INTRODUCTION
Activating RET gene fusions have been reported in < 10% of papillary thyroid cancers and in 1%-2% of non–small-cell lung cancers.1-7 In a large-scale genomic profiling study, RET gene fusions were identified in only 16 of 9,693 (0.17%) patients with breast cancer.8 Selpercatinib (LOXO-292) is a highly selective and potent, CNS-penetrant RET inhibitor that has demonstrated significant antitumor activity with a tolerable safety profile in patients with solid tumors harboring diverse RET alterations (eg, activating gene fusions, point mutations, and indels) in the ongoing registrational LIBRETTO-001 study (ClinicalTrials.gov identifier: NCT03157128).9,10 These results led to the recent approval by the US Food and Drug Administration (FDA) for the treatment of metastatic RET fusion–positive non–small-cell lung cancer and advanced or metastatic RET-mutant medullary thyroid and RET fusion–positive thyroid cancers.11 Here, we describe the first patient with RET fusion–positive breast cancer treated with selpercatinib in LIBRETTO-001.
CASE REPORT
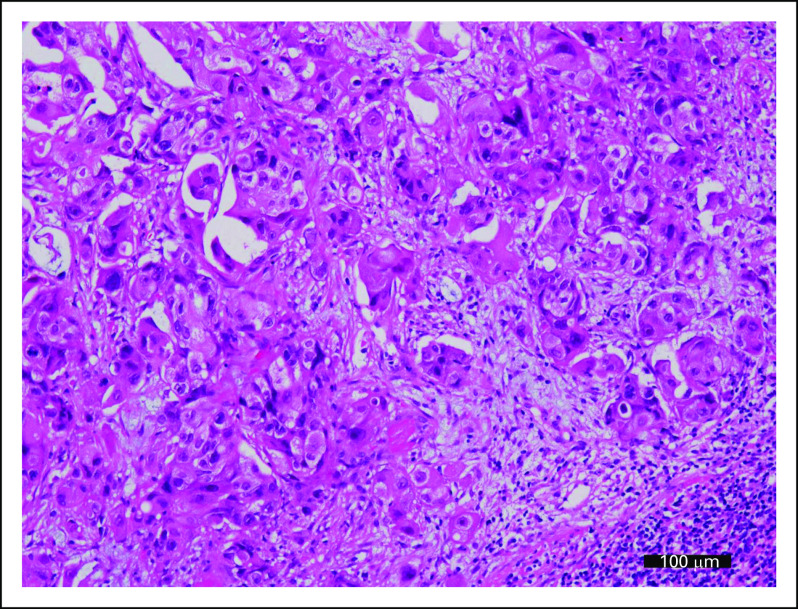
A 46-year-old premenopausal Japanese woman was referred to Kindai University Hospital with fluorodeoxyglucose-avid right axillary, right neck, and mediastinal lymphadenopathy on positron emission tomography-computed tomography (PET-CT) imaging on day 0. Ultrasound imaging identified a hypoechoic nodule in the right breast; right axillary lymph node fine-needle aspiration biopsy performed at previous hospital on day 13 revealed invasive carcinoma with a focal micropapillary pattern (Fig 1). Immunostaining of estrogen receptor (ER) and progesterone receptor (PgR) was evaluated by using the Allred score and the Allred scores were positive (proportion score [PS] 1 (< 1%), intensity score [IS] 2) and negative (PS 0, IS 0), respectively.12,13 The tumor was human epidermal growth factor 2 (HER2)–negative (immunohistochemistry [IHC] 0). Given these results, she was diagnosed with stage IV breast cancer.
FIG 1.
The biopsy of the right axial lymph node revealed invasive carcinoma with focal micropapillary pattern.
Targeted next-generation sequencing (NGS) analysis using the FoundationOne Companion Diagnostic panel (Foundation Medicine, Cambridge, MA) was performed on the right axillary lymph node specimen. The result of NGS was reported on day 58, and the NGS identified a CCDC6-RET fusion (C1; R12) with no other reported genomic alterations known to contribute to human breast tumorigenesis, including none in BRCA1 or BRCA2. CTNNB1 M739I, KEL R14H, MET L211W, and MTOR R2110Q were detected as variants of unknown significance in the patient’s tumor. Consistent with local standard-of-care guidelines, she received treatment with tamoxifen plus goserelin from day 14 to day 91, but these were discontinued due to progression in the right breast and new lesions detected in the left lower lung. Rebiopsy of the right breast tumor revealed the following results: ER Allred score 2 (PS 1 [< 1%], IS 1), PgR Allred score 2 (PS 1, IS 1), HER2 IHC 2+, HER2 fluorescence in situ hybridization negative (HER2/HER2/CEP(centromere)17 = 0.9), and programmed death ligand 1 (SP142) expression on tumor-infiltrating immune cells of 1%-4%. On day 126, she was started on treatment with selpercatinib at the recommended phase 2 dose of 160 mg twice daily in the LIBRETTO-001 study after providing written informed consent from the patient to publish information and images. She experienced rapid clinical improvement with a resolution of right breast and neck pain and erythema. Carcinoembryonic antigen levels rapidly decreased (Fig 2A), and spiral CT imaging on day 147 demonstrated a partial response by using RECIST version 1.1 (RECIST 1.1) (overall tumor reduction −30%), with a reduction in multiple right breast masses, axillary, neck and mediastinal lymphadenopathy, and left lung metastases; follow-up CT scan repeated on day 231 revealed a complete tumor response by using RECIST 1.1 (Fig 2D). At the time of this writing, she remains in complete response and on treatment for > 300 days, with all adverse event grades 1-2 (dry skin, dry mouth, weight gain, transaminitis, and blood bilirubin increased). Most adverse events recovered to baseline with medical management, and none required dose interruption or modification.
FIG 2.
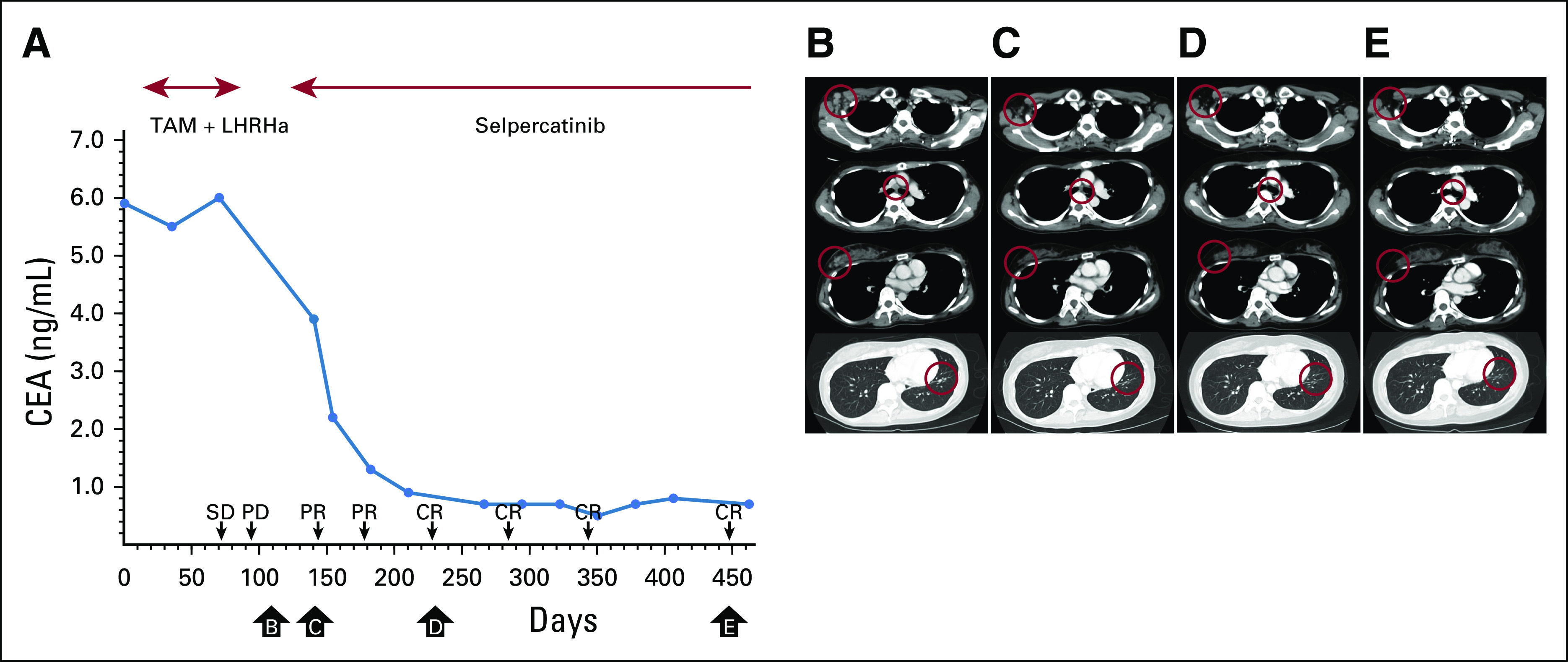
Serial monitoring of CEA and the results of response evaluation using RECIST version 1.1 (A). Each arrow (B-E) corresponds with CT imaging performed during the treatment. (B) Baseline CT scan revealed multiple right axial and mediastinal lymph node metastases, metastases in the right breast, and left lung metastases. (C) First response assessment at 21 days after treatment initiation revealed shrinkage of all the metastases, which was evaluated as partial response. (D) Repeat imaging after 3 months of the treatment showed CR. (E) The most recent CT scan revealed that the patient remains in CR. CEA, carcinoembryonic antigen; CR, complete response; CT, computed tomography; LHRHa, luteinizing hormone-releasing hormone agonist; PD, progressive disease; PR, partial response; TAM, tamoxifen.
DISCUSSION
Selpercatinib is a first-in-class selective RET inhibitor that recently received US FDA approval for the treatment of metastatic RET fusion–positive non–small-cell lung cancer and advanced or metastatic RET-altered thyroid cancers.9-11 However, for patients with RET fusion–positive breast cancer, standard of care is currently limited to hormonal therapy, chemotherapy, and anti-HER2–targeted therapies based on hormone receptor and HER2 status.
Sorafenib and vandetanib, multikinase inhibitors with preclinical inhibitory activity against RET, have been used to treat unselected patients with breast cancer, but minimal clinical activity was observed.14,15 A patient with NCOA4-RET–positive breast cancer experienced a partial response to the multikinase inhibitor cabozantinib in combination with trastuzumab and exemestane although the cabozantinib dose was reduced for toxicity, the total time on treatment was short, and the relative contribution of each agent to the overall antitumor activity was not known.8 In addition, although cabozantinib has preclinical inhibitory activity against RET, its much stronger inhibition of other kinases (eg, VEGFR2) likely accounts for its clinical activity.16,17 In contrast, in the current case, the highly selective and potent RET inhibitor selpercatinib demonstrated a durable single-agent response in a patient with RET fusion–positive breast cancer.
To our knowledge, this is the first report of a breast cancer patient with a complete and sustained response to selective, RET-targeted therapy and adds to the diversity of RET fusion–positive tumor types that may benefit from selective RET inhibition. LIBRETTO-001 continues to enroll patients with RET fusion–positive solid tumors, including breast cancer. Additionally, broad-based genomic profiling in patients with refractory breast cancer should be considered to identify potentially actionable alterations such as RET gene fusions. Continued characterization of the overall frequency of RET fusions in breast cancer and other solid tumors is warranted.
PRIOR PRESENTATION
Presented in part at the American Association for Cancer Research, Virtual Meeting, June 22-24, 2020.
SUPPORT
Supported by Loxo Oncology, Inc., a wholly owned subsidiary of Eli Lilly and Company.
AUTHOR CONTRIBUTIONS
Conception and design: Satomi Watanabe, Masayuki Takeda, Elizabeth Olek, Akihiko Ito, S. Rothenberg, Kazuhiko Nakagawa
Financial support: Elizabeth Olek
Administrative support: Elizabeth Olek
Provision of study materials or patients: Elizabeth Olek
Collection and assembly of data: Satomi Watanabe, Tomoyuki Otani, Takeshi Yoshida, Kazuko Sakai, Elizabeth Olek, S. Michael Rothenberg, Jennifer Kherani
Data analysis and interpretation: Satomi Watanabe, Elizabeth Olek, S. Michael Rothenberg, Pearl French, Kazuto Nishio, Kazuhiko Nakagawa
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Masayuki Takeda
Honoraria: AstraZeneca KK, Chugai Pharma, Bristol-Myers Squibb, Novartis, Ono Pharmaceutical
Kazuko Sakai
Honoraria: Roche Diagnostics, Bio-Rad, AstraZeneca, Chugai Pharma
Elizabeth Olek
Employment: Loxo Oncology Inc
Stock and Other Ownership Interests: Loxo Oncology, Inc
Travel, Accommodations, Expenses: Loxo Oncology, Inc
S. Michael Rothenberg
Employment: Loxo, Pfizer
Stock and Other Ownership Interests: Loxo, Pfizer
Jennifer Kherani
Employment: Loxo Oncology at Lilly, Ajax Health, Aisling Capital, Summus Global
Leadership: Ajax Health and Aisling Capital
Stock and Other Ownership Interests: Ajax Health and Aisling Capital
Consulting or Advisory Role: Ajax Health and Aisling Capital
Travel, Accommodations, Expenses: Ajax Health and Aisling Capital, Loxo
Pearl P. French
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Travel, Accommodations, Expenses: Lilly
Kazuto Nishio
Speakers' Bureau: Boehringer Ingelheim, AstraZeneca KK, Chugai Pharma, Novartis, Eisai, Otsuka, Merck Sharp & Dohme, Bristol-Myers Squibb, Ono Pharmaceutical, SymBio Pharmaceuticals, Pfizer, Sanofi, Guardant Health, Lilly, Solasia Pharma KK
Research Funding: Nippon Boehringer Ingelheim Co, Ltd, Ignyta, Inc, Korea Otsuka Pharmaceutical Co, Ltd, Lilly, Thoracic Oncology Research Group, North East Japan Study Group
Kazuhiko Nakagawa
Honoraria: Astellas Pharma, AstraZeneca KK, Ono Pharmaceutical, Daiichi Sankyo, Chugai Pharma, Nippon Boehringer Ingelheim, Lilly, Pfizer, Bristol-Myers Squibb, Novartis, CareNet, Inc, Nichi-Iko Pharmaceutical Co, Ltd, Taiho Pharmaceutical, Nanzando Co, Ltd, Yodosha CO, Ltd, Nikkei Business Publications, Inc, KYORIN Pharmaceutical Co, Ltd, Hisamitsu Pharmaceutical, Medicus Shuppan, Publishers Co, Ltd, Takeda Pharmaceutical Co, Ltd, Thermo Fisher Scientific KK, Medical Review Co, Ltd, Yomiuri Telecasting Corporation, MSD K.K, Abbvie, Kyorin Pharmaceutial.Co, Ltd, Merk Biopharma Co., Ltd., Roche Diagnosics KK, Medicus Shuppan, Publishers Co, Ltd, Bayer Yakuhin, Nippon Kayaku
Consulting or Advisory Role: Pfizer, Takeda Pharmaceutical Co, Ltd, KYORIN Pharmaceutical Co, Ltd, Lilly, Ono Pharmaceutical
Research Funding: Chugai Pharma, Ono Pharmaceutical, Daiichi Sankyo, Eisai, Pfizer, Takeda, Nippon Boehringer Ingelheim, Taiho Pharmaceutical, Bristol-Myers Squibb, Abbvie, ICON Japan KK, Lilly, Parexel International Corp, A2 Healthcare Corp, Astellas Pharma, Novartis, Iqvia Services Japan KK, SymBio Pharmaceuticals, Merck Serono, AstraZeneca KK, CMIC Shift Zero KK, Kissei Pharmaceutical Co, Ltd, Kyowa Hakko Kirin, EPS Corporation, Bayer Yakuhin, MSD KK, Pfizer R & D Japan GK, Otsuka, Syneos Health, EPS International Co, Ltd.
No other potential conflicts of interest were reported.
REFERENCES
- 1.Integrated genomic characterization of papillary thyroid carcinoma Cell 159676–6902014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Comprehensive molecular profiling of lung adenocarcinoma Nature 511543–5502014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshihara K, Wang Q, Torres-Garcia W, et al. The landscape and therapeutic relevance of cancer-associated transcript fusions Oncogene 344845–48542015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stransky N, Cerami E, Schalm S, et al. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohno T, Ichikawa H, Totoki Y, et al. KIF5B-RET fusions in lung adenocarcinoma Nat Med 18375–3772012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer Nat Med 18378–3812012 [DOI] [PubMed] [Google Scholar]
- 7.Lipson D, Capelletti M, Yelensky R, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies Nat Med 18382–3842012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paratala BS, Chung JH, Williams CB, et al. RET rearrangements are actionable alterations in breast cancer. Nat Commun. 2018;9:4821. doi: 10.1038/s41467-018-07341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drilon A, Oxnard GR, Tan DSW, et al. Efficacy of selpercatinib in RET fusion-positive non-small-cell lung cancer N Engl J Med 383(9)813–8242020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wirth LJ, Sherman E, Robinson B, et al. Efficacy of selpercatinib in RET-altered thyroid cancers N Engl J Med 383(9)825–8352020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Retevmo™ (selpercatinib) prescribing information; May, 2020. https://uspl.lilly.com/retevmo/retevmo.html#pi [Google Scholar]
- 12.Allred DC, Harvey JM, Berardo M, et al. Prognostic and predictive factors in breast cancer by immunohistochemical analysis Mod Pathol 11155–1681998 [PubMed] [Google Scholar]
- 13.Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and progesterone receptor testing in breast cancer: American society of clinical oncology/college of American pathologists guideline update. Arch Pathol Lab Med. 2020 doi: 10.5858/arpa.2019-0904-SA. [DOI] [PubMed] [Google Scholar]
- 14.Miller KD, Trigo JM, Wheeler C, et al. A multicenter phase II trial of ZD6474, a vascular endothelial growth factor receptor-2 and epidermal growth factor receptor tyrosine kinase inhibitor, in patients with previously treated metastatic breast cancer Clin Cancer Res 113369–33762005 [DOI] [PubMed] [Google Scholar]
- 15.Bronte G, Andreis D, Bravaccini S, et al: Sorafenib for the treatment of breast cancer Expert Opin Pharmacother 18621–6302017 [DOI] [PubMed] [Google Scholar]
- 16.Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: An open-label, single-centre, phase 2, single-arm trial Lancet Oncol 171653–16602016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlumberger M, Elisei R, Müller S, et al. Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma Ann Oncol 282813–28192017 [DOI] [PMC free article] [PubMed] [Google Scholar]