Supplemental Digital Content is available in the text.
Keywords: andexanet alfa, direct factor Xa inhibitor reversal, intracranial hemorrhage
Background and Purpose:
Andexanet alfa is a recombinant modified human FXa (factor Xa) developed to reverse FXa inhibition from anticoagulants. Hemostatic efficacy and reversal of anti-FXa activity with andexanet were assessed in patients from the ANNEXA-4 study (Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of FXa Inhibitors) with intracranial hemorrhage (ICrH).
Methods:
ANNEXA-4 was a single-arm study evaluating andexanet in patients presenting with major bleeding ≤18 hours after taking an FXa inhibitor. Patients received a bolus plus 2-hour infusion of andexanet. Brain imaging in patients with ICrH was performed at baseline and at 1 and 12 hours postandexanet infusion. Coprimary efficacy outcomes were change in anti-FXa activity and hemostatic efficacy at 12 hours (excellent/good efficacy defined as ≤35% increase in hemorrhage volume/thickness). Safety outcomes included occurrence of thrombotic events and death at 30 days.
Results:
A total of 227 patients with ICrH were included in the safety population (51.5% male; mean age 79.3 years) and 171 in the efficacy population (99 spontaneous and 72 traumatic bleeds). In efficacy evaluable patients, excellent/good hemostasis 12 hours postandexanet occurred in 77 out of 98 (78.6%) and in 58 out of 70 (82.9%) patients with spontaneous and traumatic bleeding, respectively. In the subanalysis by FXa inhibitor treatment group in the efficacy population, median of percent change in anti-FXa from baseline to nadir showed a decrease of 93.8% for apixaban-treated patients (n=99) and by 92.6% for rivaroxaban-treated patients (n=59). Within 30 days, death occurred in 34 out of 227 (15.0%) patients and thrombotic events occurred in 21 out of 227 (9.3%) patients (safety population).
Conclusions:
Andexanet reduced anti-FXa activity in FXa inhibitor-treated patients with ICrH, with a high rate of hemostatic efficacy. Andexanet may substantially benefit patients with ICrH, the most serious complication of anticoagulation.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02329327.
See related article, p 2106
FXa (factor Xa) inhibitors have become widely used for the prevention and treatment of venous thromboembolism, stroke prevention in atrial fibrillation, and management of patients with coronary artery disease or peripheral artery disease. Large clinical trials have demonstrated that FXa inhibitors are as effective and safer than warfarin1–3 and are now widely recommended by guidelines.4,5 Despite their superior safety, ≈0.3% to 0.5% of patients receiving FXa inhibitors suffer intracranial hemorrhage (ICrH) annually,1–3 which is associated with 30-day mortality rates of 27% to 48%.6,7
Andexanet alfa (US adopted name: coagulation factor Xa [recombinant] inactivated-zhzo) is a specific reversal agent approved by the US Food and Drug Administration and the European Medicines Agency for uncontrolled or life-threatening bleeding related to the oral FXa inhibitors apixaban and rivaroxaban.8,9 Andexanet is a modified, recombinant, enzymatically inactive form of FXa, which binds and sequesters FXa inhibitors, thereby neutralizing their anti-FXa activity.10 Studies in healthy volunteers have shown that administration of andexanet resulted in a median >92% reduction from baseline in anti-FXa activity of apixaban and rivaroxaban, with a rapid onset of effect within minutes after the start of administration.11 This was associated with a similarly rapid increase in thrombin generation that persisted up to 22 hours after the completion of andexanet.
In the ANNEXA-4 study (Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of FXa Inhibitors), patients with acute major bleeding (ICrH and gastrointestinal bleeding primarily) received andexanet.12 In a recent report on 352 patients of all bleed types in the full study population, treatment was associated with substantial reductions from baseline in anti-FXa activity. Furthermore, 82% of patients were judged by independent adjudication to have effective hemostasis (80% in patients with ICrH and 85% in patients with gastrointestinal bleeding).12 The results presented herein describe detailed efficacy and safety data from ANNEXA-4 patients with spontaneous and traumatic ICrH. These findings are important in that they summarize the bleed subtype with the greatest enrollment in the study, as well as the most severe manifestation of acute major bleeding.
Methods
Availability of Data and Material
Alexion Pharmaceuticals, Inc (Boston, MA) will consider requests for disclosure of clinical study participant-level data provided that participant privacy is assured through methods like data deidentification, pseudonymization, or anonymization (as required by applicable law) and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at https://alexion.com/our-research/research-and-development.
Link to Data Request Form (https://alexion.com/contact-alexion/medical-information).
Patients
Full details of the study design have been published previously.12 Briefly, patients aged ≥18 years were required to have an episode of acute major bleeding and to have received either apixaban, rivaroxaban, edoxaban, or enoxaparin within the preceding 18 hours. From the standpoint of ICrH, acute major bleeding was defined as the presence of blood anywhere within the intracranial vault (including intracerebral [within brain parenchyma], intraventricular [within brain ventricles], subdural, and subarachnoid bleeding).12
Patients were excluded if they were scheduled to undergo surgery within 12 hours after andexanet administration or if they had a Glasgow Coma Scale (GCS) score of <7 or an intracerebral hematoma volume >60 cc. There was no minimum volume exclusion criterion. Other exclusion criteria included expected survival of <1 month; thrombotic event within 2 weeks of enrollment; and use of vitamin K antagonists, dabigatran, prothrombin complex concentrates, recombinant factor VII, whole blood, or plasma within the preceding 7 days. Patients with advanced directives or do-not-resuscitate orders were not excluded.12 Between July 2016 and August 2017, following a recommendation from the US Food and Drug Administration, enrollment was restricted to patients with ICrH to enrich the study population for these bleeds.
Either the patient or their medical proxy (as governed by local institutional practices and laws) provided written informed consent before screening. The study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. The protocol, consent forms, and ancillary materials were approved by institutional review boards or ethics committees at each center.12
Study Design
ANNEXA-4 was a prospective, open-label, single-arm study. Upon enrollment, andexanet was administered to all patients, with a low or high dose depending on the identity, amount, and timing of the last dose of FXa inhibitor. For patients with ICrH, a computed tomography or magnetic resonance imaging scan was obtained within 2 hours before andexanet was initiated, and then at 1 hour and 12 hours after the end of treatment.12 Anatomic analysis of the hemorrhage (eg, volume, thickness, midline shift) using Quantomo software (Cybertrials, Inc, Calgary, Canada)13 was performed at a dedicated imaging core lab to support the assessment of hemostatic efficacy.
Blood samples were taken to measure anti-FXa activity and thrombin generation before and through 12 hours after andexanet administration.12 Analysis of blood samples (particularly with anti-FXa assays, which require specific methodology to properly analyze in the presence of andexanet14) has been reported previously.11
Outcomes
The coprimary end points of the study were (1) the percent change from baseline to the lowest level in anti-FXa activity measured between the start of andexanet treatment and 10 minutes after the end of the infusion (change from baseline to nadir) and (2) the proportion of patients who achieved excellent or good hemostatic efficacy at 12 hours after treatment, based on criteria established in Sarode et al15 (detailed in Table I in the Data Supplement) and assessed by an independent Endpoint Adjudication Committee. These criteria were used in the original trial because they facilitated the assessment of hemostasis of a variety of bleed types into a single metric. For patients with ICrH, a ≤20% increase in hematoma volume at both 1 and 12 hours after the end of andexanet treatment versus baseline was considered excellent hemostasis, whereas good hemostasis was defined as an increase of >20% and ≤35% in hematoma volume at 12 hours compared with baseline. Patients with a >35% increase in volume relative to baseline had poor/none hemostasis. Safety outcomes included mortality and occurrence of thrombotic events adjudicated by the Endpoint Adjudication Committee over 30 days.12
National Institutes of Health Stroke Scale (NIHSS), GCS, and modified Rankin Scale scores were assessed at baseline, 1 hour, 12 hours, and 30 days postandexanet treatment.
Statistical Analysis
For this substudy, the safety population consisted of all patients receiving any dose of andexanet. Patients were included in the efficacy population if they had a baseline anti-FXa activity of ≥75 ng/mL (≥0.25 IU/mL for enoxaparin) and had confirmed major bleeding at presentation, as determined by the adjudication committee. If the baseline anti-FXa activity was missing, the patient was excluded from the efficacy analysis. Percent change in anti-FXa activity was evaluated using a distribution-free 2-sided 95% CI for the median. Percentages of patients with excellent/good hemostasis were presented as 95% CIs calculated with the exact binomial test. In the subpopulation of patients with intracerebral/intraventricular bleeds, hemostasis and hematoma volume expansion were evaluated in the safety population to maximize sample size.
For the primary hemostatic efficacy analysis, missing values due to nonadministrative reasons were imputed as treatment failure, whereas those missing due to administrative reasons were excluded from the analysis. For postbaseline anti-FXa activity assessments, if either the end-of-bolus and end-of-infusion assessments were missing, nadir was set to the one available; if both were missing, the maximum change from baseline to nadir was replaced with zero. For the NIHSS, patients were only included in the 12-hour NIHSS evaluation if data were available at baseline and post-treatment (at 1 hour or 12 hours). If the 12-hour NIHSS was missing, the value was imputed with the 1-hour post-treatment NIHSS score, if available. If no postbaseline NIHSS score was available, the NIHSS score was treated as missing at 12 hours. For postbaseline GCS and modified Rankin Scale score, missing values were not imputed.
Results
Two hundred twenty-seven patients with ICrH were included in ANNEXA-4 and constitute the safety cohort for this analysis (Figure 1). The efficacy population included 171 patients, including 99 patients with spontaneous ICrH and 72 patients with traumatic ICrH. Three patients (1 in the spontaneous ICrH group and 2 in the trauma ICrH group) were excluded from the population evaluable for hemostatic efficacy for administrative reasons.
Figure 1.
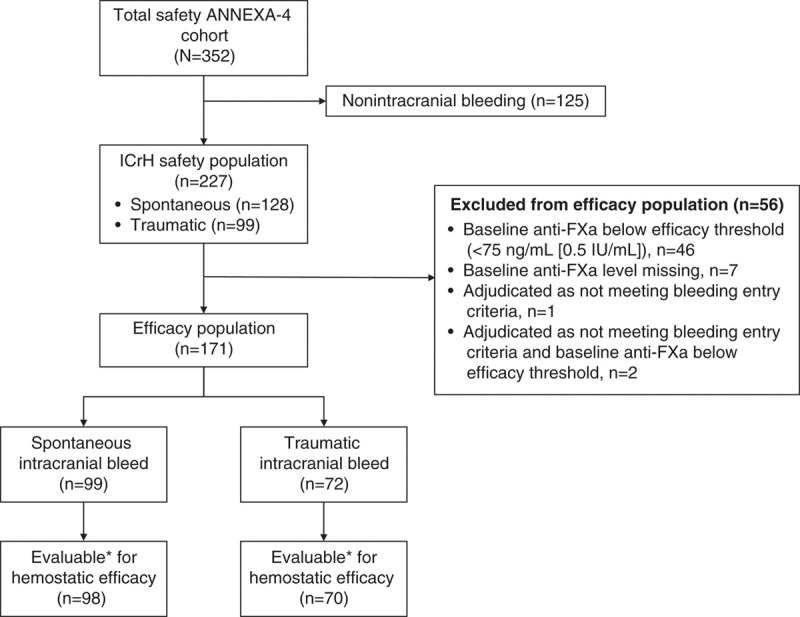
Flow chart. ANNEXA-4 indicates Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of FXa Inhibitors; and anti-FXa, anti-factor Xa activity. *Patients not evaluable due to administrative reasons were excluded: n=1 for the spontaneous intracranial hemorrhage (ICrH) population; n=2 for the traumatic ICrH population.
All 227 patients in the safety population received at least a partial dose of andexanet. Three patients were redosed with andexanet, 3 received a coagulation factor concentrate, and 2 received an antifibrinolytic (eg, tranexamic acid).
Baseline Characteristics
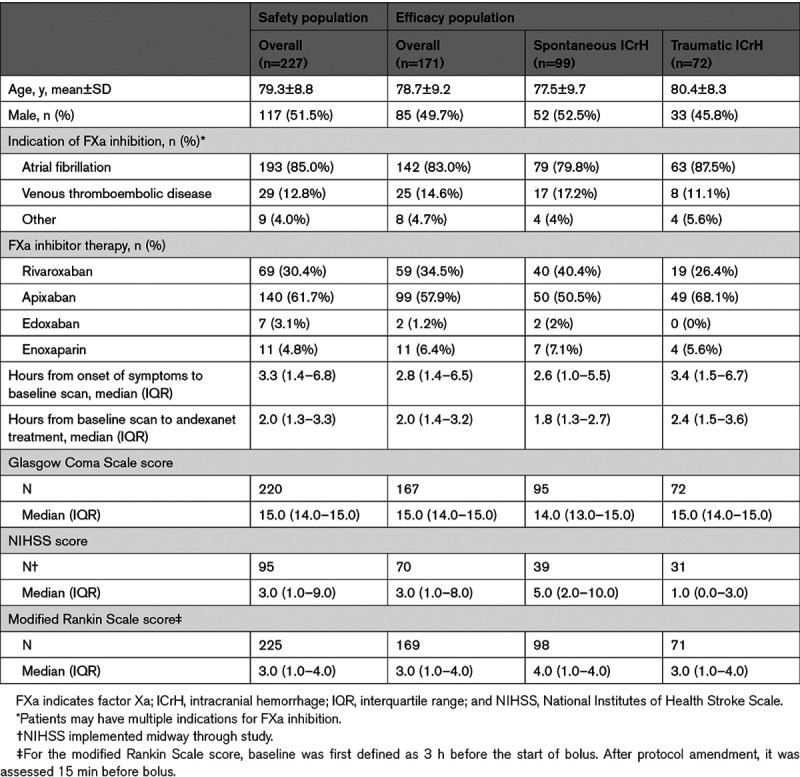
In the overall safety population, the mean age was 79.3 years, 117 (51.5%) patients were male, and 193 (85.0%) patients were receiving FXa inhibitor therapy for atrial fibrillation (Table 1). Most patients had received either apixaban (140 [61.7%]) or rivaroxaban (69 [30.4%]) before andexanet. Bleeding was spontaneous in 128 patients and the result of trauma in 99 patients. The median (IQR) time from onset of symptoms or trauma to baseline scan was 3.3 (1.4–6.8) hours, whereas the median (IQR) time from baseline scan to initiation of andexanet was 2.0 (1.3–3.3) hours (Table 1).
Table 1.
Baseline and Demographic Characteristics
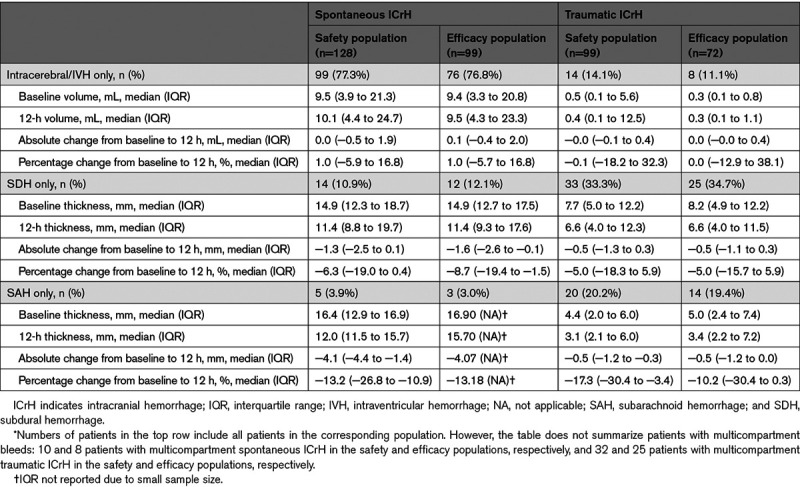
In patients with spontaneous ICrH (n=128), the compartment of bleeding was intracerebral or intraventricular in 99 patients (77.3%), subdural in 14 patients (10.9%), subarachnoid in 5 patients (3.9%; Table 2). Multicompartmental bleeding was observed in 10 patients (7.8%; intracerebral+subdural hemorrhages [n=4] and intracerebral+subarachnoid hemorrhages [n=6]). The median (IQR) hematoma volume in patients with intracerebral/intraventricular bleeding (n=99) was 9.5 (3.9–21.3) mL. In total, 27.3% and 18.2% of intracerebral/intraventricular bleeds were greater than 20 mL and 30 mL, respectively. In patients with traumatic ICrH (n=99), the compartment of bleeding was intracerebral or intraventricular in 14 patients (14.1%), subdural in 33 patients (33.3%), subarachnoid in 20 patients (20.2%; Table 2). Multicompartmental bleeding was observed in 32 patients (32.3%).
Table 2.
Change in Hematoma Characteristics*
Median (IQR) GCS, modified Rankin Scale score, and NIHSS were 14.0 ([13.0–15.0]; n=122), 4.0 [(2.0–4.0]; n=127), and 6.0 ([2.0–12.0]; n=53) in patients with spontaneous ICrH (n=128), respectively, and 15.0 ([14.0–15.0]; n=98), 3.0 ([1.0–4.0]; n=98), and 1.0 ([0.0–4.0]; n=42) in patients with traumatic ICrH (n=99), respectively (detailed in Table II in the Data Supplement).
Anti-FXa Levels for ICrH
Median baseline anti-FXa levels for patients with ICrH in the efficacy analysis receiving apixaban (n=99), rivaroxaban (n=59), and enoxaparin (n=11) were 144.2 ng/mL, 198.1 ng/mL, and 0.5 IU/mL, respectively (2 patients received edoxaban; Figure I in the Data Supplement). Median of percent change in anti-FXa from baseline to nadir showed a decrease of 93.8% for apixaban-treated patients and by 92.6% for rivaroxaban-treated patients. Over time, the anti-FXa activity remained suppressed throughout andexanet administration, increased to a level lower than baseline following the completion of treatment, then gradually decreased, presumably coincident with the elimination of the FXa inhibitor. This pattern is consistent with prior studies of andexanet in healthy volunteers11 and bleeding patients.12
Efficacy in Spontaneous ICrH Cohort
Of the 98 evaluable patients with spontaneous ICrH, 77 (78.6% [95% CI, 69.1%–86.2%]) were adjudicated to have excellent (n=70) or good (n=7) hemostasis at 12 hours following andexanet. Subgroup analyses showed a consistent effect of andexanet treatment according to the type of FXa inhibitor received and bleeding compartment (Figure 2A).
Figure 2.
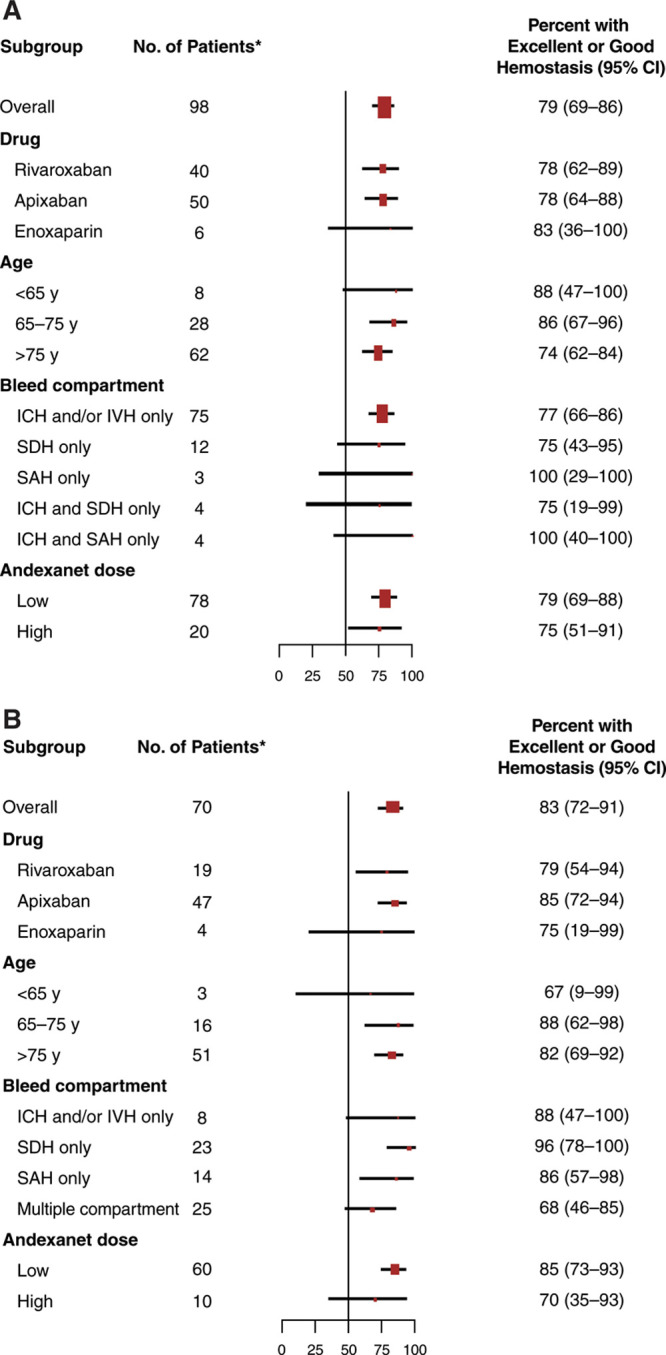
Subgroup analysis of patients achieving excellent or good hemostasis 12 h after andexanet treatment. Spontaneous (A) and traumatic (B) ICrH populations reported separately. *Evaluable patients. In A, 1 patient from the efficacy cohort of 99 patients was not evaluable. In B, 2 patients from the efficacy cohort of 72 patients were not evaluable. Sizes of squares indicate relative numbers of patients. ICH indicates intracerebral hemorrhage; ICrH, intracranial hemorrhage; IVH, intraventricular hemorrhage; SAH, subarachnoid hemorrhage; and SDH, subdural hemorrhage.
Among the 91 patients who presented with a single-compartment intracerebral/intraventricular hemorrhage (safety population-data missing for 8 patients), intracerebral/intraventricular hematoma volume expansion ≤35% between baseline and 1 hour was apparent in 75 (82.4%) patients; 73 (97.3%) of these patients maintained this status at the 12-hour scan, suggesting durability of hemostasis despite the completion of andexanet before full clearance of the FXa inhibitor (Figure II in the Data Supplement).
Median hematoma volume did not change noticeably between baseline and 12 hours in patients with single-compartment intracerebral/intraventricular bleeds (Table 2). In patients with subdural or subarachnoid bleeding, median hematoma thickness at 12 hours was modestly lower than at baseline, although the number of patients with these bleed types was limited.
Median (IQR) GCS and NIHSS scores at 12 hours in the efficacy population were 15.0 ([12.5–15.0]; n=40) and 5.5 ([1.0–15.0]; n=38), respectively, and the median (IQR) modified Rankin Scale score at 30 days was 4.0 ([1.0–5.0]; n=33).
Efficacy in Traumatic ICrH Cohort
Of the 70 evaluable patients with traumatic ICrH at 12 hours following andexanet, 58 (83% [95% CI, 72%–91%]) were adjudicated to have excellent (n=47) or good (n=11) hemostasis. As with spontaneous bleeds, subgroup analyses showed a consistent effect of andexanet treatment according to the FXa inhibitor agent and bleeding compartment (Figure 2B).
In patients with traumatic single-compartment intracerebral/intraventricular bleeding (efficacy population), median hematoma volume remained constant between baseline and 12 hours (Table 2). In patients with subdural and subarachnoid bleeding, median hematoma thickness was modestly lower at 12 hours compared with baseline, similar to patients with spontaneous ICrH.
Median (IQR) GCS and NIHSS scores at 12 hours in the efficacy population were 15.0 ([14.0–15.0]; n=29) and 1.0 ([0.0–3.5]; n=32), respectively; and the modified Rankin Scale score at 30 days was 1.0 ([0.0–4.0]; n=31).
Safety
A thrombotic event occurred in 21 of 227 (9.3%) patients in the safety population within 30 days (Table 3 and Table III in the Data Supplement). Events included deep vein thrombosis (9 patients), stroke (11 patients), myocardial infarction (2 patients), and pulmonary embolism (1 patient). No patients had a transient ischemic attack or a systemic arterial embolism. Median time to first thrombotic event was 11 days; most occurred >6 days after andexanet treatment (14 of 21 patients; 66.7%). A total of 34 (15.0%) patients died within 30 days, most (28 patients) due to cardiovascular causes. A majority of deaths (26 of 34 patients; 76.5%) occurred >6 days after andexanet administration. In patients with excellent/good hemostatic efficacy, mortality was 12.7%, whereas patients with poor/none hemostatic efficacy had a mortality rate of 26.3%. Mortality rates in patients with spontaneous and traumatic ICrH were 18.8% (24 out of 128) and 10.1% (10 out of 99), respectively. In patients with spontaneous intracerebral/intraventricular bleeding, the mortality rate in patients at the highest quartile of baseline hematoma volumes (5 of 24; 20.8%) was not dissimilar to the overall mortality rate (16 of 99; 16.2%; Figure III in the Data Supplement).
Table 3.
Safety Results
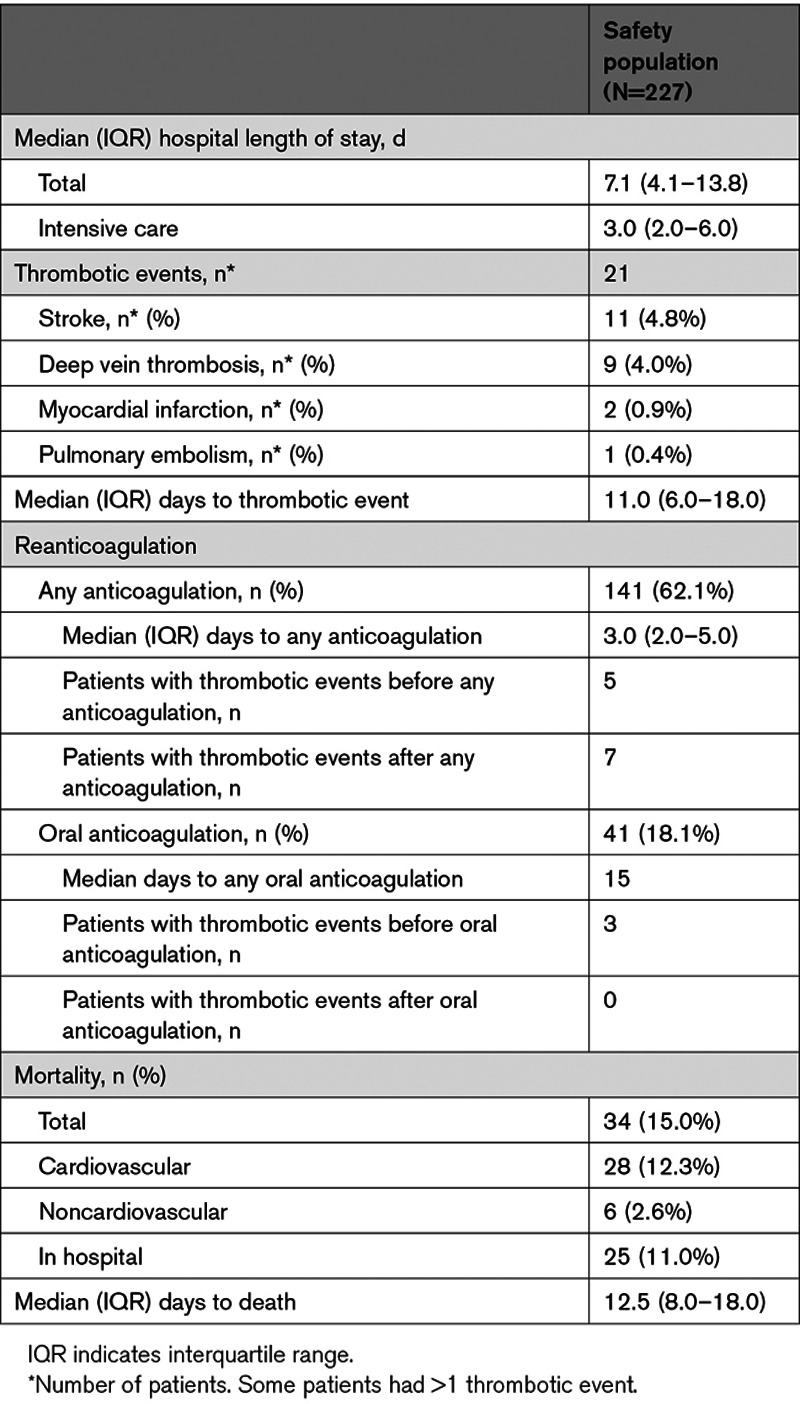
A total of 141 patients (62.1%) in the overall safety population resumed anticoagulation of any kind (any dose of intravenous or oral) after andexanet treatment, at a median of 3 days (Table 3). Of these 141 patients, 41 (29.1%) patients restarted oral anticoagulation therapy. No thrombotic events occurred after the initiation of oral anticoagulation.
Discussion
The present analysis describes the efficacy and safety of andexanet in ANNEXA-4 patients with ICrH, the most serious complication of anticoagulation. Consistent with results from the overall population, andexanet rapidly and potently decreased anti-FXa activity and resulted in effective hemostasis in 79% of patients with spontaneous ICrH and in 83% of patients with traumatic ICrH. These findings were in concert with observations that hematoma volumes and thicknesses were largely preserved over the 12 hours following andexanet treatment. Finally, the safety of andexanet, as exemplified by the 30-day rates of thrombotic events (9%) and mortality (15%), appears consistent with the bleed severity and acuteness of the study population.
Importantly, the interpretation of these data should be tempered by the study’s single-arm, uncontrolled design. One cannot evaluate the clinical benefit of andexanet in the absence of a comparator. That said, a controlled clinical trial in patients with ICrH is ongoing and will provide additional insight (ANNEXA-I study; https://www.clinicaltrials.gov; Unique identifier: NCT03661528). Furthermore, although all symptomatic ICrH bleeding (spontaneous or traumatic) is considered major bleeding by the International Society on Thrombosis and Haemostasis, the trial enrollment favored smaller hemorrhages especially in trauma patients. Patients scheduled for surgery (involving any part of the body) within 12 hours were excluded from the study as surgery limits the ability to assess for hemostatic efficacy. Nevertheless, this analysis demonstrates that the efficacy and safety findings in patients with ICrH are similar to results for the overall population (including patients without ICrH) reported in the primary ANNEXA-4 article.12
Because of the recent adoption of direct FXa inhibitors, the literature describing the natural history of ICrH in patients treated with this class of anticoagulants remains modest. In a mainly nonanticoagulated population, the risk of hematoma expansion of an intracerebral bleed has been reported to be 12% to 32%, depending on definition.16,17 Treatment with a vitamin K antagonist markedly augments this risk, with as much as a 60% incidence of hematoma expansion at 24 hours in a population of patients with intracerebral bleeding.18 In patients taking FXa inhibitors, the risk of expansion is less well characterized. However, preliminary reports have indicated a risk of 25% to 40% in patients with intracerebral hemorrhage.19–21 In patients with traumatic brain injury, the risk of hematoma expansion is reported to be 16% to 42%.22–24 It is difficult, however, to interpret these findings given differences in the definition, nature (contusion versus bleed), involved compartments, and assessment of injury in the traumatic setting. In the ANNEXA-4 efficacy population, using poor/none hemostatic efficacy as a surrogate for hematoma expansion, the risk of expansion was 21% and 17% in patients with spontaneous and traumatic ICrH, respectively. These results should be considered within the context of the relative severity of bleeding in the respective study cohorts; in particular, differences between the respective studies for various parameters (eg, age, hematoma size, intraventricular involvement, GCS, time of symptom onset) could have resulted in differences in expansion rates. Any meaningful comparison between andexanet and usual care must take these factors into account.
Given that the anticoagulant reversal effect of andexanet begins to wane once treatment is completed, the prospect of rebleeding presents a potential concern, especially in patients with higher baseline anti-FXa activity. Although it is appreciated that the risk of hematoma expansion decreases over time, the presence of therapeutic FXa inhibition likely prolongs the duration of this risk (though the magnitude of the effect is not precisely known). Nevertheless, the observation that volume expansion occurred in only 2 of 90 patients after the 1-hour postandexanet scan is reassuring.
The frequency of thrombotic events among all ICrH was similar to the 30-day thrombotic rate observed in the overall ANNEXA-4 population as well as in recent studies of bleeding occurring during FXa inhibitor treatment.25–27 The observed rate is not unexpected given the abrupt reversal of chronic anticoagulation, the high background thrombotic risk, and the physical immobility resulting from a substantial neurological insult. Many patients received reanticoagulation, with a median time to initiation of any anticoagulant of 3 days. Our data suggest that early resumption of anticoagulation to prevent thrombotic events may be performed safely in this population, albeit in selected patients.
In our study, mortality within 30 days was 18.8% in patients with spontaneous ICrH, 10.1% in traumatic ICrH, and 15.0% overall. In pivotal stroke prevention studies of oral FXa inhibitors in patients with nonvalvular atrial fibrillation, 30-day mortality rates of 27% to 48% in patients with ICrH have been reported, although the patients in these studies were unselected and ANNEXA-4 excluded patients with the most severe bleeds.6,28,29 In patients with traumatic ICrH, the mortality rate (10.1%) is not inconsistent with existing literature.23,30,31 That said, the patients evaluated in these analyses consisted of all-comers populations, with few selection criteria around baseline characteristics, extent of FXa inhibition, and severity. As with hemostatic efficacy, a properly controlled evaluation of andexanet that accounts for these factors would likely yield a meaningful understanding of mortality in this population.
Before the development of andexanet, providers were left with few options to manage bleeding patients taking FXa inhibitors. Therapeutic strategies have included supportive care, antifibrinolytic agents (aminocaproic acid, tranexamic acid), and coagulation factor therapies (prothrombin complex concentrates, plasma, factor VIII inhibitor bypassing activity). Recent reports have described the use of these agents in patients with ICrH,25,26,32,33 although the mechanistic basis for their effectiveness is controversial.34 Furthermore, their efficacy may be restricted in the presence of higher anti-FXa levels,35 and the reports provide limited or no information regarding baseline anti-FXa activity. Relative to previously available therapies, andexanet represents a therapeutic advance given its specific, rapid, and biologically relevant mechanism of action.
In summary, results of this subgroup analysis of ANNEXA-4 provide support that andexanet provides effective and safe reduction of FXa anticoagulant activity for up to 12 hours after treatment in patients with intracranial bleeding. Given its unique and specific mechanism of action in reversing the anticoagulant effects of FXa inhibitors, andexanet may confer a benefit for this severely ill and highly vulnerable population.
Acknowledgments
We acknowledge the work of the University of Calgary Stroke Imaging Core Lab Team who prepared and performed the intracranial hemorrhage volumetric measurements, including MacKenzie Horn, Ana Alvarez, Dr CK Kim, Dr Fahad Al-Ajlan, Dr Henrik Gensicke, Dr Girish Kulkarni, Dr Hyun Seok Choi, Dr Linda Kasickova, Dr Aziz Al-Sultan, Anneliese Neweduk, and Dr Eric E. Smith. We also acknowledge Cello Health Communications for editorial assistance and document formatting services. We’d like to thank Hélène Dassule, PhD (Lexington, MA), a consultant for Alexion Pharmaceuticals Inc, for her help in drafting reviewer responses and making reviewer-requested changes. Her work was paid for by Alexion Pharmaceuticals.
Sources of Funding
This study was sponsored by Portola Pharmaceuticals, Inc, South San Francisco, California, United States, now Alexion Pharmaceuticals, Inc, Boston, Massachusetts, United States, following acquisition by Alexion. Dr Milling received support for this work from a National Institutes of Health NHLBI K23 grant.
Disclosures
Drs Yue, Conley, and Curnutte are employees and stockholders of Portola. Dr Demchuk reports the following: Circle NVI (patent holder, stockholder); Portola (consultant, ANNEXA-4 [Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of FXa Inhibitors] adjudication and steering committees, personal fees). Dr Milling reports the following: CSL Behring (consulting); Portola (grant support, steering committee); Octapharma (steering committee); Population Health Research Institute, McMasters University (personal fees). Dr Goldstein reports the following: Pfizer, Octapharma, Portola (grant support); Portola, CSL Behring, Octapharma, Phillips Healthcare, NControl Therapeutics (consulting); Takeda (research, consulting). Dr Middeldorp reports the following: Daiichi Sankyo, Bayer, Aspen Pharma (grant support); Bayer, Bristol Myers Squibb/Pfizer, Boehringer Ingelheim, Portola (advisory board); Portola (lecture fees); AbbVie (adjudication committee). Dr Verhamme reports the following: Bayer Healthcare, Boehringer Ingelheim, Daiichi Sankyo, Pfizer, Bristol Myers Squibb, Leo Pharma (grant support, consulting, lecture fees); Boehringer Ingelheim (adjudication committee); Anthos, Janssen, Portola (consulting). Dr Eikelboom reports the following: AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb/Pfizer, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Janssen, Sanofi-Aventis (grant support, honoraria); Servier (personal fees). Dr Crowther reports the following: Bristol Myers Squibb Canada, Asahi Kasei, Servier Canada, Precision Biologics, Hemostasis Reference Laboratory, CSL Behring (advisory board); Alexion, Pfizer, CSL Behring, Diagnostica Stago (educational funding); Bayer, Leo Pharma (grant support); Daiichi Sankyo, Bayer, Octapharma (Data Safety Monitoring Board). Dr Connolly reports the following: Portola, Bristol Myers Squibb, Bayer, Daiichi Sankyo (grant support, consulting); Javelin (consulting); patent (US 2010/0255000); patent-pending (US 2017/0369862 A1). Dr Lopez-Sendon reports the following: Amgen, Bayer, Boehringer Ingelheim, Merck, Pfizer, Portola, Sanofi (grant support); Boehringer Ingelheim, Menarini, Pfizer (personal fees). The other authors report no conflicts. Copyright Alexion Pharmaceuticals, Inc, and the authors.
Supplemental Materials
Definitions of Thrombotic Events
Online Tables I–III
Online Figures I–III
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ANNEXA-4
- Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of FXa Inhibitors
- FXa
- factor Xa
- GCS
- Glasgow Coma Scale
- ICrH
- intracranial hemorrhage
- NIHSS
- National Institutes of Health Stroke Scale
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.120.030565.
For Sources of Funding and Disclosures, see page 2104.
Contributor Information
Patrick Yue, Email: patmanmo@gmail.com.
Elena Zotova, Email: zotovae@gmail.com.
Juliet Nakamya, Email: Juliet.Nakamya@phri.ca.
Lizhen Xu, Email: Lizhen.Xu@phri.ca.
Tomoyuki Ohara, Email: ohar21@gmail.com.
Joshua N. Goldstein, Email: jgoldstein@partners.org.
Saskia Middeldorp, Email: Saskia.Middeldorp@radboudumc.nl.
Peter Verhamme, Email: peter.verhamme@uzleuven.be.
Jose Luis Lopez-Sendon, Email: jlopezsendon@gmail.com.
Pamela B. Conley, Email: pconley@portola.com.
John T. Curnutte, Email: jcurnutte@portola.com.
John W. Eikelboom, Email: eikelbj@mcmaster.ca.
Mark Crowther, Email: crowthrm@mcmaster.ca.
Stuart J. Connolly, Email: Stuart.Connolly@phri.ca.
References
- 1.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, et al. ; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 2.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, et al. ; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638 [DOI] [PubMed] [Google Scholar]
- 3.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, et al. ; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907 [DOI] [PubMed] [Google Scholar]
- 4.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, et al. ; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 5.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, et al. ; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. doi: 10.1161/CIR.0000000000000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giugliano RP, Ruff CT, Rost NS, Silverman S, Wiviott SD, Lowe C, Deenadayalu N, Murphy SA, Grip LT, Betcher JM, et al. ; ENGAGE AF-TIMI 48 Investigators. Cerebrovascular events in 21 105 patients with atrial fibrillation randomized to edoxaban versus warfarin: effective anticoagulation with factor Xa next generation in atrial fibrillation-thrombolysis in myocardial infarction 48. Stroke. 2014;45:2372–2378. doi: 10.1161/STROKEAHA.114.006025 [DOI] [PubMed] [Google Scholar]
- 7.Held C, Hylek EM, Alexander JH, Hanna M, Lopes RD, Wojdyla DM, Thomas L, Al-Khalidi H, Alings M, Xavier D, et al. Clinical outcomes and management associated with major bleeding in patients with atrial fibrillation treated with apixaban or warfarin: insights from the ARISTOTLE trial. Eur Heart J. 2015;36:1264–1272. doi: 10.1093/eurheartj/ehu463 [DOI] [PubMed] [Google Scholar]
- 8.ANDEXXA® (coagulation factor Xa (recombinant), inactivated-zhzo). Lyophilized Powder for Solution for Intravenous Injection [package insert]. 2021. Alexion Pharmaceuticals, Inc [Google Scholar]
- 9.European Medicines Agency. Press Release: First Antidote for Reversal of Anticoagulation with Factor Xa Inhibitors Apixaban and Rivaroxaban. January 3, 2019. Accessed August 26, 2020. http://www.ema.europa.eu/en/news/first-antidote-reversal-anticoagulation-factor-xa-inhibitors-apixaban-rivaroxaban
- 10.Lu G, DeGuzman FR, Hollenbach SJ, Karbarz MJ, Abe K, Lee G, Luan P, Hutchaleelaha A, Inagaki M, Conley PB, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013;19:446–451. doi: 10.1038/nm.3102 [DOI] [PubMed] [Google Scholar]
- 11.Siegal DM, Curnutte JT, Connolly SJ, Lu G, Conley PB, Wiens BL, Mathur VS, Castillo J, Bronson MD, Leeds JM, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373:2413–2424. doi: 10.1056/NEJMoa1510991 [DOI] [PubMed] [Google Scholar]
- 12.Connolly SJ, Crowther M, Eikelboom JW, Gibson CM, Curnutte JT, Lawrence JH, Yue P, Bronson MD, Lu G, Conley PB, et al. ; ANNEXA-4 Investigators. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380:1326–1335. doi: 10.1056/NEJMoa1814051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosior JC, Idris S, Dowlatshahi D, Alzawahmah M, Tymchuk S, Hill MD. Quantomo: validation of a computer-assisted method used in the predict trial for volumetric analysis of hematoma in intracerebral hemorrhage. Stroke. 2009;40:E226–E226. Abstract [DOI] [PubMed] [Google Scholar]
- 14.Khan A, Shishko K, Arcidiacono S, Bui K, Lu G, Conley PB, Winkler A. Modified anti-FXa assays for measuring the residual activity of apixaban and rivaroxaban in andexanet alfa-containing samples on the ACL TOP Family® Coagulation analyzers. Blood. 2019;134:1153–1153. [Google Scholar]
- 15.Sarode R, Milling TJ, Jr, Refaai MA, Mangione A, Schneider A, Durn BL, Goldstein JN. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation. 2013;128:1234–1243. doi: 10.1161/CIRCULATIONAHA.113.002283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broderick JP, Diringer MN, Hill MD, Brun NC, Mayer SA, Steiner T, Skolnick BE, Davis SM; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Determinants of intracerebral hemorrhage growth: an exploratory analysis. Stroke. 2007;38:1072–1075. doi: 10.1161/01.STR.0000258078.35316.30 [DOI] [PubMed] [Google Scholar]
- 17.Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE; VISTA Collaboration. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76:1238–1244. doi: 10.1212/WNL.0b013e3182143317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steiner T, Poli S, Griebe M, Hüsing J, Hajda J, Freiberger A, Bendszus M, Bösel J, Christensen H, Dohmen C, et al. Fresh frozen plasma versus prothrombin complex concentrate in patients with intracranial haemorrhage related to vitamin K antagonists (INCH): a randomised trial. Lancet Neurol. 2016;15:566–573. doi: 10.1016/S1474-4422(16)00110-1 [DOI] [PubMed] [Google Scholar]
- 19.Purrucker JC, Haas K, Rizos T, Khan S, Wolf M, Hennerici MG, Poli S, Kleinschnitz C, Steiner T, Heuschmann PU, et al. Early clinical and radiological course, management, and outcome of intracerebral hemorrhage related to new oral anticoagulants. JAMA Neurol. 2016;73:169–177. doi: 10.1001/jamaneurol.2015.3682 [DOI] [PubMed] [Google Scholar]
- 20.Wilson D, Seiffge DJ, Traenka C, Basir G, Purrucker JC, Rizos T, Sobowale OA, Sallinen H, Yeh SJ, Wu TY, et al. ; And the CROMIS-2 collaborators. Outcome of intracerebral hemorrhage associated with different oral anticoagulants. Neurology. 2017;88:1693–1700. doi: 10.1212/WNL.0000000000003886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsivgoulis G, Lioutas VA, Varelas P, Katsanos AH, Goyal N, Mikulik R, Barlinn K, Krogias C, Sharma VK, Vadikolias K, et al. Direct oral anticoagulant- vs vitamin K antagonist-related nontraumatic intracerebral hemorrhage. Neurology. 2017;89:1142–1151. doi: 10.1212/WNL.0000000000004362 [DOI] [PubMed] [Google Scholar]
- 22.Oertel M, Kelly DF, McArthur D, Boscardin WJ, Glenn TC, Lee JH, Gravori T, Obukhov D, McBride DQ, Martin NA. Progressive hemorrhage after head trauma: predictors and consequences of the evolving injury. J Neurosurg. 2002;96:109–116. doi: 10.3171/jns.2002.96.1.0109 [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi L, Barmparas G, Bosarge P, Brown CV, Bukur M, Carrick MM, Catalano RD, Holly-Nicolas J, Inaba K, Kaminski S, et al. ; AAST Multicenter Prospective Observational Study of Trauma Patients on Novel Oral Anticoagulants Study Group. Novel oral anticoagulants and trauma: The results of a prospective American Association for the Surgery of Trauma Multi-Institutional Trial. J Trauma Acute Care Surg. 2017;82:827–835. doi: 10.1097/TA.0000000000001414 [DOI] [PubMed] [Google Scholar]
- 24.Fakharian E, Abedzadeh-Kalahroudi M, Atoof F. Effect of tranexamic acid on prevention of hemorrhagic mass growth in patients with traumatic brain injury. World Neurosurg. 2018;109:e748–e753. doi: 10.1016/j.wneu.2017.10.075 [DOI] [PubMed] [Google Scholar]
- 25.Majeed A, Ågren A, Holmström M, Bruzelius M, Chaireti R, Odeberg J, Hempel EL, Magnusson M, Frisk T, Schulman S. Management of rivaroxaban- or apixaban-associated major bleeding with prothrombin complex concentrates: a cohort study. Blood. 2017;130:1706–1712. doi: 10.1182/blood-2017-05-782060 [DOI] [PubMed] [Google Scholar]
- 26.Schulman S, Gross PL, Ritchie B, Nahirniak S, Lin Y, Lieberman L, Carrier M, Crowther MA, Ghosh I, Lazo-Langner A, et al. ; Study Investigators. Prothrombin complex concentrate for major bleeding on factor Xa inhibitors: a prospective cohort study. Thromb Haemost. 2018;118:842–851. doi: 10.1055/s-0038-1636541 [DOI] [PubMed] [Google Scholar]
- 27.Ezekwudo DE, Gbadamosi B, Chacko R, Kamidi S, Blankenship L, Jaiyesimi I, Gaikazian S. Outcome on reinstitution of anticoagulation following intracranial hemorrhage: a single institutional analysis. Blood. 2017;130:628–628. [Google Scholar]
- 28.Inohara T, Xian Y, Liang L, Matsouaka RA, Saver JL, Smith EE, Schwamm LH, Reeves MJ, Hernandez AF, Bhatt DL, et al. Association of intracerebral hemorrhage among patients taking non-vitamin K antagonist vs vitamin K antagonist oral anticoagulants with in-hospital mortality. JAMA. 2018;319:463–473. doi: 10.1001/jama.2017.21917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piccini JP, Garg J, Patel MR, Lokhnygina Y, Goodman SG, Becker RC, Berkowitz SD, Breithardt G, Hacke W, Halperin JL, et al. ; ROCKET AF Investigators. Management of major bleeding events in patients treated with rivaroxaban vs. warfarin: results from the ROCKET AF trial. Eur Heart J. 2014;35:1873–1880. doi: 10.1093/eurheartj/ehu083 [DOI] [PubMed] [Google Scholar]
- 30.Maung AA, Bhattacharya B, Schuster KM, Davis KA. Trauma patients on new oral anticoagulation agents have lower mortality than those on warfarin. J Trauma Acute Care Surg. 2016;81:652–657. doi: 10.1097/TA.0000000000001189 [DOI] [PubMed] [Google Scholar]
- 31.Prexl O, Bruckbauer M, Voelckel W, Grottke O, Ponschab M, Maegele M, Schöchl H. The impact of direct oral anticoagulants in traumatic brain injury patients greater than 60-years-old. Scand J Trauma Resusc Emerg Med. 2018;26:20. doi: 10.1186/s13049-018-0487-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerner ST, Kuramatsu JB, Sembill JA, Sprügel MI, Endres M, Haeusler KG, Vajkoczy P, Ringleb PA, Purrucker J, Rizos T, et al. ; RETRACE II (German-Wide Multicenter Analysis of Oral Anticoagulation-Associated Intracerebral Hemorrhage II) Investigators. Association of prothrombin complex concentrate administration and hematoma enlargement in non-vitamin K antagonist oral anticoagulant-related intracerebral hemorrhage. Ann Neurol. 2018;83:186–196. doi: 10.1002/ana.25134 [DOI] [PubMed] [Google Scholar]
- 33.Panos NG, Cook AM, John S, Jones GM; Neurocritical Care Society (NCS) Pharmacy Study Group. Factor Xa inhibitor-related intracranial hemorrhage: results from a multicenter, observational cohort receiving prothrombin complex concentrates. Circulation. 2020;141:1681–1689. doi: 10.1161/CIRCULATIONAHA.120.045769 [DOI] [PubMed] [Google Scholar]
- 34.Dzik WH. Reversal of oral factor Xa inhibitors by prothrombin complex concentrates: a re-appraisal. J Thromb Haemost. 2015;13(suppl 1):S187–S194. doi: 10.1111/jth.12949 [DOI] [PubMed] [Google Scholar]
- 35.Lu G, Lin J, Bui K, Curnutte JT, Conley PB. Andexanet versus prothrombin complex concentrates: Differences in reversal of factor Xa inhibitors in in vitro thrombin generation. Res Pract Thromb Haemost. 2020;4:1282–1294. doi: 10.1002/rth2.12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Alexion Pharmaceuticals, Inc (Boston, MA) will consider requests for disclosure of clinical study participant-level data provided that participant privacy is assured through methods like data deidentification, pseudonymization, or anonymization (as required by applicable law) and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at https://alexion.com/our-research/research-and-development.
Link to Data Request Form (https://alexion.com/contact-alexion/medical-information).


