Abstract
BACKGROUND
Blood pressure (BP) measured in the office setting increases from early through later adulthood. However, it is unknown to what extent out-of-office BP derived via ambulatory BP monitoring (ABPM) increases over time, and which participant characteristics and risk factors might contribute to these increases.
METHODS
We assessed 25-year change in office- and ABPM-derived BP across sex, race, diabetes mellitus (DM), and body mass index (BMI) subgroups in the Coronary Artery Risk Development in Young Adults study using multivariable-adjusted linear mixed effects models.
RESULTS
We included 288 participants who underwent ABPM at the Year 5 Exam (mean [SD] age, 25.1 [3.7]; 45.8% men) and 455 participants who underwent ABPM at the Year 30 Exam (mean [SD] age, 49.5 [3.7]; 42.0% men). Office, daytime, and nighttime systolic BP (SBP) increased 12.8 (95% confidence interval [CI], 7.6–17.9), 14.7 (95% CI, 9.7–19.8), and 16.6 (95% CI, 11.4–21.8) mm Hg, respectively, over 25 years. Office SBP increased 6.5 (95% CI, 2.3–10.6) mm Hg more among black compared with white participants. Daytime SBP increased 6.3 (95% CI, 0.2–12.4) mm Hg more among participants with a BMI ≥25 vs. <25 kg/m2. Nighttime SBP increased 4.7 (95% CI, 0.5–8.9) mm Hg more among black compared with white participants, and 17.3 (95% CI, 7.2–27.4) mm Hg more among participants with vs. without DM.
CONCLUSIONS
Office- and ABPM-derived BP increased more from early through middle adulthood among black adults and participants with DM and BMI ≥25 kg/m2.
Keywords: aging, ambulatory blood pressure monitoring, blood pressure, epidemiology, health status disparities, hypertension, risk factors
Hypertension is a leading risk factor for morbidity and mortality in the United States and globally.1–3 While blood pressure (BP) is routinely measured in an office setting, ambulatory BP monitoring (ABPM) allows characterization of several phenotypes that cannot be estimated using office measurements, including mean daytime and nighttime BP.4 Higher levels of daytime and nighttime BP are associated with increased risk for cardiovascular disease and mortality, even after adjustment for office BP.5–8
Changes in office BP over the life course are characterized by increases from early to later adulthood,9 with differences occurring by sex9–11 and race.12,13 Compared with men, women typically have lower office BP earlier in life but higher levels in later adulthood.11 Compared with white adults, black adults typically have higher office BP throughout adulthood.13 However, it is unknown whether ABPM-derived BP measurements show similar patterns. Furthermore, there are few data on the concurrent changes in both office- and ABPM-derived BP from young adulthood to midlife even though such data could provide a more comprehensive characterization of long-term BP patterns. Investigating these patterns may identify subgroups and individual risk factors associated with large increases in office- and ABPM-derived BP, which may be targets for focused interventions for primary prevention of cardiovascular disease.
The primary goal of this analysis was to describe changes in office and ambulatory BP over 25 years and to assess participant characteristics and factors associated with these changes over this time period. To accomplish this goal, we analyzed data from the Coronary Artery Risk Development in Young Adults (CARDIA) study.
METHODS
Study design and participants
CARDIA is a prospective, longitudinal cohort study of 5,115 black and white men and women aged 18–30 years, enrolled from 4 field centers in the United States (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA) in 1985 and 1986. ABPM was conducted at the Year 5 Exam in 1990–1991 among 316 participants from the Birmingham field center. Of these participants, 290 had a complete ABPM recording, defined as at least 10 daytime and 5 nighttime systolic BP (SBP) and diastolic BP (DBP) measurements (Supplementary Figure S1 online).14 Two of these participants were missing information on diabetes mellitus (DM) status at the Year 5 Exam and excluded from the analyses. ABPM was performed again at the Year 30 Exam, in 2015–2016. Of the 754 Birmingham field center participants who attended the Year 30 Exam, 496 underwent ABPM and 462 participants had a complete recording. Seven of these participants were missing information on DM status at the Year 5 Exam and excluded from the analyses. The study was approved by the University of Alabama at Birmingham institutional review board and all participants provided written informed consent at each examination.
BP measurements
Office SBP and DBP were measured by trained study staff at the Year 5 and Year 30 Exams following a standardized protocol. At the Year 5 Exam, office BP was measured on the right arm with a Hawksley random-zero sphygmomanometer using an appropriately sized cuff after 5 minutes of quiet rest in a seated position, with the participant’s back supported, and their feet flat on the floor. At the Year 30 Exam, an Omron HEM 907XL oscillometric device was used to measure office BP. The Year 30 office BP was calibrated to a random-zero sphygmomanometer to enhance comparability between the 2 visits, according to the following equations: calibrated SBP = 3.74 + 0.96 × oscillometric SBP (R2 = 0.94); calibrated DBP = 1.30 + 0.97 × oscillometric average DBP (R2 = 0.91).15 At both Exams, 3 BP measurements were taken, separated by at least 30 seconds, with the mean of the second and third measurements used for analysis.
ABPM was conducted over 24 hours following the Year 5 Exam using a Suntech Accutracker II (Morrisville, NC) and an appropriately sized cuff. BP was measured every 20 minutes between 6:00 am and 10:00 pm and every 30 minutes from 10:00 pm to 6:00 am. At the Year 30 Exam, the SpaceLabs OnTrak 90227 monitor (Snoqualmie, WA) was used and BP measurements were obtained every 30 minutes throughout the ABPM period. As awake and asleep times determined by actigraphy and sleep diary data were only available at the Year 30 Exam, we analyzed the ABPM data at both Year 5 and Year 30 using clock time to define the daytime and nighttime periods. Based on criteria from the International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcome (IDACO), daytime was defined as the period between 10:00 am and 8:00 pm while nighttime was defined as the period between midnight and 6:00 am.14 We considered 6 BP measures: office SBP and DBP; daytime SBP and DBP; and nighttime SBP and DBP.
Covariable ascertainment
Demographic, anthropometric, questionnaire, and laboratory data were collected at the Year 5 and Year 30 Exams by trained study staff following standardized protocols.16 Age, sex, and race were self-reported. Physical activity was assessed using the validated CARDIA-specific Physical Activity History questionnaire,17 with a score of 300 units approximating the recommended amount of exercise for maintaining a healthy weight by the American College of Sports Medicine. Self-reported tobacco smoking was categorized as never, former, and current smoking. Weight and height were measured during each examination and body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Cholesterol and glucose were measured using standard laboratory methods. We defined DM as a fasting glucose ≥126 mg/dl and/or self-reported use of a glucose-lowering medication.
Statistical analysis
We summarized participant characteristics at the Year 5 and Year 30 Exams as the mean and SD for continuous variables and the number and percentage for categorical variables. Pearson correlation coefficients for all pairwise comparisons of office, daytime, and nighttime SBP and DBP were calculated.
We used linear mixed effects models to estimate longitudinal changes in BP. This modeling approach included Year 5 and Year 30 Exam BP values as repeated measures which allows for the analysis of all participants with available data at the Year 5 or Year 30 Exams, increasing the sample size and statistical power. We calculated mean BP at the Year 5 and Year 30 Exams and unadjusted changes in BP over this time period, stratified by sex, race, and DM status and BMI at Year 5. We calculated the multivariable-adjusted changes in BP by including age, sex, race, DM status, and BMI at Year 5 in the mixed effects models. Age and BMI were included in the models as continuous, linear terms. Additionally, we evaluated whether several other variables measured at Year 5 were associated with longitudinal changes in BP in the mixed effects models, including education, physical activity, smoking, total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol. These variables were not independently associated with longitudinal changes in BP after adjustment for age, sex, race, DM status, and BMI, and were not included in further analyses.
We accounted for antihypertensive medication use in all analyses by adding 10 mm Hg for SBP and 5 mm Hg for DBP to the measured values of participants who reported taking antihypertensive medication at the time of the Exam, as recommended by prior reports.18,19 We assumed antihypertensive medication use would have a similar magnitude of effect on office, daytime, and nighttime SBP and DBP measures.20 We conducted 2 sensitivity analyses. First, we repeated the analyses without adding 10 and 5 mm Hg to SBP and DBP values. Second, we repeated the analyses among the subset of participants who had ABPM data at both Years 5 and 30 (n = 143). Analyses were conducted using R version 3.6.2 (R Project for Statistical Computing).
RESULTS
Characteristics of the 288 participants with ABPM data at the Year 5 Exam and 455 participants with ABPM data at the Year 30 Exam are presented in Table 1. At the Year 30 Exam, antihypertensive medication use was more common among women compared with men, black compared with white participants, those with vs. without DM, and participants with a BMI ≥25 vs. <25 kg/m2 (Supplementary Table S1 online). Correlations between office- and ABPM-derived BP measurements were higher for measurements obtained at the Year 30 Exam compared with the Year 5 Exam (Supplementary Table S2 online). Among the 143 participants who had ABPM data at both Exams, the correlations between Year 5 and Year 30 BP measurements were higher for SBP compared with DBP measurements (Supplementary Table S3 online).
Table 1.
Characteristics of CARDIA participants with ambulatory blood pressure monitoring data at the Year 5 and Year 30 Study Exams
| Variables | Year 5 Exam (n = 288) | Year 30 Exam (n = 455) |
|---|---|---|
| Age, years | 25.1 (3.7) | 49.5 (3.7) |
| Women, n (%) | 156 (54.2) | 264 (58.0) |
| Black race, n (%) | 185 (64.2) | 294 (64.6) |
| College or professional degree, n (%) | 154 (53.5) | 266 (58.5) |
| Physical activity, exercise units | 319.8 (273.0) | 232.0 (234.9) |
| Smoking, n (%) | ||
| Never | 179 (62.1) | 297 (65.3) |
| Former | 27 (9.4) | 93 (20.4) |
| Current | 82 (28.5) | 65 (14.3) |
| Body mass index, kg/m2 | 26.5 (5.3) | 31.8 (7.0) |
| Heart rate, beats per minute | 69.9 (8.6) | 67.3 (10.6) |
| Diabetes mellitus, n (%) | 15 (5.2) | 90 (19.8) |
| Total cholesterol, mg/dl | 179.5 (33.2) | 192.6 (38.5) |
| HDL cholesterol, mg/dl | 50.7 (13.9) | 58.6 (18.7) |
| LDL cholesterol, mg/dl | 111.8 (30.5) | 112.4 (33.8) |
| Antihypertensive medication use, n (%) | 2 (0.7) | 198 (43.5) |
| Systolic blood pressure, mm Hg | ||
| Office | 109.0 (10.6) | 120.4 (16.0) |
| Daytime | 121.4 (11.0) | 130.1 (16.0) |
| Nighttime | 107.0 (13.4) | 114.7 (15.8) |
| 24-Hour | 116.0 (10.7) | 124.4 (14.8) |
| Diastolic blood pressure, mm Hg | ||
| Office | 73.1 (9.7) | 73.2 (10.4) |
| Daytime | 74.4 (7.8) | 80.7 (9.6) |
| Nighttime | 59.5 (8.1) | 68.6 (9.7) |
| 24-Hour | 68.8 (6.9) | 76.2 (8.8) |
Values are mean (SD) or n (%). Abbreviations: CARDIA, Coronary Artery Risk Development in Young Adults; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
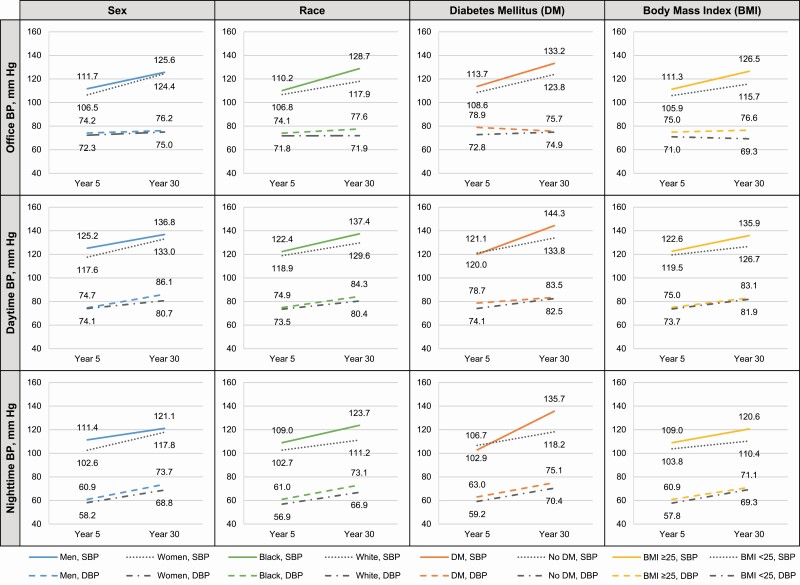
Although men had higher unadjusted mean SBP and DBP levels at the Year 5 and Year 30 Exams than women, the difference in SBP levels between men and women were smaller at the Year 30 Exam (Figure 1). Compared with white participants, black participants had higher unadjusted mean SBP and DBP levels at the Year 5 and Year 30 Exams with these differences being larger at the Year 30 Exam. Participants with DM at the Year 5 Exam had higher unadjusted daytime and nighttime SBP at the Year 30 Exam vs. participants without DM. Participants with a BMI ≥25 kg/m2 had higher unadjusted mean BP levels at the Year 5 and Year 30 Exams and the differences between SBP levels in those with vs. without a BMI ≥25 kg/m2 were larger at the Year 30 Exam.
Figure 1.
Mean office, daytime, and nighttime blood pressure at years 5 and 30 by subgroups. Solid lines indicate SBP among men, black adults, those with DM, and those with BMI ≥25 kg/m2. Dotted lines indicate SBP among women, white adults, those without DM, and those with BMI <25 kg/m2. Dashed lines indicate DBP among men, black adults, those with DM, and those with BMI ≥25 kg/m2. Dash-dotted lines indicate DBP among women, white adults, those without DM, and those with BMI <25 kg/m2. Abbreviations: BMI, body mass index; BP, blood pressure; DBP, diastolic blood pressure; DM, diabetes mellitus; SBP, systolic blood pressure.
After multivariable-adjustment, office SBP increased 6.5 mm Hg (95% confidence interval [CI], 2.3–10.6 mm Hg) more from the Year 5 to Year 30 Exam among black compared with white participants (Table 2). Additionally, office DBP increased 3.5 mm Hg (95% CI, 0.0–7.0 mm Hg) more among participants with a BMI ≥25 vs. <25 kg/m2 at the Year 5 Exam. Daytime SBP increased 6.3 mm Hg (95% CI, 0.2–12.4 mm Hg) more among participants with a BMI ≥25 vs. <25 kg/m2 at the Year 5 Exam (Table 3). Additionally, daytime DBP increased 3.9 mm Hg (95% CI, 1.3–6.5 mm Hg) more among men compared with women and 2.9 mm Hg (95% CI, 0.2–5.6) more among black compared with white participants. Nighttime SBP increased 4.7 mm Hg (95% CI, 0.5–8.9 mm Hg) more among black compared with white participants, and 17.3 mm Hg (95% CI, 7.2–27.4 mm Hg) more among those with vs. without DM at the Year 5 Exam (Table 4).
Table 2.
Mean office blood pressure at the CARDIA Year 5 and 30 Exams and difference between Year 5 and Year 30
| Office systolic BP, mm Hg | Office diastolic BP, mm Hg | |||||
|---|---|---|---|---|---|---|
| Variables | Year 5 | Year 30 | Difference | Year 5 | Year 30 | Difference |
| Overall | 112.4 (108.6, 116.1) | 125.1 (121.1, 129.1) | 12.8 (7.6, 17.9) | 76.0 (73.3, 78.6) | 73.4 (70.5, 76.3) | −2.6 (−6.3, 1.2) |
| Sex | ||||||
| Female | 109.7 (105.8, 113.6) | 123.8 (119.7, 127.8) | 14.1 (8.8, 19.4) | 74.9 (72.1, 77.6) | 72.5 (69.6, 75.4) | −2.4 (−6.2, 1.5) |
| Male | 115.0 (110.8, 119.2) | 126.5 (122.0, 130.9) | 11.5 (5.7, 17.2) | 77.0 (74.0, 80.0) | 74.3 (71.1, 77.4) | −2.8 (−7.0, 1.4) |
| Male minus female | 5.3 (2.2, 8.5) | 2.7 (−0.1, 5.5) | −2.6 (−6.6, 1.3) | 2.2 (−0.1, 4.4) | 1.8 (−0.2, 3.7) | −0.4 (−3.3, 2.5) |
| Race | ||||||
| White | 110.9 (106.5, 115.3) | 120.5 (116.0, 125.0) | 9.5 (3.6, 15.5) | 74.9 (71.8, 78.1) | 71.2 (67.9, 74.4) | −3.8 (−8.1, 0.5) |
| Black | 113.8 (110.0, 117.5) | 129.8 (125.7, 133.8) | 16.0 (10.9, 21.1) | 77.0 (74.3, 79.6) | 75.6 (72.7, 78.5) | −1.4 (−5.1, 2.4) |
| Black minus white | 2.8 (−0.5, 6.1) | 9.3 (6.4, 12.1) | 6.5 (2.3, 10.6)** | 2.1 (−0.3, 4.4) | 4.5 (2.4, 6.5) | 2.4 (−0.6, 5.4) |
| Diabetes mellitus (Year 5 Exam) | ||||||
| No | 110.3 (108.4, 112.3) | 122.2 (120.8, 123.7) | 11.9 (9.6, 14.2) | 74.1 (72.7, 75.5) | 73.9 (72.8, 74.9) | −0.2 (−1.9, 1.5) |
| Yes | 114.4 (107.3, 121.4) | 128.0 (120.2, 135.8) | 13.7 (3.8, 23.6) | 77.8 (72.8, 82.9) | 72.9 (67.3, 78.5) | −4.9 (−12.1, 2.3) |
| Yes minus no | 4.0 (−3.2, 11.2) | 5.8 (−2.1, 13.6) | 1.8 (−8.3, 11.8) | 3.8 (−1.4, 8.9) | −0.9 (−6.6, 4.7) | −4.7 (−12.0, 2.6) |
| Body mass index, kg/m2 (Year 5 Exam) | ||||||
| <25 | 108.1 (104.0, 112.2) | 118.1 (113.1, 123.1) | 10.0 (3.8, 16.2) | 73.0 (70.0, 76.0) | 68.3 (64.7, 72.0) | −4.7 (−9.2, −0.2) |
| ≥25 | 113.2 (109.3, 117.0) | 127.5 (123.5, 131.6) | 14.4 (9.1, 19.6) | 76.3 (73.5, 79.1) | 75.1 (72.2, 78.1) | −1.2 (−5.0, 2.7) |
| ≥25 minus <25 | 5.1 (1.9, 8.2) | 9.4 (5.6, 13.2) | 4.4 (−0.5, 9.2) | 3.3 (1.0, 5.6) | 6.8 (4.1, 9.5) | 3.5 (0.0, 7.0)* |
Results are adjusted for age and all other variables in the table, and are based on linear mixed model analyses of 288 participants at Year 5 (of whom 132 were male, 185 were black, 15 had diabetes mellitus, and 162 had body mass index ≥25 kg/m2) and 455 participants at Year 30 (of whom 191 were male, 294 were black, 14 had diabetes mellitus at Year 5, and 209 had body mass index ≥25 kg/m2 at Year 5). Among participants reporting use of antihypertensive medications, 10 and 5 mm Hg were added to systolic and diastolic BP values, respectively. Abbreviations: BP, blood pressure; CARDIA, Coronary Artery Risk Development in Young Adults.
For results of the 25-year difference in BP by levels of factors, P values are signified as follows: *P < 0.05; **P < 0.01; ***P < 0.001.
Table 3.
Mean daytime blood pressure at the CARDIA Year 5 and 30 Exams and difference between Year 5 and Year 30
| Daytime systolic BP, mm Hg | Daytime diastolic BP, mm Hg | |||||
|---|---|---|---|---|---|---|
| Variables | Year 5 | Year 30 | Difference | Year 5 | Year 30 | Difference |
| Overall | 122.1 (118.4, 125.8) | 136.9 (132.9, 140.8) | 14.7 (9.7, 19.8) | 76.1 (73.8, 78.5) | 83.0 (80.4, 85.5) | 6.8 (3.4, 10.2) |
| Sex | ||||||
| Female | 118.2 (114.4, 122.1) | 134.1 (130.0, 138.1) | 15.8 (10.6, 21.0) | 75.6 (73.1, 78.1) | 80.5 (77.9, 83.1) | 4.9 (1.4, 8.3) |
| Male | 126.0 (121.8, 130.2) | 139.6 (135.3, 144.0) | 13.6 (8.0, 19.3) | 76.7 (74.0, 79.3) | 85.4 (82.6, 88.2) | 8.8 (5.0, 12.5) |
| Male minus female | 7.8 (4.6, 10.9) | 5.6 (2.8, 8.3) | −2.2 (−6.1, 1.7) | 1.1 (−0.9, 3.1) | 5.0 (3.2, 6.7) | 3.9 (1.3, 6.5)** |
| Race | ||||||
| White | 120.4 (116.0, 124.7) | 133.5 (129.1, 138.0) | 13.2 (7.3, 19.0) | 75.4 (72.6, 78.2) | 80.7 (77.9, 83.6) | 5.3 (1.5, 9.2) |
| Black | 123.9 (120.2, 127.6) | 140.2 (136.2, 144.2) | 16.3 (11.2, 21.3) | 76.9 (74.5, 79.3) | 85.2 (82.6, 87.7) | 8.3 (4.9, 11.7) |
| Black minus white | 3.5 (0.2, 6.8) | 6.6 (3.8, 9.5) | 3.1 (−1.0, 7.2) | 1.5 (−0.6, 3.6) | 4.5 (2.6, 6.3) | 2.9 (0.2, 5.6)* |
| Diabetes mellitus (Year 5 Exam) | ||||||
| No | 122.4 (120.5, 124.3) | 132.6 (131.1, 134.0) | 10.2 (7.9, 12.5) | 74.3 (73.1, 75.5) | 82.5 (81.6, 83.4) | 8.2 (6.7, 9.7) |
| Yes | 121.9 (114.8, 128.9) | 141.1 (133.4, 148.9) | 19.3 (9.6, 29.0) | 78.0 (73.5, 82.5) | 83.4 (78.4, 88.4) | 5.4 (−1.1, 11.9) |
| Yes minus no | −0.6 (−7.7, 6.6) | 8.6 (0.8, 16.4) | 9.1 (−0.7, 19.0) | 3.7 (−0.9, 8.3) | 0.9 (−4.1, 5.9) | −2.8 (−9.4, 3.8) |
| Body mass index, kg/m2 (Year 5 Exam) | ||||||
| <25 | 107.5 (103.5, 111.4) | 123.1 (118.9, 127.3) | 15.6 (10.3, 20.9) | 75.4 (72.8, 78.1) | 82.2 (78.9, 85.4) | 6.7 (2.6, 10.8) |
| ≥25 | 103.7 (98.6, 108.9) | 125.7 (119.9, 131.4) | 21.9 (14.8, 29.0) | 76.2 (73.7, 78.6) | 83.0 (80.4, 85.6) | 6.8 (3.3, 10.3) |
| ≥25 minus <25 | −3.7 (−8.5, 1.0) | 2.6 (−2.1, 7.3) | 6.3 (0.2, 12.4)* | 0.7 (−1.3, 2.8) | 0.8 (−1.6, 3.3) | 0.1 (−3.0, 3.2) |
Results are adjusted for age and all other variables in the table, and are based on linear mixed model analyses of 288 participants at Year 5 (of whom 132 were male, 185 were black, 15 had diabetes mellitus, and 162 had body mass index ≥25 kg/m2) and 455 participants at Year 30 (of whom 191 were male, 294 were black, 14 had diabetes mellitus at Year 5, and 209 had body mass index ≥25 kg/m2 at Year 5). Among participants reporting use of antihypertensive medications, 10 and 5 mm Hg were added to systolic and diastolic BP values, respectively. Abbreviations: BP, blood pressure; CARDIA, Coronary Artery Risk Development in Young Adults.
For results of the 25-year difference in BP by levels of factors, P values are signified as follows: *P < 0.05; **P < 0.01; ***P < 0.001.
Table 4.
Mean nighttime blood pressure at the CARDIA Year 5 and 30 Exams and difference between Year 5 and Year 30
| Nighttime systolic BP, mm Hg | Nighttime diastolic BP, mm Hg | |||||
|---|---|---|---|---|---|---|
| Variables | Year 5 | Year 30 | Difference | Year 5 | Year 30 | Difference |
| Overall | 106.7 (102.8, 110.5) | 123.3 (119.2, 127.5) | 16.6 (11.4, 21.8) | 61.3 (58.9, 63.6) | 72.0 (69.5, 74.6) | 10.8 (7.5, 14.0) |
| Sex | ||||||
| Female | 102.1 (98.1, 106.1) | 120.2 (116.0, 124.4) | 18.1 (12.8, 23.4) | 59.6 (57.2, 62.1) | 69.2 (66.6, 71.8) | 9.6 (6.2, 12.9) |
| Male | 111.3 (106.9, 115.6) | 126.4 (121.9, 131.0) | 15.2 (9.3, 21.0) | 62.9 (60.2, 65.6) | 74.8 (72.0, 77.6) | 11.9 (8.3, 15.6) |
| Male minus female | 9.2 (5.9, 12.4) | 6.2 (3.4, 9.1) | −3.0 (−7.0, 1.0) | 3.3 (1.3, 5.3) | 5.6 (3.9, 7.4) | 2.4 (−0.2, 4.9) |
| Race | ||||||
| White | 103.7 (99.1, 108.2) | 118.0 (113.3, 122.6) | 14.3 (8.3, 20.3) | 59.2 (56.4, 62.0) | 68.9 (66.1, 71.8) | 9.7 (5.9, 13.4) |
| Black | 109.7 (105.9, 113.5) | 128.7 (124.6, 132.8) | 19.0 (13.8, 24.1) | 63.3 (60.9, 65.7) | 75.1 (72.6, 77.7) | 11.8 (8.6, 15.1) |
| Black minus white | 6.0 (2.6, 9.5) | 10.7 (7.8, 13.7) | 4.7 (0.5, 8.9)* | 4.1 (1.9, 6.2) | 6.2 (4.4, 8.1) | 2.2 (−0.5, 4.8) |
| Diabetes mellitus (Year 5 Exam) | ||||||
| No | 108.4 (106.4, 110.4) | 116.4 (114.9, 117.8) | 8.0 (5.6, 10.3) | 59.8 (58.5, 61.0) | 70.0 (69.1, 70.9) | 10.2 (8.7, 11.7) |
| Yes | 105.0 (97.7, 112.3) | 130.3 (122.2, 138.3) | 25.3 (15.3, 35.2) | 62.8 (58.3, 67.3) | 74.1 (69.1, 79.0) | 11.3 (5.0, 17.6) |
| Yes minus no | −3.4 (−10.8, 4.1) | 13.9 (5.8, 22.0) | 17.3 (7.2, 27.4)*** | 3.0 (−1.6, 7.6) | 4.1 (−0.9, 9.1) | 1.1 (−5.2, 7.4) |
| Body mass index, kg/m2 (Year 5 Exam) | ||||||
| <25 | 102.8 (98.5, 107.2) | 118.2 (113.0, 123.5) | 15.4 (9.1, 21.8) | 59.4 (56.8, 62.1) | 71.8 (68.6, 75.0) | 12.4 (8.4, 16.3) |
| ≥25 | 107.2 (103.1, 111.2) | 125.5 (121.3, 129.8) | 18.4 (12.9, 23.8) | 61.5 (59.0, 64.0) | 72.2 (69.6, 74.8) | 10.7 (7.3, 14.1) |
| ≥25 minus <25 | 4.3 (1.0, 7.7) | 7.3 (3.3, 11.2) | 2.9 (−2.1, 7.9) | 2.1 (0.1, 4.1) | 0.4 (−2.0, 2.8) | −1.7 (−4.8, 1.4) |
Results are adjusted for age and all other variables in the table, and are based on linear mixed model analyses of 288 participants at Year 5 (of whom 132 were male, 185 were black, 15 had diabetes mellitus, and 162 had body mass index ≥25 kg/m2) and 455 participants at Year 30 (of whom 191 were male, 294 were black, 14 had diabetes mellitus at Year 5, and 209 had body mass index ≥25 kg/m2 at Year 5). Among participants reporting use of antihypertensive medications, 10 and 5 mm Hg were added to systolic and diastolic BP values, respectively. Abbreviations: BP, blood pressure; CARDIA, Coronary Artery Risk Development in Young Adults.
For results of the 25-year difference in BP by levels of factors, P values are signified as follows: *P < 0.05; **P < 0.01; ***P < 0.001.
In a sensitivity analysis in which 10 and 5 mm Hg, respectively, were not added to the SBP and DBP values of participants taking antihypertensive medication, estimates of the differential change between subgroups were smaller compared with the primary analysis. For example, office SBP increased 4.8 mm Hg (95% CI, 0.7–8.8 mm Hg) more among black compared with white participants (Supplementary Table S4 online). Daytime SBP increased 2.3 mm Hg (95% CI, −2.4 to 7.0 mm Hg) more among participants with a BMI ≥25 vs. <25 kg/m2 at the Year 5 Exam (Supplementary Table S5 online). Nighttime SBP increased 3.1 mm Hg (95% CI, −0.9 to 7.1 mm Hg) more among black compared with white participants, and 15.1 mm Hg (95% CI, 5.4–24.8 mm Hg) more among those with vs. without DM at the Year 5 Exam (Supplementary Table S6 online). Results from a sensitivity analysis restricted to the 143 participants with ABPM data at both Exams appear in Supplementary Tables S7–S9 online.
DISCUSSION
In this analysis of young and middle-aged adults who had their BP measured in the office and by ABPM, the change in office- and ABPM-derived BP values over 25 years was associated with characteristics including sex, race, DM status, and BMI. Men had higher adjusted mean office, daytime, and nighttime BP values compared with women on both occasions. Black participants had higher daytime and nighttime SBP values at Year 5, and exhibited a greater 25-year increase in office and nighttime SBP compared with white participants. Additionally, participants with DM at Year 5 had larger 25-year increases in daytime and nighttime SBP compared with those without DM. Overall, daytime and nighttime SBP and DBP increased more than corresponding office values over 25 years. These findings point to several characteristics important for longitudinal changes in BP and provide a comparison of long-term, longitudinal changes in office- and ABPM-derived BP values from young adulthood to midlife among black and white adults.
There were several participant characteristics at Year 5 associated with longitudinal changes in office and out-of-office BP including age, sex, race, DM status, and BMI. These characteristics have consistently been associated with BP as measured in the office2,21–24 and outside of the office on ABPM.25–27 In the current study, female sex, black race, DM, and higher BMI were associated with larger increases in BP over 25 years. For example, SBP values among those with and without DM were similar at the Year 5 Exam, but there were steep increases by Year 30 among those with DM, particularly for daytime and nighttime SBP. Although few participants had DM at Year 5, these results suggest that frequent monitoring of BP, both in and out of the office, among young adults with DM may be warranted so that antihypertensive medication can be initiated promptly after hypertension is diagnosed. This finding supports recent recommendations by the American Diabetes Association to incorporate out-of-office BP measurements in the self-management care plan of those with DM.24
On average, men have higher office BP compared with women,28 and this difference is present across the life course until older age, when the BP levels of women often exceed that of men.9–12 The results of the current longitudinal analysis support these previous findings for both office- and ABPM-derived measurements, even after adjusting for age, race, BMI, and DM. However, SBP nearly converged over the 25-year follow-up period, characterized by a larger increase in SBP for women compared with men. Although these findings were not statistically significant, they are consistent with previous longitudinal analyses of office BP.9–11 Ji et al. documented steeper increases in office BP among women compared with men among 32,833 participants from 4 pooled community-based US cohort studies.11 The current analysis extends this information to BP as measured via ABPM, which some previous studies have analyzed using cross-sectional data.29,30 A recent longitudinal analysis among 562 participants of the PAMELA (Pressioni Monitorate E Loro Associazioni) study reported age-related 25-year increases in office and out-of-office BP, and identified steeper increases in home- and ABPM-derived BP in women compared with men.31 The potentially larger increase in BP among women compared with men could be due to several factors, including pre- and postmenopausal hormonal factors,32 autonomic function,33 or a differential vascular response to aging.34 Further research on sex differences in BP changes over the life course is warranted, especially for nighttime BP, which is strongly associated with increased risk of cardiovascular disease, independent of office and daytime BP.5,8
The prevalence of hypertension as defined by ABPM is high among black adults.35 Racial differences in ABPM have previously been reported in the CARDIA study,26,30 and were present in the current analysis. In young adulthood, black participants had higher adjusted mean office, daytime, and nighttime SBP and DBP compared with white participants. Furthermore, the difference in BP levels between black and white participants widened over follow-up. Compared with white adults, nighttime SBP increased more among black adults, while there was no evidence of a difference in the increase of daytime SBP. These findings support a prior analysis of 15-year changes in ambulatory BP among 663 black and white young adults, which reported the race difference in nighttime SBP levels and its increase with age were larger than in daytime SBP.36 Previous reports have hypothesized that cardiovascular reactivity to stress,37 depressed autonomic function,38 and disturbed sleep39 may play a role in racial disparities in BP. The current data suggest race differences in BP start early in life and the disparity increases over time. Early monitoring and lifestyle intervention in individuals at high risk for progressively higher BP, particularly black individuals, are important clinical and public health challenges. High nighttime BP is becoming increasingly recognized among black adults, in whom the prevalence of this phenotype is higher compared with white adults.26,40 More widespread ABPM assessments may be warranted in younger black adults at risk for hypertension, and further research is warranted to identify mechanisms and unique therapeutic interventions to address these disparities.
There are several strengths of the current analysis. We evaluated changes in office- and ABPM-derived BP values over a long period of time and during an essential period in the life course, from young adulthood to middle age. The CARDIA study employs rigorous standardization of data collection, which minimizes bias. However, the present study has limitations. First, we had a modest sample size. Similar longitudinal analyses of ABPM should be conducted in larger community populations as data become available to confirm and expand upon the current findings. Second, we analyzed the ABPM data at both Year 5 and Year 30 using clock time to define the daytime and nighttime periods. Third, ABPM was conducted at only 2 time points, prohibiting the analysis of BP trajectories. Additionally, office and ABPM BP measurement methods differed at the 2 time points. However, validated measurement devices were used at both visits, and the Year 30 office BP values were calibrated to a random-zero sphygmomanometer to enhance comparability with the Year 5 values.15 Fourth, we evaluated 4 factors and 6 BP measures for a total of 24 statistical comparisons. Using the Holm–Bonferroni method to adjust for multiple comparisons, the race differences in office SBP (P = 0.002) and DM differences in nighttime SBP (P < 0.001) remain statistically significant. Finally, we had longitudinal ABPM data only from the Birmingham field center. The results may not be generalizable to other regions.
In conclusion, sex, race, DM status, and BMI were associated with early-adulthood differences in BP as measured in the office and out of the office on ABPM. These factors may also be associated with larger increases in BP values, especially as measured out of the office, through middle adulthood, highlighting the importance of early monitoring and potential intervention as appropriate.
FUNDING
The current study was supported by the American Heart Association grant SFRN 15SFRN2390002 and the Coronary Artery Risk Development in Young Adults Study (CARDIA) is supported by contracts HHSN268201 800003I, HHSN268201800004I, HHSN268201800005I, HHSN268201800006I, and HHSN268201800007I from the National Heart, Lung, and Blood Institute (NHLBI). Dr Bundy was supported by the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development career development grant K12HD043451. Dr Thomas was supported by the American Heart Association grant 19CDA34660139.
DISCLOSURE
Dr Booth receives salary support for employment at CTI Clinical Trials and Consulting Services for work unrelated to the topic of this manuscript, which was completed prior to his employment.
DATA AVAILABILITY
The data underlying this article can be shared on reasonable request to the corresponding author and approval by the CARDIA study.
Supplementary Material
REFERENCES
- 1. The US Burden of Disease Collaborators. The state of US health, 1990–2016: burden of diseases, injuries, and risk factors among US states. JAMA 2018; 319:1444–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 2016; 134:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, Alexander L, Estep K, Hassen Abate K, Akinyemiju TF, Ali R, Alvis-Guzman N, Azzopardi P, Banerjee A, Bärnighausen T, Basu A, Bekele T, Bennett DA, Biadgilign S, Catalá-López F, Feigin VL, Fernandes JC, Fischer F, Gebru AA, Gona P, Gupta R, Hankey GJ, Jonas JB, Judd SE, Khang YH, Khosravi A, Kim YJ, Kimokoti RW, Kokubo Y, Kolte D, Lopez A, Lotufo PA, Malekzadeh R, Melaku YA, Mensah GA, Misganaw A, Mokdad AH, Moran AE, Nawaz H, Neal B, Ngalesoni FN, Ohkubo T, Pourmalek F, Rafay A, Rai RK, Rojas-Rueda D, Sampson UK, Santos IS, Sawhney M, Schutte AE, Sepanlou SG, Shifa GT, Shiue I, Tedla BA, Thrift AG, Tonelli M, Truelsen T, Tsilimparis N, Ukwaja KN, Uthman OA, Vasankari T, Venketasubramanian N, Vlassov VV, Vos T, Westerman R, Yan LL, Yano Y, Yonemoto N, Zaki ME, Murray CJ. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA 2017; 317:165–182. [DOI] [PubMed] [Google Scholar]
- 4. Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med 2006; 354:2368–2374. [DOI] [PubMed] [Google Scholar]
- 5. Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension 2011; 57:3–10. [DOI] [PubMed] [Google Scholar]
- 6. Roush GC, Fagard RH, Salles GF, Pierdomenico SD, Reboldi G, Verdecchiaf P, Eguchi K, Kario K, Hoshide S, Polonia J, De La Sierra A, Hermida RC, Dolank E, Zamallo H. Prognostic impact from clinic, daytime, and nighttime systolic blood pressure in nine cohorts of 13844 patients with hypertension. J Hypertens 2014; 32:2332–2340. [DOI] [PubMed] [Google Scholar]
- 7. Yano Y, Tanner RM, Sakhuja S, Jaeger BC, Booth JN III, Abdalla M, Pugliese D, Seals SR, Ogedegbe G, Jones DW, Muntner P, Shimbo D. Association of daytime and nighttime blood pressure with cardiovascular disease events among African American individuals. JAMA Cardiol 2019; 4:910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang WY, Melgarejo JD, Thijs L, Zhang ZY, Boggia J, Wei FF, Hansen TW, Asayama K, Ohkubo T, Jeppesen J, Dolan E, Stolarz-Skrzypek K, Malyutina S, Casiglia E, Lind L, Filipovský J, Maestre GE, Li Y, Wang JG, Imai Y, Kawecka-Jaszcz K, Sandoya E, Narkiewicz K, O’Brien E, Verhamme P, Staessen JA; International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes (IDACO) Investigators . Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA 2019; 322:409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wills AK, Lawlor DA, Matthews FE, Sayer AA, Bakra E, Ben-Shlomo Y, Benzeval M, Brunner E, Cooper R, Kivimaki M, Kuh D, Muniz-Terrera G, Hardy R. Life course trajectories of systolic blood pressure using longitudinal data from eight UK cohorts. PLoS Med 2011; 8:e1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheng S, Xanthakis V, Sullivan LM, Vasan RS. Blood pressure tracking over the adult life course: patterns and correlates in the Framingham heart study. Hypertension 2012; 60:1393–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ji H, Kim A, Ebinger JE, Niiranen TJ, Claggett BL, Bairey Merz CN, Cheng S. Sex differences in blood pressure trajectories over the life course. JAMA Cardiol 2020; 5:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 2019; 139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 13. Thomas SJ, Booth JN III, Dai C, Li X, Allen N, Calhoun D, Carson AP, Gidding S, Lewis CE, Shikany JM, Shimbo D, Sidney S, Muntner P. Cumulative incidence of hypertension by 55 years of age in blacks and whites: the CARDIA study. J Am Heart Assoc 2018; 7:e007988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thijs L, Hansen TW, Kikuya M, Björklund-Bodegård K, Li Y, Dolan E, Tikhonoff V, Seidlerová J, Kuznetsova T, Stolarz K, Bianchi M, Richart T, Casiglia E, Malyutina S, Filipovsky J, Kawecka-Jaszcz K, Nikitin Y, Ohkubo T, Sandoya E, Wang J, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Staessen JA, O’Brien E; IDACO Investigators . The International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcome (IDACO): protocol and research perspectives. Blood Press Monit 2007; 12:255–262. [DOI] [PubMed] [Google Scholar]
- 15. Gunderson EP, Chiang V, Lewis CE, Catov J, Quesenberry CP Jr, Sidney S, Wei GS, Ness R. Long-term blood pressure changes measured from before to after pregnancy relative to nonparous women. Obstet Gynecol 2008; 112:1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988; 41:1105–1116. [DOI] [PubMed] [Google Scholar]
- 17. Jacobs DR Jr, Hahn LP, Haskell WL, Pirie P, Sidney S. Validity and reliability of short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulm Rehabil 1989; 9:448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med 2005; 24:2911–2935. [DOI] [PubMed] [Google Scholar]
- 19. Cui JS, Hopper JL, Harrap SB. Antihypertensive treatments obscure familial contributions to blood pressure variation. Hypertension 2003; 41:207–210. [DOI] [PubMed] [Google Scholar]
- 20. Omboni S, Kario K, Bakris G, Parati G. Effect of antihypertensive treatment on 24-h blood pressure variability: pooled individual data analysis of ambulatory blood pressure monitoring studies based on olmesartan mono or combination treatment. J Hypertens 2018; 36:720–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garrison RJ, Kannel WB, Stokes J III, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med 1987; 16:235–251. [DOI] [PubMed] [Google Scholar]
- 22. Davy KP, Hall JE. Obesity and hypertension: two epidemics or one? Am J Physiol Regul Integr Comp Physiol 2004; 286:R803–R813. [DOI] [PubMed] [Google Scholar]
- 23. Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W, Sun C, Cheung M, Viikari JS, Dwyer T, Raitakari OT. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med 2011; 365:1876–1885. [DOI] [PubMed] [Google Scholar]
- 24. de Boer IH, Bangalore S, Benetos A, Davis AM, Michos ED, Muntner P, Rossing P, Zoungas S, Bakris G. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40:1273–1284. [DOI] [PubMed] [Google Scholar]
- 25. O’Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y; European Society of Hypertension Working Group on Blood Pressure Monitoring . European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 2013; 31:1731–1768. [DOI] [PubMed] [Google Scholar]
- 26. Muntner P, Lewis CE, Diaz KM, Carson AP, Kim Y, Calhoun D, Yano Y, Viera AJ, Shimbo D. Racial differences in abnormal ambulatory blood pressure monitoring measures: results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Hypertens 2015; 28:640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kario K. Nocturnal hypertension: new technology and evidence. Hypertension 2018; 71:997–1009. [DOI] [PubMed] [Google Scholar]
- 28. Ramirez LA, Sullivan JC. Sex differences in hypertension: where we have been and where we are going. Am J Hypertens 2018; 31:1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wiinberg N, Høegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, Svendsen TL, Kampmann JP, Madsen NH, Bentzon MW. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens 1995; 8:978–986. [DOI] [PubMed] [Google Scholar]
- 30. Booth JN, Anstey DE, Bello NA, Jaeger BC, Pugliese DN, Thomas SJ, Deng L, Shikany JM, Lloyd-Jones D, Schwartz JE, Lewis CE, Shimbo D, Muntner P. Race and sex differences in asleep blood pressure: the Coronary Artery Risk Development in Young Adults (CARDIA) study. J Clin Hypertens (Greenwich) 2019; 21:184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cuspidi C, Facchetti R, Dell’Oro R, Quarti-Trevano F, Tadic M, Mancia G, Grassi G. Office and out-of-office blood pressure changes over a quarter of century: findings from the PAMELA study. Hypertension 2020; 76:759–765. [DOI] [PubMed] [Google Scholar]
- 32. Coylewright M, Reckelhoff JF, Ouyang P. Menopause and hypertension: an age-old debate. Hypertension 2008; 51:952–959. [DOI] [PubMed] [Google Scholar]
- 33. Barnett SR, Morin RJ, Kiely DK, Gagnon M, Azhar G, Knight EL, Nelson JC, Lipsitz LA. Effects of age and gender on autonomic control of blood pressure dynamics. Hypertension 1999; 33:1195–1200. [DOI] [PubMed] [Google Scholar]
- 34. Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 2004; 43:1239–1245. [DOI] [PubMed] [Google Scholar]
- 35. Thomas SJ, Booth JN III, Bromfield SG, Seals SR, Spruill TM, Ogedegbe G, Kidambi S, Shimbo D, Calhoun D, Muntner P. Clinic and ambulatory blood pressure in a population-based sample of African Americans: the Jackson Heart Study. J Am Soc Hypertens 2017; 11:204–212.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang X, Poole JC, Treiber FA, Harshfield GA, Hanevold CD, Snieder H. Ethnic and gender differences in ambulatory blood pressure trajectories: results from a 15-year longitudinal study in youth and young adults. Circulation 2006; 114:2780–2787. [DOI] [PubMed] [Google Scholar]
- 37. Knox SS, Hausdorff J, Markovitz JH; Coronary Artery Risk Development in Young Adults Study . Reactivity as a predictor of subsequent blood pressure: racial differences in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Hypertension 2002; 40:914–919. [DOI] [PubMed] [Google Scholar]
- 38. Lampert R, Ickovics J, Horwitz R, Lee F. Depressed autonomic nervous system function in African Americans and individuals of lower social class: a potential mechanism of race- and class-related disparities in health outcomes. Am Heart J 2005; 150:153–160. [DOI] [PubMed] [Google Scholar]
- 39. Bowman MA, Buysse DJ, Foust JE, Oyefusi V, Hall MH. Disturbed sleep as a mechanism of race differences in nocturnal blood pressure non-dipping. Curr Hypertens Rep 2019; 21:51. [DOI] [PubMed] [Google Scholar]
- 40. Husain A, Lin FC, Tuttle LA, Olsson E, Viera AJ. The reproducibility of racial differences in ambulatory blood pressure phenotypes and measurements. Am J Hypertens 2017; 30:961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article can be shared on reasonable request to the corresponding author and approval by the CARDIA study.