INTRODUCTION
Salivary duct carcinoma (SDC) is an aggressive adenocarcinoma of the salivary gland with high rates of metastasis and mortality with 5-year survival rates between 23%-42% versus other more common, salivary gland carcinomas such as adenoid cystic carcinoma (89%) and mucoepidermoid carcinoma (79.3%).1 Even for SDCs managed with curative intent with surgery with or without adjuvant radiation, there is a high rate of locoregional recurrence (48%) or distant metastasis (48%).2 The sensitivity of advanced SDC (aSDC) to chemotherapy has been reported in the past with variable responses.3,4 However, in recent years, large numbers of somatic mutations have been identified where targeted therapies have shown great success. We explore a PIK3CA and androgen receptor (AR)-positive aSDC case that responded favorably to phosphoinositide 3-kinase (PI3K)α isoform inhibitor alpelisib and AR inhibitor bicalutamide. To the best of our knowledge, this is the first such case of aSDC treated with alpelisib and bicalutamide.
CASE HISTORY
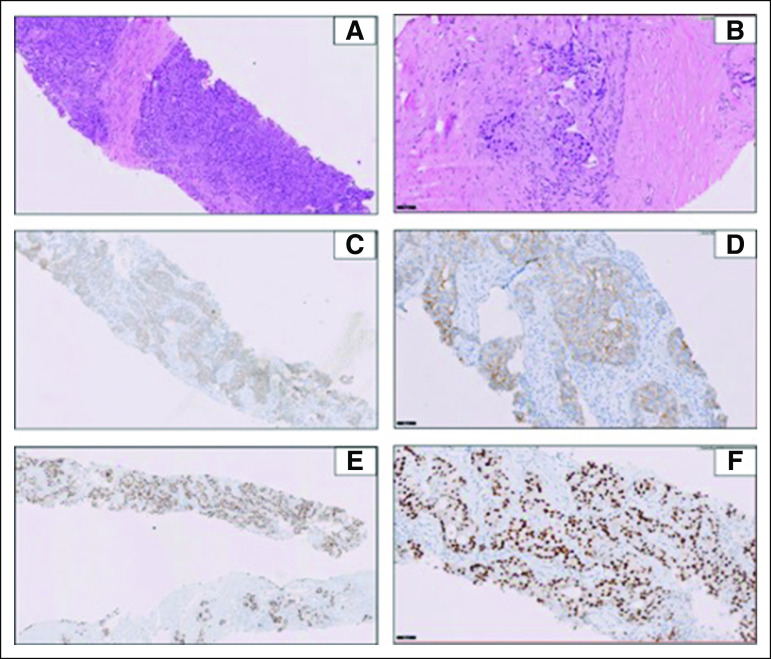
A 64-year-old gentleman, with history of hypothyroidism, presented with severe back pain and stiffness. Total spine magnetic resonance imaging with contrast showed marrow infiltration at C6, C7, D12, L1, L3, and S2 vertebrae without cord edema and a paravertebral soft tissue component. Multiple myeloma was ruled out by investigations. Whole-body positron emission tomography-computed tomography (PET-CT) scan showed a soft tissue mass involving superficial lobe of right parotid gland invading the temporalis and masseter muscles and multiple lymph nodes. Distant metastasis was reported as right upper-lobe lung nodules and multiple skeletal lesions. Biopsy of the right parotid mass showed human epidermal growth factor receptor 2 (HER2)-positive, AR-positive SDC by immunohistochemistry (Figs 1A-1F). HER2 amplification was confirmed by fluorescent in-situ hybridization. However, the tissue was insufficient for next-generation sequencing (NGS) testing. Biopsy of the sacral mass confirmed metastasis from a parotid SDC.
FIG 1.
(A) Histopathologic findings on hematoxylin and eosin (H&E) staining of the salivary ductal carcinoma case at diagnosis (5×). (B) H&E staining of the salivary ductal carcinoma case at diagnosis (20×). (C) Immunohistochemistry (IHC) analysis for human epidermal growth factor receptor 2 (HER2)-overexpression showing HER2-positive score 3+ complete membranous in more than 10% tumor at diagnosis (5×). (D) IHC analysis for HER2-overexpression showing HER2-positive tumor cells (20×). (E) Nuclear staining of androgen receptor (AR) showing strong positive AR expression in all tumor cells at diagnosis (5×). (F) Nuclear staining of AR showing strong positive AR expression in all tumor cells at diagnosis (20×).
In view of distant metastasis, the patient was not deemed fit for surgical intervention or definitive radiotherapy. Based on prior experience and published data by Limaye et al,5 the patient was started on systemic chemotherapy and targeted therapy with intravenous paclitaxel 175 mg/m2, carboplatin AUC5, and trastuzumab 8 mg/kg loading followed by 6 mg/kg q3weeks and zoledronic acid and calcium-vitamin D for bony involvement. The patient responded well to medical management of pain, and palliative radiation was not required. A reassessment scan after three cycles showed significant partial response and the same therapy was continued for six cycles followed by maintenance trastuzumab. However, after his fourth cycle of maintenance trastuzumab, a reassessment scan showed disease progression consistent with resistance to HER2-directed therapy.
To understand the cause of the newly developed HER2-targeted therapy resistance, a fresh biopsy and broad-panel NGS was performed to test for molecular alterations. The data analysis revealed genomic alterations (Table 1) including PIK3CA mutation (Fig 3A), which was considered targetable.
TABLE 1.
Summary of Molecular Analysis—Variants Identified Through NGS Analysis
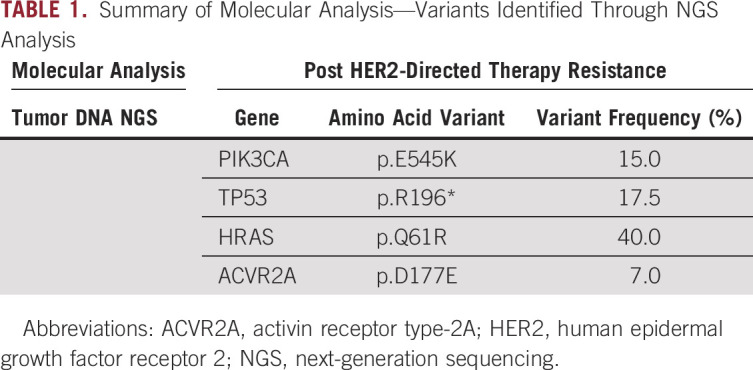
FIG 3.
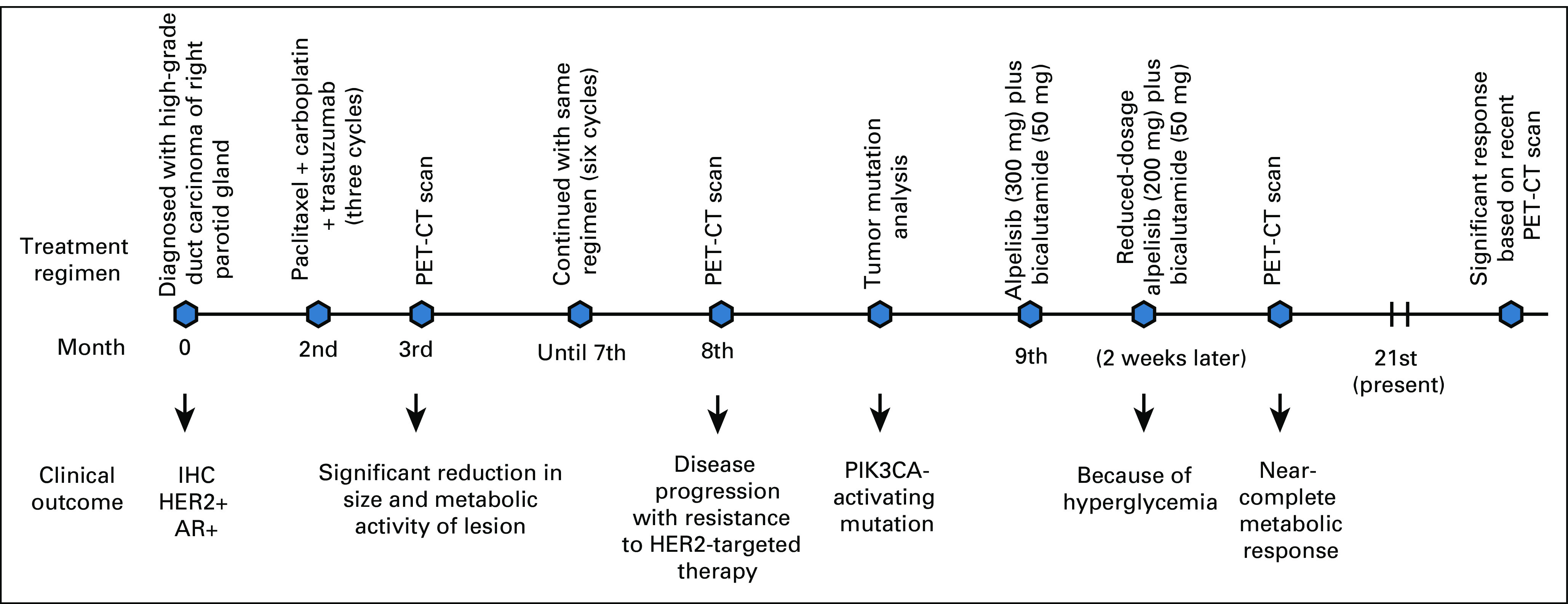
Depiction of treatment regimen and timeline of the patient with salivary duct carcinoma. AR, androgen receptor; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; PET-CT, positron emission tomography-computed tomography.
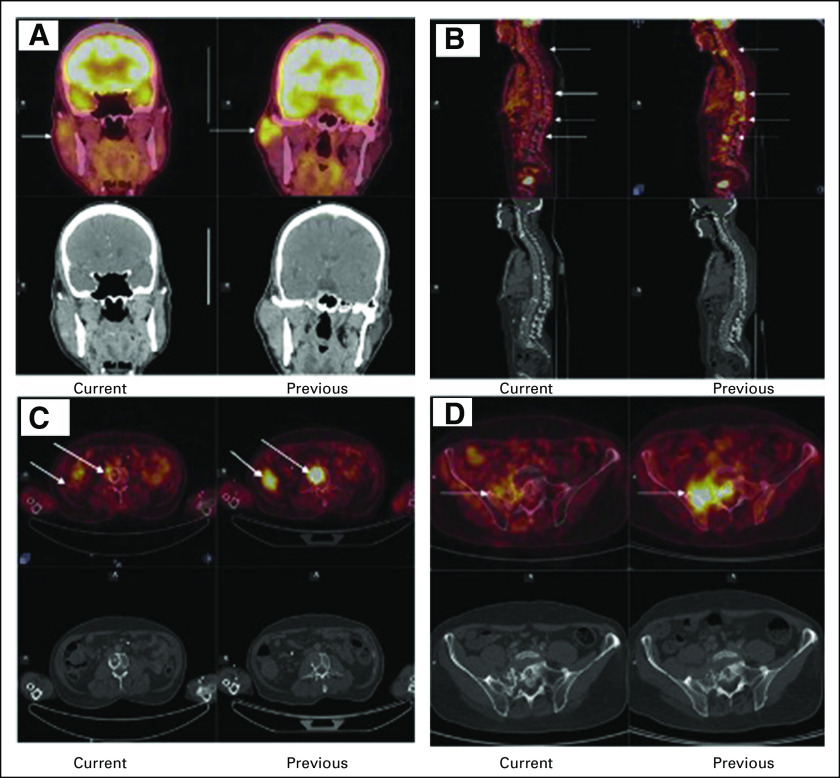
Since immunohistochemistry showed high expression of AR (Fig 1C), the patient was started on tab alpelisib 300 mg and tab bicalutamide 50 mg orally daily to target the PIK3CA and AR pathways, respectively. While on alpelisib, he developed hyperglycemia at 2 weeks of therapy requiring treatment with oral metformin and dose reduction of alpelisib from 300 mg to 250 mg and eventually to 200 mg orally daily, which was well tolerated. He has been continued on this regimen for the past 12 months, and subsequent scans have shown near-complete metabolic response to treatment with alpelisib and bicalutamide combination. Figures 2A-2C compare recent PET-CT reports with the ones done 8 months before. A timeline overview of the patient's management is summarized in Figure 3.
FIG 2.
Current and previous positron emission tomography-computed tomography scans demonstrating response to treatment from PIK3CA and androgen receptor inhibitors. (A) Arrows show near-complete metabolic resolution of the primary lesion when compared with prior scans. (B) Arrows show complete metabolic resolution of the vertebral metastasis when compared with prior scans. (C) Arrows show complete metabolic resolution of the lung and vertebral metastasis when compared with prior scans. (D) Arrows show near-complete metabolic resolution of the primary lesion when compared with prior scans.
To interrogate the PIK3CA mutation status at presentation, we located the original tissue block and performed NGS testing. The patient was found to be PIK3CA-mutated on droplet digital polymerase chain reaction (ddPCR) testing, which is plausibly one of the causative links to the HER2-directed therapy resistance.
This case report was approved by the institutional review board.
The patient has provided his consent for publishing his case in a journal, web site, or other forms of publication including images or other clinical information relating to his case. He understands that his name and initials will not be published and that his identity will not be disclosed.
DISCUSSION
SDC is an aggressive adenocarcinoma similar to high-grade ductal breast cancer.1 Local invasion may involve the extracranial portion of the facial nerve and the temporal bone via perineural spread. Distant metastases are seen in more than 50% cases with the most common being pulmonary, intracranial, bone and cutaneous sites.6 There are no established guidelines for the management of aSDC. Platinum-based anthracyclines and taxane-based chemotherapy regimens have been studied in aSDC with variable responses.3
Majority of SDC cases express AR (66.7%-96.4%) and/or HER2 (15%-44%), thus making HER2-directed therapy a viable treatment option. Studies have shown that trastuzumab with chemotherapy is superior to chemotherapy alone in the treatment of HER2-positive aSDC.5 In a single-arm phase II study, 57 patients with HER2-positive aSDC received trastuzumab and docetaxel7 with an overall response rate (ORR) of 70.2%, and median progression-free survival and overall survival of 8.9 and 39.7 months, respectively. These findings were further supported in a study by Hanna et al8 who compared the clinical outcomes of patients with aSDC receiving chemotherapy-trastuzumab combination to chemotherapy alone in an adjuvant setting along with concurrent radiation therapy with significant benefit in survival in the chemotherapy-trastuzumab arm. Li et al reported an ORR of 90% and minimal adverse events with ado-trastuzumab emtansine in 10 patients with HER2-amplified salivary gland carcinomas who had received a median of two lines of prior therapy (0-3).9
In AR-positive aSDC, androgen deprivation therapy has shown to have a better ORR and lower adverse events when compared with standard chemotherapy.11 A nationwide case series involving 35 patients with AR-positive aSDC treated with either monotherapy (luteinizing hormone-releasing hormone analogues or the AR antagonists: enzalutamide or bicalutamide) or combined androgen blockade (luteinizing hormone-releasing hormone analogue and bicalutamide) had a clinical benefit rate of 50%.10,12
Several genomic studies have reported somatic mutations associated with SDC in genes such as EGFR (20%), PDGFRA (27%), HRAS (27%), KIT (33%), PIK3CA (53%), and PTEN (53%),13 which has maximized the opportunities for potential targeted therapies. PIK3CA mutation is commonly described in several malignancies and activates PI3K-PTEN-AKT pathway causing oncogenic transformation independent of RAS or RAF mutation.14-18 Hyperactivity of PI3K signaling is associated with AKT activation leading to cell proliferation, resistance to apoptosis, and increased invasion.19 Mutations in E545 and H1047 have been linked to resistance to HER2-directed therapies in breast cancer.20
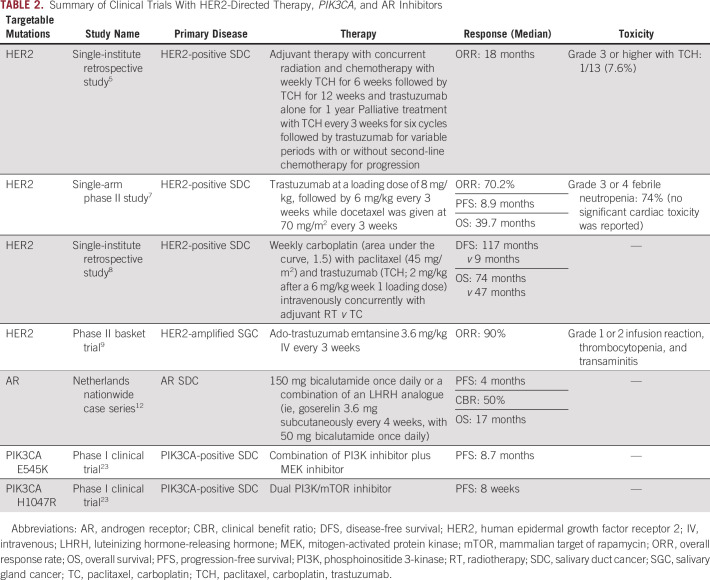
PI3K inhibitors can be broadly classified as dual PI3K and mammalian target of rapamycin (mTOR) inhibitors, pan-PI3K inhibitors, and isoform-specific inhibitors. Based on the positive results of SOLAR-1 trial, and improvement in median progression-free survival with alpelisib plus fulvestrant versus fulvestrant alone (11 v 5.7 months), alpelisib was US Food and Drug Administration–approved for hormone receptor-positive, HER2-negative, PIK3CA-mutated metastatic breast cancer (MBC) with progression on or after endocrine therapy.21 In a systemic review of targetable mutations in aSDC, two patients with PIK3CA mutation were treated with PI3K and mTOR inhibitor with limited benefit.22 Our decision to treat this case with alpelisib and endocrine therapy was based on the SOLAR-1 trial results. A summary of the clinical trials is included in Table 2.
TABLE 2.
Summary of Clinical Trials With HER2-Directed Therapy, PIK3CA, and AR Inhibitors
Studies conducted by Lehmann et al and Yadav et al demonstrated the additive effect of bicalutamide with either a pan-PI3K inhibitor or a dual PI3K and mTOR inhibitor on AR-positive triple-negative breast cancer and prostate cancer, respectively.23,24 Interestingly, a study involving AR-positive prostate cancer that focused on the role of glucocorticoid receptor expression in development of resistance to AR-inhibitor therapy demonstrated that by targeting PI3K and AKT pathway, there was increased canonical AR activity, decreased glucocorticoid receptor expression, and marked antitumor activity.25 Figure 4B depicts the rationale behind the synergy of PI3K and mTOR pathway inhibition and AR deregulation. Here, we present a case where a combination therapy with PIK3CA inhibitor alpelisib and AR inhibitor bicalutamide was considered as the most appropriate second-line therapy.
FIG 4.
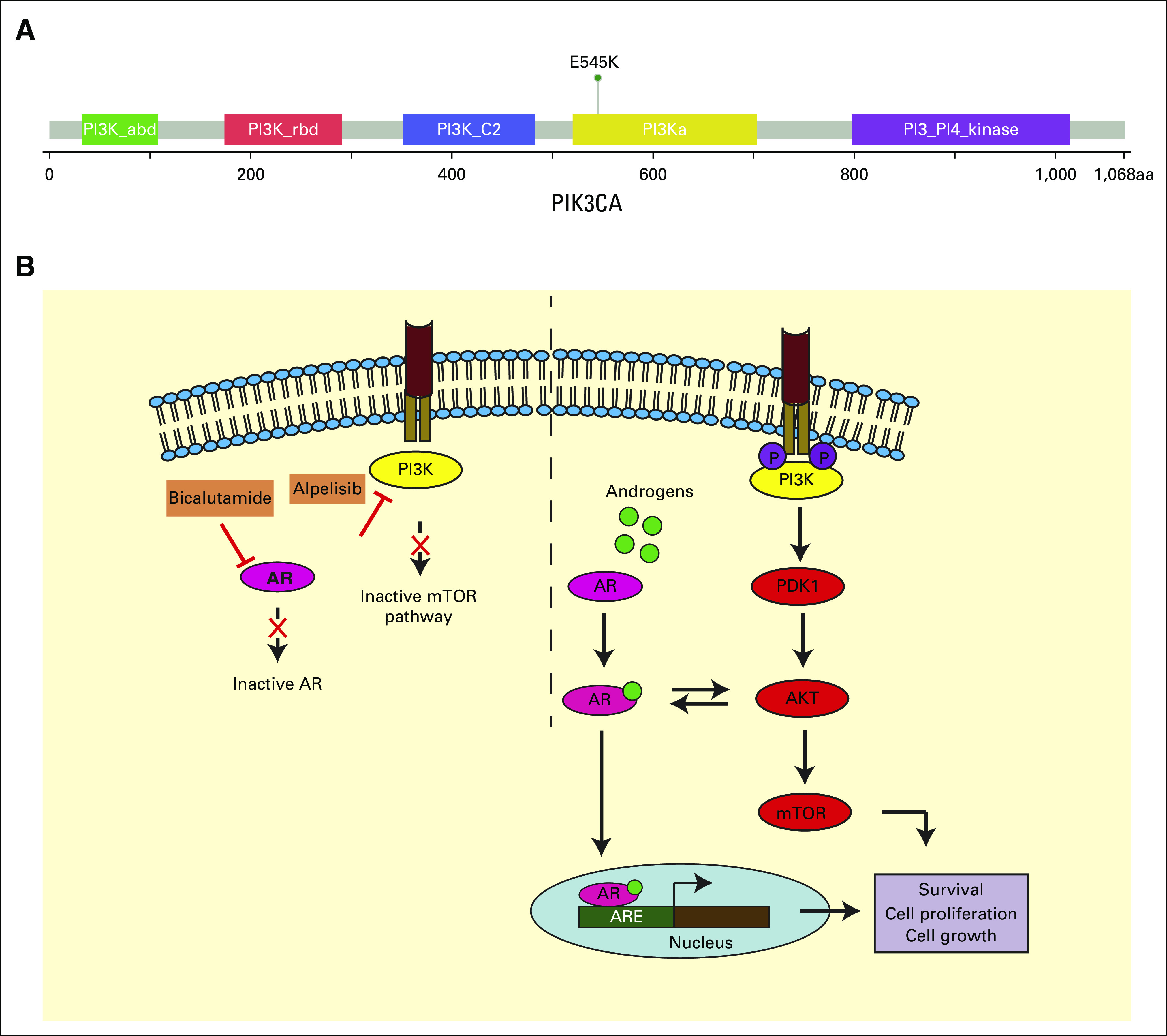
(A) Overview of PIK3CA gene with p.E545K domain-specific mutation. (B) A model of PI3K/mTOR pathway inhibition and AR deregulation in HER2-positive salivary duct carcinoma. AKT, XXX; AR, androgen receptor; ARE, androgen receptor element; CT, computed tomography; HER2, human epidermal growth factor receptor 2; mTOR, mammalian target of rapamycin; PDK1, 3 phosphoinositidine-dependent kinase 1; PET, positron emission tomography.
Initially, the patient responded well to chemotherapy and trastuzumab; however, he subsequently developed progression on maintenance trastuzumab indicating resistance to HER2-directed therapy. PIK3CA mutation has been linked to development of resistance in HER2-positive breast cancer and we believe the presence of PIK3CA mutation in this case led to the resistance to trastuzumab. Based on the available data with alpelisib and fulvestrant in MBC, we decided to treat with alpelisib and bicalutamide.19 However, one of the limitations of this study is that PIK3CA mutation analysis was unable to be performed at presentation. The other limitation is that the exact role of combination therapy versus a pure AR-based approach remains unclear. In their work on breast cancer cell lines, He et al26 have demonstrated the critical role of AR cross-talk with HER2 leading to HER2-directed therapy resistance. It was only upon progression on initial regimen that we evaluated the patient further for pathways of resistance.
To the best of our knowledge, this is the first PIK3CA-mutated, AR-overexpressed aSDC case to have excellent response to a combination therapy with alpelisib and bicalutamide in the setting of HER2-directed therapy resistance. The patient had near-complete metabolic response on follow-up PET-CT scan, which is sustained through the past several months. Further research along the lines of targeted therapies seems to be the way forward in terms of treatment options for aSDC.
Prashant Kumar
Employment: Datar Cancer Genetics
Research Funding: Datar Cancer Genetics
Patents, Royalties, Other Intellectual Property: Patent from Singapore for discovery of bladder cancer biomarker
Rajan Datar
Stock and Other Ownership Interests: Datar Cancer Genetics
Patents, Royalties, Other Intellectual Property: Multiple patents related to various technologies developed at Datar Cancer Genetics
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Hardik Sheth, Chetan Madre, Nevitha Athikari, Vishal Peshattiwar, Rajan Datar, Sewanti Limaye
Administrative support: Chetan Madre
Provision of study materials or patients: Ramya Pragya, Chetan Madre
Collection and assembly of data: Hardik Sheth, Ramya Pragya, Chetan Madre, Sewanti Limaye
Data analysis and interpretation: Hardik Sheth, Prashant Kumar, Aditya Shreenivas, Janani Sambath, Chetan Madre, Hemant Khandare, Vishal Peshattiwar, Rajan Datar, Sewanti Limaye
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Prashant Kumar
Employment: Datar Cancer Genetics
Research Funding: Datar Cancer Genetics
Patents, Royalties, Other Intellectual Property: Patent from Singapore for discovery of bladder cancer biomarker
Rajan Datar
Stock and Other Ownership Interests: Datar Cancer Genetics
Patents, Royalties, Other Intellectual Property: Multiple patents related to various technologies developed at Datar Cancer Genetics
No other potential conflicts of interest were reported.
REFERENCES
- 1.McHugh CH, Roberts DB, El-Naggar AK, et al. Prognostic factors in mucoepidermoid carcinoma of the salivary glands Cancer 1183928–39362012 [DOI] [PubMed] [Google Scholar]
- 2.Imaue S, Tomihara K, Hamashima T, et al. Successful multimodal treatment of intraoral salivary duct carcinoma in a patient with multiple lymph node metastases: A case report. World J Surg Oncol. 2017;15:18. doi: 10.1186/s12957-016-1090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellis GL.What's new in the AFIP fascicle on salivary gland tumors: A few highlights from the 4th Series Atlas Head Neck Pathol 3225–2302009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Surakanti SG, Agulnik M.Salivary gland malignancies: The role for chemotherapy and molecular targeted agents Semin Oncol 35309–3192008 [DOI] [PubMed] [Google Scholar]
- 5.Limaye SA, Posner MR, Krane JF, et al. Trastuzumab for the treatment of salivary duct carcinoma Oncologist 18294–3002013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston ML, Huang SH, Waldron JN, et al. Salivary duct carcinoma: Treatment, outcomes, and patterns of failure Head Neck 38E820–E8262016. suppl 1) [DOI] [PubMed] [Google Scholar]
- 7.Takahashi H, Tada Y, Saotome T, et al. Phase II trial of trastuzumab and docetaxel in patients with human epidermal growth factor receptor 2-positive salivary duct carcinoma J Clin Oncol 37125–1342019 [DOI] [PubMed] [Google Scholar]
- 8.Hanna GJ, Bae JE, Lorch JH, et al. The benefits of adjuvant trastuzumab for HER-2-positive salivary gland cancers Oncologist 25598–6082020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li BT, Shen R, Buonocore D, et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: Results From a phase II basket trial J Clin Oncol 36(24)2532–25372018. [Erratum: J Clin Oncol 37:362, 2019] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boon E, van Boxtel W, Buter J, et al. Androgen deprivation therapy for androgen receptor-positive advanced salivary duct carcinoma: A nationwide case series of 35 patients in The Netherlands Head Neck 40605–6132018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaspers HC, Verbist BM, Schoffelen R, et al. Androgen receptor-positive salivary duct carcinoma: A disease entity with promising new treatment options J Clin Oncol 29e473–e4762011 [DOI] [PubMed] [Google Scholar]
- 12.Uijen MJM, Lassche G, van Engen-van Grunsven ACH, et al. Systemic therapy in the management of recurrent or metastatic salivary duct carcinoma: A systematic review. Cancer Treat Rev. 2020;89:102069. doi: 10.1016/j.ctrv.2020.102069. [DOI] [PubMed] [Google Scholar]
- 13.Khoo TK, Yu B, Smith JA, et al. Somatic mutations in salivary duct carcinoma and potential therapeutic targets Oncotarget 875893–759032017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velho S, Oliveira C, Ferreira A, et al. The prevalence of PIK3CA mutations in gastric and colon cancer Eur J Cancer 411649–16542005 [DOI] [PubMed] [Google Scholar]
- 15.Spoerke JM, O'Brien C, Huw L, et al. Phosphoinositide 3-kinase (PI3K) pathway alterations are associated with histologic subtypes and are predictive of sensitivity to PI3K inhibitors in lung cancer preclinical models Clin Cancer Res 186771–67832012 [DOI] [PubMed] [Google Scholar]
- 16.Krakstad C, Birkeland E, Seidel D, et al. High-throughput mutation profiling of primary and metastatic endometrial cancers identifies KRAS, FGFR2 and PIK3CA to be frequently mutated. PLoS One. 2012;7:e52795. doi: 10.1371/journal.pone.0052795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo KT, Mao TL, Jones S, et al. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma Am J Pathol 1741597–16012009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kompier LC, Lurkin I, van der Aa MN, et al. FGFR3, HRAS, KRAS, NRAS and PIK3CA mutations in bladder cancer and their potential as biomarkers for surveillance and therapy. PLoS One. 2010;5:e13821. doi: 10.1371/journal.pone.0013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zardavas D, Phillips WA, Loi S. PIK3CA mutations in breast cancer: Reconciling findings from preclinical and clinical data. Breast Cancer Res. 2014;16:201. doi: 10.1186/bcr3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kataoka Y, Mukohara T, Shimada H, et al. Association between gain-of-function mutations in PIK3CA and resistance to HER2-targeted agents in HER2-amplified breast cancer cell lines Ann Oncol 21255–2622010 [DOI] [PubMed] [Google Scholar]
- 21.Andre F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer N Engl J Med 3801929–19402019 [DOI] [PubMed] [Google Scholar]
- 22.Nardi V, Sadow PM, Juric D, et al. Detection of novel actionable genetic changes in salivary duct carcinoma helps direct patient treatment Clin Cancer Res 19480–4902013 [DOI] [PubMed] [Google Scholar]
- 23.Lehmann BD, Bauer JA, Schafer JM, et al. PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res. 2014;16:406. doi: 10.1186/s13058-014-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yadav SS, Li J, Stockert JA, et al. Combination effect of therapies targeting the PI3K- and AR-signaling pathways in prostate cancer Oncotarget 776181–791962016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adelaiye-Ogala R, Gryder BE, Nguyen YTM, et al. Targeting the PI3K/AKT pathway overcomes enzalutamide resistance by inhibiting induction of the glucocorticoid receptor Mol Cancer Ther 191436–14472020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He L, Du Z, Xiong X, et al. Targeting androgen receptor in treating HER2 positive breast cancer. Sci Rep. 2017;7:14584. doi: 10.1038/s41598-017-14607-2. [DOI] [PMC free article] [PubMed] [Google Scholar]