Abstract
Purpose
To study postoperative Health-Related Quality of Life (HRQOL) after instrumented fusion for fresh subaxial cervical trauma and the effect of spinal cord injury (SCI).
Methods
From a total of 65 patients, 17 (26%) patients suffered on admission from SCI. Twenty-five patients underwent anterior, 25 posterior, and 15 circumferential cervical surgery for a single cervical injury. Sagittal roentgenographic parameters were measured in 65 age-matched asymptomatic controls and in patients on admission, eight months postoperatively and at final follow-up (lower C2-C7 curvature, cervical sagittal vertical axis (cSVA), spinocranial angle (SCA), T1-slope, neck tilt (NT), thorax inlet angle (TIA), cervical tilt (CT), cranial tilt (CrT), and occiput–C2 angle (C0-C2)). In the last evaluation, SCI patients were compared with their counterparts without SCI using national validated HRQOL instruments (SF-36 and neck disability index (NDI)).
Results
Fusion included an average of 3 vertebrae (range 2-4 vertebrae). All 65 patients were followed for an average of 5.5 years, (range 3-7 years) postoperatively. In the last evaluation, 10 (15.4%) patients with incomplete SCI improved postoperatively at 1-2 grades. At the last observation, patients with SCI showed poorer HRQOL scores than their counterparts without SCI. In particular, each SF-36 domain score was correlated with SCA, T1-slope, cSVA, and CT. At baseline, patients showed higher NT, CrT, and C0-C2 angle than controls. Eight months postoperatively, cSVA, NT, TIA, and cranial tilt (CrT) were increased in patients. In the last observation, there was difference in the sagittal roentgenographic parameters between patients with SCI compared to those without SCI. Patients aged ≥55 years had postoperatively increased cSVA, NT, and CrT compared to their younger counterparts.
Conclusion
At the final observation, HRQOL scores were lower in patients with SCI than in their non-SCI counterparts, obviously because of the associated neurologic impairment. SF-36 scores correlated with several sagittal roentgenographic parameters. These correlations should be taken in consideration by spine surgeons when performing cervical spine surgery for fresh cervical spine injuries.
1. Introduction
Relationships between sagittal lumbopelvic alignment and HRQOL measures have been shown in spinal deformity surgery [1]. Sagittal alignment was studied after degenerative cervical spine disease surgery [2–12], but to our knowledge, less attention was paid to the difference in postoperative HRQOL between SCI and non-SCI patients who underwent cervical spine fixation after fresh subaxial injury of surgery.
The purpose of this investigation was to correlate postoperative HRQOL and presence of SCI and postoperative sagittal cervical balance with HRQOL (NDI and SF-36).
2. Patients and Methods
In the period between January 2012 and December 2015, a total of 71 consecutive adult patients were admitted to the authors' department with fresh unstable subaxial cervical injuries. The power analysis with power 80% showed a total required number of individuals for two independent groups (patients and asymptomatic individuals) was 65. Sixty-five consecutive patients underwent primary cervical spine fixation by an experienced senior orthopedic spine surgeon in a single institution within the first 48 hours following trauma with the exception of cases of emergency life-threatening conditions in other organs that needed surgery. The inclusion criteria were as follows: unstable single subaxial cervical injury and age ≥ 18 years, while the exclusion criteria were as follows: injuries required occipitocervical and cervicothoracic fusion, nontraumatic instability, previous cervical spinal surgeries, history of degenerative cervical myelopathy, and serious psychiatric disease. If the landmarks (skull, cervicothoracic junction) necessary for distinct roentgenographic measurements were poorly discernable, these patients were excluded from the study. Four (5.6%) patients died in the first 3 months following trauma for different reasons related either to associated injuries or comorbidities and were excluded for the final evaluation. Two (2.8%) additional patients were excluded from the statistical analysis because of poorly discernable cervicothoracic junction. We conducted a retrospective radiographic and HRQOL analysis of the remainder 65 consecutive adult patients, 48 men and 17 women, who underwent anterior, posterior, or 360° surgery. The causes of cervical trauma were fall in 14 (21.5%) and traffic accident in 51 (78.5%) patients. Seventeen patients (26%) suffered on admission from SCI (ASIA A-C). There were associated injuries in 19 (28.6%) patients (pelvic, extremities, abdomen blunt, and cerebral trauma). The most common cervical injury was the AO/type-C [3] in 16 (24.6%) patients (Table 1). Standing digital cervical spine roentgenograms from 100 asymptomatic individuals, aged 52 ± 17 years derived from the database of this institution without a history of spinal injury, tumor, infection, ankylosing spondylitis, and previous spinal surgery, were subsequently selected as controls to match to patients' age. According to the roentgenographic history charts, the indications for cervical spine roentgenogram in the controls were migraine, headache, and vertigo. All spinal roentgenograms in this institution in ambulatory individuals are made in standing position with the upper extremities flexed to the shoulder in relaxed position with gaze straight forward adjust for their height. All patients with SCI were examined radiologically in sitting position postoperatively. At the final observation, the HRQOL scores of the patients with SCI were compared with those of the neurologically intact patients using validated and nationally adapted questionnaires (neck disability index (NDI) [4] and SF-36 [5]). The radiographs were taken on a digital X-ray system (eFilm Workstation 4.2 software, Merge Healthcare, Hartland, WI USA) and analyzed using commercial software that allows for measurements with 0.1 mm increments and enhancing of vertebral levels at the cervicothoracic junction. The lateral cervical roentgenographic parameters were measured in standing position in the controls and in supine position in patients on admission; 8-10 months postoperatively in standing position for ambulatory patients and sitting for SCI patients position and at the final evaluation in standing (non-SCI patients) or sitting position (SCI patients) were as follows: (1) lower C2-C7 curvature (angle formed from the lower plates of C2 and C7 vertebrae); (2) spinocranial angle (SCA): the angle is defined between the C7-slope and the straight line joining the middle of the upper C7-endplate and the middle of the sella turcica; (3) T1-slope: angle formed between a horizontal line and the superior endplate of T1-vertebra; (4) cSVA: the distance from the posterior/superior corner of C7 vertebral body to the plumbline from the C2 centroid; (5) neck tilt (NT): angle formed by the reference vertical line drawn in the upper end of the sternum and a line connecting the center of the T1-upper endplate and the upper end of the sternum; (6) thorax inlet angle (TIA): angle formed by a line perpendicular to the superior endplate of T1 and a line connecting the T1-upper endplate and the upper end of the sternum; (7) cervical tilt (CT): angle between two lines, both originating from the center of the T1-upper endplate; one is vertical to the T1-upper endplate; one is vertical to the T1-upper endplate, and the other passes through the tip of the dens; (8) cranial tilt (CrT): the angle between two lines, both originating from the center of the T1 upper endplate, with one passing through the dens and the other being a vertical line; and (9) C0-C2 (occiput–C2 angle): angle created by McGregor's line and the inferior surface of the axis (Figures 1 and 2). In the last observation, personal interview and physical examination were made in all patients. To test the inter- and intraobserver agreement, the sagittal cervical parameters were measured blinded in 30 randomly selected digital lateral radiographs from the controls and from 20 patients twice within a two-week interval by two independent orthopedic surgeons, who did not participate in the surgeries, using the Surgimap software (Surgimap Spine, Nemaris Inc., New York, USA). Additional radiological evaluation (roentgenograms, CT scans) of the cervical spine was made 8-10 months postoperatively to evaluate spinal fusion. All 65 patients were physically examined by the 4th author and completed the NDI and SF-36 questionnaires.
Table 1.
Cervical injury classification in 65 patients with fresh cervical trauma.
| Type of injury | N (patients) | % |
|---|---|---|
| AO type A4 | 3 | 4.62 |
| AO type B1 | 4 | 6.15 |
| AO type B2 | 2 | 13.85 |
| AO type B3 | 10 | 15.38 |
| AO type B4 | 9 | 13.85 |
| AO type C | 16 | 24.62 |
| AO type F4 | 10 | 15.38 |
| Combined cervical injury | 4 | 6.15 |
|
| ||
| Total | 65 | 100 |
Figure 1.
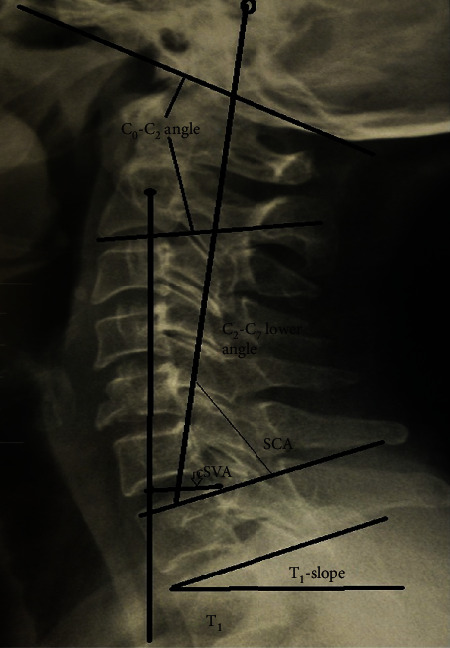
Sagittal roentgenographic parameters on standing lateral skull and cervical roentgenogram. C0-C2 = higher cervical curvature; C2-C7 = lower cervical curvature; cSVA = sagittal vertebral axis; T1-slope; SCA: spinocranial angle.
Figure 2.
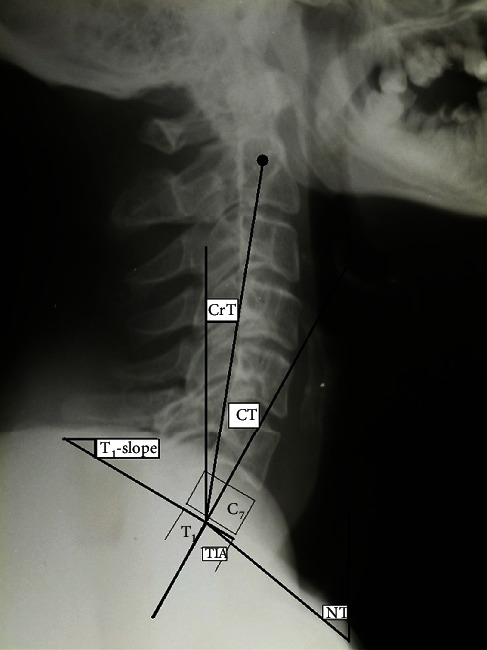
Sagittal roentgenographic parameters on standing lateral skull and cervical roentgenogram. CrT = cranial tilt; CT = cervical tilt; NT = neck tilt; TIA = thorax inlet angle.
The ethics committee of this institution approved the study protocol, and informed consent was obtained from the study participant or the person authorized to give consent. The Statistical Package for the Social Sciences (IBM SPSS Statistics version 25, SPSS Inc., Chicago, Illinois, United States) software was used. All data for the patients and controls in this study are in the central PACS documentation archives of the Orthopedic Department, General Hospital Patras Greece.
2.1. Surgical Techniques
The patient was positioned in prone position for a posterior approach with head on a headrest without skull traction or in a supine position for an anterior approach. Closed manual or reduction was applied in cases with dislocations or gross sagittal displacement under image intensifier and continuous neuromonitoring. Preoperatively cervical alignment and immobilization were maintained with a cervical orthosis. The lateral cervical alignment was controlled with a biplane image intensifier and measured intraoperatively digitally. Facet/pedicle screw fixation plus appropriately contoured rods were used for stabilization in the posterior approach. Discectomy, vertebrectomy, and a cage filled with bone graft plus lordotic contoured plate were used in the anterior surgery. A soft collar was used in all patients immediately postoperatively and a Minerva brace for mobilization of the patients for 6-8 weeks.
2.2. Statistical Data Analysis
The reproducibility and repeatability of all roentgenographic measurements were evaluated using the kappa value. The skewness and kurtosis tests were used to test the data frequency in both groups. The paired t-test was used for comparison of the same continuous variable and unpaired t-test for different continuous variable change between two periods of observation. One-way ANOVA was principally used to compare the difference of means of continuous variables between 2 or more subgroups of each categorical variable. The bivariate Pearson correlation coefficient (r) was used to correlate pairs of continuous variables.
3. Results
Interobserver and intraobserver k-values for sagittal roentgenographic parameters ranged between 0.99 and 1.0. There were no kurtosis or skewed longitudinal data.
Twenty-five (38.5%) patients underwent posterior surgery, 25 (38.5%) anterior, and the remainder 15 (23%) 360° surgery.
Three (4.6%) patients were reoperated within the first month following primary surgery for deep spinal infection.
All spines showed completed fusion 8-10 months postoperatively.
All 65 patients were available for the final evaluation in an average of 5.5, range 3-7 years postoperatively.
In the last evaluation, 7 (10.8%) patients showed residual neurologic deficit (ASIA A-C), while 10 (15.4%) patients with preoperative incomplete SIC improved at 1-2 ASIA grades postoperatively.
3.1. HRQOL (Patients)
The NDI averaged 23.8% ± 25% (range 0-72) and was not related to any sagittal parameter (Table 2).
Table 2.
Pearson correlation matrix between sagittal balance and HRQOL scores in 65 patients in the last evaluation.
| Parameters | Lower C2-C7 curvature | SCA | T1-slope | cSVA | NT | TIA | CT | CrT | C0-C2+ |
|---|---|---|---|---|---|---|---|---|---|
| NDI score | r = 0.059 | r = 0.090 | r = 0.292 | r = 0.375 | r = 0.142 | r = 0.289 | r = 0.274 | r = 0.170 | r = 0.090 |
| Physical functioning (PH)∗∗ | r = 0.0105 | r = 0.269 | r = 0.094 | r = 0.217 | r = 0.147 | r = 0.164 | r = 0.155 | r = 0.026 | r = 0.261 |
| Role limitation PH∗∗ | r = 0.052 | r = 0.251 | r = 0.482, p = 0.017 | r = 0.346 | r = 0.054 | r = 0.275 | r = 0.493, p = 0.014 | r = 0.232 | r = 0.105 |
| Limitations due to role∗∗ emotional problems | r = 0.043 | r = 0.203 | r = 0.406, p = 0.049 | r = 0.381 | r = 0.026 | r = 0.245 | r = 0.472, p = 0.020 | r = 0.125 | r = 0.011 |
| Energy/fatigue∗∗ | r = 0.199 | r = 0.148 | r = 0.275 | r = 0.468, p = 0.021 | r = 0.130 | r = 0.269 | r = 0.199 | r = 0.234 | r = 0.020 |
| Emotional well-being∗∗ | r = 0.144 | r = 0.011 | r = 0.359 | r = 0.551, p = 0.005 | r = 0.040 | r = 0.205 | r = 0.288 | r = 0.270 | r = 0.015 |
| Social functioning ∗∗ | r = 0.024 | r = 0.011 | r = 0.379 | r = 0.392 | r = 0.155 | r = 0.355 | r = 0.332 | r = 0.250 | r = 0.108 |
| Pain∗∗ | r = 0.053 | r = 0.103 | r = 0.462, p = 0.023 | r = 0.510, p = 0.011 | r = 0.093 | r = 0.256 | r = 0.414, p = 0.044 | r = 0.293 | r = 0.045 |
| General health (GH)∗∗ | r = 0.100 | r = 0.161 | r = 0.211 | r = 0.365 | r = 0.170 | r = 0.256 | r = 0.119 | r = 0.220 | r = 0.025 |
| GH change∗∗ | r = 0.125 | r = 0.480, p = 0.018 | r = 0.388 | r = 0.155 | r = 0.243 | r = 0.081 | r = 0.392 | r = 0.191 | r = 0.161 |
+ = occiput-C2 angle; ∗∗ = SF-36 domain; CT = cervical tilt; CrT = cranial tilt. p value is added in significant r values only.
SF-36 scores significantly correlated with SCA, T1-slope, cSVA, and CT (Table 2).
SCI patients showed higher NDI and lower SF-36 scores than neurologically intact patients at the final observation (Table 3).
Table 3.
NDI and SF-36 scores in neurologically intact and spinal cord injury patients in the final observation.
| Patients | NDI | Physical functioning | Role limitations due to physical health | Role limitations due to emotional problems | Energy/fatigue | Emotional well-being | Social functioning | Pain | General health | General health change |
|---|---|---|---|---|---|---|---|---|---|---|
| Neurologically intact | 13.22 ± 16 | 83.88 ± 25.9 | 93.05 ± 20.65 | 100.0 | 100.0 | 74.72 ± 10 | 85.83 ± 22.59 | 95.83 ± 22.59 | 63.88 ± 21.4 | 61.11 ± 19.59 |
| Spinal cord injury (SCI) | 47.5 ± 26 | 31.87 ± 32.9 | 46.87 ± 50.77 | 50 ± 53.45 | 34.37 ± 15.68 | 48 ± 15.26 | 33.33 ± 14.43 | 50 ± 43.58 | 25 ± 13 | 43.75 ± 16.67 |
| ∗ p value | 0.007 | 0.0022 | 0.0377 | 0.0331 | 0.00024 | 0.0011 | 0.00551 | 0.208 | 0.00001 | 0.04124 |
∗Unpaired t-test.
Significant correlations were shown in the controls between pairs of sagittal roentgenographic parameters (Table 4).
Table 4.
Pearson correlation matrix between pairs of sagittal roentgenographic parameters in 100 controls.
| Lower C2-C7 curvature | SCA | T1-slope | cSVA | NT | TIA | CT | CrT | C0-C2 angle | |
|---|---|---|---|---|---|---|---|---|---|
| Age | r = −0.476, p = 0.011 | r = −0.196 | r = 0.462, p = 0.013 | r = 0.307 | r = 0.073 | r = 0.451, p = 0.016 | r = 0.356 | r = 0.334 | r = 0.031 |
| Lower C2-C7 curvature | 1 | r = −0.159 | r = −0.524, p = 0.004 | r = 0.123 | r = 0.133 | r = −0.362 | r = −0.706, p < 0.0001 | r = 0.025 | r = 0.516, p = 0.005 |
| SCA | 1 | r = −0.321 | r = −0.643, p < 0.0001 | r = −0.232 | r = −439, p = 0.019 | r = 0.065 | r = −0.650, p < 0.0001 | r = −0.352 | |
| T1-slope | 1 | r = 0.475, p = 0.012 | r = −0.194 | r = 0.733, p < 0.0001 | r = 0.823, p < 0.01 | r = 0.651, p < 0.0001 | r = 0.603 | ||
| cSVA | 1 | r = 0.278 | r = 0.628, p < 0.0001 | r = −0.87 | r = 0.920, p < 0.0001 | r = 0.163 | |||
| NT | 1 | r = 0.525, p = 0.004 | r = −0.385, p = 0.043 | r = 0.175 | r = 0.221 | ||||
| TIA | 1 | r = 0.448, p = 0.017 | r = 0.686, p < 0.0001 | r − = 0.064 | |||||
| CT | 1 | r = 0.105 | r = −0.273 | ||||||
| CrT | 1 | r = 0.185 |
On admission, patients showed higher NT, CrT, and C0-C2 than controls (Table 5).
Table 5.
Comparative roentgenographic parameter values in 65 patients pre- and postop vs. versus 100 controls.
| Lower C2-C7 curvature | SCA | T1-slope | cSVA | NT | TIA | CT | CrT | C0-C2 | |
|---|---|---|---|---|---|---|---|---|---|
| Patients preop | −18.79 ± 12.69 | 73.72 ± 11.32 | 25.15 ± 10.6 | 2.97 ± 2.42 | 55.62 ± 10.97 | 80.77 ± 12.41 | 14.95 ± 9.6 | 10.19 ± 7.94 | 19.99 ± 7.95 |
| Controls | −19.9 ± 13 | 74.72 ± 9 | 28.6 ± 9.8 | 1.5 ± 0.83 | 48.1 ± 9.3 | 77.1 ± 13 | 18.9 ± 8 | 9.8 ± 6.5 | 19 ± 9 |
| p value pts vs. controls | 0.0134 | 0.5640 | 0.0000 | 0.1067 | 0.0032 | 0.8502 | 0.4827 | 0.0044 | 0.0000 |
| Patients postop∗ | −21.87 ± 9.5 | 73.01 ± 10.14 | 31.55 ± 12.3 | 3.3 ± 1.86 | 55.56 ± 11.39 | 86.11 ± 16.85 | 17.22 ± 9.46 | 14.32 ± 7.85 | 19.48 ± 8.70 |
| p value pts vs. controls | 0.0765 | 0.256 | 0.2168 | 0.0000 | 0.0006 | 0.0017 | 0.2549 | 0.0013 | 0.6326 |
Eight months postoperatively, cSVA, NT, TIA, and CrT were more in patients (Table 5).
Lower C2-C7 curvature (p = 0.001), T1-slope (p = 0.001), TIA (p = 0.008), and CrT (p = 0.006) increased postoperatively (Table 6).
Table 6.
Changes of sagittal roentgenographic parameters preoperatively to follow-up in 65 patients.
| Curvature lower C2-C7 | SCA | T1-slope | cSVA (cm) | NT | TIA | CT | CrT | C0-C2 | |
|---|---|---|---|---|---|---|---|---|---|
| Preop | −18.79 ± 12.69 | 73.72 ± 11.32 | 25.15 ± 10.6 | 2.97 ± 2.42 | 55.62 ± 10.97 | 80.77 ± 12.41 | 14.95 ± 9.6 | 10.19 ± 7.94 | 19.99 ± 7.95 |
| Postop | −21.87 ± 9.5 | 73.01 ± 10.14 | 31.55 ± 12.3 | 3.3 ± 1.86 | 55.56 ± 11.39 | 86.11 ± 16.85 | 17.22 ± 9.46 | 14.32 ± 7.85o | 19.48 ± 8.70 |
| p value pre/post | 0.001 | 0.852 | 0.001 | 0.4 | 0.452 | 0.008 | 0.160 | 0.006 | 0.671 |
| F/up | −16.49 ± 12.29 | 67.61 ± 17.92 | 29.85 ± 11.36 | 3.18 ± 2.10 | 53.28 ± 10.94 | 83.13 ± 14.91 | 18.44 ± 6.83 | 11.41 ± 6.91 | 23.72 ± 8.81 |
| p value post/fup | 0.820 | 0.415 | 0.716 | 0.239 | 0.608 | 0.519 | 0.223 | 0.449 | 0.029 |
| p value preop/fup | 0.91 | 0.142 | 0.04 | 0.859 | 0.37 | 0.85 | 0.101 | 0.01 | 0.523 |
Higher C0-C2 curvature (p = 0.029) increased between the follow-up of 8-10 months postoperatively to the final observation (Table 7).
Table 7.
Pearson correlation matrix between pairs of sagittal roentgenographic parameters in 65 patients at the final observation.
| SCA | T1-slope | cSVA | NT | TIA | CT | CrT | C0-C2 angle | |
|---|---|---|---|---|---|---|---|---|
| Lower C2-C7 curvature | r = 0.155 | r = −0.597, p = 0.015 | r = −0.046 | r = 0.098 | r = −0.378 | r = −0.671, p = 0.004 | r = −0.340 | r = 0.308 |
| SCA | 1 | r = −0.073 | r = 0.150 | r = −0.280 | r = −0.261 | r = −0.195 | r = 0.074 | r = 0.241 |
| T1-slope | 1 | r = 0.577, p = 0.012 | r = −0.107 | r = 0.683, p = 0.002 | r = 0.823, p < 0.0001 | r = 0.828, p < 0.0001 | r = 0.282 | |
| cSVA | 1 | r = −0.031 | r = 0.416 | r = 0.414 | r = 0.537, p = −0.022 | r = 0.594, p = 0.009 | ||
| NT | 1 | r = 0.653, p = 0.003 | r = 0.093 | r = −267 | r = 0.350 | |||
| TIA | 1 | r = 0.696, p = 0.001 | r = 0.435 | r = 0.472, p = 0.048 | ||||
| CT | 1 | r = 0.364 | r = 0.082 | |||||
| CrT | 1 | r = 0.382 |
At the final postoperative observation, T1-slope was correlated with lower C2-C7 cervical curvature (p = 0.015), cSVA (p = 0.012), TIA (p = 0.002), CT (p < 0.0001), and CrT (p < 0.0001). (Table 7). Older (≥55 years) patients showed cSVA < 4 cm but increased NT (p = 0.006) and CrT (p = 0.038) compared to their younger counterparts (≤54 years).
In the last observation, the measured sagittal roentgenographic parameters did not differ between patients with SCI and those without SCI (one-way ANOVA).
4. Discussion
Although sagittal alignment parameters associated with reconstructive surgery in cervical degenerative disease are available, to our knowledge, no postoperative sagittal alignment parameters are defined in patients with fresh subaxial cervical injuries who underwent surgical reduction and stabilization, while no data exist regarding correlation SCI and HRQOL [9].
Although some authors postulated that loss of cervical lordosis is associated with pain and disability, no correlation was found at the final observation in the patients of our series between lower C2-C7 cervical lordosis and NDI and SF-36 scores [1, 10]. In our series, 5% of the controls and 5% of the patients showed postoperatively kyphotic cervical spine.
Although several papers reported correlations between HRQOL scores and sagittal cervical spine alignment [11–13], there is no such data regarding postoperative sagittal alignment in subaxial cervical injuries. Koller et al. [9] in a series of 28 adult patients who received surgery for unstable subaxial injuries without neurological deficit reported 5.5 years postoperatively a NDI of 12.4 ± 12.7%, this being less than our NDI score of 23.8 ± 25%, with similar follow-up. This was obviously due to the inclusion of patients with SCI in our series. Although it was anticipated, in our series, patients with SCI showed higher disability NDI and lower SF-36 scores than their neurologically intact counterparts. In Koller et al.'s study, the SF-36 mean physical and mental component summary scores were better than in our patients, obviously because of the inclusion of patients with SCI in our series [9]. Postoperative SF-36 scores were higher in our patients with balanced cervical spine defined as low SCA, T1-slope, cSVA, and CT. In our series, in the final observation, disability (NDI) was much higher than the reported average of 6.98% in the general population [14]. Koller et al. [9] showed a postoperative cervical lordosis of −24.3 ± 13.3° that was close to our lower C2-C7 lordosis (−21.87 ± 9.5°).
LeHuec et al. were the first who defined the CT and SCA in cervical balance [15]. They showed that economic sagittal balance in asymptomatic population was defined by a SCA angle of 83° ± 9° [15], higher than that in both our patients (74 ± 9°) and controls (73 ± 10°). This may be due to different populations (different ethnicity, samples, etc.). In our study, we also used CT and SCA in defining sagittal balance but we selected the T1-slope instead of the C7-slope used by LeHuec et al. for sagittal cervical spine alignment [15]. In our controls and patients, T1-slope was negatively correlated with lower C2-C7 curvature. We speculate that cervical spine keeps a lordotic lower C2-C7 curvature adapting it to the T1-slope.
LeHuec et al. introduced CrT as the postural variable providing information about the spatial position of the head [15]. In our series, postoperatively CrT was increased compared to the controls, indicating no change in head spatial position following surgical stabilization of subaxial cervical spine.
We speculate that the increased cSVA, NT, and CrT in older patients in our study should be compensatory mechanisms of the cervical spine occurring with ageing.
The upper C0-C2 angle increased significantly (p = 0.029) at the final postoperative observation. The latter allows us to speculate that this is an ongoing compensatory response of the cervical spine to keep our patients the horizontal gaze. Adaptation changes at the upper C0-C2 and lower C2-C7 angles are the final “physiological” compensatory mechanism against thoracolumbar deformity of the patients and volunteers to keep horizontal gaze [16]. In our patients, the C0-C2 angle postoperatively averaged −19 ± 9°, identical with that in our controls but higher than that reported in asymptomatic individuals in other studies (14-16°) [9, 17].
Maintaining lordotic the lower C2-C7 curvature improves the NDI and SF-36 scores up to 2 years postoperatively after anterior fusion for degenerated cervical spine [17]. In our series, early reduction and fusion maintained lordotic or even improved the lower C2-C7 curvature, close to asymptomatic individuals. Some authors [9, 18] reported that cSVA and T1-slope had an impact on the NDI scores following mono- and multisegmental anterior cervical fusion for degenerative disease. Similarly, the NDI score was negatively correlated with T1-slope and cSVA.
There is a discrepancy among different reports regarding the influence of improved cervical lordosis after anterior cervical fusion and arthroplasty and HRQOL improvement [19, 20]. However, the findings of our study showed no correlation between lower C2-C7 lordosis and HRQOL scores.
There is some controversy in the literature concerning spinopelvic alterations in SCI patients with upper thoracic injury level [21, 22], while there is a paucity of literature that directly addresses sagittal imbalance in paraplegic patients. Some studies have shown significant alterations of the sagittal thoracolumbopelvic but not of the cervical alignment parameters in the nonambulatory paraplegic patients compared to ambulatory patients, depending on the level of the SCI [21–23].
There are several limitations in our study. (1) We analyzed a relatively small cohort of 65 patients. We are satisfied that we were able to identify sound statistical differences between patients and asymptomatic controls and to correlate sagittal roentgenographic parameters and HRQOL scores. (2) The smaller number of female patients was due to the fact that women rarely are involved in such injuries; (3) limitation would be that this database is surgeon maintained; however, the measurement of the digital roentgenograms was made by unbiased observes, while the validity was tested appropriately and found excellent. (4) The lack of sagittal lumbosacral roentgenographic measurements particularly in the patients with SCI was technically not possible in our facilities. (5) No sitting whole spine roentgenograms from controls and patients were taken as it would be not possible and ethical in controls.
In conclusion, NDI and SF-36 scores were lower in SCI than in their non-SCI patients, obviously because of associated neurologic impairment. SF-36 scores correlated with several sagittal roentgenographic parameters. Surgeons should take into consideration these correlations when performing cervical spine stabilization for fresh spinal injury.
Data Availability
All data for the patients and controls in this study are in the central PACS documentation archives of the Orthopedic Department, General Hospital Patras Greece.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Glassman S. D., Bridwell K., Dimar J. R., Horton W., Berven S., Schwab F. The impact of positive sagittal balance in adult spinal deformity. Spine. 2005;30(18):2024–2029. doi: 10.1097/01.brs.0000179086.30449.96. [DOI] [PubMed] [Google Scholar]
- 2.Schwab F., Patel A., Ungar B., Farcy J. P., Lafage V. Adult spinal deformity-postoperative standing imbalance. Spine. 2010;35(25):2224–2231. doi: 10.1097/BRS.0b013e3181ee6bd4. [DOI] [PubMed] [Google Scholar]
- 3.Vaccaro A. R., Koerner J. D., Radcliff K. E., et al. AOSpine subaxial cervical spine injury classification system. European Spine Journal. 2016;25(7):2173–2184. doi: 10.1007/s00586-015-3831-3. [DOI] [PubMed] [Google Scholar]
- 4.Trouli N. M., Vernon H. T., Kakavelakis K. N., Antonopoulou M. D., Paganas A. N., Lionis C. D. Translation of the neck disability index and validation of the Greek version in a sample of neck pain patients. BMC Musculoskeletal Disorders. 2008;9(1):p. 106. doi: 10.1186/1471-2474-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anagnostopoulos F., Makas D., Pappa E. Construct validation of the Greek SF-36 Health Survey. Quality of Life Research. 2005;14(8):1959–1965. doi: 10.1007/s11136-005-3866-8. [DOI] [PubMed] [Google Scholar]
- 6.Gore D. R. Roentgenographic findings in the cervical spine in asymptomatic persons. Spine. 2001;26(22):2463–2466. doi: 10.1097/00007632-200111150-00013. [DOI] [PubMed] [Google Scholar]
- 7.Gay R. E. The curve of the cervical spine: variations and significance. Journal of Manipulative and Physiological Therapeutics. 1993;16(9):591–594. [PubMed] [Google Scholar]
- 8.Hardacker J. W., Shuford R. F., Capicotto P. N., Pryor P. W. Radiographic standing cervical segmental alignment in adult volunteers without neck symptoms. Spine. 1997;22(13):1472–1479. doi: 10.1097/00007632-199707010-00009. [DOI] [PubMed] [Google Scholar]
- 9.Koller H., Reynolds J., Zenner J., et al. Mid- to long-term outcome of instrumented anterior cervical fusion for subaxial injuries. European Spine Journal. 2009;18(5):630–653. doi: 10.1007/s00586-008-0879-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAviney J., Schulz D., Bock R., Harrison D. E., Holland B. Determining the relationship between cervical lordosis and neck complaints. Journal of Manipulative and Physiological Therapeutics. 2005;28(3):187–193. doi: 10.1016/j.jmpt.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Nojiri K., Matsumoto M., Chiba K., et al. Relationship between alignment of upper and lower cervical spine in asymptomatic individuals. Journal of Neurosurgery. 2003;99(1):80–83. doi: 10.3171/spi.2003.99.1.0080. [DOI] [PubMed] [Google Scholar]
- 12.Knott P. T., Mardjetko S. M., Techy F. The use of the T1 sagittal angle in predicting overall sagittal balance of the spine. The Spine Journal. 2010;10(11):994–998. doi: 10.1016/j.spinee.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 13.Tang J. A., Scheer J. K., Smith J. S., et al. The impact of standing regional cervical sagittal alignment on outcomes in posterior cervical fusion surgery. Neurosurgery. 2012;71(3):662–669. doi: 10.1227/NEU.0b013e31826100c9. discussion 669. [DOI] [PubMed] [Google Scholar]
- 14.Kato S., Takeshita K., Matsudaira K., Tonosu J., Hara N., Chikuda H. Normative score and cut-off value of the neck disability index. Journal of Orthopaedic Science. 2012;17(6):687–693. doi: 10.1007/s00776-012-0276-y. [DOI] [PubMed] [Google Scholar]
- 15.LeHuec C. J., Demezon H., Aunoble S. Sagittal parameters of global cervical balance using EOS imaging: normative values from a prospective cohort of asymptomatic volunteers. European Spine Journal. 2015;24(1):63–71. doi: 10.1007/s00586-014-3632-0. [DOI] [PubMed] [Google Scholar]
- 16.Scheer J. K., Tang J. A., Smith J. S., et al. Cervical spine alignment, sagittal deformity, and clinical implications. Journal of Neurosurgery. Spine. 2013;19(2):141–159. doi: 10.3171/2013.4.SPINE12838. [DOI] [PubMed] [Google Scholar]
- 17.Roussouly P., Gollogly S., Noseda O., Berthonnaud E., Dimnet J. The vertical projection of the sum of the ground reactive forces of a standing patient is not the same as the C7 plumb line. Spine. 2006;31(11):E320–E325. doi: 10.1097/01.brs.0000218263.58642.ff. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y., Lan Z., Xu W. Analysis of sagittal alignment parameters following anterior cervical hybrid decompression and fusion of multilevel cervical spondylotic myelopathy. BMC Musculoskeletal Disorders. 2019;20(1):p. 1. doi: 10.1186/s12891-018-2378-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon W. K., Kim P. S., Ahn S. Y., et al. Analysis of associating factors with C2–7 sagittal vertical axis after two-level anterior cervical fusion: comparison between plate augmentation and stand-alone cages. Spine. 2017;42(5):318–325. doi: 10.1097/BRS.0000000000001776. [DOI] [PubMed] [Google Scholar]
- 20.Villavicencio A. T., Babuska J. M., Ashton A., et al. Prospective, randomized, double-blind clinical study evaluating the correlation of clinical outcomes and cervical sagittal alignment. Neurosurgery. 2011;68(5):1309–1316. doi: 10.1227/NEU.0b013e31820b51f3. [DOI] [PubMed] [Google Scholar]
- 21.Barkoh K., Lucas J. W., Lee L., Hsieh P. C., Wang J. C., Rolfe K. Paraplegic patients: how to measure balance and what is normal or functional? European Spine Journal. 2018;27(Suppl 1):109–S114. doi: 10.1007/s00586-018-5471-x. [DOI] [PubMed] [Google Scholar]
- 22.De Iure F., Chehrassan M., Cappuccio M., Amendola L. Sitting imbalance cause and consequence of post-traumatic Charcot spine in paraplegic patients. European Spine Journal. 2014;23(Suppl 6):604–609. doi: 10.1007/s00586-014-3550-1. [DOI] [PubMed] [Google Scholar]
- 23.Castro de Medeiros R., APB J., Cliquet A., Jr. Sagittal spinal alignment in paraplegics: a new paradigm for the rehabilitation under neuromuscular electrical stimulation. Spinal Cord. 2010;48(3):251–256. doi: 10.1038/sc.2009.123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data for the patients and controls in this study are in the central PACS documentation archives of the Orthopedic Department, General Hospital Patras Greece.


