Abstract
Gentiopicroside (GPS), a main active secoiridoid glucoside derived from the roots of perennial herbs in the Gentianaceae family, has antispasmodic and relaxant effects. However, the vasorelaxant effects of GPS on aortic rings and the molecular mechanisms involved in these effects are not yet clear. Therefore, we investigated whether GPS inhibits phenylephrine- (PE-) or KCl-induced contractions in isolated rat thoracic aortic rings. The present study found that GPS produced a dose-dependent relaxation in aortic rings precontracted with PE or KCl and significantly reduced CaCl2-, narciclasine- (Rho-kinase activator-), and phorbol-12,13-diacetate- (PKC activator-) induced vasocontractions. Pretreatment with NG-Nitroarginine methyl ester hydrochloride (L-NAME, NOS inhibitor), methylene blue (sGC inhibitor), indomethacin (COX inhibitor), 4-aminopyridine (KV channel inhibitor), and glibenclamide (KATP channel inhibitor) had no influence on the vasorelaxant effect of GPS, while BaCl2 (Kir channel inhibitor), tetraethylammonium chloride (KCa channel inhibitor), ruthenium red (RYR inhibitor), and heparin (IP3R inhibitor) significantly reduced GPS-induced vasorelaxation. Moreover, GPS pretreatment remarkably inhibited the influx of Ca2+ in vascular smooth muscle cells stimulated using KCl or PE-containing CaCl2 solution. Western blot analysis confirmed that GPS treatment inhibited PE-induced increases in the protein levels of p-Akt, p-myosin light chain (MLC), and p-myosin-binding subunit of myosin phosphatase 1 (MYPT1) in the aortic rings. Additionally, the vasorelaxation activity of GPS was attenuated upon pretreatment with LY294002 (PI3K/Akt inhibitor), Y27632 (Rho-kinase inhibitor), and verapamil (L-type Ca2+ channel inhibitor). These findings demonstrate that GPS exhibits endothelium-independent vasorelaxant effects through inhibition of voltage-dependent, receptor-operated, and inositol triphosphate receptor (IP3R)/ryanodine receptor- (RYR-) mediated Ca2+ channels as well as the PI3K/Akt/Rho-kinase signaling pathway.
1. Introduction
Hypertension, which is associated with vasoconstriction and vascular remodeling, is a serious threat to global public health by causing cardiovascular diseases, such as atherosclerosis, myocardial infarction, and vascular hypertrophy [1–4]. The incidence and mortality rates of hypertension are rapidly increasing worldwide; hypertension causes approximately 9 million deaths each year, and the total number of hypertensive patients in the world is expected to reach 1.5 billion by 2025 [5, 6]. Therefore, lowering blood pressure and relaxing blood vessels can greatly alleviate the risk of cardiovascular disease development caused by elevated blood pressure.
Accumulating evidence [7–9] suggests that the hypercontractility of vascular smooth muscle (VSM) is closely related to raised blood pressure, while intracellular calcium concentration ([Ca2+]in) is the primary regulator of tension in VSM. Ca2+ is a critical factor in excitation-contraction coupling in VSM, an increase in [Ca2+]in results in vasoconstriction and a decrease in [Ca2+]in results in vasodilation [10]. It is well known that the regulation of vascular tone is mainly triggered by releasing vasodilator factors [nitric oxide (NO), prostacyclin (PGI2)], changing the resting membrane potential (K+ channels), the influx of extracellular Ca2+ through receptor-operated calcium channel (ROCC) and voltage-dependent calcium channel (VDCC, including L-type Ca2+ channels), and the release of intracellular Ca2+ from sarcoplasmic reticulum [11, 12]. Additionally, many signaling pathways have been reported to play an essential role in vasoconstriction processes [13]. For example, PI3K/Akt upon activation can induce the VSM constriction by coupling membrane receptors to L-type Ca2+ channels [14, 15]. PKC and Rho-kinase Ca2+-sensitizing pathways leading to myosin phosphatase inhibition are critically involved in α1-adrenoceptor-mediated VSM contraction [16]. Although many vasodilators are commercially available such as nitroglycerin (NO donor drug), verapamil (Ca2+ channel antagonist), and fasudil (ROCK inhibitor), they are limited by their adverse effects and patient compliance [17, 18]. Therefore, the development of natural vasodilator compounds has far-reaching research significance and high relevance for the discovery of new treatment of cardiovascular diseases.
Gentiopicroside (GPS, C16H20O9, Figure 1(a)) is a secoiridoid glucoside that is isolated from the roots of perennial herbs in the Gentianaceae family, such as Gentiana straminea Maxim., Gentiana macrophylla Pall., Gentiana manshurica Kitag., Gentiana dahurica Fisch., and Gentiana scabra Bge., which are used widely as medicinal herbs in China for the treatment of rheumatoid arthritis, hemiplegia, arthralgia, stroke, and hypertension [19]. GPS has been proven to display potential protective effects against osteoarthritis, hepatitis, diabetic renal fibrosis, osteoclastogenesis, and alcoholic hepatosteatosis [20]. Kesavan et al. [21] reported that Gentiana lutea root extracts consisting of GPS significantly inhibit the proliferation of VSMCs induced by platelet-derived growth factor-BB, which may have a cardiovascular protective effect in the prevention and treatment of atherosclerosis. Given that GPS can inhibit the spontaneous contractions of smooth muscle induced by histamine, KCl, and BaCl2 in isolated pig ileum [22], we hypothesized that GPS may inhibit vascular contraction by blocking ion channels or the corresponding signal transduction pathways.
Figure 1.
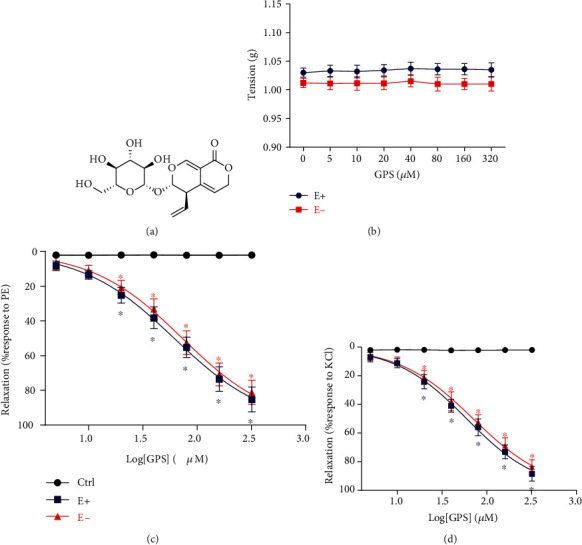
Effects of GPS on the vasorelaxation of thoracic aortic rings. (a) Chemical structure of GPS. (b) Direct effects of GPS (5, 10, 20, 40, 80, 160, and 320 μΜ) on the thoracic aorta tension. (c) Cumulative concentration-response curves of GPS (5, 10, 20, 40, 80, 160, and 320 μΜ) on endothelium-intact (E+) and endothelium-denuded (E-) aortic rings precontracted with 1 μM PE or (d) 60 mM KCl. Data are presented as mean ± SEM (n = 6). ∗p < 0.05 compared to the control group.
To the best of our knowledge, the effects of GPS on aortic rings and the molecular mechanisms involved in these effects have not yet been clarified. Therefore, this study aims to investigate the effects of GPS on the vasoconstriction of aortic rings induced by PE and KCl. We also explored the underlying mechanisms of the GPS-promoted vasodilatation effects by studying Ca2+ and K+ channels and the PI3K/Akt/Rho-kinase signaling pathway. These data could provide a novel insight into the molecular mechanisms underlying the vasodilatory effects of GPS.
2. Materials and Methods
2.1. Preparation of Rat Thoracic Aortic Rings
Fifty-one specific pathogen-free grade healthy male Sprague-Dawley rats (4-6 months old and weighing an average 250 g) were obtained from the Experimental Animal Center of Guangzhou University of Chinese Medicine (no: SCXK-2013-0020). All procedures in this study were approved by the Ethics Committee for the Use of Experimental Animals of Guangzhou University of Chinese Medicine (Permit no: 20190513056). As described previously [23], after the SD rats were euthanized, the thoracic aorta was carefully dissected and placed into ice-cold modified Krebs solution. The isolated aortas were cleaned of adipose and connective tissue and cut into 3-4 mm long rings, which were then mounted with two stainless steel hooks into an organ bath containing Krebs solution (gassed with 95% O2 and 5% CO2 at 37°C) at an initial force of 1 g tension. The alternation of isometric tension was recorded with a force-displacement transducer connected to a ML870 Power Lab Biological Signal Collection System (AD Instruments, Castle Hill, NSW, Australia). The endothelium of the aortic ring was removed carefully by rotating a manipulator inside the ring lumen, and its absence was verified by examining the capacity of 10 μM acetylcholine to induce less than 10% relaxation of rings precontracted with 1 μM PE. The endothelium was considered intact when the relaxation was more than 80% in response to acetylcholine. Only the aortic rings that met this standard were used for the subsequent experiments.
2.2. Action of GPS on Baseline Tension
After the aortic rings, with or without endothelium, were stabilized at primary 1 g tension, cumulative concentrations of GPS (0, 5, 10, 20, 40, 80, 160, and 320 μΜ) were added to the organ chambers. Changes in vascular tension were recorded, and a cumulative concentration-response curve for GPS was obtained.
2.3. Effect of GPS on Aortic Rings Precontracted with PE and KCl
After equilibration for 60 min, PE (1 μM) or KCl (60 mM) was used to induce a steady contraction in the aortic ring with or without endothelium. This was followed by the addition of cumulative concentrations of GPS (0, 5, 10, 20, 40, 80, 160, and 320 μΜ) to the ring for 20 min to verify its vasorelaxant activity. The vasodilation rate (%) was calculated as follows: Relaxation (%) = (maximal tension by PE or KCl − tension after incubation with corresponding compounds)/(maximal tension by PE or KCl − basal tension before precontraction with PE or KCl) × 100%.
2.4. Effects of Various Inhibitors on GPS-Induced Vasodilation
To elucidate the role of the endothelium, K+ channel, and PI3K/Akt/Rho-kinase pathways in GPS-mediated vasodilation, the aortic rings with intact endothelium were preincubated with nitric oxide synthase (NOS) inhibitor (100 μM L-NAME), cyclooxygenase (COX) inhibitor (100 μM indomethacin), and soluble guanylyl cyclase (sGC) inhibitor (100 μM methylene blue), respectively, for 30 min, while the aortic rings without endothelium were preincubated with different K+ channel blockers of tetraethylammonium chloride (TEA, 10 mM), 4-aminopyridine (4-AP, 1 mM), BaCl2 (1 mM), glibenclamide (0.01 mM), L-type Ca2+ channel inhibitor (100 μM verapamil), PI3K/Akt inhibitor (10 μM LY294002), and Rho-kinase inhibitor (10 μM Y27632) for 30 min (all inhibitors were obtained from MedChem Express, NJ, USA). After achieving a plateau of PE-induced contracted tension, GPS was added in a cumulative manner (20, 80, and 160 μΜ) for 20 min, and the concentration-response curves were recorded.
2.5. Effect of GPS on Extracellular Ca2+-Induced Contraction
After the aortic rings without endothelium were incubated with Ca2+-free Krebs solution [containing 0.5 mM Ethylenebis (oxyethylenenitrilo) tetraacetic acid (EGTA) and 60 mM KCl] for 30 min, the rings were treated with GPS (20, 80, and 160 μΜ) for 20 min, and subsequently, CaCl2 (0.1, 0.3, 1, 3, and 10 mM) was added cumulatively to obtain concentration-response curves.
2.6. Cell Culture
The rat thoracic aorta vascular smooth muscle A7r5 cell line was purchased from Shanghai Cell Bank (Shanghai, China). The cells were cultured in high-glucose Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin solution (all these reagents were obtained from Gibco, Grand Island, NY, USA) and incubated at 37°C with 5% CO2.
2.7. Effect of GPS on [Ca2+]in in A7r5 Cells
A7r5 cells (5 × 104 cells/well) in chamber slides were loaded with 10 μΜ Fluo-4/AM (Thermo Fisher Scientific, Grand Island, NY, USA) in Ca2+-free Krebs solution for 30 min at 37°C with 5% CO2 in the dark, as described previously [24]. A7r5 cells were then washed thrice with Ca2+-free Krebs solution and incubated with Ca2+-free Krebs solution for 20 min to generate free Fluo-4. After treatment with GPS (20, 80, and 160 μΜ) for 30 min at 37°C with 5% CO2 in the dark, 100 mM KCl in Krebs solution, or 1 μM PE-2.5 mM CaCl2 in Ca2+-free Krebs solution was added to induce fluorescence emission during detection. Fluorescence was captured using laser confocal microscopy (Zeiss 880, Jena, Germany).
2.8. Effect of GPS on Intracellular Ca2+
Aortic rings without endothelium were firstly contracted with 60 mM KCl in normal Krebs solution to assure rich Ca2+ storage in the sarcoplasmic reticulum. Following this, the rings were allowed to rest in Ca2+-free Krebs solution (containing 0.5 mM EGTA) for 30 min. (1) On one hand, the rings were incubated with 10 μM narciclasine (Rho-kinase activator) or 10 μM phorbol-12,13-diacetate (PKC activator) and then treated with GPS (20, 80, and 160 μΜ) for 20 min. (2) On the other hand, the rings were incubated with 50 mg/L heparin [inositol triphosphate receptor (IP3R) inhibitor] or 10 μM ruthenium red [ryanodine receptor (RYR) inhibitor] for 30 min (all activators and inhibitors were obtained from MedChem Express), and then, the rings precontracted with PE (1 μM) were treated with GPS (20, 80, and 160 μΜ) for 20 min, followed by recording of the concentration-response curves.
2.9. Western Blot Analysis
Isolated aortic rings without endothelium were transferred to DMEM and incubated at 37°C with 5% CO2. PE was added to the medium for 30 min, followed by GPS (160 μΜ) for 20 min. Aortic rings were snap frozen with liquid nitrogen; then, the protein was extracted using radioimmunoprecipitation assay (RIPA) lysis buffer mixed with 1% phenylmethanesulfonyl fluoride (PMSF). According to the standard WB procedure, the membranes were incubated with primary antibodies against GAPDH, Akt, p-Akt, MLC, p-MLC, MYPT1, and p-MYPT1 (1 : 1000 dilution, Cell Signaling Technologies, Beverly, MA, USA) overnight at 4°C, followed by incubation with the corresponding secondary antibody for 1 h. The protein bands were visualized using an ECL reagent (EMD Millipore) and analyzed using the Tanon 5200 image acquisition system (Tanon Science and Technology Co., Ltd., Shanghai, China).
2.10. Statistical Analysis
All data are expressed as mean ± SEM. Data were plotted using the GraphPad Prism software (version 6.0; GraphPad Software, Inc.), with sigmoidal curve fitting performed by nonlinear regression using the Prism software. The maximal relaxation or contraction response was presented as Emax, and the half-maximal effective concentration was presented as EC50. Data were analyzed by one-way and two-way analysis of variance (ANOVA), followed by Bonferroni's as a posttest, using the SPSS software (version 23.0; IBM Corp.). Differences with p < 0.05 were considered statistically significant.
3. Results
3.1. Effects of GPS on PE- or KCl-Induced Contractions in Aortic Rings
As shown in Figure 1(b), there was no change in the tension of rat thoracic aortic rings with or without endothelium after direct application of cumulative concentrations of GPS (5-320 μΜ). The results indicated that GPS has no direct vasoconstriction and vasorelaxation effects on aortic rings that sustained resting tension. However, GPS (5-320 μΜ) produced a dose-dependent relaxation in the aortic rings with or without endothelium that were precontracted with PE or KCl (Figures 1(c) and 1(d)). The half-maximal effective concentration (EC50) values of the relaxant effects of GPS for endothelium-intact and endothelium-denuded aortic rings were 76.56 ± 3.62 and 78.81 ± 3.06 μΜ, respectively, in case of PE-induced contraction, and 72.68 ± 4.18 and 75.19 ± 4.22 μΜ, respectively, in case of KCl-induced contraction. No significant difference was observed in GPS relaxing PE- or KCl-induced contractions between endothelium-intact and endothelium-denuded aortic rings (Emax/PE: E+, 85.29 ± 3.55% vs. E-, 81.32 ± 3.46%, p > 0.05; Emax/KCl: E+, 88.52 ± 2.88% vs. E-, 83.89 ± 2.69%, p > 0.05). These results showed that the vasodilation of GPS on aortic rings precontracted with PE or KCl was endothelium-independent.
3.2. Effects of Endothelial Dilated Mediators on GPS-Induced Vasorelaxation
It has been reported [25] that NOS, sGC, and COX are essential in the formation and activation of NO and PGI2, which are the main endothelium-dependent relaxing factors. Pretreatment with L-NAME (NOS inhibitor), indomethacin (COX inhibitor), and methylene blue (sGC inhibitor) had no interfering effect on GPS- (20, 80, and 160 μM) induced vasorelaxation (Emax: 72.76 ± 2.66%, 73.80 ± 2.71%, 75.28 ± 3.19%) in aortic rings precontracted with PE when compared to control group (Emax: 78.58 ± 2.13%) (p > 0.05), which further confirmed that GPS-induced vasorelaxation was endothelium-independent (Figure 2).
Figure 2.
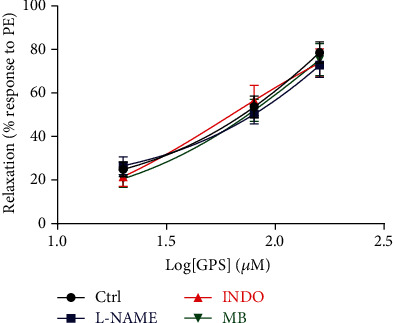
Effects of dilated mediators, including L-NAME, indomethacin (INDO), and methylene blue (MB) on GPS- (20, 80, and 160 μM) induced vasorelaxation in endothelium-intact aortic rings precontracted with PE. Data are presented as mean ± SEM (n = 6). ∗p < 0.05 compared to the control group.
3.3. Effects of K+ Channels on GPS-Induced Vasorelaxation
As shown in Figure 3, pretreatment with Gli [ATP-sensitive K+ (KATP) channel inhibitor] and 4-AP [voltage-dependent K+ (KV) channel inhibitor] had no effect on GPS- (20, 80, and 160 μM) induced vasorelaxation (Emax: 73.98 ± 3.17%, 70.29 ± 2.92%) in aortic rings without endothelium that were precontracted with PE, compared to the control group (Emax: 77.56 ± 3.03%) (p > 0.05). In contrast, pretreatment with TEA [Ca2+-activated K+ (KCa) channel inhibitor] or BaCl2 [inward rectifier K+ (Kir) channel inhibitor] significantly reduced the vasorelaxation by GPS with Emax of 25.68 ± 3.71% or 20.58 ± 3.35%, compared to the control group (p < 0.05). These results indicated that the activation of K+ channels may be related to the vasodilation of GPS.
Figure 3.
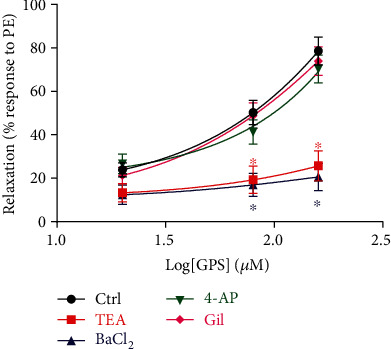
Effects of K+ channel blockers, including tetraethylammonium chloride (TEA), BaCl2, 4-aminopyridine (4-AP), and glibenclamide (Gli) on GPS- (20, 80, and 160 μM) induced vasorelaxation in endothelium-denuded aortic rings precontracted with PE. Data are presented as mean ± SEM (n = 6). ∗p < 0.05 compared to the control group.
3.4. GPS Inhibited Extracellular Ca2+ Influx in Aortic Rings and A7r5 Cells
In the high K+ and Ca2+-free Krebs solutions, the cumulative addition of CaCl2 (0.1, 0.3, 1, 3, and 10 mM) induced concentration-dependent contractions in the aortic rings without endothelium, while GPS (20, 80, and 160 μM) treatment significantly inhibited these CaCl2-induced contractions (Emax: 68.65 ± 3.89%, 56.92 ± 3.52%, 38.52 ± 2.23%, vs. 100 ± 0.01% in control group, p < 0.05) (Figure 4). To further investigate the effect of GPS on extracellular Ca2+ influx, we used a Fluo-4/AM molecular probe to observe intracellular Ca2+ fluorescence intensity in rat VSM A7r5 cells. The results revealed that pretreatment with GPS (20, 80, and 160 μM) concentration dependently and significantly reduced the fluorescence intensity of A7r5 cells stimulated with CaCl2 or KCl (Figure 5). These results confirmed that the inhibition of extracellular Ca2+ influx might be involved in the underlying mechanism of GPS in the promotion of vasorelaxation.
Figure 4.
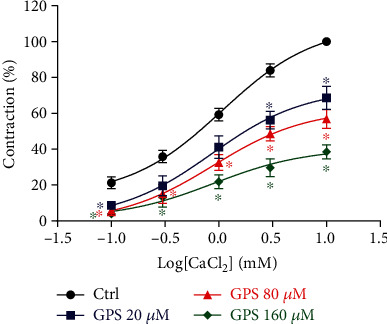
Effects of GPS (20, 80, and 160 μM) on CaCl2- (0.1, 0.3, 1, 3, and 10 mM) induced contractions of endothelium-denuded aortic rings in high K+ and Ca2+-free Krebs solution. Data are presented as mean ± SEM (n = 6). ∗p < 0.05 compared to the control group.
Figure 5.
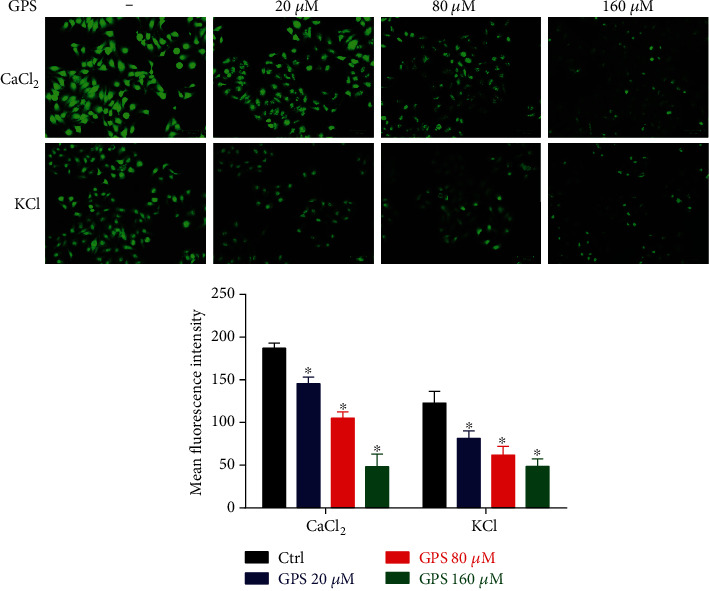
Effects of GPS (20, 80, and 160 μM) on the fluorescence intensity of vascular smooth muscle A7r5 cells stimulated using 1 μM PE-2.5 mM CaCl2 in Ca2+-free Krebs solution or 100 mM KCl in Krebs solution. Images were taken using laser confocal microscopy. Scale bar: 50 μm. Green fluorescence intensities represent the intracellular calcium concentration ([Ca2+]in) levels. Data are presented as mean ± SEM (n = 6). ∗p < 0.05 compared to the control group.
3.5. GPS Inhibited Intracellular Ca2+ Release in Aortic Rings
Preincubation with GPS (20, 80, and 160 μM) significantly decreased PE-, narciclasine- (Rho-kinase activator) or phorbol-12,13-diacetate- (PKC activator) induced contractions of aortic rings in Ca2+-free solution, compared to the control group (p < 0.05, Figures 6(a)–6(c)). The results indicated that GPS could inhibit the vasoconstriction caused by the release of intracellular Ca2+. Furthermore, pretreatment with ruthenium red (RYR antagonist) or heparin (IP3R antagonist) significantly reduced the vasodilatory effects of GPS on aortic rings precontracted with PE in Ca2+-free solution (Emax: 32.66 ± 2.91% in ruthenium red, 48.25 ± 3.22% in heparin vs. 80.58 ± 3.37% in control group, p < 0.05 in both, respectively) (Figure 6(d)). These results suggested that the mechanisms by which GPS inhibits vasoconstriction seem to be associated with the blockade of IP3R/RYR-mediated intracellular Ca2+ channels in the sarcoplasmic reticulum and the Rho-kinase-PKC induced MLC phosphorylation.
Figure 6.
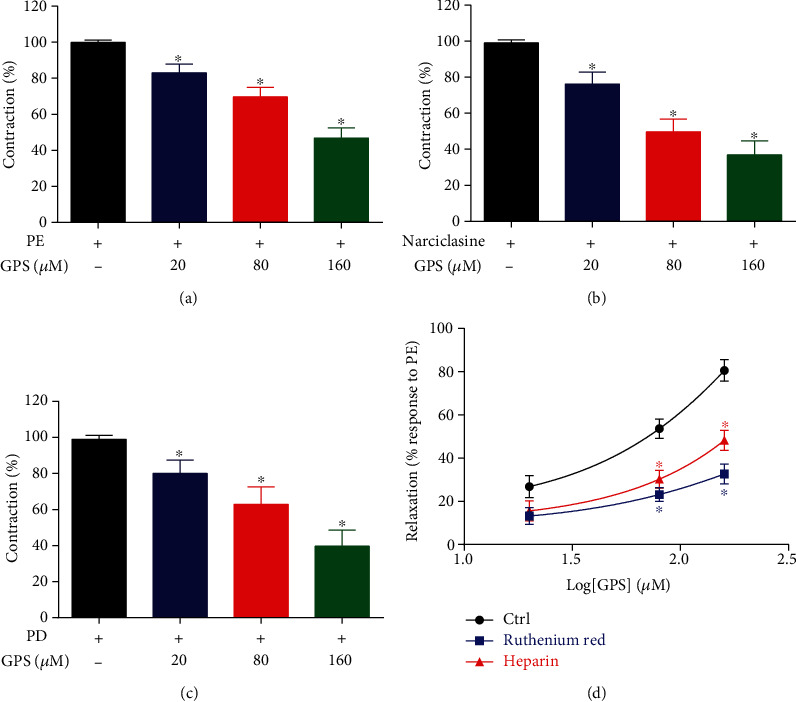
Effects of GPS treatment on intracellular Ca2+ release. (a) Endothelium-denuded aortic rings were precontracted with PE, (b) narciclasine, and (c) phorbol-12,13-diacetate (PD), followed by GPS (20, 80, and 160 μM) treatment and detection of the vasorelaxation of the rings in Ca2+-free Krebs solution. (d) Endothelium-denuded aortic rings were incubated with ruthenium red and heparin in Ca2+-free Krebs solution, and then, the rings were treated with GPS (20, 80, and 160 μΜ) after precontracted with PE. Data are presented as mean ± SEM (n = 6). ∗p < 0.05 compared to the control group.
3.6. GPS Suppressed the Activation of the PI3K/Akt/Rho-Kinase Signaling Pathway
Increasing evidence suggests that the PI3K/Akt/Rho-kinase signaling pathway plays a crucial role in vasoconstriction by stimulating the L-type Ca2+ channel [26]. Western blotting analyses showed that GPS significantly inhibited the upregulation of p-Akt, p-MYPT1, and p-MLC protein levels induced by PE in aortic rings without endothelium compared to the PE group (p < 0.05), while the levels of total Akt, MYPT1, and MLC were unchanged (Figure 7(a)). In addition, we found that pretreatment with verapamil (L-type Ca2+ channel blocker), LY294002 (PI3K/Akt inhibitor), and Y27632 (Rho-kinase inhibitor) significantly attenuated the vasodilation by GPS with Emax of 28.68 ± 3.43%, 31.52 ± 2.83%, and 39.57 ± 3.39% in aortic rings precontracted with PE, compared to the control group with Emax of 81.39 ± 3.61% (p < 0.05, Figure 7(b)). Thus, these findings confirmed that the vasorelaxation activity of GPS probably involves the PI3K/Akt/Rho-kinase signaling pathway.
Figure 7.
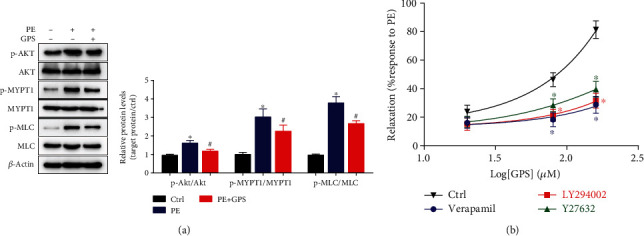
Effects of GPS on the PI3K/Akt/Rho-kinase pathway. (a) Protein expression levels of p-Akt, Akt, p-MYPT1, MYPT1, p-MLC, and MLC in aortic rings without endothelium were analyzed using western blotting. Data are presented as SEM (n = 3). ∗p < 0.05 compared to the control group; #p < 0.05 compared to the PE group. (b) Endothelium-denuded aortic rings were preincubated with verapamil, LY294002, and Y27632, and then, the rings were treated with GPS (20, 80, and 160 μΜ) after precontracted with PE. Data are presented as mean ± SEM (n = 6). ∗p < 0.05 compared to the control group.
4. Discussion
Hypertension, angina pectoris, and acute coronary syndrome are usually accompanied by the pathological characters of decreased vasodilation ability and enhanced vasoconstriction that may lead to vessel occlusion and insufficient blood supply to important organs and tissues, which serve as the main major risk factors for cardiovascular diseases [27]. Therefore, improving the vasodilation function of patients is considered an effective method for the prevention and treatment of cardiovascular diseases [28]. To date, rat thoracic aortic rings are used by many researchers as classical models to investigate the vasorelaxation effects of drugs. The present study is the first to investigate the vasodilatory effect of GPS in isolated rat thoracic aorta and to investigate the mechanism of action involved.
Vasodilation can be divided into two types: endothelium-dependent and endothelium-independent. The former is mainly related to the production of endothelium-derived relaxing factor NO and prostaglandins in the thoracic aorta, while the latter is related to a reduction in [Ca2+]in levels caused by drugs directly acting on VSM [29]. Contraction of VSM depends on the influx of extracellular Ca2+ through VDCC and ROCC in the cell membrane and the release of intracellular Ca2+ through stimulation of IP3R- and RYR-mediated Ca2+ channels in the sarcoplasmic reticulum [30]. KCl-induced contractions mainly result from membrane depolarization and openness of VDCC, while PE, an α-adrenoreceptor agonist, leads to an aortic contraction in response to extracellular Ca2+ influx through ROCC [31]. Previous studies [32, 33] have demonstrated that 4.2 mM of gentiopicroside exhibited no significant cytotoxicity to chondrocytes, and the methanol extract of Swertia corymbosa (family: Gentianaceae) did not produce any mortality and delayed toxicity orally up to 2000 mg/kg (approximately 81.45 mg/kg of GPS) when the animals were monitored for a further 14 days. The data in the present study indicates that GPS dose-dependently produced vasorelaxation effects on aortic rings with endothelium that were precontracted with PE or KCl; this effect of GPS was not significantly different in aortic rings without endothelium. Furthermore, we found that the GPS-induced vasorelaxation effect was unaffected upon pretreatment with NOS inhibitor (L-NAME), COX inhibitor (indomethacin), and sGC inhibitor (methylene blue) in aortic rings with endothelium. These results evidently indicated that the vasodilation effects of GPS on thoracic aortic rings were endothelium-independent, independent of endothelium-derived relaxing factors such as NO and PGI2, and that GPS is likely to directly act on the VSM by inhibition of VDCC and ROCC.
K+ channels play a critical role in regulating vascular tone by K+ efflux, causing cell membrane hyperpolarization, and inhibiting extracellular Ca2+ influx leading to vasodilation [34]. The four types of K+ channels including KATP, Kir, KCa, and KV on the VSM can be blocked using Gli, BaCl2, TEA, and 4-AP, respectively. These results showed that the effects of GPS in PE-precontracted rings were attenuated upon treatment with BaCl2 and TEA but not 4-AP and Gli, which manifested in the vasodilatory effects related to K+ channels. The relationship between Ca2+ channels and the vasodilation effects of GPS was further explored in this study. The data showed that GPS dose-dependently attenuated the contraction of high K+ depolarized aortic rings induced by gradually increasing CaCl2 (0.1-10 mM) input in Ca2+-free solution; in addition, GPS obviously decreased the [Ca2+]in fluorescence intensity induced by KCl and CaCl2 in A7r5 cells, which indicated that GPS inhibited extracellular calcium influx, resulting in vasodilation. PE-induced contractions in Ca2+-free solution are ascribed to intracellular Ca2+ release via activation of PKC/Rho-kinase- and IP3R/RYR-mediated Ca2+ sensitization [16, 31, 35]. Our results indicated that GPS treatment significantly decreased Rho-kinase activator- (narciclasine-) and PKC activator- (phorbol-12,13-diacetate-) mediated contraction, and the vasoconstriction of GPS was attenuated upon pretreatment with RYR inhibitor (ruthenium red) and IP3R inhibitor (heparin) in aortic rings without Ca2+. These results indicated that GPS inhibited the upregulation of [Ca2+]in via blockade of both extracellular Ca2+ influx in the cell membrane and intracellular Ca2+ release through IP3R/RYR-mediated Ca2+ channels in the sarcoplasmic reticulum by inhibition of VDCC and ROCC, and this inhibitory effect may be closely related to Rho-kinase- and PKC-induced phosphorylation of MLC [11, 36].
It is well documented that activation of the PI3K/Akt pathway significantly enhances the contraction of VSM through stimulation of the L-type Ca2+ channel and activation of Rho-kinase [37, 38]. As shown in Figure 8, Ca2+ binds to calmodulin in the cytoplasm, activates MLC kinase, phosphorylate MLC, and finally causes vasoconstriction [11, 39]. On the other hand, Rho-kinase can inhibit MLC phosphatase via phosphorylation of MYPT1, resulting in MLC constantly being in the phosphorylated state but not in the dephosphorylated state, which eventually leads to vasoconstriction [40–42]. Our results indicated that treatment with GPS significantly decreased the PE-upregulated levels of Akt, MLC, and MYPT1 phosphorylation. L-type Ca2+ channel inhibitor (verapamil), PI3K/Akt inhibitor (LY294002), and Rho-kinase inhibitor (Y27632) were used to confirm that the vasodilatory effect of GPS on aortic rings involves regulation of the PI3K/Akt/Rho-kinase signaling pathway, as evidenced by the fact that vasodilatory effects of GPS were significantly attenuated compared to the control group upon pretreatment with these three inhibitors. Therefore, we suggested that GPS inhibited PI3K/Akt/Rho-kinase signaling pathway leading to vasodilation of aortic rings.
Figure 8.
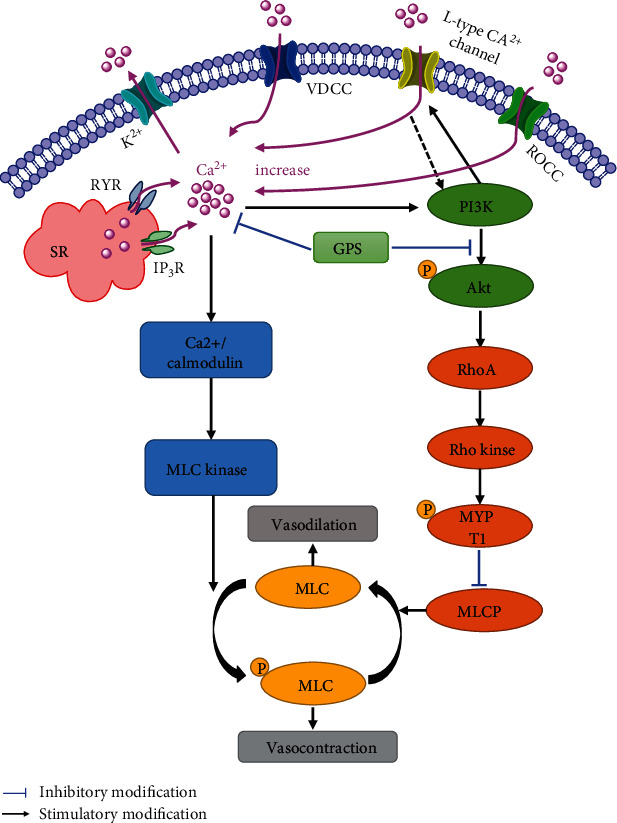
Schematic representation of the underlying mechanisms of the GPS-promoted vasodilatation effects in aortic rings. (1) GPS inhibited extracellular Ca2+ influx in the cell membrane and intracellular Ca2+ release through IP3R- and RYR-mediated Ca2+ channels in the sarcoplasmic reticulum by inhibition of VDCC and ROCC. (2) GPS may activate K+ channels. (3) GPS inhibited PI3K/Akt/Rho-kinase and L-type Ca2+ channel pathways.
5. Conclusions
In summary, the present study demonstrates for the first time that GPS has evident vasodilation effects that are endothelium-independent, and the underlying mechanisms of action for these effects involve the activation of K+ channels and inhibition of Ca2+ channels by suppressing the activation of the PI3K/Akt/Rho-kinase signaling pathway.
Acknowledgments
This project was supported by the Guangzhou University of Chinese Medicine, the Pearl River Talent Recruitment Program of Guangdong Province [grant number 2017GC010361], and the Department of Education of Guangdong Province [grant number 2016KCXTD015].
Data Availability
The research article data used to support the findings of this study are included within the article.
Conflicts of Interest
All authors declare that there is no conflict of interest.
Authors' Contributions
W.C. and R.Z. conceived and designed the experiments; S.X. and F.N coordinated the experiments and manuscript writing; J.Q. and H.H. conducted the experiments and analyzed the data.
References
- 1.Wang C., Yuan Y., Zheng M., et al. Association of age of onset of hypertension with cardiovascular diseases and mortality. Journal of the American College of Cardiology. 2020;75(23):2921–2930. doi: 10.1016/j.jacc.2020.04.038. [DOI] [PubMed] [Google Scholar]
- 2.Ning B., Chen Y., Waqar A. B., et al. Hypertension enhances advanced atherosclerosis and induces cardiac death in Watanabe heritable hyperlipidemic rabbits. American Journal of Pathology. 2018;188(12):2936–2947. doi: 10.1016/j.ajpath.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunn F. G. Hypertension and myocardial infarction. Journal of the American College of Cardiology. 1983;1(2):528–532. doi: 10.1016/S0735-1097(83)80084-9. [DOI] [PubMed] [Google Scholar]
- 4.Rosendorff C. Endothelin, vascular hypertrophy, and hypertension. Cardiovascular Drugs and Therapy. 1997;10(6):795–802. doi: 10.1007/BF00053038. [DOI] [PubMed] [Google Scholar]
- 5.Hu K., Zhou Q., Jiang Y., et al. Association between Frailty and Mortality, Falls, and Hospitalization among Patients with Hypertension: A Systematic Review and Meta-Analysis. Biomed Research International. 2021;2021:10. doi: 10.1155/2021/2690296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kearney P. M., Whelton M., Reynolds K., Muntner P., Whelton P. K., He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 7.Bohr D. F., Webb R. C. Vascular smooth muscle function and its changes in hypertension. American Journal of Medicine. 1984;77(4):3–16. doi: 10.1016/S0002-9343(84)80032-7. [DOI] [PubMed] [Google Scholar]
- 8.Dong F., Zhang J., Zhu S., Lan T., Yang J., Li L. Chrysin alleviates chronic hypoxia-induced pulmonary hypertension by reducing intracellular calcium concentration in pulmonary arterial smooth muscle cells. Journal of Cardiovascular Pharmacology. 2019;74(5):426–435. doi: 10.1097/FJC.0000000000000726. [DOI] [PubMed] [Google Scholar]
- 9.Wilson J. L., Warburton R., Taylor L., Toksoz D., Hill N., Polgar P. Unraveling endothelin-1 induced hypercontractility of human pulmonary artery smooth muscle cells from patients with pulmonary arterial hypertension. PloS One. 2018;13(4, article e0195780) doi: 10.1371/journal.pone.0195780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Y., Qu J., He L., et al. Calcium in vascular smooth muscle cell elasticity and adhesion: novel insights into the mechanism of action. Frontiers in Physiology. 2019;10:p. 852. doi: 10.3389/fphys.2019.00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z., Khalil R. A. Evolving mechanisms of vascular smooth muscle contraction highlight key targets in vascular disease. Biochemical Pharmacology. 2018;153:91–122. doi: 10.1016/j.bcp.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niazmand S., Fereidouni E., Mahmoudabady M., Hosseini M. <i>Teucrium polium</i>-induced vasorelaxation mediated by endothelium-dependent and endothelium-independent mechanisms in isolated rat thoracic aorta. Pharmacognosy Research. 2017;9(4):372–377. doi: 10.4103/pr.pr_140_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutierrez A., Contreras C., Sanchez A., Prieto D. Role of phosphatidylinositol 3-kinase (PI3K), mitogen-activated protein kinase (MAPK), and protein kinase C (PKC) in calcium signaling pathways linked to the α1-Adrenoceptor in resistance arteries. Frontiers in Physiology. 2019;10:p. 55. doi: 10.3389/fphys.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyamoto Y., Feng G. G., Satomi S., Tanaka K., Fujiwara Y., Kinoshita H. Phosphatidylinositol 3-kinase inhibition induces vasodilator effect of sevoflurane via reduction of Rho kinase activity. Life Sciences. 2017;177:20–26. doi: 10.1016/j.lfs.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Morello F., Perino A., Hirsch E. Phosphoinositide 3-kinase signalling in the vascular system. Cardiovascular Research. 2008;82(2):261–271. doi: 10.1093/cvr/cvn325. [DOI] [PubMed] [Google Scholar]
- 16.Kitazawa T., Kitazawa K. Size-dependent heterogeneity of contractile Ca2+ sensitization in rat arterial smooth muscle. Journal of Physiology. 2012;590(21):5401–5423. doi: 10.1113/jphysiol.2012.241315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siobal M. S. Pulmonary vasodilators. Respiratory Care. 2007;52(7):885–899. [PubMed] [Google Scholar]
- 18.Richards J. R., Garber D., Laurin E. G., et al. Treatment of cocaine cardiovascular toxicity: a systematic review. Clinical Toxicology. 2016;54(5):345–364. doi: 10.3109/15563650.2016.1142090. [DOI] [PubMed] [Google Scholar]
- 19.Zhou W., Ouyang J., Wang H., Wang X. Antidermatophyte activity of the Gentiopicroside-rich n-butanol fraction from Gentiana siphonantha Maxim. Root on a Guinea pig model of dermatophytosis. Complementary Medicine Research. 2019;26(1):31–38. doi: 10.1159/000492384. [DOI] [PubMed] [Google Scholar]
- 20.Chen C., Wang Y. Y., Wang Y. X., et al. Gentiopicroside ameliorates bleomycin-induced pulmonary fibrosis in mice via inhibiting inflammatory and fibrotic process. Biochemical and Biophysical Research Communications. 2018;495(4):2396–2403. doi: 10.1016/j.bbrc.2017.12.112. [DOI] [PubMed] [Google Scholar]
- 21.Kesavan R., Potunuru U. R., Nastasijević B., T A., Joksić G., Dixit M. Inhibition of vascular smooth muscle cell proliferation by Gentiana lutea root extracts. PloS One. 2013;8(4, article e61393) doi: 10.1371/journal.pone.0061393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rojas A., Bah M., Rojas J. I., Gutiérrez D. M. Smooth muscle relaxing activity of gentiopicroside isolated from Gentiana spathacea. Planta Medica. 2000;66(8):765–767. doi: 10.1055/s-2000-9774. [DOI] [PubMed] [Google Scholar]
- 23.Ferro A., Coash M., Yamamoto T., Rob J., Ji Y., Queen L. Nitric oxide-dependent beta2-adrenergic dilatation of rat aorta is mediated through activation of both protein kinase A and Akt. British Journal of Pharmacology. 2004;143(3):397–403. doi: 10.1038/sj.bjp.0705933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y., Wu X., Wu M., et al. Anti-hypertensive and vasodilatory effects of Qingda granules by suppression of calcium influx and the AKT pathway. Journal of Cardiovascular Pharmacology. 2019;74(6):549–557. doi: 10.1097/fjc.0000000000000686. [DOI] [PubMed] [Google Scholar]
- 25.Godo S., Shimokawa H. Endothelial functions. Arteriosclerosis, Thrombosis, and Vascular Biology. 2017;37(9):e108–e114. doi: 10.1161/atvbaha.117.309813. [DOI] [PubMed] [Google Scholar]
- 26.Yoshioka K., Sugimoto N., Takuwa N., Takuwa Y. Essential role for class II phosphoinositide 3-kinase alpha-isoform in Ca2+-induced, Rho- and Rho kinase-dependent regulation of myosin phosphatase and contraction in isolated vascular smooth muscle cells. Molecular Pharmacology. 2007;71(3):912–920. doi: 10.1124/mol.106.032599. [DOI] [PubMed] [Google Scholar]
- 27.Owen R. S., Carpenter J. P., Baum R. A., Perloff L. J., Cope C. Magnetic resonance imaging of angiographically occult runoff vessels in peripheral arterial occlusive disease. New England Journal of Medicine. 1992;326(24):1577–1581. doi: 10.1056/nejm199206113262428. [DOI] [PubMed] [Google Scholar]
- 28.Satoh K. Development of novel therapies for cardiovascular diseases by clinical application of basic research. Circulation Journal. 2017;81(11):1557–1563. doi: 10.1253/circj.cj-17-1029. [DOI] [PubMed] [Google Scholar]
- 29.Yang S., Xu Z., Lin C., et al. Schisantherin A causes endothelium-dependent and -independent vasorelaxation in isolated rat thoracic aorta. Life Sciences. 2020;245, article 117357 doi: 10.1016/j.lfs.2020.117357. [DOI] [PubMed] [Google Scholar]
- 30.Marks A. R. Calcium channels expressed in vascular smooth muscle. Circulation. 1992;86(6 Suppl):III61–III67. [PubMed] [Google Scholar]
- 31.Hu G. Y., Peng C., Xie X. F., Xiong L., Zhang S. Y., Cao X. Y. Patchouli alcohol isolated from _Pogostemon cablin_ mediates endothelium- independent vasorelaxation by blockade of Ca2+ channels in rat isolated thoracic aorta. Journal of Ethnopharmacology. 2018;220:188–196. doi: 10.1016/j.jep.2017.09.036. [DOI] [PubMed] [Google Scholar]
- 32.Zhao L., Ye J., Wu G. T., Peng X. J., Xia P. F., Ren Y. Gentiopicroside prevents interleukin-1 beta induced inflammation response in rat articular chondrocyte. Journal of Ethnopharmacology. 2015;172:100–107. doi: 10.1016/j.jep.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 33.Mahendran G., Thamotharan G., Sengottuvelu S., Bai V. N. Evaluation of anticonvulsant, sedative, anxiolytic, and phytochemical profile of the methanol extract from the aerial parts of Swertia corymbosa (Griseb.) wight ex C.B. Clarke. BioMed Research International. 2014;2014:9. doi: 10.1155/2014/542385.542385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dogan M. F., Yildiz O., Arslan S. O., Ulusoy K. G. Potassium channels in vascular smooth muscle: a pathophysiological and pharmacological perspective. Fundamental and Clinical Pharmacology. 2019;33(5):504–523. doi: 10.1111/fcp.12461. [DOI] [PubMed] [Google Scholar]
- 35.Wiciński M., Malinowski B., Rajewski P., et al. Resveratrol’s impact on vascular smooth muscle cells hyporeactivity: the role of Rho-kinase inhibition. BioMed Research International. 2020;2020:8. doi: 10.1155/2020/9012071.9012071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patil S. B., Bitar K. N. RhoA- and PKC-alpha-mediated phosphorylation of MYPT and its association with HSP27 in colonic smooth muscle cells. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2006;290(1):G83–G95. doi: 10.1152/ajpgi.00178.2005. [DOI] [PubMed] [Google Scholar]
- 37.Seok Y. M., Azam M. A., Okamoto Y., et al. Enhanced Ca2+-dependent activation of phosphoinositide 3-kinase class IIα isoform-Rho axis in blood vessels of spontaneously hypertensive rats. Hypertension. 2010;56(5):934–941. doi: 10.1161/hypertensionaha.110.160853. [DOI] [PubMed] [Google Scholar]
- 38.Liu H., Chen Z., Liu J., Liu L., Gao Y., Dou D. Endothelium-independent hypoxic contraction of porcine coronary arteries may be mediated by activation of phosphoinositide 3-kinase/Akt pathway. Vascular Pharmacology. 2014;61(2-3):56–62. doi: 10.1016/j.vph.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Raina H., Zacharia J., Li M., Wier W. G. Activation by Ca2+/calmodulin of an exogenous myosin light chain kinase in mouse arteries. Journal of Physiology. 2009;587(11):2599–2612. doi: 10.1113/jphysiol.2008.165258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aburima A., Wraith K. S., Raslan Z., Law R., Magwenzi S., Naseem K. M. cAMP signaling regulates platelet myosin light chain (MLC) phosphorylation and shape change through targeting the RhoA-Rho kinase-MLC phosphatase signaling pathway. Blood. 2013;122(20):3533–3545. doi: 10.1182/blood-2013-03-487850. [DOI] [PubMed] [Google Scholar]
- 41.Szasz T., Webb R. C. Rho-mancing to sensitize calcium signaling for contraction in the vasculature: role of rho kinase. Advances in Pharmacology. 2017;78:303–322. doi: 10.1016/bs.apha.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Wilson D. P., Susnjar M., Kiss E., Sutherland C., Walsh M. P. Thromboxane A2-induced contraction of rat caudal arterial smooth muscle involves activation of Ca2+ entry and Ca2+ sensitization: Rho-associated kinase-mediated phosphorylation of MYPT1 at Thr-855, but not Thr-697. Biochemicasl Journal. 2005;389(3):763–774. doi: 10.1042/bj20050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The research article data used to support the findings of this study are included within the article.


