Abstract
Background
Beta‐blockers and inhibitors of the renin‐angiotensin‐aldosterone system improve survival and reduce morbidity in people with heart failure with reduced left ventricular ejection fraction (LVEF); a review of the evidence is required to determine whether these treatments are beneficial for people with heart failure with preserved ejection fraction (HFpEF).
Objectives
To assess the effects of beta‐blockers, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, angiotensin receptor neprilysin inhibitors, and mineralocorticoid receptor antagonists in people with HFpEF.
Search methods
We updated searches of CENTRAL, MEDLINE, Embase, and one clinical trial register on 14 May 2020 to identify eligible studies, with no language or date restrictions. We checked references from trial reports and review articles for additional studies.
Selection criteria
We included randomised controlled trials with a parallel group design, enrolling adults with HFpEF, defined by LVEF greater than 40%.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included 41 randomised controlled trials (231 reports), totalling 23,492 participants across all comparisons. The risk of bias was frequently unclear and only five studies had a low risk of bias in all domains.
Beta‐blockers (BBs)
We included 10 studies (3087 participants) investigating BBs. Five studies used a placebo comparator and in five the comparator was usual care. The mean age of participants ranged from 30 years to 81 years.
A possible reduction in cardiovascular mortality was observed (risk ratio (RR) 0.78, 95% confidence interval (CI) 0.62 to 0.99; number needed to treat for an additional benefit (NNTB) 25; 1046 participants; three studies), however, the certainty of evidence was low. There may be little to no effect on all‐cause mortality (RR 0.82, 95% CI 0.67 to 1.00; 1105 participants; four studies; low‐certainty evidence). The effects on heart failure hospitalisation, hyperkalaemia, and quality of life remain uncertain.
Mineralocorticoid receptor antagonists (MRAs)
We included 13 studies (4459 participants) investigating MRA. Eight studies used a placebo comparator and in five the comparator was usual care. The mean age of participants ranged from 54.5 to 80 years.
Pooled analysis indicated that MRA treatment probably reduces heart failure hospitalisation (RR 0.82, 95% CI 0.69 to 0.98; NNTB = 41; 3714 participants; three studies; moderate‐certainty evidence). However, MRA treatment probably has little or no effect on all‐cause mortality (RR 0.91, 95% CI 0.78 to 1.06; 4207 participants; five studies; moderate‐certainty evidence) and cardiovascular mortality (RR 0.90, 95% CI 0.74 to 1.11; 4070 participants; three studies; moderate‐certainty evidence). MRA treatment may have little or no effect on quality of life measures (mean difference (MD) 0.84, 95% CI ‐2.30 to 3.98; 511 participants; three studies; low‐certainty evidence). MRA treatment was associated with a higher risk of hyperkalaemia (RR 2.11, 95% CI 1.77 to 2.51; number needed to treat for an additional harmful outcome (NNTH) = 11; 4291 participants; six studies; high‐certainty evidence).
Angiotensin‐converting enzyme inhibitors (ACEIs)
We included eight studies (2061 participants) investigating ACEIs. Three studies used a placebo comparator and in five the comparator was usual care. The mean age of participants ranged from 70 to 82 years.
Pooled analyses with moderate‐certainty evidence suggest that ACEI treatment likely has little or no effect on cardiovascular mortality (RR 0.93, 95% CI 0.61 to 1.42; 945 participants; two studies), all‐cause mortality (RR 1.04, 95% CI 0.75 to 1.45; 1187 participants; five studies) and heart failure hospitalisation (RR 0.86, 95% CI 0.64 to 1.15; 1019 participants; three studies), and may result in little or no effect on the quality of life (MD ‐0.09, 95% CI ‐3.66 to 3.48; 154 participants; two studies; low‐certainty evidence). The effects on hyperkalaemia remain uncertain.
Angiotensin receptor blockers (ARBs)
Eight studies (8755 participants) investigating ARBs were included. Five studies used a placebo comparator and in three the comparator was usual care. The mean age of participants ranged from 61 to 75 years.
Pooled analyses with high certainty of evidence suggest that ARB treatment has little or no effect on cardiovascular mortality (RR 1.02, 95% 0.90 to 1.14; 7254 participants; three studies), all‐cause mortality (RR 1.01, 95% CI 0.92 to 1.11; 7964 participants; four studies), heart failure hospitalisation (RR 0.92, 95% CI 0.83 to 1.02; 7254 participants; three studies), and quality of life (MD 0.41, 95% CI ‐0.86 to 1.67; 3117 participants; three studies). ARB was associated with a higher risk of hyperkalaemia (RR 1.88, 95% CI 1.07 to 3.33; 7148 participants; two studies; high‐certainty evidence).
Angiotensin receptor neprilysin inhibitors (ARNIs)
Three studies (7702 participants) investigating ARNIs were included. Two studies used ARBs as the comparator and one used standardised medical therapy, based on participants' established treatments at enrolment. The mean age of participants ranged from 71 to 73 years.
Results suggest that ARNIs may have little or no effect on cardiovascular mortality (RR 0.96, 95% CI 0.79 to 1.15; 4796 participants; one study; moderate‐certainty evidence), all‐cause mortality (RR 0.97, 95% CI 0.84 to 1.11; 7663 participants; three studies; high‐certainty evidence), or quality of life (high‐certainty evidence). However, ARNI treatment may result in a slight reduction in heart failure hospitalisation, compared to usual care (RR 0.89, 95% CI 0.80 to 1.00; 7362 participants; two studies; moderate‐certainty evidence). ARNI treatment was associated with a reduced risk of hyperkalaemia compared with valsartan (RR 0.88, 95% CI 0.77 to 1.01; 5054 participants; two studies; moderate‐certainty evidence).
Authors' conclusions
There is evidence that MRA and ARNI treatment in HFpEF probably reduces heart failure hospitalisation but probably has little or no effect on cardiovascular mortality and quality of life. BB treatment may reduce the risk of cardiovascular mortality, however, further trials are needed. The current evidence for BBs, ACEIs, and ARBs is limited and does not support their use in HFpEF in the absence of an alternative indication. Although MRAs and ARNIs are probably effective at reducing the risk of heart failure hospitalisation, the treatment effect sizes are modest. There is a need for improved approaches to patient stratification to identify the subgroup of patients who are most likely to benefit from MRAs and ARNIs, as well as for an improved understanding of disease biology, and for new therapeutic approaches.
Plain language summary
Beta‐blockers and inhibitors of the renin‐angiotensin aldosterone system for chronic heart failure with preserved ejection fraction
Review question
We investigated the effects of beta‐blockers (BBs), mineralocorticoid receptor antagonists (MRAs), angiotensin converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs) and angiotensin receptor neprilysin inhibitors (ARNIs) on survival, hospital admissions for heart failure, quality of life and potassium levels in people with heart failure with preserved ejection fraction (HFpEF).
Background
Heart failure is a common condition that occurs when the function of the heart muscle is impaired, being associated with symptoms of breathlessness and fatigue, and a reduction in survival. In around half of cases of heart failure, where the left ventricular ejection fraction is reduced to less than 40% (reflecting significant impairment of contractile function), several drug treatments are known to be effective at improving survival and reducing hospitalisation. In the remaining cases, where the ejection fraction is normal or only mildly reduced (HFpEF), it is not clear whether the same drug treatments are effective at improving outcomes.
Selection criteria
We sought to investigate whether treatments for heart failure with reduced ejection fraction are also effective in HFpEF. We conducted a comprehensive search for all trials investigating BBs, MRAs, ACEIs, ARBs or ARNIs (evidence current to 14 May 2020).
Results and conclusions
We included 10 studies with 3087 randomised participants for BBs, 13 studies with 4459 randomised participants for MRAs, eight studies with 2061 randomised participants for ACEIs, eight studies with 8755 randomised participants for ARBs and three studies with 7702 randomised participants for ARNIs. We combined the evidence in a pooled analysis for each drug class and for each of the outcomes assessed. Not all included studies are part of each analysis.
We found that BBs may improve cardiovascular mortality. However, the certainty of evidence was low due to small trials and uncertainty about the methods used. For MRAs, the results suggest a reduction in heart failure hospitalisation but little or no effect on cardiovascular and all‐cause mortality; however, the certainty of evidence was only moderate. For ACEIs, treatment probably has little or no effect on the outcomes of cardiovascular mortality, all‐cause mortality and heart failure hospitalisation; however, the certainty of evidence was only moderate. We found high certainty of evidence for ARB treatment, with the results suggesting little or no effect. We found that ARNI treatment has little or no effect on cardiovascular mortality (moderate‐certainty evidence), all‐cause mortality (high‐certainty evidence), or quality of life (high‐certainty evidence). ARB treatment may reduce slightly heart failure hospitalisations (moderate‐certainty evidence). Treatment with MRAs and ARBs was found to increase the risk of high potassium in the blood.
In conclusion, treatment with MRAs, and possibly ARNIs, was found to result in a slight reduction in the risk of hospitalisation due to heart failure. There was some evidence of a possible beneficial effect of BB on mortality due to cardiovascular disease. Treatment with ACEI probably has no beneficial effect in people with HFpEF, however, this remains uncertain due to a lack of evidence from clinical trials. For ARBs, the evidence suggested that treatment is of little or no benefit in people with HFpEF.
Certainty of the evidence
The certainty of evidence ranged from high to low across the outcomes and drug classes studied. With the exception of ARBs and ARNIs, there was a lack of large‐scale trials in HFpEF for the interventions and outcomes tested.
Summary of findings
Summary of findings 1. Beta‐blockers compared to placebo or no treatment for chronic heart failure with preserved ejection fraction.
| Beta‐blockers compared to placebo or no treatment for chronic heart failure with preserved ejection fraction | ||||||
| Patient or population: patients with chronic heart failure with preserved ejection fraction Setting: secondary care Intervention: beta‐blockers Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with beta‐blockers | |||||
| Cardiovascular mortality (RR) follow‐up: range 21 months to 3.2 years | Study population | RR 0.78 (0.62 to 0.99) | 1046 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Three additional studies (ELANDD; SWEDIC; Takeda 2004) reported that no deaths occurred | |
| 173 per 1000 | 135 per 1000 (107 to 171) | |||||
| Heart failure hospitalisation (RR) follow‐up: range 6 months to 3.2 years | Study population | RR 0.73 (0.47 to 1.13) | 449 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 | Follow‐up unclear for SWEDIC. ELANDD reported that no hospitalisation due to heart failure occurred | |
| 117 per 1000 | 86 per 1000 (55 to 133) | |||||
| Hyperkalaemia follow‐up: mean 3.2 years |
245 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1 6 | J‐DHF reported one participant in the intervention group (N = 120) experienced hyperkalaemia but did not report on this outcome for the control group. No further data were available from any of the other studies. | |||
| All‐cause mortality (RR) follow‐up: range 21 months to 3.2 years | Study population | RR 0.82 (0.67 to 1.00) | 1105 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Follow‐up unclear for Adamyan 2010. ELANDD, SWEDIC and Takeda 2004 reported that no deaths occurred. | |
| 243 per 1000 | 199 per 1000 (163 to 243) | |||||
| Quality of life (MLHFQ): from 0 to 105 follow‐up: mean 6 months | Mean quality of life (MLHFQ) was 24 | MD 1 lower (9.05 lower to 7.05 higher) | ‐ | 93 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 4 5 | Lower = better, 5 point difference considered to be clinically meaningful |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; MLHFQ: Minnesota Living with Heart Failure Questionnaire; RCT: Randomised controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by one level due to study limitations (unclear selection bias in most studies).
2Downgraded by one level due to imprecision (concerns about the smaller study being more precise than the larger study).
3Downgraded by two levels due to imprecision (few events and wide CI).
4Downgraded by two levels due to imprecision (very small sample size).
5Downgraded by one level due to suspected publication bias (this is a patient‐relevant outcome that is not reported in most studies).
6Downgraded by two levels due to suspected publication bias (incomplete reporting).
Summary of findings 2. Mineralocorticoid receptor antagonists (MRAs) compared to placebo or no treatment for chronic heart failure with preserved ejection fraction.
| MRAs compared to placebo or no treatment for chronic heart failure with preserved ejection fraction | ||||||
| Patient or population: patients with chronic heart failure with preserved ejection fraction Setting: secondary care Intervention: MRAs Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with MRAs | |||||
| Cardiovascular mortality (RR) follow‐up: range 12 months to 3.3 years | Study population | RR 0.90 (0.74 to 1.11) | 4070 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | Two additional trials (RAAM‐PEF; Kurrelmeyer 2014) reported that no deaths occurred | |
| 88 per 1000 | 79 per 1000 (65 to 97) | |||||
| Heart failure hospitalisation (RR) follow‐up: range 24 weeks to 3.3 years | Study population | RR 0.82 (0.69 to 0.98) | 3714 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | Three additional trials (ALDO‐DHF; Kurrelmeyer 2014; Upadhya 2017) reported that no hospitalisation due to heart failure occurred | |
| 136 per 1000 | 112 per 1000 (94 to 134) | |||||
| Hyperkalaemia follow‐up: range 24 weeks to 3.3 years | Study population | RR 2.11 (1.77 to 2.52) | 4291 (6 RCTs) | ⊕⊕⊕⊕ HIGH | Two trials defined hyperkalaemia ≥ 5.5 mEg/L | |
| 83 per 1000 | 175 per 1000 (146 to 208) | |||||
| All‐cause mortality follow‐up: range 9 months to 3.3 years | Study population | RR 0.91 (0.78 to 1.06) | 4207 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | Two additional trials (RAAM‐PEF; Kurrelmeyer 2014) reported that no deaths occurred | |
| 133 per 1000 | 121 per 1000 (104 to 141) | |||||
| Quality of life (MLHFQ): from 0 to 105 follow‐up: range 9 months to 12 months | Mean quality of life (MLHFQ) ranged from 20 to 25 | MD 0.84 higher (2.30 lower to 3.98 higher) | ‐ | 511 (3 RCTs) | ⊕⊕⊝⊝ LOW 2 3 | Lower = better, 5 points are considered a clinically significant difference We did not pre‐specify which QoL scale was to be reported in the 'Summary of findings' table. To aid comparisons among 'Summary of findings' tables we chose to include the Minnesota Living with Heart Failure questionnaire and not the SMD across two scales |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; MLHFQ: Minnesota Living with Heart Failure Questionnaire; MRAs: Mineralocorticoid receptor antagonists; RCT: Randomised controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by one level due to imprecision.
2Downgraded by one level due to study limitations (one trial was open label).
3Downgraded by one level due to imprecision (small sample size).
Summary of findings 3. Angiotensin‐converting enzyme inhibitors (ACEIs) compared to placebo or no treatment for chronic heart failure with preserved ejection fraction.
| ACEIs compared to placebo or no treatment for chronic heart failure with preserved ejection fraction | ||||||
| Patient or population: patients with chronic heart failure with preserved ejection fraction Setting: secondary care Intervention: ACEIs Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo/no treatment | Risk with ACEI | |||||
| Cardiovascular mortality (RR) follow‐up: range mean 12 months to mean 26.2 months | Study population | RR 0.93 (0.61 to 1.42) | 945 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | One additional trial (Kitzman 2010) reported that no deaths occurred | |
| 86 per 1000 | 81 per 1000 (53 to 123) | |||||
| Heart failure hospitalisation (RR) follow‐up: range 6 months to 26.2 months | Study population | RR 0.86 (0.64 to 1.15) | 1019 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 13 per 1000 | 11 per 1000 (8 to 15) | |||||
| Hyperkalaemia follow‐up: 6 months |
74 (1 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 4 | One trial (Zi 2003) reported 2 events in the intervention group (N = 36), 0 events in the control group (N = 38) (RR 5.27, 95% CI 0.26 to 106.16) | |||
| All‐cause mortality (RR) follow‐up: range mean 6 months to mean 26.2 months | Study population | RR 1.04 (0.75 to 1.45) | 1187 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | One additional trial (Kitzman 2010) reported that no deaths occurred | |
| 102 per 1000 | 106 per 1000 (77 to 148) | |||||
| Quality of life (MLHFQ): from 0 to 105 follow‐up: mean 12 months | Mean quality of life (MLHFQ) ranged from 10.9 to 29 | MD 0.09 lower (3.66 lower to 3.48 higher) | ‐ | 154 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 3 | Scale: 0 to 105, lower = better, 5 point difference considered clinically relevant One trial (SNEGOVIK) reported mean change from baseline of ‐19.8 for intervention and ‐10.7 for control |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACEIs: Angiotensin‐converting enzyme inhibitors; CI: Confidence interval; MD: Mean difference; MLHFQ: Minnesota Living with Heart Failure Questionnaire; RCT: Randomised controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by one level due to imprecision (wide CI).
2Downgraded by one level due to study limitations (risk of bias (open label)).
3Downgraded by one level due to imprecision (low sample size).
4Downgraded by one level due to study limitations (unclear selection bias).
Summary of findings 4. Angiotensin receptor blockers (ARBs) compared to placebo or no treatment for chronic heart failure with preserved ejection fraction.
| ARBs compared to placebo or no treatment for chronic heart failure with preserved ejection fraction | ||||||
| Patient or population: patients with chronic heart failure with preserved ejection fraction Setting: secondary care Intervention: ARBs Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with ARBs | |||||
| Cardiovascular mortality (RR) follow‐up: range mean 12 months to mean 49.5 months | Study population | RR 1.02 (0.90 to 1.14) | 7254 (3 RCTs) | ⊕⊕⊕⊕ HIGH | One additional trial (Parthasarathy 2009) reported that no deaths occurred | |
| 131 per 1000 | 133 per 1000 (118 to 149) | |||||
| Heart failure hospitalisation (RR) follow‐up: range mean 12 months to mean 49.5 months | Study population | RR 0.92 (0.83 to 1.02) | 7254 (3 RCTs) | ⊕⊕⊕⊕ HIGH | ||
| 171 per 1,‐000 | 157 per 1,‐000 (142 to 174) | |||||
| Hyperkalaemia follow‐up: range 36.6 months to 49.5 months | Study population | RR 1.88 (1.07 to 3.33) | 7148 (2 RCTs) | ⊕⊕⊕⊕ HIGH | ||
| 3 per 1,000 | 5 per 1,000 (3 to 8) | |||||
| All‐cause mortality (RR) follow up: range 1 years to 4.4 years | Study population | RR 1.01 (0.92 to 1.11) | 7964 (4 RCTs) | ⊕⊕⊕⊕ HIGH | One additional trial (Parthasarathy 2009) reported that no deaths occurred | |
| 72 per 1000 | 73 per 1,‐000 (66 to 80) | |||||
| Quality of life (MLHFQ): from 0 to 105 follow‐up: range mean 13.8 weeks to mean 49.5 months | Mean quality of life (MLHFQ) ranged from 10.9 to 31.6 | MD 0.41 higher (0.86 lower to 1.67 higher) | ‐ | 3117 (3 RCTs) | ⊕⊕⊕⊕ HIGH | Scale: 0 to 105, lower = better, 5 point difference considered clinically relevant |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ARBs: Angiotensin receptor blockers; CI: Confidence interval; MD: Mean difference; MLHFQ: Minnesota Living with Heart Failure Questionnaire; RCT: Randomised controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
Summary of findings 5. Angiotensin receptor neprilysin inhibitors (ARNIs) compared to usual care for chronic heart failure with preserved ejection fraction.
| ARNIs compared to usual care for chronic heart failure with preserved ejection fraction | ||||||
| Patient or population: patients with chronic heart failure with preserved ejection fraction Setting: secondary care Intervention: ARNI (sacubitril‐valsartan) Comparison: ARB (valsartan) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with ARNI | |||||
| Cardiovascular mortality (RR) Median follow‐up: 35 months |
Study population | RR 0.96 (0.79 to 1.15) | 4796 (1 study) | ⊕⊕⊕⊝ MODERATE1 | ||
| 89 per 1,000 | 85 per 1,000 (70 to 102) | |||||
| Heart failure hospitalisation, first (RR) Range of follow‐up: 24 weeks to 35 months |
Study population | RR 0.89 (0.80 to 1.00) |
7362 (2 studies) |
⊕⊕⊕⊝ MODERATE1 | ||
| 142 per 1,000 | 126 per 1,000 (113 to 142) |
|||||
| Hyperkalaemia Range of follow‐up: 36 weeks to 35 months |
Study population | RR 0.88 (0.77 to 1.01) | 5054 (2 studies) | ⊕⊕⊕⊝ MODERATE1 | ||
| 147 per 1,000 | 129 per 1,000 (113 to 148) | |||||
| All‐cause mortality (RR) Range of follow‐up: 36 weeks to 35 months |
Study population | (RR 0.97 CI 0.84 to 1.11) |
7663 (3 studies) | ⊕⊕⊕⊕ HIGH | ||
| 138 per 1,000 | 134 per 1,000 (117 to 153) | |||||
| Quality of life Range of follow‐up: 36 weeks to 35 months |
PARAMOUNT reported change from baseline for the KCCQ overall summary score for the intervention arm (n =118) as 11.25 (2.185) and the control arm (n = 116) as 11.31 (2.183) and summarised the findings as "no difference in KCCQ score between treatment groups". PARAGON‐HF reported a difference of the clinical summary score KCCQ between treatment arms as 1.0 (0.0 to 2.1). PARALLAX reported "KCCQ improved in both treatment groups, with an early benefit of S/V that was no longer significant after 24 week". |
⊕⊕⊕⊕ HIGH | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ARB: Angiotensin receptor blocker; ARNIs: Angiotensin receptor neprilysin inhibitors; CI: Confidence interval; KCCQ: Kansas City Cardiomyopathy Questionnaire; MD: Mean difference; RCT: Randomised controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by one level due to imprecision.
Background
Description of the condition
Heart failure is a clinical syndrome characterised by breathlessness and fatigue that results when abnormalities of cardiac structure and function lead to inadequate cardiac output, elevated ventricular filling pressures, or both (Ponikowski 2016). Based on available data from the United States and Europe, the prevalence of heart failure is estimated to range from 1% to 12% of the adult population and is projected to increase with populations ageing and with improved survival from cardiovascular disease (Roger 2013). Heart failure represents a significant public health problem, accounting for 5% of emergency medical admissions to hospital in the United Kingdom (NICE 2018). It is associated with significant mortality risk, with a ten‐year survival of 27%, compared with 75% in the general population, matched for age and sex (Taylor 2012). Heart failure is classified according to the left ventricular ejection fraction (LVEF) into two categories: heart failure with reduced ejection fraction (HFrEF, typically considered as LVEF less than 40%), and heart failure with preserved ejection fraction (HFpEF, typically LVEF greater than 40%). Recently, an intermediate subgroup was defined by the European Society of Cardiology as heart failure with mid‐range ejection fraction (HFmrEF) defined as LVEF 40% to 49% (Ponikowski 2016). This was defined by the American College of Cardiology as borderline HFpEF, defined as LVEF 41% to 49% (Yancy 2013). In this review, we defined HFpEF as LVEF greater than 40% because completed and ongoing HFpEF trials have used a range of LVEF cut‐offs, between 40% and 50%. HFpEF accounts for approximately half of all cases of HF; mortality outcomes are similar to those for HFrEF (Gerber 2015).
Description of the intervention
Neurohumoral inhibition with beta‐blockers (BB), angiotensin converting enzyme inhibitors (ACEIs), and mineralocorticoid receptor antagonists (MRAs) leads to improved survival and a reduction in hospitalisations for heart failure in people with HFrEF (CIBIS Investigators 1999; Consensus Trial Study Group 1987; Flather 2005; Hjalmarson 2000; Kotecha 2014; MERIT‐HF Study Group 1999; Packer 1999; Packer 2002; Packer 2001; Pitt 1999; Ponikowski 2016; SOLVD Investigators 1991; SOLVD Investigators 1992; Zannad 2011). Angiotensin receptor antagonists (ARBs) are recommended as an alternative to ACEIs in patients with intolerance to these agents or, in combination with ACEIs, for patients treated with BBs who are unable to tolerate MRAs (Ponikowski 2016). Angiotensin receptor neprilysin inhibitors (ARNIs) are recommended as an alternative to ACEIs, with superior efficacy in people with HFrEF who remain symptomatic despite optimal therapy (McMurray 2014). Although neurohumoral activation is observed in HFpEF (Hogg 2005), comparatively fewer clinical trials of neurohumoral inhibitor therapies have been performed in this population. The existing evidence from individual trials of BBs, MRAs, ACEIs, ARBs or MRAs in people with HFpEF does not support a reduction in mortality with these treatments (Ponikowski 2016). However, limited evidence indicates that candesartan (an ARB) (Yusuf 2003) and spironolactone (an MRA) (Pitt 2014) may be effective in reducing numbers of people hospitalised with HF.
This review sought to determine whether neurohumoral inhibition with therapies that improve mortality and morbidity in those with HFrEF (BBs, MRAs, ACEIs, ARBs, and ARNIs) have similar benefit in people with HFpEF.
How the intervention might work
In people with HFpEF, inadequate cardiac function triggers compensatory neurohumoral responses similar to those observed in HFrEF (Hogg 2005). Activation of the renin‐angiotensin aldosterone system (RAAS) and increased tone of the sympathetic nervous system may be adaptive in the short term; however, chronic activation is likely to be detrimental. Pre‐clinical disease models of HFpEF suggest that RAAS activation leads to maladaptive hypertrophy and fibrosis (Sharma 2014). ACEIs, ARBs or MRAs inhibit components of the RAAS system to counter the over‐activation that occurs in people with HF. ARNIs combine inhibition of RAAS with an ARB (valsartan) with augmentation of the natriuretic peptide system by inhibition of neprilysin (sacubitril). Neprilysin is a neutral endopeptidase that degrades a number of endogenous vasoactive peptides serving to counteract some of the effects of RAAS activation (McMurray 2014). The beneficial effects of BB therapy in people with HFrEF are thought to be mediated reducing the downstream effects associated with increased tone of the sympathetic nervous system, including increased heart rate, adverse myocardial energetics, and stimulation of RAAS (Sackner‐Bernstein 1995). These mechanisms may also be important in HFpEF and the effect of BBs to increase diastolic filling time may be particularly important (Sharma 2014); however, it is also possible that BB treatment may have adverse effects in this patient population. The population of people with HFpEF is heterogeneous, both with respect to disease aetiology and comorbidity. However, it is possible that neurohumoral activation represents a common pathophysiological mechanism that could be successfully targeted to improve clinical outcomes in people with heart failure across the spectrum of LVEF.
Why it is important to do this review
There is uncertainty as to whether BBs or RAAS inhibitors are effective at reducing mortality and heart failure hospitalisation and improving quality of life in people with HFpEF. Guidelines offer no specific treatment recommendations regarding the use of these therapies beyond the management of comorbidities, aside from a weak recommendation that ARBs and MRA might be considered to reduce hospitalisations (Yancy 2017). The European Society of Cardiology guidelines for the diagnosis and treatment of acute and chronic heart failure highlight a gap in the evidence for the effects of ARNIs and BBs in the treatment of HFpEF (Ponikowski 2016).
A recent systematic review and meta‐analysis of pharmacotherapy in HFpEF included BBs and RAAS inhibitors (ACEIs, ARBs and MRAs) and suggested a reduction in cardiovascular and all‐cause mortality with BB therapy (Zheng 2018). An updated review with a more comprehensive search strategy is needed to inform new guideline recommendations and to inform the conduct of further clinical trials.
Objectives
To assess the effects of BBs, ACEIs, ARBs, ARNIs, and MRAs in people with HFpEF.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) with parallel group design. We excluded cross‐over trials because we considered these to be inappropriate for our review question due to the progressive nature of HF.
Studies published in full‐text or as abstracts, or available only as unpublished data, were eligible for inclusion.
Types of participants
We included studies with adult participants (aged ≥ 18 years) with HFpEF defined by a LVEF greater than 40%. We recognised that there was likely to be significant heterogeneity among study populations, relating to the disease definition, and we summarise this narratively in the Discussion. In relation to ejection fraction, we contacted study authors to obtain data on the subgroup of interest for studies with mixed populations.
Types of interventions
We performed separate meta‐analyses of studies that compared BBs, MRAs, ACEIs, ARBs or ARNIs, in addition to standard care. For BBs, MRAs, ACEIs and ARBs, the comparator was with placebo or no‐treatment control. For ARNIs, we meta‐analysed studies that compared sacubitril‐valsartan with valsartan, an ARB.
Types of outcome measures
Reporting one of more of the listed outcomes in the trial was not an inclusion criterion for the review. We assessed outcomes at the longest reported follow‐up.
Primary outcomes
Cardiovascular mortality.
Heart failure hospitalisation (number of participants with at least one hospitalisation, analysed as risk ratios (RR), and time to first event, analysed as hazard ratios (HR)).
Hyperkalaemia.
Secondary outcomes
All‐cause mortality.
Quality of life (measured with either the 'Minnesota Living with Heart Failure Questionnaire' (MLHFQ) or the 'Kansas City Cardiomyopathy Questionnaire' (KCCQ)).
Withdrawal due to adverse event (hypotension, hyperkalaemia or renal impairment).
Search methods for identification of studies
Electronic searches
We updated the systematic searches of the following bibliographic databases on 14 May 2020:
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (Issue 5 of 12, 2020);
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and MEDLINE (Ovid, 1946 to 12 May 2020); and
Embase and Embase Classic (Ovid, 1947 to 13 May 2020) .
The search strategies used in 2017 are included in Appendix 1 and those used in 2020 are in Appendix 2. We applied the Cochrane sensitivity‐maximising RCT filter (Lefebvre 2019) to MEDLINE (Ovid). For Embase, the RCT filter with the best optimisation of sensitivity and specificity was applied, as in the previous search. (Wong 2006).
We did not impose any restriction on language of publication.
Searching other resources
We searched ClinicalTrials.gov (clinicaltrials.gov) for ongoing trials on 14 May 2020. We were unable to search the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch) as it was unavailable at the time of searching. Search terms for the trials registers are also listed in Appendix 1 (from 2017) and Appendix 2 (from 2020).
We checked all primary references of included studies and systematic reviews for additional references. For studies identified as eligible from clinical trial register records, we searched for associated publications on PubMed and in recent conference presentations.
We contacted study authors to clarify details or obtain additional information not included in the published reports.
Data collection and analysis
Selection of studies
Two review authors (KM and NM) independently screened titles and abstracts of all records identified in our search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. In the event of disagreement, a third review author was asked to arbitrate (TL). We then retrieved the full‐text study reports for records identified as eligible, potentially eligible or unclear. Two review authors (KM and NM) independently screened the full‐text articles and identified studies for inclusion. We recorded reasons for exclusion of ineligible studies. We resolved any disagreement by consensus or consulted a third review author (TL). We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report is the unit of interest in the review. We recorded the selection process and completed a PRISMA flow diagram (Figure 1) and the Characteristics of excluded studies table.
1.
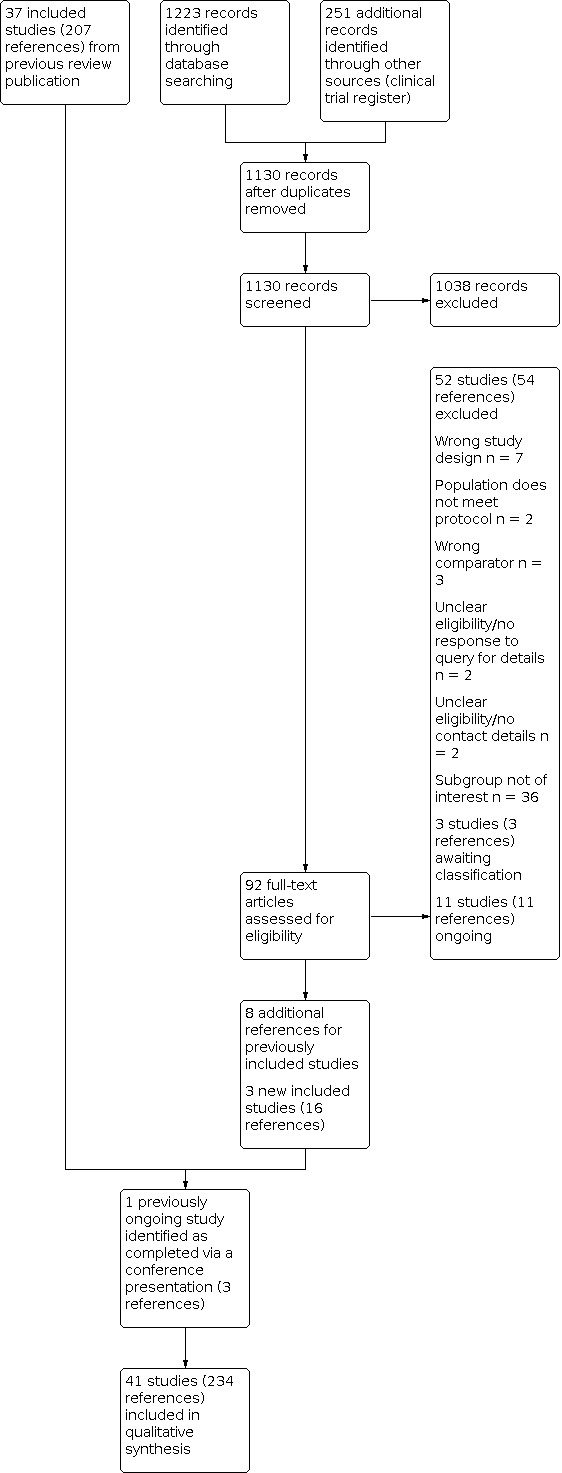
Study flow diagram
Data extraction and management
We used a data collection form to record study characteristics and outcome data from included studies, which had been piloted on two studies in the review (PEP‐CHF; TOPCAT). Some modifications were made after the pilot phase. Two review authors (NM and TL for Martin 2018, and NM and KM for this update) extracted study characteristics from included studies as follows:
Methods: study design, duration of follow‐up, details of any 'run in' period, number of study centres and location, study setting, withdrawals, and start/end date of enrolment.
Participants: number randomised/withdrawn/lost to follow‐up/analysed, mean age/age range, percent male, inclusion criteria, exclusion criteria, systolic blood pressure, heart rate, body mass index, serum creatinine, B‐type natriuretic peptide, NT pro B‐type natriuretic peptide, LVEF, New York Heart Association (NYHA) class, comorbidity (hypertension, diabetes, atrial fibrillation, hospitalisation for HF, coronary heart disease, stroke, medications at baseline).
Interventions: intervention, comparison, concomitant medications (diuretic, digoxin, BBs, ACEIs, ARBs, MRAs).
Outcomes: planned and reported.
Notes: sources of funding, and notable conflicts of interest of trial authors.
Two review authors (NM and TL for Martin 2018 and NM and KM for this update) independently extracted outcome data from included studies. Disagreements were resolved by consensus. One review author (NM) transferred data into the Review Manager 5 (RevMan 2014) file. One review author (TL) double‐checked that data were entered correctly by comparing the data presented in the systematic review with the data extraction sheet.
Assessment of risk of bias in included studies
Two review authors (NM and TL) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Disagreements were resolved by consensus. We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We judged each potential source of bias as high, low or unclear and provided quotes from study reports together with justification for our judgment in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. Where information on risk of bias related to unpublished data or correspondence with a triallist, we noted this in the 'Risk of bias' table.
When considering treatment effects in the pooled analysis, we accounted for risk of bias for the studies that contributed to each outcome tested.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported protocol deviations in the Differences between protocol and review section.
Measures of treatment effect
We analysed dichotomous data as RR with 95% confidence intervals (CIs), and continuous data as mean difference (MD) or standardized mean difference (SMD), with 95% CIs. In addition, wherever possible, we performed a pooled analysis of outcomes reported as HRs. We used SMD for one analysis when combining quality of life data reported for two different scales and followed Cohen's effect sizes of 0.2 representing a small effect, 0.5 a moderate effect and 0.8 a large effect (Cohen 1988). We entered data presented as a scale with a consistent direction of effect.
Unit of analysis issues
We included one three‐arm trial (Hong Kong DHF). Because two intervention arms contributed to two separate comparisons, no unit of analysis issue arose.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and where possible, obtain missing numerical outcome data.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. We considered possible causes in cases of substantial heterogeneity (I² = 50% to 100%).
Assessment of reporting biases
We pooled fewer than 10 trials for each comparison. Therefore, we did not examine funnel plots to explore possible small‐study biases for the primary outcomes. We plan to do so, should a sufficient number of trials become available in future updates of this review.
Data synthesis
We undertook meta‐analyses only where this was meaningful, that is, if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense. We used a fixed‐effect model in the absence of substantial heterogeneity (I² < 50%) and a random‐effects model when unexplained substantial heterogeneity was present (I² ≥ 50%). We applied a random‐effects model for quality of life analyses for MRAs to account the high heterogeneity observed for the KCCQ (Analysis 2.7; I² = 86%) and to permit a combined analysis with outcome data from the MLHFQ (Analysis 2.6; I² = 50%).
2.7. Analysis.

Comparison 2: Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 7: Quality of life (KCCQ)
2.6. Analysis.

Comparison 2: Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 6: Quality of life
We considered two relevant quality of life scales: the MLHFQ or KCCQ. The MLHFQ score has a range from 0 to 105, lower scores indicate better quality of life. The KCCQ score has a range from 0 to 100, higher scores indicate better quality of life. To account for the difference in the direction of the scale of the KCCQ, the mean values were multiplied by ‐1 (Cochrane Handbookfor Systematic Reviews of Interventions, section 9.2.3.2, Deeks 2011). For the purpose of interpretation, we considered a five point difference in score as clinically significant for the MLHFQ (Rector 1995) and KCCQ (Spertus 2005).
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
Age. The potential effect of age as an effect modifier of RAAS inhibition is not well described. We planned to explore outcomes for subgroups defined by age as less than 70 years and 70 years and over. This is based on the median age of participants in two major HFpEF trials: the TOPCAT median age was 68.7 years and the PARAGON‐HF mean age was 72 years.
Sex.
HFmrEF LVEF 40% to 49% and preserved LVEF equal to or greater than 50%.
Length of follow‐up less than 12 months and equal to or greater than 12 months.
We were unable to perform subgroup analyses, due to insufficient data (Deeks 2019).
If we had been able to conduct subgroup analyses, we had planned to use the outcomes of cardiovascular mortality and hospitalisation for heart failure and the formal test for subgroup interactions in Review Manager 5 (RevMan 2014).
Sensitivity analysis
We performed a sensitivity analysis for risk of bias by performing a pooled analysis that included only studies with a low risk of bias (where at least four of the six domains for bias assessment were judged to be low risk, and no domain was at high risk of bias).
Summary of findings and assessment of the certainty of the evidence
We created 'Summary of findings' tables for each of our five interventions and included the following outcomes: cardiovascular mortality, heart failure hospitalisation, all‐cause mortality, quality of life (when available MLHFQ, alternatively KCCQ) and hyperkalaemia. We used the five GRADE considerations (study limitations, inconsistencies, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence as it related to the studies which contributed data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEpro software (GRADEpro GDT). Four review authors assessed the certainty of evidence (TL, NM, KM, CD). We used footnotes to document our justification for decisions to downgrade the certainty of evidence.
Results
Description of studies
Results of the search
We previously included 37 studies, reported in 207 references (Martin 2018). For this update, the database searches identified 1223 new reports, and a search of ClinicalTrials.gov retrieved 251 additional records. After de‐duplication, we screened 1130 records based on the titles and abstracts. Of these, 1038 did not meet the inclusion criteria and were excluded. The remaining 92 records were assessed for eligibility in full‐text and 24 references were included. We identified eight additional references for four previously included studies (CHARM‐Preserved; STRUCTURE; TOPCAT; Yuksek 2012). We included four new studies in this updated review (McDiarmid 2020; PARAGON‐HF; PARALLAX; PARAMOUNT), reported in 17 references. PARALLAX had previously been identified as an ongoing study. We thus now include a total of 41 studies in this review
We identified 11 new ongoing studies, bringing the total of ongoing studies to 13. Three new studies were added to the category of 'Studies awaiting classification' (Botoni 2010; PER‐010‐15; Przewlocka‐Kosmala 2017) bringing the total to nine studies in this category.
Included studies
We included 41 studies (234 reports) that involved a total of 26,059 participants.
BBs
We included 10 studies (3087 participants) that investigated BBs for HFpEF. Of these, five studies compared BBs versus placebo (ELANDD; Mittal 2017; Sahoo 2016; SENIORS; SWEDIC) and five versus usual care (Adamyan 2010; Aronow 1997; J‐DHF; Shu 2005; Takeda 2004). Four studies investigated carvedilol: Adamyan 2010 (up to 50 mg daily), J‐DHF (up to 10 mg twice daily), SWEDIC (up to 25 mg twice daily or 50 mg twice daily in people weighing over 85 kg), Takeda 2004 (up to 20 mg daily). Two studies used nebivolol: ELANDD (up to 10 mg daily) and SENIORS (up to 10 mg daily). One study used propranolol: Aronow 1997 (30 mg, three times daily); and two studies investigated metoprolol succinate: Mittal 2017; Sahoo 2016 (up to 100 mg daily). Shu 2005 investigated bisoprolol (up to 10 mg daily).
Numbers of participants randomised ranged from 40 (Mittal 2017; Takeda 2004) to 643 (SENIORS).
Four were multicentre studies. ELANDD was conducted across 12 centres in eight countries in Europe; J‐DHF was assumed to have taken place in Japan; SENIORS took place in 11 countries (Czech Republic, France, Germany, Hungary, Italy, Netherlands, Romania, Spain, Switzerland, UK and Ukraine), and SWEDIC took place in 12 centres in Sweden. Mittal 2017 and Sahoo 2016 were each conducted in one centre in India. Adamyan 2010, Aronow 1997 and Shu 2005 did not report numbers of centres or countries, but we assumed that Adamyan 2010 likely took place in Armenia. Takeda 2004 was a single centre trial in Japan.
Three studies did not report LVEF of the included participants at baseline (Adamyan 2010; Shu 2005; SWEDIC). Six studies reported LVEF at baseline with a mean ranging from 56% to 63% (Aronow 1997; ELANDD; J‐DHF; Mittal 2017; Sahoo 2016; Takeda 2004). SENIORS included participants with a "clinical history of chronic HF with at least 1 of the following features: documented hospital admission within the previous 12 months with a discharge diagnosis of congestive HF or documented LVEF ≤ 35% within the previous 6 months". The SENIORS study reported a subgroup of participants with LVEF greater than 40% and these outcome data were used in our analysis (643 participants).
Most participants were NYHA class II (51% to 78%). Shu 2005 did not report participants' NYHA class at baseline. Participants' mean age ranged from 30 years to 81 years; six studies reported mean age less than 70 years (Adamyan 2010; ELANDD; Mittal 2017; Sahoo 2016; Shu 2005; SWEDIC) and four reported mean age above 70 years (Aronow 1997; J‐DHF; SENIORS; Takeda 2004).
Three studies were funded by industry (ELANDD; SENIORS; SWEDIC); two studies were funded by not‐for‐profit organisations (J‐DHF; Mittal 2017); and five did not report sources of funding (Adamyan 2010; Aronow 1997; Sahoo 2016; Shu 2005; Takeda 2004).
MRAs
We included 13 studies that investigated MRAs for HFpEF. Of these, eight compared MRAs versus placebo (ALDO‐DHF; AREA IN‐CHF; Kurrelmeyer 2014; Mottram 2004; RAAM‐PEF; STRUCTURE; TOPCAT; Upadhya 2017) and five versus usual care (Karapysh 2015; Mak 2009; McDiarmid 2020; Orea‐Tejeda 2007; Wang 2010). Ten studies investigated spironolactone (ALDO‐DHF; Kurrelmeyer 2014 (initiated at 25 mg daily and up‐titrated to a maximum of 50 mg daily); McDiarmid 2020 (25 mg daily); Mottram 2004; STRUCTURE; Upadhya 2017 (25 mg daily); Karapysh 2015; Orea‐Tejeda 2007 (25 mg daily, up‐titrated if tolerated to 50 mg daily); TOPCAT (15 mg daily, increased to a maximum of 45 mg daily); Wang 2010 (50 mg daily)). Two studies used eplerenone (Mak 2009; RAAM‐PEF (25 mg daily to a maximum of 50 mg daily)). AREA IN‐CHF investigated canrenone at a maximum dose of 50 mg daily.
Numbers of participants randomised ranged from 28 (Orea‐Tejeda 2007) to 3445 (TOPCAT). Four were multicentre trials; ALDO‐DHF included 10 centres in Germany and Austria; AREA IN‐CHF was conducted in 46 centres in Italy; STRUCTURE had centres in Poland (the number is unclear but publication states "of each centre"); and TOPCAT was conducted across 233 sites in six countries (Argentina, Brazil, Canada, Georgia, Russia, USA). Six studies were single‐centre trials; two in USA (Kurrelmeyer 2014; RAAM‐PEF), one in the UK (McDiarmid 2020), one in Australia (Mottram 2004) and one in Taiwan (Wang 2010); Mak 2009 was a single‐centre trial but the country was unspecified. Three trials did not report on numbers of centres or countries (Karapysh 2015; Orea‐Tejeda 2007; Upadhya 2017).
Two studies (Karapysh 2015; Mottram 2004) did not report participants' LVEF at baseline. AREA IN‐CHF had a mean LVEF at baseline of 39.9% (intervention) and 39.7% (control) for the overall included participants (N = 467). However, we obtained outcome data for the subgroup of participants with LVEF greater than 40% (N = 225). The LVEF in the remaining seven studies ranged from 54% to 72%.
In one study, most participants were NYHA class I (McDiarmid 2020, 61%). Most participants in five studies were NYHA class II (52% to 88%; ALDO‐DHF; Mak 2009; RAAM‐PEF; STRUCTURE; TOPCAT). Most participants in two studies were NYHA class III (58% to 64%; Kurrelmeyer 2014; Upadhya 2017). Three studies did not report NYHA class for participants eligible for inclusion in our review (AREA IN‐CHF; Karapysh 2015; Mottram 2004). Orea‐Tejeda 2007 reported that most participants in the intervention arm were NYHA class III (57.1%) and NYHA class I (75%) in the control arm.
Participants' mean age ranged from 54.5 years to 80 years; seven studies included participants whose mean age was less than 70 years (ALDO‐DHF; AREA IN‐CHF; Karapysh 2015; Mottram 2004; Orea‐Tejeda 2007; STRUCTURE; TOPCAT). In five studies, participants' mean age was over 70 years (Kurrelmeyer 2014; Mak 2009; McDiarmid 2020; RAAM‐PEF; Upadhya 2017).
AREA IN‐CHF was industry funded; seven studies were funded by not‐for‐profit organisations (ALDO‐DHF; Kurrelmeyer 2014; McDiarmid 2020; RAAM‐PEF; STRUCTURE; TOPCAT; Upadhya 2017). Five studies did not report sources of funding (Karapysh 2015; Mak 2009; Mottram 2004; Orea‐Tejeda 2007; Wang 2010).
ACEIs
We included eight studies that investigated ACEIs for HFpEF. Of these, three compared ACEIs with placebo (Kitzman 2010; PEP‐CHF; Zi 2003), and five versus usual care (Aronow 1993; Aronow 1998; Hong Kong DHF; SNEGOVIK; Yuksek 2012). Two studies investigated enalapril (Aronow 1993 up to 20 mg daily; Kitzman 2010 up to 10 mg daily). Aronow 1998 investigated benazepril (up to 40 mg daily). Two studies investigated perindopril (PEP‐CHF up to 4 mg daily; Yuksek 2012, up to 10 mg). Hong Kong DHF investigated ramipril in one of two active arms (maximum of 10 mg daily). Two studies investigated quinapril (SNEGOVIK, dose not reported; Zi 2003, up to 40 mg daily).
Numbers of participants randomised ranged from 21 (Aronow 1993) to 850 (PEP‐CHF). Two studies were reportedly multicentre trials (Hong Kong DHF; PEP‐CHF). Hong Kong DHF did not report details on the number of centres. PEP‐CHF was conducted at 53 centres in Bulgaria (3), Czech Republic (5), Hungary (10), Ireland (1), Poland (26), Russia (1), Slovakia (2), and the UK (5). Zi 2003 took place at one hospital in the UK and Yuksek 2012 was conducted in Turkey. The countries or number of centres were not reported in four studies (Aronow 1993; Aronow 1998; Kitzman 2010; SNEGOVIK).
The mean LVEF of the included participants at baseline was not reported by two studies (SNEGOVIK; Zi 2003). LVEF ranged from 61% to 69% in five studies (Aronow 1993; Aronow 1998; Hong Kong DHF; Kitzman 2010; PEP‐CHF). Most participants were classified in NYHA class II in four studies (Hong Kong DHF; Kitzman 2010; PEP‐CHF; Zi 2003) and in NYHA class III in one study (Aronow 1993). Two studies did not report participants' NYHA class at baseline (Aronow 1998; SNEGOVIK).
Participants' mean age ranged from 70 years to 82 years with all studies equal to or over a mean age of 70 years.
Four studies did not report funding sources (Aronow 1993; Aronow 1998; SNEGOVIK; Yuksek 2012). Three studies were industry‐funded (Hong Kong DHF; PEP‐CHF; Zi 2003) and one study was funded by a not‐for profit organisation (Kitzman 2010).
ARBs
We included eight studies that investigated ARBs for HFpEF. Of these, five compared ARBs versus placebo (CAN‐DHF; CHARM‐Preserved; I‐PRESERVE; Kasama 2005; Parthasarathy 2009) and three compared ARBs versus usual care (CandHeart; Hong Kong DHF; SUPPORT). Four studies investigated candesartan (CAN‐DHF; CandHeart; CHARM‐Preserved (up to 32 mg daily), Kasama 2005 (8 mg to 12 mg daily)). Two studies investigated irbesartan (one of the two active treatment arms in Hong Kong DHF (up to 75 mg daily), I‐PRESERVE (up to 300 mg)). Parthasarathy 2009 investigated valsartan (80 mg daily). SUPPORT investigated olmesartan (up to 40 mg daily).
Numbers of participants randomised ranged from 22 (CAN‐DHF) to 4128 (I‐PRESERVE). Seven were multicentre trials: CAN‐DHF was conducted at eight centres in Germany; CandHeart at 70 centres in Italy; CHARM‐Preserved was conducted at 618 centres in 26 countries; I‐PRESERVE involved 293 centres in 25 countries; Parthasarathy 2009 was conducted at five centres each in Germany and the UK; and SUPPORT was conducted at 17 centres in Japan. Hong Kong DHF was reported to be a multicentre trial but no details were provided on numbers of centres or countries. Kasama 2005 was reported to be a single‐centre trial in Japan.
The mean LVEF of the included participants at baseline was not reported by CAN‐DHF and ranged from 49% to 72% in seven studies (CandHeart; CHARM‐Preserved; Hong Kong DHF; I‐PRESERVE; Kasama 2005; Parthasarathy 2009; SUPPORT). Most participants were assessed as NYHA class II at baseline in five studies (CandHeart; CHARM‐Preserved; Hong Kong DHF; Kasama 2005; SUPPORT); NYHA class III in I‐PRESERVE; and was not reported by two studies (CAN‐DHF; Parthasarathy 2009).
Participants' mean age ranged from 61 years to 75 years. Mean age was below 70 years in six studies (CAN‐DHF; CandHeart; CHARM‐Preserved; Kasama 2005; Parthasarathy 2009; SUPPORT) and over 70 years in two studies (Hong Kong DHF; I‐PRESERVE).
Six studies were funded by industry (CAN‐DHF; CandHeart; CHARM‐Preserved; Hong Kong DHF; I‐PRESERVE; Parthasarathy 2009). SUPPORT was funded by a not‐for‐profit organisation. Kasama 2005 did not report the source of funding.
ARNIs
For this update, we elected to include three studies comparing an ARNI (sacubitril‐valsartan) with an ARB (valsartan) (PARAMOUNT and PARAGON‐HF), and one study comparing an ARNI (sacubitril‐valsartan) with individualised medical therapy whereby the comparator was specified according to the RAAS treatment status of patients at study enrolment; patients treated with ACEI at enrolment received LCZ696 or enalapril, those treated with ARB received LCZ696 or valsartan, and those without prior treatment with RAAS inhibition received LCZ696 or matching placebo) (PARALLAX).
Administration of ARBs or ACEIs in combination with sacubitril‐valsartan is contraindicated due to safety concerns. Therefore, the investigators specified the active comparator of valsartan, given that many patient with HFpEF receive ARB or ACEI treatment for hypertension (Solomon 2012). Since both treatment arms received valsartan, these studies isolate the effects of neprilysin. However, this can only be safely administered in combination with an ARB (i.e. the ARNI class of therapeutics).
Number of participants randomised ranged from 308 (PARAMOUNT) to 4822 participants (PARAGON‐HF). PARAGON‐HF was a multicentre trial across 848 centres in 43 countries and PARAMOUNT had 65 centres and 13 countries. The mean LVEF was 56 to 58% across the three trials. Most participants in these trials were assessed as NYHA class II at baseline. Participants' mean age ranged from 71 to 73 years of age. Novartis funded the three studies.
PERSPECTIVE (EUCTR2016‐001254‐17) is an ongoing RCT to examine the effect of LCZ696 compared to valsartan on cognitive outcomes in participants with HFpEF, defined as LVEF greater than 40%.
Excluded studies
We previously excluded 303 studies (324 references) based on full‐text assessment (Martin 2018). Details for the reasons for exclusion are provided in the Characteristics of excluded studies table. In this update, we have excluded 52 studies (54 references) and added these to the list for exclusions below. We referenced in the review only those that most closely missed the inclusion criteria (four references). In summary, we made exclusions based on the following considerations:
population does not meet protocol: n = 118;
wrong intervention: n = 8;
wrong comparator: n = 23;
wrong study design: n = 125;
subgroup of interest but no response to our enquiry for data: n = 8;
subgroup not of interest: n = 36;
unclear eligibility and no response to our enquiry for details: n = 12;
unclear eligibility and no current contact details: n = 15;
completed status in trial registry record but no published results and no response to our enquiry for data: n = 1;
missing data and response that no details can be provided: n = 6;
retraction: n = 1; and
did not take place as planned: n = 2.
Studies awaiting classification
We identified nine studies that are awaiting classification (Anonymous 2003d; Botoni 2010; Dielievska 2015; EUCTR2005‐001306‐87; EUCTR2005‐002109‐22Metra 1999; PER‐010‐15; Przewlocka‐Kosmala 2017; Rapezzi 1999. See Characteristics of studies awaiting classification. We are waiting to retrieve the full‐text (n = 4), await responses from translators (n = 3) and await response from the triallists to clarify eligibility (n = 2).
Ongoing studies
From the previously identified ongoing studies, two have now been included (McDiarmid 2020; PARALLAX). We identified 11 new ongoing studies, bringing the current total to 13 ongoing studies (Characteristics of ongoing studies).
Risk of bias in included studies
The 'Risk of bias' assessments are detailed in the Characteristics of included studies tables. We summarised them in the text below and in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Twelve studies reported random sequence methods and were rated as being at low risk of bias (ALDO‐DHF; CandHeart; ELANDD; Hong Kong DHF; I‐PRESERVE; McDiarmid 2020; PARAGON‐HF; PARAMOUNT; PEP‐CHF; Sahoo 2016; SENIORS; TOPCAT). We assessed 28 studies to be at unclear risk of bias for this domain because no information was provided in study reports. One study was assessed as high risk of bias in this domain as it randomised participants on the basis of admission sequence (Shu 2005).
Twelve studies used a method for allocation concealment that was judged to be at low risk of bias (ALDO‐DHF; CHARM‐Preserved; I‐PRESERVE; Kurrelmeyer 2014; McDiarmid 2020; Mittal 2017; PARAGON‐HF; PARAMOUNT; PEP‐CHF; SENIORS; STRUCTURE; TOPCAT). We assessed 29 studies to be at unclear risk of bias for this domain because no information was provided in study reports.
Blinding
We assessed 22 studies to be at low risk of bias regarding blinding of participants and personnel (ALDO‐DHF; AREA IN‐CHF; CAN‐DHF; CHARM‐Preserved; ELANDD; I‐PRESERVE; Kasama 2005; Kitzman 2010; Kurrelmeyer 2014; Mittal 2017; Mottram 2004; PARAGON‐HF; PARAMOUNT; Parthasarathy 2009; PEP‐CHF; RAAM‐PEF; SENIORS; STRUCTURE; SWEDIC; TOPCAT; Upadhya 2017; Zi 2003). Six studies were open‐label designs and therefore were judged to be at high risk of bias for this domain (CandHeart; Hong Kong DHF; J‐DHF; Mak 2009; McDiarmid 2020; SUPPORT). The remaining 13 studies were assessed at unclear risk of bias because no information was provided.
Detection bias was judged to be at low risk in 18 studies (ALDO‐DHF; CHARM‐Preserved; Hong Kong DHF; J‐DHF; Kasama 2005; Kitzman 2010; Mak 2009; Mittal 2017; Mottram 2004; Orea‐Tejeda 2007; PARAGON‐HF; PARAMOUNT; PEP‐CHF; RAAM‐PEF; Sahoo 2016; STRUCTURE; SUPPORT; TOPCAT). One study was judged to be at high risk of detection bias as outcome assessors were not blinded (McDiarmid 2020). The remaining 22 studies did not provide information and were judged to be at unclear risk of detection bias.
Incomplete outcome data
Attrition bias was judged to be at low risk in 14 studies (ALDO‐DHF; CHARM‐Preserved; ELANDD; I‐PRESERVE; J‐DHF; PARAGON‐HF; PARAMOUNT; Parthasarathy 2009; PEP‐CHF; RAAM‐PEF; SENIORS; STRUCTURE; TOPCAT; Zi 2003). We judged Kasama 2005 to be at high risk of bias for this domain because the study report did not indicate if losses to follow‐up or withdrawals occurred. Shu 2005 (unclear reporting of withdrawals) and McDiarmid 2020 (completed case analysis with uneven drop‐out in treatment arms) were also judged to be at high risk of bias. All 24 remaining studies were assessed to be at unclear risk of bias for attrition bias as no information was reported to allow judgement.
Selective reporting
We assessed 12 studies to be at low risk of reporting bias (ALDO‐DHF; CAN‐DHF; CHARM‐Preserved; ELANDD; I‐PRESERVE; J‐DHF; PARAGON‐HF; PARAMOUNT; PEP‐CHF; SENIORS; SUPPORT; TOPCAT). These 12 studies reported planned outcomes in either published protocols or clinical trial registers before enrolment started. We were unable to assess reporting bias in 29 studies either because no information was available in the form of protocols or clinical trial registry entries, or they were published/entered after enrolment was completed.
Other potential sources of bias
We judged 20 studies to be at low risk of other bias (mainly based on providing details on funding and declaring any conflict of interest by the authors) (ALDO‐DHF; AREA IN‐CHF; CHARM‐Preserved; ELANDD; I‐PRESERVE; J‐DHF; Kitzman 2010; Mak 2009; Mittal 2017; PARAGON‐HF; PARAMOUNT; Parthasarathy 2009; PEP‐CHF; RAAM‐PEF; SENIORS; STRUCTURE; SUPPORT; SWEDIC; TOPCAT; Zi 2003).
We judged six studies to be at high risk of other bias. Kurrelmeyer 2014 was originally registered as an observational study and this detail was changed after completion of the trial, but before the results were published. Five studies (Adamyan 2010; CAN‐DHF; Karapysh 2015; SNEGOVIK; Upadhya 2017) were published as conference abstracts only; withholding the full results from publication may present a form of bias. The remaining 15 studies were judged to be at unclear risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
BBs versus placebo or no treatment
We included 10 studies involving a total of 3087 participants that assessed BBs versus placebo or no treatment. The main outcomes for this comparison are included in Table 1.
Cardiovascular mortality
Six studies reported cardiovascular mortality (Aronow 1997; ELANDD; J‐DHF; SENIORS; SWEDIC; Takeda 2004). Three studies reported that no deaths occurred (ELANDD; SWEDIC; Takeda 2004). We included three studies in the meta‐analysis (Aronow 1997; J‐DHF; SENIORS) (15% of participants in the intervention arm versus 19% in the control arm; RR 0.78, 95% CI 0.62 to 0.99; NNTB 25; 1046 participants; I² = 0%; low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Beta‐blockers versus placebo or no treatment, Outcome 1: Cardiovascular mortality (RR)
J‐DHF reported cardiovascular mortality but with different numbers for events within the same table (Table 2 in the primary reference). We contacted the study authors to seek clarification but are yet to receive a response; we used the higher numbers in the analysis.
SENIORS reported a hazard ratio (HR 0.80, 95% CI 0.49 to 1.32; 643 participants).
Heart failure hospitalisation
We included five studies that reported heart failure hospitalisation (ELANDD; J‐DHF; Shu 2005; SWEDIC; Takeda 2004). ELANDD reported that no hospitalisation occurred due to HF. Data from four studies (J‐DHF; Shu 2005; SWEDIC; Takeda 2004) contributed to the meta‐analysis (RR 0.73, 95% CI 0.47 to 1.13; 449 participants; I² = 22%; very low‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Beta‐blockers versus placebo or no treatment, Outcome 2: Heart failure hospitalisation (RR)
Hyperkalaemia
J‐DHF reported that one participant in the intervention group (N = 120) experienced hyperkalaemia but did not report on this outcome for the control group (very low‐certainty evidence). No further data were available from any other studies.
All‐cause mortality
We included seven studies that reported all‐cause mortality (Adamyan 2010; Aronow 1997; ELANDD; J‐DHF; SENIORS; SWEDIC; Takeda 2004). Of these, three studies reported that no deaths occurred (ELANDD; SWEDIC; Takeda 2004). We included data from four studies in the meta‐analysis (Adamyan 2010; Aronow 1997; J‐DHF; SENIORS) (RR 0.82, 95% CI 0.67 to 1.00; 1105 participants; I² = 0%; low‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: Beta‐blockers versus placebo or no treatment, Outcome 3: All‐cause mortality (RR)
J‐DHF reported all‐cause mortality but with different numbers for events within the same table (Table 2 in the primary reference). We contacted the study authors to seek clarification but are yet to receive a response. We used the higher number of deaths in the analysis.
SENIORS reported a hazard ratio (HR 0.92, 95% CI 0.61 to 1.36; 643 participants).
Quality of life
We included two studies that reported quality of life (ELANDD; Mittal 2017). Mittal 2017 reported quality of life using SF‐36, which was not a scale we considered for our analysis. ELANDD reported end scores for the MLHFQ total score and showed MD ‐1.00 between the treatment arms, favouring the intervention (95% CI ‐9.05 to 7.05; 93 participants; very low‐certainty evidence).
Withdrawal due to adverse event
We included five studies that reported withdrawals due to adverse events (Aronow 1997; ELANDD; J‐DHF; Mittal 2017; Sahoo 2016). Mittal 2017 and Sahoo 2016 reported no withdrawals due to adverse events. Aronow 1997 reported 11 withdrawals due to "worsening CHF [chronic heart failure] in 7 patients and hypotension in 4 patients" but did not provide this information by intervention arm. Only two studies (ELANDD; J‐DHF) contributed data for meta‐analysis (9% of participants in the intervention arm versus 0% in the control arm, RR 18.07, 95% CI 2.45 to 133.04; 338 participants; I² = 0%; Analysis 1.5; number needed to harm (NNTH) = 11).
1.5. Analysis.

Comparison 1: Beta‐blockers versus placebo or no treatment, Outcome 5: Withdrawal due to adverse event
MRAs versus placebo or no treatment
We included 13 studies (4459 participants) that assessed MRAs versus placebo or no treatment. The main outcomes for this comparison are included in Table 2. The findings for this comparison were driven by one trial (TOPCAT). Four trials (Karapysh 2015; Mottram 2004; Orea‐Tejeda 2007; Wang 2010) did not contribute any outcome data of interest for this review.
Cardiovascular mortality
We included five studies that reported cardiovascular mortality (ALDO‐DHF; AREA IN‐CHF; Kurrelmeyer 2014; RAAM‐PEF; TOPCAT). Of these, two studies reported that no deaths occurred (Kurrelmeyer 2014; RAAM‐PEF). We included data from three studies in the meta‐analysis (ALDO‐DHF; AREA IN‐CHF; TOPCAT) (RR 0.90, 95% CI 0.74 to 1.11; 4070 participants; I² = 0%; moderate‐certainty evidence; Analysis 2.1).
2.1. Analysis.

Comparison 2: Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 1: Cardiovascular mortality (RR)
TOPCAT also reported a hazard ratio (HR 0.90, 95% CI 0.73 to 1.12; 3445 participants).
Heart failure hospitalisation
We included six studies that reported heart failure hospitalisation (ALDO‐DHF; AREA IN‐CHF; Kurrelmeyer 2014; RAAM‐PEF; TOPCAT; Upadhya 2017). Of these, three studies reported no hospitalisations due to heart failure(ALDO‐DHF; Kurrelmeyer 2014; Upadhya 2017). We included data from three studies in the meta‐analysis (AREA IN‐CHF; RAAM‐PEF; TOPCAT) (11% of participants in the intervention arm versus 14% in the control arm, RR 0.82, 95% CI 0.69 to 0.98; 3714 participants; NNTB 41; I² = 22%; moderate‐certainty evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2: Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 2: Heart failure hospitalisation (RR)
Hazard ratios for time to first heart failure hospitalisation were reported for two studies (AREA IN‐CHF; TOPCAT) (HR 0.82, 95% CI 0.69 to 0.98; 3670 participants; I² = 59%; Analysis 2.3). The substantial heterogeneity was explained by differences in population characteristics (TOPCAT, LVEF ≥ 45%; AREA IN‐CHF subgroup, LVEF 40% to 45%).
2.3. Analysis.

Comparison 2: Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 3: Heart failure hospitalisation (HR)
Hyperkalaemia
We included six studies that reported hyperkalaemia (ALDO‐DHF; AREA IN‐CHF; Kurrelmeyer 2014; RAAM‐PEF; STRUCTURE; TOPCAT) (16% of participants in the intervention arm versus 8% in the control arm, RR 2.11, 95% CI 1.77 to 2.51; 4291 participants; I² = 0%; high‐certainty evidence; Analysis 2.4).
2.4. Analysis.

Comparison 2: Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 4: Hyperkalaemia
All‐cause mortality
We included eight studies that reported all‐cause mortality (ALDO‐DHF; AREA IN‐CHF; Kurrelmeyer 2014; Mak 2009; RAAM‐PEF; STRUCTURE; TOPCAT; Upadhya 2017). Of these, three studies reported that no deaths occurred (Kurrelmeyer 2014; RAAM‐PEF; STRUCTURE). The meta‐analysis included data from five studies (ALDO‐DHF; AREA IN‐CHF; Mak 2009; TOPCAT; Upadhya 2017) (RR 0.91, 95% CI 0.78 to 1.06; 4207 participants; I² = 0%; moderate‐certainty evidence; Analysis 2.5).
2.5. Analysis.

Comparison 2: Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 5: All‐cause mortality (RR)
TOPCAT also reported a hazard ratio (HR 0.91, 95% CI 0.77 to 1.08; 3445 participants).
Quality of life
We included six studies that reported quality of life (ALDO‐DHF; Kurrelmeyer 2014; Mak 2009; RAAM‐PEF; TOPCAT; Upadhya 2017). TOPCAT reported quality of life in a report by Lewis 2016, but the end scores per treatment arm were not provided. We contacted the investigators and await details.
Three studies (ALDO‐DHF; Mak 2009; Upadhya 2017) reported total MLFHQ scores and were pooled for analysis (MD 0.84, 95% CI ‐2.30 to 3.98; 511 participants; I² = 0%; low‐certainty evidence; Analysis 2.8). Kurrelmeyer 2014 and RAAM‐PEF reported KCCQ results and were pooled (MD ‐0.78, 95% CI ‐28.02 to 26.46; 92 participants; I² = 86%; Analysis 2.7). The substantial heterogeneity could not be explained.
2.8. Analysis.

Comparison 2: Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 8: Quality of life (Minnesota)
All five studies that used MLHFQ and KCCQ were pooled (SMD 0.05, 95% CI ‐0.23 to 0.34; 603 participants; I² = 50%; Analysis 2.6). The substantial heterogeneity could not be explained.
Withdrawal due to adverse event
Five studies reported this outcome (ALDO‐DHF; Kurrelmeyer 2014; McDiarmid 2020; TOPCAT; Upadhya 2017) and contributed to the meta‐analysis (RR 1.10, 95% CI 1.00 to 1.21; 4037 participants; five studies; I² = 0%; Analysis 2.9).
2.9. Analysis.

Comparison 2: Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 9: Withdrawal due to adverse event
ACEIs versus placebo or no treatment
We included eight studies involving a total of 2061 participants that assessed ACEIs versus placebo or no treatment. The main outcomes for this comparison are presented in Table 3. The findings for this comparison were driven by PEP‐CHF. Two studies (Aronow 1993; Yuksek 2012) did not contribute any outcome data of interest for this review.
Cardiovascular mortality
Three studies reported cardiovascular mortality (Hong Kong DHF; Kitzman 2010; PEP‐CHF). Kitzman 2010 reported that no deaths occurred. Hong Kong DHF and PEP‐CHF contributed data to the meta‐analysis (RR 0.93, 95% CI 0.61 to 1.42; 945 participants; I² = 0%; moderate‐certainty evidence; Analysis 3.1).
3.1. Analysis.

Comparison 3: Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 1: Cardiovascular mortality (RR)
PEP‐CHF also reported a hazard ratio (HR 0.98, 95% CI 0.63 to 1.52; 850 participants).
Heart failure hospitalisation
Three studies (Hong Kong DHF; PEP‐CHF; Zi 2003) reported heart failure hospitalisation and were pooled for analysis (RR 0.86, 95% CI 0.64 to 1.15; 1019 participants; I² = 0%; moderate‐certainty evidence; Analysis 3.2).
3.2. Analysis.

Comparison 3: Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 2: Heart failure hospitalisation (RR)
PEP‐CHF also reported a hazard ratio (HR 0.86, 95% CI 0.61 to 1.20; 850 participants).
Hyperkalaemia
Zi 2003 reported hyperkalaemia (RR 5.27, 95% CI 0.26 to 106.16; 74 participants; very low‐certainty evidence; Analysis 3.3).
3.3. Analysis.

Comparison 3: Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 3: Hyperkalaemia
All‐cause mortality
We included six studies that reported all‐cause mortality (Aronow 1998; Hong Kong DHF; Kitzman 2010; PEP‐CHF; Yuksek 2012; Zi 2003). Kitzman 2010 reported that no deaths occurred. Five studies (Aronow 1998; Hong Kong DHF; PEP‐CHF; Yuksek 2012; Zi 2003) contributed to the meta‐analysis (RR 1.04, 95% CI 0.75 to 1.45; 1187 participants; five studies; I2 = 0%; moderate‐certainty evidence; Analysis 3.4).
3.4. Analysis.

Comparison 3: Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 4: All‐cause mortality (RR)
PEP‐CHF also reported a hazard ratio (HR 1.09, 95% CI 0.75 to 1.58; 850 participants).
Quality of life
Three studies reported quality of life assessed using the MLHFQ scale (Hong Kong DHF; Kitzman 2010; SNEGOVIK). SNEGOVIK reported quality of life assessment based on the MLHFQ scale as change from baseline per treatment arm (‐18.9 for intervention, ‐10.7 for control). We were unsuccessful in our attempts to contact study authors to obtain scores at the end of follow‐up. Two studies (Hong Kong DHF; Kitzman 2010) contributed to the meta‐analysis (MD ‐0.09, 95% CI ‐3.66 to 3.48; 154 participants; I² = 4%; low‐certainty evidence; Analysis 3.5).
3.5. Analysis.

Comparison 3: Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 5: Quality of life (Minnesota)
Zi 2003 assessed quality of life using the McMaster quality of life questionnaire and reported end scores at six months (12.9 ± 3.1 for the intervention and 13.1 ± 4.7 for the control arm).
Withdrawal due to adverse event
Four studies (Hong Kong DHF; PEP‐CHF; Yuksek 2012; Zi 2003) reported this outcome and were pooled for analysis (RR 2.52, 95% CI 0.49 to 12.87; 1127 participants; four studies; I² = 58%; Analysis 3.6).
3.6. Analysis.

Comparison 3: Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 6: Withdrawal due to adverse event
ARBs versus placebo or no treatment
We included eight studies involving a total of 8755 participants that assessed ARBs versus placebo or no treatment. The main outcomes for this comparison are included in Table 4. The findings for this comparison were driven by two studies (CHARM‐Preserved; I‐PRESERVE). Three trials (CAN‐DHF; CandHeart; Kasama 2005) did not contribute any outcome data of interest for this review.
Cardiovascular mortality
Four studies reported this outcome (CHARM‐Preserved; Hong Kong DHF; I‐PRESERVE; Parthasarathy 2009). Parthasarathy 2009 reported that no deaths occurred. Three studies (CHARM‐Preserved; Hong Kong DHF; I‐PRESERVE) contributed to the meta‐analysis (RR 1.02, 95% CI 0.90 to 1.14; 7254 participants; I² = 0%; high‐certainty evidence; Analysis 4.1).
4.1. Analysis.

Comparison 4: Angiotensin receptor blockers versus placebo or no treatment, Outcome 1: Cardiovascular mortality (RR)
Two studies (CHARM‐Preserved; I‐PRESERVE) were also pooled for an analysis of hazard ratio (HR 1.00, 95% CI 0.89 to 1.13; 7148 participants; Analysis 4.2).
4.2. Analysis.

Comparison 4: Angiotensin receptor blockers versus placebo or no treatment, Outcome 2: Cardiovascular mortality (HR)
Heart failure hospitalisation
Three studies (CHARM‐Preserved; Hong Kong DHF; I‐PRESERVE) reported this outcome and were pooled for analysis (RR 0.92, 95% CI 0.83 to 1.02; 7254 participants; I² = 0%; high‐certainty evidence; Analysis 4.3).
4.3. Analysis.

Comparison 4: Angiotensin receptor blockers versus placebo or no treatment, Outcome 3: Heart failure hospitalisation (RR)
Two studies (CHARM‐Preserved; I‐PRESERVE) were also pooled for analysis of hazard ratio (HR 0.90, 95% CI 0.80 to 1.01; 7148 participants; Analysis 4.4).
4.4. Analysis.

Comparison 4: Angiotensin receptor blockers versus placebo or no treatment, Outcome 4: Heart failure hospitalisation (HR)
Hyperkalaemia
Two studies reported this outcome and were pooled for analysis (CHARM‐Preserved; I‐PRESERVE) (0.9% of participants in the intervention group and 0.5% in the control group; RR 1.88, 95% CI 1.07 to 3.33; 7148 participants; high‐certainty evidence; Analysis 4.5).
4.5. Analysis.

Comparison 4: Angiotensin receptor blockers versus placebo or no treatment, Outcome 5: Hyperkalaemia
All‐cause mortality
Five studies reported this outcome (CHARM‐Preserved; Hong Kong DHF; I‐PRESERVE; Parthasarathy 2009; SUPPORT). Parthasarathy 2009 reported that no deaths occurred. Four studies (CHARM‐Preserved; Hong Kong DHF; I‐PRESERVE; SUPPORT) contributed to the meta‐analysis (RR 1.01, 95% CI 0.92 to 1.11; 7964 participants; I² = 0%; high‐certainty evidence; Analysis 4.6). For the SUPPORT trial, data for participants with LVEF ≥ 50% were analysed according to the definition of HFpEF used in this trial.
4.6. Analysis.

Comparison 4: Angiotensin receptor blockers versus placebo or no treatment, Outcome 6: All‐cause mortality (RR)
Two studies (I‐PRESERVE; SUPPORT) were also pooled for analysis of hazard ratio (HR 0.99, 95% CI 0.88 to 1.12; 4838 participants; Analysis 4.7).
4.7. Analysis.

Comparison 4: Angiotensin receptor blockers versus placebo or no treatment, Outcome 7: All‐cause mortality (HR)
Quality of life
Four studies reported this outcome (CHARM‐Preserved; Hong Kong DHF; I‐PRESERVE; Parthasarathy 2009). CHARM‐Preserved reported quality of life (MLHFQ) in a study report (Lewis 2007): however, end scores per treatment arm were not provided. Three studies (Hong Kong DHF; I‐PRESERVE; Parthasarathy 2009) contributed to the meta‐analysis for MLHFQ (MD 0.41, 95% CI ‐0.86 to 1.67; 3117 participants; I² = 19%; high‐certainty evidence; Analysis 4.8).
4.8. Analysis.

Comparison 4: Angiotensin receptor blockers versus placebo or no treatment, Outcome 8: Quality of life (Minnesota)
Withdrawal due to adverse event
Four studies (CHARM‐Preserved; Hong Kong DHF; I‐PRESERVE; Parthasarathy 2009) reported this outcome and contributed to the meta‐analysis (16% of participants in the intervention arm versus 13% in the control arm; RR 1.22, 95% CI 1.09 to 1.36; 7406 participants; I² = 0%; Analysis 4.9 NNTH = 33).
4.9. Analysis.

Comparison 4: Angiotensin receptor blockers versus placebo or no treatment, Outcome 9: Withdrawal due to adverse event
ARNIs versus usual care
We included three studies that involved a total of 7702 participants. The main outcomes for this comparison are included in Table 5.
Cardiovascular mortality
One study, PARAGON‐HF, reported proportional hazards and total event counts for CV mortality; a hazard ratio (HR) of 0.95 (95% CI 0.79 to 1.16) was reported and we calculated the risk ratio (RR), based on the total event counts, as 0.96 (95% CI 0.79 to 1.15; 4796 participants; moderate‐certainty evidence; Analysis 5.1).
5.1. Analysis.

Comparison 5: ARNI versus usual care, Outcome 1: Cardiovascular mortality (RR)
Heart failure hospitalisation
PARAGON‐HF and PARALLAX reported data for first hospitalisation due to HF, which led to a RR of 0.89 (95% CI 0.80 to 1.00; 7362 participants; I² = 56%; moderate‐certainty evidence; Analysis 5.2).
5.2. Analysis.

Comparison 5: ARNI versus usual care, Outcome 2: Heart failure hospitalisation, first (RR)
PARAGON‐HF and PARALLAX also reported HRs for time to first hospitalisation due to HF, which gives a pooled effect of HR 0.87 (95% CI 0.76 to 0.99; 7362 participants; two studies; I² = 82%; Analysis 5.3).
5.3. Analysis.

Comparison 5: ARNI versus usual care, Outcome 3: Heart failure hospitalisation (HR)
Hyperkalaemia
Two studies (PARAGON‐HF; PARAMOUNT) reported this outcome (RR 0.88, 95% CI 0.77 to 1.01; 5054 participants; two studies; I² = 8%; moderate‐certainty evidence; Analysis 5.4).
5.4. Analysis.

Comparison 5: ARNI versus usual care, Outcome 4: Hyperkalaemia
All‐cause mortality
The three studies reported data for all‐cause mortality (RR 0.97, 95% CI 0.84 to 1.11; 7663 participants; three studies; high‐certainty evidence; Analysis 5.5).
5.5. Analysis.

Comparison 5: ARNI versus usual care, Outcome 5: All‐cause mortality (RR)
Quality of life
PARAMOUNT reported change from baseline for the KCCQ overall summary score for the intervention arm (n = 118) as 11.25 (2.185) and the control arm (n = 116) as 11.31 (2.183), and summarised the findings as "no difference in KCCQ score between treatment groups".
PARAGON‐HF reported a difference of the clinical summary score KCCQ between treatment arms as 1.0 (0.0 to 2.1).
PARALLAX reported "KCCQ improved in both treatment groups, with an early benefit of S/V that was no longer significant after 24 week".
Withdrawal due to adverse event
The three studies reported withdrawals due to adverse events (RR 1.02, 95% CI 0.91 to 1.14; 7663 participants; three studies; I2 = 62%; Analysis 5.6).
5.6. Analysis.

Comparison 5: ARNI versus usual care, Outcome 6: Withdrawal due to adverse event
Subgroup analyses
We did not have sufficient data to allow for meaningful subgroup analyses.
Sensitivity analyses
We conducted a sensitivity analysis by only including studies assessed at low risk of bias. Across comparisons, the estimates were not significantly changed, with the exception of BBs, where no effect on cardiovascular mortality was observed (one low risk of bias study (SENIORS): RR 0.81, 95% CI 0.50 to 1.29; versus overall analysis of three studies: RR 0.78, 95% CI 0.62 to 0.99).
Discussion
Summary of main results
We examined the evidence for the effects of BBs and RAAS inhibitors for the treatment of HFpEF. We included 41 trials, reported in 234 publications that involved a total of 26,059 participants. We identified 13 ongoing trials with treatment arms that include interventions assessed in this review. A further nine studies await assessment.
We performed a pooled analysis for the outcomes of cardiovascular and all‐cause mortality, heart failure hospitalisation, quality of life and hyperkalaemia. For most studies, we use the MLHFQ instrument to evaluate quality of life outcomes since this was the most frequently reported measure, except for ARNI trials that used KCCQ.
Withdrawals due to adverse events were inconsistently reported; these data could not be included in 'Summary of findings' tables.
We conducted a sensitivity analysis by including only studies assessed with overall low risk of bias. The effect estimates were not significantly changed, except for BBs.
BBs
A total of 10 included studies (3087 participants) assessed BBs compared with placebo or no intervention. We performed meta‐analyses including up to four studies and 1105 participants. The results suggested that treatment may improve cardiovascular mortality, however, the quality of evidence was low due to imprecision and risk of bias. When we performed a sensitivity analysis by including only studies with a low overall risk of bias, the effects on cardiovascular mortality did not persist. The two largest studies (J‐DHF; SENIORS) reported high rates of study drug discontinuation due to intolerance rather than adverse events, which may have attenuated any true treatment effects. A recent individual participant meta‐analysis found evidence that beta‐blockers improve LVEF and prognosis in heart failure patients with LVEF 40 to 49%, but not in those with LVEF over 50% (Cleland 2018).
MRAs
A total of 13 studies (4459 participants) assessed MRA compared with placebo or no intervention. We combined evidence from up to six trials and 4291 participants in meta‐analyses. We found that treatment with MRA reduces the risk of heart failure hospitalisation but found little or no effect on cardiovascular and all‐cause mortality; however the certainty of evidence was moderate and uncertainty remains over these treatment effects. As expected, MRA treatment was associated with an increased risk of hyperkalaemia; potassium monitoring is therefore required in people being treated using MRA. A large, registry‐randomised clinical outcomes trial of spironolactone for HFpEF is ongoing and due to complete in 2022 (NCT02901184: Spironolactone Initiation Registry Randomized Interventional Trial in Heart Failure with Preserved Ejection Fraction, SPIRRIT).
ACEIs
A total of eight included studies (2062 participants) assessed ACEIs versus placebo or no intervention. We conducted a meta‐analysis of data from up to five trials and 1187 participants. We found that there was probably little or no effect on cardiovascular mortality, all‐cause mortality, heart failure hospitalisation or quality of life. Data on hyperkalaemia were limited. No large clinical trials (more than 1000 participants) were available and the certainty of evidence was assessed as moderate due to imprecision. The effectiveness of ACEI therapy in the treatment of people with HFpEF remains unclear.
ARBs
A total of eight included studies (8755 participants) assessed ARB therapy for people with HFpEF, with the evidence certainty assessed as high. We combined evidence from up to four trials and 7964 participants for meta‐analysis and found little or no overall difference on the outcomes of cardiovascular mortality, all‐cause mortality, heart failure hospitalisation or quality of life. The CHARM study found a beneficial effect on HF hospitalisation based on a time‐to‐event analysis; the strength of this association was increased in a subsequent analysis, based on recurrent events (Rogers 2014). As expected, ARB treatment was associated with an increased risk of hyperkalaemia; potassium monitoring is therefore required.
ARNIs
Three studies (7696 participants) assessed ARNI therapy for people with HFpEF. Only one study (PARAGON‐HF) provided usable data for cardiovascular mortality and found little or no difference between the treatment groups (moderate‐certainty evidence). Meta‐analysis of risk ratio for first heart failure hospitalisation from the PARAGON‐HF and PARALLAX trials indicated a probable modest benefit, however there was little or no effect on cardiovascular or all‐cause mortality.
A pooled analysis suggested that a slightly reduced risk of hyperkalaemia in ARNI versus standardized usual care or placebo comparison overall (moderate‐certainty evidence).
Overall completeness and applicability of evidence
This review provides the most comprehensive appraisal of the evidence to date. We included 41 studies (234 reports) that involved 26,059 participants. The included trials assessed BBs (10 studies, 3087 participants), MRAs (13 studies, 4459 participants), ACEIs (eight studies, 2061 participants), ARBs (eight studies, 8755 participants) and ARNIs (three studies, 7702 participants).
We searched clinical trials registries and identified 13 ongoing clinical trials, several of which have potential to influence the review findings. We also identified nine studies that were classified as Studies awaiting classification. There was insufficient information to determine whether these studies met our inclusion criteria. These studies were mostly small and it is therefore unlikely that they would influence the results of this review. In total, we identified 234 reports of 41 trials, nine studies awaiting classification, and 13 ongoing trials, compared with a total of 22 identified by Zheng 2018 for the same comparisons.
The LVEF threshold for defining the HFpEF trial populations varied among the included studies. This may contribute to indirectness, with implications for the applicability of the evidence. Nine studies included participants with an ejection fraction cut‐off of 40%, 12 used 45%, 15 used 50%, and one used 55%. Adamyan 2010 included participants with preserved ejection fraction but did not specify the cut‐off. SENIORS reported a subgroup of participants with LVEF greater than 40%, and for AREA IN‐CHF we obtained outcomes for a subgroup with LVEF greater than 40%.
The included studies had enrolment start dates from 1997 to 2014. In more recent studies, B‐type natriuretic peptides have been used as a key inclusion criterion to improve the specificity of the HFpEF population and enrich the trial populations for people at higher risk for clinical outcomes (e.g. CAN‐DHF; Mak 2009; RAAM‐PEF). Similarly, new measures of diastolic function have been included in more recent studies to increase the specificity (e.g. ELANDD; J‐DHF; PEP‐CHF). We noted considerable clinical heterogeneity among study populations with respect to comorbidities and cardiovascular therapies at baseline, which may influence the applicability of the evidence.
Quality of the evidence
We used the GRADE method to assess evidence certainty for the outcomes of cardiovascular mortality, all‐cause mortality, heart failure hospitalisation, quality of life (assessed using the MLHFQ), and hyperkalaemia. For BBs, evidence certainty for clinical outcomes (cardiovascular mortality, all‐cause mortality and heart failure hospitalisation) ranged from low to very low. In the combined analysis, most participants were from a subgroup of a single large trial; the other included studies were small, with high or unclear risk of bias.
For MRAs, the TOPCAT study contributed the majority of participants to the meta‐analysis, for which the overall evidence certainty for clinical outcomes was assessed as moderate. We noted differences in participant populations among the included studies (TOPCAT, LVEF greater than 45%; RAAM‐PEF, LVEF equal to or greater than 50%; AREA IN‐CHF, LVEF 40% to 45%) and it is reported that ejection fraction is a modifier of treatment effect for MRAs (Solomon 2016). Notably, a post hoc analysis of the TOPCAT study reported important differences in the placebo event rates among participants enrolled from the Americas (Argentina, Brazil, Canada, USA) and participants enrolled from Russia and Georgia (Pfeffer 2015). Furthermore, a pharmacology substudy of participants at 12 months (206 participants from USA and Canada; 160 participants from Russia) found that drug metabolites were undetectable in a greater proportion of participants from Russia, compared with participants from the USA and Canada (30% versus 3%, P < 0.001) (de Denus 2017). A geographical subgroup analysis suggested possible clinical benefit from spironolactone in HFpEF in participants who were enrolled in the Americas (United States, Canada, Brazil, Argentina; cardiovascular mortality HR 0.74, 95% CI 0.57 to 0.97; all‐cause mortality HR 0.83, 95% CI 0.68 to 1.02; heart failure hospitalisation HR 0.82, 95% CI 0.67 to 0.99). These findings will be investigated further in the ongoing SPIRRIT study (NCT02901184).
For ARBs, several large trials contributed a large number of events to the meta‐analysis for the clinical outcomes of mortality and heart failure hospitalisation, and the evidence certainty was high. For ACEIs, fewer trials were included in the combined analysis, event numbers were low and the evidence certainty was assessed as moderate. For BBs, the certainty of evidence was low due to small study sizes and risk of bias.
For ARNIs, the evidence certainty was classified as moderate for all outcomes except for all cause mortality and quality of life which was of high certainty; the cardiovascular mortality and hyperkalaemia outcomes were downgraded due to imprecision.
Potential biases in the review process
Although we conducted a comprehensive search of major databases, it is possible we missed studies on clinical trials registers, studies that had not been reported, or both. Where information on relevant subgroups or outcomes were not reported, we attempted to contact the study authors; however, only a limited number of responses were received. Given the small number of included studies per analysis, we were unable to formally assess the presence of publication bias.
Agreements and disagreements with other studies or reviews
The results of this study were largely consistent with those from a comprehensive review of the evidence for BBs and RAAS inhibitors in people with HFpEF (Zheng 2018); however, important differences were noted. Our analysis included more studies for each comparison, but the additional studies were small and did not significantly alter the overall effect estimates. In contrast to Zheng 2018, we did not find clear evidence of an effect of BB treatment on all‐cause mortality, after the exclusion of studies with an unclear or high risk of bias. Our findings were consistent with meta‐analyses of ACE and ARB (Khan 2017) and MRA (Li 2018).
A recent individual‐patient level analysis of RCTs of BBs in HF, across the full spectrum of LVEF, reported evidence that BBs reduced cardiovascular mortality; the benefit was observed for heart failure patients in sinus rhythm at all levels of LVEF less than 50%, including patients with LVEF 40% to 49% (Cleland 2018). Similarly, an individual patient‐level pooled analysis of PARADIGM‐HF and PARAGON‐HF found evidence of effect modification of ARNI efficacy by LVEF, but with benefit on heart failure hospitalisation and cardiovascular mortality probably extending to patients with mid‐range LVEF (40% to 49%) (Solomon 2020). Consistent with these reports, a study‐level meta‐analysis of trials of RAAS interventions found beneficial effects on heart failure hospitalisation in patients with LVEF greater than 50%; however, increasing LVEF was associated with a decreasing trend for benefit (Emdin 2015). Data from the CHARM‐preserved study suggests a similar benefit of ARB on heart failure hospitalisation in patients with LVEF 40% to 49%, compared to those with LVEF less than 40% (CHARM‐Preserved).
Taken together, these findings suggest that the beneficial effects of BBs and RAAS inhibitors may extend beyond patients with LVEF less than 40% to include patients with LVEF 40% to 49%; however, evidence for this group of patients is currently limited.
Authors' conclusions
Implications for practice.
Our findings suggest that MRA and ARNI treatments have a modest beneficial effect on the risk of heart failure hospitalisation and that BB may reduce the risk of cardiovascular mortality. We did not find evidence supporting an important beneficial effect of ARBs (high‐certainty evidence), or ACEIs (moderate‐certainty evidence) on mortality and hospitalisation outcomes. Although we were not able to stratify our analysis by LVEF, we note evidence from individual‐participant level meta‐analyses that suggest benefit from BB, MRA, and ARNI treatment in heart failure patients with LVEF 40 to 49%, in addition to those with LVEF < 40%. For all comparisons, no effect on the quality of life was observed, although the certainty of the evidence was low.
Implications for research.
This review highlights persistent uncertainty concerning the effects of BBs, MRAs, ACEIs, and ARNIs in HFpEF, defined as LVEF greater than 40%. Although we were unable to explore LVEF as an effect modifier in this study‐level review and meta‐analysis, reports of individual participant‐level pooled analyses of BBs and RAAS inhibition suggest that beneficial effects may extend beyond patients with reduced ejection fraction (LVEF less than 40%) heart failure, to include patients with heart failure with mid‐range ejection fraction (LVEF 40% to 49%; HFmrEF). It is possible that heart failure patients with normal or increased LVEF have distinct aetiopathology. For example, a recent study found that one in seven patients with HFpEF had evidence of wild‐type transthyretin amyloidosis (Gonzalez‐Lopez 2015). The application of precision phenotyping at a population‐scale may yield important information about the disease mechanisms underlying onset and progression in these patients, and provide the basis for the development of more effective therapeutic approaches.
What's new
| Date | Event | Description |
|---|---|---|
| 17 August 2020 | New citation required and conclusions have changed | Four new studies were included; three new studies contributed to the previously empty comparison of ARNIs versus usual care. |
| 17 August 2020 | New search has been performed | The review was updated with searches run on 14 May 2020. |
History
Protocol first published: Issue 7, 2017 Review first published: Issue 6, 2018
Acknowledgements
Tom Lumbers was supported by an NIHR Clinical Lectureship and, subsequently, a UKRI Health Data Research Fellowship. We are grateful to Marta Battle Lopez, Derya Senturk, Hatoko Sasaki, Teresa Cantisani, Liliya Eugenevna Ziganshina, Hanneke Dominicus, Joanna Zajac, Anna Aryee, and Vaclav Papez for their help with the full text assessment of non‐English language papers. We are also grateful to Rui Providencia and Lu Chunli for assessing and extracting data from non‐English language papers, and to Juan‐Pablo Casas for his guidance in designing this review. We are grateful for the contributions of James Thomas who contributed to the original version of the review.
The background and methods section of this review is based on a standard template provided by Cochrane Heart.
For the updated review we are grateful for input by peer reviewer Francisco Hidalgo, Department of Cardiology, Reina Sofia Hospital, Instituto Maimonides de Investigacion Biomedica (IMIBIC), Cordoba, Spain; and one other peer reviewer who wishes to remain anonymous.
Appendices
Appendix 1. Search strategies July 2017
CENTRAL
#1 MeSH descriptor: [Heart Failure] explode all trees
#2 ((heart or cardia* or myocardial) near/3 (failure or insufficienc* or decompensat*)):ab,ti,kw
#3 #1 or #2
#4 MeSH descriptor: [Ventricular Dysfunction] explode all trees
#5 MeSH descriptor: [Ventricular Function] explode all trees
#6 ((preserved or normal or greater) near/5 ("ejection fraction" or "EF" or "LVEF")):ab,ti,kw
#7 ("preserved systolic function" or "normal systolic function" or "HFpEF" or "HF‐pEF" or "HFnEF" or "HF‐nEF" or "DHF" or diastolic*):ab,ti,kw
#8 #4 or #5 or #6 or #7
#9 #3 and #8
#10 MeSH descriptor: [Adrenergic beta‐Antagonists] explode all trees
#11 (beta near/2 (antagonist* or block* or receptor*)):ab,ti,kw
#12 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone or cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxy benazepril or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol or Betapace or Blocadren or Bystolic or Cartrol or Coreg or Corgard or Inderal or Kerlone or Levatol or Lopressor or Normodyne or Sectral or Tenormin or Toprol or Trandate or Visken or Zebeta):ab,ti,kw
#13 MeSH descriptor: [Angiotensin‐Converting Enzyme Inhibitors] explode all trees
#14 ((angiotensin* or dipeptidyl* or 'kininase ii') near/3 (convert* or enzyme or inhibit* or recept* or block*)):ab,ti,kw
#15 (ace near/1 inhibit*):ab,ti,kw
#16 acei:ab,ti,kw
#17 (alacepril or altiopril or ancovenin or benazepril* or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril* or epicaptopril or fasidotril* or fosinopril or foroxymithine or gemopatrilat or idrapril or ilepatril or imidapril* or indolapril or libenzapril or lisinopril or moexipril* or omapatrilat or pentopril* or perindopril* or pivopril or quinapril* or ramipril* or rentiapril or sampatrilat or saralasin or "s nitrosocaptopril" or spirapril* or temocapril* or teprotide or trandolapril* or utibapril* or zabicipril* or zofenopril* or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril):ab,ti,kw
#18 MeSH descriptor: [Angiotensin Receptor Antagonists] explode all trees
#19 (angiotensin near/3 ('receptor antagonist*' or "receptor block*")):ab,ti,kw
#20 (arb or arbs):ab,ti,kw
#21 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or fimasartan or fonsartan or forasartan or irbesartan or "KT3‐671" or losartan or milfasartan or olmesartan or pomisartan or pratosartan or ripisartan or saprisartan or sparsentan or tasosartan or telmisartan or Losartan or zolasartan or Edarbi or Blopress or Atacand or Amias or Ratacand or Eprozar or Aprovel or Karvea or Avapro or Cozaar or Benicar or Olmecip or Micardis or Diovan):ab,ti,kw
#22 MeSH descriptor: [Neprilysin] this term only and with qualifier(s): [Antagonists & inhibitors ‐ AI]
#23 (neprilysin near/1 (inhibit* or antagonist*)):ab,ti,kw
#24 arni:ab,ti,kw
#25 (sacubitril or sacubitrilat or lbq657 or "lbq 657" or ahu377 or "ahu 377" or entresto or lcz696 or "lcz 696")
#26 MeSH descriptor: [Mineralocorticoid Receptor Antagonists] explode all trees
#27 ((mineralocorticoid or aldosterone) near/3 (antagonist* or block* or inhibit*)):ab,ti,kw
#28 ("canrenoic acid" or canrenone or eplerenone or finerenone or "oxprenoate potassium" or spironolactone or aldactone or contaren or inspra or luvion or phanurane or spiroletan):ab,ti,kw
#29 {or #10‐#28}
#30 #9 and #29
MEDLINE (Ovid)
1. exp Heart Failure/
2. ((heart or cardia* or myocardial) adj3 (failure or insufficienc* or decompensat*)).tw.
3. 1 or 2
4. exp Ventricular Dysfunction/
5. exp Ventricular Function/
6. ((preserved or normal or greater) adj5 (ejection fraction or EF or LVEF)).tw.
7. (preserved systolic function or normal systolic function or HFpEF or HF‐pEF or HFnEF or HF‐nEF or DHF or diastolic*).tw.
8. 4 or 5 or 6 or 7
9. 3 and 8
10. exp Adrenergic beta‐Antagonists/
11. (beta adj2 (antagonist* or block* or receptor*)).tw.
12. (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone or cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol or Betapace or Blocadren or Bystolic or Cartrol or Coreg or Corgard or Inderal or Kerlone or Levatol or Lopressor or Normodyne or Sectral or Tenormin or Toprol or Trandate or Visken or Zebeta).mp.
13. exp Angiotensin‐Converting Enzyme Inhibitors/
14. ((angiotensin* or dipeptidyl* or kininase ii) adj3 (convert* or enzyme or inhibit* or recept* or block*)).tw.
15. (ace adj inhibit*).tw.
16. acei.tw.
17. (alacepril or altiopril or ancovenin or benazepril* or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril* or epicaptopril or fasidotril* or fosinopril or foroxymithine or gemopatrilat or idrapril or ilepatril or imidapril* or indolapril or libenzapril or lisinopril or moexipril* or omapatrilat or pentopril* or perindopril* or pivopril or quinapril* or ramipril* or rentiapril or sampatrilat or saralasin or s nitrosocaptopril or spirapril* or temocapril* or teprotide or trandolapril* or utibapril* or zabicipril* or zofenopril* or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril).mp.
18. exp Angiotensin Receptor Antagonists/
19. (angiotensin adj3 (receptor antagonist* or receptor block*)).tw.
20. (arb or arbs).tw.
21. (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or fimasartan or fonsartan or forasartan or irbesartan or KT3‐671 or losartan or milfasartan or olmesartan or pomisartan or pratosartan or ripisartan or saprisartan or sparsentan or tasosartan or telmisartan or valsartan or zolasartan or Edarbi or Blopress or Atacand or Amias or Ratacand or Eprozar or Aprovel or Karvea or Avapro or Cozaar or Benicar or Olmecip or Micardis or Diovan).mp.
22. Neprilysin/ai [Antagonists & Inhibitors]
23. (neprilysin adj (inhibit* or antagonist*)).tw.
24. arni.tw.
25. (Sacubitril or "ahu 377" or ahu377 or Sacubitrilat or lbq657 or "lbq 657" or ahu377 or "ahu 377" or Entresto or lcz696 or "lcz 696").mp.
26. exp Mineralocorticoid Receptor Antagonists/
27. ((mineralocorticoid or aldosterone) adj3 (antagonist* or block* or inhibit*)).tw.
28. (canrenoic acid or canrenone or eplerenone or finerenone or oxprenoate potassium or spironolactone or Aldactone or Contaren or Inspra or Luvion or Phanurane or Spiroletan).mp.
29. or/10‐28
30. 9 and 29
31. randomized controlled trial.pt.
32. controlled clinical trial.pt.
33. randomized.ab.
34. placebo.ab.
35. drug therapy.fs.
36. randomly.ab.
37. trial.ab.
38. groups.ab.
39. 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38
40. exp animals/ not humans.sh.
41. 39 not 40
42. 30 and 41
Embase
#33 #31 AND #32 #32 random*:ab,ti OR placebo* OR ((double NEXT/1 blind*):ab,ti) #31 #10 AND #30 #30 #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28OR #29 #29 'canrenoic acid' OR canrenone OR eplerenone OR finerenone OR 'oxprenoate potassium' OR spironolactone OR aldactone OR contaren OR inspra OR luvion OR phanurane OR spiroletan #28 ((mineralocorticoid OR aldosterone) NEAR/3 (antagonist* OR block* OR inhibit*)):ab,ti #27 'mineralocorticoid antagonist'/exp #26 sacubitril OR sacubitrilat OR lbq657 OR 'lbq 657' OR 'ahu377' OR 'ahu 377' OR entresto OR lcz696 OR 'lcz 696' #25 arni:ab,ti #24 (neprilysin NEAR/1 (inhibit* OR antagonist*)):ab,ti #23 'enkephalinase inhibitor'/exp #22 abitesartan OR azilsartan OR candesartan OR elisartan OR embusartan OR eprosartan OR fimasartan OR fonsartan OR forasartanOR irbesartan OR 'kt3‐671' OR losartan OR milfasartan OR olmesartan OR pomisartan OR pratosartan OR ripisartan OR saprisartanOR sparsentan OR tasosartan OR telmisartan OR valsartan OR zolasartan OR edarbi OR blopress OR atacand OR amias OR ratacandOR eprozar OR aprovel OR karvea OR avapro OR cozaar OR benicar OR olmecip OR micardis OR diovan #21 arb:ab,ti OR arbs:ab,ti #20 (angiotensin NEAR/3 ('receptor antagonist*' OR 'receptor block*')):ab,ti #19 'angiotensin receptor antagonist'/exp #18 alacepril OR altiopril OR ancovenin OR benazepril* OR captopril OR ceranapril OR ceronapril OR cilazapril OR deacetylalacepril OR delapril OR derapril OR enalapril* OR epicaptopril OR fasidotril* OR fosinopril OR foroxymithine OR gemopatrilat OR idrapril OR ilepatril OR imidapril* OR indolapril OR libenzapril OR lisinopril OR moexipril* OR omapatrilat OR pentopril* OR perindopril* OR pivopril OR quinapril* OR ramipril* OR rentiapril OR sampatrilat OR saralasin OR 's nitrosocaptopril' OR spirapril* OR temocapril*OR teprotide OR trandolapril* OR utibapril* OR zabicipril* OR zofenopril* OR aceon OR accupril OR altace OR capoten OR lotensinOR mavik OR monopril OR prinivil OR univas OR vasotec OR zestril #17 acei:ab,ti #16 (ace NEAR/1 inhibit*):ab,ti #15 ((angiotensin* OR dipeptidyl* OR 'kininase ii') NEAR/3 (convert* OR enzyme OR inhibit* OR recept* OR block*)):ab,ti #14 'dipeptidyl carboxypeptidase inhibitor'/exp #13 acebutolol OR adimolol OR afurolol OR alprenolol OR amosulalol OR arotinolol OR atenolol OR befunolol OR betaxolol OR bevantololOR bisoprolol OR bopindolol OR bornaprolol OR brefonalol OR bucindolol OR bucumolol OR bufetolol OR bufuralol OR bunitrolol OR bunolol OR bupranolol OR butofilolol OR butoxamine OR carazolol OR carteolol OR carvedilol OR celiprolol OR cetamolol OR chlortalidone OR cloranolol OR cyanoiodopindolol OR cyanopindolol OR deacetylmetipranolol OR diacetolol OR dihydroalprenololOR dilevalol OR epanolol OR esmolol OR exaprolol OR falintolol OR flestolol OR flusoxolol OR hydroxybenzylpinodolol OR hydroxycarteolol OR hydroxymetoprolol OR indenolol OR iodocyanopindolol OR iodopindolol OR iprocrolol OR isoxaprolol OR labetalol OR landiolol OR levobunolol OR levomoprolol OR medroxalol OR mepindolol OR methylthiopropranolol OR metipranololOR metoprolol OR moprolol OR nadolol OR nebivolol OR nifenalol OR nipradilol OR oxprenolol OR pafenolol OR pamatolol OR penbutolol OR pindolol OR practolol OR primidolol OR prizidilol OR procinolol OR pronetalol OR propranolol OR proxodolol OR ridazolol OR salcardolol OR soquinolol OR sotalol OR spirendolol OR talinolol OR tertatolol OR tienoxolol OR tilisolol OR timolol OR tolamolol OR toliprolol OR tribendilol OR xibenolol OR betapace OR blocadren OR bystolic OR cartrol OR coreg OR corgard OR inderal OR kerlone OR levatol OR lopressor OR normodyne OR sectral OR tenormin OR toprol OR trandate OR visken OR zebeta #12 (beta NEAR/2 (antagonist* OR block* OR receptor*)):ab,ti #11 'beta adrenergic receptor blocking agent'/exp #10 #3 AND #9 #9 #4 OR #5 OR #6 OR #7 OR #8 #8 'preserved systolic function':ab,ti OR 'normal systolic function':ab,ti OR hfpef:ab,ti OR 'hf‐pef':ab,ti OR hfnef:ab,ti OR 'hf‐nef':ab,ti OR dhf:ab,ti OR diastolic*:ab,ti #7 ((preserved OR normal OR greater) NEAR/5 ('ejection fraction' OR ef OR lvef)):ab,ti #6 'systolic dysfunction'/exp #5 'diastolic dysfunction'/exp #4 'heart ventricle function'/de #3 #1 OR #2 #2 ((heart OR cardia* OR myocardial) NEAR/3 (failure OR insufficienc* OR decompensat*)):ab,ti #1 'heart failure'/exp
ClinicalTrials.gov
Advanced Search‐‐Limited to study type: interventional studies
("heart failure" AND ("preserved ejection fraction" OR "normal ejection fraction" OR "preserved systolic function" OR "normal systolic function")) OR "diastolic heart failure" OR "HFpEF" OR "HF‐pEF" OR "HFnEF" OR "HF‐nEF" OR "DHF"
WHO International Clinical Trials Registry Platform (ICTRP) Search Portal
Standard Search
heart failure AND preserved ejection fraction OR heart failure AND normal ejection fraction OR
heart failure AND preserved systolic function OR heart failure AND normal systolic function OR
diastolic heart failure OR HFpEF OR HF‐pEF OR HFnEF OR HF‐nEF OR DHF
Appendix 2. Search strategies May 2020
CENTRAL
#1 MeSH descriptor: [Heart Failure] explode all trees
#2 ((heart or cardia* or myocardial) near/3 (failure or insufficienc* or decompensat*)):ab,ti,kw
#3 #1 or #2
#4 MeSH descriptor: [Ventricular Dysfunction] explode all trees
#5 MeSH descriptor: [Ventricular Function] explode all trees
#6 ((preserved or normal or greater) near/5 ("ejection fraction" or "EF" or "LVEF")):ab,ti,kw
#7 ("preserved systolic function" or "normal systolic function" or "HFpEF" or "HF‐pEF" or "HFnEF" or "HF‐nEF" or "DHF" or diastolic*):ab,ti,kw
#8 #4 or #5 or #6 or #7
#9 #3 and #8
#10 MeSH descriptor: [Adrenergic beta‐Antagonists] explode all trees
#11 (beta near/2 (antagonist* or block* or receptor*)):ab,ti,kw
#12 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone or cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxy benazepril or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol or Betapace or Blocadren or Bystolic or Cartrol or Coreg or Corgard or Inderal or Kerlone or Levatol or Lopressor or Normodyne or Sectral or Tenormin or Toprol or Trandate or Visken or Zebeta):ab,ti,kw
#13 MeSH descriptor: [Angiotensin‐Converting Enzyme Inhibitors] explode all trees
#14 ((angiotensin* or dipeptidyl* or 'kininase ii') near/3 (convert* or enzyme or inhibit* or recept* or block*)):ab,ti,kw
#15 (ace near/1 inhibit*):ab,ti,kw
#16 acei:ab,ti,kw
#17 (alacepril or altiopril or ancovenin or benazepril* or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril* or epicaptopril or fasidotril* or fosinopril or foroxymithine or gemopatrilat or idrapril or ilepatril or imidapril* or indolapril or libenzapril or lisinopril or moexipril* or omapatrilat or pentopril* or perindopril* or pivopril or quinapril* or ramipril* or rentiapril or sampatrilat or saralasin or "s nitrosocaptopril" or spirapril* or temocapril* or teprotide or trandolapril* or utibapril* or zabicipril* or zofenopril* or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril):ab,ti,kw
#18 MeSH descriptor: [Angiotensin Receptor Antagonists] explode all trees
#19 (angiotensin near/3 ('receptor antagonist*' or "receptor block*")):ab,ti,kw
#20 (arb or arbs):ab,ti,kw
#21 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or fimasartan or fonsartan or forasartan or irbesartan or "KT3‐671" or losartan or milfasartan or olmesartan or pomisartan or pratosartan or ripisartan or saprisartan or sparsentan or tasosartan or telmisartan or Losartan or zolasartan or Edarbi or Blopress or Atacand or Amias or Ratacand or Eprozar or Aprovel or Karvea or Avapro or Cozaar or Benicar or Olmecip or Micardis or Diovan):ab,ti,kw
#22 MeSH descriptor: [Neprilysin] this term only and with qualifier(s): [Antagonists & inhibitors ‐ AI]
#23 (neprilysin near/1 (inhibit* or antagonist*)):ab,ti,kw
#24 arni:ab,ti,kw
#25 (sacubitril or sacubitrilat or lbq657 or "lbq 657" or ahu377 or "ahu 377" or entresto or lcz696 or "lcz 696")
#26 MeSH descriptor: [Mineralocorticoid Receptor Antagonists] explode all trees
#27 ((mineralocorticoid or aldosterone) near/3 (antagonist* or block* or inhibit*)):ab,ti,kw
#28 ("canrenoic acid" or canrenone or eplerenone or finerenone or "oxprenoate potassium" or spironolactone or aldactone or contaren or inspra or luvion or phanurane or spiroletan):ab,ti,kw
#29 {or #10‐#28}
#30 #9 and #29 Date added to CENTRAL trials database: 25/07/2017‐14/05/2020
MEDLINE OVID
1. exp Heart Failure/
2. ((heart or cardia* or myocardial) adj3 (failure or insufficienc* or decompensat*)).tw.
3. 1 or 2
4. exp Ventricular Dysfunction/
5. exp Ventricular Function/
6. ((preserved or normal or greater) adj5 (ejection fraction or EF or LVEF)).tw.
7. (preserved systolic function or normal systolic function or HFpEF or HF‐pEF or HFnEF or HF‐nEF or DHF or diastolic*).tw.
8. 4 or 5 or 6 or 7
9. 3 and 8
10. exp Adrenergic beta‐Antagonists/
11. (beta adj2 (antagonist* or block* or receptor*)).tw.
12. (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone or cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol or Betapace or Blocadren or Bystolic or Cartrol or Coreg or Corgard or Inderal or Kerlone or Levatol or Lopressor or Normodyne or Sectral or Tenormin or Toprol or Trandate or Visken or Zebeta).mp.
13. exp Angiotensin‐Converting Enzyme Inhibitors/
14. ((angiotensin* or dipeptidyl* or kininase ii) adj3 (convert* or enzyme or inhibit* or recept* or block*)).tw.
15. (ace adj inhibit*).tw.
16. acei.tw.
17. (alacepril or altiopril or ancovenin or benazepril* or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril* or epicaptopril or fasidotril* or fosinopril or foroxymithine or gemopatrilat or idrapril or ilepatril or imidapril* or indolapril or libenzapril or lisinopril or moexipril* or omapatrilat or pentopril* or perindopril* or pivopril or quinapril* or ramipril* or rentiapril or sampatrilat or saralasin or s nitrosocaptopril or spirapril* or temocapril* or teprotide or trandolapril* or utibapril* or zabicipril* or zofenopril* or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril).mp.
18. exp Angiotensin Receptor Antagonists/
19. (angiotensin adj3 (receptor antagonist* or receptor block*)).tw.
20. (arb or arbs).tw.
21. (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or fimasartan or fonsartan or forasartan or irbesartan or KT3‐671 or losartan or milfasartan or olmesartan or pomisartan or pratosartan or ripisartan or saprisartan or sparsentan or tasosartan or telmisartan or valsartan or zolasartan or Edarbi or Blopress or Atacand or Amias or Ratacand or Eprozar or Aprovel or Karvea or Avapro or Cozaar or Benicar or Olmecip or Micardis or Diovan).mp.
22. Neprilysin/ai [Antagonists & Inhibitors]
23. (neprilysin adj (inhibit* or antagonist*)).tw.
24. arni.tw.
25. (Sacubitril or "ahu 377" or ahu377 or Sacubitrilat or lbq657 or "lbq 657" or ahu377 or "ahu 377" or Entresto or lcz696 or "lcz 696").mp.
26. exp Mineralocorticoid Receptor Antagonists/
27. ((mineralocorticoid or aldosterone) adj3 (antagonist* or block* or inhibit*)).tw.
28. (canrenoic acid or canrenone or eplerenone or finerenone or oxprenoate potassium or spironolactone or Aldactone or Contaren or Inspra or Luvion or Phanurane or Spiroletan).mp.
29. or/10‐28
30. 9 and 29
31. randomized controlled trial.pt.
32. controlled clinical trial.pt.
33. randomized.ab.
34. placebo.ab.
35. drug therapy.fs.
36. randomly.ab.
37. trial.ab.
38. groups.ab.
39. 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38
40. exp animals/ not humans.sh.
41. 39 not 40
42. 30 and 41
43. limit 42 to ed=20170725‐20200514
Embase OVID
1 exp heart failure/
2 ((heart or cardia* or myocardial) adj3 (failure or insufficienc* or decompensat*)).ab,ti.
3 1 or 2
4 heart ventricle function/
5 exp diastolic dysfunction/
6 exp systolic dysfunction/
7 ((preserved or normal or greater) adj5 ('ejection fraction' or ef or lvef)).ab,ti.
8 ('preserved systolic function' or 'normal systolic function' or hfpef or 'hf‐pef' or hfnef or 'hf‐nef' or dhf or diastolic*).ab,ti.
9 4 or 5 or 6 or 7 or 8
10 3 and 9
11 exp beta adrenergic receptor blocking agent/
12 (beta adj2 (antagonist* or block* or receptor*)).ab,ti.
13 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantololOR bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone or cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenololOR dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranololOR metoprolol or moprolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol or betapace or blocadren or bystolic or cartrol or coreg or corgard or inderal or kerlone or levatol or lopressor or normodyne or sectral or tenormin or toprol or trandate or visken or zebeta).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
14 exp dipeptidyl carboxypeptidase inhibitor/
15 ((angiotensin* or dipeptidyl* or 'kininase ii') adj3 (convert* or enzyme or inhibit* or recept* or block*)).ab,ti.
16 (ace adj1 inhibit*).ab,ti.
17 acei.ab,ti.
18 (alacepril or altiopril or ancovenin or benazepril* or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril* or epicaptopril or fasidotril* or fosinopril or foroxymithine or gemopatrilat or idrapril or ilepatril or imidapril* or indolapril or libenzapril or lisinopril or moexipril* or omapatrilat or pentopril* or perindopril* or pivopril or quinapril* or ramipril* or rentiapril or sampatrilat or saralasin or 's nitrosocaptopril' or spirapril* or temocapril*OR teprotide or trandolapril* or utibapril* or zabicipril* or zofenopril* or aceon or accupril or altace or capoten or lotensinOR mavik or monopril or prinivil or univas or vasotec or Zestril).mp.
19 exp angiotensin receptor antagonist/
20 (angiotensin adj3 ('receptor antagonist*' or 'receptor block*')).ab,ti.
21 (arb or arbs).ab,ti.
22 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or fimasartan or fonsartan or forasartanOR irbesartan or 'kt3‐671' or losartan or milfasartan or olmesartan or pomisartan or pratosartan or ripisartan or saprisartanOR sparsentan or tasosartan or telmisartan or valsartan or zolasartan or edarbi or blopress or atacand or amias or ratacandOR eprozar or aprovel or karvea or avapro or cozaar or benicar or olmecip or micardis or diovan).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
23 exp enkephalinase inhibitor/
24 (neprilysin adj1 (inhibit* or antagonist*)).ab,ti.
25 arni.ab,ti.
26 (sacubitril or sacubitrilat or lbq657 or 'lbq 657' or 'ahu377' or 'ahu 377' or entresto or lcz696 or 'lcz 696').mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
27 exp mineralocorticoid antagonist/
28 ((mineralocorticoid or aldosterone) adj3 (antagonist* or block* or inhibit*)).ab,ti.
29 ('canrenoic acid' or canrenone or eplerenone or finerenone or 'oxprenoate potassium' or spironolactone or aldactone or contaren or inspra or luvion or phanurane or spiroletan).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
30 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29
31 10 and 30
32 random*.ab,ti. or placebo*.mp. or (double adj1 blind*).ab,ti. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
33 31 and 32
34 limit 33 to dd=20170725‐20200514
Clinicaltrials.gov
Advanced search for Study type: interventional studies
("preserved ejection fraction" OR "normal ejection fraction" OR "preserved systolic function" OR "normal systolic function") OR "diastolic heart failure" OR "HFpEF" OR "HF‐pEF" OR "HFnEF" OR "HF‐nEF" OR "DHF" | Interventional Studies | heart failure
Data and analyses
Comparison 1. Beta‐blockers versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Cardiovascular mortality (RR) | 3 | 1046 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.62, 0.99] |
| 1.2 Heart failure hospitalisation (RR) | 4 | 449 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.47, 1.13] |
| 1.3 All‐cause mortality (RR) | 4 | 1105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.67, 1.00] |
| 1.4 Quality of life (Minnesota) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.5 Withdrawal due to adverse event | 2 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 18.07 [2.45, 133.04] |
1.4. Analysis.

Comparison 1: Beta‐blockers versus placebo or no treatment, Outcome 4: Quality of life (Minnesota)
Comparison 2. Mineralocorticoid receptor antagonists versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Cardiovascular mortality (RR) | 3 | 4070 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.11] |
| 2.2 Heart failure hospitalisation (RR) | 3 | 3714 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| 2.3 Heart failure hospitalisation (HR) | 2 | 3670 | Hazard Ratio (IV, Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| 2.4 Hyperkalaemia | 6 | 4291 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.77, 2.51] |
| 2.5 All‐cause mortality (RR) | 5 | 4207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.78, 1.06] |
| 2.6 Quality of life | 5 | 603 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.23, 0.34] |
| 2.7 Quality of life (KCCQ) | 2 | 92 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐28.02, 26.46] |
| 2.8 Quality of life (Minnesota) | 3 | 511 | Mean Difference (IV, Random, 95% CI) | 0.84 [‐2.30, 3.98] |
| 2.9 Withdrawal due to adverse event | 5 | 4037 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [1.00, 1.21] |
Comparison 3. Angiotensin converting enzyme inhibitors versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Cardiovascular mortality (RR) | 2 | 945 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.61, 1.42] |
| 3.2 Heart failure hospitalisation (RR) | 3 | 1019 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.64, 1.15] |
| 3.3 Hyperkalaemia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.4 All‐cause mortality (RR) | 5 | 1187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.75, 1.45] |
| 3.5 Quality of life (Minnesota) | 2 | 154 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐3.66, 3.48] |
| 3.6 Withdrawal due to adverse event | 4 | 1127 | Risk Ratio (M‐H, Random, 95% CI) | 2.52 [0.49, 12.87] |
Comparison 4. Angiotensin receptor blockers versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Cardiovascular mortality (RR) | 3 | 7254 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.90, 1.14] |
| 4.2 Cardiovascular mortality (HR) | 2 | 7148 | Hazard Ratio (IV, Fixed, 95% CI) | 1.00 [0.89, 1.13] |
| 4.3 Heart failure hospitalisation (RR) | 3 | 7254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.83, 1.02] |
| 4.4 Heart failure hospitalisation (HR) | 2 | 7148 | Hazard Ratio (IV, Fixed, 95% CI) | 0.90 [0.80, 1.01] |
| 4.5 Hyperkalaemia | 2 | 7148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.07, 3.33] |
| 4.6 All‐cause mortality (RR) | 4 | 7964 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.92, 1.11] |
| 4.7 All‐cause mortality (HR) | 2 | 4838 | Hazard Ratio (IV, Fixed, 95% CI) | 0.99 [0.88, 1.12] |
| 4.8 Quality of life (Minnesota) | 3 | 3117 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [‐0.86, 1.67] |
| 4.9 Withdrawal due to adverse event | 4 | 7406 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.09, 1.36] |
Comparison 5. ARNI versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Cardiovascular mortality (RR) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.2 Heart failure hospitalisation, first (RR) | 2 | 7362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.80, 1.00] |
| 5.3 Heart failure hospitalisation (HR) | 2 | 7362 | Hazard Ratio (IV, Fixed, 95% CI) | 0.87 [0.76, 0.99] |
| 5.4 Hyperkalaemia | 2 | 5054 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.77, 1.01] |
| 5.5 All‐cause mortality (RR) | 3 | 7663 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.84, 1.11] |
| 5.6 Withdrawal due to adverse event | 3 | 7663 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.91, 1.14] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adamyan 2010.
| Study characteristics | ||
| Methods |
Study design: four arm factorial RCT Centres: not reported, assumed one, in Armenia Start of enrolment: not reported End of enrolment: not reported Mean follow‐up: 12 months Run‐in period: not reported |
|
| Participants |
Inclusion criteria: "III NYHA class chronic ischemic heart failure (CHF) patients (pts) with normal cholesterol who have preserved LV ejection fraction (PEF) and restrictive diastolic filling pattern" Exclusion criteria: not reported Randomised (N): 118 in total, of interest are: carvedilol, no simvastatin (N = 31) versus no carvedilol, no simvastatin (N = 28) Withdrawn (N): not reported Lost to follow‐up (N): not reported Analysed (N): not reported Age (years, mean, unspecified): 64.5, 0.3 Sex (% men): not reported Ethnicity (%): not reported Systolic blood pressure: not reported Heart rate: not reported BMI: not reported Serum creatinine: not reported B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF "preserved LV ejection fraction" but not defined NYHA class I (%): 0 NYHA class II (%): 0 NYHA class III (%): 100 NYHA class IV (%): 0 Hypertension: not reported Diabetes: not reported Atrial fibrillation: not reported Hospitalisation for heart failure: not reported Coronary heart disease: not reported Stroke: not reported Diuretic: not reported Digoxin: not reported Beta‐blockers: study drug ACEI: not reported ARB: not reported MRA: not reported |
|
| Interventions |
Intervention: carvedilol (up to 50 mg), simvastatin, carvedilol and simvastatin Comparator: not receiving carvedilol or simvastatin Concomitant medication: "in addition to ACE inhibitors, aldosterone antagonists and diuretics" |
|
| Outcomes |
Planned: not reported Reported: "prognosis, left ventricular (LV) diastolic function, plasma BNP level and inflammation status", "Assessment of relation of early (E) and late (A) diastolic filling velocities, deceleration time (DT) of E wave, levels of BNP, interleukin‐6 (IL‐6) and high sensitivity C‐reactive protein (CRP)", mortality, hospitalisation |
|
| Notes | Two conference abstracts only. Comparison between carvedilol and no treatment was of interest for this review. No outcome data relevant to this review. Trialists were contacted; no response. Source of funding: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned" but no details given |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | High risk | Published as conference abstracts only |
ALDO‐DHF.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Centres: 10 centres in Germany and Austria Start of enrolment: March 2007 End of enrolment: April 2011 Mean follow‐up: 11.6 months Run‐in period: not reported |
|
| Participants |
Inclusion criteria: "men and women aged 50 years or older were eligible to participate in the study if they had current heart failure symptoms consistent with New York Heart Association (NYHA) class II or III, left ventricular ejection fraction (LVEF) of 50% or greater, echocardiographic evidence of diastolic dysfunction (grade I) or atrial fibrillation at presentation, and maximum exercise capacity (peak VO2) of 25 mL/kg/min or less." Exclusion criteria: "Major exclusion criteria included prior documented reduced left ventricular ejection fraction (LVEF 40%), significant coronary artery disease (current angina pectoris or ischemia on stress tests; untreated coronary stenosis 50%), myocardial infarction or coronary artery bypass graft surgery 3 months or less prior to enrolment, clinically relevant pulmonary disease (vital capacity 80% or forced expiratory volume in 1 second 80% of reference values on spirometry), significant laboratory abnormalities (potassium 5.1 mmol/L; hemoglobin 11 g/dL; hematocrit 33%; serum creatinine 1.8 mg/dL; or estimated glomerular filtration rate [eGFR] 30 mL/min/1.73 m2 , calculated using the Modification of Diet in Renal Disease formula: 186 [serum creatinine {in micromoles per liter}/ 88.4] 1.154 age [in years] 0.203 1.21 [if patient is black] 0.742 [if patient is female]), known contraindications for spironolactone or known intolerance to or therapy with a mineralocorticoid receptor antagonist within the last 3 months, concomitant therapy with a potassium‐sparing diuretic (eg, triamterene, amiloride), or potassium supplementation." Randomised (N): 422 (213 intervention, 209 control) Withdrawn (N): for reasons other than death 16 (6 intervention, 10 control) Lost to follow‐up (N): 5 (2 intervention, 3 control) Analysed (N): 422 (213 intervention, 209 control) Age (years, mean, SD): intervention: 67, 8; control: 67, 8 Sex (% men): intervention: 48; control: 47 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): intervention: 135, 18; control: 135, 18 Heart rate (beats/min, mean, SD): intervention: 66, 14; control: 64, 12 BMI (mean, SD): intervention: 28.9, 3.6; control: 28.9, 3.6 Serum creatinine: not reported B‐type natriuretic peptide: not reported NT pro B‐type natriuretic peptide (pg/mL, median, IQR): intervention: 179, 81 to 276; control: 148, 80‐276 LVEF (%, mean, SD): intervention: 67, 8; control: 68, 7 NYHA class I (%): 0 NYHA class II (%): intervention: 85; control: 88 NYHA class III (%): intervention: 15; control: 12 NYHA class IV (%): 0 Hypertension (%): intervention: 92; control: 91 Diabetes (%): intervention: 17; control: 16 Atrial fibrillation (%): intervention: 6; control: 4 Hospitalisation for HF: (%): intervention: 38; control: 36 Coronary heart disease (%): intervention: 43; control: 37 Stroke (%): not reported Diuretic (%); intervention: 55; control: 52 Digoxin (%): not reported Beta‐blocker (%): intervention: 69; control: 75 ACEI (%): intervention: 78; control: 76 ARB (%): nor reported MRA (%): study drug |
|
| Interventions |
Intervention: spironolactone "The study drug could be decreased temporarily to 25 mg every other day for a potassium level greater than 5.2 mmol/L or in the presence of other reversible, non–life‐threatening adverse effects. For safety reasons, study medication was stopped for relevant hyperkalaemia (serum potassium 5.5 mmol/L) and/or hyperkalaemia‐associated clinical symptoms, significant renal impairment (serum creatinine 2.5 mg/dL; eGFR 20 mL/min/1.73m2), significant breast pain or gynaecomastia, or withdrawal of informed consent; rechallenge was encouraged wherever possible." "mean daily dose of spironolactone was 21.6 mg (95% CI, 20.8‐22.3 mg)" Comparator: matching placebo Concomitant medication: "Standard therapies for risk factor and symptom control were at the discretion of treating physicians and required to be unchanged within the 2 weeks prior to randomization." "concomitant therapy with a potassium‐sparing diuretic (eg, triamterene, amiloride), or potassium supplementation." |
|
| Outcomes |
Planned: primary outcomes: exercise capacity, left ventricular end‐diastolic pressure. Reported: all‐cause mortality, QoL, diastolic function, exercise capacity, "changes in echocardiographic measures of cardiac function and remodeling, measures of submaximal and maximal exercise capacity, serum biomarkers, and quality of life. Clinical tolerability was assessed as the safety end point. Morbidity and mortality (all‐cause and cardiovascular‐specific) were also predefined exploratory end points." |
|
| Notes | Received outcome data for CV mortality, heart failure hospitalisation and hyperkalaemia from investigators. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Pocock minimisation algorithm". |
| Allocation concealment (selection bias) | Low risk | "The allocation sequence was implemented remotely via Internet/fax by the Coordination Center for Clinical Trials Leipzig." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients, the investigator team, individuals performing the assessments, and data analysts remained blinded to the identity of treatment until after database lock". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients, the investigator team, individuals performing the assessments, and data analysts remained blinded to the identity of treatment until after database lock". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT used, except for QoL. |
| Selective reporting (reporting bias) | Low risk | primary outcomes reported as planned, some secondary outcomes not reported as planned, e.g. all‐cause mortality, cardiovascular mortality. |
| Other bias | Low risk | "Production of identical matching placebo and quality control, packaging, labelling, storage, and dispensing of both spironolactone and placebo were performed by Allphamed PHARBIL." "This work was supported by the German‐Austrian Heart Failure Study Group and the German Competence Network of Heart Failure. AldoDHF was funded by the Federal Ministry of Education and Research Grant 01GI0205 (clinical trial program Aldo‐DHF [FKZ 01KG0506]). The University of Goettingen was the formal sponsor." "The sponsor and supporters of this study had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript." |
AREA IN‐CHF.
| Study characteristics | ||
| Methods |
Study design: RCT. Centres: 46 cardiology centres in Italy. Start of enrolment: September 2002. End of enrolment: July 2005. Follow‐up: 12 months. Run‐in period: not reported. |
|
| Participants |
Inclusion criteria: aged 18 to 80 years, established evidence of NYHA class II HF, stable, optimised therapy according to European Society of Cardiology criteria, and an LV ejection fraction (EF) ≤ 45%, as measured locally up to 6 months before enrolment. Exclusion criteria: creatinine 2.5 mg/dL; K 5.0 mEq/L; valvular heart disease amenable to surgical treatment; congenital heart disease; unstable angina or acute myocardial infarction or coronary revascularisation procedure within 3 months before enrolment; intravenous therapy with inotropic drugs within 3 months before enrolment; treatment with lithium salts, Kþ‐sparing diuretics, TNF‐a antagonists, or MRA during the last 3 months; history of resuscitated ventricular arrhythmias (unless this occurred within 24 h of a previous acute myocardial infarction or in subjects with an implantable cardioverter defibrillator); other clinical or general conditions contraindicating participation in a clinical trial. Randomised (N): 467 total (225 LVEF > 40%) (231 (116) intervention, 236 (109) control) Withdrawn (N): for reasons other than death 18 (14 intervention,4 control) Lost to follow‐up: not reported Analysed: not reported Age (years, mean, SD): intervention: 62.3, 9.5; control: 62.7, 9.5 Sex (% men): intervention: 81.8; control: 85.2 Ethnicity: not reported Systolic blood pressure (mmHg, mean, SD): intervention: 127.9, 16.2; control: 128.0, 17.2 Heart rate (beats/min, mean, SD): intervention: 68.0, 11.8; control: 65.7, 10.7 BMI (mean, SD): intervention: 26.7, 3.5; control: 26.9, 3.6 Serum creatinine (mg/dL, mean, SD): intervention: 1.1, 0.3; control: 1.1, 0.2 B‐type natriuretic peptide: not reported NT pro B‐type natriuretic peptide: not reported LVEF (%, mean, SD): intervention: 39.9, 8.6; control: 39.7, 8.6 NYHA class: not reported Hypertension (%): intervention: 48.5; control: 42.4 Diabetes (%): intervention: 20.9; control: 19.9 Chronic atrial fibrillation (%): intervention: 7.4; control: 8.5 Hospitalisation for heart failure (%): intervention: 44.6; control: 49.2 Coronary heart disease: not reported Stroke (%): intervention: 1.7; control: 3 Diuretic (%); intervention: 67.8; control: 72 Digoxin: not reported Beta‐blocker (%): intervention: 81.3; control: 77.5 ACEI (%): intervention: 84.9; control: 74.6 ARB (%): intervention: 12.1; control: 24.2 MRA (%): study drug |
|
| Interventions |
Intervention: canrenone. "The dose of 25 mg/o.d. of canrenone at randomization was increased to 50 mg/o.d. after the first month, if serum Kþ was 5 mEq/L, and in the absence of deterioration in renal function. During follow‐up, if serum Kþ increased up to 5 mEq/L and/or creatinine increased up to 2.5 mg/dL, the dosage of canrenone was reduced to 25 mg/o.d. Subjects requiring down‐titration of study medications were asked to return to the outpatient clinic within 2 weeks for a supplemental visit to evaluate the effectiveness of this change in therapy. If serum Kþ remained.5.5 mEq/L, or if creatinine was 3 mg/dL or had increased by over 1 mg/dL, the study medication was discontinued and the patient managed with conventional treatment only." Comparator: placebo Concomitant medication: "Aspirin, diuretics, digoxin, nitrates, antiarrhythmic agents, oral anticoagulants, and any other therapy were allowed when indicated by the local investigators." |
|
| Outcomes |
Planned: unclear Reported: "The pre‐specified primary endpoint was the change in echocardiographic LV end‐diastolic volume (LVEDV) over 12 months, measured centrally at the Echocardiographic Reading Centre. Secondary endpoints included changes in EF, estimated diastolic filling pressure, NYHA class, BNP, cardiac mortality, hospitalization for cardiac causes, and the combination of cardiac mortality and hospitalization for cardiac causes" |
|
| Notes | Subgroup of participants of interest; response with outcome data for subgroup of participants LVEF > 40% received from trialists; baseline characteristics above are for all trial participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | double blind, placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | echocardiography data were "read at the end of the study by one experienced independent observer who was blinded to all clinical data and treatment allocation" not reported for other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT for all outcomes |
| Selective reporting (reporting bias) | Unclear risk | unable to assess as protocol and NCT record published/registered after enrolment completed |
| Other bias | Low risk | "The ANMCO Research Center coordinated the study, managed the data, and undertook analyses, under the supervision of the steering committee, who designed the AREA IN‐CHF study. The funding source (Therabel GiEnne Pharma SpA) had no role in the trial design, conduct, data collection, analyses and data interpretation." |
Aronow 1993.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Centres: not reported Start of enrolment: not reported End of enrolment: not reported Mean follow‐up: 3 months Run‐in period: 2 mornings of control period |
|
| Participants |
Inclusion criteria: "New York Heart Association functional class III CHF associated with prior myocardial infarction and normal LV ejection fraction (>50%) who were able to perform a maximal treadmill exercise test were included in the study". Exclusion criteria: "No patient had valvular heart disease, systolic blood pressure ~100 mm Hg, lung disease, hepatic disease or renal insufficiency." Randomised (N): 21 (10 intervention, 11 control) Withdrawn (N): not reported Lost to follow‐up (N): not reported Analysed (N): not reported Age (years, mean, SD): intervention: 80, 3; control: 79, 4 Sex (% men): 14.3 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): intervention: 126, 12; control: 127, 10 Heart rate (beats/min, mean, SD): intervention: 85, 6; control: 84, 3 BMI not reported Serum creatinine not reported B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): intervention: 64, 9; control: 64, 7 NYHA class I (%): 0 NYHA class II (%): 0 NYHA class III (%): 100 NYHA class IV (%): 0 Hypertension not reported Diabetes not reported Atrial fibrillation not reported Hospitalisation for heart failure: not reported Coronary heart disease not reported Stroke not reported Diuretic (%): 100 Digoxin (%): 0 Beta‐blocker (%): 0 ACEI study drug ARB not reported MRA not reported |
|
| Interventions |
Intervention: enalapril. "The initial dose of enalapril was 2.5 mg/day, which was increased to 5 mg/day during week 2, to 10 mg/day (5 mg twice daily) during week 3, to 15 mg/day (7.5 mg twice daily) during week 4 and up to a maximum of 20 mg (10 mg twice daily) during week 5, tf tolerated. If the patient developed symptomatic hypotension or an increase in serum creatinine level, the dose of enalapril was reduced to the previous dose. At the time of the follow‐up studies, 3 months after beginning enalapril, the dose of enalapril was 2.5 mg/day in 1 patient, 5 mg/day in 1 patient, 10 mg/day in 3 patients, 1.5 mg/day in 2 patients, and 20 mg/day in 3 patients." Comparator: no treatment Concomitant medication: "All patients received diuretic treatment with furosemide for ~2 weeks before the beginning of the study and a constant dose of furosemide during the study. Digitalis and other cardiac drugs (except enalapril) were not administered to any patient during the study." |
|
| Outcomes |
Planned: not reported Reported: NYHA class, blood pressure, heart rate, cardiothoracic ratio, treadmill exercise time, LVEF, peak mitral E/A ratio, left ventricular mass |
|
| Notes | no outcome data relevant for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Chest roentgenograms were interpreted by a radiologist who was unaware of the study medication. M‐mode, 2‐ dimensional and pulsed‐wave Doppler echocardiograms were interpreted by an experienced echocardiographer (IK) who was unaware of the study medication. Treadmill exercise tests were performed under the guidance of the senior author who was aware of which patients were receiving enalapril." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | not reported |
| Selective reporting (reporting bias) | Unclear risk | not reported |
| Other bias | Unclear risk | unable to assess |
Aronow 1997.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Centres: not reported Start of enrolment: not reported End of enrolment: not reported Mean follow‐up: 32 months (intervention), 31 months (control) Run‐in period: not reported |
|
| Participants |
Inclusion criteria: "≥ 62 years of age with New York Heart Association functional class II or III CHF, prior Qwave myocardial infarction, and a LV ejection fraction ≥ 40% after 2 months of treatment with diuretics and ACE inhibitors were included in the study." Exclusion criteria: "No patient had valvular heart disease, systolic blood pressure < 100 mm Hg, lung disease with bronchospasm, hepatic disease, renal insufficiency, sinus bradycardia, greater than first‐degree atrioventricular block, or severe peripheral arterial disease." Randomised (N): 158 (79 intervention, 79 control) Withdrawn (N): not reported Lost to follow‐up (N): not reported Analysed (N): 158 (79 intervention, 79 control) Age (years, mean, SD): intervention: 81, 8; control: 81, 7 Sex (% men): intervention: 29; control: 30 Ethnicity (%): not reported Systolic blood pressure not reported Heart rate not reported BMI not reported Serum creatinine not reported B‐type natriuretic peptide not reported NT pro B‐type natriuretic peptide (pg/mL): LVEF (%, mean, SD): intervention: 56, 11; control: 57, 11 NYHA class I (%): 0 NYHA class II (%): intervention: 53; control: 51 NYHA class III (%): intervention: 47; control: 49 NYHA class IV (%): 0 Hypertension (%): intervention: 67; control: 65 Diabetes: not reported Atrial fibrillation (%): intervention: 33; control: 34 Hospitalisation for HF: not reported Coronary heart disease (%): 100 Stroke not reported Diuretic (%): 100 Digoxin (%): intervention: 33; control: 34 Beta‐blocker study drug ACEI (%): 100 ARB not reported MRA not reported |
|
| Interventions |
Intervention: propranolol. "The initial dose of propranolol was 10 mg/day. This dose was increased by 10‐mg increments at 10‐day intervals until a dose of 30 mg 3 times daily was given. All patients treated with propranolol received a final daily dose of propranolol of 30 mg 3 times daily." Comparator: no treatment Concomitant medication: "All patients continued diuretic and ACE inhibitor therapy during the study. Digoxin was administered only if the patient had atrial fibrillation." |
|
| Outcomes |
Planned: no published protocol or clinical trial registry entry Reported: "total mortality and total mortality plus nonfatal myocardial infarction" |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "LV ejection fraction and LV mass were interpreted by an experienced echocardiographer (IK) who was unaware of the study medications" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analyses |
| Selective reporting (reporting bias) | Unclear risk | not reported |
| Other bias | Unclear risk | funding not reported |
Aronow 1998.
| Study characteristics | ||
| Methods |
Study design: RCT Centres: not reported Start of enrolment: not reported End of enrolment: not reported Median follow‐up: 6 months Run‐in period: not reported |
|
| Participants |
Inclusion criteria: "with New York Heart Association functional class II or III CHF associated with prior Q‐wave myocardial infarction, a normal LV ejection fraction ( 50%),7 and 30 ventricular premature complexes per hour detected by 24‐hour ambulatory electrocardiograms were included in the study." Exclusion criteria: not reported Randomised (N): 60 (30 intervention, 30 control) Withdrawn (N): not reported Lost to follow‐up (N): not reported Analysed (N): 53 completed study (27 intervention, 26 control) Age (years, mean, SD): intervention: 82, 8; control: 82, 7 Sex (% men): intervention: 27; control: 23 Ethnicity (%): not reported Systolic blood pressure not reported Heart rate not reported BMI not reported Serum creatinine not reported B‐type natriuretic peptide not reported NT pro B‐type natriuretic peptide not reported LVEF (%, median, IQR): intervention: 61, 7; control: 62, 6 NYHA class not reported Hypertension (%): intervention: 73; control: 70 Diabetes not reported Atrial fibrillation not reported Hospitalisation for HF: Coronary heart disease (%): 100 Stroke not reported Diuretic (%): 100 Digoxin not reported Beta‐blocker not reported ACEI study drug ARB not reported MRA not reported |
|
| Interventions |
Intervention: benazepril. up to 40 mg/day Comparator: no treatment Concomitant medication: not reported |
|
| Outcomes |
Planned: unclear Reported: decrease in number of ventricular premature complexes/h, decrease in ventricular couplets/h, decrease in number of runs of ventricular tachycardia/24 h |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The cardiologists interpreting the 24‐hour ambulatory electrocardiograms were blinded to the study medications" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | not reported |
| Selective reporting (reporting bias) | Unclear risk | unable to assess as we are unaware of published protocol or pre‐registered clinical trial registry entry |
| Other bias | Unclear risk | unable to assess |
CandHeart.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Centres: 70, Italy Start of enrolment: December 2005 End of enrolment: May 2008 Mean follow‐up: 48 weeks Run‐in period: not reported |
|
| Participants |
Inclusion criteria: congestive HF, "Patients aged ≥18 years, of both genders, with stable symptomatic NYHA II‐IV HF and any LVEF measured at screening visit, and who provided a written informed consent were eligible. Patients with LVEF > 40% had to be hospitalized for cardiovascular events during the past 12 months before randomisation." Exclusion criteria: "Exclusion criteria were history of prior treatment with ARBs within 2 weeks from screening; severe or malignant hypertension (SBP/DBP > 180/110 mmHg); symptomatic hypotension; prior acute myocardial infarction, stroke or transient ischemic attack (TIA), percutaneous transluminal coronary angioplasty (PTCA) or coronary artery by‐pass graft (CABG) within 1 month from screening; hemodynamically relevant arrhythmias or cardiac valvular defect; prior implant of pacemakers, cardiac resynchronization therapy or cardioverters within 6 months from randomisation; constrictive pericarditis or active myocarditis; likelihood of cardiac surgical intervention during the overall treatment period; evidence of angina pectoris in the previous month; poorly controlled diabetes mellitus (blood glucose > 140 mg/mL or HbA1c > 8%); untreated thyroid dysfunction; renal artery stenosis; angio‐edema of any etiology; significant liver (AST, ALT, total bilirubin or alkaline phosphatase > 2x the upper limit of normal range) or renal impairment (serum creatinine > 2.0 mg/dL or serum potassium > 5.0 mmol/L); anemia of any etiology (Hb <10.5 g/dL) or any other clinically relevant hematological disease; pregnant or lactating females or females at risk of pregnancy; any disease with malabsorption; presence of any non‐cardiac disease that is likely to significantly shorten life expectancy; history of chronic alcohol or drug/substance abuse, or presence of other conditions potentially able to affect study subjects’ compliance; known allergy, sensitivity or intolerance to study drugs and/or study drugs’ formulation ingredients; patients unlikely to comply with the protocol or unable to understand the nature, scope and possible consequences of the study; participation in another trial in the month preceding study entry." Randomised (N): 128 Withdrawn (N): not reported Lost to follow‐up (N): not reported Analysed (N): not reported Age (years, mean, SD): 66, 12 Sex (% men): 67.2 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): 134, 19 Heart rate (beats/min, mean, SD): 67, 14 BMI (mean, SD): 28.2, 4.5 Serum creatinine (mg/dL, mean, DS): 1.0, 0.3 B‐type natriuretic peptide (pg/mL): 163, 202 NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): 48.7, 8.2 NYHA class I (%): 0 NYHA class II (%): 71.9 NYHA class III (%): not reported NYHA class IV (%): not reported Hypertension (%): 59.8 Diabetes (%): 28.1 Atrial fibrillation (%): 21.1 Hospitalisation for HF: not reported Coronary heart disease (%): 30.7 Stroke: not reported Diuretic (%): 86.7 Digoxin (%): 26.6 Beta‐blocker (%): 76.6 ACEI (%): 88.3 ARB (%): study drug MRA (%): 32.8 |
|
| Interventions |
Intervention: candesartan cilexetil, "1 candesartan cilexetil was administered at an initial dose of 4 mg o.d. (one tablet) and, if tolerated, it was up titrated to 8 mg (one tablet o.d.) after 2 weeks of treatment, to 16 mg (one tablet o.d.) after 4 weeks of treatment, and to 32 mg (two tablets of 16 mg o.d.) after 6 weeks of treatment" Comparator: no treatment Concomitant medication: ongoing standard therapy |
|
| Outcomes |
Planned: both trial register entries were post‐hoc, unclear what was planned Reported: primary: 3‐month (12‐week) changes of BNP from baseline, "The secondary objectives of the study after a 48‐week treatment period were to assess:1) change of BNP at 48 weeks from baseline values; 2) changes from baseline of aldosterone. Other exploratory analyses included (1) changes from baseline of LVEF, LVIDD, E wave peak velocity/A wave peak velocity (E/A), deceleration time of E wave (E‐DT), atrial dimensions; (2) changes from baseline of BP and heart rate (HR); (3) persistence of active treatment and discontinuation rate; (4) quality of life by Kansas City Cardiomyopathy Questionnaire (KCCQ)." |
|
| Notes | Only subgroup of participants with LVEF > 40% of interest to this review. The above data are for this subgroup only. No outcome data reported for this review. Emailed trialists. No response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "centrally randomised" |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | open label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Thirty percent of all echocardiographic exams performed during the study were randomly selected and read at the core laboratory by an experienced cardiologist unaware of study group and visit." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | unable to assess |
| Selective reporting (reporting bias) | Unclear risk | unclear as post‐hoc trial registration and published protocol not identified |
| Other bias | Unclear risk | The study was funded by Takeda Italia Farmaceutici and endorsed by the Associazione Nazionale Medici Cardiologi Ospedalieri (ANMCO). "The study was stopped before reaching the target number of patients since an interim analysis by the DSMB showed that an unacceptable number of patients (n=1500 per group) would have been needed to show the observed difference in 3‐month change of BNP with the predefined power of 0.80, when data on 371 patients were available" |
CAN‐DHF.
| Study characteristics | ||
| Methods |
Study design: two‐arm, individual, placebo‐controlled RCT Centres: 8 sites in Germany Start of enrolment: January 2008 End of enrolment: December 2008 Follow‐up: 24 weeks Run‐in period: not reported |
|
| Participants |
Inclusion criteria: "Male or female patients of at least 45 years of age suffering from a non‐insulin dependent diabetes mellitus type 2 orally treated for at least 3 months and showing normotension or controlled hypertension with sitting systolic blood pressure (sSBP) < 140 mmHg and/or sitting diastolic blood pressure (sDBP) < 90 mmHg. Evidence of an abnormal left ventricular relaxation, diastolic distensibility or diastolic stiffness confirmed by echocardiography under the prerequisite of a preserved Left ventricular ejection fraction (LVEF) ≥ 45%. NT‐proBNP ≥ 250 pg/ml at baseline, NYHA class II or III in stable condition since 3 months, and standard HF‐ therapy with an ACE‐inhibitor alone or with further preparations in a constant regimen since at least 1 month (3 months in terms of β‐blockers). Signed written informed consent available." Exclusion criteria: "The following criteria must not be met to enrol a single patient into the study: Impaired renal function (serum creatinine > 2.2 mg/dL or > 194 µmol/l); Known bilateral renal artery stenosis (RAS) or interventional treatment for RAS in the last year; State after kidney transplantation; Serum potassium > 5.5 mmol/l or HbA1C > 9.5 %; Cor pulmonale or primary pulmonary disease with dyspnea at rest; Known disposition to episodes of symptomatic hypotension or sSBP < 95 mmHg at baseline; Acute coronary syndrome or unstable angina pectoris and any coronary artery disease that was not stable during the last 3 months prior to inclusion; CABG or PTCA (incl. stent implantation) within 3 months before inclusion; Myocardial infarction or stroke within 6 months before inclusion; Patients who are dependent on a permanently paced pacemaker (i.e. a patient with a device that is not pacing during the echocardiographic examination can enter the study); Open heart surgery for other reasons than coronary revascularization; Tachycardia at rest > 100 bpm as confirmed by ECG‐recordings; Known clinically relevant rhythm disorders (e.g. tachyarrhythmias, salves of supraventricular or ventricular extrasystoles or atrial fibrillation without ventricular rate control) or symptoms suggesting a significant rhythm disorder (e.g. recurrent syncopes); Primary valvular diseases and/or restrictive or obstructive cardiomyopathy ‐ Existing ventricular assist devices; Relevant liver diseases (cholestasis or ALAT/ASAT > 2xULN or GT > 3xULN); History of primary hyperaldosteronism; of cancer in the last 5 years (exception: nonmetastasizing skin cancer) or of another wasting disease with life expectancy of < 2 years; Known hypersensitivity to Candesartan Cilexetil; Need for maintenance therapy with NSAIDs or Cox‐2‐inhibitors; Use of other ARBs throughout the entire study period; Any history of life‐threatening diseases; History of drug addiction and/or an extensive use of alcohol; Female patients who are pregnant or breast feeding; Sexually active women of childbearing potential not consistently and correctly practicing highly effective birth control with a low failure rate (less than 1% / year) such as implants, injectables, combined oral contraceptives, hormonal intrauterine devices (IUDs), sexual abstinence or vasectomised partner; Psychological and/or emotional problems, which render the informed consent invalid or limit the ability of the patient to comply with the study requirements; Patient is an employee or at least in dependence of the investigator and/or the sponsor or of another institution directly involved in the study or other trials under the investigator's direction; Participation in another clinical investigation within 30 days prior to enrolment or for the course of the present study (incl. studies for compassionate use or experimental medical devices)." Randomised (N): 22 (11 intervention, 11 control) Withdrawn (N): for reasons other than death 14 (intervention: 3 premature study termination, 3 adverse events, 1 randomisation /enrolment error, control: 4 premature study termination, 2 adverse events, 1 randomisation / enrolment error) Lost to follow‐up (N): 0 Analysed (N): 22 (11 intervention, 11 control) Age (years, mean, SD): intervention: 67.0, 16.8; control: 69.0, 7.1 Sex (% men): intervention: 64; control: 55 Ethnicity (%): not reported Systolic blood pressure not reported Heart rate not reported BMI (mean, SD): intervention: 31.4, 5.0; control: 30.0, 5.7 Serum creatinine not reported B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF not reported NYHA class: not reported Hypertension (%): not reported Diabetes (%): not reported Atrial fibrillation (%): not reported Hospitalisation for heart failure: not reported Myocardial infarction (%): not reported Stroke (%): not reported Diuretic (%): not reported Digoxin (%): not reported Beta‐blocker (%): not reported ACEI (%): not reported ARB (%): study drug MRA (%): not reported |
|
| Interventions |
Intervention: candesartan. 8‐32mg as tolerated. "The treatment comprised a titration period of 6 weeks and a period of constant study therapy of at least 18 weeks" Comparator: placebo Concomitant medication: "in an "added" regimen to a constant background‐HF‐therapy with at least ACE‐inhibitors (or further drugs) for the treatment of symptomatic heart failure with diastolic dysfunction in diabetic and hypertensive patients" exclusion criteria: use of other ARB |
|
| Outcomes |
Planned: primary: mean change from baseline for NT‐proBNP, secondary: QoL, kidney function, NYHA, body weight, BP and echocardiographic measures, adverse events, rate of premature withdrawals Reported: as planned except QoL |
|
| Notes | This trial was terminated prematurely and the results are available via a clinical trial registry entry only. No outcome data relevant to this review reported (confirmed by sponsor Takeda via Email). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind" but no detail |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | no information |
| Selective reporting (reporting bias) | Low risk | all outcomes reported as planned except QoL |
| Other bias | High risk | "the study was terminated prematurely as a whole by the sponsor in December 2008 since randomization of patients was very poor until that date (low and slow recruitment (N = 42) with a high number (N = 20) of screening failures)" Takeda funded the study. The study results are unpublished and only available via the clinical trial registry entry. |
CHARM‐Preserved.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Centres: 618, 26 countries (Australia, Belgium, Canada, Czech Republic, Denmark, Finland, France, Germany, Hungary, Iceland, Italy, Luxembourg, Malaysia, Netherlands, Norway, Poland, Portugal, Russia, Singapore, South Africa, Spain, Sweden, Switzerland, UK/Ireland, USA) Start of enrolment: March 1999 End of enrolment: July 2000 Median follow‐up: 36.6 months Run‐in period: no |
|
| Participants |
Inclusion criteria: "Eligible patients were aged 18 years or older, had New York Heart Association functional class II–IV of at least 4 weeks’ duration, had a history of hospital admission for a cardiac reason, and had LVEF higher than 40%." Exclusion criteria: Important exclusion criteria for any of the studies include current serum‐creatinine > 265mmol/L (> 3 mg/dL); current serum‐potassium > 5.5 mmol/L (> 5.5 mEq/L) or a history of marked ACE inhibitor‐induced hyperkalemia resulting in either a serum potassium greater than or equal to 6.0 mmol/L (>6.0 mEq/L) or a life‐threatening adverse event; known bilateral renal artery stenosis; current symptomatic hypotension; persistent systolic or diastolic hypertension; stroke, acute myocardial infarction, or open heart surgery within the last 4 weeks; previous heart transplant or heart transplant expected to be performed within the next 6 months; presence of any noncardiac disease (eg, cancer) that is likely to significantly shorten life expectancy to less than 2 years. Randomised (N): 3023 (1514 intervention, 1509 control) Withdrawn (N): for reasons other than death (270 intervention, 204 control) Lost to follow‐up (N): (2 intervention, 1 control) Analysed (N): 3020 (1512 intervention, 1508 control) Age (years, mean, SD): intervention: 67.2, 11.1; control: 67.1, 11.1 Sex (% men): intervention: 60.8; control: 59.0 Ethnicity (%): intervention: European 90.8, control: European 92.3 Systolic blood pressure (mmHg, mean, SD): intervention: 136.0, 18.6; control: 136.3, 18.3 Heart rate (beats/min, mean, SD): intervention: 71.2, 12.4; control: 71.4, 12.5 BMI (mean, SD): intervention: 29.3, 5.9; control: 29.0, 5.6 Serum creatinine (mg/dL): not reported B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): intervention: 54.0, 9.4; control: 54.1, 9.4 NYHA class I (%): 0 NYHA class II (%): intervention: 61.5; control: 60.0 NYHA class III (%): intervention: 36.7; control: 38.7 NYHA class IV (%): intervention: 1.8; control: 1.3 Hypertension (%): intervention: 65.0; control: 63.6 Diabetes (%): intervention: 28.7; control: 28.0 Atrial fibrillation (%): intervention: 29.0; control: 29.3 Hospitalisation for heart failure (%): intervention: 69.6; control: 68.8 Coronary heart disease (%): intervention: 45.0; control: 43.7 Stroke (%): intervention: 9.2; control: 8.5 Diuretic (%); intervention: diuretic: 75.2, spironolactone 11.3; control: diuretic 74.3, spironolactone 12.0 Digoxin (%): intervention: 28.5; control: 27.2 Beta‐blocker (%): intervention: 55.9; control: 55.5 ACEI (%): intervention: 19.6; control: 18.6 ARB (%): study drug MRA (%): intervention: 11.3; control: 12.0 |
|
| Interventions |
Intervention: candesartan. "which could be started at 4 or 8 mg once daily, the assignment code being held by an independent centre and the data safety monitoring board. The treatment dose was doubled every 2 weeks, as tolerated, according to a forced titration protocol, with recommended monitoring of blood pressure, serum creatinine, and potassium. The target dose was 32 mg once daily from 6 weeks onwards." Comparator: "matching placebo" Concomitant medication: "physicians were free to prescribe all treatments other than angiotensin‐receptor blockers." "Initially, angiotensin converting‐enzyme inhibitors were not allowed as concomitant treatment, but after publication of the Heart Outcomes Prevention Evaluation trial results, (The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. N Engl J Med 2000; 342: 145–53.) their use was optional in appropriate patients." "By the end of the study, 298 (20%) in the candesartan and 340 (23%) in the placebo group were receiving angiotensin‐converting‐enzyme inhibitors, 712 (47%) and 748 (50%) were receiving blockers, and 136 (9%) and 201 (13%) were receiving spironolactone. Non‐study angiotensin‐receptor blockers were used in 3% of patients in each of the two groups." |
|
| Outcomes |
Planned: Planned: "The primary outcome was cardiovascular death or unplanned admission to hospital for the management of worsening CHF. Prespecified secondary outcomes were: cardiovascular death, admission to hospital for CHF, or non‐fatal myocardial infarction; cardiovascular death, admission to hospital for CHF, non‐fatal myocardial infarction, or non‐fatal stroke; cardiovascular death, admission to hospital for CHF, non‐fatal myocardial infarction, non‐fatal stroke, or coronary revascularisation; death (any cause) or admission to hospital for CHF; and development of new diabetes." Reported: as planned |
|
| Notes | The CHARM program consisted of 3 strands, one of which was CHARM‐Preserved. Contacted investigators for end scores of MLHF QoL by treatment arms and details on subgroup data by LVEF. No response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "We randomly assigned patient" |
| Allocation concealment (selection bias) | Low risk | "the assignment code being held by an independent centre and the data safety monitoring board" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "We randomly assigned patients, in a double‐blind way", "matching placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "A committee unaware of treatment assignment and which component of the CHARM programme was being undertaken adjudicated the cause of death, first myocardial infarctions, and first hospital admissions for heart failure." "All final data analyses were done by the sponsor and verified independently by the statistical centre at the London school of Hygiene and Tropical Medicine, London, UK" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "two patients who mistakenly received randomisation numbers but had no other data recorded and never received study medication" "Two candesartan patients and one placebo patient were lost to follow‐up" 207/204 withdrew due to AE "Anaysis was done by intention to treat." |
| Selective reporting (reporting bias) | Low risk | outcomes reported as planned |
| Other bias | Low risk | "MA Pfeffer, K Swedberg, CB Granger, JJV McMurray, and S Yusuf have served as consultants to or received research grants from AstraZeneca and other major cardiovascular pharmaceutical companies. J Östergren has served as a consultant and received research grants from AstraZeneca. P Held, E L Michelson, and B Olofsson are employees of AstraZeneca." "This study was supported by AstraZeneca R&D, Mölndal, Sweden" |
ELANDD.
| Study characteristics | ||
| Methods |
Study design: multicenter, double‐blind, placebo controlled, randomised, parallel group trial Centres: 12 in 8 European countries Start of enrolment: not reported End of enrolment: not reported Follow‐up: 6 months Run‐in period: not reported |
|
| Participants |
Inclusion criteria: "To be included into the study, patients had to fulfil the following criteria: willing and able to sign the informed consent form and comply with the requirements of the study, aged ≥ 40 years, have a documented history of HF and persistent symptoms during effort [New York Heart association (NYHA) class II–III ], an LVEF ≥ 45%, and LV end‐diastolic internal diameter < 3.2 cm/m2 or LV end‐diastolic volume index < 102 mL/m2 by echocardiography, radionuclide ventriculography, or nuclear magnetic imaging, or any abnormality of LV diastolic function documented by echocardiography, according to the guidelines of the European Study Group on Diastolic Heart Failure. This last inclusion criterion was revised in April 2007 following the online publication of the new consensus statement on the diagnosis of HFPEF by the European Society of Cardiology. Accordingly, an E/E ratio > 15 at tissue Doppler echocardiography was required as an inclusion criterion. Patients with an E/E‘ ratio between 8 and 15 could be included when additional abnormalities of diastolic function were found. These included an E/A ratio < 0.5 and/or a deceleration half‐time > 280 ms in patients older than 50 years, and/or a duration of reverse pulmonary vein atrial systole flow–mitral valve atrial wave flow > 30 ms, and/or a left atrial volume index > 40 mL/m2 , and/or an increased LV mass index" Exclusion criteria: "Major exclusion • Patients unable to perform 6‐mi walking test • Planned invasive cardiac procedures or cardiac surgery during the time of the study • Recent (< 3 months) acute coronary syndrome or stroke • Exercise‐induced myocardial ischaemia as main cause of exercise limitation as shown by symptoms (angina) or by previous exams (exercise test, stress echocardiography or myocardial scintigraphy) • Concomitant diseases (COPD, peripheral vasculopathy, orthopaedic disease) as main cause of exercise limitation • Major contraindications to beta‐blocker therapy (sinus bradycardia, \50/min; atrio‐ventricular block, bronchial asthma sensitive to beta‐agonists administration) • Ongoing treatment with beta‐blockers, diltiazem or verapamil • Systolic blood pressure \100 mm Hg • Pregnancy, breast feeding or childbearing potential during the study • History of alcohol or other illicit drug abuse • Expected poor compliance to drug therapy • Participation in any other clinical trial with an investigational product or scheduled to receive any such product during the study or in the 4 weeks following the study • Suffering from any other medical condition that may exclude the patient for safety reasons or interfere with the objective of the study." Randomised (N): 116 (57 intervention, 59 control) Withdrawn (N): for reasons other than death 22 (14 intervention (9 lack of tolerance, 1 protocol violation, 3 consent withdrawal, 1 other), 8 control (consent withdrawal 1, protocol violation 5, 2 other)) Lost to follow‐up (N): 1 (1 intervention, 0 control) Analysed (N): 93 (42 intervention, 51 control) Age (years, mean, SD): intervention: 66.5, 9.8; control: 65.3, 11.3 Sex (% men): intervention: 35; control: 36 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): intervention: 128, 17 (Table 3 of Conraads), 134, 21 (Table 2); control: 129, 23 (Table 3) 133, 18 (Table 2) Heart rate (beats/min, mean, SD): intervention: 76, 15 (Table 3) 73, 14 (Table 2); control: 78, 13 (Table 3), 73, 11 (Table 2) BMI (mean, SD): intervention: 30.3, 4.5; control: 30.2, 4.9 Serum creatinine (mg/dL, mean, SD): intervention: 88.5, 33.1; control: 85.8, 25.1 B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL, median, range): intervention: 147 (9‐3577); control: 126 (15‐2055) LVEF (%, mean, SD): intervention: 61.9, 7.8; control: 63.2, 9.2 NYHA class I (%): 0 NYHA class II (%): intervention: 77; control: 78 NYHA class III (%): intervention: 21; control: 22 NYHA class IV (%): 0 Hypertension (%): intervention: 86; control: 86.4 Diabetes (%): intervention: 21; control: 20 Atrial fibrillation (%): not reported Hospitalisation for heart failure: not reported Coronary heart disease (%): intervention: 17; control: 20 Stroke (%): not reported Diuretic (%); intervention: 49; control: 54 Digoxin (%): not reported Beta‐blocker (%): study drug ACEI (%): intervention: 75; control: 80 ARB (%): not reported MRA (%): not reported |
|
| Interventions |
Intervention: nebivolol. "Nebivolol was started at 2.5 mg/day and gradually up‐titrated to 10 mg/day over a period of 5 weeks. Down‐titration to lower doses was allowed if the higher dose was not tolerated. Treatment at maintenance doses was continued for an additional 21 weeks (6 months of treatment in total)." Comparator: placebo Concomitant medication: "Ongoing treatment with other drugs was maintained throughout the study." |
|
| Outcomes |
Planned: " The primary endpoint of the study is the change from baseline in the distance walked during the 6‐min walking test (6MWT) after 6 months of treatment with nebivolol versus placebo. Additional secondary endpoints are the changes from baseline after 6 months, with nebivolol versus placebo, in the following measurements: • Symptoms, assessed using a five‐level scale (extremely
worsened, moderately worsened, unchanged, moderately improved, extremely improved); • New York Heart Association (NYHA) functional class; • Minnesota living with heart failure questionnaire [21];
• Maximal exercise duration, peak oxygen consumption, [VO2] and slope of the minute ventilation [VE] to carbon dioxide [VCO2] relation, at cardiopulmonary exercise testing. • Changes in parameters related to LV diastolic function, including peak E velocity at the Doppler recording of transmitral inflow tracing, peak E0 velocity of the mitral valve annulus measured at the level of the septal and lateral wall, respectively, by tissue Doppler recording, and the E/E' ratio. Lastly, the effects of treatment on major outcomes (death, hospitalization and unexpected visit to the outpatient clinic or heart failure unit) as well as adverse events are assessed." Reported: as planned |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated 1:1 randomization" |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | reasons provided for withdrawal |
| Selective reporting (reporting bias) | Low risk | reported as planned |
| Other bias | Low risk | The trial is funded by a grant from Menarini. "We thank Joachim Klinger Director of Data Management and Statistics, Harrison Clinical Research Deutschland, and Lieven Huysse, who worked at Menarini at the time of the study, for management and statistical support." |
Hong Kong DHF.
| Study characteristics | ||
| Methods |
Study design: three‐arm, parallel RCT Centres: multicentre, no details Start of enrolment: not reported End of enrolment: not reported Mean follow‐up: 1 year Run‐in period: none |
|
| Participants |
Inclusion criteria: "The inclusion criteria were age .18 years, clinical history of heart failure within 2 months prior to screening including a chest x ray demonstrating pulmonary congestion, NYHA functional class II – IV, left ventricular ejection fraction .45% by 2D‐echocardiography or a radionuclide technique, and therapy with diuretics with stable dose.14 days prior to recruitment." Exclusion criteria: "NYHA functional class I, myocardial infarction within 3 months, unstable angina within 1 month, significant valvular heart disease, uncontrolled hypertension, serious cardiac arrhythmias, concurrent therapy with calcium channel antagonist, b‐blockers (a‐methyl dopa was used for treating hypertension if required), positive inotropic agents (except digoxin for control of atrial fibrillation) and other angiotensin converting enzyme inhibitors or receptor blockers." Randomised (N): 151 ( intervention R: 45, intervention I: 56, control: 50) Withdrawn (N): for reasons other than death: intervention R: 6 (4 persistent irritating cough, 1 uncontrolled blood pressure, 1 refused to continue), intervention I: 1 due to onset of fast atrial fibrillation, control: 3 (1 uncontrolled high blood pressure, 1 defaulted, 1 refused to continue) Lost to follow‐up (N): 0 Analysed (N): 151 (intervention R: 45, intervention I: 56, control: 50) Age (years, mean, SD): intervention R: 74, 6.1; intervention I: 75, 8.5; control: 73, 8.4 Sex (% men): intervention R: 40; intervention I: 34; control: 42 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): intervention R: 143, 22; intervention I: 145, 19; control: 145, 23 Heart rate (beats/min, mean, SD): intervention R: 79, 13; intervention I: 77, 9; control: 76, 14 BMI (mean, SD): intervention R: 26.8, 3.9; intervention I: 27.2, 4.1; control: 26.8, 4.2 Serum creatinine: not reported B‐type natriuretic peptide (pg/mL, mean, SEM): intervention R: 488, 701; intervention I: 568, 757; control: 566, 944 NT pro B‐type natriuretic peptide: not reported LVEF (%, median, IQR): intervention R: 65, 1; intervention I: 66, 1; control: 69, 2 NYHA class I (%): 0 NYHA class II (%): intervention R: 66.7; intervention I: 67.9; control: 72 NYHA class III (%): intervention R: 33.3; intervention I: 30.4; control: 28 NYHA class IV (%): 0 Hypertension (%): intervention R: 73; intervention I: 71; control: 76 Diabetes (%): intervention R: 22; intervention I: 18; control: 20 Atrial fibrillation (%): intervention R: 16; intervention I: 21; control: 10 Hospitalisation for HF: 100% as it was an inclusion criteria Coronary heart disease (%): intervention R: 18; intervention I: 11; control: 18 Stroke (%): not reported Diuretic (%): intervention R: Hydrocholorthiazide 8.9 furosemide 80 , dyazide 2.2; intervention I: Hydrocholorthiazide 10.7, furosemide 80.4 , dyazide 10.7; control: Hydrocholorthiazide 6, furosemide 68, dyazide 12 Digoxin (%): not reported Beta‐blocker (%): 0 ACEI (%): study drug (R) ARB (%): study drug (I) MRA (%): not reported |
|
| Interventions |
Intervention R: "Ramipril was started at 2.5 mg daily and similarly titrated to 10 mg daily" Intervention I: "The initial dose of irbesartan was 18.75 mg daily which was titrated at 4 and 8 weeks to 75 mg daily." Comparator: usual care, "continue with diuretics alone" Concomitant medication: "Exclusion criteria were: ...concurrent therapy with calcium channel antagonist, b‐blockers (a‐methyl dopa was used for treating hypertension if required), positive inotropic agents (except digoxin for control of atrial fibrillation) and other angiotensin converting enzyme inhibitors or receptor blockers." |
|
| Outcomes |
Planned: planned as per clinical trial registry entry: primary: 1. Number of hospital admissions for heart failure or mortality 2. Quality of life assessed by the Minnesota Quality of life Questionnaire 3. In ambulatory patients the exercise duration assessed by 6 min corridor walk test. Secondary: The incidence of side‐effects, effect on levels of natriuretic peptides, effect on doppler‐echocardiographic derived measurements of left ventricular diastolic function. Reported: cardiovascular mortality, hospitalisation for heart failure, all‐cause mortality, quality of life, 6MWT, blood pressure, NT‐proBNP, peak early diastolic mitral annular velocities, peak systolic velocity, LV mass |
|
| Notes | retrospective clinical trial registration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated using computer‐generated random numbers in blocks of 10" |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "open‐label" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All outcomes were reviewed blind to treatment allocation." "with blinded end point design" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | unable to assess |
| Selective reporting (reporting bias) | Unclear risk | retrospective clinical trial registration, no published protocol identified |
| Other bias | Unclear risk | "None of the authors received any lecture, advisory board, or consultancy fees relating to this study from the sponsors." "This study was initially supported by a small grant from the manufacturers of Irbesartan, who also donated the irbesartan medication (Sanofi‐Synthelabo). Design, conduct, retention of data, analysis and writing were all entirely independent and carried out by the authors only. Data were kept at the Chinese University of Hong Kong and are available for public scrutiny." |
I‐PRESERVE.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Centres: 293 centres in 25 countries (Argentina, Australia, Belgium, Brazil, Canada, Czech Republic, Denmark, France, Germany, Greece, Hungary, Ireland, Italy, Mexico, The Netherlands, Norway, Poland, Portugal, Russia, South Africa, Spain, Sweden, Switzerland, UK, USA) Start of enrolment: June 2002 End of enrolment: April 2005 Mean follow‐up: mean follow‐up time was 49.5 months, and the trial included 16,798 patient‐years of follow‐up Run‐in period: "Eligible patients were treated with single‐blind placebo for 1 to 2 weeks before randomization" |
|
| Participants |
Inclusion criteria: "All patients were at least 60 years of age and had heart failure symptoms and a left ventricular ejection fraction of at least 45%. In addition, we required patients to have been hospitalized for heart failure during the previous 6 months and have current New York Heart Association (NYHA) class II, III, or IV symptoms with corroborative evidence; if they had not been hospitalized, they were required to have ongoing class III or IV symptoms with corroborative evidence. Such evidence could include findings of pulmonary congestion on radiography, left ventricular hypertrophy or left atrial enlargement on echocardiography, or left ventricular hypertrophy or left bundle‐branch block on electrocardiography. Treatment with an angiotensin‐converting–enzyme (ACE) inhibitor was permitted only when such therapy was considered essential for an indication other than uncomplicated hypertension." Exclusion criteria: "Exclusion criteria included previous intolerance to an angiotensin‐receptor blocker; an alternative probable cause of the patient’s symptoms (e.g. significant pulmonary disease); any previous left ventricular ejection fraction below 40%; a history of acute coronary syndrome, coronary revascularization, or stroke within the previous 3 months; substantial valvular abnormalities; hypertrophic or restrictive cardiomyopathy; pericardial disease; cor pulmonale or other cause of isolated right heart failure; a systolic blood pressure of less than 100 mm Hg or more than 160 mm Hg or a diastolic blood pressure of more than 95 mm Hg despite antihypertensive therapy; other systemic disease limiting life expectancy to less than 3 years; substantial laboratory abnormalities (such as a hemoglobin level of less than 11 g per deciliter, a creatinine level of more than 2.5 mg per deciliter [221 µmol per liter], or liver‐function abnormalities); or characteristics that might interfere with compliance with the study protocol" Randomised (N): 4128 (2067 intervention, 2061 control) Withdrawn (N): for reasons other than death 1368 (702 intervention, 684 control) Lost to follow‐up (N): 73 (29 intervention, 44 control) Analysed (N): 4128 (2067 intervention, 2061 control) Age (years, mean, SD): intervention: 72, 7; control: 72, 7 Sex (% men): intervention: 41; control: 39 Ethnicity (%): intervention: white 94, control: white 93 Systolic blood pressure (mmHg, mean, SD): intervention: 137, 15; control: 136, 15 Heart rate (beats/min, mean, SD): intervention: 72, 11; control: 71, 10 BMI (mean, SD): intervention: 29.7, 5.2; control: 29.6, 5.3 Serum creatinine (mg/dL, mean, SD): intervention: 1.0, 0.32; control: 1.0, 0.34 B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL, median, IQR): intervention: 360, 139–987; control: 320, 131–946 LVEF (%, mean, SD): intervention: 59, 9; control: 60, 9 NYHA class I (%): 0 NYHA class II (%): intervention: 21; control: 22 NYHA class III (%): intervention: 77; control: 76 NYHA class IV (%): intervention: 3; control: 3 Hypertension (%): intervention: 89; control: 88 Diabetes (%): intervention: 28; control: 27 Atrial fibrillation (%): intervention: 29; control: 29 Hospitalisation for heart failure in the last six months (%): intervention: 44; control: 44 Myocardial infarction (%): intervention: 24; control: 23 Stroke or TIA (%): intervention: 10; control: 10 Diuretic (%): intervention: loop: 52, thiazide: 38, spironolactone 15; control: loop: 52, thiazide: 38, spironolactone 15 Digoxin (%): intervention: 14; control: 13 Beta‐blocker (%): intervention: 59; control: 58 ACEI (%): intervention: 26; control: 25 ARB (%): study drug MRA (%): intervention: 15; control: 15 |
|
| Interventions |
Intervention: irbesartan. "Patients were started on 75 mg of irbesartan or placebo once daily. The dose was doubled to 150 mg after 1 to 2 weeks and was doubled again to 300 mg after an additional 1 to 2 weeks, according to a forced‐titration protocol as tolerated." "At the end of the titration phase, 84% of the patients in the irbesartan group and 88% of those in the placebo group had reached the 300‐mg dose (mean doses, 275 mg and 284 mg, respectively)." Comparator: "matching placebo" Concomitant medication: "During the study, the proportion of patients receiving an ACE inhibitor rose from 25% in the two groups at baseline to 39% in the irbesartan group and 40% in the placebo group, the use of spironolactone rose from 15% in the two groups at baseline to 28% in the irbesartan group and 29% in the placebo group, and the use of beta‐blockers rose from 59% in the irbesartan group and 58% in the placebo group to 73% in the two groups." |
|
| Outcomes |
Planned: "The primary end point is defined as time from randomization to the first occurrence of the composite outcome of death (all cause) or cardiovascular hospitalization. [...] The endpoint additionally includes myocardial infarction or stroke occurring during any hospitalization at any point during the study. Secondary endpoints include the effect of irbesartan as compared with placebo in reducing the risk of: cardiovascular death, all‐cause mortality, combined vascular endpoint: cardiovascular death, nonfatal myocardial infarction (MI) or nonfatal stroke; or combined HF endpoint: HF mortality or hospitalizations; [...] quality of life as measured by the Minnesota Living with Heart Failure questionnaire, change in New York Heart Associatioin (NYHA) functional class, change in global assessment of symptoms, N‐terminal B‐type natriuretic peptide levels in blood." (Carson 2005) Reported: all planned outcomes reported |
|
| Notes | Emailed trialists to enquire about differing data in different publications and to ask for subgroup data. No response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "using an automated, central randomization system" |
| Allocation concealment (selection bias) | Low risk | "The randomization schedule was implemented with the use of an interactive voice‐response system." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All investigators and committee members who were involved in the conduct of the study (except for members of the data and safety monitoring board) were unaware of study‐group assignments." "double‐blind" (Carson 2005) "matching placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "blinded review of event rates in 2004" but blinding of event adjudication not specified overall |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "At the end of the study, vital‐status data were not available for 29 patients (1%) in the irbesartan group and 44 patients (2%) in the placebo group. If contact could not be made at end of study, data for these patients were censored from the analysis at the date they were last known to be alive." "Data from all patients who underwent randomization were analyzed according to the intention‐to‐treat principle." |
| Selective reporting (reporting bias) | Low risk | all planned outcomes reported (comparison between published protocol (Carson 2005) and main results paper (Massie 2008) |
| Other bias | Low risk | "Dr. Massie reports receiving grant support from Bristol‐Myers Squibb, Sanofi‐Aventis, and Merck, consulting fees from Bristol‐ Myers Squibb, Sanofi‐Aventis, Merck, Duke Clinical Research Institute, Momentum Research, Novartis, GlaxoSmithKline, Scios–Johnson & Johnson, Corthera, and Niles Therapeutics, and lecture fees from Merck; Dr. Carson, receiving consulting fees from Bristol‐Myers Squibb, Sanofi‐Aventis, and Merck and lecture fees from AstraZeneca and Novartis; Dr. McMurray, receiving support from Bristol‐Myers Squibb (to Glasgow University) for his work on this trial; Dr. Komajda, receiving consulting fees from Bristol‐Myers Squibb and Servier and lecture fees from Servier, Sanofi‐Aventis, and AstraZeneca; Dr. McKelvie, receiving consulting fees from Bristol‐Myers Squibb and Sanofi‐Aventis and lecture fees from Bristol‐Myers Squibb, Sanofi‐Aventis, Pfizer, Merck, and AstraZeneca; Dr. Zile, receiving consulting fees from Bristol‐Myers Squibb and Sanofi‐Aventis; Ms. Anderson, being employed by the Statistical Data Analysis Center at the University of Wisconsin–Madison, which conducted statistical analysis for this trial, supported by Bristol‐Myers Squibb and Sanofi‐Aventis; Drs. Donovan and Ptaszynska, being employees of and having an equity interest in Bristol‐Myers Squibb; and Dr. Staiger, being an employee of and having an equity interest in Sanofi‐Aventis." study sponsors: Bristol‐Myers Squibb and Sanofi‐Aventis. "The sponsors or a contract research organization collected the trial data, which were then analyzed at the Statistical Data Analysis Center at the University of Wisconsin, Madison, independently of the sponsors." |
J‐DHF.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Centres: "multicenter" but no further details Start of enrolment: May 2004 End of enrolment: March 2009 Mean follow‐up: 3.2 years Run‐in period: no |
|
| Participants |
Inclusion criteria: "All patients were at least 20 years of age, and had an LVEF of > 40% when diagnosed as having heart failure. Clinical diagnosis of heart failure was based on a slight modification of the Framingham criteria as previously described within the 12 months before study entry. There were no changes in baseline therapy and symptoms of heart failure within a month before study entry in any patients." Exclusion criteria: Current symptomatic hypotension, • Hypertension that has not been controlled to the satisfaction of the investigator by drugs other than β‐blocker • Hemodynamically significant (in the investigators opinion) LV outflow tract obstruction (from either aortic stenosis or ventricular hypertrophy) or mitral valve stenosis • Important aortic or mitral regurgitation in the investigator’s opinion • Heart rate < 50 beats/min • Second‐ or third‐degree heart block without permanent pacemaker in situ • Acute coronary syndrome • Arrhythmogenic right ventricular cardiomyopathy • Primary pulmonary hypertension or pulmonary hypertension not from LV dysfunction • Serious cerebrovascular disease • Acute myocardial infarction within the last 3 months • Patients who require intravenous inotropes • Cerebrovascular accident within the last 6 months • Percutaneous coronary intervention or open heart surgery within the last 3 months • On the waiting list for percutaneous coronary intervention or open heart surgery • Serum creatinine > 3.0 mg/dL or creatinine clearance ≤ 30 mL/min • Known bilateral renal artery stenosis • Serum potassium > 5.5 mEq/L • Serious liver disease • Prescription of β‐blocker within the last month or a history of a life‐threatening adverse event induced by β‐blocker • Any change in cardiovascular drug therapy within a month before randomization • History of chronic obstructive pulmonary disease or restrictive lung disease • Diabetes mellitus that has not been controlled to the satisfaction of the investigator • History of any life‐threatening noncardiac disease (eg, cancer) within 5 years • Other diseases likely to cause death or serious disability within 1 year • Patients unable to walk without personal aid • Arteriosclerosis obliterans with Fontaine Grade II or more. • Severe anemia (hemoglobin ≤ 6.0 g/dL) • Uncontrolled thyroid dysfunction Randomised (N): 245 (120 intervention, 125 control) Withdrawn (N): for reasons other than death (6 intervention, 0 control) Lost to follow‐up (N): (5 intervention, 3 control) Analysed (N): (120 intervention, 125 control) Age (years, mean, SD): intervention: 73, 10; control: 71, 11 Sex (% men): intervention: 57.5; control: 58.4 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): intervention: 134, 21; control: 133, 21 Heart rate (beats/min, mean, SD): intervention: 72, 11; control: 74, 13 BMI (mean, SD): intervention: 24.2, 4.4; control: 24.1, 4.1 Serum creatinine (mg/dL, mean, SD): intervention: 0.98, 0.37; control: 1.01, 0.45 B‐type natriuretic peptide (pg/mL, mean, SD): intervention: 219.2, 294.9; control: 234.9, 281.6 NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): intervention: 62, 10; control: 63, 11 NYHA class I (%): intervention: 18.3; control: 18.4 NYHA class II (%): intervention: 69.2; control: 75.2 NYHA class III (%): intervention: 10.8; control: 4.8 NYHA class IV (%): intervention: 1.7; control: 1.6 Hypertension (%): intervention: 80.0; control: 80.8 Diabetes (%): intervention: 27.5; control: 33.6 Atrial fibrillation (%): intervention: 50.8; control: 45.6 Hospitalisation for heart failure (%): intervention: 60.0; control: 60.0 Ischaemic heart disease (%): intervention: 28.3; control: 24.0 Stroke (%): intervention: 11.7; control: 12.8 Diuretic (%); intervention: 63.3; control: 56.8 Digoxin (%): intervention: 19.2; control: 21.6 Beta‐blocker (%): study drug ACEI (%): intervention: 24.2; control: 22.4 ARB (%): intervention: 50.8; control: 56.0 MRA (%): intervention: 20.8; control: 25.6 |
|
| Interventions |
Intervention: carvedilol. "In the carvedilol arm, carvedilol was up‐titrated from 1.25 mg twice daily to the target dose of 10 mg twice daily within 8 weeks based on tolerability. Patients were maintained at the target dose or the maximum tolerated dose for the remainder of the study." Comparator: usual care Concomitant medication: "In both arms, patients were treated with standard cardiovascular therapy excluding beta‐blockers." |
|
| Outcomes |
Planned: "The primary outcome is a composite of cardiovascular death and unplanned admission to hospital for congestive heart failure. The secondary outcomes are listed as follows: all‐cause mortality; worsening of the symptoms (defined by either a decrease by 1 Mets in the SAS questionnaire score or an increase by 1 class in the New York Heart Association functional class for at least 3 months compared with the baseline); an increase in brain natriuretic peptide by 30% of the value at the randomization in patients with brain natriuretic peptide 200 pg/mL at the randomization; unplanned admission to hospital for congestive heart failure; or a need for modification of the treatment for heart failure (changes in oral medicine for at least 1 month or addition of intravenous inotropes for at least 4 hours)." (Hori 2005) Reported: as planned |
|
| Notes | Emailed investigators on 13 November 2017 to ask about data on cardiovascular mortality and all‐cause mortality as different numbers are provided in Table 2 of Yamamoto 2013 (primary reference). No response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomized to the arm " but no details |
| Allocation concealment (selection bias) | Unclear risk | no information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "open" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Deaths and hospitalizations were adjudicated by a blinded independent Endpoint Committee, using prespecified criteria." "Outcomes were assessed by the Endpoint Committee (see Appendix) where all the committee members were blinded to the allocated group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The primary outcome was a composite of cardiovascular death and unplanned hospitalization for heart failure using a time‐to‐first‐event ana‐ lysis and the intention‐to‐treat principle." unspecified for secondary outcomes lost to follow‐up low/similar in both groups (4.2% intervention, 2.4% control) |
| Selective reporting (reporting bias) | Low risk | outcomes reported as planned in published protocol (Hori 2005) |
| Other bias | Low risk | authors CoI: "none declared" funding: "The Ministry of Health, Labor andWelfare, Japan; the Japan Heart Foundation." |
Karapysh 2015.
| Study characteristics | ||
| Methods |
Study design: RCT Centres: not reported Start of enrolment: not reported End of enrolment: not reported Follow‐up: 6 months Run‐in period: not reported |
|
| Participants |
Inclusion criteria: "patients with chronic heart failure (CHF) with preserved ejection fraction (EF)." "with stable coronary arterial disease (CAD) and mild CHF (no higher IIfunctional class (NYHA)) with preserved systolic function of the LV (EF > 45%)" Exclusion criteria: not reported Randomised (N): 79 Withdrawn (N): not reported Lost to follow‐up (N): not reported Analysed (N): not reported Age (years, mean, SD): 54.5, 10.5 Sex (% men): 61 Ethnicity (%): intervention: white, control: white Systolic blood pressure not reported Heart rate not reported BMI not reported Serum creatinine not reported B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF not reported NYHA class: not reported Diabetes not reported Atrial fibrillation not reported Hospitalisation for heart failure: not reported Coronary heart disease not reported Stroke (%): not reported Diuretic (%); not reported Digoxin (%): not reported Beta‐blocker (%): not reported ACEI (%): not reported ARB (%): not reported MRA (%): not reported |
|
| Interventions |
Intervention: spironolactone. "SPRL group was treated with the standard therapy (ACE inhibitors or angiotensin receptor blockers II, beta‐blockers,statins, antiplatelet agents) plus SPRL (25 mg/day, titrated to 50 mg/day if tolerated)" Comparator: standard therapy Concomitant medication: standard therapy (ACEI, ARB, beta‐blocker, statins, antiplatelet agents) |
|
| Outcomes |
Planned: unclear Reported: "V posterior wall thickness (LVPWT), intraventricular septal thickness (IVST), relative wall thickness (RWT) and LV mass index (LVMI)" |
|
| Notes | Intended to contact trialists to obtain missing details and to enquire whether outcomes of interest to this review were measured. This was not possible as we could not find contact details for trialists. No relevant outcome data for this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "were randomly divided" but no further detail |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | could not be assessed |
| Selective reporting (reporting bias) | Unclear risk | could not be assessed |
| Other bias | High risk | reported only as conference abstract |
Kasama 2005.
| Study characteristics | ||
| Methods |
Study design: individual, parallel RCT Centres: 1, Japan Start of enrolment: January 2002 End of enrolment: September 2003 Follow‐up: 6 months Run‐in period: not reported |
|
| Participants |
Inclusion criteria: "first episode of nonischemic heart failure and preserved LVEF. We confirmed that all patients had symptoms and signs of congestive heart failure in this study."..."they were in New York Heart Association (NYHA) functional class II or III at the time of enrollment, and all had an LVEF > 40%. " Exclusion criteria: "Patients were excluded if they had a history of myocardial infarction, coronary artery disease, congenital heart disease, primary hepatic failure, or active cancer." Randomised (N): 50 (intervention: 25, control: 25) Withdrawn (N): not reported Lost to follow‐up (N): not reported Analysed (N): not reported Age (years, mean, SD): intervention: 66, 10; control: 67, 8 Sex (% men): intervention: 68; control: 64 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): intervention: 132, 18; control: 130, 20 Heart rate (beats/min, mean, SD): intervention: 72, 12; control: 74, 14 BMI not reported Serum creatinine not reported B‐type natriuretic peptide (pg/mL): intervention: 202, 125; control: 204, 127 NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): intervention: 54, 7; control: 55, 7 NYHA class I (%): 0 NYHA class II (%): intervention: 64; control: 68 NYHA class III (%): intervention: 36; control: 32 NYHA class IV (%): 0 Hypertension (%): intervention: 64; control: 60 Diabetes (%): not reported Atrial fibrillation (%): not reported Hospitalisation for heart failure (%): 100 Coronary heart disease (%): intervention: 0; control: 0 Stroke (%): not reported Diuretic (%); intervention: 92; control: 88 Digoxin (%): not reported Beta‐blocker (%): intervention: 12; control: 12 ACEI (%): intervention: 92; control: 96 ARB (%): study drug MRA (%): intervention: 16; control: 20 |
|
| Interventions |
Intervention: candesartan. "the initial daily dose of candesartan was 2 to 4 mg, which was increased to a maintenance dose of 8 to 12 mg/day (mean 10 2 mg/day)." Comparator: placebo Concomitant medication: "in addition to baseline therapy" |
|
| Outcomes |
Planned: unclear as unaware of published protocol or pre‐registration with a clinical trial registry Reported: hemodynamics, I‐MIBG, echocardiographic findings, NYHA functional class, BNP |
|
| Notes | No outcomes reported for relevance to this review. Emailed trialist to ask for outcome data relevant to this review. No response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly classified" but no further details |
| Allocation concealment (selection bias) | Unclear risk | no information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blinded" but no further details |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "assessment was performed in a blinded fashion by two independent observers with no knowledge of the clinical status or medical therapy of the patients." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT used for all outcomes, but loss to follow‐up and withdrawals not reported |
| Selective reporting (reporting bias) | Unclear risk | unable to assess as we are not aware of a published protocol or a pre‐registration in a clinical trial registry |
| Other bias | Unclear risk | funding source not reported |
Kitzman 2010.
| Study characteristics | ||
| Methods |
Study design: individual parallel RCT Centres: not reported Start of enrolment: not reported End of enrolment: not reported Follow‐up: 12 months Run‐in period: not reported |
|
| Participants |
Inclusion criteria: "As previously described, isolated HFPEF was defined as history, symptoms, and signs of HF; a preserved LVEF (50%); and no evidence of significant coronary, valvular, or pulmonary disease or other medical condition that could mimic HF symptoms, such as anemia or thyroid dysfunction." Exclusion criteria: "Coronary disease was excluded by history, medical records, ECG, and rest and exercise echocardiogram." "Patients were excluded if they had ever been prescribed an ACEI or ARB." Randomised (N): 71 (35 intervention, 36 control) Withdrawn (N): for reasons other than death 12 (10 intervention (3 patient request, 1 pancreatitis, 1 elective rotator cuff surgery, 1 alopecia, 1 worsening cough, 1 hypotension, 1 ankle fracture, exacerbation of knee arthritis, 1 leg and hip pain and fatigue), 2 control (1 elective knee replacement surgery, no details for second participants) Lost to follow‐up (N): not reported Analysed (N): 59 completed study (25 intervention, 34 control) Age (years, mean, SD): intervention: 69, 8; control: 70, 7 Sex (% men): intervention: 20; control: 11 Ethnicity (%): intervention: black 9, control: black 6 Systolic blood pressure (mmHg, mean, SD): intervention: 143, 17; control: 144, 18 Heart rate (beats/min, mean, SD): intervention: 129, 20; control: 133, 16 BMI (mean, SD): intervention: 30, 5; control: 30, 5 Serum creatinine (mg/dL, mean, SD): intervention: 1.1, 0.2; control: 1.1, 0.2 B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): intervention: 65, 8; control: 65, 7 NYHA class I (%): 0: NYHA class II (%): intervention: 83; control: 75 NYHA class III (%): intervention: 17; control: 25 NYHA class IV (%): 0 Hypertension (%): intervention: 71; control: 75 Diabetes (%): intervention: 9; control: 17 Atrial fibrillation not reported Hospitalisation for heart failure: not reported Coronary heart disease (%): 0 Stroke not reported Diuretic (%); intervention: 49; control: 58 Digoxin (%): 0 Beta‐blocker (%): intervention: 29; control: 39 ACEI (%): study drug ARB not reported MRA not reported |
|
| Interventions |
Intervention: enalapril. "The study drug was initiated at 2.5 mg BID and titrated up to 10 mg BID as tolerated by the patient within the first 4 weeks of the study." Comparator: placebo Concomitant medication: not reported |
|
| Outcomes |
Planned: unclear as clinical trial registration was post hoc Reported: exercise capacity, aortic distensibility and LV structure and function, carotid artery stiffness, LV diastolic filling, QoL |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All investigators, staff, and patients were fully blinded to treatment group assignment throughout the entire study period." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All investigators, staff, and patients were fully blinded to treatment group assignment throughout the entire study period." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | not reported |
| Selective reporting (reporting bias) | Unclear risk | not reported |
| Other bias | Low risk | "Dr Kitzman has served as consultant for and received grant support from Synvista ($10 000), Bristol‐Meyers Squibb ($10 000), Novartis ($10 000), Boston Scientific ($10 000), Relypsa ($10 000), Forest Laboratories, and Medtronic. Dr Little has served as consultant for CorAssist Cardiovascular Ltd, Celladon, Boston Scientific, Medtronic ($10 000), Bio‐Control Medical, CVRx ($10 000), Amylin Pharmaceuticals, Gilead, and BristolMeyers Squibb ($10 000). Drs Hundley, Brubaker, and Morgan; Mr Moore; and Ms Steward report no conflicts." |
Kurrelmeyer 2014.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Centres: 1 hospital, Houston, Texas Start of enrolment: 2004 End of enrolment: 2008 Follow‐up: 6 months Run‐in period: "patients were treated with 25 mg open‐label spironolactone for 1 week before randomization to ensure drug tolerability, defined as serum potassium < 5 mEq/L and absence of other major side effects." |
|
| Participants |
Inclusion criteria: "≥ 18 years old with a previous diagnosis of HFpEF. HFpEF was defined as current New York Heart Association (NYHA) functional class II or III HF symptoms or signs, left ventricular ejection fraction (LVEF) ≥ 50% according to echocardiography, diastolic dysfunction with elevated LV filling pressure according to Dopplerechocardiograph" "the subjects had to have a blood pressure of ≤ 150/95 mm Hg for 4 weeks before enrollment and the ability to walk ≥ 50 m at the time of enrollment. Treatment with an ACEI, or ARB if ACEI intolerant, was required for ≥ 4 weeks before enrollment. " Exclusion criteria: "Exclusion criteria included current treatment with spironolactone or epleronone, previous intolerance to spironolactone, creatinine > 2.5 mg/dL, serum potassium > 5.0mEq/L, significant valvular heart disease, pericardial disease, severe chronic lung disease with cor pulmonale, unstable angina or myocardial infarction ≤ 4 weeks before enrollment, severe peripheral vascular disease with claudication that limited walking distance, presence of other severe comorbid conditions with a life expectancy < 6 months, and pregnant or lactating women." Randomised (N): 48 (24 intervention, 24 control) Withdrawn (N): for reasons other than death (3 intervention (hyperkalaemia),0 control) Lost to follow‐up (N): not reported Analysed (N): not reported Age (years, mean, SEM): intervention: 66.3, 2.2; control: 76.4, 1.6 Sex (% men): 0 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SEM): intervention: 137.0, 4.1; control: 133.1, 2.8 Heart rate (beats/min, mean, SEM): intervention: 64.2, 2.3; control: 61.1, 1.2 BMI (mean, SEM): intervention: 29.4, 2.2; control: 26.3, 1.2 Serum creatinine not reported B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SEM): intervention: 62.5, 1.2; control: 62.9, 1.2 NYHA class I (%): 0 NYHA class II (%): intervention: 33; control: 42 NYHA class III (%): intervention: 67; control: 58 NYHA class IV (%): 0 Hypertension (%): intervention: 87.5; control: 79.2 Diabetes (%): intervention: 50; control: 25 Atrial fibrillation (%): intervention: 25; control: 25 Hospitalisation for heart failure (%): intervention: 58.3; control: 54.2 Coronary heart disease (%): intervention: 37.5; control: 33.3 Stroke (%): not reported Diuretic (%): intervention: 83.3; control: 75 Digoxin (%): intervention: 12.5; control: 8.3 Beta‐blocker (%): intervention: 62.5; control: 62.5 ACEI (%): intervention: 70.8; control: 66.7 ARB (%): intervention: 29.2; control: 37.5 MRA (%): study drug |
|
| Interventions |
Intervention: Spironolactone, 25mg once daily Comparator: placebo Concomitant medication: not reported |
|
| Outcomes |
Planned: no known published protocol or pre‐enrolment clinical trial registry record Reported: 6 min walk distance, clinical composite score, doppler echocardiography, biomarkers, Kansas City Cardiomyopathy Questionnaire clinical summary score |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no further details |
| Allocation concealment (selection bias) | Low risk | "subjects were randomly allocated with the use of pharmacy‐controlled concealed randomization methods.", no further details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | double‐blind, placebo controlled, no further details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | not reported |
| Selective reporting (reporting bias) | Unclear risk | no able to assess due to lack of pre‐registration in clinical trial registry and published protocol |
| Other bias | High risk | clinical trial registry entry after start of enrolment, was originally marked as an observational study (2005‐2013) and changed to a randomised trial status in 2013. reported as a RCT in 2014 publication. funded by Women’s Fund; Houston, Texas |
Mak 2009.
| Study characteristics | ||
| Methods |
Study design: RCT Centres: 1 Start of enrolment: not reported End of enrolment: not reported Follow‐up: 12 months Run‐in period: not reported |
|
| Participants |
Inclusion criteria: heart failure with preserved systolic function, "prior New York Heart Association (NYHA) functional class IV HF admission or symptoms consistent with HF, B‐type natriuretic peptide (BNP) >100 pg/ml, left ventricular ejection fraction >45%, and evidence of diastolic dysfunction on Doppler‐echocardiographic study." Exclusion criteria: "Patients were excluded if they were clinically unstable as defined by any change in diuretic dose a month before enrollment or were already receiving eplerenone or spironolactone therapy. Other exclusion criteria were evidence of significant inflammatory disease, hepatic disease, or metabolic bone disease that may alter parameters of collagen metabolism, serum creatinine >200 mol/l, prior documented left ventricular ejection fraction <45%, hemodynamically significant valvular disease, corpulmonale, hypertrophic, restrictive, or constrictive cardiomyopathy, atrial fibrillation or flutter with resting ventricular rate >120 beats/min, severe anemia, clinically significant pulmonary disease as evidenced by hospitalizations, or use of oral corticosteroids for pulmonary decompensation within 12 months or patients who require home oxygen therapy." Randomised (N): 44 (24 intervention, 20 control) Withdrawn (N): for reasons other than death 0 Lost to follow‐up (N): 2 (0 intervention, 2 control) Analysed (N): 40 (23 intervention, 17 control) Age (years, mean, SD): intervention: 80, 7.7; control: 79, 7.9 Sex (% men): intervention: 38; control: 55 Ethnicity (%): Caucasian: 100 Systolic blood pressure (mmHg, mean, SD): intervention: 140, 20; control: 146, 20 Heart rate (beats/min, mean, SD): intervention: 69, 13; control: 66, 13 BMI (mean, SD): intervention: 31.3, 6.9; control: 31.8, 5.7 Serum creatinine: not reported B‐type natriuretic peptide (pg/mL, median, IQR): intervention: 219 (157‐317); control: 192 (132‐330) NT pro B‐type natriuretic peptide: not reported LVEF (%, mean, SD): intervention: 63, 9.0; control: 64, 9.6 NYHA class I (%): not reported NYHA class II (%): 87% NYHA class III (%): not reported NYHA class IV (%): not reported Hypertension (%): intervention: 92; control: 90 Diabetes (%): intervention: 21; control: 35 Atrial fibrillation (%): intervention: 58; control: 60 Hospitalisation for heart failure: not reported Coronary heart disease (%): not reported Stroke (%): not reported Diuretic (%); intervention: 88; control: 90 Digoxin (%): intervention: 38; control: 30 Beta‐blocker (%): intervention: 62; control: 75 ACEI (%): intervention: 67; control: 60 ARB (%): intervention: 29; control: 40 MRA (%): study drug |
|
| Interventions |
Intervention: eplerenone. "we evaluated patients with a dose of 25 mg daily for 6 months followed by a dose increment to 50 mg until the 12‐month time point" Comparator: usual heart failure treatment Concomitant medication: not reported |
|
| Outcomes |
Planned: unable to assess Reported: serum levels of markers of collagen turnover, inflammatory markers, doppler‐echocardiographic indexes, clinical and biochemical measurements, withdrawals, quality of life |
|
| Notes | Emailed investigators to ask whether ITT or PP analysis was used. No response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated" but no further detail |
| Allocation concealment (selection bias) | Unclear risk | nor reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "open label" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "all parameters were assessed by persons blinded to treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | unable to assess |
| Selective reporting (reporting bias) | Unclear risk | unable to assess as unaware of published protocol or pre‐trial registration |
| Other bias | Low risk | Dr. Mak received grant support from the Irish Heart Foundation (The Noel Hickey Bursary) sponsored by Pfizer. Drs. Ledwidge and McDonald have received honoraria from Pfizer. |
McDiarmid 2020.
| Study characteristics | ||
| Methods |
Study design: RCT, open label, parallel 2‐arm trial Centres: 1, Leeds Teaching Hosptials NHS Trust, Leeds, UK Start of enrolment: April 2014 End of enrolment: June 2016 Follow‐up: 6 months Run‐in period: no |
|
| Participants |
Inclusion criteria: physical signs consistent with HF, NYHA II‐IV, LV ejectioin fraction on clinical echocardiography of > 50%, and NT‐proBNP > 400 pg/L at routine clinic attendance. Exclusion criteria: "renal impairment with estimated flomerular filtration rate <30 mL/min per 1.73 m2, serum potassium >5.0 mmol/L at enrollment, allergy to spironolactone, inability to comply with study drug monitoring, diabetes mellitus, uncontrolled hypertension (>140 mm Hg systolic blood pressure despite medical therapy), pregnancy, breastfeeding, Addison disease, and any relative or absolute contraindication to CMR. Patients with diabetes were specifically excluded as this has been shown independently to be associated with extracellular fibrosis by CMR." Randomised (N): 51 (intervention: 27; control: 24) Withdrawn (N): for reasons other than death: 11 (reasons given for 15, including 4 that dropped out before randomisation ‐ deterioration in renal function (n=3), inability to tolerate CMR (n=1), protocol breach (n=3), withdrawal of consent (n=8)) Lost to follow‐up (N): 0 Analysed (N): 40 (intervention: 19; control 21) Age (years, mean, SD): intervention: 76.4+/‐5.4; control: 74.0+/‐8.8 Sex (% men): intervention: 53; control: 48 Ethnicity (%): not reported Systolic blood pressure (mmHG, SD): intervention: 130.8+/‐19.1; control: 129.6+/‐9.9 Heart rate (beats/minute): intervention: 77 +/‐ 10.6 (sinus), 77+/‐19.8 (atrial fibrillationi); control: 74.5+/‐12.5 (sinus), 73.8+/‐7.5 (atrial fibrillation) BMI (mean, SD): intervention: 29.8+/‐5.3; control: 29.1+/‐7.1 Serum creatinine (mg/dL, SD): intervention: 97.4 +/‐ 27.2; control: 101.33 +/‐ 38.1 B‐type natriuretic peptide: not reported NT pro B‐type natriuretic peptide (pg/mL, SD): intervention: 1667 +/‐ 1246; control: 1706 +/‐1588 LVEF (%, mean, SD): intervention: 53.5+/‐5.5; control: 54.8+/‐5.2 NYHA class I (%): 0 NYHA class II (%): intervention: 14; control: 17 NYHA class III (%): intervention: 5; control: 4 NYHA class IV (%): 0 Hypertension (%): intervention: 79; control: 62 Diabetes (%): 0 (diabetes was excluded from study) Atrial fibrillation (%): intervention: 89; control 71 Hospitalisation for heart failure: not reported Ischeamic heart disease (%): intervention: 0; control: 5 Stroke (%): cerebrovascular disease ‐ intervention: 16; control: 0 Diuretic (%): intervention: 63; control: 57 Digoxin (%): intervention: 16; control: 43 Beta‐blocker (%): intervention: 53; control: 67 ACEI/ARB (%): intervention: 58; control: 57 MRA (%): study drug |
|
| Interventions |
Intervention: Spironolactone, 25 mg once daily Comparator: no treatment Concomitant medication: not reported |
|
| Outcomes |
Planned: QoL, final myocardial ECV (%), relationship between change in myocardial tissue composition and echocardiographic measures of myocardial tissue relaxation, LV geometry, blood pressure, and circulating biomarkers. Reported: final myocardial ECV (%), relationship between change in myocardial tissue composition and echocardiographic measures of myocardial tissue relaxation, LV geometry, blood pressure, and circulating biomarkers. |
|
| Notes | Contacted trialists to ask for QoL data and other outcomes of interest to our review. No response. Funding: This study and Dr McDiarmid are funded by a British HeartFoundation (BHF) Project Grant (PG/14/10/30641). DrSwoboda is funded by a BHF Clinical Fellowship (FS/12/88/29474). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1 randomisation without stratification, using a ranodmised permuted block strategy, with a standard block size of 20 provided by a commercial online system |
| Allocation concealment (selection bias) | Low risk | 1:1 randomisation without stratification, using a ranodmised permuted block strategy, with a standard block size of 20 provided by a commercial online system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | unblinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8 participants in intervention group and 3 in control group did not complete follow‐up. Analysis was conducted as a complete case analysis. |
| Selective reporting (reporting bias) | Unclear risk | Secondary outcome: Quality of life and exercise tolerance not reported |
| Other bias | Unclear risk | No concerns |
Mittal 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Centres: 1, Cardiology Outpatient Department and HTN clinic of Postgraduate Institute of Medical Education and Research, India Start of enrolment: 15 November 2009 (from clinical trial registry) End of enrolment: not reported for pilot study, full study ongoing Follow‐up: 12 weeks Run‐in period: 2 weeks placebo run in (beta‐blocker withdrawn but co‐existing therapies were continued) |
|
| Participants |
Inclusion criteria: "18 years and above, had New York Heart Association (NYHA) functional Class II–III of at least 4 weeks' duration, LVEF ≥ 50% in a nondilated LV (LV enddiastolic volume < 97 ml/m measured by echocardiography), echocardiographic evidence of LV diastolic dysfunction, and were willing to give written informed consent." Exclusion criteria: "They were excluded if: (1) Clinically unstable as defined by any change in diuretic dose in the month before enrollment, (2) significant valvular heart disease, pericardial disease, hypertrophic or restrictive cardiomyopathy, (3) unstable angina or MI within past 4 weeks, (4) any previous LVEF below 40%, (5) any contraindication to metoprolol use, (6) patients already on beta blockers which cannot be withdrawn, (7) current participation (including prior 30 days) in any other therapeutic trial, and (8) any condition that, in the opinion of investigator, may prevent the participant from adhering to the trial protocol." Randomised (N): 40 (20 intervention, 20 control) Withdrawn (N): for reasons other than death 0 Lost to follow‐up (N): 6 (3 intervention, 3 control) Analysed (N): 40 (20 intervention, 20 control) Age (years, mean, SD): intervention: 55.2, 7.1; control: 57.2, 9.8 Sex (% men): intervention: 45; control: 50 Ethnicity (%): not reported Systolic blood pressure not reported Heart rate not reported BMI not reported Serum creatinine not reported B‐type natriuretic peptide not reported NT pro B‐type natriuretic peptide (pg/mL, median (IQR)): intervention: 238.2 (107.7‐2230.2); control: 227.6 (58.3‐3645) LVEF (%, mean, SD): intervention: 62.9, 6.2; control: 62.1, 6.57 NYHA class I (%): 0 NYHA class II (%): intervention: 55; control: 65 NYHA class III (%): intervention: 45; control: 35 NYHA class IV (%): 0 Hypertension (%): not reported Diabetes (%): not reported Atrial fibrillation (%): not reported Hospitalisation for heart failure: not reported Coronary heart disease (%): not reported Stroke (%): not reported Diuretic (%); intervention: 35; control: 40 Digoxin (%): not reported Beta‐blocker (%): intervention: 40; control: 45 ACEI (%): intervention: 15; control: 60 ARB (%): intervention: 15; control: 15 MRA (%): not reported |
|
| Interventions |
Intervention: metoprolol succinate, 25 mg. "A dose upward titration protocol with monitoring of blood pressure and heart rate (target blood pressure and heart rate as 120/80 mm Hg and 60 beats/min, respectively) was implemented for dose increments up to a maximum dose of 100 mg once daily. For patients not tolerating increased titration of drug, temporary reduction in dosage was done and decision on further escalation made on individual basis by the treating cardiologist." Comparator: placebo Concomitant medication: "During the study, calcium channel blockers were added in three patients (one in placebo and two in metoprolol group) due to high blood pressure records." coexisting therapies were continued |
|
| Outcomes |
Planned: unable to assess Reported: primary: NYHA class. Secondary: exercise capacity, diastolic dysfunction, change in LV wall thickness, LV mass, NT‐proBNP, PICP, QoL (SF‐36), adverse events, withdrawals |
|
| Notes | published results after our search date, identified via search for clinical trial registry number: CTRI/2010/091/000438 which was retrieved by search of the WHO ICTRP register Emailed investigators to ask when completion of full trial is anticipated. Response confirmed that full trial was not conducted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "block randomised" but no further details |
| Allocation concealment (selection bias) | Low risk | "Randomization and allocation sequence generation were done by investigators not directly involved in the evaluation of outcomes" From clinical trial registry: "sequentially numbered, sealed, opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind" From clinical trial registry entry: "participant and outcome assessor blinded" Unclear whether personnel was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "To avoid interobserver variability, all the echocardiographic parameters were evaluated by a single cardiologist who was blinded to study medication and the order of assessment." From clinical trial registry entry: "participant and outcome assessor blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | pilot study ‐ full study ongoing same numbers lost to follow‐up in both arms ITT and per‐protocol analysis used |
| Selective reporting (reporting bias) | Unclear risk | unable to assess due to lack of published protocol and uncertainty over trial registration date |
| Other bias | Low risk | "The study was supported by Postgraduate Institute of Medical Education and Research, Chandigarh, India." "There are no conflicts of interest" Results from pilot study only so far. |
Mottram 2004.
| Study characteristics | ||
| Methods |
Study design: individual, double‐blind, placebo‐controlled, RCT Centres: 1, Australia Start of enrolment: February 2002 End of enrolment: October 2002 Follow‐up: 6 months Run‐in period: not reported |
|
| Participants |
Inclusion criteria: "To be eligible, patients had to have hypertension requiring antihypertensive medication and report exertional dyspnea (New York Heart Association class II) but no history of angina or myocardial infarction." Exclusion criteria: "Patients taking angiotensin converting enzyme inhibitors, angiotensin‐receptor blockers, or spironolactone were excluded, as were patients with renal impairment (creatinine 0.20 mmol/dL) or hyperkalemia at baseline." "we excluded patients with evidence of pulmonary disease, ischemic heart disease, abnormal regional or global resting LV systolic function (ejection fraction < 50%), or significant (>mild) valvular dysfunction." Randomised (N): 30 (not reported by treatment arm, assumed 15 in each) Withdrawn (N): not reported Lost to follow‐up (N): 1 (intervention: 1 (migrated overseas); control: 0) Analysed (N): not reported Age (years, mean, SD): intervention: 61, 6; control: 62, 5 Sex (% men): intervention: 40; control: 34 Ethnicity (%): not reported Systolic blood pressure (mmHg): intervention: 199, 18; control: 198, 26 Heart rate (beats/min): intervention: 139, 24; control: 153, 13 BMI: intervention: 29.8, 4.7; control: 31.2, 4.6 Serum creatinine (mg/dL): intervention: 0.07, 0.01; control: 0.07, 0.01 B‐type natriuretic peptide (pg/mL) intervention: 29.3, 26.8; control: 29.7, 27.8 NT pro B‐type natriuretic peptide not reported LVEF: intervention: 68, 5; control: 67, 4 NYHA class not reported Hypertension (%): not reported Diabetes (%): intervention: 7; control: 0 Atrial fibrillation (%): not reported Hospitalisation for heart failure: not reported Coronary heart disease (%): not reported Stroke (%): not reported Diuretic (%); intervention: 40; control: 27 Digoxin (%): not reported Beta‐blocker (%): intervention: 40; control: 20 ACEI (%): not reported ARB (%): not reported MRA (%): study drug |
|
| Interventions |
Intervention: spironolactone, 25mg/d Comparator: placebo Concomitant medication: not reported |
|
| Outcomes |
Planned: unable to assess as we are not aware of a published protocol or pre‐registered clinical trial registry entry Reported: mean 24hr ambulatory blood pressure, posterior wall thickness, left atrial area, SR, peak systolic strain, and CVIB |
|
| Notes | no outcome data of interest to this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomised, but no details |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | double blind, matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Investigators remained blinded to the treatment until after analysis of results." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | not reported |
| Selective reporting (reporting bias) | Unclear risk | unable to assess |
| Other bias | Unclear risk | "This work was supported in part by a grant and scholarship from the National Heart Foundation of Australia, Melbourne, Australia, in association with a Centers of Clinical Research Excellence Award, National Health and Medical Research Council, Canberra, Australia. The authors are grateful to the Princess Alexandra Hospital Pharmacy for supervision of randomization and dispensing of active and placebo tablets." |
Orea‐Tejeda 2007.
| Study characteristics | ||
| Methods |
Study design: RCT Centres: not reported Start of enrolment: not reported End of enrolment: not reported Mean follow‐up: 13.8 months Run‐in period: not reported |
|
| Participants |
Inclusion criteria: "Patients with diastolic heart failure attending to Heart Failure Clinic were considered eligible, independently of etiology, if they had history of arterial hypertension (and/or were on antihypertensive treatment), but no history of angina, myocardial infarction or myocardial revascularization (PTCA and / or aortocoronary bypass grafting) during the 3 months previous to recruitment and they referred fatigue, dyspnea on exercise and/or orthopnea." "Diastolic dysfunction was considered when the ejection fraction was over 45%, and shortening fraction = 28%, without severe segmental dyskynesia of the left ventricle, left atrial enlargement, or increased thickness or posterior wall, interventricular septum, and left ventricular mass index." Exclusion criteria: not reported Randomised (N): 28 (14 intervention, 14 control) Withdrawn (N): not reported Lost to follow‐up (N): not reported Analysed (N): not reported Age (years, mean, SD): intervention: 63.7, 21.6; control: 64.8, 11.9 Sex (% men): intervention: 28.6; control: 71.4 Ethnicity (%): not reported Blood pressure (mmHg): intervention: 112, 12; control: 114, 8 Heart rate (beats/min): intervention: 86, 4; control: 82, 6 BMI (mean, SD): intervention: 27.5, 9.4; control: 26.9, 4.7 Serum creatinine not reported B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): intervention: 48.79, 4.65; control: 51.57, 11.71 NYHA class I (%): intervention: 42.9; control: 75.0 NYHA class II (%): intervention: 0; control: 16.7 NYHA class III (%): intervention: 57.1; control: 8.3 NYHA class IV (%): 0 Hypertension (%): intervention: 85.7; control: 92.9 Diabetes (%): intervention: 28.6; control: 64.3 Atrial fibrillation (%): not reported Hospitalisation for heart failure: not reported Ischaemic heart disease (%): intervention: 42.9; control: 57.1 Stroke (%): not reported Diuretic (%): intervention: thiazide: 76.9, loop: 5.1; control: thiazide: 62.3, loop: 13 Digoxin (%): not reported Beta‐blocker (%): intervention: 79.5; control: 79.7 ACEI (%): intervention: 38.5; control: 29 ARB (%): intervention: 69.2; control: 73.9 MRA (%): study drug |
|
| Interventions |
Intervention: spironolactone, mean dose of 37.5 mg/d (25‐50 mg once a day) Comparator: no treatment Concomitant medication: "In our study, patients with diastolic heart failure were all treated with ACE inhibitors/ARA and Beta blockers." |
|
| Outcomes |
Planned: We are not aware of a published protocol or pre‐registered clinical trial register entry Reported: echocardiographic parameters, adverse events |
|
| Notes | no outcome data of interest to this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The echocardiogram was made by a Cardiologist blinded to the clinical evaluation and treatment received." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | not reported |
| Selective reporting (reporting bias) | Unclear risk | unable to assess |
| Other bias | Unclear risk | no funding reported |
PARAGON‐HF.
| Study characteristics | ||
| Methods |
Study design: RCT Centres: 848 centres in 43 countries Start of enrolment: June 2014 End of enrolment: December 2016 Follow‐up: median 35 months Run‐in period: "Single‐blind run‐in period", Valsartan run in phase median 15 days (interquartile range, 12‐22). Sacubitril‐valsartan run in phase ‐ 19 days (interquartile range, 15 to 23 days) |
|
| Participants |
Inclusion criteria: "Eligibility requirements at screening included an age of 50 years or older, signs and symptoms of heart failure, NYHA class II to IV, an ejection faction of 45% or higher within the previous 6 months, elevated level of natriuretic peptides (with different cutoffs depending on the occurrence of recent hospitalization for heart failure and presence of atrial fibrillation or flutter), evidence of structural heart disease, and diuretic therapy." Exclusion criteria: Any prior measurement of LVEF <40%, ACS, cardiac surgery, other major CV surgery within 3 months, or urgent PCI within 3 months or and elective PCI within 30 days pror to entry, Any clinical event within 6 months prior to entry that could have reduced the LVEF (MI or CABG) unless echo confirms an EF>45%, Current acute decompensated HF requiring therapy, Patients who require treatment with 2 or more of the following: an angiotensin converting enzyme inhibitor (ACEI), an angiotensin receptor blocker (ARB) or a renin inhibitor, Alternative reason for shortness of breath such as: significant pulmonary disease or severe COPD, hemoglobin (Hgb) <10 g/dl, or body mass index (BMI) > 40 kg/m2, Systolic blood pressure (SBP) ≥ 180 mmHg at entry, or SBP >150 mmHg and <180 mmHg at entry unless the patient is receiving 3 or more antihypertensive drugs, or SBP < 110 mmHg at entry. Randomised (N): 4822 (intervention: 2419; control: 2403) Withdrawn (N): for reasons other than death: 26 (intervention: 12 patients ‐ good clinical practice violation; control: 14 patients ‐ due to good clinical practice vioation) Lost to follow‐up (N): 2 Analysed (N): 4796 (intervention: 2407; control: 2389) Age (years, mean, SD): intervention: 72.7, 8.3; control: 72.8, 8.5 Sex (% men): intervention: 48.4; control: 48.2 Ethnicity (%): intervention: White 81.6, Black 2.2, Asian 12.3, Other 4.0; control: White 81.4, Black 2.1, Asian 13.0, Other 3.6 Systolic blood pressure (mmHg, mean, SD): intervention: 130, 15.6; control: 130.6, 15.3 Heart rate (beats/minute, SD): intervention: 70.6, 12.3; control: 70.3, 12.2 BMI (mean, SD): intervention: 30.2, 4.9; control: 30.3, 5.1 Serum creatinine (mg/dL, SD): intervention: 1.1, 0.3; control: 1.1, 0.3 B‐type natriuretic peptide not reported NT pro B‐type natriuretic peptide (pg/mL, median (IQR)): intervention: 904 (475 ‐ 1596); control: 915 (453 ‐ 1625) LVEF (%, mean, SD): intervention: 57.6, 7.8; control: 57.5, 8.0 NYHA class I (%): intervention: 3; control: 2 NYHA class II (%): intervention: 77.5; control: 77 NYHA class III (%): intervention: 19; control: 19.8 NYHA class IV (%): intervention: 0.3; control: 0.5 Hypertension (%): intervention: 95.7; control: 95.4 Diabetes (%): intervention: 43.5; control: 42.5 Atrial fibrillation (%): intervention: 32.2; control: 32.5 Hospitalisation for heart failure (%): intervention: 47.2; control: 49 Coronary heart disease (%): intervention: 37.4; control: 34.5 Stroke (%): intervention: 11.1; control: 10.1 Diuretic (%): intervention: 95.3; control: 95.9 Digoxin (%): not reported Beta‐blocker (%): intervention: 79.9; control: 79.5 ACEI/ARB (%): intervention: 86.2; control: 86.4 MRA (%): intervention: 24.6; control: 27.1 |
|
| Interventions |
Intervention: Sacubitril‐valsartan (target dose, 97 mg of sacubitril with 103 mg of valsartan twice daily) Comparator: valsartan (target dose, 160 mg twice daily) Concomitant medication: From protocol: "patients will continue to take optimal background therapy to treat co‐morbid conditions, as considered appropriate by the investigator and in accordance with standard therapy guidelines, with the exception of an ACEI or ARB as this will be replaced by study drug." |
|
| Outcomes |
Planned: (from protocol): Primary objective: To compare sacubitril/valsartan to valsartan in reducing the rate of the composite endpoint of cardiovascular death and total (first and recurrent) HF hospitalizations in HF patients (NYHA functional class II to IV) with preserved EF (LVEF ≥45%). Secondary objectives: changes in the clinical summary score for HF symptoms and physical limitations, as assessed by the KCCQ), improving NYHA functional classification, delaying the time to first occurrence of a composite renal endpoint, delaying the time to all‐cause mortality Reported: "The primary outcome was a composite of total (first and recurrent) hospitalizations for heart failure and death from cardiovascular causes. Secondary outcomes were the change from baseline to 8 months in the clinical summary score on the Kansas City Cardiomyopathy Questionnaire (KCCQ)12 (scores range from 0 to 100, with higher scores indicating fewer symptoms and physical limitations); the change from baseline to 8 months in NYHA class; the first occurrence of a decline in renal function (decrease in the estimated glomerular filtration rate of ≥50%, development of end‐stage renal disease, or death due to renal failure) in a time‐to‐event analysis; and death from any cause in a time‐to‐first‐event analysis." |
|
| Notes | Funding: Novartis "The steering committee designed and oversaw the conduct of the trial and data analysis, in collaboration with the sponsor, Novartis." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | From protocol: "all eligible patients will be randomized via Interactive Response Technology (IRT) to one of the treatment arms." |
| Allocation concealment (selection bias) | Low risk | From protocol: "A patient randomization list will be produced by the IRT provider using a validated system that automates the random assignedment of patients numbers to randomization numbers. These randomization numbers are linked to the different treatment arms, which in turn are linked to medication numbers" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | From protocol: "patients, investigator staff, persons performaing the assessments, and data analysts will remain blind to the identity of the treatment from the time of randomization until database lock" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All the outcomes except KCCQ score and NYHA class were blindly adjudicated according to pre‐specified criteria. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT performed on all outcomes. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes reported as planned. |
| Other bias | Low risk | No other concerns |
PARALLAX.
| Study characteristics | ||
| Methods |
Study design: RCT Centres: not reported Start of enrolment: 22 August 2017 End of enrolment: 28 October 2019 Follow‐up: 24 weeks Run‐in period: none |
|
| Participants |
Actual enrolment: 2572 Inclusion criteria:
Exclusion criteria:
Randomised (N): 2572 (intervention: 1286; control: 1286) Withdrawn (N): intervention: 174 (patients' decision: 42, adverse events: 116, death: 7, others: 9); control: 149 (patients' decision: 45, adverse events: 87, death: 9, others: 8) Lost to follow‐up (N): not reported Analysed (N): 2566 (intervention: 1281; control: 1285) Age (years, mean, SD): intervention: 73, 8.4; control: 72, 8.6 Sex (% men): intervention: 50; control: 49 Ethnicity (%): intervention: White 87; control: White 87 Systolic blood pressure (mmHg, mean, SD): intervention: 133, 14.0; control: 134, 14.5 Heart rate (beats/minute, SD): not reported BMI (mean, SD): intervention: 31, 5.0; control: 31, 4.8 Serum creatinine (mg/dL, SD): not reported B‐type natriuretic peptide not reported NT pro B‐type natriuretic peptide (pg/mL, median (IQR)): intervention: 786 (415 ‐ 1401); control: 760 (380 ‐ 1398) LVEF (%, mean, SD): intervention: 57, 8.3; control: 56, 8.0 NYHA class I (%): intervention: 0.1; control: 0.3 NYHA class II (%): intervention: 67; control: 68 NYHA class III (%): intervention: 32; control: 31 NYHA class IV (%): intervention: 0.4; control: 0.3 Hypertension (%): intervention: 97; control: 97 Diabetes (%): intervention: 39; control: 41 Atrial fibrillation at any time in the past (%): intervention: 55; control: 54 Hospitalisation for heart failure (%): intervention: 35; control: 36 Coronary heart disease (%): not reported Stroke (%): not reported Diuretic (%): intervention: 100; control: 100 Digoxin (%): not reported Beta‐blocker (%): intervention: 84; control: 83 ACEI (%): intervention: 41; control: 42 ARB (%): intervention: 46; control: 46 MRA (%): intervention: 33; control: 31 |
|
| Interventions | Sacubitril/Valsartan uptitrated to 97/103 mg bid All patients who fulfil the inclusion/exclusion criteria were stratified before randomization based upon prior therapy for comorbidities to one of three strata: ACEi, ARB or no RASi. Patients in the ACEi strata received LCZ696 or enalapril. Patients in the ARB strata received LCZ696 or valsartan. Patients in the no RASi strata received LCZ696 or matching placebo. |
|
| Outcomes |
Primary: Change from baseline in N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) after 12 weeks Secondary:
Exploratory endpoints:
|
|
| Notes | Comparison of interest: LCZ696 or matching placebo Sponsor: Novartis Pharmaceuticals Other identifiers: CLCZ696D2302, 2016‐003410‐28 ( EudraCT Number) Results from presentation at ESC Congress 2020 included (unpublished). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not yet reported. |
| Allocation concealment (selection bias) | Unclear risk | Not yet reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not yet reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not yet reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not yet reported. |
| Selective reporting (reporting bias) | Unclear risk | Not yet reported. |
| Other bias | Unclear risk | Unable to assess |
PARAMOUNT.
| Study characteristics | ||
| Methods |
Study design: RCT Centres: 65 centres and 13 countries Start of enrolment: 2 November 2009 End of enrolment: 31 March 2011 Follow‐up: 36 weeks Run‐in period: "2‐week, single‐blind, placebo run‐in period" "continued background treatment" "ACE inhibitors and ARBs were required to be discontinued 24 h before randomisation" |
|
| Participants |
Inclusion criteria: "Men and women aged 40 years or older with a left ventricular ejection fraction (LVEF) of 45% or higher and a documented history of heart failure with associated signs or symptoms (dyspnoea on exertion, orthopnoea, paroxysmal dyspnoea, and peripheral oedema) were eligible. Patients were required to have NT‐proBNP greater than 400 pg/mL at screening, be on diuretic therapy, and have a systolic blood pressure less than 140 mm Hg, or 160 mm Hg or less if on three or more blood pressure drugs at randomisation, have an estimated glomerular filtration rate (eGFR) of at least 30 mL/min per 1·73 m2 at screening (calculated by the Modification of Diet in Renal Disease formula), and a potassium concentration of no more than 5·2 mmol/L." Exclusion criteria: "Patients were excluded if they had previous LVEF less than 45% at any time, isolated right heart failure due to pulmonary disease, dyspnoea due to non‐cardiac causes such as pulmonary disease, anaemia, or severe obesity, primary valvular or myocardial diseases, or coronary artery or cerebrovascular disease needing revascularisation within 3 months of screening or likely to need revascularisation during the trial. " Randomised (N): 308 (intervention: 149; control: 152) Withdrawn (N): for reasons other than death: 44 (intervention: 20 participants = 13 adverse events, 6 withdrawal of consent, 1 protocol violation; control: 24 participants = 16 adverse events, 8 withdrawal of consent Lost to follow‐up (N): 16 (intervention: 8 participants = 1 death, 4 lost to follow up, 3 adminstrative reasons; control: 8 participants = 1 lost to follow up, 2 death, 5 adminstrative reasons Analysed (N): depending on outcome (adverse events: ITT, others PP) Age (years, mean, SD): intervention: 70.9 ± 9.4; control: 71.2 ± 8.9 Sex (% men): intervention: 43; control: 44 Ethnicity (%): not reported Systolic blood pressure (mmHG, median, IQR): intervention: 136 (130‐145); control: 136 (126‐145) Heart rate (beats/minutes, SD): intervention: 69 ± 12; control: 70 ± 14 BMI (mean, SD): intervention: 30.1 ± 5.5; control: 29.8 ± 6.1 Serum creatinine not reported B‐type natriuretic peptide not reported NT pro B‐type natriuretic peptide (pg/mL, median (IQR)): intervention: 828 (460 ‐ 1341); control: 939 (582 ‐ 1490) LVEF (%, mean, SD): intervention: 58±7.3; control: 58 ± 8.1 NYHA class I (%): intervention: 1; control: 1 NYHA class II (%): intervention: 81; control: 78 NYHA class III (%): intervention: 19; control: 21 NYHA class IV (%): 0 Hypertension (%): intervention: 95; control: 92 Diabetes (%): intervention: 41; control: 35 Atrial fibrillation (%): intervention: 40; control: 43 Hospitalisation for heart failure (%): intervention: 40; control: 45 Coronary heart disease (myocardial infarction, %): intervention: 21; control: 20 Stroke (%): not reported Diuretic (%): 100 Digoxin (%): not reported Beta‐blocker (%): intervention: 79; control: 80 ACEI (%): intervention: 56; control: 53 ARB (%): intervention: 38; control: 41 MRA (%): intervention: 19; control: 23 |
|
| Interventions |
Intervention: LCZ696 (Sacubritril/ Valsartan), 50 mg twice daily, titated to final dose of 200 mg twice daily over 2 to 4 weeks Comparator: valsartan 40 mg twice daily, titrated to their final doses of 160 mg twice daily over 2 to 4 weeks Concomitant medication: "at the discretion of the treating physicians" |
|
| Outcomes |
Planned (from first entry on clinicaltrials.gov): primary outcome measures: change in log‐scale in NT‐proBNP. Secondary outcome measures: change in log‐scale in BNP, MR‐proBNP, cGMP; change in echocardiography parameters; class indicators of signs and symptoms of heart failure at each visit; change in the overall summary score and individual domain score of the Kansas City Cardiomyopathy questionnaire; change in clinical composite score (NYHA and global patient assessment score) Reported: "The primary study endpoint was change from baseline in NT‐proBNP assessed at 12 weeks. Secondary endpoints included changes in echocardiographic measures (left ventricular volumes and ejection fraction, left atrial volume, measures of diastolic function) and change in blood pressure, as well as change in New York Heart Association (NYHA) class, clinical composite assessment, and quality of life (Kansas City cardiomyopathy questionnaire; KCCQ). |
|
| Notes |
Funding: Novartis. "PARAMOUNT was designed jointly by the academic steering committee and the sponsor, which funded the trial. The sponsor was responsible for study management, data collection, and data analysis; all analyses were replicated by an independent statistician at the Brigham and Women's Hospital. The report was drafted by the first author and revised by all authors who have read and agree to the report as written and the decision to submit for publication. The first author had full access to and takes full responsibility for the integrity of the data." Trialists' conflict of interest: SDS, MZ, BP, AV, AS, MP, and JJVM have received research support and have consulted for Novartis. VS, TB, JG and ML are employees of Novartis. EK‐K and MT declare that they have no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random sequence with a block size of four, stratified by previous use of ACE inhibitor or ARB and region." |
| Allocation concealment (selection bias) | Low risk | "assignment used a central inter‐active voice response system with randomisation codes generated by the sponsor. The system assigned a randomisation number to each patient, which linked the patient to a treatment gorup and specified a unique drug number for study drug to be dispensed. Placebo and active treatment were identical in appearance." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "study investigators and participants were masked to treatment for the duration of the trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear ‐ sponsor was responsible for study management, data collection, and data analysis ‐ all analyses were replicated by an independent statistician at the Brigham and Women’s Hospital |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT used |
| Selective reporting (reporting bias) | Low risk | Appears all primary and secondary outcomes mentioned |
| Other bias | Low risk | No concerns |
Parthasarathy 2009.
| Study characteristics | ||
| Methods |
Study design: RCT Centres: 10 (5 in Germany, 5 in UK) Start of enrolment: December 2002 (from clinical trial registry) End of enrolment: March 2007 (from clinical trial registry) Mean follow‐up: 13.8 weeks Run‐in period: not reported |
|
| Participants |
Inclusion criteria: "Patients were ≥ 21 years of age and had the following characteristics: symptoms of breathlessness on exertion (based on patient questioning) with normal lung function at rest, an extrapolated maximum oxygen consumption (EMOC) and/or peak oxygen consumption <85% of the age‐corrected normal value on cardiopulmonary exercise testing, preserved systolic function (ejection fraction ≥ 40%) with evidence of diastolic dysfunction on echocardiography (≥ 1 of the following: abnormal flow propagation velocity, prolongation of isovolumic relaxation time, E/A ratio reversal, and abnormal E deceleration time), and ability to exercise for ≥ 3 min on a treadmill." Exclusion criteria: "Uncontrolled hypertension (sitting systolic blood pressure >160 mmHg or sitting diastolic blood pressure > 100 mmHg) Presence of clinically significant asthma or chronic obstructive pulmonary disease Abnormal lung function (forced expiratory volume in 1 s [FEV1]/ forced vital capacity [FVC] ratio < 75%) Treatment with ≥ 2 bronchodilators Exercise limiting symptomatic angina Haemodynamically significant cardiac valvular disease Documented evidence of systolic heart failure (ejection fraction <40%, fractional shortening <25%) Uncontrolled atrial fibrillation (> 100 b.p.m. at rest) History of myocardial infarction, percutaneous transluminal coronary angioplasty, or coronary artery bypass within the previous 3 months Use of ARBs within the previous 1 month" Randomised (N): 152 (70 intervention, 82 control) Withdrawn (N): for reasons other than death 5 (4 intervention (N = 2 adverse events, N = 1 protocol violation, N = 1 withdrew consent), 1 control (N = 1 protocol violation)) Lost to follow‐up (N): 0 Analysed (N): 152 (70 intervention, 82 control), except QoL: 67 intervention, 82 placebo Age (years, mean, SD): intervention: 61.0, 11.5; control: 63.1, 10.3 Sex (% men): intervention: 50; control: 50 Ethnicity (%): intervention: Caucasian: 95.6, Other: 4.4, control: Caucasian: 93.9, other: 6.1 Systolic blood pressure not reported Heart rate not reported BMI (mean, SD): intervention: 31.0, 4.7; control: 29.3, 5.3 Serum creatinine not reported B‐type natriuretic peptide (pg/mL): intervention: 93.2, 80.2; control: 120.3, 119.5 NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): intervention: 70.48, 11.43; control: 71.52, 12.08 NYHA class not reported Hypertension (%): intervention: 92.2; control: 89.0 Diabetes (%): intervention: 22.1; control: 14.6 Atrial fibrillation (%): intervention: 16.2; control: 9.8 Hospitalisation for heart failure: not reported Coronary heart disease (%): not reported Stroke (%): not reported Diuretic (%): not reported Digoxin (%): not reported Beta‐blocker (%): intervention: 33.8; control: 34.1 ACEI (%): intervention: 41.2; control: 37.8 ARB (%): study drug MRA (%): not reported |
|
| Interventions |
Intervention: valsartan. 80 mg once daily. "Study medication was force‐titrated between days 5 and 14 (Visit 3) to valsartan 160 mg daily or matching placebo, and between days 10 and 28 (Visit 4) to valsartan 320 mg daily or matching placebo. Up‐titration occurred provided the current dose was adequately tolerated. Down‐titration occurred for any of the following: evidence of persistent symptomatic hypotension, systolic blood pressure <100 mmHg or decrease of >40 mm Hg from baseline, creatinine increase of >50% from baseline, or if the investigators judged the given dose level as potentially harmful to the patient. A safety evaluation was performed between days 15 and 42 (Visit 5). After the dose‐titration period, patients received their maximum tolerated dose through to the end of the study at week 14 (+7 days) (Visit 6)." Comparator: matching placebo Concomitant medication: "Use of other ARBs as concomitant medication was prohibited, but other background medications (e.g. diuretics, calcium channel blockers) were allowed and continued throughout the study. Angiotensin‐converting enzyme inhibitors and beta‐blockers were permitted, although therapy was to be maintained at the same level throughout the study and no new treatment with one of these drugs was permitted during the trial." |
|
| Outcomes |
Planned: unclear Reported: exercise time, neurohormone levels, echocardiographic parameters, QoL, adverse events |
|
| Notes | Response to email enquiring for further details received on 2 December 2017: confirmed that no outcome data are available for heart failure hospitalisation and hyperkalaemia and provided number of centres. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Eligible patients were allocated to either the active treatment group or the placebo group according to a stratified randomization process in order to minimize the differences between study groups. Stratification was based on exercise test time at Visit 2 divided into sections of: 3–6 min, .6 min to 9 min, and.9 min, each stratum being randomized in blocks of 4." No details on how the random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind" but not specified "To maintain blinding, valsartan and placebo capsules were identical in appearance." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT, higher withdrawals in intervention group (5.7%) compared to control (1.2%) |
| Selective reporting (reporting bias) | Unclear risk | unable to assess, unaware of published protocol and clinical trial register entry (Sept 2005) after planned study start (Dec 2002) |
| Other bias | Low risk | "H.K.P. and B.P. have no conflicts of interest; C.D.A., M.W., and P.B. remain in the employ of Novartis Pharma and have no other conflicts of interest; A.D.S. received one honorarium (£1000) from Novartis for intellectual input to the rationale and the design of the study; T.M.MacD: competing interests statement Nov 2008: my department has had research grants from GSK, Aventis, Novartis, AstraZeneca, BMS, Bohringer Ingelheim, Pfizer, and Novartis, I am or have been the principal investigator on trials paid for by Pfizer and Novartis, I have been paid Consulting fees by Pfizer, Novartis, Kaiser Permanante, Takeda, Recordati, Quintiles, and Speedel." "The study was funded by Novartis." |
PEP‐CHF.
| Study characteristics | ||
| Methods |
Study design: RCT Centres: 53 centres (Bulgaria (3), Czech Republic (5), Hungary (10), Ireland (1), Poland (26), Russia (1), Slovakia (2), and the UK (5)) Start of enrolment: 2000 End of enrolment: 2003 Mean follow‐up: mean follow‐up 26.2 months (range, excluding deaths 12.0‐54.2) Run‐in period: "A 24‐h open label run in phase, during which patients will receive a single 2‐mg dose of perindopril" |
|
| Participants |
Inclusion criteria: "Patients had to be aged ≥70 years and treated with diuretics for a clinical diagnosis of CHF due to LV diastolic dysfunction as defined below and to have had a cardiovascular hospitalization within the previous 6 months. Patients had to be able to walk without the aid of another person in order to exclude very frail patients who might not respond to any treatment.""Patients had to be aged 70 years and treated with diuretics for a clinical diagnosis of CHF due to LV diastolic dysfunction as defined below and to have had a cardiovascular hospitalization within the previous 6 months. Patients had to be able to walk without the aid of another person in order to exclude very frail patients who might not respond to any treatment." "As there are no widely agreed criteria for the diagnosis of diastolic heart failure, at least three out of nine clinical and at least two out of four additional echocardiographic criteria were required. Clinical criteria were: exertional breathlessness; orthopnoea or paroxysmal nocturnal dyspnoea; ankle swelling; improved breathlessness with diuretic therapy; increased jugular venous pressure; prior episode of clinical pulmonary oedema; prior MI; cardiothoracic ratio > 0.55; and previous radiological pulmonary oedema. Echocardiographic criteria were: an LV wall motion index of 1.4–1.6 inclusive, roughly equivalent to an LVEF fraction between 40 and 50%, since abnormal diastolic dysfunction is often associated with some impairment of systolic function; a left atrial diameter > 25 mm/m2 body surface area or > 40 mm because chronic elevation of LV filling pressure should lead to atrial dilatation; an interventricular septum or posterior LV wall ≥ 12 mm in thickness suggesting hypertrophy, a common cause of impaired diastolic function or, finally, evidence of impaired LV filling by at least one of the criteria recommended by the European Society of Cardiology Study Group on Diastolic Heart Failure. These included an E/A ratio < 0.5 or deceleration time of > 280 ms from the mitral inflow pattern or an isovolumic relaxation time of > 105 ms. These criteria effectively exclude patients with atrial fibrillation (AF) and therefore, in a protocol modification early in the course of the study, this arrhythmia was counted as equivalent to evidence of impaired LV filling by Doppler." Exclusion criteria: "Patients with a wall motion index of < 1.4, roughly equivalent to an LVEF of 40%, were excluded." "Important exclusion criteria were haemodynamically significant valve disease, stroke within the previous month, sitting systolic arterial pressure < 100 mmHg, serum creatinine > 200 mmol/L or potassium > 5.4mmol/L, history of ACE‐inhibitor intolerance or use of an ACE‐inhibitor or angiotensin receptor blocker within the previous week, potassium‐sparing diuretics (other than low‐dose spironolactone), or potassium supplements." Randomised (N): 850 (424 intervention, 426 control) Withdrawn (N): due to serious adverse events 13 (9 intervention, 4 control) Lost to follow‐up (N): 4 (4 intervention, 0 control) Analysed (N): 846 (420 intervention, 426 control) Age (years, median, IQR): intervention: 75, 72–79; control:75, 72‐79 Sex (% men): intervention: 46; control: 43 Ethnicity (%): not reported Systolic blood pressure (mmHg, median, IQR): intervention: 138, 128–150; control: 140, 129–150 Heart rate (beats/min, median, IQR): intervention: 74, 66 to 81; control: 73, 66 to 82 BMI (median, IQR): intervention: 27.5, 25.1 to 30.0; control: 27.6, 25.3 to 30.7 Serum creatinine (mg/dL, median, IQR, converted from umol/L using http://www.endmemo.com/medical/unitconvert/Creatinine.php): intervention: 1.07, 0.92‐1.24; control: 1.10, 0.95‐1.26 B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL): intervention: 335, 160–1014 (for subgroup n =191); control: 453, 206–1045 (for subgroup n = 184) LVEF (%, median, IQR): intervention: 65, 56– 66; control: 64, 56– 66 NYHA class I/II (%): intervention: 77; control: 74 NYHA class III/IV (%): intervention: 23; control: 26 Hypertension (%): intervention: 79; control: 79 Diabetes (%): intervention: 21; control: 20 Atrial fibrillation (%): intervention: 19; control: 22 Hospitalisation for heart failure: not reported Coronary heart disease (%): intervention: 27; control: 26 Stroke not reported Diuretic (%); intervention: loop: 47, thiazide 54, low dose spironolactone 9; control: loop: 44, thiazide 55, low dose spironolactone 11 Digoxin (%): intervention: 11; control: 13 Beta‐blocker (%): intervention: 55; control: 54 ACEI: study drug ARB not reported MRA (%): intervention: 9; control: 11 |
|
| Interventions |
Intervention: perindopril. "Patients were reviewed weekly for the first 5 weeks to ensure that treatment was tolerated and to check serum potassium and creatinine. The dose of perindopril was increased to 4 mg once daily at the second follow‐up visit if no clinical contraindication, such as hypotension or worsening renal function existed. Study medication was reduced or discontinued if serum creatinine rose to > 250 mmol/L or by > 50 mmol/L from baseline or potassium rose to > 5.5mmol/L. Patients were reviewed at 8, 12, and every 12 weeks thereafter until 1 year follow‐up, then according to the investigator’s judgment until the end of the study." Comparator: placebo Concomitant medication: not reported |
|
| Outcomes |
Planned: "The primary end‐point of this study will be the time to first occurrence of the combined end‐point of total mortality and unplanned heart failure related hospitalisation." "Secondary 1. Death all causes 2. Death or worsening symptoms and/or signs of CHF requiring hospitalisation or an increase in diuretic treatment for CHF of >40 mg/day of frusemide compared to baseline or equivalent. This will be a time to first event analysis. 3. Cardiovascular mortality. 4. Number of days alive and out of hospital. 5. Number of days alive and not in hospital for cardiovascular reasons including CHF 6. QoL questionnaire change from baseline to 1 year. 7. CHF symptom score change from baseline to 1 year. 8. NYHA heart failure score change from baseline to 1 year." (Cleland 1999) Reported: primary outcome, all‐cause mortality, cardiovascular mortality, HF hospitalisation, days in hospital for cardiovascular reasons, days in hospital for any reason, NYHA class, 6‐min walk distance, plasma concentrations of NTproBNP, cardiovascular death or unplanned HF related hospitalisation, stroke, acute coronary syndrome, blood pressure, serum potassium and creatinine Planned but not reported: QoL |
|
| Notes | Protocol (Cleland 1999) mentions subgroup analyses by age and sex but not found in published papers. Emailed investigators. No response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients were randomly assigned from a computer‐generated list in blocks of four within treatment centres" |
| Allocation concealment (selection bias) | Low risk | "through a centrally administered process, concealed from the study investigators." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The study medication was provided in externally indistinguishable tablets." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Potential classifying events were independently classified by MT and JGFC, blind to treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/850 lost to follow up |
| Selective reporting (reporting bias) | Low risk | Primary outcomes reported, but not all secondary outcomes reported as planned (eg QoL) |
| Other bias | Low risk | "Servier funded the trial and provided site monitors for source data verification. The sponsor had access to the database and participated in the analysis under the supervision of an independent statistician (NF). The Steering Committee wrote the manuscript. Servier representatives commented on it prior to submission." |
RAAM‐PEF.
| Study characteristics | ||
| Methods |
Study design: individual, placebo‐controlled, double‐blind RCT Centres: 1 medical centre, USA Start of study: August 2004 End of Study: October 2007 Follow‐up: 24 weeks Run‐in period: "2‐week open label period of eplerenone 25 mg daily to establish tolerability" |
|
| Participants |
Inclusion criteria: "All patients were defined as having HFpEF based on the presence of all the following criteria: 1) Clinical HF for ≥ 2 months before the screening visit with New York Heart Association (NYHA) functional Class II or III HF symptoms at enrollment; 2) left ventricular ejection fraction ≥ 50% (by echocardiography, radionuclide ventriculography, or contrast angiography) within 2 months of screening; and 3) B‐type natriuretic peptide (BNP) levels ≥ 100 pg/mL within 2 months of screening. Other inclusion criteria included age ≥ 18 years, systolic blood pressure ≤150, and diastolic blood pressure ≤ 95 mm Hg for 4 weeks before and at enrollment, ability to walk ≥ 50 m, current use of angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), if tolerated, for at least 4 weeks before enrollment. Patients were expected to be euvolemic on clinical examination or all attempts were made to achieve euvolemia with change in diuretic doses prior to enrollment into the study." Exclusion criteria: "Exclusion criteria included the need for eplerenone or spironolactone for treatment of other comorbid illnesses (eg, ascites); hepatic impairment; serum creatinine > 2.5 mg/dL or serum potassium > 5.0 mEq/L; prior intolerance to eplerenone or spironolactone; significant valvular heart disease, pericardial disease or severe chronic lung disease; patients with technically inadequate echocardiographic windows; patients with severe mitral annular calcification; unstable angina or acute myocardial infarction within 4 weeks before enrollment; severe peripheral vascular disease with claudication or other physical conditions limiting the distance walked; pregnant or lactating females; history of active alcohol or substance abuse or history of repeated noncompliance; history of cancer within 3 years (other than resected cutaneous basal or squamous cell carcinoma); and participation in any other drug trial within 30 days before enrollment." Randomised (N): 46 (23 intervention, 23 control) Withdrawn (N): 0 Lost to follow‐up (N): 2 (2 intervention (relocation)) Analysed (N): 44 (21 intervention, 23 control) Age (years, mean, SD): intervention: 72.2, 9.8; control: 68.7, 9.1 Sex (% men): intervention: 95.2; control: 91.3 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): intervention: 129.7, 12.4; control: 130.6, 10.7 Heart rate (beats/min, mean, SD): intervention: 65.0, 9.3; control: 63.0, 12.1 BMI (mean, SD): intervention: 30.1, 6.1; control: 34.6, 5.8 Serum creatinine (mg/dL, mean, SD): intervention: 1.62, 0.50; control: 1.43, 0.51 B‐type natriuretic peptide (pg/mL): intervention: 254.9, 163.0; control: 283.5, 211.6 NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, median, IQR): intervention: 62.1, 5.0; control: 62.5, 7.5 NYHA class I (%): 0 NYHA class II (%): intervention: 66.7; control: 52.2 NYHA class III (%): intervention: 33.3; control: 47.8 NYHA class IV (%): 0 Hypertension (%): 100 Diabetes (%): intervention: 61.9; control: 60.9 Atrial fibrillation (%): intervention: 14.3; control: 13.0 Hospitalisation for heart failure (%): intervention: 42.9; control: 60.9 Coronary heart disease (%): intervention: 66.7; control: 47.8 Stroke (%): nor reported Diuretic (%); intervention: 95.2; control: 100 Digoxin (%): not reported Beta‐blocker (%): intervention: 76.2; control: 82.6 ACEI or ARB (%): intervention: 95.2; control: 100 MRA (%): study drug |
|
| Interventions |
Intervention: eplerenone. "After randomization, patients received study drug at a dose of 25 mg daily for 2 weeks followed by 50 mg daily for 22 weeks, if tolerated" "Study drug dose was adjusted according to the following algorithm. If the serum K+ was ≥ 5.0 mEq/L but < 5.5 mEq/L, the dose of eplerenone was not increased. If the level was ≥ 5.5 but < 6.0 mEq/L, the dose of eplerenone was reduced to half. If the serum potassium was ≥ 6.0 mEq/L eplerenone was stopped, at least transiently. If an underlying condition that was correctable was identified, the medication could be restarted at the lowest dose once the serum K+ was < 5.0 mEq/L. If no correctable cause was identified for the serum potassium ≥ 6.0 mEq/L, eplerenone was discontinued permanently and serum K+ was followed with adjustments in other medications as indicated. Oral potassium supplements were allowed if the serum potassium was < 4.0 mEq/L after the study drug was started." Comparator: matching placebo Concomitant medication: "Potassium supplements were stopped when eplerenone was initiated." |
|
| Outcomes |
Planned: in clinical trial register: all of the below, except NYHA class, hospitalisation and mortality Reported: primary: 6MWD. Secondary: echocardiographic measures of diastolic dysfunction, biomarkers including markers of collagen turnover and B‐type natriuretic peptide, HF‐related quality of life measured by the Kansas City Cardiomyopathy Questionnaire, NYHA class, hospitalisation, mortality |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind" but no details |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "all end points were evaluated blinded to treatment allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT not used, analysis based on participants that completed study, minimal loss to follow‐up (reasons reported) |
| Selective reporting (reporting bias) | Unclear risk | although there is a clinical trial registry record, it's unclear whether this was a pre‐ or post‐registration |
| Other bias | Low risk | "Supported by a VA Clinical Research Service grant # CLIN‐010‐03S (to Dr. Deswal)." "The study was sponsored by the Department of Veterans Affairs (VA). The study drug was provided by Pfizer Pharmaceuticals, but they did not provide any other funding for the study and did not have any role in the conduct and analysis of this study" originally registered as assessing spironolactone, then changed to epleronone |
Sahoo 2016.
| Study characteristics | ||
| Methods |
Study design: RCT Centres: 1, India Start of enrolment: not reported End of enrolment: not reported Mean follow‐up: 3‐6 months Run‐in period: not reported |
|
| Participants |
Inclusion criteria: "Patients with moderate or severe MR on color flow Doppler, LVEF ≥ 55%, and LV end‐systolic dimension < 40 mm were included" Exclusion criteria: "Patients with NYHA class IV symptoms, known coronary artery disease, significant other valvular disease, serum creatinine > 2.5 mg/dL, and hypertension were excluded" Randomised (N): 100 (48 intervention, 52 control) Withdrawn not reported Lost to follow‐up not reported Analysed (N): at 3 months: 100 (48 intervention, 52 control); at 6 months: 75 (39 intervention, 36 control) Age (years, mean, SD): intervention: 30.24, 12.76; control: 29.6, 15.58 Sex (% men): intervention: 35.6; control: 22.7 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): intervention: 125, 10.1; control: 124.4, 7.4 Heart rate (beats/min, mean, SD): intervention: 90.1, 11.78; control: 88.5, 18.12 BMI: intervention: 21.04, 4.74; control: 19.04, 4.7 Serum creatinine not reported B‐type natriuretic peptide (pg/mL): intervention: 194, 178.5; control: 166, 165.7 NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): intervention: 62.5, 6.5; control: 61.4, 6.9 NYHA class: "most patients were in NYHA class II (77%) while 23% were in NYHA class III" Hypertension (%): intervention: 61.1; control: 62.3 Diabetes (%): intervention: 26.9; control: 25.3 Atrial fibrillation (%): intervention: 33.8; control: 35.5 Hospitalisation for heart failure: not reported Coronary heart disease (%): intervention: 68.9; control: 67.6 Stroke (%): intervention: 0.1; control: 0 Diuretic (%): 92 Digoxin (%): 33 Beta‐blocker (%): study drug ACEI (%): 58 ARB (%): not reported MRA (%): not reported |
|
| Interventions |
Intervention: metoprolol succinate. "initiated at 12.5–25 mg/day and titrated as tolerated at 2‐week intervals to a maximum of 100 mg/day. Prior to each escalation, care was taken to ensure that resting heart rate was > 60 bpm and systolic BP > 100 mm Hg" Comparator: placebo Concomitant medication: "in addition to ongoing therapy" |
|
| Outcomes |
Planned: unclear as we did not identify a published protocol or clinical trial registry entry Reported: withdrawals due to adverse events, echocardiographic outcomes, blood pressure, MR grade, NYHA class |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "by a computerized random number generating protocol" |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Detailed echocardiography […] was performed by two operators who were blinded to the treatment protocol." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | unclear loss‐to‐follow up of 25 participants at 6 months as no reasons given |
| Selective reporting (reporting bias) | Unclear risk | unable to assess |
| Other bias | Unclear risk | "The authors declare no conflict of interest" no funding source reported |
SENIORS.
| Study characteristics | ||
| Methods |
Study design: RCT Centres: multi‐centre, international (Czech Republic, Hungary, Italy, Ukraine, UK, France, Germany, Romania, Spain, Switzerland, The Netherlands) Start of enrolment: September 2000 End of enrolment: December 2002 Mean follow‐up: 21 months Run‐in period: no |
|
| Participants |
Inclusion criteria: "To be eligible, patients had to be age ≥ 70 years, provide written informed consent, and have a clinical history of chronic HF with at least 1 of the following features: documented hospital admission within the previous 12 months with a discharge diagnosis of congestive HF or documented LVEF ≤ 35% within the previous 6 months. " Exclusion criteria: "The main exclusion criteria were new drug therapy for heart failure in the 6 weeks prior to randomization, any change in cardiovascular drug therapy in the 2 weeks prior to randomization, heart failure due primarily to uncorrected valvular heart disease, contraindication or previous intolerance to beta‐blockers (e.g. heart rate < 60 beats/min or systolic blood pressure < 90 mmHg), current use of beta‐blockers, significant hepatic or renal dysfunction, cerebrovascular accidents within the previous 3 months, and being on a waiting list for percutaneous coronary intervention or cardiac surgery or other major medical conditions that may have reduced survival during the period of the study." Randomised (N): 2128 (1067 intervention, 1061 control); subgroup of interest: 643 (nebivolol N = 320, placebo N = 323) Withdrawn not reported Lost to follow‐up (N): 37 (16 intervention, 21 control) Analysed (N): 2128 (1067 intervention, 1061 control) Age (years, mean, SD): intervention: 76.1, 4.8; control: 76.1, 4.6 Sex (% men): intervention: 61.6; control: 64.7 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): intervention: 138.6, 20.1; control: 139.5, 21.1 Heart rate (beats/min, mean, SD): intervention: 79.2, 13.6; control: 78.9, 13.7 BMI: not reported Serum creatinine (mg/dL, mean, SD)*: intervention: 1.2, 0.4; control: 1.2, 0.4 B‐type natriuretic peptide (pg/mL): not reported NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): intervention: 36, 13; control: 36, 12 NYHA class I (%): intervention: 3.0; control: 2.7 NYHA class II (%): intervention: 56.5; control: 56.3 NYHA class III (%): intervention: 38.7; control: 38.7 NYHA class IV (%): intervention: 1.8; control: 2.3 Hypertension (%): intervention: 61.1; control: 62.3 Diabetes (%): intervention: 26.9; control: 25.3 Atrial fibrillation (%): intervention: 33.8; control: 35.5 Hospitalisation for heart failure: not reported Coronary heart disease (%): intervention: 68.9; control: 67.6 Stroke (%): intervention: 0.1; control: 0 Diuretic (%); intervention: 85.8; control: 85.5 Digoxin (%): not reported Beta‐blocker (%): study drug ACEI (%): intervention: 81.7; control: 82.6 ARB (%): intervention: 6.2; control: 7.1 MRA (%): intervention: 28.8; control: 26.4 |
|
| Interventions |
Intervention: nebivolol "Nebivolol or placebo tablets were provided in identical packaging and tablet appearance. The initial dose was 1.25 mg once daily, and, if tolerated, this was increased to 2.5 and 5 mg, respectively, every 1–2 weeks, reaching a target of 10 mg once daily over a maximum of 16 weeks." Comparator: placebo Concomitant medication: exclusion criteria: current use of beta blockers |
|
| Outcomes |
Planned: planned in protocol (Shibata 2002): primary: all cause mortality and cardiovascular hospital admissions (time to first event). Secondary: all cause mortality, composite of all cause mortality or all cause hospital admissions, cardiovascular hospital admissions, cardiovascular mortality, functional capacity by NYHA class, functional capacity by 6 min walk test Reported: reported: compliance to treatment, haemodynamics, death or cardiovascular hospital admission, all cause mortality, cardiovascular hospital admissions, total mortality, cardiovascular mortality, all cause hospitalisation |
|
| Notes | subgroup of interest, partial outcome data reported baseline data for all participants, outcome data for subgroup only (nebivolol N = 320, placebo N = 323) emailed trialists to ask for details on HF hospitalisation for LVEF > 40%, withdrawal due to AE, hyperkalaemia. No response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization to nebivolol or placebo on a 1:1 basis was carried out by telephone call to a central office (Clinical Data Care, Lund, Sweden)." |
| Allocation concealment (selection bias) | Low risk | "Patients were allocated a treatment number which corresponded to the appropriate study treatment packs." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind"; no details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT used |
| Selective reporting (reporting bias) | Low risk | primary outcomes reported as planned |
| Other bias | Low risk | "Dr. van Veldhuisen has received lecture fees from Menarini and was a member of the steering committee of the SENIORS trial. Dr. Cohen‐Solal has received lecture and consultancy fees from Menarini, and was a member of the steering committee for the SENIORS trial and received lecture fees. Dr. Böhm has received speaker fees from Menarini. Dr. Anker has received speaking honoraria from Menarini Ricerche SpA, Roche, Merck, and Tanabe. Dr. Babalis’s department has received a grant from Menarini. Dr. Coats has received honoraria from Menarini. Dr. Poole‐Wilson has received honoraria from Menarini for speaking about the SENIORS trial. Dr. Flather has received research grant funding to his institution from Menarini and speaker fees from Menarini for lectures at scientific meetings and symposia. The original SENIORS trial was supported by Menarini Ricerche SpA, Italy. Funding for additional statistical analyses for the present study to the Clinical Trials and Evaluation Unit in London were obtained. All members of the Steering Committee of the SENIORS trial have received honoraria for speaking on aspects of heart failure and beta‐blockers at meetings funded by companies in the pharmaceutical industry." "SENIORS is sponsored by Menarini Ricerche SpA." |
Shu 2005.
| Study characteristics | ||
| Methods |
Study design: two‐arm, individual, RCT Centres: not reported Start of enrolment: August 2000 End of enrolment: March 2002 Follow‐up: 6‐12 months Run‐in period: not reported |
|
| Participants |
Inclusion criteria: "Patients were included in the study if they had (1) a history of uncorrected rheumatic heart valvular disease or New York Heart Association (NYHA) functional class III or IV disease, necessitating hospitalization; (2) a cardiothoracic ratio of less than 65%; (3) AF with a resting ventricular rate of 70 beats/ minute or more for at least three months, as depicted on the electrocardiogram (ECG); and (4) an echocardiogram showing a significant mitral stenosis or aortic lesions and mitral valve regurgitation." Exclusion criteria: "Patients were excluded from the study if they had uncorrected congenital heart disease, sustained ventricular tachycardia, severe liver and kidney dysfunction, chronic obstructive pulmonary disease, bronchial asthma, obstructive or restrictive cardiomyopathy or myocarditis, myocardial infarction, or unstable angina within the previous three months. Patients were also ineligible for enrollment if they required intensive care or concurrent intravenous therapy or if they were using calcium‐channel blockers, class I or III antiarrhythmic drugs, monoamine oxidase (MAO)–inhibitors or beta2‐agonists." Randomised (N): 88 (not reported by treatment arm) Withdrawn (N): 20 (did not complete the study) intervention: 11 (5 due to suspected adverse drug effects); control: 9 Lost to follow‐up (N): 14 (excluded from the evaluation at follow‐up ‐ 7 had insufficient quality of echocardiography or difficulties with telephone‐connection) Analysed (N): 67 (intervention: 33; control: 34) Age (years, mean, SD): intervention: 40.6, 6.8; control: 43.5, 7.4 Sex (% male): intervention: 36; control: 35 Ethnicity not reported Systolic blood pressure (mmHg, mean, SD): intervention: 115, 12; control: 121, 14 Heart rate not reported BMI not reported Serum creatinine not reported B‐type natriuretic peptide not reported NT pro B‐type natriuretic peptide not reported LVEF not reported NYHA class not reported Hypertension not reported Diabetes not reported Atrial fibrillation not reported Hospitalisation for heart failure: not reported Coronary heart disease not reported Stroke not reported Diuretic not reported Digoxin not reported Beta‐blocker not reported ACEI not reported ARB not reported MRA not reported |
|
| Interventions |
Intervention: Bisoprolol, "All patients in the treatment group received bisoprolol at the initial dose of 1.25 mg/day. The recommended maximal dose was 10 mg/day. The dose schedule for titration of the selective beta1 blocker was gradually increased over three to five days, by two to three weeks, to as high as 10 mg/day, with adjustments of diuretics and ACE‐inhibitors, as clinically indicated." Comparator: "control" (unspecified) Concomitant medication: "At the discretion of the treating physicians, all patients were given concomitant therapy consisting of one of the following: • diuretics, as required, to control fluid retention • digoxin, extracted from Digitalis lanata • ACE‐inhibitors (or ARBs when ACE‐inhibitors were not tolerated) unless there were specific contraindications • nitrates, depending on the presence of valvular lesions and on blood pressure readings" |
|
| Outcomes | Planned: we did not identify a published protocol or pre‐registered clinical trial register record | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "On the basis of admission sequence, patients were randomly assigned to a treatment group or a control group" |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | unclear reporting of withdrawals/loss‐to‐follow up |
| Selective reporting (reporting bias) | Unclear risk | unable to assess |
| Other bias | Unclear risk | no funding source reported |
SNEGOVIK.
| Study characteristics | ||
| Methods |
Study design: two‐arm, individual, RCT Centres: not reported Start of enrolment: not reported End of enrolment: not reported Follow‐up: 3 months Run‐in period: not reported |
|
| Participants |
Inclusion criteria: "in ambulatory patients (pts) with arterial hypertension and CHF and preserved systolic left ventricular (LV) function" "According including/exclusion criteria pts have had seated systolic BP(SBP)≤160mmHg and diastolic BP(DBP) ≤ 95mmHg at randomization." "with stable symptomatic CHF (NYHA class II‐III) as a result of arterial hypertension (AH) with preserved LV ejection fraction (EF) ≥ 50%" Exclusion criteria: not reported Randomised (N): 726 (416 intervention, 310 control) Withdrawn (N): not reported Lost to follow‐up (N): not reported Analysed (N): not reported Age not reported Sex not reported Ethnicity not reported Systolic blood pressure not reported Heart rate not reported BMI not reported Serum creatinine not reported B‐type natriuretic peptide not reported NT pro B‐type natriuretic peptide not reported LVEF not reported NYHA class not reported Hypertension not reported Diabetes not reported Atrial fibrillation not reported Hospitalisation for heart failure: not reported Coronary heart disease not reported Stroke not reported Diuretic not reported Digoxin not reported Beta‐blocker not reported ACEI not reported ARB not reported MRA not reported |
|
| Interventions |
Intervention: quinapril Comparator: "conventional treatment, recommended for CHF [congestive heart failure] and AH [arterial hypertension] treatment" Concomitant medication: not reported |
|
| Outcomes |
Planned: unclear Reported: NYHA, 6MWD, clinical status, QoL (MLHFQ), 2D echocardiography, blood pressure |
|
| Notes | Unable to find contact details to ask investigators for end scores for QoL, full publication of results and mortality data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "were randomly assigned" but no detail |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | not reported |
| Selective reporting (reporting bias) | Unclear risk | not reported |
| Other bias | High risk | published conference abstract only |
STRUCTURE.
| Study characteristics | ||
| Methods |
Study design: two‐arm, individual, RCT Centres: Poland, "of each centre" suggests multicentre trial, no details Start of enrolment: Novemer 2011 End of enrolment: February 2015 Mean follow‐up: 6 months Run‐in period: not reported |
|
| Participants |
Inclusion criteria: "Patients who presented with signs or symptoms of HF (dyspnea, fatigue, and exercise intolerance) consistent with New York Heart Association functional class II or III, with preserved LV ejection fraction (> 50%), and with evidence of diastolic dysfunction, were considered suitable for screening." Exclusion criteria: "Exclusion criteria were: Atrial fibrillation or flutter Resting heart rate > 90 beats/min Ischemic heart disease (defined by a positive coronary angiogram or inducible ischemia during exercise testing) Moderate or worse valvular heart disease Primary myocardial diseases Established or suspected pulmonary diseases (spirometry results < 80% of age‐ and sex‐specific reference values) Hemoglobin ≤ 11 g/dl Adrenocortical, hepatic, rheumatic, neoplastic, skeletal, thyroid, and renal diseases (including renal insufficiency with serum creatinine > 1.5 mg/dl [132 mmol/l]) Hyperkalemia > 5.0 mmol/l Known intolerance or treatment with an MRA within the last 3 months Concomitant therapy with a potassium‐sparing agent Current lithium use Pregnancy" Randomised (N): 150 (75 intervention, 75 control) Withdrawn (N): for reasons other than death 12 (7 intervention, 5 control) Lost to follow‐up (N): 7 (4 intervention, 3 control) Analysed (N): 131 (64 intervention, 67 control) Age (years, mean, SD): intervention: 66.3, 7.7; control: 67.6, 9.1 Sex (% men): intervention: 12; control: 19 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): intervention: 131, 15; control: 130, 18 Heart rate (beats/min, mean, SD): intervention: 72, 10; control: 73, 10 BMI (mean, SD): intervention: 30.7, 4.5; control: 29.7, 4.6 Serum creatinine (mg/dL, mean, SD): intervention: 0.99, 0.20; control: 1.03, 0.24 B‐type natriuretic peptide (pg/mL, median, IQR): intervention: 40 (26‐63); control: 54 (27‐99) NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, median, IQR): intervention: 72.6 (70.4–74.8); control: 71.4 (69.2–73.5) NYHA class I (%): 0 NYHA class II (%): intervention: 78; control: 79 NYHA class III (%): intervention: 22; control: 21 NYHA class IV (%): 0 Hypertension (%): intervention: 92; control: 91 Diabetes (%): intervention: 39; control: 40 Atrial fibrillation (%): not reported Hospitalisation for heart failure (%): intervention: 17; control: 21 Coronary heart disease (%): significant CAD excluded Stroke (%): not reported Diuretic (%); intervention: thiazides 54, loop 13; control: thiazides 46, loop 18 Digoxin (%): not reported Beta‐blocker (%): intervention: 78; control: 72 ACEI/ARB (%): intervention: 97; control: 95 MRA (%): study drug |
|
| Interventions |
Intervention: spironolactone, 25mg/day Comparator: matching placebo (120 mg/day of microcellulose) Concomitant medication: "Enrollees continued to receive other prescribed treatments throughout the study period." |
|
| Outcomes |
Planned: unclear Reported: "Coprimary outcomes were change at 6 months in exercise capacity (assessed by peak VO2) and exertional E/e' (reflecting LVFP). The secondary outcomes included change at follow‐up in exercise blood pressure (BP) response and post‐treatment global longitudinal myocardial deformation (GLS) measured by 2‐dimensional strain." |
|
| Notes | Emailed investigator to ask for additional outcome data relevant to this review. No response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The study coordinator, who was not involved in study procedures, was responsible for drug randomization and dispensing" |
| Allocation concealment (selection bias) | Low risk | "sequentially‐numbered, opaque, sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients and investigators performing the assessments and data analysis were blinded to group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "core laboratory in Hobart, Australia, for independent adjudication of the primary endpoint" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT not used, withdrawals reported with reasons, lost to follow‐up reported, similar numbers for treatment arms |
| Selective reporting (reporting bias) | Unclear risk | unclear, clinical trial registration was post‐hoc |
| Other bias | Low risk | "The authors have reported that they have no relationships relevant to the contents of this paper to disclose" "This study was funded by grants ST‐678 from Wroclaw Medical University and 13‐024 from the Royal Hobart Hospital Foundation." |
SUPPORT.
| Study characteristics | ||
| Methods |
Study design: parallel, individual RCT Centres: 17, Japan Start of enrolment: October 2006 End of enrolment: March 2010 Median follow‐up: 4.4 years Run‐in period: not reported |
|
| Participants |
Inclusion criteria: The inclusion criteria of the present study were designed to enroll symptomatic CHF patients with hypertension aged 20 to 79 years who were treated with ACEI or beta‐blocker or both. Inclusion criteria: NYHA Classes II to IV CHF, History of hypertension or treated with anti‐hypertensive medications, Aged 20 or older and, 80 years at the entry, Stable with angiotensin‐converting enzyme inhibitors and/or b‐blockers, Not treated with angiotensin II receptor blockers Exclusion criteria: The exclusion criteria were designed to exclude patients with substantive confounding medical conditions or an inability to meaningfully participate in the SUPPORT trial. Exclusion criteria: Patients who have renal dysfunction (serum creatinine ≥3.0 mg/dL), or those who are under chronic haemodialysis, Drug hypersensitivity to olmesartan, Severe liver dysfunction, History of angioedema, History of malignant tumour or life‐threatening illness of poor prognosis, Pregnant or possibly pregnant patients, Cardiovascular surgery within 6 months prior to the date of the entry, Acute myocardial infarction within 6 months prior to the date of the entry, Percutaneous coronary intervention with or without stent implantation within 6 months prior to the date of the entry. Randomised (N): 1146 (1 patient excluded prior to this for protocol violation) (578 intervention, 568 control) Withdrawn (N): for reasons other than death 9 (1 protocol violation, 8 no LVEF data) Lost to follow‐up (N): not reported Analysed (N): Total 1138 (HFpEF 709, HFrEF 429) (HFpEF 363 intervention, HFpEF 346 control) Age (years, mean, SD): intervention: 66.5, 10.1; control: 65.9, 9.7 Sex (% men): intervention: 70.2; control: 71.1 Ethnicity (%): not reported Systolic blood pressure (mmHg, mean, SD): intervention: 131.5, 17.1; control: 130.1, 17.1 Heart rate (beats/min, mean, SD): intervention: 70.6, 13.2; control: 71.4, 14.9 BMI (mean, SD): intervention: 24.4, 4.2; control: 24.8, 4.2 Serum creatinine (mg/dL, mean, SD): intervention: 0.9, 0.3; control: 0.9, 0.3 B‐type natriuretic peptide (pg/mL, median, IQR): intervention: 71.1 (30.2, 148.0); control: 58.7 (27.5, 139.0) NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): intervention: 63.8, 8.8; control: 63.1, 8.6 NYHA class I (%): 0 NYHA class II (%): intervention: 94.2; control: 93.4 NYHA class III (%): intervention: 5.5; control: 6.4 NYHA class IV (%): 0 Hypertension (%): 100 Diabetes (%): intervention: 46.6; control: 53.9 Atrial fibrillation (%): not reported Hospitalisation for heart failure (%): intervention: 52.2; control: 44.1 Coronary heart disease (%): intervention: 48.8; control: 45.1 Stroke (%): not reported Diuretic (%); intervention: 45.7; control: 48.0 Digoxin (%): not reported Beta‐blocker (%): intervention: 63.4; control: 65.4 ACEI (%): intervention: 79.9; control: 79.0 ARB (%): study drug MRA (%): intervention: 18.5; control: 22.0 |
|
| Interventions |
Intervention: olmesartan. "Olmesartan was initiated at a dose of 5–10 mg/day, and then up titrated to 40 mg/ day, if tolerable, in the olmesartan group, while no ARB use was allowed in the control group" Comparator: no treatment Concomitant medication: treated with ACEI and/or beta‐blocker in inclusion criteria, not treated with ARB |
|
| Outcomes |
Planned: Clinical trial registry entry at point of enrolment: primary outcomes all‐cause death, nonfatal acute myocardial infarction, nonfatal stroke, hospital admission due to congestive heart failure Reported: Primary Endpoint: A composite of the following outcomes: all‐cause death, non‐fatal acute myocardial infarction, non‐fatal stroke, hospital admission due to worsening heart failure Secondary Endpoints: cardiovascular death, death due to heart failure, sudden death, acute myocardial infarction, stroke, hospital admission from any cardiovascular reasons, fatal arrhythmia or appropriate ICD discharge, new‐onset diabetes, development of renal dysfunction (equal to or more than twofold increase of serum creatinine level), new‐onset atrial fibrillation, a need to modify treatment procedures for heart failure, a decrease in left ventricular ejection fraction (equal to or more than 20% decrease), an increase in B‐type natriuretic peptide levels (> 2‐fold increase if the baseline level was > 50 pg/mL and an increase of > 100 pg/mL if the baseline level was < 50 pg/mL), changes in serum markers for metabolic syndrome (high sensitive C‐reactive protein, adiponectin, microRNAs) |
|
| Notes | Emailed investigators to ask for outcome date for participants with LVEF > 40%. No response. Published (and presented above) are baseline characteristics and results for HFpEF as defined by investigators (LVEF ≥ 50%). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | open label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | blinded endpoint study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | withdrawals reported with reasons, not detailed by treatment arm ITT used, but after exclusion of some randomised patients |
| Selective reporting (reporting bias) | Low risk | primary outcomes reported as planned |
| Other bias | Low risk | "The Department of Evidence‐based Cardiovascular Medicine, Tohoku University Graduate School of Medicine, is supported in part by the unrestricted research grants from Daiichi Sankyo Co, Ltd (Tokyo, Japan), Bayer Yakuhin, Ltd (Osaka, Japan), Kyowa Hakko Kirin Co, Ltd (Tokyo, Japan), Kowa Pharmaceutical Co, Ltd (Tokyo, Japan), Novartis Pharma K.K. (Tokyo, Japan), Dainippon Sumitomo Pharma, Co, Ltd (Osaka, Japan), and Nippon Boehringer Ingelheim Co, Ltd (Tokyo, Japan). H.S. has received lecture fees from Bayer Yakuhin, Ltd (Osaka, Japan), Daiichi Sankyo Co, Ltd (Tokyo, Japan) and Novartis Pharma K.K. (Tokyo, Japan)." "This study was supported in part by the grants‐in‐aid from the Ministry of Health, Labour, and Welfare and those from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. Funding to pay the Open Access publication charges for this article was provided by the author." |
SWEDIC.
| Study characteristics | ||
| Methods |
Study design: two‐arm, individual, parallel RCT Centres: 12, Sweden Start of enrolment: not reported End of enrolment: not reported Mean follow‐up: not reported Run‐in period: not reported |
|
| Participants |
Inclusion criteria: "patients with symptoms and/or signs of HF, normal or almost normal systolic function and abnormal DF who did not have a contraindication to receiving therapy with a beta‐ adrenoceptor blocking agent were included into the study." "Major inclusion criteria were a wall motion index (WMI) ≤ 1.2, i.e akinesia of one segment or less or hypokinesia of 2 segments or less, using a 16 segment model with at least 10 segments visible, corresponding to an LVEF > 45%, and evidence of abnormal DF using at least one of the following criteria to assess diastolic dysfunction" Exclusion criteria: "Major exclusion criteria were restrictive or hypertrophic cardiomyopathies, significant uncorrected obstructive or regurgitant valvular diseases, unstable angina, active myocarditis, uncontrolled symptomatic ventricular arrhythmias, history of sick sinus syndrome, second or third degree AV‐block, heart rate less than 60 bpm, systolic blood pressure ‐85 mmHg, uncontrolled hypertension, atrial fibrillation, evidence of obstructive pulmonary disease, unstable diabetes, treatment with beta‐2‐agonists, MAO‐inhibitors, calcium channel blockers or beta‐receptor blockers" Randomised (N): 113 Withdrawn (N): for reasons other than death: "16 patients had echocardiographic data of insufficient quality and were excluded from the evaluation." Lost to follow‐up (N): 2 (reasons not reported) Analysed (N): 97 (47 intervention, 50 control) Age (years, median, IQR): intervention: 67 (48 to 81); control: 66 (48 to 84) Sex (% men): intervention: 59.6; control: 54.0 Ethnicity (%): not reported Systolic blood pressure (mmHg, median, IQR): intervention: 155 (122 to 180); control: 150 (110 to 200) Heart rate (beats/min, median, IQR): intervention: 74 (60 to 95); control: 73 (60 to 101) BMI not reported Serum creatinine not reported B‐type natriuretic peptide (pg/mL): intervention: 67.7, 76.1; control: 67.7, 67.7 NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF not reported NYHA class I (%): intervention: 40; control: 26 NYHA class II (%): intervention: 53; control: 53 NYHA class III (%): intervention: 7; control: 21 NYHA class IV (%): 0 Hypertension (%): intervention: 70.2; control: 62 Diabetes (%): intervention: 12.8; control: 16.0 Atrial fibrillation (%): 0 Hospitalisation for heart failure: not reported Coronary heart disease (%): intervention: 17; control: 6 Stroke (%): not reported Diuretic (%); not reported Digoxin (%): not reported Beta‐blocker (%): study drug ACEI (%): not reported ARB (%): not reported MRA (%): not reported |
|
| Interventions |
Intervention: carvedilol. "carvedilol or placebo twice daily in addition to their conventional treatment" "All patients were uptitrated to the maximum tolerated dose or to the target dose (25 mg b.i.d., or 50 mg b.i.d. in patients weighing 85 kg) of carvedilol or matching placebo. After completion of uptitration they were to continue on double blind medication for a 6 month maintenance period. At study end patients were withdrawn from blinded study medication in a stepwise manner over a 1–3 week period. Optimal therapy for the patient’s condition was then reinstated at the investigator’s discretion" "Overall, carvedilol was well tolerated, with 81% of patients receiving the maximum dose at the end of the uptitration phase (25 mg b.i.d. or 50 mg b.i.d.) and 82% at the end of the study" Comparator: placebo Concomitant medication: "as an addition to conventional treatment" |
|
| Outcomes |
Planned: not able to assess as we are unaware of a published protocol or pre‐registration in a clinical trial register Reported: primary: diastolic dysfunction. Secondary: Secondary endpoints were the effects of carvedilol as compared to placebo on combined all cause mortality and cardiovascular hospitalisations, combined all‐cause mortality and heart failure hospitalisation, progression of heart failure, individual cardiovascular endpoints and outcome and individual diastolic variables. Additional exploratory analyses on LV dimensions atrial size and WMI were also prespecified." |
|
| Notes | Emailed investigators for details for RoB assessment, reasons for 2 participants not completing study, start/end of enrolment, duration of follow‐up. No response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | double‐blind but no details, matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "All assessments as to whether the LV diastolic dysfunction had improved, was unchanged, or had worsened were made by two echocardiographers from the core laboratory, who were blinded to the order of the assessment and to the study medication received by the patient." only partial outcome assessment and for outcomes not relevant to this review |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | unable to assess ‐ withdrawals not reported by treatment arm ITT used |
| Selective reporting (reporting bias) | Unclear risk | unable to assess due to lack of published protocol or pre‐registration in a clinical trial register |
| Other bias | Low risk | "The study was investigator‐initiated and was partly funded by F. Hoffmann‐La Roche Ltd." |
Takeda 2004.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Centres: 1, Japan Start of enrolment: April 2000 End of enrolment: March 2001 Mean follow‐up: 12 months Run‐in period: not reported |
|
| Participants |
Inclusion criteria: "All patients met Framingham criteria for diagnosis of heart failure. LVEF, as assessed by echocardiography using Simpson’s method, was ≥ 45% in each subject at the screening examination." Exclusion criteria: "Patients with primary significant valvular disease, cor pulmonale, thyroid dysfunction, diabetes mellitus with hemoglobin A1C > 8%, alcohol abuse, other systemic diseases, obvious contraindication to carvedilol, or using angiotensin II receptor antagonists or adrenergic blockers were excluded from the initial entry." Randomised (N): 40 (19 intervention, 21 control) Withdrawn (N): not reported Lost to follow‐up (N): not reported Analysed (N): not reported Age (years, median, IQR): intervention: 69.1, 64.4‐73.7; control: 73.1, 69.7–76.4 Sex (% men): intervention: 68; control: 38 Ethnicity (%): not reported Systolic blood pressure (mmHg, median, IQR): intervention: 129.6, 124.5–134.6; control: 138.0, 130.7–145.3 Heart rate (beats/min, median, IQR): intervention: 70.6, 64.3–77.0; control: 68.9, 63.8–74.0 BMI not reported Serum creatinine not reported B‐type natriuretic peptide (pg/mL, median, IQR): intervention: 172, 135–209; control: 150, 114–186 NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, median, IQR): intervention: 55.8, 51.4–60.3; control: 57.5, 53.4–61.5 NYHA class I (%): 0 NYHA class II (%): intervention: 63.3; control: 71 NYHA class III (%): intervention: 37; control: 29 NYHA class IV (%): 0 Hypertension not reported Diabetes not reported Atrial fibrillation (%): intervention: 21; control: 38 Hospitalisation for heart failure: not reported Coronary heart disease (%): intervention: 58; control: 48 Stroke not reported Diuretic not reported Digoxin not reported Beta‐blocker study drug ACEI (%): intervention: 79; control: 86 ARB not reported MRA not reported |
|
| Interventions |
Intervention: carvedilol. "initial daily dosage of 1.25 mg in addition to conventional therapy, and the dose was doubled every week until reaching >= 5 mg/day. The decision to increase carvedilol to > 5 mg/day was made by the attending cardiologists on the basis of subjective symptoms,
physical findings, and chest roentgenography; the cardiologists were guided to increase carvedilol to 20 mg/day if the patient tolerated it." Comparator: conventional treatment Concomitant medication: not reported |
|
| Outcomes |
Planned: we are not aware of a published protocol or a pre‐registered clinical trial registry entry Reported: BNP, NYHA, exercise capacity, heart failure hospitalisations, deaths |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | not reported |
| Selective reporting (reporting bias) | Unclear risk | unable to assess |
| Other bias | Unclear risk | funding not reported |
TOPCAT.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Centres: "233 sites in 6 countries (1151 participants in the United States, 326 in Canada, 167 in Brazil, 123 in Argentina, 1066 in Russia, and 612 in Georgia)" Start of enrolment: August 2006 End of enrolment: January 2012 Mean follow‐up: 3.3 years Run‐in period: no |
|
| Participants |
Inclusion criteria: "≥ 50 years old, "had at least one sign and at least one symptom of heart failure on a prespecified list of clinically defined signs and symptoms, a left ventricular ejection fraction of 45% or more as measured at the local site by means of echocardiography or radionuclide ventriculography, controlled systolic blood pressure (defined as a target systolic blood pressure of < 140 mm Hg or ≤ 160 mm Hg if the patient was taking three or more medications to control blood pressure), and a serum potassium level of less than 5.0 mmol per liter. In addition, eligible patients had a history of hospitalization within the previous 12 months, with management of heart failure a major component of the care provided (not adjudicated by the clinical‐events adjudication committee), or an elevated natriuretic peptide level within 60 days before randomization (a brain natriuretic peptide [BNP] level ≥ 100 pg per milliliter or an N‐terminal pro‐BNP [NTproBNP] level ≥360 pg per milliliter)." Exclusion criteria: "severe systemic illness with a life expectancy of less than 3 years, severe renal dysfunction (an estimated glomerular filtration rate [GFR] of <30 ml per minute per 1.73 m2 of body‐surface area or a serum creatinine level that was ≥ 2.5 mg per deciliter [221 μmol per liter]), and specific coexisting conditions, medications, or acute events." Randomised (N): 3445 (1722 intervention, 1723 control) Withdrawn (N): 311 for reasons other than death (160 intervention, 151 control) Lost to follow‐up (N): 132 (67 intervention, 65 control) Analysed (N): 3445 (1722 intervention, 1723 control) Age (years, median, IQR): intervention: 68.7, 61.0 to 76.4; control: 68.7, 60.7 to 75.5 Sex (% men): intervention: 48.4; control: 48.5 Ethnicity (%): intervention: white 88.6, control: white 89.2 Systolic blood pressure (mmHg, median, IQR): intervention: 130,120‐139; control: 130, 120‐140 Heart rate (beats/min, median, IQR): intervention: 68, 62‐76; control: 68, 62‐76 BMI (median, IQR): intervention: 31, 27‐36; control: 31, 27‐36 Serum creatinine (mg/dL, median, IQR): intervention: 1.0, 0.9‐1.2; control: 1.1, 0.9‐1.2 B‐type natriuretic peptide (pg/mL): only in subgroup NT pro B‐type natriuretic peptide (pg/mL): only in subgroup LVEF (%, median, IQR): intervention: 56, 51‐61; control: 56, 51‐62 NYHA class I (%): intervention: 3.3; control: 3.1 NYHA class II (%): intervention: 63.3; control: 64.1 NYHA class III (%): intervention: 33.0; control: 32.1 NYHA class IV (%): intervention: 0.4; control: 0.5 Hypertension (%): intervention: 91; control: 92 Diabetes (%): intervention: 33; control: 32 Atrial fibrillation (%): intervention: 35; control: 35 Hospitalisation for heart failure: not reported Coronary heart disease (%): intervention: 26; control: 26 Stroke (%): intervention: 7; control: 8 Diuretic (%); intervention: 81; control: 82 Digoxin not reported Beta‐blocker (%): intervention: 78; control: 77 ACEI or ARB (%): intervention: 84; control: 84 MRA (%): 0 |
|
| Interventions |
Intervention: spironolactone "Study drugs were initially administered at a dose of 15 mg once daily, which was increased to a maximum of 45 mg daily during the first 4 months after randomization. Subsequent dose adjustments were made as required." Comparator: matching placebo Concomitant medication: "Study patients continued to receive other treatments for heart failure and coexisting illnesses throughout the trial." |
|
| Outcomes |
Planned: From NCT record 21 April 2006: primary outcomes: cardiovascular mortality, aborted cardiac arrest, composite of hospitalisation for the management of heart failure (ie hospitalisation for non‐fatal myocardial infarction or non‐fatal stroke). Secondary outcomes: all‐cause mortality, composite of cardiovascular mortality or cardiovascular related hospitalization (i.e. hospitalization for non‐fatal myocardial infarction, non‐fatal stroke, or the management of heart failure), hospitalization for the management of heart failure incidence rate, sudden death or aborted cardiac arrest Reported: "composite of death from cardiovascular causes, aborted cardiac arrest, or hospitalization for the management of heart failure; myocardial infarction; stroke; hospitalisation from any cause; hyperkalemia (potassium level, ≥ 5.5 mmol per liter); hypokalemia (potassium level, < 3.5 mmol per liter); an elevated serum creatinine level (≥ 2 times the baseline value and above the upper limit of the normal range); serum creatinine level of 3.0 mg per deciliter (265 μmol per liter) or higher; serious adverse events" |
|
| Notes | NCT record reports on QoL but no usable data. Hamo 2015 reports baseline QoL data but not by intervention arm. Solomon 2016 reports data for four LVEF groups for HF hospitalisation, CV death, death (table 2) ‐ 40‐49%, 50‐54.99%, 55‐59.99%, 60% and over. Data for all‐cause mortality, lost to follow up, hyperkalemia differ between Pitt 2014 and NCT results. Emailed investigators to ask for end scores for QoL KCCQ, clarification on withdrawals due to adverse events and subgroup data for primary outcomes. No response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible participants were randomly assigned to receive either spironolactone or placebo in a 1:1 ratio with the use of permuted blocks." "the randomization software will return a Treatment Allocation Code corresponding to either spironolactone or placebo" |
| Allocation concealment (selection bias) | Low risk | "The nurse coordinator will utilize a master list of Treatment Allocation Codes to determine which labelled study drug packet to provide to the subject." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Subjects and treating physicians will be blinded to whether subjects are receiving spironolactone or placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Data were collected and managed electronically by the New England Research Institutes Clinical Trial Coordinating Center, which also coordinated site monitoring and analyzed the trial results (with independent verification at Brigham and Women’s Hospital)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All randomly assigned participants were included in all analyses according to the intention‐to‐treat principle." |
| Selective reporting (reporting bias) | Low risk | reported as planned |
| Other bias | Low risk | "sponsored by National Heart, Lung and Blood Institute, National Institutes of Health" |
Upadhya 2017.
| Study characteristics | ||
| Methods |
Study design: parallel, individual, RCT Centres: not reported Start of enrolment: not reported End of enrolment: not reported Follow‐up: 9 months Run‐in period: not reported |
|
| Participants |
Inclusion criteria: "HFpEF was defined as history, symptoms, and signs of HF, a preserved LVEF of 50% or greater and no evidence of other medical condition that could mimic HF symptoms" Exclusion criteria: "Coronary disease was excluded according to history, medical record, electrocardiogram, and rest and exercise echocardiogram" "Exclusions included aldosterone antagonist use within the previous 3 months, a known contraindication, concomitant therapy with a potassium‐sparing diuretic or potassium supplementation, baseline serum potassium level greater than 5.0 mEq/L, or serum creatinine level of 2.5 mg/dL or greater." Randomised (N): 80 (42 intervention, 38 control) Withdrawn (N): for reasons other than death 9 (5 intervention (adverse event N = 1, patient choice N = 4), 4 control (patient choice N = 3, death N = 1)) Lost to follow‐up (N): not reported Analysed (N): 71 (37 intervention, 34 control) Age (years, mean, SD): intervention: 70.0, 1.1; control: 72.0, 1.2 Sex (% men): intervention: 19; control: 21 Ethnicity (%): African American: intervention: 21, control: 37 Systolic blood pressure (mmHg, mean, SD): intervention: 139, 2.7; control: 143, 3.2 Heart rate not reported BMI (mean, SD): intervention: 31.5, 0.8; control: 32.4, 1.2 Serum creatinine not reported B‐type natriuretic peptide (unit not reported): intervention: 55, 46; control: 61, 50 NT pro B‐type natriuretic peptide (pg/mL): not reported LVEF (%, mean, SD): intervention: 62.6, 1.1; control: 62.0, 1.1 NYHA class I (%): 0 NYHA class II (%): intervention: 29; control: 26 NYHA class III (%): intervention: 64; control: 63 NYHA class IV (%): 0 Hypertension (%): intervention: 83; control: 92 Diabetes (%): intervention: 17; control: 29 Atrial fibrillation (%): not reported Hospitalisation for heart failure: not reported Coronary heart disease (%): 0 Stroke (%): not reported Diuretic (%); intervention: 74; control: 71 Digoxin (%): intervention: 2; control: 0 Beta‐blocker (%): intervention: 31; control: 32 ACEI (%): not reported ARB (%): not reported MRA (%): study drug |
|
| Interventions |
Intervention: spironolactone. "The starting dose of spironolactone was 12.5 mg/d in individuals with baseline creatinine of 2.0 mg/dL or greater or potassium greater than 4.5 mEq/L; in all other participants, the starting dose was 25 mg/d. In participants who initiated therapy with the 12.5‐mg/d dose, the dose was increased to 25 mg/d once creatinine fell below 2.5 mg/dL and potassium fell below 5.0 mEq/L and maintained at that dosage as long as those levels were maintained. Spironolactone was discontinued if 1‐week creatinine was 2.5 mg/ dL or higher or potassium was 5.0 mEq/L or higher. " "The mean daily dose of spironolactone was 24.3 2.9 mg/d." Comparator: matching placebo Concomitant medication: not reported |
|
| Outcomes |
Planned: July 2005, NCT record: primary outcomes: exercise intolerance, quality of life Reported: exercise performance, aortic distensibility and LV structure and function, carotid artery stiffness, pulse wave velocity, LV diastolic filling, QoL |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The research pharmacy prepared and distributed placebo and active drug using a secure methodology. All investigators, staff, and participants were fully blinded to treatment group assignment throughout the study period" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The research pharmacy prepared and distributed placebo and active drug using a secure methodology. All investigators, staff, and participants were fully blinded to treatment group assignment throughout the study period" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | unable to assess |
| Selective reporting (reporting bias) | Unclear risk | posthoc clinical trial registration |
| Other bias | High risk | "This study was funded by the National Institutes of Health (NIH; R01AG18915), the Claude D. Pepper Older Americans Independence Center, Wake Forest University (P30AG21332), the Clinical and Translational Science Institute, Wake Forest School of Medicine (NIH UL1TR001420), and the Kermit G. Phillips Chair in Cardiovascular Medicine of Wake Forest School of Medicine" Published as conference abstract only. |
Wang 2010.
| Study characteristics | ||
| Methods |
Study design: parallel, individual, RCT Centres: 1, Taiwan Start of enrolment: not reported End of enrolment: not reported Follow‐up: at least 3 months Run‐in period: not reported |
|
| Participants |
Inclusion criteria: "hypertensive pts who had DHF, defined as the presence of HF signs/symptoms, diastolic dysfunction (mitral annular early diastolic velocity (E') < 8 cm/s), and left ventricular (LV) ejection fraction (EF) > 50%" Exclusion criteria: not reported Randomised (N): 36 (19 intervention, 17 control) Withdrawn (N): not reported Lost to follow‐up (N): not reported Analysed (N): not reported Age (years, mean, SD): not reported Sex (% men): not reported Ethnicity (%): not reported Systolic blood pressure not reported Heart rate not reported BMI not reported Serum creatinine not reported B‐type natriuretic peptide not reported NT pro B‐type natriuretic peptide not reported LVEF (%, mean, SD): intervention: 67, 7; control: 66, 7 NYHA class not reported Hypertension not reported Diabetes not reported Atrial fibrillation not reported Hospitalisation for heart failure: not reported Coronary heart disease not reported Stroke not reported Diuretic not reported Digoxin not reported Beta‐blocker not reported ACEI not reported ARB not reported MRA study drug |
|
| Interventions |
Intervention: spironolactone. 50 mg/d Comparator: no treatment control Concomitant medication: not reported |
|
| Outcomes |
Planned: we are not aware of a published protocol or pre‐registered clinical trial registry entry Reported: echo‐parameters, systolic myocardial velocities |
|
| Notes | does not contribute outcome data to this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | not reported |
| Selective reporting (reporting bias) | Unclear risk | unable to assess |
| Other bias | Unclear risk | funding not reported |
Yuksek 2012.
| Study characteristics | ||
| Methods |
Study design: parallel, individual, RCT Centres: 1, Izmir Ataturk Education and research hospital (IAERH) outpatient clinics, Turkey Start of enrolment: May 2008 End of enrolment: March 2009 Mean follow‐up: 11 months (range: 3‐16 months) Run‐in period: not reported |
|
| Participants |
Inclusion criteria: "We enrolled 108 patients with DHF [diastolic heart failure], aged ≥ 50 years, who presented to Ismir Ataturk Education and Research Hospital (IAERH) cardiology out‐patient clinics with HF symptoms and were found to have an ejection fraction (EF) of ≥ 50% on transthoracic echocardiography (TTE) accompanied by an evidence of DD on TDE. The HF diagnosis was based on Framingham heart failure criteria." Exclusion criteria: "Exclusion criteria included the following: EF <50%, patient age < 50 years, severe valvular disease on echocardiography, a history of acute coronary syndrome, atrial fibrillation, cardiomyopathy or pericardial disease, anaemia (serum haemoglobin levels < 10g/dl), hyperthyroidism, hypothyroidism, renal insufficiency (serum cretanine > 2 mg/dl or dialysis), serum potassium level > 5.5 mEg/l, moderate to servere pulmonary hypertension (sPAP > 50mmHg), and/or intolerance to angiotensin converting enzyme inhibitors (ACEIs) or angiotension receptor blockers (ARBs), bilaterial renal artery stenosis, or any kind of malignancy. Patients with decompensated HF were also excluded." Randomised (N): 108 (54 intervention, 54 control) Withdrawn (N): for reasons other than death 17 (14 intervention, 3 control) Lost to follow‐up (N): 3 (3 intervention (deaths), 0 control) Analysed (N): 88 (37 intervention, 51 control) Age (years, mean, SD): intervention: 62, 8; control: 61,8 Sex(% men): intervention: 16; control: 25 Ethnicity not reported Systolic blood pressure (mean, mmHg, SD): intervention: 126, 16; control: 123, 19 Heart rate not reported BMI (mean, SD): intervention: 33, 6; control: 33, 5 Serum creatinine (mg/dL, mean, SD): intervention: 0.29, 0.14; control: 0.98, 0.25 B‐type natriuretic peptide not reported NT pro B‐type natriuretic peptide (median, pg/mL): intervention: 114; control: 128 LVEF (%, mean, SD): intervention: 66, 7; control: 65, 8 NYHA class (%): intervention: II: 78, III: 22; control: II: 65, III: 35 Hypertension (%): intervention: 84; control: 80 Diabetes (%): intervention: 51; control: 33 Atrial fibrillation not reported Hospitalisation for heart failure not reported Coronary artery disease(%): intervention: 11; control: 22 Stroke not reported Diuretic (%): intervention: 51; control: 59 Digoxin not reported Beta‐blocker (%): intervention: 41; control: 39 ACEI study drug ARB not reported MRA not reported |
|
| Interventions |
Intervention: perindopril, "was started on oral perindopril treatment (5 mg/day)"; "perindopril dose in the study group was up‐titrated to 10mg/day at the end of one month" Comparator: "standard DHF treatment" Concomitant medication: not reported |
|
| Outcomes |
Planned: we are not aware of a published protocol or a pre‐registration in a clinical trial register Reported: primary endpoints: changes in E', A' and Sm velocities, E/E', E/A and E'/A' ratios, isovolumic relaxation time 9IVRT) and deceleration time (DT) at the end of the follow up period. Secondary endpoints included the changes in NT‐proBNP levels and NYHA functional classes, Left Atrial Volume Index. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised into two groups using a basic randomisation method" |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Per‐protocol analysis, 31% drop out from intervention group, 6% drop out of control group |
| Selective reporting (reporting bias) | Unclear risk | unable to assess |
| Other bias | Unclear risk | funding not reported |
Zi 2003.
| Study characteristics | ||
| Methods |
Study design: parallel, individual, RCT Centres: 1, Royal Liverpool and Broadgreen University Hospitals Start of enrolment: 1997 End of enrolment: 1999 Follow‐up: 6 months Run‐in period: mentioned but no details |
|
| Participants |
Inclusion criteria: "aged 65 years or older, with heart failure" "They all had left ventricular ejection fraction (LVEF) on echocardiography or radionuclide ventriculography equal or greater than 40%. Where a left ventricular ejection fraction could not be measured systolic function had to be preserved or only mildly impaired by direct visualisation of the echocardiograms" Exclusion criteria: "Patients with haemodynamically significant valvular disease, pulmonary hypertension, right ventricular systolic dysfunction, uncontrolled atrial fibrillation or flutter, unstable angina pectoris, hypotension, myocardial infarction within one month, renal failure (serum creatinine >150 mmol/L), renal‐artery stenosis, severe liver or pulmonary disease were excluded. patients treated with tetracyclines, lithium, benzodiazepines, major tranquillisers, anti‐depressants (with the exception of selective serotonin re‐uptake inhibitors) or major psychoactive drugs were also excluded." Randomised (N): 74 (36 intervention, 38 control) Withdrawn (N): for reasons other than death 4 (0 intervention, 4 control (worsening heart failure)) Lost to follow‐up (N): not reported Analysed (N): 74 (36 intervention, 38 control) Age (years, mean, SD): intervention: 77, 7; control: 78, 7 Sex (% men): intervention: 38.9; control: 31.6 Ethnicity (%): not reported Systolic blood pressure not reported Heart rate not reported BMI not reported Serum creatinine not reported B‐type natriuretic peptide not reported NT pro B‐type natriuretic peptide not reported LVEF not reported NYHA class I (%): intervention: 5.5; control: 0 NYHA class II (%): intervention: 77.8; control: 73.7 NYHA class III (%): intervention: 16.7; control: 26.3 NYHA class IV (%): 0 Hypertension (%): intervention: 27.8; control: 31.6 Diabetes (%): intervention: 11.1; control: 18.4 Atrial fibrillation (%): intervention: 38.9; control: 31.6 Hospitalisation for heart failure: not reported Coronary heart disease (%): intervention: 55.6; control: 57.9 Stroke (%): not reported Diuretic (%); intervention: 94.4; control: 97.1 Digoxin (%): intervention: 38.9; control: 26.3 Beta‐blocker (%): intervention: 19.4; control: 7.9 ACEI (%): study drug ARB (%): not reported MRA (%): not reported |
|
| Interventions |
Intervention: quinapril. "Both drugs were titrated at two‐week intervals from 5 mg to 40 mg daily or equivalent within the first six weeks." Comparator: placebo Concomitant medication: "All patients continued concomitant treatment with diuretics, nitrates, digitalis glycosides, calcium channel blockers, and beta‐blockers as appropriate without change of dose except for diuretics. Therapy with ACE inhibitors for heart failure was withdrawn at least two weeks prior to the run‐in period." |
|
| Outcomes |
Planned: we are not aware of a published protocol or pre‐registered clinical trial registry entry Reported: 6‐minutes walking distance, hypotension, worsening heart failure, changes of electrolytes, adverse events, quality of life, deaths, heart failure hospitalisation, hyperkalaemia |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomised, but no further detail |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | double‐blind, matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | withdrawals due to worsening heart failure reported |
| Selective reporting (reporting bias) | Unclear risk | unable to assess |
| Other bias | Low risk | "This study was supported by the grants from Parke Davis & Co. Ltd., UK." |
quotes are from the primary reference unless otherwise stated
* mmol/L converted to mg/dL using online converter
ACEI: angiotensin converting enzyme inhibitor
ARB: angiotensin receptor blocker
BMI: body mass index
CVD: cardiovascular disease
EF: ejection fraction
IQR: interquartile range
ITT: intention‐to‐treat
LV: left ventricular
LVEF: left ventricular ejection fraction
MRA: mineralocorticoid receptor antagonist
N: number of people
NCT: clinicaltrials.gov identifier
QoL: quality of life
RCT: randomised controlled trial
SD: standard deviation
TNF‐a: tumour necrosis factor‐alpha
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12610001087044 | Trial registry entry suggested two parts of a trial of which only the second was of interest to this review. Contact with trialists confirmed that the part of interest was registered and reported on separately (ACTRN: 12614000088640, STRUCTURE study). |
| Adgey 1992 | Population does not meet protocol |
| Ammon 2001 | Wrong study design |
| Andersson 1996 | Population does not meet protocol |
| Andersson 1999 | Population does not meet protocol |
| Andersson 2000 | Population does not meet protocol |
| Anonymous 1996 | Population does not meet protocol |
| Anonymous 1999 | Wrong intervention |
| Anonymous 2000 | Population does not meet protocol |
| Anonymous 2001 | Wrong study design |
| Anonymous 2002 | Wrong study design |
| Anonymous 2003 | Wrong comparator |
| Anonymous 2003a | Wrong study design |
| Anonymous 2003b | Wrong study design |
| Anonymous 2003c | Population does not meet protocol |
| Anonymous 2005 | Population does not meet protocol |
| Anonymous 2008 | Wrong study design |
| Anonymous 2008a | Wrong study design |
| Anonymous 2013 | Wrong study design |
| ANZ HF carvedilol | Subgroup of participants of interest (LVEF = 40‐44%). We did not receive a response from the trialists to our enquiry for details on the subgroup of interest. |
| Aoyama 2007 | Wrong study design |
| Apostolovic 2013 | Wrong comparator |
| Apostolovic 2014a | Wrong comparator |
| Apostolovic 2014b | Wrong comparator |
| Arena 2007 | Wrong study design |
| Armstrong 1999 | Wrong study design |
| Aronow 1991 | Wrong study design |
| Aronow 2001 | Wrong study design |
| Axelsson 2015 | Population does not meet protocol |
| Balaban 2007 | Turkish paper. Translated methods and data extraction. Unclear if participants had heart failure. We did not receive a response from the investigator when we asked for clarification. |
| Bao 2005 | Population does not meet protocol |
| Barr 1995 | Population does not meet protocol |
| Barrios 2009 | Population does not meet protocol |
| Barry 2003 | Wrong study design |
| Bartels 1999 | Population does not meet protocol |
| Baruch 1999 | Ineligible participants. Emailed trialists to clarify inclusion criteria. Response received: "Our study was confined to individuals with a reduced ejection fraction and the data therefore would not be applicable to your quest." |
| Baruch 2004 | Population does not meet protocol |
| Bauersachs 2004 | Wrong study design |
| Baumhakel 2008 | Wrong study design |
| Bellenger 2004 | Population does not meet protocol |
| Berry 2001 | Wrong study design |
| Bettencourt 1999 | Wrong study design |
| Beygui 2016 | Population does not meet protocol |
| Blagodar 2003 | Population does not meet protocol |
| Blomer 1990 | Wrong study design |
| Borghi 2011 | Wrong comparator |
| Borgi 1990 | Population does not meet protocol |
| Borlaug 2014 | Wrong study design |
| Bornkessel 1992 | Population does not meet protocol |
| Bounhoure 1991 | EF not reported. Could not identify current contact details. |
| Braunwald 2004 | Population does not meet protocol |
| Brilla 1989 | Wrong study design |
| Brilla 1991 | EF unclear, unclear whether allocation was random, unable to identify current contact details. |
| Bristow 1994 | Population does not meet protocol |
| Bristow 1996 | Population does not meet protocol |
| Bussmann 1987 | EF unclear. We did not receive a response to our enquiry for details. |
| Butler 2017 | Population does not meet protocol |
| Cafaro 2010 | Wrong study design |
| Cardoso 1999 | Wrong study design |
| Castagno 2010 | Population does not meet protocol |
| ChiCTR1800016350 | Wrong comparator |
| Choi 2001 | Population does not meet protocol |
| Cicoira 2002 | subgroup of participants of interest; we did not receive a response from the trialist to our enquiry for details. |
| Cleland 1984 | unclear EF. contacted trialists. no response |
| Cleland 1999 | Population does not meet protocol |
| Cleland 2001 | Population does not meet protocol |
| Cleland 2003 | Population does not meet protocol |
| Cleland 2004 | Wrong study design |
| Cleland 2006 | Wrong study design |
| Cleland 2007 | Wrong study design |
| Cleland 2010 | Wrong study design |
| Cleland 2011 | Wrong study design |
| Cleland 2013 | Wrong study design |
| Cohen‐Solal 2005 | Population does not meet protocol |
| Cohn 1993 | Wrong study design |
| Cohn 1996a | Wrong study design |
| Cohn 1996b | Wrong study design |
| Cohn 2007 | Wrong study design |
| Coletta 2008 | Wrong study design |
| Coletta 2009 | Wrong study design |
| Comin‐Colet 2002 | Wrong study design |
| CONSENSUS | EF unclear. We did not receive a response from the trialists to our enquiry for details. |
| CONSENSUS II | Mean EF suggests a subgroup of eligible participants. We did not receive a response from the trialists to our enquiry. |
| Conti 2005 | Wrong study design |
| Corder 1993 | EF unclear. Unable to identify current contact details. |
| Crouse 2011 | Wrong study design |
| Dahlstrom 2007 | Wrong study design |
| Davie 2001 | Wrong study design |
| DeBock 1994 | EF unclear. Unable to find current contact details for trialists. |
| Dekleva 2012 | Wrong comparator |
| De Melo 2011 | Wrong comparator |
| Demers 2001 | Population does not meet protocol |
| Desai 2013 | Wrong study design |
| Deswal 2010 | EF unclear. We did not receive a response from trialists to our query on the clarification of inclusion criteria. |
| de Teresa 1995 | Wrong study design |
| Ding 2008 | Wrong comparator |
| Ditiatkov 1999 | Wrong study design |
| Donal 2008 | Wrong study design |
| Dragana 2015 | Wrong comparator |
| Edner 2013 | Wrong study design |
| Eichhorn 1994 | subgroup of participants of interest; did not receive a response from trialists to our enquiry. |
| Eichhorn 2003 | Population does not meet protocol |
| Er 2005 | Wrong study design |
| Ertl 1999 | Wrong study design |
| EudraCT 2004‐004169‐13 | Trial registry record states completed but no contact details given an no published results identifiable. Sponsor: South Manchester University Hospital NHS Trust. Emailed sponsor to ask whether results are available. Unclear whether http://www.isrctn.com/ISRCTN77645264 is the same trial. Tried to contact investigator but email was undeliverable. |
| Fauchier 2009 | Wrong study design |
| Feola 2003 | Wrong study design |
| Flammer 2013 | Population does not meet protocol |
| Flather 2016 | Wrong study design |
| Flesch 2006 | Wrong study design |
| Follath 1996 | Wrong study design |
| Fonarow 2004 | Wrong study design |
| Fonarow 2007 | Population does not meet protocol |
| Fowler 1999 | Wrong study design |
| Franciosa 2002 | Wrong intervention |
| Fukunami 1991 | Wrong study design |
| Galinier 2007 | Wrong study design |
| Galloe 2006 | Potential eligible subgroup. Did not receive a response from trialists to our enquiry for outcome data for subgroup. |
| Gao 2010 | Could not obtain details on LVEF as inclusion criteria. No available subgroup data for population of interest. |
| Gardner 2003 | Wrong study design |
| Gardner 2004 | Wrong study design |
| Ghali 2002 | Population does not meet protocol |
| Gheorghiade 2009 | Population does not meet protocol |
| Good 1994 | Population does not meet protocol |
| Goodfield 1999 | Population does not meet protocol |
| Gottlieb 1996 | Population does not meet protocol |
| Grajek 2008 | Review |
| Greenberg 1996 | Population does not meet protocol |
| Gremmler 2000 | EF unclear. Unable to identify current contact details. |
| Groenning 2000 | Population does not meet protocol |
| Groenning 2001 | Wrong study design |
| Groenning 2002 | Population does not meet protocol |
| Gruner 2007 | Population does not meet protocol |
| Guazzi 1998 | Population does not meet protocol |
| Guazzi 1999 | Population does not meet protocol |
| Gøtzsche 1992 | subgroup with HF and LVEF >40%. We did not receive a response from trialists to our enquiry. |
| Hanping 1997 | Wrong study design |
| Hara 2000 | Wrong comparator |
| Hauf 1993 | Wrong study design |
| Hole 2004 | Population does not meet protocol |
| Holland 2010 | Limited information in conference abstract. Response to our enquiry for further details received: "the data you've requested was not collected on this group of patients beyond what has been published in that abstract". As we could not confirm whether the participants meet our inclusion criteria, this study was excluded. |
| Hong 2003 | Wrong intervention |
| Hoppe 2007 | Wrong study design |
| Hori 2004 | Population does not meet protocol |
| Hung 2010 | Wrong study design |
| IRIS‐HF | Response from trialists received when asked for outcome data for subgroup of interest: no data specifically for participants in subgroup of interest (LVEF 40‐45%) provided. Confirmed that QoL, mortality and HF hospitalisation were not formal endpoints. Hyperkalaemia was not shown by any participants. |
| Ito 2012 | Wrong study design |
| Jamieson 1991 | Wrong study design |
| Jellis 2014 | HF/EF unclear. No response to our enquiry for details. |
| Jessup 2003 | Wrong study design |
| Jong 2010 | Wrong study design |
| JPRN‐jRCTs051180137 | Wrong comparator |
| JPRN‐UMIN000006415 | Population does not meet protocol |
| Kanoupakis 2008 | Population does not meet protocol |
| Kapel'ko 2011 | Wrong study design |
| Kasama 2007 | Wrong comparator |
| Keren 1992 | Population does not meet protocol |
| Keren 1994 | Population does not meet protocol |
| Khalid 2013 | Wrong study design |
| Khand 2015 | Population does not meet protocol |
| Kikuchi 2016 | Population does not meet protocol |
| Kimura 2011 | Population does not meet protocol |
| Kinugawa 2007 | Wrong study design |
| Kjekshus 2007 | Wrong study design |
| Kjøller‐Hansen 1998 | Population does not meet protocol |
| Kleber 1991a | No participants with heart failure with preserved ejection fraction (confirmed by trialist via email on 20 November 2017). |
| Kleber 1991b | Heart failure was not an inclusion criteria (confirmed by trialist via email on 15 November 2017). |
| Kongstad‐Rasmussen 1998 | EF unclear ("ejection fraction measurement was not part of the protocol") |
| Krum 1996 | Population does not meet protocol |
| Krum 2015 | Population does not meet protocol |
| Kulbertus 2003 | wrong study design |
| Kuznar 2003 | Population does not meet protocol |
| Lang 1995 | cross‐over trial |
| Larsen 1996 | EF unclear. Contacted trialists. Response: data are no longer available. |
| Lechat 1993 | LVEF unclear, otherwise eligible. Emailed investigator but did not receive a response. |
| Leonetti 1999 | Population does not meet protocol |
| Lewis 1988 | EF unclear. Unable to find current contact. |
| Li 2005 | Population does not meet protocol |
| Liebson 2004 | Population does not meet protocol |
| Lindenfeld 2001 | Population does not meet protocol |
| Lindsay 1999 | Wrong study design |
| Liu 2006 | Chinese language paper. Eligibility criteria for trial unclear regarding LVEF. Emailed investigator ‐ no response. |
| Liu 2014 | Population does not meet protocol |
| Logeart 2006 | Wrong study design |
| Lopez 2000 | Wrong study design |
| Lou 2009 | Wrong study design |
| Luo 2007 | Wrong study design |
| Ma 2005 | Population does not meet protocol |
| MacGregor 2009 | Population does not meet protocol |
| Mak 2008 | EF unclear. unable to find current contact details for trialist. |
| Malnick 2007 | Population does not meet protocol |
| Maron 2018 | Heart failure not an inclusion criteria. |
| Mazayev 1998 | LVEF unclear, otherwise eligible. Unable to find current contact details for investigators. |
| McAnulty 2004 | Wrong comparator |
| McCullough 2012 | Population does not meet protocol |
| McIlwain 1997 | Wrong study design |
| McKelvie 2012 | Wrong study design |
| McMurray 2000 | Wrong study design |
| McMurray 2004 | Wrong study design |
| Melo 2011 | Wrong comparator |
| Melo 2012 | Wrong comparator |
| Messias 2016 | Wrong study design |
| Meuleman 2007 | Population does not meet protocol |
| Mitrovic 2005 | Wrong study design |
| Mochizuki 2004 | retraction |
| Morales 2011 | Population does not meet protocol |
| Murdoch 2001 | Population does not meet protocol |
| NCT00293150 | terminated due to lack of eligible participants |
| NCT00523757 | Trial did not take place as planned (as per information from trialists: "We abandoned this study as we could not adequately recruit. No results to present.") |
| NCT01691118 | Completed but no publication with results identified. Emailed trialists to ask for clarification on comparator (placebo or conventional antihypertensive treatment). No response. |
| NCT01944384 | Completed but no results found. No contact details for investigator provided. |
| NCT03882710 | Completed but no results found. No contact details for investigator provided. |
| Nodari 2003 | Wrong comparator |
| Nunez 2016 | Wrong study design |
| O'Callaghan 1995 | Wrong study design |
| O'Keefe 2008 | Population does not meet protocol |
| O'Keeffe 2015 | Wrong comparator |
| O'Meara 2012 | Population does not meet protocol |
| Ostergren 2004 | Wrong study design |
| Palazzuoli 2005 | Population does not meet protocol |
| Paolisso 1992 | Wrong study design |
| Paraskevaidis 2006 | Population does not meet protocol |
| Park 2016 | Wrong comparator |
| Patten 1997 | Population does not meet protocol |
| Pennell 2000 | Population does not meet protocol |
| Pierard 2002 | Population does not meet protocol |
| Pina 2004 | Wrong study design |
| Pitt 2005 | Population does not meet protocol |
| Pitt 2008 | Population does not meet protocol |
| Pitt 2011 | Wrong intervention |
| Pourdjabbar 2015 | Wrong study design |
| Premkumar 2016 | Population does not meet protocol |
| Quaife 1998 | Population does not meet protocol |
| Ramaswamy 2003 | Wrong study design |
| Remme 2001 | Population does not meet protocol |
| Remme 2004 | Population does not meet protocol |
| Remme 2005 | Population does not meet protocol |
| Rimatori 1990 | LVEF unclear, otherwise eligible. Unable to find current contact details for investigators. |
| Roongsritong 2005 | Population does not meet protocol |
| Rosa 2011 | Wrong intervention |
| Rosenkranz 2003 | Wrong study design |
| Rossignol 2011 | Population does not meet protocol |
| Sakai 2011 | Wrong intervention |
| Sanderson 1998 | Subgroup of interest LVEF 40‐45%. Investigator responded to our enquiry for data: "the mean EF was only 26.9% and I doubt any of the patients were in the group of EF 40% to <45%. [...] I do not have the original data now." |
| Sanghera 2011 | Wrong study design |
| Santulli 2015 | Wrong study design |
| Sardu 1991 | LVEF not specified as an inclusion criteria, mean LVEF at baseline 35.4, 4.7%. Could not find current contact details for investigator. |
| Schindler 2008 | Population does not meet protocol |
| Schwab 2009 | Wrong study design |
| Segovia 2008 | Wrong study design |
| Shimamoto 2007 | Population does not meet protocol |
| Sidorenko 2008 | Population does not meet protocol |
| Silva 2014 | Population does not meet protocol |
| Smith 2012 | Wrong study design |
| Spoto 2002 | Wrong study design |
| Stecker 2005 | Population does not meet protocol |
| Stiefelhagen 2006 | Wrong study design |
| Struthers 2004 | Wrong study design |
| Swedberg 1996 | Wrong study design |
| Swedberg 1999 | Wrong study design |
| Szajnbok 1993 | Portuguese paper. Reported outcomes not of interest but subgroup of participants eligible. Emailed investigators to ask about measured outcomes for subgroup of interest. Response: data not available. |
| Szymanski 2009 | Wrong study design |
| Taheri 2009 | subgroup of interest LVEF 40‐45%. Contacted investigators. No response. |
| Takekoshi 2004 | Wrong study design |
| Tala 2011a | Population does not meet protocol |
| Tala 2011b | Population does not meet protocol |
| Tan 2013 | Wrong study design |
| Tatsumi 2006 | Wrong study design |
| Taylor 2003 | Wrong intervention |
| Teerlink 2003 | Population does not meet protocol |
| Tereshchenko 2005 | Wrong comparator |
| Thornton 2004 | Wrong study design |
| Thune 2008 | Population does not meet protocol |
| Tinoco 2004 | Wrong study design |
| Tsutamoto 2000 | Wrong study design |
| Tsutamoto 2001 | Subgroup of interest (LVEF 40‐45%). Emailed investigators. No response. |
| Tsutamoto 2005 | Wrong study design |
| Tumasyan 2010 | Wrong comparator |
| Tumasyan 2018 | Wrong comparator |
| Umemoto 2003 | Wrong study design |
| Uusimaa 2001 | Wrong comparator |
| Van den Berg 1993 | Wrong study design |
| Van den Berg 1995 | LVEF unclear; response to our enquiry for details: cannot provide data |
| Vasiuk 2001 | Wrong study design |
| Vincent 2012 | Population does not meet protocol |
| Vizir 2000 | Population does not meet protocol |
| Vizzardi 2010 | Population does not meet protocol |
| Vizzardi 2012 | LVEF unclear. Emailed investigators. No response. |
| Vizzardi 2015a | Wrong study design |
| Vizzardi 2015b | Population does not meet protocol |
| Volpe 1992 | Subgroup of interest LVEF 40‐45%. We received a response to our enquiry for more details on the subgroup of interest confirming that the study was conducted in "patients with reduced EF". |
| Volpe 2010 | Population does not meet protocol |
| Voors 2008 | Wrong study design |
| Waagstein 2003 | Population does not meet protocol |
| Waldo 1995 | Population does not meet protocol |
| Waldo 1996 | Population does not meet protocol |
| Warner 1999 | Population does not meet protocol |
| Weinberg 2001 | Wrong study design |
| Weintraub 2005 | Population does not meet protocol |
| Weir 2011 | Population does not meet protocol |
| Wong 2002 | Population does not meet protocol |
| Wong 2004 | Population does not meet protocol |
| Woodley 1991 | Population does not meet protocol |
| Wright 2014 | Population does not meet protocol |
| Wu 2002 | LVEF unclear, otherwise eligible. Unable to find current contact details for investigators. |
| Xu 2007 | Population does not meet protocol |
| Yamamoto 2005 | Wrong study design |
| Yan 2012 | LVEF unclear, otherwise eligible. Unable to find current contact details for investigators. |
| Yoshihiro 2011 | Wrong intervention |
| Young 2004 | Population does not meet protocol |
| Zeng 2006 | Population does not meet protocol |
| Zheng 2009 | Was previously a study awaiting classification. Data extraction received from translator. Wrong patient population. |
Characteristics of studies awaiting classification [ordered by study ID]
Anonymous 2003d.
| Methods | No abstract |
| Participants | No abstract |
| Interventions | Eplerenone |
| Outcomes | No abstract |
| Notes | Could not yet obtain full text |
Botoni 2010.
| Methods | Individual, two‐arm, RCT |
| Participants | 42 |
| Interventions | Placebo versus carvedilol |
| Outcomes | QoL |
| Notes | Unclear EF/HF status |
Dielievska 2015.
| Methods | Individual, two‐arm, RCT |
| Participants | 80 participants with EHT and COPD of ll‐lll grade of bronchial obstruction (GOLD 2‐3) with chronic heart failure of the II and III NYHA classes and evidence of diastolic dysfunction |
| Interventions | Spironolactone versus standard therapy, 3 months |
| Outcomes | Left ventricular diastolic function, impaired relaxation of left ventricle, adverse events |
| Notes | Have not yet obtained full text |
EUCTR2005‐001306‐87.
| Methods | Parallel RCT, prematurely ended |
| Participants | 1500 planned; Inclusion criteria: Age ≥ 18 years; both sexes ‐ Stable, symptomatic NYHA II‐IV CHF with LVEF 40% had to be hospitalized for cardiovascular events during the past 12 months; ‐ Written informed consent Exclusion criteria: Prior treatment with Angiotensin‐Receptor Blocker (ARBs) within two weeks from visit 1; ‐ Severe or malignant hypertension (systolic blood pressure (SBP)/diastolic blood pressure (DBP)>180/110 mmHg); ‐ Symptomatic hypotension; ‐ Angina pectoris or acute myocardial infarction within one month from visit 1; ‐ Stroke or transient ischemic attack (TIA) within one month from visit 1; ‐ Percutaneous transluminal coronary angioplasty (PTCA) or coronary artery by‐pass graft (CABG) within one month from visit 1; ‐ Haemodynamically relevant arrhythmias or cardiac valvular defect; ‐ Implant of pacemakers, cardiac resynchronization therapy (CRT) or cardioverters (ICD) within 6 months prior the randomization; ‐ Constrictive pericarditis or active miocarditis; Likelihood of cardiac surgical intervention (of any type) during the overall treatment period; ‐ Poorly controlled diabetes mellitus, untreated thyroid dysfunction, renal artery stenosis, angio‐oedema of any aetiology, significant liver or renal impairment, anaemia of any aetiology or any other clinically relevant haematological disease; any disease with malabsorption ‐ Pregnant or lactating females or females at risk of pregnancy ‐ any non‐cardiac (e.g. cancer) disease likely to shorten life expectancy ‐ Chronic alcohol or drug/substance abuse ‐ Known allergy, sensitivity or intolerance to study drugs ‐ Patients unlikely to comply with the protocol ‐ Participation in another trial in the month preceding study entry |
| Interventions | Candesartan versus control 48 weeks |
| Outcomes | changes of BNP; change from baseline of aldosterone, PTX3, CRP, NYHA class, LVEF, LVIDD, E wave peak velocity/A wave peak velocity (E/A) ratio, Deceleration Time of E wave (E‐DT) time, atrial dimensions, BP, HR; persistence and discontinuation rate of active treatment; quality of life (KCCQ); adverse events |
| Notes | Need to obtain subgroup data. |
EUCTR2005‐002109‐22.
| Methods | Parallel RCT |
| Participants | 99 participants with symptomatic chronic heart failure proven by echocardiography or Tc‐Szintigraphy ( EF <45%). |
| Interventions | Telmisartan versus candesartan versus additional treatment |
| Outcomes | Insulin sensitivity; correaltion between natriuretic peptides ( as a surrogate for severity of heart failure) and impaired glucosemetabolism |
| Notes | Subgroup of interest. Trial status is completed (30 September 2008) but unable to find results. No contact details for investigator. |
Metra 1999.
| Methods | No abstract |
| Participants | No abstract |
| Interventions | No abstract |
| Outcomes | No abstract |
| Notes | Could not yet retrieve full text |
PER‐010‐15.
| Methods | Two‐arm, parallel RCT |
| Participants | 4300 overall, 30 from Peru with symptoms of heart failure and LVEF ≥45% |
| Interventions | LCZ696 versus valsartan |
| Outcomes | CV mortality, total heart failure hospitalisations, total strokes, and total myocardial infarctions, NYHA class |
| Notes | May be a trial record for an an included study ‐ checking with investigator Juan Jorge Alfredo Lema Osores: juan.lema@upch.pe. |
Przewlocka‐Kosmala 2017.
| Methods | RCT |
| Participants | 105 HFpEF |
| Interventions | spironolactone 25mg versus placebo for 6 months |
| Outcomes | UT, E/e' |
| Notes | Contact investigators to ask for full results paper. |
Rapezzi 1999.
| Methods | No abstract |
| Participants | No abstract |
| Interventions | No abstract |
| Outcomes | No abstract |
| Notes | Could not yet retrieve full text |
EF: ejection fraction
EHT: essential hypertension
HF: heart failure
RCT: randomised controlled trial
LVEF: left ventricular ejection fraction
GRK2: G protein‐coupled receptor kinase 2
mRNA: messenger RNA
COPD: chronic obstructive pulmonary disease
NYHA: New York Heart Association functional Classification of heart failure
QoL: quality of life
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐IPR‐16009507.
| Study name | Aldosterone antagonist delays the progression of diastolic dysfunction in patients with hypertension and myocardial hypertrophy: a randomised controlled clinical trial |
| Methods |
Study design: parallel RCT Anticipated completion date: 31 December 2018 |
| Participants |
Estimated enrolment: 466 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Spironolactone versus placebo |
| Outcomes | Left ventricular diastolic function (TDI: E/E’); Clinical composite end point (stage C or D of heart failure with preserved heart failure, hospitalization due to heart failure and cardiac death); LVMI; LVEF |
| Starting date | October 2016 |
| Contact information | Changqian Wang: forrestgu@sina.com |
| Notes |
ChiCTR‐TRC‐09000631.
| Study name | Treatment of diastolic heart failure: the role of blockade of the renin‐angiotensin system. A comparison of diuretics with an angiotensin converting enzyme inhibitor, angiotensin receptor blockade or diuretics alone |
| Methods |
Study design: Anticipated completion date: |
| Participants |
Estimated enrolment: 450 Inclusion criteria: "1. Signed informed consent; 2. Age >18 yrs; 3. history of heart failure for 2 months prio to screening; 4. NYHA Functional Class II‐IV; 5. LV EF >45% by ECHO or a radionucleotide technique; 6. Therapy with diuretics with stable dose >14 days prior to screening" Exclusion criteria: "1. NHYA class I; 2. Inability to answer the QOL questionnaire; 3. Myocardial infarction within 3 months; 4. unstable angina within 1 month; 5. Significant cardiac valvular heart disease; 6. Hypotension SBP <90 mmHg; 7. uncontrolled hypertension(DBP>105 ors SBP> 200 mmHg); 8. uncontrolled serious cardiac arrhythmias associated with a ventricular rate >100 bpm at study entry; 9. concurrent therapy with CCB, Betablocker, ACEI, AII or positive inotropic agents other than digoxin for control of AF" |
| Interventions | Ramipril + Diuretics vs. Irbesartan +Diuretics vs. Diuretics alone (comparison diuretics + irbesartan versus diuretics of interest to this review) |
| Outcomes | Hospital admissions for heart failure or mortality; quality of life assessed by the Minnesota Quality of life Questionnaire; exercise duration assessed by 6 min corridor walk test; side‐effects, effect on levels of natriuretic peptides, effect on doppler‐echocardiographic derived measurements of left ventricular diastolic function |
| Starting date | 13 July 1997 |
| Contact information | Skiva Chan: skivachan@cuhk.edu.hk |
| Notes | Recruitment status: completed |
CTRI/2010/091/000438.
| Study name | Evaluation of efficacy and safety of metoprolol in patients having heart failure with normal ejection fraction: a randomised, double‐blind, placebo‐controlled trial |
| Methods |
Study design: parallel RCT Anticipated completion date: November 2011 |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions | Metoprolol CR versus placebo 12 weeks |
| Outcomes | proportion of patients showing improvement of ≥ 1 in NYHA class; proportion of patients exhibiting any alteration in NYHA heart failure class from baseline; alteration in exercise capacity using exercise stress testing (treadmill test: Bruce protocol); change in tissue doppler indices of diastolic dysfunction (ratio of mitral inflow velocity to annular relaxation velocity i.e. E/E? ratio); change in left ventricular wall thickness, left ventricular mass and left atrial volume on echocardiography; change in serum NT‐proBNP levels from baseline; change in serum carboxy‐terminal propeptide of procollagen type I (PICP) from baseline; alteration in quality of life using SF‐36 questionnaire; incidence of adverse events |
| Starting date | 15 November 2009 |
| Contact information | Samir Malhotra: samirmalhotra345@yahoo.com |
| Notes |
CTRI/2017/09/009732.
| Study name | A 24‐week, randomized, double‐blind, multi‐center, parallel group, active controlled study to evaluate the effect of LCZ696 on NT‐proBNP, symptoms, exercise function and safety compared to individualized medical management of comorbidities in patients with heart failure and preserved ejection fraction |
| Methods |
Study design: parallel RCT Anticipated completion date: February 2020 |
| Participants |
Estimated enrolment: 2200 Inclusion criteria:
Exclusion criteria:
|
| Interventions | LCZ696 versus enalapril versus valsartan (of interest to this review is comparison LCZ696 versus valsartan) 24 weeks |
| Outcomes | NT‐proBNP; quality of life; |
| Starting date | 15 June 2017 |
| Contact information | Murugananthan K: murugananthan.k@novartis.com |
| Notes |
EUCTR2016‐001254‐17.
| Study name | A multicenter, randomized, double‐blind, active‐controlled study to evaluate the effects of LCZ696 compared to valsartan on cognitive function in patients with chronic heart failure and preserved ejection fraction (PERSPECTIVE) |
| Methods |
Study design: parallel RCT Anticipated completion date: not reported |
| Participants |
Estimated enrolment: 520 Inclusion criteria:
Exclusion criteria:
|
| Interventions | LCZ696 versus valsartan 3 years |
| Outcomes | CogState Global Cognitive Composite Score (GCCS); cortical composite SUVr (standardized uptake value ratio); individual cognitive domains (memory, executive function, and attention) as assessed by the individual components of the cognitive assessment battery; summary score of the instrumental activities of daily living (IADL) as assessed with Functional Activity Questionnaire (FAQ) |
| Starting date | not reported |
| Contact information | Novartis Pharmaceuticals UK Limited: medinfo.uk@novartis.com |
| Notes |
EUCTR2016‐003410‐28.
| Study name | A 24‐week, randomized, double‐blind, multi‐center, parallel group, active controlled study to evaluate the effect of LCZ696 on NT‐proBNP, exercise capacity, symptoms and safety compared to individualized medical management of comorbidities in patients with heart failure and preserved ejection fraction |
| Methods |
Study design: parallel RCT Anticipated completion date: not reported |
| Participants |
Estimated enrolment: Inclusion criteria:
Exclusion criteria:
|
| Interventions | LCZ696 versus valsartan versus enalapril |
| Outcomes | NTproBNP; six‐minute walk distance; quality of life; NYHA functional class; |
| Starting date | not reported |
| Contact information | Novartis Pharmaceuticals UK Limited: medinfo.uk@novartis.com |
| Notes |
EUCTR2017‐000697‐11.
| Study name | Spironolactone In the treatment of heart failure: a double‐blind, randomized, placebo‐controlled, parallel group, interventional phase III study to evaluate the efficacy and safety of spironolactone compared to placebo on the composite endpoint of recurrent heart failure hospitalizations and cardiovascular death in patients with heart failure with mid‐ range or preserved ejection fraction (SPIRIT‐HF) |
| Methods |
Study design: parallel RCT Anticipated completion date: not reported |
| Participants |
Estimated enrolment: 1300 Inclusion criteria: "1. Written informed consent 2. Male or female, age ≥ 50 years 3. Current symptoms of Heart Failure (NYHA ≥ II) on diuretic treatment (any) during VR 4. Symptom(s) of HF ≥ 30 days prior to VR 5. Left ventricular ejection fraction ≥ 40 % at screening measured or MRI and evidence of structural/ functional abnormalities (at least one of the following criteria): o LAVI > 34 ml/m2 o E/e’mean ≥ 13 o Mean e’ (septal and lateral) < 9 cm/s 6. Patients with at least 1 of the following: HF hospitalization (defined as HF listed as the major reason for hospitalization) within 12 months prior to Visit of Screening and NT‐proBNP >200 pg/ml for patients in SR or >600 pg/ml for patients in AF on screening visit ECG (only if NT‐proBNP is not available: BNP > 50/ 160 pg/ml), or b) NT‐proBNP >300 pg/ml for patients in SR or >900 pg/ml for patients in AF on the screening visit ECG (only if NT‐proBNP is not available: BNP > 80/ 250 pg/ml); (for entering the study a historical measurement of natriuretic peptides within the last 6 months is acceptable); 7. Controlled systolic BP: defined as a target systolic BP < 140 mm Hg. Subjects with BP up to and including 160 mm Hg are eligible for enrollment if on 3 or more medications to control BP (Patients with uncontrolled BP should be considered for Re‐Screening after optimization of antihypertensive therapy has been established) 9. Serum potassium < 5.0 mmol/L prior to randomization" Exclusion criteria: "1.hyperkalemia (potassium level ≥ 5.5 mmol/L) within the past two weeks before VR 2.Hyponatriaemia (Na < 135 mmol/L) prior to randomization 3.Severe renal dysfunction, defined as an estimated glomerular filtration rate of less than 30 mL/min/1.73m2) as calculated by the Modification in Diet in Renal Disease (MDRD) formula at VScr/VR or serum creatinine level ≥ 1,8 mg/dl (> 160 μmol/ml) 4. History of anuria or acute RF (defined by RIFLE crit. for AKI;3) within past 2 wk before VR 5. Acute CS (including MI) and elective PCI within 30 days prior to VR. 6.Cardiac surgery, other maj CV surgery, or urgent percutaneous PCI within the 3 months prior to VR 7 Current acute decompens. HF requiring augmented therapy with i.v. diuretics, i.v. vasodilators and/or i.v. inotropic drugs. Patients are eligible after initial stabilization. 8.Probable alternative diagnoses that in the opinion of the investigator could account for the patient’s HF symptoms (i.e., dyspnea, fatigue) such as significant pulmonary disease (including primary pulmonary HTN), anemia or obesity. Specifically, patients with the following are not randomized: •Severe pulmonary disease including COPD or severe asthma bronchiale as requiring continuous corticotherapy or OLD, •anemia (hemoglobin < 10 g/d ), or •body mass index (BMI) > 40 kg/m2 9. Evidence of right sided HF in the absence of left‐sided structural heart disease. 10.Specific etiologies such as infiltrative, genetic hypertrophic cardiomyopathy, pericardial constriction, sarcoidosis, amyloidosis and any other storage diseases. 11.Clinically significant congenital heart disease underlying HF. 12. Life‐threatening or uncontrolled dysrhythmia, including symptomatic or sustained ventricular tachycardia and uncontrolled persistent or permanent AF or flutter (with a heart rate > 100 beats per minute (bpm), RACE II) during VR. If AF with HR > 100/min, the patient may be rescreened after treatment for rate control. 13. Presence of significant (i.e., more than moderate) valvular heart disease expected to lead to surgery during the trial in the investigators opinion. 14. Stroke, transient ischemic attack, carotid surgery or carotid angioplasty within the 3 months prior to VR. 15. Coronary or carotid artery disease or valvular heart disease likely to require surgical or percutaneous intervention within the 6 months after VR in the investigators opinion. 16. Patients with prior major organ transplant or intent to transplant (on transplant list) or current ventricular assist device (VAD) therapy. 17. Evidence of hepatic disease as determined by any one of the following: SGOT (AST) or SGPT (ALT) values exceeding 3x the upper limit of normal (ULN), bilirubin >1.5 mg/dl at VR. 18. Presence of bilateral renal artery stenosis. 19. Known intolerance or history of hypersensitivity to the active substance (Spironolactone) or to any of the excipients of the IMP or placebo 20.Present use of any aldosterone antagonist, potassium supplements or potassium sparing diuretics at the time of enrollment. (Consider stopping these potassium sparing drugs if clinically possible and upon discussion with the patient) 21. Required treatment with prohibited Co‐medications according to the summary of product characteristics with the exception of ACE inhibitors or angiotensin receptor blockers. Concomitant use not recommended: • other potassium‐sparing diuretics (alone or combined) (amiloride, potassium canrenoate, triamterene): risk of potentially fatal hyperkalemia, particularly in renal insufficiency (additive hyperkalemic effects). • potassium chloride • cyclosporine, tacrolimus • lithium: Increased plasma lithium with signs of overdose, as with a salt‐free diet (decreased urinary lithium excretion). However, if the use of diuretics is necessary, careful monitoring of plasma lithium and dose adjustment are required. 22. Use of other investigational drugs at the time of enrollment, or within 30 days or 5 half‐lives before enrollment, whichever is longer. 23. Any condition that, in the opinion of the investigator, may prevent the subject from adhering to the study protocol. 24. History or presence of any other disease (i.e. including malignancies) with a life expectancy of < 1 years. 25. History of non‐compliance to medical regimens and patients who are considered potentially unreliable. 26. Subjects who are legally detained in an official institution. 27. Subjects who may be dependent on the sponsor, the investigator or the trial sites, have to be excluded from the trial. 28, Pregnant or nursing (lactating) women, where pregnancy is defined as the state of a female after conception and until the termination of gestation, confirmed by a positive human chorionic gonadotropin (hCG) laboratory test. 29. WOCBP, defined as all women physiologically capable of becoming pregnant, unless they are using highly effective methods of contraception during dosing and for 7 days off study drug." |
| Interventions | Spironolactone versus placebo 60 months |
| Outcomes | composite of death from cardiovascular cause or recurrent heart failure hospitalizations; recurrent rate of heart failure hospitalizations; rate of recurrent non‐fatal hospitalizations from cardiovascular (CV) cause (i.e. hospitalization for non‐fatal MI, non‐fatal stroke, or the management of heart failure); recurrent rate of hospitalizations from any cause; rate of death from cardiovascular (CV) cause; rate of death from any cause; total non‐fatal myocardial infarctions (MIs), and total non‐fatal strokes; left ventricular hypertrophy (measured by local ECG); clinical summary score for HF symptoms, physical limitations and mental dimensions of quality of life (as assessed by the KCCQ, SF‐36); NYHA functional classification; new onset atrial fibrillation (NOAF) in patients with no history of AF and without AF on ECG during VR; CV deaths and total worsening HF events |
| Starting date | not reported |
| Contact information | Burkert Pieske: burkert.pieske@charite.de |
| Notes |
NCT02901184.
| Study name | Spironolactone Initiation Registry Randomized Interventional Trial in Heart Failure With Preserved Ejection Fraction, SPIRRIT‐HFPEF |
| Methods |
Study design: parallel, open‐label, RCT Anticipated completion date: June 2022 |
| Participants |
Estimated enrolment: 3500 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Spironolactone versus standard care |
| Outcomes | Primary: Time to death from any cause [Time Frame: Collected at data base lock, five (5) years after study start] Secondary: Time to first hospitalization for heart failure [Time Frame: Collected at data base lock, five (5) years after study start ] |
| Starting date | December 2017 |
| Contact information | Inger Ekman (inger.ekman@ucr.uu.se) |
| Notes |
NCT03928158.
| Study name | LCZ696 in advanced LV hypertrophy and HFpEF |
| Methods |
Study design: parallel RCT Anticipated completion date: November 2020 |
| Participants |
Estimated enrolment: 60 Inclusion criteria:
Exclusion criteria:
|
| Interventions | LCZ696 versus valsartan 24 weeks |
| Outcomes | 6‐minute walking distance; exercise time during diastolic stress‐test; left atrial volume index (LAVI); average E/e' ratio; pulmonary artery systolic pressure (PASP); left ventricular mass index (LVMI); New York Heart Association (NYHA) functional classification; Minnesota Living With Heart Failure Questionnaire (MLHFQ) score; N‐terminal pro b‐type natriuretic peptide (NT‐proBNP); high‐sensitivity C‐reactive protein (hsCRP); carboxyterminal propeptide of type I collagen (PICP); carboxyterminal telopeptide of type I collagen (CITP); N‐Propeptide Of Type III Procollagen (PIIINP); Growth/differentiation factor 15 (GDF‐15); sST2; Galectin‐3; monocyte chemoattractant‐1 (MCP‐1) |
| Starting date | 31 May 2019 |
| Contact information | Artem Ovchinnikov: artcardio@mail.ru |
| Notes |
NCT03948685.
| Study name | Carvedilol SR study for biomarkers from blood and urine and safety of in patients with heart failure with preserved ejection fraction |
| Methods |
Study design: parallel RCT Anticipated completion date: January 2021 |
| Participants |
Estimated enrolment: 300 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Carvedilol SR versus placebo 24 weeks |
| Outcomes | NT‐proBNP; changes of maximum surrogate markers values(hsTn, hsCRP, sST2, Galectine‐3, IGFBP7, Neprilysin, D‐dimer, MMP‐2, Cystatin C, NAG, NGAL, KIM‐1, BUN, Creatinine, Chloride, Na, K, PICP and spondin‐1); degree of dyspnea using VAS questionnaire; change of body weight; frequency of symptomatic hypotension, symptomatic bradycardia and AV block above 2nd degree; frequency of hypo/hyperkalemia and worsening kidney function; all‐cause hospitalization & mortality |
| Starting date | May 2019 |
| Contact information | Seok‐Min Kang: smkang@yuhs.ac |
| Notes |
NCT03988634.
| Study name | Changes in NT‐proBNP and outcomes, safety, and tolerability in HFpEF patients with acute decompensated heart failure (ADHF) who have been stabilized during hospitalization and initiated in‐hospital or within 30 days post‐discharge (PARAGLIDE‐HF) |
| Methods |
Study design: parallel RCT Anticipated completion date: September 2021 |
| Participants |
Estimated enrolment: 800 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Sacubitril/valsartan versus valsartan 8 weeks |
| Outcomes | NT‐proBNP; composite hierarchical outcome consisting of: a) time to CV death, b) total HF hospitalizations, c) total urgent HF visits, and d) time‐averaged proportional change in NT‐proBNP; total composite events based on CV death, HF hospitalizations, and urgent HF visits; composite endpoint of worsening renal function (renal death, reaching ESRD, or decline in eGFR >/= 50%); hs‐Troponin (high sensitivity) |
| Starting date | 27 June 2019 |
| Contact information | Novartis Pharmaceuticals: novartis.email@novartis.com |
| Notes |
NCT04128891.
| Study name | Study of sacubitril/valsartan on myocardIal oxygenation and fibrosis in heart failure with preserved ejection fraction (PRISTINE‐HF) |
| Methods |
Study design: parallel RCT Anticipated completion date: 1 February 2024 |
| Participants |
Estimated enrolment: 60 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Sacubitril/valsartan versus valsartan Two years |
| Outcomes | improvement in microvascular function and ischaemia, as assessed by OS‐CMR at rest and stress; microvascular dysfunction; myocardial fibrosis; left ventricular diastolic function; NYHA class; 6‐minute walk test; heart failure related hospitalisations; cardiac mortality; all‐cause mortality |
| Starting date | 1 February 2020 |
| Contact information | Professor Selvanayagam: joseph.selva@sa.gov.au |
| Notes |
Zhou 2010.
| Study name | b‐PRESERVE |
| Methods |
Study design: "multicentre, prospective, randomized, open‐label, blinded endpoint trial" Anticipated completion date: |
| Participants |
Estimated enrolment: "A total of 1200 patients will be randomized to either b‐blocker (metoprolol succinate) or control (n = 600 per group)." Inclusion criteria: "The most essential criteria for HFNEF in this trial are: heart failure symptoms, elevated NT‐proBNP 1500 pg/mL, and LVEF 50%. In addition, age.40 years and a recent hospitalization for heart failure, but not within 3 months prior to enrolment, are required." Exclusion criteria: |
| Interventions | "The follow‐up period is a minimum of 2 years." |
| Outcomes | "The primary endpoint is a composite of hospitalization for heart failure and cardiovascular death. The secondary endpoints include cardiovascular death, heart failure mortality or hospitalization, all‐cause mortality, change in New York Heart Association class, change in left ventricular ejection fraction, increase in NT‐proBNP (by 50% of the value at randomization), b‐blocker tolerance, and premature termination of b‐blocker therapy due to adverse events" |
| Starting date | not reported |
| Contact information | Email: ge.junbo@zs‐hospital.sh.cn or jbge@zs‐hospital.sh.cn |
| Notes | Could not find the entry in the Chinese Clinical Trial Register with ID ChiCTR‐TNC‐00000144. Contacted investigators to clarify status of study. No response. |
ACEI: angiotensin‐converting‐enzyme inhibitor
ARB: angiotensin II receptor blockers
CMR: cardiac magnetic resonance
ECV: extra‐cellular volume
HTN: hypertension
KCCQ: Kansas City Cardiomyopathy Questionnaire
LVEF: left ventricular ejection fraction
NYHA: New York Heart Association Classification of heart failure
QoL: quality of life
RCT: randomised controlled trial
Differences between protocol and review
We originally planned to include participants with LVEF ≥ 40% but changed this to LVEF > 40%, since this is a more frequently used cut‐off in clinical trials.
We originally limited our population to symptomatic heart failure (NYHA class > I) at enrolment; however, this criterion was removed to include people with diagnosis of heart failure in whom symptoms had improved the functional class.
We added a clarification regarding the exclusion of cross‐over trials.
We originally planned to include withdrawals due to adverse events in the 'Summary of findings' table. This was changed because this outcome was frequently reported inconsistently, which limits the applicability of a pooled analysis. Instead, we added hyperkalaemia to the 'Summary of findings' table because this adverse event outcome has relevance for clinical decision making. For the same reason, hyperkalaemia was switched from secondary to primary outcomes and withdrawals due to adverse events was switched from primary to secondary outcomes.
We did not pre specify which scale for quality of life we would focus on in the 'Summary of findings' table. To aid comparisons across the 'Summary of findings' tables we chose to include the Minnsota Living with Heart Failure Questionnaire and not the standardized mean difference across two scales which applied only to MRA versus placebo or no treatment comparison.
Contributions of authors
NM: selection of studies, data extraction, analysis, GRADE assessment, co‐wrote the manuscript.
KM: selection of studies, GRADE assessment.
CD: contributed to review design, GRADE assessment, approved the final version.
TL: guarantor of review, designed the review, data extraction, analysis, GRADE assessment, co‐wrote the manuscript.
Sources of support
Internal sources
No sources of support provided
External sources
-
NIHR, UK
This project was supported by the NIHR via Cochrane Infrastructure and NIHR Incentive funding to Cochrane Heart. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
-
R. Thomas Lumbers, UK
R. Thomas Lumbers is supported by a UK Research and Innovation Rutherford Fellowship hosted by Health Data Research UK (MR/S003754/1); the BigData@Heart Consortium funded by the Innovative Medicines Initiative‐2 Joint Undertaking under grant agreement No. 116074; and the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Declarations of interest
NM: none known.
KM: none known.
CD: has received payment for lectures by AstraZeneca and support for attendance at a ESC‐HF meeting by Novartis.
TL: has received research grants from UK Research and Innovation, Innovative Medicines Initiative and National Institute of Health Research via his institution.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Adamyan 2010 {published data only}
- Adamyan KG, Tumasyan LR. Independent and additive influence of statin and beta-blocker on long-term prognosis in patients with chronic heart failure and preserved systolic function. European Heart Journal 2010;31:850. [Google Scholar]
- Tumasyan LR, Adamyan KG. Influence of statin and beta-blocker on long-term prognosis in patients with chronic heart failure and preserved systolic function. European Journal of Heart Failure Supplements 2010;9:S23-4. [Google Scholar]
ALDO‐DHF {published data only}
- Borlaug BA. Heart failure: Aldosterone antagonism for HFpEF. Nature Reviews Cardiology 2013;10(5):244-6. [DOI] [PubMed] [Google Scholar]
- Djawid Hashemi D, Dettmann L, Trippel TD, Bobenko A, Gelbrich G, Lindhorst R, et al. Economic impact of heart failure with preserved ejection fraction: Insights from the prospective, randomized placebo-controlled ALDO-DHF trial. European Journal of Heart Failure 2017;19:155. [Google Scholar]
- Durstewitz K, Holzendorf V, Wachter R, Gelbrich G, Stahrenberg R, Hasenfuss G, et al. Galectin-3 in heart failure with preserved ejection fraction-the effects of chronic aldosterone receptor blockade (the ALDO-DHF trial). Internist 2013;54(2):104-5. [Google Scholar]
- Edelmann F, Gelbrich G, Duvinage A, Stahrenberg R, Behrens A, Prettin C, et al. Differential interaction of clinical characteristics with key functional parameters in heart failure with preserved ejection fraction--results of the Aldo-DHF trial. International Journal of Cardiology 2013;169(6):408-17. [DOI] [PubMed] [Google Scholar]
- Edelmann F, Holzendorf V, Wachter R, Nolte K, Schmidt AG, Kraigher-Krainer E, et al. Galectin-3 in patients with heart failure with preserved ejection fraction: results from the Aldo-DHF trial. European Journal of Heart Failure 2015;17(2):214-23. [DOI] [PubMed] [Google Scholar]
- Edelmann F, Schmidt AG, Gelbrich G, Binder L, Herrmann-Lingen C, Halle M, et al. Rationale and design of the 'aldosterone receptor blockade in diastolic heart failure' trial: a double-blind, randomized, placebo-controlled, parallel group study to determine the effects of spironolactone on exercise capacity and diastolic function in patients with symptomatic diastolic heart failure (Aldo-DHF). European Journal of Heart Failure 2010;12(8):874-82. [DOI] [PubMed] [Google Scholar]
- Edelmann F, Wachter R, Pieske B. Aldosterone inhibition in patients with heart failure with preserved ejection fraction--reply. JAMA 2013;310(2):205-7. [DOI] [PubMed] [Google Scholar]
- Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA 2013;309(8):781-91. [DOI] [PubMed] [Google Scholar]
- Elguindy AM. ALDO-DHF & Paramount. Global Cardiology Science & Practice 2013;2(2012):12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EudraCT 2006-002605-31. Aldosterone receptor blockade in diastolic heart failure. A double-blind, randomised, placebo-controlled, parallel group study to determine the effects of spironolactone on exercise capacity and diastolic function in patients with symptomatic diastolic heart failure. www.clinicaltrialsregister.eu/ctr-search/trial/2006-002605-31/DE (first received 30 November 2006). [DOI] [PubMed]
- ISRCTN94726526. Aldosterone receptor blockade in diastolic heart failure: a double-blind, randomised, placebo-controlled, parallel group study to determine the effects of spironolactone on exercise capacity and diastolic function in patients with symptomatic diastolic heart failure. www.isrctn.com/ISRCTN94726526 (first received 7 November 2006).
- Lund LH, Stahlberg M. Aldosterone inhibition in patients with heart failure with preserved ejection fraction. JAMA 2013;10(2):205. [DOI] [PubMed] [Google Scholar]
- Trippel TD, Regitz-Zagrosek V, Gelbrich G, Mueller-Scholden L, Wachter R, Dungen HD, et al. Gender specific treatment effects of spironolactone in diastolic heart failure: Insight from ALDO-DHF. European Journal of Heart Failure 2016;18:130. [Google Scholar]
AREA IN‐CHF {published and unpublished data}
- Boccanelli A, Caciatore G, Mureddu GF, Simone G, Clemenza F, De Maria R, et al. Baseline characteristics of patients recruited in the AREA IN-CHF study (Antiremodelling Effect of Aldosterone Receptors Blockade with Canrenone in Mild Chronic Heart Failure). Journal of Cardiovascular Medicine 2007;8(9):683-91. [DOI] [PubMed] [Google Scholar]
- Boccanelli A, Mureddu GF, Cacciatore G, Clemenza F, Di Lenarda A, Gavazzi A, et al. Anti-remodelling effect of canrenone in patients with mild chronic heart failure (AREA IN-CHF study): final results. European Journal of Heart Failure 2009;11(1):68-76. [DOI] [PubMed] [Google Scholar]
- Cacciatore G, Boccanelli A, Mureddu GF, Maggioni AP, Latini R, Masson S, et al. The AREA IN-CHF trial (antiremodeling effect of aldosterone receptors blockade with canrenone in mild chronic heart failure): rationale and design. Italian Heart Journal 2005;6(Suppl 1):66S-74S. [PubMed] [Google Scholar]
- De Simone G, Chinali M, Mureddu GF, Cacciatore G, Lucci D, Latini R, et al. Effect of canrenone on left ventricular mechanics in patients with mild systolic heart failure and metabolic syndrome: the AREA-in-CHF study. Nutrition, Metabolism and Cardiovascular Diseases 2011;21(10):783-91. [DOI] [PubMed] [Google Scholar]
- NCT00403910. Antiremodeling effect of aldosterone receptors blockade with canrenone in mild chronic heart failure. AREA IN-CHF study [Phase 3 study of antiremodeling effect of aldosterone receptors blockade with canrenone in mild chronic heart failure]. clinicaltrials.gov/ct2/show/NCT00403910 (first received 27 November 2006.
Aronow 1993 {published data only}
- Aronow WS, Kronzon I. Effect of enalapril on congestive heart failure treated with diuretics in elderly patients with prior myocardial infarction and normal left ventricular ejection fraction. American Journal of Cardiology 1993;71(7):602-4. [DOI] [PubMed] [Google Scholar]
Aronow 1997 {published data only}
- Aronow WS, Ahn C, Kronzon I. Effect of propranolol versus no propranolol on total mortality plus nonfatal myocardial infarction in older patients with prior myocardial infarction, congestive heart failure, and left ventricular ejection fraction > or = 40% treated with diuretics plus angiotensin-converting enzyme inhibitors. American Journal of Cardiology 1997;80(2):207-9. [DOI] [PubMed] [Google Scholar]
Aronow 1998 {published data only}
- Aronow WS, Mercando AD, Epstein S. Effect of benazepril on complex ventricular arrhythmias in older patients with congestive heart failure, prior myocardial infarction, and normal left ventricular ejection fraction. American Journal of Cardiology 1998;81(11):1368-70. [DOI] [PubMed] [Google Scholar]
CandHeart {published data only}
- 2005-001306-87. Effects of Candesartan Cilexetil vs standard therapy on serum levels of brain natriuretic peptide in patients suffering from chronic heart failure with depressed and preserved systolic function. www.clinicaltrialsregister.eu/ctr-search/trial/2005-001306-87/IT (first received 2 February 2007).
- Aleksova A, Masson S, Maggioni AP, Lucci D, Urso R, Staszewsky L, et al. Effects of candesartan on left ventricular function, aldosterone and BNP in chronic heart failure. Cardiovascular Drugs and Therapy 2012;26(6):131-43. [DOI] [PubMed] [Google Scholar]
- NCT00843154. Efficacy of candesartan on brain natriuretic peptide levels in subjects with chronic heart failure (CANDHEART) [Effects of candesartan cilexetil vs standard therapy on serum levels of brain natriuretic peptide in patients suffering from chronic heart failure with depressed and preserved systolic function]. clinicaltrials.gov/ct2/show/NCT00843154 (First posted 13 February 2009).
CAN‐DHF {published data only}
- EudraCT 2007-003070-26. Candesartan “added” therapy for treatment optimization of symptomatic heart failure with diastolic dysfunction in diabetic and hypertensive patients. A randomized, placebo-controlled, double-blind, parallel-group and multicentre clinical phase III study investigating the effects on NT-proBNP over 6 months.. www.clinicaltrialsregister.eu/ctr-search/trial/2007-003070-26/DE (first entered 10 July 2007).
- NCT00775840. Efficacy of candesartan on symptomatic heart failure in treating diabetic and hypertensive patients [Candesartan "added" therapy for treatment optimization of symptomatic heart failure with diastolic dysfunction in diabetic and hypertensive patients. A randomized, placebo-controlled, double-blind, parallel-group and multicenter clinical phase III study investigating the effects on NT-proBNP over 6 months]. clinicaltrials.gov/ct2/show/NCT00775840 (first received 20 October 2008).
CHARM‐Preserved {published data only}
- Badar AA, Perez-Moreno AC, Hawkins NM, Brunton AP, Jhund PS, Wong CM, et al. Clinical characteristics and outcomes of patients with angina and heart failure in the CHARM (Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity) Programme. European Journal of Heart Failure 2015;17(2):196-204. [DOI] [PubMed] [Google Scholar]
- Bello NA, Claggett B, Desai AS, McMurray JJ, Granger CB, Yusuf S, et al. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circulation 2014;7(4):590-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagno D, Skali H, Takeuchi M, Swedberg K, Yusuf S, Granger CB, et al. Association of heart rate and outcomes in a broad spectrum of patients with chronic heart failure: results from the CHARM (Candesartan in Heart Failure: Assessment of Reduction in Mortality and morbidity) program. Journal of the American College of Cardiology 2012;59(20):1785-95. [DOI] [PubMed] [Google Scholar]
- Castagno D, Skali H, Takeuchi M, Swedberg K, Yusuf S, Granger CB, et al. Incidence and predictors of stroke in patients with chronic heart failure: Does left ventricular function Matter? Insights From the CHARM Program. Circulation 2011;124(Suppl 21):A17352. [Google Scholar]
- Chang SM, Granger CB, Johansson PA, Kosolcharoen P, McMurray JJ, Michelson EL, et al. Efficacy and safety of angiotensin receptor blockade are not modified by aspirin in patients with chronic heart failure: a cohort study from the Candesartan in Heart failure--Assessment of Reduction in Mortality and morbidity (CHARM) programme. European Journal of Heart Failure 2010;12(7):738-45. [DOI] [PubMed] [Google Scholar]
- Cohen-Solal A, McMurray JJ, Swedberg K, Pfeffer MA, Puu M, Solomon SD, et al. Benefits and safety of candesartan treatment in heart failure are independent of age: insights from the Candesartan in Heart failure--Assessment of Reduction in Mortality and morbidity programme. European Heart Journal 2008;29(24):3022-8. [DOI] [PubMed] [Google Scholar]
- Colombo GL, Caruggi M, Ottolini C, Maggioni AP. Candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) and resource utilization and costs in Italy. Vascular Health and Risk Management 2008;4(1):223-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damman K, Solomon SD, Pfeffer MA, Swedberg K, Yusuf S, Young JB, et al. Worsening renal function and outcome in heart failure patients with reduced and preserved ejection fraction and the impact of angiotensin receptor blocker treatment: data from the CHARM-study programme. European Journal of Heart Failure 2016;18(12):1508-17. [DOI] [PubMed] [Google Scholar]
- Desai AS, Claggett B, Pfeffer MA, Bello N, Finn PV, Granger C, et al. Influence of hospitalization for cardiovascular versus noncardiovascular reasons on subsequent mortality in patients with chronic heart failure across the spectrum of ejection fraction. European Journal of Heart Failure 2013;34:284. [DOI] [PubMed] [Google Scholar]
- Desai AS, Claggett B, Pfeffer MA, Bello N, Finn PV, Granger CB, et al. Influence of hospitalization for cardiovascular versus noncardiovascular reasons on subsequent mortality in patients with chronic heart failure across the spectrum of ejection fraction. Circulation 2014;7(6):895-902. [DOI] [PubMed] [Google Scholar]
- Dubrey SW. Angiotensin II receptor antagonists in the treatment of heart failure: Background to and design of the CHARM study. British Journal of Cardiology 2002;9(5):280-2,284,286. [Google Scholar]
- Fruhwald FM, Vavrovsky AD. Resource utilization and costs for candesartan in heart failure: Assessment of reduction in mortality and morbidity (CHARM) programme for the Austrian setting. Value in Health 2011;14(7):A379. [Google Scholar]
- Gerstein HC, Swedberg K, Carlsson J, McMurray JJ, Michelson EL, Olofsson B, et al. The hemoglobin A1c level as a progressive risk factor for cardiovascular death, hospitalization for heart failure, or death in patients with chronic heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Archives of Internal Medicine 2008;168(15):1699-704. [DOI] [PubMed] [Google Scholar]
- Grewal, J, McKelvie RS, Persson H, Tait P, Carlsson J, Swedberg K, et al. Usefulness of N-terminal Pro-Brain Natriuretic Peptide and Brain Natriuretic Peptide to predict cardiovascular outcomes in patients with heart failure and preserved left ventricular ejection fraction. American Journal of Cardiology 2008;102(6):733-7. [DOI] [PubMed] [Google Scholar]
- Gupta DK, Castagno D, Granger CB, Yusuf S, Swedberg K, McMurray J, et al. Prognostic impact of an ischemic etiology of chronic heart failure across the spectrum of ejection fractions in the charm program. Journal of Cardiac Failure 2012;18(8):S78. [Google Scholar]
- Hawkins NM, Wang D, McMurray JJ, Pfeffer MA, Swedberg K, Granger CB, et al. Prevalence and prognostic impact of bundle branch block in patients with heart failure: evidence from the CHARM programme. European Journal of Heart Failure 2007;9(5):510-7. [DOI] [PubMed] [Google Scholar]
- Hawkins NM, Wang D, McMurray JJ, Pfeffer MA, Swedberg K, Granger CB, et al. Prevalence and prognostic implications of electrocardiographic left ventricular hypertrophy in heart failure: evidence from the CHARM programme. Heart 2007;93(1):59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins NM, Wang D, Petrie MC, Pfeffer MA, Swedberg K, Granger CB, et al. Baseline characteristics and outcomes of patients with heart failure receiving bronchodilators in the CHARM programme. European Journal of Heart Failure 2010;12(6):557-65. [DOI] [PubMed] [Google Scholar]
- Jackson CE, Solomon SD, Gerstein HC, Zetterstrand S, Olofsson B, Michelson EL, et al. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet 2009;374(9689):543-50. [DOI] [PubMed] [Google Scholar]
- Kochsiek K. Angiotensin receptor blockers in heart failure. CHARM Study [Angiotensinrezeptorblocker bei Herzinsuffizienz]. Internist 2004;45(9):1063-7. [DOI] [PubMed] [Google Scholar]
- Kristensen SL, Kober L, Jhund PS, Solom SD, Kjekshus J, McKelvie RS, et al. International geographic variation in event rates in trials of heart failure with preserved and reduced ejection fraction. Circulation 2015;131(1):43-53. [DOI] [PubMed] [Google Scholar]
- Lund LH, Claggett B, Liu J, Lam CS, Jhund PS, Rosano GM, et al. Heart failure with mid-range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. European Journal of Heart Failure 2018;20:1230-9. [DOI] [PubMed] [Google Scholar]
- MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. European Heart Journal 2008;29(11):1377-85. [DOI] [PubMed] [Google Scholar]
- McKelvie RS. The CHARM program: the effects of candesartan for the management of patients with chronic heart failure. Expert Review Cardiovascular Therapy 2009;7(1):9-16. [DOI] [PubMed] [Google Scholar]
- McMurray J, Ostergren J, Pfeffer M, Swedberg K, Granger C, Yusuf S, et al. Clinical features and contemporary management of patients with low and preserved ejection fraction heart failure: baseline characteristics of patients in the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) programme. European Journal of Heart Failure 2003;5(3):261-70. [DOI] [PubMed] [Google Scholar]
- McMurray JJ. Angiotensin inhibition in heart failure. Journal of the Renin Angiotensin Aldosterone System 2004;5(Suppl 1):S17-22. [DOI] [PubMed] [Google Scholar]
- Meredith PA, Ostergren J, Anand I, Puu M, Solomon SD, Michelson EL, et al. Clinical outcomes according to baseline blood pressure in patients with a low ejection fraction in the CHARM (Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity) Program. Journal of the American College of Cardiology 2008;52(24):2000-7. [DOI] [PubMed] [Google Scholar]
- Meyer TE. The candesartan in heart failure-assessment of reduction in mortality and morbidity-preserved trial. Current Cardiology Reports 2004;6(3):197-8. [PubMed] [Google Scholar]
- Mitchell GF, Arnold JM, Dunlap ME, O'Brien TX, Marchiori G, Warner E, et al. Pulsatile hemodynamic effects of candesartan in patients with chronic heart failure: the CHARM Program. European Journal of Heart Failure 2006;8(2):191-7. [DOI] [PubMed] [Google Scholar]
- O'Meara E, Clayton T, McEntegart MB, McMurray JJ, Lang CC, Roger S, et al. Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: results of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation 2006;113(7):986-94. [DOI] [PubMed] [Google Scholar]
- O'Meara E, Clayton T, McEntegart MB, McMurray JJ, Pina IL, Granger CB, et al. Sex differences in clinical characteristics and prognosis in a broad spectrum of patients with heart failure: results of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 2007;115(24):3111-20. [DOI] [PubMed] [Google Scholar]
- Olsson LG, Swedberg K, Ducharme A, Granger CB, Michelson EL, McMurray JJ, et al. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. Journal of the American College of Cardiology 2006;47(10):1997-2004. [DOI] [PubMed] [Google Scholar]
- Ostergren JB. Angiotensin receptor blockade with candesartan in heart failure: findings from the Candesartan in Heart failure--assessment of reduction in mortality and morbidity (CHARM) programme. Journal of Hypertension. Supplements 2006;24(1):S3-7. [DOI] [PubMed] [Google Scholar]
- Persson H, Lonn E, Edner M, Baruch L, Lang CC, Morton JJ, et al. Diastolic dysfunction in heart failure with preserved systolic function: need for objective evidence:results from the CHARM Echocardiographic Substudy-CHARMES. Journal of the American College of Cardiology 2007;49(6):687-94. [DOI] [PubMed] [Google Scholar]
- Pocock SJ, McMurray JJ, Dobson J, Yusuf S, Granger CB, Michelson EL, et al. Weight loss and mortality risk in patients with chronic heart failure in the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) programme. European Heart Journal 2008;29(21):2641-50. [DOI] [PubMed] [Google Scholar]
- Rapp JA, Gheroghiade M. Role of neurohormonal modulators in heart failure with relatively preserved systolic function. Heart Failure Clinic 2005;1(1):77-93. [DOI] [PubMed] [Google Scholar]
- Sica DA. ACE inhibitor intolerance and lessons learned from the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) trials. Congestive Heart Failure 2004;10(3):160-4. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulationi 2005;112(24):3738-44. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation 2007;116(13):1482-7. [DOI] [PubMed] [Google Scholar]
- Somon SD, Wang D, Finn P, Skali H, Zornoff L, McMurray JJ, et al. Effect of candesartan on cause-specific mortality in heart failure patients: the Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) program.[Erratum appears in Circulation. 2005 Jan 25;111(3):378]. Circulation 2004;110(15):2180-3. [DOI] [PubMed] [Google Scholar]
- Swedberg K, Pfeffer M, Granger C, Held P, McMurray J, Ohlin G, et al. Candesartan in Heart Failure-Assessment of Reduction in Mortality and Morbidity (CHARM): Rationale and design. Journal of Cardiac Failure 1999;5(3):276-82. [DOI] [PubMed] [Google Scholar]
- Woisetschlager C. Congress of the European Society of Cardiology 2003 in Vienna. Convincing results of candesartan in heart failure. Journal fur Kardiologie 2003;10(10):453-4. [Google Scholar]
- Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003;362(9386):777-81. [DOI] [PubMed] [Google Scholar]
ELANDD {published data only}
- 2004-000746-20. Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity and left ventricular function of the patients with diastolic dysfunction (ELANDD). www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2004-000746-20 (date submitted 9 October 2006).
- Conraads VM, Metra M, Kamp O, De Keulenaer GW, Pieske B, Zamorano J, et al. Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: results of the ELANDD study. European Journal of Heart Failure 2012;14(2):219-25. [DOI] [PubMed] [Google Scholar]
- Kamp O, Metra M, De Keulenaer GW, Pieske B, Conraads V, Zamorano J, et al. Effect of the long-term administration of nebivolol on clinical symptoms, exercise capacity and left ventricular function in patients with heart failure and preserved left ventricular ejection fraction: background, aims and design of the ELANDD study. Clinical Research in Cardiology 2010;99:75-82. [DOI] [PubMed] [Google Scholar]
Hong Kong DHF {published data only}
- CUHK_CCT00035. Treatment of diastolic heart failure: the role of blockade of the renin-angiotensin system. A comparison of diuretics with an angiotensin converting enzyme inhibitor, angiotensin receptor blockade or diuretics alone. www2.ccrb.cuhk.edu.hk/registry/public/15 (trial registration 7 September 2005).
- Yip GW, Wang M, Wang T, Chan S, Fung JW, Yeung L, et al. The Hong Kong diastolic heart failure study: a randomised controlled trial of diuretics, irbesartan and ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection fraction. Heart 2008;94(5):573-80. [DOI] [PubMed] [Google Scholar]
I‐PRESERVE {published data only}
- Adabag S, Rector TS, Anand IS, McMurray JJ, Zile M, Komajda M, et al. A prediction model for sudden cardiac death in patients with heart failure and preserved ejection fraction. European Journal of Heart Failure 2014;16(11):1175-82. [DOI] [PubMed] [Google Scholar]
- Anand IS, Rector TS, Cleland JG, Kuskowski M, McKelvie RS, Persson H, et al. Prognostic value of baseline plasma amino-terminal pro-brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I-PRESERVE trial. Circulation 2011;4(5):569-77. [DOI] [PubMed] [Google Scholar]
- Anonymous. Heart failure patients with preserved left ventricular ejection fraction did not benefit from additional ARB therapy. Cardiovascular Journal of Africa 2008;19(6):337-9. [PubMed] [Google Scholar]
- Badar AA, Perez-Moreno AC, Hawkins NM, Jhund PS, Brunton AP, et al. Clinical characteristics and outcomes of patients with coronary artery disease and angina: analysis of the irbesartan in patients with heart failure and preserved systolic function trial. Circulation 2015;8(4):717-24. [DOI] [PubMed] [Google Scholar]
- Blandon JA. ARBs and the impact of worsening renal function in heart failure patients with preserved EF. Cardiology Review 2014;30(6):no pagination. [Google Scholar]
- Bohm M, Perez AC, Jhund PS, Reil JC, Komajda M, Zile MR, et al. Relationship between heart rate and mortality and morbidity in the irbesartan patients with heart failure and preserved systolic function trial (I-Preserve). European Journal of Heart Failure 2014;16(7):778-87. [DOI] [PubMed] [Google Scholar]
- Cannon JA, Shen L, Jhund PS, Anand IS, Komajda M, McKelvie RS, et al. Clinical outcomes according to QRS duration and morphology in the irbesartan in patients with heart failure and preserved systolic function (I-PRESERVE) trial. European Journal of Heart Failure 2016;18(8):1021-31. [DOI] [PubMed] [Google Scholar]
- Carson P, Anand I, Win S, Zile M, Haas M, Little W, et al. Why are patients with heart failure and preserved ejection fraction hospitalized? Data from i-preserve. Circulation 2013;128(Suppl 22):A15844. [Google Scholar]
- Carson P, Massie PM, McKelvie R, McMurray J, Komajda M, Zile M, et al. The irbesartan in heart failure with preserved systolic function (I-PRESERVE) trial: rationale and design. Journal of Cardiac Failure 2005;11(8):576-85. [DOI] [PubMed] [Google Scholar]
- Carson PE, Zile M, McMurray J, McKelvie R, Komajda M, Hetzel S, et al. Heart failure with preserved ejection fraction: Mortality risk post first hospitalization - Data from I-preserve. Journal of the American College of Cardiology 2010;55(10):A141.E1321. [Google Scholar]
- Chow SL, Krum H, O'Barr SA, Zile M, Carson P, Komajda M, et al. Markers of extracellular matrix turnover and risk of death in patients with heart failure with preserved ejection fraction: Results from I-PRESERVE. Journal of the American College of Cardiology 2015;65(10):A969. [Google Scholar]
- Damman K, Perez AC, Anand IS, Komajda M, McKelvie RS, Zile MR, et al. Worsening renal function and outcome in heart failure patients with preserved ejection fraction and the impact of angiotensin receptor blocker treatment. Journal of the American College of Cardiology 2014;64(11):1106-13. [DOI] [PubMed] [Google Scholar]
- Ezekowitz JA, McAlister FA. ACP Journal Club. Irbesartan did not reduce all-cause death or CV hospitalization in heart failure and preserved ejection fraction. Annals of Internal Medicine 2009;150(10):JC5-10. [DOI] [PubMed] [Google Scholar]
- Gandhi PU, Chow SL, Rector TS, Krum H, Gaggin HK, McMurray JJ, et al. Prognostic value of insulin-like growth factor-binding protein 7 in patients with heart failure and preserved ejection fraction. Journal of Cardiac Failure 2017;23(1):20-8. [DOI] [PubMed] [Google Scholar]
- Gandhi PU, Chow SL, Rector TS, Krum H, Gaggin HK, McMurray JJ, et al. The prognostic value of insulin-like growth factor-binding protein 7 in patients with heart failure and preserved ejection fraction: Results from irbesartan in heart failure and preserved ejection fraction (I-preserve) trial. Circulation 2015;132(Suppl 3):A14299. [Google Scholar]
- Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circulationi 2011;4(3):324-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao DP, Lewsey JD, Anand IS, Massie BM, Zile MR, Carson PE, et al. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. European Journal of Heart Failure 2015;17(9):925-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao DP, Lewsey JK, Massie BM, McMurray J, Carson PE, Anand IS, et al. Characterization of heart failure patients with preserved ejection fraction in the I-PRESERVE trial who have improved outcomes with irbesartan therapy. European Journal of Heart Failure. Supplements 2012;11:S4. [Google Scholar]
- Komajda M, Carson PE, Hetzel S, McKelvie R, McMurray J, Ptaszynska A, et al. Factors associated with outcome in heart failure with preserved ejection fraction: findings from the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-PRESERVE). Circulation 2011;4(1):27-35. [DOI] [PubMed] [Google Scholar]
- Kristensen SL, Jhund PS, Kober L, McKelvie RS, Zile MR, Anand IS, et al. Relative importance of history of heart failure hospitalization and N-Terminal pro-b-type Natriuretic Peptide level as predictors of outcomes in patients with heart failure and preserved ejection fraction. JACC Heart Failure 2015;3(6):478-86. [DOI] [PubMed] [Google Scholar]
- Kristensen SL, Jhund PS, Kober L, Preiss D, Kjekshus J, McKelvie RS, et al. Comparison of outcomes after hospitalization for worsening heart failure, myocardial infarction, and stroke in patients with heart failure and reduced and preserved ejection fraction. European Journal of Heart Failure 2015;17(2):169-76. [DOI] [PubMed] [Google Scholar]
- Kristensen SL, Kober L, Jhund PS, Solomon SD, Kjekshus J, McKelvie RS, et al. International geographic variation in event rates in trials of heart failure with preserved and reduced ejection fraction. Circulation 2015;131(1):43-53. [DOI] [PubMed] [Google Scholar]
- Kristensen SL, Mogensen UM, Jhund PS, Petrie MC, Preiss D, Win S, et al. Clinical and echocardiographic characteristics and cardiovascular outcomes according to diabetes status in patients with heart failure and preserved ejection fraction: A report from the I-Preserve Trial (Irbesartan in Heart Failure With Preserved Ejection Fraction). Circulation 2017;135(8):724-35. [DOI] [PubMed] [Google Scholar]
- Krum H, Elsik M, Schneider H, Ptaszynska A, Black M, Carson P, et al. Peripheral collagen markers predict all-cause mortality and cardiovascular hospitalisation in patients with heart failure and preserved ejection fraction: Results of the I-PRESERVE collagen sub-study. Heart, Lung and Circulation 2010;19:S77-8. [Google Scholar]
- Krum H, Elsik M, Schneider HG, Ptaszynska A, Black M, Carson PE, et al. Relation of peripheral collagen markers to death and hospitalization in patients with heart failure and preserved ejection fraction: results of the I-PRESERVE collagen substudy. Circulation 2011;4(5):561-8. [DOI] [PubMed] [Google Scholar]
- Lam CS, Carson PE, Anand IS, Rector TS, Kuskowski M, Komajda M, et al. Sex differences in clinical characteristics and outcomes in elderly patients with heart failure and preserved ejection fraction: the Irbesartan in Heart Failurewith Preserved Ejection Fraction (I-PRESERVE) trial. Circulation 2012;5(5):571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile M, et al. Irbesartan in patients with heart failure and preserved ejection fraction. New England Journal of Medicine 2008;359(23):2456-67. [DOI] [PubMed] [Google Scholar]
- McKelvie RS, Komajda M, McMurray J, Zile M, Ptaszynska A, Donovan M, et al. Baseline plasma NT-proBNP and clinical characteristics: results from the irbesartan in heart failure with preserved ejection fraction trial. Journal of Cardiac Failure 2010;16(2):128-34. [DOI] [PubMed] [Google Scholar]
- McMurray JJ, Carson PE, Komajda M, McKelvie R, Zile MR, Ptaszynska A, et al. Heart failure with preserved ejection fraction: clinical characteristics of 4133 patients enrolled in the I-PRESERVE trial. European Journal of Heart Failure 2008;10(2):149-56. [DOI] [PubMed] [Google Scholar]
- NCT00095238. Irbesartan in heart failure with preserved systolic function (I-Preserve). clinicaltrials.gov/show/NCT00095238 (first posted 2 November 2004).
- Oluleye O, Win S, Rector T, Komadja M, Zile M, McKelvie R, et al. Risk of clinical outcomes and baseline atrial fibrillation: Irbesartan in heart failure with preserved ejection fraction (I-PRESERVE) trial. Circulation 2013;128(22):A15893. [Google Scholar]
- Oluleye OW, Rector TS, Win S, McMurray JJ, Zile MR, Komajda M, et al. History of atrial fibrillation as a risk factor in patients with heart failure and preserved ejection fraction. Circulationi 2014;7(6):960-6. [DOI] [PubMed] [Google Scholar]
- Rector TS, Carson PE, Anand IS, McMurray JJ, Zile MR, McKelvie RS, et al. Assessment of long-term effects of irbesartan on heart failure with preserved ejection fraction as measured by the minnesota living with heart failure questionnaire in the irbesartan in heart failure with preserved systolic function (I-PRESERVE) trial. Circulation 2012;5(2):217-25. [DOI] [PubMed] [Google Scholar]
- Schillaci G, Pucci G, Pirro M. Irbesartan for heart failure with preserved ejection fraction. New England Journal of Medicine 2009;360(12):1258; author reply 1258-9. [PubMed] [Google Scholar]
- Schneider HG, Krum H, Elsik M, Ptaszynska A, Black M, Anand I, et al. I-preserve sub-study: Plasma collagen markers in the prediction of death and hospitalisation in patients with heart failure and preserved ejection fraction. Clinical Chemistry and Laboratory Medicine 2011;49:S337. [Google Scholar]
- Teerlink JR, Zile MR, White M, Miller AB, Lopez-Sendon J, Little WC, et al. Acute coronary syndromes in patients with heart failure with preserved ejection fraction: A harbinger of death? JACC 2010;55(10):A38.E368. [Google Scholar]
- Win S, Anand I, McMurray J, Zile M, McKelvie R, Komajda M, et al. Morbidity and mortality in diabetics with heart failure and a preserved ejection fraction: Results from the i-preserve trial. Journal of the American College of Cardiology 2013;61(10):E706. [Google Scholar]
- Zile MR, Gaasch WH, Anand I, Haass M, Little WC, Muller A, et al. Mode of death in patients with heart failure and a preserved ejection fraction: Results from irbesartan heart failure with preserved ejection fraction study (I-Preserve) trial. European Heart Journal 2009;30:867. [DOI] [PubMed] [Google Scholar]
- Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, et al. Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I-Preserve) trial. Circulation 2010;121(12):1393-405. [DOI] [PubMed] [Google Scholar]
- Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, et al. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation 2011;124(23):2491-501. [DOI] [PubMed] [Google Scholar]
J‐DHF {published data only}
- Hori M. Rationale and design of a randomized trial to assess the effects of β-blocker in diastolic heart failure; Japanese Diastolic Heart Failure Study (J-DHF). Journal of Cardiac Failure 2005;11(7):542-7. [DOI] [PubMed] [Google Scholar]
- UMIN: C000000318. Japanese Diastolic Heart Failure Study. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000000411 (Registered 1 February 2006).
- Yamamoto K, Origasa H, Hori M. Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese Diastolic Heart Failure Study (J-DHF). European Journal of Heart Failure 2013;15(1):110-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Origasa H, Hori M. Left atrial dilatation as a predictive factor of beneficial response to carvedilol in patients with HFPEF: A finding from the Japanese Diastolic Heart Failure study (J-DHF). European Heart Journal 2013;34:161. [Google Scholar]
- Yamamoto K, Origasa H, Suzuki Y, Takahashi T, Shinozaki T, Watanabe T, et al. Relation of risk factors with response to carvedilol in heart failure with preserved ejection fraction - a report from the Japanese Diastolic Heart Failure Study (J-DHF). Journal of Cardiology 2014;63(6):424-31. [DOI] [PubMed] [Google Scholar]
Karapysh 2015 {published data only}
- Karapysh VA, Vatutin NT, Shevelok AN. Effects of spironolactone on the left ventricular hypertrophy in chronic heart failure with preserved ejection fraction. European Journal of Heart Failure 2015;17:48. [Google Scholar]
Kasama 2005 {published data only}
- Kasama S, Toyama T, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, et al. Effects of candesartan on cardiac sympathetic nerve activity in patients with congestive heart failure and preserved left ventricular ejection fraction. Journal of the American College of Cardiology 2005;45(5):661-7. [DOI] [PubMed] [Google Scholar]
Kitzman 2010 {published data only}
- Kitzman DW, Hundley WG, Brubaker PH, Morgan TM, Moore JB, Stewart KP, et al. A randomized double-blind trial of enalapril in older patients with heart failure and preserved ejection fraction: effects on exercise tolerance and arterial distensibility. Circulation 2010;3(4):477-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01411735. Evaluation of enalapril versus placebo in patients with diastolic heart failure (PIE-I). clinicaltrials.gov/show/NCT01411735 (first posted 8 August 2011).
Kurrelmeyer 2014 {published and unpublished data}
- Kurrelmeyer KM, Ashton Y, Xu J, Nagueh SF, Torre-Amione G, Deswal A. Effects of spironolactone treatment in elderly women with heart failure and preserved left ventricular ejection fraction. Journal of Cardiac Failure 2014;20(8):560-8. [DOI] [PubMed] [Google Scholar]
- NCT00206232. Novel treatment for diastolic heart failure in women. clinicaltrials.gov/show/NCT00206232 (first posted 21 September 2005).
Mak 2009 {published data only}
- Mak G, Ledwidge M, Murphy NF, Phelan D, Dawkins I, Watson C, et al. Eplerenone favourably alters collagen metabolism in diastolic heart failure. European Heart Journal 2009;30:866. [Google Scholar]
- Mak GJ, Ledwidge MT, Watson CJ, Phelan DM, Dawkins IR, Murphy N, et al. Natural history of markers of collagen turnover in patients with early diastolic dysfunction and impact of eplerenone. Journal of the American College of Cardiology 2009;54(18):1674-82. [DOI] [PubMed] [Google Scholar]
- NCT00505336. The effect of eplerenone and atorvastatin on markers of collagen turnover in diastolic heart failure. clinicaltrials.gov/show/NCT00505336 (first posted 23 July 2007).
McDiarmid 2020 {published data only}
- EudraCT 2013-000867-10. Effects of spironolactone in heart failure with preserved ejection fraction: measured by CMR, echo, excercise capacity and quality of life questionaire [Effects of aldosterone antagonism in heart failure with preserved ejection fraction (HF-PEF): cardiac MRI, echocardiography, exercise physiology & quality of life assessment]. www.clinicaltrialsregister.eu/ctr-search/trial/2013-000867-10/GB (first received 13 February 2014).
- McDiarmid AK, Swoboda PP, Erhayiem B, Bounford KA, Bijstervel P, Tyndall K, et al. Myocardial effects of aldosterone antagonism in heart failure with preserved ejection fraction. Journal of the American Heart Association 2020;9:e011521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mittal 2017 {published and unpublished data}
- CTRI/2010/091/000438. Evaluation of efficacy and safety of metoprolol in patients having heart failure with normal ejection fraction: a randomised, double-blind, placebo-controlled trial [A clinical trial to study the efficacy and safety of metoprolol in heart failure with normal ejection fraction]. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=1626 (registered on 19 May 2010).
- Mittal N, Shafiq N, Reddy S, Malhotra S, Kumari S, Varma S. Evaluation of efficacy of metoprolol in patients having heart failure with preserved ejection fraction: A randomized, double-blind, placebo-controlled pilot trial. Perspectives in Clinical Research 2017;8(3):124-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mottram 2004 {published data only}
- Mottram PM, Haluska B, Leano R, Cowley D, Stowasser M, Marwick TH. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation 2004;110:558-65. [DOI] [PubMed] [Google Scholar]
- Mottram PM, Haluska BA, Leano R, Cowley D, Stowasser M, Marwick TH. Myocardial dysfunction in hypertensive patients with isolated diastolic heart failure is reversible.A randomized trial of aldosterone antagonism. European Heart Journal 2004;25(Suppl S):47-8. [DOI] [PubMed] [Google Scholar]
Orea‐Tejeda 2007 {published data only}
- Orea-Tejeda A, Colin-Ramirez E, Castillo-Martinez L, Asensio-Lafuente E, Corzo-Leon D, Gonzalez-Toledo R, et al. Aldosterone receptor antagonists induce favorable cardiac remodeling in diastolic heart failure patients. Revista de Investigacion Clinica 2007;59(2):103-7. [PubMed] [Google Scholar]
PARAGON‐HF {published data only}
- Chandra A, Vaduganathan M, Lewis EF, Claggett BL, Rizkala AR, Wang W, et al. Health-related quality of life in heart failure with preserved ejection fraction: the PARAGON-HF trial. JACC: Heart Failure 2019;7(10):862-74. [DOI] [PubMed] [Google Scholar]
- EUCTR2013-001747-31. A multicenter, randomized, double-blind, parallel group, active-controlled study to evaluate the efficacy and safety of LCZ696 compared to valsartan, on morbidity and mortality in heart failure patients (NYHA Class II-IV) with preserved ejection fraction. www.clinicaltrialsregister.eu/ctr-search/trial/2013-001747-31/ES (first entered 10 January 2014).
- McMurray JJ, Jackson AM, Lam CS, Redfield MM, Anand IS, Ge J, et al. Effects of sacubitril-valsartan versus valsartan in women compared with men with heart failure and preserved ejection fraction: insights from PARAGON-HF. Circulation 2020;141:338-51. [DOI] [PubMed] [Google Scholar]
- NCT01920711. Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction (PARAGON-HF). clinicaltrials.gov/ct2/show/NCT01920711 (first posted 12 August 2013).
- Solomon SD, McMurray JJ, Anand IS, Ge J, Lam CS, Maggioni AP, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. New England Journal of Medicine 2019;381(17):1609-20. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Rizkala AR, Gong J, Wang W, Anand IS, Ge J, et al. Angiotensin receptor neprilysin inhibition in heart failure with preserved ejection fraction: rational and design of the PARAGON-HF trial. JACC: Heart Failure 2017;5(7):471-82. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Rizkala AR, Lefkowitz MP, Shi VC, Gong J, Anavekar N, et al. Baseline characteristics of patients with heart failure and preserved ejection fraction in the PARAGON-HF trial. Circulation: Heart Failure 2018;11:e004962. [DOI] [PubMed] [Google Scholar]
- Vardeny O, Claggett B, Anand I, Vaduganathan M, Pfeffer MA, Zannand F, et al. Influence of age on efficacy and safety of sacubitril/valsartan in heart failure with preserved ejection fraction. JACC 2020;75(11):910. [Google Scholar]
PARALLAX {published data only}
- NCT03066804. A randomized, double-blind controlled study comparing LCZ696 to medical therapy for comorbidities in HFpEF patients (PARALLAX) [A 24-week, randomized, double-blind, multi-center, parallel group, active controlled study to evaluate the effect of LCZ696 on NT-proBNP, symptoms, exercise function and safety compared to individualized medical management of comorbidities in patients with heart failure and preserved ejection fraction]. clinicaltrials.gov/show/NCT03066804 (first posted 28 February 2017).
- Pieske B. PARALLAX (slideshow). ESC Congress 2020 - The Digital Experience 30 August 2020.
- Wachter R, Shah SJ, Cowie MR, Szecsody P, Shi V, Ibram G, et al. Angiotensin receptor neprilysin inhibition versus individualized RAAS blockade: design and rationale of the PARALLAX trial. ESC Heart Failure 2020;7:856-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
PARAMOUNT {published data only}
- Biering-Sorensen T, Shah A, Claggett B, Zile M, Pieske B, Pieske-Kraigher E, et al. The angiotensin receptor neprilysin inhibitor (ARNI), sacubitril/valsartan, improves left ventricular myocardial deformation in heart failure with preserved ejection fraction (PARAMOUNT trial). JACC 2018;71(11):2665. [Google Scholar]
- EudraCT 2009-010208-27. A twelve-week, randomized, double-blind, multi-center,parallel group, active controlled study to evaluate the efficacy, safety and tolerability of LCZ696 compared to valsartan in patients with chronic heart failure and preserved left-ventricular ejection fraction. www.clinicaltrialsregister.eu/ctr-search/trial/2009-010208-27/IT (first entered 6 May 2009).
- Januzzi JL, Claggett B, Liu J, Shah A, Zile M, Pieske B, et al. Abstract 17434: insulin-like growth factor binding protein-7 as a biomarker of diastolic dysfunction: results from the prospective comparison of ARNI with ARB on management of heart failure with preserved ejection fraction (PARAMOUNT) study. Circulation 2017;136:A17434. [Google Scholar]
- NCT00887588. LCZ696 compared to valsartan in patients with chronic heart failure and preserved left-ventricular ejection fraction. clinicaltrials.gov/ct2/show/NCT00887588 (first posted 13 August 2015).
- Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012;380:1387-95. [DOI] [PubMed] [Google Scholar]
- Zile MR, Jhund PS, Baicu CF, Claggett BL, Pieske B, Voors AA, et al. Plasma biomarkers reflecting profibrotic processes in heart failure with a preserved ejection fraction: data from the prospective comparison of ARNI with ARB on management of heart failure with preserved ejection fraction study. Circulation. Heart Failure 2016;9(1):e002551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parthasarathy 2009 {published and unpublished data}
- CVAL489B2401. Novartis Clinical Trial Results Database. www.novctrd.com/CtrdWeb/displaypdf.nov?trialresultid=2460 (date accessed 30 November 2017).
- NCT00171106. Efficacy and safety of valsartan versus placebo on exercise tolerance in patients with heart failure [A double-blind, randomised, placebo-controlled, parallel group study to determine the effects of valsartan on exercise time in subjects with symptomatic diastolic heart failure]. clinicaltrials.gov/show/NCT00171106 (first posted 15 September 2005).
- Parthasarathy HK, Pieske B, Weisskopf M, Andrews CD, Brunel P, Struthers AD, et al. A randomized, double-blind, placebo-controlled study to determine the effects of valsartan on exercise time in patients with symptomatic heart failure with preserved ejection fraction. European Journal of Heart Failure 2009;11(10):980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
PEP‐CHF {published data only}
- Cleland JG, Taylor J, Freemantle N, Goode KM, Rigby AS, Tendera M. Relationship between plasma concentrations of N-terminal pro brain natriuretic peptide and the characteristics and outcome of patients with a clinical diagnosis of diastolic heart failure: a report from the PEP-CHF study. European Journal of Heart Failure 2012;14(5):487-94. [DOI] [PubMed] [Google Scholar]
- Cleland JG, Tendera M, Adamus J, Freemantle N, Gray CS, Lye M, et al. Perindopril for elderly people with chronic heart failure: the PEP-CHF study. European Journal of Heart Failure 1999;1(3):211-7. [DOI] [PubMed] [Google Scholar]
- Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. European Heart Journal 2006;27(16):2338-45. [DOI] [PubMed] [Google Scholar]
- McMurray J. Renin angiotensin blockade in heart failure with preserved ejection fraction: The signal gets stronger. European Heart Journal 2006;27(19):2257-9. [DOI] [PubMed] [Google Scholar]
- Widimsky J. Managing diastolic heart failure. Results of PEP-CHF [Lecba diastolickeho srdecniho selhani. Vysledky studie PEP-CHF]. Cor Vasa 2006;48(11):403-7. [Google Scholar]
RAAM‐PEF {published and unpublished data}
- Deswal A, Richardson P, Bozkurt B, Mann DL. Results of the Randomized Aldosterone Antagonism in Heart Failure with Preserved Ejection Fraction trial (RAAM-PEF). Journal of Cardiac Failure 2011;17(8):634-42. [DOI] [PubMed] [Google Scholar]
- NCT00108251. Aldosterone antagonism in diastolic heart failure. clinicaltrials.gov/ct2/show/NCT00108251 (first posted 15 April 2005).
Sahoo 2016 {published data only}
- Sahoo D, Kapoor A, Sinha A, Khanna R, Kumar S, Garg N, et al. Targeting the sympatho-adrenergic link in chronic rheumatic mitral regurgitation: assessing the role of oral beta-blockers. Cardiovascular Therapeutics 2016;34(4):261-7. [DOI] [PubMed] [Google Scholar]
SENIORS {published data only}
- Cohen-Solal A, Kotecha D, Veldhuisen DJ, Babalis D, Bohm M, Coats AJ, et al. Efficacy and safety of nebivolol in elderly heart failure patients with impaired renal function: insights from the SENIORS trial. European Journal of Heart Failure 2009;11(9):872-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer RA, Doehner W, Horst IC, Anker SD, Babalis D, Rougthon M, et al. Influence of diabetes mellitus and hyperglycemia on prognosis in patients > or =70 years old with heart failure and effects of nebivolol (data from the Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors with heart failure [SENIORS]). American Journal of Cardiology 2010;106(1):78-86. [DOI] [PubMed] [Google Scholar]
- Flather MD, Shibata MC, Coats AJS, Van Veldhuisen DJ, Parkhomenko A, Borbola J, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). European Heart Journal 2005;26:215-25. [DOI] [PubMed] [Google Scholar]
- Ghio S, Magrini G, Serio A, Klersy C, Fucili A, Ronaszeki A, Karpati P, et al. Effects of nebivolol in elderly heart failure patients with or without systolic left ventricular dysfunction: results of the SENIORS echocardiographic substudy. European Heart Journal 2006;27(5):562-8. [DOI] [PubMed] [Google Scholar]
- Lindsey ML, Freeman GL. Beta-blockade in heart failure: adding SENIORS to the mix. European Heart Journal 2006;27(5):506-7. [DOI] [PubMed] [Google Scholar]
- Montero-Perez-Barquero M, Flather M, Roughton M, Coats A, Bohm M, Van Veldhuisen DJ, et al. Influence of systolic blood pressure on clinical outcome in elderly heart failure patients with preserved and reduced ejection fraction treated with nebivolol: data from SENIORS trial. European Heart Journal 2014;35:339. [DOI] [PubMed] [Google Scholar]
- Montero-Perez-Barquero M, Flather M, Roughton M, Coats A, Bohm M, Van Veldhuisen DJ, et al. Influence of systolic blood pressure on clinical outcomes in elderly heart failure patients treated with nebivolol: data from the SENIORS trial. European Journal of Heart Failure 2014;16(9):1009-15. [DOI] [PubMed] [Google Scholar]
- Mulder BA, Veldhuisen DJ, Crijns HJ, Bohm M, Cohen-Solal A, Babalis D, et al. Effect of nebivolol on outcome in elderly patients with heart failure and atrial fibrillation: insights from SENIORS. European Journal of Heart Failure 2012;14(10):1171-8. [DOI] [PubMed] [Google Scholar]
- Shibata MC, Flather MD, Bohm M, Borbola J, Cohen-Solal A, Dumitrascu D, et al. Study of the effects of nebivolol intervention on outcomes and reshospitalisation in seniors with heart failure (SENIORS). International Journal of Cardiology 2002;86:77-85. [DOI] [PubMed] [Google Scholar]
- Veldhuisen DJ, Cohen-Solal A, Bohm M, Anker SD, Babalis D, Roughton M, et al. Beta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: Data From SENIORS (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure). Journal of the American College of Cardiology 2009;53(23):2150-8. [DOI] [PubMed] [Google Scholar]
- Von Haehling S, Babalis D, Roghton M, Van Veldhuisen DJ, Poole-Wilson PA, Flather M, et al. Prevalence of anaemia and the effects of nebivolol on haemoglobin values in patients with chronic heart failure: Results from the SENIORS database. Journal of Cardiac Failure 2009;15(6):S96. [Google Scholar]
- Haehling S, Veldhuisen DJ, Roughton M, Babalis D, Boer RA, Coats AJ, et al. Anaemia among patients with heart failure and preserved or reduced ejection fraction: results from the SENIORS study. European Journal of Heart Failure 2011;13(6):656-63. [DOI] [PubMed] [Google Scholar]
Shu 2005 {published data only}
- Shu M, Xi R, Zhang P, He G, Song Z, Chi L, et al. Short-term and long-term effects of bisoprolol on chronic heart failure related to rheumatic heart disease and atrial fibrillation. Pharmacy and Therapeutics 2005;30(7):400-7. [Google Scholar]
SNEGOVIK {published data only}
- Mareev V, Skvortsov A, Danielyan M, Belenkov YU. Quinapril in treatment of ambulatory patients with arterial hypertension and congestive heart failure and preserved systolic left ventricular function. (Results from the SNEGOVIK Study). European Journal of Heart Failure. Supplement 2011;10:S78. [Google Scholar]
STRUCTURE {published data only}
- ACTRN12614000088640. Spironolactone in myocardial dysfunction with reduced exercise capacity [Effects of spironolactone or control on LV filling pressure and exercise capacity in patients with myocardial dysfunction with reduced exercise capacity]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=365563 (data submitted 6 January 2014).
- Kosmala W, Rojek A, Przewlocka-Kosmala M, Wright L, Mysiak A, Marwick TH. Beneficial effect of aldosterone antagonism on exercise tolerance in heart failure with preserved ejection fraction-SpironolacTone in myocaRdial dysfunction with reduced exercise capacity (STRUCTURE). European Heart Journal 2016;37:795. [Google Scholar]
- Kosmala W, Rojek A, Przewlocka-Kosmala M, Wright L, Mysiak A, Marwick TH. Effect of aldosterone antagonism on exercise tolerance in heart failure with preserved ejection fraction. Journal of the American College of Cardiology 2016;68(17):1823-34. [DOI] [PubMed] [Google Scholar]
- Kosowski W, Kabaj M, Przewlocka-Kosmala M, Mysiak A, Marwick T, Kosmala W. Usefulness of myocardial work measurement in the assessment of left ventricular systolic reserve response to spironolactone in heart failure with preserved ejection fraction. Kardiologia Polska 2018;76(Suppl 1):120-1. [DOI] [PubMed] [Google Scholar]
SUPPORT {published data only}
- Miura M, Sakata Y, Miyata S, Shiba N, Takahashi J, Nochioka K, et al. Influence of left ventricular ejection fraction on the effects of supplemental use of angiotensin receptor blocker olmesartan in hypertensive patients with heart failure. Circulation Journal 2016;80(10):2155-64. [DOI] [PubMed] [Google Scholar]
- Miura M, Sakata Y, Miyata S, Tadaki S, Ushigome R, Yamouchi T, et al. The supplemental benefit of an angiotensin receptor blocker in hypertensive patients with stable heart failure using olmesartan (SUPPORT) trial. Journal of Cardiac Failure 2015;21(10):S159. [DOI] [PubMed] [Google Scholar]
- NCT00417222. Supplemental benefit of angiotensin receptor blocker in hypertensive patients with stable heart failure using olmesartan (SUPPORT). clinicaltrials.gov/ct2/show/NCT00417222 (first posted 29 December 2006).
- Sakata Y, Shiba N, Takahashi J, Miyata S, Nochioka K, Miura M, et al. Clinical impacts of additive use of olmesartan in hypertensive patients with chronic heart failure: The supplemental benefit of an angiotensin receptor blocker in hypertensive patients with stable heart failure using olmesartan (SUPPORT) trial. European Heart Journal 2015;36(15):915-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
SWEDIC {published data only}
- Bergstrom A, Andersson B, Edner M, Nylander E, Persson H, Dahlstrom U. Effect of carvedilol on diastolic function in patients with diastolic heart failure and preserved systolic function. Results of the Swedish Doppler-echocardiographic study (SWEDIC). European Journal of Heart Failure 2004;6(4):453-61. [DOI] [PubMed] [Google Scholar]
Takeda 2004 {published data only}
- Takeda Y, Fukutomi T, Suzuki S, Yamamoto K, Ogata M, Kondo H, et al. Effects of carvedilol on plasma B-type natriuretic peptide concentration and symptoms in patients with heart failure and preserved ejection fraction. American Journal of Cardiology 2004;94:448-53. [DOI] [PubMed] [Google Scholar]
TOPCAT {published data only}
- Anand IS, Claggett B, Liu J, Shah AM, Rector TS, Shah SJ, et al. Interaction between spironolactone and natriuretic peptides in patients with heart failure and preserved ejection fraction: from the TOPCAT trial. JACC. Heart Failure 2017;5(4):241-52. [DOI] [PubMed] [Google Scholar]
- Anand IS, Solomon SD, Claggett B, Shah SJ, O'Meara E, Boineau R, et al. Prognostic value of baseline BNP and NT-proBNP and its interaction with spironolactone in patients with heart failure and preserved ejection fraction in the TOPCAT trial. Circulation 2015;132:no pagination. [Google Scholar]
- Bajaj NS, Claggett B, Lewis E, Desai A, Fang J, Omeara E, et al. Influence of left ventricular ejection fraction on cause-specific mortality in heart failure with preserved ejection fraction: The TOPCAT trial. Journal of the American College of Cardiology 2017;69(11):884. [Google Scholar]
- Bristow MR, Enciso JS, Gersh BJ, Grady C, Rice MM, Singh S, et al. Detection and management of geographic disparities in the TOPCAT trial: lessons learned and derivative recommendations. JACC: Basic to Translational Science 2016;1(3):180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, et al. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. American Heart Journal 2011;162(6):966-72. [DOI] [PubMed] [Google Scholar]
- Desai AS, Liu J, Claggett B, Bristow M, Fleg J, Lewis EF, et al. Incidence and predictors of doubling of serum creatinine during treatment of heart failure and preserved ejection fraction with spironolactone. European Heart Journal 2015;36:662-3. [Google Scholar]
- Girerd N, Ferreira JP, Rossignol P, Zannad F. A tentative interpretation of the TOPCAT trial based on randomized evidence from the brain natriuretic peptide stratum analysis. European Journal of Heart Failure 2016;18(12):1411-4. [DOI] [PubMed] [Google Scholar]
- Hamo CE, Heitner JF, Pfeffer MA, Kim HY, Kenwood CT, Assmann SF, et al. Baseline distribution of participants with depression and impaired quality of life in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial. Circulation 2015;8(2):268-77. [DOI] [PubMed] [Google Scholar]
- Hegde S, Claggett B, Shah AM, Lewis EF, Anand IS, Shah SJ, et al. Physical activity and prognosis in the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) trial. Circulation 2017;21:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J, Claggett BC, Anand IS, Fleg JL, Huynh T, Desai AS, et al. QRS duration is a predictor of adverse outcomes in heart failure with preserved ejection fraction. JACC. Heart Failure 2016;4(6):477-86. [DOI] [PubMed] [Google Scholar]
- Joseph J, Claggett BL, Anand IS, Fleg JL, Huynh T, Desai AS, et al. Prolonged QRS duration predicts outcomes in heart failure with preserved ejection fraction. Circulation 2015;132(Suppl 3):A11159. [Google Scholar]
- Kao DP, Flint KM, Merrill M. Spironolactone reduces all-cause mortality in women but not men with heart failure with preserved ejection fraction enrolled in TOPCAT from the Americas. European Journal of Heart Failure 2017;19:530-1. [Google Scholar]
- Kelly J, Granger CB. Spironolactone did not reduce cardiac outcomes in symptomatic heart failure with preserved ejection fraction. Annals of Internal Medicine 2014;161(8):JC6. [DOI] [PubMed] [Google Scholar]
- Kelsey Fling K, Merrill M, Kao D. Sex differences in long-term health status in heart failure with preserved ejection fraction. European Journal of Heart Failure 2017;19:530-1. [Google Scholar]
- Kitai T, Grodin J, Wilson Tang WH. Paradoxical impact of proportional pulse pressure on mortality in patients with heart failure with preserved ejection fraction. Journal of the American College of Cardiology 2017;69(11):882. [Google Scholar]
- Kristensen SL, Kober L, Jhund PS, Solomon SD, Kjekshus J, McKelvie RS, et al. International geographic variation in event rates in trials of heart failure with preserved and reduced ejection fraction. Circulation 2015;131(1):43-53. [DOI] [PubMed] [Google Scholar]
- Merrill M, Sweitzer NK, Lindenfeld JA, Kao KP. Sex differences in outcomes and responses to spironolactone in heart failure with preserved ejection fraction: a secondary analysis of TOPCAT trial. JACC Heart Failure 2019;7(3):228-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawietz H, Bornstein SR. Spironolactone for heart failure with preserved ejection fraction. New England Journal of Medicine 2014;371(2):181. [DOI] [PubMed] [Google Scholar]
- NCT00094302. Aldosterone Antagonist Therapy for Adults With Heart Failure and Preserved Systolic Function (TOPCAT) [Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT)]. clinicaltrials.gov/ct2/show/NCT00094302 (First posted 15 October 2004).
- Neefs J, Van Den Berg NW, Berger WR, Meulendijks ER, Groot JR. Risk of atrial fibrillation in heart failure with preserved ejection fraction: results from the treatment of cardiac function with an aldosterone antagonist (TOPCAT). European Heart Journal 2017;38(Suppl 1):759-60. [Google Scholar]
- Neefs J, den Berg NW, Krul SP, Boekholdt SM, Groot JR. Effect of spironolactone on atrial fibrillation in patients with heart failure with preserved ejection fraction: post-hoc analysis of the randomized, placebo-controlled TOPCAT trial. American Journal of Cardiovascular Drugs 2020;20:73-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Meara E, Kenwood CT, Anand IS, Desai AS, De Denus S, Sweitzer NK, et al. Anemia in heart failure with preserved ejection fraction: insights from the Topcat trial. Circulation 2015;132(Suppl 3):A17975. [Google Scholar]
- O'Neal WT, Sandesara P, Patel N, Venkatesh S, Samman-Tahhan A, Hammadah M, et al. Echocardiographic predictors of atrial fibrillation in patients with heart failure with preserved ejection fraction. European Heart Journal - Cardiovascular Imaging 2017;30:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal WT, Sandesara PB, Samman-Tahhan A, Kelli HM, Hammadah M, Soliman EZ. Heart rate and the risk of adverse outcomes in patients with heart failure with preserved ejection fraction. European Journal of Preventive Cardiology 2017;24(11):1212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer MA, Braunwald E. Treatment of heart failure with preserved ejection fraction: reflections on its treatment with an aldosterone antagonist. JAMA Cardiology 2016;1(1):7-8. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardia Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015;131(1):34-42. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, McKinlay S, Pitt B. Treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT). Circulation 2013;128(24):2709. [Google Scholar]
- Pitt B, Pfeffer MA, Assmann S, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. New England Journal of Medicine 2014;370(15):1383-92. [DOI] [PubMed] [Google Scholar]
- Sandesara PB, Hammadah M, Samman-Tahhan A, Kelli HM, O'Neal WT. Peripheral artery disease and risk of adverse outcomes in heart failure with preserved ejection fraction. Clinical Cardiology 2017;26:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos AB, Roca GQ, Claggett B, Sweitzer NK, Shah SJ, Anand IS, et al. Prognostic relevance of left atrial dysfunction in heart failure with preserved ejection fraction. Circulation 2016;9(4):e002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, et al. Impaired systolic function assessed by strain imaging predicts cardiovascular morbidity and mortality in heart failure with preserved ejection fraction. Circulation 2014;130(Suppl 1):A16989. [Google Scholar]
- Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, et al. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation 2015;132(5):402-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O'Meara E, et al. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction. Circulation. Heart Failure 2014;7(5):740-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AM, Claggett B, Sweitzer NK, Shah SJ, Deswal A, Anand IS, et al. Prognostic importance of changes in cardiac structure and function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation 2015;8(6):1052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AM, Shah SJ, Anand IS, Sweitzer NK, O'Meara E, Heitner JF, et al. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circulation 2014;7(1):104-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SJ, Heitner JF, Sweitzer NK, Anand IS, Kim HY, Harty B, et al. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circulation 2013;6(2):184-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK, et al. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. European Heart Journal 2016;37(5):455-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardeny O, Claggett B, Vaduganathan M, Beldhuis I, Rouleau J, O'Meara E, et al. Influence of age on efficacy and safety of spironolactone in heart failure. JACC: Heart Failure 2019;7(12):1022-8. [DOI] [PubMed] [Google Scholar]
- Vardeny O, Vaduganathan M, Claggett B, Desai AS, Anand IS, Shah SJ, et al. Influence of age on the efficacy and safety of spironolactone in heart failure with preserved ejection fraction. Journal of Cardiac Failure 2019;25(8S):S12. [Google Scholar]
Upadhya 2017 {published and unpublished data}
- NCT00123955. PIE II: pharmacological intervention in the elderly II [Exercise intolerance in elderly diastolic heart failure]. clinicaltrials.gov/show/NCT00123955 (first posted 26 July 2005).
- Upadhya B, Hundley WG, Brubaker PH, Morgan TM, Stewart KP, Kitzman DW. Effect of spironolactone on exercise tolerance and arterial function in older adults with heart failure with preserved ejection fraction. Journal of the American Geriatric Society 2017;19:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang YC, Yu CC, Chiu FC, Tsai CT, Lai LP, Hwang JJ, et al. Aldosterone antagonism increases post-exercise recruitment of systolic myocardial motion over left ventricular lateral wall in hypertensive patients with diastolic heart failure. Circulation 2010;122(2):P1059. [Google Scholar]
Yuksek 2012 {published data only}
- Yuksek U, Cerit L, Eren NK, Ergene O. The effect of perindopril on echocardiographic parameters, NYHA functional class and serum NT-proBNP values in patients with diastolic heart failure. Cardiovascular Journal of Africa 2019;30(4):222-7. [DOI] [PubMed] [Google Scholar]
- Yuksek U, Hamza D, Zehra A, Ugur K, Nihan KE, Rida B, et al. The effect of perindopril treatment on echocardiographic diastolic and systolic functional parameters and serum NT-proBNP values in diastolic heart failure patients. European Journal of Heart Failure. Supplements 2012;11:S231. [Google Scholar]
Zi 2003 {published data only}
- Zi M, Carmichael N, Lye M. The effect of quinapril on functional status of elderly patients with diastolic heart failure. Cardiovascular Drugs and Therapy 2003;17:133-9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12610001087044 {published data only}
- ACTRN12610001087044. Use of exercise and medical therapies to improve cardiac function among patients with exertional shortness of breath due to lung congestion. www.anzctr.org.au/ACTRN12610001087044.aspx (date received 13 December 2010).
- Kosmala W, Holland DJ, Rojek A, Wright L, Przewlocka-Kosmala M, Marwick TH. Effect of If-channel inhibition on hemodynamic status and exercise tolerance in heart failure with preserved ejection fraction: a randomized trial. Journal of the American College of Cardiology 2013;62(15):1330-8. [DOI] [PubMed] [Google Scholar]
Adgey 1992 {published data only}
- Adgey J, Clarke M, Raza A, Meddis D. Study of the safety and efficacy of ACE inhibitors and their effects on 24-hour electrocardiographic monitoring in the treatment of moderate-to-severe heart failure: an interim analysis. American Journal of Cardiology 1992;70(10):142C-4C. [DOI] [PubMed] [Google Scholar]
Ammon 2001 {published data only}
- Ammon S. Managing patients with heart failure. American Journal of Nursing 2001;101(12):34-40. [DOI] [PubMed] [Google Scholar]
Andersson 1996 {published data only}
- Andersson B, Caidahl K, di Lenarda A, Warren SE, Goss F, Waldenstrom A, et al. Changes in early and late diastolic filling patterns induced by long-term adrenergic beta-blockade in patients with idiopathic dilated cardiomyopathy. Circulation 1996;94(4):673-82. [DOI] [PubMed] [Google Scholar]
Andersson 1999 {published data only}
- Andersson B, Stromblad SO, Lomsky M, Waagstein F. Heart rate dependency of cardiac performance in heart failure patients treated with metoprolol. European Heart Journal 1999;20(8):575-83. [DOI] [PubMed] [Google Scholar]
Andersson 2000 {published data only}
- Andersson B, Waagstein F, Caidahl K, Eurenius I, Tang MS, Wikh R. Early changes in longitudinal performance predict future improvement in global left ventricular function during long term beta adrenergic blockade. Heart 2000;84(6):599-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anonymous 1996 {published data only}
- Anonymous. Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]). American Journal of Cardiology 1996;78(8):902-7. [DOI] [PubMed] [Google Scholar]
Anonymous 1999 {published data only}
- Anonymous. Heart failure management in primary care: implications of the ATLAS study. International Journal of Clinical Practice. Supplement 1999;100:25-30. [PubMed] [Google Scholar]
Anonymous 2000 {published data only}
- Anonymous. Effects of metoprolol CR in patients with ischemic and dilated cardiomyopathy: the randomized evaluation of strategies for left ventricular dysfunction pilot study. Circulation 2000;101(4):378-84. [DOI] [PubMed] [Google Scholar]
Anonymous 2001 {published data only}
- Anonymous. Carvedilol saves lives - new data from landmark trials prove survival benefits in heart failure and post myocardial infarction. Cardiovascular Journal of South Africa 2001;12(2):122-3. [PubMed] [Google Scholar]
Anonymous 2002 {published data only}
- Anonymous. Stress intolerance in diastolic dysfunction. A case for AT1 blocker? MMW Fortschritte der Medizin 2002;144(1-2):69. [PubMed] [Google Scholar]
Anonymous 2003 {published data only}
- Anonymous. When after myocardial infarction a pump weakness remains. ACE inhibitors and AT-1 blockers equivalent. MMW Fortschritte der Medizin 2003;145(49):18-9. [PubMed] [Google Scholar]
Anonymous 2003a {published data only}
- Anonymous. CHARM shows benefits of Atacand for symptomatic heart failure. Cardiovascular Journal of South Africa 2003;14(5):284. [PubMed] [Google Scholar]
Anonymous 2003b {published data only}
- Anonymous. Heart failure symptoms despite normal pump function. Beta blockers also improve diastole. MMW Fortschritte der Medizin 2003;145(26):58. [PubMed] [Google Scholar]
Anonymous 2003c {published data only}
- Anonymous. NMR shows how beta blockers change the failing ventricle. Decreased size and better output performance. MMW Fortschritte der Medizin 2003;145(25):56. [PubMed] [Google Scholar]
Anonymous 2005 {published data only}
- Anonymous. Heart failure after acute myocardial infarct. Early aldosterone blockade increases survival rate. MMW Fortschritte der Medizin 2005;147(27-28):44. [PubMed] [Google Scholar]
Anonymous 2008 {published data only}
- Anonymous. Diastolic heart failure: AT1 blocker without effect. MMW Fortschritte der Medizin 2008;150(47):13. [PubMed] [Google Scholar]
Anonymous 2008a {published data only}
- Anonymous. Misdiagnosed emergency: heart failure symptoms after infarct. Rapid aldosterone block can safe the tired heart. MMW Fortschritte der Medizin 2008;150(22):48-9. [PubMed] [Google Scholar]
Anonymous 2013 {published data only}
- Anonymous. Spironolactone made no difference to patients with mild diastolic heart failure. BMJ 2013;346:f1290. [DOI] [PubMed] [Google Scholar]
ANZ HF carvedilol {published data only}
- Australia/New Zealand Heart Failure Research Collaborative Group. Randomised, placebo-controlled trial of carvedilol in patients with congestive heart failure due to ischaemic heart disease. Lancet 1997;349(9049):375-80. [PubMed] [Google Scholar]
- Australia–New Zealand Heart Failure Research Collaborative Group. Effects of carvedilol, a vasodilator-beta-blocker, in patients with congestive heart failure due to ischemic heart disease. Australia-New Zealand Heart Failure Research Collaborative Group. Circulation 1995;92(2):212-8. [PubMed] [Google Scholar]
- Doughty RN, Whalley GA, Gamble G, MacMahon S, Sharpe N. Effects of carvedilol on left ventricular regional wall motion in patients with heart failure caused by ischemic heart disease. Journal of Cardiac Failure 2000;6(1):11-8. [DOI] [PubMed] [Google Scholar]
- Doughty RN, Whalley GA, Gamble G, MacMahon S, Sharpe N. Left ventricular remodeling with carvedilol in patients with congestive heart failure due to ischemic heart disease. Australia-New Zealand Heart Failure Research Collaborative Group. Journal of the American College of Cardiology 1997;29(5):1060-6. [DOI] [PubMed] [Google Scholar]
- Krum H, Shusterman N, MacMahon S, Sharpe N. Efficacy and safety of carvedilol in patients with chronic heart failure receiving concomitant amiodarone therapy. Australia/New Zealand Heart Failure Research Collaborative Group. Journal of Cardiac Failure 1998;4(4):281-8. [DOI] [PubMed] [Google Scholar]
- Krum H, Tonkin A, Trotter A, Burton R, Garrett J, Lane G, et al. Effects of carvedilol, a vasodilator-β-blocker, in patients with congestive heart failure due to ischemic heart disease. Circulation 1995;92(2):212-8. [PubMed] [Google Scholar]
- Richards AM, Doughty R, Nicholls MG, Macmahon S, Ikram H, Sharpe N, et al. Neurohumoral prediction of benefit from carvedilol in ischemic left ventricular dysfunction. Circulation 1999;99(6):768-92. [DOI] [PubMed] [Google Scholar]
- Richards AM, Doughty R, Nicholls MG, MacMahon S, Sharpe N, Murphy J, et al. Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin: prognostic utility and prediction of benefit from carvedilol in chronic ischemic left ventricular dysfunction. Journal of the American College of Cardiology 2001;37(7):1781-7. [DOI] [PubMed] [Google Scholar]
- Sharpe N, Doughty RN. Left ventricular remodelling and improved long-term outcomes in chronic heart failure. European Heart Journal 1998;19(Suppl B):B36-9. [PubMed] [Google Scholar]
Aoyama 2007 {published data only}
- Aoyama N. Effect of combination therapy with angiotensin II type I receptor and aldosterone receptor blockers in heart failure. Nippon Rinsho 2007;65(Suppl 5):145-9. [PubMed] [Google Scholar]
Apostolovic 2013 {published data only}
- Apostolovic S, Stanojevic D, Jankovic-Tomasevic R, Salinger-Martinovic S, Djordjevic-Radojkovic D, Pavlovic M, et al. Influence of optimal beta blocker therapy on biomarkers of inflammation and proliferation in elderly patients with chronic stable heart failure. European Journal of Heart Failure 2013;12:S200. [Google Scholar]
Apostolovic 2014a {published data only}
- Apostolovic S, Stanojevic D, Jankovic-Tomasevic R, Salinger-Martinovic S, Pavlovic M, Djordjevic-Radojkovic D, et al. Beta-blockers in heart failure-influence on left ventricle remodeling. CIBIS ELD sub-study. European Heart Journal Cardiovascular Imaging 2014;15:ii213. [Google Scholar]
Apostolovic 2014b {published data only}
- Apostolovic S, Stanojevic D, Salinger-Martinovic S, Jankovic- Tomasevic R, Djordjevic-Radojkovic D, Pavlovic M, et al. Influence of optimization of beta blocker therapy in heart failure patients on natriuretic peptide levels and exercise capacity. European Heart Journal: Acute Cardiovascular Care 2014;3(2):176. [Google Scholar]
Arena 2007 {published data only}
- Arena RA, Guazzi M, Myers J, Abella J. The prognostic value of ventilatory efficiency with beta-blocker therapy in heart failure. Medicine & Science in Sports & Exercise 2007;39(2):213-9. [DOI] [PubMed] [Google Scholar]
Armstrong 1999 {published data only}
- Armstrong PW. Improved outcome in heart failure. International Journal of Clinical Practice. Supplement 1999;100:10-1. [PubMed] [Google Scholar]
Aronow 1991 {published data only}
- Aronow WS. Digoxin or angiotensin converting enzyme inhibitors for congestive heart failure in geriatric patients. Which is the preferred treatment? Drugs and Aging 1991;1(2):98-103. [DOI] [PubMed] [Google Scholar]
Aronow 2001 {published data only}
- Aronow WS, Ahn C, Kronzon I. Effect of beta blockers alone, of angiotensin-converting enzyme inhibitors alone, and of beta blockers plus angiotensin-converting enzyme inhibitors on new coronary events and on congestive heart failure in older persons with healed myocardial infarcts and asymptomatic left ventricular systolic dysfunction. American Journal of Cardiology 2001;88(11):1298-300. [DOI] [PubMed] [Google Scholar]
Axelsson 2015 {published data only}
- Axelsson A, Iversen K, Vejlstrup N, Ho C, Havndrup O, Jensen M K, et al. The effect of losartan on diastolic function, exercise capacity and symptoms in hypertrophic cardiomyopathy. JACC 2015;65(10):A857. [Google Scholar]
Balaban 2007 {published data only}
- Balaban Y, Ozhan H, Albayrak S, Kaya A, Ordu, Yazici M. Efficacy of spiranolactone therapy in patients with impaired left ventricular filling [Sol ventrikul doluzu bozulmus hastalarda spironolakton tedavisinin etkinligi]. MN Kardiyoloji 2007;14(3):182-6. [Google Scholar]
Bao 2005 {published data only}
- Bao ZH, Han Y, Sheng ZS. Carvedilol for the intervention of the cardiac function and exercise tolerance in patients with congestive heart failure. Chinese Journal of Clinical Rehabilitation 2005;9(15):53-5. [Google Scholar]
Barr 1995 {published data only}
- Barr CS, Lang CC, Hanson J, Arnott M, Kennedy N, Struthers AD. Effects of adding spironolactone to an angiotensin-converting enzyme inhibitor in chronic congestive heart failure secondary to coronary artery disease. American Journal of Cardiology 1995;76(17):1259-65. [DOI] [PubMed] [Google Scholar]
Barrios 2009 {published data only}
- Barrios V, Escobar C, Echarri R. Letter by Barrios et al regarding article, "Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial". Circulation 2009;120(5):e31. [DOI] [PubMed] [Google Scholar]
Barry 2003 {published data only}
- Barry WH, Gilbert EM. How do beta-blockers improve ventricular function in patients with congestive heart failure? Circulation 2003;107(19):2395-7. [DOI] [PubMed] [Google Scholar]
Bartels 1999 {published data only}
Baruch 1999 {published data only}
- Baruch L, Anand I, Cohen IS, Ziesche S, Judd D, Cohn JN. Augmented short- and long-term hemodynamic and hormonal effects of an angiotensin receptor blocker added to angiotensin converting enzyme inhibitor therapy in patients with heart failure. Vasodilator Heart Failure Trial (V-HeFT) Study Group. Circulation 1999;99(20):2658-64. [DOI] [PubMed] [Google Scholar]
Baruch 2004 {published data only}
- Baruch L, Glazer RD, Aknay N, Vanhaecke J, Heywood JT, Anand I, et al. Morbidity, mortality, physiologic and functional parameters in elderly and non-elderly patients in the Valsartan Heart Failure Trial (Val-HeFT). American Heart Journal 2004;148(6):951-7. [DOI] [PubMed] [Google Scholar]
Bauersachs 2004 {published data only}
- Bauersachs J. Aldosterone antagonism in heart failure: improvement of cardiac remodelling, endothelial dysfunction and platelet activation. European Journal of Clinical Investigation 2004;34(10):649-52. [DOI] [PubMed] [Google Scholar]
Baumhakel 2008 {published data only}
- Baumhakel M, Muller U, Bohm M. Valsartan improves symptoms and quality of life in patients with chronic heart failure. MMW Fortschritte der Medizin 2008;150(Suppl 1):48-53. [PubMed] [Google Scholar]
Bellenger 2004 {published data only}
- Bellenger NG, Rajappan K, Rahman SL, Lahiri A, Raval U, Webster J, et al. Effects of carvedilol on left ventricular remodelling in chronic stable heart failure: a cardiovascular magnetic resonance study. Heart 2004;90(7):760-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berry 2001 {published data only}
- Berry C, Norrie J, McMurray JJV. Are angiotensin II receptor blockers more efficacious than placebo in heart failure? Implications of ELITE-2. American Journal of Cardiology 2001;87(5):606-7. [DOI] [PubMed] [Google Scholar]
Bettencourt 1999 {published data only}
- Bettencourt P. Treating diastolic heart failure: the role of ACE inhibitors. Revista Portuguesa de Cardiologia 1999;18(Suppl 5):V101-6. [PubMed] [Google Scholar]
Beygui 2016 {published data only}
- Beygui F, Cayla G, Roule V, Roubille F, Delarche N, Silvain J, et al. Early aldosterone blockade in acute myocardial infarction: The ALBATROSS randomized clinical trial. Journal of the American College of Cardiology 2016;67(16):1917-27. [DOI] [PubMed] [Google Scholar]
Blagodar 2003 {published data only}
- Blagodar VN, Petrii VV, Makolkin VI. Effect of lisinopril on cardiac remodeling in patients with cardiosclerosis after myocardial infarction and signs of chronic heart failure. Kardiologiia 2003;43(9):17-20. [PubMed] [Google Scholar]
Blomer 1990 {published data only}
- Blomer H. Treatment of heart failure: what - when - how? Verhandlungen Der Deutschen Gesellschaft Fur Innere Medizin 1990;96:157-67. [PubMed] [Google Scholar]
Borghi 2011 {published data only}
- Borghi C. Double-blind, randomized comparison of zofenopril vs. ramipril in MI patients treated with ASA: The SMILE 4 study and subgroup analysis. Circulation 2011;124(21):no pagination. [Google Scholar]
Borgi 1990 {published data only}
- Borgi C, Magelli C, Costa FV, Magnani B, Ambrosioni E. Captopril improves hemodynamic response to static exercise in patients with congestive heart failure: a double-blind, placebo-controlled, randomized trial. Clinical Cardiology 1990;13(5):329-34. [DOI] [PubMed] [Google Scholar]
Borlaug 2014 {published data only}
- Borlaug BA. MY APPROACH to heart failure with preserved ejection fraction. Trends in Cardiovascular Medicine 2014;24(8):369-70. [DOI] [PubMed] [Google Scholar]
Bornkessel 1992 {published data only}
- Bornkessel B, Heinzl S. ACE-inhibitors and heart failure. Results of studies of left ventricular dysfunction. Medizinische Monatsschrift für Pharmazeuten 1992;15(3):76-80. [PubMed] [Google Scholar]
Bounhoure 1991 {published data only}
- Bounhoure J P. Perindopril and chronic heart failure. Archives Des Maladies Du Coeur Et Des Vaisseaux 1991;84(Spec No 4):89-92. [PubMed] [Google Scholar]
Braunwald 2004 {published data only}
- Braunwald E, Domanski MJ, Fowler SE, Geller NL, Gersh BJ, Hsia J, et al. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. New England Journal of Medicine 2004;351(20):2058-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brilla 1989 {published data only}
- Brilla CG, Kramer B, Hoffmeister HM, Muller-Schauenburg W, Risler T, Seipel L. Low-dose enalapril in severe chronic heart failure. Cardiovascular Drugs and Therapy 1989;3(2):211-8. [DOI] [PubMed] [Google Scholar]
Brilla 1991 {published data only}
- Brilla CG, Kramer B, Risler T, Seipel L. Low-dose enalapril in severe chronic heart failure [Niedrigdosiertes Enalapril bei schwerer chronischer Herzinsuffizienz]. Zeitschrift für Kardiologie 1991;80(Suppl 2):44-9. [PubMed] [Google Scholar]
Bristow 1994 {published data only}
- Bristow MR, O'Connell JB, Gilbert EM, French WJ, Leatherman G, Kantrowitz NE, et al. Dose-response of chronic beta-blocker treatment in heart failure from either idiopathic dilated or ischemic cardiomyopathy. Bucindolol Investigators. Circulation 1994;89(4):1632-42. [DOI] [PubMed] [Google Scholar]
Bristow 1996 {published data only}
- Bristow MR, Gilbert EM, Abraham WT, Adams KF, Fowler MB, Hershberger RE, et al. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation 1996;94(11):2807-16. [DOI] [PubMed] [Google Scholar]
Bussmann 1987 {published data only}
- Bussmann WD, Storger H, Hadler D, Reifart N, Fassbinder W, Jungmann E, et al. Long-term treatment of severe chronic heart failure with captopril: a double-blind, randomized, placebo-controlled, long-term study. Journal of Cardiovascular Pharmacology 1987;9(Suppl 2):S50-60. [DOI] [PubMed] [Google Scholar]
Butler 2017 {published data only}
- Butler J, Anstrom KJ, Felker GM, Givertz MM, Kalogeropoulos AP, Konstam MA, et al. Efficacy and safety of spironolactone in acute heart failure: the ATHENA-HF randomized clinical trial. JAMA Cardiology 2017;2(9):950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J, Hernandez AF, Anstrom KJ, Kalogeropoulos A, Redfield MM, Konstam MA, et al. Rationale and design of the ATHENA-HF trial: aldosterone targeted neurohormonal combined with natriuresis therapy in heart failure. Journal of the American College of Cardiology. Heart Failure 2016;4(9):726-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene SJ, Felker M, Giczewska A, Kalogeropoulos AP, Ambrosy AP, Chakraborty H, et al. Spironolactone in acute heart failure patients with renal dysfunction in risk factors for diuretic resistance: from the ATHENA-HF trial. Canadian Journal of Cardiology 2019;35:10978-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02235077. Study of high-dose spironolactone vs placebo therapy in acute heart failure (ATHENA-HF). clinicaltrials.gov/show/NCT02235077 (first received 9 September 2014).
Cafaro 2010 {published data only}
- Cafaro B, Barr S, Brand JL. Question: does beta-blocker therapy improve outcomes in patients with congestive heart failure with normal left ventricular function (diastolic dysfunction)? Journal of the Oklahoma State Medical Association 2010;103(2):51-2. [PubMed] [Google Scholar]
Cardoso 1999 {published data only}
- Cardoso JS. Treating diastolic heart failure: the role of beta-blockers. Revista Portuguesa de Cardiologia 1999;18(Suppl 5):V97-9. [PubMed] [Google Scholar]
Castagno 2010 {published data only}
- Castagno D, Jhund PS, Weir CJ, Colucci WS, Lopez Sendon JL, Remme WJ, et al. Effect of carvedilol in patients with or without renal impairment after myocardial infarction:analysis of the carvedilol post-infarct survival control in left ventricular dysfunction (CAPRICORN) study. European Heart Journal 2010;31:571. [Google Scholar]
ChiCTR1800016350 {published data only}
- ChiCTR1800016350. Effect of allisartan isoproxil tablets on ventricular remodeling and cardiac function in patients with chronic congestive heart failure. www.chictr.org.cn/showprojen.aspx?proj=27554 (date of registration 26 May 2018).
Choi 2001 {published data only}
- Choi JY, Lee KH, Hong KP, Kim BT, Seo JD, Lee WR, et al. Iodine-123 MIBG imaging before treatment of heart failure with carvedilol to predict improvement of left ventricular function and exercise capacity. Journal of Nuclear Cardiology 2001;8(1):4-9. [DOI] [PubMed] [Google Scholar]
Cicoira 2002 {published data only}
- Cicoira M, Rossi A, Bonapace S, Zanolla L, Perrot A, Francis A, et al. Effects of ACE gene insertion/deletion polymorphism on response to spironolactone in patients with chronic heart failure. American Journal of Medicine 2004;116(10):657-61. [DOI] [PubMed] [Google Scholar]
- Cicoira M, Zanolla L, Rossi A, Golia G, Franceschini L, Brighetti G, et al. Long-term, dose-dependent effects of spironolactone on left ventricular function and exercise tolerance in patients with chronic heart failure. Journal of the American College of Cardiology 2002;40(2):304-10. [DOI] [PubMed] [Google Scholar]
Cleland 1984 {published data only}
- Cleland JG, Dargie HJ, Hodsman GP, Ball SG, Robertson JI, Morton JJ, et al. Captopril in heart failure. A double blind controlled trial. British Heart Journal 1984;52(5):530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cleland 1999 {published data only}
- Cleland JG, Pennel D, Ray S, Murray G, MacFarlane P, Cowley A, et al. The carvedilol hibernation reversible ischaemia trial; marker of success (CHRISTMAS). The CHRISTMAS Study Steering Committee and Investigators. European Journal of Heart failure 1999;1(2):191-6. [DOI] [PubMed] [Google Scholar]
Cleland 2001 {published data only}
- Cleland JG. ACE inhibitors for 'diastolic' heart failure? reasons not to jump to premature conclusions about the efficacy of ACE inhibitors among older patients with heart failure. European Journal of Heart Failure 2001;3(6):637-9. [DOI] [PubMed] [Google Scholar]
Cleland 2003 {published data only}
- Cleland JG, Pennell DJ, Ray SG, Coats AJ, Macfarlane PW, Murray GD, et al. Myocardial viability as a determinant of the ejection fraction response to carvedilol in patients with heart failure (CHRISTMAS trial): randomised controlled trial. Lancet 2003;362(9377):14-21. [DOI] [PubMed] [Google Scholar]
Cleland 2004 {published data only}
- Cleland JG, Huan Loh P, Freemantle N, Clark AL, Coletta AP. Clinical trials update from the European Society of Cardiology: SENIORS, ACES, PROVE-IT, ACTION, and the HF-ACTION trial. European Journal of Heart Failure 2004;6(6):787-91. [DOI] [PubMed] [Google Scholar]
Cleland 2006 {published data only}
- Cleland JG, Coletta AP, Clark AL. Clinical trials update from the joint European Society and World Congress of Cardiology meeting: PEP-CHF, ACCLAIM and the HHH study. European Journal of Heart Failure 2006;8(6):658-61. [DOI] [PubMed] [Google Scholar]
Cleland 2007 {published data only}
- Cleland JGF, Coletta AP, Clark AL. Clinical trials update from the American College of Cardiology 2007: ALPHA, EVEREST, FUSION II, VALIDD, PARR-2, REMODEL, SPICE, COURAGE, COACH, REMADHE, pro-BNP for the evaluation of dyspnoea and THIS-diet. European Journal of Heart Failure 2007;9(6-7):740-5. [DOI] [PubMed] [Google Scholar]
Cleland 2010 {published data only}
- Cleland JG, Coletta AP, Freemantle N, Ahmed D, Rubis P, Clark AL. Clinical trials update from the American Heart Association meeting 2009: HEAAL, FAIR-HF, J-CHF, HeartMate II, PACE and a meta-analysis of dose-ranging studies of beta-blockers in heart failure. European Journal of Heart Failure 2010;12(2):197-201. [DOI] [PubMed] [Google Scholar]
Cleland 2011 {published data only}
- Cleland JGF, Coletta AP, Cullington D, Castiello T, De Boer RA, Clark AL. Clinical trials update from the European Society of Cardiology Meeting 2011: ARISTOTLE, SMART-AV: QLV substudy, SHIFT: Echocardiography and quality of life substudies, European CRT Survey, and Basic Science Update. European Journal of Heart Failure 2011;13(12):1376-80. [DOI] [PubMed] [Google Scholar]
Cleland 2013 {published data only}
- Cleland JG, Clark AL, Costanzo P, Francis DP. Diabetes, aliskiren, and heart failure: Let's bring ASTRONAUT down to earth. European Heart Journal 2013;34(40):3097-9. [DOI] [PubMed] [Google Scholar]
Cohen‐Solal 2005 {published data only}
- Cohen-Solal A, Rouzet F, Berdeaux A, Le Guludec D, Abergel E, Syrota A, et al. Effects of carvedilol on myocardial sympathetic innervation in patients with chronic heart failure. Journal of Nuclear Medicine 2005;46(11):1796-803. [PubMed] [Google Scholar]
Cohn 1993 {published data only}
- Cohn JN. ACE inhibitors. Cardiovascular Drugs and Therapy 1993;7(3):379. [DOI] [PubMed] [Google Scholar]
Cohn 1996a {published data only}
- Cohn JN. Slowing the progression of heart failure. European Heart Journal 1996;17(11):1609-11. [DOI] [PubMed] [Google Scholar]
Cohn 1996b {published data only}
- Cohn JN. The management of chronic heart failure. New England Journal of Medicine 1996;335(7):490-8. [DOI] [PubMed] [Google Scholar]
Cohn 2007 {published data only}
- Cohn JN. Myocardial structural effects of aldosterone receptor antagonism in heart failure. JACC 2007;50(7):597-9. [DOI] [PubMed] [Google Scholar]
Coletta 2008 {published data only}
- Coletta AP, Cullington D, Clark AL, Cleland JGF. Clinical trials update from European Society of Cardiology meeting 2008: TIME-CHF, BACH, BEAUTIFUL, GISSI-HF, and HOME-HF. European Journal of Heart Failure 2008;10(12):1264-7. [DOI] [PubMed] [Google Scholar]
Coletta 2009 {published data only}
- Coletta AP, Clark AL, Cleland JG. Clinical trials update from the Heart Failure Society of America and the American Heart Association meetings in 2008: SADHART-CHF, COMPARE, MOMENTUM, thyroid hormone analogue study, HF-ACTION, I-PRESERVE, beta-interferon study, BACH, and ATHENA. European Journal of Heart Failure 2009;11(2):214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Comin‐Colet 2002 {published data only}
- Comin-Colet J, Sanchez-Corral MA, Manito N, Gomez-Hospital JA , Roca J, Fernandez-Nofrerias E, et al. Effect of carvedilol therapy on functional mitral regurgitation, ventricular remodeling, and contractility in patients with heart failure due to left ventricular systolic dysfunction. Transplantation Proceedings 2002;34(1):177-8. [DOI] [PubMed] [Google Scholar]
CONSENSUS {published data only}
- Eriksson SV, Caidahl K, Hall C, Eneroth P, Kjekshus J, Offstad J, et al. Atrial natriuretic peptide ANP(1-98) and ANP(99-126) in patients with severe chronic congestive heart failure: relation to echocardiographic measurements. A subgroup analysis from the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Journal of Cardiac Failure 1995;1(2):109-16. [DOI] [PubMed] [Google Scholar]
- Ljungman S, Kjekshus J, Swedberg K. Renal function in severe congestive heart failure during treatment with enalapril (the Cooperative North Scandinavian Enalapril Survival Study Trial). American Journal of Cardiology 1992;70(4):479-87. [DOI] [PubMed] [Google Scholar]
- Swedberg K, Kjekshus J, CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). American Journal of Cardiology 1988;62(2):60A-66A. [DOI] [PubMed] [Google Scholar]
- The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). New England Journal of Medicine 1987;316(23):1429-35. [DOI] [PubMed] [Google Scholar]
CONSENSUS II {published data only}
- Edner M, Bonarjee VV, Nilsen DW, Berning J, Carstensen S, Caidahl K. Effect of enalapril initiated early after acute myocardial infarction on heart failure parameters, with reference to clinical class and echocardiographic determinants. CONSENSUS II Multi-Echo Study Group. Clinical Cardiology 1996;19(7):543-8. [DOI] [PubMed] [Google Scholar]
Conti 2005 {published data only}
- Conti CR. Management of heart failure and left ventricular systolic dysfunction following acute myocardial infarction. Clinical Cardiology 2005;28(1):1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corder 1993 {published data only}
- Corder CN, Rubler S, Deere LF, Puls A, Peguero-Rivera A, Nagarajan R, et al. Effect of cilazapril on exercise tolerance in congestive heart failure. Pharmacology 1993;46(3):148-54. [DOI] [PubMed] [Google Scholar]
Crouse 2011 {published data only}
- Crouse M, Flack D, Kerns JW, Martin L, Pham D, Sudireddy R, et al. Clinical inquiries. Which medications benefit patients with diastolic heart failure? Journal of Family Practice 2011;60(2):101-2, 108. [PubMed] [Google Scholar]
Dahlstrom 2007 {published data only}
- Dahlstrom U, Boman K, Brodin L A, Hagerman I, Willenheimer R. Treatment of heart failure and preserved systolic function. Lakartidningen 2007;104(34):2348-50. [PubMed] [Google Scholar]
Davie 2001 {published data only}
- Davie AP, Rumley A, Lowe GD, McMurray JJ. Effect of chronic angiotensin II type I receptor antagonism and angiotensin converting enzyme inhibition on plasma fibrinolytic variables in patients with heart failure. Thrombosis and Haemostasis 2001;86(6):1585-6. [PubMed] [Google Scholar]
DeBock 1994 {published data only}
- DeBock V, Mets T, Romagnoli M, Derde MP. Captopril treatment of chronic heart failure in the very old. Journal of Gerontology 1994;49(3):M148-52. [DOI] [PubMed] [Google Scholar]
Dekleva 2012 {published data only}
- Dekleva M, Dungen HD, Gelbrich G, Incrot S, Suzic Lazic J, Pavlovic Kleut M, et al. Beta blockers therapy is associated with improved left ventricular systolic function and sustained exercise capacity in elderly patients with heart failure. CIBIS-ELD sub-study. Aging Clinical and Experimental Research 2012;24(6):675-81. [DOI] [PubMed] [Google Scholar]
De Melo 2011 {published data only}
- De Melo D, Barretto AC, Ramires JF. The impact of the rapid use of beta-blockers on ventricular remodeling and mortality in end-stage heart failure. JACC 2011;57(14):E259. [Google Scholar]
Demers 2001 {published data only}
- Demers C, McKelvie RS, Negassa A, Yusuf S, Investigators Resolvd Pilot Study. Reliability, validity, and responsiveness of the six-minute walk test in patients with heart failure. American Heart Journal 2001;142(4):698-703. [DOI] [PubMed] [Google Scholar]
Desai 2013 {published data only}
- Desai AS. Heart failure with preserved ejection fraction: Time for a new approach? JACC 2013;62(4):272-4. [DOI] [PubMed] [Google Scholar]
Deswal 2010 {published data only}
- Deswal A, Richardson P, Bozkurt B, Mann DL. Randomized trial of aldosterone antagonism in diastolic heart failure (RAAM-DHF). Journal of Cardiac Failure 2010;16(8):S7. [DOI] [PubMed] [Google Scholar]
de Teresa 1995 {published data only}
- Teresa E, Espinosa JS, Gomez Doblas JJ. Cardiovascular pharmacology (IX). Angiotensin-converting enzyme inhibitors in hypertension and heart failure. Revista Espanola de Cardiologia 1995;48(2):128-41. [PubMed] [Google Scholar]
Ding 2008 {published data only}
- Ding P, Li L, Xu ZY, Han L, He B, Hu Y. Ramipril in combination with irbesartan for treatment of chronic heart failure in patients with rheumatic heart disease. Academic Journal of Second Military Medical University 2008;29(6):675-8. [Google Scholar]
Ditiatkov 1999 {published data only}
- Ditiatkov AE, Radzevich AE, Tikhonov VA, Gal IG. Ramipril treatment of heart failure in disseminated forms of pulmonary tuberculosis. Problemy Tuberkuleza 1999;2:31-2. [PubMed] [Google Scholar]
Donal 2008 {published data only}
- Donal E. Heart failure with preserved ejection fraction. La Revue de Médecine Interne 2008;29(Suppl 1):6-7. [DOI] [PubMed] [Google Scholar]
Dragana 2015 {published data only}
- Dragana Stanojevic D, Apostolovic S, Salinger-Martinovic S, Djordjevic-Radojkovic D, Jankovic-Tomasevic R, Pavlovic M, et al. Beta blockers in heart failure with preserved left ventricle ejection fraction. European Journal of Heart Failure 2015;17:364. [Google Scholar]
Edner 2013 {published data only}
- Edner M, Lund LH. Renin-angiotensin system antagonists associated with reduced [corrected] mortality in diastolic heart failure. Lakartidningen 2013;110(7):331. [PubMed] [Google Scholar]
Eichhorn 1994 {published data only}
- Eichhorn EJ, Hatfield B, Marcoux L, Risser RC. Functional importance of myocardial relaxation in patients with congestive heart failure. Journal of Cardiac Failure 1994;1(1):45-56. [DOI] [PubMed] [Google Scholar]
Eichhorn 2003 {published data only}
- Eichhorn EJ, Grayburn PA, Mayer SA, St John Sutton M, Appleton C, Plehn J, et al. Myocardial contractile reserve by dobutamine stress echocardiography predicts improvement in ejection fraction with beta-blockade in patients with heart failure: the Beta-Blocker Evaluation of Survival Trial (BEST). Circulation 2003;108(19):2336-41. [DOI] [PubMed] [Google Scholar]
Er 2005 {published data only}
- Er F, Hoppe UC. Ivabradine -- a novel approach for heart rate lowering. Deutsche Medizinische Wochenschrift 2005;130(24):1501-2. [DOI] [PubMed] [Google Scholar]
Ertl 1999 {published data only}
- Ertl G, Hu K. Left ventricular remodelling during treatment with beta-blockers. Journal of Cardiovascular Risk 1999;6(3):145-50. [DOI] [PubMed] [Google Scholar]
EudraCT 2004‐004169‐13 {published data only}
- EudraCT 2004-004169-13. A Pilot study to assess the effects of beta-blockade on exercise capacity and BNP levels in patients with predominantly diastolic heart failure. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2004-004169-13 (first received 21 April 2005).
Fauchier 2009 {published data only}
- Fauchier L, Grimard C, Pierre B, Nonin E, Gorin L, Rauzy B, et al. Comparison of beta blocker and digoxin alone and in combination for management of patients with atrial fibrillation and heart failure. American Journal of Cardiology 2009;103(2):248-54. [DOI] [PubMed] [Google Scholar]
Feola 2003 {published data only}
- Feola M, Menardi E, Ribichini F, Vado A, Deorsola A, Ferrero V, et al. Effects of the addition of a low dose of spironolactone on brain natriuretic peptide plasma level and cardiopulmonary function in patients with moderate congestive heart failure. Medical Science Monitor 2003;9(8):CR341-5. [PubMed] [Google Scholar]
Flammer 2013 {published data only}
- Flammer A, Sudano I, Enseleit F, Luscher TF, Noll G, Ruschitzka F. Mineralocorticoid receptor antagonism in patients with coronary artery disease and preserved ejection fraction-a randomized, double-blind trial. European Journal of Heart Failure 2013;12:S222-3. [Google Scholar]
Flather 2016 {published data only}
- Flather MD, Gollop ND. Understanding mechanisms of action of beta-blockers in heart failure with reduced and preserved ejection fraction. JACC Heart Failure 2016;4(2):150-1. [DOI] [PubMed] [Google Scholar]
Flesch 2006 {published data only}
- Flesch M. Combined administration of AT1-receptor antagonists and beta-blockers in patients with chronic heart insufficiency. Internistische Praxis 2006;46(4):829-32. [Google Scholar]
Follath 1996 {published data only}
- Follath F. Beta-blockers in cardiac insufficiency. Time to reconsider? Schweizer Medizinische Wochenschrift 1996;126(18):747-9. [PubMed] [Google Scholar]
Fonarow 2004 {published data only}
- Fonarow G, Cannon C, Epstein A. Questions and answers. Journal of Invasive Cardiology 2004;16 Suppl E:16E-7E. [PubMed] [Google Scholar]
Fonarow 2007 {published data only}
- Fonarow GC, Lukas MA, Robertson M, Colucci WS, Dargie HJ. Effects of carvedilol early after myocardial infarction: analysis of the first 30 days in Carvedilol Post-Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN). American Heart Journal 2007;154(4):637-44. [DOI] [PubMed] [Google Scholar]
Fowler 1999 {published data only}
- Fowler MB. The influence of beta-adrenergic blocking drugs on morbidity and mortality in heart failure. Journal of Cardiovascular Risk 1999;6(3):141-4. [DOI] [PubMed] [Google Scholar]
Franciosa 2002 {published data only}
- Franciosa JA, Taylor AL, Cohn JN, Yancy CW, Ziesche S, Olukotun A, et al. African-American Heart Failure Trial (A-HeFT): rationale, design, and methodology. Journal of Cardiac Failure 2002;8(3):128-35. [DOI] [PubMed] [Google Scholar]
Fukunami 1991 {published data only}
- Fukunami M, Hashimura K, Ohmori M, Ikeda T, Umemoto K, Kumagai K, et al. Effectiveness of long-term beta-blocker therapy for dilated cardiomyopathy--echocardiographical follow-up. Cardiovascular Drugs and Therapy 1991;5(2):463-9. [DOI] [PubMed] [Google Scholar]
Galinier 2007 {published data only}
- Galinier M. Treatment of heart failure with preserved ejection fraction. Archives des Maladies du Coeur et des Vaisseaux - Pratique 2007;158:17-21. [Google Scholar]
Galloe 2006 {published data only}
- Galloe AM, Skagen K, Christensen NJ, Nielsen SL, Frandsen EK, Bie P, et al. Dosage dependent hormonal counter regulation to combination therapy in patients with left ventricular dysfunction. Journal of Clinical Pharmacology and Therapy 2006;31(2):139-47. [DOI] [PubMed] [Google Scholar]
Gao 2010 {published data only}
- Gao WQ, Han CG, Zhao YX, Wang Q, Zhu P, Yang TS, et al. Effect of metoprolol on the expression of GRK2 in lymphocyte of advanced elderly patients with chronic heart failure. Nan Fang Yi Ke Da Xue Xue Bao 2010;30(5):1132-3. [PubMed] [Google Scholar]
Gardner 2003 {published data only}
- Gardner RS, Martin W, Carter R, McDonagh TA. Importance of beta blockade in the treatment of advanced heart failure. Heart 2003;89(12):1442-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gardner 2004 {published data only}
- Gardner RS, McDonagh TA. The treatment of chronic heart failure due to left ventricular systolic dysfunction. Clinical Medicine 2004;4(1):18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghali 2002 {published data only}
- Ghali JK, Pina IL, Gottlieb SS, Deedwania PC, Wikstrand JC, Group Merit-Hf Study. Metoprolol CR/XL in female patients with heart failure: analysis of the experience in Metoprolol Extended-Release Randomized Intervention Trial in Heart Failure (MERIT-HF). Circulation 2002;105(13):1585-91. [DOI] [PubMed] [Google Scholar]
Gheorghiade 2009 {published data only}
- Gheorghiade M, Khan S, Blair JE, Harinstein ME, Krum H, Mukherjee R, et al. The effects of eplerenone on length of stay and total days of heart failure hospitalization after myocardial infarction in patients with left ventricular systolic dysfunction. American Heart Journal 2009;158(3):437-43. [DOI] [PubMed] [Google Scholar]
Good 1994 {published data only}
- Good JM, Brady AJ, Noormohamed FH, Oakley CM, Cleland JG. Effect of intense angiotensin II suppression on the diuretic response to furosemide during chronic ACE inhibition. Circulation 1994;90(1):220-4. [DOI] [PubMed] [Google Scholar]
Goodfield 1999 {published data only}
- Goodfield NE, Newby DE, Ludlam CA, Flapan AD. Effects of acute angiotensin II type 1 receptor antagonism and angiotensin converting enzyme inhibition on plasma fibrinolytic parameters in patients with heart failure. Circulation 1999;99(23):2983-5. [DOI] [PubMed] [Google Scholar]
Gottlieb 1996 {published data only}
- Gottlieb SS, Singh S, Munger M, Eichhorn EJ, Ilgenfritz J, Hanyok J. Hemodynamic effects of the class III antiarrhythmic drug, d-sotalol, in patients with congestive heart failure. American Journal of Cardiology 1996;78(12):1411-5. [DOI] [PubMed] [Google Scholar]
Grajek 2008 {published data only}
- Grajek S. Do angiotensin receptor blockers increased the risk of myocardial infarction? The landscape after ONTARGET study. Kardiologia Polska 2008;66(12):1313-24. [PubMed] [Google Scholar]
Greenberg 1996 {published data only}
- Greenberg BH, Quinones MA, Koilpillai C, Limacher M, Shindler D, Benedict C, et al. Left ventricular dysfunction: Effects of long-term enalapril therapy on remodeling. Cardiology Review 1996;13(7):40-6. [Google Scholar]
Gremmler 2000 {published data only}
- Gremmler B, Kunert M, Schleiting H, Ulbricht LJ. Improvement of cardiac output in patients with severe heart failure by use of ACE-inhibitors combined with the AT1-antagonist eprosartan. European Journal of Heart Failure 2000;2(2):183-7. [DOI] [PubMed] [Google Scholar]
Groenning 2000 {published data only}
- Groenning BA, Nilsson JC, Sondergaard L, Fritz-Hansen T, Larsson HB, Hildebrandt PR. Antiremodeling effects on the left ventricle during beta-blockade with metoprolol in the treatment of chronic heart failure. Journal of the American College of Cardiology 2000;36(7):2072-80. [DOI] [PubMed] [Google Scholar]
Groenning 2001 {published data only}
- Groenning BA, Nilsson JC, Sondergaard L. Antiremodeling effects on the left ventricle during beta-blockage with metaprolol in the treatment of chronic heart failure. Congestive Heart Failure 2001;7(1):58. [Google Scholar]
Groenning 2002 {published data only}
- Groenning BA, Nilsson JC, Hildebrandt PR, Kjaer A, Fritz-Hansen T, Larsson HB, et al. Neurohumoral prediction of left-ventricular morphologic response to beta-blockade with metoprolol in chronic left-ventricular systolic heart failure. European Journal of Heart Failure 2002;4(5):635-46. [DOI] [PubMed] [Google Scholar]
Gruner 2007 {published data only}
- Gruner Svealv B, Tang M S, Waagstein F, Andersson B. Pronounced improvement in systolic and diastolic ventricular long axis function after treatment with metoprolol. European Journal of Heart Failure 2007;9(6-7):678-83. [DOI] [PubMed] [Google Scholar]
Guazzi 1998 {published data only}
- Guazzi M, Pontone G, Trevisi N, Lomanto M, Matturri M, Agostoni P. A failed improvement in pulmonary function and exercise capacity with carvedilol in congestive heart failure despite an excellent effect on left ventricular function. Cardiologia 1998;43(2):181-7. [PubMed] [Google Scholar]
Guazzi 1999 {published data only}
- Guazzi M, Agostoni P, Matturri M, Pontone G, Guazzi M D. Pulmonary function, cardiac function, and exercise capacity in a follow-up of patients with congestive heart failure treated with carvedilol. American Heart Journal 1999;138(3 Pt 1):460-7. [DOI] [PubMed] [Google Scholar]
Gøtzsche 1992 {published data only}
- Gøtzsche CO, Søgaard P, Ravkilde J, Thygesen K. Effects of captopril on left ventricular systolic and diastolic function after acute myocardial infarction. American Journal of Cardiology 1992;70(2):156-60. [DOI] [PubMed] [Google Scholar]
Hanping 1997 {published data only}
- Hanping Z, Guolong Y, Jing L, Jin H. The changes of PRA, ATII, ALD, ET and ANP in patients with left ventricular and intervention with enalapril. Bulletin of Hunan Medical University 1997;22(4):323-6. [PubMed] [Google Scholar]
Hara 2000 {published data only}
- Hara Y, Hamada M, Shigematsu Y, Suzuki M, Kodama K, Kuwahara T, et al. Effect of beta-blocker on left ventricular function and natriuretic peptides in patients with chronic heart failure treated with angiotensin-converting enzyme inhibitor. Circulation Journal – Official Journal of the Japanese Circulation Society 2000;64(5):365-9. [DOI] [PubMed] [Google Scholar]
Hauf 1993 {published data only}
- Hauf GF, Roskamm H. Heart failure in coronary heart disease. Internist 1993;34(10):953-60. [PubMed] [Google Scholar]
Hole 2004 {published data only}
- Hole T, Frøland G, Gullestad L, Offstad J, Skjaerpe T. Metoprolol CR/XL improves systolic and diastolic left ventricular function in patients with chronic heart failure. Echocardiography 2004;21(3):215-23. [DOI] [PubMed] [Google Scholar]
Holland 2010 {published data only}
- Holland DJ, Mottram PM, Hare JL, Jenkins C, Marwick TH. Extended duration of aldosterone blockade fails to improve diastolic dysfunction over the effects of blood pressure in patients with heart failure and normal ejection fraction. European Heart Journal 2010;31:731-2. [Google Scholar]
Hong 2003 {published data only}
- Hong Y, Li Y, Wang XM, Liu XS, Li YN, Ouyang WM, et al. Effects of perindopril on plasma soluble TRAIL and receptor soluble DR5 in patients with congestive heart failure. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2003;19(4):366-8. [PubMed] [Google Scholar]
Hoppe 2007 {published data only}
- Hoppe UC. Treatment of heart failure with ACE inhibitors and beta-blockers: what is next? AT1-receptor antagonists? Clinical Research in Cardiology 2007;96(4):196-8. [DOI] [PubMed] [Google Scholar]
Hori 2004 {published data only}
- Hori M, Sasayama S, Kitabatake A, Toyo-oka T, Handa S, Yokoyama M, et al. Low-dose carvedilol improves left ventricular function and reduces cardiovascular hospitalization in Japanese patients with chronic heart failure: the Multicenter Carvedilol Heart Failure Dose Assessment (MUCHA) trial. American Heart Journal 2004;147(2):324-30. [DOI] [PubMed] [Google Scholar]
Hung 2010 {published data only}
- Hung CL, Verma A, Uno H, Shin SH, Bourgoun M, Hassanein AH, et al. Longitudinal and circumferential strain rate, left ventricular remodeling, and prognosis after myocardial infarction. Journal of the American College of Cardiology 2010;56(22):1812-22. [DOI] [PubMed] [Google Scholar]
IRIS‐HF {published data only}
- ACTRN12609000789268. Non-invasive cardiac imaging in the detection and assessment of subclinical diabetic heart disease [Non-invasive cardiac imaging in the detection and assessment of subclinical diabetic heart disease in type 2 diabetes mellitus patients - outcome of antifibrotic therapy with spironolactone]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=308109 (date received 10 September 2009).
- Doehner W, Todorovic J, Kennecke C, Rauchhaus M, Sandek A, Lainscak M, et al. Improved insulin sensitivity by the angiotensin receptor antagonist irbesartan in patients with systolic heart failure: a randomized double-blinded placebo-controlled study. International Journal of Cardiology 2012;161(3):137-42. [DOI] [PubMed] [Google Scholar]
- NCT00347087. Effect of irbesartan on insulin sensitivity in chronic heart failure [Effect of the angiotensin II receptor antagonist irbesartan on insulin sensitivity and metabolic profile in patients with chronic heart failure]. clinicaltrials.gov/ct2/show/NCT00347087 (first received 4 July 2006).
Ito 2012 {published data only}
- Ito H, Ishii K, Kihara H, Kasayuki N, Nakamura F, Shimada K, et al. Adding thiazide to a renin-angiotensin blocker improves left ventricular relaxation and improves heart failure in patients with hypertension. Hypertension Research 2012;35(1):93-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jamieson 1991 {published data only}
- Jamieson MJ, Webster J, Fowler G, Rawles J, Smith FW, Petrie JC. A comparison of the chronic effect of oral xamoterol and enalapril on blood pressure and renal function in mild to moderate heart failure. British Journal of Clinical Pharmacology 1991;31(3):305-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jellis 2014 {published data only}
- Jellis CL, Sacre JW, Wright J, Jenkins C, Haluska B, Jeffriess L, et al. Biomarker and imaging responses to spironolactone in subclinical diabetic cardiomyopathy. European Heart Journal 2014;15:776-86. [DOI] [PubMed] [Google Scholar]
- Jellis CL, Wright J, Sacre J, Kennedy D, Jeffriess L, Fenwick J, et al. Backscatter, T1 mapping or pro-collagen biomarkers for non-invasive assessment of treatment response to anti-fibrotic therapy in subclinical diabetic cardiomyopathy? A randomized trial. Journal of the American College of Cardiology 2012;59(13):E1076. [Google Scholar]
Jessup 2003 {published data only}
- Jessup M. Aldosterone blockade and heart failure. New England Journal of Medicine 2003;348(14):1380-2. [DOI] [PubMed] [Google Scholar]
Jong 2010 {published data only}
- Jong P, McKelvie R, Yusuf S. Should treatment for heart failure with preserved ejection fraction differ from that for heart failure with reduced ejection fraction? BMJ 2010;341:c4202. [DOI] [PubMed] [Google Scholar]
JPRN‐jRCTs051180137 {published data only}
- JPRN-jRCTs051180137. J-TASTE trial. rctportal.niph.go.jp/en/detail?trial_id=jRCTs051180137 (date registered 18 March 2019).
JPRN‐UMIN000006415 {published data only}
- JPRN-UMIN000006415. The multicenter study for effects of eplerenone on cardiac diastolic dysfunction in hypertensive patients. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000006415 (date of registration 27 September 2011).
Kanoupakis 2008 {published data only}
- Kanoupakis EM, Manios EG, Mavrakis HE, Kallergis EM, Lyrarakis GM, Koutalas EP, et al. Electrophysiological effects of carvedilol administration in patients with dilated cardiomyopathy. Cardiovascular Drugs and Therapy 2008;22(3):169-76. [DOI] [PubMed] [Google Scholar]
Kapel'ko 2011 {published data only}
- Kapel'ko VI. Diastolic dysfunction. Kardiologiia 2011;51(1):79-90. [PubMed] [Google Scholar]
Kasama 2007 {published data only}
- Kasama S, Toyama T, Sumino H, Matsumoto N, Sato Y, Kumakura H, et al. Additive effects of spironolactone and candesartan on cardiac sympathetic nerve activity and left ventricular remodeling in patients with congestive heart failure. Journal of Nuclear Medicine 2007;48(12):1993-2000. [DOI] [PubMed] [Google Scholar]
Keren 1992 {published data only}
- Keren G, Pardes A, Eschar Y, Hansch E, Scherez J, Laniado S. Left ventricular filling dynamics by Doppler echocardiography in dilated cardiomyopathy: one-year follow-up in patients treated with captopril compared to placebo. Cardiology 1992;81(4-5):196-206. [DOI] [PubMed] [Google Scholar]
Keren 1994 {published data only}
- Keren G, Pardes A, Eschar Y, Koifman B, Scherez J, Geleranter I, et al. One-year clinical and echocardiographic follow-up of patients with congestive cardiomyopathy treated with captopril compared to placebo. Israel Journal of Medical Sciences 1994;30(1):90-8. [PubMed] [Google Scholar]
Khalid 2013 {published data only}
- Khalid U, Deswal A. Lack of definitive evidence for the use of renin-angiotensin system antagonists for heart failure with preserved ejection fraction. Evidence Based Medicine 2013;18(6):226-7. [DOI] [PubMed] [Google Scholar]
Khand 2015 {published data only}
- Khand AU, Chew PG, Douglas H, Jones J, Jan A, Cleland JG. The effect of carvedilol on B-type natriuretic peptide and cardiac function in patients with heart failure and persistent atrial fibrillation. Cardiology 2015;130(3):153-8. [DOI] [PubMed] [Google Scholar]
Kikuchi 2016 {published data only}
- Kikuchi N, Jujo K, Yamaguchi J, Ogawa H, Hagiwara N. Impact of left ventricular ejection function on blood pressure-lower ing therapy in hypertensive patients with coronary artery disease. Journal of Hypertension 2016;34(5):1011-8. [DOI] [PubMed] [Google Scholar]
Kimura 2011 {published data only}
- Kimura M, Ogawa H, Wakeyama T, Takaki A, Iwami T, Hadano Y, et al. Effects of mineralocorticoid receptor antagonist spironolactone on atrial conduction and remodeling in patients with heart failure. Journal of Cardiology 2011;57(2):208-14. [DOI] [PubMed] [Google Scholar]
Kinugawa 2007 {published data only}
- Kinugawa S. Diabetic heart disease. Nippon Rinsho 2007;65(Suppl 5):465-9. [PubMed] [Google Scholar]
Kjekshus 2007 {published data only}
- Kjekshus J. Prescription of beta-blockers in patients with advanced heart failure and preserved left ventricular ejection fraction. Clinical implications and survival. European Journal of Heart Failure 2007;9(9):962; author reply 962-3. [DOI] [PubMed] [Google Scholar]
Kjøller‐Hansen 1998 {published data only}
- Kjøller-Hansen L, Steffensen R, Grande P. The Angiotensin Converting Enzyme Inhibition Post Revascularization Study (APRES). Effects of ramipril in patients with reduced left ventricular function. Rationale, design, methods, baseline characteristics and first-year experience. Scandinavian Cardiovascular Journal 1998;32(4):225-32. [DOI] [PubMed] [Google Scholar]
Kleber 1991a {published data only}
- Kleber FX, Doering W. Prognosis of mild chronic heart failure: effects of the ACE inhibitor captopril [Prognose bei leichter chronischer Herzinsuffizienz: Einflüsse des ACE-Hemmers Captopril]. Herz 1991;16(Spec No 1):283-93. [PubMed] [Google Scholar]
Kleber 1991b {published data only}
- Kleber FX, Nussberger J, Niemoller L, Doering W, Brunner H. Development of heart failure after myocardial infarct: changes in plasma renin activity, angiotensin II, catecholamines and atrial natriuretic peptide [Entwicklung der Herzinsuffizienz nach Myokardinfarkt: Veränderungen von Plasma-Reninaktivität, Angiotensin II, Katecholaminen und atrialem natriuretischen Peptid]. Zeitschrift für Kardiologie 1991;80(Suppl 8):111-2. [PubMed] [Google Scholar]
Kongstad‐Rasmussen 1998 {published data only}
- Kongstad-Rasmussen O, Blomstrand M, Dahlstrom U, Wranne B. Treatment with ramipril improves systolic function even in patients with mild systolic dysfunction and symptoms of heart failure after acute myocardial infarction. Clinical Cardiology 1998;21(11):807-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krum 1996 {published data only}
- Krum H, Gu A, Wilshire-Clement M, Sackner-Bernstein J, Goldsmith R, Medina N, et al. Changes in plasma endothelin-1 levels reflect clinical response to beta-blockade in chronic heart failure. American Heart Journal 1996;131(2):337-41. [DOI] [PubMed] [Google Scholar]
Krum 2015 {published data only}
- Krum H, McMurray JJ, Abraham WT, Dickstein K, Kober L, Desai AS, et al. The Aliskiren Trial to Minimize OutcomeS in Patients with HEart failure trial (ATMOSPHERE): revised statistical analysis plan and baseline characteristics. European Journal of Heart Failure 2015;17(10):1075-83. [DOI] [PubMed] [Google Scholar]
Kulbertus 2003 {published data only}
- Kulbertus H. Clinical study of the month. The CHARM study. Revue Medicale de Liege 2003;58(10):646-52. [PubMed] [Google Scholar]
Kuznar 2003 {published data only}
- Kuznar W. Blocking aldosterone reduces deaths in post-MI heart failure. Cardiology Review 2003;20(6):1+14. [Google Scholar]
Lang 1995 {published data only}
- Lang CC, McAlpine HM, Kennedy N, Rahman AR, Lipworth BJ, Struthers AD. Effects of lisinopril on congestive heart failure in normotensive patients with diastolic dysfunction but intact systolic function. European Journal of Clinical Pharmacology 1995;49(1-2):15-9. [DOI] [PubMed] [Google Scholar]
Larsen 1996 {published data only}
- Larsen J, Sykulski R, Jensen G, Dossegger L, Trimarco B, Moccetti T, et al. Adaptive changes in the acute haemodynamic effects of cilazapril during chronic treatment. Comparison with long-term clinical effect. European Journal of Clinical Pharmacology 1996;50(6):433-41. [DOI] [PubMed] [Google Scholar]
Lechat 1993 {published data only}
- Bounhoure JP, Bottineau G, Lechat P, Garnham J, Lapeyre G. Contribution of perindopril to the treatment of chronic congestive cardiac insufficiency. Multicenter pilot double blind study versus placebo. Archives des Maladies du Coeur et des Vaisseaux 1989;82(Spec No 1):73-8. [PubMed] [Google Scholar]
- Bounhoure JP, Bottineau G, Lechat P, Garnham J, Lapeyre G. Value of perindopril in the treatment of chronic congestive heart failure. Multicenter double-blind placebo-controlled study. Clinical and experimental hypertension. Part A, Theory and practice 1989;11(Suppl 2):575-86. [PubMed] [Google Scholar]
- Bounhoure JP. Perindopril and chronic cardia failure. Archives des Maladies du Coeur et des Vaisseaux 1991;84(Spec Iss IV):89-92. [PubMed] [Google Scholar]
- Lechat P, Garnham SP, Desche P, Bounhoure JP. Efficacy and acceptability of perindopril in mild to moderate chronic congestive heart failure. American Heart Journal 1993;126(3 II Suppl):798-806. [DOI] [PubMed] [Google Scholar]
Leonetti 1999 {published data only}
- Leonetti Luparini R, Celli V, Piccirillo G, Guidi V, Cacciafesta M, Marigliano V. Carvedilol in elderly patients with chronic heart failure, a 12 weeks randomized, placebo controlled open trial. Archives of Gerontology and Geriatrics 1999;29(3):275-82. [DOI] [PubMed] [Google Scholar]
Lewis 1988 {published data only}
- Lewis GR. Lisinopril versus placebo in older congestive heart failure patients. American Journal of Medicine 1988;85(3B):48-54. [DOI] [PubMed] [Google Scholar]
Li 2005 {published data only}
- Li ZK, Mei X, Zhu SJ, Wang J, Tian Y, Zhou YZ, et al. Ventricular remodeling and cardiovascular events in patients with chronic heart failure and the interventional effects of metoprolol. Chinese Journal of Clinical Rehabilitation 2005;9(35):28-30. [Google Scholar]
Liebson 2004 {published data only}
- Liebson PR. VALIANT and EUROPA. Preventive Cardiology 2004;7(1):42-4. [DOI] [PubMed] [Google Scholar]
Lindenfeld 2001 {published data only}
- Lindenfeld J, Robertson AD, Lowes BD, Bristow MR, Investigators Mocha. Aspirin impairs reverse myocardial remodeling in patients with heart failure treated with beta-blockers. JACC 2001;38(7):1950-6. [DOI] [PubMed] [Google Scholar]
Lindsay 1999 {published data only}
- Lindsay J, Freemantle N, Nazareth I. Beta-blockers in heart failure. Lancet 1999;353(9157):1011-2. [DOI] [PubMed] [Google Scholar]
Liu 2006 {published data only}
- Liu G, Ji Z, Liu K. Effects of spironolactone in treatment of elderly hypertensive patients with diastolic heart failure. Chinese Journal of New Drugs and Clinical Remedies 2006;25(8):567-70. [Google Scholar]
Liu 2014 {published data only}
- Liu X, Zhong C, Zhao P, Zhang Z, Jia N, Su S, et al. Analysis of therapeutic effect and safety of target-dose metoprolol in the treatment of patients with diabetes mellitus with chronic heart failure. Pakistan Journal of Medical Sciences 2014;30(1):7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Logeart 2006 {published data only}
- Logeart D. Diastolic heart failure. Archives des Maladies du Coeur et des Vaisseaux - Pratique 2006;147:23-7. [Google Scholar]
Lopez 2000 {published data only}
- Lopez Herrero F, Pardo Alvarez J. Spironolactone in heart failure. Atención Primaria 2000;25(4):281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lou 2009 {published data only}
- Lou YF, Shi XP, Tong H, Yu GY, Yao GD, Dong K. Re: Effect of carvedilol on plasma adiponectin concentration in patients with chronic heart failure. Circulation Journal 2009;73(12):2363; author reply 2364. [DOI] [PubMed] [Google Scholar]
Luo 2007 {published data only}
- Luo M, Bi Y, Xu YX. Effects of metoprolol on beta1 adrenergic receptor polymorphism and receptor density in urban Chinese patients with heart failure. Chinese Medical Journal 2007;120(19):1720-3. [PubMed] [Google Scholar]
Ma 2005 {published data only}
- Ma HY. Effect of valsartan and hydrochloric benazepril on ventricular remodeling and cardiac function of patients with heart failure. Journal of Clinical Rehabilitative Tissue Engineering Research 2005;9(47):22-4. [Google Scholar]
MacGregor 2009 {published data only}
- MacGregor JF, Wachter SB, Munger M, Stoddard G, Bristow MR, Gilbert EM. Carvedilol produces sustained long-term benefits: follow-up at 12 years. Congestive Heart Failure 2009;15(1):5-8. [DOI] [PubMed] [Google Scholar]
Mak 2008 {published data only}
- Mak G, Murphy NM, Phelan D, Watson C, O'Loughlin C, Baugh J, et al. The effects of low dose eplerenone on markers of collagen turnover in diastolic heart failure. Irish Journal of Medical Science 2008;177(Suppl 12):S398. [Google Scholar]
Malnick 2007 {published data only}
- Malnick SD, Somin M. The VALIDD study. Lancet 2007;370(9591):931. [DOI] [PubMed] [Google Scholar]
Maron 2018 {published data only}
- Maron M, Kerur B, Chan RH, McGraw AP, Qiao X, Paruchuri V, et al. Abstract 16910: can spironolactone mitigate myocardial fibrosis and alter sudden death risk and heart failure symptoms in patients with hypertrophic cardiomyopathy?: a prospective, randomized trial. Circulation 2013;128(Suppl 22):Abstract 16910. [Google Scholar]
- Maron MS, Chan RH, Kapur NK, Jaffe IZ, McGraw AP, Kerur R, et al. Effect of spironolactone on myocardial fibrosis and other clinical variables in patients with hypertrophic cardiomyopathy. American Journal of Medicine 2018;131:837-41. [DOI] [PubMed] [Google Scholar]
- NCT00879060. Clinical and therapeutic implications of fibrosis in hypertrophic cardiomyopathy. clinicaltrials.gov/ct2/show/NCT00879060 (first posted 9 April 2009).
Mazayev 1998 {published data only}
- Mazayev VP, Fomina IG, Kazakow EN, Sulimov VA, Zvereva TV, Lyusov VA, et al. Valsartan in heart failure patients previously untreated with an ACE inhibitor. International Journal of Cardiology 1998;65(3):239-46. [DOI] [PubMed] [Google Scholar]
McAnulty 2004 {published data only}
- McAnulty JH Jr. Valsartan, captopril, or both in myocardial infarction. New England Journal of Medicine 2004;350(9):943-5; author reply 943. [DOI] [PubMed] [Google Scholar]
McCullough 2012 {published data only}
- McCullough PA, Cowan S. Mineralocorticoid receptor antagonists and mortality in heart failure with concurrent atrial fibrillation. Circulation 2012;5(5):550-1. [DOI] [PubMed] [Google Scholar]
McIlwain 1997 {published data only}
- McIlwain JS. HCQIP Project Report--findings released in Use of Ace Inhibitors in Heart Failure Project. Journal of the Mississippi State Medical Association 1997;38(7):282. [PubMed] [Google Scholar]
McKelvie 2012 {published data only}
- McKelvie RS. Heart failure. American Family Physician 2012;86(2):182-4. [PubMed] [Google Scholar]
McMurray 2000 {published data only}
- McMurray J. AT(1) receptor antagonists-beyond blood pressure control: possible place in heart failure treatment. Heart 2000;84 Suppl 1:i42-5: discussion i50. [DOI] [PMC free article] [PubMed] [Google Scholar]
McMurray 2004 {published data only}
- McMurray JJ, Pfeffer MA, Swedberg K, Dzau VJ. Which inhibitor of the renin-angiotensin system should be used in chronic heart failure and acute myocardial infarction? Circulation 2004;110(20):3281-8. [DOI] [PubMed] [Google Scholar]
Melo 2011 {published data only}
- Melo DSB, Barretto ACP, Oliveira AI, Uchida AH, Ochiai ME, Cardoso JN, et al. The impact of the rapid use of beta blockers on ventricular mortality and remodeling in and-stage heart failure. European Journal of Heart Failure. Supplement 2011;10:S173. [Google Scholar]
Melo 2012 {published data only}
- Melo DB, Barretto AP, Ochiai M, Cardoso J, Oliveira A, Melo F, et al. The impact of the rapid use of beta-blockers on ventricular remodeling and mortality in end-stage heart failure (the FAST study). Circulation 2012;125(19):e754. [Google Scholar]
Messias 2016 {published data only}
- Messias L R, Ferreira A G, Miranda S M, Teixeira J A, Azevedo J C, Messias A C, et al. Effect of Nebivolol on MIBG Parameters and Exercise in Heart Failure with Normal Ejection Fraction. Arquivos Brasileiros de Cardiologia 2016;106(5):358-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meuleman 2007 {published data only}
- Meuleman C. Diastolic dysfunction in heart failure with CHARMES (candesartan in heart failure assessment of reduction in mortality and morbidity) preserved systolic function: Results of the CHARM echocardiographic sub-study. Medecine Therapeutique - Cardio 2007;3(5):346-9. [Google Scholar]
Mitrovic 2005 {published data only}
- Mitrovic V. Conclusions from CHARM interview with Prof. Veselin Mitrovich, Bad Nauheim. Strong evidence: more help for weak hearts. MMW Fortschritte der Medizin 2005;147(13):48-9. [PubMed] [Google Scholar]
Mochizuki 2004 {published data only}
- Mochizuki S, Shimizu M, Taniguchi I, Kanae K, Yoshida S, Tajima N, et al. JIKEI HEART Study--a morbi-mortality and remodeling study with valsartan in Japanese patients with hypertension and cardiovascular disease. Cardiovascular Drugs and Therapy 2004;18(4):305-9. [DOI] [PubMed] [Google Scholar]
Morales 2011 {published data only}
- Morales H, Espinoza F, Larrea R, Gonzalez B, Puga L, Vukusich A, et al. A randomized, double-blind, placebo-controlled trial of spironolactone on diastolic dysfunction in diabetic patients. Diabetes 2011;60:A562. [Google Scholar]
Murdoch 2001 {published data only}
- Murdoch DR, McDonagh TA, Farmer R, Morton JJ, McMurray JJ, Dargie HJ. ADEPT: Addition of the AT1 receptor antagonist eprosartan to ACE inhibitor therapy in chronic heart failure trial: hemodynamic and neurohormonal effects. American Heart Journal 2001;141(5):800-7. [DOI] [PubMed] [Google Scholar]
NCT00293150 {published data only}
- NCT00293150. Reversing endothelial and diastolic dysfunction and improving collagen turnover in diastolic heart failure (PREDICT). clinicaltrials.gov/show/NCT00293150 (first posted 17 February 2006).
NCT00523757 {published data only}
- NCT00523757. Aldosterone blockade in heart failure (ARCTIC-D) [Aldosterone-blockade randomized controlled trial In CHF - diastolic]. clinicaltrials.gov/show/NCT00523757 (first received 31 August 2007).
NCT01691118 {published data only}
- NCT01691118. A trial of fimasartan for early diastolic heart failure (FINE) [Fimasartan for improvement of diastolic dysfunction in hypertensive patients]. clinicaltrials.gov/show/NCT01691118 (first posted 24 September 2012).
NCT01944384 {published data only}
- NCT01944384. Impacts of aldosterone blockade on myocardial remodeling in hypertensive patients with diastolic failing heart. clinicaltrials.gov/ct2/show/NCT01944384 (first posted 17 September 2013).
NCT03882710 {published data only}
- NCT03882710. Metoprolol XR in heart failure with normal ejection fraction (HFNEF). clinicaltrials.gov/ct2/show/NCT03882710 (first posted 20 March 2019).
Nodari 2003 {published data only}
- Nodari S, Metra M, Dei Cas L. Beta-blocker treatment of patients with diastolic heart failure and arterial hypertension. A prospective, randomized, comparison of the long-term effects of atenolol vs. nebivolol. European Journal of Heart Failure 2003;5(5):621-7. [DOI] [PubMed] [Google Scholar]
Nunez 2016 {published data only}
- Nunez J, Nunez E, Sanchis J. Spironolactone in patients with heart failure and preserved ejection fraction. Revista Clinica Espanola 2016;216(2):111. [DOI] [PubMed] [Google Scholar]
O'Callaghan 1995 {published data only}
- O'Callaghan PA, Walsh MJ. ACE inhibitors in the management and prevention of heart failure. Irish Medical Journal 1995;88(2):48,50. [PubMed] [Google Scholar]
O'Keefe 2008 {published data only}
- O'Keefe JH, Abuissa H, Pitt B. Eplerenone improves prognosis in postmyocardial infarction diabetic patients with heart failure: results from EPHESUS. Diabetes Obesity and Metabolism 2008;10(6):492-7. [DOI] [PubMed] [Google Scholar]
O'Keeffe 2015 {published data only}
- O'Keeffe St, Sharma N, Donnellan Ca, Lye M. Does stopping diuretics prevent first dose hypotension in older heart failure patients commencing ACE inhibitor therapy? European Geriatric Medicine 2015;6(4):366-7.
O'Meara 2012 {published data only}
- O'Meara E, Khairy P, Blanchet MC, Denus S, Pedersen OD, Levesque S, et al. Mineralocorticoid receptor antagonists and cardiovascular mortality in patients with atrial fibrillation and left ventricular dysfunction: insights from the Atrial Fibrillation and Congestive Heart Failure Trial. Circulation 2012;5(5):586-93. [DOI] [PubMed] [Google Scholar]
Ostergren 2004 {published data only}
- Ostergren J. Candesartan for the treatment of hypertension and heart failure. Expert Opinion in Pharmacotherapy 2004;5(7):1589-97. [DOI] [PubMed] [Google Scholar]
Palazzuoli 2005 {published data only}
- Palazzuoli A, Quatrini I, Vecchiato L, Calabria P, Gennari L, Martini G, et al. Left ventricular diastolic function improvement by carvedilol therapy in advanced heart failure. Journal of Cardiovascular Pharmacology 2005;45(6):563-8. [DOI] [PubMed] [Google Scholar]
Paolisso 1992 {published data only}
- Paolisso G, Gambardella A, Marrazzo G, Verza M, Teasuro P, Varricchio M, et al. Metabolic and cardiovascular benefits deriving from beta-adrenergic blockade in chronic congestive heart failure. American Heart Journal 1992;123(1):103-10. [DOI] [PubMed] [Google Scholar]
Paraskevaidis 2006 {published data only}
- Paraskevaidis IA, Tsiapras D, Karavolias G, Adamopoulos S, Dodouras T, Cokkinos P, et al. The effect of carvedilol therapy on myocardial functional reserve in patients with advanced heart failure caused by nonischemic dilated cardiomyopathy. Journal of the American Society of Echocardiography 2006;19(5):529-35. [DOI] [PubMed] [Google Scholar]
Park 2016 {published data only}
- Park K, Park TH. Comparative effects of nebivolol and carvedilol on left ventricular diastolic function in older heart failure patients with preserved ejection fraction: study protocol for a randomized controlled trial. Trials 2016;17(1):530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patten 1997 {published data only}
- Patten RD, Kronenberg MW, Benedict CR, Udelson JE, Kinan D, Stewart D, et al. Acute and long-term effects of the angiotensin-converting enzyme inhibitor, enalapril, on adrenergic activity and sensitivity during exercise in patients with left ventricular systolic dysfunction. American Heart Journal 1997;134(1):37-43. [DOI] [PubMed] [Google Scholar]
Pennell 2000 {published data only}
- Pennell DJ, Ray SG, Davies G, Burgess M, Webster J, Slomka P, et al. The carvedilol hibernation reversible ischaemia trial, marker of success (CHRISTMAS) study. Methodology of a randomised, placebo controlled, multicentre study of carvedilol in hibernation and heart failure. International Journal of Cardiology 2000;72(3):265-74. [DOI] [PubMed] [Google Scholar]
Pierard 2002 {published data only}
- Pierard L. Clinical study of the month. Effects of valsartan in chronic heart failure: the VAL-HeFT study. Revue Medical de Liege 2002;57(1):57-9. [PubMed] [Google Scholar]
Pina 2004 {published data only}
- Pina IL. Valsartan in acute myocardial infarction trial. Current Cardiology Reports 2004;6(3):159-60. [PubMed] [Google Scholar]
Pitt 2005 {published data only}
- Pitt B, White H, Nicolau J, Martinez F, Gheorghiade M, Aschermann M, et al. Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. JACC 2005;46(3):425-31. [DOI] [PubMed] [Google Scholar]
Pitt 2008 {published data only}
- Pitt B, Bakris G, Ruilope LM, DiCarlo L, Mukherjee R, Investigators Ephesus. Serum potassium and clinical outcomes in the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS). Circulation 2008;118(16):1643-50. [DOI] [PubMed] [Google Scholar]
Pitt 2011 {published data only}
- Pitt B, Latini R, Maggioni AP, Solomon SD, Smith BA, Wright M, et al. Neurohumoral effects of aliskiren in patients with symptomatic heart failure receiving a mineralocorticoid receptor antagonist: the Aliskiren Observation of Heart Failure Treatment study. European Journal of Heart Failure 2011;13(7):755-64. [DOI] [PubMed] [Google Scholar]
Pourdjabbar 2015 {published data only}
- Pourdjabbar A, Dwivedi G, Haddad R, Saikali A, Mielniczuk L, Haddad H. Heart rate control in patients with left ventricular systolic dysfunction and heart failure. International Journal of Cardiology 2015;184:276-7. [DOI] [PubMed] [Google Scholar]
Premkumar 2016 {published data only}
- Premkumar M, Rangegowda D, Shasthry SM, Vyas TS, Goyal R, Singh JK, et al. Targeted heart rate reduction using carvedilol ivabradine improves left ventricular diastolic dysfunction,clinical progression and survival in cirrhosis. Indian Journal of Gastroenterology 2016;35(1):A46-7. [DOI] [PubMed] [Google Scholar]
Quaife 1998 {published data only}
- Quaife RA, Christian PE, Gilbert EM, Datz FL, Volkman K, Bristow MR. Effects of carvedilol on right ventricular function in chronic heart failure. American Journal of Cardiology 1998;81(2):247-50. [DOI] [PubMed] [Google Scholar]
Ramaswamy 2003 {published data only}
- Ramaswamy K. Beta blockers improve outcome in patients with heart failure and atrial fibrillation: U.S. carvedilol study. Cardiac Electrophysiology Review 2003;7(3):229-32. [DOI] [PubMed] [Google Scholar]
Remme 2001 {published data only}
- Remme WJ, Committee Carmen Steering, Investigators. The Carvedilol and ACE-Inhibitor Remodelling Mild Heart Failure EvaluatioN trial (CARMEN)--rationale and design. Cardiovascular Drugs and Therapy 2001;15(1):69-77. [DOI] [PubMed] [Google Scholar]
Remme 2004 {published data only}
- Remme WJ, Riegger G, Hildebrandt P, Komajda M, Jaarsma W, Bobbio M, et al. The benefits of early combination treatment of carvedilol and an ACE-inhibitor in mild heart failure and left ventricular systolic dysfunction. The carvedilol and ACE-inhibitor remodelling mild heart failure evaluation trial (CARMEN). Cardiovascular Drugs and Therapy 2004;18(1):57-66. [DOI] [PubMed] [Google Scholar]
Remme 2005 {published data only}
- Remme WJ. Could beta-blockers precede or replace angiotensin-converting enzyme inhibitors in heart failure? Heart Failure Clinic 2005;1(1):67-75. [DOI] [PubMed] [Google Scholar]
Rimatori 1990 {published data only}
- Rimatori C, Zayat M. Captopril in the early phase of cardiac failure: effects on left ventricular diastolic function. Journal of International Medicine Supplement 1990;228(733):58. [Google Scholar]
Roongsritong 2005 {published data only}
- Roongsritong C, Sutthiwan P, Bradley J, Simoni J, Power S, Meyerrose GE. Spironolactone improves diastolic function in the elderly. Clinical Cardiology 2005;28(10):484-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosa 2011 {published data only}
- Rosa MLD. Effect of aliskiren and antihypertensive drugs on diastolic function in hypertensives with diastolic dysfunction: A randomised study. Journal of Clinical Hypertension 2011;13(4):A81. [Google Scholar]
Rosenkranz 2003 {published data only}
- Rosenkranz S, Erdmann E. Hemodynamic effects of the beta blocker nebivolol. Internist 2003;44(4):481-2. [DOI] [PubMed] [Google Scholar]
Rossignol 2011 {published data only}
- Rossignol P, Manard J, Fay R, Gustafsson F, Pitt B, Zannad F. Eplerenone survival benefits in heart failure patients post-myocardial infarction are independent from its diuretic and potassium-sparing effects: Insights from an EPHESUS (Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study) substudy. JACC 2011;58(19):1958-66. [DOI] [PubMed] [Google Scholar]
Sakai 2011 {published data only}
- Sakai H, Tsutamoto T, Kawahara C, Fujii M, Yamaji M, Ohnishi M, et al. Effects of aliskiren on Left ventricular remodeling in patients with dilated cardiomyopathy. European Heart Journal 2011;32:787. [Google Scholar]
Sanderson 1998 {published data only}
- Sanderson JE, Chan SKW, Yu CM, Yeung LYC, Chan WM, Raymond K, et al. Beta blockers in heart failure: a comparison of a vasodilating beta blocker with metoprolol. Heart 1998;79:86-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanghera 2011 {published data only}
- Sanghera KM. Keep patients with heart failure out of hospital: Ensure they get target doses. Pharmaceutical Journal 2011;286(7658):727-8. [Google Scholar]
Santulli 2015 {published data only}
- Santulli G. beta-Blockers in diabetic patients with heart failure. JAMA Internal Medicine 2015;175(4):657. [DOI] [PubMed] [Google Scholar]
Sardu 1991 {published data only}
- Sardu G. Treatment of mild-to-moderate congestive heart failure with the angiotensin converting enzyme inhibitor quinapril. Advances in Therapy 1991;8(3):124-32. [Google Scholar]
Schindler 2008 {published data only}
- Schindler B. Angiotensin receptor antagonists: No advantage for hypertensive patients with diastolic dysfunction. Medizinische Monatsschrift fur Pharmazeuten 2008;31(5):195-6. [PubMed] [Google Scholar]
Schwab 2009 {published data only}
- Schwab J, Schneider M P, Pauschinger M, Schmieder R E. Hypertension and diastolic dysfunction. MMW Fortschritte der Medizin 2009;151(23):41-3. [DOI] [PubMed] [Google Scholar]
Segovia 2008 {published data only}
- Segovia J, Bermejo J, Alfonso F. Summary of the clinical studies reported in the 57th Scientific Session of the American College of Cardiology (Chicago, USA, 30 march-2 april 2008). Revista Espanola de Cardiologia 2008;61(7):726-37. [PubMed] [Google Scholar]
Shimamoto 2007 {published data only}
- Shimamoto K, Kawana M. The Carvedilol and ACE-Inhibitor Remodelling Mild Heart Failure Evaluation Trial. Nippon Rinsho 2007;65 Suppl 4:531-6. [PubMed] [Google Scholar]
Sidorenko 2008 {published data only}
- Sidorenko BA, Bugrimova MA, Iosava IK, Pataraia SA, Preobrazhenskii DV. Controlled release metoprolol succinate in MERIT-HF. Analysis of patients subgroups. Kardiologiia 2008;48(3):85-8. [PubMed] [Google Scholar]
Silva 2014 {published data only}
- Silva MC, Rassi CH, Meira ZM, Giannetti JG, Vainzof M, Zatz M, et al. Progression of myocardial fibrosis by magnetic resonance imaging in patients with duchenne and becker muscular dystrophy and preserved left ventricular ejection fraction-a randomized clinical trial for treatment with ACE inhibitors. Journal of Cardiovascular Magnetic Resonance 2014;16:P311. [Google Scholar]
Smith 2012 {published data only}
- Smith JG, Kohl S, Kornhall B, Ekmehag B. Congestive heart failure, part 2: treatment. Lakartidningen 2012;109(41):1829-34. [PubMed] [Google Scholar]
Spoto 2002 {published data only}
- Spoto S, Palma Modoni A, De Galasso L. Diastolic cardiac insufficiency. La Clinica Terapeutica 2002;153(5):355-7. [PubMed] [Google Scholar]
Stecker 2005 {published data only}
- Stecker EC, McAnulty JH. Letter regarding article by Weintraub et al, "Cost-effectiveness of eplerenone compared with placebo in patients with myocardial infarction complicated by left ventricular dysfunction and heart failure". Circulation 2005;112(5):e74. [DOI] [PubMed] [Google Scholar]
Stiefelhagen 2006 {published data only}
- Stiefelhagen P. New study results in cardiology. Internist 2006;47(3):311-6. [DOI] [PubMed] [Google Scholar]
Struthers 2004 {published data only}
- Struthers AD. Aldosterone in heart failure: pathophysiology and treatment. Current Heart Failure Reports 2004;1(4):171-5. [DOI] [PubMed] [Google Scholar]
Swedberg 1996 {published data only}
- Swedberg K, Sharpe N. The value of angiotensin converting enzyme inhibitors for the treatment of patients with left ventricular dysfunction, heart failure or after acute myocardial infarction. European Heart Journal 1996;17(9):1306-11. [DOI] [PubMed] [Google Scholar]
Swedberg 1999 {published data only}
- Swedberg K. Beta-blockers and heart failure. Journal of Cardiovascular Risk 1999;6(3):129-30. [DOI] [PubMed] [Google Scholar]
Szajnbok 1993 {published data only}
- Szajnbok FE, Barretto AC, Mady C, Parga Filho J, Gruppi C, Alfieri RG, et al. Beneficial effects of enalapril on the diastolic ventricular function in Chagas myocardiopathy. Arquivos Brasileiros de Cardiologia 1993;60(4):273-8. [PubMed] [Google Scholar]
Szymanski 2009 {published data only}
- Szymanski P, Klisiewicz A, Hoffman P. Therapeutic options for systemic right ventricular failure. Heart 2009;95(23):1950-1; author reply 1951. [DOI] [PubMed] [Google Scholar]
Taheri 2009 {published data only}
- Taheri S, Mortazavi M, Shahidi S, Pourmoghadas A, Garakyaraghi M, Seirafian S, et al. Spironolactone in chronic hemodialysis patients improves cardiac function. Saudi Journal of Kidney Diseases and Transplantation 2009;20(3):392-7. [PubMed] [Google Scholar]
Takekoshi 2004 {published data only}
- Takekoshi N, Asaji T, Tada N. Hypertension in patients with heart failure. Nippon Rinsho 2004;62 Suppl 3:478-83. [PubMed] [Google Scholar]
Tala 2011a {published data only}
- Tala S, Rossignol P, Fay R, Pitt B, Zannad F. Relation of loop diuretic dose to morbimortality in patients with heart failure and left ventricular systolic dysfunction: Insights from EPHESUS. European Journal of Heart Failure. Supplement 2011;10:S114-5. [Google Scholar]
Tala 2011b {published data only}
- Tala S, Rossignol P, Fay R, Pitt B, Zannad F. Relation of loop diuretic dose to morbimortality in patients with heart failure and left ventricular systolic dysfunction: Insights from the eplerenone post-acute myocardial infarction heart failure efficacy and survival study (EPHESUS). Fundamental and Clinical Pharmacology 2011;25:2. [Google Scholar]
Tan 2013 {published data only}
- Tan LB, Schlosshan D, Hall AS. Cardiac functional benefits of ivabradine therapy in patients with severe heart failure. International Journal of Cardiology 2013;165(2):389-90. [DOI] [PubMed] [Google Scholar]
Tatsumi 2006 {published data only}
- Tatsumi T, Matsubara H. Cardioprotective effect of aldosterone antagonists for ventricular remodeling. Nippon Rinsho 2006;64 Suppl 5:524-9. [PubMed] [Google Scholar]
Taylor 2003 {published data only}
- Taylor AL. The African-American Heart Failure Trial (A-HeFT): rationale and methodology.[Erratum appears in J Card Fail. 2003 Dec;9(6):481 Note: Dosage error in article text]. Journal of Cardiac Failure 2003;9(5 Suppl Nitric Oxide):S216-9. [DOI] [PubMed] [Google Scholar]
Teerlink 2003 {published data only}
- Teerlink JR, Massie BM. Late breaking heart failure trials from the 2003 ACC meeting: EPHESUS and COMPANION. Journal of Cardiac Failure 2003;9(3):158-63. [DOI] [PubMed] [Google Scholar]
Tereshchenko 2005 {published data only}
- Tereshchenko SN, Kositsyna IV, Dzhaiani NA, Golubev AV, Kochetov AG. The use of esmolol in patients with myocardial infarction complicated with acute left ventricular failure. Kardiologiia 2005;45(6):19-22. [PubMed] [Google Scholar]
Thornton 2004 {published data only}
- Thornton PL, Ahmed A. Angiotensin-converting enzyme inhibitors, beta-blockers, and mortality in systolic heart failure. JACC 2004;43(7):1333; author reply 1333-4. [DOI] [PubMed] [Google Scholar]
Thune 2008 {published data only}
- Thune JJ, Signorovitch J, Kober L, Velazquez EJ, McMurray JJ, Califf RM, et al. Effect of antecedent hypertension and follow-up blood pressure on outcomes after high-risk myocardial infarction. Hypertension 2008;51(1):48-54. [DOI] [PubMed] [Google Scholar]
Tinoco 2004 {published data only}
- Tinoco Mesquita E, Socrates J, Rassi S, Villacorta H, Mady C. Heart failure with preserved systolic function. Arquivos Brasileiros de Cardiologia 2004;82(5):494-500. [DOI] [PubMed] [Google Scholar]
Tsutamoto 2000 {published data only}
- Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, et al. Spironolactone inhibits the transcardiac extraction of aldosterone in patients with congestive heart failure. JACC 2000;36(3):838-44. [DOI] [PubMed] [Google Scholar]
Tsutamoto 2001 {published data only}
- Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, et al. Effect of spironolactone on plasma brain natriuretic peptide and left ventricular remodeling in patients with congestive heart failure. Journal of the American College of Cardiology 2001;37(5):1228-33. [DOI] [PubMed] [Google Scholar]
Tsutamoto 2005 {published data only}
- Tsutamoto T, Horie M, Hayashi M. Left ventricular remodeling post myocardial infarction. Nippon Rinsho 2005;63 Suppl 3:323-9. [PubMed] [Google Scholar]
Tumasyan 2010 {published data only}
- Tumasyan LR, Adamyan KG. Comparative efficacy of combined therapy with angiotensin converting enzyme inhibitor and angiotensin receptor blocker and direct renin inhibitor in patients with severe chronic heart failure. European Heart Journal 2010;31:463-4. [Google Scholar]
Tumasyan 2018 {published data only}
- Tumasyan LR, Adamyan KG. Comparative efficacy of renin-angiotensin system modulators on prognosis, right and left heart functional parameters in chronic heart failure in relation to mid-ranged or preserved ejection fraction. European Journal of Heart Failure 2018;20(Suppl 1):75. [Google Scholar]
Umemoto 2003 {published data only}
- Umemoto S, Kawahara S, Hashimoto R, Matsuzaki M. Angiotensin receptor blockers in chronic heart failure. Nippon Rinsho 2003;61(9):1683-9. [PubMed] [Google Scholar]
Uusimaa 2001 {published data only}
- Uusimaa P, Tokola H, Ruskoaho H, Vuolteenaho O, Risteli J, Ylitalo A, et al. Vasoactive peptides and procollagen propeptides in patients with hypertension in relation to cardiac hypertrophy and diastolic heart failure: design of the study and patient characteristics. Journal of Human Hypertension 2001;15 Suppl 1:S19-22. [DOI] [PubMed] [Google Scholar]
Van den Berg 1993 {published data only}
- Van den Berg MP, Veldhuisen DJ, Crijns HJ, Lie KI. Reversion of tachycardiomyopathy after beta-blocker. Lancet 1993;341(8861):1667. [DOI] [PubMed] [Google Scholar]
Van den Berg 1995 {published data only}
- Van den Berg MP, Crijns HJ, Van Veldhuisen DJ, Grief N, De Kam PJ, Lie KI. Effects of lisinopril in patients with heart failure and chronic atrial fibrillation. Journal of Cardiac Failure 1995;1(5):355-63. [DOI] [PubMed] [Google Scholar]
Vasiuk 2001 {published data only}
- Vasiuk IA, Kopelev MV, Khadzegova AB, Krikunov PV, Iushchuk EN, Sologub KN. The role of beta-blockers in the treatment of chronic cardiac failure. Klinicheskaia Meditsina 2001;79(1):5-8. [PubMed] [Google Scholar]
Vincent 2012 {published data only}
- Vincent J, Sutradhar S, Beckerman B, Pitt B, Zannad F. Changes in sodium and renal function may contribute to the beneficial effects of eplerenone on mortality and morbidity in the EPHESUS trial. Circulation 2012;125(19):e745. [Google Scholar]
Vizir 2000 {published data only}
- Vizir VA, Berezin AE. Losartan in therapy of chronic heart failure. Klinicheskaia Meditsina 2000;78(2):36-9. [PubMed] [Google Scholar]
Vizzardi 2010 {published data only}
- Vizzardi E, D'Aloia A, Giubbini R, Bordonali T, Bugatti S, Pezzali N, et al. Effect of spironolactone on left ventricular ejection fraction and volumes in patients with class I or II heart failure. American Journal of Cardiology 2010;106(9):1292-6. [DOI] [PubMed] [Google Scholar]
Vizzardi 2012 {published data only}
- Vizzardi E, D'Aloia A, Della Pina P, Lombardi C, Bonadei I, Rovetta R, et al. The effect of aldosterone-antagonist therapy on aortic elastic properties in patients with moderate heart failure. European Heart Journal 2012;33:934. [Google Scholar]
Vizzardi 2015a {published data only}
- Vizzardi E, Sciatti E, Bonadei I, D'Aloia A, Tartiere-Kesri L, Tartiere J M, et al. Effects of spironolactone on ventricular-arterial coupling in patients with chronic systolic heart failure and mild symptoms. Clinical Research in Cardiology 2015;104(12):1078-87. [DOI] [PubMed] [Google Scholar]
Vizzardi 2015b {published data only}
- Vizzardi E, Pina P D, Caretta G, Bonadei I, Sciatti E, Lombardi C, et al. The effect of aldosterone-antagonist therapy on aortic elastic properties in patients with nonischemic dilated cardiomyopathy. Journal of Cardiovascular Medicine 2015;16(9):597-602. [DOI] [PubMed] [Google Scholar]
Volpe 1992 {published data only}
- Volpe M, Tritto C, DeLuca N, Rubattu S, Mele AF, Lembo G, et al. Angiotensin converting enzyme inhibition restores cardiac and hormonal responses to volume overload in patients with dilated cardiomyopathy and mild heart failure. Circulation 1992;86(6):1800-9. [DOI] [PubMed] [Google Scholar]
Volpe 2010 {published data only}
- Volpe M, Taddei S. The HEAAL study. Giornale Italiano di Cardiologia 2010;11(9):625-9. [PubMed] [Google Scholar]
Voors 2008 {published data only}
- Voors AA, Jong RM. Treating diastolic heart failure. Heart 2008;94(8):971-2. [DOI] [PubMed] [Google Scholar]
Waagstein 2003 {published data only}
- Waagstein F, Stromblad O, Andersson B, Bohm M, Darius M, Delius W, et al. Increased exercise ejection fraction and reversed remodeling after long-term treatment with metoprolol in congestive heart failure: a randomized, stratified, double-blind, placebo-controlled trial in mild to moderate heart failure due to ischemic or idiopathic dilated cardiomyopathy. European Journal of Heart Failure 2003;5(5):679-91. [DOI] [PubMed] [Google Scholar]
Waldo 1995 {published data only}
- Waldo AL, Camm AJ, deRuyter H, Freidman PL, MacNeil DJ, Pitt B, et al. Survival with oral d-sotalol in patients with left ventricular dysfunction after myocardial infarction: rationale, design, and methods (the SWORD trial). American Journal of Cardiology 1995;75(15):1023-7. [DOI] [PubMed] [Google Scholar]
Waldo 1996 {published data only}
- Waldo AL, Camm AJ, deRuyter H, Friedman PL, MacNeil DJ, Pauls JF, et al. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol.[Erratum appears in Lancet 1996 Aug 10;348(9024):416]. Lancet 1996;348(9019):7-12. [DOI] [PubMed] [Google Scholar]
Warner 1999 {published data only}
- Warner JG Jr, Metzger DC, Kitzman DW, Wesley DJ, Little WC. Losartan improves exercise tolerance in patients with diastolic dysfunction and a hypertensive response to exercise. JACC 1999;33(6):1567-72. [DOI] [PubMed] [Google Scholar]
Weinberg 2001 {published data only}
- Weinberg EO, Herzig JW. Management of hypertension and heart failure with AT1 receptor blockade. JPMA 2001;51(2):81-5. [PubMed] [Google Scholar]
Weintraub 2005 {published data only}
- Weintraub WS, Zhang Z, Mahoney EM, Kolm P, Spertus JA, Caro J, et al. Cost-effectiveness of eplerenone compared with placebo in patients with myocardial infarction complicated by left ventricular dysfunction and heart failure. Circulation 2005;111(9):1106-13. [DOI] [PubMed] [Google Scholar]
Weir 2011 {published data only}
- Weir RA, Tsorlalis IK, Steedman T, Dargie HJ, Fraser R, McMurray JJ, et al. Aldosterone and cortisol predict medium-term left ventricular remodelling following myocardial infarction. European Journal of Heart Failure 2011;13(12):1305-13. [DOI] [PubMed] [Google Scholar]
Wong 2002 {published data only}
- Wong M, Staszewsky L, Latini R, Barlera S, Volpi A, Chiang YT, et al. Valsartan benefits left ventricular structure and function in heart failure: Val-HeFT echocardiographic study. JACC 2002;40(5):970-5. [DOI] [PubMed] [Google Scholar]
Wong 2004 {published data only}
- Wong M, Staszewsky L, Latini R, Barlera S, Glazer R, Aknay N, et al. Severity of left ventricular remodeling defines outcomes and response to therapy in heart failure: Valsartan heart failure trial (Val-HeFT) echocardiographic data. JACC 2004;43(11):2022-7. [DOI] [PubMed] [Google Scholar]
Woodley 1991 {published data only}
- Woodley SL, Gilbert EM, Anderson JL, O'Connell JB, Deitchman D, Yanowitz FG, et al. Beta-blockade with bucindolol in heart failure caused by ischemic versus idiopathic dilated cardiomyopathy. Circulation 1991;84(6):2426-41. [DOI] [PubMed] [Google Scholar]
Wright 2014 {published data only}
- Wright LM, Jellis C, Kosmala W, Marwick T. Use of plasma markers of fibrosis to predict responders to the use of spironolactone in stage b heart failure: A strain analysis. JACC 2014;63(12):A1127. [Google Scholar]
Wu 2002 {published data only}
- Wu DQ, Yang YJ. Improving effect of carvedilol on cardiac function and exercise tolerance in patients with congestive heart failure of dilated cardiomyopathy. Chinese Journal of Clinical Rehabilitation 2002;6(15):2342-3. [Google Scholar]
Xu 2007 {published data only}
- Xu JL, Zuo XH, Sha YB, Han CY, Liu XY, Zhang CJ. Effect of carvedilol on left ventricular function of patients with congestive heart failure evaluated by Doppler image. Journal of Clinical Rehabilitative Tissue Engineering Research 2007;11(13):2537-9. [Google Scholar]
Yamamoto 2005 {published data only}
- Yamamoto T, Yano M. Aldosterone antagonist therapy for chronic heart failure. Nippon Naika Gakkai Zasshi 2005;94(2):262-9. [DOI] [PubMed] [Google Scholar]
Yan 2012 {published data only}
- Yan ZL, Wei L, Zhong YM, Xie DM, Yang YH. Effects of benazepril on left ventricular remodelling and exercise tolerance in patients with valvular heart failure. Heart 2012;98:E262. [Google Scholar]
Yoshihiro 2011 {published data only}
- Yoshihiro I, Takaki T, Akira Y. The addition of direct renin inhibitor aliskiren to standard therapy suppresses enhanced cardiac sympathetic activity in patients with systolic heart failure. Circulation 2011;124(21):A8406. [Google Scholar]
Young 2004 {published data only}
- Young JB, Dunlap ME, Pfeffer MA, Probstfield JL, Cohen-Solal A, Dietz R, et al. Mortality and morbidity reduction with Candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trials. Circulation 2004;110(17):2618-26. [DOI] [PubMed] [Google Scholar]
Zeng 2006 {published data only}
- Zeng QL, He L. Effectiveness of losartan and benazepril in improving diastolic function of left ventricle in patients with heart failure and hypertension. Pharmaceutical Care and Research 2006;6(4):294-6. [Google Scholar]
Zheng 2009 {published data only}
- Zheng Y, Meng L, Dong JS, Wu JY. Effect of carvedilol on heart function and glucolipometabolic in elder patients with diastolic heart failure. Journal of Jilin University (Medicine Edition) 2009;35(6):1111-4. [Google Scholar]
References to studies awaiting assessment
Anonymous 2003d {published data only}
- Anonymous. Selective aldosterone blocking in heart failure. Eplerenone reduces the risk after infarction. MMW Fortschritte der Medizin 2003;145(44):42-3. [PubMed] [Google Scholar]
Botoni 2010 {published data only}
- Botoni F, Poole-Wilson PPW, Ribeiro ALP, Guedes VC, Oliveira BMR, Teixeira MM, et al. Assessment of the Quality of Life (SF36) after optimized cardiovascular therapy in Chagas cardiomyopathy. European Journal of Heart Failure Supplement 2010;9:S108. [Google Scholar]
Dielievska 2015 {published data only}
- Dielievska VY. The effect of aldosterone receptor blockade in diastolic heart failure in patients with arterial hypertension combined with chronic obstructive pulmonary disease. General Medicine 2015;17(3):3-7. [Google Scholar]
EUCTR2005‐001306‐87 {published data only}
- EUCTR2005-001306-87. Effects of candesartan cilexetil vs standard therapy on serum levels of brain natriuretic peptide in patients suffering from chronic heart failure with depressed and preserved systolic function. www.clinicaltrialsregister.eu/ctr-search/trial/2005-001306-87/IT#P (first entered 2 February 2007).
EUCTR2005‐002109‐22 {published data only}
- EUCTR2005-002109-22. Comparative effects of telmisartan and candesartan on glucose metabolism in patients with chronic heart failure. www.clinicaltrialsregister.eu/ctr-search/trial/2005-002109-22/AT (first entered 27 October 2005).
Metra 1999 {published data only}
- Metra M, Nodari S, Bordonali T, Cagnazzi E, Boldi E, Dei Cas L. ACE-inhibitors, AT1 receptor antagonists and diastolic dysfunction. Cardiologia 1999;44(Suppl 1):53-8. [PubMed] [Google Scholar]
PER‐010‐15 {published data only}
- PER-010-15. A multicenter, randomized, double blind, parallel group, active controlled study to evaluate the efficacy and safety of LCZ696 compared to valsartan, on morbidity and mortality in heart failure patients (NYHA class II-IV) with preserved ejection fraction. www.ins.gob.pe/ensayosclinicos/rpec/recuperarECPBNuevo.asp?ver=EN&numEc=010-15 (registration date 9 June 2015).
Przewlocka‐Kosmala 2017 {published data only}
- Przewlocka-Kosmala M, Kosmala W, Marwick TWH. Effect of aldosterone antagonism on active and passive determinants of LV diastolic filling and exercise intolerance in heart faillure with preserved ejection fraction. European Heart Journal Supplements 2017;18(Suppl 3):iii223. [Google Scholar]
Rapezzi 1999 {published data only}
- Rapezzi C, Perugini E, Amati S, Branzi A. Beta blockers and calcium antagonists in diastolic dysfunction. Cardiologia 1999;44(Suppl 1):61-4. [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR‐IPR‐16009507 {published data only}
- ChiCTR-IPR-16009507. Aldosterone antagonist delays the progression of diastolic dysfunction in patients with hypertension and myocardial hypertrophy: a randomised controlled clinical trial. www.chictr.org.cn/showprojen.aspx?proj=16311 (date of registration 20 October 2016).
ChiCTR‐TRC‐09000631 {published data only}
- ChiCTR-TRC-09000631. Treatment of diastolic heart failure: the role of blockade of the renin-angiotensin system. A comparison of diuretics with an angiotensin converting enzyme inhibitor, angiotensin receptor blockade or diuretics alone. www.chictr.org.cn/showprojen.aspx?proj=8904 (date of registration 21 September 2014).
CTRI/2010/091/000438 {published data only}
- CTRI/2010/091/000438. Evaluation of efficacy and safety of metoprolol in patients having heart failure with normal ejection fraction: a randomised, double-blind, placebo-controlled trial. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=1626&EncHid=&modid=&compid=%27,%271626det%27 (registered 19 May 2010).
CTRI/2017/09/009732 {published data only}
- CTRI/2017/09/009732. A 24-week, randomized, double-blind, multi-center, parallel group, active controlled study to evaluate the effect of LCZ696 on NT-proBNP, symptoms, exercise function and safety compared to individualized medical management of comorbidities in patients with heart failure and preserved ejection fraction. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=19155&EncHid=&modid=&compid=%27,%2719155det%27 (registered 12 September 2017).
EUCTR2016‐001254‐17 {published data only}
- EUCTR2016-001254-17. A multicenter, randomized, double-blind, active-controlled study to evaluate the effects of LCZ696 compared to valsartan on cognitive function in patients with chronic heart failure and preserved ejection fraction (PERSPECTIVE). www.clinicaltrialsregister.eu/ctr-search/trial/2016-001254-17/GB (first entered 11 August 2016).
EUCTR2016‐003410‐28 {published data only}
- EUCTR2016-003410-28. A 24-week, randomized, double-blind, multi-center, parallel group, active controlled study to evaluate the effect of LCZ696 on NT-proBNP, exercisecapacity, symptoms and safety compared to individualized medical management of comorbidities in patients with heart failure and preserved ejection fraction. www.clinicaltrialsregister.eu/ctr-search/search?query=2016-003410-28 (first entered 12 May 2017).
EUCTR2017‐000697‐11 {published data only}
- EUCTR2017-000697-11. Spironolactone In the treatment of heart failure: a double-blind, randomized, placebo-controlled, parallel group, interventional phase III study to evaluate the efficacy and safety of spironolactone compared to placebo on the composite endpoint of recurrent heart failure hospitalizations and cardiovascular death in patients with heart failure with mid- range or preserved ejection fraction (SPIRIT-HF). www.clinicaltrialsregister.eu/ctr-search/trial/2017-000697-11/DE (first entered 8 December 2017).
NCT02901184 {published data only}
- NCT02901184. Spironolactone initiation registry randomized interventional trial in heart failure with preserved ejection fraction (SPIRRIT). clinicaltrials.gov/show/NCT02901184 (first posted 15 September 2016).
NCT03928158 {published data only}
- NCT03928158. LCZ696 in advanced LV hypertrophy and HFpEF. clinicaltrials.gov/ct2/show/NCT03928158 (first posted 26 Aopril 2019).
NCT03948685 {published data only}
- NCT03948685. Carvedilol SR study for biomarkers from blood and urine and safety of in patients with heart failure with preserved ejection fraction. clinicaltrials.gov/ct2/show/NCT03948685 (first posted 14 May 2019).
NCT03988634 {published data only}
- NCT03988634. Changes in NT-proBNP and outcomes, safety, and tolerability in HFpEF patients with acute decompensated heart failure (ADHF) who have been stabilized during hospitalization and initiated in-hospital or within 30 days post-discharge (PARAGLIDE-HF). clinicaltrials.gov/ct2/show/NCT03988634 (first posted 17 June 2019).
NCT04128891 {published data only}
- NCT04128891. Study of sacubitril/valsartan on myocardIal oxygenation and fibrosis in heart failure with preserved ejection fraction (PRISTINE-HF). clinicaltrials.gov/ct2/show/NCT04128891 (first posted 16 October 2019).
Zhou 2010 {published data only}
- Zhou J, Shi H, Zhang J, Lu Y, Fu M, Ge J, beta-PRESERVE Study Investigators. Rationale and design of the beta-blocker in heart failure with normal left ventricular ejection fraction (beta-PRESERVE) study. European Journal of Heart Failure 2010;12(2):181-5. [DOI] [PubMed] [Google Scholar]
Additional references
CIBIS Investigators 1999
- II CIBIS. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353(9146):9-13. [PubMed] [Google Scholar]
Cleland 2018
- Cleland JG, Buntin KV, Flather MD, Altman DG, Holmes J, Coats AJ, et al. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. European Heart Journal 2018;39(1):26-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc., 1988. [Google Scholar]
Consensus Trial Study Group 1987
- Group CONSENSUS Trial Study. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). New England Journal of Medicine 1987;316(23):1429-35. [DOI] [PubMed] [Google Scholar]
de Denus 2017
- Denus S, O'Meara E, Desai AS, Claggett B, Lewis EF, Leclair G, et al. Spironolactone metabolites in TOPCAT - new insights into regional variation. New England Journal of Medicine 2017;376(17):1690-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editors(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. [Google Scholar]
Emdin 2015
- Emdin CA, Callender T, Cao J, McMurray JJ, Rahimi K. Meta-analysis of large-scale randomized trials to determine the effectiveness of inhibition of the renin-angiotensin aldosterone system in heart failure. American Journal of Cardiology 2015;116(1):155-61. [DOI] [PubMed] [Google Scholar]
Flather 2005
- Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, et al, SENIORS Investigators. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). European Heart Journal 2005;26(3):215-25. [DOI] [PubMed] [Google Scholar]
Gerber 2015
- Gerber Yariv, Weston Susan A, Redfield Margaret M, Chamberlain Alanna M, Manemann Sheila M, Jiang Ruoxiang, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Internal Medicine 2015;175(6):996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzalez‐Lopez 2015
- Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G, Haro-Del Moral FJ, Cobo-Marcos M, Robles C, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. European Heart Journal 2015;36(38):1285-94. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed prior to 12 February 2018. Hamilton, ON: McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. [Google Scholar]
Hjalmarson 2000
- Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA 2000;283(10):1295-302. [DOI] [PubMed] [Google Scholar]
Hogg 2005
- Hogg K, McMurray J. Neurohumoral pathways in heart failure with preserved systolic function. Progress in Cardiovascular Diseases 2005;47(6):357-66. [DOI] [PubMed] [Google Scholar]
Khan 2017
- Khan MS, Fonarow GC, Kahn H, Greene SJ, Anker SD, Gheorghiade M, et al. Renin-angiotensin blockade in heart failure with preserved ejection fraction: a systematic review and meta-analysis. ESC Heart Failure 2017;4:402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kotecha 2014
- Kotecha D, Holmes J, Krum H, Altman DG, Manzano L, Cleland JGF, et al, Beta-Blockers in Heart Failure Collaborative Group. Efficacy of β blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis. Lancet 2014;384(9961):2235-43. [DOI] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, Noel-Storr A, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.. [Google Scholar]
Li 2018
- Li S, Zhang X, Dong M, Gong S, Shang Z, Jia X, et al. Effects of spironolactone in heart failure with preserve ejection fraction: a meta-analysis of randomized controlled trials. Medicine 2018;97:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
McMurray 2014
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al, PARADIGM-HF Investigators and Committees. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. New England Journal of Medicine 2014;371(11):993-1004. [DOI] [PubMed] [Google Scholar]
MERIT‐HF Study Group 1999
- MERIT-HF. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353(9169):2001-7. [PubMed] [Google Scholar]
NICE 2018
- NICE. Chronic heart failure in adults: diagnosis and management. www.nice.org.uk/guidance/ng106 (accessed 2 October 2020).
Packer 1999
- Packer M, Poole-Wilson PA, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, et al. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation 1999;100(23):2312-8. [DOI] [PubMed] [Google Scholar]
Packer 2001
- Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, et al. Effect of carvedilol on survival in severe chronic heart failure. New England Journal of Medicine 2001;344(22):1651-8. [DOI] [PubMed] [Google Scholar]
Packer 2002
- Packer M, Fowler MB, Roecker EB, Coats AJS, Katus Hugo A, Krum H, et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 2002;106(17):2194-9. [DOI] [PubMed] [Google Scholar]
Pfeffer 2015
- Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac FunctionHeart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015;131(1):34-42. [DOI] [PubMed] [Google Scholar]
Pitt 1999
- Pitt Bertram, Zannad Faiez, Remme Willem J, Cody Robert, Castaigne Alain, Perez Alfonso, et al. The Effect of Spironolactone on Morbidity and Mortality in Patients with Severe Heart Failure. New England Journal of Medicine 1999;341(10):709-17. [DOI] [PubMed] [Google Scholar]
Pitt 2014
- Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. New England Journal of Medicine 2014;370(15):1383-92. [DOI] [PubMed] [Google Scholar]
Ponikowski 2016
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al, Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal 2016;37(27):2129-200. [DOI] [PubMed] [Google Scholar]
Rector 1995
- Rector T S, Tschumperlin L K, Kubo S H, Bank A J, Francis G S, McDonald K M, et al. Use of the Living With Heart Failure questionnaire to ascertain patients' perspectives on improvement in quality of life versus risk of drug-induced death. Journal of Cardiac Failure 1995;1(3):201-6. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration, 2014.
Roger 2013
- Roger VL. Epidemiology of heart failure. Circulation Research 2013;113(6):646-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rogers 2014
- Rogers JK, Pocock SJ, McMurray JJ, Granger CB, Michelson EL, Östergren J, et al. Analysing recurrent hospitalizations in heart failure: a review of statistical methodology, with application to CHARM-Preserved. European Journal of Heart Failure 2014;16(1):33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sackner‐Bernstein 1995
- Sackner-Bernstein JD, Mancini DM. Rationale for treatment of patients with chronic heart failure with adrenergic blockade. JAMA 1995;274(18):1462-7. [PubMed] [Google Scholar]
Sharma 2014
- Sharma K, Kass DA. Heart failure with preserved ejection fraction mechanisms, clinical features, and therapies. Circulation Research 2014;115:79-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomon 2012
- Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, et al. The angiotensin receptor neprilysin inhibitor LCZ696 inheart failure with preserved ejection fraction: a phase 2double-blind randomised controlled trial. Lancet 2012;380:1387-95. [DOI] [PubMed] [Google Scholar]
Solomon 2016
- Solomon Scott D, Claggett Brian, Lewis Eldrin F, Desai Akshay, Anand Inder, Sweitzer Nancy K, et al. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. European Heart Journal 2016;37(5):455-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomon 2020
- Solomon SD, Vaduganathan M, Claggett BL, Packer M, Zile M, Swedberg K, et al. Sacubitril/Valsartan across the spectrum of ejection fraction in heart failure. Circulation 2020;141(5):352-61. [DOI] [PubMed] [Google Scholar]
SOLVD Investigators 1991
- SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. New England Journal of Medicine 1991;325(5):293-302. [DOI] [PubMed] [Google Scholar]
SOLVD Investigators 1992
- SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. New England Journal of Medicine 1992;1992(327):685-91. [DOI] [PubMed] [Google Scholar]
Spertus 2005
- Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, et al. Cardiovascular Outcomes Research Consortium. Monitoring clinical changes in patients with heart failure: a comparison of methods. American Heart Journal 2005;150:707-15. [DOI] [PubMed] [Google Scholar]
Taylor 2012
- Taylor CJ, Roalfe AK, Iles R, Hobbs FD. Ten-year prognosis of heart failure in the community: follow-up data from the Echocardiographic Heart of England Screening (ECHOES) study. European Journal of Heart Failure 2012;14(2):176-84. [DOI] [PubMed] [Google Scholar]
Wong 2006
- Wong SS, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. Journal of the Medical Library Association 2006;94(1):41-7. [PMC free article] [PubMed] [Google Scholar]
Yancy 2013
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardioliology 2013;62(16):e147-239. [DOI] [PubMed] [Google Scholar]
Yancy 2017
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey Jr DE, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017;136(6):e137-61. [DOI] [PubMed] [Google Scholar]
Yusuf 2003
- Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJV, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003;362(9386):777-81. [DOI] [PubMed] [Google Scholar]
Zannad 2011
- Zannad F, McMurray JJV, Krum H, Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. New England Journal of Medicine 2011;364(1):11-21. [DOI] [PubMed] [Google Scholar]
Zheng 2018
- Zheng SL, Chan FT, Nabeebaccus AA, Shah AM, McDonagh T, Okonko DO, et al. Drug treatment effects on outcomes in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Heart 2018;104:407-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Lumbers 2017
- Lumbers RT, Martin N, Manoharan K, Nyong J, Thomas J, Casas JP, et al. Beta-blockers and inhibitors of the renin-angiotensin aldosterone system for chronic heart failure with preserved ejection fraction. Cochrane Database of Systematic Reviews 2017, Issue 7. Art. No: CD012721. [DOI: 10.1002/14651858.CD012721] [DOI] [Google Scholar]
Martin 2018
- Martin N, Manoharan K, Thomas J, Davies C, Lumbers RT. Beta-blockers and inhibitors of the renin-angiotensin aldosterone system for chronic heart failure with preserved ejection fraction. Cochrane Database of Systematic Reviews 2018, Issue 6. Art. No: CD012721. [DOI: 10.1002/14651858.CD012721.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


