ABSTRACT
Chlamydia suis intestinal infection of single-animal experimental groups of gnotobiotic newborn piglets was previously reported to cause severe, temporary small intestinal epithelium damage. We investigated archived intestinal samples for pro-inflammatory nuclear factor kappa B (NF-κB) activation, Interleukin (IL)-6 and IL-8 production and immune cell influx. Samples were collected 2, 4 and 7 days post-inoculation with C. suis strain S45/6 or mock inoculum (control). Increased nuclear localization of epithelial NF-κB, representative of activation, in the jejunum and ileum of C. suis-infected animals, compared to uninfected controls, began by 2 days post-infection (dpi) and persisted through 7 dpi. Infected animals showed increased production of IL-8, peaking at 2 dpi, compared to controls. Infection-mediated CD45-positive immune cell influx into the jejunal lamina propria peaked at 7 dpi, when epithelial damage was largely resolved. Activation of NF-κB appears to be a key early event in the innate response of the unprimed porcine immune system challenged with C. suis. This results in an acute phase, coinciding with the most severe clinical symptoms, diarrhea and weight loss. Immune cells recruited shortly after infection remain present in the lamina propria during the recovery phase, which is characterized by reduced chlamydial shedding and restored intestinal epithelium integrity.
Keywords: gnotobiotic piglets, enteric infection, Chlamydia suis, NF-κB, IL-8, histopathology, immunohistochemistry, ELISA
Chlamydia suis intestinal infection of gnotobiotic piglets is associated with intestinal NF-κB activation, which appears to be important in the early innate unprimed porcine immune response to C. suis.
INTRODUCTION
Chlamydiae are Gram-negative, obligate intracellular bacteria that can cause a variety of diseases in humans and animals. In humans, ocular serovars of Chlamydia trachomatis cause conjunctivitis and are the leading infectious cause of blindness in developing countries (Hu et al. 2010). Moreover, genital serovars of C. trachomatis are responsible for the most common bacterial sexually transmitted infections worldwide and may result in infertility and ectopic pregnancy (Haggerty et al. 2010; Mishori, McClaskey and Winklerprins 2012; Satterwhite et al. 2013; WHO 2016). Infections with other chlamydial species have a negative impact on the health and productivity of livestock resulting in significant economic losses. The most commonly found chlamydial species in livestock are Chlamydia pecorum, Chlamydia abortus and Chlamydia suis, leading to asymptomatic infections or causing encephalomyelitis, polyarthritis, endometritis and enteritis in cattle and pneumonia, pericarditis and abortion in pigs (Schautteet and Vanrompay 2011). Chlamydial abortion in pigs has been associated with C. abortus and mixed infections of C. pecorum and C. suis (Schiller et al. 1997). C. suis has been identified on farms worldwide, and is considered endemic in domestic pigs (Schautteet and Vanrompay 2011; De Puysseleyr et al. 2014).
The prevalence of enteric chlamydial infection has been reported as approximately 30% and 15% in finishing and suckling pigs, respectively (Zahn et al. 1995; Szeredi et al. 1996), and chlamydial infection was similarly prevalent (15%) in both healthy and diarrheic piglets (Nietfeld et al. 1997). However, intestinal pathology caused by C. suis includes diarrhea, epithelial sloughing and villus atrophy and chronic wasting both under experimental conditions (Rogers and Andersen 1996; Guscetti et al. 1998, 2009) and in naturally infected pigs under farm settings (Nietfeld et al. 1993; Szeredi et al. 1996). Chlamydia suis has also been isolated from conjunctival and pharyngeal swabs and stool samples from healthy pigs and farm workers (De Puysseleyr et al. 2017), and has been identified as infectious agent in conjunctivitis cases (Donati et al. 2014; Chahota et al. 2018). The chlamydial prevalence in Swiss fattening pig farms is high; evaluation of 636 pigs from 29 farms showed that all farms were positive for Chlamydiaceae whilst C. suis was detected in 96% fecal swabs (Hoffmann et al. 2015). Oral transmission of Chlamydia is considered a likely scenario in both animals and humans and it was postulated that the GI tract could act as reservoir for Chlamydia (Turner 2009; Yeruva et al. 2013; Bavoil et al. 2017).
The GI tract is able to host a myriad of commensal bacteria without mounting an immune response (Turner 2009; Peterson and Artis 2014). This tolerance is crucial to maintain a healthy balance of microbes, which in turn protect the intestinal barrier from pathogenic invasion (Pickard et al. 2017). In order to decipher the course of chlamydial intestinal infection, several studies have used gnotobiotic piglets as an infection model. While this approach is inarguably more cumbersome, it provides a unique insight into the infection characteristics of a single pathogen regardless of any other confounding factors such as commensal microbiota. The enteral pathogenicity of different chlamydial strains in gnotobiotic piglets was studied previously by Guscetti et al. (1998, 2009). Oral infection with C. abortus induced active intestinal shedding of bacterial organisms but only the newborn gnotobiotic piglets displayed mild pathological changes in the intestinal lining and mild clinical signs such as intermittent watery diarrhea between 8 and 12 days post-inoculation (dpi; Guscetti et al. 1998). A more severe clinical course was observed when gnotobiotic piglets were orally infected with C. suis. Clinical signs such as diarrhea, failure to gain weight and enlarged mesenteric lymph nodes as well as histopathological intestinal lesions were apparent after 2 days of infection and lasted until the end of the experiment at 13 days post inoculation resulting in severe lesions of the intestinal epithelium, fecal shedding of bacteria, weight loss and diarrhea (Guscetti et al. 2009).
The intestinal damage caused by infection with C. suis is a hallmark of enteropathogenic microorganisms. In contrast to commensal bacteria, enteropathogens activate a plethora of immune reactions, with the first line of defense being the epithelial cells and their potential to release cytokines, chemokines and danger signals, leading to the recruitment of immune cells (Tomasello and Bedoui 2013). Chlamydia, generally studied in the context of human pathogens and infection models, is particularly associated with robust neutrophil infiltration at the site of infection, and the ensuing inflammatory response is both essential for clearing the infection and responsible for disease pathology (Barteneva et al. 1996; Stephens 2003; Lijek et al. 2018). An important effector of pro-inflammatory signaling in this context is nuclear factor kappa B (NF-κB). This transcription factor is activated by Toll-like receptors (TLR), nucleotide oligomerization domain proteins (NODs) and other innate immune receptors, and results in the transcription of mostly pro-inflammatory effectors and initiation of apoptosis (Hayden, West and Ghosh 2006). NF-κB is associated with induction of intestinal epithelial Interleukin (IL)-6 and IL-8 cytokine production (Stadnyk 2002). IL-6 has both inflammatory and anti-inflammatory effects and may play a role in recovery from intestinal injury (Jin et al. 2010). IL-8 is an important neutrophil attractant and is implicated in bacteria-induced inflammatory intestinal pathology (Singer and Sansonetti 2004). Activation of NF-κB in response to infection with Chlamydia has been studied using mostly strains associated with human disease in rodent and in vitro models.
Previous infection studies in pigs have demonstrated that the porcine chlamydial strain C. suis can cause intestinal inflammation and severe diarrhea (Guscetti et al. 2009). In vitro studies suggests that the NF-κB pathway is activated and IL-6 and IL-8 are induced upon infection with Chlamydia (Buchholz and Stephens 2006; Leonard, Schoborg and Borel 2017). Additionally, NF-κB was recently shown to be activated in the jejunum and ileum in a piglet model of Salmonella induced diarrhea (Xia et al. 2020). To our knowledge, the intestinal immune response to C. suis related to NF-κB activation has not been studied in vivo. Therefore, using archived samples from a previous study, we aimed to investigate whether NF-κB is associated with the intestinal inflammation observed in C. suis experimentally-infected gnotobiotic piglets, and which effector cells and cytokines play a role during the acute phase of infection. Furthermore, the localization and time-course of the inflammation in correlation with NF-κB activation in epithelial cells and the recruitment of other innate immune cells was elucidated in this study.
MATERIAL AND METHODS
Inoculum
Lyophilized C. suis was used to generate an inoculum for the infection trial as described previously (Guscetti et al. 1998, 2009). Briefly, after resuspension of the lyophilisate in sucrose–phosphate–glutamate medium, C. suis was propagated in specific pathogen-free embryonated chicken eggs. The protocol for harvesting, determining the titers and controlling for contamination of the inocula was described in detail in the original publication of this study (Guscetti et al. 1998).
Animals and experimental design
Intestinal specimens consisting of formalin-fixed and paraffin-embedded (FFPE) or fresh frozen tissues (−80°C) for immunohistochemical and ELISA analysis, respectively, were collected during a previously performed and published study (Guscetti et al. 1998, 2009). In brief, gnotobiotic landrace piglets were obtained through closed hysterectomy and maintained in isolators for the duration of the trial. The diet consisted of sterile evaporated milk powder and feedings were given four times daily and increased up to eight times daily if watery diarrhea was present. The subgroup that was further analyzed for the current study received an intragastric dose of 5 × 106 inclusion forming units of C. suis strain S45/6 between 47 and 55 h postpartum and was sacrificed at day 2, 4 and 7 post-inoculation (dpi). This subgroup consisted of a single animal per treatment condition and, due to this, only semi-quantitative methods, and not statistical analyses, have been applied to describe observed effects reported here and results should thus be interpreted conservatively. The animal experiments were performed according to relevant Swiss law and were approved by ethical committee (authorization no. 229/93, Veterinary Office of the Canton Zurich).
Immunohistochemistry
Sections of the small intestine, namely the proximal jejunum (Jej 2), the distal jejunum (Jej 4) and the ileum, previously fixed in 4% buffered formaldehyde for 24 h followed by embedding in paraffin according to routine procedure, were deparaffinized using xylol and rehydrated in a degressive alcohol series. After blocking with DAKO blocking buffer (S2023, Agilent Technologies, Basel, Switzerland) for 10 min, tissues were labeled for 1 h at room temperature using antibodies directed against NF-κB (8242S, rabbit monoclonal, Cell signaling, Leiden, The Netherlands) and CD45 (ab10559, rabbit polyclonal, Abcam, Cambridge, UK) at a dilution of 1:400 and 1:500, respectively. The secondary antibody (Dako K4003, Agilent Technologies) was incubated for 30 min at room temperature and substrate chromogen (Dako K3464) applied for 10 min. Slides were briefly counterstained for 2 s with hematoxylin solution modified according to Gill II (Merck, Darmstadt, Germany), dehydrated and mounted with Tissue Tek media (Sakura Alphen aan den Rijn, The Netherlands). Detection of chlamydial antigen was performed using a mouse monoclonal Chlamydiaceae family-specific antibody directed against the chlamydial lipopolysaccharide (LPS; Clone ACI-P, Progen, Heidelberg, Germany) and was performed as described previously (Guscetti et al. 2009). Negative control sections without primary antibodies were processed in parallel.
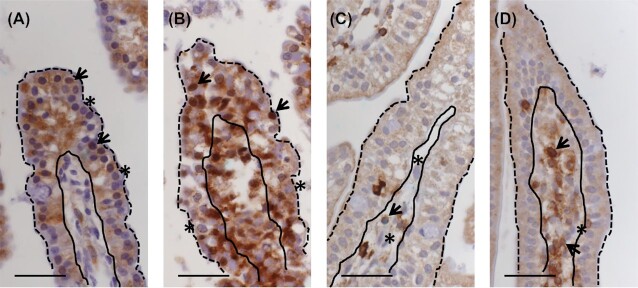
Sections were scanned using the Hamamatsu NanoZoomer 2.0-HT digital slide scanner and further evaluation of immunohistochemical sections was performed on scanned slides. Hematoxylin counterstaining allows visual differentiation of the lamina epithelialis (epithelial cells), the layer of cells in direct contact with the intestinal lumen, and the lamina propria, the underlying connective tissue containing blood vessels and lymphatics, within the tunica mucosa of the small intestine. Examples of lumen/epithelial cell borders and epithelial cell/lamina propria borders have been denoted in Fig. 1A–D. From each section, at least 10 full-length, randomly chosen cross-sectioned villi were evaluated, and approximately 1000 epithelial cells were counted for each condition. Cells were considered to contain active NF-κB if the nucleus displayed brown labeling, regardless of labeling intensity relative to cytoplasmic labeling intensity. In cases where the nucleus was blue (counterstain) and only the cytoplasm was labeled for NF-κB, this was not considered active NF-κB. Thus, in our counting scheme, NF-κB activation or lack of activation is determined by presence or absence of nuclear NF-κB localization, respectively. The number of cells with labeled nuclear NF-κB per 100 counted epithelial cells was plotted in a graph to compare levels of NF-κB activation between animals from different treatment days and between C. suis-infected piglets and non-infected controls. This counting scheme was established to quantify the difference in cell activation status that is apparent when looking at the histological image. An example of cells considered positive and negative is shown in Fig. 1A and B.
Figure 1.
Semi-quantitative counting scheme to determine activation of NF-κB in epithelial cells and recruitment of CD45-positive cells into the lamina propria. Example of images used to quantify epithelial cells with labeled nuclear NF-kB (arrowheads) or no nuclear NF-κB labeling (asterisks) in jejunum of uninfected (A) and C. suis infected (B) piglets. Example of images used to quantify CD45-positive (arrowheads) and CD45-negative (asterisks) labeling in cells of the lamina propria in jejunum of uninfected (C) and C. suis infected (D) piglets (solid black line indicates boundary of epithelium and lamina propria, dashed black line delimits epithelium). Scale bar 40 µm.
A similar strategy was chosen for the quantification of CD45 positive cells. Although an increase in the size and cell numbers of the lamina propria was observed in infected animals, this effect was variable across the individual section. The evaluation of at least 10 randomly chosen villi in multiple locations on each slide aimed to give a more accurate picture of the overall activation of CD45-positive cells. At least 350 lamina propria cells were counted for each condition. Total cells and CD45-positive cells in the lamina propria were counted, and an example of which cells were counted and considered positive is shown in Fig. 1C and D.
Tissue homogenate ELISA
At the time of tissue collection, macroscopic examination revealed little luminal content and pieces of intestine of approximately 1 cm were snap frozen without washing out the lumen (Guscetti et al. 2009). Portions of these pieces of intestine were harvested, on dry ice to prevent thawing, from the frozen material in order to represent approximately equivalent weights of intestinal tissues and processed for ELISA analysis. To quantify pro-inflammatory cytokines, about 150 mg of archived fresh frozen Jej 2 and Jej 4 tissues were transferred to tubes equipped with ceramic beads and 1.5 ml of RIPA buffer (Sigma, Buchs, Switzerland) containing complete mini protease inhibitor cocktail without EDTA (Roche Diagnostics, Rotkreuz, Switzerland). Tissues were homogenized using the MagNA Lizer Instrument at 6500 rpm for 50 s, and centrifuged at 10 000 × g for 5 min. The resulting Jej 2 and Jej 4 supernatants were pooled for each animal, and stored at −20°C. Protein concentration was measured using a commercially available BCA kit (ThermoFisher Scientific, Reinach, Switzerland). Porcine IL-6 and IL-8 ELISA kits (Sigma, Buchs SG, Switzerland) were used to determine cytokine concentration in pig tissues. Prior to performing ELISAs, each sample concentration was adjusted to 780 µg/mL protein concentration using RIPA buffer or sample diluent supplied with the respective ELISA kit. Incubation steps were carried out according to the manufacturer's recommendations, chemiluminescent reading was performed using BioTek Gen5 microplate reader (BioTek, Luzern, Switzerland) and analysis was performed by using standard dilution curves to determine sample cytokine concentrations (pg/mL).
RESULTS
The impact of C. suis infection on newborn gnotobiotic piglets has been described previously, and included clinical signs such as diarrhea starting between 2–4 dpi, accompanied by weight loss after 3 dpi (Guscetti et al. 2009). Histopathological changes in the villous structure were present from early stages on and included shortening and fusion of villi, erosion of villous epithelium and accumulation of lymphocytes in the lamina propria. Furthermore, lymphatic vessels in the tunica submucosa were engorged and filled with macrophages and neutrophils. These histopathological changes described in the previous report (Guscetti et al. 2009) were observed, as expected, during the course of our investigation (see Fig. 2 as an example of villi with normal appearance in an uninfected animal (A) or villous epithelial erosion in a C. suis-infected animal (D)). Based on our recent findings showing an activation of NF-κB and its downstream effector IL-6 in HeLa cells in response to C. pecorum (Leonard, Schoborg and Borel 2017), we hypothesized that NF-κB activation is also a driver for pro-inflammatory signaling in response to C. suis in vivo. We herein analyzed existing FFPE samples from the previous infection trial of gnotobiotic piglets for the presence of NF-κB activation and CD45-positive effector cells, namely leukocytes.
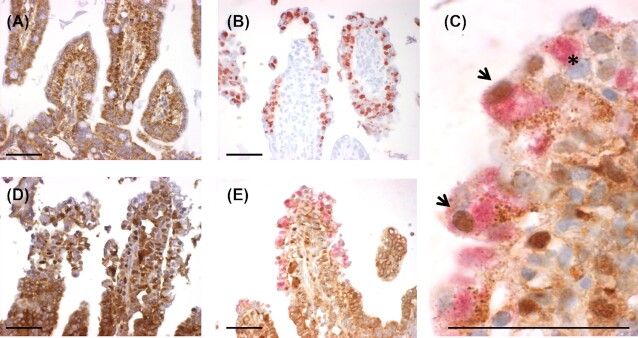
Figure 2.
NF-κB staining and C. suis staining in sections of the jejunum. The jejunum of an uninfected animal (A) or an animal infected with C. suis(B–E) at 2 days post-inoculation (dpi) is shown. Panels A and D show NF-κB single staining (brown), B shows C. suis single staining (red), C and E show NF-κB (brown) staining and C. suis (red) double staining. Panel C is a detail of Panel E. Scale bar 50 µm. Arrowheads indicate C. suis-infected cells with labeled nuclear NF-κB and asterisk indicates C. suis-infected cell with no labeled nuclear NF-κB.
Confirmation of chlamydial infection
According to the previous study evaluating chlamydial infection in the tissues we evaluated here, the small intestinal locations were heavily infected; the highest number of intraepithelial chlamydial inclusions was found approximately 2 days post-inoculation, with another slight increase in the distal small intestine between 5–7 dpi (Guscetti et al. 2009). Our qualitative immunohistochemical analysis of chlamydial infection in the archived FFPE small intestine samples confirmed the expected presence of chlamydial inclusions in the small intestine (Fig. 2B).
Intestinal NF-κB activation
NF-κB activation, determined by nuclear localization of NF-κB, was present in intestinal epithelial and lamina propria cells of neonatal gnotobiotic piglets, both in uninfected animals and in those infected with C. suis (Fig. 2). Compared to uninfected animals (Fig. 2A), the epithelial lining of C. suis infected piglets (Fig. 2B–E) showed loss of integrity and loss of typical epithelial organization, and showed NF-κB activation in the apical portions of the villi. Quantification of epithelial cell (but not lamina propria cell) NF-κB activation was performed by counting NF-κB labeled nuclei in epithelial cells in relation to overall number of epithelial cell nuclei, to generate % of cells with active NF-κB (Fig. 1A and B). All regions of the small intestine were affected by severe tissue damage and NF-κB activation, with the ileum displaying highest % of cells with NF-κB activation at 2 dpi (Fig. 3). Chlamydial infection and tissue damage, at the animal/tissue level, correlated with NF-κB activation, but no apparent peak in activation was noted for any of the specific time points. At the cellular level, chlamydial infection was not observed to correlate with NF-κB nuclear localization/activation and both uninfected and infected cells could be observed with or without NF-κB nuclear localization/activation (Fig. 2C). Though evaluation of statistical significance of C. suis-associated NF-κB activation was precluded due to single-animal experimental groups, the trend observed represented increases of at least 80% in the % of cells with NF-κB activation for all intestinal sections on all days evaluated (uninfected animals’ range was 1.8–14.4% of epithelial cells with NF-κB activation, C. suis-infected animals’ range was 21.5–39.2% of epithelial cells with NF-κB activation, % increase range was 80–1294%; Fig. 3). Although increase in NF-κB activation in all intestinal sections evaluated was relatively robust, the most modest increases particularly, corresponding to increases from 10–15% in the control animal to approximately 25% NF-κB activation in the C. suis-infected animal, as seen at 7 dpi, must necessarily be interpreted conservatively, especially with regards to potential biological relevance of these observations.
Figure 3.
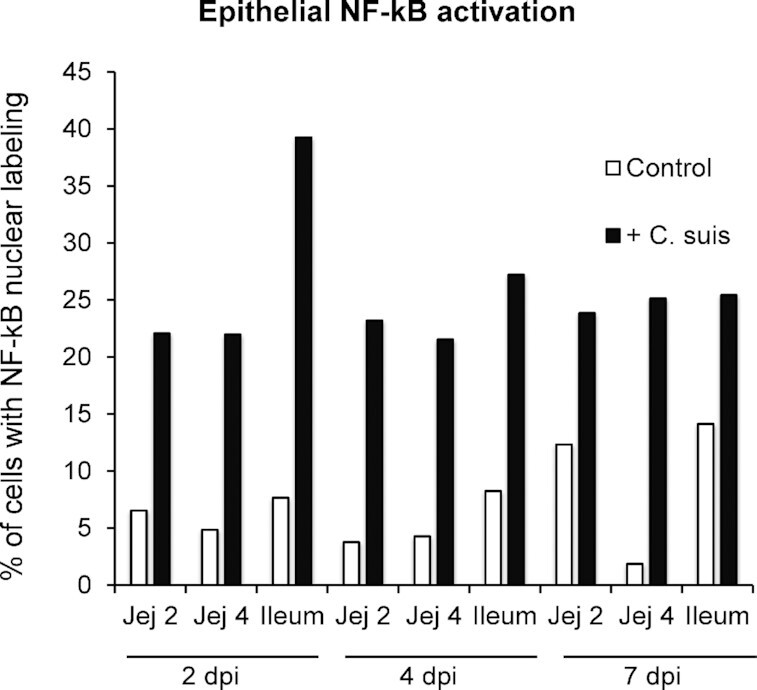
Quantification of epithelial NF-κB activation in the small intestine in response to infection with C. suis at 2, 4 and 7 days post-inoculation (dpi). Cells demonstrating nuclear NF-κB localization were considered to have active NF-κB, while those with only cytoplasmic NF-ΚB localization were not. The percentage of cells with active NF-κB, relative to total epithelial cells counted, is shown.
Recruitment of CD45-positive cells to the proximal jejunum
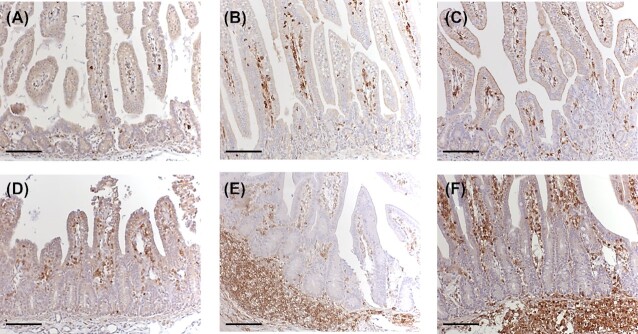
Even in uninfected animals, cells positive for CD45, a general leukocyte marker, were present in the lamina propria, but the morphology of villi and lamina propria was different when compared to the C. suis-infected piglets (Fig. 4). In uninfected neonates, villi appeared long, highly structured and the lamina propria in villus tips was narrow. In infected animals, the architecture of the villi was severely disturbed. Shortening and fusion of villi were evident as previously described (Guscetti et al. 2009). Quantification of CD45-positive cells was performed by counting CD45-labeled cells, in relation to overall number of cells, in the lamina propria to generate % of cells positive for CD45 labeling (Fig. 1C and D). Proximal jejunal % of lamina propria cells positive for CD45 was lowest, in both uninfected and C. suis-infected animals, at 2 dpi and increased from 2 to 4 and 4 to 7 dpi (Fig. 5). There was an apparent recruitment of cells positive for CD45 into the proximal jejunal lamina propria of infected piglets at 7 dpi, whereas at earlier stages of infection no such influx was clearly apparent. Though statistical significance of C. suis-associated CD45-positive cell influx was precluded, the largest observed increase in % of lamina propria cells positive for CD45 labeling, at the 7 dpi time point, was a 61% increase in the C. suis-infected animal compared to the control uninfected animal, with 69% of the cells being CD45-positive in the infected animal. Changes in % of cells positive for CD45, associated with C. suis infection, were smaller at 2 and 4 dpi and were not thus considered to represent an influx of CD45-positive cells (an increase of 36% at 2 dpi and a decrease of 33% at 4 dpi; Fig. 4).
Figure 4.
Sections of jejunum stained for presence of CD45-positive cells. The jejuna of uninfected animals (A–C) or animals infected with C. suis(D–F) at 2 days post-inoculation (dpi) (A and D), 4 dpi (B and E) and 7 dpi (C and F) are shown. Scale bar 100 µm.
Figure 5.
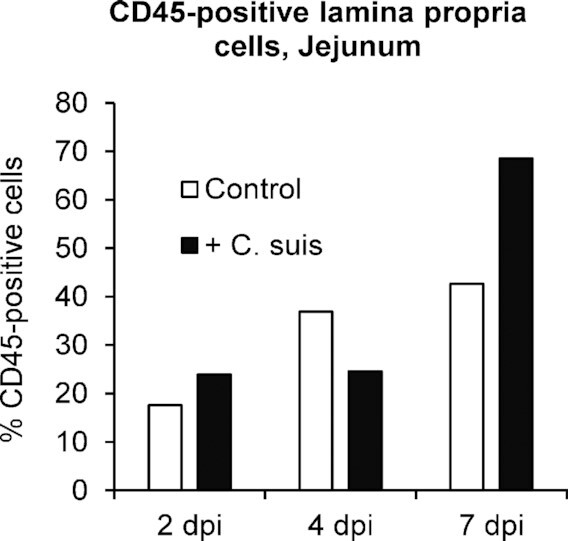
Quantification of CD45-positive cells in the lamina propria of the proximal jejunum in response to infection with C. suis at 2, 4 and 7 days post-inoculation (dpi). The percentage of cells positive for the general leukocyte marker CD45, relative to total lamina propria cells counted, is shown.
Jejunal expression of IL-8
Archived fresh frozen tissues were used to generate pooled proximal and distal jejunal homogenates for evaluation of jejunal expression of IL-6 and IL-8. Jejunal IL-8 expression was highest, in both uninfected and C. suis-infected animals, at 2 dpi. IL-8 expression decreased from 2–4 dpi for uninfected animals (no further decrease from 4 to 7 dpi) and decreased from 2 to 4 and 4 to 7 dpi for infected animals (Fig. 6). For each time point evaluated, C. suis-infected piglets had higher levels of IL-8 expression than corresponding uninfected animals (Fig. 6). C. suis-associated relative increase in IL-8 expression, compared to corresponding uninfected animals, was 89% at 2 dpi, 518% at 4 dpi and 50% at 7 dpi. The lowest IL-8 expression levels overall were 158 and 165 pg IL-8/mg of total protein for uninfected animals at 7 and 4 dpi, respectively; while the highest IL-8 expression level was 1556 pg IL-8/mg of total protein for the C. suis-infected animal at 2 dpi (Fig. 5). Jejunal IL-6 expression showed no apparent trends with regards to dpi or C. suis infection ( Suppl. data). Again, because the experimental groups consist of single animals, the apparent trend toward increased IL-8 expression, and the lack of observation of a similar trend for IL-6 expression, must be interpreted conservatively.
Figure 6.
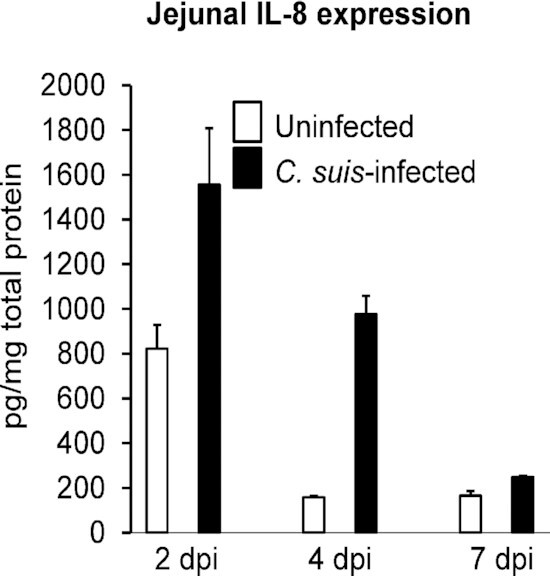
Jejunal IL-8 expression in response to infection with C. suis at 2, 4 and 7 days post-inoculation (dpi). Jejunal homogenates generated from archived fresh frozen tissues were evaluated by commercial IL-8 ELISA assay and IL-8 pg/mg of total protein is shown (mean + standard deviation of 4 replicate wells).
DISCUSSION
During the early phase of infection, intestinal epithelial cells are invaded by C. suis resulting in the formation of inclusions of characteristic morphology. Based on our observations regarding the course and site of infection, we hypothesized that the presence of luminal/extracellular and intraepithelial C. suis activates TLRs and intracellular PRRs in epithelial cells which leads to nuclear translocation of activated NF-κB, thereby triggering a pro-inflammatory signaling cascade. The findings presented here support this hypothesis, as far as this is possible given the single-animal groups available for analysis in this study. At 2, 4 and 7 dpi, a higher % of intestinal epithelial cells had active, nuclear localized NF-κB in C. suis-infected animals compared to uninfected animals, across all evaluated intestinal sections (proximal and distal jejunum and ileum). Determination of NF-κB activation by nuclear localization, regardless of staining intensity, was employed as a proxy for NF-κB activation independent of transcriptional or translational regulation of NF-κB levels. We employed this strategy for evaluation of NF-κB activity because nuclear localization is required for NF-κB activity as a transcription factor, while increased presence of NF-κB is not indicative of transcription factor activity. Double staining for chlamydial infection and NF-κB nuclear localization/activation showed that while NF-κB activation appears to be associated with chlamydial infection at the tissue level in the porcine small intestine, chlamydial infection of an individual cell did not exclusively result in NF-κB activation of the cell. This observation is similar to that previously reported in C. pecorum-infected human epithelial cells in vitro (Leonard, Schoborg and Borel 2017) and represents the general phenomenon of NF-κB activation in individual uninfected/unexposed ‘bystander cells’ cells in the setting of bacterial infection or ionizing irradiation, respectively (Kasper et al. 2010; Hellweg et al. 2016).
Leukocytes (CD45-positive) were present in the lamina propria of the proximal jejunum in uninfected and C. suis-infected animals from 2 dpi on, but showed potential recruitment associated with C. suis infection at 7 dpi. Tissue damage was reversed and epithelial cell integrity was almost fully restored at day 7, while the leukocyte population in the lamina propria was still increased at this time point. Jejunal IL-8 expression, directly measured by cytokine ELISA, was increased in C. suis-infected animals compared to uninfected animals at all time points evaluated, and may represent both epithelial and leukocyte sources of IL-8. However, IL-8 levels were highest at 2 dpi (for both infected and uninfected animals), with a trend toward decreasing levels over time, suggesting epithelial cell-dependent, not leukocyte-dependent, generation of this inflammatory cytokine. IL-6 expression, conversely, was not apparently associated with C. suis infection, though our previous in vitro study demonstrated that chlamydial infection can induce IL-6 production in cultured human cervical epithelial cells (Leonard, Schoborg and Borel 2017).
The tissues investigated in the present study originated from a previous infection trial (Guscetti et al. 2009), and limited animal numbers and sample availability correspondingly limited the types and extent of analysis we could undertake. Therefore, we emphasize that the results presented herein must be interpreted conservatively, and individual animal-specific findings cannot be reliably applied to a broader context. Despite these methodological limitations, we were able to gain insight, though limited, into the host-pathogen-interactions using an experimental pig model. The initial phase of infection in neonatal pigs is characterized by a high load of chlamydial antigen, in particular in the small intestine. The presence of Chlamydia in epithelial cells correlated with an apparent increase in NF-κB-positive nuclei in the same regions where chlamydial inclusions prevailed, namely the villus tips. The ubiquitous nature of NF-κB activation makes it difficult to pinpoint the direct pathway involved, since a number of factors such as IL-1, TNFα but also LPS and reactive oxygen species have been shown to activate NF-κB through receptors or directly (Hoesel and Schmid 2013). NF-κB plays an important role in in vivo and in vitro chlamydial infection (Cocchiaro and Valdivia 2009), in human necrotizing colitis (Hunter and De Plaen 2014) and in acute enteritis in pigs (Wang et al. 2018), but had not been evaluated for a role in intestinal in vivo C. suis infections. Here we showed that C. suis infection has the potential to induce NF-κB activation in the small intestine of gnotobiotic piglets.
The innate immune response to intestinal pathogens is crucially dependent on the presence of rapidly activated pro-inflammatory cytokines and signaling to their downstream effectors. IL-6, known for both pro- and anti-inflammatory effects, has been shown to activate NF-κB in epithelial cells, leading to the increased expression of ICAM-1 which enables interaction with immune cells (Wang et al. 2003), and to inhibit apoptosis and to lead to the recruitment of myeloid cells (i.e. macrophages and neutrophils; Grivennikov et al. 2009). Additionally, following the acute inflammatory phase, IL-6 promotes epithelial cell proliferation and repair after injury, as demonstrated in a mouse model of intestinal inflammation (Kuhn et al. 2014). The role of IL-6 in chlamydial infections in vivo has remained unclear, and both protective and damaging effects have been attributed to this cytokine. As demonstrated in the C. muridarum mouse model of chlamydial vaginal infection, for example, low versus high chlamydial infective dose may influence the protective or damaging role of IL-6. Site of infection, which varies widely in natural human and animal infections, is also likely to exert an influence. In our current findings, IL-6 expression did not appear to show any trend related to dpi or C. suis infection, so we were not able to gain insight into a potential role for IL-6 in the porcine in vivo/C. suis setting. NF-κB-induced IL-8 has been specifically implicated in gastrointestinal inflammatory processes with pathogenic outcomes (Cotton et al. 2016). In vitro, C. trachomatis has been shown to induce IL-8 expression (Buchholz and Stephens 2006), and IL-8 expression has been shown to increase in pig small intestine with both day post-weaning and bacterial lipopolysaccharide treatment (Jaime Parra et al. 2013), but a role for IL-8 in C. suis in vivo infection had not been evaluated. Here we showed that C. suis infection may induce IL-8 expression in the small intestine of gnotobiotic piglets.
A potential advantage of our study design was the use of gnotobiotic piglets, which eliminates other commensal or pathogenic bacteria as potential activators of NF-κB. Moreover, gnotobiotic piglets are more sensitive to infection with pathogens as compared to conventionally-reared piglets allowing robust experimental infections. A previously published gnotobiotic piglet infection model using Salmonella typhimurium was used to evaluate the effect of both commensal and pathogenic bacteria in the gut (Splichalova et al. 2011). When gnotobiotic pigs were inoculated with probiotic strains of Escherichia coli or Bifidobacterium choerinum prior to Salmonella infection, they did not develop clinical signs of infection and had lower levels of inflammatory cytokines IL-10 and TNF alpha present in the plasma and the intestine as compared to gnotobiotic pigs that had not been exposed to probiotic bacteria before. Interestingly, the probiotic bacteria elicited intestinal IL-8 production, and this was associated with a protective effect that reduced Salmonella infection. The researchers conducting the study speculated that an enterocyte-specific production of IL-8 in response to the probiotic bacteria, along with upregulation of tight-junction proteins in the epithelial barrier was sufficient to control the growth and prevent the invasion of pathogenic Salmonella (Splichalova et al. 2011). This priming effect appears to be crucial for an effective and immediate response of the innate immune system. In our model, the absence of commensal or probiotic bacteria likely contributed to the susceptibility to infection with Chlamydia and the severe histopathological and clinical signs observed. In line with the previous finding that bacteria can induce IL-8 expression in the intestine of gnotobiotic piglets (Splichalova et al. 2011), our findings showed that IL-8 is potentially induced by C. suis in the small intestine of gnotobiotic piglets. The previous and current studies suggest that IL-8 induction can be beneficial, or at least present in a setting indicative of recovery, respectively. Importantly, a recent weaned piglet diarrhea model (not gnotobiotic), using Salmonella oral infection, also showed that pathogenic infection can induce intestinal NF-κB activation in the setting of a more natural microbiome (Xia et al. 2020).
Despite the pronounced epithelial response, we noted that the observed apparent NF-κB activation in the underlying lamina propria was limited, most likely due to the early stage of infection. The tissue damage caused by Chlamydia infection is most prominent after 2–4 days of infection, and is already reversed at day 7. This suggests that despite the pathogenic nature of Chlamydia, the host seems to be capable to limit the extent of the infection and to initiate epithelial regeneration very soon after the peak of infection. This might indicate that the tissue damage observed in the piglets is not due to an uncontrolled or exaggerated activation of NF-κB. Possibly, the detachment of epithelial cells expels intracellular Chlamydia through the induction of apoptosis. The proposed mechanisms leading to a significant decrease in chlamydial load after 7d of infection are (1) expelling of infected epithelial cells and thereby limiting release of newly formed Chlamydia and re-infection of adjacent enterocytes, and (2) activation of pro-inflammatory innate immune pathways with recruitment of immune effector cells.
In summary, we can support our previously reported in vitro finding (Leonard, Schoborg and Borel 2017) which identified NF-κB as target and driver of pro-inflammatory signaling in response to infection with Chlamydia. In gnotobiotic piglets, C. suis appeared to trigger NF-κB activation in intestinal epithelial cells of the small intestine, which was accompanied by an early, though brief, apparent increase in IL-8 expression and accumulation of CD45-positive cells in the lamina propria.
Supplementary Material
ACKNOWLEDGEMENTS
This work was funded by grant 310030_147026, Swiss National Science Foundation, NB.
Contributor Information
Helen Aumayer, Department of Pathobiology, Institute of Veterinary Pathology, Vetsuisse Faculty, University of Zurich, Winterthurerstrasse 268, CH-8057 Zurich, Switzerland.
Cory Ann Leonard, Department of Pathobiology, Institute of Veterinary Pathology, Vetsuisse Faculty, University of Zurich, Winterthurerstrasse 268, CH-8057 Zurich, Switzerland.
Theresa Pesch, Department of Pathobiology, Institute of Veterinary Pathology, Vetsuisse Faculty, University of Zurich, Winterthurerstrasse 268, CH-8057 Zurich, Switzerland.
Barbara Prähauser, Department of Pathobiology, Institute of Veterinary Pathology, Vetsuisse Faculty, University of Zurich, Winterthurerstrasse 268, CH-8057 Zurich, Switzerland.
Sabina Wunderlin, Department of Pathobiology, Institute of Veterinary Pathology, Vetsuisse Faculty, University of Zurich, Winterthurerstrasse 268, CH-8057 Zurich, Switzerland.
Franco Guscetti, Department of Pathobiology, Institute of Veterinary Pathology, Vetsuisse Faculty, University of Zurich, Winterthurerstrasse 268, CH-8057 Zurich, Switzerland.
Nicole Borel, Department of Pathobiology, Institute of Veterinary Pathology, Vetsuisse Faculty, University of Zurich, Winterthurerstrasse 268, CH-8057 Zurich, Switzerland.
Conflicts of interests
None declared.
REFERENCES
- Barteneva N, Theodor I, Peterson EMet al. . Role of neutrophils in controlling early stages of a Chlamydia trachomatis infection. Infect Immun. 1996;64:4830–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavoil PM, Marques PX, Brotman Ret al. . Does active oral sex contribute to female infertility? J Infect Dis. 2017;216:932–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz KR, Stephens RS. Activation of the host cell proinflammatory interleukin-8 response by Chlamydia trachomatis. Cell Microbiol. 2006;8:1768–79. [DOI] [PubMed] [Google Scholar]
- Chahota R, Ogawa H, Ohya Ket al. . Involvement of multiple Chlamydia suis genotypes in porcine conjunctivitis. Transbound Emerg Dis. 2018;65:272–7. [DOI] [PubMed] [Google Scholar]
- Cocchiaro JL, Valdivia RH. New insights into Chlamydia intracellular survival mechanisms. Cell Microbiol. 2009;11:1571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton JA, Platnich JM, Muruve DAet al. . Interleukin-8 in gastrointestinal inflammation and malignancy: induction and clinical consequences. Int J Interf Cytokine Mediat Res. 2016;8:13. [Google Scholar]
- De Puysseleyr K, De Puysseleyr L, Dhondt Het al. . Evaluation of the presence and zoonotic transmission of Chlamydia suis in a pig slaughterhouse. BMC Infect Dis. 2014;14:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Puysseleyr L, De Puysseleyr K, Braeckman Let al. . Assessment of Chlamydia suis infection in pig farmers. Transbound Emerg Dis. 2017;64:826–33. [DOI] [PubMed] [Google Scholar]
- Donati M, Huot-Creasy H, Humphrys Met al. . Genome sequence of Chlamydia suis MD56, isolated from the conjunctiva of a weaned piglet. Genome Announc. 2014;2:e00425–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S, Karin E, Terzic Jet al. . IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guscetti F, Schiller I, Sydler Tet al. . Experimental Chlamydia psittaci serotype 1 enteric infection in gnotobiotic piglets: histopathological, immunohistochemical and microbiological findings. Vet Microbiol. 1998;62:251–63. [DOI] [PubMed] [Google Scholar]
- Guscetti F, Schiller I, Sydler Tet al. . Experimental enteric infection of gnotobiotic piglets with Chlamydia suis strain S45. Vet Microbiol. 2009;135:157–68. [DOI] [PubMed] [Google Scholar]
- Haggerty CL, Gottlieb SL, Taylor BDet al. . Risk of squelae after Chlamydia trachomatis genital infection in women. J Infect Dis. 2010;201:134–55. [DOI] [PubMed] [Google Scholar]
- Hayden MS, West AP, Ghosh S. NF-κB and the immune response. Oncogene. 2006;25:6758–80. [DOI] [PubMed] [Google Scholar]
- Hellweg CE, Spitta LF, Henschenmacher Bet al. . Transcription factors in the cellular response to charged particle exposure. Front Oncol. 2016;6:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K, Schott F, Donati Met al. . Prevalence of Chlamydial Infections in fattening pigs and their influencing factors. PLoS One. 2015;10:e0143576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CJ, De Plaen IG. Inflammatory signaling in NEC: role of NF-κB, cytokines and other inflammatory mediators. Pathophysiology. 2014;21:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu VH, Harding-Esch EM, Burton MJet al. . Epidemiology and control of trachoma: systematic review. Trop Med Int Heal. 2010;15:673–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaime Parra S, Jorge Agudelo T, David Sanín Pet al. . Intestinal expression of pro-inflammatory cytokines induced by oral intake of lipopolysaccharide (LPS) from E. Coli in weaned pigs. Rev Colomb Ciencias Pecu. 2013;26:108–18. [Google Scholar]
- Jin X, Zimmers TA, Zhang Zet al. . Interleukin-6 is an important in vivo inhibitor of intestinal epithelial cell death in mice. Gut. 2010;59:186–96. [DOI] [PubMed] [Google Scholar]
- Kasper CA, Sorg I, Schmutz Cet al. . Cell-cell propagation of NF-κB transcription factor and MAP kinase activation amplifies innate immunity against bacterial infection. Immunity. 2010;33:804–16. [DOI] [PubMed] [Google Scholar]
- Kuhn KA, Manieri NA, Liu T-Cet al. . IL-6 stimulates intestinal epithelial proliferation and repair after injury. PLoS One. 2014;9:e114195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CA, Schoborg RV, Borel N. Productive and penicillin-stressed Chlamydia pecorum infection induces nuclear factor Kappa B activation and Interleukin-6 secretion in vitro. Front Cell Infect Microbiol. 2017;7, DOI: 10.3389/fcimb.2017.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijek RS, Helble JD, Olive AJet al. . Pathology after Chlamydia trachomatis infection is driven by nonprotective immune cells that are distinct from protective populations. Proc Natl Acad Sci. 2018;115:2216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishori R, McClaskey EL, Winklerprins VJ. Chlamydia trachomatis infections: screening, diagnosis, and management. Am Fam Physician. 2012;86:1127–32. [PubMed] [Google Scholar]
- Nietfeld JC, Janke BH, Leslie-Steen Pet al. . Small intestinal Chlamydia infection in piglets. J Vet Diagn Invest. 1993;5:114–7. [DOI] [PubMed] [Google Scholar]
- Nietfeld JC, Leslie-Steen P, Zeman DHet al. . Prevalence of antestinal chlamydial infection in pigs in the midwest, as determined by immunoperoxidase staining. Am J Vet Res. 1997;58:260–4. [PubMed] [Google Scholar]
- Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–53. [DOI] [PubMed] [Google Scholar]
- Pickard JM, Zeng MY, Caruso Ret al. . Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 2017;279:70–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DG, Andersen AA. Intestinal lesions caused by two swine chlamydial isolates in gnotobiotic pigs. J Vet Diagn Invest. 1996;8:433–40. [DOI] [PubMed] [Google Scholar]
- Satterwhite CL, Torrone E, Meites Eet al. . Sexually transmitted infections among US women and men. Sex Transm Dis. 2013;40:187–93. [DOI] [PubMed] [Google Scholar]
- Schautteet K, Vanrompay D. Chlamydiaceae infections in pig. Vet Res. 2011;42:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller I, Koesters R, Weilenmann Ret al. . Mixed infections with porcine Chlamydia trachomatis/pecorum and infections with ruminant Chlamydia psittaci serovar 1 associated with abortions in swine. Vet Microbiol. 1997;58:251–60. [DOI] [PubMed] [Google Scholar]
- Singer M, Sansonetti PJ. IL-8 Is a key chemokine regulating neutrophil recruitment in a new mouse model of Shigella-induced colitis. J Immunol. 2004;173:4197–206. [DOI] [PubMed] [Google Scholar]
- Splichalova A, Trebichavsky I, Rada Vet al. . Interference of Bifidobacterium choerinum or Escherichia coli Nissle 1917 with Salmonella Typhimurium in gnotobiotic piglets correlates with cytokine patterns in blood and intestine. Clin Exp Immunol. 2011;163:242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadnyk AW. Intestinal epithelial cells as a source of inflammatory cytokines and chemokines. Can J Gastroenterol. 2002;16:241–6. [DOI] [PubMed] [Google Scholar]
- Stephens RS. The cellular paradigm of chlamydial pathogenesis. Trends Microbiol. 2003;11:44–51. [DOI] [PubMed] [Google Scholar]
- Szeredi L, Schiller I, Sydler Tet al. . Intestinal Chlamydia in finishing pigs. Vet Pathol. 1996;33:369–74. [DOI] [PubMed] [Google Scholar]
- Tomasello E, Bedoui S. Intestinal innate immune cells in gut homeostasis and immunosurveillance. Immunol Cell Biol. 2013;91:201–3. [DOI] [PubMed] [Google Scholar]
- Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. [DOI] [PubMed] [Google Scholar]
- Wang L, Qiao X, Zhang Set al. . Porcine transmissible gastroenteritis virus nonstructural protein 2 contributes to inflammation via NF-κB activation. Virulence. 2018;9:1685–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Walia B, Evans Jet al. . IL-6 Induces NF-κB activation in the intestinal epithelia. J Immunol. 2003;171:3194–201. [DOI] [PubMed] [Google Scholar]
- WHO . WHO Guidelines for the Treatment of Chlamydia Trachomatis. World Health Organization, 2016. [PubMed] [Google Scholar]
- Xia B, Yu J, He Tet al. . Lactobacillus johnsonii L531 ameliorates enteritis via elimination of damaged mitochondria and suppression of SQSTM1-dependent mitophagy in a Salmonella infantis model of piglet diarrhea. FASEB J. 2020;34:2821–39. [DOI] [PubMed] [Google Scholar]
- Yeruva L, Spencer N, Bowlin AKet al. . Chlamydial infection of the gastrointestinal tract: a reservoir for persistent infection. Pathog Dis. 2013;68:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn I, Szeredi L, Schiller Iet al. . Immunohistochemical determination of Chlamydia Psittaci/Pecorum and C. trachomatis in the piglet gut. Zentralbl Vet B. 1995;42:266–76. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.