Abstract
Introduction:
Kidney cancer incidence is increasing among Hispanics but rate differences by distinct group, such as Cuban, Puerto Rican, and Mexican have not been studied. To fill this knowledge gap, we use mortality data, reflecting fatal kidney cancers, to examine patterns by race-ethnicity, including detailed Hispanic groups, and correlate the mortality rates with each group’s prevalence of known kidney cancer risk factors: smoking, obesity, hypertension, and diabetes.
Methods:
We used individual-level death data for California, Florida, and New York (2008-2018), and population prevalence data from the National Health Interview Surveys (2008-2018). Age-adjusted mortality rates (AAMRs) and regression-derived mortality rate ratios (MRRs) were computed. Pearson correlation analyses assessed the extent to which group-specific risk factor prevalence explained variability in observed AAMRs.
Results:
US-born Mexican Americans and American Indians had the highest rates and MRRs compared to Whites: 1.44 (95%CI: 1.35-1.53) and 1.51 (1.38-1.64) for Mexican American men and women, respectively, and 1.54 (95%CI: 1.25-1.89) and 1.53 (95%CI: 1.15-2.04) for American Indians. In contrast, non-Mexican Hispanics had lower rates than Whites. Among males, positive correlations between AAMRs and smoking, obesity, and chronic kidney disease prevalence by race-ethnicity were found.
Conclusion:
Mexican Americans and American Indians are high-risk for fatal kidney cancer. Disparities are only partially attributable to higher smoking and obesity prevalence, and more so among men than women. A shared risk factor profile, as well as possible genetic similarities, may explain their disproportionately higher kidney cancer mortality, but further research is warranted.
Keywords: Mexican, Cuban, American Indian, Smoking, Obesity
Introduction
In the United States (US), nearly 74,000 new cases and 14,000 deaths from kidney cancer are reported annually. Kidney cancer is one of the most rapidly increasing cancers, particularly among men, for which incidence is increasing 1.5% annually [1]. However, the rising incidence has largely been attributed to inadvertent detection during imaging, including CT, MRI, and ultrasound scans, for other conditions [2, 3]; most of these tumors are diagnosed at an early/localized stage. In contrast, kidney cancer mortality is decreasing: 2.4% annually in men and 1.4% in women [4]. Fewer than 1 in 5 patients diagnosed with kidney cancer die from it. Prognosis is relatively good given that almost 85% of kidney cancer cases survive 5 years or more [5] and a large proportion of kidney cancer cases are effectively cured. The favorable kidney cancer mortality trend can be explained both by tumor detection at earlier stages [6] and by improved therapy after diagnosis. In particular, the discovery of anti-angiogenic treatment with tyrosine kinase inhibitors for renal cell carcinoma has significantly extended the survival time for patients with advanced kidney cancer [7–9].
American Indians are the racial-ethnic group at the highest risk for kidney cancer [1, 10]. Hispanics, non-Hispanic Whites, and non-Hispanic Blacks all have similar incidence, while kidney cancer risk is lowest among the Asian/Pacific Islander population. Nonetheless, among Hispanics, kidney cancer is the most rapidly increasing cancer with nearly 8,000 new kidney cancer cases and 1,300 deaths reported each year. In Hispanic men, kidney cancer is now the fourth most common cancer after prostate, lung, and colorectal [1]. Moreover, the annual decreases in mortality for Hispanics (at 0.8% and 0.7% annually in males and females, respectively) lag behind those observed among Whites and Blacks [4]. Despite this burden, little is known about kidney cancer patterns among specific Hispanic groups. Cubans, Mexicans, Puerto Ricans, and others are remarkably heterogeneous in genetic, socioeconomic, cultural dimensions. The prevalence of smoking, hypertension, obesity, and diabetes, the main risk factors for kidney cancer [11–16], vary substantially between these groups [17, 18].
Studying kidney cancer patterns by distinct Hispanic group has the potential to reveal specific predispositions due to complex genetic and environmental factors which can modulate public health awareness and provide opportunities for targeted interventions. Currently, it is not feasible to analyze kidney incidence rates for distinct Hispanic groups because cancer surveillance registry data is incomplete for detailed Hispanic ethnicity [19]. In contrast, mortality data is highly complete in Hispanic group affiliation [19]. Additionally, mortality rates more readily translate the overall kidney cancer burden on a population basis, effectively encapsulating the experience of the more aggressive forms of kidney cancer, where interventions may have the strongest impact in reducing mortality disparities [6].
To address this knowledge gap, we aim to study kidney cancer among all major racial-ethnic groups and specific Hispanic groups in the US. In the current study, we analyze individual-level kidney cancer mortality data for three diverse states: California, Florida, and New York. Lastly, we assess the extent to which differences in rates by race-ethnicity can be explained by the distinct prevalence of the main risk factors for kidney cancer: smoking, obesity, hypertension, and diabetes.
Materials and Methods
All cancer deaths in California and Florida (2008-2018) and New York (2008-2017) were obtained from the Department of Public Health in California and the Departments of Health in Florida and New York. Decedents with kidney cancer (International Classification of Diseases ICD-10 code C64) listed as the cause of death were selected for inclusion in the current study. Five mutually exclusive major racial-ethnic groups were analyzed: White, Black, Asian/Pacific Islander (API), and American Indian, all non-Hispanic; and Hispanic, of any race. Since individual-level mortality data for these three states is highly complete for race-ethnicity (over 99%) and birthplace (over 98%), a specific Hispanic group is ascertainable for 96% of all Hispanic decedents [19]. Accordingly, the broad Hispanic group was disaggregated into the following detailed groups: Mexican, Puerto Rican, Cuban, Dominican, Central American, South American, Spanish (from Spain; rates not reported due to low numbers), and unknown. For the largest Hispanic group in the US, Mexicans, we formed two groups for analysis: Mexican Americans (Mexican origin, born in the US) and Mexican Immigrant (Mexican origin, born abroad, regardless of citizenship), given past evidence of substantial differences in cancer mortality between these two groups [20, 21]. Kidney cancer deaths among individuals with unknown race-ethnicity or more than two reported races were excluded from analyses (N=123). Detailed population denominators corresponding to the death data for each analyzed racial-ethnic and Hispanic group for each state were obtained from single-year American Community Survey data from 2008-2018 for California and Florida and 2008-2017 for New York [22]. Combined, they have robust populations-at-risk for each major racial-ethnic group (and distinct Hispanic group), with at least 1,000,000 people annually for all groups except American Indians (220,000) and Dominicans (992,000) [22].
Age-adjusted mortality rates (AAMR) stratified by sex were calculated per 100,000 persons, annualized and age-standardized to the 2000 US Standard Population using eighteen age bands (all 5-year except the last, 85 and older) for each racial-ethnic group studied. Gamma intervals modification was used to calculate 95% CIs for AAMRs (Table 1). Mortality rate ratios (MRRs) comparing each population to Whites as the reference were computed using negative binomial regression (Table 2). Median age (in years) at death and age-specific sex-combined mortality rates per 100,000 were computed (Figure 1). In addition, to assess how possible differences in kidney cancer rates by Hispanic group impact different rates for all Hispanics combined at the state level, we retrieved data on AAMRs for non-Hispanic Whites (hereafter, Whites) and Hispanics for the eight states with the largest numbers of Hispanic population (all greater than 1 million) from the Centers for Disease Control and Prevention database, for 2008-2016 [23], These include four states bordering Mexico (Arizona, California, New Mexico, and Texas) as well as four non-border states (Florida, Illinois, New Jersey, and New York) (Supplementary Table 1).
Table 1.
Total population at risk and number of kidney cancer deaths by race-ethnicity and sex; California and Florida (2008-2018), New York (2008-2017).
| Population Data | Cancer Deaths | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Males | Females | ||||||||
| Annualized Total Populationa | Number | % Born in US | AAMR | 95%CI | Number | % Born in US | AAMR | 95%CI | |
| WHITE | 37,223,149 | 15,192 | 91 | 5.3 | (5.26-5.43) | 7,947 | 90 | 2.2 | (2.17-2.27) |
| BLACK | 8,646,537 | 1,631 | 85 | 4.7 | (4.49-4.98) | 904 | 88 | 1.9 | (1.78-2.04) |
| HISPANIC | 23,429,193 | 3,465 | 38 | 4.7 | (4.54-4.88) | 1,933 | 38 | 2.1 | (1.97-2.16) |
| Mexican | 14,065,644 | 2,075 | 48 | 5.7 | (5.42-5.95) | 1,166 | 49 | 2.8 | (2.61-2.94 |
| Mexican American | 9,014,209 | 1,073 | 100 | 7.4 | (6.91-7.85) | 600 | 100 | 3.4 | (3.14-3.71) |
| Mexican Immigrant | 5,051,435 | 1,002 | 0 | 4.6 | (4.31-4.97) | 566 | 0 | 2.4 | (2.15-2.60) |
| Puerto Rican | 2,306,260 | 315 | 54 | 3.6 | (3.17-4.00) | 193 | 51 | 1.7 | (1.45-1.93) |
| Cuban | 1,594,218 | 485 | 5 | 4.6 | (4.16-4.99) | 231 | 5 | 1.6 | (1.41-1.86) |
| Dominican | 992,017 | 57 | 18 | 1.8 | (1.35-2.43) | 35 | 11 | 0.8 | (0.53-1.07) |
| Central American | 2,389,329 | 196 | 8 | 3.3 | (2.76-3.85) | 145 | 1 | 1.5 | (1.28-1.80) |
| South American | 1,801,645 | 290 | 3 | 4.4 | (3.85-4.93) | 139 | 4 | 1.5 | (1.23-1.74) |
| ASIAN/PACIFIC ISLANDER | 8,409,458 | 1,111 | 15 | 2.9 | (2.74-3.10) | 627 | 12 | 1.3 | (1.17-1.37) |
| AMERICAN INDIAN | 220,446 | 91 | 100 | 7.9 | (6.24-9.90) | 48 | 100 | 3.4 | (2.52-4.60) |
Includes all 3 states
Abbreviations: AAMR, Age-adjusted mortality rate
Table 2.
MRRs for kidney cancer by race-ethnicity and sex.
| Males | Females | |
|---|---|---|
| MRR (95% CI) | MRR (95% CI) | |
| WHITE(referent) | 1 | 1 |
| BLACK | 0.88 (0.83-0.93) | 0.84 (0.79-0.91) |
| HISPANIC | 0.89 (0.86-0.93) | 0.92 (0.88-0.97) |
| Mexican American | 1.44 (1.35-1.53) | 1.51 (1.38-1.64) |
| Mexican Immigrant | 0.85 (0.80-0.91) | 1.07 (0.98-1.16) |
| Puerto Rican | 0.65 (0.58-0.73) | 0.74 (0.64-0.85) |
| Cuban | 0.85 (0.78-0.93) | 0.72 (0.63-0.83) |
| Dominican | 0.33 (0.25-0.43) | 0.32 (0.22-0.45) |
| Central American | 0.62 (0.53-0.72) | 0.65 (0.55-0.77) |
| South American | 0.79 (0.71-0.89) | 0.63 (0.44-0.89) |
| ASIAN/PACIFIC ISLANDER | 0.55 (0.52-0.59) | 0.57 (0.53-0.62) |
| AMERICAN INDIAN | 1.54 (1.25-1.89) | 1.53 (1.15-2.04) |
Abbreviations: MRR, Mortality Rate Ratios20
Figure 1.
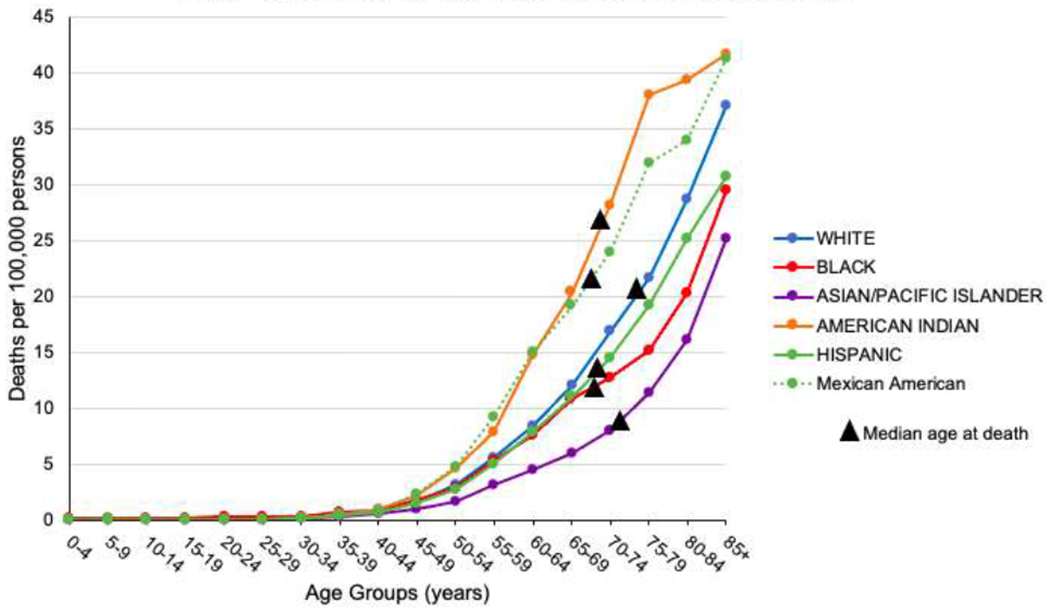
Age-specific mortality rates by select racial-ethnic groups
Lastly, prevalence data for five major kidney cancer risk factors - smoking, obesity, hypertension, diabetes (both types I and II), and chronic kidney disease (CKD) - were obtained for adults aged 50 and older (eight age bands) from the 2008-2018 National Health Interview Surveys (NHIS) [18], As CKD data is not directly available on NHIS, we followed the convention of previous research studies [24, 25] and used the question on “weak or failing kidneys” diagnosed by a physician or other health care provider in the 12 months preceding the interview as a proxy to characterize this important risk factor. The average prevalence across all available survey years was computed by age group, sex, and racial-ethnic group, including for the distinct Hispanic groups [18] (Supplementary Table 2). Pearson correlation coefficients and corresponding 95% confidence intervals (CI) were computed to examine the associations between AAMRs and the prevalence of each risk factor for each racial-ethnic group (Figure 2). For Central and South Americans, composite AAMRs were computed for the correlation analysis because NHIS groups data on risk factors from both populations.
Figure 2. Correlation between AAMRs and prevalence of smoking, obesity, and chronic kidney disease by race-ethnicity and sex.
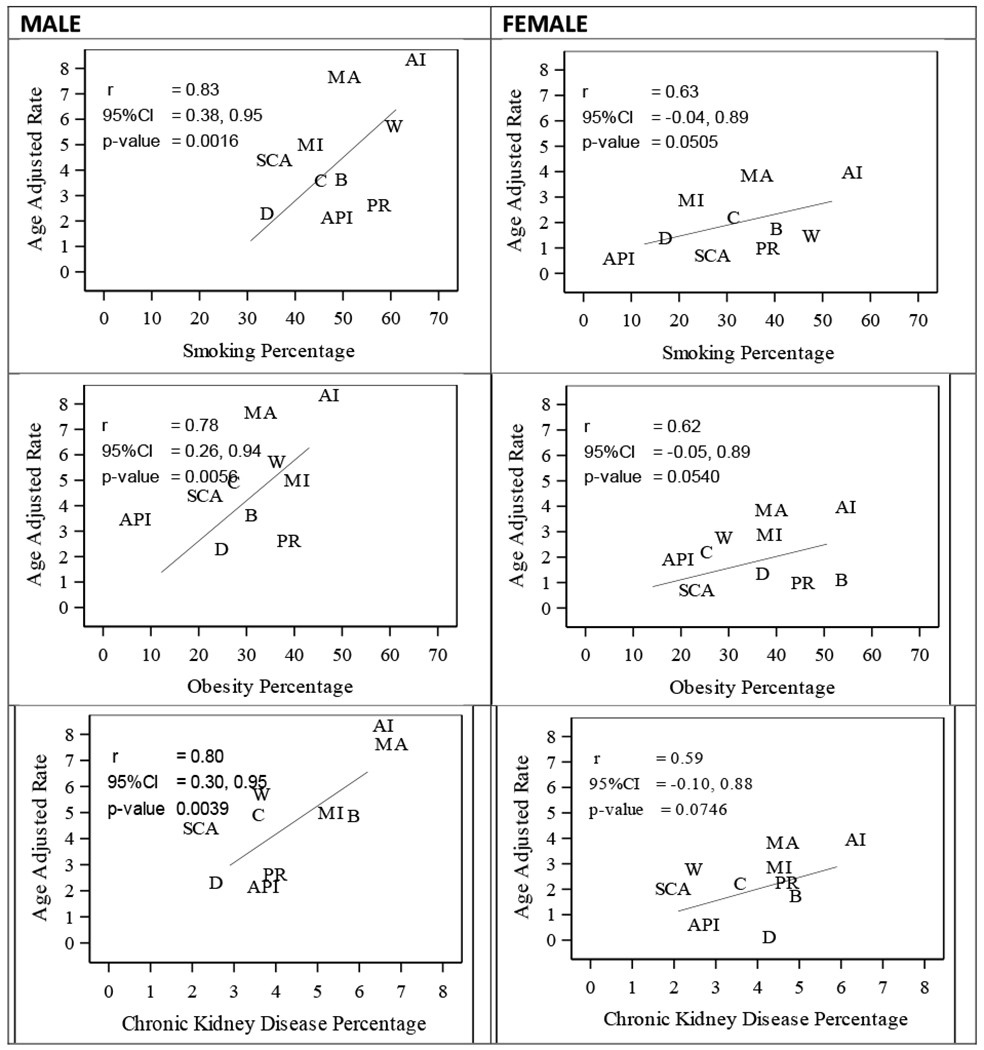
Abbreviations: AAMR, Age-adjusted mortality rate; AI, American Indian; API, Asian/Pacific Islander; C, Cuban; B, Black; D, Dominican; MA, Mexican American; MI, Mexican Immigrant; PR, Puerto Rican; SCA, South/Central American; W, White
SAS v9.4 and SPSS v25 were used for data management and statistical analyses. Ethical reviews were undertaken by the University of Miami Miller School of Medicine, the New York Department of Health, and the California Health and Human Services Agency.
Results
A total of 33,064 kidney cancer deaths from the three states for which granular, individual-level cancer mortality data were available were analyzed (Table 1). Among Whites, 23,139 deaths were recorded; with 5,398 among Hispanics, 2,535 among Blacks, 1,738 among Asian/Pacific Islanders, and 139 among American Indians. Sixty percent (n=3,241) of all Hispanic decedents were of Mexican origin, followed by Cubans (n=716; 13.3%), and Puerto Ricans (n=508; 9.4%) (Table 1). The median age of death from kidney cancer varied substantially by group, from 66 years old among Dominicans and Central Americans to 74 years old among Whites (Figure 1).
For all racial-ethnic groups, kidney cancer AAMRs for males were consistently higher, over twice that of their female counterparts (Table 1). American Indians showed the highest mortality, 54% (MRR 1.54; 95%CI: 1.25-1.89) and 53% (MRR 1.53; 95%CI: 1.15-2.04) higher for men and women, respectively, than their White counterparts (Table 2). Conversely, APIs showed significantly lower mortality than Whites, 45% lower for males and 43% for females. Kidney cancer mortality among Blacks and all-combined Hispanics was also significantly lower than among Whites, but by smaller margins: 12% and 16% lower for Black males and females, 11% and 8% lower Hispanic males and females, respectively.
When examined by distinct Hispanic group, kidney cancer mortality varied significantly (Table 1). Mexican Americans had the highest mortality rate of all the Hispanic groups (7.4 and 3.4 per 100,000 in males and females, respectively; 44% and 51% higher than White men and women). Rates for Mexican American men and women were statistically similar to those observed among the most afflicted non-Hispanic group, American Indians. Besides Mexican Americans, all other analyzed Hispanic groups had lower mortality rates than their White counterparts, except for Mexican Immigrant females, whose rates were not statistically significantly different from White females. After Mexican Americans, Mexican Immigrant, Cuban, and South American males had similar rates, followed by Puerto Ricans, Central Americans, and Dominicans. Among women, the kidney cancer mortality rate for Mexican Immigrants was higher than all other Hispanic groups, except Mexican Americans; Puerto Rican, Cuban, Central and South American rates were similar, while Dominican women, as with men, had the lowest kidney cancer mortality, one-third or less than that of Whites (Table 1). By age, excess mortality among American Indians and Mexican Americans in comparison to Whites is evident across all age groups above 50 years old, particularly among those aged 60-74 (Figure 1).
By geographic region, age-adjusted MRRs comparing Hispanics to Whites as the reference group, showed higher mortality for Hispanics, greater than 1, for all border states; Arizona had the highest mortality difference with Hispanics showing 38% greater kidney cancer mortality (MRR 1.38; 95%CI: 1.25-1.53). In non-border states, Hispanics had significantly lower rates than Whites for all four states examined; the lowest was in New York, where Hispanics had a 49% lower mortality rate (MRR 0.51; 95% CI: 0.46-0.56) than Whites (Supplementary Table 1).
Lastly, among males, positive univariable statistically significant correlations between kidney cancer AAMRs and the prevalence of smoking (r=0.83; 95%CI 0.38,0.95; p<0.01), obesity (r=0.78; 95%CI 0.26,0.94; p<0.01), and CKD (r=0.80; 95%CI 0.30,0.95; p<0.01) by racial-ethnic group were documented (Figure 2). Among females, results were suggestive of similar correlations but did not achieve statistical significance: smoking (r=0.63; p=0.051), obesity (r=0.62; p=0.054), and CKD (r=0.59; p=0.074). No significant correlations were found between the prevalence of hypertension or diabetes and kidney cancer AAMRs by race-ethnicity for either males or females.
Discussion
Among US Hispanics, kidney cancer mortality rates vary substantially by specific group. We found that Mexican-origin Hispanics had significantly higher kidney cancer mortality than any other Hispanic group. Within the Mexican population, country of birth was also an important determinant of kidney cancer mortality: Mexican Americans showed significantly higher mortality rates than Mexican Immigrants, 60% and 40% higher among males and females, respectively. In contrast, all non-Mexican Hispanic groups had lower kidney cancer mortality rates. These findings explain our own results by geographic region which similarly to previous reports [26] show higher rates of kidney cancer in border states where Hispanics are largely of Mexican origin in contrast to lower rates in non-border states such as Florida and New York where Cubans and Puerto Ricans are the predominant Hispanic group, respectively.
For non-Hispanics, our study confirmed previous reports that show a disproportionately high kidney cancer mortality burden among the American Indian population, higher than all other major racial-ethnic groups in the US [1, 5, 10, 13]. For Blacks, rates were lower than Whites in contrast with national reports, likely a result of the known lower kidney cancer rates among Afrocaribbeans in Florida and New York who make up a meaningful proportion of Blacks in these states [27, 28]. However, by disaggregation of Hispanic groups, we now convincingly demonstrate, for the first time, that Mexican Americans share a similarly high and disproportionate burden of kidney cancer mortality. This disparity suggests similarities in the epidemiology of kidney cancer between Mexican Americans and Americans Indians, two understudied populations.
Although American Indians and Mexican Americans have profoundly different historical experiences in the US, they also share commonalities that may explain why both are so afflicted by fatal kidney cancer. Both groups share social, cultural, historical, and economic life experiences derived from their minority status in the US and the concomitant impacts of discrimination and prejudice, such as low rankings for major socioeconomic status indicators, including education and income [29–31]. Moreover, in addition to these two groups sharing a high prevalence of known risk factors for kidney cancer (Supplementary Table 2), Mexican Americans also have a high American Indian genomic contribution [32]. Combined, these characteristics may represent common factors impacting cancer outcomes in these populations.
From a public health standpoint, our results from the correlation analyses that highlighted the impact of the population prevalence of smoking, obesity, and CKD on kidney cancer mortality are critically important. These findings suggest that fatal kidney cancer may be largely preventable with targeted and culturally specific population-level programs that effectively modify key lifestyle factors and result in lower obesity and smoking prevalence among these high-risk racial-ethnic groups. However, it is notable that the Black population, another historically marginalized group that shares a poor socioeconomic profile as well as high prevalence of both smoking and obesity, does not demonstrate high kidney cancer mortality, despite often showing the highest burden for other cancers [1, 5, 27].
In addition to the exceedingly high kidney cancer mortality rates in Mexican Americans, Mexican Immigrants also showed higher rates than all other female and most other male Hispanic groups. These relatively high rates among Mexican Immigrants, particularly among females, where the smoking prevalence is the lowest of all Hispanics could indicate a higher predisposition to fatal kidney cancer in Mexican origin groups compared to non-Mexican origin Hispanics. While studies describing the association between mutations in the von-Hippel Lindau and Polybromo-1 (PBRM1) genes and clear cell carcinomas have been conducted in the population at large, molecular genomic studies among American Indian and Hispanic (even in aggregate) populations are scarce [26]. From a population perspective, our findings highlight the importance of studying genetic and epigenetic features of fatal kidney cancer with Mexican origin populations. Acculturation factors impacting second and third generation Mexican Americans, including a higher prevalence of smoking and obesity compared to Mexican Immigrants, may well exacerbate an underlying genetic predisposition for fatal kidney cancers among Mexican-origin groups, a predisposition that may be shared by American Indians. Moreover, beyond targeted kidney cancer prevention programs, genetic studies on these populations may be highly valuable in decreasing mortality after a kidney cancer diagnosis, since the greatest improvements in prognosis and clinical outcomes will likely result from early detection as well as efforts in precision medicine to target specific genomic variations.
In the broader context of highlighting disparities in cancer outcomes, the shared disadvantage in fatal kidney cancer between Mexican Hispanics and American Indians has also been observed for other cancers, especially liver cancer [10, 33]. These patterns, often invisible when analyzing only large racial-ethnic groups, i.e., Hispanics in aggregate, are quite distinct from the more extensively studied cancer disparities documented for Blacks in relation to Whites. Therefore, this suggests that further investigation of cancer epidemiology patterns for these two similarly disadvantaged groups are warranted.
In this population-based study, we found low rates of kidney cancer among Dominicans and high rates among Mexicans, which is consistent with previous research conducted with hospital-based patients through the national cancer database [34]. The same report also suggested a lower age of onset for kidney cancer among Hispanic populations [34]. In the current study, the median age at death from kidney cancer was indeed lower among all minority populations than their White counterparts. However, the age-specific mortality rates in our study and particularly the excessive rates among American Indians and Mexican Americans in relation to Whites were not markedly different by age group. This demonstrates that the observed younger age at death for kidney cancer is more likely a reflection of the underlying population age structure rather than an increased mortality risk for younger minority populations, as has been suggested for incident kidney cancer [34, 35].
The population-based nature of the current study is its greatest strength as this eliminates selection bias. We also chose to conduct the study with states that provide a full representation of all major racial-ethnic groups in the US as well as the remarkable diversity within the Hispanic population. Nonetheless, some limitations should be noted. Our population-based mortality rates depend mostly on incidence patterns; however, racial-ethnic differences can also be affected by survival, which has not been assessed by distinct Hispanic group thus far. Individual-level data on kidney cancer risk factors, including obesity, smoking, and CKD are not available in mortality data; therefore, ecological data were used instead, with some inherent degree of ecological fallacy. Moreover, interpretation of the correlations for each distinct risk factor should take into account that these often occur simultaneously as comorbidities. Obesity, smoking, and CKD are risk factors but also potentially prognostic factors for kidney cancer; each has the potential to impact mortality rates through both risk and prognosis. However, with the current data, ascertaining the separate impact of each is not possible; as such, clarification on the detailed effects, including the potential paradoxical effect of obesity [36–38] as a protective effect in kidney cancer survival, is not possible. Additionally, mortality data does not have information on tumor morphology so we were unable to examine the possible impact of the documented higher propensity for clear cell carcinomas among Whites and Hispanics, in contrast to the higher prevalence of papillary renal carcinomas among Blacks [34, 39, 40], To potentially supplement our mortality data with histology information, we explored available cancer registry data. However, on a populational basis, over 50% of incident cases with kidney cancer as the cause of death were recorded with a non-informative “renal cell carcinoma” histology or as “unspecified” carcinomas. Therefore, conducting rigorous population-based analyses of histologies such as clear cell, papillary or chromophobe by detailed racial-ethnic group is currently not feasible. Additionally, individual-level data on stage at diagnosis, another important determinant of mortality, is also not readily available in vital statistics death data. American Indian populations are heterogeneous in terms of prevalence of smoking and obesity; this variability is not examined [10], Lastly, as with most mortality studies, the Salmon bias, in which foreign-born populations emigrate back home prior to death and are undercounted, could potentially deflate mortality rates in some of the majority foreign-born populations studied here [41].
In conclusion, our descriptive epidemiological profile for kidney cancer mortality by race-ethnicity offers several novel findings. We found that Mexican American men and women join American Indians as the two groups most disproportionately burdened by fatal kidney cancer in the US, with over 50% higher mortality than Whites. Smoking and obesity prevalence differences were able to explain some of the variation by race-ethnicity in kidney cancer mortality rates; thus, targeted kidney cancer prevention programs could focus on these disadvantaged groups. The high rates among these two high-risk populations, with no disparity seen for Blacks, combined with the persistent disparity for Mexican Immigrant women with low smoking prevalence, warrant the more detailed study of genetic factors among American Indian and Mexican-origin Hispanics contributing to kidney cancer in order to best prepare for improving kidney cancer prognosis and reducing mortality. Our study highlights the importance of clinicians and researchers alike no longer treating these diverse racial/ethnic populations in a monolithic manner: risk factors, risk, and prognoses vary by country of origin, culture, and geography.
Supplementary Material
Highlights:
Mexican Hispanics and American Indians are at high risk for fatal kidney cancer.
Strong correlation between kidney cancer mortality and smoking/obesity prevalence.
For Hispanics, birthplace impacts cancer patterns, should be included in studies.
Acknowledgments
Financial Disclosure:
Supplemental funding was provided by the Sylvester Comprehensive Cancer Center at University of Miami Miller School of Medicine. Research reported in this publication was also supported by the National Cancer Institute of the National Institutes of Health under Award Number P30CA240139. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
List of Abbreviations
- US
United States
- ICD
International Classification of Diseases
- API
Asian/Pacific Islander
- AAMR
Age-adjusted mortality rate
- CI
Confidence interval
- MRR
Mortality rate ratio
- NHIS
National Health Interview Survey
- PBRM1
Polybromo-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors declare no conflicts of interest.
References
- [1].Ward EM, Sherman RL, Henley SJ, Jemal A, Siegel DA, Feuer EJ, Firth AU, Kohler BA, Scott S, Ma J, Anderson RN, Benard V, Cronin KA, Annual Report to the Nation on the Status of Cancer, Featuring Cancer in Men and Women Age 20-49 Years, J Natl Cancer Inst 111(12) (2019) 1279–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chow WH, Dong LM, Devesa SS, Epidemiology and risk factors for kidney cancer, Nat Rev Urol 7(5) (2010) 245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Morris CR, Lara PN Jr., Parikh-Patel A, Kizer KW, Kidney Cancer Incidence in California: End of the Trend?, Kidney Cancer 1(1) (2017) 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Henley SJ, Ward EM, Scott S, Ma J, Anderson RN, Firth AU, Thomas CC, Islami F, Weir HK, Lewis DR, Sherman RL, Wu M, Benard VB, Richardson LC, Jemal A, Cronin K, Kohler BA, Annual report to the nation on the status of cancer, part I: National cancer statistics, Cancer 126(10) (2020) 2225–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov ) SEER*Stat Database: Incidence - SEER Research Data, 9 Registries, Nov 2019 Sub (1975-2017) - Linked To County Attributes - Time Dependent (1990-2017) Income/Rurality, 1969-2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2020, based on the November 2019 submission.
- [6].Palumbo C, Pecoraro A, Knipper S, Rosiello G, Luzzago S, Deuker M, Tian Z, Shariat SF, Simeone C, Briganti A, Saad F, Berruti A, Antonelli A, Karakiewicz P.l., Contemporary Age-adjusted Incidence and Mortality Rates of Renal Cell Carcinoma: Analysis According to Gender, Race, Stage, Grade, and Histology, European Urology Focus (2020). [DOI] [PubMed] [Google Scholar]
- [7].Choueiri TK, Motzer RJ, Systemic Therapy for Metastatic Renal-Cell Carcinoma, N Engl J Med 376(4) (2017) 354–366. [DOI] [PubMed] [Google Scholar]
- [8].Li P, Wong YN, Armstrong K, Haas N, Subedi P, Davis-Cerone M, Doshi JA, Survival among patients with advanced renal cell carcinoma in the pretargeted versus targeted therapy eras, Cancer Med 5(2) (2016) 169–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pal SK, Ghate SR, Li N, Swallow E, Peeples M, Zichlin ML, Perez JR, Agarwal N, Vogelzang NJ, Real-World Survival Outcomes and Prognostic Factors Among Patients Receiving First Targeted Therapy for Advanced Renal Cell Carcinoma: A SEER-Medicare Database Analysis, Clin Genitourin Cancer 15(4) (2017) e573–e582. [DOI] [PubMed] [Google Scholar]
- [10].Melkonian SC, Weir HK, Jim MA, Preikschat B, Haverkamp D, White MC, Incidence and Trends of the Leading Cancers with Elevated Incidence Among American Indian and Alaska Native Populations, 2012-2016, Am J Epidemiol (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hunt JD, van der Hel OL, McMillan GP, Boffetta P, Brennan P, Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies, Int J Cancer 114(1) (2005) 101–8. [DOI] [PubMed] [Google Scholar]
- [12].Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M, Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies, Lancet 371(9612) (2008) 569–78. [DOI] [PubMed] [Google Scholar]
- [13].Chow WH, Gridley G, Fraumeni JF Jr., Järvholm B, Obesity, hypertension, and the risk of kidney cancer in men, N Engl J Med 343(18) (2000) 1305–11. [DOI] [PubMed] [Google Scholar]
- [14].Weikert S, Boeing H, Pischon T, Weikert C, Olsen A, Tjonneland A, Overvad K, Becker N, Linseisen J, Trichopoulou A, Mountokalakis T, Trichopoulos D, Sieri S, Palli D, Vineis P, Panico S, Peeters PH, Bueno-de-Mesquita HB, Verschuren WM, Ljungberg B, Hallmans G, Berglund G, González CA, Dorronsoro M, Barricade A, Tormo MJ, Allen N, Roddam A, Bingham S, Khaw KT, Rinaldi S, Ferrari P, Norat T, Riboli E, Blood pressure and risk of renal cell carcinoma in the European prospective investigation into cancer and nutrition, Am J Epidemiol 167(4) (2008) 438–46. [DOI] [PubMed] [Google Scholar]
- [15].Macleod LC, Hotaling JM, Wright JL, Davenport MT, Gore JL, Harper J, White E, Risk factors for renal cell carcinoma in the VITAL study, J Urol 190(5) (2013) 1657–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cumberbatch MG, Rota M, Catto JW, La Vecchia C, The Role of Tobacco Smoke in Bladder and Kidney Carcinogenesis: A Comparison of Exposures and Meta-analysis of Incidence and Mortality Risks, Eur Urol 70(3) (2016) 458–66. [DOI] [PubMed] [Google Scholar]
- [17].Siegel RL, Fedewa SA, Miller KD, Goding-Sauer A, Pinheiro PS, Martinez-Tyson D, Jemal A, Cancer statistics for Hispanics/Latinos, 2015, CA Cancer J Clin 65(6) (2015) 457–480. [DOI] [PubMed] [Google Scholar]
- [18].National Center for Health Statistics, Centers for Disease Control and Prevention. National Health Interview Survey Public Use Data File 2020.Atlanta, GA: : National Center for Health Statistics, Centers for Disease Control and Prevention; 2020. [Google Scholar]
- [19].Pinheiro PS, Callahan KE, Kobetz EN, Disaggregated Hispanic Groups and Cancer: Importance, Methodology, and Current Knowledge, in: Ramirez AG, Trapido EJ (Eds.), Advancing the Science of Cancer in Latinos, Springer International Publishing, Cham, 2020, pp. 17–34. [PubMed] [Google Scholar]
- [20].Pinheiro PS, Callahan KE, Gomez SL, Marcos-Gragera R, Cobb TR, Roca-Barcelo A, Ramirez AG, High cancer mortality for US-born Latinos: evidence from California and Texas, BMC Cancer 17(1) (2017) 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pinheiro PS, Callahan KE, Stern MC, de Vries E, Migration from Mexico to the United States: A high-speed cancer transition, Int J Cancer 142(3) (2018) 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ruggles S, Flood S, Goeken R, Grover J, Meyer E, Pacas J, Sobek M, IPUMS USA: Version 10.0 [dataset]. Minneapolis, MN: IPUMS, 2020. 10.18128/D010.V10.0. [DOI] [Google Scholar]
- [23].United States Cancer Statistics - Incidence: 1999-2017, WONDER Online Database. United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2020. https://wonder.cdc.gov/wonder/help/cancer-v2017.html.Accessed November 22, 2020. [Google Scholar]
- [24].Choi NG, Sullivan JE, DiNitto DM, Kunik ME, Health Care Utilization Among Adults With CKD and Psychological Distress, Kidney Medicine 1(4) (2019) 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Salifu I, Tedla F, Pandey A, Ayoub I, Brown C, McFarlane S.l., Jean-Louis G, Sleep duration and chronic kidney disease: analysis of the national health interview survey, Cardiorenal Med 4(3-4) (2014) 210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Batai K, Bergersen A, Price E, Hynes K, Ellis NA, Lee BR, Clinical and Molecular Characteristics and Burden of Kidney Cancer Among Hispanics and Native Americans: Steps Toward Precision Medicine, Clin Genitourin Cancer 16(3) (2018) e535–e541. [DOI] [PubMed] [Google Scholar]
- [27].Pinheiro PS, Medina H, Callahan KE, Kwon D, Ragin C, Sherman R, Kobetz EN, Jemal A, Cancer mortality among US blacks: Variability between African Americans, Afro-Caribbeans, and Africans, Cancer Epidemiol 66 (2020) 101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pinheiro PS, Callahan KE, Ragin C, Hage RW, Hylton T, Kobetz EN, Black Heterogeneity in Cancer Mortality: US-Blacks, Haitians, and Jamaicans, Cancer Control 23(4) (2016) 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jones DS, The persistence of American Indian health disparities, Am J Public Health 96(12) (2006) 2122–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sarche M, Spicer P, Poverty and health disparities for American Indian and Alaska Native children: current knowledge and future prospects, Ann N Y Acad Sci 1136 (2008) 126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Vega WA, Rodriguez MA, Gruskin E, Health disparities in the Latino population, Epidemiol Rev 31 (2009) 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bryc K, Velez C, Karafet T, Moreno-Estrada A, Reynolds A, Auton A, Hammer M, Bustamante CD, Ostrer H, Colloquium paper: genome-wide patterns of population structure and admixture among Hispanic/Latino populations, Proc Natl Acad Sci USA 107 Suppl 2(Suppl 2) (2010) 8954–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pinheiro PS, Callahan KE, Jones PD, Morris C, Ransdell JM, Kwon D, Brown CP, Kobetz EN, Liver cancer: A leading cause of cancer death in the United States and the role of the 1945-1965 birth cohort by ethnicity, JHEP Reports 1(3) (2019) 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Batai K, Harb-De la Rosa A, Zeng J, Chipollini JJ, Gachupin FC, Lee BR, Racial/ethnic disparities in renal cell carcinoma: Increased risk of early-onset and variation in histologic subtypes, Cancer Med 8(15) (2019) 6780–6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Suarez-Sarmiento A, Yao X, Hofmann JN, Syed JS, Zhao WK, Purdue MP, Chow W-H, Corley D, Shuch B, Ethnic disparities in renal cell carcinoma: An analysis of Hispanic patients in a single-payer healthcare system, International Journal of Urology 24(10) (2017) 765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kim LH, Doan P, He Y, Lau HM, Pleass H, Patel MI, A Systematic Review and Meta-Analysis of the Significance of Body Mass Index on Kidney Cancer Outcomes, J Urol 205(2) (2021) 346–355. [DOI] [PubMed] [Google Scholar]
- [37].Sanchez A, Furberg H, Kuo F, Vuong L, Ged Y, Patil S, Ostrovnaya I, Petruzella S, Reising A, Patel P, Mano R, Coleman J, Russo P, Liu CH, Dannenberg AJ, Chan TA, Motzer R, Voss MH, Hakimi AA, Transcriptomic signatures related to the obesity paradox in patients with clear cell renal cell carcinoma: a cohort study, The Lancet Oncology 21(2) (2020) 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Choi Y, Park B, Jeong BC, Seo SI, Jeon SS, Choi HY, Adami HO, Lee JE, Lee HM, Body mass index and survival in patients with renal cell carcinoma: a clinical-based cohort and meta-analysis, Int J Cancer 132(3) (2013) 625–34. [DOI] [PubMed] [Google Scholar]
- [39].Olshan AF, Kuo TM, Meyer AM, Nielsen ME, Purdue MP, Rathmell WK, Racial difference in histologic subtype of renal cell carcinoma, Cancer Med 2(5) (2013) 744–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mafolasire A, Yao X, Nawaf C, Suarez-Sarmiento A, Chow WH, Zhao W, Corley D, Hofmann JN, Purdue M, Adeniran AJ, Shuch B, Racial disparities in renal cell carcinoma: a single-payer healthcare experience, Cancer Med 5(8) (2016) 2101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pinheiro PS, Morris CR, Liu L, Bungum TJ, Altekruse SF, The impact of follow-up type and missed deaths on population-based cancer survival studies for Hispanics and Asians, J Natl Cancer Inst Monogr 2014(49) (2014) 210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


