Supplemental Digital Content is available in the text.
Keywords: coronavirus, cytokines, embolic stroke, ischemic stroke, von Willebrand Factor
Background and Purpose:
Reports indicate an increased risk of ischemic stroke during coronavirus disease 2019 (COVID-19) infection. We aimed to identify patients with COVID-19 and ischemic stroke and explore markers of inflammation, hypercoagulability, and endotheliopathy, a structural and functional disturbance of the vascular endothelium due to a stressor.
Methods:
This was a retrospective, observational cohort study comparing acute ischemic stroke patients with and without COVID-19 across 3 hospitals. Timing of stroke onset during COVID-19 course and markers of inflammation, hypercoagulability, and endothelial activation were evaluated by COVID-19 status and stroke cause.
Results:
Twenty-one patients with ischemic stroke were diagnosed with COVID-19 during the study period. Patients with COVID-19 had a similar age and burden of vascular risk factors compared with the control cohort (n=168). We identified a temporal correlation between stroke onset and the peak of acute phase reactants, including CRP (C-reactive protein), ferritin, and d-dimer. In subsets of patients with labs available, embolic stroke of undetermined source was associated with elevated IL (interleukin)-6 (median, 171 [interquartile range, 13–375] versus 8 [4–11], P<0.01) and sIL (soluble IL)-2 receptor (1972 [1525–4720] versus 767 [563–1408.5], P=0.05) levels. Stroke patients with COVID-19 demonstrated elevated levels of endothelial activation markers compared with non-COVID-19 stroke controls (median von Willebrand activity 285.0% [interquartile range, 234%–382%] versus 150% [128%–183%], P=0.034; von Willebrand antigen 330.0% [265%–650%] versus 152% [130%–277%], P=0.007, and factor VIII 301% [289%–402%] versus 49% [26%–94%], P<0.001).
Conclusions:
Ischemic stroke in patients with COVID-19 is associated with endotheliopathy and a systemic inflammatory response in patients with vascular risk factors. Further research evaluating endothelial and inflammatory markers in the setting of ischemic stroke and COVID-19 in larger, prospective cohorts is needed to validate the findings.
Novel coronavirus disease 2019 (COVID-19) secondary to severe acute respiratory syndrome coronavirus 2 is associated with a diverse array of neurological complications, including ischemic stroke.1–4 Postulated mechanisms of ischemic stroke in COVID-19 include hypercoagulability and endothelial injury,5 although laboratory evidence of these pathways is sparse in patients with stroke. We compare clinical and laboratory characteristics of contemporaneous ischemic stroke patients with and without COVID-19. We analyze relationships between levels of serological markers and the timing and subtype of ischemic stroke among patients with COVID-19.
Methods
Full methodological details are provided in the Expanded Materials and Methods (Material in the Data Supplement). The data set compiled for this study will be made available by the corresponding author upon reasonable request. This was a retrospective, observational study of individuals with ischemic stroke and COVID-19 between March 1 and June 6, 2020, across 2 primary stroke centers and 1 comprehensive stroke center in Connecticut. Stroke patients with COVID-19 (n=21) were compared with stroke patients without COVID-19 (n=168) who presented in an overlapping time period. Severe acute respiratory syndrome coronavirus 2 infection was diagnosed by nasopharyngeal reverse transcriptase polymerase chain reaction. We analyzed data from a subset of patients for whom levels of acute phase reactants, inflammation, and hypercoagulability were collected for clinical care; temporal patterns in these laboratory values were analyzed if collected serially in the COVID-19 group (n=18) and analyzed for associations with stroke onset and cause. Laboratory studies for endothelial injury were analyzed on frozen plasma samples from both COVID-19 (n=8) and a subgroup of control patients who donated blood for research immediately before the pandemic (n=13) using the same clinical laboratory platform. The Institutional Review Board approved the study and all patients were cross-referenced with the institution’s research exemption database.
Results
Patient Characteristics
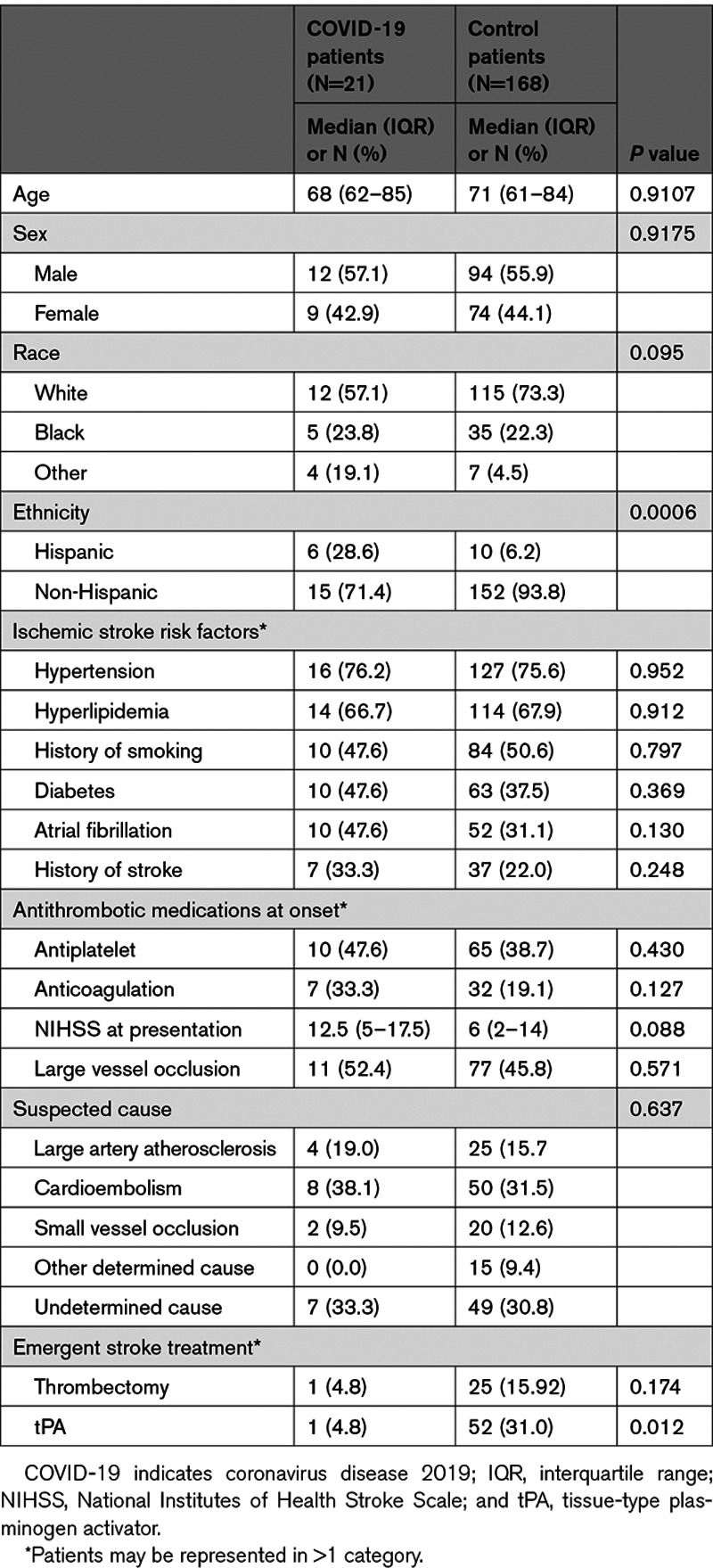
There were 21 stroke patients with COVID-19 and 168 without COVID-19 included in the study. Clinical characteristics are shown in Table 1. There was a significantly higher proportion of stroke patients with COVID-19 who were Hispanic in comparison to the control patients. Traditional stroke risk factors were similar between stroke patients with COVID-19 and control patients. There was no difference in large vessel occlusion or stroke cause between groups. Patients with COVID-19 were significantly less likely to receive tPA (tissue-type plasminogen activator) during the acute pandemic. Among the stroke patients with COVID-19, the median time from COVID-19 symptom onset to ischemic stroke was 7 (interquartile range, 0–13) days. Five patients were asymptomatic from COVID-19 when presenting with a stroke. Nearly 43% developed an additional nonstroke thrombosis (Table I in the Data Supplement).
Table 1.
Clinical Characteristics of Patients Presenting With Ischemic Stroke With and Without COVID-19
Inflammatory and Hypercoagulability Markers
Levels of acute phase reactants were serially measured in stroke patients with COVID-19 according to hospital protocols that were evolving over the course of the pandemic. Measures of inflammation were markedly elevated at the time of stroke presentation in the stroke patients with COVID-19, including CRP (C-reactive protein), D-dimer, ferritin, lactate dehydrogenase, sIL-2R, and IL-6 (Table 2). We hypothesized that risk of stroke would increase contemporaneously with increasing inflammation in patients hospitalized with COVID-19. We, therefore, examined temporal correlations between laboratory values and stroke onset (n=18). There was a temporal correlation between onset of stroke and the peak of ferritin, CRP, and D-dimer levels (but not other laboratory measures tested), with median and mode times from stroke to peak ranging from 0 to 1 days (Figure [A]).
Table 2.
Laboratory Values in Stroke Patients With and Without COVID-19
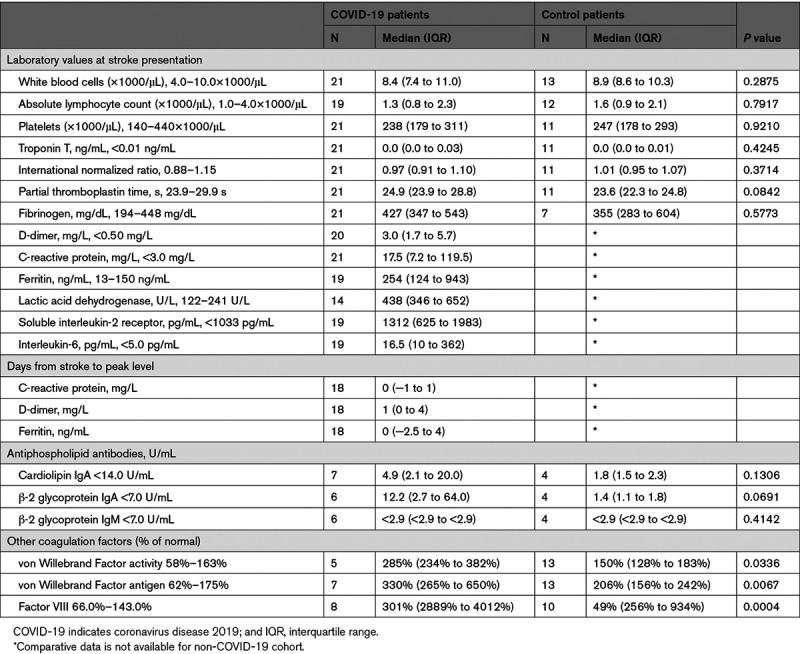
Figure.
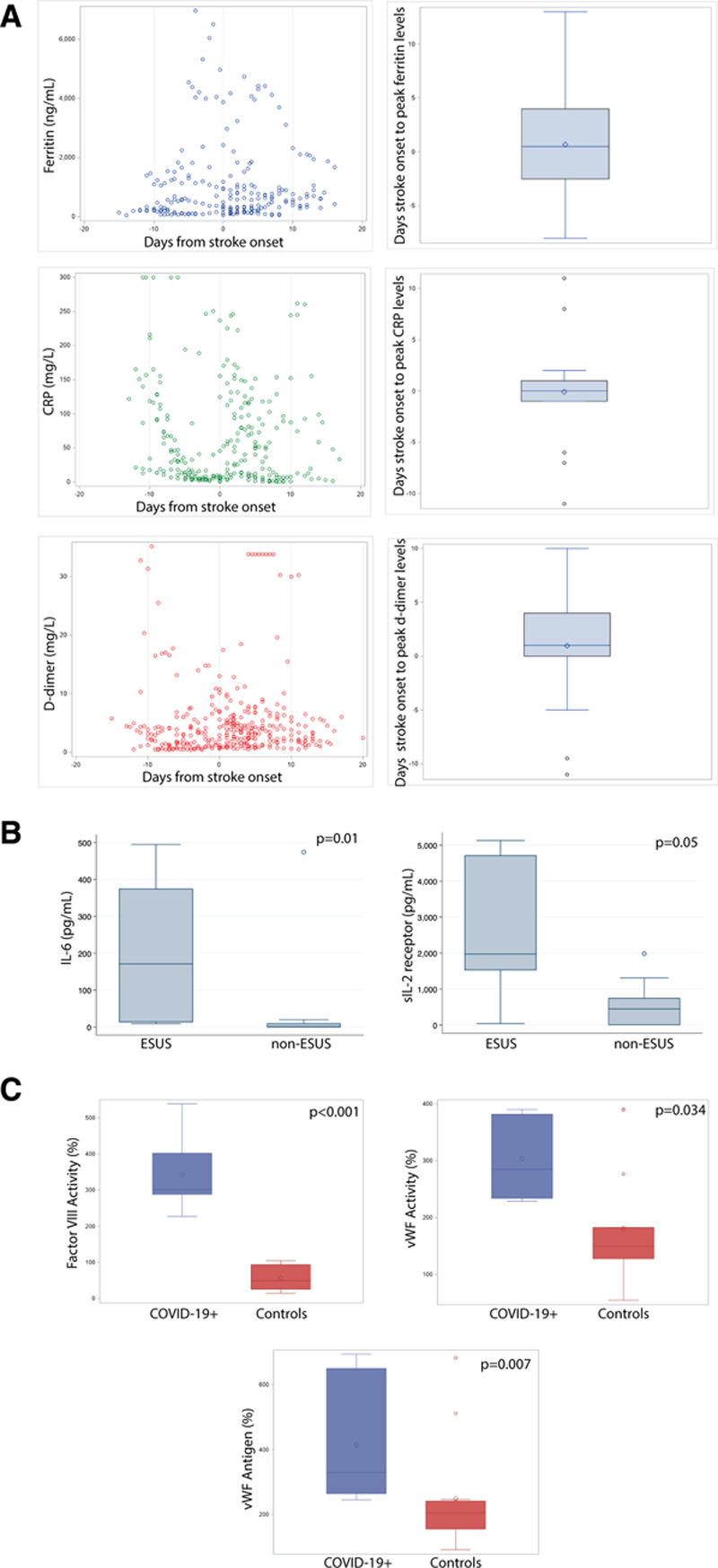
Laboratory values temporally associate with stroke onset and cause. A, Ferritin, CRP (C-reactive protein), and D-dimer levels among patients with coronavirus disease 2019 (COVID-19; n=21) as a function of days from stroke (left). Distribution of days from stroke (n=18) to peak levels (right). Comparative data not available for control patients. B, IL (Interleukin)-6 and sIL (soluble interleukin)-2R levels and embolic stroke of undetermined source (ESUS; n=7) vs non-ESUS (n=12) causes. C, Endotheliopathy markers in stroke patients with COVID-19 (n=5–8) compare to stroke patients without COVID-19 (n=10–13).
Given the emerging consideration of hypercoagulability as an cause for COVID-19 complications and the high rate of thrombosis in our cohort, we then asked whether hypercoagulability and inflammatory markers were associated with embolic stroke of undetermined source (ESUS) in the stroke patients with COVID-19. All 7 cases of cryptogenic stroke in the COVID-19 cohort met criteria for ESUS. Elevated IL-6 and sIL-2R (but not other inflammatory markers) at the time of stroke were significantly associated with ESUS (Figure [B]).
Routine testing for VWF (von Willebrand Factor) activity, VWF antigen, and factor VIII in patients with COVID-19 began prospectively midway during the study period. In the resulting subset of patients with these endotheliopathy laboratory values (n=8), all stroke patients with COVID-19 showed marked elevations at the time of stroke presentation (Table 2). To determine whether these elevations were specific to COVID-19 infection or due to endothelial activation in the setting of stroke or vascular disease, laboratory results from the stroke patients with COVID-19 were compared with a control group of stroke patients without COVID-19 who had donated blood samples for stroke research in the months immediately before a pause in research blood collection due to the pandemic. These stroke patients without COVID-19 (n=13) had similar demographic and clinical factors as the patients with COVID-19 (Table II in the Data Supplement). There was no difference in the days from stroke onset (or last known well) and sample collection between the groups (median days COVID-19 patients 1 [interquartile range, 0–3] versus controls 1 [1–1], P=0.77. Stroke patients with COVID-19 demonstrated elevated levels of endothelial activation compare to non-COVID-19 stroke controls (median von Willebrand activity 285% [234%–382%] versus 150% [128%–183%], P=0.034, von Willebrand antigen 330% [265%–650%] versus 152% [130%–277%], P=0.007, and factor VIII 301% [288.5%–401.8%] versus 49.2% [25.7%–93.6%], P<0.001; Figure [C]).
Discussion
This study describes the clinical characteristics of COVID-19 patients with ischemic stroke compared with control stroke patients. Patients in the 2 cohorts had similar age and high prevalence of vascular risk factors but a higher percentage of stroke patients with COVID-19 were Hispanic. The lower rate of tPA administration in the stroke patients with COVID-19 is likely explained by the fact that most stroke patients with COVID-19 presented after the tPA treatment window. Stroke patients with COVID-19 had a rate of large vessel occlusions comparable to control patients. Higher levels of IL-6 and sIL-2R were observed in patients with COVID-19 and ESUS. We found markedly elevated VWF activity, VWF antigen, and factor VIII activity in stroke patients with COVID-19. Based on data from this and other studies,6 we hypothesize ischemic stroke is due to an underlying endotheliopathy and thrombosis in COVID-19 distinct from disseminated intravascular coagulation or antiphospholipid antibody syndrome. Both atrial fibrillation and anticoagulation tended to be more prevalent in patients with COVID-19, although these were not significant. Atrial arrhythmias have been associated with severe COVID-19 infection,7 and a higher prevalence of anticoagulation in patients with COVID-19 could be related to comorbid thrombotic complications and protocolized use of antithrombotics in patients with severe inflammation.
In COVID-19, acute phase reactants and cytokines are markers of systemic inflammation and are not typical of control stroke patients. An increase in acute phase reactants was associated with an increase in procoagulants in COVID-19 in a recent study.8 We demonstrated the peak of acute phase reactants correlated with onset of stroke and found 42.9% of patients had additional comorbid thromboses. Furthermore, there was a strong correlation between IL-6 and sIL-2R levels with ESUS suggesting inflammation-associated hypercoagulability as a cause for embolic-appearing strokes. While a subset of our COVID-19 cohort was found to have abnormal antiphospholipid syndrome antibodies, particularly IgA, the clinical significance of IgA antibodies is unclear. We noted elevations in labs associated with endothelial cell activation in all stroke patients with COVID-19 tested compared with non-COVID-19 stroke controls with similar demographic and clinical characteristics. Endothelial cells express angiotensin-converting enzyme 2 and, when activated by infection and inflammation, release VWF.9,10 A recent study analyzed markers of endothelial cell activation or damage in patients with COVID-19, including soluble P-selectin, soluble thrombomodulin, and VWF, and identified endotheliopathy as a common pathophysiology of COVID-19 disease, particularly as patients become critically ill.6 Endothelial dysfunction and VWF release can occur in critically ill patients with acute respiratory distress syndrome in the absence of COVID-19.11 However, our subset of stroke patients with elevated VWF and factor VIII did not suffer a critical illness resulting in intubation, acute respiratory distress syndrome, or sepsis. The majority of our patients had preexisting vascular risk factors, suggesting prior endothelial dysfunction, perhaps rendering them more susceptible to developing an endotheliopathy and thrombosis in the setting of COVID-19. However, our results demonstrate the endotheliopathy observed in patients with COVID-19 is not present in the majority of stroke patients without COVID-19. The only 2 patients in our COVID-19 cohort with small vessel infarcts developed stroke >50 days from COVID-19 symptom onset and had persistently elevated VWF, factor VIII, and acute phase reactants, suggesting endotheliopathy may be longstanding after infection. Together, these findings support the proposed theory that COVID-19 disease triggers a systemic inflammatory response, which results in endothelial damage, activation, and hypercoagulability, significantly increasing the risk for thrombosis as observed in nonstroke cohorts.6,12–14 If confirmed, such evidence may support initiating prophylactic antithrombotic therapy in response to elevated inflammatory markers to prevent thrombotic complications of COVID-19, including ischemic stroke.
The limited number of patients with full laboratory panels is a weakness of this study. This was dictated by clinical care and the numbers of patients with stroke during the pandemic in our region; our results should be interpreted as hypothesis-generating. In addition, the sample size of 21 stroke patients with COVID-19 limited our power to detection of only large differences between the patient groups. Our data highlight the need for prospective, longitudinal analyses exploring endothelial activation, inflammation, and hypercoagulability in larger cohorts of patients with ischemic stroke and COVID-19, including broader panels of endotheliopathy laboratory studies, standardized timing of measurements, and follow-up testing to determine the persistence of abnormalities in this patient population.
Conclusions
In a convenience cohort during a pandemic, we provide evidence of endotheliopathy and systemic inflammation due to COVID-19 in ischemic stroke patients with vascular risk factors when compared with contemporaneous controls. The results provide insight on potential pathways to be investigated future prospective studies evaluating endothelial injury and inflammation in the setting of COVID-19 to optimize therapies for stroke prevention and treatment.
Sources of Funding
Dr Sansing is supported by the National Institutes of Health (NIH) R01NS097728, R01NS095993, U01NS113445, and American Heart Association EIA34770133, Dr Sheth by NIH U01NS106513-02S1, RO1NR018335, R01NS110721, RO3NS112859, U01NS106513, UO1NS113445, U24NS107215, U24NS107136, and American Heart Association 20SRG35540018 and 17CSA33550004.
Disclosures
Dr Sheth reports grants from Bard, Novartis, Biogen, and Hyperfine, equity in Alva, Chair of DSMB for Zoll study and provides consulting for NControl and Ceribell. Dr Chun reports grants and personal fees from Pfizer and personal fees from Astra Zeneca outside the submitted work. LHS reports funding from the National Institutes of Health (NIH).
Supplemental Materials
Expanded Materials and Methods
Online Tables I–II
Supplementary Material
Nonstandard Abbreviations and Acronyms
- COVID-19
- coronavirus disease 2019
- CRP
- C-reactive protein
- ESUS
- embolic stroke of undetermined source
- IL
- interleukin
- sIL
- soluble interleukin
- tPA
- tissue-type plasminogen activator
- VWF
- von Willebrand Factor
R. Sharma and L.H. Sansing contributed equally.
This manuscript was sent to Marc Fisher, Senior Guest Editor, for review by expert referees, editorial decision, and final disposition.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.120.031971.
For Sources of Funding and Disclosures, see page e237.
Contributor Information
Lindsay S. McAlpine, Email: lindsay.mcalpine@yale.edu.
Adeel S. Zubair, Email: adeel.zubair@yale.edu.
Ilavarasy Maran, Email: Ilavarasy.maran@yale.edu.
Pola Chojecka, Email: pola.chojecka@gmail.com.
Paul Lleva, Email: paul.lleva@yale.edu.
Adam S. Jasne, Email: adam.jasne@yale.edu.
Dhasakumar Navaratnam, Email: Dhasakumar.Navaratnam@yale.edu.
Charles Matouk, Email: charles.matouk@yale.edu.
Joseph Schindler, Email: joseph.schindler@yale.edu.
Kevin N. Sheth, Email: kevin.sheth@yale.edu.
Hyung Chun, Email: hyung.chun@yale.edu.
Alfred I. Lee, Email: alfred.lee@yale.edu.
Serena Spudich, Email: serena.spudich@yale.edu.
Richa Sharma, Email: richa.sharma@yale.edu.
References
- 1.Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of Coronavirus Disease 2019: a review. JAMA Neurol. 2020;77:1018–1027. doi: 10.1001/jamaneurol.2020.2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K, Henninger N, Trivedi T, Lillemoe K, Alam S, et al. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51:2002–2011. doi: 10.1161/STROKEAHA.120.030335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merkler AE, Parikh NS, Mir S, Gupta A, Kamel H, Lin E, Lantos J, Schenck EJ, Goyal P, Bruce SS, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (covid-19) vs patients with influenza. JAMA Neurol. 2020;77:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothstein A, Oldridge O, Schwennesen H, Do D, Cucchiara BL. Acute cerebrovascular events in hospitalized COVID-19 patients. Stroke. 2020;51:e219–e222. doi: 10.1161/STROKEAHA.120.030995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hess DC, Eldahshan W, Rutkowski E. COVID-19-related stroke. Transl Stroke Res. 2020;11:322–325. doi: 10.1007/s12975-020-00818-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goshua G, Pine AB, Meizlish ML, Chang CH, Zhang H, Bahel P, Baluha A, Bar N, Bona RD, Burns AJ, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colon CM, Barrios JG, Chiles JW, McElwee SK, Russell DW, Maddox WR, Kay GN. Atrial arrhythmias in COVID-19 patients. JACC Clin Electrophysiol. 2020;6:1189–1190. doi: 10.1016/j.jacep.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masi P, Hékimian G, Lejeune M, Chommeloux J, Desnos C, Pineton De Chambrun M, Martin-Toutain I, Nieszkowska A, Lebreton G, Bréchot N, et al. Systemic inflammatory response syndrome is a major contributor to COVID-19-associated coagulopathy: insights from a prospective, single-center cohort study. Circulation. 2020;142:611–614. doi: 10.1161/CIRCULATIONAHA.120.048925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plautz WE, Matthay ZA, Rollins-Raval MA, Raval JS, Kornblith LZ, Neal MD. Von Willebrand factor as a thrombotic and inflammatory mediator in critical illness. Transfusion. 2020;60(suppl 3):S158–S166. doi: 10.1111/trf.15667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varatharajah N, Rajah S. Microthrombotic complications of COVID-19 are likely due to embolism of circulating endothelial derived ultralarge Von Willebrand factor (eULVWF) decorated-platelet strings. Fed Pract. 2020;37:e1–e2. [PMC free article] [PubMed] [Google Scholar]
- 13.Iba T, Levy JH, Connors JM, Warkentin TE, Thachil J, Levi M. The unique characteristics of COVID-19 coagulopathy. Crit Care. 2020;24:360. doi: 10.1186/s13054-020-03077-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A, Peyvandi F, Tripodi A. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


