Abstract
Social behaviors, such as mating, fighting and parenting, are fundamental to the survival of any vertebrate species. All members of a species express social behaviors in a stereotypical and species-specific way without training thanks to developmentally hardwired neural circuits dedicated to these behaviors. Despite being innate, social behaviors are flexible. The readiness to interact with a social target or engage in specific social acts can vary widely based on reproductive state, social experience and many other internal and external factors. Such high flexibility renders vertebrates the ability to release the relevant behavior at the right moment and towards the right target. This maximizes the reproductive success while minimizing the cost and risk associated with behavioral expression. Decades of research have revealed the basic neural circuits underlying each innate social behavior. The neural mechanisms that support behavioral plasticity also started to emerge. Here, we will first provide an overview of these social behaviors and their underlying neural circuits, and then discuss in detail, the recent findings regarding the neural processes that support the flexibility of innate social behaviors.
In Brief
Social behaviors such as aggression are innate yet flexible. In this review, Wei et. al. summarize our current understanding of the neural circuits underlying social behaviors in rodents and the plasticity in the circuits that supports the behavioral flexibility.
Innate Social Behaviors
Throughout its lifetime an organism will be exposed to a plethora of internal and external stimuli. It will respond to such stimuli in order to survive and thrive. At times, the organism must respond in a social context, via multimodal forms of communication with conspecifics. For instance, as an infant, it may derive food from its parents to satiate its hunger. As a juvenile, it may indulge in play behavior with its siblings. As an adult, it may mate with conspecifics, defend its territory and resources from conspecific intruders and take care of its young to ensure the continuation of its line. Such social behaviors are instinctive, can be initiated without being explicitly taught and consist of a series of robust, stereotypical action progressions. Their implementation is driven by developmentally hardwired neural circuits and constrained by anatomical and morphological features, which evolve simultaneously. These behavioral solutions are retained through generations due to their contribution in ensuring the continuation of the species (Kappeler et al., 2013).
Much thought has been put into characterizing the components of innate social behaviors. Famed ethologists, Konrad Lorenz and Nikolaas Tinbergen proposed that instinctive behaviors start with an appetitive phase (Hashikawa et al., 2016; Hogan and Bolhuis, 2009; Lorenz, 1981). The initial component of the appetitive phase is detecting and subsequently approaching the social stimulus, or conversely, soliciting a conspecific. A subsequent component of the appetitive phase involves the investigation or exploration of the social stimulus. The appetitive phase is followed by a stereotyped consummatory phase which consists of a series of fixed motor patterns (Hashikawa et al., 2016; Hogan and Bolhuis, 2009; Lorenz, 1981). For the purposes of the review, we divide innate social behaviors into four instead of two phases to better describe the points of change observed in the progression of a particular social behavior. These four phases are detection, approach, investigation and consummation or action phases (Figure 1). The first three phases can be considered together as an identification phase that brings the animal to a close proximity of the target and determine its identity so that appropriate actions can be taken.
Figure 1. The four proposed phases of innate social behaviors.
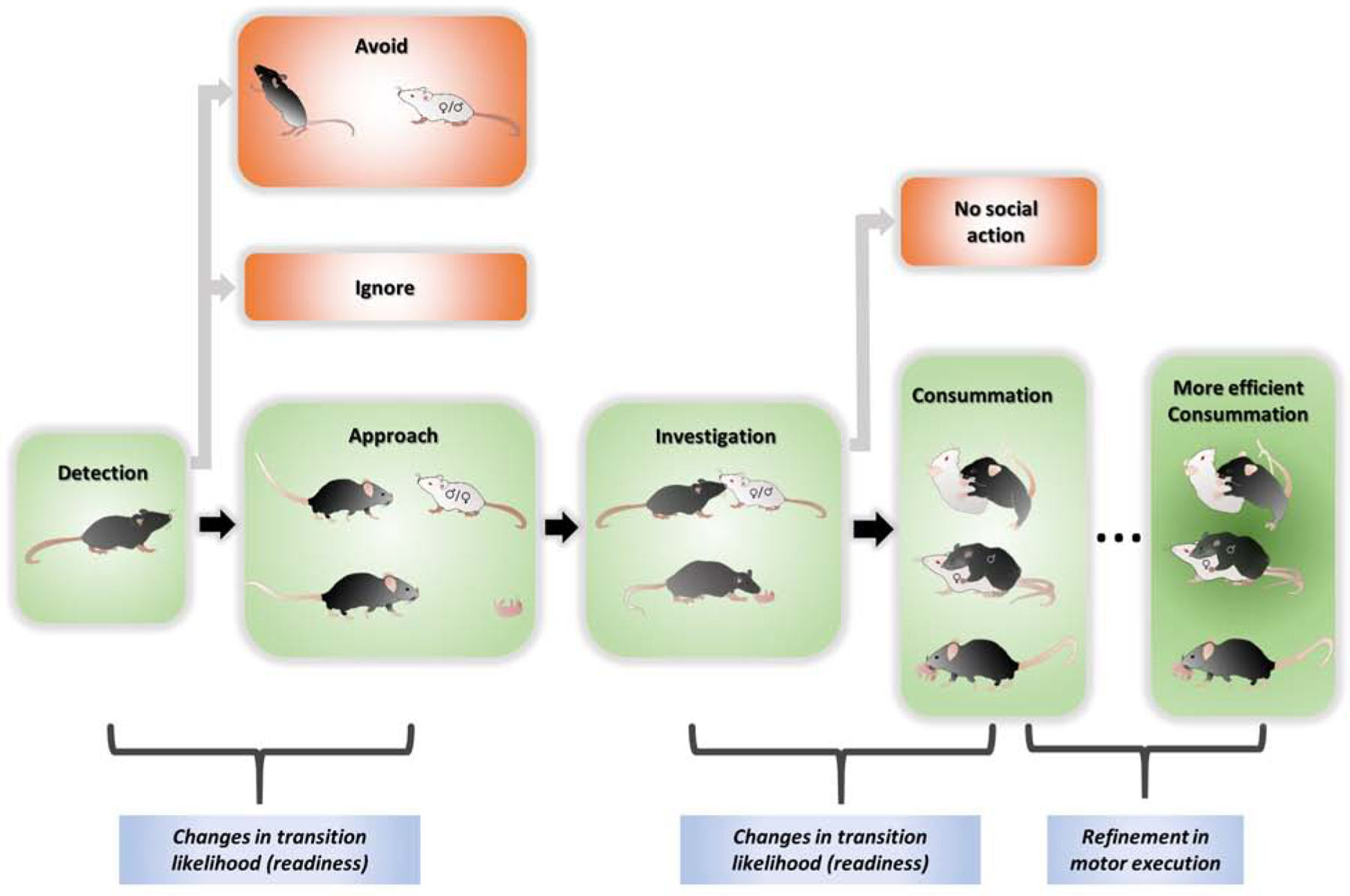
The four phases, including detection, approach, investigation and consummation, are illustrated in green. The readiness to transition from detection to approach and from investigation to consummation is subject to change based on the animal’s experience, internal state and external factors. The motor execution of the consummatory phase can also be refined with experience.
Below, we will describe common innate social behaviors seen in vertebrates. We will particularly focus on rodents as they have been at the center stage of the biological research and display a complex array of innate social behaviors.
Detection Phase
In this phase, an individual aims to identify the presence and location of a distant social target via unique sensory cues emitted by the target. In rodents, such cues are mainly olfactory in nature, such as the vaginal secretions of females or the urine markings of males. The chemical cues are particularly useful for species that live in relative isolation due to their long lasting nature. Animals can also locate the social target using species-specific acoustic or visual cues, for instance, rodent pups emit ultrasonic vocalizations when they are out of their nest and cold (Hofer et al., 2002). These pup calls trigger approach in dams and stop when pups are in contact with their mother or litter – an effect known as contact quieting (Hofer et al., 2002).
Upon sensing conspecific olfactory cues, a rodent will decrease its velocity, twitch its nose rhythmically, whisker and bob its head, all phase-locked with the respiratory cycle, while slowly changing its orientation (Kurnikova et al., 2017). During this process, the breathing rate increases from a basal level of 1–3 Hz to 4–12 Hz (Deschênes et al., 2016). These well-coordinated actions allow the rodent to maximally sample the odor and determine the direction of its source before making a move towards, or in some cases away from it.
Approach Phase
The purpose of the approach phase is to reduce the distance between an individual and a distal social stimulus. It is a prerequisite of social investigation and consummatory actions. The motor outputs that define approach are similar for different types of innate social behaviors, which could involve walking, running, flying or swimming toward the social stimulus.
Approach towards a social target is a consequence of an individual’s internal readiness to engage with conspecifics. Most vertebrates and invertebrates are socially inclined. For some, the mere proximal presence of a conspecific or the presence of conspecific cue is rewarding. For instance, dams are willing to give up food or cocaine in order to be close to pups, specifically in early postpartum (Fleming et al., 1994; Trezza et al., 2011). Naïve female mice developed a preference for the chamber where they were exposed to male-soiled bedding (Martínez-García et al., 2009). More importantly, approach provides a gateway for the individual to carry out rewarding consummatory actions endemic to innate social behaviors such as sexual intercourse, aggression, parenting and social play (Golden et al., 2016; Tenk et al., 2009; Trezza et al., 2011). The opportunity for a two-fold reward, companion of a conspecific and the subsequent consummatory act, is what drives an individual to approach a conspecific.
Investigation Phase
The investigation phase is defined as close exploration of the social stimulus, aiming at gathering information about the conspecific. This phase helps the individual collect enough evidence to proceed to the appropriate, potentially rewarding, consummatory phase.
The motor output during the investigatory phase involves orienting towards the stimulus and examining a part of its body. In many mammals, including rodents and primates, such examination is carried out via sniffing (Jänig et al., 2018; Kepecs et al., 2005). Sniffing is often directed towards facial and anogenital areas where the pheromones are enriched (Liberles, 2014). In addition to sampling chemical cues, the action of sniffing itself could signal the social status of the two animals involved. Specifically, subordinate male rats reliably reduce their sniffing frequency upon being investigated by the dominant rats and failure to do so shortens the latency of the dominant rat to initiate attacks (Wesson, 2013).
Approach and investigation phases precede the consummatory phase of innate social behaviors and are therefore independent of it. The expression of approach and investigation can thus reflect an individual’s internal readiness to engage with a social stimulus even if the subsequent consummatory phase is blocked. The social preference test (conducted mostly in rodents) (Kim et al., 2019a) measures the relative time a test animal spends approaching and investigating two social targets or a social and non-social target under a cup. This assesses the animal’s internal readiness to engage with the stimulus, commonly referred to as social interest. When consummatory behaviors are not blocked, however, the animal investigates and then carries out the appropriate actions towards the social stimulus. A quick transition from investigation to consummation phase reflects a high level of internal readiness to release the consummatory actions.
Consummation (Action) Phase
The consummation phases of different social behaviors differ, as different innate social behaviors aim to accomplish different goals. Below, we describe the consummation phases seen in goal directed innate social behaviors such as sexual behavior, aggression, and infant and parental behavior.
Sexual Behavior
Sexual behaviors are necessary for species survival. Vast majority of vertebrates reproduce sexually. The consummation phase of mating in male rodents consists of three stages (Hull and Dominguez, 2007). The first stage is mounting, where the male pushes his forepaws against the female’s flanks and gives her several shallow thrusts. During the second stage or intromission he detects the female’s vagina with his penis and gives several deeper thrusts. The third stage, or ejaculation, is the expelling of sperm into the vagina following rounds of intromission. Males emit ultrasonic vocalization in conjunction with the copulation to facilitate sexual responsiveness of the female (McIntosh et al., 1978). The female, typically in the proestrous or estrous stage of her reproductive cycle (labeled “receptive”), solicits sexual behavior from the male (Beach, 1976). She then allows the male to mount her with relative ease and assumes a position known as lordosis. Lordosis is characterized by an arched back allowing for vaginal penetration by the male. Despite being thought of as relatively quiescent during mating, female rodents play an active role in copulation. This could be either by pacing the male’s mounts or by adjusting their positions to allow for more efficient intromissions (McClintock and Adler, 1978).
Aggressive Behavior
Aggression is an important and ubiquitous method to defend or compete for territory, resources, mates and to protect oneself and one’s family against potential threats. For rodents, biting is one of the main strategies to inflict pain upon their opponents. A series of motor actions such as aggressive grooming, chasing, lunging and sideway threats are employed to gain access to a favorable target of attack, e.g. the back of an opponent before a bite is delivered (Blanchard and Caroline Blanchard, 1977; Takahashi and Miczek, 2014). In rodents, males are typically more aggressive than females although the motor patterns of attack are largely similar between sexes (Hashikawa et al., 2018). Upon being attacked, the other animal employs a different set of defensive actions, such as jumping, dashing, standing upright and pushing to fend off the bites and escape from the aggressor (Blanchard et al., 1979; Wang et al., 2019).
Infant and Parental Behavior
Infants depend on their parents for food, shelter and protection. Accordingly, a set of innate behaviors have evolved in both the young and the parents to ensure that the needs of the young are met. Most infant mammals show suckling behaviors upon birth in order to derive milk from their mother (German and Crompton, 2000) and the initiation of suckling depends on the olfactory cues around the nipples (Logan et al., 2012). With regards to parenting, starting from early pregnancy, females increase their nest building behavior (Auclair et al., 2014). After giving birth, female mice spend an average of 10–15 hours a day in the nest to care for the young (Auclair et al., 2014). Dams assume a crouching posture over the pups, with all four limbs supported and a slightly arched back, so that the pups can access their ventral surface to suckle the milk. Dams also lick and groom the pups extensively to keep them clean and retrieve the pups if they accidently wander off the nest. While females are the main care givers, fathers, especially those of monogamous species, express many of the same parental behaviors as mothers (Dulac et al., 2014; Kohl et al., 2017).
Circuitry of Innate Social Behaviors
Below, we describe the circuitry that underlies the detection, approach, investigation and consummation phases of social behaviors. Our focus shall be on subcortical circuitries involved in generating sexual, aggressive and parental behaviors. Much of our knowledge is obtained in rodents although these circuitries are likely highly conserved across vertebrate species (Goodson, 2005; O’Connell and Hofmann, 2011). Cortical regions, such as prefrontal cortex, encode complex social information, which could be used to modulate social behaviors (Behrens et al., 2008; Kingsbury et al., 2020; Kingsbury et al., 2019; Nelson et al., 2019; Wang et al., 2011; Zhou et al., 2017). Readers interested in this topic should refer to several excellent reviews (Gangopadhyay et al., 2021; Krueger et al., 2009; Watanabe, 2017).
Circuitry underlying the Detection Phase
In rodents, olfaction is the most crucial sensory modality of communication, although they also use auditory and visual cues to facilitate the localization of the social target (Ryan et al., 2008; Smotherman et al., 1974; Strasser and Dixon, 1986). Volatiles emitted from distant animals are detected by the olfactory sensory neurons (OSNs) in the main olfactory epithelium (MOE) and are transferred to the main olfactory bulb (MOB), where the signal is further distributed to multiple regions, including olfactory tubercle (OT), anterior olfactory nucleus (AON), cortical amygdala (anterior and posterolateral part) (CoAa and COApl), piriform cortex (Pir) and entorhinal cortex (Ent) (Spehr et al., 2006)(Figure 2). During sniffing, a process by which an individual actively acquires the olfactory cues, the animal exerts a series of micro-movements, including fastened breathing, recurrent protraction and retraction of mystacial vibrissae, repeated retraction and protraction of the tip of the snout, and up and down head movements (Deschênes et al., 2016). These movements are generated by a medullary circuit, including the pre-Botzinger complex (preBotC), the core respiratory generator (Feldman and Kam, 2015). How the odor cues reach the preBotC to accelerate respiration and generate coordinated micro-movements remains largely unknown (Deschênes et al., 2016). Along with these nose and head micro-movements, the animals gradually orientate towards the odor source based on the small difference of the odor inputs to the left vs. the right nostril (Rajan et al., 2006). The neural circuit that supports odor orientation is also poorly understood but likely involves inputs from the AON (Kikuta et al., 2010).
Figure 2. The circuitry underlying detection of olfactory and auditory cues and approach.
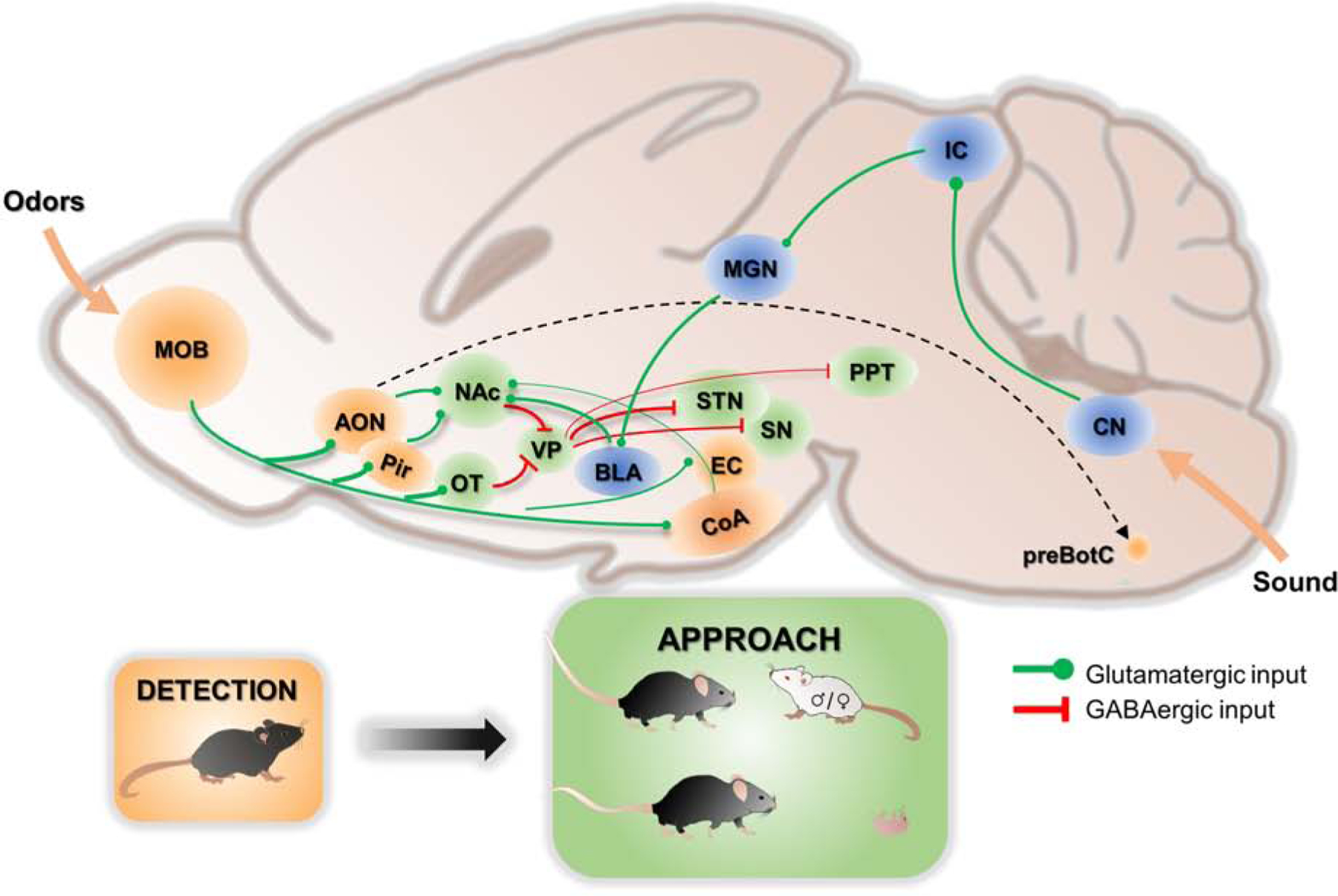
Schematics showing the brain regions involved in detecting olfactory (orange) and auditory (blue) social cues and approaching toward the cues (green) and the relevant connections. The dotted line represents a putative connection that could be indirect. The width of the lines indicates the connection strength. AON: anterior olfactory nucleus; BLA: basolateral amygdala; CN: cochlear nucleus; CoA: cortical amygdala; EC: entorhinal cortex; IC: inferior colliculus; MGN: medial geniculate nucleus; MOB: main olfactory bulb; NAc: nucleus accumbens; OT: olfactory tubercle; Pir: piriform cortex; PPT: pedunculopontine nucleus; preBotC: pre-Botzinger complex; SN: substantia nigra; STN: subthalamic nucleus; VP: ventral pallidum.
Circuitry underlying the Approach Phase
The nucleus accumbens (NAc) has been firmly established as a key region in mediating goal-directed approach behaviors, including approach towards social targets (Blaiss and Janak, 2009; Floresco, 2015; Hamel et al., 2017). Bilateral inactivation of NAc impairs preferential social approach towards stressed juveniles in rats (Rogers-Carter et al., 2019). Administration of an oxytocin receptor antagonist into NAc decreases social approach in monogamous California mice and mandarin voles (Williams et al., 2020; Yu et al., 2016). Conversely, enhancing serotonin release in NAc rescues social approach deficit in mouse autism models (Walsh et al., 2018). Recently, Scribner et al. performed in vivo miniscope imaging and revealed that distinct ensembles of NAc cells are activated during approach towards partners and novel conspecifics in prairie voles (Scribner et al., 2020). Importantly, NAc cells become active prior to approach, supporting its potential function in driving the behavior (Scribner et al., 2020). Dopaminergic cells in ventral tegmental area (VTA) project densely to NAc and are known to modulate synaptic transmission onto striatal medial spiny neurons (MSN) (Tritsch and Sabatini, 2012). Consistent with a role of VTA-NAc dopaminergic cells in social approach, the cells increase activity during the behavior and optogenetic activation of the cells facilitates social approach (Gunaydin et al., 2014). NAc likely connects to the motor system through its GABAergic efferent to the ventral pallidum (VP) (Mogenson and Yang, 1991; Richard et al., 2016). VP in turn projects to the brainstem motor output such as pedunculopontine tegmentum (PPT), substantia nigra (SN), and subthalamic nucleus (STN) to direct locomotion (Smith et al., 2009) (Figure 2).
Multiple regions along the main olfactory pathway can potentially send conspecific olfactory information to NAc (Figure 2). Tracing studies revealed that both AON and Pir provide strong inputs to NAc, especially its core region (Li et al., 2018). Pir is the primary olfactory cortex serving broad functions in odor recognition and discrimination (Bekkers and Suzuki, 2013). Very little is known regarding the function of AON. Two studies involving electrophysiological recordings of AON cells in anesthetized mice and rats showed that AON cells integrate inputs from diverse chemical classes and some cells are specifically tuned to socially relevant olfactory cues (e.g. soiled bedding) (Lei et al., 2006; Tsuji et al., 2019). COApl also sends weak projection to NAc and is preferentially activated by innately attractive odors. Reactivation of COApl cells that are responsive to attractive orders recapitulate the approach behavior (Root et al., 2014).
One additional region along the main olfactory pathway that is worth mentioning is OT. OT is composed of primarily GABAergic cells and considered as a part of the ventral striatum, just like NAc (Yamaguchi, 2017). OT receives projections mainly from the ventral side of MOB where social information is encoded (Martel and Baum, 2007; Scott et al., 1980) and projects strongly to the VP (Wesson and Wilson, 2011). Thus, OT is well positioned to form a circuit parallel to NAc to drive approach based on conspecific olfactory inputs. Consistent with this possibility, recent functional studies showed that lesion or pharmacogenetic inactivation of OT abolished the attraction of male cues to female mice (Agustín-Pavón et al., 2014; DiBenedictis et al., 2015).
Prosocial USVs also activate NAc cells and increase dopamine level in the area (Sadananda et al., 2008; Willuhn et al., 2014). The precise pathway that passes USVs to NAc remains incompletely understood but basolateral amygdala (BLA), a region receives extensive auditory inputs and projects heavily to NAc, is likely a key relay. Electrophysiological recording found that a majority of BLA cells are responsive to social USVs (Parsana et al., 2012).
Circuitry underlying the Investigation Phase
For rodents, once the social target is in close proximity, the approaching animal closely investigates the target to acquire additional information regarding the latter’s social identity. During nasal contact with a conspecific, nonvolatile pheromones are actively pumped into the vomeronasal organ (VNO) and then bind to highly specific receptors on the vomeronasal sensory neurons (VSNs). VSNs then pass the Information to the accessory olfactory bulb (AOB) which in turn projects to medial amgydala (MeA), posterior part of bed nucleus of stria terminalis (BNSTp) and posteromedial cortical amygdala (COApm) (Spehr et al., 2006). From there, the pheromone information is delivered to a series of medial hypothalamic nuclei that are essential for generating the social behaviors, as detailed subsequently (Figure 3).
Figure 3. Schematics showing neural circuits of social behaviors.
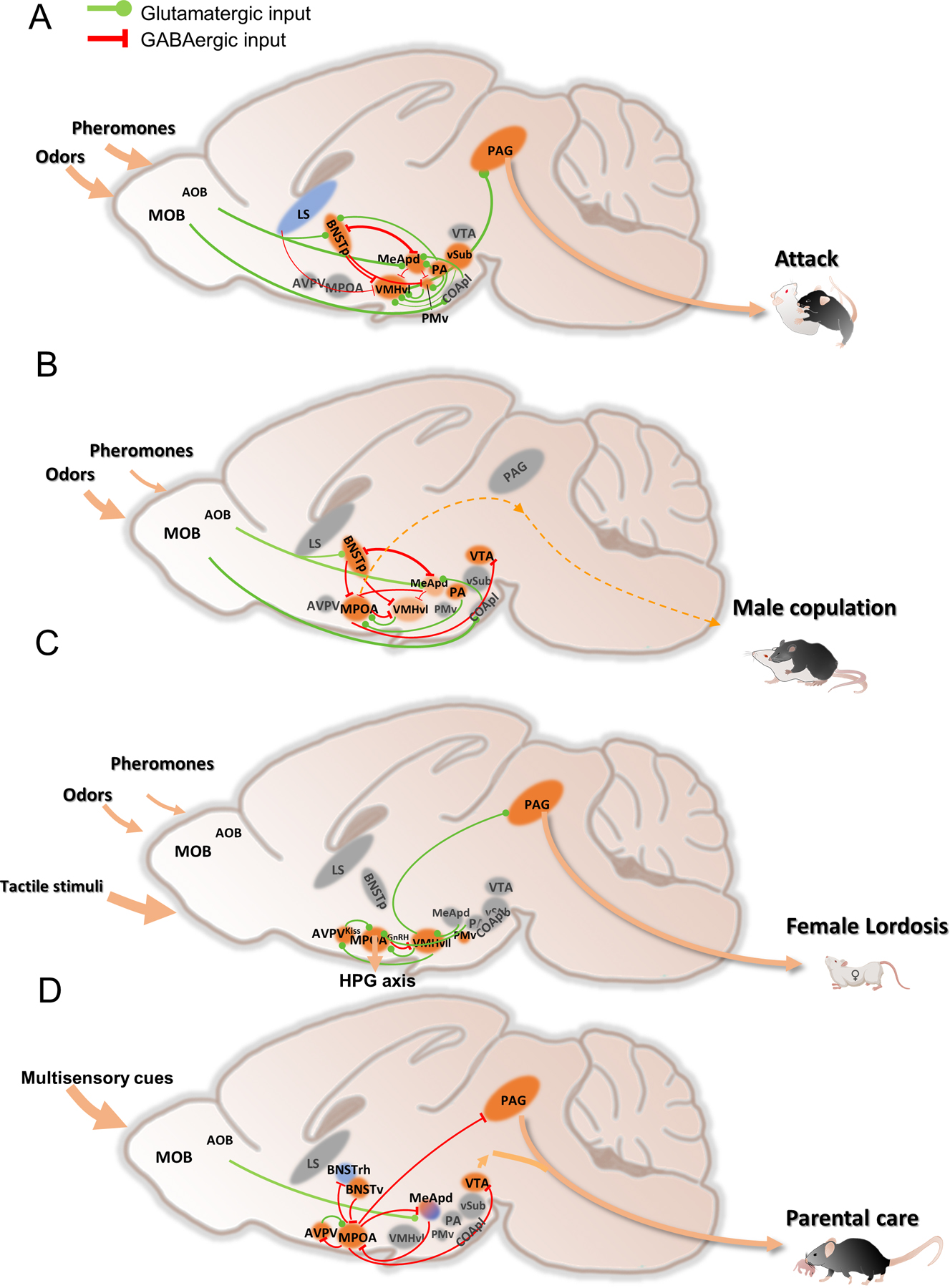
(A) aggression circuit;
(B) male sexual behavior circuit;
(C) female sexual behavior circuit;
(D) parental care circuit.
Solid lines denote known pathways that are involved in mediating the behaviors. Dashed lines denote potential pathways to be further explored. Blue colored regions suppress the behavior while orange colored regions promote the behaviors. Light orange marks regions that play minor roles in the behavior. Gray colored regions are not essential for the behaviors or not yet studied. Arrow size indicates importance of the sensory input. Line width indicates connection strength. Not all the known connections are shown. AOB: accessory olfactory bulb; AVPV: anteroventral periventricular nucleus; BNSTp: posterior part of the bed nucleus of stria terminalis; BNSTrh: rhomboid nucleus of the bed nucleus of stria terminalis; BNSTv: ventral part of the bed nucleus of stria terminalis; COApl: posterolateral cortical amygdala; LS: lateral septum; MeA: medial amygdala; MPOA: medial preoptic area; PA: posterior amygdala; PAG: periaqueductal gray; PMv: ventral premammillary nucleus; VMHvl: ventrolateral part of the ventromedial hypothalamus; VMHvll: lateral subdivision of the VMHvl; vSUB: ventral subiculum; VTA: ventral tegmental area.
Unique to VNO is its ability to accumulate sensory cues. With each bout of sniffing, the pheromone concentration in VNO increases and more VSNs and their downstream cells are activated (He et al., 2010). The information conveyed by VNO is essential for precise social identification (Pankevich et al., 2006) although its exact role in behavior initiation varies based on the nature of social behaviors. VNO inputs appears to be indispensable for aggression initiation. Blocking or genetically impairing VNO severely suppresses aggressive behaviors in both male and female mice in nearly all studies (Chamero et al., 2011; Kimchi et al., 2007; Leypold et al., 2002; Stowers et al., 2002). In contrast, the impacts of VNO deficits on male sexual behavior are variable. VNO ablation diminished mating in some males but not others (Clancy et al., 1984; Powers and Winans, 1975; Winans and Powers, 1977). A careful survey revealed that sexually experienced males are less prone to mating deficits after VNO removal (Meredith, 1986; Saito and Moltz, 1986). Transgenic mice with deficits in subsets of VNO cells showed differential changes in sexual behaviors based on the mutated genes (Yu, 2015). Male and female mice that are deficient for Trpc2, an ion channel essential for VNO sensory transduction, mount intruder males and females excessively and indiscriminately (Kimchi et al., 2007; Leypold et al., 2002; Stowers et al., 2002). Deletion of a cluster of V1R genes caused reduced mounting in males (Del Punta et al., 2002) while disruption of a signal molecule, Gαi2, did not alter male sexual behaviors (Norlin et al., 2003). Thus, VNO inputs appear to facilitate male sexual behaviors but is not absolutely required and inputs from some VNO cells are likely more important than others. In females, VNO ablation experiments support a facilitating role of VNO inputs in female sexual receptivity although Trpc2−/− females show no decrease in lordosis (Kimchi et al., 2007; Lepri and Wysocki, 1987; Mackay-Sim and Rose, 1986; Rajendren et al., 1990).
It is worth noting that during investigation, olfactory signals continue to be detected by MOE, which pass through MOB and converge with vomeronasal inputs onto MeA cells either directly or via COApl (Figure 3)(Martinez-Marcos, 2009). Genetically or chemically disabling MOE cells completely abolished aggression in both sexes and sexual behaviors in male mice (Mandiyan et al., 2005; Wang et al., 2006). The manipulation also reduced lordosis in sex hormone primed females by approximately 50% (Keller et al., 2006). These deficits are unlikely to be solely caused by a lack of close interaction as the MOE defective males failed to copulate with the females even after 10 days of co-housing in a standard shoe box-sized cage. Instead, it suggests that olfactory inputs are indispensable for the transition from investigation to consummatory actions related to aggression and mating.
Circuitry underlying the Consummation Phase
Decades of research have established the medial hypothalamus as a key region where social behavior-promoting cells reside. For nearly a hundred years, neuroscientists have been captivated by the strikingly natural-looking social behaviors elicited by artificial activation of the medial hypothalamic cells (Hashikawa et al., 2017b; Kohl and Dulac, 2018; Siegel et al., 1999; Sternson, 2013). Conversely, blocking activity of this area by permanent lesions or transient inactivation impaired those behaviors (Falkner and Lin, 2014; Kohl and Dulac, 2018; Sternson, 2013). Studies in the last decade further elaborated the hypothalamic regions and their related circuits underlying social behaviors and those findings were covered extensively in several recent reviews (Chen and Hong, 2018; Hashikawa et al., 2016; Ishii and Touhara, 2019; Kohl et al., 2017; Lenschow and Lima, 2020; Micevych and Meisel, 2017; Sternson, 2013). Here, we attempt to provide a synthesized overview of these results, highlighting the differences among those circuits.
The neural circuitry of attack
The ventrolateral part of ventromedial hypothalamus (VMHvl) and the ventral premammilary nucleus (PMv), two hypothalamic nuclei with cells that are almost exclusively glutamatergic, have emerged as the key neural substrates for generating attack (Falkner et al., 2014; Falkner et al., 2016; Hashikawa et al., 2017a; Lee et al., 2014; Lin et al., 2011; Motta et al., 2013; Stagkourakis et al., 2018; Yang et al., 2013; Yang et al., 2017) (Figure 3A). Both VMHvl and PMv receive inputs from MeA and BNSTp with more dense inputs to PMv than VMHvl (Canteras et al., 1995; Dong and Swanson, 2004). The projections from MeA and BNSTp to VMHvl and PMv are primarily GABAergic. Nevertheless, these connections promote aggression, presumably by increasing activity in VMHvl and PMv through a disinhibition mechanism. Another major upstream of PMv and VMHvl is posterior amygdala (PA). PA is largely glutamatergic and PA cells that project to VMHvl are essential for generating male aggression (Canteras et al., 1992b). Unlike MeA and BNSTp, the PA receives inputs mainly from the ventral hippocampus (Yamaguchi et al., 2020; Zha et al., 2020). Lateral septum (LS) receives massive and topographically arranged inputs from hippocampus and provides GABAergic inputs to VMHvl (Leroy et al., 2018; Swanson and Cowan, 1977; Wong et al., 2016). Inactivation of the LS causes an increase in aggression, namely “septal rage”, suggesting that LS exerts a tonic inhibition on the aggression circuit (Albert and Chew, 1980). Lastly, the ventral hippocampus also provides a moderate projection to VMHvl directly, and pharmacogenetic activation of this pathway promotes attack in male mice (Chang and Gean, 2019). Within the hypothalamus, both VMHvl and PMv densely and reciprocally connect with the medial preoptic nucleus (MPN), forming the “medial hypothalamic reproductive system” (Canteras, 2002), although the functional importance of the MPN in aggression remains unclear. Between VMHvl and PMv, PMv provides more inputs to VMHvl than the other way around (Canteras et al., 1992a; Canteras et al., 1994; Lo et al., 2019).
At the output level, PMv primarily targets regions within the hypothalamus whereas VMHvl projects densely to the midbrain, especially the periaqueductal gray (PAG), a premotor region essential for the final execution of attack (Falkner et al., 2020). Thus, the sensory information roughly flows from the early olfactory relays to MeA, PMv, VMHvl and then PAG to generate attack. Regions that are essential for male aggression play similarly important roles in female aggression although quantitative differences of aggression cells between males and females clearly exist (Hashikawa et al., 2018; Motta et al., 2013; Yang et al., 2013). In females, aggression-related cells are mostly concentrated in the medial subdivision of VMHvl (VMHvlm) while the cells are distributed throughout VMHvl in males (Hashikawa et al., 2018; Hashikawa et al., 2017a).
The neural circuitry of male copulation
The consummation phase of sexual behaviors differs qualitatively between sexes and unsurprisingly is supported by sex-specific neural circuits. In males, medial preoptic area (MPOA) has long been recognized as a key region for sexual behaviors based on numerous lesion, activation and recording experiments (Hull and Dominguez, 2007) (Figure 3B). Of note, MPOA is a heterogeneous region that includes MPN, several other nuclei and unnamed regions between anterior commission and the nuclei (www.brain-map.org). In this review, we will use MPOA unless the original study mapped the target precisely to MPN. VMHvl also plays a role in male sexual behaviors, though likely a minor one (Yang et al., 2013). In vivo recordings reveal clear differences between VMHvl and MPOA cell responses during sexual behaviors: while MPOA cells increase activity as the sexual behaviors advance, the VMHvl cells are only transiently activated during the initial mounting and then become suppressed for the rest of the behavior (Lin et al., 2011; Shimura et al., 1994). Interestingly, cells that drive male-style mounting appear to exist in female MPOA and VMHvl, since optogenetic activation of estrogen receptor alpha positive (Esr1+) cells in these regions can elicit time-locked mounting in mice of both sexes (Hashikawa et al., 2017a; Wei et al., 2018). However, the male-style mounting in females is generally considered a dominance behavior for maintaining social status instead of being a form of female sexual behaviors (Fang and Clemens, 1999).
Surprisingly, little is known regarding the outputs from MPOA and VMHvl that drive motor actions during male copulation. One early study that systematically lesioned midbrain regions downstream of MPOA, suggested the dorsolateral tegmentum (DLT) as a key region for male sexual behaviors (Brackett et al., 1986), but a later study failed to confirm this result (Romero-Carbente et al., 2006). Instead, the central tegmental field, a region adjacent to DLT, appears to impair sexual behaviors post-lesion, but the effect is incomplete and transient (Romero-Carbente et al., 2007). The projection from MPOA to the VTA, has received some attention although it is not essential for copulation itself (Hull and Dominguez, 2007; Iyilikci et al., 2016). MPOA-VTA pathway is likely to be responsible for dopamine release in NAc during sexual behaviors which may reinforce the preference towards female cues in sexually experienced males as detailed later (Fujiwara and Chiba, 2018; Iyilikci et al., 2016; Sun et al., 2018; Wang et al., 1995).
MeA, BNST and PA are three major regions upstream of both MPOA and VMHvl. MeA contains an abundance of cells responsive to female pheromone cues (Li et al., 2017) but surprisingly recent cell type-specific inactivation and ablation experiments found a relatively small functional role of MeA in male sexual behaviors (Hong et al., 2014; Unger et al., 2015). In contrast, PA cells that project to MPN are indispensable for male sexual behaviors. Inactivation of PAEsr1 to MPN projecting cells abolished nearly all aspects of male sexual behaviors (Yamaguchi et al., 2020). Similarly, inactivation of BNSTp cells that express aromatase caused clear deficits in male sexual behaviors (Bayless et al., 2019).
The neural circuitry of female copulation
In contrast to males, VMHvl plays a pivotal role in mediating female sexual behaviors (Lenschow and Lima, 2020; Micevych and Meisel, 2017)(Figure 3C). VMHvl is sexually dimorphic. Female VMHvl contains a lateral subdivision (VMHvll) that mediates sexual behaviors (Hashikawa et al., 2017a), which likely does not exist in males based on a lack of many VMHvll-specific genes in male VMHvl (Kim et al., 2019b). The female VMHvll, but not VMHvlm, projects densely to the anteroventral periventricular nucleus (AVPV), a region enriched of kisspeptin (Kiss) expressing cells (Hashikawa et al., 2017a), which are in turn important for regulating GnRH secretion from MPOA cells (Gottsch et al., 2004). While VMHvll-AVPVKiss-MPOAGnRH circuit likely controls the neuroendocrine responses associated with mating, VMHvll-PAG projection has been suggested to mediate lordosis (Lonstein et al., 1998; Sakuma and Pfaff, 1979; Yamada and Kawata, 2014). PMv, the third component of the “medial hypothalamic reproductive system”, is positioned as an interface between the metabolic state, the environmental cues, and the female reproductive circuit. Specifically, leptin, a hormone released from the fat cells, can activate the PMv leptin receptor expressing cells to promote the activity of GnRH expressing cells in females (Donato and Elias, 2011). In birds, PMv contains a population of light-sensitive cells that can detect the day length change and activate the hypothalamus-pituitary-gonad (HPG) axis in preparation for seasonal reproduction (Kang et al., 2007).
Both BNSTp and MeA aromatase cells are non-essential for female sexual receptivity, i.e. lordosis (Kim et al., 2019b; Unger et al., 2015). While it remains possible that other cells in BNSTp and MeA, especially those receiving information from MOB, contribute to the behavior, it is worth noting that tactile cues e.g. palpation of the flanks and perineal contact, appear to be a key sensory trigger of the action phase of female behavior. In female rats, manual vaginal cervical stimulation (VSC) is sufficient to elicit lordosis and induces strong c-Fos expression in PAG, VMHvl and MPOA (Auger et al., 1996; Pfaus et al., 1996). Future studies are needed to delineate the pathway that delivers the tactile information to the hypothalamus.
The neural circuitry of parental care
Although the readiness to show parental behaviors could differ between sexes, the motor outputs of the behaviors, except nursing, are largely the same in males and females. Correspondingly, the neural circuits underlying parental behaviors are qualitatively similar between sexes (Figure 3D)(Dulac et al., 2014). Starting from a lesion study that was performed nearly half a century ago, numerous studies have demonstrated a central role of the MPOA in parental care (Numan, 2006). More recently, studies that focus on genetically identified subpopulations revealed that galanin expressing cells in MPOA (MPOAGal) mediate pup grooming while Esr1 expressing cells in MPOA (MPOAEsr1), which partially overlap with the galanin expressing cells, are essential for pup retrieval in both sexes (Bloch et al., 1992; Fang et al., 2018; Kohl et al., 2018; Wu et al., 2014). In vivo electrophysiological recordings have further revealed important features of MPOA cell responses (Fang et al., 2018). Firstly, many MPOA cells are activated during pup investigation, suggesting that pup sensory cues are key inputs that drive MPOA cells. Secondly, MPOA cells that respond to adults and pups are largely non-overlapping, suggesting that MPOA contains subpopulations with distinct social functions. Consistent with the recording data, single cell RNAseq of MPOA cells revealed that cells activated during different social behaviors are genetically distinguishable (Moffitt et al., 2018). Thirdly, some MPOA cells are activated exclusively during pup retrieval but not pup investigation and the rise of the cell activity precedes the retrieval itself, which is consistent with the response patterns of premotor cells. Fourthly, the MPOA cells that are activated during pup retrieval, grooming and nest building are largely distinct, giving credence to the hypothesis that there are multiple MPOA populations, each of which drive one aspect of parental behavior. Consistent with this, activating MPOAGal projection to PAG, VTA and MeA elicits grooming, pup-seeking and suppression of infanticide respectively, whereas activating MPOAEsr1+ projection to VTA elicits pup approach and retrieval (Fang et al., 2018; Kohl et al., 2018). In addition to the direct behavioral control, MPOA also modulates the neuroendocrine responses related to parental behaviors through its projection to the vasopressin, oxytocin and corticotropin-releasing hormone (CRH) expressing cells in the paraventricular nucleus (PVN) (Kohl et al., 2018; Scott et al., 2015) as well as neuroendocrine dopaminergic (NEDA) neurons in the arcuate nucleus (ARC)(Esteves et al., 2019). NEDA cells are essential for parental behaviors as it controls the release of prolactin, which directly activates MPOA cells and promotes parental behaviors (Brown et al., 2017; Gudelsky, 1981; Stagkourakis et al., 2020a). Interestingly, a recent study found that differential NEDA cell activity caused species difference in paternal behaviors. In male rats, NEDA cells are synchronized, resulting in a high level of dopamine, a low level of prolactin and poor paternal behaviors while NEDA cells in male mice fire asynchronously, leading to low dopamine, high prolactin, high MPOAGal cell activity and paternal care (Stagkourakis et al., 2020a). In contrast to a pivotal role of MPOA in parental care behaviors, VMHvl appears to be completely dispensable. Inactivation of VMHvl did not cause any deficit in maternal care and in vivo recording found no activity change of VMHvl cells during maternal care (Hashikawa et al., 2017a).
While much progress has been made regarding the circuit downstream of MPOA, the parental circuit upstream of MPOA remains elusive. While VNO input that relays through MeA has been traditionally considered as a negative regulator of parental behaviors based on many lesion and inactivation studies (Fleming et al., 1979; Fleming et al., 1980; Numan et al., 1993; Sheehan et al., 2001; Tachikawa et al., 2013), a recent study showed that optogenetic activation of MeA GABAergic cells (the major population in MeA) can elicit both parental, i.e. grooming, and infanticidal behavior depending on the stimulation intensity (Chen et al., 2019). Similarly, disruption of specific subregions of BNST can either facilitate or suppress parental behaviors (Numan and Numan, 1996; Tsuneoka et al., 2015). Thus, it is likely that MeA and BNST contain cell groups that play opposite roles in parental behaviors and their precise molecular identities await to be identified in future studies.
An alternative strategy to identify the parental circuit upstream of MPOA is to understand the sensory trigger of the behavior. In studies that systematically alter the females’ senses or the physical properties of pups (e.g. temperature, smell, skin texture, shape et. al), it was found that none of the sensory modalities are indispensable (Beach and Jaynes, 1956). Anosmic, blind, deaf or anaptic lactating females all retrieved the pups indistinguishably from intact females (Beach and Jaynes, 1956). The abnormality of pup cues in any sensory modality (e.g. painting the pup with a foreign odor) reduces the efficiency of the pup to elicit parental behaviors but rarely abolishes the behaviors. This multi-sensory strategy may be in place to ensure the parental behaviors are readily and robustly released or it may be simply due to a lack of pup-specific pheromones (Isogai et al., 2018). Nevertheless, the diverse sensory cues that can trigger the parental behaviors suggest that the circuitry underlying pup cue processing is likely to be complex. Future studies will be needed to further understand how this multi-modal sensory information reaches MPOA and ultimately releases parental behaviors.
Summary of the neural circuits underlying the consummation phase
By examining various social behavioral circuits all at once, some general principles emerge. First, social behaviors that differ qualitatively in motor patterns between sexes (e.g. sexual behaviors) are mediated by sex-specific circuits whereas social behaviors that only differ quantitatively in their motor patterns between sexes (e.g. aggressive behavior) are mediated by largely the same circuit in both sexes.
Second, in rodents, the sensory modality of proximate cues that trigger the consummation phase of social behaviors differ across the behaviors. While aggression relies heavily on olfactory cues and pheromones, tactile cues likely play a bigger role in female copulation. As such, MeA and BNST, two important relays in the accessory olfactory system, are essential for aggression but dispensable for female sexual behaviors.
Third, the medial hypothalamus is essential for all innate social behaviors examined so far and each behavior is likely to be mediated by a distinct neural population in the region. Specifically, VMHvl is essential for aggression and female sexual behaviors whereas MPOA is essential for parental behaviors and male sexual behavior.
Fourth, VMHvl and MPOA recruit both motor-action pathways, at least partly through PAG, and neuroendocrine pathways. The neuroendocrine pathway is particularly developed for female reproductive circuit, likely due to the significant physical changes required for pregnancy as a result of copulation.
Innate Social Behaviors are Flexible
Social behaviors are innate and are expressed with a stereotypical set of movements, however, they are far from fixed. All animals navigate a dynamic world. Therefore, the ability to adjust their social behaviors accordingly, in both the short and long term, is crucial for their survival and reproductive success. In this section, we will provide an overview of the plasticity seen in social behaviors. Later, we will delve deeper into the mechanisms that make the plasticity of innate social behavior possible.
Plasticity in the Readiness to express Innate Social Behaviors
The readiness or tendency to express a social behavior, is highly plastic and is the biggest source of variability in social behaviors across individuals. For example, in a classic resident-intruder assay where a single-housed resident male mouse encounters a male intruder, some resident mice initiate attack within seconds while others never do. However, when VMHvl is artificially activated in non-aggressive mice, they attack the intruder in a way that is indistinguishable from a naturally occurring attack, suggesting a fully developed motor circuit for executing the behavior (Yang et al., 2017). Thus, the lack of attack in the non-aggressive males is primarily a result of low readiness to engage in the behavior.
Why does the readiness to express a social behavior vary so widely? In short, it is because innate social behaviors are costly. While social stimuli and social behaviors are intrinsically rewarding, the individual must balance these payoffs with a potentially substantial trade-off: cost.
The basic costs of innate social behaviors are the opportunity cost and the metabolic cost. The metabolic cost refers to the energy spent on performing a certain task. The energy level and health of an individual determines whether it can afford the associated metabolic cost. The opportunity cost refers to the loss of potential gain from carrying out alternative behaviors. For instance, an individual carrying out a social behavior could have competing needs in life such as feeding, and must adjust its behavior accordingly after a cost-benefit evaluation. Additionally, engaging in agonistic social behaviors, i.e. aggression, could be particularly costly, i.e. have a risk cost. It could inflict physical damage, and in extreme cases, lead to death. Thus, the risk to engage in aggressive behaviors needs to be calculated and one should only take on such a risk if there is a reasonable chance of winning. As the experience is a good predictor of the future outcome, the willingness to engage in a fight adjusts based on the previous fighting experience (Rutte et al., 2006). In addition to costs that incur in the immediate context, social behaviors often have a long-term cost. For example, sexual behavior could result in pregnancy and birth that requires abundant food and energy to be sustained. Thus, in this case, the frequency of sexual behavior needs to be adjusted to suit the organisms’ environment.
Many factors influence the weights of trade-offs and pay-offs and subsequently the individual’s readiness to exhibit innate social behaviors. These factors include
Internal factors that are defined as scalable and persistent factors internal to an individual, such as sex, age, metabolic level, circadian state, and reproductive state;
External factors that are associated with the environment, such as food availability, territory and population density;
Experiential factors such as sexual experiences and winning or losing experiences.
These factors collectively determine an individual’s readiness to express social behaviors by modulating the probability of sensory-motor transition at two time points (Figure 1). The first point is when the animal initiates a reaction to a distal social stimulus, i.e. transition from detection to approach phase. Internal factors, external factors and the individual’s experience collectively determine if and how quickly an individual approaches, avoids or ignores the social cue upon its detection. The second point is when the animal initiates a reaction to a proximal conspecific, i.e. transition from investigation to consummation phase. Similar to a perceptual decision-making process, the sensory information is gathered and accumulated during investigation. Once and if a specific internal threshold is reached, the relevant consummation (e.g. attack) is initiated. The external, internal and experiential factors can all influence the set point of the threshold that triggers the action.
Plasticity in the Motor Performance of Consummatory Actions
While the consummation phase of social behaviors is highly stereotyped, there is room for improvement in making the actions more efficient. The consummation aspects of social behaviors follow the same “practice makes perfect” rule that applies to complex learned motor actions (Papale and Hooks, 2018) (Figure 1).
The winner effect, found in rodents, humans and fish among others, states that the probability of winning a fight increases after repeated winning experiences (Beaugrand et al., 1991; Hsu and Wolf, 2001; Oyegbile and Marler, 2005; Zilioli and Watson, 2014). This increase in winning probability is likely not only due to an increase in willingness to fight but also in the actual fighting ability, meaning fighting with high efficiency (minimum movement to strike), high accuracy (accurate targeting of the vulnerable body parts of the opponent) and high precision (consistency of the behavior) (Briffa and Lane, 2017). As a result, experienced winners can defeat opponents that are bigger in size, even though size is a strong predictor of the fighting outcome (Oyegbile and Marler, 2005). To date, detailed and quantitative measures of attacking movements remain lacking. Future studies combining high speed video recording, wireless EMG recording, and machine learning based fine movement characterization will allow a quantitative assessment of motor actions during attack and its potential refinement with experience (Wiltschko et al., 2015).
Repeated sexual experience also quickens and refines actions performed during mating. Sexually experienced male mice and rats show a shorter latency to mount and intromit and a higher chance to achieve ejaculation (Sulain et al., 2011; Swaney et al., 2012). On the other hand, sexually inexperienced male hamsters often orient themselves incorrectly, resulting in ectopic mounts of the female’s head (Miller et al., 1977). Sexual experience in females also contributes to a higher success of mating. When naïve male hamsters mount sexually experienced female hamsters, they have a higher chance to turn mounts to intromissions (hit rate – 70%) in comparison to mounting towards naïve female hamsters (hit rate – 50%) (Bradley et al., 2005).
Neural mechanism mediating the plasticity of social behaviors
The plasticity in social behaviors must be supported by changes in the underlying circuits. The circuit changes could occur at the level of individual cells or communication between cells, i.e. synapses, and ultimately the input-output relationship of the circuit (Figure 4). As mentioned earlier, changes in innate social behaviors mainly occur at two sensory-motor transformation points: the first is when the animal responds to a distant social cue and approaches it, and the second is when the animal responds to proximate cues and initiates specific consummatory actions. Here, we will discuss the neural mechanisms underlying the social behavioral changes at these two time points with specific examples. Sex hormones, neuromodulators and spike-timing dependent plasticity all contribute to the changes. While the efficiency and precision of actions endemic to social behaviors can change with experience (another form of plasticity), its underlying mechanisms remain unexplored and will not be discussed in the current review.
Figure 4. An overview of the neural mechanisms underlying the plasticity of innate social behaviors.
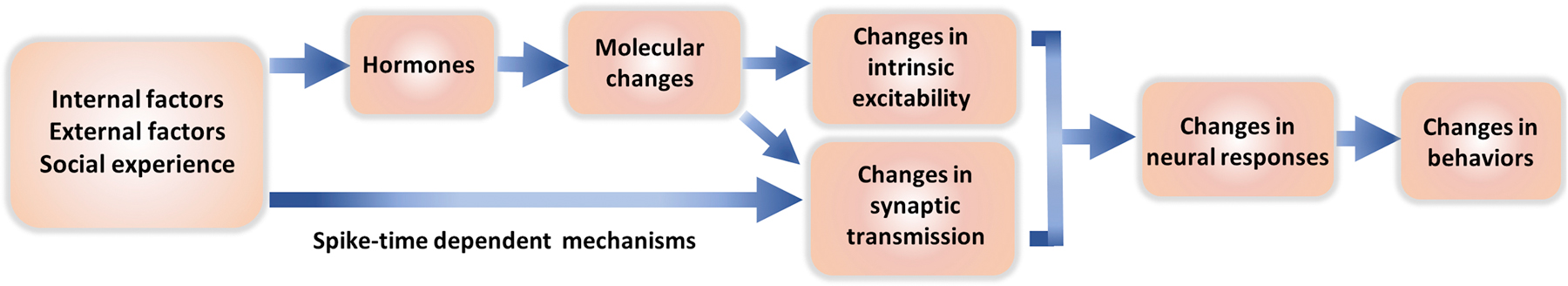
Internal factors (e.g. reproductive state), external factors (e.g. population density), and social experience (e.g. winning) can all lead to changes in hormonal levels or Hebbian spike timing dependent plasticity. Hormones induce changes in the molecular composition of cells and subsequently lead to changes in cell morphology, excitability and synaptic strength. Hebbian mechanisms can lead to potentiation or depression of specific synapses. Together, these physiological changes alter the cell responses to conspecific sensory inputs and the readiness to express social behaviors.
Changes in females’ readiness to approach males with estrus cycle
Female sexual motivation is synchronized with the ovulation period in most species. In rodents, females show low interest towards the male cues during diestrous when progesterone level is high and estrogen level is low. In contrast, the females’ interest towards males increases during proestrus and estrus when estrogen level surges and the progesterone level is low. Sex hormones play a pivotal role in changing female sexual interest by acting on multiple nodes in the circuit (Erskine, 1989; Mhaouty-Kodja et al., 2018) (Figure 5A).
Figure 5. Neural mechanisms underlying reproductive state and experience induced changes in social approach.
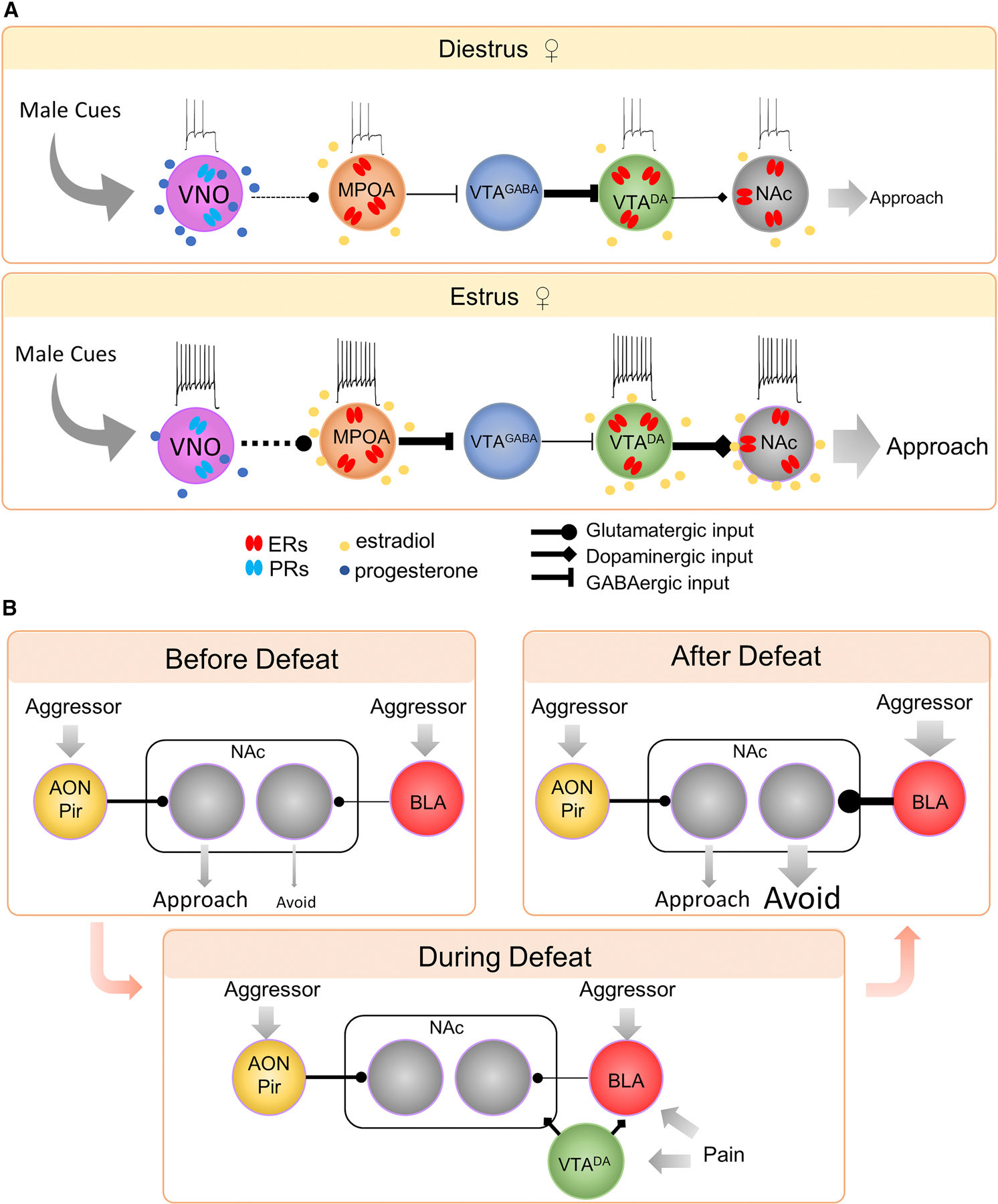
(A) Schematics showing how females’ readiness to approach males changes with the estrus cycle. Progesteone level during diestrus is high, causing a decrease in VNO cell responses to males cues. In contrast, during estrus, estrodial level is high, causing an increase in intrinsic excitability of MPNNts cells, which leads to decreased inhibitory tone within the VTA and increased DA transmission to the NAc. Estrodial also acts on VTA DA neurons and NAc MSNs cells to increase the dopamine release from the VTA and the overall responses of NAc MSNs. (B) A proposed circuit underlying the defeat induced social avoidance. Before defeat, male cues, including cues of the aggressor, dominantly activate the AON/Pir-NAc circuit and drive approach. During acute defeat, the pairing of the sensory cues of the aggressor and the pain causes a potentiation of the aggressor input to BLA. The pairing of the DA release in the NAc and BLA inputs causes a potentiation of BLA synaptic inputs to the NAc cells. Collectively, the aggressor cues activate the BLA-NAc circuit post-defeat to drive social avoidance while other male cues continue to primarily activate the AON/Pir-NAc circuit to drive approach.
Size of the arrows and line width indicate the strength of the inputs and outputs while the font size indicates the readiness of approach. Dashed lines indicate multi-synaptic connections.
In an elegant study, Dey et. al. found that VNO cells lost their responses to male mouse pheromones -- major urinary proteins (MUPs) during diestrus thanks to the high progesterone level (Dey et al., 2015). Progesterone suppresses VNO cell responsiveness by acting on non-canonical progesterone receptor which then recruits phospholipase C β2 (PLCβ2) that is abundantly expressed in MUP-responsive but not MUP-nonresponsive VNO cells (Dey et al., 2015). Thus, the rise of progesterone in diestrus causes the females to become selectively “anosmic” to male pheromones.
Sex hormones, especially estradiol, also act centrally, e.g. NAc, to increase females’ readiness to approach males (Figure 5A). First, estradiol can directly modulate the activity of NAc MSNs through estrogen receptors (Almey et al., 2015). Application of estradiol can rapidly change the intrinsic excitability and miniature excitatory postsynaptic current (mEPSC) of NAc MSNs and is essential for the estrus cycle-dependent fluctuation of the intrinsic properties of MSNs (Krentzel et al., 2019; Proano et al., 2018). Second, estradiol can also modulate MSNs through its influence on dopamine transmission. For example, during estrus when estradiol level is high, VTA dopamine cells show higher firing rate and stimulation of VTA dopaminergic terminals elicits a higher dopamine release in NAc in comparison to diestrus females (Calipari et al., 2017). The enhanced VTA-NAc dopamine level in turn promotes activation of D1R MSN cells and favors social approach (Gunaydin et al., 2014; Tritsch and Sabatini, 2012; Yoest et al., 2014). Third, estradiol can act on hypothalamic neurons to enhance the dopamine release specifically in response to social cues. Estradiol is found to increase the excitability of the neurotensin expressing MPOA cells (MPOANts) in ovariectomized (OVX) female mice (McHenry et al., 2017). Since MPOANts cells are preferentially responsive to male cues, project to VTA and directly induce dopamine release in NAc, an increase in excitability of these cells during proestrus is expected to increase dopamine release in NAc to male odors (McHenry et al., 2017).
Altogether, sex hormone fluctuation during the estrous cycle changes the female’s sensitivity and interest to male cues by modulating the cell responsiveness at multiple regions along the detection, approach and investigation circuits.
Changes in partner preference with sexual experience
The experience of positive consummatory social behaviors could lead to changes in the preference to the cues associated with the experience, i.e. the readiness to approach the cue. In rats and mice, naïve males show no preference between cues of males, estrous females, and diestrous females, while sexually experienced males strongly prefer the estrous female cues (Fujiwara and Chiba, 2018; Hayashi and Kimura, 1974; Hosokawa and Chiba, 2005; Landauer et al., 1977). In the case of monogamous prairie voles, mating induces a strong and enduring preference towards the mated partner, a phenomenon known as pair bonding (Young et al., 2011). Several lines of evidence suggest that just like other forms of reward associative learning, VTA dopaminergic neurons and NAc play important roles in mating induced partner preference. In vivo microdialysis, fast-cyclic voltammetry and optical recordings using genetically encoded dopamine sensors all revealed an increase of dopamine in NAc during sexual behaviors (Pleim et al., 1990; Sun et al., 2018). The dopamine release is locked to individual mating episodes and reaches its maximum during ejaculation (Sun et al., 2018). Blocking the dopamine action in NAc by injecting D2 receptor antagonist prior to the mating period blocks the partner preference induced by mating in prairie voles (Aragona et al., 2006; Aragona and Wang, 2009; Gingrich et al., 2000) whereas activation of D2 receptors within the rostral NAc shell facilitates partner preference, supporting an important role of dopamine in mating-induced partner preference (Aragona et al., 2006).
Dopamine is not the only neuromodulator essential for social reward learning. Oxytocin, a neuropeptide hormone widely indicated in social behaviors, is abundantly released during mating in both rodents and humans (Borrow and Cameron, 2012). Blockade of OXTR in NAc prevented partner preference induced by mating whereas administration of oxytocin in NAc is sufficient to induce partner preference in female prairie voles (Liu and Wang, 2003). The facilitating effect of oxytocin and dopamine on partner preference is interdependent as D2 antagonist in NAc abolished partner preference induced by oxytocin and vice versa although the precise mechanisms remain unclear (Liu and Wang, 2003; Young et al., 2001). In addition to dopamine, oxytocin also interact with serotonin to induce plasticity in NAc. Dölen et. al. found that oxytocin release at NAc core during social interaction is indispensable for the social interaction induced conditioned preference (Dolen et al., 2013). Oxytocin acts on the presynaptic terminals of serotonin axon terminals from dorsal raphe, causing an increase in serotonin release, which in turn induces long term depression (LTD) of the glutamatergic synaptic inputs onto MSNs. The induced synaptic plasticity is essential for the social interaction conditioned preference as either OXTR knockdown or antagonizing 5HT1B in the NAc abolished the preference to the social interaction conditioned chamber (Dolen et al., 2013).
Thus, mating induced partner preference, either to a specific animal or ones alike, could be considered as an associative learning process that is supported by synaptic plasticity and resulted from combined actions of various neuromodulators, including dopamine, oxytocin and perhaps many more.
Social defeat induced social avoidance
While social stimuli are intrinsically attractive, negative social experiences can completely override this attraction (Figure 5B). When a male mouse is defeated by another male conspecific for as short as 10 minutes, the defeated animal shows avoidance towards the defeater for at least three days (Qi et al., 2018). The avoidance behavior is not simply a lack of social approach although it is often quantified in such a way, instead it is an active behavior that involves freezing, defensive crouching, stretched approaches (risk assessment) and running away from the defeater (Toth and Neumann, 2013). The one-time defeat induced avoidance is target-specific. The defeated animals will only avoid the defeater but not unknown conspecifics (Toth and Neumann, 2013). The specificity of the avoidance behavior suggests that the behavioral change is likely a result of the associative learning process during which the cues of a specific conspecific becomes tightly associated with the painful experience of defeat (Diaz and Lin, 2020). The BLA, a region that is well studied for its role in fear conditioning, has been found essential for acute defeat-induced social avoidance. Inactivation, blocking protein synthesis, or blocking NMDA receptors into BLA before defeat all reduced social avoidance 24 hours later (Day et al., 2011; Jasnow and Huhman, 2001; Markham and Huhman, 2008; Markham et al., 2010). Conversely, increasing the cAMP-responsive element binding protein (CREB), a transcription factor essential for memory formation, in BLA enhanced social avoidance (Jasnow et al., 2005). Inhibiting BLA immediately before the post-defeat social avoidance test also reduced the avoidant behavior, supporting a role of BLA in both social avoidance acquisition and expression (Jasnow and Huhman, 2001).
While NAc is essential for driving social approach, paradoxically, it is also a region essential for social avoidance. Pharmacological inhibition of NAc 24 hours after defeat reverses social avoidance (Luckett et al., 2012). Injecting a dopamine receptor antagonist, flupenthixol, into NAc before defeat also blocks social avoidance in defeated hamsters 24 hours later, suggesting that the defeat-induced avoidance is dependent on the DA signaling in NAc (Gray et al., 2015; Luckett et al., 2012). Consistently, microdialysis revealed an increase in dopamine level in NAc during defeat (Holly et al., 2015). NAc is a major area downstream of BLA. A recent study reported that direct optogenetic activation of BLA-NAc pathway increases social avoidance in undefeated mice (Folkes et al., 2020). Altogether, these data support a model that repeated association between the aggressor cues and the painful experience during defeat causes an increased response of BLA cells to the aggressor, while repeated pairing of BLA glutamatergic inputs and dopamine release in NAc potentiates the BLA-NAc pathway and leads to social avoidance to the defeater (Figure 5B).
How NAc cells drives both social approach and social avoidance remains incompletely understood but it is likely that NAc contains subpopulations of cells with distinct functions. Indeed, the responses and functions of NAc cells vary with their anatomical location in NAc as well as MSN type (D1R vs. D2R) (Al-Hasani et al., 2015; de Jong et al., 2019; Hikida et al., 2010; Kravitz et al., 2012; Pecina and Berridge, 2005; Yang et al., 2018). Roughly speaking, NAc D1R cells are approach-promoting and essential for reward learning whereas NAc D2R cells are aversion-promoting and essential for aversive learning (Hikida et al., 2010; Hikida et al., 2013; Kravitz et al., 2012; Zalocusky et al., 2016). Thus, it is possible that the relative activity of the approach-driving and avoidance-driving cells in NAc determines the final behavior output. It is worth noting that BLA-NAc is likely not the only pathway to drive defeat-induced social avoidance, other pathways involving BNST and hypothalamus may also play a role (Diaz and Lin, 2020; Duque-Wilckens et al., 2020; Steinman et al., 2019).
Unlike acute defeat, chronic (10 continuous days) social defeat leads to generalized social avoidance as well as a suite of depression-like behaviors, including a decreased preference to attractants, e.g. sucrose, in a subset of animals (called susceptible animals) (Kudryavtseva et al., 1991; Rygula et al., 2005; Toth and Neumann, 2013; Wells et al., 2017). These profound behavioral changes appear to be critically dependent on an increase in BDNF, but not DA, in NAc that is originated from VTA terminals (Berton et al., 2006; Wook Koo et al., 2016). Specifically, after chronic defeat, VTA dopamine cells that project to NAc show an enhancement in tonic firing while the corticotropin releasing factor (CRF) level in NAc is simultaneously increased, resulting in an activity-dependent and CRF-gated upregulation of BDNF secretion in NAc (Krishnan et al., 2007; Walsh et al., 2014). BDNF appears to preferentially act on TrkB receptors of MSN D1 cells, causing phosphorylation of extracellular signal-regulated kinase (ERK), which is linked to a reduction in MSN D1 cell activity (Koo et al., 2019; Lobo et al., 2010; Wook Koo et al., 2016). Furthermore, chronic defeat reduces the excitatory synaptic inputs to MSN D1 cells, further leading to reduced activities of MSN D1 cells (Francis et al., 2015). Taken together, these results suggest a model that chronic defeat dampens NAc MSN D1 cell responses by increasing BDNF level in NAc and decreasing the excitatory synaptic drive of the cells. The blunted MSN D1 responses cause a suppression of approaching behaviors towards various hedonic stimuli. Consistent with this model, artificial activation of MSN D1 cells, but not D2 cells, reverses social avoidance and restores sucrose preference in susceptible mice (Francis et al., 2015).
Changes in the animals’ readiness to execute consummatory social behaviors with reproductive state
Reproductive state influences the readiness to express social behaviors profoundly. Perhaps, the most extreme example comes from the behavioral changes during parenthood. In mothers, sexual behavior and infanticide are virtually abolished while maternal behavior and aggression towards intruders increase dramatically. These behavioral changes are a result of orchestrated hormonal changes during pregnancy, starting with surges of ovarian hormones, such as estrogen and progesterone, before parturition and followed by the rise of pituitary hormones, such as prolactin and oxytocin, after parturition to elicit fast onset of behavioral changes (Bridges et al., 1985; Rosenblatt et al., 1988). All key brain regions in the social behavior circuit (see above) are enriched of receptors that sense estrogen, progesterone, oxytocin, prolactin and many others. Thus, the hormone waves during pregnancy induce a suite of “pre-programmed” changes in these areas to support the behavioral changes. Indeed, emerging evidence support plasticity in core social regions at the molecular, morphological and physiological levels during parenthood. Here we will discuss these changes, highlighting results in MPOA -- an essential region for parental behaviors. At the morphological level, Keyser-Marcus et al. showed that rat MPOA cells increase in neuronal soma size by 50% as parturition approaches. During the postpartum period, the neuronal soma returns to a pre-pregnancy level but the basal dendritic length and branching number remain increased (Keyser-Marcus et al., 2001). At the molecular level, systematic comparison of gene expression patterns at the MPOA has identified difference in the expression of hundreds of genes (Akbari et al., 2013; Gammie et al., 2005). More specifically, receptors for estrogen, progesterone, prolactin, oxytocin, neurotensin are all upregulated towards the end of pregnancy and during early postpartum period (Driessen et al., 2014; Francis et al., 2002; Grattan et al., 2001; Meddle et al., 2007; Numan et al., 1999; Wagner and Morrell, 1996; Young et al., 1997). A recent comprehensive study using single cell RANseq further revealed that molecularly distinct clusters of cells in the preoptic area are additionally activated in mothers and fathers in comparison to virgin females during pup interaction (Moffitt et al., 2018). At the physiological level, our recent in vivo optical recordings revealed increased Ca2+ responses (∆F/F) of MPOAEsr1+ cells during pup approach and retrieval in lactating females in comparison to prepartum female mice (Fang et al., 2018). Interestingly, in vivo electrophysiological recording showed that the spiking activity during pup-directed behaviors did not increase in mothers, instead the basal firing rate of MPOA cells decreased by 50%, resulting in an effective increase in signal to noise ratio (Fang et al., 2018). How do the electrophysiological changes link to the hormonal and molecular changes during parenthood? A direct answer to this question at the level of MPOA remains unavailable, but some clues have been provided by studies in other brain regions. In vitro slice recordings in the hippocampal and the auditory cortex showed that oxytocin increased signal to noise ratio of pyramidal neurons by elevating the basal firing of GABAergic interneurons (Marlin et al., 2015; Owen et al., 2013). Due to the enhanced inhibitory tone, pyramidal neurons in the auditory cortex show a decrease in spontaneous firing but an increase in evoked response to pup ultrasonic vocalization possibly due to usage-dependent fatigue of interneurons (Marlin et al., 2015). Future studies will elucidate whether a similar mechanism is responsible for an enhanced response to pup cues in MPOA and other brain regions during parenthood. Nevertheless, given the large array of molecular changes, multiple signaling pathways likely work in concert to change MPOA cell responses and cause the suite of behavioral changes during parenthood.
As a second example, the readiness to engage in female sexual behaviors varies with the estrous cycle. Female rats in estrus solicit males by ear wiggling and hopping and readily show lordosis upon being mounted whereas female rats in diestrus actively avoid male rats and rebuff their mounting attempts by kicking them and rolling on their backs (Donna Fitzroy, 1972). These behavioral changes are causally linked to the cyclic change of estrogen and progesterone as applying estrogen and progesterone in a pattern similar to the hormone changes during proestrus and estrus is sufficient to enhance female sexual receptivity in OVX female rats (Powers, 1970). VMHvl, the essential region for female sexual behavior, shows striking plasticity in morphology and electrophysiological responses with estrus cycle or sex hormone injections. Specifically, during proestrus when the estrogen level is high, VMHvl cells in female rats increase in somatic size, dendritic length and spine density (Madeira et al., 2001; Sa and Madeira, 2005). Application of estrogen to OVX females induces a similar set of morphology changes in VMHvl (Calizo and Flanagan-Cato, 2000). The increases in spine density during estrus suggests an increase in inputs from upstream regions. Indeed, electrophysiological recordings found that a higher firing rate increase of VMHvl cells during male investigation in estrous females than that in diestrus females (Hashikawa et al., 2017a; Nomoto and Lima, 2015). A recent study further revealed changes in axon morphology of VMHvl cells with estrus cycle. Inoue et. al. found that the terminal density of progesterone expressing VMHvl (VMHvlPR) cells in AVPV increases by approximately 3 folds during estrus in comparison to diestrus in female mice. This morphological change is functional as supported by a 3-fold increase in the EPSC magnitude of AVPV cells upon optogenetic activation of VMHvlPR axon terminals in vitro (Inoue et al., 2019). Taken together, the rise of sex hormones during proestrus enhances both the synaptic inputs and outputs of VMHvl cells, which leads to an increase in the probability to express female sexual behaviors in response to male cues.
Increase in the readiness to attack with winning experience
Social experience can change an animal’s readiness to engage in social behaviors in both the short and long terms. In the short term, for example, a single attack or even a brief exposure to a same-sex conspecific causes a transient increase in the readiness to attack, manifested as a decreased latency to attack, a phenomenon known as “aggression priming” (Miczek et al., 2013; Potegal, 1992; Potegal et al., 1996). In the long term, repeated winning permanently increases animal’s readiness to engage in a fight, i.e. winner effect (Hsu et al., 2006). While the neuroendocrine events that are relevant for the winner effect, e.g. post-winning testosterone surge, have been widely studied (Hsu et al., 2006), the neural mechanisms underlying the winner effect only began to be elucidated (Figure 6).
Figure 6. Neural mechanisms underlying winning caused increase in the readiness to attack.
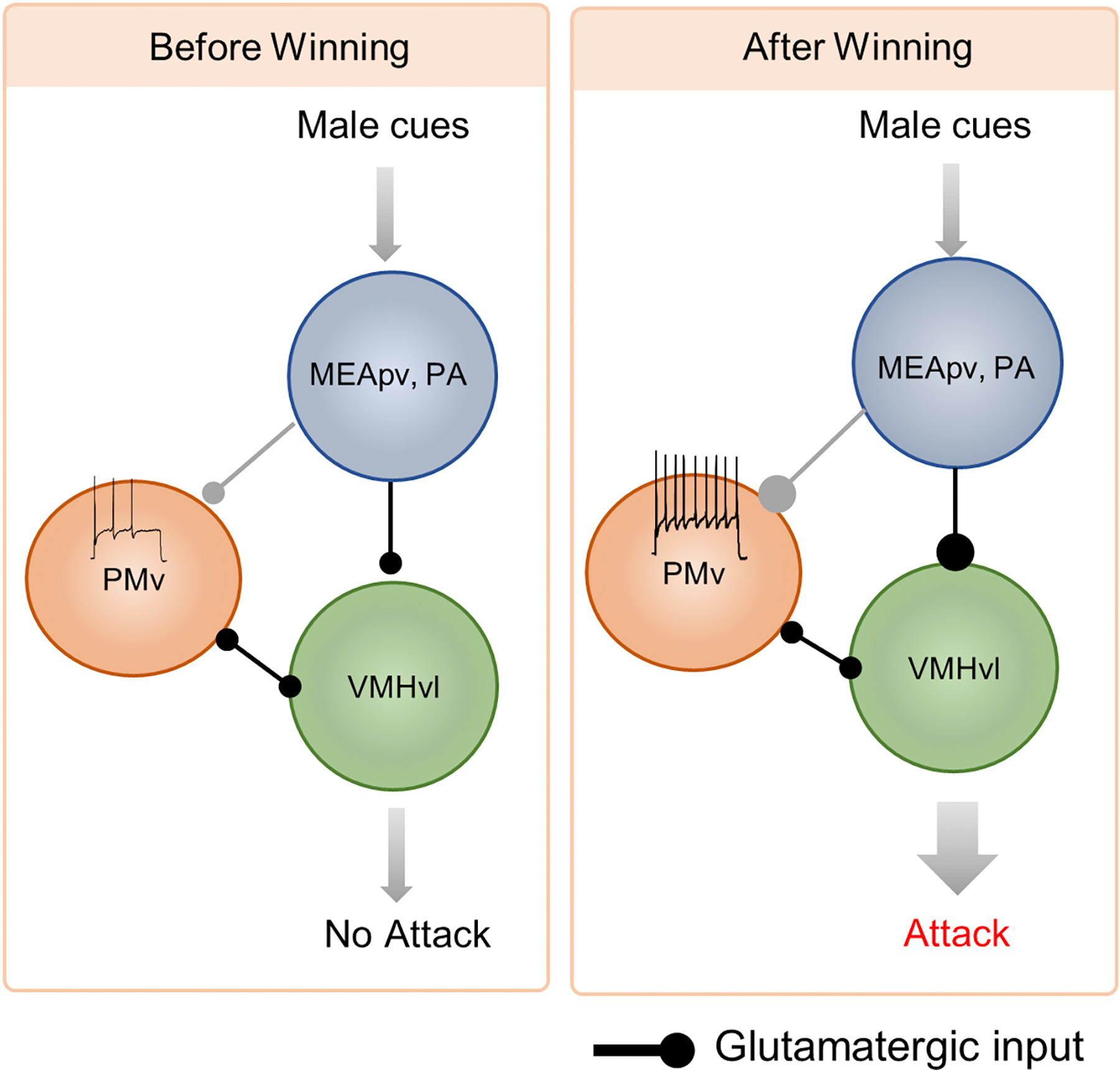
After winning, the synaptic connection between MEApv/PA and VMHvl increases while the PMv excitability also increases, causing an overall enhanced response of VMHvl cells to male cues and thus an increase in readiness to attack. The MEApv/PA to PMv pathway may also be potentiated after winning. Size of the lines and heads indicates the strength of the inputs and outputs.
Nordman et. al. recently reported that the pathway from MeA to VMHvl and BNST can undergo long term potentiation (LTP) and is essential for aggression priming (Nordman et al., 2020). They found that after a short period of attack, MeA stimulation evoked field excitatory postsynaptic potential (fEPSP) at VMHvl and BNST increases. This increase is blocked by NMDA receptor antagonist or a low-frequency stimulation protocol that is known to induce long-term depression (LTD). Importantly, when LTP is blocked, so is the aggression priming. Conversely, when MeA is stimulated at high frequency (100 Hz), MeA-VMHvl pathway is potentiated and the aggression level increases.
The synaptic potentiation between the amygdala to VMHvl could also be an essential mechanism for winner effect. In a recent study, Stagkourakis et. al. reported a significant increase in the spontaneous EPSCs (sEPSCs) and the spine density of VMHvl cells in experienced winners in comparison to naïve animals (Stagkourakis et al., 2020b). The authors then focused on the projection from PA -- a major source of excitatory input to VMHvl. Strikingly, the AMPA/NMDA ratio (EPSCs mediated by AMPA receptors vs. NMDA receptors) of VMHvl cells in response to optogenetic stimulation of PA is over 3 times higher in experienced winners than that in naïve or non-aggressive animals, indicating insertion of AMPAR into postsynaptic membrane after winning. Additionally, winning animals showed facilitating synaptic integration whereas naïve animals showed depressing synaptic integration. Altogether, these results suggest that the communication from PA to VMHvl becomes much more efficient after repeated winning. PA aggression responsive cells also projects extensively to PMv, another aggression promoting medial hypothalamic region, and it remains to be investigated whether the synapse between PA and PMv is also potentiated after winning (Yamaguchi et al., 2020) (Figure 6).
The winner effect likely also involves changes in the intrinsic properties of cells in the aggression circuit. PMv dopamine transporter (DAT) expressing cells in winners are more excitable than those in non-aggressive animals as reflected by a higher percentage of spontaneously active cells and a higher resting membrane potential (Stagkourakis et al., 2018). As PMv DAT cells form strong connections with each other and cells in VMHvl, an increase in excitability of PMv cells can lead to an overall higher activity in the circuit in response to an excitatory input. In this study, however, the authors did not examine PMv cell property in naïve animals thus it remains unclear whether the winning experience increases PMv cell excitability or if the excitability difference exists prior to winning and animals with higher PMv cell excitability are predisposed to become aggressive.
The testosterone surge after winning has been shown to be essential for the post-winning behavioral change (Oyegbile and Marler, 2005; Trainor et al., 2004). Is the testosterone increase also causally linked to changes in the neural circuit? Answers to this question remains incompletely understood, but is likely to be yes, at least partially. Stagkourakis et. al. found that the testosterone level in non-aggressive males was low and VMHvl cells in those animals failed to show LTP (Stagkourakis et al., 2020b). When the non-aggressive males were supplemented with testosterone, LTP was then induced reliably, suggesting a permissive role of the testosterone in synaptic potentiation at VMHvl. Additionally, testosterone is expected to change the aggression circuit through non-Hebbian spike-timing dependent mechanisms given that testosterone supplement alone is sufficient to increase aggression without winning experience. Such mechanism perhaps involves a series of genomic events triggered by sex hormones that causes changes in the molecular composition and physiological properties of cells. This non-Hebbian plasticity might explain the “cross-talk” of social experience. For example, sexual experience that also induces testosterone surge, can lead to an increase in aggression (Gleason et al., 2009). Future studies will be needed to understand the full impact of the testosterone on the neural circuit of aggression.
In sum, the experience-dependent change of social behavior likely involves both changes in synaptic transmission and intrinsic biophysical properties of cells in the relevant circuit. These changes could be mediated by Hebbian mechanisms as well as pre-programmed hormonal actions. While we focused on reproductive state and social experience induced changes here, other internal and external factors, such as hunger state, stress, phase of circadian clock and population density, could all modulate the social behaviors presumably through their influences on the relevant social circuit. While the ventral striatum, the amygdala and the hypothalamus are likely the key sites for plasticity, similar social experience induced physiological changes have been reported in the thalamic-prefrontal cortex pathway (Nelson et al., 2019; Zhou et al., 2017) as well as the accessory olfactory bulb microcircuit (Gao et al., 2017), and is likely a wide-spread phenomenon in the central nervous system.
Concluding remarks and future directions
All animals possess a repertoire of social behaviors that can be initiated without being taught. These behaviors are central for the survival and continuation of any species. They are triggered by a set of species-specific cues and are expressed by all members of a species in a stereotypical way. It is important to recognize that different social behaviors rely on cues of a certain sensory modality to different extents. For example, while olfactory cues and pheromones are indispensable to aggression, they are dispensable for parental behaviors in rodents. Future studies should carefully determine the contributions of each cue to a given social behavior. Such an understanding will be instructive in identifying the relevant brain regions for the behavior.
Social behaviors can be roughly separated into four phases: detection, approach, investigation and action. The first three phases could be collectively considered as a process to determine the identity of a target. Decades of research have identified key neural substrates for each stage of social behaviors, with NAc and medial hypothalamus emerging as essential areas to drive approach and specific consummatory actions, respectively. Despite our fast-growing knowledge, many nodes in each of the behavior circuit remain missing. For example, what are the upstream regions of VMHvl essential for female sexual behaviors? What are the pathways that carry auditory or somatosensory information to the hypothalamus? Is PAG the only key premotor region downstream of hypothalamus essential for the motor execution of social behaviors? Additionally, different social behaviors often involve the same brain region. For example, female sexual behaviors, aggression and social defense are all critically dependent on VMHvl activity. How cells in the same region drive different and often incompatible behaviors? Does it involve the same set of cells or distinct cells with different molecular identity and connectivity? As our knowledge of the circuit grows, it becomes clear that the same behavior, e.g. aggression, can be triggered from multiple regions. How does the information transform among those regions? Noticeably, many regions in the circuit are reciprocally connected, indicating that the information is likely distributed instead of flowing unidirectionally.
While social behaviors are innate, they are flexible: the same social cues could evoke vastly different or even opposite social responses from the same individual at different time points in life. This behavioral flexibility is accomplished by changes in two sensory-motor transition points, one from detecting a distant cue to approaching a conspecific; and the other from sampling proximal cues to undertaking consummatory actions. Our understanding of how the social behavior circuit change as a function of various factors just start to emerge and many studies shall follow in the near future. It becomes increasingly clear that the social behavior circuits are plastic and changes are likely to occur in various nodes along the circuit. It undergoes not only spike-timing dependent plasticity but is also subjective to preprogrammed hormonal actions. The combination of learning-dependent and independent modulatory mechanisms makes the seemingly stereotypical social behaviors incredibly flexible and adaptive, ensuring the continuation of life cycles.
Acknowledgments
This work was supported by the Irma T. Hirschl Trust, R01MH101377, 1R01HD092596, 1U19NS107616 and 5U01NS113358 (D.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agustín-Pavón C, Martínez-García F, and Lanuza E (2014). Focal lesions within the ventral striato pallidum abolish attraction for male chemosignals in female mice. Behavioural brain research 259, 292–296. [DOI] [PubMed] [Google Scholar]
- Akbari EM, Shams S, Belay HT, Kaiguo M, Razak Z, Kent CF, Westwood T, Sokolowski MB, and Fleming AS (2013). The effects of parity and maternal behavior on gene expression in the medial preoptic area and the medial amygdala in postpartum and virgin female rats: A microarray study. Behav Neurosci 127, 913–922. [DOI] [PubMed] [Google Scholar]
- Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, Pyo CO, Park SI, Marcinkiewcz CM, Crowley NA, et al. (2015). Distinct Subpopulations of Nucleus Accumbens Dynorphin Neurons Drive Aversion and Reward. Neuron 87, 1063–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert DJ, and Chew GL (1980). The septal forebrain and the inhibitory modulation of attack and defense in the rat. A review. Behavioral and neural biology 30, 357–388. [DOI] [PubMed] [Google Scholar]
- Almey A, Milner TA, and Brake WG (2015). Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm Behav 74, 125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, and Wang Z (2006). Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci 9, 133–139. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, and Wang Z (2009). Dopamine regulation of social choice in a monogamous rodent species. Front Behav Neurosci 3, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auclair Y, König B, Ferrari M, Perony N, and Lindholm AK (2014). Nest attendance of lactating females in a wild house mouse population: benefits associated with communal nesting. Animal Behaviour 92, 143–149. [Google Scholar]
- Auger AP, Moffatt CA, and Blaustein JD (1996). Reproductively-relevant stimuli induce Fos-immunoreactivity within progestin receptor - containing neurons in localized regions of female rat forebrain. Journal of neuroendocrinology 8, 831–838. [DOI] [PubMed] [Google Scholar]
- Bayless DW, Yang T, Mason MM, Susanto AA, Lobdell A, and Shah NM (2019). Limbic Neurons Shape Sex Recognition and Social Behavior in Sexually Naive Males. Cell 176, 1190–1205. e1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach FA (1976). Sexual attractivity, proceptivity, and receptivity in female mammals. Horm Behav 7, 105–138. [DOI] [PubMed] [Google Scholar]
- Beach FA, and Jaynes J (1956). Studies of Maternal Retrieving in Rats. Iii. Sensory Cues Involved in the Lactating Female’s Response To Her Young 1. Behaviour 10, 104–124. [Google Scholar]
- Beaugrand J, Goulet C, and Payette D (1991). Outcome of dyadic conflict in male green swordtail fish, Xiphophorus helleri: Effects of body size and prior dominance. Animal Behaviour 41, 417–424. [Google Scholar]
- Behrens TE, Hunt LT, Woolrich MW, and Rushworth MF (2008). Associative learning of social value. Nature 456, 245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers JM, and Suzuki N (2013). Neurons and circuits for odor processing in the piriform cortex. Trends Neurosci 36, 429–438. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, DiLeone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, et al. (2006). Essential Role of BDNF in the Mesolimbic Dopamine Pathway in Social Defeat Stress. Science 311, 864–868. [DOI] [PubMed] [Google Scholar]
- Blaiss CA, and Janak PH (2009). The nucleus accumbens core and shell are critical for the expression, but not the consolidation, of Pavlovian conditioned approach. Behav Brain Res 200, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, and Caroline Blanchard D (1977). Aggressive behavior in the rat. Behavioral Biology 21, 197–224. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, O’Donnell V, and Caroline Blanchard D (1979). Attack and defensive behaviors in the albino mouse. Aggressive behavior 5, 341–352. [Google Scholar]
- Bloch GJ, Kurth SM, Akesson TR, and Micevych PE (1992). Estrogen-concentrating cells within cell groups of the medial preoptic area: sex differences and co-localization with galanin-immunoreactive cells. Brain research 595, 301–308. [DOI] [PubMed] [Google Scholar]
- Borrow AP, and Cameron NM (2012). The role of oxytocin in mating and pregnancy. Horm Behav 61, 266–276. [DOI] [PubMed] [Google Scholar]
- Brackett NL, Iuvone PM, and Edwards DA (1986). Midbrain lesions, dopamine and male sexual behavior. Behav Brain Res 20, 231–240. [DOI] [PubMed] [Google Scholar]
- Bradley KC, Haas AR, and Meisel RL (2005). 6-Hydroxydopamine Lesions in Female Hamsters (Mesocricetus auratus) Abolish the Sensitized Effects of Sexual Experience on Copulatory Interactions With Males. Behavioral Neuroscience 119, 224–232. [DOI] [PubMed] [Google Scholar]
- Bridges RS, DiBiase R, Loundes DD, and Doherty PC (1985). Prolactin stimulation of maternal behavior in female rats. Science 227, 782–784. [DOI] [PubMed] [Google Scholar]
- Briffa M, and Lane SM (2017). The role of skill in animal contests: a neglected component of fighting ability. Proceedings Biological sciences 284, 20171596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RSE, Aoki M, Ladyman SR, Phillipps HR, Wyatt A, Boehm U, and Grattan DR (2017). Prolactin action in the medial preoptic area is necessary for postpartum maternal nursing behavior. Proc Natl Acad Sci U S A 114, 10779–10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, Roman-Ortiz C, Ramakrishnan C, Deisseroth K, Han MH, et al. (2017). Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun 8, 13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calizo LH, and Flanagan-Cato LM (2000). Estrogen selectively regulates spine density within the dendritic arbor of rat ventromedial hypothalamic neurons. J Neurosci 20, 1589–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras N, Simerly R, and Swanson L (1992a). Projections of the ventral premammillary nucleus. Journal of Comparative Neurology 324, 195–212. [DOI] [PubMed] [Google Scholar]
- Canteras NS (2002). The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol Biochem Behav 71, 481–491. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, and Swanson LW (1992b). Connections of the posterior nucleus of the amygdala. J Comp Neurol 324, 143–179. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, and Swanson LW (1994). Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol 348, 41–79. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, and Swanson LW (1995). Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol 360, 213–245. [DOI] [PubMed] [Google Scholar]
- Chamero P, Katsoulidou V, Hendrix P, Bufe B, Roberts R, Matsunami H, Abramowitz J, Birnbaumer L, Zufall F, and Leinders-Zufall T (2011). G protein G(alpha)o is essential for vomeronasal function and aggressive behavior in mice. Proc Natl Acad Sci U S A 108, 12898–12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, and Gean PW (2019). The Ventral Hippocampus Controls Stress-Provoked Impulsive Aggression through the Ventromedial Hypothalamus in Post-Weaning Social Isolation Mice. Cell Rep 28, 1195–1205 e1193. [DOI] [PubMed] [Google Scholar]
- Chen P, and Hong W (2018). Neural Circuit Mechanisms of Social Behavior. Neuron 98, 16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PB, Hu RK, Wu YE, Pan L, Huang S, Micevych PE, and Hong W (2019). Sexually Dimorphic Control of Parenting Behavior by the Medial Amygdala. Cell 176, 1206–1221.e1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy A, Coquelin A, Macrides F, Gorski R, and Noble E (1984). Sexual behavior and aggression in male mice: involvement of the vomeronasal system. The Journal of Neuroscience 4, 2222–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DE, Cooper MA, Markham CM, and Huhman KL (2011). NR2B subunit of the NMDA receptor in the basolateral amygdala is necessary for the acquisition of conditioned defeat in Syrian hamsters. Behav Brain Res 217, 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong JW, Afjei SA, Pollak Dorocic I, Peck JR, Liu C, Kim CK, Tian L, Deisseroth K, and Lammel S (2019). A Neural Circuit Mechanism for Encoding Aversive Stimuli in the Mesolimbic Dopamine System. Neuron 101, 133–151 e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Punta K, Leinders-Zufall T, Rodriguez I, Jukam D, Wysocki CJ, Ogawa S, Zufall F, and Mombaerts P (2002). Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature 419, 70–74. [DOI] [PubMed] [Google Scholar]
- Deschênes M, Kurnikova A, Elbaz M, and Kleinfeld D (2016). Circuits in the ventral medulla that phase-lock motoneurons for coordinated sniffing and whisking. J Neural Plasticity 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey S, Chamero P, Pru JK, Chien MS, Ibarra-Soria X, Spencer KR, Logan DW, Matsunami H, Peluso JJ, and Stowers L (2015). Cyclic Regulation of Sensory Perception by a Female Hormone Alters Behavior. Cell 161, 1334–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz V, and Lin D (2020). Neural circuits for coping with social defeat. Current Opinion in Neurobiology 60, 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBenedictis BT, Olugbemi AO, Baum MJ, and Cherry JA (2015). DREADD-Induced Silencing of the Medial Olfactory Tubercle Disrupts the Preference of Female Mice for Opposite-Sex Chemosignals(1,2,3). eNeuro 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Darvishzadeh A, Huang KW, and Malenka RC (2013). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato J Jr., and Elias CF (2011). The ventral premammillary nucleus links metabolic cues and reproduction. Front Endocrinol (Lausanne) 2, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, and Swanson LW (2004). Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol 471, 396–433. [DOI] [PubMed] [Google Scholar]
- Donna Fitzroy H (1972). Sexual Behavior in Continuously Cycling Rats. Behaviour 41, 288–297. [DOI] [PubMed] [Google Scholar]
- Driessen TM, Zhao C, Whittlinger A, Williams H, and Gammie SC (2014). Endogenous CNS expression of neurotensin and neurotensin receptors is altered during the postpartum period in outbred mice. PLoS One 9, e83098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C, O’Connell LA, and Wu Z (2014). Neural control of maternal and paternal behaviors. Science 345, 765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Wilckens N, Torres LY, Yokoyama S, Minie VA, Tran AM, Petkova SP, Hao R, Ramos-Maciel S, Rios RA, Jackson K, et al. (2020). Extrahypothalamic oxytocin neurons drive stress-induced social vigilance and avoidance. Proc Natl Acad Sci U S A 117, 26406–26413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine MS (1989). Solicitation behavior in the estrous female rat: a review. Horm Behav 23, 473–502. [DOI] [PubMed] [Google Scholar]
- Esteves FF, Matias D, Mendes AR, Lacoste B, and Lima SQ (2019). Sexually dimorphic neuronal inputs to the neuroendocrine dopaminergic system governing prolactin release. J Neuroendocrinol 31, e12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner AL, Dollar P, Perona P, Anderson DJ, and Lin D (2014). Decoding ventromedial hypothalamic neural activity during male mouse aggression. Journal of Neuroscience 34, 5971–5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner AL, Grosenick L, Davidson TJ, Deisseroth K, and Lin D (2016). Hypothalamic control of male aggression-seeking behavior. Nature neuroscience 19, 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner AL, and Lin D (2014). Recent advances in understanding the role of the hypothalamic circuit during aggression. Front Syst Neurosci 8, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner AL, Wei D, Song A, Watsek LW, Chen I, Chen P, Feng JE, and Lin D (2020). Hierarchical Representations of Aggression in a Hypothalamic-Midbrain Circuit. Neuron 106, 637–648 e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, and Clemens LG (1999). Contextual determinants of female-female mounting in laboratory rats. Anim Behav 57, 545–555. [DOI] [PubMed] [Google Scholar]
- Fang YY, Yamaguchi T, Song SC, Tritsch NX, and Lin D (2018). A Hypothalamic Midbrain Pathway Essential for Driving Maternal Behaviors. Neuron 98, 192–207 e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, and Kam K (2015). Facing the challenge of mammalian neural microcircuits: taking a few breaths may help. J Physiol 593, 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A, Vaccarino F, Tambosso L, and Chee P (1979). Vomeronasal and olfactory system modulation of maternal behavior in the rat. Science 203, 372–374. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Korsmit M, and Deller M (1994). Rat pups are potent reinforcers to the maternal animal: Effects of experience, parity, hormones, and dopamine function. Psychobiology 22, 44–53. [Google Scholar]
- Fleming AS, Vaccarino F, and Luebke C (1980). Amygdaloid inhibition of maternal behavior in the nulliparous female rat. Physiol Behav 25, 731–743. [DOI] [PubMed] [Google Scholar]
- Floresco S.B.J.A.r.o.p. (2015). The nucleus accumbens: an interface between cognition, emotion, and action. Annual review of psychology 66, 25–52. [DOI] [PubMed] [Google Scholar]
- Folkes OM, Baldi R, Kondev V, Marcus DJ, Hartley ND, Turner BD, Ayers JK, Baechle JJ, Misra MP, Altemus M, et al. (2020). An endocannabinoid-regulated basolateral amygdala-nucleus accumbens circuit modulates sociability. J Clin Invest 130, 1728–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis K, Meddle SL, Bishop VR, and Russell JA (2002). Progesterone receptor expression in the pregnant and parturient rat hypothalamus and brainstem. Brain Res 927, 18–26. [DOI] [PubMed] [Google Scholar]
- Francis TC, Chandra R, Friend DM, Finkel E, Dayrit G, Miranda J, Brooks JM, Iniguez SD, O’Donnell P, Kravitz A, et al. (2015). Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol Psychiatry 77, 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara M, and Chiba A (2018). Sexual odor preference and dopamine release in the nucleus accumbens by estrous olfactory cues in sexually naive and experienced male rats. Physiol Behav 185, 95–102. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Hasen NS, Awad TA, Auger AP, Jessen HM, Panksepp JB, and Bronikowski AM (2005). Gene array profiling of large hypothalamic CNS regions in lactating and randomly cycling virgin mice. Brain Res Mol Brain Res 139, 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangopadhyay P, Chawla M, Dal Monte O, and Chang SWC (2021). Prefrontal-amygdala circuits in social decision-making. Nat Neurosci 24, 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Budlong C, Durlacher E, and Davison IG (2017). Neural mechanisms of social learning in the female mouse. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German RZ, and Crompton AW (2000). CHAPTER 14 - The Ontogeny of Feeding in Mammals. In Feeding K. Schwenk, ed. (San Diego: Academic Press; ), pp. 449–457. [Google Scholar]
- Gingrich B, Liu Y, Cascio C, Wang Z, and Insel TR (2000). Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster). Behav Neurosci 114, 173–183. [DOI] [PubMed] [Google Scholar]
- Gleason ED, Fuxjager MJ, Oyegbile TO, and Marler CA (2009). Testosterone release and social context: when it occurs and why. Front Neuroendocrinol 30, 460–469. [DOI] [PubMed] [Google Scholar]
- Golden SA, Heshmati M, Flanigan M, Christoffel DJ, Guise K, Pfau ML, Aleyasin H, Menard C, Zhang H, Hodes GE, et al. (2016). Basal forebrain projections to the lateral habenula modulate aggression reward. Nature 534, 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL (2005). The vertebrate social behavior network: evolutionary themes and variations. Horm Behav 48, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, and Steiner RA (2004). A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145, 4073–4077. [DOI] [PubMed] [Google Scholar]
- Grattan DR, Pi XJ, Andrews ZB, Augustine RA, Kokay IC, Summerfield MR, Todd B, and Bunn SJ (2001). Prolactin receptors in the brain during pregnancy and lactation: implications for behavior. Horm Behav 40, 115–124. [DOI] [PubMed] [Google Scholar]
- Gray CL, Norvelle A, Larkin T, and Huhman KL (2015). Dopamine in the nucleus accumbens modulates the memory of social defeat in Syrian hamsters (Mesocricetus auratus). Behav Brain Res 286, 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudelsky GA (1981). Tuberoinfundibular dopamine neurons and the regulation of prolactin secretion. Psychoneuroendocrinology 6, 3–16. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, et al. (2014). Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel L, Thangarasa T, Samadi O, and Ito R (2017). Caudal nucleus accumbens core is critical in the regulation of cue-elicited approach-avoidance decisions. Eneuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashikawa K, Hashikawa Y, Falkner A, and Lin D (2016). The neural circuits of mating and fighting in male mice. Current opinion in neurobiology 38, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashikawa K, Hashikawa Y, Lischinsky J, and Lin D (2018). The Neural Mechanisms of Sexually Dimorphic Aggressive Behaviors. Trends Genet 34, 755–776. [DOI] [PubMed] [Google Scholar]
- Hashikawa K, Hashikawa Y, Tremblay R, Zhang J, Feng JE, Sabol A, Piper WT, Lee H, Rudy B, and Lin D (2017a). Esr1(+) cells in the ventromedial hypothalamus control female aggression. Nat Neurosci 20, 1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashikawa Y, Hashikawa K, Falkner AL, and Lin D (2017b). Ventromedial hypothalamus and the generation of aggression. Frontiers in systems neuroscience 11, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, and Kimura T (1974). Sex-attractant emitted by female mice. Physiol Behav 13, 563–567. [DOI] [PubMed] [Google Scholar]
- He J, Ma L, Kim S, Schwartz J, Santilli M, Wood C, Durnin MH, and Yu CR (2010). Distinct signals conveyed by pheromone concentrations to the mouse vomeronasal organ. J Neurosci 30, 7473–7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kimura K, Wada N, Funabiki K, and Nakanishi S (2010). Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron 66, 896–907. [DOI] [PubMed] [Google Scholar]
- Hikida T, Yawata S, Yamaguchi T, Danjo T, Sasaoka T, Wang Y, and Nakanishi S (2013). Pathway-specific modulation of nucleus accumbens in reward and aversive behavior via selective transmitter receptors. Proc Natl Acad Sci U S A 110, 342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer MA, Shair HN, and Brunelli SA (2002). Ultrasonic vocalizations in rat and mouse pups. Curr Protoc Neurosci Chapter 8, Unit 8.14. [DOI] [PubMed] [Google Scholar]
- Hogan JA, and Bolhuis JJ (2009). Tinbergen’s four questions and contemporary behavioral biology. In Tinbergen’s Legacy: Function and Mechanism in Behavioral Biology, Bolhuis J, and Verhulst S, eds. (Cambridge: Cambridge University Press; ), pp. 25–34. [Google Scholar]
- Holly EN, DeBold JF, and Miczek KA (2015). Increased mesocorticolimbic dopamine during acute and repeated social defeat stress: modulation by corticotropin releasing factor receptors in the ventral tegmental area. Psychopharmacology (Berl) 232, 4469–4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W, Kim D-W, and Anderson DJ (2014). Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell 158, 1348–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa N, and Chiba A (2005). Effects of sexual experience on conspecific odor preference and estrous odor-induced activation of the vomeronasal projection pathway and the nucleus accumbens in male rats. Brain Res 1066, 101–108. [DOI] [PubMed] [Google Scholar]
- Hsu Y, Earley RL, and Wolf LL (2006). Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol Rev Camb Philos Soc 81, 33–74. [DOI] [PubMed] [Google Scholar]
- Hsu Y, and Wolf LL (2001). The winner and loser effect: what fighting behaviours are influenced? Animal Behaviour 61, 777–786. [Google Scholar]
- Hull EM, and Dominguez JM (2007). Sexual behavior in male rodents. Horm Behav 52, 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Yang R, Tantry A, Davis C. h., Yang T, Knoedler JR, Wei Y, Adams EL, Thombare S, Golf SR, et al. (2019). Periodic Remodeling in a Neural Circuit Governs Timing of Female Sexual Behavior. Cell 179, 1393–1408.e1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii KK, and Touhara K (2019). Neural circuits regulating sexual behaviors via the olfactory system in mice. Neurosci Res 140, 59–76. [DOI] [PubMed] [Google Scholar]
- Isogai Y, Wu Z, Love MI, Ahn MH, Bambah-Mukku D, Hua V, Farrell K, and Dulac C (2018). Multisensory Logic of Infant-Directed Aggression by Males. Cell 175, 1827–1841 e1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyilikci O, Balthazart J, and Ball GF (2016). Medial Preoptic Regulation of the Ventral Tegmental Area Related to the Control of Sociosexual Behaviors. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig S, Weiß BM, and Widdig A (2018). Comparing the sniffing behavior of great apes. American journal of primatology 80, e22872. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, and Huhman KL (2001). Activation of GABA(A) receptors in the amygdala blocks the acquisition and expression of conditioned defeat in Syrian hamsters. Brain Res 920, 142–150. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Shi C, Israel JE, Davis M, and Huhman KL (2005). Memory of social defeat is facilitated by cAMP response element-binding protein overexpression in the amygdala. Behav Neurosci 119, 1125–1130. [DOI] [PubMed] [Google Scholar]
- Kang S, Thayananuphat A, Bakken T, and El Halawani M (2007). Dopamine-melatonin neurons in the avian hypothalamus controlling seasonal reproduction. Neuroscience 150, 223–233. [DOI] [PubMed] [Google Scholar]
- Kappeler PM, Barrett L, Blumstein DT, and Clutton-Brock TH (2013). Constraints and flexibility in mammalian social behaviour: introduction and synthesis. Philos Trans R Soc Lond B Biol Sci 368, 20120337–20120337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Douhard Q, Baum MJ, and Bakker J (2006). Destruction of the main olfactory epithelium reduces female sexual behavior and olfactory investigation in female mice. Chem Senses 31, 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, and Mainen ZF (2005). The Sniff as a Unit of Olfactory Processing. Chemical Senses 31, 167–179. [DOI] [PubMed] [Google Scholar]
- Keyser-Marcus L, Stafisso-Sandoz G, Gerecke K, Jasnow A, Nightingale L, Lambert KG, Gatewood J, and Kinsley CH (2001). Alterations of medial preoptic area neurons following pregnancy and pregnancy-like steroidal treatment in the rat. Brain Res Bull 55, 737–745. [DOI] [PubMed] [Google Scholar]
- Kikuta S, Sato K, Kashiwadani H, Tsunoda K, Yamasoba T, and Mori K (2010). From the Cover: Neurons in the anterior olfactory nucleus pars externa detect right or left localization of odor sources. Proc Natl Acad Sci U S A 107, 12363–12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DG, Gonzales EL, Kim S, Kim Y, Adil KJ, Jeon SJ, Cho KS, Kwon KJ, and Shin CY (2019a). Social Interaction Test in Home Cage as a Novel and Ethological Measure of Social Behavior in Mice. Exp Neurobiol 28, 247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DW, Yao Z, Graybuck LT, Kim TK, Nguyen TN, Smith KA, Fong O, Yi L, Koulena N, Pierson N, et al. (2019b). Multimodal Analysis of Cell Types in a Hypothalamic Node Controlling Social Behavior. Cell 179, 713–728 e717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi T, Xu J, and Dulac C (2007). A functional circuit underlying male sexual behaviour in the female mouse brain. Nature 448, 1009–1014. [DOI] [PubMed] [Google Scholar]
- Kingsbury L, Huang S, Raam T, Ye LS, Wei D, Hu RK, Ye L, and Hong W (2020). Cortical Representations of Conspecific Sex Shape Social Behavior. Neuron 107, 941–953 e947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury L, Huang S, Wang J, Gu K, Golshani P, Wu YE, and Hong W (2019). Correlated Neural Activity and Encoding of Behavior across Brains of Socially Interacting Animals. Cell 178, 429–446 e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl J, Autry AE, and Dulac C (2017). The neurobiology of parenting: A neural circuit perspective. Bioessays 39, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl J, Babayan BM, Rubinstein ND, Autry AE, Marin-Rodriguez B, Kapoor V, Miyamishi K, Zweifel LS, Luo L, Uchida N, et al. (2018). Functional circuit architecture underlying parental behaviour. Nature 556, 326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl J, and Dulac C (2018). Neural control of parental behaviors. Curr Opin Neurobiol 49, 116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Chaudhury D, Han MH, and Nestler EJ (2019). Role of Mesolimbic Brain-Derived Neurotrophic Factor in Depression. Biol Psychiatry 86, 738–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, and Kreitzer AC (2012). Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci 15, 816–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentzel AA, Barrett LR, and Meitzen J (2019). Estradiol rapidly modulates excitatory synapse properties in a sex- and region-specific manner in rat nucleus accumbens core and caudate-putamen. J Neurophysiol 122, 1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, et al. (2007). Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404. [DOI] [PubMed] [Google Scholar]
- Krueger F, Barbey AK, and Grafman J (2009). The medial prefrontal cortex mediates social event knowledge. Trends Cogn Sci 13, 103–109. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN, Bakshtanovskaya IV, and Koryakina LA (1991). Social model of depression in mice of C57BL/6J strain. Pharmacology Biochemistry and Behavior 38, 315–320. [DOI] [PubMed] [Google Scholar]
- Kurnikova A, Moore JD, Liao SM, Deschenes M, and Kleinfeld D (2017). Coordination of Orofacial Motor Actions into Exploratory Behavior by Rat. Curr Biol 27, 688–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landauer MR, Wiese RE, and Carr WJ (1977). Responses of sexually experienced and naive male rats to cues from receptive vs. nonreceptive females. Animal Learning & Behavior 5, 398–402. [Google Scholar]
- Lee H, Kim DW, Remedios R, Anthony TE, Chang A, Madisen L, Zeng H, and Anderson DJ (2014). Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature 509, 627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H, Mooney R, and Katz LC (2006). Synaptic integration of olfactory information in mouse anterior olfactory nucleus. J Neurosci 26, 12023–12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow C, and Lima SQ (2020). In the mood for sex: neural circuits for reproduction. Curr Opin Neurobiol 60, 155–168. [DOI] [PubMed] [Google Scholar]
- Lepri JJ, and Wysocki CJ (1987). Removal of the vomeronasal organ disrupts the activation of reproduction in female voles. Physiol Behav 40, 349–355. [DOI] [PubMed] [Google Scholar]
- Leroy F, Park J, Asok A, Brann DH, Meira T, Boyle LM, Buss EW, Kandel ER, and Siegelbaum SA (2018). A circuit from hippocampal CA2 to lateral septum disinhibits social aggression. Nature 564, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, and Axel R (2002). Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci U S A 99, 6376–6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Mathis A, Grewe BF, Osterhout JA, Ahanonu B, Schnitzer MJ, Murthy VN, and Dulac C (2017). Neuronal Representation of Social Information in the Medial Amygdala of Awake Behaving Mice. Cell 171, 1176–1190 e1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Chen Z, Fan G, Li A, Yuan J, and Xu T (2018). Cell-Type-Specific Afferent Innervation of the Nucleus Accumbens Core and Shell. Front Neuroanat 12, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberles SD (2014). Mammalian Pheromones. Annual Review of Physiology 76, 151–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein E, Perona P, and Anderson DJ (2011). Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, and Wang ZX (2003). Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121, 537–544. [DOI] [PubMed] [Google Scholar]
- Lo L, Yao S, Kim DW, Cetin A, Harris J, Zeng H, Anderson DJ, and Weissbourd B (2019). Connectional architecture of a mouse hypothalamic circuit node controlling social behavior. Proc Natl Acad Sci U S A 116, 7503–7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Covington HE 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, et al. (2010). Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330, 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan DW, Brunet LJ, Webb WR, Cutforth T, Ngai J, and Stowers L (2012). Learned recognition of maternal signature odors mediates the first suckling episode in mice. Current biology : CB 22, 1998–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, Simmons DA, and Stern JM (1998). Functions of the caudal periaqueductal gray in lactating rats: kyphosis, lordosis, maternal aggression, and fearfulness. Behav Neurosci 112, 1502–1518. [DOI] [PubMed] [Google Scholar]
- Lorenz KZ (1981). The Foundations of Ethology (Vienna: : Springer Vienna; ). [Google Scholar]
- Luckett C, Norvelle A, and Huhman K (2012). The role of the nucleus accumbens in the acquisition and expression of conditioned defeat. Behav Brain Res 227, 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay-Sim A, and Rose JD (1986). Removal of the vomeronasal organ impairs lordosis in female hamsters: effect is reversed by luteinising hormone-releasing hormone. Neuroendocrinology 42, 489–493. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Ferreira-Silva L, and Paula-Barbosa MM (2001). Influence of sex and estrus cycle on the sexual dimorphisms of the hypothalamic ventromedial nucleus: stereological evaluation and Golgi study. J Comp Neurol 432, 329–345. [DOI] [PubMed] [Google Scholar]
- Mandiyan VS, Coats JK, and Shah NM (2005). Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci 8, 1660–1662. [DOI] [PubMed] [Google Scholar]
- Markham CM, and Huhman KL (2008). Is the medial amygdala part of the neural circuit modulating conditioned defeat in Syrian hamsters? Learning & memory 15, 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham CM, Taylor SL, and Huhman KL (2010). Role of amygdala and hippocampus in the neural circuit subserving conditioned defeat in Syrian hamsters. Learn Mem 17, 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin BJ, Mitre M, D’Amour J A, Chao MV, and Froemke RC (2015). Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520, 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel KL, and Baum MJ (2007). Sexually dimorphic activation of the accessory, but not the main, olfactory bulb in mice by urinary volatiles. Eur J Neurosci 26, 463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-García F, Martínez-Ricós J, Agustín-Pavón C, Martínez-Hernández J, Novejarque A, and Lanuza E (2009). Refining the dual olfactory hypothesis: Pheromone reward and odour experience. Behavioural Brain Research 200, 277–286. [DOI] [PubMed] [Google Scholar]
- Martinez-Marcos A (2009). On the organization of olfactory and vomeronasal cortices. Prog Neurobiol 87, 21–30. [DOI] [PubMed] [Google Scholar]
- McClintock MK, and Adler NT (1978). The role of the female during copulation in wild and domestic Norway rats (Rattus norvegicus). J Behaviour 67, 67–95. [Google Scholar]
- McHenry JA, Otis JM, Rossi MA, Robinson JE, Kosyk O, Miller NW, McElligott ZA, Budygin EA, Rubinow DR, and Stuber GD (2017). Hormonal gain control of a medial preoptic area social reward circuit. Nat Neurosci 20, 449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh TK, Barfield RJ, and Geyer LA (1978). Ultrasonic vocalisations facilitate sexual behaviour of female rats. Nature 272, 163–164. [DOI] [PubMed] [Google Scholar]
- Meddle SL, Bishop VR, Gkoumassi E, van Leeuwen FW, and Douglas AJ (2007). Dynamic changes in oxytocin receptor expression and activation at parturition in the rat brain. Endocrinology 148, 5095–5104. [DOI] [PubMed] [Google Scholar]
- Meredith M (1986). Vomeronasal organ removal before sexual experience impairs male hamster mating behavior. J Physiology behavioral and brain sciences 36, 737–743. [DOI] [PubMed] [Google Scholar]
- Mhaouty-Kodja S, Naule L, and Capela D (2018). Sexual Behavior: From Hormonal Regulation to Endocrine Disruption. Neuroendocrinology 107, 400–416. [DOI] [PubMed] [Google Scholar]
- Micevych PE, and Meisel RL (2017). Integrating Neural Circuits Controlling Female Sexual Behavior. Front Syst Neurosci 11, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, De Boer SF, and Haller JJP (2013). Excessive aggression as model of violence: a critical evaluation of current preclinical methods. 226, 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LL, Whitsett JM, Vandenbergh JG, and Colby DR (1977). Physical and behavioral aspects of sexual maturation in male golden hamsters. J Comp Physiol Psychol 91, 245–259. [DOI] [PubMed] [Google Scholar]
- Moffitt JR, Bambah-Mukku D, Eichhorn SW, Vaughn E, Shekhar K, Perez JD, Rubinstein ND, Hao J, Regev A, Dulac C, et al. (2018). Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, and Yang CR (1991). The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Advances in experimental medicine and biology 295, 267–290. [DOI] [PubMed] [Google Scholar]
- Motta SC, Guimarães CC, Furigo IC, Sukikara MH, Baldo MV, Lonstein JS, and Canteras NS (2013). Ventral premammillary nucleus as a critical sensory relay to the maternal aggression network. Proceedings of the National Academy of Sciences 110, 14438–14443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AC, Kapoor V, Vaughn E, Gnanasegaram JA, Rubinstein ND, Murthy VN, and Dulac C (2019). Molecular and Circuit Architecture of Social Hierarchy. BioRxiv, 838664.
- Nomoto K, and Lima SQ (2015). Enhanced male-evoked responses in the ventromedial hypothalamus of sexually receptive female mice. Curr Biol 25, 589–594. [DOI] [PubMed] [Google Scholar]
- Nordman JC, Ma X, Gu Q, Potegal M, Li H, Kravitz AV, and Li Z (2020). Potentiation of Divergent Medial Amygdala Pathways Drives Experience-Dependent Aggression Escalation. J Neurosci 40, 4858–4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norlin EM, Gussing F, and Berghard A (2003). Vomeronasal phenotype and behavioral alterations in G alpha i2 mutant mice. Curr Biol 13, 1214–1219. [DOI] [PubMed] [Google Scholar]
- Numan M (2006). Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behav Cogn Neurosci Rev 5, 163–190. [DOI] [PubMed] [Google Scholar]
- Numan M, and Numan M (1996). A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Dev Psychobiol 29, 23–51. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, and English JB (1993). Excitotoxic amino acid injections into the medial amygdala facilitate maternal behavior in virgin female rats. Hormones and behavior 27, 56–81. [DOI] [PubMed] [Google Scholar]
- Numan M, Roach JK, del Cerro MC, Guillamon A, Segovia S, Sheehan TP, and Numan MJ (1999). Expression of intracellular progesterone receptors in rat brain during different reproductive states, and involvement in maternal behavior. Brain Res 830, 358–371. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, and Hofmann HA (2011). The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol 519, 3599–3639. [DOI] [PubMed] [Google Scholar]
- Owen SF, Tuncdemir SN, Bader PL, Tirko NN, Fishell G, and Tsien RW (2013). Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature 500, 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyegbile TO, and Marler CA (2005). Winning fights elevates testosterone levels in California mice and enhances future ability to win fights. Hormones and Behavior 48, 259–267. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Cherry JA, and Baum MJ (2006). Effect of vomeronasal organ removal from male mice on their preference for and neural Fos responses to female urinary odors. Behav Neurosci 120, 925–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papale AE, and Hooks BM (2018). Circuit changes in motor cortex during motor skill learning. Neuroscience 368, 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsana AJ, Li N, and Brown TH (2012). Positive and negative ultrasonic social signals elicit opposing firing patterns in rat amygdala. Behav Brain Res 226, 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, and Berridge KC (2005). Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci 25, 11777–11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaus JG, Marcangione C, Smith WJ, Manitt C, and Abillamaa H (1996). Differential induction of Fos in the female rat brain following different amounts of vaginocervical stimulation: modulation by steroid hormones. Brain Res 741, 314–330. [DOI] [PubMed] [Google Scholar]
- Pleim ET, Matochik JA, Barfield RJ, and Auerbach SB (1990). Correlation of dopamine release in the nucleus accumbens with masculine sexual behavior in rats. Brain Res 524, 160–163. [DOI] [PubMed] [Google Scholar]
- Potegal M (1992). Time course of aggressive arousal in female hamsters and male rats. Behavioral and neural biology 58, 120–124. [DOI] [PubMed] [Google Scholar]
- Potegal M, Ferris CF, Hebert M, Meyerhoff J, and Skaredoff L (1996). Attack priming in female Syrian golden hamsters is associated with a c-fos-coupled process within the corticomedial amygdala. Neuroscience 75, 869–880. [DOI] [PubMed] [Google Scholar]
- Powers JB (1970). Hormonal control of sexual receptivity during the estrous cycle of the rat. Physiol Behav 5, 831–835. [DOI] [PubMed] [Google Scholar]
- Powers JB, and Winans SS (1975). Vomeronasal organ: critical role in mediating sexual behavior of the male hamster. Science 187, 961–963. [DOI] [PubMed] [Google Scholar]
- Proano SB, Morris HJ, Kunz LM, Dorris DM, and Meitzen J (2018). Estrous cycle-induced sex differences in medium spiny neuron excitatory synaptic transmission and intrinsic excitability in adult rat nucleus accumbens core. J Neurophysiol 120, 1356–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi CC, Wang QJ, Ma XZ, Chen HC, Gao LP, Yin J, and Jing YH (2018). Interaction of basolateral amygdala, ventral hippocampus and medial prefrontal cortex regulates the consolidation and extinction of social fear. Behav Brain Funct 14, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan R, Clement JP, and Bhalla US (2006). Rats smell in stereo. Science 311, 666–670. [DOI] [PubMed] [Google Scholar]
- Rajendren G, Dudley CA, and Moss RL (1990). Role of the vomeronasal organ in the male-induced enhancement of sexual receptivity in female rats. Neuroendocrinology 52, 368–372. [DOI] [PubMed] [Google Scholar]
- Richard JM, Ambroggi F, Janak PH, and Fields HL (2016). Ventral Pallidum Neurons Encode Incentive Value and Promote Cue-Elicited Instrumental Actions. Neuron 90, 1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers-Carter MM, Djerdjaj A, Gribbons KB, Varela JA, and Christianson JP (2019). Insular Cortex Projections to Nucleus Accumbens Core Mediate Social Approach to Stressed Juvenile Rats. J Neurosci 39, 8717–8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Carbente JC, Camacho FJ, and Paredes RG (2006). The role of the dorsolateral tegmentum in the control of male sexual behavior: a reevaluation. Behav Brain Res 170, 262–270. [DOI] [PubMed] [Google Scholar]
- Romero-Carbente JC, Hurtazo EA, and Paredes RG (2007). Central tegmental field and sexual behavior in the male rat: effects of neurotoxic lesions. Neuroscience 148, 867–875. [DOI] [PubMed] [Google Scholar]
- Root CM, Denny CA, Hen R, and Axel R (2014). The participation of cortical amygdala in innate, odour-driven behaviour. Nature 515, 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt JS, Mayer AD, and Giordano AL (1988). Hormonal basis during pregnancy for the onset of maternal behavior in the rat. Psychoneuroendocrinology 13, 29–46. [DOI] [PubMed] [Google Scholar]
- Rutte C, Taborsky M, and Brinkhof MWG (2006). What sets the odds of winning and losing? Trends in Ecology & Evolution 21, 16–21. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Young NB, Moy SS, and Crawley JN (2008). Olfactory cues are sufficient to elicit social approach behaviors but not social transmission of food preference in C57BL/6J mice. Behav Brain Res 193, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, and Havemann-Reinecke U (2005). Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res 162, 127–134. [DOI] [PubMed] [Google Scholar]
- Sa SI, and Madeira MD (2005). Estrogen modulates the sexually dimorphic synaptic connectivity of the ventromedial nucleus. J Comp Neurol 484, 68–79. [DOI] [PubMed] [Google Scholar]
- Sadananda M, Wohr M, and Schwarting RK (2008). Playback of 22-kHz and 50-kHz ultrasonic vocalizations induces differential c-fos expression in rat brain. Neurosci Lett 435, 17–23. [DOI] [PubMed] [Google Scholar]
- Saito TR, and Moltz H (1986). Copulatory behavior of sexually naive and sexually experienced male rats following removal of the vomeronasal organ. Physiol Behav 37, 507–510. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, and Pfaff DW (1979). Mesencephalic mechanisms for integration of female reproductive behavior in the rat. Am J Physiol 237, R285–290. [DOI] [PubMed] [Google Scholar]
- Scott JW, McBride RL, and Schneider SP (1980). The organization of projections from the olfactory bulb to the piriform cortex and olfactory tubercle in the rat. J Comp Neurol 194, 519–534. [DOI] [PubMed] [Google Scholar]
- Scott N, Prigge M, Yizhar O, and Kimchi T (2015). A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature 525, 519–522. [DOI] [PubMed] [Google Scholar]
- Scribner JL, Vance EA, Protter DSW, Sheeran WM, Saslow E, Cameron RT, Klein EM, Jimenez JC, Kheirbek MA, and Donaldson ZR (2020). A neuronal signature for monogamous reunion. Proc Natl Acad Sci U S A 117, 11076–11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan T, Paul M, Amaral E, Numan M, and Numan M (2001). Evidence that the medial amygdala projects to the anterior/ventromedial hypothalamic nuclei to inhibit maternal behavior in rats. Neuroscience 106, 341–356. [DOI] [PubMed] [Google Scholar]
- Shimura T, Yamamoto T, and Shimokochi M (1994). The medial preoptic area is involved in both sexual arousal and performance in male rats: re-evaluation of neuron activity in freely moving animals. Brain Res 640, 215–222. [DOI] [PubMed] [Google Scholar]
- Siegel A, Roeling TA, Gregg TR, and Kruk MR (1999). Neuropharmacology of brain-stimulation evoked aggression. Neurosci Biobehav Rev 23, 359–389. [DOI] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, and Berridge KC (2009). Ventral pallidum roles in reward and motivation. Behavioural brain research 196, 155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotherman WP, Bell RW, Starzec J, Elias J, and Zachman TA (1974). Maternal responses to infant vocalizations and olfactory cues in rats and mice. Behav Biol 12, 55–66. [DOI] [PubMed] [Google Scholar]
- Spehr M, Spehr J, Ukhanov K, Kelliher KR, Leinders-Zufall T, and Zufall F (2006). Parallel processing of social signals by the mammalian main and accessory olfactory systems. Cell Mol Life Sci 63, 1476–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagkourakis S, Smiley KO, Williams P, Kakadellis S, Ziegler K, Bakker J, Brown RSE, Harkany T, Grattan DR, and Broberger C (2020a). A Neuro-hormonal Circuit for Paternal Behavior Controlled by a Hypothalamic Network Oscillation. Cell 182, 960–975 e915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagkourakis S, Spigolon G, Liu G, and Anderson DJ (2020b). Experience-dependent plasticity in an innate social behavior is mediated by hypothalamic LTP. Proc Natl Acad Sci U S A 117, 25789–25799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagkourakis S, Spigolon G, Williams P, Protzmann J, Fisone G, and Broberger C (2018). A neural network for intermale aggression to establish social hierarchy. Nature neuroscience 21, 834. [DOI] [PubMed] [Google Scholar]
- Steinman MQ, Duque-Wilckens N, and Trainor BC (2019). Complementary Neural Circuits for Divergent Effects of Oxytocin: Social Approach Versus Social Anxiety. Biol Psychiatry 85, 792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM (2013). Hypothalamic survival circuits: blueprints for purposive behaviors. Neuron 77, 810–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, and Koentges G (2002). Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science 295, 1493–1500. [DOI] [PubMed] [Google Scholar]
- Strasser S, and Dixon AK (1986). Effects of visual and acoustic deprivation on agonistic behaviour of the albino mouse (M. musculus L.). Physiol Behav 36, 773–778. [DOI] [PubMed] [Google Scholar]
- Sulain M, Sulaiman SA, D’Souza U, Jaafar H, and Jamalullai SMSS (2011). Revising the Sexual Behavior of Naive and Experienced Male Sprague Dawley Rats. International Medical Journal 18, 8–13. [Google Scholar]
- Sun F, Zeng J, Jing M, Zhou J, Feng J, Owen SF, Luo Y, Li F, Wang H, Yamaguchi T, et al. (2018). A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice. Cell 174, 481–496 e419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney WT, Dubose BN, Curley JP, and Champagne FA (2012). Sexual experience affects reproductive behavior and preoptic androgen receptors in male mice. Hormones and behavior 61, 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L, and Cowan W (1977). An autoradiographic study of the organization of the efferet connections of the hippocampal formation in the rat. journal of Comparative Neurology 172, 49–84. [DOI] [PubMed] [Google Scholar]
- Tachikawa KS, Yoshihara Y, and Kuroda KO (2013). Behavioral transition from attack to parenting in male mice: a crucial role of the vomeronasal system. J Neurosci 33, 5120–5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, and Miczek KA (2014). Neurogenetics of aggressive behavior: studies in rodents. Curr Top Behav Neurosci 17, 3–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenk CM, Wilson H, Zhang Q, Pitchers KK, and Coolen LM (2009). Sexual reward in male rats: effects of sexual experience on conditioned place preferences associated with ejaculation and intromissions. Horm Behav 55, 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth I, and Neumann ID (2013). Animal models of social avoidance and social fear. Cell Tissue Res 354, 107–118. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Bird IM, Marler CAJH, and Behavior (2004). Opposing hormonal mechanisms of aggression revealed through short-lived testosterone manipulations and multiple winning experiences. Hormones and Behavior 45, 115–121. [DOI] [PubMed] [Google Scholar]
- Trezza V, Campolongo P, and Vanderschuren LJMJ (2011). Evaluating the rewarding nature of social interactions in laboratory animals. Developmental Cognitive Neuroscience 1, 444–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, and Sabatini BL (2012). Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 76, 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Tsuji C, Lozic M, Ludwig M, and Leng G (2019). Coding of odors in the anterior olfactory nucleus. Physiol Rep 7, e14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuneoka Y, Tokita K, Yoshihara C, Amano T, Esposito G, Huang AJ, Yu LM, Odaka Y, Shinozuka K, McHugh TJ, et al. (2015). Distinct preoptic-BST nuclei dissociate paternal and infanticidal behavior in mice. EMBO J 34, 2652–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger EK, Burke KJ Jr., Yang CF, Bender KJ, Fuller PM, and Shah NM (2015). Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep 10, 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CK, and Morrell JI (1996). Levels of estrogen receptor immunoreactivity are altered in behaviorally-relevant brain regions in female rats during pregnancy. Brain Res Mol Brain Res 42, 328–336. [DOI] [PubMed] [Google Scholar]
- Walsh JJ, Christoffel DJ, Heifets BD, Ben-Dor GA, Selimbeyoglu A, Hung LW, Deisseroth K, and Malenka RC (2018). 5-HT release in nucleus accumbens rescues social deficits in mouse autism model. Nature 560, 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JJ, Friedman AK, Sun H, Heller EA, Ku SM, Juarez B, Burnham VL, Mazei-Robison MS, Ferguson D, Golden SA, et al. (2014). Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci 17, 27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CT, Huang RL, Tai MY, Tsai YF, and Peng MT (1995). Dopamine release in the nucleus accumbens during sexual behavior in prenatally stressed adult male rats. Neurosci Lett 200, 29–32. [DOI] [PubMed] [Google Scholar]
- Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, and Hu H (2011). Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science 334, 693–697. [DOI] [PubMed] [Google Scholar]
- Wang L, Talwar V, Osakada T, Kuang A, Guo Z, Yamaguchi T, and Lin D (2019). Hypothalamic control of conspecific self-defense. Cell reports 26, 1747–1758. e1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Balet Sindreu C, Li V, Nudelman A, Chan GC, and Storm DR (2006). Pheromone detection in male mice depends on signaling through the type 3 adenylyl cyclase in the main olfactory epithelium. J Neurosci 26, 7375–7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M (2017). The prefrontal cortex as an executive, emotional, and social brain (New York, NY: Springer Berlin Heidelberg; ). [Google Scholar]
- Wei YC, Wang SR, Jiao ZL, Zhang W, Lin JK, Li XY, Li SS, Zhang X, and Xu XH (2018). Medial preoptic area in mice is capable of mediating sexually dimorphic behaviors regardless of gender. Nat Commun 9, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AM, Ridener E, Bourbonais CA, Kim W, Pantazopoulos H, Carroll FI, Kim KS, Cohen BM, and Carlezon WA Jr. (2017). Effects of Chronic Social Defeat Stress on Sleep and Circadian Rhythms Are Mitigated by Kappa-Opioid Receptor Antagonism. J Neurosci 37, 7656–7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson Daniel W. (2013). Sniffing Behavior Communicates Social Hierarchy. Current Biology 23, 575–580. [DOI] [PubMed] [Google Scholar]
- Wesson DW, and Wilson DA (2011). Sniffing out the contributions of the olfactory tubercle to the sense of smell: hedonics, sensory integration, and more? Neurosci Biobehav Rev 35, 655–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AV, Duque-Wilckens N, Ramos-Maciel S, Campi KL, Bhela SK, Xu CK, Jackson K, Chini B, Pesavento PA, and Trainor BC (2020). Social approach and social vigilance are differentially regulated by oxytocin receptors in the nucleus accumbens. Neuropsychopharmacology 45, 1423–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I, Tose A, Wanat MJ, Hart AS, Hollon NG, Phillips PE, Schwarting RK, and Wohr M (2014). Phasic dopamine release in the nucleus accumbens in response to pro-social 50 kHz ultrasonic vocalizations in rats. J Neurosci 34, 10616–10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltschko AB, Johnson MJ, Iurilli G, Peterson RE, Katon JM, Pashkovski SL, Abraira VE, Adams RP, and Datta SR (2015). Mapping Sub-Second Structure in Mouse Behavior. Neuron 88, 1121–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans SS, and Powers JB (1977). Olfactory and vomeronasal deafferentation of male hamsters: histological and behavioral analyses. Brain Res 126, 325–344. [DOI] [PubMed] [Google Scholar]
- Wong LC, Wang L, D’amour JA, Yumita T, Chen G, Yamaguchi T, Chang BC, Bernstein H, You X, and Feng JE (2016). Effective modulation of male aggression through lateral septum to medial hypothalamus projection. Current biology 26, 593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wook Koo J, Labonte B, Engmann O, Calipari ES, Juarez B, Lorsch Z, Walsh JJ, Friedman AK, Yorgason JT, Han MH, et al. (2016). Essential Role of Mesolimbic Brain-Derived Neurotrophic Factor in Chronic Social Stress-Induced Depressive Behaviors. Biol Psychiatry 80, 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, and Dulac CG (2014). Galanin neurons in the medial preoptic area govern parental behaviour. Nature 509, 325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, and Kawata M (2014). Identification of neural cells activated by mating stimulus in the periaqueductal gray in female rats. Front Neurosci 8, 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M (2017). Functional Sub-Circuits of the Olfactory System Viewed from the Olfactory Bulb and the Olfactory Tubercle. Front Neuroanat 11, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wei D, Song SC, Lim B, Tritsch NX, and Lin D (2020). Posterior amygdala regulates sexual and aggressive behaviors in male mice. Nat Neurosci [DOI] [PMC free article] [PubMed]
- Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, Juntti SA, Unger EK, Wells JA, and Shah NM (2013). Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell 153, 896–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, de Jong JW, Tak Y, Peck J, Bateup HS, and Lammel S (2018). Nucleus Accumbens Subnuclei Regulate Motivated Behavior via Direct Inhibition and Disinhibition of VTA Dopamine Subpopulations. Neuron 97, 434–449 e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Yang CF, Chizari MD, Maheswaranathan N, Burke KJ Jr., Borius M, Inoue S, Chiang MC, Bender KJ, Ganguli S, et al. (2017). Social Control of Hypothalamus-Mediated Male Aggression. Neuron [DOI] [PMC free article] [PubMed]
- Yoest KE, Cummings JA, and Becker JB (2014). Estradiol, dopamine and motivation. Cent Nerv Syst Agents Med Chem 14, 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, and Wang Z (2011). The neurobiology of pair bonding: insights from a socially monogamous rodent. Front Neuroendocrinol 32, 53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, and Insel TR (2001). Cellular mechanisms of social attachment. Horm Behav 40, 133–138. [DOI] [PubMed] [Google Scholar]
- Young LJ, Muns S, Wang Z, and Insel TR (1997). Changes in oxytocin receptor mRNA in rat brain during pregnancy and the effects of estrogen and interleukin-6. J Neuroendocrinol 9, 859–865. [DOI] [PubMed] [Google Scholar]
- Yu CJ, Zhang SW, and Tai FD (2016). Effects of nucleus accumbens oxytocin and its antagonist on social approach behavior. Behav Pharmacol 27, 672–680. [DOI] [PubMed] [Google Scholar]
- Yu CR (2015). TRICK or TRP? What Trpc2(−/−) mice tell us about vomeronasal organ mediated innate behaviors. Front Neurosci 9, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalocusky KA, Ramakrishnan C, Lerner TN, Davidson TJ, Knutson B, and Deisseroth K (2016). Nucleus accumbens D2R cells signal prior outcomes and control risky decision-making. Nature 531, 642–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha X, Wang L, Jiao ZL, Yang RR, Xu C, and Xu XH (2020). VMHvl-Projecting Vglut1+ Neurons in the Posterior Amygdala Gate Territorial Aggression. Cell Rep 31, 107517. [DOI] [PubMed] [Google Scholar]
- Zhou T, Zhu H, Fan Z, Wang F, Chen Y, Liang H, Yang Z, Zhang L, Lin L, Zhan Y, et al. (2017). History of winning remodels thalamo-PFC circuit to reinforce social dominance. Science 357, 162–168. [DOI] [PubMed] [Google Scholar]
- Zilioli S, and Watson NV (2014). Testosterone across successive competitions: Evidence for a ‘winner effect’ in humans? Psychoneuroendocrinology 47, 1–9. [DOI] [PubMed] [Google Scholar]


