Abstract
PURPOSE
Current guidelines for postoperative management of patients with stage I-IIA cutaneous melanoma (CM) do not recommend routine cross-sectional imaging, yet many of these patients develop metastases. Methods that complement American Joint Committee on Cancer (AJCC) staging are needed to improve identification and treatment of these patients. A 31-gene expression profile (31-GEP) test predicts metastatic risk as low (class 1) or high (class 2). Prospective analysis of CM outcomes was performed to test the hypotheses that the 31-GEP provides prognostic value for patients with stage I-III CM, and that patients with stage I-IIA melanoma and class 2 31-GEP results have metastatic risk similar to patients for whom surveillance is recommended.
MATERIALS AND METHODS
Two multicenter registry studies, INTEGRATE (ClinicalTrials.gov identifier:NCT02355574) and EXPAND (ClinicalTrials.gov identifier:NCT02355587), were initiated under institutional review board approval, and 323 patients with stage I-III CM and median follow-up time of 3.2 years met inclusion criteria. Primary end points were 3-year recurrence-free survival (RFS), distant metastasis-free survival (DMFS), and overall survival (OS).
RESULTS
The 31-GEP was significant for RFS, DMFS, and OS in a univariate analysis and was a significant, independent predictor of RFS, DMFS, and OS in a multivariable analysis. GEP class 2 results were significantly associated with lower 3-year RFS, DMFS, and OS in all patients and those with stage I-IIA disease. Patients with stage I-IIA CM and a class 2 result had recurrence, distant metastasis, and death rates similar to patients with stage IIB-III CM. Combining 31-GEP results and AJCC staging enhanced sensitivity over each approach alone.
CONCLUSION
These data provide a rationale for using the 31-GEP along with AJCC staging, and suggest that patients with stage I-IIA CM and a class 2 31-GEP signature may be candidates for more intense follow-up.
INTRODUCTION
Cutaneous melanoma (CM) has one of the fastest rising incidence rates of cancers in the United States.1 National Comprehensive Cancer Network guidelines recommend clinical management decisions be based on an individual patient’s recurrence risk, including decisions for a sentinel lymph node biopsy (SLNB), metastatic surveillance, referral to oncology, adjuvant therapy, and clinical trial participation. Moreover, these guidelines indicate that gene expression profiling (GEP) can provide recurrence risk information as an adjunct to staging.2 Although approximately 70% of patients with CM are diagnosed with stage I-II disease, this group contributes the most deaths in patients with melanoma without distant metastasis at diagnosis.3,4 Thus, relying upon traditional staging factors, including Breslow thickness, presence of ulceration, and sentinel lymph node (SLN) involvement is insufficient to identify all patients at risk for metastasis and death. Therefore, improved prognostication is needed to reduce treatment in patients less likely to progress and escalate treatment for patients more likely to progress.
CONTEXT
Key Objective
Can the 31-gene expression profile (31-GEP), which assesses the primary tumor's molecular biology, add prognostic value to relevant clinicopathologic features? This work builds upon previous studies that identified the prognostic relevance of the 31-GEP and assesses the potential of the 31-GEP to complement the American Joint Committee on Cancer staging in the context of national guidelines for melanoma surveillance.
Knowledge Generated
We showed that the 31-GEP is a significant, independent prognostic factor for 3-year recurrence-free survival, distant metastasis-free survival, and overall survival in a prospectively enrolled cohort of patients diagnosed with stage I-III melanoma. Moreover, patients diagnosed with stage I-IIA melanoma who receive a high-risk 31-GEP result (class 2) have survival outcomes like those with stage IIB-III melanoma, for whom national guidelines recommend more intense follow-up.
Relevance
31-GEP testing can refine American Joint Committee on Cancer patient risk assessment, identifying those who may benefit from more or less intense follow-up.
Recently, tumor tissue–based GEP has refined clinicopathologic prognosis and helped inform the treatment of solid tumors. GEP tests for uveal and breast cancer have demonstrated clinical utility in avoiding unnecessary surgical intervention in low-risk patients and are often used in staging.5,6 A 21-GEP test identifies patients with estrogen receptor-positive human epidermal growth factor receptor 2-negative breast cancer with low risk for recurrence who could avoid chemotherapy.7,8 The prognostic accuracy of this breast cancer GEP led to its incorporation into American Joint Committee on Cancer 8th edition staging guidelines (AJCC 8th ed).9,10 In uveal melanoma, a prognostic 15-GEP test identifies metastatic risk, helping to avoid high-intensity surveillance for low-risk patients and targeting frequent surveillance and adjuvant therapy consideration to high-risk patients,11,12 and is now recommended by AJCC and National Comprehensive Cancer Network guidelines.13,14
A 31-GEP test (DecisionDx-Melanoma) was developed and validated to assess tumor biology–based CM risk.15 The test evaluates 28 discriminating genes and 3 control genes from formalin-fixed, paraffin-embedded tissue to stratify patient risk into class 1, including class 1A (lowest risk) and class 1B (low risk); or class 2, including class 2A (increased risk) and class 2B (highest risk). Eight peer-reviewed studies have shown that the 31-GEP is an independent, significant predictor of disease progression.13-21 However, current staging systems and guidelines do not recommend its use. We used clinical outcomes data for patients with stage I-III CM from two prospective registry studies to evaluate the hypotheses that the 31-GEP test has independent prognostic value, and that patients with stage I-IIA melanoma and a 31-GEP class 2 result have metastatic risk similar to patients with stage IIB-III disease.
MATERIALS AND METHODS
Study Design and Enrollment
The study design and methods have been previously described.17 Patients were enrolled in one of two prospective studies, EXPAND and INTEGRATE (ClinicalTrials.gov identifiers:NCT02355587 and NCT02355574), which have the same enrollment criteria and aims but differ in the extent of data collection to accommodate private and academic center resources. A combined analysis of the studies was preplanned.
Eleven US dermatologic and surgical centers participated after receiving institutional review board approval of the protocol. Patients ≥ 16 years of age diagnosed with stage I-III CM who had successful 31-GEP testing and no prior history of melanoma were consented and enrolled between 2013 and 2019. These registry studies were designed to enroll 6,672 patients to account for inclusion that satisfied all primary objectives, including comparison of class 1 and class 2 risk profiles. The primary end point of risk stratification was met after enrollment of 334 patients. Other primary end points remain to be assessed. The 31-GEP test was performed in a CAP- and CLIA-accredited laboratory using published protocols.15
Each patient’s clinicopathologic data, as defined by the AJCC 8th ed were entered into a secure case report form, and clinical outcomes were collected and entered at 6-month intervals. The last censor date for clinical data was December 15, 2018. Cases were excluded if they did not have at least one follow-up visit, if multiple primary melanomas or distant metastasis were present at diagnosis, or if the patient’s outcome was unverifiable by the enrolling center (Fig 1).
FIG 1.
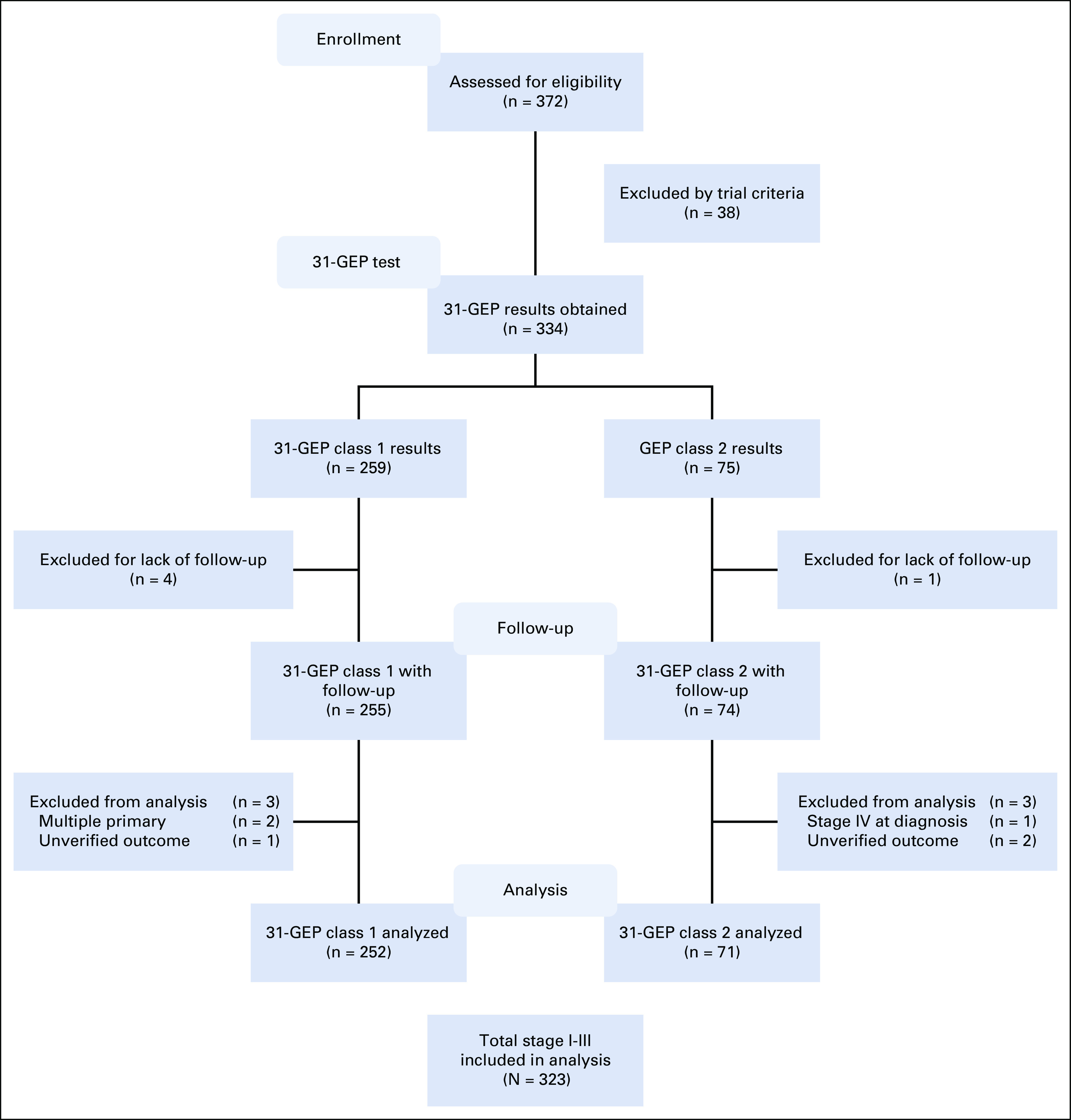
Study design for INTEGRATE and EXPAND registries. Enrollment and eligibility of 372 consecutively tested patients with 31-GEP in INTEGRATE and EXPAND registries. Inclusion criteria for enrollment were ≥ 16 years of age and no prior history of cancer. Exclusion criteria for analysis were < 1 follow-up visit, multiple primary melanomas present at diagnosis, distant metastasis present at diagnosis, or patients with unverifiable outcomes. GEP, gene expression profile.
Statistical Analyses
Survival end points of 3-year recurrence-free survival (RFS, time from diagnosis to regional or distant metastasis), distant metastasis-free survival (DMFS, time from diagnosis to any metastatic event beyond the regional nodal basin), and overall survival (OS, time from diagnosis to documented death of any cause) were assessed using Kaplan-Meier analysis with logrank test. Univariate Cox regression analysis was used to determine significant variables from continuous mitotic rate, age, and categorical sex, tumor location, AJCC stage, and GEP result. Multivariable Cox regression analysis was used to compare the prognostic impact of the 31-GEP relative to other significant (P < .01) variables identified from the univariate analysis for RFS, DMFS, and OS. Prognostic accuracy of the 31-GEP test and AJCC staging was measured by sensitivity, specificity, positive predictive value, and negative predictive value (NPV).
As this analysis follows an interim analysis, to reduce the chance of a type I error, the critical α level was established at .04 because an α level of .01 was already spent on the interim analysis, summing to the traditional .05.17,22 Statistical associations were evaluated using Wilcoxon and Pearson's chi-squared tests where appropriate. Statistical analyses were performed in R version 3.6.3 (University of Auckland, Auckland, New Zealand) according to prespecified analysis plans.
RESULTS
Patient Demographics
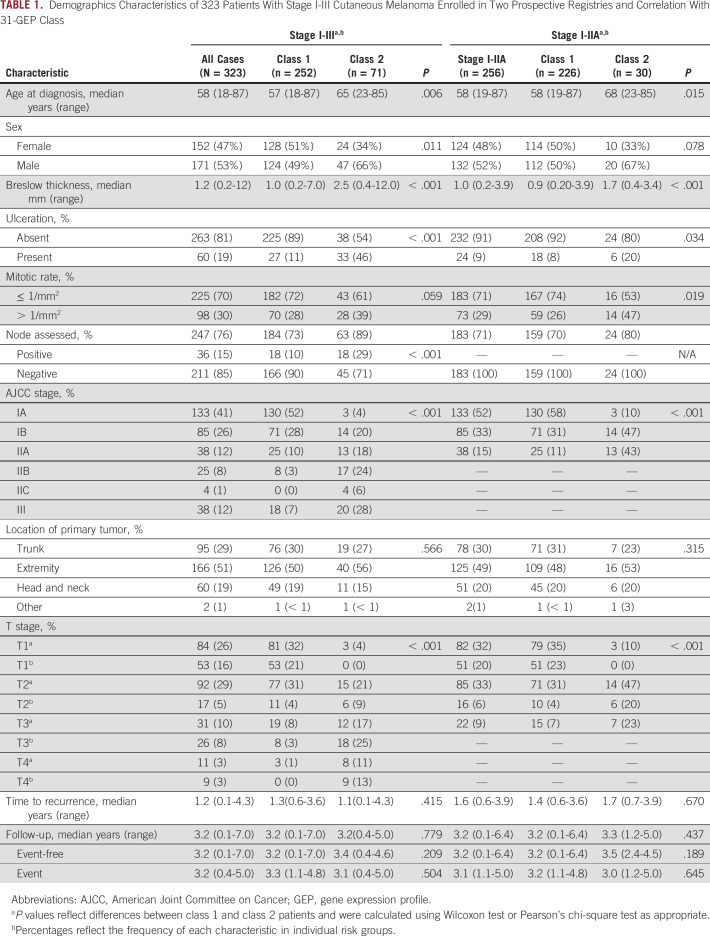
A total of 372 patients were assessed for eligibility, and 334 who met enrollment criteria and had 31-GEP test results were enrolled. Eleven patients were excluded from the analysis, leaving 323 patients who met enrollment and analysis inclusion criteria (Fig 1). Median age at diagnosis was 58 (range, 18-87) years with a median follow-up of 3.2 years (range, 0.1-7.0 years; Table 1). Median follow-up time was 3.2 years in patients without an event and was similar between class 1 and class 2 patients (3.2 [range, 0.1-7.0] years and 3.4 [range, 0.4-4.6] years, respectively; P = .209). For patients who experienced an event, the median follow-up time was 3.2 years overall, 3.3 (range, 1.1-4.8) years for class 1, and 3.1 (range, 0.4-5.0) years for class 2 (P = .504). Class 2 tumors were associated with increased high-risk clinicopathologic features compared with class 1 tumors, including significantly increased age (P = .006), proportion of males (P = .011), median Breslow thickness (P < .001), presence of ulceration (P < .001), and proportion with positive SLN (P < .001; Table 1). Of all patients at diagnosis, 79% (256) were stage I-IIA, 76% (246) had T1-T2 melanomas, 81% (263) did not exhibit ulceration, and 85% (211/247) were pathologically SLN-negative, indicating that most patients in this cohort would be considered low risk and to have good prognosis according to traditional clinicopathologic risk factors (Table 1).
TABLE 1.
Demographics Characteristics of 323 Patients With Stage I-III Cutaneous Melanoma Enrolled in Two Prospective Registries and Correlation With 31-GEP Class
3-Year Clinical Outcomes in the Stage I-III Population
To determine the utility of the 31-GEP test in predicting CM progression risk, we compared 3-year RFS, DMFS, and OS of patients with class 1 (n = 252) and class 2 (n = 71) disease. Patients with a class 2 result had significantly lower 3-year RFS (66% [95% CI, 56 to 78] v 95% [95% CI, 92 to 98], P < .0001), DMFS (79% [95% CI, 70 to 90] v 97% [95% CI, 94 to 99], P < .0001), and OS (81% [95% CI, 72 to 91] v 97% [95% CI, 95 to 100], P < .0001; Fig 2) than patients with a class 1 result. Furthermore, the outcomes were analyzed by 31-GEP subclass (class 1A, class 1B, class 2A, or class 2B), defined by the linear probability score as previously described.23 The highest-risk (class 2B) designation had significantly lower 3-year RFS (60% [95% CI, 47 to 76] v 96% [95% CI, 93 to 99], P < .0001), DMFS (78% [95% CI, 67 to 91] v 98% [95% CI, 96 to 100], P < .0001), and OS (74% [95% CI, 62 to 88] v 98% [95% CI, 97 to 100], P < .0001) than the lowest-risk (class 1A) designation. Patients with intermediate-risk (class 1B or 2A) 31-GEP results had intermediate survival rates (Appendix Fig A1). For nine of the 25 deaths in this cohort, distant metastasis was observed, but melanoma was not specified as the cause of death. Seven of those nine cases (78%) had a class 2 result (6/7 were class 2B). These results are similar to all-cause mortality rates in which 68% (17/25) of deaths had class 2 biology (15/17 were class 2B).
FIG 2.
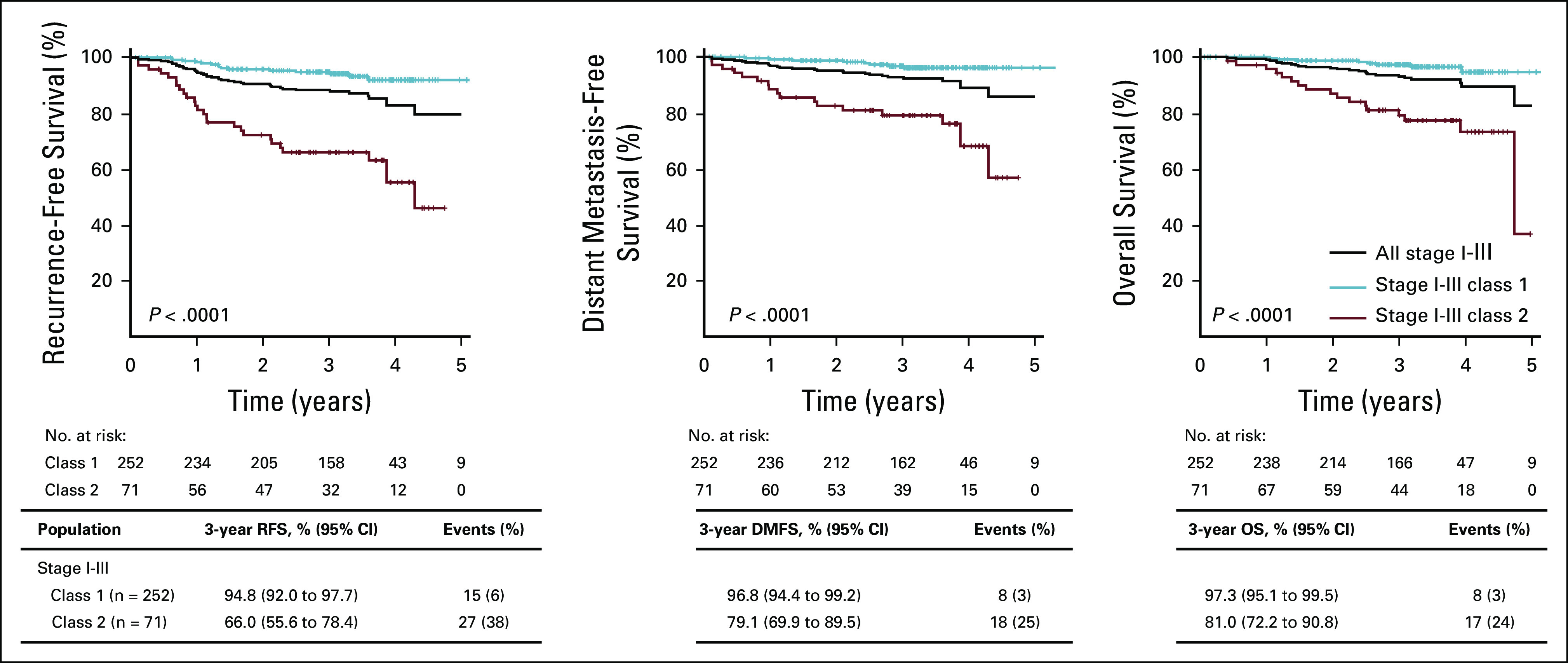
Survival outcomes of patients with stage I-III CM by 31-GEP results. RFS, DMFS, and OS were estimated by Kaplan-Meier analysis and P values determined by logrank test. Tables beneath the graphs show survival rates and number of events in each GEP class. CM, cutaneous melanoma; DMFS, distant metastasis-free survival; GEP, gene expression profile; OS, overall survival; RFS, recurrence-free survival.
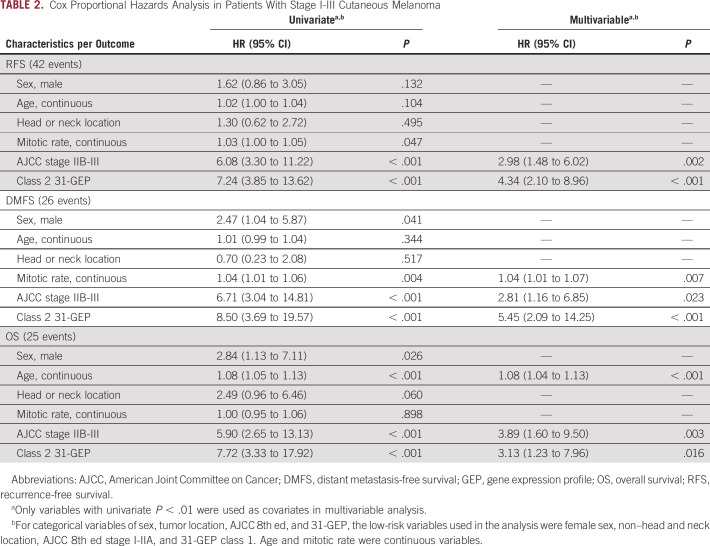
In a univariate analysis including class 2 (v class 1) 31-GEP results, continuous age, male (v female) sex, head and neck (v non–head and neck) tumor location, continuous mitotic rate, and high-risk stage IIB-III (v stage I-IIA) AJCC 8th ed staging, only GEP class 2 and high-risk AJCC stages reached a threshold of P < .01 for all three end points; whereas, mitotic rate was significant for DMFS, and age was significant for OS (Table 2). Similarly, subclass analysis of the 31-GEP demonstrated that classes 1B, 2A, and 2B were significant predictors of recurrence risk; whereas, classes 2A and 2B were significant predictors of distant metastasis, and class 2B was a significant predictor of mortality (Appendix Table A1).
TABLE 2.
Cox Proportional Hazards Analysis in Patients With Stage I-III Cutaneous Melanoma
In a multivariable analysis, 31-GEP class 2 (hazard ratio [HR], 4.34 [95% CI, 2.10 to 8.96], P < .001) and AJCC stage IIB-III (HR, 2.98 [95% CI, 1.48 to 6.02], P = .002) were independent, significant predictors of RFS. For 3-year DMFS, 31-GEP class 2 (HR, 5.45 [95% CI, 2.09 to 14.25], P < .001), AJCC stage IIB-III (HR, 2.81 [95% CI, 1.16 to 6.58], P = .023), and mitotic rate (HR, 1.04 [95% CI, 1.01 to 1.07], P = .007) were significant. 31-GEP class 2 (HR, 3.13 [95% CI, 1.23 to 7.96], P = .016), AJCC stage IIB-III (HR, 3.89 [95% CI, 1.60 to 9.50], P = .003), and age (HR, 1.08 [95% CI, 1.04 to 1.13], P < .001) were significant for OS (Table 2).
3-Year Clinical Outcomes in AJCC Low- and High-Risk Populations
We evaluated the prognostic performance of 31-GEP in patients categorized as low risk (AJCC 8th ed stage I-IIA).2 In patients with stage I-IIA disease, class 2 (n = 30) results accounted for 42% of the total class 2 population. Patients with class 2 results had significantly lower 3-year RFS (83% [95% CI, 71 to 98] v 97% [95% CI, 94 to 99], P < .0001), DMFS (87% [95% CI, 75 to 100] v 99% [95% CI, 97 to 100], P < .0001), and OS (90% [95% CI, 80 to 100] v 98% [95% CI, 96 to 100], P = .01; Fig 3) than those with class 1 results (n = 226). Moreover, survival rates for stage I-IIA class 2 and all stage IIB-III cases were not significantly different. Subgroup analysis of survival rates for RFS, DMFS, and OS end points are presented in Appendix Figure A2. Stage I-IIA class 2B event rates (29%) were similar to stage IIB-III rates (36%), as well as stage IIB-IIC (21% at 24 months) and stage IIC-III (23% at 31 months) rates in previously published cohorts.24,25 Similarly, patients with stage I-IIA CM with class 2B results had rates of distant metastasis and death that were not different from the rates in patients with stage IIB-III CM in this cohort (21% v 24%, and 29% v 22%, respectively; Appendix Fig A2).
FIG 3.
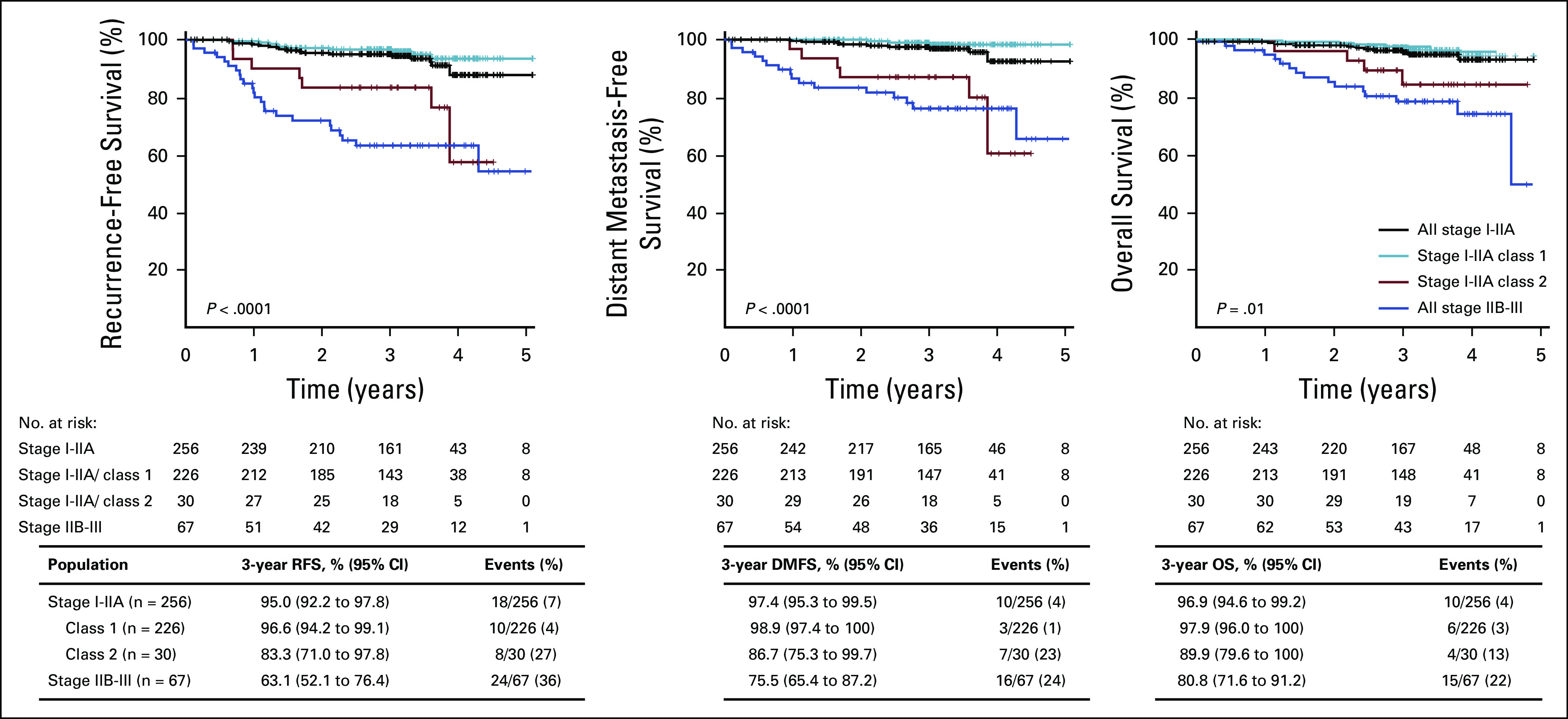
Survival outcomes of patients with stage I-IIA CM by 31-GEP results compared with stage IIB-III overall. RFS, DMFS, and OS were estimated by Kaplan-Meier analysis and P values determined by logrank test. Tables beneath the graphs show survival rates and number of events in each GEP class. CM, cutaneous melanoma; DMFS, distant metastasis-free survival; GEP, gene expression profile; OS, overall survival; RFS, recurrence-free survival.
Similarly, the 31-GEP test stratified risk in the AJCC stage IIB-III population. Patients with stage IIB-III CM and a class 1 result (n = 26) had a significantly higher 3-year RFS (79% [95% CI, 65 to 97] v 52% [95% CI, 38 to 71], P = .020), a nonsignificantly higher 3-year DMFS (79% [95% CI, 68 to 93] v 74% [95% CI, 61 to 89]; P = .400), and a significantly higher 3-year OS (91% [95% CI, 81 to 100] v 74% [95% CI, 61 to 89], P = .020) compared with patients with a class 2 result (n = 41). These results suggest that the 31-GEP adds prognostic value to AJCC 8th ed staging.
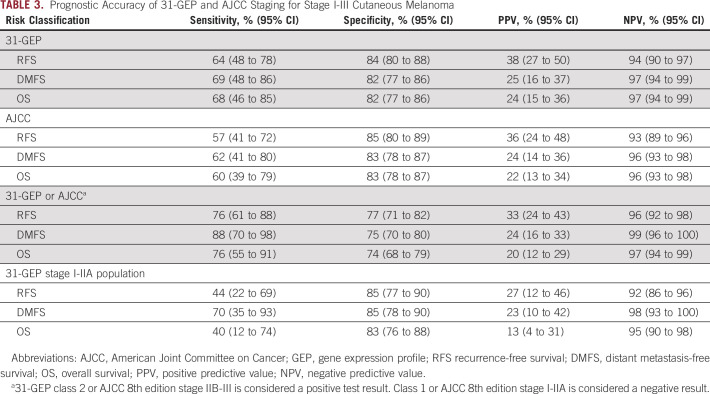
Prognostic Accuracy of 31-GEP and Clinicopathologic Features
Class 2 31-GEP results were more sensitive in detecting recurrence, distant metastasis, and death than AJCC staging alone (Table 3). Class 1 31-GEP results had a high NPV for 3-year RFS (94%), DMFS (97%), and OS (97%), indicating good survival outcomes in patients with a class 1 result. When identifying high-risk patients by either 31-GEP class 2 result or AJCC high-risk category, sensitivity was enhanced for 3-year RFS (76%), DMFS (88%), and OS (76%) compared with AJCC alone with sensitivities of 57% (RFS), 62% (DMFS), and 60% (OS) or 31-GEP status alone with sensitivities of 64% (RFS), 69% (DMFS), and 68% (OS). Class 2 31-GEP results identified AJCC stage I-IIA patients with increased risk for recurrence, distant metastasis, and death with 44%, 70%, and 40% sensitivity, respectively; whereas a class 1 result confirmed a low risk of recurrence, distant metastasis, and death in this population with an NPV of 92%, 98%, and 95%, respectively (Table 3).
TABLE 3.
Prognostic Accuracy of 31-GEP and AJCC Staging for Stage I-III Cutaneous Melanoma
DISCUSSION
We have recently seen the emergence of the 31-GEP test, which further stratifies patient risk for melanoma metastasis.15-20,26,27 The 31-GEP has been validated in retrospective and prospective studies, including an interim analysis of the present cohort.17 The potential value of a reliable tissue-based prognostic test is to identify patients who are considered low risk by current AJCC staging, but who are biologically at high risk of metastasis and death. Such patients may benefit from more aggressive follow-up and consideration for clinical trials with adjuvant therapy. We performed a prospective analysis of outcomes in a multicenter CM cohort to test the hypotheses that patients with stage I-IIA melanoma with class 2 31-GEP results have metastatic risk similar to stage IIB-III patients, and that the 31-GEP has independent prognostic value in addition to current AJCC staging factors.
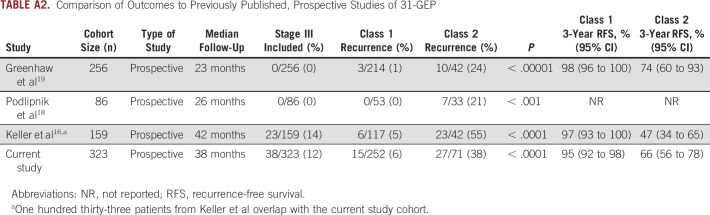
In the full cohort, we found that patients with a class 2 result had significantly worse 3-year RFS, DMFS, and OS than those with class 1 results. The observed rates of recurrence are consistent with previously reported prospective cohorts, demonstrating the objective accuracy and consistency of the 31-GEP test (Appendix Table A2).16,18,19 Median time to recurrence and distant metastasis have been reported at 1.9 years and 2.1 years, respectively, indicating that the median follow-up time of 3.2 years in this study is sufficient to detect the majority of expected events.28,29 Class 2 patients have a 3-year survival rate of 81%, which is lower than 5-year melanoma-specific survival for stage IIB-IIC patients (87% and 82%, respectively) for which AJCC 8th ed recommends increased imaging surveillance. These data suggest that class 2 patients may benefit from increased surveillance management for early detection of recurrence and metastasis.
In stage I-IIA patients, considered to have low risk of disease progression, patients with class 2 results had significantly lower 3-year RFS, DMFS, and OS than class 1 patients. In this cohort, the 3-year rates of recurrence, distant metastasis, and death in patients with stage I-IIA disease and a class 2 result were equivalent to that of stage IIB-III patients traditionally considered high risk. Because a substantial number of patients who die from melanoma each year are initially diagnosed with stage I-IIA disease, the data suggest that class 2, stage I-IIA patients would benefit from increased surveillance as is recommended for stage IIB-III. In this cohort, 7% (18/256) of stage I-IIA patients had recurrence, and 4% (10/256) had distant metastasis at 3 years. The 31-GEP further refined the risk of this subset of patients by identifying 44% (8/18) of patients with recurrence and 70% (7/10) of patients with distant metastasis with a class 2 result within this traditionally low-risk subset of patients (Fig 3). Thus, combining the 31-GEP with AJCC staging reduced the misclassification of high-risk patients by 44% and 70%, respectively.
Most patients with a low-risk AJCC stage I designation received a low-risk class 1 31-GEP result (n = 201) and had good survival outcomes (NPV of 96%). However, the patients with stage I disease and a class 2 result (n = 17) had increased rates of recurrence (18% v 4%), distant metastasis (18% v 1%), and death (6% v 2%) compared to those with class 1 tumors. These results align with previous studies that have demonstrated that patients with stage I CM and a class 2 31-GEP result have a significantly worse prognosis than those with a class 1 result.20 Previous work has shown that class 1A 31-GEP results correlate with a negative SLNB and, although not the focus of this study, adds further support for recommending the 31-GEP test to patients with a clinical stage I CM diagnosis.
When analyzing prognostic accuracy for 3-year RFS, DMFS, and OS, 31-GEP had higher sensitivity and NPV than AJCC high-risk stages alone. In concordance with other studies, combining 31-GEP and AJCC 8th ed stage yielded higher sensitivity than either approach alone.16,20 Thus, for patients with a class 2 result, added information from AJCC staging increases sensitivity to identify patients at high risk of progression. Also, the high NPV of a 31-GEP class 1 result provides reassurance that these patients have a favorable prognosis. These data highlight the prognostic value added by the 31-GEP when combined with AJCC staging to improve risk assessment for patients with CM.
This study’s outcomes are based on a median follow-up time of 3.2 years for those without an event, which should have captured most events. However, future additional events may have a small effect on recurrence rates and survival estimates, as previously seen from archival cohorts with longer follow-up.20,26 Additionally, the number of DMFS and OS events was limited relative to the number of significant variables in the univariate analysis. Thus, Breslow, ulceration, and SLN status were condensed into stage, and inclusion criteria in the multivariable model were stringent (P < .01). In addition, OS was assessed in place of disease-specific survival because the cause of death was not documented in some cases. Finally, many of the patients in this study underwent SLNB. Although it is unlikely SLNB changed the course of the patients’ disease, risk stratification by 31-GEP class in this study is consistent with outcomes in a study in which the majority of patients did not undergo SLNB.19
In conclusion, the 31-GEP is an independent predictor of disease progression that adds prognostic information to clinical and pathologic staging criteria. This study confirms the clinical validity of the 31-GEP test in patients with stage I-IIA CM with class 2 GEP results who may benefit from more intense follow-up. Patients with a class 2 31-GEP, including patients with stage I-IIA CM, have 3-year survival rates similar to those for patients with stage IIB-III CM. Moreover, the combination of GEP testing with AJCC staging improves the accuracy of prognosis. These data provide a rationale for using the 31-GEP test in conjunction with AJCC staging to obtain an optimal prognosis for patients with CM.
ACKNOWLEDGMENT
The authors would like to thank the patients who participated in this study, the physicians and clinical staff who contributed to the study, and Olga Zolochevska, PhD, Christine Bailey, MPH, and Brian Martin, PhD, who contributed to the editing and writing of this manuscript.
Appendix
FIG A1.
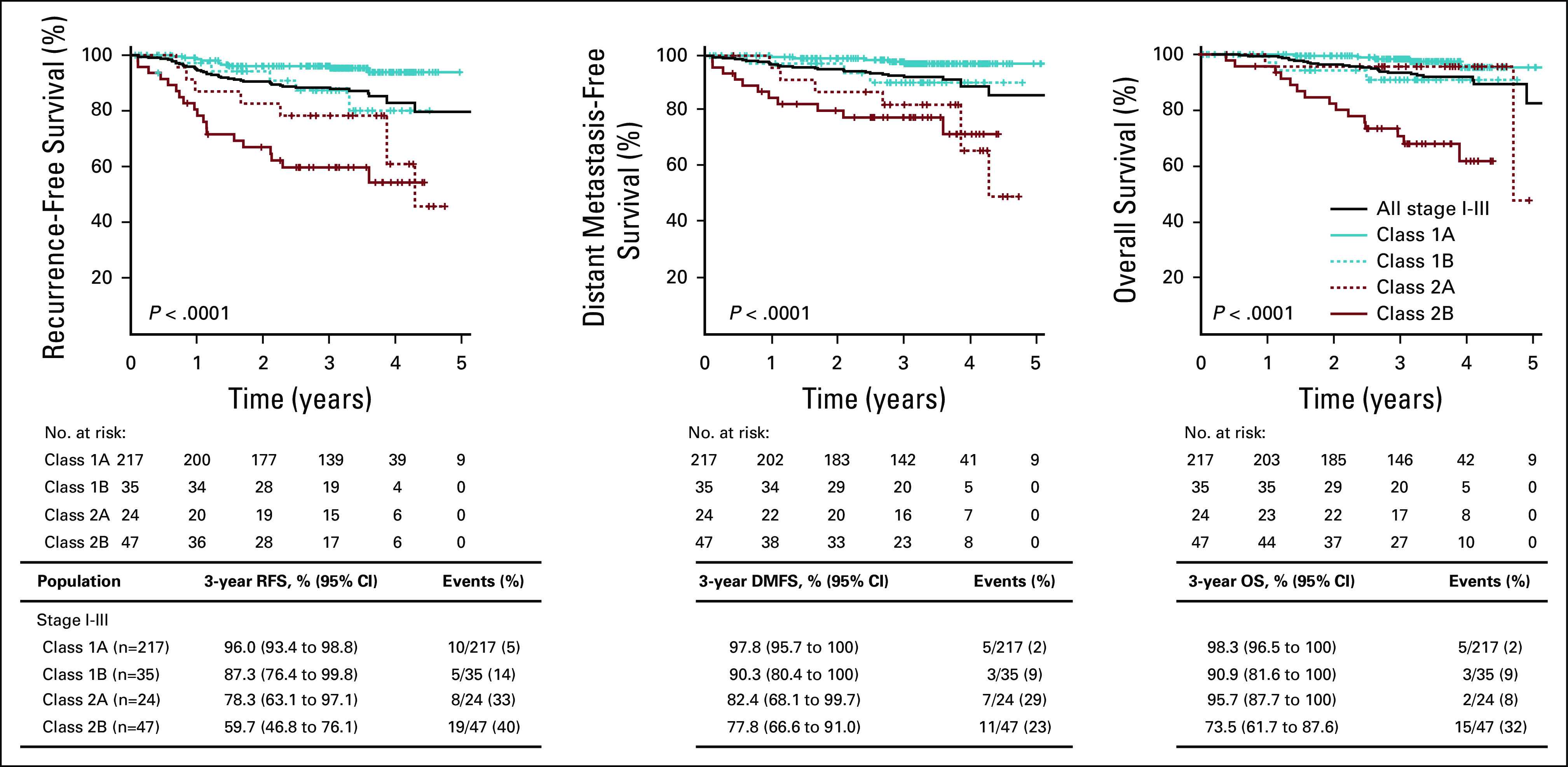
Survival outcomes of patients with stage I-III CM by 31-GEP subclass. RFS, DMFS, and OS were estimated by Kaplan-Meier analysis and P values determined by logrank test. Tables beneath the graphs show survival rates and number of events in each GEP subclass. CM, cutaneous melanoma; DMFS, distant metastasis-free survival; GEP, gene expression profile; OS, overall survival; RFS, recurrence-free survival.
FIG A2.
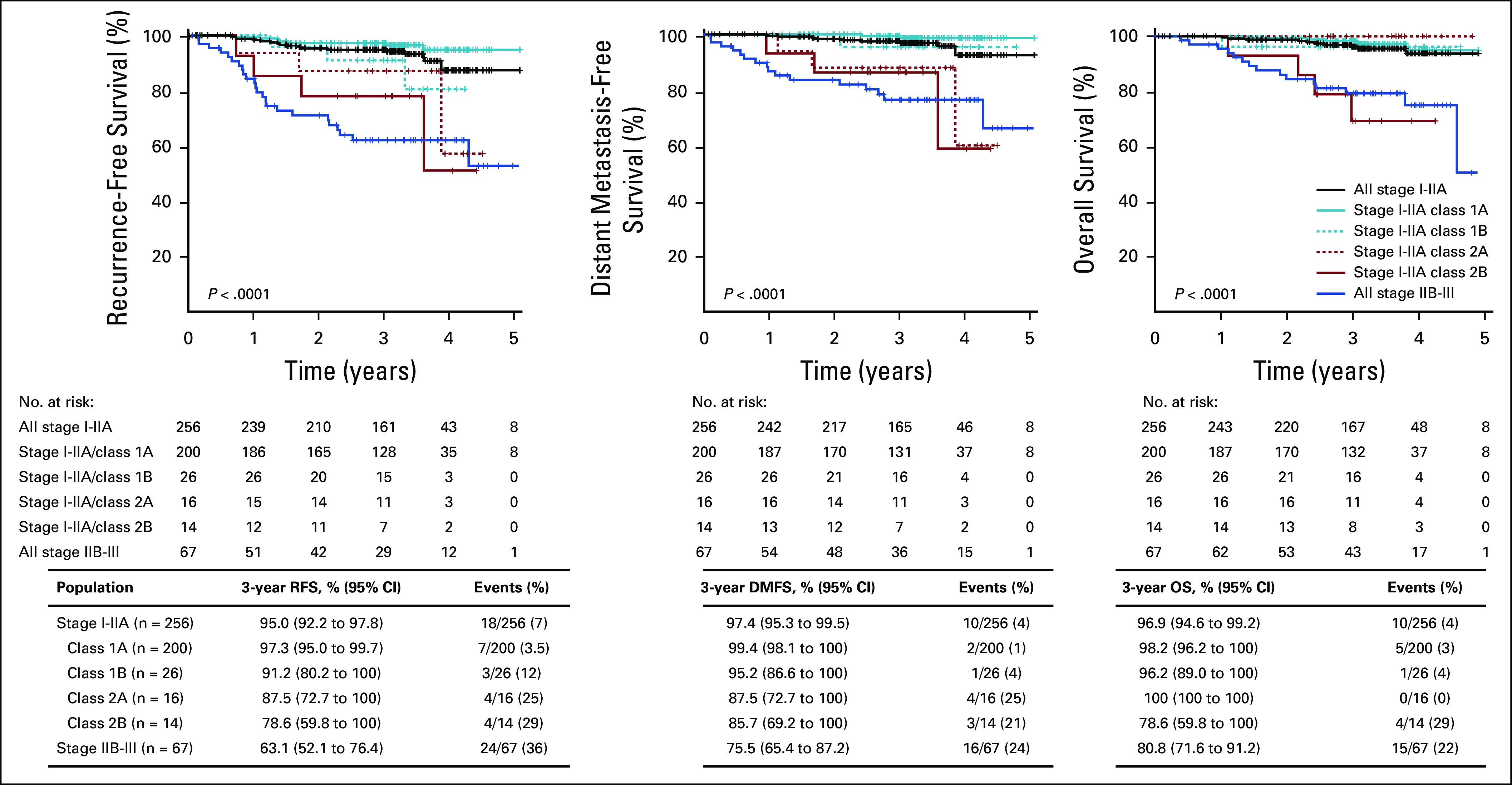
Survival outcomes of patients with stage I-IIA CM by 31-GEP subclass. RFS, DMFS, and OS were estimated by Kaplan-Meier analysis and P values for statistical differences between GEP subclasses determined by logrank test. Table beneath the curve show the number of patients at risk each year, 3-year survival, and event rates for each population. CM, cutaneous melanoma; DMFS, distant metastasis-free survival; GEP, gene expression profile; OS, overall survival; RFS, recurrence-free survival.
TABLE A1.
Cox Proportional Hazards Analysis With Expanded Variables in Patients With Stage I-III Cutaneous Melanoma
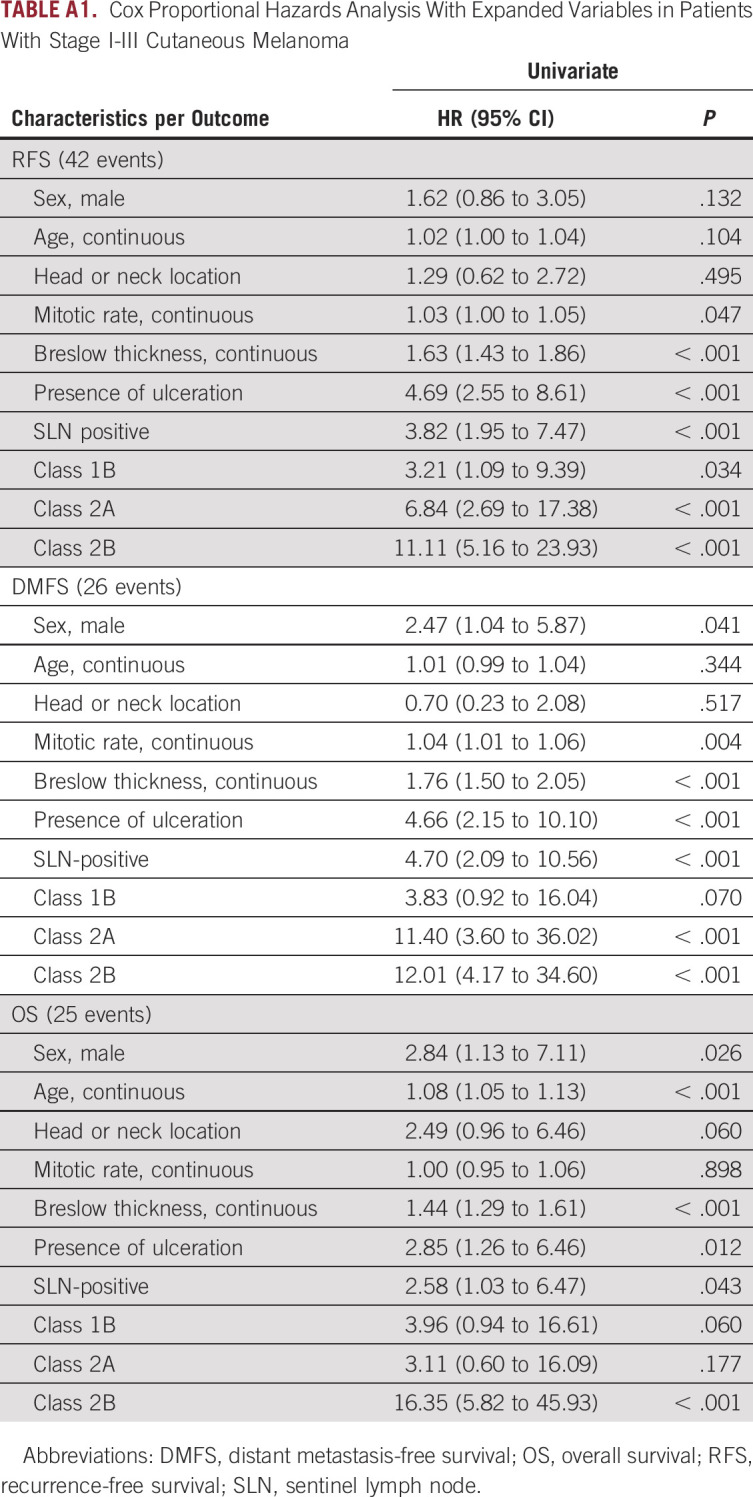
TABLE A2.
Comparison of Outcomes to Previously Published, Prospective Studies of 31-GEP
Eddy C. Hsueh
Speakers' Bureau: Amgen, Castle Biosciences
Jeffrey J. Sussman
Consulting or Advisory Role: Castle Biosciences
Kyle R. Covington
Employment: Castle Biosciences
Stock and Other Ownership Interests: Castle Biosciences
Patents, Royalties, Other Intellectual Property: Gene expression profile tests
Travel, Accommodations, Expenses: Castle Biosciences
Hillary G. Caruso
Employment: Castle Biosciences
Stock and Other Ownership Interests: Castle Biosciences
Patents, Royalties, Other Intellectual Property: Chimeric antigen receptors (CARs) and CAR-expressing T cells are provided that can specifically target cells that express an elevated level of a target antigen. Likewise, methods for specifically targeting cells that express elevated levels of antigen (eg, cancer cells) with CAR T-cell therapies are provided
Travel, Accommodations, Expenses: Castle Biosciences
Ann P. Quick
Employment: Castle Biosciences
Stock and Other Ownership Interests: Castle Biosciences
Travel, Accommodations, Expenses: Castle Biosciences
Robert W. Cook
Employment: Castle Biosciences
Stock and Other Ownership Interests: Castle Biosciences
Patents, Royalties, Other Intellectual Property: Castle Biosciences related patents
Travel, Accommodations, Expenses: Castle Biosciences
Craig L. Slingluff
Consulting or Advisory Role: Immatics, Polynoma, CureVac
Research Funding: GlaxoSmithKline, Merck Sharp & Dohme, 3M, Theraclion, Celldex
Patents, Royalties, Other Intellectual Property: Licensing and Ventures Group of the University of Virginia
Travel, Accommodations, Expenses: Polynoma
Uncompensated Relationships: Agenus
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part as an abstract in the J Clin Oncol 37, 2019 (suppl; abstr 9519), doi: 10.1200/JCO.2019.37.15_suppl.9519 and presented at a poster presentation at the ASCO Annual Meeting, Chicago, IL, May 31-June 4, 2019.
SUPPORT
This study was partially sponsored by Castle Biosciences, Inc, which provided financial compensation to those who provided melanoma tissue for this study.
AUTHOR CONTRIBUTIONS
Conception and design: Robert W. Cook
Financial support: Kyle R. Covington
Provision of study materials or patients: Eddy C. Hsueh, Craig L. Slingluff
Collection and assembly of data: Eddy C. Hsueh, James R. DeBloom, Jonathan H. Lee, Jeffrey J. Sussman, Robert W. Cook, Craig L. Slingluff, Kelly M. McMasters
Data analysis and interpretation: Eddy C. Hsueh, Jonathan H. Lee, Jeffrey J. Sussman, Kyle R. Covington, Hillary G. Caruso, Ann P. Quick, Robert W. Cook
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Eddy C. Hsueh
Speakers' Bureau: Amgen, Castle Biosciences
Jeffrey J. Sussman
Consulting or Advisory Role: Castle Biosciences
Kyle R. Covington
Employment: Castle Biosciences
Stock and Other Ownership Interests: Castle Biosciences
Patents, Royalties, Other Intellectual Property: Gene expression profile tests
Travel, Accommodations, Expenses: Castle Biosciences
Hillary G. Caruso
Employment: Castle Biosciences
Stock and Other Ownership Interests: Castle Biosciences
Patents, Royalties, Other Intellectual Property: Chimeric antigen receptors (CARs) and CAR-expressing T cells are provided that can specifically target cells that express an elevated level of a target antigen. Likewise, methods for specifically targeting cells that express elevated levels of antigen (eg, cancer cells) with CAR T-cell therapies are provided
Travel, Accommodations, Expenses: Castle Biosciences
Ann P. Quick
Employment: Castle Biosciences
Stock and Other Ownership Interests: Castle Biosciences
Travel, Accommodations, Expenses: Castle Biosciences
Robert W. Cook
Employment: Castle Biosciences
Stock and Other Ownership Interests: Castle Biosciences
Patents, Royalties, Other Intellectual Property: Castle Biosciences related patents
Travel, Accommodations, Expenses: Castle Biosciences
Craig L. Slingluff
Consulting or Advisory Role: Immatics, Polynoma, CureVac
Research Funding: GlaxoSmithKline, Merck Sharp & Dohme, 3M, Theraclion, Celldex
Patents, Royalties, Other Intellectual Property: Licensing and Ventures Group of the University of Virginia
Travel, Accommodations, Expenses: Polynoma
Uncompensated Relationships: Agenus
No other potential conflicts of interest were reported.
REFERENCES
- 1.Cronin KA, Lake AJ, Scott S, et al. Annual report to the nation on the status of cancer, part I: National cancer statistics: Annual report national cancer statistics Cancer 1242785–28002018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network . Cutaneous Melanoma (Version 1.2020) 2020. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma_blocks.pdf [Google Scholar]
- 3.Shaikh WR, Dusza SW, Weinstock MA, et al. Melanoma thickness and survival trends in the United States, 1989 to 2009. J Natl Cancer Inst. 2016;108:djv294. doi: 10.1093/jnci/djv294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whiteman DC, Baade PD, Olsen CM.More people die from thin melanomas (1 mm) than from thick melanomas (>4 mm) in Queensland, Australia J Invest Dermatol 1351190–11932015 [DOI] [PubMed] [Google Scholar]
- 5.Eggener S, Karsh LI, Richardson T, et al. A 17-gene panel for prediction of adverse prostate cancer pathologic features: Prospective clinical validation and utility Urology 12676–822019 [DOI] [PubMed] [Google Scholar]
- 6.Chang EM, Punglia RS, Steinberg ML, et al. Cost effectiveness of the oncotype DX genomic prostate score for guiding treatment decisions in patients with early stage prostate cancer Urology 12689–952019 [DOI] [PubMed] [Google Scholar]
- 7.Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer N Engl J Med 3732005–20142015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer N Engl J Med 379111–1212018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giuliano AE, Connolly JL, Edge SB, et al. Breast cancer-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual: Updates to the AJCC breast TNM staging system CA Cancer J Clin 67290–3032017 [DOI] [PubMed] [Google Scholar]
- 10.Wang M, Wu K, Zhang P, et al. The prognostic significance of the oncotype DX recurrence score in T1-2N1M0 estrogen receptor-positive HER2-negative breast cancer based on the prognostic stage in the updated AJCC 8th edition Ann Surg Oncol 261227–12352019 [DOI] [PubMed] [Google Scholar]
- 11.Kivela T, Simpson ER, Grossniklaus HE, et al. Uveal melanoma. In: Amin MB, Edge MB, Greene FL, et al., editors. American Joint Committee on Cancer (AJCC) Staging Manual. Volume 8. Cham, Switzerland: Springer; 2017. [Google Scholar]
- 12.Onken MD, Worley LA, Char DH, et al. Collaborative ocular oncology group report number 1: Prospective validation of a multi-gene prognostic assay in uveal melanoma Ophthalmology 1191596–16032012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerami P, Cook RW, Wilkinson J, et al. Development of a prognostic genetic signature to predict the metastatic risk associated with cutaneous melanoma Clin Cancer Res 21175–1832015 [DOI] [PubMed] [Google Scholar]
- 14.Gerami P, Cook RW, Russell MC, et al. Gene expression profiling for molecular staging of cutaneous melanoma in patients undergoing sentinel lymph node biopsy J Am Acad Dermatol 72780–785.e32015 [DOI] [PubMed] [Google Scholar]
- 15.Gastman BR, Gerami P, Kurley SJ, et al. Identification of patients at risk of metastasis using a prognostic 31-gene expression profile in subpopulations of melanoma patients with favorable outcomes by standard criteria J Am Acad Dermatol 80149–157.e42019 [DOI] [PubMed] [Google Scholar]
- 16.Keller J, Schwartz TL, Lizalek JM, et al. Prospective validation of the prognostic 31‐gene expression profiling test in primary cutaneous melanoma Cancer Med 82205–22122019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsueh EC, DeBloom JR, Lee J, et al. Interim analysis of survival in a prospective, multi-center registry cohort of cutaneous melanoma tested with a prognostic 31-gene expression profile test. J Hematol Oncol. 2017;10:152. doi: 10.1186/s13045-017-0520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Podlipnik S, Carrera C, Boada A, et al. Early outcome of a 31-gene expression profile test in 86 AJCC stage IB-II melanoma patients. A prospective multicentre cohort study J Eur Acad Dermatol Venereol 33857–8622019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenhaw BN, Zitelli JA, Brodland DG.Estimation of prognosis in invasive cutaneous melanoma: An independent study of the accuracy of a gene expression profile test Dermatol Surg 441494–15002018 [DOI] [PubMed] [Google Scholar]
- 20.Zager JS, Gastman BR, Leachman S, et al. Performance of a prognostic 31-gene expression profile in an independent cohort of 523 cutaneous melanoma patients. BMC Cancer. 2018;18:130. doi: 10.1186/s12885-018-4016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenhaw BN, Covington KR, Kurley SJ, et al. Molecular risk prediction in cutaneous melanoma: A meta-analysis of the 31-gene expression profile prognostic test in 1,479 patients J Am Acad Dermatol 83745–7532020 [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Chakraborty BS.Interim analysis: A rational approach of decision making in clinical trial J Adv Pharm Technol Res 7118–1222016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cook RW, Middlebrook B, Wilkinson J, et al. Analytic validity of DecisionDx-Melanoma, a gene expression profile test for determining metastatic risk in melanoma patients. Diagn Pathol. 2018;13:13. doi: 10.1186/s13000-018-0690-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurtz J, Beasley GM, Agnese D, et al. Surveillance strategies in the follow-up of melanoma patients: Too much or not enough? J Surg Res 21432–372017 [DOI] [PubMed] [Google Scholar]
- 25.von Schuckmann LA, Hughes MCB, Ghiasvand R, et al. Risk of melanoma recurrence after diagnosis of a high-risk primary tumor JAMA Dermatol 155688–6932019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gastman BR, Zager JS, Messina JL, et al. Performance of a 31-gene expression profile test in cutaneous melanomas of the head and neck Head Neck 41871–8792019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vetto JT, Hsueh EC, Gastman BR, et al. Guidance of sentinel lymph node biopsy decisions in patients with T1-T2 melanoma using gene expression profiling Future Oncol 151207–12172019 [DOI] [PubMed] [Google Scholar]
- 28.Vallet A, Oriano B, Mortier L, et al. Association of time from primary diagnosis to first distant relapse of metastatic melanoma with progression of disease and survival JAMA Dermatol 155673–6782019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tas F, Erturk K.Relapse patterns in patients with local and regional cutaneous melanoma Clin Transl Oncol 21412–4192019 [DOI] [PubMed] [Google Scholar]