Abstract
Purpose of Review:
Sleep and obesity share a bidirectional relationship, and weight loss has been shown to enhance sleep. Aiming to extend sleep on its own or as part of a lifestyle intervention may attenuate health consequences of short sleep. This review highlights several sleep extension approaches, discusses feasibility of each, and summarizes findings relevant to obesity.
Recent Findings:
Sleep extension in response to experimental sleep restriction demonstrates partial rescue of cardiometabolic dysfunction in some but not all studies. Adequate sleep on a nightly basis may be necessary for optimal health. While, initial sleep extension interventions in habitually short sleepers have been met with obstacles, preliminary findings suggest that sleep extension or sleep hygiene interventions may improve glycemic control, decrease blood pressure, and enhance weight loss.
Summary:
Sleep extension has the potential to attenuate obesity risk and cardiometabolic dysfunction. There is tremendous opportunity for future research that establishes a minimum threshold for sleep extension effectiveness and addresses logistical barriers identified in seminal studies.
Keywords: insulin sensitivity, blood pressure, sleep recover, sleep extension, catch-up sleep, sleep hygiene
Introduction
Short sleep duration constitutes a strong relationship with obesity and affects a large proportion of adults [1, 2]. Experts recommend at least 7 hours of nightly sleep [3]. Yet, 35% of US adults consistently fall short of this target [2] and an overwhelming majority (~70%) report sleep deficits occurring monthly [4].
Short sleeping adults are 55% more likely to have obesity [5, 6], are more susceptible to weight gain over time [7, 8], and appear to accumulate greater visceral and ectopic fat [9]. Causation cannot be determine from observations alone and limited experimental studies make it difficult to ascertain whether sleep restriction specifically causes weight gain [10, 11]. Despite this controversy, a collection of short-term experimental studies (<2 weeks) align with the concept that insufficient sleep enhances obesity risk [11, 12]. Excessive energy intake is a likely cause [11–13] with heightened hunger [11], hormonal and neurological responses [11, 14, 15], and eating later in the day [16, 17] offering potential mechanisms that serve to further increase risk. Insufficient sleep is also associated with obesity co-morbidities, such as, hypertension [18], insulin resistance and diabetes [19, 20], dyslipidemia [21], and markers of inflammation [22]. Several experimental studies demonstrate diminished insulin sensitivity [11] and heightened inflammation with insufficient sleep [23]. As such, short sleep is well suited to perpetuate cardiometabolic dysfunction and promote chronic disease in obesity.
Sleep is more complex than duration alone. Timing, quality, and satisfaction are important components to healthy sleep [24, 25], and health consequences of irregular sleep have recently come to light [26–29]. Irregular sleep patterns may induce circadian desynchrony [30] and associate with obesogenic dietary patterns [31, 32]. The importance of sleep quality has also been demonstrated experimentally with selective suppression of slow wave sleep eliciting detrimental effects on glucose homeostasis [33]. Bidirectionally, obesity presents unique barriers to sleep quality. Obstructive sleep apnea (OSA), as an example, disproportionately affects people with obesity [34] and is prevalent in 86% of people with obesity and diabetes [35]. OSA not only diminishes sleep quality, it also elicits cardiometabolic dysfunction [36]. These findings underscore the complexity of the bidirectional relationship between sleep and obesity.
The sleep-obesity paradigm is indeed compelling; yet, little is known about how to address the consequences. Emerging literature points to the need for cross-discipline approaches. For example, secondary analyses from weight loss interventions suggest that optimal sleep (e.g. adequate sleep duration, regular sleep, and good sleep quality) promotes weight loss [37–41] while enhancing body composition [39, 41]. Reciprocally, indicators of sleep quality and duration seem to also improve with weight loss [42–44]. Here we offer our perspective on acute sleep recovery following sleep restriction and focus on lifestyle approaches targeting nighttime sleep extension, sleep hygiene, and combined therapies to prevent or attenuate obesity and cardiometabolic dysfunction (Figure 1).
Figure 1.
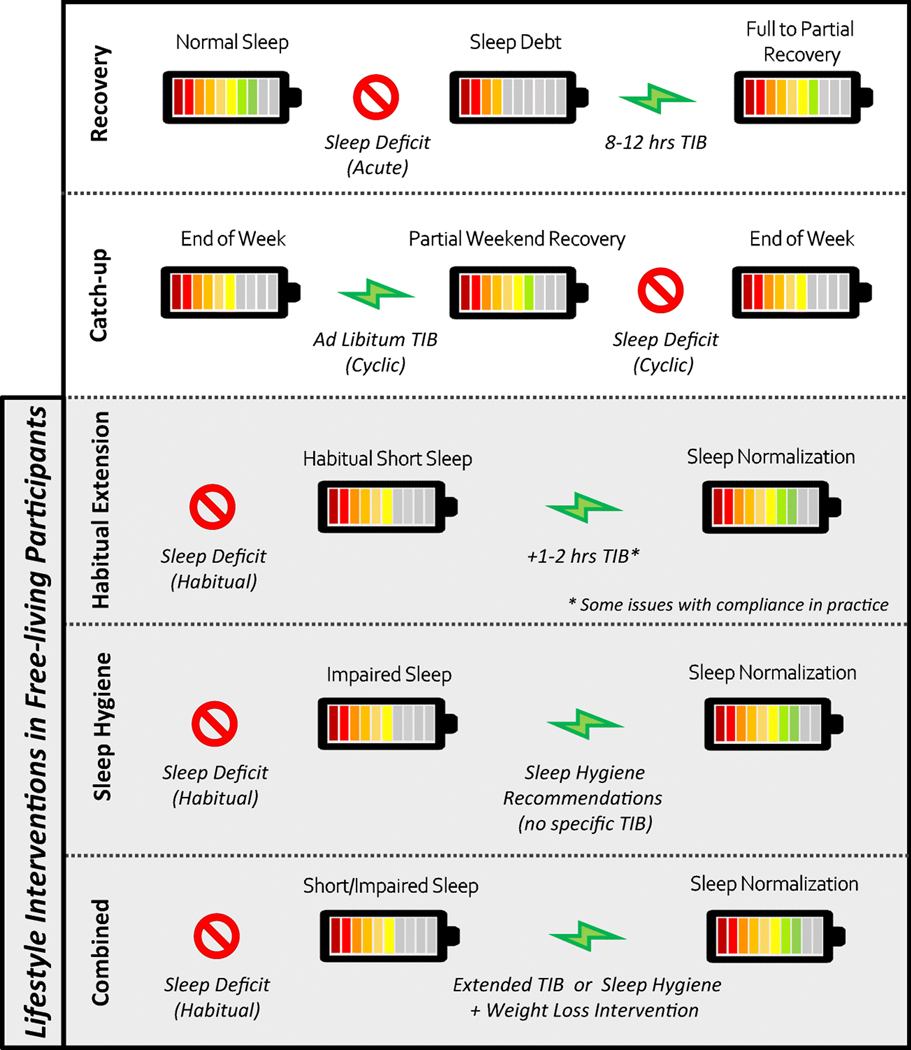
Description of sleep extension approaches following a sleep deficit
Sleep Recovery
Sleep recovery extends sleep in response to sleep restriction and is an intuitive countermeasure to alleviate accumulated sleep pressure. Several studies under review highlight the transient nature of cardiometabolic heath with night-time sleep recovery (8–12 hours of time spent in bed [TIB]) following acute sleep restriction (4–6 hours TIB).
Seminal work by Spiegel et al. [45, 46] attempted to fully restore sleep debt accumulated over 6 nights of sleep restriction (4 hours TIB) with 7 sleep recovery nights (12 hours TIB). At the end of the sleep recovery phase participants averaged about 9 hours of nightly sleep [45]. Sleep restriction impaired glycemic control assessed with a 2-hour intravenous glucose tolerance test (ivGTT); however, insults to glucose clearance, insulin independent glucose disposal, and the acute insulin response to glucose were attenuated with sleep recovery. Insulin sensitivity assessed using minimal-model analysis remained similar between the two sleeping conditions. The glycemic response at breakfast also decreased with sleep recovery, but lunch and dinner responses after recovery were comparable to meal responses during sleep restriction [45]. Bear in mind, it is unknown if sleep recovery fully restored glycemic control as sleep recovery was not compared to pre-sleep restriction values. A separate analysis of the same study included a normal sleeping comparison condition (8 hours TIB), and observed higher leptin and thyroid stimulating hormone (TSH) concentrations during sleep recovery compared to sleep restriction [46]. Leptin signals negative energy balance from adipose tissue, and commonly decreases with weight loss [47]. The concurrent decrease observed in leptin and TSH suggests an effect of sleep recovery on the hypothalamo-pituitary-adrenal axis that may influence energy balance through regulation of appetite and energy expenditure [47]. Interestingly, normal sleep appeared to have intermediate leptin, cortisol, and insulin resistance values between sleep restriction and sleep recovery [46], suggesting a potential dose-response to sleep duration. Another study coupled longer exposure to sleep restriction (3 weeks of 5.6 hours TIB) with forced circadian desynchrony (28.5-hour day) [48]. Three participants (n=21) met clinical criteria for pre-diabetes following sleep restriction and circadian desynchrony, but after 9 days of sleep recovery (10 hours TIB) no participants displayed a prediabetic glucose response to a meal. Post-prandial glucose and insulin levels returned to baseline values, but a sub-group of older participants showed a sustained elevation in peak glucose concentration. Other subclinical indicators of obesity risk showed only partial rescue with sleep recovery. For example, resting metabolic rate decreased by 8% with sleep restriction, and did not fully recover with 9 days of sleep extension. Elevated ghrelin, a signal for hunger, and depressed leptin across 24-hours persisted following recovery as well [48]. While these findings indicate potential benefits of a sleep extension countermeasure to acute sleep restriction, physiological disturbances persist and could be more pronounced in older people or those also experiencing circadian desynchrony.
Less ambitious sleep recovery approaches allowed 10–12 hours TIB across 2–3 nights or 8–9 hours TIB for up to 5 nights in the inpatient setting. Two studies allowed ad libitum intake and reported normalization of energy intake when switching from sleep restriction to sleep recovery [49, 50]. Only Markwald et al. observed a return to baseline body weight with 5 days of 9 hours TIB following an increase in body weight that occurred during sleep restriction (5 days, 5 hours TIB) [49]. This finding could be explained by the longer 5-day recovery period. It should also be noted that the 9-hour TIB condition was designed to be a normal sleeping condition. However, half of the group completed the 9-hour condition immediately following sleep restriction, and this sequential order could be interpreted as sleep recovery. Other studies performed under controlled dietary conditions point to weight-independent effects of sleep recovery on cardiometabolic outcomes. Broussard et al. [51] evaluated 2 nights of sleep recovery (12 hours TIB on the first night and 10 hours TIB on the second night) after 4 nights of sleep restriction (4.5 hours TIB). Participants averaged 9.7 hours of nightly recovery sleep. Glucose homeostasis was evaluated via ivGTT after normal, restricted, and recovered sleep. The acute insulin response to glucose was similar across the three conditions. Insulin sensitivity and the disposition index were impaired with sleep restriction, but returned to normal sleep values upon recovery. Shorter sleep recovery approaches (8–9 hours TIB) also have the potential to restore glucose homeostasis. Three nights of recovery sleep (8 hours TIB) normalized fasting insulin and fasting insulin resistance [52]. Slightly longer TIB (9 hours) across 3 days of sleep recovery indicated restoration of insulin sensitivity via an oral glucose tolerance test (OGTT). The same study, conversely, revealed persistent detriments to insulin sensitivity demonstrated by ivGTT performed 2 days later [53]. Other indicators of cardiometabolic risk may persist after sleep recovery as elevations in heart rate and inflammatory markers compared to baseline values have been reported [54]. In contrast, assessment of IL-6 across 24 hours demonstrated a return to baseline [55], and highlights discrepancies that may arise due to different assessment approaches. Additionally, salivary cortisol secretion patterns were influenced by altered bed and wake times during sleep restriction and recovery [55, 56], indicating potential circadian ramifications.
These studies differ by the number of sleep recovery nights and duration of TIB. Nevertheless, sleep recovery studies suggest that even partial sleep recovery may mitigate some, but not all, of the obesity and cardiometabolic risks from acute sleep restriction. An ideal degree of sleep recovery needed to counter acute sleep restriction needs to be established to inform evidenced-based recommendations.
Catch-up Sleep
Ongoing application of sleep recovery following repeated bouts of sleep restriction would likely result in a cyclic pattern of catch-up sleep. As a real-life example, catch-up sleep with extended TIB on the weekends could theoretically repay accumulated sleep debt acquired during weekdays [57]. Both inpatient and free-living studies indicate that 1–4 hours/day of additional time in bed on the weekends only partially repays total sleep debt [58–60] and may be more difficult to achieve in females [60]. Even hourly increases in weekend sleep are associated with lower BMI [71] and incremental increases in weekly sleep have also been connected with a greater reduction in fat mass during energy restriction [39, 61]. On the other hand, a cyclic pattern of sleep restriction and recovery would result in variable sleep which may actually be detrimental to outcomes related to weight loss, weight maintenance [72, 62], and cardiometabolic health [63, 64, 62]. As such, experimental studies are needed to determine causation.
Short-term evaluation of weekend catch-up sleep has been performed in normal sleepers in the inpatient setting. Depner et al. [60] allowed ad libitum food intake across 9 days of a sleep condition. One cycle of catch-up sleep was compared to continuous sleep restriction or normal sleep. During the catch-up sleep condition, participants underwent sleep restriction (5 hours TIB) across a 5-day ―work-week‖, followed by 2 days of ad libitum ―weekend‖ sleep, and then 2 additional days of sleep restriction to represent a return to work. Catch-up sleep over the weekend was not protective against weight gain as both sleeping groups gained a similar amount of body weight. This weight gain may be explained by increased energy intake noted during sleep restriction in both sleeping conditions. Consistent with other sleep restriction literature [65, 49], snacks were the primary source for increased energy intake. Importantly, weekend catch-up sleep appeared to reduce the tendency to snack throughout the week, and snacking over the weekend was unchanged from baseline snaking habits during catch-up sleep [60]. Despite no difference in body weight between continuous restriction and catch-up sleep over the short 9-day study, a reduction in snacking could lead to long-term obesity risk mitigation. Under an energy deficit in free living participants, Wang et al. [66] showed differential responses to 8 weeks of a weight loss intervention in normal sleepers compared to those assigned to weekend catch-up sleep. While weight loss was similar between groups, the weekend catch-up sleepers lost proportionally less fat mass compared to normal sleeping participants. Normal sleepers also experienced a decrease in respiratory quotient, suggesting a greater reliance on fat stores during energy restriction [66]. Even though the catch-up participants extended their sleep on the weekends, they still carried sleep debt throughout the study [69]. In agreement with Wang et al., an energy restricted inpatient study under constant sleep restriction reported a similar decrease in body weight with suboptimal alterations in body composition and fat utilization during weight loss under sleep restriction compared to normal sleep [67].
The potential benefits of catch-up sleep on cardiometabolic outcomes vary by study. In one example, men who habitually practiced weekend catch-up sleep experienced better metabolic function after continuing with their normal weekend sleep extension (10 hours TIB) compared to undergoing continuous sleep restriction (6 hours TIB) [58]. This was characterized by decreased fasting insulin, C-peptide, and insulin resistance (HOMA-IR) along with enhanced insulin sensitivity determined via a 2-hour OGTT. Glucose area under the curve was unchanged between catch-up sleep and continuous restriction for the entire cohort, but a sub-analysis of only young men in the study (<35 years) showed a decrease following weekend catch-up sleep [58]. Unlike Spiegel et al. who reported an increase in mean 24-hour leptin during sleep recovery in habitually normal sleepers [46], Killick et al. observed a decrease in fasting leptin in habitually sleep-restricted participants. The disagreement between studies may reflect differential responses to sleep extension based on an individual’s habitual sleep pattern [68]. For example, 3 days of treatment with continuous positive airway pressure in patients with obstructive sleep apnea, which would improve sleep quality, also decreased leptin [69]. Additionally, PYY, an appetite hormone signaling fullness, was increased with catch-up sleep, and ghrelin, signaling hunger, was unchanged [58]. Others suggest that benefits to glycemic control may be short-lived and that alterations in circadian rhythm may introduce metabolic dysfunction. In the previously discussed study by Depner et al. [60], whole body insulin sensitivity was diminished during both consistent sleep restriction (13%) and catch-up sleep (27%) using the hyperinsulinemic-euglycemic clamp method compared to a normal sleeping control. Upon adjusting for body weight, the insult to whole body insulin sensitivity only remained with continuous sleep restriction. However, tissue-specific blunting of insulin sensitivity at the level of the liver and muscle occurred only with catch-up sleep [60]. This could be attributed to altered circadian rhythm due to variable sleeping patterns [30]. In agreement with this theory, catch-up sleepers in Depner et al. self-selected later bedtimes when allowed ad libitum sleep despite having just experienced a week of sleep restriction [60]. This behavior was consistent with observed delays in dim light melatonin onset and offset [60]. Another study [59] assessed catch-up sleep in free-living habitual short sleepers (<6 hours average weekday sleep). Blood pressure was the only cardiometabolic outcome reported, and there was no difference between habitual sleep restriction and extended weekend sleep [59]. These limited studies reveal a potential glycemic benefit of weekend catch-up sleep that could be overshadowed by damaging consequences of circadian desynchrony induced by variable sleeping patterns [70].
As such, these findings support the notion that some sleep extension, even if inconsistent, may be better than no sleep extension at all. Nonetheless, the long-term safety of cyclic sleep restriction and recovery is questionable. Continued work should rigorously evaluate the effects of catch-up sleep in short sleepers and focus specifically on how cyclic sleep extension may influence cardiometabolic health in short sleepers with obesity over time.
Ongoing Sleep Extension
Acute sleep extension following bouts of sleep restriction may not reflect physiological adaptations to habitual sleep extension. Ongoing sleep extension (Figure 1), as an intervention to habitual short sleep, is an emerging area of research with clinical and public health implications. At this time, the literature consists of primarily short-term pilot studies evaluating the feasibility of sleep extension or hygiene as either stand-alone treatments or in combination with weight loss interventions (Table 1).
Table 1.
Ongoing sleep extension in free living participants
| Study | Design | Participants | Intervention | Sleep | Cardiometabolic Outcome* |
|---|---|---|---|---|---|
| Reynold AM, 2014 [71] | Parallel Arm N= 14; 6 control | Adults (6–9 h nightly sleep) | Sleep target: +3 h TIB Control: Habitual sleep Duration: 1 week | Baseline TIB: 7 h 56 min TST: 6 h 47 min Achieved TIB: +2 h 7 min TST: +1 h 59 min | ∅ SBP ∅ DBP ∅ Heart rate ∅ TNF-α ∅ Adiponectin ∅ CRP ↑ IL-6 |
| Stock AA, 2020 [72] | Single Arm N=53 | College students (6–8 h nightly sleep) | Sleep target: + 1 h TIB Control: Baseline Duration: 1 week | Baseline TIB: 6–8 h TST: 7 h 19 min Achieved TST: + 43 min | ↓SBP# ∅ DBP# ∅ Heart rate# |
| Al Khatib HK, 2018 [73] | Parallel Arm N=42; 21 control | Short sleepers (5–7 h nightly sleep) | Sleep target: +1–1.5 h TIB Control: Habitual Sleep Duration: 4 weeks | Baseline TIB: 7 h 3 min TST: 5 h 28 min Achieved TIB: +55 min TST: +21 min | ↓ Free sugar intake ∅ Fasting ghrelin ∅ Fasting leptin ∅ Glucose ∅ Insulin ∅ Blood lipids ∅ BP ∅ Body weight ∅ Body composition ∅ Energy expenditure |
| Tasali E, 2014 [74] | Single Arm N=10 | Adults with overweight BMI and short sleep (< 6.5 h nightly sleep) | Sleep target: 8.5 h TIB via individualized behavioral counseling and sleep hygiene recommendations Control: Baseline Duration: 2 weeks | Baseline TIB:6 h 24 min TST: 5 h 36 min Achieved TIB:+1h48 min TST: +1 hr 36 min | ↓ Overall appetite# ↓ Desire for sweet and salty foods# ∅ Desire for fruits, vegetables, and protein- rich food# |
| Leproult R, 2015 [75] | Single Arm N=16 | Adults (≤ 7 h nightly sleep) | Sleep target: +1 h TIB and sleep hygiene recommendations Control: Baseline Duration: 6 weeks | Baseline TST: 5 h 59 min Achieved TST: +44 min | ∅ Body weight#∅ Glucose#∅ Insulin#∅ Insulin sensitivity# (Positive associations between TST and glycemic control) |
| So-Ngern A, 2019 [76] | Cross-Over N=21 | Short sleepers (≤ 6 h nightly sleep) | Sleep target: + 1 h TIB and sleep hygiene recommendations Control: Habitual sleep and sleep hygienerecommendations Duration: 2 weeks | Baseline TST: 5 h 18 mins Achieved TST: +36 mins | Intention to Treat ∅ Body weight ∅ Self-selected diet ∅ Glucose ∅ Insulin resistance Per-protocol ↓ Insulin resistance ↑ Early insulin secretion ↑ β-cell function |
| Haack M, 2013 [77] | Parallel Arm N=22; 9 control | Short sleepers with elevated blood pressure (< 7 h nightly sleep or >1 h less than self-perceived ideal sleep) | Sleep target: +1 h TIB and sleep hygiene recommendations Control: Habitual sleep and sleep hygiene recommendations Duration: 6 weeks | Baseline TST: 6 h 18 mins Achieved TST: +35 mins (+4 min of TST in control) | ∅ Body weight ∅ Body composition ∅ Self-selected diet ∅ SBP ∅ DBP ∅ Inflammatory markers |
| Cizza G, 2014 [79]; Lucassen EA, 2014 [80] | Observational N=125 | Short sleepers with obesity (< 6.5 h nightly sleep) | Sleep target: Analysis of participants awaiting randomization into sleep extension or habitual sleep arm Duration: 81 days median observation time | Baseline TST: 5 h 44 min Achieved TST: + 14 min | ∅ Body weight#↓ Waist circumference# ↓ Glucose#↓ Insulin#↑ Insulin sensitivity#↓ Total cholesterol#↓ LDL cholesterol#↓ HDL cholesterol#↓ Triglycerides#∅ Fasting ghrelin# |
| Baron Kelly G, 2019 [81] | Parallel Arm N=16; 5 control | Short sleepers with elevated blood pressure (<7 h nightly sleep) | Sleep target: Up to 8 h TIB or +1 h TIB via technology supported sleep hygiene education and coaching Control: Habitual Sleep Duration: 6 weeks | Baseline TIB: 7 h 2 min TST: 6 h 7 min Achieved TIB: +34 min TST: +34 min | ↓ 24-h SBP ↓ 24-h DBP |
| McGrath ER, 2017 [82] | Parallel Arm N=134; 67 Control) | Impaired sleepers with elevated blood pressure ( >30 min to fall asleep and waking >1 nightly awakening) | Sleep target: Sleep hygiene (education and CBT) and vascular risk education Control: Vascular risk education Duration: 6–8 weeks | Baseline TST: 6 h 56 min Achieved TST: No Change ↑ Sleep Quality | ∅ 24-h SBP ∅ 24-h DBP |
| Sawamoto R, 2016 [89] | Single Arm N=90 | Women with elevated BMI (sleep duration eligibility not specified) | Diet & Sleep Target: Diet (−500 kcal/day) and exercise (>8,000 steps/day) lifestyle program with CBT for insomnia Control: Baseline Duration: 7 months | Baseline TST: 5 h 32 min Achieved TST: +14 min (trend) | ↓ Body weight#↓ Body fat#↓ Waist circumference# ↓ Hip circumference# ↑ Adiponectin# |
| Logue EE, 2012 [90] | Parallel Arm N=46;23 control | Adults with elevated BMI (sleep duration eligibility not specified) | Diet & Sleep Target: Diet and exercise CBT via group sessions with sleep education and hygiene recommendations 4 weeks into program. Control: Diet and exercise CBT via group sessions Duration: 12 weeks | Baseline Sleep Efficiency: 85% Achieved Sleep Efficiency: 93% (Change similar to control) | ↑ Weight loss |
| Demos KE, 2015 [91] | Parallel Arm N=25; 13 control | Adults with elevated BMI (≤7 h nightly sleep) | Diet & Sleep Target: 8 h TIB with eating at least every 4 hours across 3–5 meals/day then initiation of energy and dietary fat restricted diet (1,200–1,500 kcal/day) Control: 4 weeks of diet and exercise education then initiation of energy and dietary fat restricted diet (1,200–1,500 kcal/day) Duration: 4 weeks of sleep intervention or general diet education prior to 18 weeks of a weight loss intervention | Baseline TST: 6 h 48 min Achieved TST: ∅ (Objectively TST: +37 min (Self-reported) | ↓ Weight loss (6 weeks) ∅ Weight loss (18 weeks) |
| MorenoFrais C, 2020 [92] | Parallel Arm N=52 (27 control) | Adolescents with obesity (Sleep criteria not specified) | Diet & Sleep Target: +1 h TIB and −500 kcal/day Control: −500 kcal/day Duration: 4 weeks | Baseline TIB: 7 h 48 min TST: 7 h 36 min Achieved TIB: 8 h 54 min TST: 8 h 42 min (+30 min in control group) | Sleep Group: ↓ Body weight#↓ Waist circumference# ↓ Energy intake ∅ Glucose#↓ Insulin#∅ Insulin resistance#∅ HDL cholesterol#∅ Non-HDL cholesterol# ∅ Triglycerides#∅ Leptin#↓ IL=6#∅ TNF-a#∅ Cortisol#Compared to Control: ↓ Body weight ↓ Waist circumference |
Hour (h); Minute (min); Time in bed (TIB); Total sleep time (TST); Cognitive behavioral therapy (CBT); Systolic blood pressure (SBP); Diastolic blood pressure (DBP); Not reported (NR); Kilocalorie (kcal)
Comparing sleep target to control as defined in intervention column unless otherwise specified by #
Indicates changes from baseline
Extension of Habitual Sleep
Weeklong sleep extension interventions aiming for 1–3 hours of additional time in bed in normal and short sleeping adults demonstrate the feasibility of short-term sleep extension. Reynold et al. [71] asked participants to spend 3 additional hours in bed each night. Despite falling short of this request, participants extended TIB by 2 hours 7 minutes and achieved just under 2 hours of actual sleep extension. Blood pressure, heart rate, and inflammation were similar in the sleep extension (n=8) group compared to their baseline values and were no different than the habitual sleeping controls (n=6). The authors also noted large effect sizes in inflammatory markers indicating potentially negative consequences of sleep extension. This interpretation, however, should be viewed cautiously as baseline sleep duration ranged widely (6 to 9 hours) and the sample was quite small. Only an hour of additional TIB was required by Stock et al. [72]. Participants (n=53) who previously slept between 6 to 8 hours a night increased average sleep duration by 43 minutes. Systolic blood pressure decreased by 7 mmHg while diastolic blood pressure and heart rate remained unchanged. No other cardiometabolic outcomes were assessed which limits clinical interpretation. The majority of the participants (83%) reported some degree of extended sleep, while a smaller proportion (66%) attained at least 30 minutes of nightly sleep. Importantly, 93% of habitual short sleepers (< 7 hours) at baseline (n=14) achieved optimal sleep duration [72]. These initial studies indicate that moderate habitual sleep extension (1–2 hours TIB) is possible and holds potential to improve blood pressure.
Interventions recommending at least an hour of extended TIB and lasting 2 to 6 weeks have been performed in habitually short sleepers [73–77], but there appears to be obstacles related to compliance. Tasali et al. [74] achieved an impressive 1.6 hours of nightly sleep extension across a 2-week intervention via individual counseling that focused on extending total sleep duration. A longer study lasting 4 weeks only averaged 21 minutes of sleep extension and less than 10% of the cohort reached 7 hours of nightly sleep with a target TIB extension of 1 to 1.5 hours [73]. Study duration may have played a role in compliance as sleep extension may decrease over the course of an intervention [75]. In another small (n=21) 2-week study, only 8 of the participants achieved 6 or more hours of total nightly sleep [76]. Indeed, compliance presents a potential barrier to habitual sleep extension. The shortest sleepers require the greatest degree of sleep extension to normalize sleep duration, and baseline sleep quality may influence an individual’s capacity to successfully extend sleep [76]. Furthermore, dedicating time to sleep may conflict with daily obligations necessitating short sleep to begin with [78]. Sleep research may also be especially vulnerable to observational effects. For example, Cizza et al. [79] noted a significant increase in sleep duration (14 minutes) in participants waiting to be randomized into either a sleep extension or control group. Echoing this, the control group from Haack et al. [77] experienced a small increase in sleep (4 minutes).
Preliminary findings are mixed related to obesity risk. Tasali et al. [74] reported decreases in overall appetite with a diminished desire for sweet and salty foods–key characteristics of junk food. In support of this, Al Khatib et al. [73] observed a decrease in the intake of sugar while fasting ghrelin and leptin concentrations were unchanged. Other studies reported no effect of sleep extension on diet selection [77, 76], fasting ghrelin [80], or fasting leptin [73]. Changes in body weight [73, 75–77], body composition [77, 73], and energy expenditure [73] have also gone undetected in a handful of studies; albeit, no studies have utilized gold standard techniques with sensitivity to detect small changes in body composition or energy balance.
Cardiometabolic findings from Cizza et al. [79] are perhaps the most intriguing as unintentional sleep extension occurred (14 min) between screening and randomization (81 days median) in habitually short sleeping participants with obesity. Despite the intervention having not yet started, concurrent improvements in glucose (−7%), insulin (−26%), insulin sensitivity (8%), and triglycerides (−10%) along with modest decreases in waist circumference and total cholesterol concentrations occurred independent of weight loss. Others have demonstrated improvements in blood pressure. Ambulatory blood pressure assessed across 24-hours by Haack et al. [77] in hypertensive and pre-hypertensive participants revealed a clinically significant drop in systolic (14 mmHg) and diastolic blood pressures (8 mmHg) from baseline. In the same study [77], no changes were noted for inflammatory markers assessed at a single time point. Decreases in systolic and diastolic blood pressures (~7%, all) have also been noted following a technology-supported sleep extension intervention in a small pilot (n=16) of hypertensive participants who extended their sleep an average of 34 minutes [81]. A discernable increase in sleep duration may be required to influence blood pressure as a sleep hygiene only intervention in those with elevated blood pressure did not extend sleep duration and did not observe a change in 24-hour blood pressure [82]. Several studies in lean and healthy participants report no change in blood pressure [73] or fasting values for glucose [75, 73, 76], insulin [75, 73], or blood lipid concentrations with sleep extension. It is possible that clinical benefits may require a minimum threshold of sleep duration. For example, a cross-over study by So-Ngern et al. [76] reported a 17% reduction in insulin resistance in only those attaining at least 6 hours of sleep. Likewise, Leproult et al. [75] reported no change in fasting glucose or insulin concentrations but correlations uncovered favorable associations between relative sleep extension and change in fasting insulin (r=−0.6), insulin resistance (r=−0.5), and insulin sensitivity (r=0.76). Given the connection between cardiometabolic disease and short sleep, sleep extension may be a promising therapeutic in at-risk populations that are able to successfully extend sleep duration.
Combined Sleep and Weight Loss Approaches
Behavioral interventions support the efficacy of combined lifestyle approaches incorporating diet, exercise, and stress management techniques [83]. Poor sleep has been shown to promote fatigue and depressive symptoms [84] while hindering impulse control [85] and enhancing appetite [65, 86]. Therefore, it is reasonable to theorize that the inclusion of sleep hygiene or sleep extension tactics within a weight loss study could enhance compliance and promote health benefits.
While rarely discussed, program curricula from landmark lifestyle interventions incorporated sleep-related learning objectives [87, 88]. These pioneering programs recognized sleep as an important aspect to achieve weight loss targets of ≥7%. There have been many iterations of these programs, and Sawamoto et al. [89] is the only current example to emphasize sleep. Their 7-month program employed cognitive behavioral therapy (CBT) for insomnia but did not detail specific sleep extension recommendations. There was a trend for increased nightly sleep duration (14 minutes; p=0.070), but this trend is comparable to increases in sleep duration observed during traditional weight loss regimens [42] and it is similar to the small degree of spontaneous sleep extension noted by Cizza et al. [79]. Baseline sleep was very low—averaging just 5.5 hours/night, and even a small increase in sleep would not reach expert recommendations (≥ 7 hours/night). Participants lost 15% of their initial body weight, while experiencing an improvement in body composition and increases in adiponectin, a cardioprotective adipokine that increases with weight loss. Multiple regression analysis revealed a positive effect on change in sleep and adiponectin concentrations [89]. This supports the notion that sleep extension may play a role in beneficial alterations to body composition during weight loss.
Interventions employing a control group undergoing only a weight loss intervention are emerging in the literature and offer insight regarding the potential utility of sleep extension during lifestyle interventions. Logue et al. [90] designed a 12-week CBT program (n= 46), during which two intervention arms were provided group counseling to enhance diet, physical activity, and coping. One group (n=23) was assigned to also incorporate sleep hygiene after 4 weeks of the CBT intervention. There were no predetermined targets for energy intake or sleep duration. At 12 weeks there was a greater decline in body weight in the group partaking in sleep counseling (5%) compared to CBT only (2%). Improved coping was reported by the sleep group and may have aided compliance to diet and physical activity goals. Demos et al. [91] designated a prerequisite 4 weeks to improve sleep (8 hrs TIB) and eating regularity prior to initiating an 18-week energy restriction diet. Only 60% of the participants were compliant with their sleep schedule at 4 weeks and there was no objective increase in nightly sleep duration. Unexpectedly, participants assigned to sleep extension experienced less weight loss after 6 weeks of dieting compared to a habitual sleeping control. This difference in weight loss was no longer apparent at 18 weeks [91]. Only one study included simultaneous energy restriction and sleep extension targets [92], during which, adolescents with obesity underwent energy restriction for 4 weeks. One group was asked to maintain habitual sleep and the other was asked to extend weekday sleep by 1 hour/night. The habitual sleeping control extended their sleep by 0.5 hours/night and the sleep extension group extended sleep by 1.2 hours/night. Both groups lost a small amount of body weight amounting to 1% and 2%, respectively. The sleep extension group also experienced decreased waist circumference, fasting insulin, and inflammation. Statistical differences favoring sleep extension were observed for changes in body weight and waist circumference, but change across 4 weeks was similar between groups for all other outcomes [92]. Taken together, the effect of sleep extension in people concurrently engaging in dietary interventions is unclear and early studies question practicality. Continued research should determine whether sleep extension has an additive benefit to obesity treatment, and next steps should explore ways to make such interventions manageable to the general population.
Conclusions
Studies discussed in this review show promise for different sleep extension approaches to counter acute sleep restriction, improve cardiometabolic health in habitually short sleepers, or complement weight loss interventions. Nonetheless, evidence backing sleep as an obesity treatment target remains sparse–leaving interpretations incomplete. While examples of acute sleep extension conducted in highly controlled inpatient settings are important and clearly demonstrate a physiological effect, the application of sleep extension as a viable, scalable, and long-term intervention remains undetermined. Evidence in favor of sleep extension in free living participants are often from pilot studies and subject to small sample sizes, rudimentary measurements, and some compliance issues. Nonetheless, preliminary findings are encouraging and demonstrate an effect of free-living sleep extension on obesity and cardiometabolic risk factors. Continued investigation via randomized clinical trials should primarily focus on short sleeping individuals, especially, those with obesity, hypertension, or poor glycemic control. There is tremendous opportunity for researchers to embrace cross-discipline collaborations to examine sleep extension across the spectrum of translational science. Precise sleep extension targets must be determined to elicit a health benefit, and special attention must be paid to the complexity of sleep extension in regards to participant selection and compliance. Research pairing energy restriction with sleep extension to enhance weight loss needs to establish whether the burden of modifying sleep offers a worthwhile advantage for obesity treatment. A clear understanding of the health benefits and behavior considerations of sleep extension stand to inform future public health recommendations and practitioner decision making.
Acknowledgments
Funding
This research was supported in part by National Institute of Health grants F31HL151232 (KSP), P20GM109036 (LAB) and R01DK091718 (LAB), and U54GM104940 (JPK).
Footnotes
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Kristin K. Hoddy, Kaitlin S. Potts, Lydia A. Bazzano, and John P. Kirwan each declare no potential conflicts of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES:
- 1.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Medicine. 2004;1:210–7. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of Healthy Sleep Duration among Adults — United States, 2014. MMWR Morbidity and mortality weekly report. 2016;65:137–41. doi: 10.15585/mmwr.mm6506a1. [DOI] [PubMed] [Google Scholar]
- 3.Consensus Conference Panel, Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM et al. Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion. Sleep. 2015;38:1161–83. doi: 10.5665/sleep.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Perceived Insufficient Rest or Sleep Among Adults—United States, 2008. In: Morbidity and Mortality Weekly Report. 2008. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5842a2.htm. Accessed 12/26/2019 2019. [PubMed] [Google Scholar]
- 5.Ford ES, Li C, Wheaton AG, Chapman DP, Perry GS, Croft JB. Sleep duration and body mass index and waist circumference among Us adults. Obesity. 2014;22:598–607. doi: 10.1002/oby.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jean-Louis G, Williams NJ, Sarpong D, Pandey A, Youngstedt S, Zizi F et al. Associations between inadequate sleep and obesity in the US adult population: Analysis of the national health interview survey (1977–2009). BMC public health. 2014;14:290. doi: 10.1186/1471-2458-14-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaput J-P, Després J-P, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep. 2008;31:517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasler G, Buysse DJ, Klaghofer R, Gamma A, Ajdacic V, Eich D et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661–6. [DOI] [PubMed] [Google Scholar]
- 9.Chaput JP, Bouchard C, Tremblay A. Change in sleep duration and visceral fat accumulation over 6 years in adults. Obesity (Silver Spring, Md). 2014;22(5):E9–12. doi: 10.1002/oby.20701. [DOI] [PubMed] [Google Scholar]
- 10.•.Yu H, Lu J, Jia P, Liu C, Cheng J. Experimental sleep restriction effect on adult body weight: a meta-analysis. Sleep and Breathing. 2019;23(4):1341–50. doi: 10.1007/s11325-019-01828-0.A meta-analysis including six randomized controlled trials reporting body weight outcomes during sleep restriction compared to normal sleep. No difference in body weight or composition were found; however, sex specific differences may exist.
- 11.•.Zhu B, Shi C, Park CG, Zhao X, Reutrakul S. Effects of sleep restriction on metabolism-related parameters in healthy adults: A comprehensive review and meta-analysis of randomized controlled trials. Sleep Medicine Reviews. 2019;45:18–30. doi: 10.1016/j.smrv.2019.02.002.A meta-analysis of 41 randomized controlled trials showing increased body weight, hunger, and brain activity related to food stimuli were increased along with a decrease in insulin sensitivity during sleep restriction.
- 12.•.Al Khatib HK, Harding SV, Darzi J, Pot GK. The effects of partial sleep deprivation on energy balance: a systematic review and meta-analysis. European Journal of Clinical Nutrition. 2017;71(5):614–24. doi: 10.1038/ejcn.2016.201.A meta-analysis of 11 randomized controlled trials reporting that the positive energy balance observed during sleep restriction likely comes from an excessive increase in energy intake.
- 13.Shechter A, O’Keeffe M, Roberts AL, Zammit GK, RoyChoudhury A, St-Onge M-P. Alterations in sleep architecture in response to experimental sleep curtailment are associated with signs of positive energy balance. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2012;303(9):R883–R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.•.Bhutani S, Howard JD, Reynolds R, Zee PC, Gottfried J, Kahnt T. Olfactory connectivity mediates sleep-dependent food choices in humans. Elife. 2019;8. doi: 10.7554/eLife.49053.This study provides human evidence supporting that increased energy intake following sleep restriction is influenced by food-based decision making via effects of the the endocannabinoid system.
- 15.St-Onge MP, Wolfe S, Sy M, Shechter A, Hirsch J. Sleep restriction increases the neuronal response to unhealthy food in normal-weight individuals. International journal of obesity (2005). 2014;38(3):411–6. doi: 10.1038/ijo.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spaeth AM, Dinges DF, Goel N. Effects of Experimental Sleep Restriction on Weight Gain, Caloric Intake, and Meal Timing in Healthy Adults. Sleep. 2013;36(7):981–90. doi: 10.5665/sleep.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baron KG, Reid KJ, Kern AS, Zee PC. Role of Sleep Timing in Caloric Intake and BMI. Obesity. 2011;19(7):1374–81. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Mei H, Jiang YR, Sun WQ, Song YJ, Liu SJ et al. Relationship between duration of sleep and hypertension in adults: A meta-analysis. Journal of Clinical Sleep Medicine. 2015;11:1047–56. doi: 10.5664/jcsm.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shan Z, Ma H, Xie M, Yan P, Guo Y, Bao W et al. Sleep duration and risk of type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care. 2015;38:529–37. doi: 10.2337/dc14-2073. [DOI] [PubMed] [Google Scholar]
- 20.Lee SWH, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: A systematic review and meta-analysis. Sleep Medicine Reviews. 2017;31:91–101. doi: 10.1016/j.smrv.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Grandner MA, Chakravorty S, Perlis ML, Oliver L, Gurubhagavatula I. Habitual Sleep Duration Associated with Self-Reported and Objectively-Determined Cardiometabolic Risk Factors. Sleep medicine. 2014;15:42. doi: 10.1016/J.SLEEP.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, Tracy R et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irwin MR, Olmstead R, Carroll JE. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biological psychiatry. 2016;80(1):40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17. doi: 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czeisler CA. Duration, timing and quality of sleep are each vital for health, performance and safety. Sleep Health. 2015;1(1):5–8. doi: 10.1016/j.sleh.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Rosique-Esteban N, Papandreou C, Romaguera D, Warnberg J, Corella D, Martinez-Gonzalez MA et al. Cross-sectional associations of objectively-measured sleep characteristics with obesity and type 2 diabetes in the PREDIMED-Plus trial. Sleep. 2018;41(12). doi: 10.1093/sleep/zsy190. [DOI] [PubMed] [Google Scholar]
- 27.••.Lunsford-Avery JR, Engelhard MM, Navar AM, Kollins SH. Validation of the Sleep Regularity Index in Older Adults and Associations with Cardiometabolic Risk. Scientific Reports. 2018;8(1):14158. doi: 10.1038/s41598-018-32402-5.An observational study using a sleep regularity index connecting sleep variabity with cardiometabolic risk and psychological distress.
- 28.Kobayashi D, Takahashi O, Shimbo T, Okubo T, Arioka H, Fukui T. High sleep duration variability is an independent risk factor for weight gain. Sleep and Breathing. 2013;17:167–72. doi: 10.1007/s11325-012-0665-7. [DOI] [PubMed] [Google Scholar]
- 29.Zhou M, Lalani C, Banda JA, Robinson TN. Sleep duration, timing, variability and measures of adiposity among 8- to 12-year-old children with obesity. Obesity Science & Practice. 2018;4:535–44. doi: 10.1002/osp4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.•.Phillips AJK, Clerx WM, O’Brien CS, Sano A, Barger LK, Picard RW et al. Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Scientific Reports. 2017;7(1):3216. doi: 10.1038/s41598-017-03171-4.Findings indicate that sleep varibility represents characteristics of sleep that may not be reflected by sleep duration alone and demonstrates the physiological importance of sleep regularity via a circadian link.
- 31.Mota MC, Silva CM, Balieiro LCT, Gonçalves BF, Fahmy WM, Crispim CA. Association between social jetlag food consumption and meal times in patients with obesity-related chronic diseases. PloS one. 2019;14(2):e0212126. doi: 10.1371/journal.pone.0212126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuraikat FM, Makarem N, Liao M, St-Onge MP, Aggarwal B. Measures of Poor Sleep Quality Are Associated With Higher Energy Intake and Poor Diet Quality in a Diverse Sample of Women From the Go Red for Women Strategically Focused Research Network. J Am Heart Assoc. 2020;9(4):e014587. doi: 10.1161/jaha.119.014587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(3):1044–9. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Resta O, Foschino-Barbaro MP, Legari G, Talamo S, Bonfitto P, Palumbo A et al. Sleep-related breathing disorders, loud snoring and excessive daytime sleepiness in obese subjects. International Journal of Obesity. 2001;25:669–75. doi: 10.1038/sj.ijo.0801603. [DOI] [PubMed] [Google Scholar]
- 35.Foster GD, Sanders MH, Millman R, Zammit G, Borradaile KE, Newman AB et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes care. 2009;32:1017–9. doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malhotra A, White DP. Obstructive sleep apnoea. The Lancet. 2002;360(9328):237–45. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 37.Elder CR, Gullion CM, Funk KL, Debar LL, Lindberg NM, Stevens VJ. Impact of sleep, screen time, depression and stress on weight change in the intensive weight loss phase of the LIFE study. International Journal of Obesity. 2012;36:86–92. doi: 10.1038/ijo.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomson CA, Morrow KL, Flatt SW, Wertheim BC, Perfect MM, Ravia JJ et al. Relationship between sleep quality and quantity and weight loss in women participating in a weight-loss intervention trial. Obesity. 2012;20:1419–25. doi: 10.1038/oby.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaput J-P, Tremblay A. Sleeping Habits Predict the Magnitude of Fat Loss in Adults Exposed to Moderate Caloric Restriction. Obesity Facts. 2012;5:561–6. doi: 10.1159/000342054. [DOI] [PubMed] [Google Scholar]
- 40.. Sawamoto R, Nozaki T, Furukawa T, Tanahashi T, Morita C, Hata T et al. Higher sleep fragmentation predicts a lower magnitude of weight loss in overweight and obese women participating in a weight-loss intervention. Nutrition &Amp; Diabetes. 2014;4:e144. doi:10.1038/nutd.2014.4110.1038/nutd.2014.41https://www.nature.com/articles/nutd201441https://www.nature.com/articles/nutd201441 - supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.••.Papandreou C, Bulló M, Díaz-López A, Martínez-González MA, Corella D, Castañer O et al. High sleep variability predicts a blunted weight loss response and short sleep duration a reduced decrease in waist circumference in the PREDIMED-Plus Trial. International Journal of Obesity. 2019;44:330–9. doi: 10.1038/s41366-019-0401-5.First study to objectively measure sleep varaibility during a weight loss intervention. Findings indicated that sleep varaiblity was assoaciated with weight loss after 12 months and assocaited sleep dration with greater decreases in waist circumference.
- 42.Alfaris N, Wadden TA, Sarwer DB, Diwald L, Volger S, Hong P et al. Effects of a 2 year behavioral weight loss intervention on sleep and mood in obese individuals treated in primary care practice. Obesity. 2015;23(3):558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaput J-P, Drapeau V, Hetherington M, Lemieux S, Provencher V, Tremblay A. Psychobiological impact of a progressive weight loss program in obese men. Physiology & behavior. 2005;86(1):224–32. [DOI] [PubMed] [Google Scholar]
- 44.Martin CK, Bhapkar M, Pittas AG, Pieper CF, Das SK, Williamson DA et al. Effect of calorie restriction on mood, quality of life, sleep, and sexual function in healthy nonobese adults the CALERIE 2 randomized clinical trial. JAMA Internal Medicine. 2016;176:743–52. doi: 10.1001/jamainternmed.2016.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. The Lancet. 1999;354(9188):1435–9. doi: 10.1016/S01406736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 46.Spiegel K, Leproult R, L’Hermite-Bal riaux M, Copinschi G, Penev PD, an Cauter E. Leptin Levels Are Dependent on Sleep Duration: Relationships with Sympathovagal Balance, Carbohydrate Regulation, Cortisol, and Thyrotropin. The Journal of Clinical Endocrinology & Metabolism. 2004;89(11):5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 47.Wauters M, Considine RV, Van Gaal LF. Human leptin: from an adipocyte hormone to an endocrine mediator. European journal of endocrinology. 2000;143(3):293–311. doi: 10.1530/eje.0.1430293. [DOI] [PubMed] [Google Scholar]
- 48.Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4(129):129ra43. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proceedings of the National Academy of Sciences. 2013;110(14):5695. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Calvin AD, Carter RE, Adachi T, Macedo PG, Albuquerque FN, van der Walt C et al. Effects of Experimental Sleep Restriction on Caloric Intake and Activity Energy Expenditure. Chest. 2013;144(1):79–86. doi: 10.1378/chest.12-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Broussard JL, Wroblewski K, Kilkus JM, Tasali E. Two Nights of Recovery Sleep Reverses the Effects of Short-term Sleep Restriction on Diabetes Risk. Diabetes Care. 2016;39(3):e40. doi: 10.2337/dc15-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Leeuwen W, Hublin C, Sallinen M, Härmä M, Hirvonen A, Porkka-Heiskanen T. Prolonged sleep restriction affects glucose metabolism in healthy young men. International journal of endocrinology. 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eckel Robert H, Depner Christopher M, Perreault L, Markwald Rachel R, Smith Mark R, McHill Andrew W et al. Morning Circadian Misalignment during Short Sleep Duration Impacts Insulin Sensitivity. Current Biology. 2015;25(22):3004–10. doi: 10.1016/j.cub.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 54.van Leeuwen WMA, Lehto M, Karisola P, Lindholm H, Luukkonen R, Sallinen M et al. Sleep Restriction Increases the Risk of Developing Cardiovascular Diseases by Augmenting Proinflammatory Responses through IL-17 and CRP. PloS one. 2009;4(2):e4589. doi: 10.1371/journal.pone.0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pejovic S, Basta M, Vgontzas AN, Kritikou I, Shaffer ML, Tsaoussoglou M et al. Effects of recovery sleep after one work week of mild sleep restriction on interleukin-6 and cortisol secretion and daytime sleepiness and performance. American Journal of Physiology-Endocrinology and Metabolism. 2013;305(7):E890–E6. doi: 10.1152/ajpendo.00301.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Leeuwen WMA, Sallinen M, Virkkala J, Lindholm H, Hirvonen A, Hublin C et al. Physiological and autonomic stress responses after prolonged sleep restriction and subsequent recovery sleep in healthy young men. Sleep and biological rhythms. 2018;16(1):45–54. doi: 10.1007/s41105-017-0122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monk TH, Buysse DJ, Rose LR, Hall JA, Kupfer DJ. The sleep of healthy people--a diary study. Chronobiol Int. 2000;17(1):49–60. doi: 10.1081/cbi-100101031. [DOI] [PubMed] [Google Scholar]
- 58.Killick R, Hoyos CM, Melehan KL, Dungan GC 2nd, Poh J, Liu PY. Metabolic and hormonal effects of ‘catch-up’ sleep in men with chronic, repetitive, lifestyle-driven sleep restriction. Clinical endocrinology. 2017;83(4):498–507. doi: 10.1111/cen.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kubo T, Takahashi M, Sato T, Sasaki T, Oka T, Iwasaki K. Weekend sleep intervention for workers with habitually short sleep periods. Scandinavian Journal of Work, Environment & Health. 2011(5):418–26. doi: 10.5271/sjweh.3162. [DOI] [PubMed] [Google Scholar]
- 60.••.Depner CM, Melanson EL, Eckel RH, Snell-Bergeon JK, Perreault L, Bergman BC et al. Ad libitum Weekend Recovery Sleep Fails to Prevent Metabolic Dysregulation during a Repeating Pattern of Insufficient Sleep and Weekend Recovery Sleep. Current Biology. 2019;29(6):957–67.e4. doi: 10.1016/j.cub.2019.01.069.Weekend sleep extension in response to weekday sleep restriction does not sufficently repay sleep debt and only partially mitigates obeisty risk and cardiometabolic dysfunction as determined with hyperinsulemic-euglycemic clamp.
- 61.Im HJ, Baek SH, Chu MK, Yang KI, Kim WJ, Park SH et al. Association Between Weekend Catch-up Sleep and Lower Body Mass: Population-Based Study. Sleep. 2017;40(7). doi: 10.1093/sleep/zsx089. [DOI] [PubMed] [Google Scholar]
- 62.Larsen SC, Horgan G, Mikkelsen M-LK, Palmeira AL, Scott S, Duarte C et al. Consistent sleep onset and maintenance of body weight after weight loss: An analysis of data from the NoHoW trial. PLOS Medicine. 2020;17(7):e1003168. doi: 10.1371/journal.pmed.1003168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Papandreou C, Bulló M, Díaz-López A, Martínez-González MA, Corella D, Castañer O et al. High sleep variability predicts a blunted weight loss response and short sleep duration a reduced decrease in waist circumference in the PREDIMED-Plus Trial. International Journal of Obesity. 2020;44(2):330–9. doi: 10.1038/s41366-019-0401-5. [DOI] [PubMed] [Google Scholar]
- 64.Lunsford-Avery JR, Engelhard MM, Navar AM, Kollins SH. Validation of the Sleep Regularity Index in Older Adults and Associations with Cardiometabolic Risk. Scientific reports. 2018;8:14158. doi: 10.1038/s41598-018-32402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. The American Journal of Clinical Nutrition. 2009;89(1):126–33. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.••.Wang X, Sparks JR, Bowyer KP, Youngstedt SD . Influence of Sleep Restriction on Weight Loss Outcomes Associated with Caloric Restriction. Sleep. 2018. doi: 10.1093/sleep/zsy027.First weightloss intervention to evaluate changes in body composition and substrate utilization under habitual short sleeping conditions. Results indicate similar overall weight loss but blunted fat mass loss in the sleep restricted group compared to normal sleeping group.
- 67.Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient Sleep Undermines Dietary Efforts to Reduce Adiposity. Annals of Internal Medicine. 2010;153. doi: 10.7326/0003-4819-153-7-201010050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12(3):197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 69.Chen X, Niu X, Xiao Y, Dong J, Lu M, Kong W. Effect of continuous positive airway pressure on leptin levels in patients with obstructive sleep apnea: a meta-analysis. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2015;152(4):610–8. doi: 10.1177/0194599814562719. [DOI] [PubMed] [Google Scholar]
- 70.Mason IC, Qian J, Adler GK, Scheer FAJL. Impact of circadian disruption on glucose metabolism: implications for type 2 diabetes. Diabetologia. 2020. doi: 10.1007/s00125-019-05059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reynold AM, Bowles ER, Saxena A, Fayad R, Youngstedt SD. Negative Effects of Time in Bed Extension: A Pilot Study. J Sleep Med Disord. 2014;1(1). [PMC free article] [PubMed] [Google Scholar]
- 72.•.Stock AA, Lee S, Nahmod NG, Chang AM. Effects of sleep extension on sleep duration, sleepiness, and blood pressure in college students. Sleep Health. 2020;6(1):32–9. doi: 10.1016/j.sleh.2019.10.003.This study showed significant decreases in systolic blood pressure with moderate sleep extension in a group of normal and short sleeping young adults without hypertension.
- 73.•.Al Khatib HK, Hall WL, Creedon A, Ooi E, Masri T, McGowan L et al. Sleep extension is a feasible lifestyle intervention in free-living adults who are habitually short sleepers: a potential strategy for decreasing intake of free sugars? A randomized controlled pilot study. The American Journal of Clinical Nutrition. 2018;107(1):43–53. doi: 10.1093/ajcn/nqx030.One of the few free-living sleep extention interventions in habaitually short sleepers. Body weight and objective indicatiors of hunger were unchanged, but diet was improved by reduced sugar intake.
- 74.Tasali E, Chapotot F, Wroblewski K, Schoeller D. The effects of extended bedtimes on sleep duration and food desire in overweight young adults: a home-based intervention. Appetite. 2014;80:220–4. doi: 10.1016/j.appet.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leproult R, Deliens G, Gilson M, Peigneux P. Beneficial impact of sleep extension on fasting insulin sensitivity in adults with habitual sleep restriction. Sleep. 2015;38(5):707–15. doi: 10.5665/sleep.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.So-Ngern A, Chirakalwasan N, Saetung S, Chanprasertyothin S, Thakkinstian A, Reutrakul S. Effects of Two-Week Sleep Extension on Glucose Metabolism in Chronically Sleep-Deprived Individuals. J Clin Sleep Med. 2019;15(5):711–8. doi: 10.5664/jcsm.7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haack M, Serrador J, Cohen D, Simpson N, Meier-Ewert H, Mullington JM. Increasing sleep duration to lower beat-to-beat blood pressure: a pilot study. Journal of sleep research. 2013;22(3):295–304. doi: 10.1111/jsr.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Basner M, Fomberstein KM, Razavi FM, Banks S, William JH, Rosa RR et al. American time use survey: sleep time and its relationship to waking activities. Sleep. 2007;30(9):1085–95. doi: 10.1093/sleep/30.9.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.. Cizza G, Piaggi P, Rother KI, Csako G. Hawthorne effect with transient behavioral and biochemical changes in a randomized controlled sleep extension trial of chronically short-sleeping obese adults: implications for the design and interpretation of clinical studies. PloS one. 2014;9(8):e104176. doi: 10.1371/journal.pone.0104176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lucassen EA, Piaggi P, Dsurney J, de Jonge L, Zhao XC, Mattingly MS et al. Sleep extension improves neurocognitive functions in chronically sleep-deprived obese individuals. PloS one. 2014;9(1):e84832. doi: 10.1371/journal.pone.0084832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.•.Baron Kelly G, Duffecy J, Richardson D, Avery E, Rothschild S, Lane J. Technology Assisted Behavior Intervention to Extend Sleep Among Adults With Short Sleep Duration and Prehypertension/Stage 1 Hypertension: A Randomized Pilot Feasibility Study. Journal of Clinical Sleep Medicine. 2019;15(11):1587–97. doi: 10.5664/jcsm.8018.A small pilot study reporting marked improvements in blood pressure after a sleep hygiene intervention that resulted in clinically relevant increases in sleep duration.
- 82.McGrath ER, Espie CA, Power A, Murphy AW, Newell J, Kelly C et al. Sleep to Lower Elevated Blood Pressure: A Randomized Controlled Trial (SLEPT). American journal of hypertension. 2017;30(3):319–27. doi: 10.1093/ajh/hpw132. [DOI] [PubMed] [Google Scholar]
- 83.. Mudaliar U, Zabetian A, Goodman M, Echouffo-Tcheugui JB, Albright AL, Gregg EW et al. Cardiometabolic Risk Factor Changes Observed in Diabetes Prevention Programs in US Settings: A Systematic Review and Meta-analysis. PLoS medicine. 2016;13(7):e1002095-e. doi: 10.1371/journal.pmed.1002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3(5):519–28. [PMC free article] [PubMed] [Google Scholar]
- 85.Krause AJ, Simon EB, Mander BA, Greer SM, Saletin JM, Goldstein-Piekarski AN et al. The sleep-deprived human brain. Nature Reviews Neuroscience. 2017;18(7):404–18. doi: 10.1038/nrn.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Broussard JL, Kilkus JM, Delebecque F, Abraham V, Day A, Whitmore HR et al. Elevated ghrelin predicts food intake during experimental sleep restriction. Obesity (Silver Spring, Md). 2016;24(1):132–8. doi: 10.1002/oby.21321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes care. 2002;25(12):2165–71. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24(5):610–28. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 89.Sawamoto R, Nozaki T, Furukawa T, Tanahashi T, Morita C, Hata T et al. A change in objective sleep duration is associated with a change in the serum adiponectin level of women with overweight or obesity undergoing weight loss intervention. Obesity science & practice. 2016;2(2):180–8. doi: 10.1002/osp4.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Logue EE, Bourguet CC, Palmieri PA, Scott ED, Matthews BA, Dudley P et al. The better weight-better sleep study: a pilot intervention in primary care. Am J Health Behav. 2012;36(3):319–34. doi: 10.5993/ajhb.36.3.4. [DOI] [PubMed] [Google Scholar]
- 91.Demos KE, Leahey TM, Hart CN, Trautvetter J, Coward PR, Duszlak J et al. A pilot randomized controlled trial testing the effects of a routine-based intervention on outcomes in a behavioural weight loss programme. Obesity Science & Practice. 2015;1(2):110–8. doi: 10.1002/osp4.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.••.Moreno-Frias C, Figueroa-Vega N, Malacara JM. Sleep Extension Increases the Effect of Caloric Restriction Over Body Weight and Improves the Chronic Low-Grade Inflammation in Adolescents With Obesity. J Adolesc Health. 2020. doi: 10.1016/j.jadohealth.2019.11.301.First weight loss intervention to show that sleep extension in conjuction with weight loss in adolescents may elicit superlative weightloss outcomes related to cardiometabolic health.


