Abstract
Objective:
Intervening illnesses and injuries have pronounced deleterious effects on functional status in older persons, but have not been carefully evaluated after critical illness. We set out to evaluate the functional effects of intervening illnesses and injuries in the year after critical illness.
Design:
Prospective longitudinal study of 754 nondisabled community-living persons, aged 70 years or older.
Setting:
Greater New Haven, Connecticut, from March 1998 to December 2018.
Patients:
The analytic sample included 250 intensive care unit (ICU) admissions from 209 community-living participants who were discharged from the hospital.
Interventions:
None.
Measurements and Main Results:
Functional status (13 activities) and exposure to intervening illnesses and injuries leading to hospitalization, emergency department (ED) visit, or restricted activity were assessed each month. Comprehensive assessments (for covariates) were completed every 18 months. In the year after critical illness, recovery of premorbid function was observed for 169 (67.6%) of the ICU admissions, and the mean (sd) number of episodes of functional decline (from one month to the next) was 2.2 (1.6). The adjusted hazard ratios (95% CI) for recovery were 0.18 (0.09–0.39), 0.46 (0.17–1.26), and 0.75 (0.48–1.18) for intervening hospitalizations, ED visits, and restricted activity, respectively. For functional decline, the corresponding odds ratios (95% CI) were 2.06 (1.56–2.73), 1.78 (1.12–2.83), and 1.25 (0.92–1.69). The effect sizes for hospitalization and ED visit were larger than those for any of the covariates.
Conclusions:
In the year after critical illness, intervening illnesses and injuries leading to hospitalization and ED visit are strongly associated with adverse functional outcomes, with effect sizes larger than those of traditional risk factors. To improve functional outcomes, more aggressive efforts will be needed to prevent and manage intervening illnesses and injuries after critical illness.
Keywords: critical illness, functional status, disability, hospitalization, cohort study, risk factor
In 2015, there were approximately 1.9 million hospitalizations for critical illness among persons 65 or older in the US (1). This value will increase considerably in the coming years based on projections that the number of persons 65 or older will nearly double from 50.9 million in 2017 to 94.7 million in 2060 (2). One of the most feared complications of critical illness is loss of independence. Prior research has shown that critical illness is a potent precipitant of functional decline and disability in older persons (3–6). In the setting of critical illness, the level of disability in daily activities increases in about three-quarter of the cases within the first month (7), and the likelihood of developing severe disability, defined as the need for personal assistance with ≥3 essential activities, is increased more than 1000-fold among previously nondisabled older persons (5).
The capacity to recover after a disabling event, including critical illness, is high (7–9). Among older persons who survive a critical illness, more than half recover to their pre-morbid level of function within six months (7). Increasing evidence, however, suggests that recovery after a disabling event is often followed by recurrent disability and functional decline (8, 10). The factors associated with functional recovery and functional decline after a disabling event are well established and include (among others) older age, frailty, cognitive impairment, multimorbidity, depressive symptoms, sensory impairments, and obesity (7, 11–13). In contrast, far less is known about the functional effects of illnesses/injuries that occur after critical illness. We hypothesized that functional outcomes after critical illness are adversely affected by subsequent illnesses/injuries and that these effects persist after accounting for traditional risk factors.
To test our hypothesis, we used high quality data from a unique longitudinal study of community-living older persons that includes monthly assessments of functional status and intervening illnesses/injuries, along with a large array of covariates that were assessed every 18 months for 20 years. Our objective was to evaluate the functional effects of intervening illnesses/injuries in the year after critical illness. The results of this study may provide additional insights on how functional outcomes after critical illness can be improved in community-living older persons.
MATERIALS AND METHODS
Study Population
Participants were members of an ongoing longitudinal study of 754 community-living persons, 70 years or older, who were initially nondisabled in their essential activities of daily living (8, 14). Potential participants were members of a large health plan and were excluded for significant cognitive impairment with no available proxy (15), life expectancy less than 12 months, plans to move out of the area, or inability to speak English. Enrollment occurred from March 1998 to October 1999, and the participation rate was 75.2%. The study was approved by the Yale Human Investigation Committee, and all participants provided informed consent.
Data Collection
Comprehensive assessments were completed by trained nurse researchers at baseline and every 18 months, while telephone interviews were completed monthly by a separate team of researchers through December 2018. For participants who had significant cognitive impairment or were otherwise unavailable, a proxy informant was interviewed using a rigorous protocol (15). In the current study, 18.1% of the monthly interviews were completed by a proxy. Deaths were ascertained from an informant during a subsequent interview and/or by review of local obituaries. Completion of data collection has been high, and attrition has been low (16).
Descriptive Characteristics and Covariates
During the comprehensive assessments, data were collected on demographic characteristics, nine self-reported, physician-diagnosed chronic conditions, body mass index, cognitive status (17), depressive symptoms (18), functional self-efficacy (19), hearing (20), vision (21), and frailty (22). Additional operational details are provided in Table 1.
Table 1.
Characteristics of Analytic Samples for Critical Illness According to Type of Functional Outcome
| Overall * | Functional Recovery | Functional Decline | |||||
|---|---|---|---|---|---|---|---|
| Characteristic b | N=250 | Yes N=169 | No N=81 | P-value | Yes N=212 | No N=38 | P-value |
| Age in years, mean ± sd | 82.3 ± 5.7 | 81.9 ± 5.7 | 83.1 ± 5.6 | .125 | 82.2 ± 5.8 | 82.4 ± 5.4 | .840 |
| Female sex, n (%) | 146 (58.4) | 98 (58.0) | 48 (59.3) | .848 | 124 (58.5) | 22 (57.9) | .945 |
| Non-Hispanic white, n (%) | 220 (88.0) | 146 (86.4) | 74 (91.4) | .258 | 185 (87.3) | 35 (92.1) | .397 |
| Lives alone, n (%) | 98 (39.2) | 66 (39.1) | 32 (39.5) | .945 | 83 (39.2) | 15 (39.5) | .970 |
| Education in years, mean ± sd | 12.0 ± 2.8 | 12.1 ± 2.9 | 11.9 ± 2.7 | .621 | 12.0 ± 2.8 | 11.9 ± 2.9 | .915 |
| Number of chronic conditions c, mean ± sd | 2.5 ± 1.3 | 2.3 ± 1.3 | 2.7 ± 1.3 | .024 | 2.5 ± 1.3 | 2.2 ± 1.3 | .123 |
| Body mass index (kg/m2), mean ± sd | 26.2 ± 5.2 | 26.4 ± 5.4 | 25.6 ± 4.9 | .237 | 26.2 ± 5.3 | 25.9 ± 5.2 | .777 |
| Cognitive impairment d, n (%) | 44 (17.6) | 26 (15.4) | 18 (22.2) | .184 | 37 (17.5) | 7 (18.4) | .885 |
| Depressive symptoms e, n (%) | 43 (17.2) | 26 (15.4) | 17 (21.0) | .271 | 38 (17.9) | 5 (13.2) | .473 |
| Functional self-efficacy f, mean ± sd | 27.2 ± 8.9 | 28.5 ± 8.8 | 24.5 ± 8.6 | .001 | 27.0 ± 8.7 | 28.5 ± 9.8 | .353 |
| Hearing impairment g, n (%) | 80 (32.0) | 46 (27.2) | 34 (42.0) | .019 | 69 (32.6) | 11 (29.0) | .661 |
| Visual impairment h, n (%) | 116 (46.4) | 73 (43.2) | 43 (53.1) | .142 | 98 (46.2) | 18 (47.4) | .897 |
| Frailty phenotype i, n (%) | .015 | .291 | |||||
| Non-frail | 33 (13.2) | 28 (16.6) | 5 (6.2) | 27 (12.7) | 6 (15.8) | ||
| Pre-frail | 109 (43.6) | 77 (45.6) | 32 (39.5) | 89 (42.0) | 20 (52.6) | ||
| Frail | 108 (43.2) | 64 (37.9) | 44 (54.3) | 96 (45.3) | 12 (31.6) | ||
| Number of disabilities j, mean ± sd | 4.1 ± 3.3 | 3.6 ± 3.2 | 5.0 ± 3.3 | .002 | 4.1 ± 3.2 | 3.8 ± 4.0 | .600 |
| Mechanical ventilation, n (%) | 29 (11.7) | 20 (11.8) | 9 (11.3) | .893 | 24 (11.3) | 5 (13.5) | .701 |
| Length of ICU stay, days, mean ± sd | 3.0 ± 4.3 | 2.8 ± 4.6 | 3.4 ± 3.6 | .253 | 2.9 ± 4.5 | 3.1 ± 2.8 | .841 |
Abbreviation: ICU, intensive care unit; sd, standard deviation.
The 250 observations were contributed by 209 community-living participants, who survived to their first interview after hospital discharge.
Data on chronic conditions, body mass index, cognitive impairment, depressive symptoms, functional self-efficacy, hearing and visual impairment, and frailty phenotype were collected during the prior comprehensive assessment, while data on number of disabilities were collected during the prior monthly interview.
Included hypertension, myocardial infarction, congestive heart failure, stroke, diabetes mellitus, arthritis, hip fracture, chronic lung disease, and cancer.
Defined as score < 24 on Folstein Mini-Mental State Examination.
Defined as score ≥ 20 on Center for Epidemiological Studies-Depression Scale.
Score on the Modified Self-Efficacy Scale: 0 (low) to 40 (high), where higher scores indicate greater confidence performing activities (19).
Assessed using a handheld Audioscope, with severe impairment defined as 4 out of 4 tones missed, based on 1000- and 2000-Hz measurements for the left and right ears (20).
Defined as ≥ 5% when assessed with a Jaeger card and use of corrective lenses, if applicable (7).
Based on number of frailty criteria met (22).
Of 13 possible: 4 essential, 5 instrumental, and 4 mobility.
Intervening Events
The intervening illnesses/injuries, i.e. events, included hospitalizations, emergency department (ED) visits, and episodes of restricted activity. The primary source of information on hospitalizations and ED visits was linked Medicare claims data, which were available for nearly all hospitalizations and for ED visits among fee-for-service participants (23). For periods when participants had managed Medicare, hospitalizations were ascertained using Medicare Provider and Analysis Review files, while information on ED visits and some hospitalizations (i.e. those without a Medicare record) was obtained during the monthly interviews. Participants were asked whether they had visited an ED or stayed overnight in a hospital since the last interview. The accuracy of self-reported hospitalizations, based on an independent review of hospital records, and ED visits, based on Medicare claims data, was high, with Kappa=0.92 (95% CI, 0.90-0.95) and Kappa=0.80 (0.78-0.82) (24), respectively. For descriptive purposes, the reasons for hospitalizations and ED visits were subsequently grouped into distinct diagnostic categories using revised versions of a previously described protocol (24, 25).
To ascertain less potent intervening events, participants were asked two questions related to restricted activity (14): 1) “Since we last talked on (date of last interview), have you cut down on your usual activities due to an illness, injury or other problem?” and 2) “Since we last talked on (date of last interview), have you stayed in bed for at least ½ day due to an illness, injury or other problem?” Participants were considered to have restricted activity during a specific month if they answered “Yes” to one or both of the questions. These participants were subsequently asked to identify the reason(s) for their restricted activity using a standardized protocol that included 24 prespecified problems and an open-ended response.
The intervening events were organized into three mutually exclusive hierarchical categories: hospitalization, ED visit without hospitalization, and restricted activity alone (5).
Disability Assessments
Complete details regarding the assessment of disability are provided elsewhere (8, 15). Each month, participants were asked, “At the present time, do you need help from another person to (complete the task)?” for each of four essential activities (bathing, dressing, walking, and transferring), five instrumental activities (shopping, housework, meal preparation, taking medications, and managing finances), and three mobility activities (walk ¼ mile, climb flight of stairs, and lift/carry ten pounds). For these 12 activities, disability was operationalized as the need for personal assistance. Participants were also asked about a fourth mobility activity, “Have you driven a car during the past month?” Participants who responded “No” (including never drivers) were considered to be “disabled” in driving (26). To address the small amount of missing data on disability (1% of observations), multiple imputation was used with 100 random draws per missing observation (27).
Ascertainment of Critical Illness
As previously described (7), admissions to the ICU were ascertained from two sources. The primary source was linked Medicare claims data. An ICU admission was defined as any critical care revenue code, including general, specialty, and coronary care units, but excluding psychiatric or intermediate critical care. For periods when participants had managed Medicare, hospitalizations ascertained from the monthly interviews were evaluated for ICU admission through review of the corresponding medical records. Information was also obtained from these two sources on use of mechanical ventilation (7, 28) and length of ICU stay (7).
Functional Outcomes
The two outcomes included functional recovery and functional decline. Functional recovery was defined as the return, within 12 months of the first interview after hospital discharge from critical illness, to a total disability count (out of 13) less than or equal to that from the month immediately prior to the ICU admission (29). Functional decline was defined as an increase in the disability count of 1 or more essential activities (out of 4) or 2 or more instrumental or mobility activities (out of 9) from one month to the next after hospital discharge from critical illness for up to 12 months (30, 31).
Assembly of analytic samples
ICU admissions were included through December 2017. Participants could contribute more than one observation to the analysis based on the following criteria: (1) only the first ICU admission within an 18-month interval was eligible; (2) participant was not admitted from a nursing home; (3) participant was not disabled in all 13 activities prior to the critical illness; (4) participant was not discharged from the hospital on hospice; (5) at least one interview was completed after hospital discharge; and (6) participant did not contribute an observation within the prior 12 months. As described in Figure 1, the analytic sample included 250 ICU admissions, which were contributed by 209 community-living participants. The reasons for these admissions were grouped into distinct diagnostic categories as described earlier.
Figure 1.
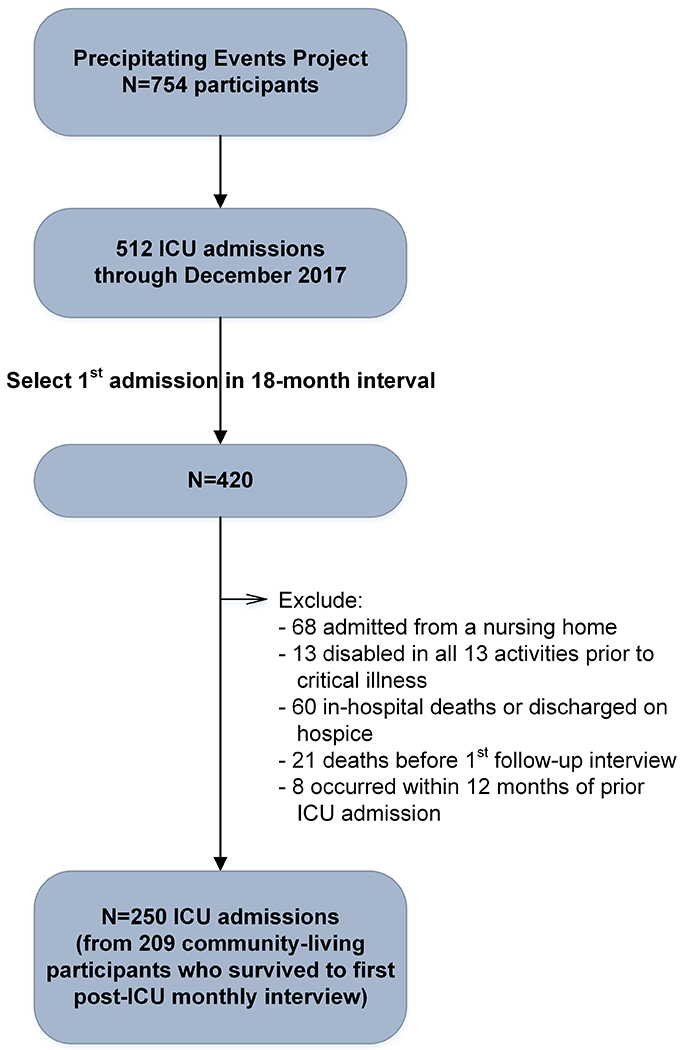
Assembly of Analytic Sample from Parent Cohort. Of the 512 ICU admissions through December 2017, 420 represented the first admission within an 18-month interval. Of these, 170 were excluded: 68 were admitted from a nursing home, 13 were disabled in all 13 activities, 60 died in the hospital or were discharged on hospice, 21 died before their first interview after hospital discharge, and 8 followed a prior ICU admission within 12 months. The remaining 250 ICU admissions, which were contributed by 209 community-living participants, formed the final sample.
Statistical Analysis
The characteristics of the analytic samples, assessed at the start of each 18-month interval, along with information on the ICU admissions, were summarized according to the type of functional outcome. For each outcome, exposure to the intervening events was calculated per 100-person months using an intercept-only Poisson model with generalized estimating equations (GEE).
A Cox model for recurrent events was used to evaluate the bivariate and multivariable associations between each of the three intervening events and time to functional recovery (32). Participants who had not recovered were censored at time of death, withdrawal from study, or end of 12-month follow-up period. The multivariable model included each of the eight factors that were in a previously published functional recovery model—age, sex, race/ethnicity, body mass index, hearing impairment, visual impairment, functional self-efficacy, and number of disabilities in the month prior to ICU admission (7), along with number of months to ICU admission from start of 18-month interval and an indicator for calendar time. Several other factors (Table 1) were evaluated in the multivariable model using backwards selection, but only length of ICU stay was retained based on a P-value < 0.20. For each of the intervening events, adjusted hazard ratios (HR) and 95% confidence intervals (CIs) were calculated with use of a robust sandwich variance estimator for standard errors (32).
Functional decline was evaluated as a recurrent event during the 12-month follow-up period. Months were not included when a further decline in function was not possible. A GEE logistic regression model was used to evaluate the bivariate and multivariable relationships between each of the intervening events and functional decline on a monthly basis (33). Adjusted odds ratios (OR) and 95% CIs were estimated using the same set of covariates as in the earlier multivariable Cox model. A first-order autoregressive covariance structure was used to account for intercorrelations among repeated observations contributed by the same participants (33).
RESULTS
Table 1 provides the characteristics of the analytic samples. Overall, the mean age was 82.3 years, 58.4% were female, and 88.0% were Non-Hispanic white. More than one of six had cognitive impairment, while more than four of ten were frail. The mean number of disabilities was 4.1. About 12% were mechanically ventilated during their critical illness. The characteristics were generally worse among the 81 (32.4%) without recovery than the 169 (67.6%) with recovery. Differences were less pronounced according to functional decline, and none was statistically significant. As shown in Supplemental Digital Content 1, the most common reasons for the ICU hospital admissions were cardiac and infection.
Over the 12-month follow-up period, the mean (sd) time to functional recovery was 3.2 (2.5) months, and mean (sd) number of subsequent episodes, i.e. months, with functional decline was 2.2 (1.6). Of the 541 episodes, 174 (32.2%), 226 (41.8%), and 141 (26.1%) were attributable to decline in essential activities alone, instrumental and/or mobility activities alone, and both.
The overall exposure rates (95% CI) per 100-person months to the intervening hospitalizations, ED visits, and restricted activity were 17.2 (14.3–20.8), 3.8 (2.7–5.2), and 12.1 (9.7–15.1) for the functional recovery analysis and 12.1 (10.3–14.3), 3.1 (2.4–4.0), 11.5 (9.7–13.6) for the functional decline analysis. As shown in Table 2, exposure to intervening hospitalizations was nearly 3-fold higher in the absence versus presence of functional recovery. Exposure to intervening ED visits and restricted activity were modestly higher in the absence versus presence of functional recovery, but these differences were not statistically significant. Differences in exposure to the intervening events were less pronounced based on the presence/absence of functional decline, and none of the differences were statistically significant. The reasons for the intervening hospitalizations, ED visits, and restricted activity are provided in Supplemental Digital Content 2, 3 and 4, respectively. For each, the most common reasons were infection and cardiac for hospitalization; cardiac, musculoskeletal, and infection for ED visit; and fatigue and dizziness/unsteadiness on feet for restricted activity. In absolute terms, differences between the no functional recovery and functional decline groups were most pronounced for infection (for hospitalization), cardiac (for ED visit), and problem with memory/difficulty thinking (for restricted activity).
Table 2.
Exposure to Intervening Events in the Year after Critical Illness by Functional Outcome a
| Outcome | Hospitalization | Emergency Department Visit | Restricted Activity |
|---|---|---|---|
| Rate per 100-person months (95% CI) | |||
| Functional recovery | |||
| Yes | 9.4 (7.3–12.0) | 3.0 (1.8–4.9) | 10.5 (7.9–14.0) |
| No | 24.2 (19.2–30.5) | 4.6 (3.1–6.7) | 13.6 (9.9–18.5) |
| P-value | <.001 | .172 | .246 |
| Functional decline | |||
| Yes | 12.3 (10.4–14.6) | 3.2 (2.5–4.1) | 11.5 (9.7–13.7) |
| No | 10.8 (6.8–17.3) | 2.5 (1.1–5.4) | 11.4 (6.9–18.8) |
| P-value | .610 | .523 | .914 |
Abbreviations: CI, confidence interval; sd, standard deviation.
The three intervening events are mutually exclusive and hierarchical, as described in the Methods. The mean (sd) duration of follow-up was 4.8 (3.9) months for functional recovery and 10.5 (3.2) months for functional decline.
Figure 2 provides the bivariate and multivariable associations between the intervening events and functional outcomes. An intervening hospitalization was strongly and significantly associated with reduced recovery in both bivariate and multivariable analyses, with an adjusted hazard ratio of 0.18. Recovery was also reduced in the setting of an ED visit and restricted activity, respectively, but none of the bivariate or multivariable associations were statistically significant. In the multivariable analyses, the likelihood of functional decline was increased more than 2-fold in the setting of a hospitalization, nearly 80% in the setting of an ED visit, and 25% in the setting of restricted activity, although the latter difference was not statistically significant. In the same multivariable model, the effect sizes for hospitalization on both functional outcomes were much larger than those for any of the covariates, including traditional risk factors such as functional self-efficacy and hearing impairment, while the effect size for ED visit on functional decline was modestly higher than those for the covariates (Table 3).
Figure 2.
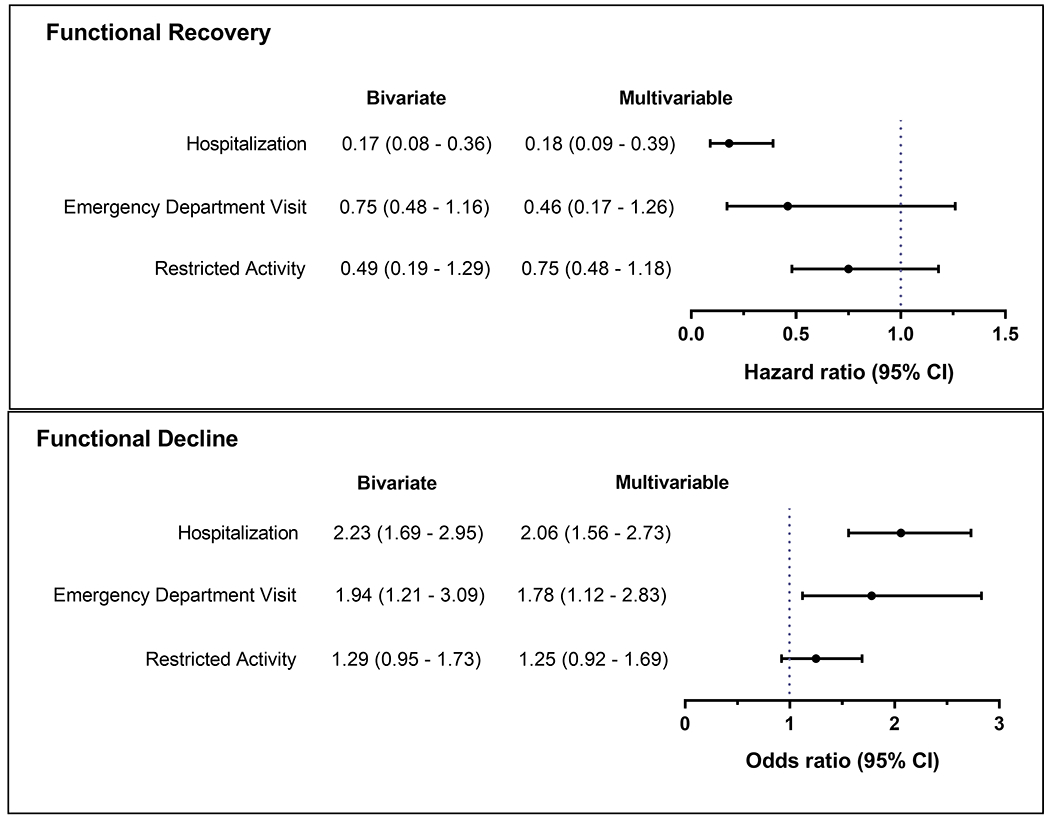
Bivariate and Multivariable Associations Between the Intervening Events and Functional Outcomes. Each of the models included the three intervening events, which were mutually exclusive and hierarchical, as described in the Methods. The multivariable models also included age, sex, race/ethnicity, body mass index, functional self-efficacy, hearing impairment, visual impairment, number of disabilities in the month prior to critical illness, length of ICU stay, number of months to ICU admission from start of 18-month interval, and number of the specific 18-month interval (to account for calendar time). CI, confidence interval.
Table 3.
Multivariable Associations Between Covariates and Functional Outcomes a
| Functional Recovery | Functional Decline | |
|---|---|---|
| Covariate b | Hazard Ratio (95% CI) | Odds Ratio (95% CI) |
| Age, per year | 1.00 (0.97–1.03) | 1.00 (0.98–1.02) |
| Female sex | 0.96 (0.73–1.27) | 0.84 (0.68–1.04) |
| Non-Hispanic white | 0.98 (0.66–1.47) | 1.02 (0.75–1.38) |
| Body mass index, kg/m2 | 1.02 (1.00–1.05) | 0.98 (0.96–1.00) |
| Functional self-efficacy c | 1.04 (1.02–1.06) | 0.98 (0.97–0.99) |
| Hearing impairment d | 0.72 (0.52–0.99) | 1.02 (0.82–1.30) |
| Visual impairment e | 1.00 (1.00–1.01) | 0.79 (0.64–0.98) |
| Disabilities f, per each number | 1.04 (0.98–1.10) | 1.03 (0.99–1.07) |
| Length of ICU stay, per day | 0.97 (0.93–1.01) | 0.99 (0.97–1.01) |
Abbreviation: CI, confidence interval; ICU, intensive care unit
The results are from the same multivariable models that are described in Figure 2. The models included the three intervening events, which were mutually exclusive and hierarchical, and also adjusted for number of months to ICU admission from start of 18-month interval, and number of the specific 18-month interval (to account for calendar time). The 250 observations were contributed by 209 community-living participants.
Data on body mass index, functional self-efficacy, hearing impairment, and visual impairment were collected during the prior comprehensive assessment, while data on number of disabilities were collected during the prior monthly interview
Per point on scale from 0 to 40, where higher scores indicate greater confidence performing activities.
Assessed using a handheld Audioscope, with severe impairment defined as 4 out of 4 tones missed, based on 1000- and 2000-Hz measurements for the left and right ears.
Defined as > 26% when assessed with a Jaeger card and use of corrective lenses, if applicable.
Of 13 possible: 4 essential, 5 instrumental, and 4 mobility.
DISCUSSION
In this prospective study of community-living older ICU survivors, we evaluated the effects of intervening illnesses/injuries on two distinct, but related functional outcomes in the year after hospital discharge from a critical illness. Four main findings warrant comment. First, about a third of participants did not recover their premorbid level of function and most experienced at least one subsequent episode of functional decline in the year after their critical illness. Second, intervening illnesses/injuries leading to hospitalization, ED visit or restricted activity were common in the year after critical illness. Third, in multivariable analyses that included a comprehensive array of demographic, clinical and geriatric covariates, intervening hospitalizations were significantly associated with a lower likelihood of functional recovery, while intervening hospitalizations and ED visits were each significantly associated with a higher likelihood of subsequent functional decline. Finally, the magnitude of these associations was larger than that of the covariates, including traditional risk factors. These findings suggest that attention to intervening illnesses/injuries will be necessary to improve functional outcomes after critical illness in community-living older persons.
Prior studies of functional outcomes after critical illness have largely focused on factors available at the time of hospital discharge (7, 13, 34–36). While several of these factors were evaluated in the current study, their effect on the two functional outcomes was generally much smaller than that of the intervening hospitalizations and ED visits. Because the rate of hospitalization after critical illness was higher than that of ED visit without hospitalization, strategies for improving functional outcomes after critical illness might focus most intently on intervening hospitalizations, including minimizing preventable illnesses/injuries leading to hospitalization (37–41), decreasing the adverse functional consequences of hospitalization (42–46), bolstering restorative therapies after hospitalization (47, 48), and substituting hospital-at-home for traditional inpatient care (49). However, because the reasons for intervening hospitalizations were quite varied, with no single diagnostic category comprising more than 30% of the reasons, interventions delivered during or after these hospitalizations may have the greatest opportunity for success. Although only a minority of hospitalizations occurred within the first month of discharge after critical illness (24.6% for functional recovery and 15.4% for functional decline), facilitating safe and effective discharges, perhaps coupled with follow-up in a post-ICU clinic (50), may improve short- and long-term functional outcomes. Unfortunately, information was not available in the current study on post-hospital outpatient care.
A unique feature of our study is the availability of data from monthly interviews, which allowed us to more accurately determine changes in functional status and more completely ascertain exposure to intervening illnesses/injuries. The frequency of our assessments increases the likelihood that the intervening events preceded the functional outcomes, thereby strengthening temporal precedence and supporting causal associations (25). Other strengths include assessment of a comprehensive set of essential, instrumental and mobility activities, which allowed us to more precisely determine recovery of premorbid function and ascertain clinically meaningful declines in functional status; the availability of detailed data on a large array of demographic, clinical and geriatric factors, which were reassessed every 18 months; and complete ascertainment of ICU admissions, using several different sources of information.
Our findings should be interpreted in the context of several potential limitations. First, information was not available on ICU-specific factors that cannot be reliably drawn from administrative data, such as delirium and severity of illness. To partially address this issue, the multivariable models included ICU length of stay and use of mechanical ventilation. Second, as evidenced by the wide confidence intervals, power was limited to detect statistically significant differences for associations that are likely clinically meaningful, such as the 54% reduction in functional recovery in the setting of an ED visit. Third, because this was an observational study, the reported associations cannot be construed as causal. Even if the associations were causal, whether functional outcomes could be improved through currently available interventions is uncertain. Fourth, because our participants were members of a single health plan in a small urban area, our findings may not be generalizable to older persons in other settings. Generalizability, however, depends not only on the choice of the study sample but also on the stability of the sample over time (51). One of the great strengths of our study is the low attrition rate. The generalizability of our findings is also enhanced by our high participation rate, which was greater than 75%.
CONCLUSIONS
Intervening illnesses/injuries are common in the year after critical illness and those leading to hospitalization and ED visit are strongly associated with adverse functional outcomes, with effect sizes larger than those of traditional risk factors. To improve functional outcomes among older persons, more aggressive efforts will be needed to prevent and manage intervening illnesses/injuries after critical illness.
Supplementary Material
1. Reasons for ICU Hospital Admissions
2. Reasons for Intervening Hospitalizations
3. Reasons for Intervening Emergency Department Visits
4. Reasons for Intervening Restricted Activity
Acknowledgments:
We thank Denise Shepard, BSN, MBA, Andrea Benjamin, BSN, Barbara Foster, and Amy Shelton, MPH, for assistance with data collection; Geraldine Hawthorne, BS, for assistance with data entry and management; Peter Charpentier, MPH, for design and development of the study database and participant tracking system; and Joanne McGloin, MDiv, MBA, for leadership and advice as the Project Director. Each of these persons were paid employees of Yale School of Medicine during the conduct of this study.
Funding: This research was supported by a grant from the National Institute on Aging (NIA) (R01AG017560). Dr. Gill is supported by the Yale Claude D. Pepper Older Americans Independence Center (P30AG021342). Dr. Ferrante is supported by a Paul B. Beeson Emerging Leaders in Aging Research Career Development Award from the NIA (K76AG057023).
Copyright form disclosure: Drs. Gill, Murphy, and Ferrante’s institution received funding from the National Institutes of Health (NIH)/National Institute on Aging (NIA). Drs. Gill, Han, Leo-Summers, Murphy, and Ferrante received support for article research from the NIH. Dr. Han’s institution received funding from NIA R01AG017560 and Yale Claude D. Pepper Older Americans Independence Center (P30AG021342). Dr. Gahbauer disclosed that he does not have any potential conflicts of interest.
Footnotes
Conflicts of interest: none
REFERENCES
- 1.Weissman GE, Kerlin MP, Yuan Y, Gabler NB, et al. : Population trends in intensive care unit admissions in the United States among Medicare beneficiaries, 2006-2015. Ann Intern Med 2019; 170(3):213–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Administration on Aging, Administration for Community Living, U.S. Department of Health and Human Services: 2018 Profile of Older Americans. https://acl.gov/sites/default/files/Aging%20and%20Disability%20in%20America/2018OlderAmericansProfile.pdf. April 2018
- 3.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, et al. : The association of frailty with post-ICU disability, nursing home admission, and mortality: a longitudinal study. Chest 2018; 153(6):1378–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, et al. : Functional trajectories among older persons before and after critical illness. JAMA Intern Med 2015; 175(4):523–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gill TM, Han L, Gahbauer EA, Leo-Summers L, et al. : Risk factors and precipitants of severe disability: a cohort study of community-living older persons. JAMA Netw Open 2020; 3(6):e206021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brummel NE, Balas MC, Morandi A, Ferrante LE, et al. : Understanding and reducing disability in older adults following critical illness. Crit Care Med 2015; 43(6):1265–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, et al. : Factors associated with functional recovery among older intensive care unit survivors. Am J Respir Crit Care Med 2016; 194(3):299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardy SE, Gill TM: Recovery from disability among community-dwelling older persons. JAMA 2004; 291(13):1596–1602 [DOI] [PubMed] [Google Scholar]
- 9.Loyd C, Beasley TM, Miltner RS, Clark D, et al. : Trajectories of community mobility recovery after hospitalization in older adults. J Am Geriatr Soc 2018; 66(7):1399–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prvu Bettger JA, Coster WJ, Latham NK, Keysor JJ: Analyzing change in recovery patterns in the year after acute hospitalization. Arch Phys Med Rehabil 2008; 89(7):1267–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stuck AE, Walthert JM, Nikolaus T, Bula CJ, et al. : Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med 1999; 48(4):445–469 [DOI] [PubMed] [Google Scholar]
- 12.Hardy SE, Gill TM: Factors associated with recovery of independence among newly disabled older persons. Arch Intern Med 2005; 165(1):106–112 [DOI] [PubMed] [Google Scholar]
- 13.Ferrante LE, Murphy TE, Leo-Summers LS, Gahbauer EA, et al. : The combined effects of frailty and cognitive impairment on post-ICU disability among older ICU survivors. Am J Respir Crit Care Med 2019; 200(1):107–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill TM, Desai MM, Gahbauer EA, Holford TR, et al. : Restricted activity among community-living older persons: incidence, precipitants, and health care utilization. Ann Intern Med 2001; 135(5):313–321 [DOI] [PubMed] [Google Scholar]
- 15.Gill TM, Hardy SE, Williams CS: Underestimation of disability among community-living older persons. J Am Geriatr Soc 2002; 50(9):1492–1497 [DOI] [PubMed] [Google Scholar]
- 16.Gill TM, Han L, Gahbauer EA, Leo-Summers L, et al. : Cohort Profile: The Precipitating Events Project (PEP Study). Journal of Nutrition, Health and Aging 2020; 24(4):438–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR: “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12(3):189–198 [DOI] [PubMed] [Google Scholar]
- 18.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J: Two shorter forms of the CES-D Depression Symptoms Index. J Aging Health 1993; 5(2):179–193 [DOI] [PubMed] [Google Scholar]
- 19.Reid MC, Williams CS, Gill TM: The relationship between psychological factors and disabling musculoskeletal pain in community-dwelling older persons. J Am Geriatr Soc 2003; 51(8):1092–1098 [DOI] [PubMed] [Google Scholar]
- 20.Lichtenstein MJ, Bess FH, Logan SA: Validation of screening tools for identifying hearing-impaired elderly in primary care. JAMA 1988; 259(19):2875–2878 [PubMed] [Google Scholar]
- 21.Spaeth EB, Fralick FB, Hughes WF: Estimates of loss of visual efficiency. Arch Ophthalmol 1955; 54:462–468 [DOI] [PubMed] [Google Scholar]
- 22.Fried LP, Tangen CM, Walston J, Newman AB, et al. : Frailty in older adults: evidence for a phenotype. J Gerontol Med Sci 2001; 56A(3):M146–M156 [DOI] [PubMed] [Google Scholar]
- 23.Gill TM: Disentangling the disabling process: insights from the Precipitating Events Project. Gerontologist 2014; 54(4):533–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagurney JM, Fleischman W, Han L, Leo-Summers L, et al. : Emergency department visits without hospitalization are associated with functional decline in older persons. Ann Emerg Med 2017; 69(4):426–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gill TM, Allore HG, Holford TR, Guo Z: Hospitalization, restricted activity, and the development of disability among older persons. JAMA 2004; 292(17):2115–2124 [DOI] [PubMed] [Google Scholar]
- 26.Gill TM, Gahbauer EA, Murphy TE, Han L, et al. : Risk factors and precipitants of long-term disability in community mobility: a cohort study of older persons. Ann Intern Med 2012; 156(2):131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gill TM, Guo Z, Allore HG: Subtypes of disability in older persons over the course of nearly 8 years. J Am Geriatr Soc 2008; 56(3):436–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sjoding MW, Prescott HC, Wunsch H, Iwashyna TJ, et al. : Longitudinal changes in ICU admissions among elderly patients in the United States. 2016; 1(7):1353–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becher RD, Murphy TE, Gahbauer EA, Leo-Summers L, et al. : Factors associated with functional recovery among older survivors of major surgery. Ann Surg 2020; 272:92–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kempen GI, Myers AM, Powell LE: Hierarchical structure in ADL and IADL: analytical assumptions and applications for clinicians and researchers. J Clin Epidemiol 1995; 48(11):1299–1305 [DOI] [PubMed] [Google Scholar]
- 31.Finch M, Kane RL, Philp I: Developing a new metric for ADLs. J Am Geriatr Soc 1995; 43(8):877–884 [DOI] [PubMed] [Google Scholar]
- 32.Therneau TM, Grambsch PM: Modeling survival data: extending the Cox model. New York, Springer, 2000 [Google Scholar]
- 33.Diggle PJ, Heagerty PJ, Liang K-Y, Zeger SL: Analysis of longitudinal data. Second Edition. New York, Oxford University Press, 2002 [Google Scholar]
- 34.Neuman MD, Eckenhoff RG: Functional outcomes after critical illness in the elderly. Crit Care Med 2015; 43(6):1340–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baldwin MR: Measuring and predicting long-term outcomes in older survivors of critical illness. Minerva Anestesiol 2015; 81(6):650–661 [PMC free article] [PubMed] [Google Scholar]
- 36.Herridge MS, Chu LM, Matte A, Tomlinson G, et al. : The RECOVER Program: disability risk groups and 1-year outcome after 7 or more days of mechanical ventilation. Am J Respir Crit Care Med 2016; 194(7):831–844 [DOI] [PubMed] [Google Scholar]
- 37.Tinetti ME: Clinical practice. Preventing falls in elderly persons. N Engl J Med 2003; 348(1):42–49 [DOI] [PubMed] [Google Scholar]
- 38.Straus SE, Majumdar SR, McAlister FA: New evidence for stroke prevention: clinical applications. JAMA 2002; 288(11):1396–1398 [DOI] [PubMed] [Google Scholar]
- 39.Kim DK, Hunter P: Recommended adult immunization schedule, United States, 2019. Ann Intern Med 2019; 170(3):182–192 [DOI] [PubMed] [Google Scholar]
- 40.Kastner M, Cardoso R, Lai Y, Treister V, et al. : Effectiveness of interventions for managing multiple high-burden chronic diseases in older adults: A systematic review and meta-analysis. CMAJ 2018; 190(34):E1004–E1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pritchard C, Ness A, Symonds N, Siarkowski M, et al. : Effectiveness of hospital avoidance interventions among elderly patients: A systematic review. CJEM Canadian Journal of Emergency Medical Care 2020:1–10 [DOI] [PubMed] [Google Scholar]
- 42.Rich MW: Heart failure in the 21st century: a cardiogeriatric syndrome. J Gerontol Med Sci 2001; 56A(2):M88–M96 [DOI] [PubMed] [Google Scholar]
- 43.Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, et al. : A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med 1995; 332(20):1338–1344 [DOI] [PubMed] [Google Scholar]
- 44.Cohen HJ, Feussner JR, Weinberger M, Carnes M, et al. : A controlled trial of inpatient and outpatient geriatric evaluation and management. N Engl J Med 2002; 346(12):905–912 [DOI] [PubMed] [Google Scholar]
- 45.Inouye SK: Delirium in older persons. N Engl J Med 2006; 354(11):1157–1165 [DOI] [PubMed] [Google Scholar]
- 46.Detsky AS, Krumholz HM: Reducing the trauma of hospitalization. JAMA 2014; 311(21):2169–2170 [DOI] [PubMed] [Google Scholar]
- 47.Tinetti ME, Baker D, Gallo WT, Nanda A, et al. : Evaluation of restorative care vs usual care for older adults receiving an acute episode of home care. JAMA 2002; 287(16):2098–2105 [DOI] [PubMed] [Google Scholar]
- 48.Hoenig H, Nusbaum N, Brummel-Smith K: Geriatric rehabilitation: state of the art. J Am Geriatr Soc 1997; 45(11):1371–1381 [DOI] [PubMed] [Google Scholar]
- 49.Federman AD, Soones T, DeCherrie LV, Leff B, et al. : Association of a bundled hospital-at-home and 30-day postacute transitional care program with clinical outcomes and patient experiences. JAMA Intern Med 2018; 178(8):1033–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuehn BM: Clinics Aim to Improve Post-ICU Recovery. JAMA 2019; 321(11):1036–1038 [DOI] [PubMed] [Google Scholar]
- 51.Szklo M: Population-based cohort studies. Epidemiol Rev 1998; 20:81–90 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1. Reasons for ICU Hospital Admissions
2. Reasons for Intervening Hospitalizations
3. Reasons for Intervening Emergency Department Visits
4. Reasons for Intervening Restricted Activity


