Abstract
Background
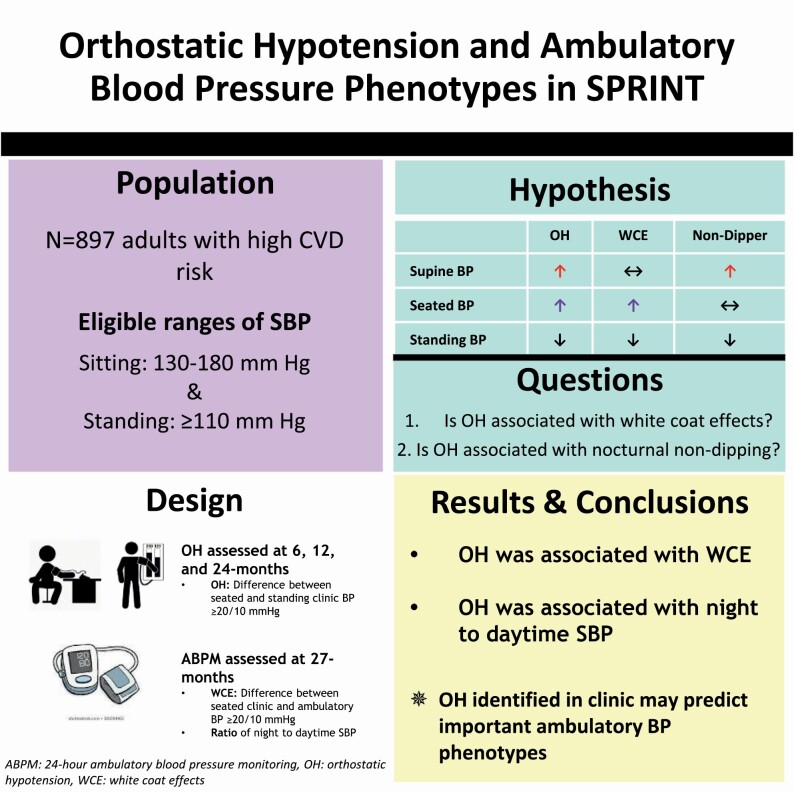
Clinic blood pressure (BP) when measured in the seated position, can miss meaningful BP phenotypes, including low ambulatory BP (white coat effects [WCE]) or high supine BP (nocturnal non-dipping). Orthostatic hypotension (OH) measured using both seated (or supine) and standing BP, could identify phenotypes poorly captured by seated clinic BP alone.
Methods
We examined the association of OH with WCE and night-to-daytime systolic BP (SBP) in a subpopulation of SPRINT, a randomized trial testing the effects of intensive or standard (<120 vs. <140 mm Hg) SBP treatment strategies in adults at increased risk of cardiovascular disease. OH was assessed during follow-up (6, 12, and 24 months) and defined as a decrease in mean seated SBP ≥20 or diastolic BP ≥10 mm Hg after 1 min of standing. WCE, based on 24-hour ambulatory BP monitoring performed at 27 months, was defined as the difference between 27-month seated clinic and daytime ambulatory BP ≥20/≥10 mm Hg. Reverse dipping was defined as a ratio of night-to-daytime SBP >1.
Results
Of 897 adults (mean age 71.5±9.5 years, 29% female, 28% black), 128 had OH at least once. Among those with OH, 15% had WCE (vs. 7% without OH). Moreover, 25% of those with OH demonstrated a non-dipping pattern (vs. 14% without OH). OH was positively associated with both WCE (OR=2.24; 95%CI: 1.28, 4.27) and reverse dipping (OR=2.29; 95% CI: 1.31, 3.99).
Conclusions
The identification of OH in clinic was associated with two BP phenotypes often missed with traditional seated BP assessments. Further studies on mechanisms of these relationships are needed.
Clinical trials registration
Trial Number NCT03569020.
Keywords: ambulatory blood pressure monitoring, blood pressure, hypertension, nocturnal dipping status, orthostatic hypotension, white coat effects
Graphical Abstract
Graphical Abstract.
Hypertension is endemic in the United States and updated guidelines recommend treating to blood pressure (BP) goals based on seated, clinic-based measurements.1,2 However, clinic-based BP measurements often did not agree with 24-hour ambulatory BP monitoring (ABPM) measurements in Systolic Blood Pressure Intervention Trial (SPRINT).3 Furthermore, hypotensive events were among the most common complications of intensive BP treatment in SPRINT.3,4 One limitation of clinic-based BP assessments are that measurements are performed in a single, rested position. This misses important fluctuations in BP that occur in standing or supine positions assumed by patients in home settings throughout the day and night.
Orthostatic hypotension (OH) is a clinic-based measure of BP change that involves measuring BP in at least 2 positions—supine or seated and then standing. OH is defined by a large drop in systolic BP (SBP) ≥20 mm Hg or diastolic BP (DBP) ≥10 mm Hg,5,6 and is common among older adults7 with hypertension8 and in the setting of hypertension treatment.9 Moreover, we recently demonstrated that OH was an important predictor of hypotension outside of the clinic in SPRINT.10 Given that OH is a measure of discordant physiology between seated (or supine) and standing (or upright BP), it is possible that it could be used to detect discordance between seated clinic BP measurements and low ambulatory BP (i.e., white coat effects [WCEs]) or high supine BP (i.e., nocturnal nondipping). However, this has never been examined in SPRINT.
The purpose of this study was to compare the association of OH measured during 6-, 12-, and 24-month follow-up visits with the following patterns of 24-hour ABPM at 27 months in SPRINT: (i) WCE, i.e., the ambulatory minus clinic BP and (ii) night-to-daytime BP. We hypothesized that BP differences observed after transitioning from seated to standing positions would be associated with WCE and elevated nocturnal (supine) BP.
METHODS
Study overview
The study design and methods of SPRINT have been previously published.4,11,12 In brief, SPRINT was a NIH-funded, prospective, randomized, controlled, and open-label outcome trial with blinded end point determination performed at 102 clinical sites in the United States and Puerto Rico from November 2010 to August 2015. SPRINT compared intensive treatment to a SBP goal <120 mm Hg and standard treatment to a SBP goal <140 mm Hg. Institutional Review Boards at each clinical site approved the original study protocol, including subsequent analyses. All SPRINT participants provided written informed consent. A subset of the SPRINT population at 15 sites also consented to an IRB-approved, 24-hour ABPM ancillary study performed at the 27-month follow-up visit.13
Study participants
SPRINT recruited 9,361 participants, who were at least 50 years old with clinic SBP of 130–180 mm Hg, depending on number of antihypertensive medications they were taking during the screening visit. Participants had at least 1 cardiovascular disease (CVD) risk factor: presence of clinical or subclinical CVD other than stroke, an estimated glomerular filtration rate (based on the Modification of Diet in Renal Disease study equation) of 20–59 ml/min/1.73 m2, Framingham 10-year risk score ≥15%, or age ≥75 years. Exclusion criteria included diabetes mellitus, previous stroke, symptomatic heart failure in the past 6 months, advanced chronic kidney disease (estimated glomerular filtration rate <20 ml/min/1.73 m2), left ventricular ejection fraction <35%, any organ transplant, dialysis, proteinuria >1 g/day, dementia, and SBP <110 mm Hg after 1 minute of standing at a screening visit.
Our study population was restricted to participants who completed the ABPM ancillary study. This ancillary excluded participants for the following reasons: their arm circumference was >50 cm, they were a shift worker or worked regularly at night, they had a history of breast cancer requiring mastectomy or radiation on the nondominant arm and needed to avoid frequent BP measurements due to lymphedema, or they had end-stage renal disease. Ultimately, of the 1,003 participants who consented to ABPM measurements, 925 participants were eligible and underwent ABPM, and 897 had complete ABPM data as defined below.
Office BP measurement
Seated office BP was measured 3 times at 1-minute intervals after a 5-minute rest period, using a validated automated oscillometric measurement device (HEM-907XL, Omron Healthcare, Lake Forest, IL) and standardized procedures.11,14,15
Orthostatic hypotension
OH was assessed at screening, baseline, 1-, 6-, and 12-month visits, and then annually thereafter. Participants were instructed to stand after seated office BP measurement, and after 1 minute of standing, BP was measured again.10,16,17 OH was defined using the consensus definition of a decrease in SBP ≥20 mm Hg or DBP ≥10 mm Hg from the seated to standing positions.5,6 For our main analysis, we assessed for presence of OH at 6, 12, or 24 months. OH presence was defined as: never, isolated (1 occurrence), or recurrent (2 or 3 occurrences). OH was also defined as a dichotomous outcome (present at any of 6-, 12-, or 24-month visits). Given that ABPM was performed in the setting of SPRINT’s treatment protocol, we excluded the screening and baseline OH assessments to avoid introducing pretreatment effects on OH occurrence.18 We also excluded the 1-month visit as BP treatment was actively titrated during this time4 and excluded OH assessments after ABPM was performed. We also performed a sensitivity analysis defining OH based on the 24-month visit alone. This approach was not used in our primary analysis as OH is a recurrent event and there were fewer adults with OH at the 24-month visit.
Ambulatory BP
ABPM was conducted over 24 hours within 3 weeks of the 27-month study visit using a validated device, SpaceLabs 90207 (Snoqualmie, WA).19 The monitor was placed on the participants’ nondominant arm and configured to measure BP every 30 minutes. An ABPM recording period was deemed to be complete if there were ≥14 readings between 6:00 am and 12:00 am and ≥6 readings between 12:00 am and 6:00 am.13,20,21 Daytime SBP and daytime DBP were defined as the mean of all SBP readings and DBP readings, respectively, during the 9:00 am to 9:00 pm window; nighttime SBP and nighttime DBP were defined as the average of all SBP readings and DBP readings, respectively, during the 1:00 am to 6:00 am window.22 Twenty-four hour SBP and daytime DBP were defined as the mean of all SBP readings and DBP readings, respectively, over the entire monitoring period.
Our primary outcomes were: (i) WCE defined as a difference between clinic and daytime SBP ≥20 mm Hg or daytime DBP ≥10 mm Hg and (ii) nocturnal dipping status defined as the ratio of night-to-daytime SBP, which was further classified as extreme dipping <0.8, normal dipping 0.8 to <0.9, nondipping 0.9 to 1, and reverse dipping >1.23 Note that WCE based on SBP is presented in the main text, while WCE based on DBP is presented in Supplementary Material online. We also examined white coat hypertension defined as clinic BP ≥140/90 mm Hg and daytime ambulatory BP <135/85 mm Hg.24 Other BP phenotypes of interest derived from ABPM were: (i) masked hypertension (clinic BP <140/90 mm Hg and daytime ambulatory BP ≥135/85 mm Hg), (ii) controlled hypertension (clinic BP <140/90 mm Hg and daytime ambulatory BP <135/85 mm Hg), and (iii) sustained hypertension (clinic BP ≥140/90 mm Hg and daytime ambulatory BP ≥135/85 mm Hg).
Covariates
In general, we used covariate information assessed in closest proximity to the 27-month ABPM measurement (i.e., the 24-month visit). However, some data were only assessed at baseline, which was used if 24-month data was not available. Age, sex, race/ethnicity (black, white, other, Hispanic), and baseline smoking status (never, current, former) were self-reported at baseline. Body mass index was determined using height and weight measurements at the 24-month visit. Chronic kidney disease was based on measured creatinine at baseline and the 24-month visit and defined as an estimated glomerular filtration rate <60 ml/min/1.73 m2, using the 4 variable Modification of Diet in Renal Disease equation. High-density lipoprotein cholesterol and total cholesterol were measured in serum using standard assays during the 24-month visit. History of CVD was based on self-reported CVD at baseline or incident cases between baseline and the 24-month visit. Diabetes was based on self-report at the 24-month visit. Prior stroke was determined based on adjudicated events up through the 24-month visit.3,4,10,11,13
Statistical analysis
We compared baseline characteristics between participants with and without OH, using means and proportions. We used logistic regression for WCE as a dichotomous variable and linear regression for WCE as a continuous variable to examine the association with OH, postural change in BP, and orthostatic hypertension. Models were adjusted for age, sex, and race/ethnicity (model 1). We additionally adjusted for smoking status, chronic kidney disease (at baseline and at 24 months), body mass index, high-density lipoprotein cholesterol, total cholesterol, CVD, and treatment group (model 2). A test for interaction was performed to examine effect modification by treatment group. Given the absence of a statistical interaction between exposures and treatment group, all analyses are primarily presented using the pooled study population, combining intensive and standard BP treatment groups, while results by treatment assignment are presented in Supplementary Material online.
Models were repeated for night-to-daytime SBP and DBP ratio, using linear regression. We also used multinomial logistic regression to study the association between OH BP measures and dipping categories (dippers, extreme dippers, nondippers, and reverse dippers) with dippers serving as the reference group.
In sensitivity analyses, we defined OH, postural change in BP, and orthostatic hypertension using the 24-month visit assessment alone. We also characterized mean seated, standing and ambulatory SBP and DBP by OH, WCE, and nondipping status. Analyses were conducted using the R Statistical Computing Environment. P values were 2 sided and not adjusted for multiple comparisons.
RESULTS
Overall, the mean age of the 897 SPRINT participants in our analyses at the 27-month follow-up visit was 71.5 ± 9.5 years; 28.7% were female, and 28.0% were black (Table 1). The mean 27-month clinic SBP was 127.6 ± 15.6 mm Hg and the mean 27-month clinic DBP was 69.7 ± 12.0 mm Hg; 128 participants (14.3%) had OH during follow-up visits prior to the 27-month visit (Table 2). Among those assigned to intensive treatment, 12.6% had OH during follow-up visits prior to the 27-month visit, while 16.0% had OH among those assigned to standard treatment. Notably there was no evidence of a difference between treatment groups for the incidence of OH prior to the 27-month study visit; thus both treatment groups were combined in ensuing analyses.
Table 1.
Characteristics of SPRINT (Systolic Blood Pressure Intervention Trial) participants in the ambulatory blood pressure ancillary study by orthostatic hypotension history at the 6-, 12-, or 24-month SPRINT study visit, mean (SD) or %
| Variable | Total | Orthostatic hypotension at least once during 6-, 12-, and 24-month visits | No occurrence of orthostatic hypotension |
|---|---|---|---|
| N = 897 | N = 128 | N = 769 | |
| Intensive treatment group, % | 50.5 | 44.5 | 51.5 |
| Standard treatment group, % | 49.5 | 55.4 | 48.5 |
| Age, years (27-mo) | 71.5 (9.5) | 72.8 (9.9) | 71.3 (9.4) |
| Female, % | 28.7 | 25.0 | 29.3 |
| Race/ethnicity, % | |||
| Black | 28.0 | 23.4 | 28.7 |
| White | 67.3 | 70.3 | 66.8 |
| Other | 2.3 | 4.7 | 2.0 |
| Hispanic | 2.3 | 1.6 | 2.5 |
| Body mass index, kg/m2 (24-mo) | 29.5 (5.6) | 27.9 (5.0) | 29.8 (5.7) |
| Smoking, % | |||
| Never | 46.2 | 39.1 | 47.4 |
| Former | 43.6 | 46.1 | 43.2 |
| Current | 10.2 | 14.8 | 9.4 |
| History of CKD (baseline), % | 29.2 | 30.2 | 29.1 |
| History of CVD (baseline), % | 21.7 | 25.8 | 21.1 |
| Experienced CVD event before ABPMa, % | 3.2 | 3.1 | 3.3 |
| Diabetes (24-mo), % | 2.3 | 2.3 | 2.3 |
| Stroke (24-mo), % | 0.1 | 0.0 | 0.1 |
| CKD (24-mo), % | 32.4 | 33.6 | 32.2 |
| HDL, mg/dl (24-mo) | 53.1 (17.0) | 56.2 (18.2) | 52.6 (16.7) |
| Total cholesterol, mg/dl (24-mo) | 181.0 (39.4) | 180.3 (40.5) | 181.1 (39.2) |
24-mo, data collected at 24-month annual visit; 27-mo, data collected at 27-month study visit. Abbreviations: ABPM, ambulatory BP monitoring; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate based on the Modification of Diet in Renal Disease study equation; HDL, high-density lipoprotein.
aAfter randomization.
Table 2.
Blood pressure characteristics of SPRINT (Systolic Blood Pressure Intervention Trial) participants in the ambulatory blood pressure ancillary study by orthostatic hypotension history at the 6-, 12-, or 24-month SPRINT study visit, mean (SD) or %
| Variable | Total | Orthostatic hypotension at least once during 6-, 12-, and 24-month visits | No occurrence of orthostatic hypotension |
|---|---|---|---|
| N = 897 | N = 128 | N = 769 | |
| Clinic SBP, mm Hg (27-mo) | 127.6 (15.6) | 129.2 (17.1) | 127.4 (15.3) |
| Clinic DBP, mm Hg (27-mo) | 69.7 (12.0) | 69.7 (13.1) | 69.7 (11.8) |
| White coat effect, % | 8.3 | 14.8 | 7.2 |
| Dipping status, % | |||
| Extreme dipper | 10.3 | 3.1 | 11.4 |
| Dipper | 35.3 | 28.1 | 36.5 |
| Nondipper | 38.8 | 43.8 | 38.0 |
| Reverse dipper | 15.6 | 25.0 | 14.0 |
| BP phenotypes, % | |||
| White coat hypertension | 5.7 | 10.2 | 4.9 |
| Masked hypertension | 26.2 | 25.0 | 26.4 |
| Controlled hypertension | 51.1 | 48.4 | 51.6 |
| Sustained hypertension | 17.0 | 16.4 | 17.1 |
| N antihypertensive medications (27-mo) | 2.3 (1.3) | 2.4 (1.3) | 2.3 (1.3) |
24-mo, data collected at 24-month annual visit; 27-mo, data collected at 27-month study visit. Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; N antihypertensive medication, number of antihypertensive medications; SBP, systolic blood pressure. Participant BP phenotypes were defined as follows: (i) white-coat hypertension (clinic BP ≥140/90 mm Hg and daytime ambulatory BP <135/85 mm Hg), (ii) masked hypertension (clinic BP <140/90 mm Hg and daytime ambulatory BP ≥135/85 mm Hg), (iii) controlled hypertension (clinic BP <140/90 mm Hg and daytime ambulatory BP <135/85 mm Hg), and (iv) sustained hypertension (clinic BP ≥140/90 mm Hg and daytime ambulatory BP ≥135/85 mm Hg).
The following OH BP measurements were significantly associated with a higher odds of WCE: any OH (odds ratio [OR] 2.34; 95% confidence interval [CI]: 1.28, 4.27), isolated OH (OR 2.36; 95% CI: 1.22, 4.55), and postural change per 1 mm Hg (OR 1.06; 95% CI: 1.02, 1.11) (Table 3, Supplementary Tables ST1 and ST2 online). In contrast, change in DBP and orthostatic hypertension was not associated with WCE. Sensitivity analyses with WCE modeled as a continuous variable based on SBP were confirmatory, in that any OH, isolated OH, and postural change in SBP were all associated with WCE (Supplementary Table ST3 online). In contrast, sensitivity analyses with WCE as a continuous variable based on DBP were generally attenuated (Supplementary Table ST4 online). Furthermore, in a sensitivity analysis examining white coat hypertension, OH, and postural change in SBP were associated with white coat hypertension (Supplementary Table ST5 online).
Table 3.
Association of orthostatic hypotension, postural change in SBP and DBP, and orthostatic hypertension (at 6, 12, or 24 months) with white coat effect as a dichotomous outcome variable defined as the difference between clinic and ABPM daytime SBP ≥20 mm Hg or DBP ≥10 mm Hg
| Odds ratio [95% CI] | Crude | Model 1 | Model 2 |
|---|---|---|---|
| Orthostatic hypotension (6, 12, and 24 months; N = 128) | 2.26 [1.29, 3.95] | 2.30 [1.31, 4.05] | 2.33 [1.28, 4.25] |
| Orthostatic hypotension | |||
| Never (N = 769) | Reference | Reference | Reference |
| Isolated (N = 100) | 2.11 [1.13, 3.96] | 2.16 [1.15, 4.07] | 2.35 [1.22, 4.53] |
| Recurrent (N = 28) | 2.82 [1.03, 7.70] | 2.84 [1.03, 7.83] | 2.27 [0.72, 7.11] |
| Postural change in SBPa (for every −1 mm Hg) | 1.06 [1.02, 1.11] | 1.06 [1.01, 1.10] | 1.10 [1.04, 1.17] |
| Postural change in DBPa (for every −1 mm Hg) | 1.05 [0.97, 1.13] | 1.05 [0.98, 1.13] | 1.05 [0.97, 1.13] |
| Orthostatic hypertension (6, 12, and 24 months; N = 348) | 1.02 [0.62, 1.65] | 0.99 [0.61, 1.63] | 0.97 [0.57, 1.64] |
| Orthostatic hypertension | |||
| Never (N = 549) | Reference | Reference | Reference |
| Isolated (N = 225) | 1.09 [0.63, 1.89] | 1.09 [0.62, 1.89] | 1.08 [0.59, 1.94] |
| Recurrent (N = 123) | 0.88 [0.42, 1.86] | 0.84 [0.40, 1.78] | 0.79 [0.35, 1.79] |
Model 1: age, sex, race/ethnicity. Model 2: smoking status (baseline), chronic kidney disease (baseline), chronic kidney disease (24 months), BMI (baseline), HDL (24 months), total cholesterol (24 months), cardiovascular disease (between baseline and 24 months), treatment group. Abbreviations: ABPM, ambulatory BP monitoring; BMI, body mass index; BP, blood pressure; CI, confidence interval; DBP, diastolic blood pressure; HDL, high-density lipoprotein; SBP, systolic blood pressure.
aPostural change = average of the difference of standing − seated BP at 6, 12, and 24 months.
Any OH was positively associated with a higher night-to-daytime SBP ratio (β = 0.04; 95% CI: 0.02, 0.06) (Table 4, Supplementary Tables ST6 and ST7 online). Moreover, isolated and recurrent OH were also associated with a higher night-to-daytime SBP ratio (β = 0.03; 95% CI: 0.01, 0.05 and β = 0.08; 95% CI: 0.05, 0.12, respectively). Every 1 mm Hg postural reduction in SBP or DBP was associated with higher night-to-daytime SBP ratio (β = 0.003; 95% CI: 0.001, 0.004 and β = 0.004; 95% CI: 0.002, 0.006, respectively). Recurrent orthostatic hypertension was inversely associated with night-to-daytime SBP ratio (β = −0.04; 95% CI: −0.05, −0.02). Analyses were repeated with night-to-daytime DBP ratio and were similar with any OH, recurrent OH, and postural change in SBP or DBP being associated with high night-to-day DBP ratio (Supplementary Table ST8 online). Similarly, recurrent orthostatic hypertension was inversely associated with night-to-day DBP ratio.
Table 4.
Association of orthostatic hypotension, postural change in SBP and DBP, and orthostatic hypertension (at 6, 12, or 24 months) with ratio of night-to-daytime SBP
| Beta coefficient [95% CI] | Crude | Model 1 | Model 2 |
|---|---|---|---|
| Orthostatic hypotension (6, 12, and 24 months; N = 128) | 0.04 [0.02, 0.06] | 0.04 [0.02, 0.06] | 0.04 [0.02, 0.06] |
| Orthostatic hypotension | |||
| Never (N = 769) | Reference | Reference | Reference |
| Isolated (N = 100) | 0.03 [0.01, 0.05] | 0.03 [0.01, 0.05] | 0.03 [0.01, 0.05] |
| Recurrent (N = 28) | 0.08 [0.05, 0.12] | 0.08 [0.05, 0.12] | 0.08 [0.05, 0.12] |
| Postural change in SBPa (for every −1 mm Hg) | 0.003 [0.002, 0.004] | 0.003 [0.001, 0.004] | 0.003 [0.001, 0.004] |
| Postural change in DBPa (for every −1 mm Hg) | 0.003 [0.002, 0.005] | 0.004 [0.002, 0.005] | 0.004 [0.002, 0.006] |
| Orthostatic hypertension (6, 12, and 24 months; N = 348) | −0.008 [−0.02, 0.008] | −0.013 [−0.03, 0.003] | −0.0013 [−0.03, 0.003] |
| Orthostatic hypertension (6, 12, and 24 months) | |||
| Never (N = 549) | Reference | Reference | Reference |
| Isolated (N = 225) | −0.006 [−0.02, 0.008] | −0.007 [−0.02, 0.007] | −0.005 [−0.01, 0.009] |
| Recurrent (N = 123) | −0.03 [−0.05, −0.01] | −0.03 [−0.05, −0.02] | −0.04 [−0.05, −0.02] |
Model 1: age, sex, and race/ethnicity. Model 2: smoking status (baseline), chronic kidney disease (baseline), chronic kidney disease (24 months), BMI (baseline), HDL (24 months), total cholesterol (24 months), cardiovascular disease (between baseline and 24 months), and treatment group. Abbreviations: BMI, body mass index; BP, blood pressure; CI, confidence interval; DBP, diastolic blood pressure; HDL, high-density lipoprotein; SBP, systolic blood pressure.
aPostural change = average of the difference of standing − seated BP at 6, 12 and 24 months.
Reverse dippers were associated with OH (OR 2.27; 95% CI: 1.30, 3.97), recurrent OH (OR 7.68; 95% CI 2.32, 25.5), postural change in SBP (OR 1.08; 95% CI: 1.04, 1.12), and postural change in DBP (OR 1.13; 95% CI: 1.05, 1.21) (Table 5, Supplementary Tables ST9 and ST10 online). Orthostatic hypertension was not associated with nocturnal dipping categories after adjustment (Supplementary Tables ST11 and ST12 online).
Table 5.
Association of orthostatic hypotension, categories of orthostatic hypotension (at 6, 12, or 24 months), and postural change in BP with nocturnal dipping categories
| OR [95% CI] | Crude (Ref: dipper, n = 317) | Model 1 (Ref: dipper, n = 317) | Model 2 (Ref: dipper, n = 317) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Extreme dippers (n = 92) |
Nondipper (n = 348) |
Reverse dippers (n = 140) |
Extreme dippers (n = 92) |
Nondipper (n = 348) |
Reverse dippers (n = 140) |
Extreme dippers (n = 92) |
Nondipper (n = 348) |
Reverse dippers (n = 140) |
|
| Orthostatic hypotension (6, 12, and 24 months; N = 128) | 0.31 [0.12, 1.02] | 1.04 [0.95, 2.35] | 2.31 [1.37, 3.91] | 0.36 [0.12, 1.03] | 1.54 [0.97, 2.43] | 2.42 [1.42, 4.15] | 0.29 [0.01, 0.86] | 1.38 [0.86, 2.21] | 2.29 [1.31, 3.99] |
| Orthostatic hypotension | |||||||||
| Never (N = 769) | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Isolated (N = 225) | 0.39 [0.14, 1.16 | 1.32 [0.82, 2.14] | 1.62 [0.89, 2.96] | 0.41 [0.14, 1.19] | 1.38 [0.84, 2.25] | 1.71 [0.93, 3.15] | 0.35 [0.12, 1.03] | 1.22 [0.74, 2.02] | 1.64 [0.87, 3.08] |
| Recurrent (N = 123) | 0.002 [3 × 10−23, 7 × 1016] | 2.89 [0.92, 9.07] | 7.81 [2.47, 24.8] | 4 × 10−6 [4 × 10−6, 4 × 10−6] | 2.83 [0.89, 8.96] | 8.11 [2.51, 26.12] | 10−6 [10−6, 10−6] | 2.65 [0.82, 8.51] | 7.70 [2.32, 25.6] |
| Postural Change in SBPa (for every −1 mm Hg) | 0.97 [0.93, 1.02] | 1.03 [1.00, 1.06] | 1.08 [1.04, 1.12] | 0.98 [0.93, 1.02] | 1.03 [0.99, 1.06] | 1.08 [1.04, 1.12] | 0.96 [0.92, 1.01] | 1.03 [1.00, 1.06] | 1.08 [1.04, 1.12] |
| Postural Change in DBPa (for every −1 mm Hg) | 0.98 [0.91, 1.05] | 1.03 [0.99, 1.09] | 1.12 [1.05, 1.19] | 0.98 [0.931 1.05] | 1.04 [0.99, 1.09] | 1.13 [1.05, 1.21] | 0.96 [0.89, 1.04] | 1.05 [1.00, 1.04] | 1.13 [1.05, 1.21] |
Note: Dipping categories were defined as extreme dipper <0.8, normal dipper 0.8 to <0.9, nondipper 0.9–1, and reverse dipper >1. Model 1: age, sex, race/ethnicity. Model 2: smoking status (baseline), chronic kidney disease (baseline), chronic kidney disease (24 months), BMI (baseline), HDL (24 months), total cholesterol (24 months), cardiovascular disease (between baseline and 24 months), and treatment group. Abbreviations: BMI, body mass index; BP, blood pressure; CI, confidence interval; DBP, diastolic blood pressure; HDL, high-density lipoprotein; OR, odds ratio; SBP, systolic blood pressure.
Sensitivity analyses with OH restricted to the 24-month SPRINT study visit were consistent, but attenuated due to the small number with OH (N = 70) (Supplementary Tables ST13–ST16 online).
Characterization of seated and standing BP by OH, WCE, and dipping status showed that seated SBP and DBP were substantially greater among those with WCE vs. those with nondipping status, regardless of OH (Supplementary Table ST17 online).
Discussion
In this study of hypertensive, middle-aged and older adults, OH was associated with low BP outside of clinic (WCE) and high supine BP (reverse nocturnal dipping). These findings suggest a potential role of OH in identifying 2 BP phenotypes related to complications of BP treatment and CVD events.23,25
WCEs are prevalent in older adults,26,27 and represent an important challenge to BP treatment. In fact, hypotension events outside of the clinic setting were among the most common complications of intensive treatment in SPRINT, and OH was a strong predictor of hypotension events.4,10 While WCEs have been attributed to a number of factors, including the clinic environment, BP measurement technique, or even physiologic responses,28,29 it is also possible that BP measurement in the seated position simply misses BP excursions associated with standing that are captured with an ambulatory protocol. It is also biologically plausible that both OH and WCE are related via shared autonomic dysfunction.30 Further research is needed to elucidate mechanisms of the relationship between OH and WCE.
OH is a dynamic clinical BP assessment long associated with autonomic regulation.5 However, emerging evidence has shown that OH is a strong predictor of CVD events.7,31–35 Mechanisms of this association have often focused on poor heart rate augmentation or endothelial stiffness underlying BP drops after standing.36 Our study highlights sleep-time elevations in BP as another mechanism by which OH may be related to CVD events. BP fluctuates over a 24-hour period with a general trend toward lower BPs during sleep.37 This reduction in sleep-time BP is associated with a lower risk of CVD events, while nondipping or reverse dipping have been associated with a higher risk of CVD events.23 Some have even considered the absence of diurnal variation in BP to explain the higher risk of CVD among evening shift workers.38 However, nondipping or even reverse dipping patterns cannot be diagnosed without ABPM, causing these conditions to go undetected in many patients. Our study demonstrates that OH was strongly associated with day-to-nighttime sleep ratio and reverse dipping status. Thus, OH may represent a useful clinic-based tool for identifying elevated BP at night.
Our study has limitations. OH and ABPM were not measured concurrently, which may have attenuated the association between OH BP measures and ABPM. As a result, the observed associations between OH and BP phenotypes may be even stronger than suggested by our report. Furthermore, ABPM was performed in the setting of BP treatment and thus some BP phenotypes (e.g., white coat hypertension) were rare due to treatment protocols in the trial. In addition, OH was not very common, which may be due to SPRINT’s seated (vs. supine) BP measurements that were delayed beyond 1 minute. We have shown that earlier OH assessments and symptoms may be more strongly associated with adverse clinical events.39,40 Moreover, by 27 months SPRINT participants were familiar with the study staff and protocol, which may underestimate the prevalence of WCEs. Another limitation is that we did not have ecologic data on the participant’s position at the time of each ABPM measurements. Finally, our study was observational and thus is subject to residual confounding.
Our study also has strengths. SPRINT represents one of the largest studies of both OH and ABPM in adults with hypertension. There are few studies with both OH and ABPM assessments, mostly in populations with established dementia (largest N = 200).41–46 Further, BP was assessed in a standardized, rigorous fashion in clinic, minimizing imprecision. Finally, OH was measured multiple times, allowing us to examine recurrent and isolated OH phenotypes.
Our study has clinical implications. While 24-hour ABPM continues to be viewed as the gold standard BP assessment,2,47 it remains inaccessible to many patients due to costs and logistics, often requiring 2 in-person visits. This is particularly problematic for adults in rural communities or lacking healthcare access. Our study demonstrates how a BP assessed in 2 positions in clinic can help identify 2 important ABPM phenotypes. However, these findings require replication and assume that clinic BP is measured according to guidelines,48 while in routine practice the quality of BP measurements varies greatly.49,50 Adding standing BP to routine clinic visits might be challenging in the clinical setting (time, training), but should be the focus of subsequent studies to determine whether OH assessments might improve BP treatment.
In conclusion, in this population of middle-aged and older hypertensive adults, OH was associated with WCE and elevated sleep-time BP. OH may represent a practical, clinic-based approach for detecting BP phenotypes outside of the clinic when ABPM is not available, but these findings require replication.
Supplementary Material
Acknowledgments
The authors thank the SPRINT participants for their important contributions. For a full list of contributors to SPRINT, please visit www.sprinttrial.org.
SUPPLEMENTARY MATERIAL
Supplementary data are available at American Journal of Hypertension online.
Funding
The SPRINT ambulatory blood pressure ancillary study was supported by grants from the National Institutes of Health (NIH, R03DK100530 and R03DK105314) and the University of Minnesota Chronic Kidney Disease Research Fund. Other support includes R01AG055606 (N.M.P.), R01HL136679 (P.E.D., N.M.P.), and K23HL135273 (S.P.J.). The Systolic Blood Pressure Intervention Trial is funded with Federal funds from the NIH, including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268200900040C, HHSN268200 900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the U.S. Department of Veterans Affairs, or the United States Government. For a full list of contributors to SPRINT, please see the supplementary acknowledgement list: https://www.sprinttrial.org/public/dspScience.cfm. We also acknowledge the support from the following CTSAs funded by NCATS: CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134 and UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073, and UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017-06, University of Utah: UL1TR000105-05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of CA, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433, Tulane University: P30GM103337 COBRE Award NIGMS, Wake Forest University: UL1TR001420.
DISCLOSURE
The authors declared no conflict of interest.
References
- 1. Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr, Whelton PK. Potential U.S. population impact of the 2017 ACC/AHA High Blood Pressure Guideline. J Am Coll Cardiol 2018; 71:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 3. Ghazi L, Pajewski NM, Rifkin DE, Bates JT, Chang TI, Cushman WC, Glasser SP, Haley WE, Johnson KC, Kostis WJ, Papademetriou V, Rahman M, Simmons DL, Taylor A, Whelton PK, Wright JT, Bhatt UY, Drawz PE. Effect of intensive and standard clinic-based hypertension management on the concordance between clinic and ambulatory blood pressure and blood pressure variability in SPRINT. J Am Heart Assoc 2019; 8:e011706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT; SPRINT Research Group . A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res 1996; 6:125–126. [DOI] [PubMed] [Google Scholar]
- 6. Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorff R, Stewart JM, van Dijk JG. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011; 21:69–72. [DOI] [PubMed] [Google Scholar]
- 7. Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS. Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension 1992; 19:508–519. [DOI] [PubMed] [Google Scholar]
- 8. Press Y, Punchik B, Freud T. Orthostatic hypotension and drug therapy in patients at an outpatient comprehensive geriatric assessment unit. J Hypertens 2016; 34:351–358. [DOI] [PubMed] [Google Scholar]
- 9. Di Stefano C, Milazzo V, Totaro S, Sobrero G, Ravera A, Milan A, Maule S, Veglio F. Orthostatic hypotension in a cohort of hypertensive patients referring to a hypertension clinic. J Hum Hypertens 2015; 29:599–603. [DOI] [PubMed] [Google Scholar]
- 10. Juraschek SP, Taylor AA, Wright JT Jr, Evans GW, Miller ER III, Plante TB, Cushman WC, Gure TR, Haley WE, Moinuddin I, Nord J, Oparil S, Pedley C, Roumie CL, Whittle J, Wiggers A, Finucane C, Anne Kenny R, Appel LJ, Townsend RR; SPRINT Research Group . Orthostatic hypotension, cardiovascular outcomes, and adverse events: results from SPRINT. Hypertension 2020; 75:660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC Jr, Johnson KC, Killeen AA, Lewis CE, Oparil S, Reboussin DM, Rocco MV, Snyder JK, Williamson JD, Wright JT Jr, Whelton PK; SPRINT Study Research Group . The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014; 11:532–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, Kitzman DW, Kostis JB, Krousel-Wood MA, Launer LJ, Oparil S, Rodriguez CJ, Roumie CL, Shorr RI, Sink KM, Wadley VG, Whelton PK, Whittle J, Woolard NF, Wright JT Jr, Pajewski NM; SPRINT Research Group . Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drawz PE, Pajewski NM, Bates JT, Bello NA, Cushman WC, Dwyer JP, Fine LJ, Goff DC Jr, Haley WE, Krousel-Wood M, McWilliams A, Rifkin DE, Slinin Y, Taylor A, Townsend R, Wall B, Wright JT, Rahman M. Effect of intensive versus standard clinic-based hypertension management on ambulatory blood pressure: results from the SPRINT (Systolic Blood Pressure Intervention Trial) Ambulatory Blood Pressure Study. Hypertension 2017; 69:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Omboni S, Riva I, Giglio A, Caldara G, Groppelli A, Parati G. Validation of the Omron M5-I, R5-I and HEM-907 automated blood pressure monitors in elderly individuals according to the International Protocol of the European Society of Hypertension. Blood Press Monit 2007; 12:233–242. [DOI] [PubMed] [Google Scholar]
- 15. El Assaad MA, Topouchian JA, Darné BM, Asmar RG. Validation of the Omron HEM-907 device for blood pressure measurement. Blood Press Monit 2002; 7:237–241. [DOI] [PubMed] [Google Scholar]
- 16. Townsend RR, Chang TI, Cohen DL, Cushman WC, Evans GW, Glasser SP, Haley WE, Olney C, Oparil S, Del Pinto R, Pisoni R, Taylor AA, Umanath K, Wright JT Jr, Yeboah J; SPRINT Study Research Group . Orthostatic changes in systolic blood pressure among SPRINT participants at baseline. J Am Soc Hypertens 2016; 10:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnson KC, Whelton PK, Cushman WC, Cutler JA, Evans GW, Snyder JK, Ambrosius WT, Beddhu S, Cheung AK, Fine LJ, Lewis CE, Rahman M, Reboussin DM, Rocco MV, Oparil S, Wright JT Jr; SPRINT Research Group . Blood pressure measurement in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension 2018; 71:848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Juraschek SP, Hu J-R, Cluett JL, Ishak A, Mita C, Lipsitz LA, Appel LJ, Beckett NS, Coleman RL, Cushman WC, Davis BR, Grandits G, Holman RR, Miller ER, Peters R, Staessen JA, Taylor AA, Thijs L, Wright JT, Mukamal KJ. Effects of intensive blood pressure treatment on orthostatic hypotension: a systematic review and individual participant-based meta-analysis. Ann Intern Med 2020. (doi: 10.7326/M20-4298). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O’Brien E, Mee F, Atkins N, O’Malley K. Accuracy of the SpaceLabs 90207 determined by the British Hypertension Society protocol. J Hypertens 1991; 9:573–574. [DOI] [PubMed] [Google Scholar]
- 20. Drawz PE, Alper AB, Anderson AH, Brecklin CS, Charleston J, Chen J, Deo R, Fischer MJ, He J, Hsu CY, Huan Y, Keane MG, Kusek JW, Makos GK, Miller ER III, Soliman EZ, Steigerwalt SP, Taliercio JJ, Townsend RR, Weir MR, Wright JT Jr, Xie D, Rahman M; Chronic Renal Insufficiency Cohort Study Investigators . Masked hypertension and elevated nighttime blood pressure in CKD: prevalence and association with target organ damage. Clin J Am Soc Nephrol 2016; 11:642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gabbai FB, Rahman M, Hu B, Appel LJ, Charleston J, Contreras G, Faulkner ML, Hiremath L, Jamerson KA, Lea JP, Lipkowitz MS, Pogue VA, Rostand SG, Smogorzewski MJ, Wright JT, Greene T, Gassman J, Wang X, Phillips RA; African American Study of Kidney Disease and Hypertension (AASK) Study Group . Relationship between ambulatory BP and clinical outcomes in patients with hypertensive CKD. Clin J Am Soc Nephrol 2012; 7:1770–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parati G, Stergiou G, O’Brien E, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y; European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability . European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens 2014; 32:1359–1366. [DOI] [PubMed] [Google Scholar]
- 23. Yang WY, Melgarejo JD, Thijs L, Zhang ZY, Boggia J, Wei FF, Hansen TW, Asayama K, Ohkubo T, Jeppesen J, Dolan E, Stolarz-Skrzypek K, Malyutina S, Casiglia E, Lind L, Filipovský J, Maestre GE, Li Y, Wang JG, Imai Y, Kawecka-Jaszcz K, Sandoya E, Narkiewicz K, O’Brien E, Verhamme P, Staessen JA; International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes (IDACO) Investigators . Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA 2019; 322:409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005; 111:697–716. [DOI] [PubMed] [Google Scholar]
- 25. Lee KW, Blann AD, Lip GY. High pulse pressure and nondipping circadian blood pressure in patients with coronary artery disease: relationship to thrombogenesis and endothelial damage/dysfunction. Am J Hypertens 2005; 18:104–115. [DOI] [PubMed] [Google Scholar]
- 26. Franklin SS, Thijs L, Asayama K, Li Y, Hansen TW, Boggia J, Jacobs L, Zhang Z, Kikuya M, Björklund-Bodegård K, Ohkubo T, Yang WY, Jeppesen J, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Filipovský J, Imai Y, Wang JG, O’Brien E, Staessen JA; IDACO Investigators . The cardiovascular risk of white-coat hypertension. J Am Coll Cardiol 2016; 68:2033–2043. [DOI] [PubMed] [Google Scholar]
- 27. Tanner RM, Shimbo D, Seals SR, Reynolds K, Bowling CB, Ogedegbe G, Muntner P. White-coat effect among older adults: data from the Jackson Heart Study. J Clin Hypertens (Greenwich) 2016; 18:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manios ED, Koroboki EA, Tsivgoulis GK, Spengos KM, Spiliopoulou IK, Brodie FG, Vemmos KN, Zakopoulos NA. Factors influencing white-coat effect. Am J Hypertens 2008; 21:153–158. [DOI] [PubMed] [Google Scholar]
- 29. Pickering TG, Gerin W, Schwartz AR. What is the white-coat effect and how should it be measured? Blood Press Monit 2002; 7:293–300. [DOI] [PubMed] [Google Scholar]
- 30. Lantelme P, Milon H, Gharib C, Gayet C, Fortrat JO. White coat effect and reactivity to stress: cardiovascular and autonomic nervous system responses. Hypertension 1998; 31:1021–1029. [DOI] [PubMed] [Google Scholar]
- 31. Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O. Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (The Malmo Preventive Project). Eur Heart J 2010; 31:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Juraschek SP, Daya N, Appel LJ, Miller ER, McEvoy JW, Matsushita K, Ballantyne CM, Selvin E. Orthostatic hypotension and risk of clinical and subclinical cardiovascular disease in middle-aged adults. J Am Heart Assoc 2018; 7 (doi: 10.1161/JAHA.118.008884). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Verwoert GC, Mattace-Raso FU, Hofman A, Heeringa J, Stricker BH, Breteler MM, Witteman JC. Orthostatic hypotension and risk of cardiovascular disease in elderly people: the Rotterdam study. J Am Geriatr Soc 2008; 56:1816–1820. [DOI] [PubMed] [Google Scholar]
- 34. Rose KM, Tyroler HA, Nardo CJ, Arnett DK, Light KC, Rosamond W, Sharrett AR, Szklo M. Orthostatic hypotension and the incidence of coronary heart disease: the Atherosclerosis Risk in Communities study. Am J Hypertens 2000; 13:571–578. [DOI] [PubMed] [Google Scholar]
- 35. Fedorowski A, Hedblad B, Melander O. Early postural blood pressure response and cause-specific mortality among middle-aged adults. Eur J Epidemiol 2011; 26:537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mattace-Raso FU, van der Cammen TJ, Knetsch AM, van den Meiracker AH, Schalekamp MA, Hofman A, Witteman JC. Arterial stiffness as the candidate underlying mechanism for postural blood pressure changes and orthostatic hypotension in older adults: the Rotterdam Study. J Hypertens 2006; 24:339–344. [DOI] [PubMed] [Google Scholar]
- 37. O’Brien E, Kario K, Staessen JA, de la Sierra A, Ohkubo T. Patterns of ambulatory blood pressure: clinical relevance and application. J Clin Hypertens (Greenwich) 2018; 20:1112–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yamasaki F, Schwartz JE, Gerber LM, Warren K, Pickering TG. Impact of shift work and race/ethnicity on the diurnal rhythm of blood pressure and catecholamines. Hypertension 1998; 32:417–423. [DOI] [PubMed] [Google Scholar]
- 39. Juraschek SP, Daya N, Rawlings AM, Appel LJ, Miller ER III, Windham BG, Griswold ME, Heiss G, Selvin E. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Intern Med 2017; 177:1316–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Juraschek SP, Longstreth WT Jr, Lopez OL, Gottdiener JS, Lipsitz LA, Kuller LH, Mukamal KJ. Orthostatic hypotension, dizziness, neurology outcomes, and death in older adults. Neurology 2020; 95:e1941–e1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Costa A, Bosone D, Ramusino MC, Ghiotto N, Guaschino E, Zoppi A, D’Angelo A, Fogari R. Twenty-four-hour blood pressure profile, orthostatic hypotension, and cardiac dysautonomia in elderly type 2 diabetic hypertensive patients. Clin Auton Res 2016; 26:433–439. [DOI] [PubMed] [Google Scholar]
- 42. Vallelonga F, Romagnolo A, Merola A, Sobrero G, Di Stefano C, Milazzo V, Burrello J, Burrello A, Zibetti M, Milan A, Veglio F, Maule S. Detection of orthostatic hypotension with ambulatory blood pressure monitoring in Parkinson’s disease. Hypertens Res 2019; 42:1552–1560. [DOI] [PubMed] [Google Scholar]
- 43. Cappelleri C, Janoschka A, Berli R, Kohler S, Braun-Dullaeus RC, Heuss LT, Wolfrum M. Twenty-four-hour ambulatory blood pressure monitoring in very elderly patients: comparison of in-hospital versus home follow-up results. Medicine (Baltimore) 2017; 96:e7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vichayanrat E, Low DA, Iodice V, Stuebner E, Hagen EM, Mathias CJ. Twenty-four-hour ambulatory blood pressure and heart rate profiles in diagnosing orthostatic hypotension in Parkinson’s disease and multiple system atrophy. Eur J Neurol 2017; 24:90–97. [DOI] [PubMed] [Google Scholar]
- 45. Kang SJ, Ahn JY, Kim JS, Cho JW, Kim JY, Choi YY, Kim HT. 24-Hour ambulatory blood pressure monitoring in SWEDDs patients with parkinsonism. Can J Neurol Sci 2016; 43:390–397. [DOI] [PubMed] [Google Scholar]
- 46. Voichanski S, Grossman C, Leibowitz A, Peleg E, Koren-Morag N, Sharabi Y, Shamiss A, Grossman E. Orthostatic hypotension is associated with nocturnal change in systolic blood pressure. Am J Hypertens 2012; 25:159–164. [DOI] [PubMed] [Google Scholar]
- 47. Leung AA, Nerenberg K, Daskalopoulou SS, McBrien K, Zarnke KB, Dasgupta K, Cloutier L, Gelfer M, Lamarre-Cliche M, Milot A, Bolli P, Tremblay G, McLean D, Tobe SW, Ruzicka M, Burns KD, Vallée M, Prasad GV, Lebel M, Feldman RD, Selby P, Pipe A, Schiffrin EL, McFarlane PA, Oh P, Hegele RA, Khara M, Wilson TW, Penner SB, Burgess E, Herman RJ, Bacon SL, Rabkin SW, Gilbert RE, Campbell TS, Grover S, Honos G, Lindsay P, Hill MD, Coutts SB, Gubitz G, Campbell NR, Moe GW, Howlett JG, Boulanger JM, Prebtani A, Larochelle P, Leiter LA, Jones C, Ogilvie RI, Woo V, Kaczorowski J, Trudeau L, Petrella RJ, Hiremath S, Drouin D, Lavoie KL, Hamet P, Fodor G, Grégoire JC, Lewanczuk R, Dresser GK, Sharma M, Reid D, Lear SA, Moullec G, Gupta M, Magee LA, Logan AG, Harris KC, Dionne J, Fournier A, Benoit G, Feber J, Poirier L, Padwal RS, Rabi DM; CHEP Guidelines Task Force . Hypertension Canada’s 2016 Canadian Hypertension Education Program Guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol 2016; 32:569–588. [DOI] [PubMed] [Google Scholar]
- 48. Muntner P, Einhorn PT, Cushman WC, Whelton PK, Bello NA, Drawz PE, Green BB, Jones DW, Juraschek SP, Margolis KL, Miller ER III, Navar AM, Ostchega Y, Rakotz MK, Rosner B, Schwartz JE, Shimbo D, Stergiou GS, Townsend RR, Williamson JD, Wright JT Jr, Appel LJ; 2017 National Heart, Lung, and Blood Institute Working Group . Blood pressure assessment in adults in clinical practice and clinic-based research: JACC Scientific Expert Panel. J Am Coll Cardiol 2019; 73:317–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Drawz PE, Beddhu S, Kramer HJ, Rakotz M, Rocco MV, Whelton PK. Blood pressure measurement: a KDOQI perspective. Am J Kidney Dis 2020; 75:426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Drawz PE, Agarwal A, Dwyer JP, Horwitz E, Lash J, Lenoir K, McWilliams A, Oparil S, Rahbari-Oskoui F, Rahman M, Parkulo MA, Pemu P, Raj DS, Rocco M, Soman S, Thomas G, Tuot DS, Whelton PK, Pajewski NM. Concordance between blood pressure in the systolic blood pressure intervention trial and in routine clinical practice. JAMA Intern Med 2020; 180:1655–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.