SUMMARY
We previously tested HER2-targeted antibody drug conjugates (ADC) in immunocompromised (SCID) mice, precluding evaluation of host immunity, impact on cancer stem cells (CSCs), and potential benefit when combined with PD-L1 blockade. In this study, we tested HER2-targeted ADC in two immunocompetent mouse tumor models. HER2-targeted ADC specifically inhibited the growth of HER2-expressing tumors, prolonged animal survival, and reduced HER2+ and PD-L1+ cells. ADC plus anti-PD-L1 antibody augmented therapeutic efficacy, modulated immune gene signatures, increased the number and function of CD3+ and CD19+ tumor-infiltrating lymphocytes (TILs), induced tumor antigen specific immunological memory, stimulated B cell activation, differentiation, and IgG1 production both systemically and in the tumor microenvironment. In addition, ADC therapy modulated T cell subsets and their activation in TILs. Furthermore, HER2-targeted ADC reduced the number and tumorigenicity of ALDHhi CSCs. This study demonstrates that HER2-targeted ADC effectively targets ALDHhi CSCs and this effect is augmented by co-administration of anti-PD-L1 antibody.
eTOC Blurb
A major challenge for current cancer management is the relapse of the disease due to CSCs that are resistant to traditional therapies. Here, Xia and colleagues show that HER2-targeted ADC targets both bulk tumor cells and ALDHhigh CSCs via inducing anti-tumor cellular and humoral immune responses and immunological memory.

INTRODUCTION
Aberrant expression and activation of human epidermal growth factor receptor 2 (HER2) is found in 20%-25% of breast cancer (BC) patients and is associated with aggressive disease and historically had a poor prognosis (Roskoski, 2014a; Roskoski, 2014b; Takada et al., 2018; Yarden and Sliwkowski, 2001). However, development of HER2 targeted therapies over the past several decades has had a dramatic effect on improving patient outcome. The efficacy of HER2 antibodies such as trastuzumab were improved by conjugating them to cytotoxic payloads. The first generation of HER2-targeted antibody drug conjugates (ADCs), Ado-trastuzumab emtansine (T-DM1), prolonged overall survival of patients by 5-6 months with an objective response rate of 44% (Ma et al., 2016). Second generation HER2-targeted ADCs include DS-8201 in which anti-HER2 antibody is conjugated to an active metabolite of irinotecan, a topoisomerase I inhibitor. Among 57 evaluable patients, DS-8201 demonstrated a confirmed overall response rate (ORR) of 61.4% (35 of 57 patients) and a disease control rate of 94.7% (54 of 57 patients) in patients with HER2-positive metastatic BC previously treated with T-DM1(Baselga et al., 2018). Our group previously reported that the biparatopic HER2-targeted ADC, MEDI4276, demonstrated significant activity toward both HER2+ and HER2low BCs, including T-DM1 resistant tumors (Li et al., 2016). However, that study was performed in immunocompromised (SCID) mice, which precluded evaluation of the role of host immunity in mediating antitumor effects. These models also did not permit assessment of addition of immune checkpoint blockade to ADCs efficacy.
Immune checkpoint molecules play critical roles in balancing host immunity. PD-L1 expression on tumor cells can result in the escape of the tumor from immune attack by converting PD-1+ immune effector cells into anergic cells (Xu-Monette et al., 2017). We reported that PD-L1 blockade significantly enhanced the therapeutic efficacy of cancer stem cell (CSC)-based dendritic cell (DC) vaccines in the adjuvant setting after surgical removal of an established head and neck cancer (Hu et al., 2016). Tumor cells with PD-L1 expression may have a reduced immunogenic response to ADC (D’Amico et al., 2019). Therefore, we hypothesize that the efficacy of HER2-targeted ADC could be enhanced by PD-1/PD-L1 blockade in immunocompetent hosts. Currently, the role of host immunity invoked by biparatopic ADCs is largely unknown, due to the testing of HER2-targeted ADCs in immunocompromised hosts (Li et al., 2016). In the present study, we established two human HER2-expressing murine BC models in syngeneic immunocompetent mice and evaluated both host cellular and humoral anti-tumor immune responses modulated by biparatopic anti-HER2 ADC administration.
In addition to studying the role of anti-HER2 ADC in modulating the immune system, we examined the capacity of these agents to target CSCs. There is growing evidence that CSCs are responsible for cancer relapse and metastasis (Huang and Rofstad, 2017; Iqbal et al., 2016; Schulenburg et al., 2015). The resistance of CSCs to standard-of-care therapies may play an important role in cancer relapse (Guo, 2014; Luo et al., 2015). Experimental evidence has indicated that HER2 serves as an important regulator of CSCs in BC (Baselga et al., 2018; Huang et al., 2016; Nami and Wang, 2017). Our group previously described that HER2 overexpression increases, and HER2 blockade decreases, the CSC population in BC cell lines and mouse xenografts (Ithimakin et al., 2013). Also, HER2-overexpressing CSCs are resistant to a number of chemotherapy drugs (Ithimakin et al., 2013; Liu et al., 2018). These findings suggest that targeting HER2 may impact the number and function of CSCs, and may therefore benefit BC patients by reducing the number and the bioactivity of BC stem cells (BCSCs) (Wang et al., 2013). However, the effect of HER2-targeted ADCs and the role of host immune responses on CSCs has not yet been described. In the present study, we assessed host anti-CSC immunity conferred by biparatopic ADC administration during the treatment of HER2-expressing murine breast tumors in syngeneic immunocompetent hosts as well as the efficacy of combining HER2-targeted ADC with PD-1/PD-L1 blockade.
RESULTS
Murine breast cancer cell lines transduced with human HER2 are capable of generating tumors in immunocompetent Balb/c mice
To test whether HER2-targeted ADC could induce host immunity, we first established immunocompetent animal models in which human HER2-expressing murine tumor cells can form tumors. To this end, we used mouse breast tumor D2/F2 and the triple-negative BC (TNBC) 4T1 with enforced expression of human HER2, designated D2F2/E2 and HER2-4T1, respectively. More than 65% of the D2F2/E2 cells and more than 75% of the HER2-4T1 cells expressed HER2 (Figure 1A).
Figure 1. Human HER2-transduced murine cell lines generated tumors in wild type Balb/c mice.

(A) Both transduced D2F2/E2 & HER2-4T1 cell lines express HER2. Human HER2 surface expression on transduced D2F2/E2 and HER2-4T1 cell lines vs. parental D2F2 and 4T1 cell lines. Numbers represent the percentage of HER2 positive tumor cells in total cultured cells. One of three experiments is shown.
(B) Growth of HER2 transfected murine cells in immunocompetent mice. Equal numbers of D2F2 and D2F2/E2 cells were inoculated in the opposite fat pads of the same mouse. The 4T1 vs. HER2-4T1 tumor model was constructed in the same way. D2F2/E2 and HER2-4T1 cells produced progressively growing tumors in the wild type Balb/c mice.
(C) Harvested HER2-transduced tumors vs. parental tumors. One of two experiments is shown.
(D) D2F2/E2 and HER2-4T1 fresh tumor cells harvested from B express HER2. Numbers represent the percentage of HER2 positive tumor cells of total tumor cells. One of three experiments is shown.
(E) Confirmation of HER2 expression on D2F2/E2 and HER2-4T1 fresh tumor cells by immunohistochemistry as compared to fresh D2F2 and 4T1 parental tumors respectively.
Equal numbers of D2F2 and HER2-expressing D2F2/E2 cells were injected into opposite fat pads of the same syngeneic Balb/c mouse, and tumor growth was monitored. At a higher cell number, (3x106), the growth of D2F2/E2 tumors was delayed compared to D2F2; whereas at a lower cell number (1x106), the growth of D2F2/E2 tumors was identical to that of D2F2 tumors (Figure 1B). In the HER2-4T1 model, at both the higher (3x106) and lower (1x106) cell inoculation, the growth of human HER2-expressing HER2-4T1 tumors was delayed compared to 4T1, but still generated large tumors (Figure 1B). These experiments showed that both human HER2-expressing D2F2/E2 and HER2-4T1 cells were immunologically tolerated and could generate tumors in immunocompetent Balb/c mice, thus allowing us to evaluate host anti-tumor and anti-CSC immunity in subsequent experiments.
The percentage of HER2-positive cells was assessed in harvested fresh tumors as shown in Figure 1C to confirm that the tumor models could be used for HER2-targeted ADC therapy. Flow cytometry analysis revealed that >60% of the cells were HER2+ in freshly harvested D2F2/E2 tumors, and nearly 25% were HER2+ in freshly harvested HER2-4T1 tumors (Figure. 1D). The expression of HER2 protein in D2F2/E2 and HER2-4T1 tumor tissue was confirmed by IHC (Figure. 1E). Of note, the human HER2-targeted ADC does not cross-react with or recognize murine HER2, requiring the use of these human HER2-expressing tumor models for human HER2-targeted ADC evaluation (Li et al., 2016). Together, these experiments validated the use of these two animal models to evaluate the efficacy, specificity, and host immune responses of human HER2-targeted ADC in immunocompetent mice.
Human HER2-targeted ADC effectively and specifically inhibits the growth of HER2-exressing tumors in immunocompetent hosts
Experiments were performed to test the efficacy and specificity of HER2-targeted ADC in the immunocompetent D2F2/E2 and HER2-4T1 tumor models. The structures of ADC and control antibodies are shown in Figure 2A. MEDI4276 ADC is a biparatopic anti-HER2 antibody conjugated with the AZ1508 tubulysin payload, which releases the AZ9185 warhead when taken up by cells and processed in the lysosome. Controls included unconjugated MEDI4276 without AZ1508 tubulysin and mixed with AZ9185 at a ratio of 1:4 and an isotype control IgG recognizing an irrelevant viral antigen conjugated with the same payload as MEDI4276.
Figure 2. HER2-targeted ADC demonstrated efficacy and specificity in immunocompetent hosts.

(A) The structure of ADC and controls.
(B) HER2-targeted ADC significantly inhibited D2F2/E2 tumor growth in a dose-dependent manner. D2F2/E2 tumors were treated as indicated. One of two representative experiments is shown. Data represent mean ± SEM.
(C) HER2-targeted ADC specifically inhibited D2F2/E2 tumor growth, but not parental D2F2 tumors. Both D2F2/E2 and D2F2 tumors were treated as indicated. One of two experiments is shown. Data represent mean ± SEM.
(D) The efficacy and specificity of the HER2-targeted ADC were confirmed in the HER2-4T1 tumor model vs. parental 4T1 tumors as shown. One of two experiments is shown. Data represent mean ± SEM.
Balb/c mice were inoculated into the mammary fat pad with human HER2 transduced D2F2/E2 cells. After 14 days, tumor-bearing mice were administered 1 mg or 3 mg/kg of ADC or control Abs intravenously once a week for a total of three weeks as indicated in Figure 2B. HER2-targeted ADC significantly inhibited D2F2/E2 tumor growth in a dose-dependent manner (Figure 2B). In separate experiments, Balb/c mice were inoculated into the mammary fat pads with D2F2/E2 cells or D2E2 cells respectively. Tumor-bearing mice were then treated with ADC or controls as indicated in Figure 2C at 3 mg/kg once a week for four weeks. While HER2-targeted ADC significantly inhibited D2F2/E2 tumor growth, it had no effect on the parental D2F2 tumors that were not transduced with human HER2, demonstrating the specificity of the HER2-targeted ADC for human HER2-expressing tumors (Figure 2C).
We validated these results in a second tumor model, HER2-4T1. Human HER2-transduced 4T1 tumor cells or parental 4T1 cells were injected into the mammary fat pad of Balb/c mice and ADC or controls administered as indicated in Figure 2D. HER2-targeted ADC significantly inhibited HER2-4T1 tumor growth, but had no effect on the growth of parental 4T1 tumors (Figure 2D), confirming the efficacy and specificity of the ADC for human HER2-expressing tumors. Together, data obtained in these two models support the conclusion that HER2-targeted ADC could effectively and specifically inhibit the growth of HER2-expressing tumors in immunocompetent hosts.
HER2-targeted ADC treatment reduces the number of HER2 positive D2F2/E2 cells and ALDHhi D2F2/E2 cells which are highly tumorigenic CSCs
To investigate the efficacy of HER2 targeting by HER2-targeted ADC, we determined the percent of HER2-expressing D2F2/E2 cells in residual tumors harvested from each treated group in Figure 2B. HER2-expressing cells were significantly reduced from 63.4% in the PBS-treated control group to 45.7% and 39% in groups treated with 1 mg/kg or 3 mg/kg ADC, respectively (Figure 3A).
Figure 3. The reduction of HER2-expressing cells and ALDHhi cells after HER2-targeted ADC treatment.

(A) HER2-targeted ADC significantly reduced HER2-expressing tumor cells in vivo. HER2-expressing tumor cells in tumor cell suspensions prepared from tumors harvested after treatment with ADC and controls from Figure 2B. One of two experiments is shown.
(B) HER2-targeted ADC significantly reduced ALDHhi cells in vivo. ALDHhi tumor cells were assessed in freshly harvested tumors after treatment with ADC and controls from Figure 2B.
(C) Tumorigenicity of ALDHhi D2F2/E2 cells in syngeneic immunocompetent Balb/c mice.
(D) Pictures of tumors from mice engrafted with 10,000 ALDHhi vs. 10,000 ALDHlow D2F2/E2 cells.
We have previously utilized high ALDH activity as a marker to isolate CSCs both from human (Ginestier et al., 2007) and mouse tumors (Ning et al., 2012). Other investigators have used ALDH as a marker to isolate and characterize CSCs from nearly 20 different tumor types (Cheung et al., 2007; Clay et al., 2010; Deng et al., 2010; Ginestier et al., 2007; Jiang et al., 2009; Kim et al., 2011; Li et al., 2010; Ma et al., 2008; Ning et al., 2012; Prince et al., 2016; Rasper et al., 2010; Shenoy et al., 2012; Shimamura et al., 2014; Su et al., 2010; Wang et al., 2011; Wang et al., 2017). To evaluate the effect of HER2-targeted ADC on ALDHhi cells, we measured ALDHhi cells in residual tumors harvested in each treated group from Figure 2B. Treatment with 1 mg/kg or 3mg/kg ADC significantly reduced ALDHhi tumor cells from 5.6% in the PBS-treated group to 1.9% and 1.3% respectively (Figure 3B).
We evaluated the tumorigenicity of ALDEFLUOR+ (ALDHhi) D2F2/E2 cells as we previously described (Ning et al., 2012). Briefly, an equal number, e.g. 500 or 2,500 or 10,000, of ALDHhi D2F2/E2 cells and ALDHlow D2F2/E2 cells were injected into the opposite mammary fat pads of the same mouse, and tumor growth monitored. Whereas 10,000 ALDHhi D2F2/E2 cells generated large tumors, an equal number of ALDHlow D2F2/E2 cells injected into the opposite side of the same mouse developed much smaller tumors (Figure 3C). Separate mice were injected with lower numbers of ALDHhi D2F2/E2 cells as indicated. As few as 500 injected ALDHhi cells formed large tumors. In contrast, as many as 10,000 ALDHlow cells failed to generate larger tumors in 66 days (Figure 3C and 3D). These results indicate that ALDHhi D2F2/E2 cells are highly tumorigenic compared to ALDHlow D2F2/E2 cells, thus reflecting their tumor initiating potential by the considered “gold standard” assay for CSCs. Similarly, we characterized the stemness of ALDHhi 4T1 cells (data not shown).
Collectively, these data indicate that HER2-targeted ADC treatment reduced the number of HER2 positive D2F2/E2 cells as well as the proportion of ALDHhi D2F2/E2 CSCs.
Anti-PD-L1 mAb significantly augments the therapeutic efficacy of HER2-targeted ADC
We recently reported that co-administration of anti-PD-L1 significantly augmented the efficacy of a CSC-based vaccine in a murine squamous cell cancer model (Hu et al., 2016). To investigate the feasibility of this approach, we first examined PD-L1 expression on D2F2/E2 and HER2-4T1 fresh tumor cells and cell lines. More than 3% of PD-L1+ cells were detected in D2F2/E2 cell line, and the PD-L1+ cells increased to nearly 8% in fresh tumor suspension (Figure 4A). Similarly, about 3% of PD-L1+ cells were found in the HER2-4T1 cell line, and the PD-L1+ population increased to almost 9% in HER2-4T1 fresh tumor suspension (Figure 4A). These data support the potential utility of adding PD-L1 blockade to HER2-targeted ADC therapy.
Figure 4. The anti-tumor efficacy of HER2-targeted ADC was enhanced by anti-PD-L1 administration in HER2-expressing tumor-bearing immunocompetent mice.

(A) PD-L1 expression in D2F2/E2 and HER2-4T1 cell lines vs. freshly dissociated tumor cells. One of three experiments is shown.
(B) HER2-targeted ADC inhibited tumor growth from injected ALDHhi D2F2/E2 CSCs. ALDHhi D2F2/E2 cells were injected and treated as indicated. One of two experiments is shown. Data represent mean ± SEM.
(C) HER2-targeted ADC combined with anti-PD-L1 significantly inhibited D2F2/E2 tumor growth. D2F2/E2 cells were injected and treated with ADC and/or anti-PD-L1 Ab as indicated. One of two experiments is shown. Data represent mean ± SEM.
(D) The synergistic efficacy of HER2-targeted ADC with anti-PD-L1 was verified in the HER2-4T1 tumor model. On day 14, after HER2-4T1 cell injection, animals were treated with ADC and/or anti-PD-L1 as indicated. One of two experiments is shown. Data represent mean ± SEM.
(E) Animal survival was significantly prolonged by HER2-targeted ADC and ADC + anti-PD-L1 treatment. Balb/c mice were inoculated with ALDHhi D2F2/E2 cells on day 0 and treatment started on the same day with HER2-targeted ADC alone, or in combination with anti-PD-L1 as described in Methods. Survival was recorded up to day 100.
(F) Tumor re-challenge demonstrated immunological memory formation. Tumor-free animals were generated from D2F2/E2 tumor-bearing mice subjected to HER2-targeted ADC or ADC + anti-PD-L1 therapy. On day 35, these tumor-free animals were re-challenged with the relevant tumor D2F2/E2 (3x105 cells/mouse) or irrelevant tumor WT 4T1 cells (3x105 cells/mouse) respectively in the mammary fat pad. Tumor size was then recorded for 30 days post re-challenge with D2F2/E2 or WT 4T1 cells. Age-matched naïve Balb/c mice were used as additional controls. Brackets indicate the previous treatments, and parentheses represent the tumor cells used for re-challenge.
To test the direct effect of HER2-targeted ADC and/or anti-PD-L1 on ALDHhi D2F2/E2 CSCs, we injected ALDHhi D2F2/E2 cells instead of unselected D2F2/E2 cells and started treatment with HER2-targeted ADC and/or anti-PD-L1 on the same day as ALDHhiD2F2/E2 cell inoculation (Figure 4B). We found that the HER2-targeted ADC dosed at 1 mg/kg almost completely inhibited D2F2/E2 tumor growth even without anti-PD-L1 treatment. Anti-PD-L1 monotherapy failed to depress tumor growth and showed no additive or synergistic effect when combined with the ADC (Figure 4B). These experiments indicate that the HER2-targeted ADC directly limited the tumorigenicity of ALDHhi D2F2/E2 CSCs when HER2-targeted ADC was administrated at the same time when ALDHhi D2F2/E2 cells were inoculated.
To examine the potential synergistic effect of HER2-targeted ADC with anti-PD-L1 in established tumors, anti-HER2 ADC was injected 14 days after D2F2/E2 tumor cell inoculation, once a week for 4 weeks, followed by anti-PD-L1 administration as indicated in Figure 4C. ADC or anti-PD-L1 monotherapy each showed anti-tumor activity (Figure 4C p<0.05 compared with PBS control). Importantly, the combination group demonstrated more potent tumor growth inhibition (p<0.01 compared with each monotherapy). This synergistic effect was confirmed in the HER2-4T1 tumor model, where the anti-tumor efficacy of HER2-targeted ADC was significantly (p<0.01) enhanced by anti-PD-L1 administration (Figure 4D).
To assess the long-term therapeutic effect of HER2-targeted ADC with vs. without anti-PD-L1, we monitored mice for 100 days using the same treatment protocol as in Figure 4B. As demonstrated in Figure 4E, HER2-targeted ADC alone and ADC + anti-PD-L1 treatments both significantly prolonged animal survival compared to PBS controls (p=0.0046 and p=0.0126 respectively). Combination therapy with HER2-targeted ADC + anti-PD-L1 significantly improved survival compared to anti-PD-L1 monotherapy (p=0.0136). However, at day 100, we found no significant difference between HER2-targeted ADC treatment alone vs. ADC + anti-PD-L1 immunotherapy (p=0.7015). This is consistent with the tumor growth data as presented in Figure 4B.
In addition, we investigated the immunological memory formation using a tumor re-challenge model in tumor-free animals generated as described in Methods. Tumor-free mice from both ADC alone and ADC + anti-PD-L1 dual therapy groups were re-challenged with either relevant D2F2/E2 cells or irrelevant WT 4T1 (HER2 negative) cells. As shown in Figure 4F, while tumor grew rapidly in all 3 groups after challenge with the irrelevant 4T1 tumor cells, HER2-targeted ADC-treated tumor-free animals re-challenged with relevant D2F2/E2 demonstrated significantly slower tumor growth compared with the age-matched naive mice (p<0.0001). Furthermore, this protection was significantly (p=0.0078) enhanced in ADC + anti-PD-L1-treated tumor-free animals compared to ADC-treated tumor-free animals, demonstrating HER2-specific immunological memory conferred by HER2-targeted ADC and ADC + anti-PD-L1 therapy.
Together, these data clearly show that immune checkpoint blockade with anti-PD-L1 significantly augmented the therapeutic efficacy of HER2-targeted ADC in immunocompetent hosts in established tumors engrafted with HER2-expression. This was revealed in both D2F2/E2 and HER2-4T1 tumor models. In addition, anti-PD-L1 significantly enhanced the immunological memory conferred by HER2-targeted ADC.
HER2-targeted ADC modulates immune response gene signatures in the tumor microenvironment
In order to evaluate the host anti-tumor immunity induced by the HER2-targeted ADC, we measured the expression of 92 genes related to immune responses using fresh tumor tissues harvested from the animals subjected to PBS, ADC, anti-PD-L1, or ADC plus anti-PD-L1 treatments (Figure 5A). ADC plus anti-PD-L1 treatment up-regulated genes related to T cell activation including CD28, CD3e, CD8a, CXCL11, GZMB, IL-2, IL-2RA, ICOS, Perforin1, SELP, STAT1/4, TBX21, TNFrsf18, and antigen presentation related MHC-II molecules H2-Eb1 and H2-Ea, compared with the treatment with ADC alone (Figure 5A, 5B). Likewise, the expression of STAT1, GZMB, CXCL11, CD28, SELP, Perforin1, IL-2, IL-2RA, ICOS, H2-Eb1, and H2-Ea genes in the ADC plus anti-PD-L1 combination group was higher than that in the anti-PD-L1 monotherapy group (Figure 5A, 5B). Thus, the ADC plus anti-PD-L1 combination significantly enhanced the expression of genes related to immune responses compared to monotherapies. In contrast, ADC alone inhibited the expression of immune-suppressive factors including IL-6, IL-4, IL-1b, IL-1a, IL-17a, IL-10, and depressed tumor metastasis-related genes, such as VCAM1 and ICAM1 (Figure 5A, 5C). The combined treatment further decreased the expression of HMOX1, IL-10, IL-1a, IL-4, IL-6, compared with the treatment with ADC alone (Figure 5A, 5C). These results demonstrate that the strategy of a HER2 targeted ADC combined with inhibition of PD-L1 activates T lymphocytes while simultaneously decreasing the expression of genes that regulate immunosuppressive pathways in the tumor microenvironment.
Figure 5. HER2-targeted ADC alone or combined with anti-PD-L1 modulated gene signatures involved in immune responses.
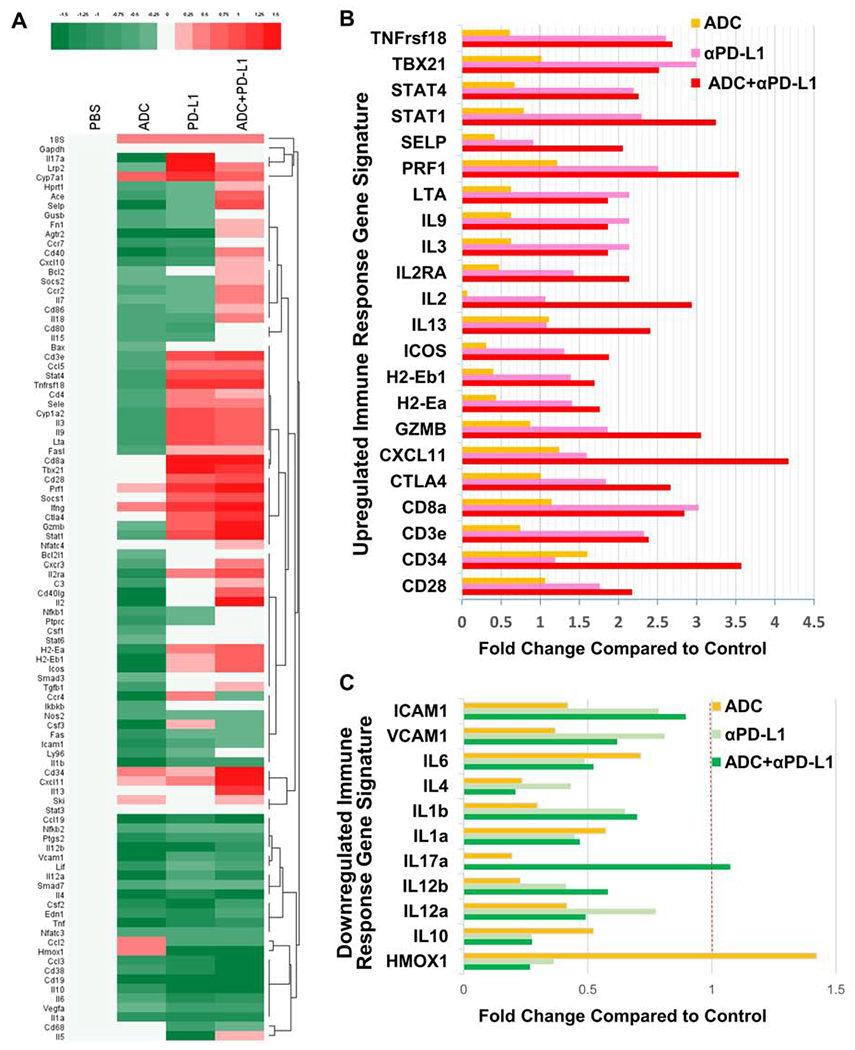
(A) Taqman qPCR analysis of D2F2/E2 fresh tumors harvested from animals treated with PBS, ADC, anti-PD-L1, or ADC + anti-PD-L1. The heat map indicates immune response signature genes with a log2 transformed relative fold change (log2scale)
(B) Top upregulated immune-related genes in tumors from mice subjected to HER2-targeted ADC and/or anti-PD-L1 treatment.
(C) Top downregulated immune-suppressive genes in tumors from mice treated with HER2-targeted ADC and/or anti-PD-L1.
HER2-targeted ADC increases anti-tumor immunity of TILs and splenocytes
To further characterize the host anti-tumor immunity induced by HER2-targeted ADC with or without anti-PD-L1, we isolated CD3+ tumor-infiltrating lymphocytes (TILs) and isolated CD8+ cells to test their CTL activity; collected IgG from the supernatants of expanded CD19+ TILs to detect the IgG production; assessed the immune serum binding to tumor cells and examined the consequence of such binding. In addition, we tested the activation and differentiation of various T and B cell subsets.
We first assessed the percentage of CD3+ and CD19+ TILs from animals treated with PBS, ADC, anti-PD-L1, and combination therapy. ADC monotherapy expanded CD19+ B cells in the TILs over PBS controls, increasing their frequency approximately two-fold, while anti-PD-L1 therapy increased the frequency of CD3+ T lymphocytes. Combination therapy balanced the B and T cell responses by increasing CD19+ B cells over anti-PD-L1 treatment alone (from 2.5% to 3.9%) and CD3+ T cells over ADC monotherapy (from 72% to 77%) (Figure 6A).
Figure 6. HER2-targeted ADC plus anti-PD-L1 administration modulated anti-tumor function both systematically and in the tumor microenvironment.

(A) CD19+ vs. CD3+ lymphocytes in the TILs. Cells were gated on live, CD45+ lymphocytes. Dot plots represent concatenates of 3-5 mice per group.
(B) The cytotoxicity of CD8+ cells isolated from expanded TILs obtained from different treatment groups against HER2-expressing D2F2/E2 vs. parental D2F2 tumor cells by LDH assay. The experiment was repeated three times. Data represent mean ± SEM.
(C) Levels of IgG secreted into cell culture supernatants by expanded CD19+ TILs harvested from D2F2 engrafted mice treated with ADC vs. CD19+ TILs harvested from D2F2/E2 bearing mice treated with ADC, anti-PD-L1, or ADC + anti-PD-L1, tested by ELISA. The experiment was repeated three times. Data represent mean ± SEM.
(D) The binding to D2F2/E2 tumor cells by antibodies in immune serum from D2F2/E2 tumor-bearing mice subjected to treatments as indicated. Serum dilutions at 1:200, 1:400, 1:800, 1:1600, and 1:3200 were used to detect Ab binding, and results from the 1:800 dilution are shown.
(E) ADCC assays were performed to test immune serum IgG-mediated cytotoxicity against D2F2/E2 tumor cells. The experiment was repeated three times. Data represent mean ± SEM.
(F) B cell differentiation in TILs and spleen. CD19+ cells were gated on CD38+CD138−GL7+ germinal center B cells or CD38+CD138+ plasma cells. Dot plots represent concatenates of 3-5 mice per group.
(G) Corresponding bar graphs for dot plots in panel F. Bar graphs display mean plus SEM. Data are from one representative experiment of two performed.
(H) B cell activation in TILs and spleens. Total CD19+ B cells were gated for Ki67 expression in the first and third row dot plots, while CD19+GL7+ germinal center B cells were gated for CD86 expression in the second and fourth rows. Dot plots represent concatenates of 3-5 mice per group.
(I) Corresponding bar graphs for dot plots in panel H. Bar graphs display mean plus SEM. Data are from one representative experiment of two performed.
(J) ADC and/or anti-PD-L1 treatment-induced antibody isotypes in mouse serum. Bar graph displays mean plus SEM.
(K) T cell subset analysis in the TILs. T cells were gated on live, CD45+CD19− lymphocytes, followed by CD8 vs. CD4 expression. All dot plots represent concatenates of 3-5 mice per group.
(L) Bar graphs displaying data for CD4+FoxP3+ Tregs and CD8+PD-1+ T cells. Bar graphs display mean plus SEM. Data are from one representative experiment of three performed.
(M) T cell activation assessed by IFNγ, Granzyme B and Ki67 expression on CD8+ T cells. Bar graphs display mean plus SEM. Data are from one representative experiment of three performed.
We then examined the CTL activity of CD3+ TILs. CD8+ lymphocytes were isolated from expanded CD3+ TILs and tested for their killing of D2F2 and D2F2/E2 cells, respectively. As shown in Figure 6B, CD8+ TILs isolated from mice treated with HER2-targeted ADC showed significantly (p<0.05) higher killing of HER2-expressing D2F2/E2 cells compared to parental HER2-negative D2F2 cells, demonstrating the HER2 specificity of these CD8+ TILs. Moreover, anti-PD-L1 treatment significantly (p<0.01) enhanced the killing activity of these CD8+ TILs against D2F2/E2 cells.
To determine if ADC or ADC plus PD-L1 inhibition activated the humoral response we measured IgG production from cultured CD19+ TILs harvested from the D2F2-bearing mice subjected to ADC treatment or CD19+ TILs from the D2F2/E2-bearing mice subjected to ADC, anti-PD-L1, and ADC + anti-PD-L1 treatments. As shown in Figure 6C, CD19+ B cells of the TILs from HER2-targeted ADC plus anti-PD-L1 combined treatment produced more IgG than all the other groups.
To provide further evidence that HER2-targeted ADC could induce humoral anti-HER2 immunity, we collected the sera from the treated mice and assessed binding of serum antibodies to D2F2/E2 cells. D2F2/E2 cells were incubated with equal quantities of serum from each group followed by incubation with a FITC-conjugated anti-mouse IgG secondary antibody and then assessed by flow cytometry. The sera from mice that received HER2-targeted ADC (9.64%) and anti-PD-L1(17.5%) treatment bound to D2F2/E2 cells more effectively than serum collected from control mice (PBS, 5.85%). Importantly, ADC + anti-PD-L1 treatment further enhanced the binding of serum antibody to D2F2/E2 cells (21.1%) (Figure 6D). To determine the potential consequence of serum Ab binding to tumor cells, we performed serum IgG-mediated antibody-dependent cellular cytotoxicity (ADCC) assays. Antibodies in serum collected from treated D2F2/E2 tumor-bearing mice were bound to D2F2/E2 target cells, followed by addition of peripheral blood mononuclear cells (PBMC) as effector cells. While serum from ADC or anti-PD-L1 treated mice mediated minimal cytotoxicity to D2F2/E2 cells compared with control serum from PBS treated mice, serum from the ADC plus anti-PD-L1 group mediated significantly (p<0.05) higher cytotoxicity to the D2F2/E2 cells via ADCC compared with each monotherapy (Figure 6E).
To elucidate the mechanisms of anti-tumor humoral immunity, we evaluated the differentiation and activation of B cells in the different treatment groups. As shown in Figure 6F and 6G, in TILs of ADC + anti-PD-L1-treated animals, we found significantly (p=0.027) more CD19+ B cells expressing GL7, a marker used to identify germinal center B cells with greater propensity to produce antibody and present antigen (Krishnamurty et al., 2016 & Cervenak et al., 2001). In the spleen, the frequency of GL7+ B cells was also significantly (p=0.027) increased after combination therapy, in addition to increased CD38+CD138+ plasma cells (Rodríguez-Alba et al., 2008 & McCarron et al., 2017 & Krishnamurty et al., 2016). To assess B cell activation, we used the proliferation marker Ki67 and activation marker CD86 (Gerdes et al., 1984 & Wang et al., 2005 & Ma et al., 2013). ADC + anti-PD-L1 treatment significantly increased the frequencies of CD19+Ki67+ cells over PBS controls both in TILs (p=0.014) and in the spleen (p=0.014) (Figure 6H and 6I). In addition, we found statistically significant increases in CD86 expression on CD19+GL7+ germinal center B cells in both TILs (p=0.041) and spleens (p=0.048) after combination therapy. Together, these results indicate that B cells are more differentiated and proliferative after ADC + anti-PD-L1 dual therapy.
We demonstrated immune serum IgG-mediated cytotoxicity against D2F2/E2 tumor cells via ADCC assays (Figure 6E). We characterized the isotypes of the serum antibody following ADC and/or anti-PD-L1 therapy. As shown in Figure 6J, IgG1 is the predominant isotype found in the serum of treated mice vs. IgG2a, IgG2b and IgG3. Importantly, ADC + anti-PD-L1 combination therapy extensively (p=0.0698) increased the amount of IgG1 found in the serum compared to PBS controls. IgG2a, IgG2b and IgG3 were found at much lower serum levels and revealed no significant differences among treatment groups.
In parallel, we analyzed the T cell subsets as well as their activation status in TILs during the same animal study conducted for assessing B cell differentiation and activation as described above. TILs were stained as described in Methods. As shown in Figure 6K, ADC monotherapy increased the frequency of CD4+ T cells (from 46% to 52%), while anti-PD-L1 alone increased CD8+ T cells (from 31% to 37%) over PBS controls. Combination therapy resulted in the largest T cell expansion with a 10% increase specifically in CD8 T cells (from 31% to 41%). In addition, ADC monotherapy and ADC + anti-PD-L1 significantly reduced immunosuppressive CD4+FoxP3+ Tregs (p=0.0008 and p=0.005 respectively) vs. PBS controls (Figure 6L). Furthermore, anti-PD-L1 increased the frequency of highly activated CD8+PD-1+ cells (Oba et al., 2020). However, only the combination therapy decreased the frequency of Tregs (p=0.005) and increased the expression of PD-1 on CD8+ T cells (p=0.077) simultaneously. In addition, combination therapy resulted in significantly (p=0.001) higher frequency of CD8+IFNγ+ T cells and the most proliferative CD8+ T cell population (p=0.005) as measured by Ki67 expression, compared to PBS controls (Figure 6M). We found extremely low numbers of CD8+ cells expressing Granzyme B, which made it difficult to compare the CD8+Granzyme B+ cells among the different treatment groups.
Collectively, these data indicate that HER2-targeted ADC therapy can induce specific anti-HER2 host humoral as well as cellular immunity by inducing B cell activation, differentiation, and IgG1 production both systemically and in the tumor microenvironment. In addition, ADC therapy modulated T cell subsets as well as their activation status in TILs. Co-administration of anti-PD-L1 significantly augmented these effects.
HER2-targeted ADC reduces the number of ALDHhi CSCs and this effect was significantly enhanced by anti-PD-L1 co-administration
To evaluate the potential anti-CSC effect of HER2-targeted ADC and anti-PD-L1, we measured surface protein expression of HER2 and PD-L1 on ALDHhi D2F2/E2 CSCs by flow cytometry and compared it with the unsorted D2F2/E2 cells. We found that nearly 80% of the ALDHhi D2F2/E2 CSCs express HER2, and approximately 20% of the ALDHhi D2F2/E2 cells express PD-L1 (Figure 7A). There was a statistically significant increase in PD-L1 expression in ALDHhi D2F2/E2 CSCs compared to unsorted D2F2/E2 cells (Figure. 7B). Flow cytometry results were confirmed by western blot where the level of HER2 protein expression was similar among ALDHhi, ALDHlow, and unsorted D2F2/E2 cells, whereas the PD-L1 expression of ALDHhi D2F2/E2 CSCs was significantly higher than ALDHlow or unsorted D2F2/E2 cells (Figure. 7C and 7D).
Figure 7. Targeting of ALDHhi CSCs by HER2-targeted ADC.

(A) Surface expression of HER2 and PD-L1 on ALDHhi D2F2/E2 CSCs. One of three experiments is shown.
(B) Comparison of HER2 and PD-L1 expression on ALDHhi D2F2/E2 CSCs vs. unsorted D2F2/E2 cells. Flow cytometry was repeated three times. Data represent mean ± SEM.
(C and D) Confirmation of HER2 and PD-L1 expression on ALDHhi D2F2/E2 CSCs, ALDHlow and unsorted D2F2/E2 cells by western blot (C) with statistics (D). One of three experiments is shown.
(E) Expression of HER2 and PD-L1 on freshly harvested D2F2/E2 tumors after treatment indicated treatment. One of two experiments is shown.
(F) Evaluation of ALDHhi D2F2/E2 CSCs frequencies in fresh tumor after indicated treatment. One of two experiments is shown.
(G) Tumorigenicity of ALDHhi cells. After treatment with anti-PD-L1 and/or HER2-Targeted ADC, ALDHhi D2F2/E2 tumor cells were engrafted in equal numbers to naïve mice and tumor growth was monitored. One of two experiments is shown. Data represent mean ± SEM.
HER2-targeted ADC therapy markedly reduced HER2-expressing tumor cells by nearly half from 68.3% in the PBS treated control group to 36.8% in the ADC group, and ADC combined with anti-PD-L1 demonstrated a similar reduction of HER2-expressing cells (34.4%) (Figure 7E). Similarly, HER2-targeted ADC therapy markedly reduced PD-L1-expressing tumor cells by about a third from 6.48% in the PBS treated control group to 4.82%, with a similar decrease in the ADC + anti-PD-L1 group (4.63%) (Figure 7E). These results suggest that HER2-targeted ADC therapy kills ALDHhi D2F2/E2 CSCs by targeting HER2-expressing tumor cells and PD-L1-expressing cells.
To provide direct evidence that HER2-targeted ADC therapy killed ALDHhi D2F2/E2 CSCs, we assessed the percentage of ALDHhi cells in the tumors harvested from animals subjected to PBS, ADC, anti-PD-L1, and ADC + anti-PDL1 treatment. ADC or anti-PD-L1 treatment alone each reduced ALDHhi cells from 2.26% in the PBS control group to 1.71% and 1.14%, respectively (Figure 7F). Importantly, ALDHhi cells were reduced markedly to 0.29% (by ~90% relative to PBS control) in the combined group of ADC plus anti-PD-L1 therapy (Figure 7F). These studies provided the first direct evidence that HER2-targeted ADC can target ALDHhi CSCs, and this effect is significantly enhanced by blocking PD-L1. Furthermore, we tested the tumorigenicity of ALDHhi cells isolated from residual D2F2/E2 tumors harvested from treated animals. Figure 7G shows that the tumor growth from identical numbers of engrafted ALDHhi D2F2/E2 cells from animals that received HER2-targeted ADC therapy or ADC + anti-PD-L1 therapy was significantly (p<0.05) reduced compared with the engraftment of the same number of ALDHhi D2F2/E2 cells from mice administered with PBS or anti-PD-L1 mAb. Together, these data indicate that ADC and ADC + anti-PD-L1 therapy not only reduces the number of ALDHhi CSCs but also reduces their tumorigenicity.
DISCUSSION
ADCs mediate the targeted delivery of cytotoxic drugs to tumor cells by selective binding to tumor antigens, followed by subsequent internalization, lysosomal trafficking, and ultimately release of the cytotoxic warhead within the tumor cells. T-DM1 is the first FDA approved HER2-targeting ADC to treat HER2-positive metastatic BC patients. Although the development of TDM1 represents an important clinical advance, over 50% of HER2+ BC patients display inherent resistance to T-DM1 (Sakai et al., 2018). Previous data generated by our group indicated that a biparatopic HER2 ADC could induce complete tumor regression in HER2+ JIMT-1 BC-bearing immunodeficient mice, which were resistant to T-DM1(Li et al., 2016). Moreover, the biparatopic ADC induced robust and sustained tumor regression of tumors relapsed from the repeated T-DM1 treatment, showing promise as an effective therapy for the relapsed/refractory patients. Li et al. also evaluated the biparatopic ADC against a panel of primary tumor xenograft models derived from TNBC and HER2−/ER+ which are not candidates for T-DM1 and demonstrated that the biparatopic ADC induced tumor regressions in a majority of the models, including tumors that express low levels of HER2 (Li et al., 2016). Together, these studies demonstrated that the biparatopic HER2-targeted ADC binding to two non-overlapping epitopes on HER2 shows significantly higher therapeutic activity in BC models representing a wide range of HER2 expression and resistance to TDM1 (Leal et al., 2018; Li et al., 2016).
Despite the promising results of previous studies, all of the experiments to date evaluating the efficacy of the biparatopic HER2 targeted ADC were conducted using human xenografts of primary tumor-derived models in immunodeficient mice, thus limiting the ability to assess immunomodulatory functions (Li et al., 2016). ADCs may show greater antitumor activity in immunocompetent versus immunodeficient mice due to immune contributions to the antitumor activity of ADCs (Rios-Doria et al., 2017). In addition, to our knowledge, HER2-targeted ADCs have not been examined for their ability to target CSCs. In the present work, we established two human HER2-expressing murine BC models that were capable of growing in syngeneic immunocompetent host mice. This allowed us to examine whether the biparatopic HER2 ADC possessed the potential to induce host immunity against human HER2-expressing tumors; target CSCs, and to evaluate any synergistic effects when used in combination with other immunological approaches, e.g., anti-PD-L1.
Hollingsworth and co-workers reported that EphA2-targeted ADC conjugated with tubulysin payloads induced immunogenic cell death (Rios-Doria et al., 2017). In the CT26 model, depletion of CD8+ T cells abrogated the activity of EphA2-targeted ADCs when used alone or in combination with a PD-L1 antibody (Rios-Doria et al., 2017). Expanded CD3+ TILs from the tumor subjected to HER2-targeted ADC treatment were capable of directly killing tumor cells. Importantly, immune sera collected from the host subjected to HER2-targeted ADC treatment could specifically bind to tumor cells, resulting in cytotoxicity of the tumor cells via ADCC. Moreover, we showed that HER2-targeted ADC, either alone or in combination with anti-PD-L1, modulated immune response gene signatures in the tumor microenvironment. Of particular note, the combination treatment elicited a gene signature that was distinct from either monotherapy treatment and was not a simple aggregate of each treatment. This observation suggests that the effects of the combination treatment are not merely additive, but may represent a synergistic effect on immune response gene signatures. Together, our data demonstrate the immunomodulatory function induced by biparatopic HER2-targeted ADC in immunocompetent hosts, as evident by the regulation of immune gene signatures and induction of anti-tumor CD19+ and CD3+ TILs. As a result, HER2-targeted ADC significantly inhibited the growth of HER2-expressing tumors by reducing the number of HER2-expressing cells via stimulating the host anti-tumor immunity to both directly (via T cells) and indirectly (via ADCC) kill HER2-expressing tumor cells.
To evaluate the mechanisms of HER2-targeted ADC therapy-induced anti-HER2 humoral as well as cellular immunity, we performed extensive immune phenotyping (Mueller et al., 2015). Focusing on CD4, CD8, and B cells as the major populations, we used CD45 to separate immune cells from tumor cells in all the flow data. Following CD45 gating, we used CD19 for B cells and CD3 for T cells. Additional markers have included GL7, CD138, CD38, Ki67, CD86 to show B cell differentiation and activation. We also used Foxp3, PD-1, Granzyme B, and IFNγ for T cell subsets and activation analyses. As a result, we found that HER2-targeted ADC induced B cell activation and differentiation both systemically and in the tumor microenvironment. We identified IgG1 as the predominant isotype found in the serum of ADC-treated mice. Furthermore, ADC therapy modulated T cell subsets as well as their activation in TILs. Co-administration of anti-PD-L1 significantly augmented these effects.
PD-L1 expression has been reported in both HER2+ and triple-negative BC (TNBC) subtypes (Bertucci et al., 2016; Li et al., 2018), and this expression was associated with CSC resistance to immunological control (Hsu et al., 2018). Indeed, we observed that PD-L1 is expressed on BC cells, and the expression is significantly higher on ALDHhi BCSCs compared to the cells comprising the tumor bulk. Since tumor cells with PD-L1 expression may escape immune attack by converting PD-1+ immune effector cells into anergic cells, we hypothesized that there may be a synergistic effect between HER2-targeted ADC and anti-PD-L1. We found that the efficacy of HER2-targeted ADC was significantly enhanced by addition of anti-PD-L1 in both D2F2/E2 and HER2-4T1 tumor models. In line with our findings, a recent study demonstrated that T-DM1-induced immune responses in BC patients reveal synergistic antitumor activity when combined with PD-1/PD-L1 pathway blockade in vivo (Muller et al., 2015; Rios-Doria et al., 2017) and (https://www.abstracts2view.com/sabcs18/view.php?nu=SABCS18L_1253&terms). Delivering ADC while blocking PD-L1 has potential advantages for improving patient outcomes: (1) Combinatorial therapy attacks cancer cells by multiple mechanisms, thereby decreasing the risk of tumor resistance emerging; (2) Combinatorial therapy allows for dose reduction of each modality, which could potentially decrease the toxicities inherently associated with each monotherapy (Adams et al., 2016). Data from our D2F2/E2 immunocompetent model demonstrated that both numbers and the function of the CD3+ and CD19+ TILs were improved significantly by ADC combined with anti-PD-L1 compared to ADC alone. Currently, there are 5 ongoing phase I and phase II clinical trials testing the combination of HER2 targeting ADCs with either anti-PD-1 or PD-L1 antibodies in BC patients. Results from these trials will determine the clinical utility of this approach (Muller et al., 2018).
We also determined the efficacy of HER2-targeted ADC alone or with anti-PD-L1 to target CSCs in 2 tumor models. Importantly, we find that both ALDHhi D2F2/E2 CSCs and ALDHhi 4T1 CSCs express high levels of HER2 and PD-L1 comparing with ALDHlow cells. HER2-targeted ADC treatment reduced the percentage of ALDHhi D2F2/E2 CSCs and this effect was significantly enhanced by addition of anti-PD-L1 (the reduction was nearly 90%). Previously, it was reported that HER2-associated activation of STAT3 signaling could induce EMT of normal mammary epithelial cells and contribute to metastatic BCSCs in HER2+ subtypes (Geng et al., 2014). Our group reported that HER2 plays a critical role in regulating the CSC population in HER2− BC and HER2 is highly expressed on ALDHhi CSCs even in tumors with heterogeneous expression of HER2 (Ithimakin et al., 2013). We replicated these findings in animal studies by showing that ALDHhi 4T1 cells preferentially express high levels of HER2, even though 4T1 is classified as TNBC. These findings may explain why HER2low tumors may benefit from HER2 targeted therapy. In these tumors, anti-HER2 therapy may be capable of effectively targeting the HER2 expressing CSCs within these tumors classified as HER2low (Wang et al., 2013). This is supported by our finding demonstrating increased HER2 expression in ALDHhi 4T1 cells. These cells also expressed increased PD-L1 perhaps accounting for efficacy of adding anti-PD-L1 for immune checkpoint blockade.
In summary, this study represents a continued investigation evaluating a HER2-targeted ADC (MEDI4276) in both HER2high (D2F2/E2) and HER2low (HER2-4T1) murine BCs in immunocompetent hosts. Using the human HER2-expressing D2F2/E2 and HER2-4T1 murine breast tumor models, we demonstrated that HER2-targeted ADC treatment induces both cellular and humoral anti-HER2 host adaptive immunity which effectively targeted BCSCs as well as bulk tumor cells. Moreover, we demonstrated that addition of immune checkpoint blockade with anti-PD-L1 further increased the effectiveness of ADC. The increased effectiveness of this combined therapy may allow for dose reduction of each component potentially limiting toxicity. The ability of HER2-targeted ADC to kill CSCs in breast tumors currently classified as HER2low greatly expands the spectrum of breast tumors amenable to this therapy. The biparatopic HER2 ADC also has a higher binding avidity to HER2 due to the tetravalent nature of the antibody. This may also enhance the uptake of the HER2 ADC in tumor cells expressing lower levels of HER2 as demonstrated in our previous publication (Li et al., 2016). Together, these results support the use of HER2-targeted ADCs for the therapy of both HER2high and HER2low patients; combined with PD-1/PD-L1 blockade to enhance host immunity to eliminate CSCs. Furthermore, since HER2 may be expressed in subsets of other solid tumors this approach may have wider clinical utility.
SIGNIFICANCE
The remarkable clinical efficacy of HER2 targeting agents including ADCs may relate to their ability to target HER2-expressing CSCs. However, the role of host immunity in mediating these responses remains unclear. Utilizing immunocompetent mouse models, we demonstrate that HER2-targeted ADC treatment induced both cellular and humoral anti-HER2 host adaptive immunity which effectively targeted both bulk tumor and cancer stem cells. We developed an approach to improve the potent antitumor response of HER2-targeted ADC by combining it with anti-PD-L1 administration. These results shed light on the mechanisms underlying the efficacy of HER2-targeted ADCs and demonstrate that these effects are further augmented by simultaneous PD-1/PD-L1 blockade to enhance the host anti-tumor immunity and effective targeting of CSCs.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for reagents should be directed to the corresponding author Qiao Li (qiaoli@umich.edu).
Materials availability
Reagents generated in this study are available on request with a completed Materials Transfer Agreement.
Data and code availability
All software and parameters used are described, and custom scripts and functions will be made available upon request.
EXPERIMANTAL MODEL DETAILS
Mouse Studies
Six to eight-week-old female Balb/c mice were purchased from the Jackson Laboratories (Bar Harbor, ME) and maintained in a pathogen-free environment. Healthy littermates were randomly assigned to experimental groups and socially housed under a 12-hour light dark cycle in ventilated racks; mice in the same experimental group were housed together. Animals weighted approximately 19 to 21 grams at the initiation of all experiments. For tumor models, mice were euthanized when tumors reached humane endpoints. Daily animal care and husbandry was provided by the University of Michigan’s Unit for Laboratory Animal Medicine (ULAM). NIH guidelines of laboratory animal care were followed and the animal protocols were approved by the University of Michigan’s ULAM and the Institutional Animal Care & Use Committee (IACUC).
Cell Lines
Murine mammary tumor line, D2F2 syngeneic to Balb/c mice, was cloned from a prolactin-induced, spontaneous mammary tumor. Stable transfection of D2F2 with wild-type human HER2 resulted in the cell line D2F2/E2. Both D2F2 and D2F2/E2 cell lines were kindly provided by Prof. Wei-Zen Wei (Wayne State University, Detroit, MI) and were grown in DMEM (Gibco, Grand Island, NY) containing 10% FBS (Gibco) at 37°C. The HER2 expression on D2F2/E2 was selected in a medium supplemented with 0.6 mg/ml G418 (Gibco).
4T1 cell line is a murine mammary carcinoma syngeneic to Balb/c mice which were obtained from Dr. M. Sabel from the University of Michigan and were grown in complete RPMI1640 medium. HER2-4T1 cells were derived from parental 4T1 by stable transduction with wild-type human HER2 and were kindly provided by Herbert Kim Lyerly and Zachary C. Hartman (Duke University, Durham, NC, USA) and Dr. Michael Kershaw (Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia). HER2-4T1 cells were maintained in DMEM with 4.5 g/L (high) glucose containing 10% FBS and Pen/Strep.
METHOD DETAILS
Flow cytometry Analysis
Cell surface expression of HER2, PD-L1, CD3, CD19 were analyzed by flow cytometry. Cells were suspended in 200μL flow cytometry buffer (PBS with 1% FBS) containing fluorochrome-conjugated antibodies and incubated at 4°C for 45 minutes. Cells were then washed in flow cytometry buffer, re-suspended, and analyzed using a BD LSR II flow cytometer (BD Biosciences, Franklin Lakes, NJ). Isotype control antibody staining was used to define gates for positive and negative cells. FlowJo software (version 10.0) was used for all flow data analysis.
Growth of human HER2 transfected murine breast cancer cells in immunocompetent mice
Equal numbers of D2F2 and D2F2/E2 cells were inoculated into the opposite fat pads of the same Balb/c mouse. High (3x106) and low (1x106) dosages of cells were tested for tumor growth in immunocompetent mice. The HER2-4T1 tumor model was established in Balb/c mice in the same way. HER2-transfected D2F2/E2 and HER2-4T1 tumors were compared with parental D2F2 and 4T1 cells for tumor growth in immunocompetent mice.
Preparation of tumor cell suspensions
Harvested tumors were cut into small pieces (1-2mm) using sterile ophthalmic surgical scissors and then washed with HBSS three times, resuspended in 10 mL mixture of 1X Collagenase/Hyaluronidase (StemCell Technologies, Canada), digested for 30-45min on a Corning stirrer at room temperature. Digestion was stopped by adding FBS to 5% final concentration. Samples were then filtered through a 70 μm sterile cell strainer, washed, and re-suspended in HBSS for future use.
Immunohistochemistry (IHC) to test HER2 expression
IHC was performed on 10% neutral buffered formalin-fixed, paraffin-embedded (FFPE) tissue. Sections (5-μm thickness) were cut from FFPE tumor blocks and routinely deparaffinized and rehydrated. Anti-HER2 (monoclonal, Thermo Fisher Scientific, Grand Island, NY) at a dilution of 1:100 was used to detect the expression of HER2 on fresh D2F2, D2F2/E2, 4T1, and HER2-4T1 tumors by IHC. HER2 was visualized using the supersensitive streptavidin-biotin-peroxidase complex (Biogenex, Fremont, CA). For antigen retrieval, slides were heated in a microwave oven for 30 min in citrate buffer solution (pH=7.4) and cooled slowly to room temperature. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 8 min. Sections were then incubated with the anti-HER2 antibody overnight (>12 h). Slides were subsequently rinsed in phosphate-buffered saline (PBS) three times and incubated with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies. After incubation, slides were washed again with PBS and visualized using diaminobenzidine. Mayer’s hematoxylin was used to counterstain sections, which were dehydrated and mounted.
Tumor treatment
Healthy 6-8-week-old Balb/c mice were inoculated with indicated numbers of D2F2, D2F2/E2, 4T1, HER2-4T1 cells, respectively, into the mammary fat pad. 14 days after tumor inoculation, tumor-bearing mice were treated with tail vein injection of ADC and controls, once a week for a total of 3 or 4 weeks. In additional experiments, tumor-bearing animals were treated with ADC as above, followed by twice-weekly intraperitoneal (i.p.) injections of anti-PD-L1 clone 80 (0.05mg) administered in 0.2 ml of PBS after each ADC injection. When tumors spread beyond the limit of the largest diameter of 2cm or 1 week after the last ADC or PD-L1 administration, mice were sacrificed and samples harvested for further experiments.
To test the direct effect of HER2-targeted ADC on ALDHhi CSCs, we injected ALDHhi D2F2/E2 cells (5,000 cells/mouse) into the mammary fat pad of Balb/c mice on day 0, and treated mice by injecting HER2-targeted ADC (3 mg/kg) via tail vein on day 0, 7, 14, 21, accompanied by twice-weekly anti-PD-L1 (0.05 mg/mouse) i.p. administration on day 0, 2, 7, 9, 14, 16, 21, and 23. Tumor growth was then monitored. In separate experiment using the same treatment protocol, animal survival was monitored for 100 days. Furthermore, tumor-free mice were used for re-challenge experiment to test the immunological memory formation.
ALDEFLUOR assay
The ALDEFLUOR™ Kit (StemCell Technologies, British Columbia, Canada) was used to stain ALDHhi cells generated from tumors following the manufacturer’s protocol. Briefly, cancer cells isolated from treated mice were suspended in ALDEFLUOR Assay Buffer (AAB), and then incubated with activated ALDEFLUOR Reagent at 37°C for 45 minutes, washed, re-suspended, and analyzed using BD LSR II flow cytometer (BD Biosciences, Franklin Lakes, NJ) or sorted using MoFlo Astrios flow cytometer (Beckman Coulter, Indianapolis, IN). FlowJo software (version 10.0) was used for all flow data analysis.
Testing of the tumorigenicity of ALDHhi cells
To test the tumorigenicity of ALDHhi cells isolated from D2F2/E2, equal numbers of ALDHhi or ALDHlow tumor cells mixed with Matrigel (BD Biosciences; 1:1) were injected into the opposite side of the mammary fat pad of healthy 6-8-week-old Balb/c mice. Tumor size was measured every 3 or 4 days.
To test the tumorigenicity of ALDHhi cells isolated from the residual D2F2/E2 tumors harvested from treated animals, we sorted ALDHhi cells from D2F2/E2 tumor suspension prepared from the tumor harvested from the animals treated with PBS, ADC, anti-PD-L1, and ADC + anti-PDL1 using the ALDEFLUOR assay as described. We then injected ALDHhi cells into the mammary fat pad. Tumors size was then monitored every two days.
Assessing lymphocyte differentiation and activation in the tumor and periphery
We inoculated Balb/c mice on day -7 with 3x105 D2F2/E2 tumor cells. On day 0, we began weekly ADC (3 mg/kg) treatment, and twice weekly anti-PD-L1 (0.05 mg/mouse) therapy for 4 weeks. The experiment was terminated on day 28. Spleens and tumors were processed to generate single cell suspension. Splenocytes and TILs were stained with Invitrogen’s Live/Dead Near IR Fixable Dye and FcR block for 10 minutes at room temperature in the dark. Cells were washed and incubated with surface antibodies for 30 minutes at 4°C. Cell surface markers included CD45, CD19, CD3, CD38, CD138, GL7, CD86. After incubation with surface antibodies, cells were washed and spun. For staining intracellular targets, including Ki67, eBioscience Transcription Factor Staining Buffer Set was used according to the kit’s instructions. Flow cytometry was performed at the University of Michigan’s Flow Core on a Cytek Aurora. B cells were gated as Live/Dead NIR−, CD45+, CD19+, CD3−. Subpopulations for analysis included CD19+CD138+ plasma cells, CD19+CD138−GL7+ germinal center B cells, CD19+Ki67+ proliferating B cells, and CD19+CD138−GL7+CD86+ germinal center B cells.
To address T cell subsets and activation status, we used the cell surface markers CD45, CD19, CD4, CD8, and PD-1 and the intracellular markers FoxP3, IFNγ, Granzyme B and Ki67. T cells were gated as Live/Dead NIR−, CD45+, CD19−, followed by CD4 or CD8 expression examination. Subpopulations included CD4+ T cells, CD4+FoxP3+ Tregs, CD8+ T cells, CD8+PD-1+, CD8+IFNγ+, CD8+Granzyme B+, and CD8+Ki67+ T cells.
CD3+ TIL expansion and CTL cytotoxicity assay
To expand CD3+ TILs from tumors, we cut tumor tissues into small chunks of 1-2 mm in each dimension. 5-10 tumor fragments were placed into wells of 6-well tissue culture plate with 6mL complete medium (CM) including 2000IU/mL of rmIL-2 (Catalog # 550069, BD Bioscience), and the plates were placed in a 37°C incubator with 5% CO2. The plates were monitored every two days, and half of the medium was replaced every 3-4 days. 10 days after culture initiation, cells were harvested, counted, and stored for further experiments.
Expanded TILs from tumor tissue were separated into CD8+ TILs using mouse CD8 MicroBeads (130-049-401, Miltenyi Biotec, Gaithersburg, MD). CD8+ TILs were used as effector cells in killing assays. Cell cytotoxicity was assessed by measuring the release of cytoplasmic lactate dehydrogenase (LDH) into cell culture supernatant according to the manufacturer’s protocol. CD8+ TILs separated from different groups were used as effector cells, D2F2/E2 cells were used as target cells, and D2F2 cells were used as control. 1x105 D2F2 and D2F2/E2 cells were plated in a well of a 24 well tissue culture plate and co-incubated with 1x106 CD8+ TILs isolated from expanded TILs of different groups. After 4 h of incubation, 100 μl of the supernatant from each well were transferred in triplicate to a 96-well tissue culture plate before adding to each well 50μl of the substrate mix, then the plate was incubated for 30 min at room temperature in the dark (CytoTox 96 Non-Radioactive Cytotoxicity Assay, Catalog # G1780, Promega, Madison, WI). 50μL stop solution was added into each well before LDH measurement. Maximal release of LDH was performed as positive control by incubating target cells with the lysis solution. Target cells alone were served as spontaneous release control. Absorbance was measured at 490nm using a 96-well plate reader.
CD19+ TILs expansion and IgG production
CD19+ TILs were isolated from TILs using mouse CD19 MicroBeads (130-052-201, Miltenyi Biotec,), and were expanded in rmIL-2. Then, supernatants from the expanded CD19+ TILs were collected for IgG detection using a mouse IgG ELISA Quantitation Set (Catalog # E90-131, Bethyl Laboratories, TX). Detection of IgG was performed as follows: add 100ul of diluted coating antibody to each well, incubate at room temperature (20-25°C) for 1 h. After blocking, anti-mouse IgG-HRP (1:8000) (Bethyl Laboratories, Inc. Montgomery, TX, USA) was added and incubated at room temperature for 2 h. After every step, the plate was washed three times. After adding TMB substrate solution, the reaction was visualized with chromogen/substrate solution (0.05 M citrate/citric acid, 4.0 mg O-phenylenediamine, and 4.0 μL 30% H2O2). The reaction was stopped with H2SO4, and the absorbance was measured at 490 nm on an automatic ELISA reader (EL 800, Bio-Tek Instruments, Winooski, VT, USA). The cut-off value was calculated as the mean from the negative samples plus three times the standard deviation.
IgG binding experiment
Sera were collected from D2F2/E2-bearing mice treated with PBS, ADC, anti-PD-L1, or ADC + anti-PD-L1. D2F2/E2 cells were incubated with equal quantities of serum from different groups followed by incubation with FITC-conjugated anti-mouse IgG second antibody, followed by assessment using flow cytometry.
Antibody-dependent cellular cytotoxicity (ADCC)
In order to evaluate serum IgG-medicated ADCC, serum collected from D2F2/E2-bearing mice treated as indicated were co-cultured with D2F2/E2 (2x104) cells overnight in a 96-well culture plate at 37°C (5% CO2, humidified atmosphere), followed by the addition of PBMCs collected from healthy mice as effector cells (4:1 as the effector: target ratio). The plate was further incubated overnight at 37°C in a 5% CO2 humidified atmosphere. 100 μl of the supernatant from each well was transferred in triplicate to a 96-well tissue culture plate. Then the IgG-medicated ADCC was assessed by measuring the release of LDH into cell culture supernatant according to the LDH cytotoxicity assay protocol.
Characterization of the isotypes of anti-tumor antibodies
To assess the isotypes of ADC and/or anti-PD-L1 therapy-induced anti-tumor antibodies, we collected the whole blood at the same time as spleens and tumors were collected for the B cell differentiation and activation studies as described above. Blood samples were left standing for 30 minutes to clot. After clotting, samples were spun at 3000 rpm for 15 minutes at room temperature. Sera were collected and stored at −20°C until isotype analysis was performed. Millipore Sigma’s Milliplex Map Mouse Immunoglobulin Isotyping Magnetic Bead Panel was used according to the kit’s instructions and isotype analysis was performed by the University of Michigan’s Immunology Core.
Western blot
Cells were lysed on ice in cold RIPA lysis buffer (50 mM Tris-HCl, pH8.0; 1% Triton X-100; 150 mM NaCl; 0.1% SDS; 0.5% Na-Deoxycholate and protease inhibitor cocktail). The lysates were centrifuged at 14,000 rpm for 30 min at 4°C to remove debris. Protein concentration was measured by Enhanced BCA Protein Assay Kit (Bio-Rad Laboratories, Inc. Hercules, CA). Protein samples were mixed with Laemmli sample buffer (Bio-Rad Laboratories, Inc. Hercules, CA) including reducing agent 2-mercaptoethanol, and heated at 100°C for 5 min. Equal amounts of protein (50 μg) were loaded and separated by SDS-PAGE and transferred onto PVDF membranes. Membranes were blocked with buffer (5% BSA) for 1 h at room temperature. Then, incubated overnight at 4°C with primary antibodies (anti-HER2/ErbB2, anti-PD-L1). After washing with TBS-T, HRP-labelled goat anti-rabbit IgG (H+L) was used as a secondary antibody. Membrane-bound immune complexes were developed by incubating the blots with ECL select kit. The radiographic films were scanned and blot quantification of protein bands density was calculated.
TaqMan Gene Expression Assay
For comparison of immune response-related gene expression among D2F2/E2-bearing mice treated with PBS, ADC, anti-PD-L1, or ADC+ anti-PD-L1, total RNA of tumor cells from the different groups were extracted and reverse transcribed into cDNA according to the protocol. Mix cDNA with TaqMan Fast Advanced Master Mix gently and ensure that the final cDNA concentration per well is 100ng for 96-well (Mouse Immune Response TaqMan Array 96-Well FAST Plate). Seal and centrifuge the plate to collect the contents in the bottom of the wells. Using QuantStudio systems to perform PCR amplification according to the manufacture’s instruction. Relative fold-changes (FC) in mRNA expression levels compared to control samples were calculated using the 2−ΔΔCT method. Then relative FC was log2 transformed (log2 scale) and shown as Signature Genes Heat map (Figure 5A). The heat map was performed with Hemi 1.0 software. Signature Genes with a relative FC of greater than 2 over the PBS group to be considered significant. The relative FC in mRNA expression levels of treated groups over the PBS group was calculated.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistics were analyzed using the mean ± standard error. The significance of differences in tumor size and tumorigenicity was determined using Repeated measured ANOVA. The significance of differences in IgG binding and ADCC was determined using one-way ANOVA. Log-rank (Mantel-Cox) test with Bonferroni’s correction was used to determine significance for survival experiments. An unpaired two tailed t test was used for remaining flow cytometry analysis. P<0.05 was considered statistically significant between the experimental groups. Statistical analyses were performed using Graph-Pad Prism 8.0.
Highlights.
HER2-targeted ADC shows anti-tumor activity in immune-competent mouse models
HER2-targeted ADC induces humoral and cellular immunity and immunological memory
The ADC inhibits tumor growth and prolongs survival by targeting ALDHhigh CSCs
HER2-targeted ADC augments the therapeutic efficacy of anti-PD-L1 antibody
ACKNOWLEDGMENTS
This work was supported by AstraZeneca, Gaithersburg, Maryland, and partially supported by the Gillson Longenbaugh Foundation. LX was partially supported by The third affiliated hospital of Anhui Medical University, Hefei, China, and LW and YQ were both partially supported by the Cancer Center, Union Hospital, Wuhan, China. MSW was supported by R35 CA197585, and by the Breast Cancer Research Foundation (BCRF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
Steven R. Coats, Frank I. Comer, Mary Jane Hinrichs, and Michael Oberst are employees of AstraZeneca, with stock ownership and/or stock options or interests in AstraZeneca.
REFERENCES
- Adams SR, Yang HC, Savariar EN, Aguilera J, Crisp JL, Jones KA, Whitney MA, Lippman SM, Cohen EE, Tsien RY, and Advani SJ (2016). Anti-tubulin drugs conjugated to anti-ErbB antibodies selectively radiosensitize. Nat Commun 7, 13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga J, Tamura H, Yamashita T, Modi S, Tokunaga E, Ito Y, Iwata H, Charif M, Caleb C Lee CC, Sugihara M, et al. (2018). A phase 2, multicenter, open-label study of trastuzumab deruxtecan (DS-8201a) in subjects with HER2-positive, unresectable and/or metastatic breast cancer previously treated with T-DM1. Journal of Clinical Oncology 36, suppl, TPS1102–TPS1102. [Google Scholar]
- Bertucci F, Finetti P, Birnbaum D, and Mamessier E (2016). The PD1/PDL1 axis, a promising therapeutic target in aggressive breast cancers. Oncoimmunology 5, e1085148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenak L, Magyar A, Boja R, László G (2001). Differential expression of GL7 activation antigen on bone marrow B cell subpopulations and peripheral B cells. Immunol Lett. 78, 89–96. [DOI] [PubMed] [Google Scholar]
- Cheung AM, Wan TS, Leung JC, Chan LY, Huang H, Kwong YL, Liang R, and Leung AY (2007). Aldehyde dehydrogenase activity in leukemic blasts defines a subgroup of acute myeloid leukemia with adverse prognosis and superior NOD/SCID engrafting potential. Leukemia 21, 1423–1430. [DOI] [PubMed] [Google Scholar]
- Clay MR, Tabor M, Owen JH, Carey TE, Bradford CR, Wolf GT, Wicha MS, and Prince ME (2010). Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck 32, 1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico L, Menzel U, Prummer M, Müller P, Buchi M, Kashyap A, Haessler U, Yermanos A, Gébleux R, Briendl M, Hell T, Wolter FI, Beerli RR, Truxova I, Radek Š, Vlajnic T, Grawunder U, Reddy S, Zippelius A (2019). A novel anti-HER2 anthracycline-based antibody-drug conjugate induces adaptive anti-tumor immunity and potentiates PD-1 blockade in breast cancer. J Immunother Cancer 7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S, Yang X, Lassus H, Liang S, Kaur S, Ye Q, Li C, Wang LP, Roby KF, Orsulic S, et al. (2010). Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS One 5, e10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng SQ, Alexandrou AT, and Li JJ (2014). Breast cancer stem cells: Multiple capacities in tumor metastasis. Cancer Lett 349, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H (1984). Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 133, 1710–1715. [PubMed] [Google Scholar]
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, et al. (2007). ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1, 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W (2014). Concise review: breast cancer stem cells: regulatory networks, stem cell niches, and disease relevance. Stem Cells Transl Med 3, 942–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JM, Xia W, Hsu YH, Chan LC, Yu WH, Cha JH, Chen CT, Liao HW, Kuo CW, Khoo KH v et al. (2018). STT3-dependent PD-L1 accumulation on cancer stem cells promotes immune evasion. Nat Commun 9, 1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Lu L, Xia Y, Chen X, Chang AE, Hollingsworth RE, Hurt E, Owen J, Moyer JS, Prince ME et al. (2016). Therapeutic Efficacy of Cancer Stem Cell Vaccines in the Adjuvant Setting. Cancer Res 76, 4661–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, and Rofstad EK (2017). Cancer stem cells (CSCs), cervical CSCs and targeted therapies. Oncotarget 8, 35351–35367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WC, Hung CM, Wei CT, Chen TM, Chien PH, Pan HL, Lin YM, and Chen YJ (2016). Interleukin-6 expression contributes to lapatinib resistance through maintenance of stemness property in HER2-positive breast cancer cells. Oncotarget 7, 62352–62363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal W, Alkarim S, AlHejin A, Mukhtar H, and Saini KS (2016). Targeting signal transduction pathways of cancer stem cells for therapeutic opportunities of metastasis. Oncotarget 7, 76337–76353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ithimakin S, Day KC, Malik F, Zen Q, Dawsey SJ, Bersano-Begey TF, Quraishi AA, Ignatoski KW, Daignault S, Davis A, et al. (2013). HER2 drives luminal breast cancer stem cells in the absence of HER2 amplification: implications for efficacy of adjuvant trastuzumab. Cancer Res 73, 1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, Xing L, Wang H, Liu Z, Su Y, Stass SA, and Katz RL (2009). Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res 7, 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw MH, Jackson JT, Haynes NM, Teng MW, Moeller M, Hayakawa Y, Street SE, Cameron R, Tanner JE, Trapani JA, Smyth MJ, Darcy PK (2004). Gene-engineered T cells as a superior adjuvant therapy for metastatic cancer. J Immunol 173, 2143–2150. [DOI] [PubMed] [Google Scholar]
- Kim MP, Fleming JB, Wang H, Abbruzzese JL, Choi W, Kopetz S, McConkey DJ, Evans DB, and Gallick GE (2011). ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma. PLoS One 6, e20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurty AT, Thouvenel CD, Portugal S, Keitany GJ, Kim KS, Holder A, Crompton PD, Rawlings DJ, Pepper M (2016). Somatically Hypermutated Plasmodium-Specific IgM(+) Memory B Cells Are Rapid, Plastic, Early Responders upon Malaria Rechallenge. Immunity 45, 402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal AD, Krishnamurthy A, Head L, and Messersmith WA (2018). Antibody drug conjugates under investigation in phase I and phase II clinical trials for gastrointestinal cancer. Expert Opin Investig Drugs. [DOI] [PubMed] [Google Scholar]
- Li JY, Perry SR, Muniz-Medina V, Wang X, Wetzel LK, Rebelatto MC, Hinrichs MJ, Bezabeh BZ, Fleming RL, Dimasi N, et al. (2016). A Biparatopic HER2-Targeting Antibody-Drug Conjugate Induces Tumor Regression in Primary Models Refractory to or Ineligible for HER2-Targeted Therapy. Cancer Cell 29, 117–129. [DOI] [PubMed] [Google Scholar]
- Li T, Su Y, Mei Y, Leng Q, Leng B, Liu Z, Stass SA, and Jiang F (2010). ALDH1A1 is a marker for malignant prostate stem cells and predictor of prostate cancer patients’ outcome. Lab Invest 90, 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Opyrchal M, Yao S, Peng X, Yan L, Jabbour H, and Khoury T (2018). The role of programmed death ligand-1 and tumor-infiltrating lymphocytes in breast cancer overexpressing HER2 gene. Breast Cancer Res Treat 170, 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Lee JS, Jie C, Park MH, Iwakura Y, Patel Y, Soni M, Reisman D, and Chen H (2018). HER2 Overexpression Triggers an IL1alpha Proinflammatory Circuit to Drive Tumorigenesis and Promote Chemotherapy Resistance. Cancer Res 78, 2040–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Clouthier SG, Deol Y, Liu S, Nagrath S, Azizi E, and Wicha MS (2015). Breast cancer stem cells: current advances and clinical implications. Methods Mol Biol 1293, 1–49. [DOI] [PubMed] [Google Scholar]
- Ma B, Ma Q, Wang H, Zhang G, Zhang H, and Wang X (2016). Clinical efficacy and safety of T-DM1 for patients with HER2-positive breast cancer. Onco Targets Ther 9, 959–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Chan KW, Lee TK, Tang KH, Wo JY, Zheng BJ, and Guan XY (2008). Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res 6, 1146–1153. [DOI] [PubMed] [Google Scholar]
- Ma Y, Xiang D, Sun J, Ding C, Liu M, Hu X, Li G, Kloecker G, Zhang HG, Yan J (2013). Targeting of antigens to B lymphocytes via CD19 as a means for tumor vaccine development. J Immunol. 190, 5588–5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron MJ, Park PW, Fooksman DR (2017). CD138 mediates selection of mature plasma cells by regulating their survival. Blood 129, 2749–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P, Kreuzaler M, Khan T, Thommen DS, Martin K, Glatz K, Savic S, Harbeck N, Nitz U, Gluz O, et al. (2015). Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci Transl Med 7, 315ra188. [DOI] [PubMed] [Google Scholar]
- Müller P, Rios-Doria J, Harper J, and Cao A (2018). Combining ADCs with Immuno-Oncology Agents. 11–44. [Google Scholar]
- Nami B, and Wang Z (2017). HER2 in Breast Cancer Stemness: A Negative Feedback Loop towards Trastuzumab Resistance. Cancers (Basel) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning N, Pan Q, Zheng F, Teitz-Tennenbaum S, Egenti M, Yet J, Li M, Ginestier C, Wicha MS, Moyer JS, et al. (2012). Cancer stem cell vaccination confers significant antitumor immunity. Cancer Res 72, 1853–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oba T, Long MD, Keler T, Marsh HC, Minderman H, Abrams SI, Liu S, Ito F (2020). Overcoming primary and acquired resistance to anti-PD-L1 therapy by induction and activation of tumor-residing cDC1s. Nat Commun. 11, 5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince MEP, Zhou L, Moyer JS, Tao H, Lu L, Owen J, Etigen M, Zheng F, Chang AE, Xia J, et al. (2016). Evaluation of the immunogenicity of ALDH(high) human head and neck squamous cell carcinoma cancer stem cells in vitro. Oral Oncol 59, 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasper M, Schafer A, Piontek G, Teufel J, Brockhoff G, Ringel F, Heindl S, Zimmer C, and Schlegel J (2010). Aldehyde dehydrogenase 1 positive glioblastoma cells show brain tumor stem cell capacity. Neuro Oncol 12, 1024–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios-Doria J, Harper J, Rothstein R, Wetzel L, Chesebrough J, Marrero A, Chen C, Strout P, Mulgrew K , McGlinchey K, et al. (2017). Antibody-Drug Conjugates Bearing Pyrrolobenzodiazepine or Tubulysin Payloads Are Immunomodulatory and Synergize with Multiple Immunotherapies. Cancer Res 77, 2686–2698. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Alba JC, Moreno-García ME, Sandoval-Montes C, Rosales-Garcia VH, Santos-Argumedo L (2008). CD38 induces differentiation of immature transitional 2 B lymphocytes in the spleen. Blood 111, 3644–3652. [DOI] [PubMed] [Google Scholar]
- Roskoski R Jr. (2014a). The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res 79, 34–74. [DOI] [PubMed] [Google Scholar]
- Roskoski R Jr. (2014b). ErbB/HER protein-tyrosine kinases: Structures and small molecule inhibitors. Pharmacol Res 87, 42–59. [DOI] [PubMed] [Google Scholar]
- Sabel MS, Su G, Griffith KA, Chang AE (2010). Intratumoral delivery of encapsulated IL-12, IL-18 and TNF-alpha in a model of metastatic breast cancer. Breast Cancer Res Treat 122, 325–336. [DOI] [PubMed] [Google Scholar]
- Sakai H, Tsurutani J, Iwasa T, Komoike Y, Sakai K, Nishio K, and Nakagawa K (2018). HER2 genomic amplification in circulating tumor DNA and estrogen receptor positivity predict primary resistance to trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer. Breast Cancer 25, 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenburg A, Blatt K, Cerny-Reiterer S, Sadovnik I, Herrmann H, Marian B, Grunt TW, Zielinski CC, and Valent P (2015). Cancer stem cells in basic science and in translational oncology: can we translate into clinical application? J Hematol Oncol 8, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy A, Butterworth E, and Huang EH (2012). ALDH as a marker for enriching tumorigenic human colonic stem cells. Methods Mol Biol 916, 373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura M, Nagayama Y, Matsuse M, Yamashita S, and Mitsutake N (2014). Analysis of multiple markers for cancer stem-like cells in human thyroid carcinoma cell lines. Endocr J 61, 481–490. [DOI] [PubMed] [Google Scholar]
- Su Y, Qiu Q, Zhang X, Jiang Z, Leng Q, Liu Z, Stass SA, and Jiang F (2010). Aldehyde dehydrogenase 1 A1-positive cell population is enriched in tumor-initiating cells and associated with progression of bladder cancer. Cancer Epidemiol Biomarkers Prev 19, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K, Kashiwagi S, Goto W, Asano Y, Takahashi K, Takashima T, Tomita S, Ohsawa M, Hirakawa K, and Ohira M (2018). Use of the tumor-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting treatment responses to combination therapy with pertuzumab, trastuzumab, and docetaxel for advanced HER2-positive breast cancer. J Transl Med 16, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Park P, Zhang H, La Marca F, and Lin CY (2011). Prospective identification of tumorigenic osteosarcoma cancer stem cells in OS99-1 cells based on high aldehyde dehydrogenase activity. Int J Cancer 128, 294–303. [DOI] [PubMed] [Google Scholar]
- Wang SH, Lu L, Fan Y, Wicha MS, Cao Z, Chang AE, Xia JC, Baker JR Jr., and Li Q (2013). Characterization of a novel transgenic mouse tumor model for targeting HER2+ cancer stem cells. Int J Biol Sci 10, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Li Y, Liu N, Gao Y, and Li L (2017). MiR-23b controls ALDH1A1 expression in cervical cancer stem cells. BMC Cancer 17, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Carter RH,. (2005). CD19 regulates B cell maturation, proliferation, and positive selection in the FDC zone of murine splenic germinal centers. Immunity 22, 749–761. [DOI] [PubMed] [Google Scholar]
- Wei WZ, Shi WP, Galy A, Lichlyter D, Hernandez S, Groner B, Heilbrun L, Jones RF (1999). Protection against mammary tumor growth by vaccination with full-length, modified human ErbB-2 DNA. Int J Cancer 81, 748–754. [DOI] [PubMed] [Google Scholar]
- Xu-Monette ZY, Zhang M, Li J, and Young KH (2017). PD-1/PD-L1 Blockade: Have We Found the Key to Unleash the Antitumor Immune Response? Front Immunol 8, 1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y, and Sliwkowski MX (2001). Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2, 127–137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All software and parameters used are described, and custom scripts and functions will be made available upon request.


