Abstract
Introduction:
Normal placental vascular development is influenced by inflammatory, angiogenic and apoptotic processes, which may be modulated by choline through its role in membrane biosynthesis, cellular signaling and gene expression regulation. The current study examined the effect of maternal choline supplementation (MCS) on placental inflammatory, angiogenic and apoptotic processes during murine pregnancy.
Method:
Pregnant dams were randomized to receive 1, 2 or 4 times (X) the normal choline content of rodent diets, and tissues were harvested on embryonic day (E) 10.5, 12.5, 15.5 or 18.5 for gene expression, protein abundance and immunohistochemical analyses.
Results:
The choline-induced changes in the inflammatory and angiogenic markers were a function of fetal sex. Specifically, 4X (versus 1X) choline reduced the transcript (P ≤ 0.05) and protein (P ≤ 0.06) expression of TNF-a and IL-1β in the male placentas at E10.5 and E18.5, respectively. In the female placentas, 4X (versus 1X) choline modulated the transcript expression of Il1b in a biphasic pattern with reduced Il1b at E12.5 (P = 0.045) and E18.5 (P = 0.067) but increased Il1b at E15.5 (P = 0.031). MCS also induced an upregulation of Vegfa expression in the female placentas at E15.5 (P = 0.034; 4X versus 2X) and E18.5 (P = 0.026; 4X versus 1X). MCS decreased (P = 0.011; 4X versus 1X) placental apoptosis at E10.5. Additionally, the luminal area of the maternal spiral arteries was larger (P ≤ 0.05; 4X versus 1X) in response to extra choline throughout gestation.
Discussion:
MCS during murine pregnancy has fetal sex-specific effects on placental inflammation and angiogenesis, with possible consequences on placental vascular development.
Keywords: Choline, Placenta, Inflammation, Angiogenesis, Fetal Sex
INTRODUCTION
The placenta is the organ of pregnancy that mediates nutrient and oxygen supply to the developing fetus, and is therefore a critical determinant of fetal growth and development. Efficient placental transport requires proper remodeling of the maternal uterine spiral arteries and the development of a vascular network within the chorionic villi (in human placenta) or labyrinth (in mouse placenta) [1, 2]. When placental vascularization is compromised, the placenta is unable to provide sufficient nutrients and oxygen to the developing fetus, which increases the risk of fetal growth restriction and abnormal birth weight [1].
Normal placental vascular development is influenced by the balance of pro- and anti-angiogenic factors. Pro-angiogenic factors such as vascular endothelial growth factor (VEGF) and placental growth factor (PGF) play a regulatory role in the growth and proliferation of endothelial cells, angiogenesis and vasodilation while anti-angiogenic factors such as soluble fms-like tyrosine kinase-1 (sFLT1) and soluble endoglin (sENG) interfere with normal pro-angiogenic signaling, disrupt endothelial tube formation and damage the placental vasculature [3, 4]. The inflammatory milieu also plays a role in placental vascular development. Heightened levels of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-a) and interleukin 6 (IL-6) have been shown to cause endothelial cell dysfunction, reduce vascular relaxation, inhibit trophoblast invasion into the maternal decidua and adversely affect placental vascularization [4-6].
Abnormal angiogenesis and inflammation may be causal in pregnancy disorders such as preeclampsia. Aberrant expression of these proteins and others including interleukin 1 beta (IL-1β) and interleukin 10 (IL-10) is detected among women with placental dysfunction [7-11]. Recent work also reveals that placental angiogenesis and inflammation may be a sexual dimorphic phenomenon, underscoring the importance of considering fetal sex when studying these placental markers [12-14].
Choline is an essential micronutrient required for membrane biosynthesis and cellular signaling, and plays a regulatory role in gene expression via epigenetic processes (e.g., DNA and histone methylation) [15]. Consequently, choline may modulate physiological processes such as inflammation, angiogenesis and apoptosis that are central to placental function and fetal development [15-17]. Notably, we have shown an effect of choline on these processes in a cell culture model of extravillous human trophoblast cells where increasing choline concentrations decreased the abundance of pro-inflammatory, anti-angiogenic and pro-apoptotic markers [18]. Similarly, we found that supplementing the maternal diet of healthy pregnant women with extra choline (930 vs. 480 mg/d) throughout the third trimester of pregnancy decreased placental production and circulating concentrations of sFLT1 [19]. However, apart from the choline-induced reduction in placental sFLT1 expression, it is unknown whether maternal choline supplementation (MCS) can influence inflammatory, angiogenic and apoptotic processes in an in vivo model of normal pregnancy. A better understanding of the functional role of choline in placental vascular development is also needed. Accordingly, we conducted a choline supplementation study in pregnant mice and examined biomarkers of placental inflammation, angiogenesis, and apoptosis at four gestational time points. We also conducted a preliminary histological investigation to examine the effect of MCS on vascular indicators within the maternal decidua and the feto-placental unit.
MATERIALS AND METHODS
Mice and diets
All animal protocols and procedures used in this study were approved by the Institutional Animal Care and Use Committees at Cornell University and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals. Adult male and female non-Swiss Albino (NSA) mice were purchased from Harlan (Indianapolis, IN). The animals were housed in microisolator cages (Ancare) in an environmentally-controlled room (22-25°C and 70% humidity) with a 12-hour light-dark cycle. The mice in the breeding colonies were given ad libitum access to a commercially available rodent chow and water. After weaning at 3 weeks of age, both females and males were given ad libitum access to the AIN-93G purified rodent diet (Dyets no. 103345; Dyets, Bethlehem, PA) containing 1.4g choline chloride/kg diet (1X choline diet). This dietary regimen was continued until five days prior to mating at which time female mice were randomized to the 1X choline diet, a 2X choline diet containing 2.8g choline chloride/kg diet (Dyets no. 103346; Dyets, Bethlehem, PA), or a 4X choline diet containing 5.6g choline chloride/kg diet (Dyets no. 103347; Dyets, Bethlehem, PA). These dosages were selected based on our studies conducted in third-trimester pregnant women showing a choline lowering effect on sFLT1 with 2X choline supplementation [19] and evidence from rodent studies reporting improvements in brain development in the adult offspring whose mothers were supplemented with 4X choline [20]. Day of conception was determined by the presence of a vaginal plug and was defined as gestational day (E) 0.5. The female mice continued to consume their assigned diet until they were euthanized at one of four gestational time points (i.e., E10.5, E12.5, E15.5 or E18.5; n=6-8 dams/treatment group/time point).
Tissue collection and processing
Maternal blood was collected by cardiac puncture into microtainer collection tubes with clot activator and SST gel (Becton Dickinson, Franklin Lakes, NJ), and was allowed to clot at room temperature for one hour. The sample was then centrifuged at 14,000 rpm for 6 minutes, and the serum was collected and stored at −80°C. Maternal liver was removed, immediately frozen in liquid nitrogen and stored at −80°C. The gravid uterus was removed, the fetuses and placentas were then carefully dissected and weighed. One-third of the placental disks were fixed in 10% formalin for histology analysis, while the remaining placental disks were cut in half across the chorionic plate and placed in RNAlater or immediately frozen in liquid nitrogen and stored at −80°C. The fetuses were imaged to obtain crown rump measurements using the Image J Analysis Software (NIH). Fetal DNA was extracted and subjected to PCR using a commercial kit (Qiagen) for sex determination (Supplemental Table 1).
Measurement of choline metabolites in maternal liver
The concentrations of choline and its metabolic derivatives [betaine, dimethylglycine (DMG) and trimethylamine N-oxide (TMAO)] were measured in maternal liver obtained at the last study time point (i.e.: E18.5) by LC/MS according to the method of Holm et al [21] with modifications based on our equipment [22].
Quantification of placental transcript abundance
Total RNA was extracted from the placental tissues fixed in RNAlater by TRIzol reagent (Invitrogen). Reverse transcription was performed using ImProm-II Reverse Transcription System (Promega) with the following reaction conditions: 25°C for 10 minutes, 42°C for 40 minutes and 95°C for 5 minutes. Quantitative PCR was performed using the SYBR Green system in Roche LightCycler480. All primers for the targeted genes (Tnf, Il1b, Il6, Il10, Nfkb1, Vegfa, Pgf, sFlt1, Eng, Mmp14) were designed using Primer-BLAST available on the NCBI website (Supplemental Table 1). These genes were selected because of their importance in placental development and association with adverse pregnancy outcomes [5, 7-11, 23, 24] and their responsiveness to choline in prior investigations [18, 19]. The reaction conditions were as follows: 95°C for 5 minutes, followed by 40 cycles with 15 sec at 95°C, 30 sec at 63°C, and 30 sec at 72°C. To ensure the specificity of the PCR product, a dissociation stage was included at the end of the amplification cycles. Data are expressed by the ΔΔCt method, in which the expression level of the gene of interest is normalized by the expression level of the housekeeping gene as fold change before comparison between samples. TATA box binding protein, Tbp, was selected as the housekeeping gene because its expression is stable in placental tissue [25] and remains unchanged under different choline intake levels [17].
Quantification of placental protein abundance
To evaluate the protein abundance of IL-1β, TNF-a and NF-κB in the placenta, frozen placental samples were homogenized in ten volumes of buffer [50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, and 0.5% IGEPAL CA-630 (Santa Cruz Biotechnology)] containing protease inhibitor cocktails (Sigma-Aldrich). The homogenates were centrifuged at 13,200 rpm for 25 minutes at 4°C. The total protein concentration in the supernatant was quantified by the Bradford assay (Thermo Scientific Pierce). Protein was loaded onto SDS-PAGE gel, subjected to electrophoresis, and then transferred onto Immobilon FL PVDF membranes (EMD Millipore). Membranes were blocked in blocking buffer (LI-COR). The membranes were then incubated overnight with primary antibodies for IL-1β (1:200; Santa Cruz Biotechnology), TNF-a, NF-κB or β-actin (1:200, 1:1000 and 1:5000, respectively; Cell Signaling Technology), after which secondary antibodies (IRDye 800CW goat anti-rabbit and IRDye 680RD goat anti-mouse (LI-COR), 1:10,000) were added to the membranes. Protein bands were visualized and quantified by the Odyssey imaging system (LI-COR). Data are expressed as the ratio of the intensity of targeted protein to the intensity of β-actin and compared between samples.
Measurement of circulating angiogenic factors in maternal serum
Circulating concentrations of sFLT1 and sENG in the maternal serum were measured using commercial ELISA kits according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA).
Assessment of placental apoptosis
The placental tissues were fixed in 10% formalin, paraffin embedded and sectioned at 10μm. The terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was conducted using a commercial kit (Millipore, Billerica, MA) to assess placental apoptosis. The total number of cells and the number of TUNEL-positive cells in the placenta were quantified by the Aperio ImageScope software to determine the percentage of TUNEL-positive cells.
Assessment of maternal spiral artery area and placental labyrinth vasculature
Some formalin-fixed sections were subjected to immunohistochemistry as described previously [26]. To identify maternal spiral arteries for area evaluation, the placental sections were incubated with a smooth muscle actin (SMA) antibody (1:50, DakoCytomatin, Glostrup, Denmark), followed by incubation with a secondary antibody. All stained sections were imaged on an Aperio Scanscope (Vista, CA). The maternal spiral arteries were defined manually, and their areas were quantified using the Aperio ImageScope software. Data on the spiral artery area are presented as a ratio of the luminal area to the total vessel area.
To evaluate the vascular structure in the placental labyrinth, the placental sections were incubated with isolectin (1:100, Vector Laboratories, Burlingame, CA), which is a marker of the endothelial cells and has been used to stain the vasculature in other mouse tissues [27, 28], and then counterstained with hematoxylin. The placental labyrinth compartment was defined manually, and the intensity of the isolectin staining was determined using the Aperio ImageScope software. Data are expressed as the staining intensity per unit area of placental labyrinth.
Statistical analysis
Fetal measurements and the placental transcript and protein data were analyzed separately for each gestational day and fetal sex using a mixed linear model. Because some fetuses were fixed in formalin together with their placentas, fetal DNA was degraded and was not available for sex genotyping. Therefore, histology data were analyzed without stratifying by fetal sex. All mixed linear models included choline treatment as an independent fixed effect and maternal identification as an independent random effect. Litter size was included in the model as a covariate when it achieved P ≤ 0.05. For the maternal measurements, data were analyzed separately for each gestational day using one-way ANOVA. The model included choline treatment as an independent fixed effect, and litter size as a covariate when it had a P ≤ 0.05. Correlations between the choline metabolites in maternal liver and placental inflammatory or angiogenic markers at E18.5 were assessed using Pearson’s correlation analysis (with log-transformed variables as needed). Bonferroni correction was used to adjust for multiple comparisons. Data are presented as means ± SEM. SPSS software, Version 23 (SPSS Inc, Chicago, IL) was used to perform the statistical analysis and differences were considered statistically significant when Padjusted ≤ 0.05. Given that we hypothesized (a priori) that supplementing the maternal diet with extra choline would influence the outcome variables, unadjusted P-values (Punadjusted) are also presented for variables whose significance was lost after adjusting for multiple testing.
RESULTS
Concentrations of choline (and its metabolites) in the maternal liver
Maternal liver concentration of choline was higher in response to MCS, but only the difference between 1X and 4X choline groups remained significant after adjusting for multiple testing (4X vs 1X choline: Padjusted ≤ 0.001; 4X vs 2X choline: Punadjusted = 0.02, Padjusted = 0.06; 2X vs 1X choline: Punadjusted = 0.032, Padjusted = 0.09; Figure 1A). Maternal liver concentrations of betaine, DMG and TMAO were higher in response to 2X and 4X choline (Padjusted < 0.05 vs 1X choline; Figure 1B-1D).
Figure 1.
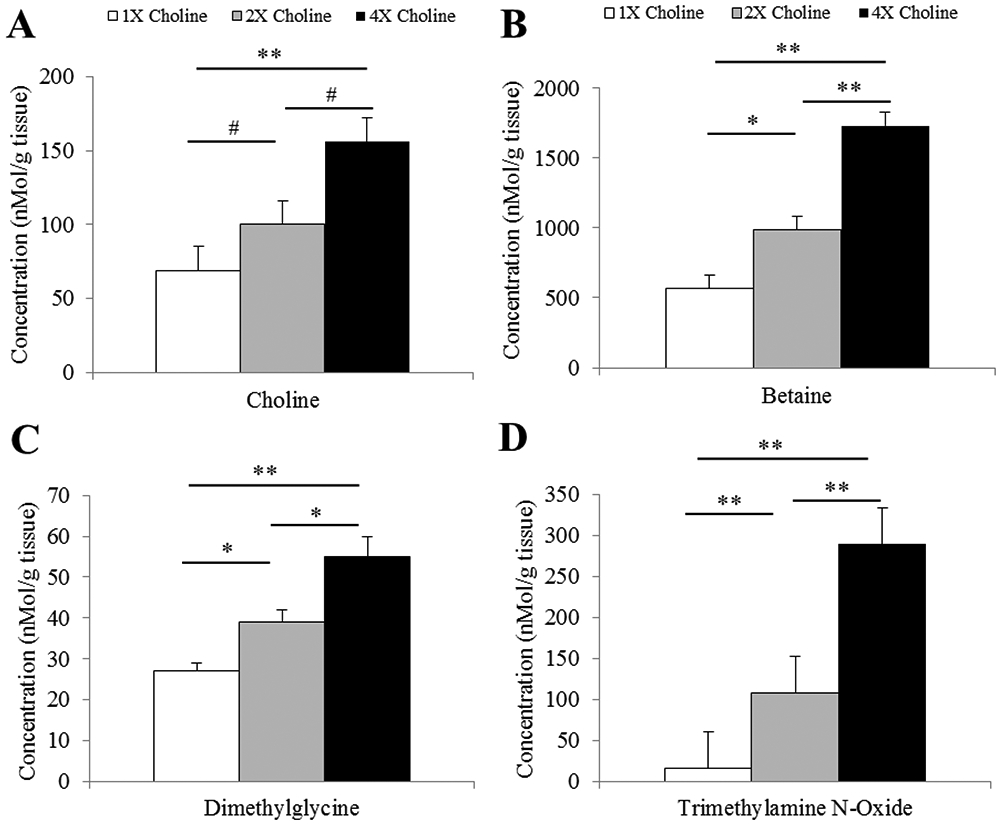
Maternal hepatic concentrations of A) choline, B) betaine, C) dimethylglycine and D) trimethylamine N-oxide at E18.5 in response to three different choline treatments (1X, 2X and 4X). Data were analyzed using ANOVA followed by post-hoc Bonferroni corrections. Values are presented as mean ± SEM. *P ≤ 0.05, **P ≤ 0.001. # Punadjusted ≤ 0.05, Padjusted > 0.05.
Placental inflammation
In the female placentas, MCS influenced the transcript abundance of Il1b with the 4X choline group having lower abundance at E12.5 (Padjusted = 0.045 vs 1X choline) and higher abundance at E15.5 (Padjusted = 0.031 vs 1X choline; Padjusted = 0.006 vs 2X choline). A lower Il1b transcript abundance in response to 4X choline was also detected at E18.5 (Punadjusted = 0.022 vs 1X choline), but this difference was lost after adjusting for multiple testing (Padjusted = 0.067 vs 1X choline) (Figure 2A). Protein concentrations of IL-1β exhibited expression patterns that mirrored those of mRNA abundance at E12.5 (4X vs 1X choline: Punadjusted = 0.039, Padjusted = 0.11), E15.5 (4X vs 2X choline: Punadjusted = 0.041, Padjusted = 0.12), and E18.5 (4X vs 1X choline: Punadjusted = 0.022, Padjusted = 0.065) (Figure 2B-C).
Figure 2.
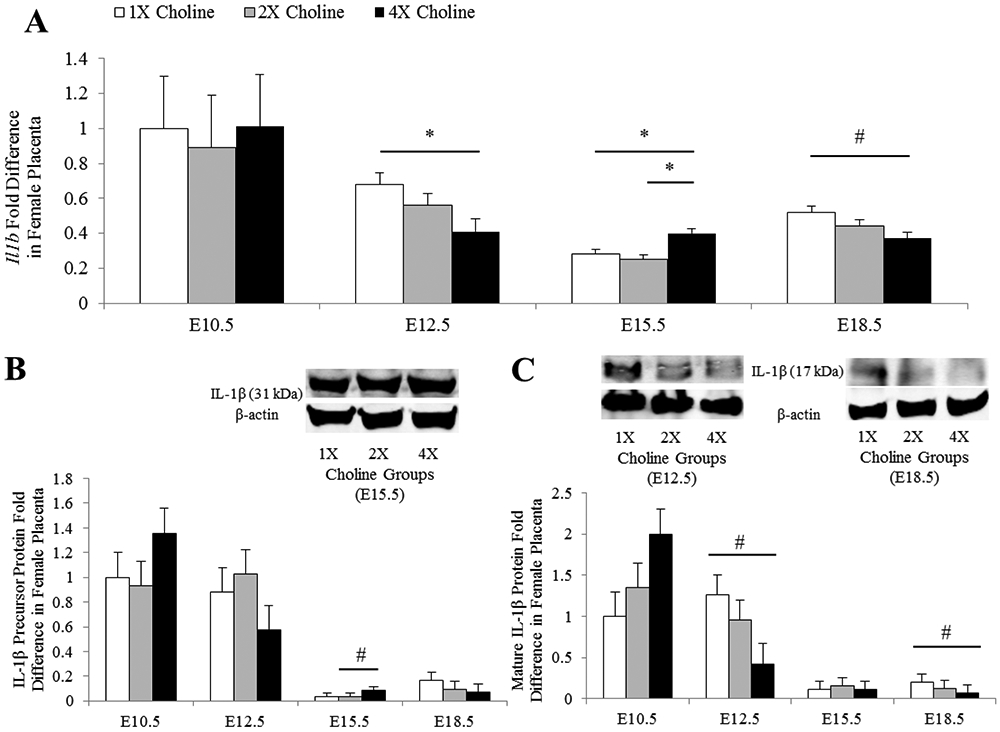
A) Transcript and B-C) protein abundance of IL-1β in the female placentas obtained from dams receiving 1X, 2X or 4X choline treatments at E10.5, E12.5, E15.5 and E18.5. The transcript data are expressed as fold-change relative to the housekeeping gene Tbp and the protein data are expressed relative to β-actin. After normalization, the mean value of the control group at E10.5 was assigned a value of 1 and the mean values of the other groups were presented as a fraction of this value. Statistical analysis was done using the mixed linear model followed by post-hoc Bonferroni corrections. Values are given as mean ± SEM. *P ≤ 0.05. #Punadjusted ≤ 0.05, Padjusted > 0.05.
Nfkb1 transcript abundance in the female placentas was higher at E18.5 in the 4X choline group (Padjusted = 0.014 vs 1X choline). Protein concentration of NF-κB exhibited an expression pattern similar to mRNA abundance but did not achieve statistical significance (4X vs 1X choline: Punadjusted = 0.059, Padjusted = 0.177) (Figure 3A-B). MCS had no detectable effects on the transcript abundance of Tnf, Il6 and Il10 (P ≥ 0.12) in the female placentas at any time points. Correlation analyses indicated a modest but significant negative correlation (r = −0.54, P = 0.02) of Il1b abundance in the E18.5 placentas with TMAO concentration in the maternal liver. The placental Nfkb1 transcript abundance at E18.5 was also positively associated with the concentrations of choline (r = 0.7, P = 0.001), betaine (r = 0.65, P = 0.004) and DMG (r = 0.48, P = 0.044) in the maternal liver.
Figure 3.
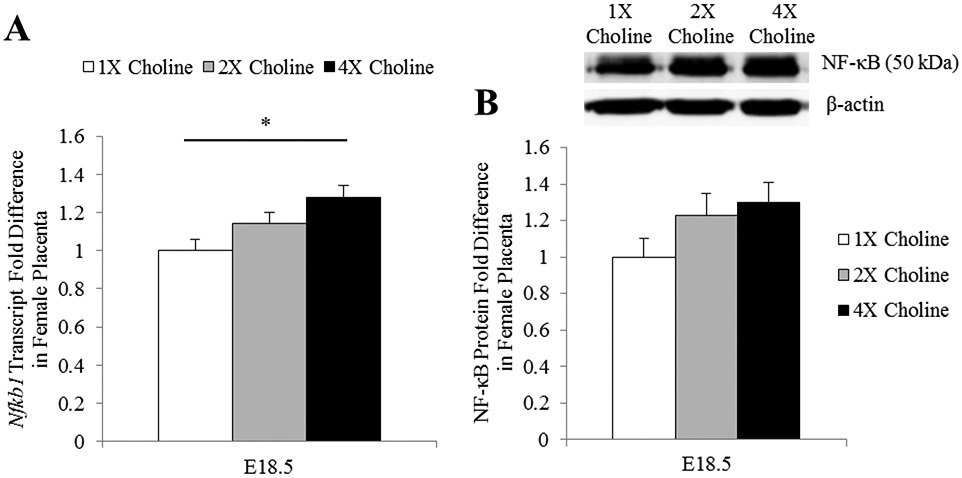
A) Transcript and B) protein expression of NF-κB in the E18.5 female placentas obtained from dams receiving 1X, 2X or 4X choline treatments. The transcript data are expressed as fold-change relative to the housekeeping gene Tbp and the protein data are expressed relative to β-actin. After normalization, the mean value of the control group was assigned a value of 1 and the mean values of the treatment groups were presented as a fraction of this value. Statistical analysis was done using the mixed linear model followed by post-hoc Bonferroni corrections. Values are given as mean ± SEM. *P ≤ 0.05.
In the male placentas, 4X choline decreased the transcript abundance of Il1b at E18.5 (Padjusted = 0.035 vs 1X choline) (Figure 4A). The protein abundance of the precursor form of IL-1β was also reduced in the 4X choline group (Padjusted = 0.01 vs 1X choline). Similarly, a reduction in the mature form of IL-1β was detected in the 4X choline group (Punadjusted = 0.035 vs 1X choline) but statistical significance was lost after adjusting for multiple testing (Padjusted = 0.1 vs 1X choline; Figure 4B).
Figure 4.
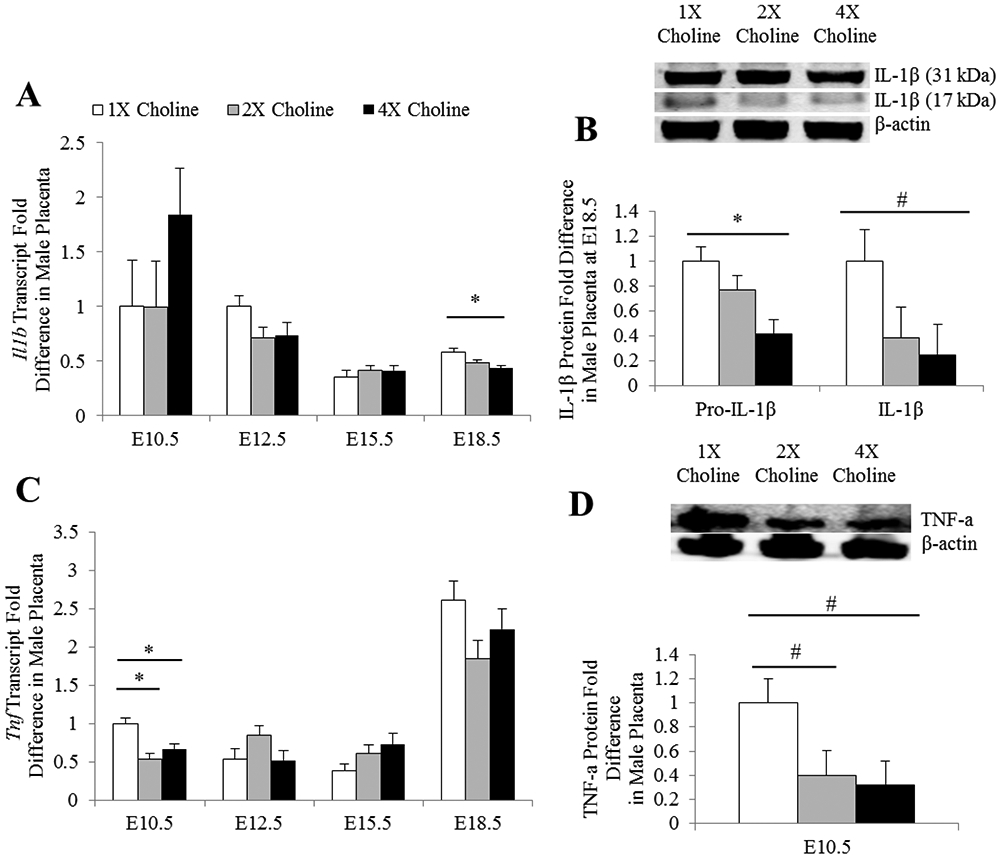
mRNA and protein abundance of A-B) IL-1β and C-D) TNF-a in the male placentas obtained from dams receiving 1X, 2X or 4X choline treatments. The transcript data are expressed as fold-change relative to the housekeeping gene Tbp and the protein data are expressed relative to β-actin. After normalization, the mean value of the control group at E10.5 (for mRNA data) or the mean value of the control group (for protein data) was assigned a value of 1 and the mean values of the other groups were presented as a fraction of this value. Statistical analysis was done using the mixed linear model followed by post-hoc Bonferroni corrections. Values are given as mean ± SEM. *P ≤ 0.05. #Punadjusted ≤ 0.05, Padjusted > 0.05.
The male placentas in the 2X and 4X choline groups also had lower (Padjusted = 0.008 and 0.033 vs 1X choline, respectively) transcript abundance of Tnf at E10.5. Similarly, the protein concentration of TNF-a was lower in the 2X and 4X choline groups at E10.5 (Punadjusted = 0.05 and 0.02 vs 1X choline, respectively) but statistical significance was lost after adjusting for multiple testing (Padjusted = 0.15 and 0.06 vs 1X choline, respectively) (Figure 4C-D). MCS had no effects (P ≥ 0.1) on the transcript abundance of Il6, Il10 and Nfkb1 or the protein concentration of NF-κB in the male placentas.
Placental angiogenic markers
In the female placentas, a higher expression of Vegfa was observed at E15.5 (Padjusted = 0.034 vs 2X choline) and E18.5 (Padjusted = 0.026 vs 1X choline) in response to 4X choline (Figure 5). Correlation analyses showed significant modest correlations between Vegfa abundance in the E18.5 placentas and all four choline metabolites in the maternal liver (choline: r = 0.57, P = 0.014; betaine: r = 0.48, P = 0.045; DMG: r = 0.51, P = 0.032; TMAO: r = 0.58, P = 0.011). MCS had no detectable effects on the transcript abundance of Pgf, sFlt1, Mmp14 and Eng (P ≥ 0.1).
Figure 5.
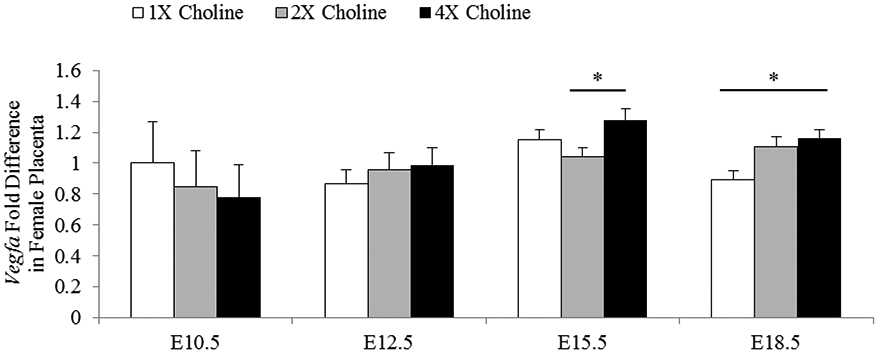
mRNA abundance of Vegfa in the female placentas obtained from dams receiving 1X, 2X or 4X choline treatments at E10.5, E12.5, E15.5 and E18.5. Data are expressed as fold-change relative to the housekeeping gene Tbp. After normalization, the mean value of the control group at E10.5 was assigned a value of 1 and the mean values of the other groups were presented as a fraction of this value. Statistical analysis was done using the mixed linear model followed by post-hoc Bonferroni corrections. Values are given as mean ± SEM. *P ≤ 0.05.
In the male placentas, sFlt1 transcript abundance tended to be lower in response to 2X and 4X choline (Punadjusted = 0.07 vs 1X choline) at E18.5; however, this tendency was not detected after adjusting for multiple testing (Padjusted = 0.22 vs 1X choline). Other angiogenic factors in the male placentas remained unchanged (P ≥ 0.1) in response to MCS.
Maternal circulating concentration of sFLT1 and sENG
4X choline decreased sFLT1 concentration in the maternal serum at E18.5 (Punadjusted = 0.05 vs 1X choline), but this difference was lost after adjusting for multiple testing (Padjusted = 0.15 vs 1X choline). MCS did not affect the concentration of sENG (P ≥ 0.5) in the maternal serum.
Placental apoptosis
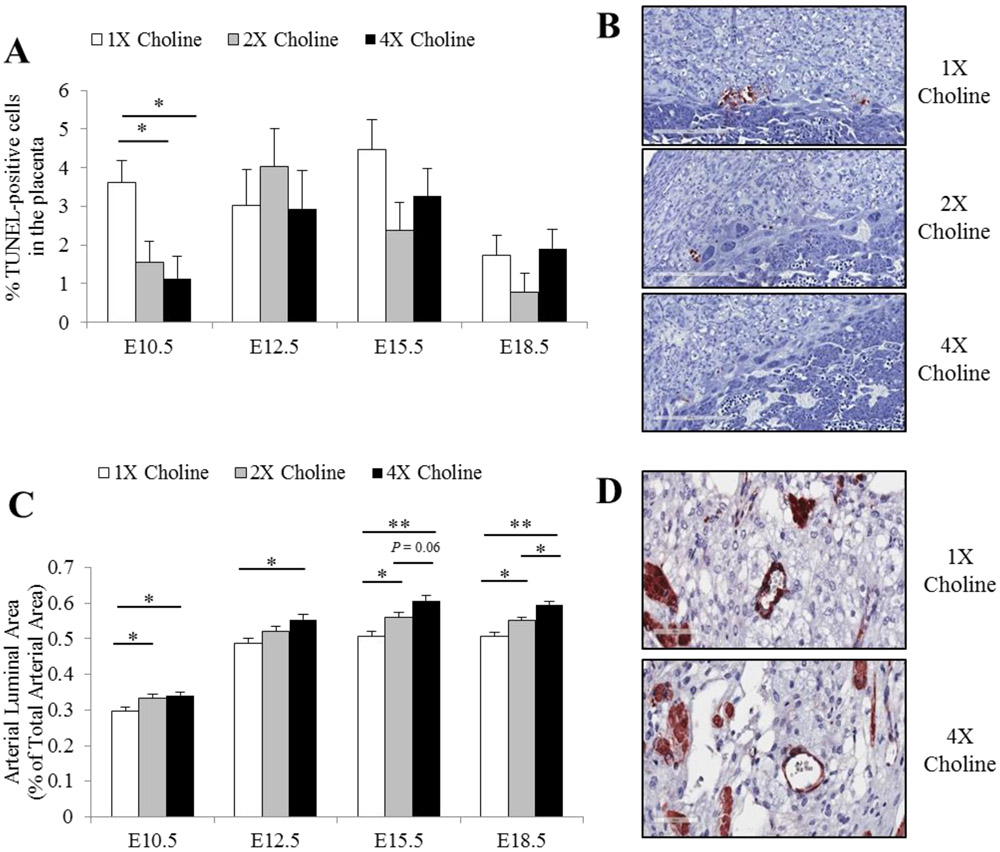
Fewer TUNEL-positive cells were detected in the placentas of the 2X (Padjusted = 0.04 vs 1X choline) and 4X choline (Padjusted = 0.011 vs 1X choline) groups at E10.5 (Figure 6A-B). No effects of MCS (P ≥ 0.18) were detected on the apoptotic index in the placentas at any other time points (Figure 6A).
Figure 6.
A) The percentage of TUNEL-positive cells in the placenta from dams receiving 1X, 2X or 4X choline treatments at E10.5, E12.5, E15.5 and E18.5. B) Representative images of the apoptotic nuclei within the E10.5 placentas are shown. C) Arterial luminal area in the maternal decidua from dams receiving 1X, 2X or 4X choline treatments at E10.5, E12.5, E15.5 and E18.5. D) Representative images of the smooth muscle actin staining within the maternal decidua are shown. Data were analyzed using the mixed linear model followed by post-hoc Bonferroni corrections. Values are presented as mean ± SEM. *P ≤ 0.05, **P ≤ 0.001.
Placental vasculature
Placentas from the 2X and 4X choline groups exhibited a larger (Padjusted ≤ 0.05) maternal spiral artery luminal area across all four gestational time points as compared to the 1X choline group (Figure 6C-D). The isolectin staining intensity in the placental labyrinth did not differ in response to MCS at any of the gestational time points (P ≥ 0.13).
Phenotypic measurements of the fetus and the placenta
Fetal weight and crown rump measurements were not affected (P ≥ 0.28 and P ≥ 0.6, respectively) by MCS. Maternal choline treatment also had no effects on placental weight (P ≥ 0.23) or placental efficiency (the ratio of fetal weight to placental weight; P ≥ 0.19) (Supplemental table 2).
DISCUSSION
Previous investigations from our group have shown that extra dietary choline during the third trimester of human pregnancy suppresses placental production of an anti-angiogenic factor sFLT1 [19] while choline inadequacy in a cell culture model leads to a molecular profile that impairs trophoblast function and in vitro angiogenesis [18]. In the current study, we show effects of MCS on placental markers of inflammation, angiogenesis and apoptosis, all of which can influence placental vascular development. We also demonstrate that most of these choline-induced effects manifest in a fetal sex- and gestational day-dependent manner. Finally, we present preliminary in vivo evidence suggesting that a higher maternal choline intake during murine pregnancy improves remodeling of the maternal spiral arteries, a finding that merits additional investigation in the future.
MCS alters the placental abundance of inflammatory and angiogenic markers in a fetal sex- and gestational day-dependent manner
Choline is an essential nutrient known to have an important role in fetal development [15]. In rodent studies, offspring from dams who received 4X choline (as compared to 1X choline) during pregnancy have improved cognitive function and attenuated age-related memory decline [20]. These neuroprotective consequences of extra maternal choline have been associated with inflammatory and angiogenic processes in the nervous system [17, 29]. We extend these findings to the mouse placenta whereby maternal choline supply modulated these same biological processes but in a manner that was dependent on fetal sex and gestational time point. Because aberrant expression of the inflammatory and angiogenic markers is associated with placental dysfunction, the choline-induced changes of these markers shown in the present study may have important clinical implications on pregnancy outcomes.
One striking difference between male and female placentas in response to MCS was the expression of the pro-inflammatory cytokine Il1b. In the female placentas, 4X (versus 1X) choline induced a 40% reduction at E12.5, a 43% increase at E15.5, and a 30% reduction at E18.5. Although statistical significance was not achieved after adjusting for multiple testing, IL-1β protein abundance exhibited an expression pattern that paralleled those of the transcript. In contrast, IL-1β expression remained largely unchanged in the male placentas until E18.5, when 4X choline yielded a 26% reduction in Il1b transcript abundance and a 55% reduction in IL-1β protein abundance as compared to 1X choline. As some immune responses are shown to be more active and stronger in females compared to males [30], we speculate that the less pronounced effects of MCS on the expression of IL-1β in the male placentas may relate to the sex-specific differences in immune regulation. Notably, however, the sex-specific immune response to maternal choline may also be cytokine dependent, as suggested by the downregulation of Tnf expression in the male placentas at E10.5, but not in the female placentas, in response to 4X choline supplementation.
The observed choline-induced downregulation of placental pro-inflammatory cytokines at several gestational time points may be beneficial to placental development. Excessive production of placental TNF-a and IL-1β have been shown to impair vascular remodeling [31] and increase the risk of adverse pregnancy outcomes in both animals [5] and humans [9, 32, 33]. Consistent with these data, pharmacological targeting of these pro-inflammatory cytokines in animal models reverses some of the placental vascular abnormalities and improves pregnancy outcomes [34, 35]. Therefore, supplementing the maternal diet with extra choline may be a nutritional strategy for lowering the risk of developing pregnancy disorders characterized by an intensified placental pro-inflammatory response.
As our prior investigation in extravillous human trophoblasts [18] found that cells cultured in a medium deficient in choline have an increased abundance of NF-κB, we expected that placental Nfkb1 expression would be downregulated in response to MCS in the present study. Contrary to our hypothesis, the transcript abundance of Nfkb1 at E18.5 was 28% higher in the 4X choline group, and this change was detected only in the female placentas. Although statistical significance was not achieved, the protein abundance in these placentas also showed a 30% increase, which was comparable to the change detected at the transcript level. The reason for this sex-specific difference and choline-induced upregulation of placental Nfκb1 is unclear. However, consistent with the greater investment of female placentas in the maintenance of pregnancy [36], we hypothesize this induction of a pro-inflammatory state during late gestation may facilitate nutrient transport to the rapidly growing fetus [37].
MCS also affected the transcript abundance of angiogenic proteins in the female placentas, as evidenced by an approximately 30% upregulation of the pro-angiogenic factor Vegfa in late gestation. VEGF promotes endothelial cell proliferation and new blood vessel formation, and stimulates relaxation of the vascular system by increasing the production of nitric oxide [4]. Notably, reduced expression of VEGF is observed in placentas from preeclamptic women as compared to placentas from normotensive women [38], and adenoviral-mediated delivery of Vegf in a mouse model of preeclampsia resolves the maternal preeclamptic phenotype [23]. Taken together, these data suggest that the choline-induced increase of Vegfa may beneficially influence placental angiogenic balance, vascular development and pregnancy outcome.
Although the objectives of the present study did not focus on exploring the mechanisms by which extra choline affects placental inflammatory and angiogenic processes, we suggest that some of these choline-induced effects are mediated by signaling pathways related to acetylcholine and protein kinase C (PKC). Choline is a precursor of acetylcholine and we have shown that MCS during pregnancy increased the placental concentration of acetylcholine and upregulated placental cholinergic receptor muscarinic 4 (CHRM4) expression [19, 22]. Others have shown that acetylcholine can signal through the alpha-7 nicotinic acetylcholine receptor, resulting in the recruitment of VEGF and blood vessel formation [39] as well as the reduction of pro-inflammatory cytokines [40]. Furthermore, biosynthesis of phosphatidylcholine from choline can prevent the accumulation of diacylglycerol and subsequent activation of PKC [15] which induces TNF-a production [41] and attenuates the actions of VEGF [42, 43]. In previous work, we demonstrated that the addition of a PKC inhibitor partially rescues aberrant IL1B expression induced by choline inadequacy in a cell culture model of extravillous human trophoblasts [18]. Because choline can be oxidized to generate the methyl donor betaine, it may be possible that an epigenetic mechanism is also involved in mediating these choline-induced effects.
The reasons for the sexual dimorphic placental response to MCS are also unclear, but it may relate to different rates of fetal development and different strategies to meet nutrient demands [44]. Regardless, these observations are consistent with the theory of fetal programming suggesting that female fetuses tend to generate a more adaptive response to environmental triggers (such as maternal diet) and invest more resources in developing their placentas [44, 45].
MCS decreases placental apoptosis in early gestation
Consistent with our prior investigation in extravillous human trophoblasts [18], we found that supplementing the maternal diet with 2X and 4X choline decreased placental apoptosis at E10.5 by 57% and 68%, respectively. This reduction may be beneficial because it could increase the survival of trophoblasts and endothelial cells thereby enhancing the development of the placental vasculature.
MCS increases the luminal area of the maternal spiral arteries
In the present study, we found that placentas from the 4X choline supplemented groups exhibited larger maternal spiral artery luminal areas than the 1X choline group. To the best of our knowledge, these data are the first in vivo evidence indicating extra maternal choline may improve remodeling of the maternal spiral arteries. Nonetheless, blood flow measurements are needed to determine if the choline-induced increase in luminal area leads to enhanced uteroplacental perfusion.
Conclusion
Supplementing the maternal diet of mice with extra choline influences placental inflammatory, angiogenic and apoptotic processes, with possible consequences on placental vascular development. Of note, most of these choline-induced effects occur in a fetal sex- and gestational day-dependent manner, highlighting the importance of these variables in studies that examine the effects of dietary manipulation on placental development. A higher maternal choline intake also increased the luminal area of the maternal spiral arteries, which may influence placental perfusion. Overall, our data provide additional support for increasing maternal choline intake during normal pregnancy as a nutritional strategy to improve placenta-related pregnancy outcomes.
Supplementary Material
Highlights:
Choline alters the abundance of inflammatory, angiogenic and apoptotic markers in the mouse placenta.
The placental responses to maternal choline supplementation are fetal sex- and gestational day-dependent.
Maternal choline supplementation increases the maternal spiral artery luminal area, which may enhance placental perfusion.
Acknowledgments
Funding: This work was supported by USDA/NIFA Grant (M.A.C.; Grant Number: USDA 2012-67017-30176) and Egg Nutrition Center Dissertation Fellowship (S.T.K.). The funders had no role in the study design, data collection, data analysis and interpretation or preparation of this manuscript.
Abbreviations:
- Vegfa
vascular endothelial growth factor
- Pgf
placental growth factor
- sFLT1
soluble fms-like tyrosine kinase-1
- sENG
soluble endoglin
- Tnf
tumor necrosis factor alpha
- Il6
interleukin 6
- Il1b
interleukin 1 beta
- Il10
interleukin 10
- MCS
maternal choline supplementation
- NSA
non-Swiss Albino
- DMG
dimethylglycine
- TMAO
trimethylamine N-oxide
- Nfkb1
nuclear factor of kappa light polypeptide gene enhancer in B-cells
- Eng
endoglin
- Mmp14
matrix metalloproteinase 14
- Tbp
TATA box binding protein
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- SMA
smooth muscle actin
REFERENCES
- [1].Reynolds LP, Borowicz PP, Caton JS, Vonnahme KA, Luther JS, Buchanan DS, Hafez SA, Grazul-Bilska AT, Redmer DA, Uteroplacental vascular development and placental function: an update, The International journal of developmental biology 54(2-3) (2010) 355–66. [DOI] [PubMed] [Google Scholar]
- [2].Rossant J, Cross JC, Placental development: lessons from mouse mutants, Nature reviews. Genetics 2(7) (2001) 538–48. [DOI] [PubMed] [Google Scholar]
- [3].Hod T, Cerdeira AS, Karumanchi SA, Molecular Mechanisms of Preeclampsia, Cold Spring Harbor perspectives in medicine 5(10) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ali SM, Khalil RA, Genetic, immune and vasoactive factors in the vascular dysfunction associated with hypertension in pregnancy, Expert opinion on therapeutic targets 19(11) (2015) 1495–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cotechini T, Komisarenko M, Sperou A, Macdonald-Goodfellow S, Adams MA, Graham CH, Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia, The Journal of experimental medicine 211(1) (2014) 165–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lash GE, Ernerudh J, Decidual cytokines and pregnancy complications: focus on spontaneous miscarriage, J Reprod Immunol 108 (2015) 83–9. [DOI] [PubMed] [Google Scholar]
- [7].Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D'Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA, Soluble endoglin contributes to the pathogenesis of preeclampsia, Nature medicine 12(6) (2006) 642–9. [DOI] [PubMed] [Google Scholar]
- [8].Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA, Circulating angiogenic factors and the risk of preeclampsia, N Engl J Med 350(7) (2004) 672–83. [DOI] [PubMed] [Google Scholar]
- [9].Dong W, Yin L, Expression of lipoxin A4, TNFalpha and IL-1beta in maternal peripheral blood, umbilical cord blood and placenta, and their significance in pre-eclampsia, Hypertension in pregnancy 33(4) (2014) 449–56. [DOI] [PubMed] [Google Scholar]
- [10].Lau SY, Guild SJ, Barrett CJ, Chen Q, McCowan L, Jordan V, Chamley LW, Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: a systematic review and meta-analysis, Am J Reprod Immunol 70(5) (2013) 412–27. [DOI] [PubMed] [Google Scholar]
- [11].Jakovljevic A, Bogavac M, Lozanov-Crvenkovic Z, Milosevic-Tosic M, Nikolic A, Mitic G, Early pregnancy angiogenic proteins levels and pregnancy related hypertensive disorders, J Matern Fetal Neonatal Med (2016) 1–6. [DOI] [PubMed] [Google Scholar]
- [12].Scott NM, Hodyl NA, Murphy VE, Osei-Kumah A, Wyper H, Hodgson DM, Smith R, Clifton VL, Placental cytokine expression covaries with maternal asthma severity and fetal sex, Journal of immunology (Baltimore, Md. : 1950) 182(3) (2009) 1411–20. [DOI] [PubMed] [Google Scholar]
- [13].Muralimanoharan S, Maloyan A, Myatt L, Evidence of sexual dimorphism in the placental function with severe preeclampsia, Placenta 34(12) (2013) 1183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Andersen LB, Jorgensen JS, Herse F, Andersen MS, Christesen HT, Dechend R, The association between angiogenic markers and fetal sex: Implications for preeclampsia research, J Reprod Immunol 117 (2016) 24–9. [DOI] [PubMed] [Google Scholar]
- [15].Kwan ST, King JH, Caudill MA, Choline and Placental Trophoblast Development, in: Duttaroy AK, Basak S (Eds.), Human Placental Trophoblasts: Impact of Maternal Nutrition, CRC Press; 2015, pp. 209–230. [Google Scholar]
- [16].Zeisel SH, da Costa KA, Choline: an essential nutrient for public health, Nutrition reviews 67(11) (2009) 615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mehedint MG, Craciunescu CN, Zeisel SH, Maternal dietary choline deficiency alters angiogenesis in fetal mouse hippocampus, Proc Natl Acad Sci U S A 107(29) (2010) 12834–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jiang X, Jones S, Andrew BY, Ganti A, Malysheva OV, Giallourou N, Brannon PM, Roberson MS, Caudill MA, Choline inadequacy impairs trophoblast function and vascularization in cultured human placental trophoblasts, Journal of cellular physiology 229(8) (2014) 1016–27. [DOI] [PubMed] [Google Scholar]
- [19].Jiang X, Bar HY, Yan J, Jones S, Brannon PM, West AA, Perry CA, Ganti A, Pressman E, Devapatla S, Vermeylen F, Wells MT, Caudill MA, A higher maternal choline intake among third-trimester pregnant women lowers placental and circulating concentrations of the antiangiogenic factor fms-like tyrosine kinase-1 (sFLT1), FASEB J 27(3) (2013) 1245–53. [DOI] [PubMed] [Google Scholar]
- [20].Blusztajn JK, Mellott TJ, Neuroprotective actions of perinatal choline nutrition, Clinical chemistry and laboratory medicine : CCLM / FESCC 51(3) (2013) 591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Holm PI, Ueland PM, Kvalheim G, Lien EA, Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry, Clin Chem 49(2) (2003) 286–94. [DOI] [PubMed] [Google Scholar]
- [22].Yan J, Jiang X, West AA, Perry CA, Malysheva OV, Devapatla S, Pressman E, Vermeylen F, Stabler SP, Allen RH, Caudill MA, Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans, Am J Clin Nutr 95(5) (2012) 1060–71. [DOI] [PubMed] [Google Scholar]
- [23].Woods AK, Hoffmann DS, Weydert CJ, Butler SD, Zhou Y, Sharma RV, Davisson RL, Adenoviral delivery of VEGF121 early in pregnancy prevents spontaneous development of preeclampsia in BPH/5 mice, Hypertension 57(1) (2011) 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kaitu'u-Lino TJ, Palmer KR, Whitehead CL, Williams E, Lappas M, Tong S, MMP-14 is expressed in preeclamptic placentas and mediates release of soluble endoglin, Am J Pathol 180(3) (2012) 888–94. [DOI] [PubMed] [Google Scholar]
- [25].Meller M, Vadachkoria S, Luthy DA, Williams MA, Evaluation of housekeeping genes in placental comparative expression studies, Placenta 26(8-9) (2005) 601–7. [DOI] [PubMed] [Google Scholar]
- [26].Berghorn KA, Clark PA, Encarnacion B, Deregis CJ, Folger JK, Morasso MI, Soares MJ, Wolfe MW, Roberson MS, Developmental expression of the homeobox protein Distal-less 3 and its relationship to progesterone production in mouse placenta, The Journal of endocrinology 186(2) (2005) 315–23. [DOI] [PubMed] [Google Scholar]
- [27].Walchli T, Mateos JM, Weinman O, Babic D, Regli L, Hoerstrup SP, Gerhardt H, Schwab ME, Vogel J, Quantitative assessment of angiogenesis, perfused blood vessels and endothelial tip cells in the postnatal mouse brain, Nature protocols 10(1) (2015) 53–74. [DOI] [PubMed] [Google Scholar]
- [28].Ernst C, Christie BR, Isolectin-IB 4 as a vascular stain for the study of adult neurogenesis, Journal of neuroscience methods 150(1) (2006) 138–42. [DOI] [PubMed] [Google Scholar]
- [29].Guseva MV, Hopkins DM, Scheff SW, Pauly JR, Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury, Journal of neurotrauma 25(8) (2008) 975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hewagama A, Patel D, Yarlagadda S, Strickland FM, Richardson BC, Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis, Genes and immunity 10(5) (2009) 509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wu ZM, Yang H, Li M, Yeh CC, Schatz F, Lockwood CJ, Di W, Huang SJ, Pro-inflammatory cytokine-stimulated first trimester decidual cells enhance macrophage-induced apoptosis of extravillous trophoblasts, Placenta 33(3) (2012) 188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Steinborn A, Gunes H, Roddiger S, Halberstadt E, Elevated placental cytokine release, a process associated with preterm labor in the absence of intrauterine infection, Obstet Gynecol 88(4 Pt 1) (1996) 534–9. [DOI] [PubMed] [Google Scholar]
- [33].Rinehart BK, Terrone DA, Lagoo-Deenadayalan S, Barber WH, Hale EA, Martin JN Jr., Bennett WA, Expression of the placental cytokines tumor necrosis factor alpha, interleukin 1beta, and interleukin 10 is increased in preeclampsia, Am J Obstet Gynecol 181(4) (1999) 915–20. [DOI] [PubMed] [Google Scholar]
- [34].Carpentier PA, Dingman AL, Palmer TD, Placental TNF-alpha signaling in illness-induced complications of pregnancy, Am J Pathol 178(6) (2011) 2802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Girard S, Tremblay L, Lepage M, Sebire G, IL-1 receptor antagonist protects against placental and neurodevelopmental defects induced by maternal inflammation, Journal of immunology (Baltimore, Md. : 1950) 184(7) (2010) 3997–4005. [DOI] [PubMed] [Google Scholar]
- [36].Buckberry S, Bianco-Miotto T, Bent SJ, Dekker GA, Roberts CT, Integrative transcriptome meta-analysis reveals widespread sex-biased gene expression at the human fetal-maternal interface, Mol Hum Reprod 20(8) (2014) 810–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Redman CW, Sargent IL, Pre-eclampsia, the placenta and the maternal systemic inflammatory response--a review, Placenta 24 Suppl A (2003) S21–7. [DOI] [PubMed] [Google Scholar]
- [38].Weel IC, Baergen RN, Romao-Veiga M, Borges VT, Ribeiro VR, Witkin SS, Bannwart-Castro C, Peracoli JC, De Oliveira L, Peracoli MT, Association between Placental Lesions, Cytokines and Angiogenic Factors in Pregnant Women with Preeclampsia, PloS one 11(6) (2016) e0157584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Arias HR, Richards VE, Ng D, Ghafoori ME, Le V, Mousa SA, Role of non-neuronal nicotinic acetylcholine receptors in angiogenesis, The international journal of biochemistry & cell biology 41(7) (2009) 1441–51. [DOI] [PubMed] [Google Scholar]
- [40].de Jonge WJ, Ulloa L, The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation, British journal of pharmacology 151(7) (2007) 915–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Reiner G, Oliver M, Skamene E, Radzioch D, Induction of tumor necrosis factor alpha gene expression by lipoprotein lipase requires protein kinase C activation, Journal of lipid research 35(8) (1994) 1413–21. [PubMed] [Google Scholar]
- [42].Rask-Madsen C, King GL, Differential regulation of VEGF signaling by PKC-alpha and PKC-epsilon in endothelial cells, Arterioscler Thromb Vasc Biol 28(5) (2008) 919–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shizukuda Y, Tang S, Yokota R, Ware JA, Vascular endothelial growth factor-induced endothelial cell migration and proliferation depend on a nitric oxide-mediated decrease in protein kinase Cdelta activity, Circulation research 85(3) (1999) 247–56. [DOI] [PubMed] [Google Scholar]
- [44].Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJ, Boys live dangerously in the womb, American journal of human biology : the official journal of the Human Biology Council 22(3) (2010) 330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mao J, Zhang X, Sieli PT, Falduto MT, Torres KE, Rosenfeld CS, Contrasting effects of different maternal diets on sexually dimorphic gene expression in the murine placenta, Proc Natl Acad Sci U S A 107(12) (2010) 5557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.