Abstract
The most common form of esophageal cancer (EC), esophageal squamous cell carcinoma (ESCC), is prevalent in many unindustrialized societies, among people with lower socioeconomic status and those who frequently use tobacco and alcohol. In some areas, ESCC mortality ranked top among all cancer. In this review, we begin with discussions of the extensive research on EC in Linxian in northern China that started 60 years ago and the recent studies in Kenya from our personal perspectives. Based on the results obtained from these studies and information from the literature, we summarize our current understanding about the risk factors for ESCC including lifestyle factors (smoking, alcohol, consumption of food and beverages at high temperature and other unhealthy habits), poor diet and nutritional insufficiencies and genetic susceptibility. Elimination or minimization of these environmental risk factors, as well as early detection and treatment of precancerous lesions, would be effective means for the prevention of ESCC. Current knowledge of molecular alterations in ESCC (gene mutations, hypermethylation and amplification or overexpression), as well as treatment of ESCC and the potential of targeted therapy, are also discussed. Finally, we propose effective approaches for the prevention of ESCC by adapting a healthy lifestyle, including a healthy diet that would also prevent other diseases. Community outreach, public education and international collaboration are important for achieving this public health goal.
Keywords: esophageal cancer, molecular alterations, prevention, risk factors, therapy
Abbreviations
- ADH
alcohol dehydrogenase
- ALDH2
aldehyde dehydrogenase 2
- CICAMS
Cancer Institute of the Chinese Academy of Medical Sciences
- EC
esophageal cancer
- ESCC
esophageal squamous cell carcinoma
- GCC
gastric cardia cancer
- GWAS
genome‐wide significant association study
- IARC
International Agency for Research on Cancer
- LNIT
Linxian Nutritional Intervention Trial
- NCI
National Cancer Institute
- NGS
NextGen sequencing
- NMBA
N‐nitrosomethylbenzylamine
- NRF2
nuclear factor erythroid‐derived 2
- OESO
World Organization for Specialized Studies on Diseases of the Esophagus
- PAHs
polyaromatic hydrocarbons
- PLCE1
phospholipase c epsilon 1
- SNP
single nucleotide polymorphism
- TMR
Taihang Mountain Range
1. INTRODUCTION
Esophageal cancer (EC) is one of the deadliest diseases in many parts of the world. There were an estimated 572 034 new cases and 508 585 cancer deaths in 2018, with almost half of new cases occurring in China. 1 The most common form, esophageal squamous cell carcinoma (ESCC), accounting for ~90% of all the cases of EC, occurs at high incidence in certain areas, such as the central Asia EC belt ‐ starting from the Taihang Mountain Range (TMR) in northern China to Central Asia and extending to Iran, and the African EC corridor in eastern and southern Africa. In Western countries, the incidence of ESCC has significantly declined in the past decades. Instead, another type of EC, esophageal adenocarcinoma, which is caused mainly by gastroesophageal reflux, has become the major form of EC. 2 This review article will focus on ESCC.
In China, most of the research activities on EC were historically centered in Linxian County (now named Linzhou City) of the Henan Province, located in the TMR. In this area, the EC mortality rate presented itself as concentric belts from a high incidence rate of >80/100 000 and descending to regions of >40, >20 and then <20/100 000. In August 1979, supported by a grant from the International Agency for Research on Cancer (IARC) and hosted by the Cancer Institute of the Chinese Academy of Medical Sciences (CICAMS) in Beijing, coauthor Chung S. Yang visited China to learn about EC research. Based on what he learned from Beijing and later in Linxian, Chung S. Yang wrote a review article titled “Research on Esophageal Cancer in China: A Review.” 3
The young ESCC cases reported by Dr Russell White in the 13th World Conference of OESO (World Organization for Specialized Studies on Diseases of the Esophagus) caught the attention of coauthor Xiaoxin Luke Chen. He wrote a review article on EC in Kenya 4 and visited Kenyatta National Hospital in Nairobi and Tenwek Hospital in Western Kenya in December 2018 to gather first‐hand information on EC in Kenya.
In this article, we will first review the research activities in China and Kenya from the authors' personal perspectives. Then, based on available information in the literature, we will discuss our current understanding of the risk factors, molecular alterations, prevention and treatment of ESCC. Effective approaches for the prevention of ESCC and needs for future research will also be discussed.
2. ESOPHAGEAL CANCER IN LINXIAN
2.1. Early studies in Linxian
Linxian was a rural county with beautiful mountains, hard‐working people and extensively cultivated land. Since the late 1950s, the EC problem had received attention from the Chinese central government. In 1959, many medical doctors and researchers from different areas were assigned to work on EC in Linxian. By 1979, much information on the risk factors had been accumulated, based primarily on observational epidemiology and information from the literature. 3 To avoid “eating moldy food,” “using pond water” and “ingesting hot food and beverages” were promoted as measures for EC prevention. Moldy food could contain carcinogenic mycotoxins and precursors for N‐nitrosamines, such as amines and amides. The nitrosating agent—nitrite could be derived from drinking water sources—usually from ponds. Nitrosamines, such as N‐nitrosomethylbenzylamine (NMBA), can readily induce EC in rats 5 and were believed to be the leading candidate carcinogen of human EC. 3 A new nitrosamine, N‐1‐methylacetonyl‐N‐3‐methylbutylnitrosamine, was found in moldy cornbread at the level of 0.2 to 0.3 ppm. 6 Polyaromatic hydrocarbons (PAHs), generated from stoves near the bed (to keep warm in the winter), were also discussed as possible carcinogens for EC. 3
A highly suspected moldy food was “pickled vegetables,” which were commonly consumed in that area. 3 The presence of N‐nitroso compounds in pickled vegetables and their mutagenic or cell transformation activity was later demonstrated. 7 , 8 The correlation between pickled vegetable consumption and EC mortality was observed in a large study in 1973. 3 However, some follow‐up studies were said to be uninformative.
The importance of the treatment of precancerous lesions was recognized at that time. Esophagitis was found to be quite prevalent and was considered to be a precursor of EC by some scientists, but there was no real evidence that this condition itself leads to EC. Besides, there was no effective way of preventing or treating it, except “improve the eating habit.” However, “early detection and early treatment” was well recognized. Of note was the outstanding work of Professor Qiong Shen who pioneered the use of balloon cytology in China for the screening, diagnosis and even localization of EC (Figure 1). Because of the economical strain, only some of the EC were treated by surgery (usually proficiently performed) or by chemotherapy or radiation therapy (not as advanced as the Western world). Balloon cytology also could pick up dysplastic cells (reflecting a precancerous state) occasionally, but there was no effective way of treating esophageal dysplasia at that time. Dr Shen was so dedicated to research on EC and moved to Linxian so he could be fully devoted to EC research. He once said, “We should try to solve people's problems with cheap and convenient means.” This statement is still of great value to scientists and medical doctors now, especially with the current “for‐profit” mindset.
FIGURE 1.
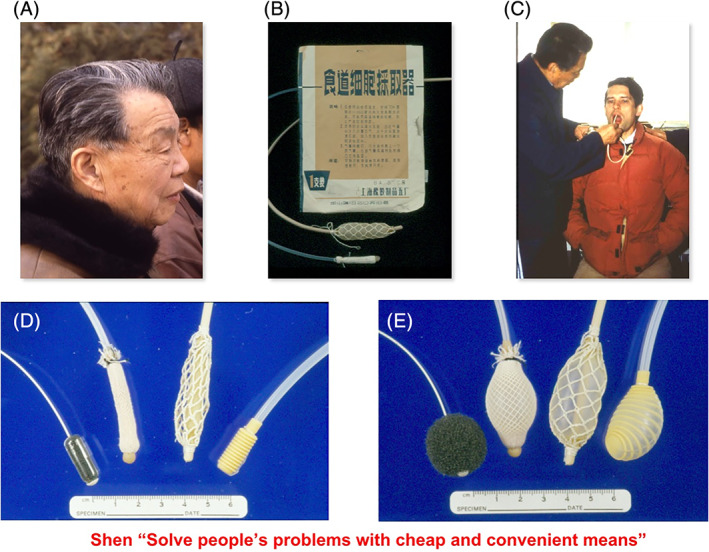
Professor Qiong Shen and balloon cytology. A, A photo of Professor Qiong Shen; B, balloons used in the 1970s for balloon cytology. C, Professor Shen performing balloon cytology on Dr Sanford Dawsey of the National Cancer Institute (NCI). D,E, Newer versions of the balloons used for cytology and a quotation from Dr Shen. (thank to Dr Dawsey for providing these photos)
Cigarette smoking and alcohol drinking were recognized as the major risk factors for ESCC in many parts of the world. 2 In Linxian, they were not because most people could not afford cigarettes or alcoholic beverages. A possible risk factor was the poor nutritional status of the population due to a monotonous diet with corn as the major staple. In 1979, peasants ate their share of grains and other food that were collectively produced locally. Commercial food items and refrigerators were not available. Consumption of meat, eggs, fish and dairy products was very low. The consumption of vegetables and fruits was low and seasonal and thus insufficiencies in micronutrients were suspected. People were lean; however, signs of overt nutritional deficiencies were rare. This encouraged us to pursue a biochemical approach to further assess the nutritional status of the population. 9
In 1980, supported by a grant from the US National Academy of Sciences, Chung S. Yang worked in Linxian with a collaborative group from the Henan Tumor Institute and Henan Medical College to measure blood levels of ascorbic acid and carotenoids and accessed riboflavin nutritional status by the glutathione reductase assay. The study confirmed the suspected lower nutritional status or insufficiency of vitamin C, pro‐vitamin A and riboflavin. 9 With these results, the idea of a nutritional intervention study was discussed among collaborators in Linxian, Zhengzhou and Beijing, and then with Dr William Blot of the US National Cancer Institute (NCI). At that time, Dr Blot was visiting China and was interested in the idea. United States and China had recently established a formal diplomatic relationship, and the NCI was interested in establishing meaningful collaborative cancer research projects in China. In 1981, Dr Junyao Li, the chief epidemiologist from the CICAMS, was invited to the NCI to discuss US‐China collaboration on cancer research. The US‐China collaborative intervention studies would start a few years later.
2.2. Nutritional interventional studies in Linxian
The hypothesis that supplementation with micronutrients would reduce EC risk in humans was tested in the 1980s in a large‐scale intervention study on gastroesophageal cancer, the US‐China cooperative Linxian Nutritional Intervention Trial (LNIT). This trial was conducted jointly by the CICAMS and NCI. NCI provided expertise in epidemiology and intervention trials as well as most of the funding. For example, Dr. Sanford Dawsey, providing expertise in pathology, worked meticulously with Chinese collaborators to establish the standard of diagnosis and conducted other key studies. CICAMS provided administrative support, medical doctors, pathologists and organized a large team of local paramedics to conduct the field work, including physical examinations, interviews for dietary and health information, delivery of intervention pills, compliance assessment and data entry (Figure 2). Chung S. Yang contributed his expertise in nutrition and cancer, conducted key studies and facilitated many of the research interactions. The advantages of conducting a nutritional intervention trial in Linxian were (a) the high mortality rates of EC, especially in the northern part of Linxian; (b) the known nutritional insufficiencies and stable dietary pattern; (c) the stable population—a person can be found either at home or in the field; and (d) the well‐structured society—people followed the instructions from authorities.
FIGURE 2.

Photos of the Linxian Nutritional Intervention Trials. A, Site visit of National Cancer Institute (NCI) and other investigators to Linxian before launching the intervention. B,C, Interviewing and physical examination at the beginning of the trial. (Photos taken by Chung S. Yang.) D, Chung S. Yang (in striped shirt) observing pill counting for compliance analysis
After many planning meetings and several site visits to Linxian starting in 1982, the study was launched in 1985. Because many micronutrients had been implicated in gastroesophageal cancer, there were many different opinions on what nutrients to use. Finally, a compromise was made that nine nutrients were grouped into four sets (factors): (A) retinol and zinc; (B) riboflavin and niacin; (C) ascorbate and molybdenum; and (D) α‐tocopherol (α‐T), β‐carotene, and selenium (as selenium yeast), with each nutrient at 2 to 3 times the dose of the US Recommended Daily Allowance. These Sets of nutrients were combined in a factorial design in eight groups: placebo, AB, AC, AD, BC, BD, CD and ABCD. The study involved 29 584 adults (aged 40‐69) who were given daily supplementation as pills for 63 months (1985‐1991). The CICAMS established a fieldwork team stationed in Linxian and the US collaborators routinely visited Linxian, at least once or twice a year, to check the progress and provide expertise in different areas. During the trial, blood samples from some of the subjects were collected for analysis of compliance and for establishing a serum bank for future nested case‐control studies.
There were 2127 deaths during the trial period; 32% were due to ESCC and gastric cancer—mainly gastric cardia cancer (GCC). Gastric cardia is located just below the esophagus and GCC was also considered as “esophageal cancer” by residents in Linxian, where GCC accounted for 40% of the “esophageal cancer” and the other 60% was ESCC. Factor D, a combination of α‐T (50 mg), β‐carotene (15 mg), and selenium (50 μg), was found to decrease mortality from gastric cancer (mainly GCC) by 20% and total cancer mortality by 13%, while the ESCC mortality was not affected. 10 Other nutrient combinations in Factor A, B or C, however, did not show any significant effect on the endpoints measured. Subsequent nested case‐control studies showed that the baseline serum levels of α‐T and selenium were low, and were each inversely associated with gastroesophageal cancer risk. 11 , 12 The result is consistent with the concept that the low antioxidant (vitamin E and selenium) nutritional status makes the individuals susceptible to inflammation and carcinogenesis. 13 Under these conditions, supplementation with vitamin E (α‐T) and selenium attenuated carcinogenesis.
Furthermore, results from a 10‐year follow‐up showed that the preventive effect of the combination of α‐T/β‐carotene/selenium on gastric cancer and total mortality was sustained and that this nutrient combination also protected against ESCC in younger subjects—when they were enrolled in the trial at ages 55 or younger, but not in those older than 55. 14 It is possible that the intervention was ineffective in older subjects because they already had higher‐grade precancerous lesions. This concept is consistent with the result of a parallel trial in Linxian on subjects with esophageal dysplasia, showing a lack of beneficial effect by supplementation with a combination of micronutrients. 15 In the 25‐year post‐trial follow‐up of the LNIT, the previously observed protective effects of α‐T/selenium/β‐carotene were attenuated or lost. 16 However, an apparent new protective effect against ESCC was found for Factor B (riboflavin and niacin).
The above cancer‐preventive effect of α‐T/selenium is supported by studies in a rat model demonstrating that a diet with insufficiencies in α‐T/selenium enhanced NMBA‐induced esophageal carcinogenesis, and that supplementation of α‐T/selenium at the early stage of the experiment was more effective than that at the late stage. 17 These experiments demonstrate the cancer‐preventive activity of antioxidant nutrients (α‐T and selenium) when supplemented to a human population or rats with low antioxidant nutritional status.
2.3. Socioeconomic development and lowered EC risk in Linzhou
Living in the mountain range, the men in Linxian (the old name) were traditionally skilled at stonework and in modern days, the skill has been transformed into construction work. During the past 40 years, there has been tremendous construction work in China and the construction teams from Linzhou worked all over the country. This resulted in the accumulation of capital in Linzhou and helped the rapid socioeconomic development in Linzhou; the GDP per capita increased from ¥186 in 1978 to ¥68 501 in 2017 according to data from the National Bureau of Statistics of China. When Chung S. Yang revisited Linzhou City in 2019, the city was well developed with many shops and restaurants. This urbanization was in sharp contrast to 40 years ago.
The Linzhou Cancer Hospital is now a modern hospital in a big high‐rise building. In the conference room of the hospital, Vice Director Dr Zhicai Liu showed that the age‐adjusted incidence rate of EC (including GCC) for males had decreased from approximately 110/100 000 to about 55/100 000 from 1988 to 2013. The age‐adjusted death rate was slightly lower and showed a similar decrease. The incidence and death rates for females were significantly lower than men and also showed a similar decrease by about 50% from 1988 to 2013. Apparently, the improvement of diet, nutrition, quality of water and oral hygiene due to economic development had contributed to the decrease of EC in Linxian and some other areas, but such an extensive decrease has not occurred in some economically less developed areas in China. 18 , 19 , 20 Ironically, the increased consumption of alcohol and cigarettes in Linzhou and other areas nowadays is expected to compromise the beneficial effect of economic development on EC.
Of note is that during the past 40 years, especially after 2000, the overall cancer rate has markedly increased in Linzhou—among them cancers of the lung, breast, ovary, liver and the colorectum. The rate of cardiovascular diseases also increased. These follow the same disease pattern of China as a country, 21 possibly due to economic development and commercialization; the consumption of meat, fat, refined grain and total calories as well as cigarette and alcoholic drinks had increased, while physical activities had decreased. Does this mean we expel one devil and invite another? A recent 26‐year follow‐up study of LNIT based on food frequency questionnaires in 1984‐1991 found that higher intake of grains, vegetables, beans, fruits and nuts was associated with a lower risk of gastric (cardia) cancer, heart disease and stroke mortality. 22 Apparently, the best approach to prevent EC is to adapt a healthy lifestyle, including a healthy diet, which will also reduce the risk of other chronic diseases.
3. STUDIES ON EC IN KENYA
EC (mostly ESCC) is ranked the third in its incidence among all cancers in Kenya. It ranked second after prostate cancer in men and third after breast cancer and cervical cancer in women. Yet, EC is the number one cancer killer in Kenya. In 2018, among 33 978 cancer deaths, 4351 (16%) were due to EC. 23
ESCC in Kenya shares many features of ESCC in China. The proportion of Kenyans living below the international poverty line was 46.8% in 2005/2006 and 36.1% in 2015/2016. Kenya belongs to a high incidence region known as the African EC corridor Rift Valley in Western and Central Kenya, similar to the North Central TMR in China. Kenya shares many recognized risk factors of ESCC, which include tobacco smoking, alcohol drinking, scalding hot food or drink, deficiency of micronutrients, food and drink containing carcinogens, familial history of cancer—especially ESCC. 2 , 4 Liquor (Chang'aa, containing >50% alcohol) is a popular social drink in rural Western Kenya where binge drinking is a very common practice. A recent epidemiological study showed that alcohol consumption, particularly of Chang'aa, contributes to half of the ESCC burden in western Kenya. 24 Human papilloma virus infection is not likely associated with the ESCC in Kenya, nor in other parts of the world. 25 In Kenya, 70% to 80% of cancer cases are diagnosed in late stages, and only a few hospitals treat EC patients. Self‐expandable metallic stents applied under endoscopy are the most frequently used form of palliative treatment.
Nevertheless, ESCC in Kenya has some unique features. NextGen sequencing (NGS) of human ESCC samples from Malawi (a neighboring country on the African EC corridor) identified a unique mutation signature characterized by C → A transversion and C → T transition, suggesting a unique etiology of ESCC in this region. 26 A large‐scale sequencing project supported by Cancer Research UK (Mutographs of Cancer) may provide important insights into the etiology.
3.1. Young EC
ESCC is rare among young people (<30 years) in most countries, 0.7% in northern China, 1% in northeastern Iran and 0.5% in the United States. Most ESCC patients are diagnosed at the age of >50 with an average of ~65 years old. However, in Kenya, one study in 2010 from Tenwek Hospital reported 6.3% (58/914 cases) occurred in patients <30 and 1% (9/914 cases) in patients <20. 27 An earlier report in 2002 from the same hospital reported even 11% (26/227 cases) of ESCC patients aged <30, and 2% (4/227 cases) aged <20. 28 The youngest case was at the age of 8 in this hospital. Unfortunately, ESCC in many young patients has already progressed to the late stage and long‐term survival is rare. 29
3.2. Kalenjin ethnicity
Kenya has 42 ethnic groups among which the Kalenjin (~12% of the population) have a strikingly high incidence of young ESCC. The Kalenjin constitute the largest community residing in the northern and southern parts of Rift Valley. The main economic activity in the region is farming. According to a study from Tenwek Hospital, the proportions of young ESCC among Kalenjin cases and non‐Kalenjin cases within the catchment area were 8.8% and 3.9%, respectively, and the proportions of young ESCC among Kalenjins and non‐Kalenjins outside the catchment area were 11.1% and 1.1%. Young ESCC was strongly associated with Kalenjin ethnicity, but not with a residency in the catchment area. However, being Kalenjin was not associated with an increased risk of ESCC if cases of all ages were pooled together. 27 Kalenjins may have unique genetic makeup and they are well known to produce many top marathon athletes. At Tenwek Hospital, in 60 cases of young ESCC, tobacco smoking was only observed in 15% (9/60) and alcohol drinking in 15% (9/60). Whereas among 49 young ESCC cases with family history data, 21 cases (43%) had a family history of ESCC, and 5 cases had multiple cases in the family. These data suggest that genetic factors and shared environmental factors, but not smoking and drinking, are important in causing ESCC in Kalenjins, in particular, young Kalenjins. 29
3.3. Mursik consumption
Fermented milk (Mursik) is a traditional and popular drink in Kenya. Many Kenyans do not like the flavor of fresh milk and ~80% Africans cannot tolerate milk due to lactose intolerance. 30 Every tribe has its own unique method of production. For example, in Kalenjin tribe, the inside of calabash gourds is first coated with charcoal dust. Whole cow milk is boiled, poured into the gourd, mixed with left‐over fermented milk (as a starter) and left for fermentation for several days at room temperature. Small quantities of cow blood may be added as a source of iron to facilitate microbial metabolism. Sometimes mursik may be stored for over a year before consumption. Refrigerators are not available in most of these households.
Most mursik starter culture collected from Kalenjin families contained mutagenic levels of acetaldehyde (>100 μM; sometimes >1000 μM) in addition to >100 mM of ethanol and potentially PAHs from the charcoal. 31 Regular consumption of mursik by young children may contribute to young ESCC in Kalenjins. According to a survey of 383 Kalenjin families in 2013, 32% of households fed their children (<5 years) with mursik with a mean quantity of 250 mL per week. 32 There is an incentive to let the children consume more mursik due to nutritional needs and a lack of other dairy products. It is well known that acetaldehyde induces nasal and respiratory tract squamous cell carcinoma in rats and hamsters when inhaled. 33 , 34 Mechanistically, as a highly reactive compound, acetaldehyde attacks nucleic acids and amino acids and causes DNA mutations and protein dysfunction. 35 , 36 , 37 However, it remains unclear how acetaldehyde exposure may cause ESCC in humans.
3.4. Scalding hot tea drinking
Tea (chai) drinking is part of the Kenyan lifestyle. Black tea with milk is usually served right away from a boiling tea kettle 2‐3 times every day. At Tenwek Hospital, a recent study showed that 99% (99/100) of local healthy participants preferred tea at the temperature of >65°C, a temperature classified by IARC as possibly carcinogenic (Group 2A) to the esophagus. 38 In Western Kenya, ESCC cases were 12.3 times more likely to drink scalding hot food compared to the controls. 39 It is known that hot water (65°C) significantly increased the number of tumors and the incidence of ESCC in NMBA‐exposed rats. 40
3.5. Khat chewing
Chewing khat (Catha edulis) leaves for the pleasurable stimulant effect is a widespread habit in East Africa. Khat is one of the most popular and lucrative, yet legal, drugs in Kenya. Other than its systemic effects, chronic khat chewing has been associated with periodontitis, leukoplakia, oral cancer, esophagitis and EC, 41 , 42 , 43 , 44 similar to betel quid (Group 2A) chewing in other regions.
3.6. Cultural taboo
In rural Kenya, most ESCC patients came for medical care at the late stage. In the surrounding communities that Tenwek Hospital serves, there is a false conception that medical treatment by surgery or esophageal stent causes death in months. Herbal therapy is often believed as the best treatment option. Patients are fear of being diagnosed and feel shameful in seeking medical care because EC is thought to be contagious. Thus, delay in hospital visits or diagnosis is a common problem.
3.7. Poverty
Lower socioeconomic status has long been associated with higher risk and mortality of ESCC according to many studies in different parts of the world including China. 18 , 19 , 20 Such health disparity can be expressed in sex differences, racial and ethnic differences and urban‐rural differences. In fact, poverty explains many risk factors of ESCC in Kenya.
4. RISK FACTORS AND PREVENTION OF ESCC: WORLD PERSPECTIVE
Numerous reports indicate that environmental and lifestyle factors, which are related to lower socioeconomic status, are the major causes of ESCC worldwide. 2 , 3 , 18 , 19 , 20 However, the relative importance of these factors may vary in different populations. In the Western world, tobacco and alcohol are more important risk factors for ESCC than in most high‐incidence areas in the developing countries. For example, in a recent cohort study in northeastern Iran, a 10‐year follow‐up showed that 76% of EC was attributable to the combination of the following risk factors: unpiped water, opium smoking, tooth loss, low intake of vegetables and fruits, hot tea and indoor pollution. 45 Because of the economic development, the diet may improve, but smoking and drinking may become more prevalent; the relative contribution of smoking and drinking to ESCC is expected to increase. These and other important etiological factors are summarized as follows.
4.1. Tobacco, alcohol and other unhealthy substances
Tobacco smoking and chewing are recognized as major risk factors for ESCC in many areas of the world. 2 , 46 , 47 , 48 The risk for ESCC is usually threefold to ninefold higher in current smokers compared to nonsmokers. Tobacco and tobacco smoke contain numerous carcinogens such as nitrosamines, PAHs and reactive aldehydes—existed in tobacco or produced during smoking.
Alcoholic beverage consumption has consistently been found to increase the risk of ESCC in most parts of the world, and IARC has causally linked alcohol to ESCC. 2 Alcohol is metabolically converted to acetaldehyde, which is recognized as a class I carcinogen. 36 , 37 , 38 , 49 Genetic polymorphisms associated with lower ALDH activity are associated with increased risk of ESCC. 50 Some alcoholic beverages and fermented foods also contain acetaldehyde. Tobacco and alcohol may interact synergistically to increase the risk of ESCC, 51 partially due to ethanol enhanced absorption of carcinogens by epithelial cells.
Betel quid chewing used to be common in the south and southeast Asian countries, such as India and Taiwan. 52 In Taiwan, lime (calcium hydroxide) was added, and chewing was said to produce stimulants. Upon chewing the nuts in the presence of lime, the polyphenolic compounds from betel are rapidly oxidized to produce reactive oxygen species that damage oral and esophageal epithelial cells to induce mutations and cancer. The IARC has confirmed betel quid as a risk factor for ESCC and betel chewing and smoking may have a synergistic effect to induce ESCC. 52 The association of khat chewing and opium smoking (produces PAHs) with ESCC was found in Kenya and Iran, respectively.
4.2. Dietary and nutritional factors
Studies in the TMR areas in northern China, Iran, South Africa and other areas indicated that ESCC was associated with a poor monotonous diet. The consumption of vegetables and fruits, legumes, meat and dairy products was usually low. Nutritional insufficiencies are highly suspected to be linked to ESCC. 10 , 11 , 12 , 53 , 54 , 55 , 56 , 57 Insufficiency of folic acid, which affects one‐carbon metabolism and may increase the promoter hypermethylation leading to the silencing of many tumor suppressors and receptors, may also be a contributing factor to ESCC, especially in individuals with low methylenetetrahydrofolate reductase activity. 58 Other micronutrient insufficiencies may also increase the risk for ESCC and other cancers, but the evidence is not as strong as that shown in the LNIT. 57 , 59
Even though mycotoxins and N‐nitroso compounds are highly suspected as carcinogens for ESCC, 3 strong evidence in humans is still lacking. The pickled vegetables from Linxian were shown or suspected to contain these types of carcinogens; however, there was limited evidence in humans to support the carcinogenicity of pickled vegetables. 60
4.3. Other risk factors
Eating foods and beverages at a high temperature has been demonstrated to be an etiological factor for oral cancer and EC. This includes studies in China, Kenya and Iran, and the temperature may exceed 70°C. 38 , 45 , 61 , 62 Hot temperature can cause thermal irritation of the esophageal epithelium, which may promote inflammation and enhance the action of chemical carcinogenesis in animal models. 63 The high incidence of EC in South America due to the consumption of hot maté may be due to the combined action of the high temperature and the carcinogenic PAHs in maté. 64 , 65
Early studies in Linxian suspected that poor oral hygiene leading to tooth loss might cause the individual to chew hard food insufficiently and irritate the esophageal mucosa. The association between tooth loss and EC was confirmed in a prospective study in Linxian. 66 Regular tooth brushing has been reported to be associated with reduced risk of ESCC in many studies including those conducted in China, Iran, Kenya and India. 2 A possible mechanism for the association is that poor oral hygiene could enhance inflammation associated with dental disease or affect oral microbiota. 2 , 67
4.4. Genetic susceptibility
Since 2010, there have been several papers on genome‐wide significant association study (GWAS) based on the analysis of single nucleotide polymorphism (SNP) assessing the possible association between high‐prevalence but low‐penetrance genetic variations with EC in China. Susceptibility loci at 10q23 associated with phospholipase c epsilon 1 (PLCE1), was found for both ESCC and GCC by two research groups, independently. 68 , 69 The functional importance of PLCE1 has been elucidated. 70 In rodent models, knockout of PLCE1 decreased susceptibility to chemically or genetically induced ESCC, skin or colon cancer. 71 , 72 , 73 Other studies and joint analyses of data from different GWAS revealed many other susceptibility loci. 74 , 75 , 76 , 77 Some of these variants, with low frequency or low risk association, may only have a limited role in ESCC. In combination with environmental factors, however, some of the genetic polymorphisms could play an important role. For example, variants at the 4q23 locus, which includes the alcohol dehydrogenase (ADH) cluster, each had a significant interaction with alcohol drinking in their association with ESCC risk. The known association of the aldehyde dehydrogenase 2 (ALDH2) locus on 12q22 with ESCC 50 was confirmed, and drinkers with both the ADH1B and ALDH2 risk alleles had a fourfold increased risk for ESCC in comparison to drinkers without these risk alleles. 75 An rs138478634‐GG phenotype, most likely due to a higher capacity of variant CYP26B1 to catabolites trans‐retinoic acid, a possible anti‐cancer nutrient, enhanced the risk for developing ESCC. 78 The risk may be further increased in individuals with low vitamin A nutritional status. More studies on the genetic susceptibility or heritability of ESCC are needed.
4.5. Prevention of EC—Healthy lifestyle and screening for precancerous lesions
We propose three approaches for the prevention of ESCC: (a) Remove or avoid risk factors, such as tobacco, betel quid, khat, opium, food that may contain carcinogens, excessive alcohol, hot food and beverages and poor oral hygiene. (b) Eat a healthy diet that provides sufficient nutrients. This is especially true for the populations in the high EC incidence areas, where consumption of vegetables and fruits are low. (c) Early detection and treatment can reduce the incidence and mortality of ESCC, as demonstrated in several screening studies in China. For example, in an endoscopic screen study of ESCC in Linzhou, residents age 40 to 69 years were recruited from communities with high rates of ESCC and 3319 volunteers were screened in the intervention group during 2000 to 2001. Those with dysplasia or occult cancer were treated. During the 10‐year follow‐up, 652 incident cases and 542 fatal ESCC were identified. The cumulative incidence and mortality in the intervention group (4.17% and 3.35%, respectively) were significantly lower than the control group (5.92% and 5.05%, respectively), which consisted of residents who did not receive the endoscopic screening in villages that were geographically adjacent to the intervention villages. 79
With economic development in China, endoscopic examination becomes affordable and endoscopic screening in a high incidence area is a practical approach. The National Cohort of Esophageal Cancer‐Prospective Cohort Study of Esophageal Cancer and Precancerous Lesions based on High‐Risk Populations (NCEC‐HRP), an open‐ended prospective cohort study of screening supported by the National Key R&D Program, was launched in eight areas around China in June 2017 and a total of 100 000 participants will be enrolled by December 2020; the screening protocol was published. 80 In a recently published large population‐based prospective study, 21 111 participants aged 40 to 69 years from three high‐risk areas in China were endoscopic screened in 2005 to 2009 and followed through 2016. Increasing grade of esophageal squamous dysplasia was found to be associated with increased risk for ESCC incidence and mortality. The study suggests that in high‐risk areas in China, dysplasia/carcinoma in situ and moderate dysplasia should receive therapy. The first screening should start at age 50 years old, and endoscopic surveillance intervals for moderate and mild dysplasia should take place in 3 and 5 years, respectively. 81
5. RESEARCH ON MOLECULAR ALTERATIONS AND TREATMENT OF ESCC
5.1. Early studies on molecular alterations
In the 1990s, in collaboration with Dr Lidong Wang of Henan Medical University, Chung S. Yang studied the molecular events in the genesis of ESCC using resected samples or biopsies obtained from hospitals in Linxian and nearby areas. We found TP53 mutation was a common and early event, occurring even in precancerous lesions. 82 , 83 , 84 , 85 Loss of heterozygosity of the tumor suppressor Rb gene, correlated with loss of Rb protein expression, was also a frequent event. 86 , 87 Hypermethylation was found to be a mechanism for silencing the expression of HGMT, RARβ, p15, p14, hMLH1, FHIT and HLA Class I genes and other genes. 88 , 89 , 90 , 91 It is interesting that during the progression of carcinogenesis, generally the frequency of hypermethylation of a particular gene increased and additional genes became hypermethylated. We hypothesized that in combination with TP53 mutation, the promoter hypermethylation profile of genes could be used as biomarkers of the progression of ESCC and would be useful to identify precancerous lesions and predict the development and progression of ESCC. However, we were unable to fully test this hypothesis.
5.2. Mutations and other genetic changes
With the advancement of the high‐throughput sequencing technology, large‐scale comprehensive analyses of ESCC have been conducted. Ohashi et al 92 summarized four large studies on ESCC in China. The most frequently mutated gene was TP53 (59%‐93%), confirming the results of many previous studies, and suggesting its important role in the development of ESCC. Mutation of genes that are involved in cell cycle regulation (CDKN2A, RB1, NFE2L2, CHEK1 and CHEK2) or cell differentiation (NOTCH1 and NOTCH3) have been detected at frequencies of 2% to 10%. Many cell cycle regulating genes were also amplified (CCND1 at 46.4%, CDK4‐CDK6 at 23.6% and MDM2 at 5.7%). EGFR overexpression in ESCC is often accompanied with amplifications in downstream signaling molecules, such as RAS and AKT pathways. 92 A more recent comprehensive molecular analysis of 164 ESCC samples found frequent genomic amplifications of CCND1, SOX2 and TP63. 93
Up to now, more than a dozen of NGS studies have been performed on ESCC from China and elsewhere, generated a lot of information on genetic alterations and shed light on etiology, carcinogenesis and therapeutic targets. 94 In addition to a list of genetic and epigenetic alterations as briefly described above, there is a much in‐depth understanding of molecular subtyping, 93 racial differences, 93 , 95 clonal evolution from precancerous lesions, 96 , 97 genetic heterogeneity and lymphatic metastasis. 98 , 99 Based on these studies, a list of potential therapeutic targets has been proposed for further studies: for example, receptor tyrosine kinases (EGFR, FGFR1, PI3K/AKT), nuclear factor erythroid‐derived 2 (NRF2) pathway, squamous differentiation mechanism (SOX2‐TP63, NOTCH), cell cycle control (TP53), WNT pathway, Hippo pathway, nuclear exportin (XPO1) and DNA repair pathway (BRCA1).
Of note is that somatic mutations of cancer‐related genes do not necessarily lead to the development of ESCC. Several groups discovered that histologically normal esophagus has a relatively higher mutation burden than most other tissues. The mutation burden was associated with both age and cell proliferation rate, suggesting that mutations in the esophagus accumulate over time with cell division. 100 Mutant clones can appear in early childhood, then increase in number and size with aging, and ultimately replace larger areas of the esophageal epithelium in the elderly. Exposure to environmental factors, heavy smoking and drinking, further speeds up this process. 101 Surprisingly, the prevalence of NOTCH1 mutations in the histologically normal esophagus was much higher than that in ESCC. It was suggested that the expansion of multiple NOTCH1‐mutated clones made epithelium prone to carcinogenesis by exposure to tobacco and alcohol. 101 , 102
5.3. Therapy for ESCC
While esophagectomy remains the cornerstone, endoscopic procedures for early lesions, neoadjuvant chemoradiotherapy and minimally invasive esophagectomy have been successfully and widely used for clinical management of ESCC. 103 , 104 , 105 However, early lymphatic metastasis in some cases remains a challenge and makes outcomes unpredictable. Patient‐derived cell lines, xenograft models, and three‐dimensional organoids have been tested for selecting sensitive chemotherapeutic agents and determining drug resistance before treatment. 106 , 107 , 108 For the clinical follow‐up, circulating tumor DNA analysis has emerged as a potentially valuable tool when used together with existing tools. 109
In the era of personalized medicine, targeted therapy is a very promising treatment modality when used in combination with other therapies. 110 , 111 Anti‐PDL1 immunotherapy with pembrolizumab (Keytruda) has been approved by US FDA for locally advanced or metastatic ESCC that has progressed after treatment with one or more lines of standard therapy. In patients with high levels of PD‐L1, a response rate of ~20% was achieved in two clinical trials. 112 , 113 Anti‐EGFR therapy failed to show much clinical benefit, although EGFR overexpression is commonly seen in ESCC. 114
Apart from these molecular targets shared with other cancers, some other targets are relatively specific for ESCC and require extensive basic research to develop corresponding drugs. Xiaoxin Luke Chen and associates have focused on NRF2 as a drug target for ESCC with NRF2 hyperactivation. 115 As a major cellular defense mechanism, the NRF2 signaling pathway is known to regulate the expression of antioxidant and detoxification enzymes. However, hyperactive NRF2 also helps cancer cells survive chemoradiation‐induced oxidative stress and accelerates drug metabolism thus contributing to chemoradioresistance and poor prognosis, as well as metabolic reprogramming. 116 , 117 Several NRF2 inhibitors have been identified through high‐throughput screening and these inhibitors have shown promising in vitro and in vivo efficacy in preclinical models.
6. CONCLUDING REMARKS
During the past 40 years, much has been learned about the etiology and molecular alterations in the development of ESCC associated with lifestyle and nutrition. These would help us design strategies for the prevention, early detection, diagnosis and treatment of EC. From a socioeconomic point of view, a public health approach in the prevention of EC is of fundamental importance, and it requires planning and commitment from the government and the people. Within the research community, a multidisciplinary approach is needed to unite all relevant specialties to deliver cost‐effective strategies against EC.
6.1. Healthy lifestyle and healthy diet
It is important to adopt a healthy lifestyle by avoiding the use of tobacco and other unhealthy substances, limiting the use of alcohol, maintaining good oral health and avoiding the consumption of hot food and beverages. Of equal importance is to eat a healthy diet that would provide sufficient nutrients, including eating plenty of vegetables and fruits. All of these depend on socioeconomic development so people can afford the needed food and have the information and household appliances to make healthy choices. As we learned from the example in Linzhou and other areas, as the economy developed, even though the occurrence of EC decreased, many other cancers (such as lung, colon and breast cancers), cardiovascular diseases and other diseases increased, possibly due to the increased consumption of cigarette, alcohol, fat, meat and sugar as well as reduced physical activity. Therefore, healthy lifestyle and healthy diet should be consistently emphasized.
6.2. Community outreach
Studies in China have proved that extensive community outreach is the essential foundation for the fight against EC. Health education through the education system, community activities and news media will be essential in delivering accurate and useful information on EC, avoiding risk factors, and recruiting subjects for screening and clinical trials.
6.3. Future research
Even with the many important discoveries in mechanisms and therapy on EC, many research questions remain unanswered and require extensive research. Epidemiological research has suggested modifiable risk factors; high‐quality research epidemiological research is still needed in developing countries to better clarify the etiology of EC. How to reduce cancer risk based on this information through intervention remains a big challenge and needs more research. GWAS has identified SNPs significantly associated with cancer risk; the functional roles of these SNPs and their interactions with environmental factors remain to be further elucidated. NGS has revealed potential drug targets and much research efforts are needed for developing targeted therapy. Further research is needed to increase our understanding of the development, biochemistry and molecular biology of the esophageal squamous epithelium and to develop effective means for drug screening.
6.4. International collaboration
International collaboration is essential for the progress of EC research. This is illustrated by the extensive collaboration between China and United States during the past decades. 118 These collaborations greatly enhanced our understanding of EC from prevention to treatment and helped train many researchers. It is encouraging that lately an African Esophageal Cancer Consortium was formed to help Africa in fighting EC. 119 OESO has organized a global education platform in esophagology to help developing countries and share experiences globally. More such collaborations are needed.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
ACKNOWLEDGMENTS
We would like to thank colleagues from China and Kenya who provided many of the information, and thank Vi Dan for her capable assistance in the preparation of this manuscript. The funding of the early work by Chung S. Yang was supported by International Agency for Research on Cancer and the US National Academy of Sciences. The Linxian Nutritional Intervention Studies was supported by a contract N01‐CP‐41019 from the National Cancer Institute to the CICAMS (subcontract to Chung S. Yang). The studies on molecular alterations of ESCC were supported by National Institutes of Health (NIH) grant CA65781 (to Chung S. Yang). Xiaoxin Luke Chen is supported by NIH grants (R01 CA244236, R21 AA028047, R01 DK113144, U54 MD012392, U54 AA019765, U54 CA156735).
Yang CS, Chen XL. Research on esophageal cancer: With personal perspectives from studies in China and Kenya. Int. J. Cancer. 2021;149:264–276. 10.1002/ijc.33421
Funding information National Cancer Institute, Grant/Award Number: N01‐CP‐41019; National Institutes of Health, Grant/Award Numbers: AA019765, AA028047, CA156735, CA244236, CA65781, DK113144, MD012392; US National Academy of Sciences; International Agency for Research on Cancer
Contributor Information
Chung S. Yang, Email: csyang@pharmacy.rutgers.edu.
Xiaoxin Luke Chen, Email: lchen@nccu.edu.
REFERENCES
- 1. Cancer IAfRo . World Fact Sheet, 2018.
- 2. Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154:360‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang CS. Research on esophageal cancer in China: a review. Cancer Res. 1980;40:2633‐2644. [PubMed] [Google Scholar]
- 4. Odera JO, Odera E, Githang'a J, et al. Esophageal cancer in Kenya. Am J Dig Dis (Madison). 2017;4:23‐33. [PMC free article] [PubMed] [Google Scholar]
- 5. Magee PN, Barnes JM. Carcinogenic nitroso compounds. Adv Cancer Res. 1967;10:163‐246. [DOI] [PubMed] [Google Scholar]
- 6. Lu SX, Li MX, Ji C, Wang MV, Wang VL, huang L. A new N‐nitroso compound, N‐3‐methylbutyl‐N‐1‐methylacetonylnitrosamine, in corn bread inoculated with fungi. Sci Sin. 1979;22:601‐608. [PubMed] [Google Scholar]
- 7. Cheng SJ, Sala M, Li MH, Wang MY, Pot‐Deprun J, Chouroulinkov I. Mutagenic, transforming and promoting effect of pickled vegetables from Linxian county, China. Carcinogenesis. 1980;1:685‐692. [DOI] [PubMed] [Google Scholar]
- 8. Lu SH, Camus AM, Tomatis L, Bartsch H. Mutagenicity of extracts of pickled vegetables collected in Linhsien County, a high‐incidence area for esophageal cancer in northern China. J Natl Cancer Inst. 1981;66:33‐36. [PubMed] [Google Scholar]
- 9. Yang CS, Miao J, Yang W, et al. Diet and vitamin nutrition of the high esophageal cancer risk population in Linxian, China. Nutr Cancer. 1982;4:154‐164. [DOI] [PubMed] [Google Scholar]
- 10. Blot WJ, Li JY, Taylor PR, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease‐specific mortality in the general population. J Natl Cancer Inst. 1993;85:1483‐1492. [DOI] [PubMed] [Google Scholar]
- 11. Taylor PR, Qiao YL, Abnet CC, et al. Prospective study of serum vitamin E levels and esophageal and gastric cancers. J Natl Cancer Inst. 2003;95:1414‐1416. [DOI] [PubMed] [Google Scholar]
- 12. Mark SD, Qiao YL, Dawsey SM, et al. Prospective study of serum selenium levels and incident esophageal and gastric cancers. J Natl Cancer Inst. 2000;92:1753‐1763. [DOI] [PubMed] [Google Scholar]
- 13. Yang CS, Luo P, Zeng Z, Wang H, Malafa M, Suh N. Vitamin E and cancer prevention: studies with different forms of tocopherols and tocotrienols. Mol Carcinog. 2020;59:365‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qiao YL, Dawsey SM, Kamangar F, et al. Total and cancer mortality after supplementation with vitamins and minerals: follow‐up of the Linxian General Population Nutrition Intervention Trial. J Natl Cancer Inst. 2009;101:507‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li JY, Taylor PR, Li B, et al. Nutrition intervention trials in Linxian, China: multiple vitamin/mineral supplementation, cancer incidence, and disease‐specific mortality among adults with esophageal dysplasia. J Natl Cancer Inst. 1993;85:1492‐1498. [DOI] [PubMed] [Google Scholar]
- 16. Wang SM, Taylor PR, Fan JH, et al. Effects of nutrition intervention on total and cancer mortality: 25‐year post‐trial follow‐up of the 5.25‐year Linxian Nutrition Intervention Trial. J Natl Cancer Inst. 2018;110:1229‐1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang H, Fang J, Jia X, et al. Chemopreventive effects of early‐stage and late‐stage supplementation of vitamin E and selenium on esophageal carcinogenesis in rats maintained on a low vitamin E/selenium diet. Carcinogenesis. 2011;32:381‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao P, Yang X, Suo C, et al. Socioeconomic status is inversely associated with esophageal squamous cell carcinoma risk: results from a population‐based case‐control study in China. Oncotarget. 2018;9:6911‐6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kou K, Baade PD, Guo X, et al. Area socioeconomic status is independently associated with esophageal cancer mortality in Shandong, China. Sci Rep. 2019;9:6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xie SH, Lagergren J. Social group disparities in the incidence and prognosis of oesophageal cancer. United European Gastroenterol J. 2018;6:343‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun D, Cao M, Li H, He S, Chen W. Cancer burden and trends in China: a review and comparison with Japan and South Korea. Chin J Cancer Res. 2020;32:129‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang JB, Fan JH, Dawsey SM, et al. Dietary components and risk of total, cancer and cardiovascular disease mortality in the Linxian Nutrition Intervention Trials cohort in China. Sci Rep. 2016;6:22619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oketch A. Cancer of food pipe is leading killer in Kenya. Daily Nation, November 27, 2018.
- 24. Menya D, Kigen N, Oduor M, et al. Traditional and commercial alcohols and esophageal cancer risk in Kenya. Int J Cancer. 2019;144:459‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. White RE, Mungatana C, Mutuma G, et al. Absence of human papillomavirus in esophageal carcinomas from southwestern Kenya. Dis Esophagus. 2005;18:28‐30. [DOI] [PubMed] [Google Scholar]
- 26. Liu W, Snell JM, Jeck WR, et al. Subtyping sub‐Saharan esophageal squamous cell carcinoma by comprehensive molecular analysis. JCI Insight. 2017;2:e98457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parker RK, Dawsey SM, Abnet CC, White RE. Frequent occurrence of esophageal cancer in young people in western Kenya. Dis Esophagus. 2010;23:128‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. White RE, Abnet CC, Mungatana CK, Dawsey SM. Oesophageal cancer: a common malignancy in young people of Bomet District, Kenya. Lancet. 2002;360:462‐463. [DOI] [PubMed] [Google Scholar]
- 29. Dawsey SP, Tonui S, Parker RK, et al. Esophageal cancer in young people: a case series of 109 cases and review of the literature. PLoS One. 2010;5:e14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Segal I. Physiological small bowel malabsorption of carbohydrates protects against large bowel diseases in Africans. J Gastroenterol Hepatol. 2002;17:249‐252. [DOI] [PubMed] [Google Scholar]
- 31. Nieminen MT, Novak‐Frazer L, Collins R, et al. Alcohol and acetaldehyde in African fermented milk mursik—a possible etiologic factor for high incidence of esophageal cancer in western Kenya. Cancer Epidemiol Biomarkers Prev. 2013;22:69‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kirui E, Nguka G. Factors that negatively influence consumption of traditionally fermented milk (Mursik) among preschool children (1‐5 years old) in Kapseret location ‐Uasin Gishu county, Kenya. Afr J Food Agric Nutr Dev. 2017;17:12295‐12310. [Google Scholar]
- 33. Feron VJ, Kruysse A, Woutersen RA. Respiratory tract tumours in hamsters exposed to acetaldehyde vapour alone or simultaneously to benzo(a)pyrene or diethylnitrosamine. Eur J Cancer Clin Oncol. 1982;18:13‐31. [DOI] [PubMed] [Google Scholar]
- 34. Woutersen RA, Appelman LM, Van Garderen‐Hoetmer A, Feron VJ. Inhalation toxicity of acetaldehyde in rats. III. Carcinogenicity study. Toxicology 1986;41: 213–31. [DOI] [PubMed] [Google Scholar]
- 35. Mizumoto A, Ohashi S, Hirohashi K, Amanuma Y, Matsuda T, Muto M. Molecular mechanisms of acetaldehyde‐mediated carcinogenesis in squamous epithelium. Int J Mol Sci. 2017;18:1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Garaycoechea JI, Crossan GP, Langevin F, et al. Alcohol and endogenous aldehydes damage chromosomes and mutate stem cells. Nature. 2018;553:171‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tan SLW, Chadha S, Liu Y, et al. A class of environmental and endogenous toxins induces BRCA2 haploinsufficiency and genome instability. Cell. 2017;169:1105‐18.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mwachiro MM, Parker RK, Pritchett NR, et al. Investigating tea temperature and content as risk factors for esophageal cancer in an endemic region of Western Kenya: validation of a questionnaire and analysis of polycyclic aromatic hydrocarbon content. Cancer Epidemiol. 2019;60:60‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Patel K, Wakhisi J, Mining S, Mwangi A, Patel R, Cancer E. The topmost cancer at MTRH in the Rift Valley, Kenya, and its potential risk factors. ISRN Oncol. 2013;2013:503249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li ZG, Shimada Y, Sato F, et al. Promotion effects of hot water on N‐nitrosomethylbenzylamine‐induced esophageal tumorigenesis in F344 rats. Oncol Rep. 2003;10:421‐426. [PubMed] [Google Scholar]
- 41. Balint EE, Falkay G, Balint GA. Khat ‐ a controversial plant. Wien Klin Wochenschr. 2009;121:604‐614. [DOI] [PubMed] [Google Scholar]
- 42. Kassie F, Darroudi F, Kundi M, Schulte‐Hermann R, Knasmüller S. Khat (Catha edulis) consumption causes genotoxic effects in humans. Int J Cancer. 2001;92:329‐332. [DOI] [PubMed] [Google Scholar]
- 43. Gunaid AA, Sumairi AA, Shidrawi RG, et al. Oesophageal and gastric carcinoma in the Republic of Yemen. Br J Cancer. 1995;71:409‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Soufi HE, Kameswaran M, Malatani T. Khat and oral cancer. J Laryngol Otol. 1991;105:643‐645. [DOI] [PubMed] [Google Scholar]
- 45. Sheikh M, Poustchi H, Pourshams A, et al. Individual and combined effects of environmental risk factors for esophageal cancer based on results from the Golestan cohort study. Gastroenterology. 2019;156:1416‐1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nasrollahzadeh D, Kamangar F, Aghcheli K, et al. Opium, tobacco, and alcohol use in relation to oesophageal squamous cell carcinoma in a high‐risk area of Iran. Br J Cancer. 2008;98:1857‐1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dar NA, Bhat GA, Shah IA, et al. Hookah smoking, nass chewing, and oesophageal squamous cell carcinoma in Kashmir, India. Br J Cancer. 2012;107:1618‐1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yuan JM, Knezevich AD, Wang R, Gao YT, Hecht SS, Stepanov I. Urinary levels of the tobacco‐specific carcinogen N'‐nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis. 2011;32:1366‐1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu Y, Chen H, Sun Z, Chen X. Molecular mechanisms of ethanol‐associated oro‐esophageal squamous cell carcinoma. Cancer Lett. 2015;361:164‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yokoyama T, Yokoyama A, Kato H, et al. Alcohol flushing, alcohol and aldehyde dehydrogenase genotypes, and risk for esophageal squamous cell carcinoma in Japanese men. Cancer Epidemiol Biomarkers Prev. 2003;12:1227‐1233. [PubMed] [Google Scholar]
- 51. Prabhu A, Obi KO, Rubenstein JH. The synergistic effects of alcohol and tobacco consumption on the risk of esophageal squamous cell carcinoma: a meta‐analysis. Am J Gastroenterol. 2014;109:822‐827. [DOI] [PubMed] [Google Scholar]
- 52. Wen CP, Tsai MK, Chung WS, et al. Cancer risks from betel quid chewing beyond oral cancer: a multiple‐site carcinogen when acting with smoking. Cancer Causes Control. 2010;21:1427‐1435. [DOI] [PubMed] [Google Scholar]
- 53. Wei WQ, Abnet CC, Qiao YL, et al. Prospective study of serum selenium concentrations and esophageal and gastric cardia cancer, heart disease, stroke, and total death. Am J Clin Nutr. 2004;79:80‐85. [DOI] [PubMed] [Google Scholar]
- 54. Steevens J, van den Brandt PA, Goldbohm RA, Schouten LJ. Selenium status and the risk of esophageal and gastric cancer subtypes: The Netherlands cohort study. Gastroenterology. 2010;138:1704‐1713. [DOI] [PubMed] [Google Scholar]
- 55. Cai X, Wang C, Yu W, et al. Selenium exposure and cancer risk: an updated meta‐analysis and meta‐regression. Sci Rep. 2016;6:19213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Seifried HE, McDonald SS, Anderson DE, Greenwald P, Milner JA. The antioxidant conundrum in cancer . Cancer Res. 2003;63:4295‐4298. [PubMed] [Google Scholar]
- 57. Mayne ST, Ferrucci LM, Cartmel B. Lessons learned from randomized clinical trials of micronutrient supplementation for cancer prevention. Annu Rev Nutr. 2012;32:369‐390. [DOI] [PubMed] [Google Scholar]
- 58. Wang LD, Guo RF, Fan ZM, et al. Association of methylenetetrahydrofolate reductase and thymidylate synthase promoter polymorphisms with genetic susceptibility to esophageal and cardia cancer in a Chinese high‐risk population. Dis Esophagus. 2005;18:177‐184. [DOI] [PubMed] [Google Scholar]
- 59. Angelo G, Drake VJ, Frei B. Efficacy of multivitamin/mineral supplementation to reduce chronic disease risk: a critical review of the evidence from observational studies and randomized controlled trials. Crit Rev Food Sci Nutr. 2015;55:1968‐1991. [DOI] [PubMed] [Google Scholar]
- 60. World Health Organization . World Health Organization International Agency for Research on Cancer Pickled Vegetables IARC Monographs Evaluating the Carcinogenic Risks to Humans. Vol 56. Geneva, Switzerland: World Health Organization; 1993:83‐113. [Google Scholar]
- 61. Gao YT, McLaughlin JK, Blot WJ, Ji BT, Dai Q, Fraumeni JF Jr. Reduced risk of esophageal cancer associated with green tea consumption. J Natl Cancer Inst. 1994;86:855‐858. [DOI] [PubMed] [Google Scholar]
- 62. Munishi MO, Hanisch R, Mapunda O, et al. Africa's oesophageal cancer corridor: do hot beverages contribute? Cancer Causes Control. 2015;26:1477‐1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yioris N, Ivankovic S, Lehnert T. Effect of thermal injury and oral administration of N‐methyl‐N'‐nitro‐N‐nitrosoguanidine on the development of esophageal tumors in Wistar rats. Oncology. 1984;41:36‐38. [DOI] [PubMed] [Google Scholar]
- 64. Loria D, Barrios E, Zanetti R. Cancer and yerba mate consumption: a review of possible associations. Rev Panam Salud Publica. 2009;25:530‐539. [DOI] [PubMed] [Google Scholar]
- 65. Kamangar F, Schantz MM, Abnet CC, Fagundes RB, Dawsey SM. High levels of carcinogenic polycyclic aromatic hydrocarbons in mate drinks. Cancer Epidemiol Biomarkers Prev. 2008;17:1262‐1268. [DOI] [PubMed] [Google Scholar]
- 66. Abnet CC, Qiao YL, Mark SD, Dong ZW, Taylor PR, Dawsey SM. Prospective study of tooth loss and incident esophageal and gastric cancers in China. Cancer Causes Control. 2001;12:847‐854. [DOI] [PubMed] [Google Scholar]
- 67. Gao S, Li S, Ma Z, et al. Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infect Agent Cancer. 2016;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang LD, Zhou FY, Li XM, et al. Genome‐wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies susceptibility loci at PLCE1 and C20orf54. Nat Genet. 2010;42:759‐763. [DOI] [PubMed] [Google Scholar]
- 69. Abnet CC, Freedman ND, Hu N, et al. A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinoma. Nat Genet. 2010;42:764‐767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wei L, Shao M, Zhao Y, et al. Functional role of PLCE1 intronic insertion variant associated with susceptibility to esophageal squamous cell carcinoma. Carcinogenesis. 2018;39:191‐201. [DOI] [PubMed] [Google Scholar]
- 71. Guo Y, Bao Y, Ma M, et al. Clinical significance of the correlation between PLCE 1 and PRKCA in esophageal inflammation and esophageal carcinoma. Oncotarget. 2017;8:33285‐33299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bai Y, Edamatsu H, Maeda S, et al. Crucial role of phospholipase Cepsilon in chemical carcinogen‐induced skin tumor development. Cancer Res. 2004;64:8808‐8810. [DOI] [PubMed] [Google Scholar]
- 73. Li M, Edamatsu H, Kitazawa R, Kitazawa S, Kataoka T. Phospholipase Cepsilon promotes intestinal tumorigenesis of Apc(Min/+) mice through augmentation of inflammation and angiogenesis. Carcinogenesis. 2009;30:1424‐1432. [DOI] [PubMed] [Google Scholar]
- 74. Wu C, Hu Z, He Z, et al. Genome‐wide association study identifies three new susceptibility loci for esophageal squamous‐cell carcinoma in Chinese populations. Nat Genet. 2011;43:679‐684. [DOI] [PubMed] [Google Scholar]
- 75. Wu C, Kraft P, Zhai K, et al. Genome‐wide association analyses of esophageal squamous cell carcinoma in Chinese identify multiple susceptibility loci and gene‐environment interactions. Nat Genet. 2012;44:1090‐1097. [DOI] [PubMed] [Google Scholar]
- 76. Abnet CC, Wang Z, Song X, et al. Genotypic variants at 2q33 and risk of esophageal squamous cell carcinoma in China: a meta‐analysis of genome‐wide association studies. Hum Mol Genet. 2012;21:2132‐2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wu C, Wang Z, Song X, et al. Joint analysis of three genome‐wide association studies of esophageal squamous cell carcinoma in Chinese populations. Nat Genet. 2014;46:1001‐1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chang J, Zhong R, Tian J, et al. Exome‐wide analyses identify low‐frequency variant in CYP26B1 and additional coding variants associated with esophageal squamous cell carcinoma. Nat Genet. 2018;50:338‐343. [DOI] [PubMed] [Google Scholar]
- 79. Wei WQ, Chen ZF, He YT, et al. Long‐term follow‐up of a community assignment, one‐time endoscopic screening study of esophageal cancer in China. J Clin Oncol. 2015;33:1951‐1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chen R, Ma S, Guan C, et al. The national cohort of esophageal cancer‐prospective cohort study of esophageal cancer and precancerous lesions based on high‐risk population in China (NCEC‐HRP): study protocol. BMJ Open. 2019;9:e027360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wei WQ, Hao CQ, Guan CT, et al. Esophageal histological precursor lesions and subsequent 8.5‐year cancer risk in a population‐based prospective study in China. Am J Gastroenterol. 2020;115:1036‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wang LD, Hong JY, Qiu SL, Gao H, Yang CS. Accumulation of p53 protein in human esophageal precancerous lesions: a possible early biomarker for carcinogenesis. Cancer Res. 1993;53:1783‐1787. [PubMed] [Google Scholar]
- 83. Gao H, Wang LD, Zhou Q, Hong JY, Huang TY, Yang CS. p53 tumor suppressor gene mutation in early esophageal precancerous lesions and carcinoma among high‐risk populations in Henan, China. Cancer Res. 1994;54:4342‐4346. [PubMed] [Google Scholar]
- 84. Wang LD, Shi ST, Zhou Q, et al. Changes in p53 and cyclin D1 protein levels and cell proliferation in different stages of human esophageal and gastric‐cardia carcinogenesis. Int J Cancer. 1994;59:514‐519. [DOI] [PubMed] [Google Scholar]
- 85. Shi ST, Feng B, Yang GY, Wang LD, Yang CS. Immunohistoselective sequencing (IHSS) of p53 tumor suppressor gene in human oesophageal precancerous lesions. Carcinogenesis. 1996;17:2131‐2136. [DOI] [PubMed] [Google Scholar]
- 86. Yang G, Zhang Z, Liao J, et al. Immunohistochemical studies on Waf1p21, p16, pRb and p53 in human esophageal carcinomas and neighboring epithelia from a high‐risk area in northern China. Int J Cancer. 1997;72:746‐751. [DOI] [PubMed] [Google Scholar]
- 87. Xing EP, Yang GY, Wang LD, Shi ST, Yang CS. Loss of heterozygosity of the Rb gene correlates with pRb protein expression and associates with p53 alteration in human esophageal cancer. Clin Cancer Res. 1999;5:1231‐1240. [PubMed] [Google Scholar]
- 88. Xing EP, Nie Y, Song Y, et al. Mechanisms of inactivation of p14ARF, p15INK4b, and p16INK4a genes in human esophageal squamous cell carcinoma. Clin Cancer Res. 1999;5:2704‐2713. [PubMed] [Google Scholar]
- 89. Xing EP, Nie Y, Wang LD, Yang GY, Yang CS. Aberrant methylation of p16INK4a and deletion of p15INK4b are frequent events in human esophageal cancer in Linxian, China. Carcinogenesis. 1999;20:77‐84. [DOI] [PubMed] [Google Scholar]
- 90. Nie Y, Yang G, Song Y, et al. DNA hypermethylation is a mechanism for loss of expression of the HLA class I genes in human esophageal squamous cell carcinomas. Carcinogenesis. 2001;22:1615‐1623. [DOI] [PubMed] [Google Scholar]
- 91. Wang Y, Fang MZ, Liao J, et al. Hypermethylation‐associated inactivation of retinoic acid receptor beta in human esophageal squamous cell carcinoma. Clin Cancer Res. 2003;9:5257‐5263. [PubMed] [Google Scholar]
- 92. Ohashi S, Miyamoto S, Kikuchi O, Goto T, Amanuma Y, Muto M. Recent advances from basic and clinical studies of esophageal squamous cell carcinoma. Gastroenterology. 2015;149:1700‐1715. [DOI] [PubMed] [Google Scholar]
- 93. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lin DC, Wang MR, Koeffler HP. Genomic and epigenomic aberrations in esophageal squamous cell carcinoma and implications for patients. Gastroenterology. 2018;154:374‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Deng J, Chen H, Zhou D, et al. Comparative genomic analysis of esophageal squamous cell carcinoma between Asian and Caucasian patient populations. Nat Commun. 2017;8:1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Liu X, Zhang M, Ying S, et al. Genetic alterations in esophageal tissues from squamous dysplasia to carcinoma. Gastroenterology. 2017;153:166‐177. [DOI] [PubMed] [Google Scholar]
- 97. Chen XX, Zhong Q, Liu Y, et al. Genomic comparison of esophageal squamous cell carcinoma and its precursor lesions by multi‐region whole‐exome sequencing. Nat Commun. 2017;8:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yan T, Cui H, Zhou Y, et al. Multi‐region sequencing unveils novel actionable targets and spatial heterogeneity in esophageal squamous cell carcinoma. Nat Commun. 2019;10:1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yuan W, Liu Z, Wang Y, et al. Clonal evolution of esophageal squamous cell carcinoma from normal mucosa to primary tumor and metastases. Carcinogenesis. 2019;40:1445‐1451. [DOI] [PubMed] [Google Scholar]
- 100. Yizhak K, Aguet F, Kim J, et al. RNA sequence analysis reveals macroscopic somatic clonal expansion across normal tissues. Science. 2019;364:eaaw0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yokoyama A, Kakiuchi N, Yoshizato T, et al. Age‐related remodelling of oesophageal epithelia by mutated cancer drivers. Nature. 2019;565:312‐317. [DOI] [PubMed] [Google Scholar]
- 102. Martincorena I, Fowler JC, Wabik A, et al. Somatic mutant clones colonize the human esophagus with age. Science. 2018;362:911‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Triantafyllou T, Wijnhoven BPL. Current status of esophageal cancer treatment. Chin J Cancer Res. 2020;32:271‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Fatehi Hassanabad A, Chehade R, Breadner D, Raphael J. Esophageal carcinoma: towards targeted therapies. Cell Oncol (Dordr). 2020;43:195‐209. [DOI] [PubMed] [Google Scholar]
- 105. Yang H, Liu H, Chen Y, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open‐label clinical trial. J Clin Oncol. 2018;36:2796‐2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Su D, Zhang D, Jin J, et al. Identification of predictors of drug sensitivity using patient‐derived models of esophageal squamous cell carcinoma. Nat Commun. 2019;10:5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lee NP, Chan CM, Tung LN, Wang HK, Law S. Tumor xenograft animal models for esophageal squamous cell carcinoma. J Biomed Sci. 2018;25:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kijima T, Nakagawa H, Shimonosono M, et al. Three‐dimensional organoids reveal therapy resistance of esophageal and oropharyngeal squamous cell carcinoma cells. Cell Mol Gastroenterol Hepatol. 2019;7:73‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Azad TD, Chaudhuri AA, Fang P, et al. Circulating tumor DNA analysis for detection of minimal residual disease after chemoradiotherapy for localized esophageal cancer. Gastroenterology. 2020;158:494‐505.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kang X, Chen K, Li Y, Li J, D'Amico TA, Chen X. Personalized targeted therapy for esophageal squamous cell carcinoma. World J Gastroenterol. 2015;21:7648‐7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Liu Y, Xiong Z, Beasley A, D'Amico T, Chen XL. Personalized and targeted therapy of esophageal squamous cell carcinoma: an update. Ann N Y Acad Sci. 2016;1381:66‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Shah MA, Kojima T, Hochhauser D, et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: the phase 2 KEYNOTE‐180 study. JAMA Oncol. 2019;5:546‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Yamamoto S, Kato K. Immuno‐oncology for esophageal cancer. Future Oncol. 2020. 10.2217/fon-2020-0545. [DOI] [PubMed] [Google Scholar]
- 114. Dutton SJ, Ferry DR, Blazeby JM, et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double‐blind, placebo‐controlled randomised trial. Lancet Oncol. 2014;15:894‐904. [DOI] [PubMed] [Google Scholar]
- 115. Ma S, Paiboonrungruan C, Yan T, Williams KP, Major MB, Chen XL. Targeted therapy of esophageal squamous cell carcinoma: the NRF2 signaling pathway as target. Ann N Y Acad Sci. 2018;1434:164‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Fu J, Xiong Z, Huang C, et al. Hyperactivity of the transcription factor Nrf2 causes metabolic reprogramming in mouse esophagus. J Biol Chem. 2019;294:327‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cui Y, Chen H, Xi R, et al. Whole‐genome sequencing of 508 patients identifies key molecular features associated with poor prognosis in esophageal squamous cell carcinoma. Cell Res. 2020;30:902‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Klingelhofer D, Zhu Y, Braun M, Bruggmann D, Schoffel N, Groneberg DA. A world map of esophagus cancer research: a critical accounting. J Transl Med. 2019;17:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Van Loon K, Mwachiro MM, Abnet CC, et al. The African esophageal cancer consortium: a call to action. J Glob Oncol. 2018;4:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]


