INTRODUCTION
Colorectal carcinoma (CRC) has the third highest incidence and the second highest mortality across all types of cancer worldwide.1 In 2015, there were 388,000 new CRC cases and 187,000 deaths in China.2 With advances in combining chemotherapy with vascular endothelial growth factor or epidermal growth factor receptor inhibitors, the median overall survival for patients with metastatic colorectal carcinoma (mCRC) is approximately 30 months.3 Recent next-generation sequencing (NGS) has uncovered several novel potential molecular targets in mCRC, such as RET, ROS1, NTRK, and ALK.4-11 Based on basket trials that screen for the off-label use of a targeted drug in patients with the same genomic alterations,12 NGS-guided therapy could yield clinical benefits and provide novel insights into optimal clinical management for intractable mCRC.
ALK gene fusions have been successfully exploited as therapeutic targets in non–small-cell lung cancer (NSCLC) using the ALK inhibitors crizotinib and lorlatinib.13,14 However, knowledge on the efficacy of targeted therapy for ALK gene fusion in mCRC remains rare. To our knowledge, only two patients have been described who harbored ALK gene fusions and responded to ceritinib and entrectinib, respectively.8,10
A diagnosis of leptomeningeal metastasis (LM) carries a poor prognosis with a median survival of only 2-4 months.15 Few cases of LM caused by CRC have been reported.16 Recently, it was found that CSF circulating tumor DNA (ctDNA) could better reflect the molecular characteristics than plasma ctDNA in patients with NSCLC harboring ALK rearrangement and may be useful in identifying drug targets and guiding treatment.17
In this case study, we describe the first instance of ALK rearrangement in CRC detected using NGS of CSF ctDNA, as well as a case of lasting objective tumor response to crizotinib, alectinib, and lorlatinib therapy.
CASE REPORT
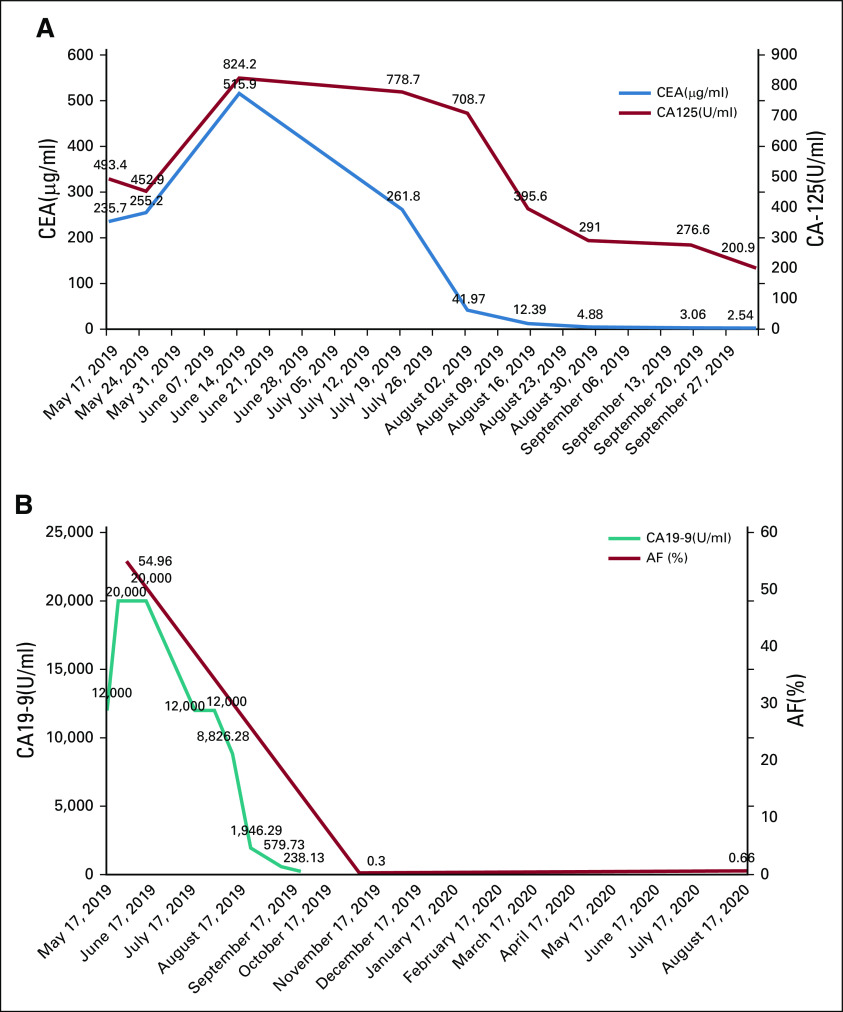
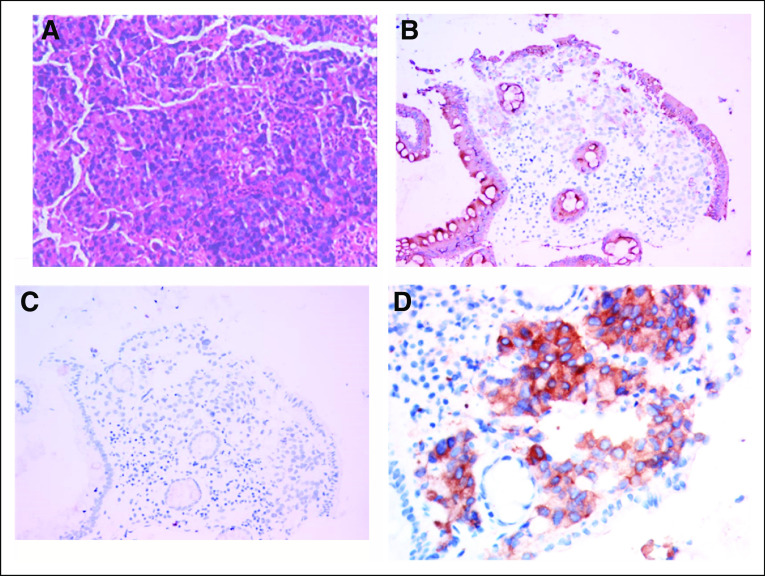
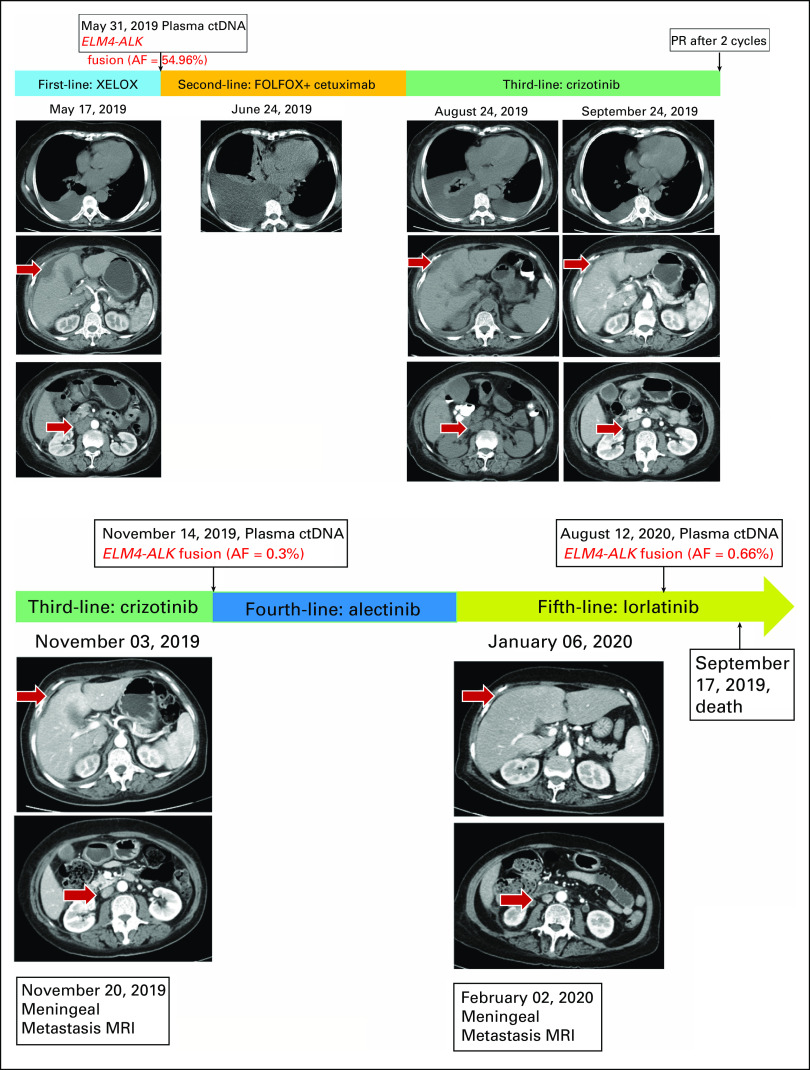
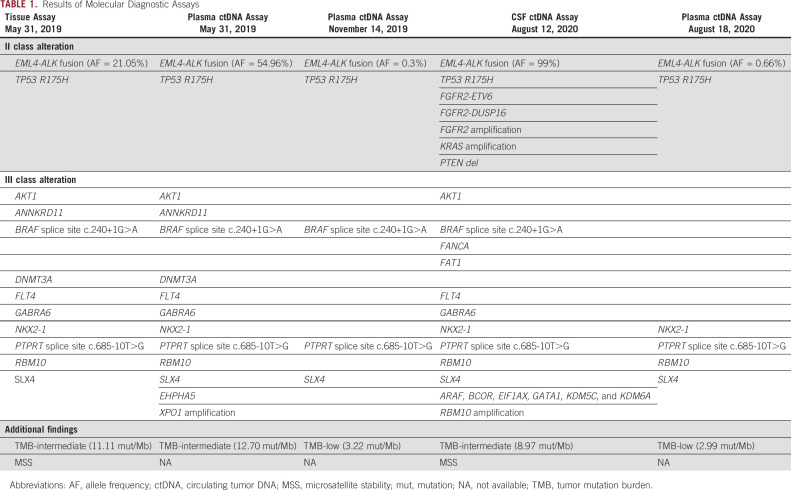
A 70-year-old female arrived at our clinic with abdominal pain present for 3 months. A computed tomography scan revealed a mass in the ascending colon accompanied by liver, peritoneum, and pleura metastases. Serum tumor markers including carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 125, and CA19-9 significantly increased (Figs 1A and 1B). Colonoscopy pathology reported moderately to poorly differentiated adenocarcinoma (Fig 2A), and immunohistochemistry (IHC) demonstrated positivity for CK20 (Fig 2B). Formalin-fixed paraffin-embedded specimens from colonoscopy biopsy were subjected to NGS using the OncoScreen plus (Burning Rock Dx, Irvine, CA) assay platform, including 520 cancer-related genes and plasma ctDNA. The tissue-based NGS identified 12 genomic alterations: EML4-ALK fusion (E21;A20) (Fig 3A) and mutation in TP53 R157H, AKT1, ANNKRD11, BRAF, DNMT3A, FLT4, GABRA6, NKX2-1, PTPRT, RBM10, and SLX4. No BRAF V600E or KRAS mutations were identified. The IHC results showing strong D5F3 anti-ALK antibody of Ventana staining verified ALK overexpression as a result of EML4-ALK fusion (Fig 2D). The first XELOX (capecitabine, 1.5g, d1-14 + oxaliplatin, 200mg, d1, q3w) cycle was interrupted because of the patient’s intestinal obstruction after she had taken oral capecitabine for 1 week. She was subsequently switched to mFOLFOX6 (oxaliplatin, 130 mg, d1 + 5-fluorouracil, 500 mg, d1 leucovorin 500mg, d1 + maintenance dose of 3g, 5-fluorouracil for 46 hours, q2w) + cetuximab (700mg, d1, q2w), but the regimen was terminated after nine days because of dyspnea resulting from hydrothorax. Because of the presence of EML4-ALK fusion, she was treated with oral crizotinib (250 mg, twice a day) on July 10, 2019. The treatment resulted in clinical benefit with the disappearance of tumor-related abdominal pain. After a month, computed tomography scans revealed partial response (PR) for retroperitoneal lymphadenectasis and liver metastases based on RECIST 1.1 (Fig 4) and concomitant decrease in serum CEA and CA 19-9 (Figs 1A and 1B). After four months of treatment, LM symptoms appeared, accompanied by continuous elevation of serum CA 19-9. Brain MRI demonstrated diffuse linear enhancement of the cerebral sulci (Fig 4). The second ctDNA NGS test was implemented, but no resistant mutations were found except for a lower allele frequency of EML4-ALK fusion (0.3%) and TP53 mutation (0.3%) (Table 1 and Fig 1A). The patient accepted alectinib (600 mg twice a day), and the LM symptoms were slightly relieved, but did not entirely disappear after 2 weeks. Thus, lorlatinib, a third-generation tyrosine kinase inhibitor, was recommended as fifth-line therapy with a dose of 75 mg qd beginning December 9, 2019. As the patient’s LM symptoms gradually improved, we increased the dose to 100 mg qd. The progression-free survival (PFS) on lorlatinib was 11.5 months. Because of the increasing serum CEA and CA 19-9 and stable extracranial lesions, her oncologist opted for a lumbar puncture to obtain her CSF to implement the third ctDNA test with CSF and plasma samples on August 13, 2020. It is surprising that high allele frequency of EML4-ALK (99%) and other gene alterations, such as FGFR2 mutations, KRAS amplification, and PTEN deletion, appeared in CSF (Table 1). No evidence was confirmed regarding the progressive index related to ALK alterations, and thus, lorlatinib was still retained. The patient died on September 17, 2020, because of progression of LM.
FIG 1.
(A) Fluctuation of CEA (normal value: 0-5 μg/L) and CA 125 (normal value: 0-35 U/L) levels in the blood. (B) Fluctuation of CA 19-9 (normal value: 0-39 U/mL) levels and AF of ctDNA ELM4-ALK fusion in the blood. AF, allele frequency; CA, carbohydrate antigen; CEA, carcinoembryonic antigen; ctDNA, circulating tumor DNA.
FIG 2.
H&E staining (A), 100×, and IHC, (B) for CK20 (+), (C) for Ki-67 (−), and (D) for IHC with D5F3 anti-ALK Ventana antibody showing strong staining and verifying ALK overexpression as a result of EML4-ALK fusion. ALK, anaplastic lymphoma kinase; H&E, hematoxylin and eosin; IHC, immunohistochemistry.
FIG 3.
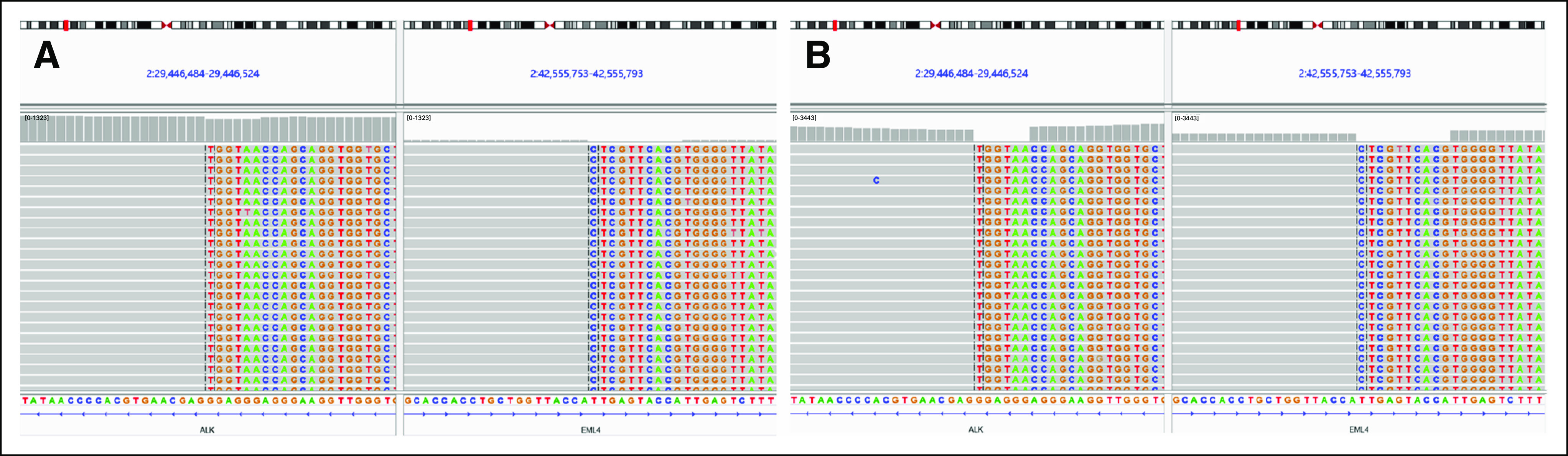
NGS showing EML4-ALK fusion (E21;A20) on (A) FFP and (B) CSF, in which the AF is 21.5% and 99%, respectively. AF, allele frequency; ctDNA, circulating tumor DNA; FFP, fresh frozen plasma; NGS, next-generation sequencing; PR, partial response.
FIG 4.
mCRC treatment using different regimens and results of NGS ctDNA monitoring in plasma. AF, allele frequency; ctDNA, circulating tumor DNA; mCRC, metastatic colorectal carcinoma; MRI, magnetic resonance imaging; NGS, next-generation sequencing; PR, partial response.
TABLE 1.
Results of Molecular Diagnostic Assays
DISCUSSION
In this report, we show the clinical efficacy of crizotinib, alectinib, and lorlatinib in an ALK-rearrangement mCRC with LM. This case had several uncommon features: the presence of ALK fusions that are rarely present in mCRC; the first report of a patient with ALK-rearranged mCRC who showed a good response to crizotinib, alectinib, and lorlatinib therapy; and the first case of ALK fusion detected in a patient with mCRC through CSF ctDNA.
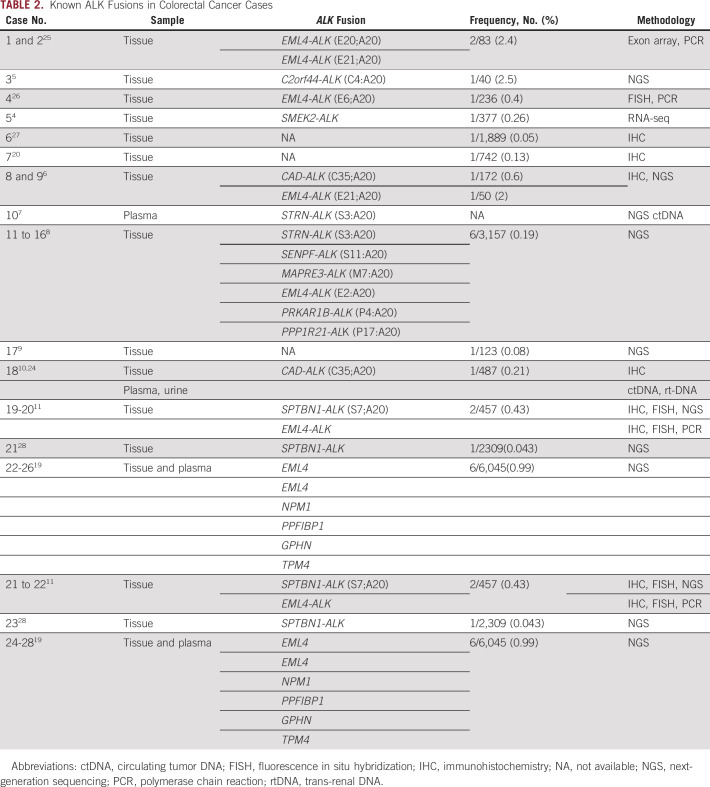
ALK fusion activates downstream signaling pathways without ligand binding, including phospholipase Cγ, Janus kinase-signal transducer and activator of transcription, and PI3K-AKT-mTOR signaling cascades, which regulate proliferation, growth, invasion, and antiapoptotic signaling. In epithelial tumors, ALK gene rearrangements are most common in lung carcinomas, with an incidence rate varying from 3% to 7%, and are rare in CRCs.18The oncogenic ALK rearrangements were reported to have frequencies varying from 0.04% to 2.5% and in 23 cases of CRC with various fusion partners (Table 2). Sheng et al19 reported more than 40,000 Chinese cancer tissue or blood sample subjected to NGS for ALK rearrangement. The frequency of ALK fusion in CRC is 0.99%. Based on the data available from The Cancer Genome Atlas and Burning Rock datasets, the rates of ALK fusion in CRC cases are estimated to be 0.17% and 0.16%, respectively.
TABLE 2.
Known ALK Fusions in Colorectal Cancer Cases
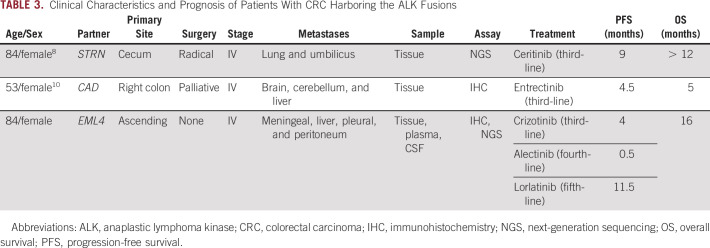
Several studies have investigated the effects of ALK inhibitors on CRC in vitro. It was shown that crizotinib or entrectinib could inhibit the phosphorylation of ALK protein tumor cell line derived from EML4-ALK fusion CRC.6 It was noted that C10 cells, harboring the ALK rearrangement, were sensitive to crizotinib, which downregulates MAPK and PI3K pathways.20 To date, only three patients have been responsive to ALK inhibitor, including our patient. Yakirevich et al8 reported an 84-year-old male presenting with an STRN-ALK fusion who achieved clinical benefit for 9 months after treatment with ceritinib, a second-generation ALK/ROS1 inhibitor. Another case study also reported an objective response to the ALK/ROS1/NTRK inhibitor entrectinib in a patient with CRC harboring a CAD-ALK fusion.10 Interestingly, nivolumab, a PD-1 inhibitor, also remained PR in a patient with dMMR and high PD-L1 (> 50%) CRC harboring EML4-ALK fusion more than 9 months.21 The most common ALK-dependent resistance mechanisms of crizotinib are L1196M and of alectinib and ceritinib are G1269A and G1202R,22 yet no secondary resistant mutations were found in our second ctDNA NGS. Lorlatinib was designed to cross the blood-brain barrier and had potent antitumor activity in preclinical study23 result in durable control of LMs in our case (Table 3).
TABLE 3.
Clinical Characteristics and Prognosis of Patients With CRC Harboring the ALK Fusions
Sample diversity makes ctDNA-based liquid biopsies not less limited to plasma, such as urine. Based on the urine sample of patient who has objective response to entrectinib,10 Siravegna et al24 showed that detection of the CAD-ALK gene fusion in urine trans-renal DNA anticipated CRC response to entrectinib.
In conclusion, we have reported on an elderly patient with ALK-fusion mCRC who was treated with crizotinib, alectinib, and lorlatinib and achieved PR with the PFS of 3, 0.5, and 11.5 months, respectively. The case provides a new potential treatment strategy for patients with CRC who did not respond to standard treatment with ALK rearrangement but still poses a few questions. Are there any other targetable ALK-fusion partners in patients with mCRC? What are the biological characteristics in such patients harboring ALK fusions? Translational studies and the establishment of a database will be instrumental for addressing many of these unanswered questions.
The patient provided written informed consent and gave permission for the use of biopsies and the publication of case details. This study was approved by the Ethical Committee of the Changzheng Hospital of Naval Medical University. Data and materials in the current study are not available to any readers as they contain the patient's personal details.
ACKNOWLEDGMENT
The authors thank our patient for sharing her presentation for this work.
Footnotes
X.H. and X.-D.J. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Xi He, Xiao-Dong Jiao, Ke Liu, Yuan-Sheng Zang
Administrative support: Ying Wu, Yan Ling, Yuan-Sheng Zang
Financial support: Xi He
Provision of study materials or patients: Ying Wu, Yan Ling, A-Qiao Xu, Kun Song, Yuan-Sheng Zang
Collection and assembly of data: Xi He, Xiao-Dong Jiao, Ke Liu, Ying Wu, Yan Ling, Jun Liu, A-Qiao Xu
Data analysis and interpretation: Xi He, Xiao-Dong Jiao, Bao-Dong Qin, Ying Wu, Yan Ling, Kun Song, Yuan-Sheng Zang
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 68394–4242018 [DOI] [PubMed] [Google Scholar]
- 2.Zheng R, Sun K, Zhang S, et al. Report of cancer epidemiology in China, 2015 Zhonghua Zhong Liu Za Zhi 4119–282019 [DOI] [PubMed] [Google Scholar]
- 3.Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: A randomized clinical trial JAMA 3172392–24012017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stransky N, Cerami E, Schalm S, et al. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipson D, Capelletti M, Yelensky R, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies Nat Med 18382–3842012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J, Kim HC, Hong JY, et al. Detection of novel and potentially actionable anaplastic lymphoma kinase (ALK) rearrangement in colorectal adenocarcinoma by immunohistochemistry screening Oncotarget 624320–243322015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai AZ, Schrock AB, Erlich RL, et al. Detection of an ALK fusion in colorectal carcinoma by hybrid capture-based assay of circulating tumor DNA Oncologist 22774–7792017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yakirevich E, Resnick MB, Mangray S, et al. Oncogenic ALK fusion in rare and aggressive subtype of colorectal adenocarcinoma as a potential therapeutic target Clin Cancer Res 223831–38402016 [DOI] [PubMed] [Google Scholar]
- 9.Martinelli E, Sforza V, Cardone C, et al. Clinical outcome and molecular characterisation of chemorefractory metastatic colorectal cancer patients with long-term efficacy of regorafenib treatment. ESMO Open. 2017;2:e000177. doi: 10.1136/esmoopen-2017-000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amatu A, Somaschini A, Cerea G, et al. Novel CAD-ALK gene rearrangement is drugable by entrectinib in colorectal cancer Br J Cancer 1131730–17342015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ying J, Lin C, Wu J, et al. Anaplastic lymphoma kinase rearrangement in digestive tract cancer: Implication for targeted therapy in Chinese population. PLoS One. 2015;10:e0144731. doi: 10.1371/journal.pone.0144731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin BD, Jiao XD, Liu K, et al. Basket trials for intractable cancer. Front Oncol. 2019;9:229. doi: 10.3389/fonc.2019.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: Results from a global phase 2 study Lancet Oncol 191654–16672018 [DOI] [PubMed] [Google Scholar]
- 14.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer N Engl J Med 3712167–21772014 [DOI] [PubMed] [Google Scholar]
- 15.Thakkar JP, Kumthekar P, Dixit KS, et al. Leptomeningeal metastasis from solid tumors. J Neurol Sci. 2020;411:116706. doi: 10.1016/j.jns.2020.116706. [DOI] [PubMed] [Google Scholar]
- 16.Giglio P, Weinberg JS, Forman AD, et al. Neoplastic meningitis in patients with adenocarcinoma of the gastrointestinal tract Cancer 1032355–23622005 [DOI] [PubMed] [Google Scholar]
- 17.Zheng MM, Li YS, Jiang BY, et al. Clinical utility of cerebrospinal fluid cell-free DNA as liquid biopsy for leptomeningeal metastases in ALK-rearranged NSCLC J Thorac Oncol 14924–9322019 [DOI] [PubMed] [Google Scholar]
- 18.Hallberg B, Palmer RH.Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology Nat Rev Cancer 13685–7002013 [DOI] [PubMed] [Google Scholar]
- 19.Sheng Y, Gong FY, Wang GQ, et al. Novel ALK fusions are detected in patients with not only NSCLC but also other solid tumors NGS fusion assay is an optional method for screening novel fusion. Presented at the ASCO 2020 (poster 3555)
- 20.Medico E, Russo M, Picco G, et al. The molecular landscape of colorectal cancer cell lines unveils clinically actionable kinase targets. Nat Commun. 2015;6:7002. doi: 10.1038/ncomms8002. [DOI] [PubMed] [Google Scholar]
- 21.Pietrantonio F, Di Nicolantonio F, Schrock AB, et al. ALK, ROS1, and NTRK rearrangements in metastatic colorectal cancer. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djx089. [DOI] [PubMed] [Google Scholar]
- 22.Lin JJ, Riely GJ, Shaw AT.Targeting ALK: Precision medicine takes on drug resistance Cancer Discov 7137–1552017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw AT, Bauer TM, De Marinis F, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer N Engl J Med 3832018–20292020 [DOI] [PubMed] [Google Scholar]
- 24.Siravegna G, Sartore-Bianchi A, Mussolin B, et al. Tracking a CAD-ALK gene rearrangement in urine and blood of a colorectal cancer patient treated with an ALK inhibitor Ann Oncol 281302–13082017 [DOI] [PubMed] [Google Scholar]
- 25.Lin E, Li L, Guan Y, et al. Exon array profiling detects EML4-ALK fusion in breast, colorectal, and non-small cell lung cancers Mol Cancer Res 71466–14762009 [DOI] [PubMed] [Google Scholar]
- 26.Aisner DL, Nguyen TT, Paskulin DD, et al. ROS1 and ALK fusions in colorectal cancer, with evidence of intratumoral heterogeneity for molecular drivers Mol Cancer Res 12111–1182014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houang M, Toon CW, Clarkson A, et al. ALK and ROS1 overexpression is very rare in colorectal adenocarcinoma Appl Immunohistochem Mol Morphol 23134–1382015 [DOI] [PubMed] [Google Scholar]
- 28.Cocco E, Benhamida J, Middha S, et al. Colorectal carcinomas containing hypermethylated MLH1 promoter and wild-type BRAF/KRAS are enriched for targetable kinase fusions Cancer Res 791047–10532019 [DOI] [PMC free article] [PubMed] [Google Scholar]