Abstract
PURPOSE
To assess the association between the Oncotype DX Genomic Prostate Score (GPS) result and long-term oncological outcomes following radical prostatectomy (RP).
METHODS
We evaluated the association of the GPS result assayed from the index lesion from RP tissue with the risk of distant metastases (DM) and prostate cancer–specific mortality (PCSM) over the 20 years following RP in a stratified cohort sample of 428 patients from 2,641 treated between 1987 and 2004. Cox regression of cause-specific hazards was used to estimate the absolute risk of both end points, with death from other causes treated as a competing risk. A correction for regression to the mean (RM) was applied since the GPS test was developed using this cohort. Exploratory analysis using presurgical parameters and the GPS test as prognostic variables was performed to assess the additional value of the GPS test on 20-year risk of DM and PCSM. Model discrimination was measured using the area under the receiver operating characteristic curve.
RESULTS
The GPS test appears to be independently associated with both 20-year risk of DM and PCSM with a low false discovery rate. Per 20-unit increase in GPS, multivariable analysis with RM correction estimated hazard ratios of 2.24 (95% CI, 1.49 to 3.53) and 2.30 (95% CI, 1.45 to 4.36) for DM and PCSM, respectively. Accuracy of models including clinical risk factors alone appeared to improve when including the GPS test in assessing risk of both end points.
CONCLUSION
The results suggest that the GPS test provides information on the risk for the meaningful long-term outcomes of DM and PCSM.
INTRODUCTION
Long-term cancer outcomes are an important consideration when deciding between active surveillance (AS) and immediate treatment for newly diagnosed prostate cancer. Multiple prospective AS studies that predominantly include patients at the lowest risk of progression have demonstrated a low risk of distant metastasis (DM) and prostate cancer–specific mortality (PCSM) with extended follow-up.1,2 However, these excellent outcomes with AS require strict selection criteria and stringent follow-up with frequent repeat prostate biopsies. Based on these studies, the current use of AS for newly diagnosed men is increasing and now includes expanded selection criteria, including younger men with longer life expectancy and those with biopsy-defined pathologic features that fall outside eligibility criteria of older studies.3 It is unknown whether the excellent long-term AS outcomes observed for low-risk disease can be maintained in this expanded group using only traditional clinical variables and existing surveillance strategies. For example, although both PIVOT and ProtecT showed no statistically significant difference in PCSM between observation and immediate treatment for intermediate-risk patients with up to a 12.7-year follow-up,4,5 the confidence intervals for the hazard ratio (HR) do not exclude substantial benefit of radical prostatectomy (RP). Furthermore, the long-term results of Scandinavian Prostate Cancer Group Trial 4 demonstrated an 11.7% absolute risk reduction in PCSM with RP, corresponding to a relative risk of 0.55, compared with conservative management.6 With up to a 29-year follow-up, the results of this study are more reflective of the lifetime risk of cancer recurrence and death in men with intermediate- or high-risk features. As such, development of tools that assess long-term risk for meaningful clinical events such as DM and PCSM more accurately than clinical variables alone is needed.
CONTEXT
Key Objective
Localized prostate cancer has an extended natural history, making immediate treatment decisions difficult without the understanding of long-term cancer risks. Risk calculators based on clinical variables alone are imperfect. We sought to evaluate the association between the Genomic Prostate Score (GPS) test and long-term prostate cancer outcomes: distant metastasis and prostate cancer–specific mortality following radical prostatectomy.
Knowledge Generated
The GPS test appeared to be associated with both distant metastasis and prostate cancer–specific mortality at a 20-year follow-up in both univariable and multivariable models, where model discrimination was improved when the GPS results were included.
Relevance
The use of the GPS test can provide risk assessment of long-term prostate cancer outcomes, beyond clinical factors alone, for patients with localized prostate cancer.
Multiple studies have validated the ability of the biopsy-based 17-gene expression profile Genomic Prostate Score (GPS, Genomic Health, a wholly owned subsidiary of Exact Sciences) test to improve accuracy over traditionally used clinical information in assessing risk of adverse pathology (AP) at RP.7,8 Van Den Eeden et al9 established the association of the GPS test with risk of DM and PCSM over 10 years. In this report, we sought to evaluate whether the GPS test is also associated with 20-year risk of DM and PCSM and whether its use improves risk assessment for these end points compared with clinical variables alone.
METHODS
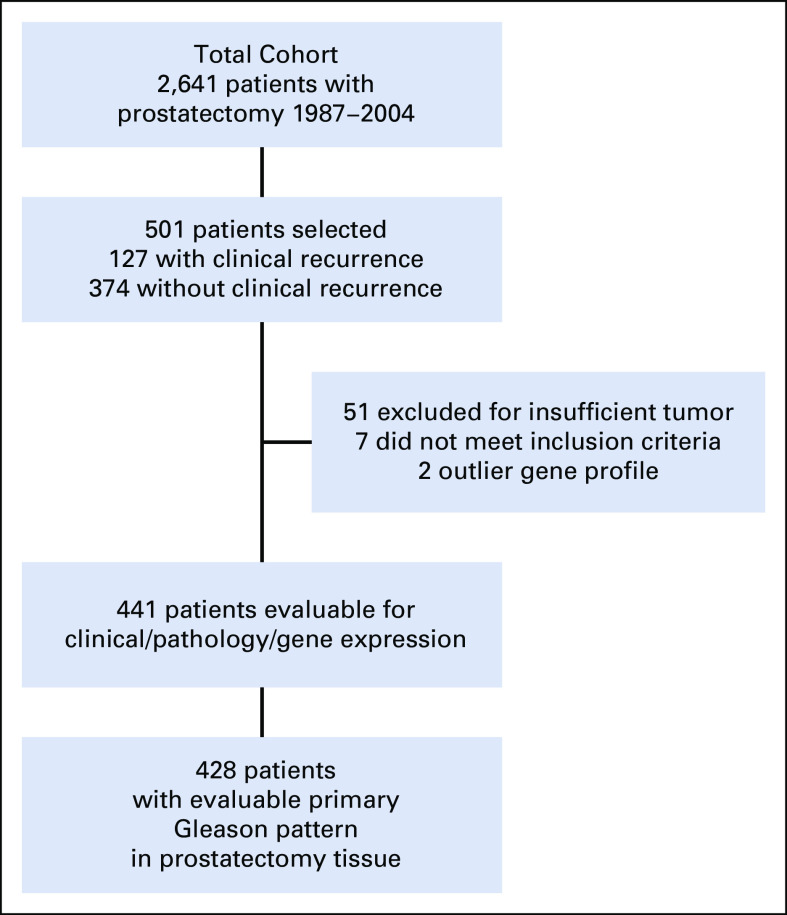
Using a stratified cohort sampling design, so that a weighted analysis of the study cohort is representative of all patients (N = 2,641) who underwent RP between 1987 and 2004 at our institution, we selected 501 patients for inclusion. This design was chosen because at the time of the original study, it was not feasible to perform the GPS test on all 2,641 patients. A 1:3 sampling was used, where all patients who experienced clinical recurrence (local recurrence determined by biopsy and distant metastasis determined by imaging or biopsy) were included, and controls were selected at random from the 2,514 patients who did not experience clinical recurrence, stratifying by surgery year (1987-1992 v 1993-2004), clinical T-Stage (T1 v T2), and surgical Gleason Score (≤ 7 v > 7). Therefore, the stratified cohort included all 127 patients who did experience clinical recurrence and 374 patients who did not have a recurrence.10,11 Because of variability in its clinical significance, biochemical recurrence (which occurred in 31.5% of the cohort, a rate typical for those treated by RP in this era) was not included as an end point. Fifty-four percent of the patients in the cohort underwent surgery in the year 2000 or later (see Table 1 for patient characteristics). The index lesion from the RP specimen was used for determination of the GPS test result. Sixty patients were then excluded because of pathology or laboratory failures. Among the remaining 441 patients, the final analysis cohort comprised the 428 patients with evaluable primary Gleason pattern in prostatectomy tissue (Fig 1).
TABLE 1.
Characteristics of the Patients in the Cohort Sample
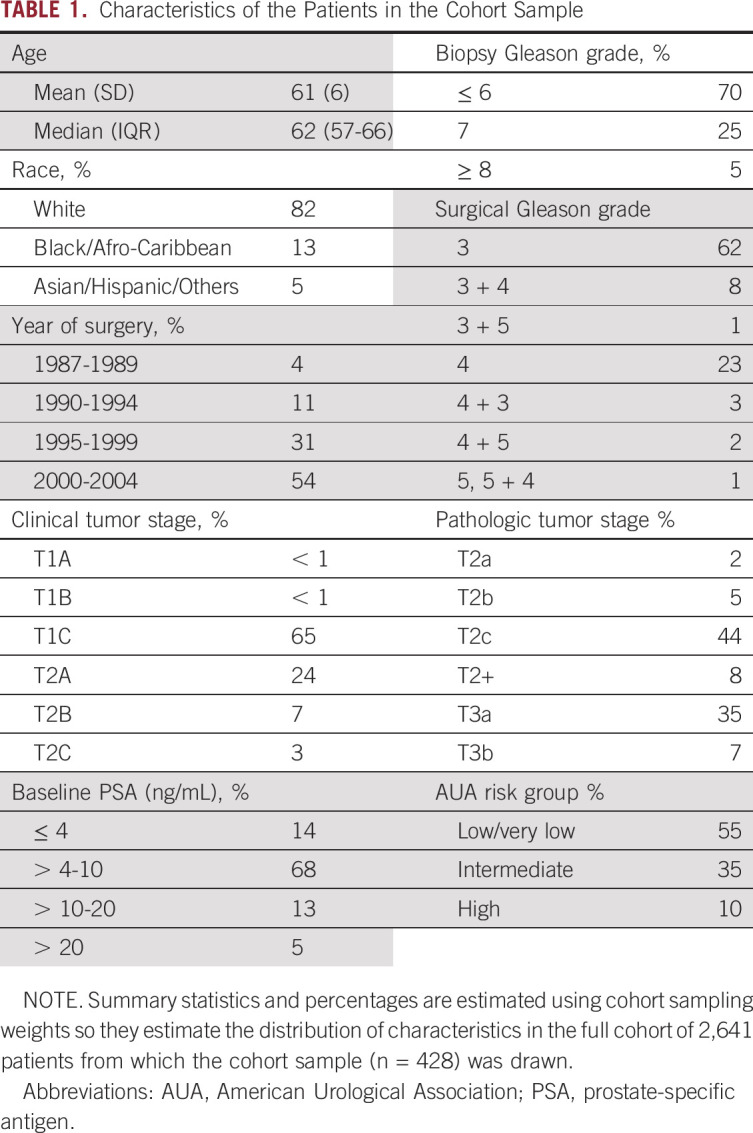
FIG 1.
CONSORT diagram of the patient cohort. Of 2,641 patients who underwent RP between 1987 and 2004, a cohort of 501 patients was selected using a 1:3 sampling design, which included all 127 patients who did and 374 patients who did not have a clinical recurrence. The final analysis cohort consisted of 428 patients with evaluable primary Gleason pattern in the RP tissue and valid GPS results after excluding pathological and laboratory failures. GPS, Genomic Prostate Score; RP, radical prostatectomy.
All patients were selected from our prospective Institutional Review Board (IRB)–approved database that includes clinical staging, pathology from biopsy and RP, and follow-up information. Follow-up and outcome data were obtained through subsequent clinic visits, telephone calls, and semiannual follow-up letters obtained through August 1, 2019, approximately 10 years after the previously reported cutoff.7 Multiple data reviews and quality checks were performed to ensure fidelity of the data set.
High grade at RP was defined as grade group 3 or above (Gleason Score 4 + 3 or higher). High stage was defined as nonorgan-confined disease including extraprostatic extension, seminal vesicle invasion, or lymph node metastases. Pathologic staging was determined by central review of RP specimens by an expert genitourinary pathologist (C.M.-G.).
Statistical analysis was performed by M.C. and independently verified by J.L. All statistical analysis was performed using SAS version 9.4 with SAS/Stat Version 14.1. Weighted Cox proportional hazards regression models were used to analyze time to DM and PCSM. The time to event in patients who died without an event or who were alive without an event at the end of follow-up was considered censored in these analyses. Each patient was weighted using the inverse sampling fraction in his stratum. Lin and Wei’s robust variance estimate was used.12
In the multivariable analysis, the GPS result, preoperative prostate–specific antigen (PSA), clinical stage, and biopsy grade were used as covariables. The HR for continuous GPS results was reported per 20-GPS unit increase, which approximated the difference between the average GPS value in the highest 25% and the average GPS value in the lowest 25% of patients based on the GPS value distribution from previous studies.7,13 Preoperative PSA value was included in the model as Log2(PSA value). As previously described,7 follow-up data available from this cohort at the time were used in the development of the GPS test. Although the current analyses include approximately 10 years of additional follow-up, there is still a strong potential for overoptimism of effect estimates involving the GPS test. Therefore, all estimates using the GPS test as a covariable were corrected for regression to the mean (RM)14 and false discovery rates (FDRs) were reported rather than P values (Data Supplement). For other variables, two-sided P values were calculated using the Wald test. P values < .05 were considered statistically significant.
Cox regression of cause-specific hazards13 was used to estimate the absolute risk of each outcome, with other-cause mortality considered a competing risk. The cause-specific models for other-cause mortality each had a time-dependent covariate for current age, computed as age at surgery plus elapsed time since surgery. Applying the RM correction for GPS test, the absolute risk estimates were computed as the weighted average of the absolute risk estimates over the study population patient age for each AP status, with CIs derived using the delta method. Receiver operating characteristic (ROC) curve estimates were based on these absolute risk estimates, correcting for bias inherent in ROC curves (Data Supplement), with and without the GPS results as a covariable. Differences between models in the ROC area under the curve (AUC) were tested for significance using the bootstrap.
RESULTS
The overall cohort consisted of predominantly American Urological Association (AUA) low- and intermediate-risk patients with prostate cancer, 55% and 35%, respectively (Table 1). Of the 428 patients in the cohort, 105 patients experienced DM and 53 patients experienced PCSM during follow-up. The median follow-up for censored patients was 15.5 years (IQR, 14.6-16.6 years). The median GPS result was 26 (IQR, 19-39 units). The distribution of GPS results, accounting for the stratified cohort sampling weighting, in the overall population and by biopsy Gleason grade is shown in Figure 2.
FIG 2.
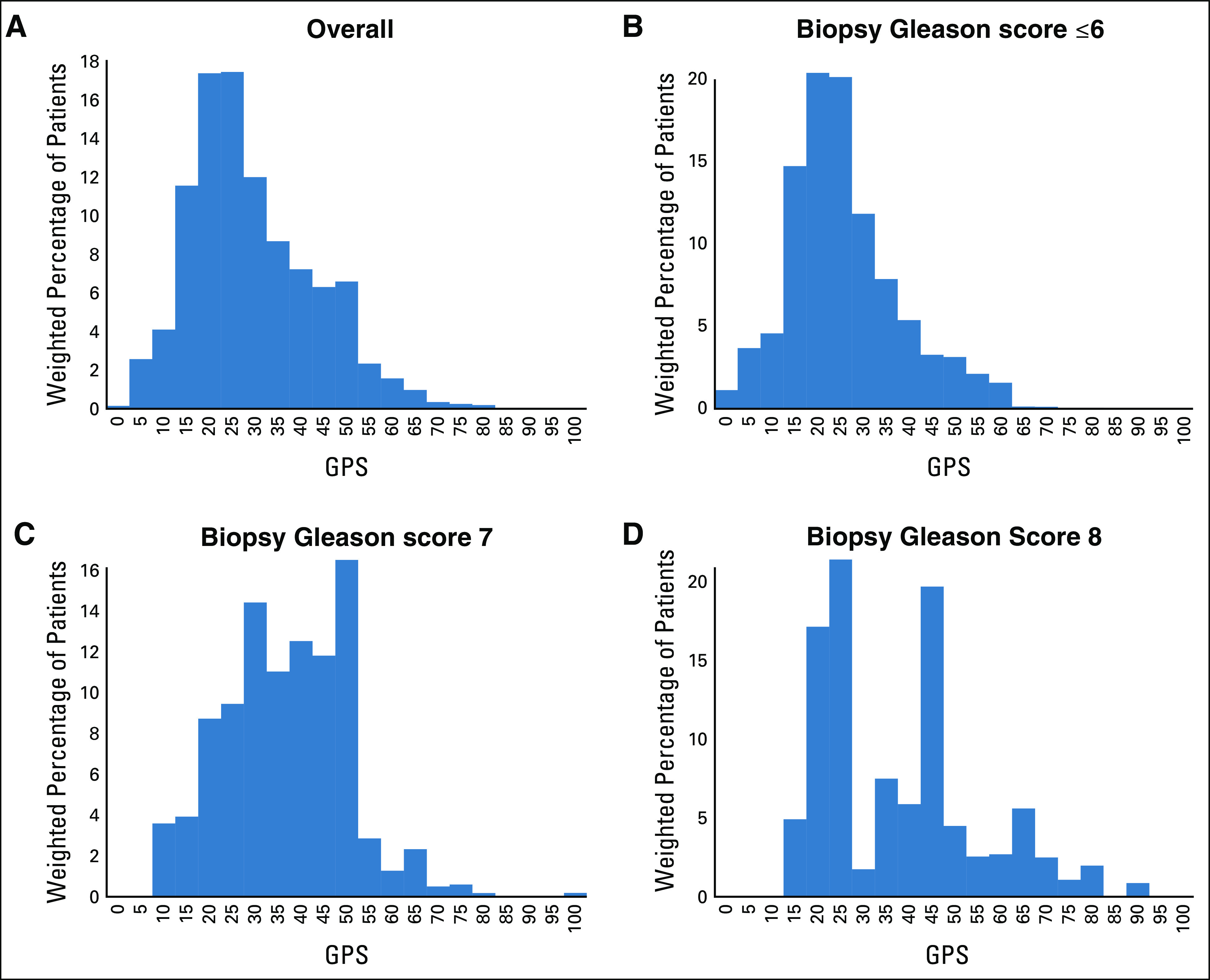
Histogram of the GPS results in study population, accounting for cohort sampling weighting in the overall population and by Biopsy Gleason score. GPS, Genomic Prostate Score.
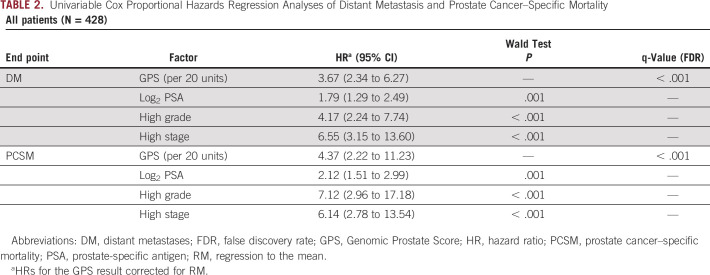
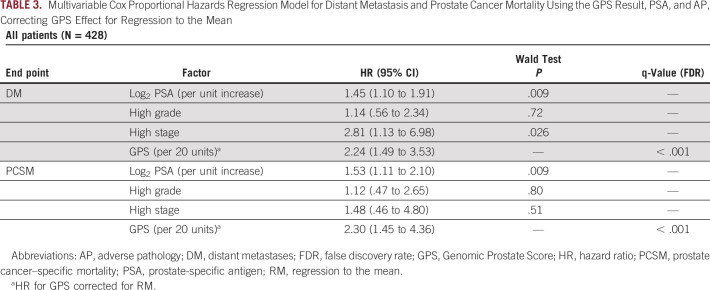
The GPS result appeared to be highly associated with both DM and PCSM at 20 years of follow-up. On univariable analysis, the RM-corrected HR for GPS results per 20 units was 3.67 (95% CI, 2.34 to 6.27) and 4.37 (95% CI, 2.22 to 11.23) for DM and PCSM, respectively (q-value < 0.001, both). Additionally, Log2PSA, High Grade, and High Stage were each associated with both outcomes on univariable analysis (Table 2). On multivariable analysis, the GPS test appeared to remain highly associated with both DM and PCSM (RM-corrected HR, 2.24; 95% CI, 1.49 to 3.53, HR, 2.30; 95% CI, 1.45 to 4.36, respectively), whereas among the clinical variables, only Log2PSA and High Stage remained significantly associated with DM (HR per unit increase, 1.45; 95% CI, 1.10 to 1.91, HR, 2.81; 95% CI, 1.13 to 6.98, respectively) and only Log2PSA remained significantly associated with PCSM (HR, 1.53; 95% CI, 1.11 to 2.10) (Table 3).
TABLE 2.
Univariable Cox Proportional Hazards Regression Analyses of Distant Metastasis and Prostate Cancer–Specific Mortality
TABLE 3.
Multivariable Cox Proportional Hazards Regression Model for Distant Metastasis and Prostate Cancer Mortality Using the GPS Result, PSA, and AP, Correcting GPS Effect for Regression to the Mean
RM-corrected estimates of the 20-year absolute risk of DM and PCSM as a function of the GPS result indicated a low risk of both outcomes with the GPS result < 20 and a large increase in risk with the GPS result > 40 (Fig 3). When the model assessing risk of DM included the GPS results in addition to Log2PSA, High Grade, and High Stage, the ROC AUC appeared to improve to an estimated 0.824 compared with an AUC of 0.772 for the model with clinical factors alone (two-sided P < .005 based on 400 bootstrap replications; Fig 4A). Similarly, the model discrimination for PCSM appeared to improve the AUC to an estimated 0.822 from 0.762 when including the GPS results as a prognostic variable (two-sided P < .005 based on 400 bootstrap replications; Fig 4B).
FIG 3.
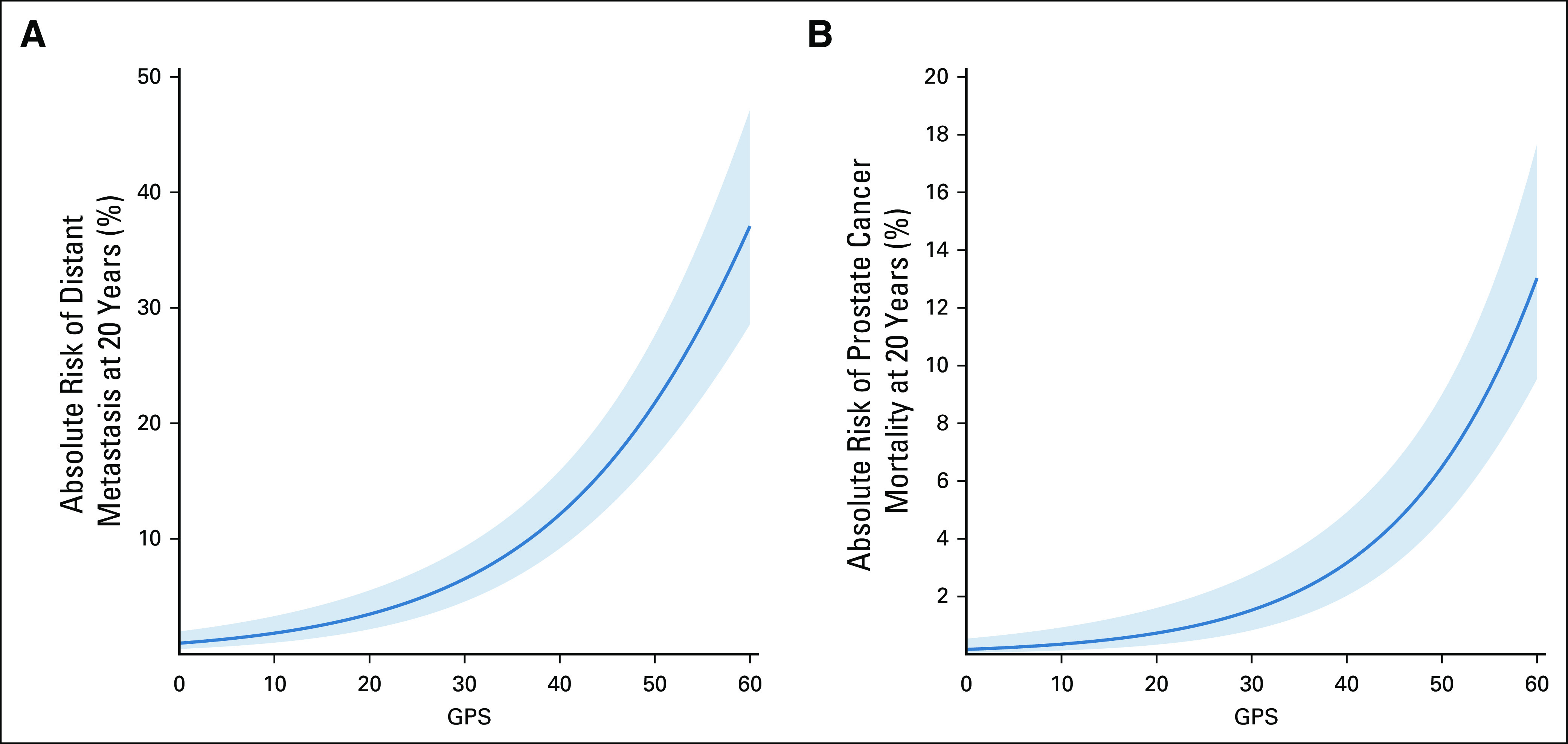
RM-corrected estimates with 95% confidence intervals of the 20-year absolute risk of distant metastasis (A) and prostate cancer–specific mortality (B) as a function of the GPS result. GPS, Genomic Prostate Score; RM, regression to the mean.
FIG 4.
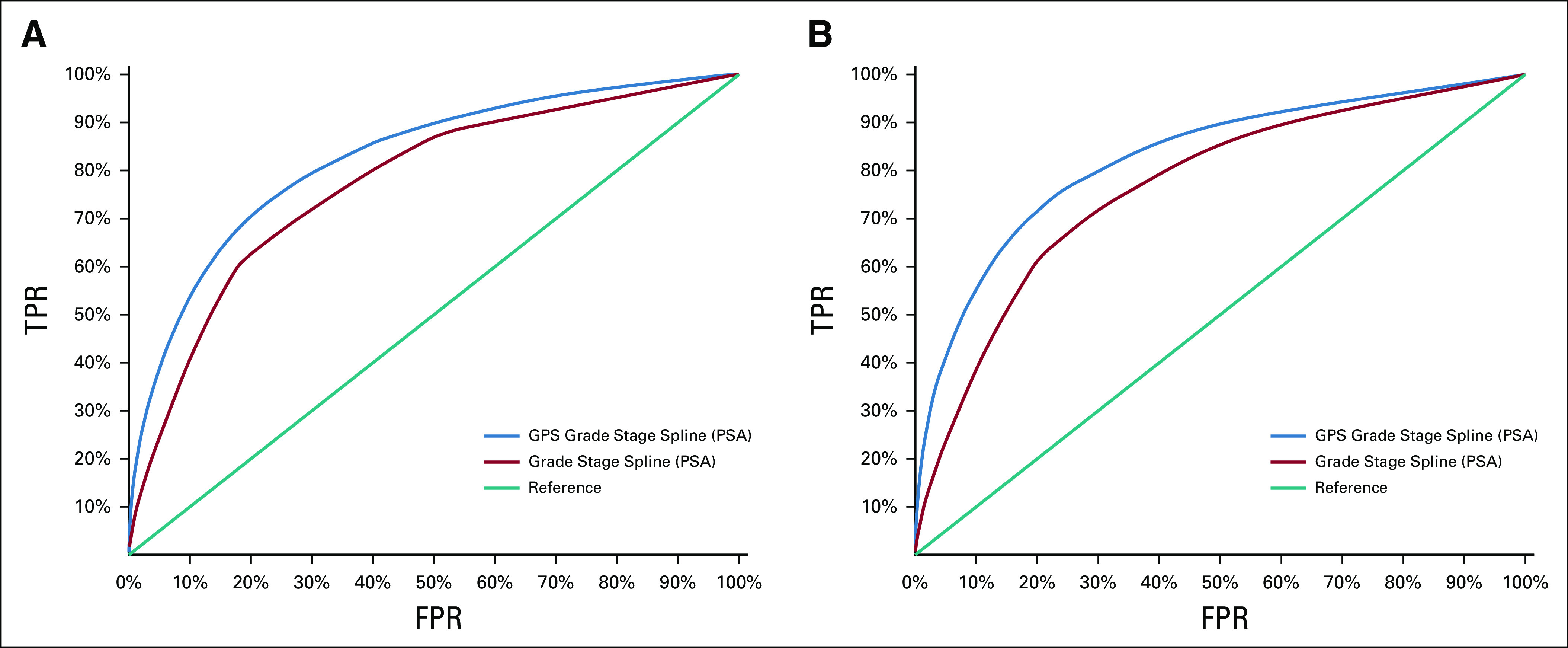
Model-based RM-corrected, bias-corrected ROC curve for DM (A) and PCSM (B) within 20 years of surgery with covariables: the GPS result, high stage, high grade, and log2 PSA. The AUC is 0.824 (DM) and 0.822 (PCSM) for the GPS result, grade, stage, and 2 df natural cubic spline applied to log2 PSA, compared with 0.772 (DM) and 0.762 (PCSM) for grade, stage, and 2 df natural cubic spline applied to log2 PSA (both P < .005 based on 400 bootstrap replications). AUC, area under the curve; DM, distant metastases; FPR, false positive rate; GPS, Genomic Prostate Score; PCSM, prostate cancer–specific mortality; PSA, prostate-specific antigen; RM, regression to the mean; ROC, receiver operating characteristic; TPR, true positive rate.
Among the 428 patients in the analysis data set, there were 239 in the AUA low-risk group and favorable intermediate–risk group (an estimated 86% of the overall population accounting for the cohort sampling weights), 33 of whom experienced distant metastasis and 16 who died because of prostate cancer. The distribution of the GPS results among AUA low- and intermediate-risk patients is shown in the Data Supplement. On univariable analysis, the RM-corrected HRs for the GPS result per 20 units were 3.12 (95% CI, 2.40 to 9.30) and 2.86 (95% CI, 2.13 to 11.88) for DM and PCSM, respectively (q-value < 0.001, both). RM-corrected estimates of the 20-year absolute risk of DM and PCSM as a function of GPS result among AUA low-risk patients and favorable intermediate–risk patients have functional form similar to the estimates for the overall population, with a large increase in risk for the GPS result > 40 (Data Supplement). As expected, the risk of these events in the AUA low- and intermediate-risk patients is substantially lower than that in the overall population. The number of events in this subgroup was insufficient for multivariable analysis.
DISCUSSION
Previous validation studies have clearly shown the GPS test and other prostate biopsy–based gene expression profiles to predict the presence of AP (grade group 3 or higher or extraprostatic disease) in RP specimens or serial needle biopsies in men on AS or post-RP.7,8,15 Although AP on biopsy or RP is prognostic regarding risk of recurrence, DM, and PCSM,16 its use as a surrogate end point for these outcomes in men considering starting or staying on surveillance has been questioned with no clear short-term alternative.17 In this study, we sought to assess whether the GPS result, based on pathologic evaluation of the index lesion on RP, in the original discovery cohort of this biomarker appeared to be associated with the more clinically meaningful outcomes of DM and PCSM at long-term follow-up. Indeed, we observed that with a follow-up extending to 20 years (median, 15.5; IQR, 14.6-16.6), the GPS test was highly associated with risk of DM and PCSM. Absolute risks of these outcomes, considering death from causes other than prostate cancer as a competing risk, showed a low-slope relationship with the GPS result from 1 to 29, with an inflection point evident at a score ≥ 30, above which the risk of DM or PCSM increased substantially, reaching an absolute risk of 38% for DM and 13% for PCSM at a score of 60 (Fig 3). Furthermore, we found that including the GPS results in models assessing risk of DM and PCSM at 20 years improved discrimination compared with using clinical variables alone (Tables 2 and 3; Fig 4A and 4B). These results confirm and extend a previous independent validation of these end points in a large US Healthcare system with shorter follow-up (median 9.8 years) that demonstrated that the GPS test was also highly associated with both risk of and time to DM and PCSM, with added prognostic value for both end points over both AUA and CAPRA risk stratifications.9 In that study, the multivariable HR/20 GPS units for DM was 2.34 (95% CI, 1.42 to 3.86) and the HR/20 GPS units for PCSM was 2.69 (95% CI, 1.50 to 4.82), similar to those observed in this study (HR, 2.24; 95% CI, 1.49 to 3.53 and HR, 2.30; 95% CI, 1.45 to 4.36, respectively), suggesting that these risk estimates are robust across independent cohorts. Other commercially available gene expression profiles have reported similar findings, albeit at earlier time points.18-21
Because key drivers of biological progression in patients on AS are yet to be elucidated, an open question is what aspect of tumor biology commercially available gene expression profiles may be measuring. Detailed pathological analysis of the Canary-PASS study, a large, well-characterized AS cohort, identified the presence of cribriform or stromagenic histology as the strongest predictor of cancer recurrence after treatment in men who progressed to requiring therapy.22 The presence of cribriform glands has been shown in other studies to be associated with higher rates of biochemical recurrence after RP or radiation, as well as both DM and PCSM,23,24 likely driven by the observation that these glands exhibit genomic features characteristic of aggressive disease.25 In a recent study of 194 men with National Comprehensive Cancer Network very low-, low-, or intermediate-risk disease considering AS, we observed that only those with the GPS results above 29 had cribriform histology present on biopsy (Falzarano et al, manuscript submitted for publication). Furthermore, higher GPS scores have also been shown to be associated with the presence of stromagenic features.26 Kornberg et al27 observed that a GPS result ≥ 29 was associated with grade reclassification on subsequent biopsy in a cohort of low- and intermediate-risk men on AS, whereas in an underpowered study using a cohort of lower-risk men whose mean GPS results were 21, no association with grade progression was observed.17 Together, these observations suggest that the higher GPS results reflect the presence of genomic drivers of tumor progression.
We recognize that there is debate over whether biopsy-based gene expression profiles can inform decisions on AS and that their utility may be limited in cases where a low score may not fully represent tumors that are clonally distinct. However, a number of published observations support a role for decision making in men with higher scores: (1) Multiple biopsy-based studies have validated that higher GPS scores are associated with traditional histologic measures of AP (defined as grade group ≥ 3 or extraprostatic disease, both of which are associated with worse outcomes)7-9; (2) The association of higher GPS scores with histologic variants (cribriform [Falzarano et al, manuscript submitted for publication] and stromagenic26 histology) shown to be associated with higher rates of recurrence in an AS cohort;22 and (3) Long-term data from the current study that link higher GPS scores to the risk of meaningful clinical end points (DM and PCSM). Thus, a higher GPS score on a biopsy with low-grade tumor could prompt a more thorough look for higher-grade disease that might have been missed on initial biopsy, prompt closer follow-up of men who stay on AS, or result in a decision to proceed to definitive treatment.
Strengths of this study include that the data were sourced from a prospectively maintained patient registry with locked baseline clinical, pathologic, and gene expression information; centralized expert pathology review; 20-year follow-up, the longest reported in any similar study; and independent verification of the statistical analysis. There are two limitations: (1) changes in standardized biopsy grading and stage migration since initial patient enrollment, although we note that all RP specimens were reviewed by an expert GU pathologist after the majority of substantive changes in prostate cancer grading occurred and (2) that this should be considered an exploratory analysis since the GPS test was developed in this cohort. Although the study by Van Den Eeden et al9 largely replicates the results of this analysis, we believe that further validation in other cohorts is important to firmly establish our conclusions.
In conclusion, with long-term follow-up, the GPS test appears to be associated with both DM and PCSM and improves the accuracy of models containing clinical variables alone. These findings suggest that genomic changes in the tumor tissue, quantified by the GPS test, provide additional biological insight into the long-term risk of DM and PCSM. This information may be valuable to those considering AS. Prospective studies should be pursued to validate these results.
AUTHOR CONTRIBUTIONS
Conception and design: Michael Crager, Ruixiao Lu, John Abran, Tamer Aboushwareb, Eric A. Klein
Financial support: Eric A. Klein
Administrative support: Tamer Aboushwareb, Eric A. Klein
Provision of study materials or patients: Eric A. Klein
Collection and assembly of data: Cristina Magi-Galluzzi, John Abran, Eric A. Klein
Data analysis and interpretation: Michael Brooks, Lewis Thomas, Jianbo Li, Michael Crager, Ruixiao Lu, John Abran, Tamer Aboushwareb, Eric A. Klein
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Michael Crager
Employment: Exact Sciences
Stock and Other Ownership Interests: Exact Sciences
Travel, Accommodations, Expenses: Genomic Health
Ruixiao Lu
Employment: Genomic Health, Exact Sciences
Stock and Other Ownership Interests: Genomic Health, Exact Sciences
Consulting or Advisory Role: Mission Bio
Patents, Royalties, Other Intellectual Property: Patent co-inventor on 17-Gene Prostate assay with Genomic Health, Inc
John Abran
Employment: Exact Sciences
Stock and Other Ownership Interests: Exact Sciences
Tamer Aboushwareb
Employment: Exact Sciences
Stock and Other Ownership Interests: Exact Sciences
Eric A. Klein
Consulting or Advisory Role: Grail
No other potential conflicts of interest were reported.
REFERENCES
- 1.Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer J Clin Oncol 33272–2772015 [DOI] [PubMed] [Google Scholar]
- 2.Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and longer-term outcomes from a prospective active-surveillance program for favorable-risk prostate cancer J Clin Oncol 333379–33852015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahal AR, Butler S, Franco I, et al. Conservative management of low-risk prostate cancer among young versus older men in the United States: Trends and outcomes from a novel national database Cancer 1253338–33462019 [DOI] [PubMed] [Google Scholar]
- 4.Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer N Engl J Med 3751415–14242016 [DOI] [PubMed] [Google Scholar]
- 5.Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer N Engl J Med 377132–1422017 [DOI] [PubMed] [Google Scholar]
- 6.Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in prostate cancer—29-year follow-up N Engl J Med 3792319–23292018 [DOI] [PubMed] [Google Scholar]
- 7.Klein EA, Cooperberg MR, Magi-Galluzzi C, et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling Eur Urol 66550–5602014 [DOI] [PubMed] [Google Scholar]
- 8.Cullen J, Rosner IL, Brand TC, et al. A biopsy-based 17-gene genomic prostate score predicts recurrence after radical prostatectomy and adverse surgical pathology in a racially diverse population of men with clinically low- and intermediate-risk prostate cancer Eur Urol 68123–1312015 [DOI] [PubMed] [Google Scholar]
- 9.Van Den Eeden SK, Lu R, Zhang N, et al. A biopsy-based 17-gene genomic prostate score as a predictor of metastases and prostate cancer death in surgically treated men with clinically localized disease Eur Urol 73129–1382018 [DOI] [PubMed] [Google Scholar]
- 10.Gray RJ.Weighted analyses for cohort sampling designs Lifetime Data Anal 1524–402009 [DOI] [PubMed] [Google Scholar]
- 11.Chen K, Lo SH.Case-cohort and case-control analysis with Cox's model Biometrika 86755–7641999 [Google Scholar]
- 12.Lin DY, Wei LJ.The robust inference for the Cox proportional hazards model J Am Stat Assoc 841074–10781989 [Google Scholar]
- 13.Prentice RL, Kalbfleisch JD, Peterson AV, et al. The analysis of failure times in the presence of competing risks Biometrics 34541–5541978 [PubMed] [Google Scholar]
- 14.Crager MR.Gene identification using true discovery rate degree of association sets and estimates corrected for regression to the mean Stat Med 2933–452010 [DOI] [PubMed] [Google Scholar]
- 15.Kim HL, Li P, Huang HC, et al. Validation of the Decipher Test for predicting adverse pathology in candidates for prostate cancer active surveillance Prostate Cancer Prostatic Dis 22399–4052019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eggener SE, Scardino PT, Walsh PC, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy J Urol 185869–8752011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin DW, Zheng Y, McKenney JK, et al. 17-gene Genomic Prostate Score test results in the Canary Prostate Active Surveillance Study (PASS) cohort J Clin Oncol 381549–15572020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spratt DE, Yousefi K, Deheshi S, et al. Individual patient-level meta-analysis of the performance of the Decipher genomic classifier in high-risk men after prostatectomy to predict development of metastatic disease J Clin Oncol 351991–19982017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein EA, Yousefi K, Haddad Z, et al. A genomic classifier improves prediction of metastatic disease within 5 years after surgery in node-negative high-risk prostate cancer patients managed by radical prostatectomy without adjuvant therapy Eur Urol 67778–7862015 [DOI] [PubMed] [Google Scholar]
- 20.Cooperberg MR, Davicioni E, Crisan A, et al. Combined value of validated clinical and genomic risk stratification tools for predicting prostate cancer mortality in a high-risk prostatectomy cohort Eur Urol 67326–3332015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canter DJ, Freedland S, Rajamani S, et al. Analysis of the prognostic utility of the cell cycle progression (CCP) score generated from needle biopsy in men treated with definitive therapy Prostate Cancer Prostatic Dis 23102–1072020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKenney JK, Wei W, Hawley S, et al. Histologic grading of prostatic adenocarcinoma can be further optimized: Analysis of the relative prognostic strength of individual architectural patterns in 1275 patients from the canary retrospective cohort Am J Surg Pathol 401439–14562016 [DOI] [PubMed] [Google Scholar]
- 23.Kweldam CF, Wildhagen MF, Steyerberg EW, et al. Cribriform growth is highly predictive for postoperative metastasis and disease-specific death in Gleason score 7 prostate cancer Mod Pathol 28457–4642015 [DOI] [PubMed] [Google Scholar]
- 24.Kweldam CF, Kümmerlin IP, Nieboer D, et al. Prostate cancer outcomes of men with biopsy Gleason score 6 and 7 without cribriform or intraductal carcinoma Eur J Cancer 6626–332016 [DOI] [PubMed] [Google Scholar]
- 25.Böttcher R, Kweldam CF, Livingstone J, et al. Cribriform and intraductal prostate cancer are associated with increased genomic instability and distinct genomic alterations. BMC Cancer. 2018;18:8. doi: 10.1186/s12885-017-3976-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenland NY, Cowan JE, Chan E, et al. Prostate biopsy histopathologic features correlate with a commercial gene expression assay's reclassification of patient NCCN risk category Prostate 801421–14282020 [DOI] [PubMed] [Google Scholar]
- 27.Kornberg Z, Cooperberg MR, Cowan JE, et al. A 17-gene genomic prostate score as a predictor of adverse pathology in men on active surveillance J Urol 202702–7092019 [DOI] [PubMed] [Google Scholar]