PURPOSE
MET dysregulation is an oncogenic driver in non–small-cell lung cancer (NSCLC), as well as a mechanism of TKI (tyrosine kinase inhibitor) resistance in patients with epidermal growth factor receptor (EGFR)–mutated disease. This study was conducted to determine safety and preliminary efficacy of the combination EGFR and MET inhibitors as a strategy to overcome and/or delay EGFR-TKI resistance.
METHODS
A standard 3 + 3 dose-escalation trial of capmatinib in combination with erlotinib in patients with MET-positive NSCLC was used. Eighteen patients in the dose-escalation cohort received 100-600 mg twice daily of capmatinib with 100-150 mg daily of erlotinib. There were two dose-expansion cohorts. Cohort A included 12 patients with EGFR-mutant tumors resistant to TKIs. Cohort B included five patients with EGFR wild-type tumors. The primary outcome was to assess safety and determine the recommended phase II dose (RP2D) of the combination.
RESULTS
The most common adverse events of any grade were rash (62.9%), fatigue (51%), and nausea (45.7%). Capmatinib exhibited nonlinear pharmacokinetics combined with erlotinib, while showing no significant drug interactions. The RP2D was 400 mg twice daily capmatinib tablets with 150 mg daily erlotinib. The overall response rate (ORR) and DCR in dose-expansion cohort A was 50% and 50%, respectively. In cohort B, the ORR and disease control rate were 75% and 75%.
CONCLUSION
Capmatinib in combination with erlotinib demonstrated safety profiles consistent with prior studies. We observed efficacy in specific patient populations. Continued evaluation of capmatinib plus EGFR-TKIs is warranted in patients with EGFR activating mutations.
INTRODUCTION
Aberrant MET signaling plays a role in tumor invasion, progression, metastasis, and survival.1-5 MET amplification is a well-established mechanism of resistance to first-generation EGFR-TKIs, occurring in 5%-22% of patients with EGFR-mutated non–small-cell lung cancer (NSCLC).6-8 In addition, MET amplification is a common resistance mechanism after treatment with the third-generation EGFR TKI osimertinib, ranging in frequency from 14% to 30%.9-12 De novo MET amplification and MET exon 14 splicing mutations are known independent oncogenic drivers.4,13,14 Preclinically, MET protein overexpression has been shown to be oncogenic, but its transformative potential in human tumors is controversial.15,16 Overall, MET dysregulation plays a significant biologic role in lung cancer, making it an ideal drug target.
CONTEXT
Key Objective
Aberrant MET signaling plays a role in tumor invasion, progression, metastasis, and survival in non–small-cell lung cancer. It is a common resistance mechanism to third-generation EGFR TKIs, and MET amplification and exon 14 mutations are independent oncogenic drivers. Because of overlapping signaling pathways, we sought to determine whether the combination of capmatinib with erlotinib would be safe and demonstrate an efficacy signal in patients with EGFR- or MET-altered tumors.
Knowledge Generated
We demonstrated that the combination of capmatinib and erlotinib is safe. Furthermore, we demonstrated an overall response rate of 50% in patient with EGFR mutations and acquired resistance to EGFR TKIs and further demonstrated the efficacy of capmatinib in patients with MET exon 14 mutations.
Relevance
The use of combination targeted therapy, capmatinib, and EGFR TKI may help to treat patients with resistance to frontline therapy and is important to consider as frontline therapy to provide prolonged responses.
Several MET inhibitors have been evaluated in patients with lung cancer. Early studies evaluated onartuzumab (an anti-MET monoclonal antibody) in combination with erlotinib in recurrent NSCLC.16,17 In the phase II study, patients with MET 2+ or 3+ by immunohistochemistry (IHC) demonstrated a significant improvement in survival end points compared with low MET expression. Despite this, the phase III trial was negative.18 Tivantinib, a non-ATP competitive small molecule MET inhibitor, was evaluated in combination with erlotinib in a phase II trial of unselected pretreated patients. The combination did not meet its overall efficacy goal; however, a planned subset analysis showed a trend toward prolonged progression-free survival (PFS) in patients with MET amplification.19 This lack of efficacy may be due to the lack of specificity of tivantinib for the MET pathway compared with other MET inhibitors.20
Capmatinib (INC280) is a highly potent and selective oral MET inhibitor that has recently been approved for the treatment of tumors with MET exon 14 mutations.21 In the phase I study, antitumor activity was observed in pretreated patients with EGFR wild-type (WT) tumors with MET dysregulation. The most frequent adverse effects were anorexia (33%), nausea (30%), vomiting (27%), and fatigue (27%). The recommended phase II dose was 600-mg capsules twice daily.22
Preclinically, capmatinib demonstrated minimal single-agent cytotoxicity, despite potent inhibition of MET kinase activity. However, it restored sensitivity to erlotinib and promoted apoptosis in NSCLC models rendered erlotinib resistant by HGF.23 On the basis of the data available during trial development, we propose that the combination of capmatinib plus erlotinib would be safe and show efficacy in patients with EGFR-mutated tumors experiencing disease progression on erlotinib due to MET activating bypass pathways. In addition, it may increase the duration of response in TKI-naïve patients with EGFR WT tumors with MET dysregulation.
METHODS
This trial was conducted at the University of California, Davis (UCD) and the University of California, San Francisco (UCSF). A standard 3 + 3 dose-escalation design was used, as illustrated in Figure 1. The initial phase of the study was a dose escalation to determine the maximum tolerated dose (MTD) of capmatinib plus erlotinib; there was no intrapatient dose escalation. This was followed by two expansion cohorts; cohort A consisted of patients with EGFR-mutated tumors resistant to erlotinib, and cohort B enrolled erlotinib-naïve patients with WT EGFR tumors. Cohort B was designed based on preclinical and clinical data indicating that MET alterations serve as an oncogenic driver in a subset of patients with WT EGFR tumors.17,19 While the study was ongoing, MET exon 14 mutations emerged as a predictive marker for TKI response, and additional studies clarified the role of MET alterations as oncogenic drivers.18,24-26 Because of slowed enrollment resulting from changes in standard-of-care treatment in the EGFR-mutated population and the US Food and Drug Administration withdrawal of erlotinib in the EGFR WT population, the study was terminated before enrollment was completed in cohort B.
FIG 1.
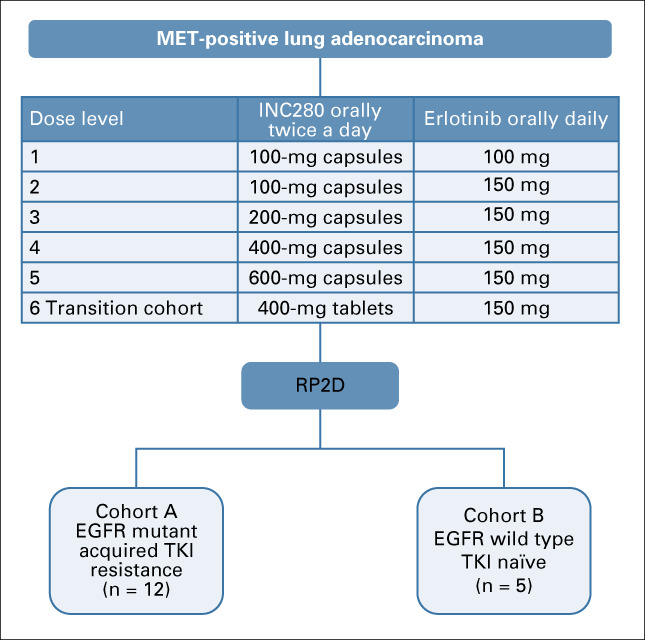
Consort diagram of clinical trial. EGFR, epidermal growth factor receptor; RP2D, recommended phase II dose; TKI, tyrosine kinase inhibitor.
This study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization harmonized Tripartite Guidelines for good clinical practice, and US 21 code of federal regulations. Site-specific institutional review boards approved the study protocol and amendments. All patients provided written informed consent. The study is registered at www.ClinicalTrials.gov (ClinicalTrials.gov identifier: NCT01911507).
Patients
Key eligibility criteria included advanced/metastatic NSCLC with measurable disease, age ≥ 18 years, Eastern Cooperative Oncology Group performance status (ECOG PS) 0-2, MET increased copy number by fluorescence in situ hybridization (FISH; CNG or MET/CEN7 ratio outside of normal range), MET IHC 2-3+, positive reverse transcriptase polymerase chain reaction (RT-PCR) value, or an exon 14 splice site mutation (Data Supplement). Patients in cohort A must have an EGFR activating mutation and a biopsy at the time of progression that shows evidence of MET positivity.
Treatment
Capmatinib capsules were administered orally every 12 hours of a 28-day cycle at 100-600 mg. Dose level 6 was used to determine whether tablets demonstrated a similar safety profile as capsules. The expansion cohort was based on information provided by the sponsor that 400-mg tablets demonstrated equivalent pharmacokinetics (PK) to 600-mg capsules. In addition, the capsule formulation required patients to take up to eight capsules twice daily. For these reasons, the formulation was shifted to tablets during the study. Erlotinib was administered at 100 mg orally once daily in dose level 1 and escalated to 150 mg orally daily in dose levels 2-6. Dose modifications were made independently for each drug on the basis of specific toxicities, and treatment was administered as per study protocol (Data Supplement).
Assessments
Adverse events (AEs) were assessed for severity using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTACAE version 4.0).
In dose escalation, all evaluable patients were included in the dose-escalation decisions except for patient 15 (dose level 5), who discontinued the study after 2 days and was not replaced because of the opening of dose level 6, a pharmacologically equivalent dose. A dose-limiting toxicity (DLT) was defined as per the study protocol (Data Supplement).
PK blood samples were collected for patients in the dose-escalation cohort on cycle 1, day 15; cycle 2, days 1 and 15; and cycles 3 and 4, day 1. PK analysis details are listed in the Data Supplement.
Overall response rate (ORR), complete response (CR), partial response (PR), stable disease (SD), and progressive disease were evaluated by the treating physician using the revised RECIST guideline (version 1.1).27
Biomarker Analysis
Patients were selected based on Clinical Laboratory Improvement Amendments–certified laboratory testing for MET alterations by RT-PCR, IHC, FISH, or next-generation sequencing (NGS; MET exon 14 alteration or amplification). Additional testing was done by Foundation Medicine (Boston, MA) for NGS and Clarient (Aliso Viejo, CA) for IHC and FISH. MET IHC positivity was defined as a score of 2+ or 3+, indicating ≥ 50% of tumor cells with moderate- or strong-intensity staining, respectively. Analysis of HGF levels was conducted in duplicate by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN; Cat DHG00) as per the manufacturer’s instructions.
Statistical Methods
All patients who had at least one dose of study therapy were included in the analyses. Data were summarized by study phase and dose group. DLT meetings were conducted to determine whether to proceed to the next dose level. For continuous variables, summary statistics included mean, median, standard deviation, and range. Categorical end points were summarized as frequency and percentages.
RESULTS
From August 2013 to August 2017, 35 patients with MET-positive, stage IV NSCLC were enrolled at UCD and UCSF. Eighteen patients were enrolled into six dose-escalation cohorts. Twelve and five patients were enrolled in cohorts A and B, respectively (Fig 1).
Patient Demographics and Disease Characteristics
Patient demographics and disease characteristics are listed in Table 1. The median age was 65 years, and most patients were female (60%), white (80%), and had an ECOG PS of 0 (65.7%).
TABLE 1.
Patient Demographics and Clinical Characteristics (N = 35)

In the dose-escalation cohorts, nine (50%) patients had EGFR-positive tumors and two had T790M alterations (Fig 2). EGFR alterations are listed in Figure 2.
FIG 2.
Swimmer’s plot demonstrating the cohort, MET, and epidermal growth factor receptor (EGFR) status of each patient and corresponding response status and progression-free survival of each patient. Patient 30 was found to have an EGFR T790M mutation on a biopsy sample taken after EGFR tyrosine kinase inhibitor (TKI) treatment; however, on immediate pretreatment biopsy this mutation was not detected. Patient 15 came off the trial after 2 days. amp, amplification; CR, complete response; IHC, immunohistochemistry; NA, not applicable; Neg, negative; NGS, next-generation sequencing; PD, progressive disease; Pos, positive; PR, partial response; RT-PCR, reverse transcriptase polymerase chain reaction; SD, stable disease; Unk, unknown; WT, wild type.
Prior treatments are outlined in Table 1. In the dose-escalation cohort and cohort A, 67% (patients 1 and 3-13) and 92% (all except patient 29) had prior treatment with at least one EGFR TKI. Three patients (patients 26, 33, and 28) had prior immunotherapy. Across the cohorts, two patients (patients 7 and 8) received prior treatment with a MET-targeted agent (cabozantinib).
For all patients with EGFR activating mutations (n = 21), except patients 7, 9, 23, and 33, the immediate prior treatment regimen was EGFR TKI monotherapy or in combination with other treatments (pembrolizumab, n = 2; MK2206 [AKT inhibitor], n = 1; cabozantinib, n = 2).
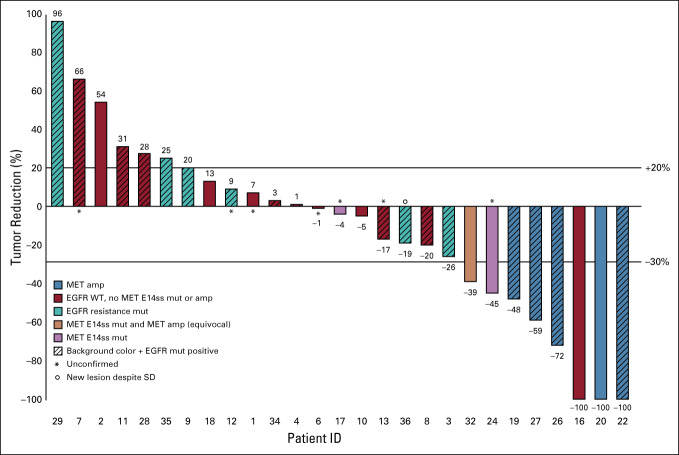
In cohort A, six (50%) patients had tumors with 3+ MET by IHC and five (42%) had 2+ IHC expression (Fig 2). Patient 22’s tumor showed MET amplification by NGS (> 10 copies); however, by FISH the tumor had equivocal amplification, with MET/CEN7 ratio of 1.1. In patient 21, FISH demonstrated a MET/CEN7 ratio of 3.4, consistent with the amplification observed by NGS. MET amplification was not observed in tumor samples from the other nine patients in cohort A. In cohort B, three of the five patients had MET exon 14 mutations (patients 24, 25, and 32), and one patient (patient 20), had MET amplification by NGS; however, this was equivocal by FISH (MET/CEN7 = 1.2; Fig 2). A waterfall plot of best response for all evaluable patients is shown in Figure 3.
FIG 3.
Waterfall plot of response. amp, amplification; EGFR, epidermal growth factor receptor; mut, mutation; SD, stable disease; WT, wild type.
AEs
The AEs that were possibly, probably, or definitely attributed to the study drugs are shown in Table 2 for all dose levels. Thirty-two patients developed an AE (91.4%) of any grade, with 12 (34.2%) patients developing a grade 3 or higher AE. The most common AEs of any grade were acneiform rash (62.9%), fatigue (51%), nausea (45.7%), diarrhea, lower extremity edema, vomiting, and hypoalbuminemia (37% each). The most common grade 3 or higher AEs were anorexia and increased lipase (5.7% each). In patients who received the recommended phase II dose (RP2D), the most common AEs were acneiform rash (65%), fatigue and nausea (both 60%), vomiting (55%), and hypoalbuminemia and edema (both 50%); additional AEs are detailed in Appendix Table A1. There were no grade 5 toxicities related to the drug combination.
TABLE 2.
Treatment-Related Adverse Events

DLT and MTD
In dose level 5, one patient developed grade 3 neutropenia possibly related to treatment. Dose level 6 served as the expansion of dose level 5, given that 600-mg capsules were previously shown to be pharmacokinetically equivalent to 400-mg tablets. No patients in dose level 6 experienced a DLT. The RP2D was capmatinib 400-mg tablets twice a day with erlotinib 150 mg daily.
Dose Modification
During study procedures, 15 patients received dose modifications. Six patients received dose modifications of capmatinib due to nausea (2), ALT abnormalities (1), edema (1), low neutrophil count (1), and elevated amylase (1). Five patients received dose modifications of erlotinib due to paronychia (2), acneiform rash (1), creatinine increase (1), and diarrhea (1). Four patients received dose modifications of both drugs for lipase elevation (1), creatinine increase (1), lymphopenia (1), and lung inflammation/pneumonitis (1).
PK
The PK properties of erlotinib and capmatinib were examined during dose escalation on cycle 1, day 15 at multiple time points after drug administration. The PK profiles of erlotinib and capmatinib are presented in Appendix Figure A1, and estimated PK parameters are shown in Appendix Table A2. Capmatinib exposure appears proportional with an increase of dose from 100 to 200 mg twice a day dose range, but were not dose proportional from 100 to 600 mg (Appendix Fig A1A and Appendix Table A2) in the presence of 150 mg of erlotinib. Coadministration of erlotinib slightly reduced systemic exposure to capmatinib; however, it was not statistically significant. The same 400-mg dose of capmatinib in the tablet formulation demonstrates a higher drug exposure with greater variation (Appendix Table A2) compared with the capsule formulation, although it was not statistically significant. In addition, erlotinib showed a dose-dependent change of systemic drug exposure (150 mg v 100 mg; Appendix Fig A1B and Appendix Table A2); coadministration with capmatinib did not have significant impact on erlotinib PK (Appendix Table A2). Overall, the range of erlotinib exposures during dose escalation was similar to previously published results.28
Efficacy
Across all evaluable patients, the ORR was 31% (8/26; Fig 3). Six of the eight patients with a PR or CR had MET amplification by FISH, NGS, or both. Two of the six patients with MET amplification also had MET exon 14 mutations. Of the eight patients with PR or CR, the four EGFR-positive patients had MET amplification by FISH, NGS, or both. The remaining one patient (patient 17) with MET exon 14 mutation and equivocal amplification had SD.
Across all patients, the median PFS was 3 months. Among patients with PR or CR, the responses were prolonged, apart from patient 24, who discontinued study after 3 months because of pneumonia/pneumonitis; investigators could not rule out possible drug-related pneumonitis, and thus the patient was discontinued from study. Two responders were treatment naïve (20 and 24); the remainder had at least one prior regimen (Table 1).
Dose-Escalation Cohort
Fifteen of the 18 patients in the dose-escalation cohort were evaluable. For two patients (patients 5 and 14), the only RECIST measurable lesions became unmeasurable during treatment. The third patient (patient 15) developed symptomatic brain metastases after one dose of study drug and discontinued study. Patient 16 (EGFR WT, MET IHC 3+) on dose level 6 had a CR. Ten patients demonstrated SD. Of the evaluable patients in the dose-escalation cohort, the ORR was 7% (n = 1/15), and the disease control rate (DCR) was 73% (n = 11/15; Table 3).
TABLE 3.
Efficacy Data Across Dose-Escalation Cohort
Expansion Cohorts
In expansion cohort A, eight of 12 patients with EGFR activating mutations with acquired resistance to an EGFR TKI were response evaluable. Two patients (patients 21 and 23) withdrew from the study by individual choice, one patient (patient 33) developed new brain metastasis and withdrew, and one patient (patient 30) had a prolonged hospitalization for a cerebrovascular event deemed unrelated to study drugs. Despite the small numbers of patients, both the ORR and DCR in this group were 50% (four patients; Table 4). Patients were on treatment of a median of 2 cycles (range, 1-33 cycles). Patient 22, who had a CR, harbored an EGFR L858R mutation and MET amplification and an IHC 3+ score. Patients 26 and 27 both had PRs with EGFR alterations, exon 19 deletion and L858R, respectively, and IHC 3+ but did not demonstrate MET amplification.
TABLE 4.
Efficacy Data Across Dose-Expansion Cohorts

Five EGFR-WT patients were enrolled in cohort B; one patient (patient 25) was not evaluable because of death during cycle 1 deemed unrelated to the study drugs. The ORR and DCR were both 75% (3/4 patients), and patients were on treatment of a median of three cycles (range, 1-45 cycles; Table 4). All three responding patients had a MET IHC score of 3+, two had MET exon 14 mutations (patients 24 and 32), and one had MET amplification (patient 20; Figs 2 and 3). Notably, patient 32, with a MET exon 14 mutation, is still on treatment with response.
DISCUSSION
MET alterations are well-established mediators of EGFR TKI resistance. Given that MET and EGFR have overlapping and complementary activation of growth and proliferation pathways, the clinical evaluation of the combination of MET and EGFR TKIs as a potential strategy to improve EGFR TKI activity and prevent/overcome MET-driven EGFR-TKI resistance in EGFR-mutant NSCLC is rational. In this study, we demonstrated that standard-dose erlotinib can be safely combined with capmatinib.
At the recommended phase II dosing, responses were seen in patients with MET amplification by NGS and/or 3+ IHC expression and those with MET exon 14 mutations, but not in patients with 2+ IHC expression. A study in Chinese patients with gefitinib plus capmatinib also reported that 2+ IHC expression was not predictive of response unless it was accompanied by a gene copy number of ≥ 5.29 The MET/CEN7 ratios were not fully reported in this study, but a ratio > 2.2 is considered positive.29 In addition, recently presented data demonstrated an ORR of 29% in previously treated and 40% in first-line patients with MET amplification treated with capmatinib.30 These collective findings demonstrate that MET amplification is a predictive biomarker of response to MET-TKIs.
Since initiation of the current study, MET exon 14 mutations were demonstrated to be predictive of response to crizotinib as well as capmatinib.26,31-34 Given this, cohort B, which was originally designed to determine whether there was a signal for response in EGFR mutant–negative, MET-positive patients, evolved to focus on cases with MET exon 14 mutation. The therapeutic contribution of erlotinib in these patients, if any, cannot be disentangled and should be evaluated in a larger study. Overall, our study is consistent with several reports showing efficacy in EGFR WT patients with MET exon 14 mutation or MET amplification.
Hepatocyte growth factor is the principal ligand for the MET receptor. Serial blood HGF levels were measured to evaluate baseline levels and treatment-related changes in patients over time; however, no overt correlations with baseline HGF levels and treatment outcomes were observed (data not shown).
The patient population with the greatest potential to benefit from the combination treatment was cohort A, which enrolled EGFR-positive patients who had experienced progression on EGFR TKIs. Four of nine evaluable patients showed durable benefit from combination capmatinib and erlotinib. These results are consistent with those from the TATTON trial (ClinicalTrials.gov identifier: NCT02143466) of osimertinib plus savolitinib (a selective and potent MET inhibitor) in patients with EGFR-mutated, T790M-negative tumors who have failed an EGFR TKI and with evidence of MET dysregulation.35,36 Our slightly lower ORR can be attributed to the inclusion of four patients with T790M and/or C797S resistance mutations, all of whom did poorly.
Limitations to this study include the study design, which was executed before the more nuanced recognition of the best methods for detecting predictive MET abnormalities and before the oncogenic activity of MET exon 14 mutations was fully clarified.37,38 Second, we cannot delineate whether there is any additional benefit from the addition of erlotinib to single-agent capmatinib in patients with EGFR WT NSCLC with MET amplification and exon 14 mutations. Finally, we acknowledge that differences in testing modalities and “positivity” determinations remain a challenge for MET amplification.14
In conclusion, we have demonstrated that full doses of capmatinib and erlotinib can be safely co-administered. More work is needed to determine whether combination therapies are effective in TKI-naïve patients. As MET amplification is a major mechanism of osimertinib resistance, the evaluation of capmatinib with osimertinib may provide more durable response to treatment as frontline therapy than monotherapy. Additional evaluation of an EGFR-TKI plus a MET-TKI to overcome this common resistance mechanism is warranted.
ACKNOWLEDGMENT
We thank patients and their families for participation in this study.
Appendix
FIG A1.

Pharmacokinetic profiles of patients in dose escalation. (A) Capmatinib (C) concentration as a function of time and erlotinib (E) dosing. (B) E concentration as a function of time and dose of C. BID, twice a day; D, daily.
TABLE A1.
Treatment-Related Adverse Events by Cohort and Dose Level
TABLE A2.
Pharmacokinetic Parameters
PRIOR PRESENTATION
Presented at the International Association for the Study of Lung Cancer 16th World Conference on Lung Cancer, Denver, CO, September 6-9, 2015.
SUPPORT
Supported by Novartis Pharmaceuticals.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Caroline E. McCoach, Aiming Yu, David R. Gandara, Philip C. Mack, Laurel A. Beckett, Karen Kelly
Provision of study material or patients: David R. Gandara, Jonathan W. Riess, Tiahong Li, Primo N. Lara, Matthew Gubens, Frances Lara
Collection and assembly of data: Caroline E. McCoach, Jonathan W. Riess, Daniel P. Vang, Tiahong Li, Primo N. Lara, Matthew Gubens, Frances Lara, Philip C. Mack, Karen Kelly
Data analysis and interpretation: Caroline E. McCoach, Aiming Yu, Jonathan W. Riess, Tiahong Li, Primo N. Lara, Philip C. Mack, Laurel A. Beckett, Karen Kelly
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Caroline E. McCoach
Honoraria: Novartis, Genentech, Guardant Health
Consulting or Advisory Role: AstraZeneca
Speaker’s Bureau: Novartis
Research Funding: Novartis, Revolution Medicine
Travel, Accommodations, Expenses: Loxo, Eli Lilly, Takeda Pharmaceuticals
Aiming Yu
Stock and Other Ownership Interests: Johnson & Johnson
Patents, Royalties, Other Intellectual Property: Patents: (1) Yu AM, Wang WP, Chen QX, Li MM. Hybrid tRNA/pre-miRNA molecules and methods of use. US Patent No. 10619156, Issue date: April 14, 2020. European Patent No. 3150980, Grant date: May 20, 2020. (2) Yu AM. Method for detection of RNase activity. US Patent No. 10422003. Issue date: September 24, 2019. (3) Yu AM, Jilek JL, Zhang QY, Ho PY, Tu MJ. tRNA/pre-miRNA compositions and methods for treating hepatocellular carcinoma. US Patent Application No. 62/660919, filed on April 20, 2018. (4) Yu AM, Ho PY, Tu MJ, Jilek JL, Zhang QY, Petrek HE. tRNA/pre-miRNA compositions and use in treating cancer. US Patent Application No. 62/674939, filed on May 22, 2018.
David R. Gandara
Honoraria: AstraZeneca
Consulting or Advisory Role: AstraZeneca (I), Guardant Health (I), OncoCyte (I), Amgen, IO Biotech (I), Merck, Roche/Genentech, Boehringer Ingelheim, Inivata, Novartis
Research Funding: Roche/Genentech (I), Merck (I), Amgen (I)
Travel, Accommodations, Expenses: Boehringer Ingelheim
Jonathan W. Riess
Consulting or Advisory Role: Celgene, Medtronic, Spectrum Pharmaceuticals, Loxo, Boehringer Ingelheim, Novartis, Blueprint Medicines, Genentech
Research Funding: Merck (I), AstraZeneca/MedImmune (I), Spectrum Pharmaceuticals (I), Boehringer Ingelheim (I), Novartis (I)
Tiahong Li
Consulting or Advisory Role: Foundation Medicine, Archer Diagnostics, Eisai
Research Funding: Pfizer (I), Hengrui Therapeutics (I), Eureka (I), Merck (I), OncoImmune (I)
Primo N. Lara
Consulting or Advisory Role: Janssen
Research Funding: Aragon Pharmaceuticals (I), Janssen Biotech (I), Tracon Pharma (I), Merck (I), Pharmacyclics (I), Incyte (I), Taiho Pharmaceutical (I)
Matthew Gubens
Consulting or Advisory Role: Bristol Myers Squibb, Roche/Genentech, AstraZeneca, Heron, Boehringer Ingelheim, Takeda Pharmaceuticals, BeyondSpring Pharmaceuticals, Inivata
Research Funding: Celgene (I), Merck (I), Novartis (I), Roche/Genentech (I), OncoMed (I)
Philip C. Mack
Honoraria: Guardant Health
Consulting or Advisory Role: AstraZeneca, Guardant Health, Amgen
Research Funding: Boehringer Ingelheim
Karen Kelly
Honoraria: Merck
Consulting or Advisory Role: Roche/Genentech, Regeneron, AbbVie, EMD Serono, Merck, Pfizer, AstraZeneca, Novartis, Symphogen, Inivata, Bristol Myers Squibb, Takeda Pharmaceuticals, Eli Lilly, Amgen, Genmab, Targeted Oncology, Genentech
Research Funding: EMD Serono (I), Genentech (I), AbbVie (I), Five Prime Therapeutics (I), Regeneron (I), Astellas Pharma (I), Tizona Therapeutics, (I) Eli Lilly (I), Novartis (I), Amgen (I)
Patents, Royalties, Other Intellectual Property: Author royalties for UpToDate, an evidence-based, peer-reviewed information resource, available via the web, desktop, and PDA
Travel, Accommodations, Expenses: AstraZeneca, Roche/Genentech, Merck, Regeneron, Eli Lilly, AbbVie, EMD Serono
No potential conflicts of interest were reported.
REFERENCES
- 1.Finocchiaro G, Toschi L, Gianoncelli L, et al. : Prognostic and predictive value of MET deregulation in non-small cell lung cancer. Ann Transl Med 3:83, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Go H, Jeon YK, Park HJ, et al: High MET gene copy number leads to shorter survival in patients with non-small cell lung cancer. J Thorac Oncol 5:305-313, 2010. [DOI] [PubMed]
- 3.Dimou A, Non L, Chae YK, et al. : MET gene copy number predicts worse overall survival in patients with non-small cell lung cancer (NSCLC); a systematic review and meta-analysis. PLoS One 9:e107677, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. doi: 10.1158/1078-0432.CCR-15-2061. Tong JH, Yeung SF, Chan AW, et al: MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res 22:3048-3056, 2016. [DOI] [PubMed] [Google Scholar]
- 5. doi: 10.1038/nrc3205. Gherardi E, Birchmeier W, Birchmeier C, et al: Targeting MET in cancer: Rationale and progress. Nat Rev Cancer 12:89-103, 2012 [Erratum: Nat Rev Cancer 12:637, 2012] [DOI] [PubMed] [Google Scholar]
- 6.Engelman JA, Zejnullahu K, Mitsudomi T, et al. : MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316:1039-1043, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Bean J, Brennan C, Shih JY, et al. : MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA 104:20932-20937, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cappuzzo F, Jänne PA, Skokan M, et al. : MET increased gene copy number and primary resistance to gefitinib therapy in non-small-cell lung cancer patients. Ann Oncol 20:298-304, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Piotrowska Z, Thress KS, Mooradian M, et al: MET amplification (amp) as a resistance mechanism to osimertinib. J Clin Oncol 35, 2017 (15_suppl; abstr 9020) [Google Scholar]
- 10.Wang Y, Li L, Han R, et al. : Clinical analysis by next-generation sequencing for NSCLC patients with MET amplification resistant to osimertinib. Lung Cancer 118:105-110, 2018 [DOI] [PubMed] [Google Scholar]
- 11. doi: 10.1038/ncomms11815. Chabon JJ, Simmons AD, Lovejoy AF, et al: Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat Commun 7:11815, 2016 [Erratum: Nat Commun 7:13513, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. doi: 10.1158/1078-0432.CCR-18-1542. Le X, Puri S, Negrao MV, et al: Landscape of EGFR-dependent and -independent resistance mechanisms to osimertinib and continuation therapy beyond progression in EGFR-mutant NSCLC. Clin Cancer Res 24:6195-6203, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. doi: 10.1038/nature13385. Cancer Genome Atlas Research Network: Comprehensive molecular profiling of lung adenocarcinoma. Nature 511:543-550, 2014 [Erratum: Nature 599:E12, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. doi: 10.1016/j.jtho.2016.10.014. Drilon A, Cappuzzo F, Ou SI, et al: Targeting MET in lung cancer: Will expectations finally be MET? J Thorac Oncol 12:15-26, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patanè S, Avnet S, Coltella N, et al. : MET overexpression turns human primary osteoblasts into osteosarcomas. Cancer Res 66:4750-4757, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Wang R, Ferrell LD, Faouzi S, et al. : Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol 153:1023-1034, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spigel DR, Ervin TJ, Ramlau RA, et al. : Randomized phase II trial of onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol 31:4105-4114, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spigel DR, Edelman MJ, O’Byrne K, et al. : Results from the phase III randomized trial of onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIB or IV non-small-cell lung cancer: METLung. J Clin Oncol 35:412-420, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Sequist LV, von Pawel J, Garmey EG, et al. : Randomized phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small-cell lung cancer. J Clin Oncol 29:3307-3315, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Calles A, Kwiatkowski N, Cammarata BK, et al. : Tivantinib (ARQ 197) efficacy is independent of MET inhibition in non-small-cell lung cancer cell lines. Mol Oncol 9:260-269, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. doi: 10.1158/1078-0432.CCR-11-1157. Liu X, Wang Q, Yang G, et al: A novel kinase inhibitor, INCB28060, blocks c-MET-dependent signaling, neoplastic activities, and cross-talk with EGFR and HER-3. Clin Cancer Res 17:7127-7138, 2011. [DOI] [PubMed] [Google Scholar]
- 22. Bang Y-J, Su W-C, Nam D-H, et al: Phase I study of the safety and efficacy of INC280 in patients with advanced MET-dependent solid tumors. J Clin Oncol 32, 2014 (15_suppl; abstr 2520) [Google Scholar]
- 23.Lara MS, Holland WS, Chinn D, et al. : Preclinical evaluation of MET inhibitor INC-280 with or without the epidermal growth factor receptor inhibitor erlotinib in non-small-cell lung cancer. Clin Lung Cancer 18:281-285, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paik PK, Drilon A, Fan PD, et al. : Correction: Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov 6:330, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Paik PK, Drilon A, Fan PD, et al. : Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov 5:842-849, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jorge SE, Schulman S, Freed JA, et al. : Responses to the multitargeted MET/ALK/ROS1 inhibitor crizotinib and co-occurring mutations in lung adenocarcinomas with MET amplification or MET exon 14 skipping mutation. Lung Cancer 90:369-374, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Fukudo M, Ikemi Y, Togashi Y, et al. : Population pharmacokinetics/pharmacodynamics of erlotinib and pharmacogenomic analysis of plasma and cerebrospinal fluid drug concentrations in Japanese patients with non-small cell lung cancer. Clin Pharmacokinet 52:593-609, 2013 [DOI] [PubMed] [Google Scholar]
- 29. doi: 10.1200/JCO.2018.77.7326. Wu YL, Zhang L, Kim DW, et al: Phase Ib/II study of capmatinib (INC280) plus gefitinib after failure of epidermal growth factor receptor (EGFR) inhibitor therapy in patients with EGFR-mutated, MET factor-dysregulated non-small-cell lung cancer. J Clin Oncol 36:3101-3109, 2018 [Erratum: J Clin Oncol 37:261, 2019] [DOI] [PubMed] [Google Scholar]
- 30. Wolf J, Overbeck TR, Han J, et al: Capmatinib in patients with high-level MET-amplified advanced non–small cell lung cancer (NSCLC): Results from the phase 2 GEOMETRY mono-1 study. J Clin Oncol 38, 2020 (15_suppl; abstr 9509) [Google Scholar]
- 31. Camidge DR, Otterson GA, Clark JW, et al: Crizotinib in patients (pts) with MET-amplified non-small cell lung cancer (NSCLC): Updated safety and efficacy findings from a phase 1 trial. J Clin Oncol 36, 2018 (15_suppl; abstr 9062) [Google Scholar]
- 32. doi: 10.1016/j.jtho.2016.09.116. Caparica R, Yen CT, Coudry R, et al: Responses to crizotinib can occur in high-level MET-amplified non-small cell lung cancer independent of MET exon 14 alterations. J Thorac Oncol 12:141-144, 2017. [DOI] [PubMed] [Google Scholar]
- 33. doi: 10.1016/j.jtho.2018.09.001. Lin JJ, Chin E, Yeap BY, et al: Increased hepatotoxicity associated with sequential immune checkpoint inhibitor and crizotinib therapy in patients with non-small-cell lung cancer. J Thorac Oncol 14:135-140, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wolf J, Seto T, Han J, et al: Results of the GEOMETRY mono-1 phase II study for evaluation of the MET inhibitor capmatinib (INC280) in patients with METΔex14 mutated advanced non-small cell lung cancer. Presented at ESMO 2018 Congress, Munich, Germany, October 19-23, 2018; Munich, Germany. [Google Scholar]
- 35. Yu H, Ahn M, Kim S, et al: CT032 - TATTON phase Ib expansion cohort: Osimertinib plus savolitinib for patients (pts) with EGFR-mutant, MET-amplified NSCLC after progression on prior first/second-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI). Presented at AACR Annual Meeting 2019, Atlanta, GA, March 31, 2019. [Google Scholar]
- 36. Sequist L, Lee JS, Han J, et al: CT033 - TATTON phase Ib expansion cohort: Osimertinib plus savolitinib for patients (pts) with EGFR-mutant, MET-amplified NSCLC after progression on prior third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI). Presented at AACR Annual Meeting 2019, Atlanta, GA, March 31, 2019. [Google Scholar]
- 37. Paik PK, Veillon R, Cortot AB, et al: Phase II study of tepotinib in NSCLC patients with METex14 mutations. J Clin Oncol 37, 2019 (15_suppl; abstr 9005) [Google Scholar]
- 38. Wolf J, Seto T, Han J, et al: Capmatinib (INC280) in METΔex14-mutated advanced non-small cell lung cancer (NSCLC): Efficacy data from the phase II GEOMETRY mono-1 study. J Clin Oncol 37, 2019 (15_suppl; abstr 9004) [Google Scholar]