Abstract
For therapeutic materials to be successfully delivered to the heart, several barriers need to be overcome, including the anatomical challenges of access, the mechanical force of the blood flow, the endothelial barrier, the cellular barrier and the immune response. Various vectors and delivery methods have been proposed to improve the cardiac-specific uptake of materials to modify gene expression. Viral and non-viral vectors are widely used to deliver genetic materials, but each has its respective advantages and shortcomings. Adeno-associated viruses have emerged as one of the best tools for heart-targeted gene delivery. In addition, extracellular vesicles, including exosomes, which are secreted by most cell types, have gained popularity for drug delivery to several organs, including the heart. Accumulating evidence suggests that extracellular vesicles can carry and transfer functional proteins and genetic materials into target cells and might be an attractive option for heart-targeted delivery. Extracellular vesicles or artificial carriers of non-viral and viral vectors can be bioengineered with immune-evasive and cardiotropic properties. In this Review, we discuss the latest strategies for targeting and delivering therapeutic materials to the heart and how the knowledge of different vectors and delivery methods could successfully translate cardiac gene therapy into the clinical setting.
The ageing population and improvements in medical care for acute cardiac conditions mean that the numbers of patients with chronic cardiovascular diseases are increasing worldwide1. Although several effective drugs such as angiotensin-converting enzyme inhibitors and β-blockers are available, cardiovascular diseases remain the major cause of morbidity and mortality worldwide2,3. Novel therapies with different mechanisms of action might change this situation.
The past three or four decades have seen a huge growth in our knowledge of the molecular biology of healthy and diseased hearts. Detailed signalling pathways that promote cardiac pathology have been unravelled, and laboratory science continues to discover important molecular targets that have important roles in these pathways. Therapies directed at modifying intracellular gene expression hold substantial promise because they can modify the deranged intracellular signalling that is often difficult to target using traditional drug therapies. A large number of preclinical studies have indicated that targeted delivery of genes to cardiac cells can improve heart function4–7. To modify gene expression, therapeutic genes can be delivered as plasmids or using various types of vector to induce overexpression (Box 1). By contrast, delivery of short interfering RNA (siRNA) or small hairpin RNA (shRNA) suppresses gene expression. Non-coding RNA overexpression or its inhibition by antisense oligonucleotides might be used to regulate the expression of several genes in an organized manner. Direct delivery of modified mRNA8,9 is a new approach to cardiac gene delivery and has rapidly progressed to a clinical trial10.
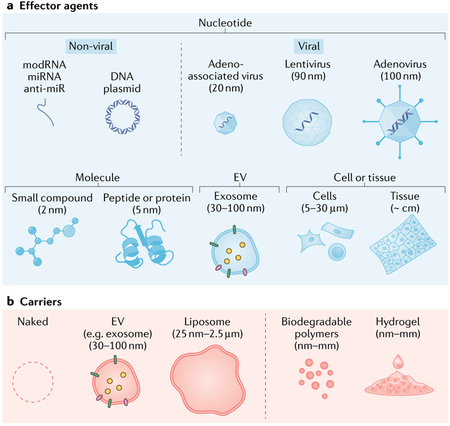
Box 1 |. Types of therapeutic agent for delivering genetic materials.
Effector agents (see the figure, part a) primarily deliver their therapeutic materials to the target location and can be administered on their own. Carriers (see the figure, part b) facilitate the delivery and targeting of an effector agent.
Effector agents
Effector agents can be classified as nucleotides23, molecules, extracellular vesicles (EVs), cells and tissues.
Modified mRNA (modRNA): a single-stranded mRNA with modified nucleotides, which achieves immediate and short-term expression (~2 weeks), with a low immune response.
MicroRNA (miRNA): a short, single-stranded, non-coding RNA that regulates gene expression; stability varies widely.
Anti-microRNA (anti-miR): an antisense inhibitor of a specific miRNA.
DNA plasmids: produce short-term expression, with a moderate immune response.
Adeno-associated viruses: contain single-stranded DNA and produce long-term expression, with a low immune response.
Lentiviruses: contain single-stranded RNA and produce long-term expression, with a mild immune response.
Adenoviruses: contain double-stranded DNA and produce short-term (1–4 weeks) expression, with a strong immune response.
Small compounds: a tetracycline or doxycycline system is commonly used in experimental studies.
Peptides or proteins: several cytokines (such as fibroblast growth factor and erythropoietin) have been tested in the treatment of cardiovascular diseases.
EVs, such as exosomes: vesicles containing therapeutic nucleic acids and/or proteins that produce short-term expression, with a low immune response.
Cells or tissues: intracoronary administration of large or clustered cells confers a risk of microvascular plugging. Most of the cells are cleared within a few hours but the remaining cells might engraft and exert long-term effects. Tissues can be made from different types of stem cell but implantation requires epicardial surgical access.
Carriers
Carriers can facilitate the delivery of effector agents.
EVs, such as exosomes: can be used as carriers by encapsulating viruses such as adeno-associated viruses159; EVs are non-immunogenic, and surface modifications and/or bioengineered donor cells can be used.
Liposomes: phospholipid bilayer capsules that are heterogeneous in size, have a low transduction efficacy and have low target specificity.
Biodegradable polymers: polylactic acid and poly(lactic acid-co-glycolic acid) are widely used.
Hydrogels: hydrophilic colloidal gels that can retain viral vectors, proteins and even cells, allowing controlled, localized release.
Extracellular vesicles (EVs), including exosomes (EVs of ~30–100 nm in diameter), are another new and promising vehicle that can carry genetic material by themselves or through packaging of other viral or non-viral vectors. Although cardiac delivery of stem cells seemed to be less effective than initially expected, successes in gene-modified cell delivery, for example chimeric antigen receptor T cell therapy in the field of oncology11, have renewed our interest in cardiac cell-based therapies.
Over the past 5–6 years, clinical trials have demonstrated the efficacy of several gene-modification approaches targeting various organs and diseases12. For example, adeno-associated virus (AAV)-mediated gene therapy has been approved by the FDA for Leber congenital amaurosis13 and spinal muscular atrophy14. A lipid nanoparticle-based siRNA (siRNA for mutant and wild-type transthyretin) for hereditary transthyretin amyloidosis15 was also approved in 2018. As discussed below, exosome-based therapies that involve cardiovascular interventions are now being actively studied in clinical trials. Early-phase clinical trials continue to show positive results in retinal diseases16, metabolic disorders and blood disorders17. Further clinical application of gene-modification approaches are expected in the coming years, and the field of gene therapy continues to attract researchers, industries and investors18.
Despite these examples of successful gene-targeting therapies in other disease areas, clinical translation of similar therapies for cardiac diseases remains slow. Indeed, large randomized clinical trials of cardiac gene therapy focusing on angiogenesis did not demonstrate efficacy19. Subsequently, three clinical trials of gene therapy focused on various targets in patients with heart failure similarly did not meet their primary efficacy end points20–22. These neutral outcomes indicate that substantial hurdles need to be overcome before clinical gene therapy for cardiac diseases can be achieved. Although limited data are available, the lack of efficacy seems to be at least partly associated with the low cardiac specificity of currently available therapeutic materials and vectors and inefficient methods for delivering these materials specifically to the heart (TABLE 1). Indeed, analysis of cardiac tissues from patients enrolled in the phase IIb CUPID trial23 suggested that little gene expression was achieved using the delivery method that had been efficacious in preclinical animal models. Understanding the factors that regulate cardiac uptake of therapeutic materials in humans is essential to overcoming the current inefficiency.
Table 1 |.
Barriers to cardiac-targeted delivery of therapeutic agents
| Barrier | Description | Methods for overcoming the barriers | Examples |
|---|---|---|---|
| Anatomy | Difficulty in gaining access | Cardiac-specific delivery | Surgical or catheter-based delivery |
| Cardiac-directed delivery | Microbubble delivery | ||
| A strong cardiotropic vector | Adeno-associated viruses or bioengineered vectors | ||
| Degradation | Endonucleases and antibodies | Vector modifications | Modified mRNA, lipids and polymers |
| Anti-immune therapies | Plasmapheresis to remove antibodies | ||
| Patient screening | Antibody screening | ||
| Endothelium | Blocks entry of foreign bodies from the blood | Intramyocardial injection | Epicardial or endocardial intramyocardial injection |
| Agents that increase vascular permeability | Vascular endothelial growth factor or nitroglycerin | ||
| Cell entry | Requires efficient cell-entry mechanisms | Cardiotropic vector | Viral vectors |
| Packaging | Extracellular vesicles | ||
| Surface modification | Bioengineered vectors | ||
| Intracellular trafficking | Appropriate intracellular localization is necessary | Viral vector | Adenovirus, adeno-associated virus or lentivirus |
| mRNA (cell entry is sufficient) | Modified mRNA |
In this Review, we discuss the current understanding and challenges in heart-targeted delivery of therapeutic materials, with a focus on those agents directed at modifying gene expression. Characteristics of gene delivery vectors, emerging approaches to the use of biological materials for efficient cardiac uptake and features of different delivery methods for heart-specific targeting are highlighted.
Genetic therapeutic agents
Therapeutic genes can be delivered either in the form of naked genetic material, by shielding the DNA or RNA constructs with synthetic materials (carriers) or by packaging them in viral vectors (BOX 1).
Naked nucleic acids
Naked nucleic acids, including DNAs, mRNAs, microRNAs (miRNAs) and siRNAs, are compatible with the delivery of large genes in high quantities from mass production. However, low stability and low cellular internalization are common issues with all these molecules because of a lack of protection from endonuclease degradation and their uncondensed shape and poly-anionic charge. Typically, the half-life of plasmid DNA is approximately 10 min after systemic injection into mice24. Chemical modifications to mRNA (modified mRNA) reduce activation of the immune system and improve stability when delivered in vivo25, and modified mRNAs are attractive agents for short-term gene delivery to the myocardium26. Having shown efficient gene transfection in the human skin27, modified mRNA encoding vascular endothelial growth factor A is now being tested in patients with ischaemic heart failure undergoing coronary artery bypass graft surgery28.
Non-viral approaches
To overcome the low transduction efficiency as well as the safety, immunogenicity and manufacturing limitations of naked nucleic acids, lipids and chemical-based nanoparticles (polymers and hydrogels) have been used. Injectable hydrogels, porous scaffolds, cellular and acellular material-based scaffolds, and artificially synthesized nanobiologics29 are some of the clinically compatible approaches that are currently being investigated for cardiac delivery of therapeutic agents. In a number of these systems, nucleic acids (such as plasmid DNA or RNA interference materials) were encapsulated in hydrogels, supramolecular hydrogels (that actively respond to external stimuli), nanogels (nano-sized hydrogels), nanoparticles or other scaffolding materials, either as conjugates or as polyplex particles, to achieve controlled, local release and to reduce adverse effects and increase in vivo efficacy30. In one study, an injectable and biocompatible hydrogel successfully achieved intramyocardial delivery of a nanocomplex containing graphene oxide and the VEGF gene in rats31. Unconventional approaches include the use of magnetic nanoparticles32, polymer-lipid (lipopolyplex) and gold-lipid hybrid nanoparticles33,34 for cardiac delivery of nucleic acids, drugs or stem cells. However, questions remain about the biocompatibility, targeting efficiency, immunogenicity, pro-inflammatory effects, degradation rates, clearance and medical safety of these materials, which need to be carefully evaluated before developing clinical applications.
Small-molecule drugs, especially to treat arrhythmias and cardiac contractile dysfunction, are of particular interest because they are suited for oral administration and can be chemically synthesized. Moreover, synthetically designed molecules to modulate miRNAs have emerged as a promising approach to treat various diseases, including cancer and cardiovascular diseases35. One strategy is to restore the activity of miRNAs using synthetic double-stranded miRNA mimics that imitate mature miRNA duplexes and can stimulate miRNA pathways of interest (miRNA mimics). Conversely, single-stranded antisense oligonucleotides can be used to inhibit miRNAs (anti-miRs). Anti-miRs have been successfully used to modulate miRNA levels in the heart in preclinical studies with therapeutic benefits, including in small-animal and large-animal models of heart failure36,37. Locked nucleic acid-based antisense inhibitors of miRNAs (such as anti-miR-132 (REF.36), anti-miR-21 (REF.37) and anti-miR-34 (REF.38)) administered in vitro or in vivo to rodents and clinically relevant pig models have shown cardioprotective, antifibrotic and immunomodulatory effects and have demonstrated that the approach is safe and has favourable pharmacokinetics. The diversity of these systems highlights the progress of gene-based therapy using non-viral approaches. However, the regulatory process for the development of new treatment modalities can be protracted, complex and expensive.
Viral vectors
Non-viral gene delivery has the advantage of being able to deliver large genes, but the efficacy of gene transduction is generally low and the expression period is short. These limitations are mostly caused by the presence of intracellular and extracellular barriers that impede cellular uptake and transfection. By contrast, viral vectors have the inherent capacity to enter cells and can effectively deliver their DNA or RNA cargo into the nucleus, with greater efficiency than non-viral vectors. The duration of expression varies depending on the vector of choice. In addition, some viral vectors have tropism to the heart, making them a promising tool for cardiac targeting.
Initially, adenoviruses were the major viral vector used for gene therapy39,40. Adenoviruses carry double-stranded DNA and offer efficient transduction of various cell types, including cardiomyocytes. Gene expression is fast and peaks within a few days after delivery, then diminishes gradually and ceases after approximately 4 weeks41. However, the immune response to the adenovirus vector was a major concern, even after removal of all the viral genes42. AAVs emerged as an alternative option owing to their low immunogenicity, their prolonged and high level of transgene expression43–45 and the cardiotropism shown by some of the serotypes46. These features increased the safety of gene therapy, and AAVs became the preferred choice of vector for organ-specific gene delivery in various laboratories. AAVs carry single-stranded DNA, and the gene construct stays as an episome for >24 months after transduction47, which makes AAVs highly suitable for the treatment of chronic heart failure. Transgene expression peaks around 2–4 weeks after delivery, probably owing to second-strand synthesis. The initially slow gene-expression profile can be improved by delivering a self-complementary gene construct48, but this approach halves the deliverable gene size, which is already small for AAVs (~4.7 kb).
Among several naturally occurring AAV serotypes, AAV9 has been shown to have the highest cardiac gene-transduction efficacy in mice and rats with either systemic46 or direct cardiac injection49. However, other studies have suggested that this finding might not be the case for larger mammals. AAV6 was found to be a more effective vector than AAV9 for cardiac gene transduction when injected directly into the myocardium of pigs50, dogs51 and non-human primates52, with a similar level of liver transduction52. Of note, no studies have directly compared the efficacy of intracoronary delivery of commonly used AAV serotypes in large animals. More studies in large animals, and ideally in humans, are needed to determine the optimal AAV serotypes for cardiac gene transfer for clinical translation.
Innovative concepts of AAV vectorization of CRISPR gene-editing technologies have been adopted to correct variants in DMD, the gene encoding dystrophin, to restore its cardiac and skeletal expression53 and improve muscle function54 in preclinical studies in large animals. These emerging approaches pave the way for new AAV-mediated treatment approaches for patients with genetic disorders.
Bioengineered capsid viruses are being designed with favourable transduction tropism and immunogenic profiles55. For example, AAV2i8 has been developed by site-directed mutagenesis of AAV capsids50 and is now being tested in humans. This vector has a similar degree of cardiotropism to that of AAV9, but has reduced liver tropism56, which offers improved cardiac-specific gene transduction. Unique capsid profiles also alter its antigenicity and might contribute to the low prevalence of neutralizing antibodies generated to the vector56. Although expanding the repertoire of AAVs increases our options for cardiac targeting, results obtained in rodents require validation in large animals. Moreover, direct comparisons between native and bioengineered AAV serotypes in clinical trials might be needed to determine their relative clinical efficacies.
Lentiviral vectors also enable transduction of non-dividing cells and long-term transgene expression by integrating the delivered genes into the host genome. Lentiviruses deliver single-stranded RNA with a packaging capacity of approximately 9 kb, and the expression peaks after 4–6 days57. The immune response to the vector is low, but safety concerns about potential insertional mutagenesis and off-target gene expression remain58. Lack of vector cardiotropism is another limitation for heart-specific delivery. Owing to its high efficiency in transducing non-dividing cells, the lentivirus vector is increasingly being used for ex vivo gene delivery. Although lentivirus gene therapy for the treatment of non-cardiac diseases has been tested in humans59,60, its use for the treatment of cardiac diseases is yet to reach the clinical stage, and only a few studies in rats have been published57,61. Additional safety and efficacy data from animal models are needed before clinical translation of this vector for the treatment of cardiac diseases.
In summary, although viral vectors offer efficient gene transduction compared with non-viral vector delivery, their package capacity is limited, and expression profiles differ according to the vector used. Off-target expression, particularly liver transduction, is generally high in large mammals, even with cardiotropic AAV serotypes, and additional approaches to confine gene expression to the heart are necessary. A humoral immune response associated with previous exposure to the vector is also a major problem because it reduces the patient population that is treatable and prevents repeat administration.
Biological therapeutic agents
Cell therapies
The human heart has limited capacity for endogenous repair and regeneration, especially after a catastrophic insult such as myocardial infarction or scar formation. Exogenous supplementation of stem cells (such as mesenchymal stem cells (MSCs), cardiospheres, induced pluripotent stem cells, endothelial progenitor cells or CD34+ haematopoietic stem cells) is a promising therapeutic approach to augment the reparative and regenerative potential and to improve the function of an injured heart. Several clinical trials have evaluated the efficacy of stem cells derived from autologous (such as CD34+ stem cells62,63) or allogenic (such as cardiosphere-derived cells64 or MSCs60,65) sources to improve cardiac remodelling in patients with ischaemic or non-ischaemic cardiomyopathy. Although the stem cell transplantations were reported to be safe, only modest improvements were observed in patient functional capacity, quality of life and ventricular remodelling. Major obstacles to the success of stem cell therapies are the low engraftment and survival rates of transplanted cells in the harmful microenvironment of the host cardiac tissue and the paucity of endogenous cells with endogenous repair capacity66. Newer approaches to improving the delivery and retention of stem cells in the ischaemic myocardium include combining cell therapy with tissue engineering strategies. Remarkably, many studies have now shown that the original concept of stem cell engraftment and differentiation into myocardial cells has little role in these settings67,68, leading to an alternative hypothesis that paracrine factors from the stem cells are beneficial to the myocardium69.
Extracellular vesicles
A large number of studies have shown that exosomes, a type of nano-sized EV, are a major functional component of the paracrine factors secreted by most of the stem cells70. Exosomes carry selective biomolecules from their cell of origin and deliver them to recipient cells, thereby mediating intercellular communication without direct cell-to-cell contact70–72. Exosomes from various stem cells and other biological sources73,74 have been shown to be pro-angiogenic75 and cardioprotective76,77, making them ideal therapeutic candidates.
Characteristics of EVs.
Owing to their ideal native structure, biocompatibility and other characteristics, EVs have many advantages over cells and other available drug-delivery vehicles. These advantages include their small size that is compatible with deep penetration into tissues, slightly negative zeta potential for long circulation, deformable structure and their similarity to cell membranes, which might allow the EVs to pass through natural barriers such as the blood-brain barrier78. In addition, depending on their cell of their origin, some EVs can evade clearance by the immune system79 or can modulate the immune system. For example, EVs released by MSCs express immunosuppressive factors (such as IL-10, indoleamine 2,3-dioxygenase, prostaglandin E2 and transforming growth factor β1)80,81. In addition, EVs from breast milk82 and from certain cancer cells83 are reported to be immunosuppressive. By contrast, EVs from antigen-presenting cells carry MHC class I and class II molecules and can stimulate CD8+ and CD4+ T cells, respectively80.
Several clinical trials have already demonstrated that EVs are safe for immunotherapy in humans84,85. Although EVs hold immense potential for therapeutics and drug delivery (BOX 2), clinical application crucially depends on the development of scalable production and isolation techniques, approaches for efficient drug loading either before or after isolation of the EVs, and improved methods for modifying their in vivo biodistribution and to deliver them to the target tissues79.
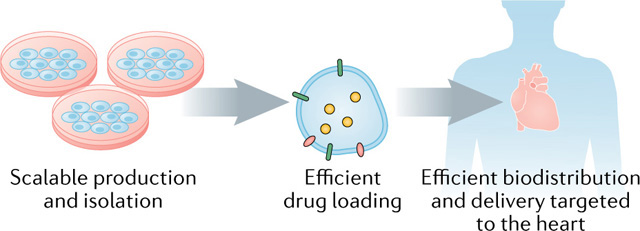
Box 2 |. Development of EVs for clinical application.
Substantial progress has been made in the development of natural extracellular vesicles (EVs) as therapeutic agents and drug-delivery vehicles (see the figure).
Scalable production and isolation
The low yield of EVs produced by mammalian cells remains an obstacle to large-scale production. Therefore, EV-smimetic nanovesicles, which have structural and physical features that resemble those of EVs, that are produced from broken cells with substantially greater yield have attracted attention160.
Efficient drug loading
Therapeutic agents can be incorporated into exosomes, other EVs or EV mimetics using either passive or active encapsulation, which results in different loading efficiencies and stabilities of the drugs in the vesicles161. Passive cargo loading methods use simple incubation of drugs and loading materials with EVs or EV-producing cells, often resulting in low loading capacity. Active encapsulation uses mechanical forces, extrusion, temperature, pH, membrane permeabilizers, electroporation, chemical agents or antibody-based approaches, with varying results102.
Efficient biodistribution and delivery targeted to the heart
Bioengineering of exosomes and EV mimetics further highlights the unique advantages of exosome-based nanoplatforms for cargo delivery. Similarly, several small molecules, either hydrophobic or hydrophilic, have been incorporated into exosomes with the use of the loading methods listed above. In general, exosomal delivery leads to improved drug stability and blood circulation time and increased drug accumulation in target cells, thereby improving the potency of drugs and lowering the dosage required79.
Following in vivo delivery, EVs are quickly taken up by recipient cells75. Some studies indicate that the half-life of EVs in the circulation might be only 2–4 min, and in mice EVs are mainly distributed to the liver after intravenous administration86. The use of various labelling methods (such as fluorescence75 or iron oxide particles combined with MRI87) has shown that EVs are cleared from the injection site within approximately 24 h of administration. Interestingly, our studies have shown different degrees of EV uptake by different cell types in hindlimb muscle (more efficient uptake by endothelial cells than by smooth muscle cells or fibroblasts)75 and in the heart in vivo, whereas differential uptake of EVs was not observed in vitro using primary or cultured cells (S.S., unpublished observations). However, therapeutically targeting EVs to specific cell types in a target organ has not yet been demonstrated. The uptake of EVs at a remote location is thought to depend on a combination of specific molecules on the surface of the EV that can be recognized by receptor molecules on the surface of the target cell88,89. The nature of the targeting molecules, which probably consist of proteins and lipids on the EV surface, remains a central question in the field. Investigations into the differential uptake of EVs, their biodistribution and pharmacokinetics are important to establish their biological roles, develop exosome-based therapeutics, and define the optimal timing and route of delivery.
Bioengineering of EVs.
Several approaches to augmenting the therapeutic efficacy of EVs for the treatment of cardiovascular diseases have been described. These include surface modifications using chemical conjugation (with cholesterol, recombinant proteins, lipid anchors and intercalating dyes) and the addition of pH-sensitive peptides to improve their bioactivity, targeting, trafficking and internalization (reviewed previously90). Another approach includes the use of exosomes secreted from cardiospheres bioengineered to express LAMP2B (an exosomal membrane protein) fused to a cardiomyocyte-specific peptide, which results in increased cardiac retention of the exosomes in mice91. Tannic acid modification has been shown to increase the binding of proteins, peptides and viruses to the myocardium92. Further investigations are needed to demonstrate the clinical safety, utility and efficacy of these approaches. Modifying the contents of progenitor cell-derived EVs on the basis of predictive computational models and loading them with exogenous nucleic acids or drugs to develop exosome mimics is an interesting approach that might resolve some of the mass-production problems associated with EV therapy93.
EV-associated AAVs.
Interestingly, many parallels exist between EVs and viruses — in their physical and chemical properties, biogenesis and incorporation of biological materials (such as proteins and fragments of RNA) — and they might actually be close relatives94. EVs can have an important role in either facilitating or suppressing viral infection, depending on the proteins and genetic material incorporated inside them94. Moreover, EVs generated by either enveloped or non-enveloped virus-infected cells can incorporate viral proteins and fragments of viral RNA, making them indistinguishable from defective (non-infectious) viruses.
The discovery that EVs can carry various different types of intact virus, such as hepatitis A virus95 and AAVs96, led to the concept of using EVs as gene-delivery agents. Hybrid approaches have been developed and are being pursued in our laboratory97 and by others to deliver genes to the myocardium and to other organs, such as the liver98 or retina99, using exosome-associated AAVs. Unpublished data from our laboratory suggest that exosomes carrying AAVs can facilitate cardiotropic delivery of genetic material97. Moreover, EVs that carry AAVs are more resistant to AAV-neutralizing antibodies (Fig. 1), increasing their efficiency as gene-delivery vectors and therapeutic agents96,100. This approach might also allow multiple therapeutic doses to be given as well as the treatment of patients who have AAV-neutralizing antibodies, the presence of which has been the major reason for exclusion of individuals from previous clinical trials of AAV gene therapy.
Fig. 1 |. Exosomes can envelope AAV vectors to shield them from neutralizing antibodies.
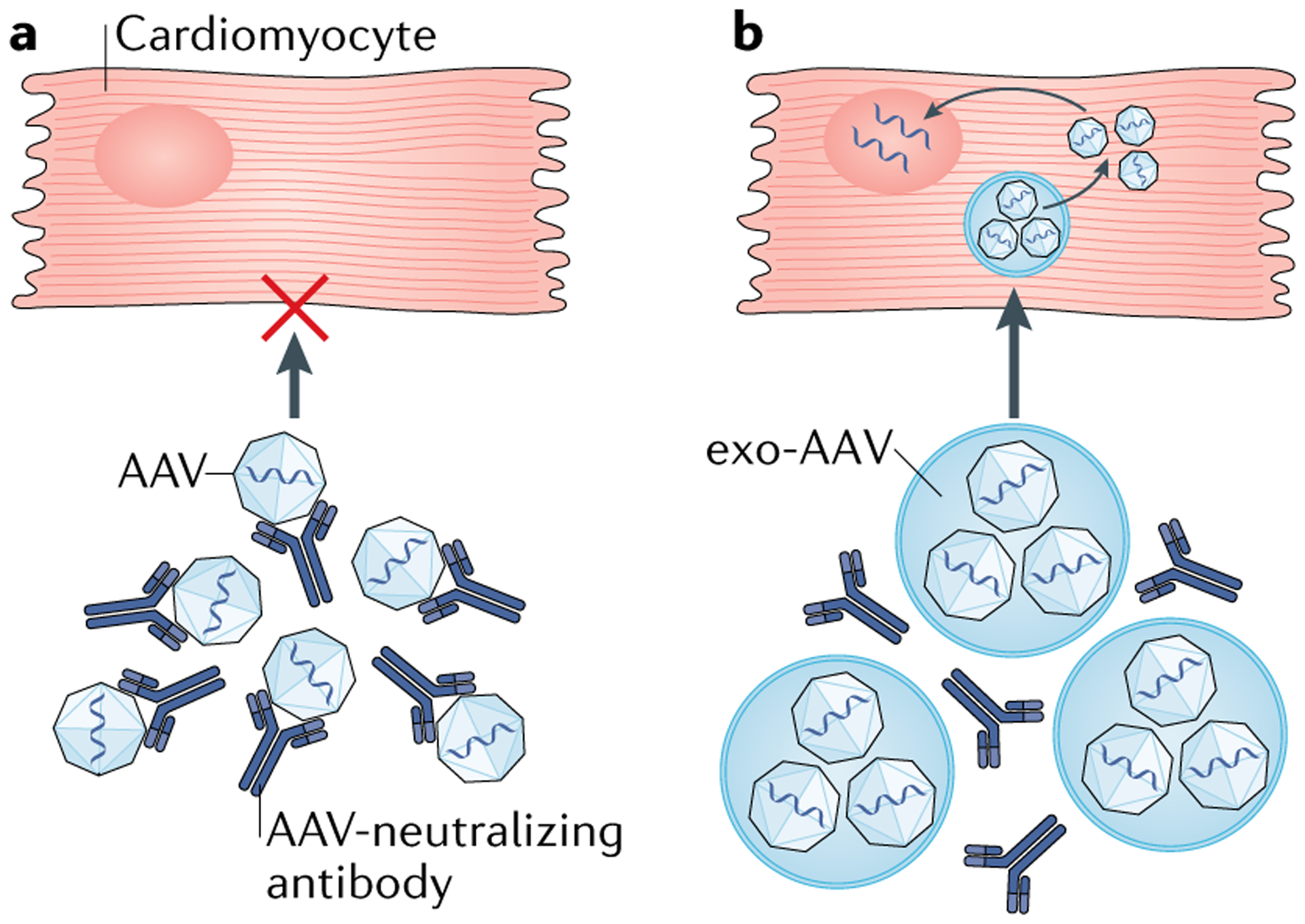
a | Neutralizing antibodies bind to adeno-associated viruses (AAVs) and prevent the uptake by cardiomyocytes of AAVs containing therapeutic genetic material. b | Exosome-associated AAVs (exo-AAVs) are more resistant to AAV-neutralizing antibodies because the exosome encapsulates the AAVs and shields them from neutralizing antibody-mediated detection and degradation. Exo-AAVs have a longer half-life in the circulation than AAVs and can penetrate deep tissues. Bioengineered surface and/or content modifications of exosomes could further improve the transduction efficacy of exo-AAVs.
Clinical trials of EVs.
Approximately 20 clinical studies involving EVs in cardiovascular diseases are listed on ClinicalTrials.gov, predominantly focusing on cardiovascular diagnostics; only two of the studies are on cardiovascular therapeutics. In one study101, the use of allogenic MSC-derived exosomes enriched with miR-124 is being investigated in patients with acute ischaemic stroke. In the other study102, the safety and efficacy of the intravenous delivery of MSC-derived exosomes is being investigated for the treatment of multiple organ dysfunction syndrome after surgical repair of acute type A aortic dissection. The vast majority of the 95 clinical trials listed on ClinicalTrials.gov involving EVs or exosomes are still in progress. Many of these studies are evaluating the safety and feasibility of EVs, specifically exosomes, for clinical use. Of note, the production of a fairly homogeneous population of EVs in accordance with Good Manufacturing Practices remains a challenge.
Delivery methods targeting the heart
In preclinical studies, some therapeutic materials have been shown to accumulate in the heart or in the injured myocardium after intravenous administration. For example, AAV9 has a high tropism towards the myocardium in rodents and can effectively transduce cardiomyocytes after injection into a tail vein103. Stem cells and stem cell-derived exosomes might target the site of injury and promote myocardial repair after myocardial infarction104,105. Nevertheless, most therapeutic agents have poor specificity to the heart when administered systemically, especially in larger animals. Therefore, these materials require direct or cardiac-targeted delivery to increase both specificity and efficacy. Importantly, each delivery approach is characterized by respective advantages and disadvantages associated with the route and method of delivery (TABLE 2). In this section, we describe the delivery methods that are commonly used for cardiac targeting of therapeutic materials (FIG. 2).
Table 2 |.
Advantages and disadvantages of different delivery methods to the heart
| Method | Advantages | disadvantages |
|---|---|---|
| Surgical | ||
| Cell sheet106–108, tissue strip109 or patch110,111 | Direct visualization of the target site helps to deliver the material precisely; can be used with large materials; can be combined with cardiac surgery | Needs open-chest surgery; can require mechanical circulatory support when cardiac displacement is needed; fixation of materials requires suture or tissue glue |
| Epicardial injection10,112–116,143 | Direct visualization of the target site helps to deliver the agent precisely; gradient administration can be achieved by number of injections per area; can be combined with cardiac surgery122,144 | Needs open-chest surgery and general anaesthesia; heterogeneous distribution; myocardial injury from needle puncture; leakage (therapeutic agent loss) from the needle holes145; can require mechanical circulatory support when cardiac displacement is needed |
| Painting121 | Effective gene transfer to thin walls (for example, the atrial wall), which can be targeted specifically; can be combined with cardiac surgery | Needs open-chest surgery; limited transmurality for thick walls; trypsin exposure needed to increase transmurality |
| Catheter-based | ||
| Antegrade intracoronary administration, with139 or without146 balloon occlusion | Less invasive than surgical approaches; homogeneous distribution; devices and techniques used clinically for coronary angiography and interventions can be used; global cardiac distribution is achievable; coronary sinus balloon occlusion can facilitate transduction140 | Delivery depends on antegrade coronary perfusion — low delivery in the presence of a stenotic coronary artery or when selection of an arterial branch is difficult; systemic off-target transduction; large materials can cause microinfarctions; balloon occlusion can cause haemodynamic deterioration associated with myocardial ischaemia |
| Coronary artery intervention with a drug-eluting147 or gene-eluting148,149 stent or a drug-eluting balloon150 | Sophisticated techniques and devices; less invasive than surgical approaches; gene-eluting stent provides slow and continuous release of genetic material | For stents, the scaffold stays permanently and can cause in-stent thrombosis; distribution can be localized |
| Retrograde coronary sinus access: intracoronary with balloon occlusion at coronary sinus124,125 or transcoronary126 | Retrograde perfusion124,125 can achieve fairly homogeneous distribution in the presence of coronary artery stenosis; applicable during open-chest cardiac surgery with retrograde cardioplegia administration in a closed circuit151 | Difficult to target the right ventricle because of challenges in selecting right coronary veins; systemic off-target transduction |
| Endocardial injection152–154 | Direct targeting regardless of coronary distribution; endocardial approach can avoid interference from epicardial vessels | Requires a mapping system for precise targeting152; difficult to administer to thin walls |
| Pericardial delivery131 | Percutaneous transcatheter approach can deliver larger materials than is possible with intracoronary or myocardial injection approaches | Cardiac uptake can vary; epicardial administration can limit transmural transduction; puncture might cause myocardial injury and subsequent cardiac tamponade |
| Other | ||
| Systemic administration | Less invasive than either catheter-based or surgical approaches; universally applicable | Can cause off-target uptake in other organs and systems; an appropriate drug-delivery system (such as nanoparticle155,156, antibody157, ultrasound-targeted microbubble destruction133,158) should be implemented to increase organ specificity |
Fig. 2 |. Delivery methods targeting the heart.
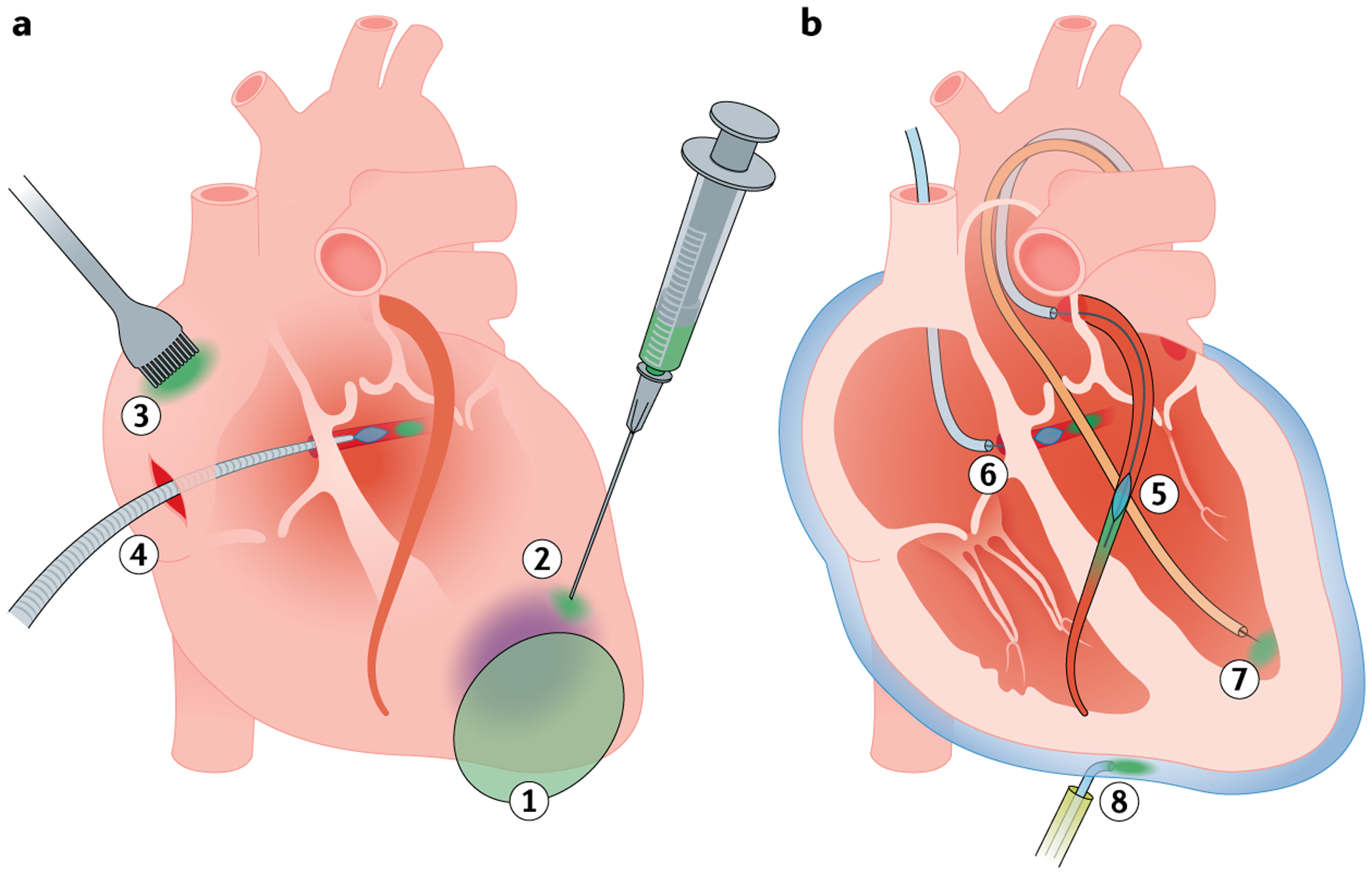
The therapeutic agent is depicted in green. a | Surgical approaches. (1) A cell sheet, tissue strip or biomaterial patch is about to be applied to a diseased area of the myocardium, such as an infarcted region (purple). (2) An epicardial injection is applied to the border zone of the diseased area. (3) Painting is applied on the right atrium. (4) Using cardiopulmonary bypass, retrograde recirculation via the coronary sinus allows cardiac-targeted delivery. b | Catheter-based approaches. (5) The coronary arteries are accessed using a guidewire and a guiding catheter (light blue), and coronary balloon occlusion is incorporated to facilitate transduction of the therapeutic agent. (6) The coronary sinus is selected using a guidewire and a balloon catheter (light blue), and the therapeutic agent is injected retrogradely in a coronary vein. (7) Transvalvular endocardial injection from the left ventricle. (8) Pericardial injection is administered using a percutaneous access sheath and an injection catheter (light blue).
Surgical approaches
Physical access to the heart requires open-chest and open-pericardial surgery but offers direct visualization and manipulation during the delivery of materials. This method is the only way to deliver large materials that do not fit inside catheters, such as cell sheets106–108, tissue strips109 and therapeutic patches110,111. These materials can be sutured or glued onto the myocardium, but compression of the epicardial coronary arteries needs to be avoided to prevent disturbance of coronary blood flow.
Intramyocardial injection from the epicardial side using small needles is another popular method that has been used in preclinical studies and some clinical trials to deliver genes112–114 or cells10,115,116. Direct visualization helps to determine the infarct border zone after myocardial infarction, but the accessible injection sites might be limited depending on the surgical window. For instance, the myocardium directly under the epicardial vessels or on the opposite side of the surgical window and the ventricular septum might not be readily accessible. Needle sizes of 27–30 gauge are commonly used for injection, but challenges remain in keeping all the injected material inside the myocardium. Injection of an excessive volume can lead to leakage from the needle holes117. Venous drainage is also an important factor that can reduce retention of injected material118,119 (FIG. 3). Adding mattress sutures around the injection site might increase the retention of injected material120; however, the therapeutic efficacy remains to be examined.
Fig. 3 |. Factors that influence cardiac uptake of therapeutic agents.
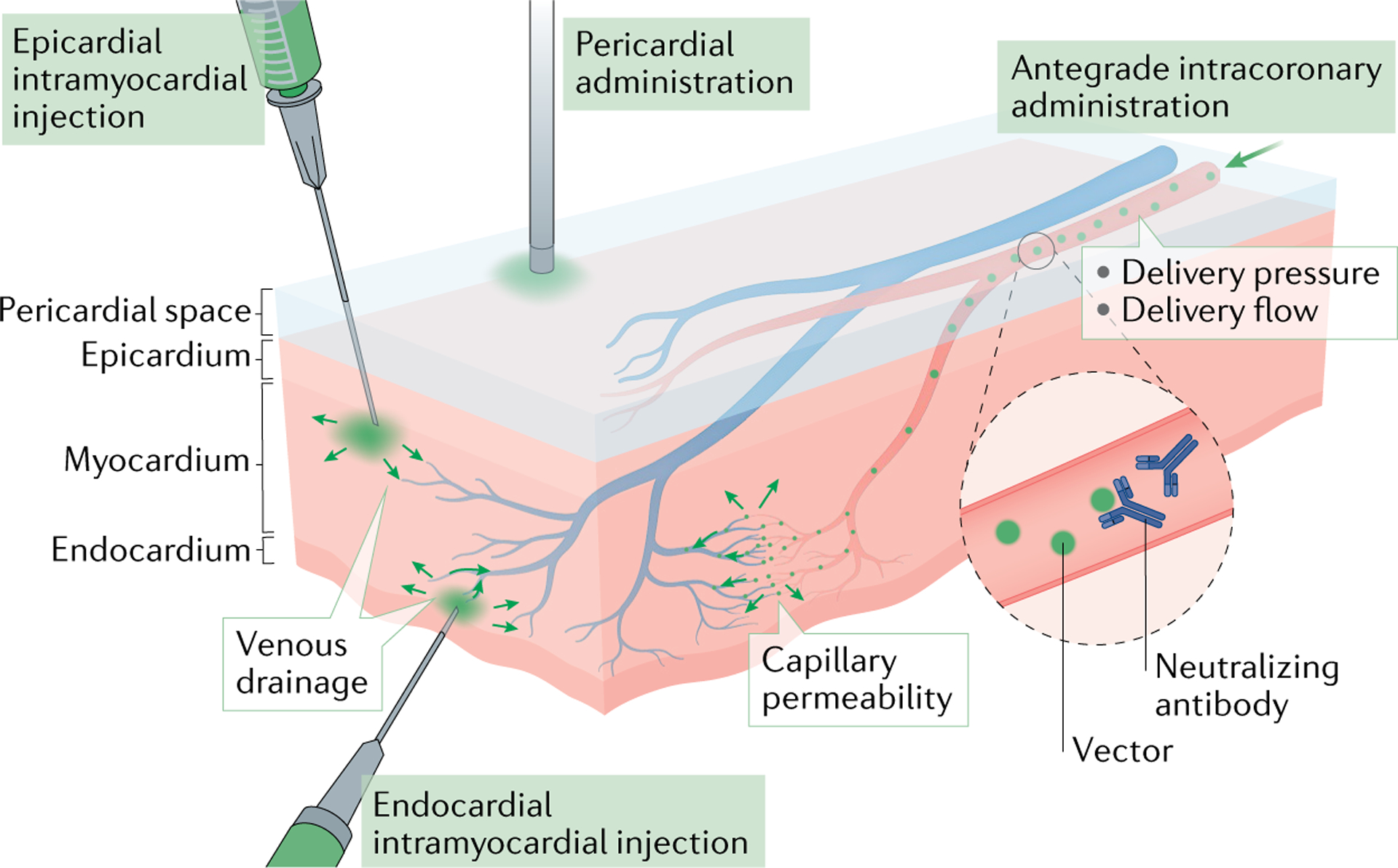
Several factors influence the cardiac uptake of therapeutic agents including the type, dose and modification of the vector. Modifications include conjugation, coating and encapsulation (BOX 1). The delivery route should be selected on the basis of the therapeutic agent (epicardial intramyocardial injection, endocardial intramyocardial injection, antegrade intracoronary administration and pericardial administration are shown). For intracoronary administration, delivery pressure, delivery flow, capillary permeability and venous drainage influence the transduction efficacy. Venous drainage also influences direct injections. Neutralizing antibodies can reduce the effective titre of some classes of vector.
Atrial painting is a unique surgical approach that was developed for delivering genes. Kikuchi and colleagues painted adenovirus on the surface of porcine atria and achieved transmural gene expression when trypsin was used together with the virus121. However, the transmurality of gene expression in the atria was limited without the addition of trypsin, and the thick wall of the left ventricle precluded complete transmural gene transduction even with trypsin. In summary, although surgical approaches are usually highly invasive, these procedures that allow the controlled delivery of materials and the capacity to deliver large materials are an attractive option particularly for patients who are already scheduled to undergo open-chest surgery122.
Catheter-based approaches
Catheter-based delivery approaches are generally less invasive than surgical approaches and can be safely used in patients with advanced cardiac dysfunction. Intracoronary delivery uses the same techniques that have been developed for coronary angiography and intervention. Catheter-based injection into the coronary artery allows homogeneous distribution, in contrast to the more focal distribution achieved by intramyocardial injection123. Because the antegrade flow to the ischaemic myocardium is limited in patients with severely narrowed or occluded coronary arteries, a method of retroperfusion from the coronary sinus side has been proposed to deliver vectors124 or cells125,126. Data on whether injections from these different directions affect therapeutic efficacy are limited, but the distribution of injected materials seems to result in more basal and epicardial expression with retrograde delivery than with antegrade delivery127–129.
Endocardial intramyocardial injection catheters allow direct injection of therapeutic materials using a percutaneous approach. In general, catheters are retrogradely advanced into the left ventricle through the aortic valve, and a small needle or screw tip is inserted into the myocardium to inject the material. Various imaging devices have been used to guide these catheters, including an electromechanical mapping guidance system (NOGA), radiography and MRI. The NOGA system allows the detection of scarred tissue by finding areas of low electrical signal, which facilitates targeted injection to the infarct border zones130. Unlike surgical injection, epicardial vessels do not interfere with the injection site, and the ventricular septum is also accessible. However, other areas might be difficult to target, depending on the design of the catheter, such as the myocardium below the valves. Endocardial injection has been reported to be better than either intracoronary or surgical approaches for delivering MSCs10, but further studies are needed for the delivery of other materials.
Although less frequently attempted, the pericardial space can also be targeted using catheter-based approaches. Access to the pericardium can be established by subxiphoid puncture, and therapeutic materials can be administered into the pericardial space131. Owing to the epicardial barrier and lymphatic drainage132, cardiac uptake varies depending on the properties of the delivered materials.
Other approaches
A few approaches have been proposed to direct therapeutic agents to the heart after systemic injection. The microbubble destruction method increases cardiac-targeted delivery with the use of ultrasound to destroy the microbubbles that coat or conjugate therapeutic agents133. Minor injury induced by bubble destruction can increase the uptake of therapeutic materials. Similarly, delivery materials conjugated to magnetic particles can be directed to the heart using magnets or MRI134,135.
Factors affecting cardiac uptake
These delivery methods differ not only in their route of cardiac access but also in the mechanical and biological factors associated with the cardiac uptake of therapeutic materials. For example, intramyocardial injection overcomes blood interaction and the endothelial barrier by delivering therapeutic material directly into the myocardial interstitial space. However, needle injury is an important concern with this method52. As discussed above, the volume injected is an important determinant of leakage during intramyocardial injection. Studies using microspheres have shown that larger volumes result in lower uptake efficiency owing to leakage from the needle hole and venous drainage117. However, unlike microspheres, gene-delivery vectors enter cells and might benefit from increased intramyocardial pressure during injection when a large volume is used (FIG. 3). Indeed, up to 1 ml per injection has been used in a clinical trial of gene therapy136. Few studies have directly compared the cardiac uptake efficiency of actual therapeutic materials using different injection volumes.
One important factor that needs attention when delivering materials through the coronary vasculature is the size of the therapeutic materials. Coronary microvessels have diameters of approximately 7–10 μm, and larger materials can cause microvascular plugging137, which can lead to micro-infarctions138. Other factors that have been shown to influence the cardiac uptake of injected materials are shown in FIG. 3. Studies suggest that the efficacy and retention of materials injected via the coronary route might be dependent on the therapeutic material. Adenoviral vector delivery to a coronary artery distal to an inflated balloon was reported to improve transgene expression139. Indeed, adding coronary sinus blockade to coronary artery blockade might further improve adenoviral gene transduction140. By contrast, the retention of stem cells does not seem to be altered by these approaches141,142. Whether this distinction is because of differences in size, biological properties or other factors is uncertain. Nevertheless, the optimal combination of therapeutic material, modification and delivery method is highly likely to be specific for each therapeutic agent. Therefore, thorough testing is necessary for each therapeutic material to maximize cardiac uptake before clinical translation.
Conclusions
Cardiac-specific delivery of therapeutic agents remains a challenge. Establishing specific approaches to target the heart is as important as identifying novel therapeutic agents. In addition to efforts to increase the cardiac specificity and retention of therapeutic agents, a programme that is more focused on targeted delivery to the heart might be required to advance this field. Targeted drug delivery is one of the most important and unresolved problems in pharmacology. By contrast, viruses have developed unique and highly specific tropisms towards their cellular targets by incorporating specific binding proteins over the course of their evolution. Incorporation of these viral proteins into the plasma membrane of EVs might facilitate EV-mediated delivery of drugs to specific cells. In addition, deciphering the structure, cargo and mechanisms of exosome-cell interactions and their uptake might facilitate the design of bioengineered exosomes and other EVs that can be used as suitable vehicles for targeted drug delivery. Together with identifying the optimal vector for each therapeutic material, minimally invasive yet highly specific delivery methods are the key to successful clinical translation of cardiac therapeutics.
Key points.
Therapies directed at modifying gene expression are emerging and have shown positive results for non-cardiac diseases in clinical trials; clinical translation of these therapies for cardiac diseases remains slow.
Currently, cardiac-specific delivery of therapeutic materials in large mammals requires invasive approaches, and the patterns of distribution depend on the delivery method used.
Vector options for gene delivery are increasing; adeno-associated viruses provide safe gene delivery but their gene-transduction efficacy in the human heart remains suboptimal.
Extracellular vesicles hold immense potential for the delivery of therapeutic agents; their clinical applications depend on their efficient isolation, scalability, drug loading, biodistribution and tissue targeting.
Next-generation cardiovascular therapeutics might include bioengineered macromolecules, viruses, nanobiologics and extracellular vesicles.
Acknowledgements
The authors are supported by grants NIH R01HL140469, R01HL124187 and R01HL148786 and New York Stem Cell Science (NYSTEM) C32562GG to S.S., and NIH R01HL139963 and AHA-SDG 17SDG33410873 to K.I. The authors acknowledge the Gene Therapy Resource Program (GTRP) of the National Heart, Lung, and Blood Institute.
Glossary
- Polyplex particles
Any complex of a polymer and a nucleic acid (DNA or RNA interference molecules) formed through electrostatic interactions between cationic groups of the polymer and the negatively charged nucleic acids.
- Episome
A segment of DNA that exists independently of a chromosome.
- Second-strand synthesis
DNA synthesis to form double-stranded DNA after delivery of single-stranded DNA.
- Zeta potential
A measure of the effective electric charge on the surface of an extracellular vesicle (EV) (or nanoparticle); the potential is calculated by quantifying the electrophoretic mobility of EVs in liquid between electrodes when a field is applied.
- Retroperfusion
Injection through the coronary sinus (vein).
Footnotes
Competing interests
The authors declare no competing interests.
Peer review information
Nature Reviews Cardiology thanks C. Emanueli, C. Kupatt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
References
- 1.Mattiuzzi C & Lippi G Worldwide disease epidemiology in the older persons. Eur. Geriatr. Med 11, 147–153 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 139, e56–e528 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Mensah GA, Roth GA & Fuster V The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J. Am. Coll. Cardiol 74, 2529–2532 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Miyamoto MI et al. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc. Natl Acad. Sci. USA 97, 793–798 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kho C et al. SUMO1-dependent modulation of SERCA2a in heart failure. Nature 477, 601–605 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishikawa K et al. Cardiac gene therapy in large animals: bridge from bench to bedside. Gene Ther. 19, 670–677 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain K, Riyad JM & Weber T Cardiac gene therapy with adeno-associated virus-based vectors. Curr. Opin. Cardiol 32, 275–282 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zangi L et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol 31, 898–907 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlsson L et al. Biocompatible, purified VEGF-A mRNA improves cardiac function after intracardiac injection 1 week post-myocardial infarction in swine. Mol. Ther. Methods Clin. Dev 9, 330–346 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanelidis AJ, Premer C, Lopez J, Balkan W & Hare JM Route of delivery modulates the efficacy of mesenchymal stem cell therapy for myocardial infarction: a meta-analysis of preclinical studies and clinical trials. Circ. Res 120, 1139–1150 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson HJ, Rafiq S & Brentjens RJ Driving CAR T-cells forward. Nat. Rev. Clin. Oncol 13, 370–383 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goswami R et al. Gene therapy leaves a vicious cycle. Front. Oncol 9, 297 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell S et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet 390, 849–860 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowes LP et al. Impact of age and motor function in a phase 1/2A study of infants with SMA type 1 receiving single-dose gene replacement therapy. Pediatr. Neurol 98, 39–45 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Adams D et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med 379, 11–21 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Prado DA, Acosta-Acero M & Maldonado RS Gene therapy beyond luxturna: a new horizon of the treatment for inherited retinal disease. Curr. Opin. Ophthalmol 31, 147–154 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Curtis M & Philipson R Cell & gene therapy commercial insight – December 2019. Cell Gene Ther. Insights 6, 69–83 (2020). [Google Scholar]
- 18.Lambert JL State of the industry: the financial, clinical, and scientific landscape for cell and gene therapies. Cell Gene Ther. Insights 6, 47–54 (2020). [Google Scholar]
- 19.Yla-Herttuala S & Baker AH Cardiovascular gene therapy: past, present, and future. Mol. Ther 25, 1095–1106 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenberg B et al. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet 387, 1178–1186 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Chung ES et al. Changes in ventricular remodelling and clinical status during the year following a single administration of stromal cell-derived factor-1 non-viral gene therapy in chronic ischaemic heart failure patients: the STOP-HF randomized phase II trial. Eur. Heart J 36, 2228–2238 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammond HK et al. Intracoronary gene transfer of adenylyl cyclase 6 in patients with heart failure: a randomized clinical trial. JAMA Cardiol. 1, 163–171 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hulot JS, Ishikawa K & Hajjar RJ Gene therapy for the treatment of heart failure: promise postponed. Eur. Heart J 37, 1651–1658 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nyamay’Antu A, Dumont M, Kedinger V & Erbacher P Non-viral vector mediated gene delivery: the outsider to watch out for in gene therapy. Cell Gene Ther. Insights 5, 51–57 (2019). [Google Scholar]
- 25.Yin H et al. Non-viral vectors for gene-based therapy. Nat. Rev. Genet 15, 541–555 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Zangi L & Hajjar RJ Synthetic microRNAs stimulate cardiac repair. Circ. Res 120, 1222–1223 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gan LM et al. Intradermal delivery of modified mRNA encoding VEGF-A in patients with type 2 diabetes. Nat. Commun 10, 871 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anttila V et al. Synthetic mRNA encoding VEGF-A in patients undergoing coronary artery bypass grafting: design of a phase 2a clinical trial. Mol. Ther. Methods Clin. Dev 18, 464–472 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen CW et al. Human pericytes for ischemic heart repair. Stem Cell 31, 305–316 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fliervoet LAL, Engbersen JFJ, Schiffelers RM, Hennink WE & Vermonden T Polymers and hydrogels for local nucleic acid delivery. J. Mater. Chem. B 6, 5651–5670 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Paul A et al. Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair. ACS Nano 8, 8050–8062 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng K et al. Magnetic antibody-linked nanomatchmakers for therapeutic cell targeting. Nat. Commun 5, 4880 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Ma A & Shang L Conjugating existing clinical drugs with gold nanoparticles for better treatment of heart diseases. Front. Physiol 9, 642 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei J et al. Differential m6A, m6Am, and m1A demethylation mediated by FTO in the cell nucleus and cytoplasm. Mol. Cell 71, 973–985.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Rooij E & Kauppinen S Development of microRNA therapeutics is coming of age. EMBO Mol. Med 6, 851–864 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foinquinos A et al. Preclinical development of a miR-132 inhibitor for heart failure treatment. Nat. Commun 11, 633 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hinkel R et al. AntimiR-21 prevents myocardial dysfunction in a pig model of ischemia/reperfusion injury. J. Am. Coll. Cardiol 75, 1788–1800 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Bernardo BC et al. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc. Natl Acad. Sci. USA 109, 17615–17620 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barr E et al. Efficient catheter-mediated gene transfer into the heart using replication-defective adenovirus. Gene Ther. 1, 51–58 (1994). [PubMed] [Google Scholar]
- 40.Stratford-Perricaudet LD, Makeh I, Perricaudet M & Briand P Widespread long-term gene transfer to mouse skeletal muscles and heart. J. Clin. Invest 90, 626–630 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu D et al. Direct comparison of efficiency and stability of gene transfer into the mammalian heart using adeno-associated virus versus adenovirus vectors. J. Thorac. Cardiovasc. Surg 126, 671–679 (2003). [DOI] [PubMed] [Google Scholar]
- 42.Douglas JT Adenoviral vectors for gene therapy. Mol. Biotechnol 36, 71–80 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Flotte TR & Berns KI Adeno-associated virus: a ubiquitous commensal of mammals. Hum. Gene Ther 16, 401–407 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Herzog RW et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat. Med 5, 56–63 (1999). [DOI] [PubMed] [Google Scholar]
- 45.Wang Z et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat. Biotechnol 23, 321–328 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Zincarelli C, Soltys S, Rengo G & Rabinowitz JE Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther 16, 1073–1080 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Penaud-Budloo M et al. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. J. Virol 82, 7875–7885 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCarty DM, Monahan PE & Samulski RJ Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 8, 1248–1254 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Bish LT et al. Adeno-associated virus (AAV) serotype 9 provides global cardiac gene transfer superior to AAV1, AAV6, AAV7, and AAV8 in the mouse and rat. Hum. Gene Ther 19, 1359–1368 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gabisonia K et al. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature 569, 418–422 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bish LT et al. Percutaneous transendocardial delivery of self-complementary adeno-associated virus 6 achieves global cardiac gene transfer in canines. Mol. Ther 16, 1953–1959 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Gao G et al. Transendocardial delivery of AAV6 results in highly efficient and global cardiac gene transfer in rhesus macaques. Hum. Gene Ther 22, 979–984 (2011). [DOI] [PubMed] [Google Scholar]
- 53.Amoasii L et al. Gene editing restores dystrophin expression in a canine model of Duchenne muscular dystrophy. Science 362, 86–91 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moretti A et al. Somatic gene editing ameliorates skeletal and cardiac muscle failure in pig and human models of Duchenne muscular dystrophy. Nat. Med 26, 207–214 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li C & Samulski RJ Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet 21, 255–272 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Ishikawa K et al. Cardiac I-1c overexpression with reengineered AAV improves cardiac function in swine ischemic heart failure. Mol. Ther 22, 2038–2045 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fleury S et al. Multiply attenuated, self-inactivating lentiviral vectors efficiently deliver and express genes for extended periods of time in adult rat cardiomyocytes in vivo. Circulation 107, 2375–2382 (2003). [DOI] [PubMed] [Google Scholar]
- 58.Di Pasquale E, Latronico MV, Jotti GS & Condorelli G Lentiviral vectors and cardiovascular diseases: a genetic tool for manipulating cardiomyocyte differentiation and function. Gene Ther. 19, 642–648 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Campochiaro PA et al. Lentiviral vector gene transfer of endostatin/angiostatin for macular degeneration (GEM) study. Hum. Gene Ther 28, 99–111 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hare JM et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA 308, 2369–2379 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niwano K et al. Lentiviral vector-mediated SERCA2 gene transfer protects against heart failure and left ventricular remodeling after myocardial infarction in rats. Mol. Ther 16, 1026–1032 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Povsic TJ et al. The RENEW trial: efficacy and safety of intramyocardial autologous CD34+ cell administration in patients with refractory angina. JACC Cardiovasc. Interv 9, 1576–1585 (2016). [DOI] [PubMed] [Google Scholar]
- 63.Henry TD et al. Autologous CD34+ cell therapy improves exercise capacity, angina frequency and reduces mortality in no-option refractory angina: a patient-level pooled analysis of randomized double-blinded trials. Eur. Heart J 39, 2208–2216 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Makkar RR et al. Intracoronary ALLogeneic heart STem cells to Achieve myocardial Regeneration (ALLSTAR): a randomized, placebo-scontrolled, double-blinded trial. Eur. Heart J 41, 3451–3458 (2020). [DOI] [PubMed] [Google Scholar]
- 65.Mathiasen AB et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial). Eur. Heart J 36, 1744–1753 (2015). [DOI] [PubMed] [Google Scholar]
- 66.Madonna R et al. ESC working group on cellular biology of the heart: position paper for cardiovascular research: tissue engineering strategies combined with cell therapies for cardiac repair in ischaemic heart disease and heart failure. Cardiovasc. Res 115, 488–500 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kanisicak O, Vagnozzi RJ & Molkentin JD Identity crisis for regenerative cardiac cKit+ cells. Circ. Res 121, 1130–1132 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Berlo JH et al. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 509, 337–341 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vagnozzi RJ et al. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature 577, 405–409 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sahoo S et al. Exosomes from human CD34+ stem cells mediate their proangiogenic paracrine activity. Circ. Res 109, 724–728 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sahoo S & Losordo DW Exosomes and cardiac repair after myocardial infarction. Circ. Res 114, 333–344 (2014). [DOI] [PubMed] [Google Scholar]
- 72.Adamiak M & Sahoo S Exosomes in myocardial repair: advances and challenges in the development of next-generation therapeutics. Mol. Ther 26, 1635–1643 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vicencio JM et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J. Am. Coll. Cardiol 65, 1525–1536 (2015). [DOI] [PubMed] [Google Scholar]
- 74.Beltrami C et al. Human pericardial fluid contains exosomes enriched with cardiovascular-expressed microRNAs and promotes therapeutic angiogenesis. Mol. Ther 25, 679–693 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mathiyalagan P et al. Angiogenic mechanisms of human cd34+ stem cell exosomes in the repair of ischemic hindlimb. Circ. Res 120, 1466–1476 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tseliou E et al. Fibroblasts rendered antifibrotic, antiapoptotic, and angiogenic by priming with cardiosphere-derived extracellular membrane vesicles. J. Am. Coll. Cardiol 66, 599–611 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gallet R et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J 38, 201–211 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rupert DLM, Claudio V, Lasser C & Bally M Methods for the physical characterization and quantification of extracellular vesicles in biological samples. Biochim. Biophys. Acta Gen. Subj 1861, 3164–3179 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Vader P, Mol EA, Pasterkamp G & Schiffelers RM Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev 106, 148–156 (2016). [DOI] [PubMed] [Google Scholar]
- 80.Robbins PD & Morelli AE Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol 14, 195–208 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mokarizadeh A et al. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol. Lett 147, 47–54 (2012). [DOI] [PubMed] [Google Scholar]
- 82.Admyre C et al. Exosomes with immune modulatory features are present in human breast milk. J. Immunol 179, 1969–1978 (2007). [DOI] [PubMed] [Google Scholar]
- 83.Hellwinkel JE et al. Glioma-derived extracellular vesicles selectively suppress immune responses. Neuro. Oncol 18, 497–506 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Escudier B et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J. Transl. Med 3, 10 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dai S et al. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Ther 16, 782–790 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morishita M, Takahashi Y, Nishikawa M & Takakura Y Pharmacokinetics of exosomes-an important factor for elucidating the biological roles of exosomes and for the development of exosome-based therapeutics. J. Pharm. Sci 106, 2265–2269 (2017). [DOI] [PubMed] [Google Scholar]
- 87.Hu L, Wickline SA & Hood JL Magnetic resonance imaging of melanoma exosomes in lymph nodes. Magn. Reson. Med 74, 266–271 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Horibe S, Tanahashi T, Kawauchi S, Murakami Y & Rikitake Y Mechanism of recipient cell-dependent differences in exosome uptake. BMC Cancer 18, 47 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Margolis L & Sadovsky Y The biology of extracellular vesicles: the known unknowns. PLoS Biol. 17, e3000363 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Abreu RC et al. Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat. Rev. Cardiol 17, 685–697 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mentkowski KI & Lang JK Exosomes engineered to express a cardiomyocyte binding peptide demonstrate improved cardiac retention in vivo. Sci. Rep 9, 10041 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shin M et al. Targeting protein and peptide therapeutics to the heart via tannic acid modification. Nat. Biomed. Eng 2, 304–317 (2018). [DOI] [PubMed] [Google Scholar]
- 93.Trac D et al. Predicting functional responses of progenitor cell exosome potential with computational modeling. Stem Cell Transl. Med 8, 1212–1221 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nolte-’t Hoen E, Cremer T, Gallo RC & Margolis LB Extracellular vesicles and viruses: are they close relatives? Proc. Natl Acad. Sci. USA 113, 9155–9161 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Feng Z et al. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature 496, 367–371 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gyorgy B, Fitzpatrick Z, Crommentuijn MH, Mu D & Maguire CA Naturally enveloped AAV vectors for shielding neutralizing antibodies and robust gene delivery in vivo. Biomaterials 35, 7598–7609 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liang Y et al. AAV-containing exosomes as a novel vector to improve AAV-mediated myocardial gene delivery in resistance to neutralizing antibody [abstract]. Circulation 136, A15439 (2017). [Google Scholar]
- 98.Meliani A et al. Enhanced liver gene transfer and evasion of preexisting humoral immunity with exosome-enveloped AAV vectors. Blood Adv. 1, 2019–2031 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wassmer SJ, Carvalho LS, Gyorgy B, Vandenberghe LH & Maguire CA Exosome-associated AAV2 vector mediates robust gene delivery into the murine retina upon intravitreal injection. Sci. Rep 7, 45329 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gyorgy B et al. Rescue of hearing by gene delivery to inner-ear hair cells using exosome-associated AAV. Mol. Ther 25, 379–391 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03384433 (2020). [DOI] [PubMed]
- 102.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04356300 (2020). [DOI] [PubMed]
- 103.Pacak CA et al. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ. Res 99, e3–e9 (2006). [DOI] [PubMed] [Google Scholar]
- 104.Arslan F et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 10, 301–312 (2013). [DOI] [PubMed] [Google Scholar]
- 105.Luger D et al. Intravenously delivered mesenchymal stem cells: systemic anti-inflammatory effects improve left ventricular dysfunction in acute myocardial infarction and ischemic cardiomyopathy. Circ. Res 120, 1598–1613 (2017). [DOI] [PubMed] [Google Scholar]
- 106.Terajima Y et al. Autologous skeletal myoblast sheet therapy for porcine myocardial infarction without increasing risk of arrhythmia. Cell Med 6, 99–109 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Masumoto H et al. Human iPS cell-engineered cardiac tissue sheets with cardiomyocytes and vascular cells for cardiac regeneration. Sci. Rep 4, 6716 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kang K et al. Exosomes secreted from CXCR4 overexpressing mesenchymal stem cells promote cardioprotection via akt signaling pathway following myocardial infarction. Stem Cell Int. 2015, 659890 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weinberger F et al. Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci. Transl. Med 8, 363ra148 (2016). [DOI] [PubMed] [Google Scholar]
- 110.Wei K et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 525, 479–485 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Serpooshan V et al. The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction. Biomaterials 34, 9048–9055 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Svensson EC et al. Efficient and stable transduction of cardiomyocytes after intramyocardial injection or intracoronary perfusion with recombinant adeno-associated virus vectors. Circulation 99, 201–205 (1999). [DOI] [PubMed] [Google Scholar]
- 113.Fortuin FD et al. One-year follow-up of direct myocardial gene transfer of vascular endothelial growth factor-2 using naked plasmid deoxyribonucleic acid by way of thoracotomy in no-option patients. Am. J. Cardiol 92, 436–439 (2003). [DOI] [PubMed] [Google Scholar]
- 114.Ishikawa K et al. Stem cell factor gene transfer improves cardiac function after myocardial infarction in swine. Circ. Heart Fail 8, 167–174 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Losordo DW et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation 115, 3165–3172 (2007). [DOI] [PubMed] [Google Scholar]
- 116.Kulandavelu S et al. Pim1 kinase overexpression enhances c-kit+ cardiac stem cell cardiac repair following myocardial infarction in swine. J. Am. Coll. Cardiol 68, 2454–2464 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Grossman PM, Han Z, Palasis M, Barry JJ & Lederman RJ Incomplete retention after direct myocardial injection. Catheter. Cardiovasc. Interv 55, 392–397 (2002). [DOI] [PubMed] [Google Scholar]
- 118.van den Akker F et al. Intramyocardial stem cell injection: go(ne) with the flow. Eur. Heart J 38, 184–186 (2017). [DOI] [PubMed] [Google Scholar]
- 119.Dow J, Simkhovich BZ, Kedes L & Kloner RA Washout of transplanted cells from the heart: a potential new hurdle for cell transplantation therapy. Cardiovasc. Res 67, 301–307 (2005). [DOI] [PubMed] [Google Scholar]
- 120.Chong JJ et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510, 273–277 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kikuchi K, McDonald AD, Sasano T & Donahue JK Targeted modification of atrial electrophysiology by homogeneous transmural atrial gene transfer. Circulation 111, 264–270 (2005). [DOI] [PubMed] [Google Scholar]
- 122.Yau TM et al. Intramyocardial injection of mesenchymal precursor cells and successful temporary weaning from left ventricular assist device support in patients with advanced heart failure: a randomized clinical trial. JAMA 321, 1176–1186 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li J et al. Comparative study of catheter-mediated gene transfer into heart. Chin. Med. J 115, 612–613 (2002). [PubMed] [Google Scholar]
- 124.von Degenfeld G et al. Selective pressure-regulated retroinfusion of fibroblast growth factor-2 into the coronary vein enhances regional myocardial blood flow and function in pigs with chronic myocardial ischemia. J. Am. Coll. Cardiol 42, 1120–1128 (2003). [DOI] [PubMed] [Google Scholar]
- 125.Tuma J et al. Safety and feasibility of percutaneous retrograde coronary sinus delivery of autologous bone marrow mononuclear cell transplantation in patients with chronic refractory angina. J. Transl. Med 9, 183 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thompson CA et al. Percutaneous transvenous cellular cardiomyoplasty. A novel nonsurgical approach for myocardial cell transplantation. J. Am. Coll. Cardiol 41, 1964–1971 (2003). [DOI] [PubMed] [Google Scholar]
- 127.Hou D et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation 112, I150–I156 (2005). [DOI] [PubMed] [Google Scholar]
- 128.Hoshino K et al. Three catheter-based strategies for cardiac delivery of therapeutic gelatin microspheres. Gene Ther 13, 1320–1327 (2006). [DOI] [PubMed] [Google Scholar]
- 129.Hong SJ et al. Intracoronary and retrograde coronary venous myocardial delivery of adipose-derived stem cells in swine infarction lead to transient myocardial trapping with predominant pulmonary redistribution. Catheter. Cardiovasc. Interv 83, E17–E25 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vale PR et al. Left ventricular electromechanical mapping to assess efficacy of phVEGF(165) gene transfer for therapeutic angiogenesis in chronic myocardial ischemia. Circulation 102, 965–974 (2000). [DOI] [PubMed] [Google Scholar]
- 131.Ladage D et al. Delivery of gelfoam-enabled cells and vectors into the pericardial space using a percutaneous approach in a porcine model. Gene Ther 18, 979–985 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vogiatzidis K et al. Physiology of pericardial fluid production and drainage. Front. Physiol 6, 62 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sun W, Li Z, Zhou X, Yang G & Yuan L Efficient exosome delivery in refractory tissues assisted by ultrasound-targeted microbubble destruction. Drug Deliv. 26, 45–50 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mah C et al. Improved method of recombinant AAV2 delivery for systemic targeted gene therapy. Mol. Ther 6, 106–112 (2002). [DOI] [PubMed] [Google Scholar]
- 135.Delyagina E, Li W, Ma N & Steinhoff G Magnetic targeting strategies in gene delivery. Nanomedicine 6, 1593–1604 (2011). [DOI] [PubMed] [Google Scholar]
- 136.Penn MS et al. An open-label dose escalation study to evaluate the safety of administration of nonviral stromal cell-derived factor-1 plasmid to treat symptomatic ischemic heart failure. Circ. Res 112, 816–825 (2013). [DOI] [PubMed] [Google Scholar]
- 137.Ly HQ et al. In vivo myocardial distribution of multipotent progenitor cells following intracoronary delivery in a swine model of myocardial infarction. Eur. Heart J 30, 2861–2868 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ishikawa K Intracoronary injection of large stem cells: size matters. Circ. Cardiovasc. Interv 8, e002648 (2015). [DOI] [PubMed] [Google Scholar]
- 139.Emani SM et al. Catheter-based intracoronary myocardial adenoviral gene delivery: importance of intraluminal seal and infusion flow rate. Mol. Ther 8, 306–313 (2003). [DOI] [PubMed] [Google Scholar]
- 140.Hayase M et al. Catheter-based antegrade intracoronary viral gene delivery with coronary venous blockade. Am. J. Physiol. Heart Circ. Physiol 288, H2995–H3000 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Keith MC et al. Effect of the stop-flow technique on cardiac retention of c-kit positive human cardiac stem cells after intracoronary infusion in a porcine model of chronic ischemic cardiomyopathy. Basic Res. Cardiol 110, 503 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Musialek P et al. Randomized transcoronary delivery of CD34+ cells with perfusion versus stop-flow method in patients with recent myocardial infarction: early cardiac retention of 99mTc-labeled cells activity. J. Nucl. Cardiol 18, 104–116 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Losordo DW et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ. Res 109, 428–436 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Karantalis V et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: the Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ. Res 114, 1302–1310 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Teng CJ, Luo J, Chiu RC & Shum-Tim D Massive mechanical loss of microspheres with direct intramyocardial injection in the beating heart: implications for cellular cardiomyoplasty. J. Thorac. Cardiovasc. Surg 132, 628–632 (2006). [DOI] [PubMed] [Google Scholar]
- 146.Shah AS et al. Intracoronary adenovirus-mediated delivery and overexpression of the β2-adrenergic receptor in the heart: prospects for molecular ventricular assistance. Circulation 101, 408–414 (2000). [DOI] [PubMed] [Google Scholar]
- 147.James SK et al. Long-term safety and efficacy of drug-eluting versus bare-metal stents in Sweden. N. Engl. J. Med 360, 1933–1945 (2009). [DOI] [PubMed] [Google Scholar]
- 148.Sharif F, Daly K, Crowley J & O’Brien T Current status of catheter- and stent-based gene therapy. Cardiovasc. Res 64, 208–216 (2004). [DOI] [PubMed] [Google Scholar]
- 149.Lekshmi KM, Che HL, Cho CS & Park IK Drug- and gene-eluting stents for preventing coronary restenosis. Chonnam. Med. J 53, 14–27 (2017).28184335 [Google Scholar]
- 150.Bukka M, Rednam PJ & Sinha M Drug-eluting balloon: design, technology and clinical aspects. Biomed. Mater 13, 032001 (2018). [DOI] [PubMed] [Google Scholar]
- 151.Fargnoli AS et al. A pharmacokinetic analysis of molecular cardiac surgery with recirculation mediated delivery of βARKct gene therapy: developing a quantitative definition of the therapeutic window. J. Card. Fail 17, 691–699 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Krause KT et al. Percutaneous endocardial injection of erythropoietin: assessment of cardioprotection by electromechanical mapping. Eur. J. Heart Fail 8, 443–450 (2006). [DOI] [PubMed] [Google Scholar]
- 153.Tao Z et al. HGF percutaneous endocardial injection induces cardiomyocyte proliferation and rescues cardiac function in pigs. J. Biomed. Res 24, 198–206 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Traverse JH et al. First-in-man study of a cardiac extracellular matrix hydrogel in early and late myocardial infarction patients. JACC Basic Transl. Sci 4, 659–669 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Matoba T & Egashira K Nanoparticle-mediated drug delivery system for cardiovascular disease. Int. Heart J 55, 281–286 (2014). [DOI] [PubMed] [Google Scholar]
- 156.Matoba T, Koga JI, Nakano K, Egashira K & Tsutsui H Nanoparticle-mediated drug delivery system for atherosclerotic cardiovascular disease. J. Cardiol 70, 206–211 (2017). [DOI] [PubMed] [Google Scholar]
- 157.Lee RJ et al. Antibody targeting of stem cells to infarcted myocardium. Stem Cell 25, 712–717 (2007). [DOI] [PubMed] [Google Scholar]
- 158.Chen S & Grayburn PA in Cardiac Gene Therapy: Methods and Protocols (ed. Ishikawa K) 205–218 (Springer, 2017). [Google Scholar]
- 159.Hudry E et al. Exosome-associated AAV vector as a robust and convenient neuroscience tool. Gene Ther. 23, 380–392 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jang SC et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano 7, 7698–7710 (2013). [DOI] [PubMed] [Google Scholar]
- 161.Luan X et al. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin 38, 754–763 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]


