Abstract
The aim of the present study was to develop, optimize brucine-loaded transliposomes (BRC-TL) formulation for dermal delivery of brucine for skin cancer. The BRC-TL formulations were evaluated for vesicle size, entrapment efficiency, and in vitro drug release. The optimized formulation was further evaluated for skin penetration by confocal laser microscopy and dermatokinetic study. The optimized BRC-TL formulation presented sealed lamellar shaped vesicles, with vesicles size, polydispersity index, entrapment efficiency, and in vitro drug release of 136.20 ± 2.87 nm, 0.354 ± 0.02, 86.01 ± 1.27%, and 83.09 ± 2.07%, respectively. Ex vivo permeation study showed that, developed BRC-TL formulation had a 2.4-fold increment in permeation as compared to BRC suspension. Texture analysis showed that the BRC-TL gel presented firmness of 158.91 g, consistency of 615.03 g/s, cohesiveness of − 115.26 g and a viscosity index of − 472.05 g/s. The confocal images of rat skin clearly showed the deeper penetration of rhodamine B-loaded TL formulation as compared to the Rhodamine B-hydro alcoholic solution. The optimized BRC-TL formulation demonstrated significantly higher cytotoxicity than placebo liposome and BRC suspension (P < 0.05). Further, the BRC-TL nanogel treated rat skin showed a substantial increase in CSkin max and AUC0–8 in comparison to rat skin treated with BRC conventional gel (P < 0.05). The data revealed that the developed TLs formulation could be a promising drug nanocarrier for brucine dermal delivery in the treatment of skin cancer.
Keywords: Brucine, Transliposomes, Dermal, Dermatokinetics
Introduction
Skin cancer starts with excessive cell growth in the epidermis, the skin’s outermost layer; this is caused by unrepaired DNA damage in cells, which causes mutations, causing skin cells to multiply rapidly and develop malignant tumors. Squamous cell carcinoma (SCC), basal cell carcinoma (BCC), melanoma, and Merkel cell carcinoma are the four major forms of skin cancer (MCC). Two main causes of skin cancer are the sun’s harmful ultraviolet (UV) rays and the use of UV tanning machines.
Targeted drug delivery systems (TDDS) are potentially valuable in allowing drugs to be delivered specifically to tumours cells, while sparing normal cells. In these systems, the drug is encapsulated into a nanocarrier such as ethosomes, liposomes, niosomes, nanoemulsions, or nanoparticles. TDDS reduce drug toxicity and increase drug efficacy, overcoming a wide range of problems such as drug solubility and instability (Mohamed et al. 2019).
Brucine (2,3-dimethoxystrychnidin-10-one), a toxic alkaloid extracted from the seeds of Strychnos nux-vomica L. and belong to Loganiaceae Family, was shown to have inhibitory effects against various tumors (Guo et al. 2018; Li et al. 2018; Qin et al. 2018). It inhibited the proliferation of HepG2 cells (Shu et al. 2013) and tumor angiogenesis, growth, and bone metastasis in a mouse model of metastatic breast cancer (Li et al. 2012). It also inhibited the growth and migration of colorectal cancer cells (Shi et al. 2018). The clinical application of brucine has been hindered due to poor aqueous solubility, narrow therapeutic window, short half‑life and high toxicity Oral administration of brucine causes GI irritation and systemic toxicity (Lu et al. 2020; Li and Wang 2017). To overcome these problems, there is a quest for development of innovative drug delivery system of brucine. Several researchers had developed liposomal delivery systems for brucine, where safety and efficacy of the developed liposomal brucine deliveries were depicted (Qin et al. 2007; Li and Wang 2017). Active targeting of the liposomal delivery was reported to facilitate tumor-targeted delivery of brucine for effective control of the tumor diameter (Li and Wang 2017). However, major limitations of these liposomal deliveries are the stability and encapsulation efficiency of these formulations, (Sharma and Sharma 1997). Alternatively, transfersomes were utilised as delivery vehicle for brucine, where transdermal index and absorption of brucine were reported to be improved (Wu et al. 2016). Similarly, polyethylene glycol-tagged nanoemulsion approach for delivering brucine had recently been made for significant inhibition of cancer cell growth in in vitro and in vivo models (Elsewedy et al. 2021). Instead, to avoid associated limitations of lipidic carriers, researchers had developed polymeric nanoparticular deliveries of brucine. Development of immune-nanoparticle of brucine was reported to be stable, which possessed significant efficacy against liver cancer cells (Qin et al. 2012). Approaches of developing chitosan-nanoparticles or PLGA-nanoparticles (Elsewedy et al. 2020) had also been reported towards effective delivery of brucine in the control of tumor growth; however, scalability and toxicity aspects of these nanoparticular deliveries limits its application potential (Khan et al. 2019).
Therefore, in the present study an approach has been made to develop brucine-loaded transliposomes (BRC-TL) for the dermal delivery of BRC. This TL (Transliposomes) delivery is a novel approach combining the advantages of liposome and transferosomes. Since TL vesicles contained edge activator, that showed better permeation ability and deposition characteristics and presented increase solubility, stability, and permeation of drugs (Gupta et al. 2020). The transformation of TL into a gel could also increase the viscosity of the formulation, thus enhancing the formulation residence time at the application site.
The objective of the present study was to develop and optimized of BRC-TL (Brucine-loaded transliposomes) modifying independent variables such as the content of lipoid S100, cholesterol and sodium cholate. The effects on the dependent variables, namely vesicle size, entrapment efficiency and in vitro drug release were examined using Box–Behnken design (BBD). Furthermore, the optimized BRC-TL formulation was analysed for vesicles morphology, skin permeation and penetration characteristics, antioxidant, cytotoxicity and dermatokinetic study.
Materials and methods
Materials
Brucine, Cholesterol, Triethanolamine were purchased from Sigma-Aldrich, (St. Louis, MO, USA). Lipoid S100 was obtained from lipoid GmbH (Germany). Sodium cholate was purchased from Thomas Baker, USA. Carbopol 940 and polyethylene glycol 400 were purchased from Merck, USA. Other chemicals were procured from Merck, USA. All solvents and chemicals used were of analytical grade and HPLC water was used for all experiments.
Preparation of brucine-TL formulation
For the development of brucine-TLs, defined quantities of lipoid S100, cholesterol, sodium cholate and brucine were mixed with chloroform:ethanol (2:1, v/v) in a flask, and subjected to solvent removal in a rotary evaporator for 4 h. The resultant dried, precipitated lipid thin film was rehydrated with saline (pH 7.4) solution for 1 h with rotation at 150 rpm under ambient temperature. Then the obtained dispersions were placed in a probe sonicator for 3 min to produce a small vesicle size. The TLs were characterized based on vesicle size, % entrapment efficiency, in vitro drug release and permeation through excised skin. A Box–Behnken design using three-factors and three-levels was applied; selected independent and dependent responses are shown in Table 1.
Table 1.
Box–Behnken design (BBD) independent and dependent variables for the development and optimization of BRC-TL
| Variables | Used levels | ||
|---|---|---|---|
| Low (− 1) | Medium (0) | High (+ 1) | |
| Independent variables | |||
| X1 = lipoid S100 (mg) | 80 | 100 | 120 |
| X2 = cholesterol (mg) | 10 | 15 | 20 |
| X3 = sodium cholate (mg) | 5 | 10 | 15 |
| Dependent variables | |||
| Y1 = vesicles size (nm) | |||
| Y2 = entrapment efficient (%) | |||
| Y3 = in vitro release (%) | |||
Optimization of brucine-TL formulation using Box–Behnken design (BBD) software
The 3-factor, 3-levels BBD was applied for the optimization of the brucine-TL formulation. As per the design expert software, 17 brucine-TL formulations (Table 2) were obtained and evaluated. The independent responses selected were lipoid S100 (X1), cholesterol (X2) and sodium cholate (X3) and the dependent responses were vesicle size (Y1), entrapment efficiency (Y2) and in vitro release (Y3) (Table 1).
Table 2.
Observed responses in BBD software for the optimization of BRC-TL formulation and summary of results of regression analysis for responses Y1, Y2 and Y3 for fitting to quadratic model
| Formulations | Independent variables | Dependent variables | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | Y2 | Y3 | |
| 1 | 120 | 10 | 10 | 181.82 ± 2.45 | 70.14 ± 1.05 | 58.24 ± 3.22 |
| 2 | 100 | 15 | 10 | 136.22 ± 3.17 | 84.28 ± 0.58 | 82.22 ± 1.54 |
| 3 | 100 | 15 | 10 | 135.74 ± 1.74 | 84.88 ± 1.48 | 81.55 ± 1.68 |
| 4 | 100 | 15 | 10 | 137.55 ± 3.27 | 86.57 ± 0.85 | 82.14 ± 2.12 |
| 5 | 100 | 20 | 15 | 167.59 ± 4.18 | 62.48 ± 2.12 | 71.14 ± 2.48 |
| 6 | 100 | 15 | 10 | 136.20 ± 2.87 | 86.01 ± 1.27 | 83.14 ± 3.42 |
| 7 | 80 | 15 | 15 | 151.22 ± 3.74 | 65.88 ± 0.68 | 62.47 ± 2.22 |
| 8 | 80 | 20 | 10 | 166.14 ± 1.22 | 55.58 ± 1.52 | 59.22 ± 2.17 |
| 9 | 100 | 10 | 15 | 152.67 ± 3.75 | 68.55 ± 1.12 | 78.55 ± 1.62 |
| 10 | 120 | 15 | 15 | 188.64 ± 2.18 | 68.14 ± 1.62 | 56.07 ± 2.95 |
| 11 | 80 | 15 | 5 | 152.44 ± 3.15 | 60.74 ± 0.48 | 52.47 ± 2.88 |
| 12 | 100 | 10 | 5 | 155.84 ± 2.54 | 65.25 ± 1.14 | 75.25 ± 2.25 |
| 13 | 120 | 15 | 5 | 198.47 ± 2.62 | 63.59 ± 1.72 | 46.28 ± 3.25 |
| 14 | 80 | 10 | 10 | 149.57 ± 1.58 | 61.87 ± 0.85 | 65.55 ± 2.18 |
| 15 | 100 | 20 | 5 | 169.48 ± 2.94 | 60.14 ± 0.38 | 68.55 ± 2.04 |
| 16 | 120 | 20 | 10 | 214.55 ± 1.84 | 64.55 ± 1.27 | 55.57 ± 3.11 |
| 17 | 100 | 15 | 10 | 137.14 ± 1.48 | 85.68 ± 0.68 | 82.66 ± 2.14 |
| Quadratic model | R2 | Adjusted R2 | Predicted R2 | SD | % CV |
|---|---|---|---|---|---|
| Response (Y1) | 0.9932 | 0.9844 | 0.8942 | 2.96 | 1.84 |
| Response (Y2) | 0.9870 | 0.9703 | 0.8180 | 1.84 | 2.63 |
| Response (Y3) | 0.9880 | 0.9725 | 0.8157 | 2.04 | 2.99 |
X1 = lipoid S100 (mg), X2 = cholesterol (mg), X3 = sodium cholate (mg), Y1 = vesicles size (nm), Y2 = entrapment efficient (%) and Y3 = in vitro release (%)
TL vesicle size and polydispersity index (PDI)
The vesicle size and PDI of the BRC-TLs were estimated using a zetasizer (Nano ZSP, Malvern, Worcestershire, United Kingdom) at 25 ± 1 °C. For this study, the formulations were dispersing in saline (pH −7.4) and the measurements were made in triplicate.
Entrapment efficiency (% EE)
The % EE of the developed TL formulation was estimated by ultracentrifugation. Brucine-TL (2 ml) was placed in the centrifuge tube and centrifuged at 25,000 rpm for 1 h at 4 °C to separate the free brucine. The collected supernatant was diluted, filtered, and the brucine concentration was determined at 264 nm by UV spectroscopy (Qin et al. 2012). The entrapment efficiency (%) was calculated using the following equation.
where % EE = percentage entrapment efficiency.
In vitro drug release
The release of drug from BRC-TLs and from a BRC suspension (BRC-CF) was determined using the dialysis bag diffusion technique. The optimized formulations were poured into the dialysis bags, which were then each immersed in 50 ml phosphate buffer (pH 7.4) at ambient temperature. 1 ml samples were removed at 0, 0.25, 0.5, 1, 2, 4, 6, 12 and 24 h and replaced by the same volume of dissolution medium. The brucine concentration was determined in a UV spectrometer at a wavelength of 264 nm (Mohammed and Urszula 2014). The in vitro drug release data were estimated using various mathematical models (Ahad et al. 2016).
Morphological analysis of BRC-TL formulation
Transmission electron microscopy (JEOL, JEM 1010, Tokyo, Japan) was used for morphological analysis of the brucine-TL formulation. A small drop of diluted sample was placed on a copper grid, allowed to dry and stained with 1% w/v of phosphotungstic acid (Ahad et al. 2018b, c).
Antioxidant properties
The antioxidant effect of brucine was determined using the DPPH (2,2-diphenyl-1-picryl-hydrazyl) method at ambient temperature (Gupta et al. 2020). At room temperature, DPPH free radical solution has a violet colour which becomes colourless when reacting with an antioxidant. The brucine-TL (0.5 ml) formulation was dissolved in alcohol (3 ml), mixed with DPPH ethanolic solution (0.3 ml), and placed in the dark for 100 min. The colour change was assessed by spectroscopy at 517 nm. The blank sample comprised 3.5 ml of 70% ethanol and 0.3 ml of DPPH solution t (Garcia et al. 2012; Moolakkadath et al. 2019). The sample free radical scavenging potential was described as % antioxidant activity (Mensor et al. 2001). The percent antioxidant activity was calculated using the following formula where Abs. denotes Absorbance, (Moolakkadath et al. 2019).
Preparation of BRC-TL gel
BRC-TL formulation could quickly detach from the skin; therefore, the BRC-TL was converted into a gel formulation. 1% w/w of carbopol 934 (100 mg) was mixed in double distilled water (10 ml) to obtain a uniform distribution, and kept overnight to allow complete swelling of the carbopol mixture. 15% w/w of polyethylene glycol 400 and 0.1% of chlorocresol (as preservative) were then added to the dispersion, followed by the addition of triethanolamine as a pH adjusting agent. Finally, the optimized BRC-TL was added dropwise into this preformed gel based with constant agitation to produce a homogeneous gel formulation (Ahad et al. 2014a, b).
Estimation of pH and the analysis of texture of the BRC-TL gel formulation
The pH of prepared BRC-TL gel formulations was estimated using pH meter (Mettler Instrument, Germany). The texture of gel formulation was then determined using a software-regulated texture analyser (Texture Analyzer, Stable Micro Systems Ltd., Surrey, UK). 50 g of the gel formulation (brucine-TL carbopol gel) was placed in a 100 ml beaker, while avoiding the development of air bubbles inside and ensuring a smooth surface. The gel formulation was then compacted with the analytical probe to a depth of 15 mm at a rate of 2 mm/s rate on two successive occasions, with a delay of 20 s between the end of the first and the beginning of the second compression. A texture analysis curve was obtained that indicated the mechanical properties of the gel such as firmness, consistency, cohesiveness, and index of viscosity (Moolakkadath et al. 2019; Gupta et al. 2020).
Skin permeation study
The skin permeation study was determined using Franz diffusion cells. One gram of the BRC-TL gel was placed in the donor compartment of the diffusion cell on a sample of excised rat skin in a non-occlusive manner (The animal studies for the test was approved by Research Ethics Committee, Reference No: PH-1442-39, Faculty of Pharmacy, King Abdulaziz University, Jeddah, KSA). The receiver vehicle, composed of phosphate buffer pH 7.4, was constantly stirred (600 rpm) at 37 ± 1 °C throughout the experiment (Mura et al. 2009). Samples (1 ml) were withdrawn from the receiver compartment through the sampling port at various time intervals (0, 0.25, 0.5, 1, 2, 4, 6, and 12 h); the withdrawn sample was immediately replenished with 1 ml of fresh vehicle. The brucine content was determined using HPLC method with UV detection at 264 nm (Chen et al. 2013).
Determination of depth of skin penetration
The depth of skin penetration from the formulation was determined using confocal laser scanning microscopy (CLSM). The BRC-TL gel loaded with rhodamine B or rhodamine B in hydro alcoholic solution were homogeneously and non-occlusively applied on excised rat skin mounted on two different Franz diffusion cells and left for 8 h at 37 °C. The rat skin treated with either BRC-TL gel-loaded rhodamine B or with rhodamine B hydro alcoholic solution were washed with distilled water to remove excess BRC-TL gel or hydro alcoholic solution. The rat skin samples were then dissected into small pieces and immediately placed on glass slides facing the stratum corneum upwards, which were observed under confocal laser scanning microscope (Leica TC SPE-IIw, DMI 4000 RGBV Leica Microsystems, Germany). The optical scanning of rat skin was undertaken using the z-axis of the confocal microscope with 5 µm increments. The argon laser beam for the optical excitation and detection of fluorescence emission was carried out at 488 nm, and 532 nm, respectively (Moolakkadath et al. 2018).
Dermatokinetic study
The content of drug in a different layer of skin at different times was determined by applying the BRC-TL gel to rat skin mounted in Franz diffusion cells as described above under the in vitro skin permeation study. However, for this study, the whole skin was removed from the Franz diffusion cell at different times (0, 1, 2, 4, 6 and 8 h) (Negi et al. 2015). The skin was washed with saline (pH 7.4) to remove any adhering formulation and then dipped in warm water at 60 °C for 2–3 min. The epidermis and dermis layers of the skin sample were separated using forceps. For the extraction of BRC, the separated skin layers were cut into small pieces and placed in 5 ml of methanol for 24 h. The subsequently obtained methanolic was passed through a membrane filter and the brucine concentration was determined using HPLC as described above (Chen et al. 2013). A curve was plotted for epidermis and dermis separately between brucine content per cm2 of skin vs time. Tskinmax, Cskinmax, AUC0–8 h and Ke dermatokinetic parameters were evaluated.
In vitro cytotoxicity study
The cell viability assay of BRC-TL, placebo TL and BRC suspension in murine melanoma cell lines (B16-F10) were performed. The mature cells were seeded in 96-well plates at a density of 5 × 103 cells/wells in 100 µl of Dulbecco’s Modified Eagle’s Medium (DMEM), further added on 10% foetal bovine serum, 1% l-glutamine, 1% sodium pyruvate, 1% non-essential amino acids and streptomycin (100 mg/ml) and penicillin (100 U/ml) to allow cell adherence. Now, the cells incubated in humidified chamber, at 37 °C and 100% relative humidity furnish with 5% CO2. At the end of 24 h, culture medium was discarded and treated with varying concentrations (5, 10, 25, 50 and 100 µm) of different formulations. After 48 h of treatment 250 µl of MTT reagent [3(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was added into well plate and incubated for 4 h. Now, 150 µl of DMSO was added to dissolve formazan crystals indicating purple colour and absorbance of the samples were examined on Microplate Reader (BioTek Synergy HT) at 550 nm.
Statistical analysis
The experiments were done three times and the results are given as mean standard deviation. One-way ANOVA was used to assess statistical significance, followed by the Tukey multiple comparison test, with a P value of 0.05 was considered significant.
Results and discussion
Optimization of BRC-TL by Box–Behnken design (BBD)
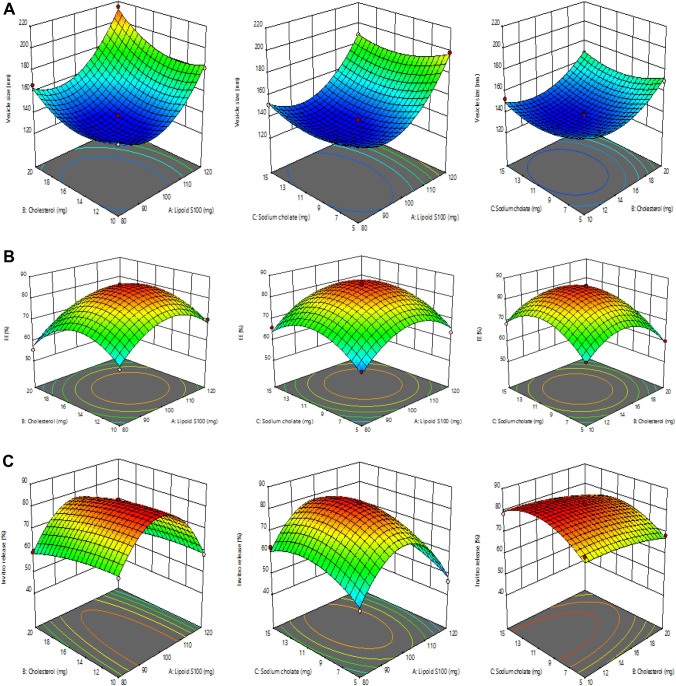
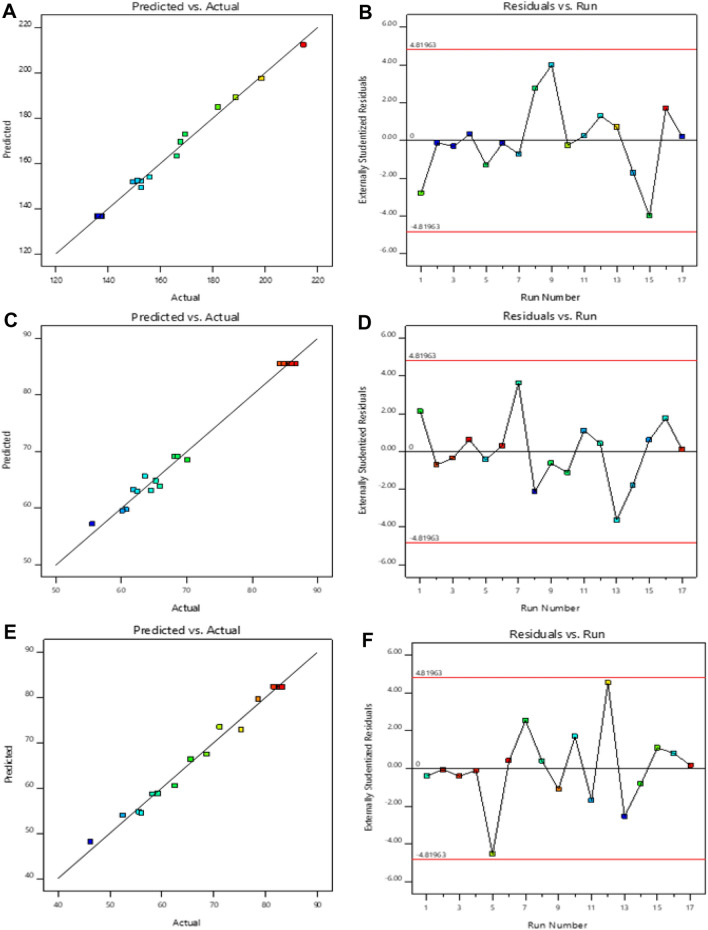
The BBD software had designed 17 runs for the development of formulations with 3 center points. The quadratic model was found to be the most fitted model for the responses of all the 17 runs. The independent responses selected were lipoid S100 (X1), cholesterol (X2) and sodium cholate (X3) and the dependent responses were vesicle size (Y1), entrapment efficiency (Y2) and in vitro release (Y3). The three responses R2, SD and %CV values are shown in Table 2. The effect of the selected independent variables on vesicles size, entrapment efficiency and in vitro release is represented by the 3D graph (Fig. 1). The resultant experimental values of the responses with that of the predicted values are quantitatively compared in Fig. 2.
Fig. 1.
a Representation of 3D surface plot on the effect of independent variables on vesicle size, b representation of 3D surface plot on the effect of independent variables on entrapment efficiency, c representation of 3D surface plot on the effect of independent variables on in vitro release characteristics
Fig. 2.
The linear correlation plots (a, c, e) between actual predicted vs actual values and corresponding residual plots (b, d, f) for responses vesicle size and entrapment efficiency and in vitro release of BRC-TL
Response-1 (Y1): effect of independent variables on vesicle size
The average vesicle size shown by all 17 runs was 160.66 nm of (range 135.74 nm to 214.55 nm) (Table 2).
The above equation shows that the other two independent variables, lipoid S100 and cholesterol, had a positive effect on vesicle size. Thus, increases in the concentrations of lipoid S100 and cholesterol resulted in increases in vesicle size; this is because it increases the width of bilayer (Mohammed et al. 2013).
The quantity of sodium cholate is also essential for vesicle formation Increases in the concentration of sodium cholate from 5 to 15 mg produced a decrease in size of the brucine-TL vesicles (from 152.44 ± 3.15 nm to 151.22 ± 3.74 nm, 198.47 ± 2.62 nm to 188.64 ± 2.18 nm, 155.84 ± 2.54 nm to 152.67 ± 3.75 nm and 169.48 ± 2.94 nm to 167.59 ± 4.18 nm) as seen in formulation numbers 11 and 7, 13 and 10, 12 and 9, 15 and 5, respectively.
Response-2 (Y2): effect of independent variables on entrapment efficiency (EE)
The EE (%) in all the 17 runs ranged from 55.58 to 86.57%, with an average value of 70.25% (Table 2).
| 1 |
Lipoid S100 and sodium cholate had a significant effect on entrapment efficiency. There was a positive correlation between the concentrations of lipoid S100 and sodium cholate and entrapment efficiency of brucine in the TL vesicles. An increase in the concentration of sodium cholate (5–15 mg) slightly increases the entrapment efficiency from 60.74 ± 0.48% to 65.88 ± 0.68%, 63.59 ± 1.72% to 68.14 ± 1.62%, 65.25 ± 1.14% to 68.55 ± 1.12% and 60.14 ± 0.38% to 62.48 ± 2.12%, as seen in formulations 11 and 7, 13 and 10, 12 and 9, 15 and 5, respectively. Similarly, increasing the concentration of lipoid S100 from 80 to 120 mg improved the percentage entrapment efficiency from 61.87 ± 0.85% to 70.14 ± 1.05% as seen in formulation 14 and 1, respectively. This is mostly due to the large number of TL vesicles formed, which increases the dimensions of the domain, and makes space for entrapment of drug (Shah et al. 2019).
Cholesterol was included in the formulation to produce stable vesicles; it mainly prevents leakage, stabilizes bilayers and retards permeation of solutes present in the aqueous core of the vesicles (Ahad et al. 2018a, b, c, d). From the above equation, it was concluded that cholesterol resulted in a negative effect on entrapment efficiency. When the cholesterol concentration was increased from 10 to 20 mg, the entrapment efficiency of the brucine-TL was reduced from 61.87 ± 0.85% to 55.58 ± 1.52%, as seen in the formulations 14 and 8, respectively. The current data are in agreement with previous reports (Mohammed et al. 2013; Auda et al. 2016). Above a certain concentration, cholesterol can interrupt the regular bilayered structure of the vesicular membranes resulting in drug loss from the vesicles (Mohammed et al. 2013).
Response-3 (Y3): effect of independent variables on in vitro drug release
The in vitro drug release from all 17 formulations ranged from 46.28 to 83.14% (Table 2).
| 2 |
The above equation indicated that sodium cholate produced a positive effect on in vitro release. When the concentration of sodium cholate was increased from 5 to 15 mg, the in vitro release of brucine increased from 52.47 ± 2.88% to 62.47 ± 2.22% as seen in formulation 11 and 7, respectively.
On the other hand, both lipoid S100 and cholesterol exerted a negative impact on in vitro drug release. When the concentration of cholesterol was increased from 10 to 20 mg there was a decrease in drug release from 65.55 ± 2.18% to 59.22 ± 2.17%, as seen in formulation 14 and 8, respectively.
The point prediction method of the BBD software was utilized for the optimization of the brucine-TL formulation. Using this approach, BRC-TLs containing lipoid S100 (100 mg), cholesterol (15 mg) and sodium cholate (10 mg) fulfilled the conditions of an optimized formulation.
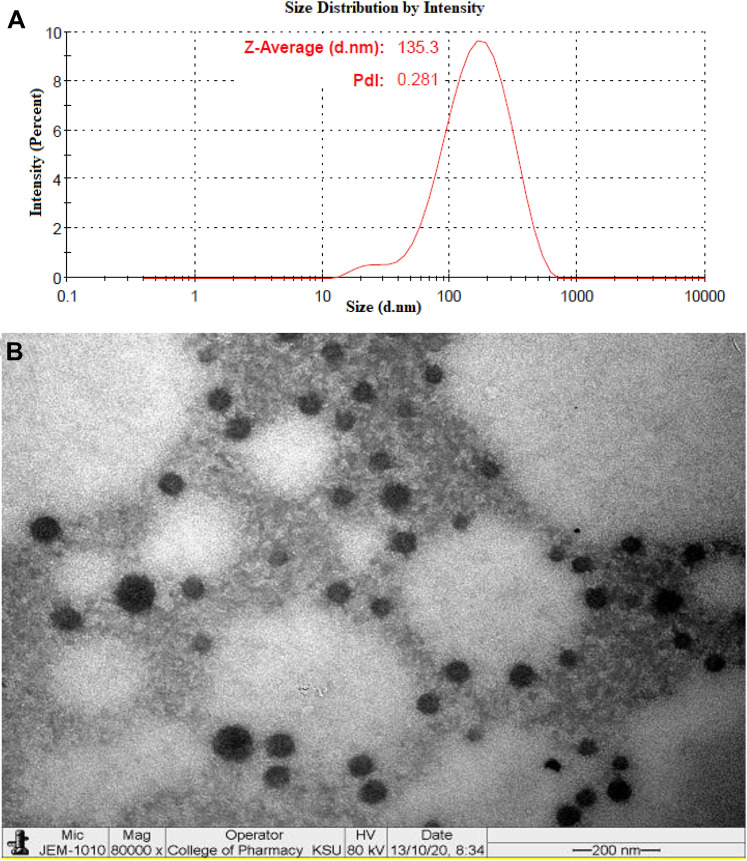
The optimized BRC-TL formulation showed a vesicle size of 136.20 ± 2.87 nm (Fig. 3a), an entrapment efficiency of 86.01 ± 1.27% and an in vitro release of 83.14 ± 3.42%. The values generated by the Design Expert software were close to the predicted values for vesicle size (136.57 nm), entrapment efficiency (85.48%) and in vitro release (82.34%). Additionally, the polydispersity index (PDI) value of the optimized formulation was found to be 0.354. The optimized BRC-TL formulation was further evaluated for vesicle morphology, in vitro drug release, antioxidant activity, pH and texture, skin permeation, and depth of skin penetration, as well as undergoing a dermatokinetic study.
Fig. 3.
a Average vesicle size using zetasizer, b transmission electron micrograph of optimised BRC-TLs formulation
Morphology of BRC-TL
The transmission electron microscopy (TEM) investigation image of optimized BRC-TL formulation confirmed that the prepared vesicles are well-identified sealed structure with spherical in shapes and uniform size distribution (Fig. 3b). The size of the vesicle determined by Zetasizer instrument using dynamic light scattering method also revealed similar size pattern as depicted in Fig. 3a. The TEM micrograph represented the absence of any crystalline drug, which represent complete entrapment of brucine within the vesicular structure.
In vitro release
The release behaviour of the prepared optimized BRC-TL and BRC-CF to 37 ± 0.5 °C under continuous stirring at 100 RPM using dialysis bag method showed low percentage of drug release 46.17 ± 1.27% from BRC-CF as compared to optimized BRC-TL which showed higher release up to 83.09 ± 2.07% (Fig. 4). Our approach of delivering brucine using this TL platform indicates controlled release of entrapped drug over a period of 24 h, which is quite similar to the release pattern of brucine from liposomal deliveries as reported in the literature (Elsewedy et al. 2020; Li and Wang 2017). Alternatively, the release pattern of BRC from the BRC-CF was found to be significantly low in aqueous environment (46.17 ± 1.27%) within our limits of experimental design, which indicated superiority of the developed BRC-TL formulation for delivering BRC. Data obtained by in vitro release study were set to several models like zero order, First order, Higuchi, Korsmeyer Peppas model. The correlation coefficient (R2) in highest value was preferred for selecting order of release. The highest correlation coefficient value in case of optimized BRC-TL was found for Higuchi model (R2 = 0.8379), followed by first order (R2 = 0.7709) and zero order (R2 = 0.6304) model. The optimized BRC-TL was found highest value of correlation coefficient which showed the Higuchi’s model as a best fit model. The release behaviour of BRC from optimized BRC-TL was examined by fitting value in Korsmeyer–peppas model, the R2 value was found to be 0.9289, and the n value was found 0.55 which indicates that the release of BRC from the optimized BRC-TL follow fickian diffusion.
Fig. 4.
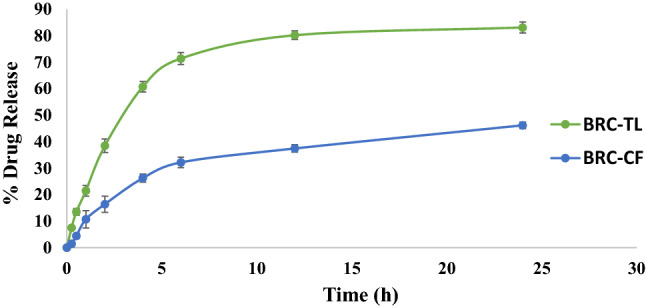
In vitro drug release of BRC- TL and BRC-CF suspension at pH 7.4. BRC-TL showed statistically significant difference in % drug release as compared to BRC-CF (P < 0.05)
Antioxidant activity
Antioxidant potential of BRC is well established, researchers had shown the antioxidant efficacy of this agent through inhibition of generated free radicals and preventing lipid peroxidation (Saraswati et al. 2013). The optimized BRC-TL formulation was compared with a standard ascorbic acid solution for antioxidant potency. Ascorbic acid solution showed an antioxidant effect of 92.08% while that of the BRC-TL optimized formulation was 72.15%. This observation confirms the antioxidant ability of the brucine-TL gel formulation. It was concluded that the antioxidant potency of BRC was unchanged after incorporation into the TL gel formulation. Further, retained antioxidant potency of BRC will simultaneously increase the cellular catalase, superoxide dismutase activity and glutathione peroxidase levels to promote blocking of tumor progression (Saraswati et al. 2013).
Texture analysis and pH of the optimized BRC-TL gel
The pH of the formulation intended for transdermal application is an important determinant, particularly when the topical delivery of designed for cancer treatment. Normally, the pH of the cell and extracellular region possess near to neutral pH (pH 7.2); however, cancerous area is differentiated by mild acidic pH (Carvalho et al. 2018). Thus, the observed 6.2 pH value of BRC-TL gel formulation was within the acceptable range for topical application in skin cancer condition. Texture analysis showed that the BRC-TL gel presented firmness of 158.91 g, consistency of 615.03 g/sec, cohesiveness of − 115.26 g and a viscosity index of − 472.05 g/s. From the findings, it can be inferred that the BRC-TL gel formulation are possessing homogenous appearance and texture where smooth consistency of the formulation was found to contain no lumps (Moolakkadath et al. 2019).
Skin permeation study
Cumulative brucine permeation from the BRC-CF was only 30.57 ± 3.77%, compared to optimized brucine-TL gel (73.19 ± 5.87%) at 12 h. This significant increase in penetration through the rat skin could be explained by the squeezing of the elastic structure of TLs through the stratum corneum, where edge activators facilitate such permeation. The difference in transpore hydrostatic force between the two sides of skin is liable of such penetration due to the creation of hydrotaxis, where this permeation of TLs is controlled by the principles of elastomechanics. The elasticity of the vesicular structure reversibly changes the membrane fluidity to facilitate transportation of the vesicles through the pores (Rai et al. 2017).
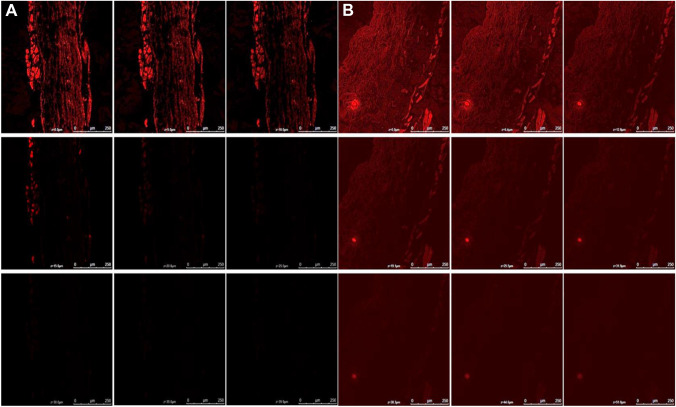
Depth of skin penetration using CLSM
Confocal laser scanning microscopy showed that while a hydro-alcoholic solution of rhodamine B was retained in the top layers of the skin, (penetrating only to a depth of 10 µm, Fig. 5a), when rhodamine B was included in the brucine-TL gel it was well absorbed and showed deeper penetration up to a depth of 44.6 µm (Fig. 5b). The gel formulation showed maximum fluorescence intensity in the middle portion of the skin, indicating that the gel penetrated in a higher amount in the lower epidermis portion after bypassing the top layers. Hence it was concluded that the developed BRC-TL gel successfully transported rhodamine B dye into the deeper layers of the rat skin. This could also be explained by the existence of misdeeds within the lipid packing on animal skins within the subcutaneous layer, which might be utilised by the BRC-TL while penetrating through the skin (Rai et al. 2017). Overall, this specially designed vesicular lipid particle possesses the property to deliver the entrapped drug within the subcutaneous region when applied transdermally.
Fig. 5.
Confocal laser microscopy of Rhodamine B solution (a) and Rhodamine B loaded TL (b) showing a depth of penetration 10 μm and 44.6 μm across rat skin
Dermatokinetic study
The relative content of brucine in layers of rat skin dermis and epidermis after treatment with BRC-CF and BRC-TL gel formulation at various time intervals is illustrated in Fig. 6, with the statistical analysis using one factor ANOVA presented in Table 3. Comparison of the epidermis and dermis of rat skin treated with brucine-TL and brucine-CF gel showed that the brucine-TL gel produced significantly higher concentrations of brucine as measured both by CSkin max and AUC0–8 (Table 3). The maximum retaining of BRC-TL gel could be due to the potency of vesicles to augment the partitioning through the skin lipid bilayers. The TSkin max of the brucine-TL gel was like that of the BRC-CF gel in the epidermis. From the findings, the concentration of BRC is measurable following 30 min of application to the skin. Compared to the commercial formulation, the BRC-TL delivery showed rapid absorption of the drug, reaching the Cmax within 1.5 h and 2 h in dermis and epidermis, respectively, following topical application. The concentration of BRC was found to be decreased over time until 8 h of experiment, where the concentration of the drug was found to be measurable. Above findings of the TL delivery suggest increased penetration of the delivery tool when applied topically, which might be correlated to the previous results on skin permeation study and confocal laser microscopic study, where the increased penetration of the formulation was evident because of its elasticity of the formulation and the presence of edge activator.
Fig. 6.
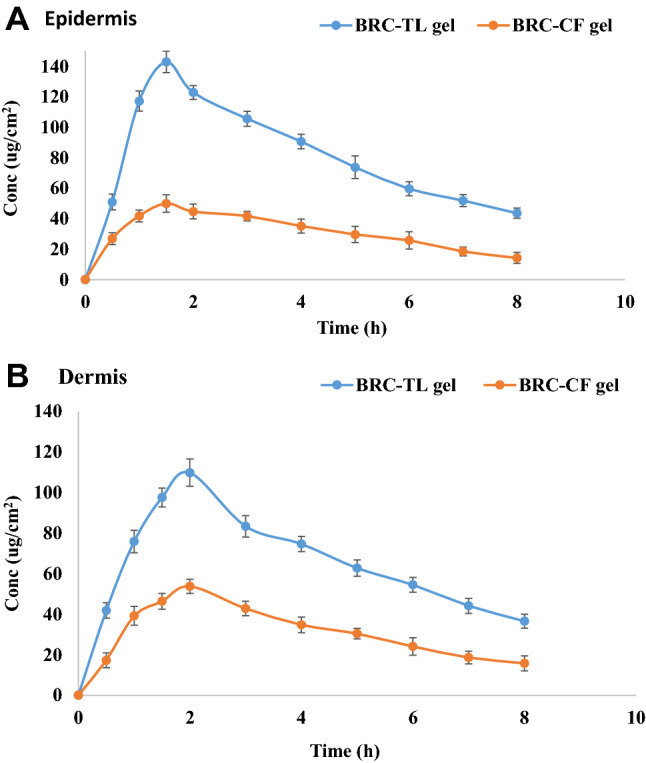
BRC concentration on a epidermis and b dermis after topical application of BRC-TL gel and BRC-CF gel on excised rat skin. The BRC-TL showed statistically significant difference in concentration as compared to BRC-CF (P < 0.05) in epidermis and dermis
Table 3.
Dermatokinetic parameters of brucine conventional formulation (BRC-CF) gel and brucine transliposomes (BRC-TL) gel
| Dermatokinetics parameters | BRC-CF gel | BRC-TL gel | ||
|---|---|---|---|---|
| Epidermis | Dermis | Epidermis | Dermis | |
| Tskin max (h) | 1.5 ± 0.2 | 2 ± 0.1 | 1.5 ± 0.1 | 2 ± 0.15 |
| Cskin max (µg/cm2) | 50.01 ± 5.73 | 53.73 ± 3.50 | 142.9 ± 7.06 | 109.8 ± 6.74 |
| AUC0–8 (µg/cm2h) | 250.8 ± 3.93 | 250.1 ± 20.6 | 651.2 ± 23.8 | 527.5 ± 23.7 |
| Ke (h− 1) | 0.13 ± 0.01 | 0.10 ± 0.02 | 0.10 ± 0.01 | 0.08 ± 0.005 |
Tskin max, time to maximum concentration; Cskin max, maximum concentration; AUC, area under curve; Ke, elimination rate constant
Cytotoxicity study
Viability of B16-F10 cells determined in colorimetric MTT assay upon incubation with placebo-TL, BRC-TL and BRC suspension was represented in Fig. 7. It is clearly noticeable a dose-dependent decrease in cell viability due to the action of BRC from the suspension and the TL delivery, when compared to the control group. The blank formulation did not show any significant reduction in cell viability, which represent safety of the delivery system. Dose-dependent decrease in cell viability showed the IC50 values of 37.23 µm and 74.31 µm for the BRC-TL and BRC suspension, respectively. The significant reduction of IC50 value by the action of BRC-TL representing superiority of BRC delivery using the TL platform. Our findings on decreased IC50 value of BRC using the lipid nanocarrier when compared to the free drug suspension are in agreement to the previous reports in literature (Elsewedy et al. 2021). The rapid control of cancerous cells using this TLs formulation over the suspension might be explained by the increased solubility of the drug into the vesicular structure, which further facilitate rapid penetration into the cell and controlled release of the entrapped drug.
Fig. 7.
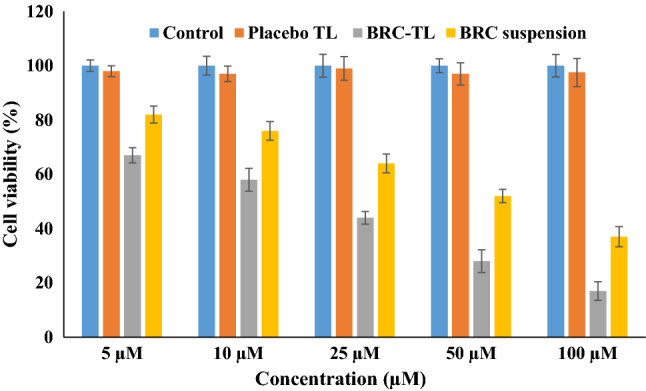
Representation of MTT assay results of cell viability following 48 h incubation of B16-F10 cells with placebo-TL, BRC-TL and BRC suspension. Results are expressed as the mean ± SD, (n = 3). Statistical inferences: no statistically significant difference between control vs placebo (P > 0.05); statistically significant difference between BRC-TL compared to control group, BRC suspension and placebo-TL (P < 0.05) at every concentration
Conclusion
In the present study, BRC-TL formulations were optimized using BBD software. The optimized BRC-TL formulation had a vesicle size in the nano range and showed good entrapment efficiency and in vitro drug release. Confocal laser scanning microscopy showed that the BRC-TL gel resulted in better penetration of rhodamine across rat skin compared with rhodamine control solution. Moreover, the dermatokinetic study revealed better penetration of brucine from brucine-TL gel than from the brucine-CF gel formulation. The antioxidant effects of brucine were retained, even after incorporation into TL vesicles. BRC-TL showed higher cytotoxicity when compared to BRC suspension. Typically, TL vesicular systems produce depots in the deeper skin layers and continuously release the drug over time, which has the advantage of reducing application frequency. The present results demonstrate that the developed TL formulation is a potentially useful drug carrier for dermal delivery of brucine which help in the management of skin cancer. However, detailed in vivo animal studies are required to confirm the therapeutic ability of brucine-loaded transferosome in the treatment of skin cancer.
Acknowledgements
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. (RG-11-166-38). The authors, therefore, acknowledge with thanks DSR technical and financial support.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest. This article does not contain any studies with human participants performed by any of the authors.
References
- Ahad A, Aqil M, Ali A. Investigation of antihypertensive activity of carbopol valsartan transdermal gel containing 1,8-cineole. Int J Biol Macromol. 2014;64:144–149. doi: 10.1016/j.ijbiomac.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Ahad A, Aqil M, Kohli K, Sultana Y, Mujeeb M. Design, formulation and optimization of valsartan transdermal gel containing iso-eucalyptol as novel permeation enhancer: preclinical assessment of pharmacokinetics in Wistar albino rats. Expert Opin Drug Deliv. 2014;11:1149–1162. doi: 10.1517/17425247.2014.914027. [DOI] [PubMed] [Google Scholar]
- Ahad A, Aqil M, Kohli K, Sultana Y, Mujeeb M. The ameliorated longevity and pharmacokinetics of valsartan released from a gel system of ultradeformable vesicles. Artif Cells Nanomed Biotechnol. 2016;44:1457–1463. doi: 10.3109/21691401.2015.1041638. [DOI] [PubMed] [Google Scholar]
- Ahad A, Al-Saleh AA, Al-Mohizea AM, et al. Formulation and characterization of novel soft nanovesicles for enhanced transdermal delivery of eprosartan mesylate. Saudi Pharm J. 2017;25:1040–1046. doi: 10.1016/j.jsps.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahad A, Al-Saleh AA, Al-Mohizea AM, et al. Formulation and characterization of Phospholipon 90 G and tween 80 based transfersomes for transdermal delivery of eprosartan mesylate. Pharm Dev Technol. 2018;23:787–793. doi: 10.1080/10837450.2017.1330345. [DOI] [PubMed] [Google Scholar]
- Ahad A, Raish M, Ahmad A, Al-Jenoobi FI, Al-Mohizea AM. Development and biological evaluation of vesicles containing bile salt of telmisartan for the treatment of diabetic nephropathy. Artif Cells Nanomed Biotechnol. 2018;46:532–539. doi: 10.1080/21691401.2018.1430700. [DOI] [PubMed] [Google Scholar]
- Ahad A, Raish M, Ahmad A, Al-Jenoobi FI, Al-Mohizea AM. Eprosartan mesylate loaded bilosomes as potential nano-carriers against diabetic nephropathy in streptozotocin-induced diabetic rats. Eur J Pharm Sci. 2018;111:409–417. doi: 10.1016/j.ejps.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Ahad A, Raish M, Al-Jenoobi FI, Al-Mohizea AM. Sorbitane Monostearate and Cholesterol based Niosomes for Oral Delivery of Telmisartan. Curr Drug Deliv. 2018;15:260–266. doi: 10.2174/1567201814666170518131934. [DOI] [PubMed] [Google Scholar]
- Auda SH, Fathalla D, Fetih G, El-Badry M, Shakeel F. Niosomes as transdermal drug delivery system for celecoxib: in vitro and in vivo studies. Polym Bull. 2016;73:1229–1245. doi: 10.1007/s00289-015-1544-8. [DOI] [Google Scholar]
- Carvalho SM, Mansur AA, Capanema NS, et al. Synthesis and in vitro assessment of anticancer hydrogels composed by carboxymethylcellulose-doxorubicin as potential transdermal delivery systems for treatment of skin cancer. J Mol Liq. 2018;266:425–440. doi: 10.1016/j.molliq.2018.06.085. [DOI] [Google Scholar]
- Chen J, Hu W, Qu YQ. Evaluation of the pharmacodynamics and pharmacokinetics of brucine following transdermal administration. Fitoterapia. 2013;86:193–201. doi: 10.1016/j.fitote.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Elsewedy HS, Dhubiab BEA, Mahdy MA, Elnahas HM. Development, optimization, and evaluation of PEGylated brucine-loaded PLGA nanoparticles. Drug Del. 2020;27:1134–1146. doi: 10.1080/10717544.2020.1797237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsewedy HS, Al Dhubiab BE, Mahdy MA, Elnahas HM. Brucine PEGylated Nanoemulsion: In vitro and In vivo Evaluation. Colloids Surf, A Physicochem Eng Asp. 2021;608:125618. doi: 10.1016/j.colsurfa.2020.125618. [DOI] [Google Scholar]
- Garcia EJ, Oldoni TL, Alencar SM, Reis A, Loguercio AD, Grande RH. Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Braz Dent J. 2012;23:22–27. doi: 10.1590/S0103-64402012000100004. [DOI] [PubMed] [Google Scholar]
- Guo R, Wang T, Zhou G et al (2018) Botany, Phytochemistry, Pharmacology and Toxicity of Strychnos nux-vomica L.: A Review Am J Chin Med 46:1–23. [DOI] [PubMed]
- Gupta DK, Aqil M, Ahad A, et al. Tailoring of berberine loaded transniosomes for the management of skin cancer in mice. J Drug Deliv Sci Technol. 2020;60:102051. doi: 10.1016/j.jddst.2020.102051. [DOI] [Google Scholar]
- Khan I, Saeed K, Khan I. Nanoparticles: Properties, applications and toxicities. Arab J Chem. 2019;12:908–931. doi: 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- Li S, Wang XP. In vitro and in vivo evaluation of novel NGR-modified liposomes containing brucine. Int J Nanomedicine. 2017;12:5797–5804. doi: 10.2147/IJN.S136378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li P, Zhang M, Ma W, Sun X, Jin F. Effects of brucine on vascular endothelial growth factor expression and microvessel density in a nude mouse model of bone metastasis due to breast cancer. Chin J Integr Med. 2012;18:605–609. doi: 10.1007/s11655-012-1184-x. [DOI] [PubMed] [Google Scholar]
- Li M, Li P, Zhang M, Ma F. Brucine suppresses breast cancer metastasis via inhibiting epithelial mesenchymal transition and matrix metalloproteinases expressions. Chin J Integr Med. 2018;24:40–46. doi: 10.1007/s11655-017-2805-1. [DOI] [PubMed] [Google Scholar]
- Lu L, Huang R, Wu Y, et al. Brucine: A Review of Phytochemistry, Pharmacology, and Toxicology. Front Pharmacol. 2020;11:377. doi: 10.3389/fphar.2020.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensor LL, Menezes FS, Leitao GG, et al. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- Mohammed H, Urszula D. PLGA biodegradable nanoparticles containing perphenazine or chlorpromazine hydrochloride: effect of formulation and release. Int J Mol Sci. 2014;15:23909–23923. doi: 10.3390/ijms151223909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed N, Rejinold NS, Mangalathillam S, et al. Fluconazole loaded chitin nanogels as a topical ocular drug delivery agent for corneal fungal infections. J Biomed Nanotechnol. 2013;9:1521–1531. doi: 10.1166/jbn.2013.1647. [DOI] [PubMed] [Google Scholar]
- Mohamed F, Nicolas A, Justine W, et al. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol. 2019;71:1185–1198. doi: 10.1111/jphp.13098. [DOI] [PubMed] [Google Scholar]
- Moolakkadath T, Aqil M, Ahad A, et al. Development of transethosomes formulation for dermal fisetin delivery: Box-Behnken design, optimization, in vitro skin penetration, vesicles-skin interaction and dermatokinetic studies. Artif Cells Nanomed Biotechnol. 2018;46:755–765. doi: 10.1080/21691401.2018.1469025. [DOI] [PubMed] [Google Scholar]
- Moolakkadath T, Aqil M, Ahad A, et al. Fisetin loaded binary ethosomes for management of skin cancer by dermal application on UV exposed Rat. Int J Pharm. 2019;560:78–91. doi: 10.1016/j.ijpharm.2019.01.067. [DOI] [PubMed] [Google Scholar]
- Moolakkadath T, Aqil M, Ahad A, et al. Preparation and optimization of fisetin loaded glycerol based soft nanovesicles by Box-Behnken design. Int J Pharm. 2020;578:119125. doi: 10.1016/j.ijpharm.2020.119125. [DOI] [PubMed] [Google Scholar]
- Mura S, Manconi M, Sinico C, Valenti D, Fadda AM. Penetration enhancer-containing vesicles (PEVs) as carriers for cutaneous delivery of minoxidil. Int J Pharm. 2009;380:72–79. doi: 10.1016/j.ijpharm.2009.06.040. [DOI] [PubMed] [Google Scholar]
- Negi P, Singh B, Sharma G, Beg S, Katare OP. Biocompatible lidocaine and prilocaine loaded-nanoemulsion system for enhanced percutaneous absorption: QbD-based optimisation, dermatokinetics and in vivo evaluation. J Microencapsul. 2015;32:419–431. doi: 10.3109/02652048.2015.1046513. [DOI] [PubMed] [Google Scholar]
- Qin XQ, Yuan Y, Liu CS, et al. Preparation of liposomal brucine and its pharmaceutical/pharmacodynamic characterization. Acta Pharmacol Sin. 2007;28:1851–1858. doi: 10.1111/j.1745-7254.2007.00654.x. [DOI] [PubMed] [Google Scholar]
- Qin J, Pei-Hao Y, Qi L, et al. Anti-tumor effects of brucine immuno-nanoparticles on hepatocellular carcinoma. Int J Nanomed. 2012;7:369–379. doi: 10.2147/IJN.S27226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Yang L, Sheng X, et al. Antitumor effects of brucine immuno-nanoparticles on hepatocellular carcinoma in vivo. Oncol Lett. 2018;15:6137–6146. doi: 10.3892/ol.2018.8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai S, Pandey V, Rai G. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: the state of the art. Nano Rev Exp. 2017;8:1325708. doi: 10.1080/20022727.2017.1325708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraswati S, Alhaider AA, Agrawal SS. Anticarcinogenic effect of brucine in diethylnitrosamine initiated and phenobarbital-promoted hepatocarcinogenesis in rats. Chem Biol Interact. 2013;206:214–221. doi: 10.1016/j.cbi.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Shah H, Nair AB, Shah J, Bharadia P, Al-Dhubiab BE. Proniosomal gel for transdermal delivery of lornoxicam: optimization using factorial design and in vivo evaluation in rats. Daru. 2019;27:59–70. doi: 10.1007/s40199-019-00242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Sharma US. Liposomes in drug delivery: progress and limitations. Int J Pharm. 1997;154:123–140. doi: 10.1016/S0378-5173(97)00135-X. [DOI] [Google Scholar]
- Shi L, Li Y, Fei L, Lv S. Herbal Textual Research on Strychnos nuxvomica. Res Pract Chin Medicines. 2017;31:6–10. [Google Scholar]
- Shi X, Zhu M, Kang Y, et al. Wnt/bcatenin signaling pathway is involved in regulating the migration by an effective natural compound brucine in LoVo cells. Phytomedicine. 2018;46:85–92. doi: 10.1016/j.phymed.2018.04.019. [DOI] [PubMed] [Google Scholar]
- Shu G, Mi X, Cai J, et al. Brucine, an alkaloid from seeds of Strychnos nux-vomica Linn., represses hepatocellular carcinoma cell migration and metastasis: the role of hypoxia inducible factor 1 pathway. Toxicol Lett. 2013;222:91–101. doi: 10.1016/j.toxlet.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Wu Y, Chen J, Fang Y, Dong J. In vitro transdermal permeation and penetration properties for transfersomes of brucine. China J Chin Materia Med. 2016;41:3009–3015. doi: 10.4268/cjcmm20161611. [DOI] [PubMed] [Google Scholar]