body sensations; Restrict = restricting amount of food eaten; Safe = feeling one’s body is a safe place; Tension = ignoring physical tension/discomfort; Judge = overvaluation of weight; Othersee = uncomfortable with others seeing shape.
Discussion
The present study used network analysis to identify how dimensions of IA are connected to ED symptoms and explored whether central and bridge symptoms predicted outcome at discharge from intensive treatment. The most central node in the IA-ED network was having a strong desire to lose weight. The primary symptom that connected IA and ED symptoms was (not) feeling one’s body is a safe place, from the MAIA Trusting subscale. This suggests that body (mis)trust may be the most relevant aspect of IA for understanding EDs, and that this construct may be important for understanding the overlap between IA and EDs. Network structure did not differ significantly between adolescents and adults. Importantly, the central symptom of having a strong desire to lose weight significantly predicted lower likelihood of remission at discharge, whereas bridge symptoms did not.
Results demonstrating the centrality of desire to lose weight are consistent with previous network analyses using the EDE-Q in clinical samples (Forrest et al., 2018; Levinson et al., 2017), and research demonstrating that weight and shape concerns are central to ED networks (DuBois et al., 2017; Forbush et al., 2016; Forrest et al., 2018; Forrest et al., 2019; Levinson et al., 2017; Vanzhula et al., 2019). These results also provide further support for the enhanced cognitive behavioral therapy (CBT-E) model of EDs (Fairburn, 2008) in a transdiagnostic sample of adults and adolescents seeking treatment at higher levels of care, given a primary aim is to disrupt overvaluation of weight and shape.
Bridge analyses revealed that IA was linked to ED symptoms through low levels of body trust. In particular, feeling unsafe in one’s body represented the strongest link between the clusters and was most strongly connected to weight and shape concerns (i.e., overvaluation of weight, desire for a flat stomach). The IA item (mis)trust in body sensations also demonstrated relatively strong bridge EI and was also most strongly connected to ED items related to weight and shape (i.e., shape dissatisfaction, distress about weighing). These results are consistent with previous research linking deficits in body trust and interoception to body image and drive for thinness (Brown et al., 2017; Denny, Loth, Eisenberg, & Neumark-Sztainer, 2013; Duffy, Rogers, Joiner, et al., 2019). Body (mis)trust characterizes EDs (Brown et al., 2017;Khalsa et al., 2015) as well as several other clinical conditions, including suicidality (Duffy, Rogers, Gallyer, & Joiner, 2019; Duffy, Rogers, & Joiner, 2018), panic disorder (Clark et al., 1997), complex trauma (Gene-Cos, Fisher, Ogden, & Cantrell, 2016), and somatoform disorders (Kalisvaart et al., 2012). However, based on our results, the presence of weight and shape concerns (e.g., overvaluation of weight and desire for a flat stomach) appears to distinguish EDs from other disorders characterized by low body trust. Indeed, individuals who overvalue weight in their self-evaluation, desire a flat stomach, and experience low body trust may be more likely to rely on external body standards and engage in body checking behaviors to ensure/reassure that they have not gained weight. However, if the perceptual information they receive about their body is distorted or inaccurate, this may exacerbate feeling unsafe in one’s body and lead to greater body monitoring and avoidance of food in an attempt to prevent disproportionate and unexpected weight gain.
Consistent with our previous research supporting the relevance of the MAIA Not Distracting subscale for ED symptoms (Brown et al., 2017), in addition to body trust, ignoring physical discomfort or tension until it becomes severe also demonstrated relatively strong bridge EI. This aspect of IA was most strongly connected to restraint (desire for an empty stomach) and eating concerns (fear of losing control). This finding is also consistent with previous research documenting elevated pain threshold and tolerance in EDs (de Zwaan, Biener, Schneider, & Stacher, 1996) and research linking pain insensitivity with ED psychopathology (Smith et al., 2013). Indeed, the ability to ignore physical sensations of discomfort may help maintain restrictive eating to avoid fullness or cognitive concerns about losing control. Alternatively, restriction may lead to greater ability to ignore physical discomfort over time.
Low body trust also may be influenced by valenced judgements of internal experiences. For example, research on other forms of psychopathology characterized by altered interoceptive processing (e.g., panic disorder) supports the common experience of interpretations/judgements of body signals as dangerous or aversive—a tendency termed anxiety sensitivity (Reiss et al., 1986). Although tentative, initial research also indicates elevated anxiety sensitivity in EDs (Anestis, Selby, Fink, & Joiner, 2007; Fulton et al., 2012; Thompson-Brenner, Boswell, Espel-Huynh, Brooks, & Lowe, 2018), suggesting that this construct may influence an individual’s self-reported bodily trust. For example, a patient may feel full, bloated, or notice their clothing tighten after eating and may (mis)interpret these sensations as indicating that they are becoming fat. This misinterpretation may occur, in part, due to believing that these body sensations are abnormal and/or dangerous, given their perceived association with weight gain. Importantly, the directionality of associations between an individual’s experience of an interoceptive sensation, their negative judgements of interoceptive sensations, and their level of body trust remains unclear. Future research is needed to gain a more complete understanding of these phenomena. Given documented comorbidities between gastrointestinal disorders and EDs, future research may also benefit from exploring whether body mistrust may connect these disorders.
Critically, our finding that desire to lose weight predicted lower likelihood of remission at discharge supports the posited role of central symptoms in network theory and adds to a growing literature focused on identifying symptoms with prognostic value using network analysis (Elliot et al., 2018; Olatunji et al., 2019; Olatunji et al., 2018; Rodebaugh et al., 2018). This result in a transdiagnostic sample aligns with previous network analytic research in AN (Elliot et al., 2018), where desire to lose weight was among the strongest predictors of recovery status and clinical impairment at 12-month follow-up from outpatient treatment. Given the limited and often inconsistent literature on predictors of ED treatment outcomes, it is notable that the predictive ability of desire to lose weight replicates across at least two independent samples. In contrast, bridge symptoms did not significantly predict remission status. However, this is consistent with network theory, which suggests that bridge symptoms should help explain co-occurrence between symptoms networks, rather than demonstrating predictive validity (Borsboom & Cramer, 2013; Borsboom, 2017). While results suggest that targeting bodily trust may not impact likelihood of remission, based on network theory, intervening on this pathway should theoretically disrupt the connections among IA and ED symptoms, thereby weakening the overall comorbidity network.
Although one might expect network relationships between IA and ED symptoms to be stronger in adults (e.g., given longer duration of illness), we did not find significant differences in network structure between adolescents and adults. The present study found that ED psychopathology was more severe in adults, and a previous study from our group found that adolescents scored significantly higher than adults on the MAIA Trusting subscale. However, mean differences in ED symptoms or IA dimensions across age groups does not necessarily reflect differences in the nature of the associations between these constructs. Thus, the relationship between IA and ED may not differ across adolescents and adults even if adults exhibit greater severity in certain IA dimensions and ED symptoms. Notably, given recent network analysis research demonstrating more tightly connected ED symptoms in older compared to younger individuals with ED (Christian et al., in press), future research should continue to compare networks across ages.
Clinical Implications
The current results support that desire to lose weight may be a salient ED treatment target and reinforce the importance of targeting weight/shape concerns. Cognitive-behavioral interventions (e.g., CBT-E) aim to target weight-focused cognitions and desires via cognitive (e.g., pie chart) and behavioral (e.g., reducing body checking and body avoidance) strategies. Additionally, recent evidence suggests that dissonance-based techniques focused on challenging social pressures to lose weight/be thin may be helpful in ED populations (Stice, Rohde, Butryn, Menke, & Marti, 2015); however, additional research is needed.
Results also suggest that targeting body mistrust may be helpful in improving body image in patients with EDs by disrupting the IA-ED network. In the anxiety field, mistrust of interoceptive signals has been targeted using interoceptive exposure. In recent years, researchers have begun to examine the efficacy of ED-specific interoceptive exposures (Boswell et al., 2019; Hildebrandt, Bacow, Greif, & Flores, 2014; Zucker et al., 2017), such as rapidly drinking water to simulate fullness/bloating or wearing tight clothing to elicit body image concerns. Initial research supports that interoceptive exposure may help improve ED symptoms and the tendency to perceive body signals as dangerous (Boswell et al., 2019; Thompson-Brenner et al., 2018), suggesting that this may be one way to challenge body mistrust.
Body mistrust and ignoring physical discomfort until severe could also be targeted through mindfulness-based approaches. In a prior study, IA was found to mediate the relationship between mindfulness and disordered eating behaviors in an at-risk non-clinical sample (Lattimore et al., 2017). Thus, mindfulness interventions focused on non-judgmentally noticing uncomfortable body sensations without acting on them or distracting (e.g., noticing fullness and the urge to purge without acting) may be useful. Further, mindfulness may increase awareness of hunger/fullness sensations (Kristeller & Hallett, 1999). However, it is unclear whether mindfulness may influence body trust, even if it does increase IA. Therefore, more research on this topic is needed.
Strengths & Limitations
The present study has notable strengths, including the large transdiagnostic clinical sample of adolescent and adult patients with EDs, implementation of methods to reduce network collinearity, examination of network and bridge stability, and examination of central and bridge symptom predictive. These strengths notwithstanding, there were also limitations. First, smaller sample sizes within specific diagnostic subgroups precluded examination of whether the IA-ED symptoms associations differed across ED diagnoses. Further, given differences in how diagnoses were assessed, inter-rater reliability was not established across diagnoses. Second, data for the present study came from a treatment-seeking sample that was predominately female, Caucasian, and non-Hispanic. Thus, results may not generalize to other demographic groups. Third, network analyses are inherently limited by the items included in the models. Although we used an empirical approach for item selection to help reduce item redundancy, this did result in the removal of certain IA and ED items, and items were not selected based on clinical usefulness or relevance, which may have influenced our results. Results may have differed if other items were or were not included. Additionally, objectively measurable mechanisms that may explain the link between “feeling unsafe in one’s body” and ED symptoms should be the focus of future studies.
Conclusions
The present study utilized network analysis to more precisely characterize associations between ED symptoms and multiple dimensions of IA. Results support that feeling unsafe in one’s body may be one factor that maintains associations between IA and ED symptoms and could represent an important focus for future research. Results further underscore the importance of weight and shape concerns in network models of eating pathology and suggest that targeting desire to lose weight may be helpful in promoting symptom remission across ED diagnoses. Future longitudinal research clarifying the nature of body mistrust in EDs will be essential to appropriately inform ED interventions that may target altered IA.
Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Preparation of this manuscript was supported by the Hilda and Preston Davis Foundation (Dr. Erin Reilly), National Institute of Mental Health (NIMH) (R01MH113588 and R21MH118409; Dr. Christina E. Wierenga), NIMH training grant (F32MH108311; Dr. Laura A. Berner). Portions of this work were presented at the International Conference on Eating Disorders in March 2019.
Research ethics committee approval: This study, “Outcomes of Eating Disorder Treatment” was approved by the University of California, San Diego Institutional Review Board.
Footnotes
To provide context for severity of the sample, ED symptoms were assessed using the full global EDE-Q score.
References
- Anestis MD, Selby EA, Fink EL, & Joiner TE (2007). The multifaceted role of distress tolerance in dysregulated eating behaviors. International Journal of Eating Disorders, 40, 718–726. doi: 10.1002/eat.20471 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-V (5th ed.). Washington, DC. [Google Scholar]
- Bardone-Cone AM, Harney MB, Maldonado CR, Lawson MA, Robinson DP, Smith R, & Tosh A (2010). Defining recovery from an eating disorder: Conceptualization, validation, and examination of psychosocial functioning and psychiatric comorbidity. Behaviour Research and Therapy, 48, 194–202. doi: 10.1016/j.brat.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KC, Peterson CB, Frazier P, & Crow SJ (2011). Convergence of scores on the interview and questionnaire versions of the Eating Disorder Examination: A meta-analytic review. Psychological Assessment, 23, 714. doi: 10.1037/a0023246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizeul C, Sadowsky N, & Rigaud D (2001). The prognostic value of initial EDI scores in anorexia nervosa patients: a prospective follow-up study of 5–-10 years. Eating Disorder Inventory. European Psychiatry, 16, 232–238. doi: 10.1016/S0924-9338(01)00570-3 [DOI] [PubMed] [Google Scholar]
- Borsboom D (2017). A network theory of mental disorders. World Psychiatry, 16, 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsboom D, & Cramer AO (2013). Network analysis: an integrative approach to the structure of psychopathology. Annual Review of Clinical Psychology, 9, 91–121. doi: 10.1146/annurev-clinpsy-050212-185608 [DOI] [PubMed] [Google Scholar]
- Boswell JF, Anderson LM, Oswald JM, Reilly EE, Gorrell S, & Anderson DA (2019). A preliminary naturalistic clinical case series study of the feasibility and impact of interoceptive exposure for eating disorders. Behaviour Research and Therapy, 117, 54–64. doi: 10.1016/j.brat.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA, Berner LA, Jones MD, Reilly EE, Cusack A, Anderson LK, … Wierenga CE (2017). Psychometric Evaluation and Norms for the Multidimensional Assessment of Interoceptive Awareness (MAIA) in a Clinical Eating Disorders Sample. European Eating Disorders Review, 25, 411–416. doi: 10.1002/erv.2532 [DOI] [PubMed] [Google Scholar]
- Bruch H (1962). Perceptual and conceptual disturbances in anorexia nervosa. Psychosomatic Medicine, 24, 187–194. doi: 10.1097/00006842-196203000-00009 [DOI] [PubMed] [Google Scholar]
- Carter JC, Blackmore E, Sutandar-Pinnock K, & Woodside DB (2004). Relapse in anorexia nervosa: a survival analysis. Psychological Medicine, 34, 671–679. doi: 10.1017/S0033291703001168 [DOI] [PubMed] [Google Scholar]
- Clark DM, Salkovskis PM, Öst L-G, Breitholtz E, Koehler KA, Westling BE, … Gelder M (1997). Misinterpretation of body sensations in panic disorder. Journal of Consulting and Clinical Psychology, 65, 203–213. doi: 10.1037/0022-006X.65.2.203 [DOI] [PubMed] [Google Scholar]
- Christian C, Perko VL, Vanzhula IA, Tregarthen JP, Forbush KT, & Levinson CA (in press). Eating disorder core symptoms and symptom pathways across developmental stages: A network analysis. Journal of Abnormal Psychology. [DOI] [PubMed] [Google Scholar]
- Cramer AO, Waldorp LJ, van der Maas HL, & Borsboom D (2010). Complex realities require complex theories: Refining and extending the network approach to mental disorders. Behavioral and Brain Sciences, 33, 178–193. doi: 10.1017/S0140525X10000920 [DOI] [Google Scholar]
- de Zwaan M, Biener D, Schneider C, & Stacher G (1996). Relationship between thresholds to thermally and to mechanically induced pain in patients with eating disorders and healthy subjects. Pain, 67, 511–512. [DOI] [PubMed] [Google Scholar]
- Denny KN, Loth K, Eisenberg ME, & Neumark-Sztainer D (2013). Intuitive eating in young adults. Who is doing it, and how is it related to disordered eating behaviors? Appetite, 60, 13–19. doi: 10.1016/j.appet.2012.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedenhofen B, & Musch J (2015). cocor: A comprehensive solution for the statistical comparison of correlations. PLoS One, 10, e0121945. doi: 10.1371/journal.pone.0121945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois RH, Rodgers RF, Franko DL, Eddy KT, & Thomas JJ (2017). A network analysis investigation of the cognitive-behavioral theory of eating disorders. Behaviour Research and Therapy, 97, 213–221. doi: 10.1016/j.brat.2017.08.004 [DOI] [PubMed] [Google Scholar]
- Duffy ME, Rogers ML, Gallyer AJ, & Joiner TE (2019). Body Trust and Agitation: Pathways to Suicidal Thoughts and Behaviors. Archives of Suicide Research, 1–15. [DOI] [PubMed] [Google Scholar]
- Duffy ME, Rogers ML, & Joiner TE (2018). Body trust as a moderator of the association between exercise dependence and suicidality. Comprehensive psychiatry, 85, 30–35. [DOI] [PubMed] [Google Scholar]
- Duffy ME, Rogers ML, Joiner TE, Bergen AW, Berrettini W, Bulik CM, … Fichter M (2019). An investigation of indirect effects of personality features on anorexia nervosa severity through interoceptive dysfunction in individuals with lifetime anorexia nervosa diagnoses. International Journal of Eating Disorders, 52, 200–205. [DOI] [PubMed] [Google Scholar]
- Elliot H, Jones PJ, & Schmidt U (2018). Central Symptoms Predict Post-Treatment Outcomes and Clinical Impairment in Anorexia Nervosa: A Network Analysis. PsyArXiv. [Google Scholar]
- Epskamp S, Borsboom D, & Fried EI (2018). Estimating psychological networks and their accuracy: A tutorial paper. Behavior Research Methods, 50, 195–212. doi: 10.31234/osf.io/hw2dz [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epskamp S, Cramer AO, Waldorp LJ, Schmittmann VD, & Borsboom D (2012). qgraph: Network visualizations of relationships in psychometric data. Journal of Statistical Software, 48, 1–18. doi: 10.18637/jss.v048.i04 [DOI] [Google Scholar]
- Epskamp S, & Fried EI (2018). A tutorial on regularized partial correlation networks. Psychological Methods, 23, 617–634. doi: 10.1037/met0000167 [DOI] [PubMed] [Google Scholar]
- Eshkevari E, Rieger E, Longo MR, Haggard P, & Treasure J (2012). Increased plasticity of the bodily self in eating disorders. Psychological Medicine, 42, 819–828. doi: 10.1017/S0033291711002091 [DOI] [PubMed] [Google Scholar]
- Fairburn CG (2008). Cognitive behavior therapy and eating disorders: Guilford Press. [Google Scholar]
- Fairburn CG, & Beglin SJ (1994). Assessment of eating disorders: Interview or self‐report questionnaire? International Journal of Eating Disorders, 16, 363–370. [PubMed] [Google Scholar]
- First MB, Williams J, & Spitzer RL (2015). Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). Arlington, VA: American Psychiatric Association, 2015. [Google Scholar]
- Forbush KT, Siew CS, & Vitevitch MS (2016). Application of network analysis to identify interactive systems of eating disorder psychopathology. Psychological Medicine, 46, 2667–2677. doi: 10.1017/S003329171600012X [DOI] [PubMed] [Google Scholar]
- Forrest LN, Jones PJ, Ortiz SN, & Smith AR (2018). Core psychopathology in anorexia nervosa and bulimia nervosa: A network analysis. International Journal of Eating Disorders, 51, 668–679.doi: 10.1002/eat.22871 [DOI] [PubMed] [Google Scholar]
- Forrest LN, Sarfan LD, Ortiz SN, Brown TA, & Smith AR (2019). Bridging eating disorder symptoms and trait anxiety in patients with eating disorders: A network approach. International Journal of Eating Disorders, 52, 701–711. doi: 10.1002/eat.23070 [DOI] [PubMed] [Google Scholar]
- Frank GK, Collier S, Shott ME, & O’reilly RC (2016). Prediction error and somatosensory insula activation in women recovered from anorexia nervosa. Journal of Psychiatry & Neuroscience, 41, 304–311. doi: 10.1503/jpn.150103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton JJ, Lavender JM, Tull MT, Klein AS, Muehlenkamp JJ, & Gratz KL (2012). The relationship between anxiety sensitivity and disordered eating: The mediating role of experiential avoidance. Eating Behaviors, 13, 166–169. doi: 10.1016/j.eatbeh.2011.12.003 [DOI] [PubMed] [Google Scholar]
- Garner DM (1991). Eating Disorder Inventory-2. Professional Manual. Odessa, FL: Psychological Assessment Research, Inc. [Google Scholar]
- Gene-Cos N, Fisher J, Ogden P, & Cantrell A (2016). Sensorimotor psychotherapy group therapy in the treatment of complex PTSD. Annals of Psychiatry and Mental Health, 4, 1080. [Google Scholar]
- Groothuis-Oudshoorn K, & Van Buuren S (2011). Mice: Multivariate imputation by chained equations in R. Journal of Statistical Software, 45, 1–67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- Heaner MK, & Walsh BT (2013). A history of the identification of the characteristic eating disturbances of Bulimia Nervosa, Binge Eating Disorder and Anorexia Nervosa. Appetite, 71, 445–448. doi: 10.1016/j.appet.2013.06.001 [DOI] [PubMed] [Google Scholar]
- Hildebrandt T, Bacow T, Greif R, & Flores A (2014). Exposure-Based Family Therapy (FBT-E): An open case series of a new treatment for anorexia nervosa. Cognitive and Behavioral Practice, 21, 470–484. doi: 10.1016/j.cbpra.2013.10.006 [DOI] [Google Scholar]
- Jones P (2017). networktools: Tools for identifying important nodes in networks. R package version 1.1.0. [Google Scholar]
- Jones PJ, Mair P, & McNally RJ (2018). Visualizing psychological networks: A tutorial in R. Frontiers in Psychology: Quantitative Psychology and Measurement, 9, 1742. doi: 10.3389/fpsyg.2018.01742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisvaart H, van Broeckhuysen S, Bühring M, Kool MB, van Dulmen S, & Geenen R (2012). Definition and structure of body-relatedness from the perspective of patients with severe somatoform disorder and their therapists. PLoS One, 7, e42534. doi: 10.1371/journal.pone.0042534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye WH, Fudge JL, & Paulus M (2009). New insights into symptoms and neurocircuit function of anorexia nervosa. Nature Reviews Neuroscience, 10, 573–584. doi: 10.1038/nrn2682 [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Craske MG, Li W, Vangala S, Strober M, & Feusner JD (2015). Altered interoceptive awareness in anorexia nervosa: Effects of meal anticipation, consumption and bodily arousal. International Journal of Eating Disorders, 48, 889–897. doi: 10.1002/eat.22387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Adolphs R, Cameron OG, Critchley HD, Davenport PW, Feinstein JS, … Mehling WE (2017). Interoception and Mental Health: a Roadmap. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristeller JL, & Hallett CB (1999). An exploratory study of a meditation-based intervention for binge eating disorder. Journal of Health Psychology, 4, 357–363. doi: 10.1177/135910539900400305 [DOI] [PubMed] [Google Scholar]
- Lattimore P, Mead B, Irwin L, Grice L, Carson R, & Malinowski P (2017). ‘I can’t accept that feeling’: Relationships between interoceptive awareness, mindfulness and eating disorder symptoms in females with, and at-risk of an eating disorder. Psychiatry Research, 247, 163–171. doi: 10.1016/j.psychres.2016.11.022 [DOI] [PubMed] [Google Scholar]
- Levinson CA, Brosof LC, Vanzhula I, Christian C, Jones P, Rodebaugh TL, … Weeks JW (2018). Social anxiety and eating disorder comorbidity and underlying vulnerabilities: Using network analysis to conceptualize comorbidity. International Journal of Eating Disorders, 51, 693–709. doi: 10.1002/eat.22890 [DOI] [PubMed] [Google Scholar]
- Levinson CA, Vanzhula I, & Brosof LC (2018). Longitudinal and personalized networks of eating disorder cognitions and behaviors: Targets for precision intervention a proof of concept study. International Journal of Eating Disorders, 51, 1233–1243. doi: 10.1002/eat.22952 [DOI] [PubMed] [Google Scholar]
- Levinson CA, Zerwas S, Calebs B, Forbush K, Kordy H, Watson H, … Peat C (2017). The core symptoms of bulimia nervosa, anxiety, and depression: A network analysis. Journal of Abnormal Psychology, 126, 340–354. doi: 10.1037/abn0000254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machorrinho J, Veiga G, Fernandes J, Mehling W, & Marmeleira J (2019). Multidimensional assessment of interoceptive awareness: Psychometric properties of the Portuguese version. Perceptual and Motor Skills, 126, 87–105. doi: 10.1177/0031512518813231 [DOI] [PubMed] [Google Scholar]
- McNally RJ (2016). Can network analysis transform psychopathology? Behaviour Research and Therapy, 86, 95–104. doi: 10.1016/j.brat.2016.06.006 [DOI] [PubMed] [Google Scholar]
- Mehling WE, Price C, Daubenmier JJ, Acree M, Bartmess E, & Stewart A (2012). The Multidimensional Assessment of Interoceptive Awareness (MAIA). PLoS One, 7, e48230. doi: 10.1371/journal.pone.0048230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merwin RM, Zucker NL, Lacy JL, & Elliott CA (2010). Interoceptive awareness in eating disorders: Distinguishing lack of clarity from non-acceptance of internal experience. Cognition and Emotion, 24, 892–902. doi: 10.1080/02699930902985845 [DOI] [Google Scholar]
- Mond JM, Hay PJ, Rodgers B, & Owen C (2006). Eating Disorder Examination Questionnaire (EDE-Q): norms for young adult women. Behaviour Research and Therapy, 44, 53–62. doi: 10.1016/j.brat.2004.12.003 [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Christian C, Brosof L, Tolin DF, & Levinson CA (2019). What is at the Core of OCD? A Network Analysis of Selected Obsessive-Compulsive Symptoms and Beliefs. Journal of Affective Disorders, 257, 45–54. doi: 10.1016/j.jad.2019.06.064 [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Levinson C, & Calebs B (2018). A network analysis of eating disorder symptoms and characteristics in an inpatient sample. Psychiatry Research, 262, 270–281. doi: 10.1016/j.psychres.2018.02.027 [DOI] [PubMed] [Google Scholar]
- Perez ME, Coley B, Crandall W, Di Lorenzo C, & Bravender T (2013). Effect of nutritional rehabilitation on gastric motility and somatization in adolescents with anorexia. Journal of Pediatrics, 163, 867–872. doi: 10.1016/j.jpeds.2013.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva G, Gaudio S, & Dakanalis A (2014). I’m in a virtual body: a locked allocentric memory may impair the experience of the body in both obesity and anorexia nervosa. Eating and Weight Disorders-Studies on Anorexia, Bulimia and Obesity, 19, 133–134. doi: 10.1007/s40519-013-0066-3 [DOI] [PubMed] [Google Scholar]
- Rodebaugh TL, Tonge NA, Piccirillo ML, Fried E, Horenstein A, Morrison AS, … Fernandez KC (2018). Does centrality in a cross-sectional network suggest intervention targets for social anxiety disorder? Journal of Consulting and Clinical Psychology, 86, 831–844. doi: 10.1037/ccp0000336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DM, Mizes JS, & Epstein EM (2005). Empirical classification of eating disorders. Eating Behaviors, 6, 53–62. doi: 10.1016/j.eatbeh.2004.06.002 [DOI] [PubMed] [Google Scholar]
- Smith AR, Fink EL, Anestis MD, Ribeiro JD, Gordon KH, Davis H, … Klein MH (2013). Exercise caution: over-exercise is associated with suicidality among individuals with disordered eating. Psychiatry research, 206(2–3), 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Rohde P, Butryn M, Menke KS, & Marti CN (2015). Randomized controlled pilot trial of a novel dissonance-based group treatment for eating disorders. Behaviour Research and Therapy, 65, 67–75. doi: 10.1016/j.brat.2014.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Brenner H, Boswell JF, Espel-Huynh H, Brooks G, & Lowe MR (2018). Implementation of transdiagnostic treatment for emotional disorders in residential eating disorder programs: A preliminary pre-post evaluation. Psychotherapy Research, 1–17. doi: 10.1080/10503307.2018.1446563 [DOI] [PubMed] [Google Scholar]
- van Borkulo C, Boschloo L, Borsboom D, Penninx BW, Waldorp LJ, & Schoevers RA (2015). Association of symptom network structure with the course of depression. JAMA Psychiatry, 72, 1219–1226. doi: 10.1001/jamapsychiatry.2015.2079 [DOI] [PubMed] [Google Scholar]
- Van Dyck Z, Schulz A, Blechert J, Herbert B, & Vögele C (2016). Gastric interoception and gastric myoelectrical activity in bulimia nervosa and binge eating disorder. European Health Psychologist, 18(S), 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanzhula IA, Calebs B, Fewell L, & Levinson CA (2019). Illness pathways between eating disorder and post‐traumatic stress disorder symptoms: Understanding comorbidity with network analysis. European Eating Disorders Review, 27, 147–160. doi: 10.1002/erv.2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker N, Mauro C, Craske M, Wagner HR, Datta N, Hopkins H, … Egger H (2017). Acceptance-based interoceptive exposure for young children with functional abdominal pain. Behaviour Research and Therapy, 97, 200–212. doi: 10.1016/j.brat.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]


