Abstract
Background
Mechanical circulatory support (MCS) with either extracorporeal membrane oxygenation or Impella has shown potential as a salvage therapy for patients with refractory out-of-hospital cardiac arrest (OHCA). The objective of this study was to describe the gradual implementation, survival and adherence to the national consensus with respect to use of MCS for OHCA in Denmark, and to identify factors associated with outcome.
Methods
This retrospective, observational cohort study included patients receiving MCS for OHCA at all tertiary cardiac arrest centers (n = 4) in Denmark between July 2011 and December 2020. Logistic regression and Kaplan–Meier survival analysis were used to determine association with outcome. Outcome was presented as survival to hospital discharge with good neurological outcome, 30-day survival and predictors of 30-day mortality.
Results
A total of 259 patients were included in the study. Thirty-day survival was 26%. Sixty-five (25%) survived to hospital discharge and a good neurological outcome (Glasgow–Pittsburgh Cerebral Performance Categories 1–2) was observed in 94% of these patients. Strict adherence to the national consensus showed a 30-day survival rate of 30% compared with 22% in patients violating one or more criteria. Adding criteria to the national consensus such as signs of life during cardiopulmonary resuscitation (CPR), pre-hospital low-flow < 100 min, pH > 6.8 and lactate < 15 mmol/L increased the survival rate to 48%, but would exclude 58% of the survivors from the current cohort. Logistic regression identified asystole (RR 1.36, 95% CI 1.18–1.57), pulseless electrical activity (RR 1.20, 95% CI 1.03–1.41), initial pH < 6.8 (RR 1.28, 95% CI 1.12–1.46) and lactate levels > 15 mmol/L (RR 1.16, 95% CI 1.16–1.53) as factors associated with increased risk of 30-day mortality. Patients presenting signs of life during CPR had reduced risk of 30-day mortality (RR 0.63, 95% CI 0.52–0.76).
Conclusions
A high survival rate with a good neurological outcome was observed in this Danish population of patients treated with MCS for OHCA. Stringent patient selection for MCS may produce higher survival rates but potentially withholds life-saving treatment in a significant proportion of survivors.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-021-03606-5.
Keywords: Out-of-hospital cardiac arrest, Mechanical circulatory support, Extracorporeal membrane oxygenation, Impella, Cardiopulmonary resuscitation
Background
Out-of-hospital cardiac arrest (OHCA) is a time-critical condition associated with a high mortality worldwide. Despite various initiatives to improve public engagement and ensure access to a sufficient number of external defibrillators, survival rates remain poor [1]. Short-term mechanical circulatory support (MCS) with extracorporeal membrane oxygenation (ECMO) or Impella devices has emerged as a rescue therapy in adult patients with OHCA that is refractory to conventional cardiopulmonary resuscitation (CPR). MCS may ensure life-saving organ perfusion, lending clinicians crucial time to identify and treat the underlying cause of cardiac arrest. Extracorporeal cardiopulmonary resuscitation (ECPR) refers to the rapid application of ECMO in the setting of refractory cardiac arrest. Several observational studies and recently one randomized clinical trial have demonstrated encouraging results after ECPR [2–6]. In recent years, the use of Impella devices as MCS for refractory OHCA have also shown potential in this high-risk population [7, 8]. Although the field of MCS for refractory cardiac arrest has evolved rapidly over the past decades, identifying optimal candidates is still an inevitable challenge.
MCS for refractory OHCA has been introduced gradually over the past ten years in Denmark, and a national consensus was adopted in February 2018 [9]. However, detailed knowledge of the full cohort treated remains scarce. Regional differences in triage of patients with OHCA may influence the availability of MCS, which may in turn affect patient selection and outcome. The aims of this study were to describe temporal trends and regional variation in the use of MCS in Denmark, to evaluate adherence to the national consensus on ECPR use and to identify factors associated with outcome.
Methods
This nationwide retrospective, observational cohort study was conducted at four tertiary cardiac arrest centers in Denmark (Aalborg University Hospital, Aarhus University Hospital, Odense University Hospital and Copenhagen University Hospital). In Denmark, MCS for refractory OHCA is performed at these four centers exclusively.
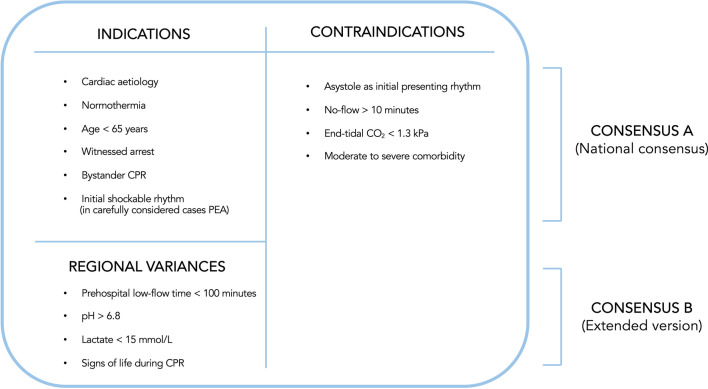
National consensus
The Danish national consensus on the use of ECPR in patients with refractory OHCA was adopted in February 2018 [9] (Fig. 1, Consensus A—National consensus). The most consistent criteria for inclusion were normothermic cardiac arrest with a presumed cardiac origin, age < 65 years, an initial shockable rhythm, witnessed arrest, bystander CPR and end-tidal CO2 > 1.3 kPa. Regional differences in the inclusion criteria were present during the study period as shown in Fig. 1, Consensus B—Extended version and Additional file 1: Table S1.
Fig. 1.
National consensus and extended consensus with regional variances for selection of patients with refractory OHCA and possible candidates for mechanical circulatory support
Study population
The study population included all patients aged ≥ 18 years receiving MCS for refractory OHCA, which was defined as absence of return of spontaneous circulation (ROSC) despite resuscitation efforts for more than 15 min. Patients treated between July 2011 and December 2020 were identified from local MCS databases and medical records. Due to regional differences in MCS availability and updates to databases, the data collection period differed for each hospital: Aalborg University Hospital (February 2016–December 2020), Aarhus University Hospital (July 2011–December 2020), Odense University Hospital (November 2015–December 2020) and Copenhagen University Hospital (November 2016–December 2020).
Study setting
Patient selection, triage, and implementation of either Impella or ECPR were performed at the discretion of the treating ECPR team at the individual centers. The specialized ECPR teams, including anaesthesiologists, cardiothoracic surgeons, perfusionists, and invasive and general cardiologists managed all patients upon arrival at the catherization laboratory. Venoarterial ECMO cannulations were inserted percutaneously using the Seldinger technique with ultrasound guidance. In case of unsuccessful percutaneous cannulation, open cut-down technique with direct visualization of the femoral vein and artery was performed. Vascular access was achieved by 15F-23F arterial cannulas and 19F-26F venous cannulas. In the majority of patients, a distal arterial perfusion cannula was inserted to ensure antegrade limb perfusion. Circuit flow was titrated until effective circulatory response was achieved. Fluid, inotropes and vasopressors were applied if necessary. ECPR was achieved in most cases; however, in a minority of patients, the Impella device was initially employed if visible spontaneous cardiac contractility was observed on echocardiography during rhythm check. The Impella device was placed percutaneously in the femoral artery, and correct positioning was then confirmed by fluoroscopy or echocardiography. ECPR in combination with Impella was applied in patients with failing cardiac recovery immediately after ECPR initiation or within 24 h after. Combined use of ECPR and Impella was initiated to ensure aortic valve opening and sufficient left ventricular unloading.
For both ECPR- and Impella-treated patients, unfractionated heparin was administrated routinely to avoid systemic clotting and aiming for an activated partial thromboplastin time of 60–80 s or an activated clotting time of 160–180 s.
Post-resuscitation care and management were performed according to local intensive care unit (ICU) standard protocols including targeted temperature management (TTM), neurological prognostication and procedures for withdrawal of treatment. Weaning from MCS was done if cardiac and respiratory function were considered to have recovered sufficiently or if further treatment was deemed futile [10].
Data collection
Study data were recorded in a uniform national database. A study coordinator from each hospital was assigned to manage the data collection. According to the Utstein recommendation for data collection [11], information on cardiac arrest was acquired from the pre-hospital emergency medical service logistic systems and included information on: time of cardiac arrest, witnessed arrest, bystander CPR, initial rhythm and pre-hospital care comprising inotropic usage and intubation. Patient demographics and in-hospital data on clinical parameters, known comorbidities, laboratory tests, intervention and outcome data were obtained from patient records.
Study end-points
The primary end-point was 30-day survival. Secondary end-points included survival to hospital discharge, neurological outcome at hospital discharge and regional differences in triage and outcome. Neurological outcome was evaluated by the Glasgow–Pittsburgh Cerebral Performance Categories (CPC), and a favorable outcome was defined as CPC scores 1 and 2 [12].
Statistical analysis
Continuous data are presented as median and interquartile range (IQR, P25-P75) and categorical data as number and percentages. The Mann–Whitney U test and the Kruskal–Wallis H test were used for comparison of continuous data, whereas the chi-squared test and Fisher’s exact test were used for categorical data. Logistic regression was performed to assess the association of risk factors on 30-day mortality. Results are expressed as risk ratio (RR) and 95% confidence interval (CI). Risk factors were identified a priori based on their clinical relevance and previously published literature. Survival analysis results are presented as Kaplan–Meier curves for various subgroups of patients and compared with the log rank test. In case of missing values, patients were excluded from the statistical analysis. Two-sided p-values of < 0.05 were considered statistically significant. Statistical tests were performed using STATA/IC 16, College Station TX77845, USA, for Mac.
Results
Additional file 2: Figure S1 demonstrates the gradual implementation of MCS in Denmark. Between July 2011 and December 2020, a total of 259 patients treated with MCS for OHCA were enrolled in the study: (Aalborg University Hospital, n = 34, Aarhus University Hospital, n = 138, Odense University Hospital, n = 55, and Copenhagen University Hospital, n = 32). Survival to day 30 was seen in 67 (26%). A total of 65 (25%) patients survived to hospital discharge, and 61 (94%) of these patients were discharged with a CPC of 1–2.
Baseline and cardiac arrest characteristics
Baseline and arrest characteristics are summarized in Table 1. The median age of the study population was 53 years (IQR, 45–60 years), and 79% were men. No significant differences in comorbidities were seen between survivors and non-survivors. In most cases, arrest aetiology was of cardiac origin; and the three dominant causes were acute myocardial infarction (n = 142, 55%), primary arrhythmia (n = 42, 16%) and pulmonary embolism (n = 24, 9%). Significantly more survivors than non-survivors presented an initial shockable rhythm (p = 0.002) and signs of life during conventional CPR (p < 0.001). Witnessed cardiac arrest was present in 223 (86%) of the patients, and 246 (95%) patients received bystander CPR initiated immediately after recognition of arrest with a median no-flow time of 0 min (IQR, 0–1 min). Survivors experienced a significantly shorter total low-flow time from cardiac arrest to MCS initiation than non-survivors (94 min versus 107 min, p = 0.002).
Table 1.
Baseline characteristics and pre-hospital data stratified by 30-day survival status
| Variable | Total (n = 259) |
Survivors (n = 67) |
Non-survivors (n = 192) |
P-value |
|---|---|---|---|---|
| Age (years) | 53 [45–60] | 54 [46–62] | 53 [43–59] | 0.19 |
| Age ≤ 65 years | 221 (85) | 56 (84) | 165 (86) | 0.64 |
| Male sex | 205 (79) | 50 (75) | 155 (81) | 0.29 |
| Comorbidities | ||||
| History of ischemic heart disease | 30 (12) | 9 (13) | 21 (11) | 0.58 |
| Previous myocardial infarction | 28 (11) | 8 (12) | 20 (11) | 0.75 |
| History of congestive heart disease | 19 (7) | 4 (6) | 15 (8) | 0.62 |
| Hypertension | 65 (25) | 18 (27) | 47 (25) | 0.73 |
| Type 2 diabetes | 26 (10) | 3 (5) | 23 (12) | 0.06 |
| Peripheral vascular disease | 11 (4) | 4 (6) | 7 (4) | 0.31 |
| Previous chronic kidney disease | 8 (3) | 2 (3) | 6 (3) | 0.65 |
| Previous stroke | 10 (4) | 0 (0) | 10 (5) | 0.05 |
| Cause of cardiac arrest | ||||
| Acute myocardial infarction | 142 (55) | 42 (63) | 100 (52) | 0.13 |
| Pulmonary embolism | 24 (9) | 8 (12) | 16 (8) | 0.26 |
| Primary arrhythmia | 42 (16) | 10 (15) | 32 (17) | 0.85 |
| Chronic heart disease | 5 (2) | 1 (2) | 4 (2) | 1.00 |
| Cerebral | 6 (2) | 0 (0) | 6 (3) | 0.34 |
| Toxic | 10 (4) | 3 (5) | 7 (4) | 0.72 |
| Other | 20 (8) | 4 (6) | 16 (8) | 0.79 |
| Unknown | 13 (4) | 0 (0) | 13 (5) | 0.02 |
| Witnessed arrest | 223 (86) | 60 (90) | 163 (85) | 0.34 |
| Bystander CPR | 246 (95) | 64 (96) | 182 (95) | 0.94 |
| Transient ROSC | 48 (19) | 27 (40) | 21 (11) | < 0.001 |
| Signs of life during CPR | 100 (39) | 45 (67) | 55 (29) | < 0.001 |
| Initial presenting rhythm | ||||
| Shockable (VT/VF) | 173 (67) | 55 (82) | 118 (62) | 0.002 |
| PEA | 57 (22) | 10 (15) | 47 (25) | 0.10 |
| Asystole | 28 (11) | 2 (3) | 26 (14) | 0.02 |
| End-tidal CO2 | 3.6 [2.8–5.0] | 3.6 [2.9–5.0] | 3.5 [2.5–5.0] | 0.99 |
| Mechanical compression (LUCAS) | 235 (91) | 61 (91) | 174 (91) | 0.97 |
| No-flow (min) | 0 [0–1] | 0 [0–2] | 0 [0–1] | 0.77 |
| Pre-hospital low-flow (min) | 72 [58–90] | 67 [46–90] | 75 [60–90] | 0.06 |
| Total low-flow (min) | 105 [86–125] | 94 [73–120] | 107 [90–127] | 0.002 |
Abbreviations: CPR Cardiopulmonary resuscitation; ROSC Return of spontaneous circulation; VT Ventricular tachycardia; VF Ventricular fibrillation; PEA Pulseless electrical activity; LUCAS Lund University cardiopulmonary assist system
Values are stated as medians and interquartile range [IQR] or numbers and percentages. A p value < 0.05 is considered significant
In-hospital and outcome characteristics
In-hospital and outcome data are shown in Table 2. ECPR was established in 225 (86.9%) patients, whereas Impella assistance was commenced in 12 (4.6%) patients. Twenty-two patients (8.5%) received concomitant support (ECPR + Impella). Among the 247 patients treated with ECPR, cannulation was done percutaneously in 224 patients and by cut-down in 23 patients. Distal perfusion was established in 179 of the ECPR patients. Acute coronary angiography was performed in 234 (90%) patients, and 124 (48%) of the patients received percutaneous coronary intervention. Survivors more likely presented advantageous blood gas analysis with higher median pH levels (7.01 versus 6.88, p < 0.001) and lower serum lactate levels (12.0 mmol/L versus 15.0 mmol/L, p < 0.001) prior to MCS implantation. The majority of the patients (n = 218; 84%) were admitted directly to the ICU after MCS commencement. However, in 41 (16%) patients, further resuscitation efforts were deemed futile and treatment was withdrawn before admission to the ICU. The main reasons for terminating treatment were anoxic brain injury after cardiac arrest or intracerebral haemorrhage, extensive bleeding due to either acute aortic dissection or complication of Lund University Cardiopulmonary Assist System (LUCAS) with spleen or liver rupture, and severe heart failure despite inotropes and vasopressor support.
Table 2.
In-hospital data stratified by 30-day survival status
| Variable | Total (n = 259) |
Survivors (n = 67) |
Non-survivors (n = 192) |
P-value |
|---|---|---|---|---|
| ECMO (only) | 225 (86.9) | 55 (82) | 170 (89) | 0.18 |
| Impella (only) | 12 (4.6) | 6 (9) | 6 (3) | 0.05 |
| ECMO + Impella | 22 (8.5) | 6 (9) | 16 (8) | 0.88 |
| Laboratory data upon arrival | ||||
| pH | 6.90 [6.82–7.02] | 7.01 [6.92–7.15] | 6.88 [6.80–6.98] | < 0.001 |
| Lactate (mmol/L) | 14.4 [11.4–17.0] | 12.0 [9.1–14.7] | 15.0 [12.0–19.0] | < 0.001 |
| Potassium (mmol/L) | 4.4 [3.7–5.4] | 4.1 [3.6–4.9] | 4.6 [3.8–5.5] | 0.06 |
| Hemoglobin (mmol/L) | 8.6 [7.5–9.6] | 8.7 [8.1–9.6] | 8.6 [7.3–9.6] | 0.22 |
| Creatinine (mmol/L) | 114 [98–130] | 109 [95–131] | 115 [99–130] | 0.86 |
| CAG performed | 234 (90) | 62 (93) | 172 (90) | 0.48 |
| Coronary intervention (PCI/stent) | 124 (48) | 37 (55) | 87 (46) | 0.20 |
| Left main | 22 (9) | 5 (8) | 17 (10) | 0.73 |
| Left anterior descending | 76 (33) | 27 (44) | 49 (29) | 0.02 |
| Left circumflex | 8 (3) | 2 (3) | 6 (4) | 0.96 |
| Right coronary artery | 30 (13) | 9 (15) | 21 (12) | 0.58 |
| Intensive care stay | ||||
| No. of patients admitted to ICU | 218 (84) | 67 (100) | 151 (79) | < 0.001 |
| TTM | 152 (70) | 46 (69) | 106 (70) | 0.46 |
| Renal replacement therapy | 91 (42) | 32 (48) | 59 (31) | 0.27 |
| ICU length of stay (hours) | 53 [13–238] | 284 [163–528] | 19 [10–63] | < 0.001 |
| ECPR-/Impella-related complications | ||||
| Bleeding at cannulation site | 76 (29) | 32 (48) | 44 (23) | 0.01 |
| Limb ischemia | 24 (9) | 5 (8) | 19 (10) | 0.31 |
| Gastrointestinal bleeding | 33 (13) | 10 (15) | 23 (12) | 0.90 |
| Gastrointestinal ischemia | 22 (9) | 4 (6) | 18 (9) | 0.20 |
| Time on ECPR (hours) | 50 [27–95] | 67 [40–98] | 37 [8–77] | 0.002 |
| Time on Impella (hours) | 74 [28–165] | 62 [50–165] | 84 [10–166] | 0.46 |
| Hospital length of stay (hours) | 23 [7–358] | 687 [496–1060] | 13 [4–46] | < 0.001 |
Abbreviations: ECMO Extracorporeal membrane oxygenation; CAG Coronary angiogram; PCI Primary coronary intervention; ICU Intensive care unit; TTM Target temperature management
Values are stated as medians and interquartile range (IQR) or numbers and percentages. A p- value of < 0.05 is considered significant
No differences between the groups were seen regarding renal replacement therapy or TTM, (p = 0.27 and p = 0.46, respectively). MCS duration and ICU length of stay were longer in survivors, indicating rapid withdrawal of support in the case of treatment futility. Bleeding at the cannulation site was observed in 76 (29%) and limb ischemia was seen in 24 (9%) of the patients. None of the patients without distal perfusion had limb ischemia. The main cause of withdrawal of life-sustaining treatment in non-survivors was severe brain injury (n = 88, 46%), no cardiac recovery (n = 25, 13%), device failure (n = 4, 2%), multiorgan failure (n = 49, 26%) and other (n = 26, 14%).
Predictors of 30-day mortality and survival outcomes
Table 3 shows results from the binary logistic regression. Thirty-day mortality was significantly associated with initial presenting rhythm with asystole (RR 1.36, 95% CI 1.18–1.57, p < 0.001), pulseless electrical activity (PEA) (RR 1.20, 95% CI 1.03–1.41, p = 0.02), low pH levels < 6.8 (RR 1.28, 95% CI 1.12–1.46, p < 0.001) and high lactate levels > 15 mmol/L (RR 1.33, 95% CI 1.16–1.53, p < 0.001). Signs of life during CPR (RR 0.63, 95% CI 0.52–0.76, p < 0.001) and transient ROSC (RR 0.54, 95% CI 0.39–0.76, p < 0.001) were both associated with a lower risk of mortality.
Table 3.
Binary logistic regression analysis of risk factors associated with 30-day mortality
| Variables | Number of valid cases | Univariate analysis | ||
|---|---|---|---|---|
| Total (n = 259) | RR | 95% CI | P-value | |
| Age (years) | 259 | 1.00 | (0.99–1.00) | 0.21 |
| Male sex | 259 | 1.10 | (0.90–1.34) | 0.34 |
| Witnessed arrest | 259 | 0.91 | (0.76–1.08) | 0.28 |
| Bystander CPR | 259 | 0.98 | (0.70–1.38) | 0.93 |
| Initial presenting rhythm | 258 | |||
| VT/VF* | 1.00 | – | – | |
| PEA | 1.20 | (1.03–1.41) | 0.02 | |
| Asystole | 1.36 | (1.18–1.57) | < 0.001 | |
| Signs of life during CPR | 251 | 0.63 | (0.52–0.76) | < 0.001 |
| Transient ROSC | 252 | 0.54 | (0.39–0.76) | < 0.001 |
| End-tidal CO2 | 224 | 0.82 | (0.65–1.01) | 0.07 |
| Pre-hospital low-flow ≤ 60 min | 259 | 0.80 | (0.67–0.95) | 0.02 |
| pH ≤ 6.8 | 236 | 1.28 | (1.12–1.46) | < 0.001 |
| Lactate ≥ 15 mmol/L | 251 | 1.33 | (1.16–1.53) | < 0.001 |
Abbreviations: CPR Cardiopulmonary resuscitation; VT Ventricular tachycardia; VF Ventricular fibrillation; PEA Pulseless electrical activity; ROSC Return of spontaneous circulation; RR Risk ratio; CI Confidence interval
*Reference group
Kaplan–Meier curves and the log rank test demonstrated similar results (Additional file 3: Figure S2). Patients presenting signs of life during CPR had a higher survival rate compared to patients without signs of life (45% versus 13%, p < 0.001), Fig. S2, B. A favorable 30-day survival was also seen in patients with a pre-hospital low-flow time < 60 min and in patients with a pre-hospital low-flow time > 80 min (40% versus 26%), Fig. S2, C. Patients with a prolonged pre-hospital low-flow time (> 80 min) had a higher rate of signs of life during CPR than patients with a pre-hospital low-flow time of 60–80 min (41% versus 31%, p = 0.14). For other subgroups, please refer to Additional files 2, 3: Figure S2 and Figure S3 for further 30-day survival data.
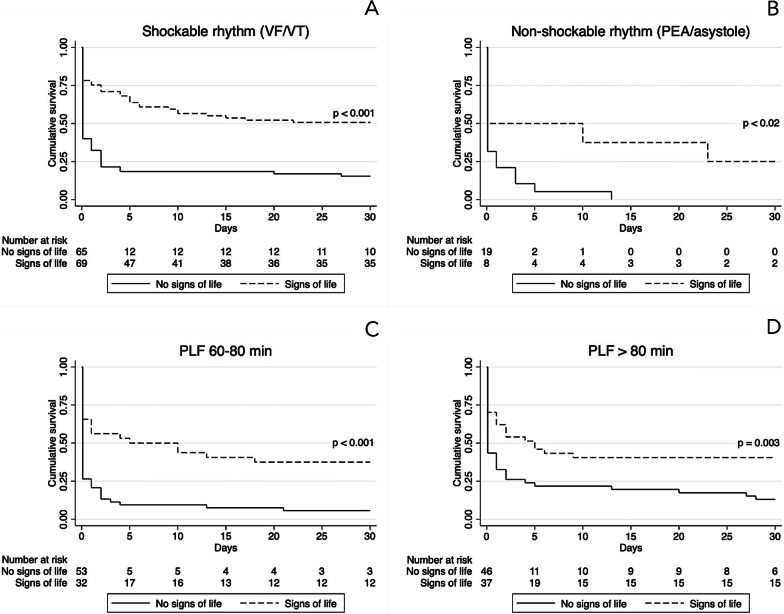
Figure 2 demonstrates Kaplan–Meier curves for patients with and without signs of life during CPR and their 30-day survival with respect to initial presenting rhythm and pre-hospital low-flow times. Patients with signs of life had significantly higher survival rates compared to patients with no signs of life for both entities. Patients presenting non-shockable rhythm without signs of life had no survivors at day 30.
Fig. 2.
Kaplan–Meier survival curves for patients with and without signs of life during cardiopulmonary resuscitation with respect to initial presenting rhythm and pre-hospital low-flow times. a Patients with initial shockable rhythm with signs of life and no signs of life. b Patients with non-shockable rhythm with signs of life and no signs of life. c Patients with pre-hospital low flow (PLF) 60–80 min with signs of life and no signs of life. d Patients with PLF > 80 min with signs of life and no signs of life
Regional differences
Regional differences in patient selection and outcome are shown in Table 4. The ECPR activity per million inhabitants differed between centers. In most centers, younger age was a predominant factor for triage; nevertheless, 38 (15%) patients with age > 65 years did receive ECPR. Initial presenting rhythm differed significantly between centers (p = 0.003). Pre-hospital low-flow times were significantly longer (77 min versus 60 min, p = 0.02) in hospitals serving patients in remote and rural areas with a distance to center > 100 km (p = 0.001). Thirty-day survival between centers varied from 15 to 28%; however, this difference did not reach statistical significance, p = 0.41.
Table 4.
Regional differences in triage and outcome of patients treated with extracorporeal cardiopulmonary resuscitation or Impella for refractory cardiac arrest
| Variable | Aalborg University Hospital (n = 34) |
Aarhus University Hospital (n = 138) |
Odense University Hospital (n = 55) |
Copenhagen University Hospital (n = 32) |
P-value |
|---|---|---|---|---|---|
| No. of MCS/mio-inhabitants*year | 11.6 | 11.2 | 8.9 | 2.8 | < 0.001 |
| Age < 65 years | 26 (76) | 113 (82) | 51 (93) | 31 (97) | 0.03 |
| Initial presenting rhythm | 0.003 | ||||
| Shockable VT/VF | 19 (56) | 82 (59) | 44 (80) | 28 (88) | |
| PEA | 11 (32) | 38 (28) | 4 (7) | 4 (13) | |
| Asystole | 3 (9) | 18 (13) | 7 (13) | 0 (0) | |
| Unknown | 1 (3) | 0 (0) | 0 (0) | 0 (0) | |
| Witnessed arrest | 31 (91) | 114 (83) | 49 (89) | 29 (91) | 0.38 |
| Bystander CPR | 31 (91) | 134 (97) | 52 (95) | 29 (91) | 0.42 |
| No-flow ≥ 10 min | 0 (0) | 10 (7) | 0 (0) | 1 (3) | 0.07 |
| Pre-hospital low-flow (min) | 60 [43–77] | 75 [60–90] | 77 [65–99] | 60 [48–70] | < 0.001 |
| Total low-flow (min) | 90 [62–110] | 105 [88–125] | 119 [105–127] | 94 [81–130] | < 0.001 |
| Distance to center ≥ 100 km | 2 (6) | 21 (15) | 19 (35) | 0 (0) | 0.001 |
| 30-day survival | 5 (15) | 38 (28) | 14 (25) | 9 (28) | 0.41 |
Abbreviations: MCS Mechanical circulatory support; VT Ventricular tachycardia; VF Ventricular fibrillation; PEA Pulseless electrical activity; CPR Cardio pulmonary resuscitation
Values are stated as medians and interquartile range (IQR) or numbers and percentages. A p value of < 0.05 is considered significant
Analysis of selection criteria in the Danish national consensus
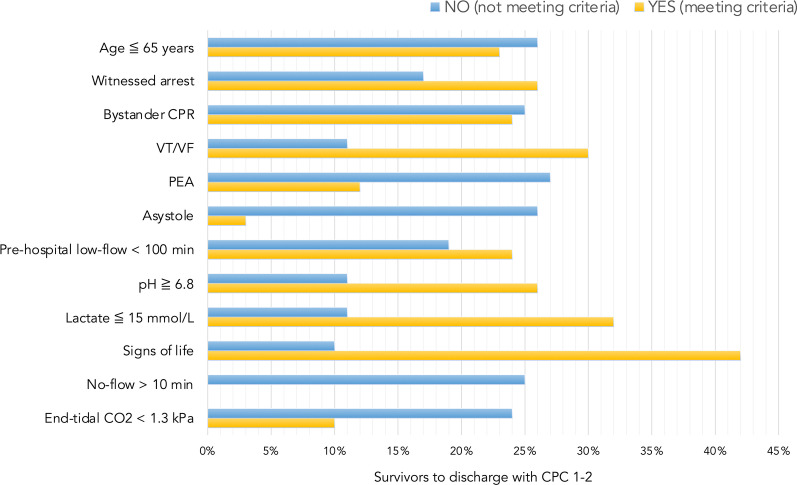
Figure 3 illustrates the survival rates of survivors to discharge with CPC 1–2 (n = 61) in regards to each selection criteria of the Danish 2018 national ECPR consensus. Poorest outcomes were seen in patients with initial non-shockable rhythm, no-flow times > 10 min and end-tidal CO2 < 1.3 kPa.
Fig. 3.
The impact of each selection criteria of the Danish national ECPR consensus in relation to survival to discharge with favorable neurological outcome (CPC 1–2). Survivors to discharge with CPC 1–2 in percentage when meeting selection criteria and failing to meet criteria of the national consensus
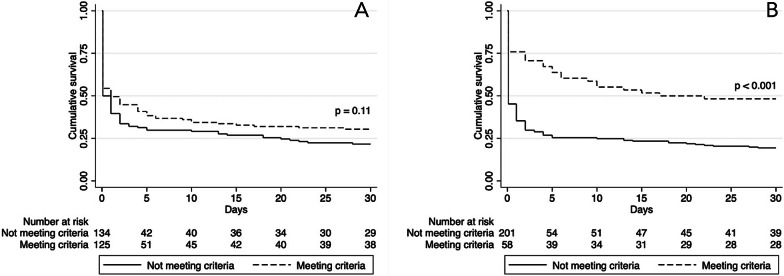
Patients meeting all of the selection criteria of the Danish 2018 national ECPR consensus with respect to normothermic arrest of presumed cardiac origin, age < 65 years, witnessed arrest, bystander CPR, initial shockable rhythm, no-flow time < 10 min and end-tidal CO2 < 1.3 kPa (n = 125, 48%) were compared with those who failed to meet one or more of these parameters (n = 134, 52%) (Fig. 4). Thirty-day survival was 30% in patients who met the selection criteria compared with 22% in patients who failed to meet one or more criteria (p = 0.11).
Fig. 4.
Kaplan–Meier survival curve for patients meeting the selection criteria and patients failing to meet the criteria. a Consensus A (National consensus): Survival analysis between patients meeting the Danish national consensus selection criteria in regards to younger age < 65 years, witnessed arrest, bystander CPR, initial shockable rhythm, no-flow < 10 min and end-tidal CO2 < 1.3 kPa, and patients failing to meet one or more criteria. b Consensus B (Extended version): Survival analysis based on a more refined assessment of selection criteria. Patients meeting all the selection criteria in the Danish national consensus and additionally one or more of following parameters: signs of life during CPR, pH > 6.8, lactate < 15 mmol/L and a pre-hospital low-flow < 100 min
A more refined assessment of selection criteria including parameters such as signs of life during CPR, pre-hospital low-flow < 100 min, initial pH > 6.8 and lactate ≤ 15 mmol/L in addition to the consensus criteria (Consensus B) showed significantly higher 30-day survival rates in patients meeting one or more of the extended criteria than in patients failing to comply with the criteria (48% versus 19%, p < 0.001) (Fig. 4). However, this also implied that 58% (39/67) of the 30-day survivors in this cohort would fail to meet the extended consensus.
Discussion
The present study is the first nationwide multicenter study of outcomes in patients receiving MCS for refractory OHCA in Denmark. The main findings showed that one in four MCS patients survive to hospital discharge with a good neurological outcome. Outcome was significantly associated with initial presenting rhythm, signs of life during CPR, transient ROSC, pre-hospital low-flow time, initial pH and lactate levels. By offering MCS only to patients meeting the strictest criteria, survival exceeded 48%, but this occurred at the cost of withholding life-saving treatment in the majority of patients saved by MCS in the cohort. Thus, our data rightly questions the validity of stringent patient selection for MCS.
ECPR has emerged as a salvage therapy for patients suffering from refractory OHCA. Although resource demanding, ECPR has been shown to be both feasible and cost effective [13]. Moreover, recent small reports have described the potential of the Impella device as an alternative rescue therapy in cardiac arrest patients [7, 8]. Several previous observational studies have demonstrated encouraging survival rates and a favorable neurological outcome from applying strict inclusion and exclusion criteria in distinctive patient populations [14–18]. The first randomized clinical trial recently published by Yannopoulos et al. (the ARREST trial) revealed superiority of ECMO-facilitated resuscitation with a survival rate of 43% compared with 7% with standard advanced life support [6]. Survival at six months was also greater in the ECMO group (hazard ratio (HR) 0.16, 95% CI 0.06–0.41, p = 0.0001). Whereas the trial was well-designed and supports use of ECPR in refractory OHCA, apparent limitations are present as this was an open-labelled single-center study with a relatively small and highly selected patient population.
In Denmark, a number of initiatives to improve pre-hospital quality of care for OHCA patients have produced a remarkable increase in 30-day survival rates from 3.9% in 2001 to 16% in 2018 [19]. Concurrently, MCS with ECMO and in limited cases the Impella device, has been evolving steadily and is now an established treatment for selected patients with refractory OHCA in all Danish regions. However, given the formal inclusion and exclusion criteria defined in the Danish national ECPR consensus, one might assume that the results of our study would reflect a more homogeneous population. Despite our intention to select qualified candidates, violation of the national consensus was still seen in 52%. In this population, the fatal consequence of treatment not being provided, opted some ECPR teams to perform MCS despite adverse conditions. Selection of appropriate candidates for MCS is evidently a challenge in real clinical practise. Despite growing interest in and a growing body of literature on MCS for refractory OHCA, robust evidence on patient eligibility is still lacking.
In the present study, the majority of patients treated with MCS were younger (< 65 years) in compliance with the national consensus. This may explain why no statistically significant difference was detected in age between survivors and non-survivors. One may have predicted a worse prognosis in patients above 65 years of age, but survival to hospital discharge with good neurological outcome was similar in this group compared with the younger population < 65 years (26% versus 23%). Previous studies have proposed advanced age as a predictor of a poor outcome in patients with ECPR [20], and some studies have even suggested an age of > 75 years as a contraindication for ECPR [21], which is in line with our results. In our cohort, no one above 72 years of age survived to discharge. However, there is limited and conflicting evidence that older age is associated with poor outcome [22, 23]. The dilemma of initiating ECPR in the elderly remains controversial and more evidence is needed to confirm, whether to proceed or stop advanced life support based on age limits.
Pre-hospital parameters are crucial in the selection of suitable candidates for ECPR. Initial shockable rhythm, transient ROSC and signs of life during CPR are considered favorable prognostic factors in ECPR [24–26]. In a prospective registry study, Bougouin et al. compared conventional CPR with ECPR in 13,191 consecutive patients with OHCA [27]. Prognostic factors in the ECPR group comprised an initial shockable rhythm (OR 3.9, 95% CI 1.5–10.3) and transient ROSC (OR 2.3, 95% CI 1.1–4.7) prior to ECPR implementation. One of the main findings in our study was that patients with signs of life during CPR had a threefold higher survival rate than patients without signs of life during CPR. This is in correlation with a study recently published by Debaty et al. [28] The authors found that any signs of life before or during CPR substantially improved 30-day survival with favorable neurological outcome in a multivariable prognostic model (OR 7.35, 95% CI 2.71–19.97). These results are supported by our data. Signs of life was a highly significant prognostic factor in relation to initial presenting rhythm and low-flow times. In the present cohort, a pre-hospital low-flow time < 60 min was associated with an increased survival rate; however, patients exceeding pre-hospital low-flow times > 80 min also had an advantageous outcome. Patients with pre-hospital low-flow times > 80 min had a higher rate of signs of life during CPR than patients with a pre-hospital low-flow time of 60–80 min. The presence of signs of life increased survival rates substantially in both groups. Collectively, these observations support the evidence of incorporating signs of life as an important factor in the selection of patients for ECPR. Our results suggest that prolonged resuscitation efforts in the field may not be futile, especially in patients presenting favorable circumstances such as signs of life and where ECPR can be established within a reasonable timeframe. The present findings do not allow us to determine whether any of the patients would have survived without MCS. However, the long total low-flow times observed makes this unlikely.
Historically, the arrest-to-perfusion time has been linked to survival [16, 18, 29, 30]. Wengenmayer et al. reported that among 133 patients with cardiac arrest treated with ECPR, low-flow time was an independent predictor of mortality [30]. Bartos et al. demonstrated a significant association between time from arrest to sufficient ECPR flow and neurological outcome in a cohort of 160 patients [18]. These results are similar to ours. In the present study, hospitals serving patients in remote and rural areas had longer arrest-to-perfusion time due to longer distances to the invasive center. Implementation of systematic pre-hospital ECPR calls, more rapid allocation of helicopter-mediated transport and direct triage to the catheterization laboratory may improve the performance and facilitate a reduction in system delay for these patients. In this setting, pre-hospital ECPR may also shorten the interval from collapse to onset of ECPR [17].
The predictive value of pH and lactate levels in patients with cardiac arrest is well established. Controversy still exists regarding the ECPR population. In our study, initial arterial pH and lactate levels were found to be associated with mortality. This finding is consistent with previous findings [14, 31]. Jung et al. retrospectively reviewed 93 patients with cardiac arrest undergoing ECPR and found results similar to our results [32]. On the contrary, Leick et al. found no association between elevated lactate levels and mortality [33]. Our results support the inclusion of pH and lactate into our decision-making when considering patients for MCS, whereas specific cutoffs still need conformation in other cohorts. Importantly, a stringent use of pH > 6.8 and lactate < 15 mmol/L as selection criteria, may result in denying life-saving therapy to a considerable number of the survivors present in this cohort.
In the present study, we assessed pre-hospital and in-hospital factors in relation to outcome, which may come in benefit for clinicians in the selection of appropriate candidates for MCS. High mortality rates were seen among patients with initial non-shockable rhythm, no-flow times > 10 min and end-tidal CO2 < 1.3 kPa. On the contrary, our results suggest that a more refined assessment of the inclusion criteria, comprising additional criteria such as signs of life during CPR and lactate levels, may improve survival rates in patients receiving MCS. Nevertheless, one must recognize that limiting patient selection to strict pre-defined criteria will inevitably exclude some patients in whom MCS would have bought valuable time until the reversible cause could be treated. The fact that the national consensus was violated in 52% of patients, of whom 20% survived to hospital discharge with a good neurological outcome in 94% of the cases, indicates that there must be room for individual decision-making, especially in the young patients. Patient selection for MCS continues to be a challenging part of real-world clinical practice and further randomized clinical trials are warranted.
Limitations
The present study has several limitations. Its retrospective nature makes it subject to patient selection bias. The national consensus was available and adopted to some extent in all centers. This produces risk of bias in the evaluation of the associations with outcome. Although we conducted a multicenter study using nationwide registry data, the heterogeneity of the study population with a mixed cohort of patients with OHCA hampers generalization of the results. Neurological outcome at hospital discharge is a fairly crude measure; it is, however, broadly used in cardiac arrest studies. Studies assessing long-term survival and neurological outcome are necessary.
Conclusion
Patients receiving MCS for refractory OHCA presented promising survival rates with a favorable neurological outcome at hospital discharge. Even though a more stringent patient selection with additional criteria may produce higher survival rates, this would also limit the number of candidates and possibly exclude half of the survivors from treatment, why optimization of the selection criteria is still of essence in the future.
Supplementary Information
Additional file 1. Table S1: National and regional indications for mechanical circulatory support in refractory normothermic cardiac arrest with presumed cardiac origin.
Additional file 2. Figure S1: National trend in the use of mechanical circulatory support for OHCA in Denmark.
Additional file 3. Figure S2: Kaplan-Meier survival curves of patients who had out-of-hospital cardiac arrest and received mechanical circulatory support.
Additional file 4. Figure S3: Kaplan-Meier survival curves stratified by groups.
Acknowledgements
Not applicable.
Abbreviations
- CPC
Cerebral performance category
- CPR
Cardiopulmonary resuscitation
- ECMO
Extracorporeal membrane oxygenation
- ECPR
Extracorporeal cardiopulmonary resuscitation
- ICU
Intensive care unit
- LUCAS
Lund university cardiopulmonary assist system
- MCS
Mechanical circulatory support
- OHCA
Out-of-hospital cardiac arrest
- PEA
Pulseless electrical activity
- ROSC
Return of spontaneous circulation
- TTM
Targeted temperature management
- VF
Ventricular fibrillation
- VT
Ventricular tachycardia
Authors' contributions
SRM, CS, SC, and CJT conceived and designed the study. SRM, LL, JBA, and EG collected data. SRM performed statistical analysis, interpreted the data, and drafted the manuscript. CS, SC and CJT supervised the study. All authors read and approved the final manuscript.
Funding
This work was supported by the Danish Heart Foundation [20-R142-A9498-22178]; and Health Research Foundation of Central Denmark Region [R64-A3178-B1349]; and Danish Helicopter Emergency Medical Service Research Fund; and Aase og Ejnar Danielsens Fond; and Snedkermester Sophus Jacobsen og hustru Astrid Jacobsens Fond; and Henry og Astrid Møllers Fond; and a research grant from Aarhus University to Dr. Mørk; and Lundbeck Foundation [R186-2015–2132] to Dr. Hassager. None of the mentioned sources listed participated in design of the study, study collection, analysis, interpretation of data, or writing of the manuscript.
Availability of data and materials
The data underlying this article were provided by the administrative Regions of Denmark under license from the Danish Data Protection Agency and the Danish Patient Safety Authority and cannot be shared publicly due to Danish regulations for data protection. Data are however available from the authors upon reasonable request and with permission from the five administrative Regions of Denmark.
Declarations
Ethical approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the Danish Data Protection Agency (Ref. 1-16-02-383-18) and the Danish Patient Safety Authority (Ref. 3-3013-2696/1). In Denmark, informed consent or ethical approval is not required for registry-based research.
Consent for publication
Not applicable.
Competing interests
Dr. Stengaard reports speaker’s fees from Rosche Diagnostics, outside the submitted work. Dr. Møller reports personal fees and grants from Orion Pharma, Novartis, Astra Zeneca, Abbott and Abiomed and served at scientific advisory board for Boehringer Ingelheim, outside the submitted work. Dr. Freeman reports grants from St. Jude and Astra Zeneca and personal fees from Meril Lifesciences and Edwards Lifesciences, outside the submitted work. Dr. Hassager reports grants from the Lundbeck Foundation and speaker’s honoraria from Abiomed, outside the submitted work. Dr. Kjaergaard reports non-financial participation in the advisory board for the CoCa Trial. Dr. Terkelsen is supported by an unrestricted research grant from the Danish Heart Foundation. The remaining authors have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grasner JT, Wnent J, Herlitz J, Perkins GD, Lefering R, Tjelmeland I, Koster RW, Masterson S, Rossell-Ortiz F, Maurer H, Bottiger BW, Moertl M, Mols P, Alihodzic H, Hadzibegovic I, Ioannides M, Truhlar A, Wissenberg M, Salo A, Escutnaire J, Nikolaou N, Nagy E, Jonsson BS, Wright P, Semeraro F, Clarens C, Beesems S, Cebula G, Correia VH, Cimpoesu D, Raffay V, Trenkler S, Markota A, Stromsoe A, Burkart R, Booth S, Bossaert L. Survival after out-of-hospital cardiac arrest in Europe: results of the EuReCa TWO study. Resuscitation. 2020;148:218–226. doi: 10.1016/j.resuscitation.2019.12.042. [DOI] [PubMed] [Google Scholar]
- 2.Haneya A, Philipp A, Diez C, Schopka S, Bein T, Zimmermann M, Lubnow M, Luchner A, Agha A, Hilker M, Hirt S, Schmid C, Muller T. A 5-year experience with cardiopulmonary resuscitation using extracorporeal life support in non-postcardiotomy patients with cardiac arrest. Resuscitation. 2012;83:1331–1337. doi: 10.1016/j.resuscitation.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Sakamoto T, Morimura N, Nagao K, Asai Y, Yokota H, Nara S, Hase M, Tahara Y, Atsumi T and Group S-JS Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out-of-hospital cardiac arrest: a prospective observational study. Resuscitation. 2014;85:762–768. doi: 10.1016/j.resuscitation.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 4.Fjolner J, Greisen J, Jorgensen MR, Terkelsen CJ, Ilkjaer LB, Hansen TM, Eiskjaer H, Christensen S, Gjedsted J. Extracorporeal cardiopulmonary resuscitation after out-of-hospital cardiac arrest in a Danish health region. Acta Anaesthesiol Scand. 2017;61:176–185. doi: 10.1111/aas.12843. [DOI] [PubMed] [Google Scholar]
- 5.Dennis M, McCanny P, D'Souza M, Forrest P, Burns B, Lowe DA, Gattas D, Scott S, Bannon P, Granger E, Pye R, Totaro R, Sydney ERIG. Extracorporeal cardiopulmonary resuscitation for refractory cardiac arrest: A multicentre experience. Int J Cardiol. 2017;231:131–136. doi: 10.1016/j.ijcard.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Yannopoulos D, Bartos J, Raveendran G, Walser E, Connett J, Murray TA, Collins G, Zhang L, Kalra R, Kosmopoulos M, John R, Shaffer A, Frascone RJ, Wesley K, Conterato M, Biros M, Tolar J, Aufderheide TP. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet. 2020;396:1807–1816. doi: 10.1016/S0140-6736(20)32338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vase H, Christensen S, Christiansen A, Therkelsen CJ, Christiansen EH, Eiskjaer H, Poulsen SH. The Impella CP device for acute mechanical circulatory support in refractory cardiac arrest. Resuscitation. 2017;112:70–74. doi: 10.1016/j.resuscitation.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Panagides V, Vase H, Shah SP, Basir MB, Mancini J, Kamran H, Batra S, Laine M, Eiskjaer H, Christensen S, Karami M, Paganelli F, Henriques JPS and Bonello L. Impella CP Implantation during Cardiopulmonary Resuscitation for Cardiac Arrest: A Multicenter Experience. J Clin Med. 2021;10. [DOI] [PMC free article] [PubMed]
- 9.Danish National Consensus for ECPR (Danish Society of Cardiology). 2018:https://www.cardio.dk/media/com_reditem/files/customfield/item/6866/DCS%20Holdningspapir%201-18.pdf.
- 10.Mork SR, Frederiksen CA, Nielsen RR, Lichscheidt E, Christensen S, Greisen JR, Tang M, Vase H, Logstrup BB, Mellemkjaer S, Wiggers HS, Molgaard H, Poulsen SH, Terkelsen CJ, Eiskjaer H. A systematic approach to weaning from extracorporeal membrane oxygenation in patients with refractory cardiac failure. Acta Anaesthesiol Scand. 2021;00:1–8. doi: 10.1111/aas.13814. [DOI] [PubMed] [Google Scholar]
- 11.Idris AH, Bierens J, Perkins GD, Wenzel V, Nadkarni V, Morley P, Warner DS, Topjian A, Venema AM, Branche CM, Szpilman D, Morizot-Leite L, Nitta M, Lofgren B, Webber J, Grasner JT, Beerman SB, Youn CS, Jost U, Quan L, Dezfulian C, Handley AJ, Hazinski MF. 2015 revised Utstein-style recommended guidelines for uniform reporting of data from drowning-related resuscitation: An ILCOR advisory statement. Resuscitation. 2017;118:147–158. doi: 10.1016/j.resuscitation.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 12.Brain Resuscitation Clinical Trial IISG A randomized clinical study of a calcium-entry blocker (lidoflazine) in the treatment of comatose survivors of cardiac arrest. N Engl J Med. 1991;324:1225–1231. doi: 10.1056/NEJM199105023241801. [DOI] [PubMed] [Google Scholar]
- 13.Matsuoka Y, Goto R, Atsumi T, Morimura N, Nagao K, Tahara Y, Asai Y, Yokota H, Ariyoshi K, Yamamoto Y, Sakamoto T and Group S-JS Cost-effectiveness of extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest: A multi-centre prospective cohort study. Resuscitation. 2020;157:32–38. doi: 10.1016/j.resuscitation.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Debaty G, Babaz V, Durand M, Gaide-Chevronnay L, Fournel E, Blancher M, Bouvaist H, Chavanon O, Maignan M, Bouzat P, Albaladejo P, Labarere J. Prognostic factors for extracorporeal cardiopulmonary resuscitation recipients following out-of-hospital refractory cardiac arrest. A systematic review and meta-analysis . Resuscitation. 2017;112:1–10. doi: 10.1016/j.resuscitation.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Dennis M, Buscher H, Gattas D, Burns B, Habig K, Bannon P, Patel S, Buhr H, Reynolds C, Scott S, Nair P, Hayman J, Granger E, Lovett R, Forrest P, Coles J, Lowe DA, Sydney ERIG. Prospective observational study of mechanical cardiopulmonary resuscitation, extracorporeal membrane oxygenation and early reperfusion for refractory cardiac arrest in Sydney: the 2CHEER study. Crit Care Resusc. 2020;22:26–34. doi: 10.51893/2020.1.oa3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lunz D, Calabro L, Belliato M, Contri E, Broman LM, Scandroglio AM, Patricio D, Malfertheiner M, Creteur J, Philipp A, Taccone FS, Pappalardo F. Extracorporeal membrane oxygenation for refractory cardiac arrest: a retrospective multicenter study. Intensive Care Med. 2020;46:973–982. doi: 10.1007/s00134-020-05926-6. [DOI] [PubMed] [Google Scholar]
- 17.Lamhaut L, Hutin A, Puymirat E, Jouan J, Raphalen JH, Jouffroy R, Jaffry M, Dagron C, An K, Dumas F, Marijon E, Bougouin W, Tourtier JP, Baud F, Jouven X, Danchin N, Spaulding C, Carli P. A Pre-Hospital Extracorporeal Cardio Pulmonary Resuscitation (ECPR) strategy for treatment of refractory out hospital cardiac arrest: an observational study and propensity analysis. Resuscitation. 2017;117:109–117. doi: 10.1016/j.resuscitation.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Bartos JA, Grunau B, Carlson C, Duval S, Ripeckyj A, Kalra R, Raveendran G, John R, Conterato M, Frascone RJ, Trembley A, Aufderheide TP, Yannopoulos D. Improved survival with extracorporeal cardiopulmonary resuscitation despite progressive metabolic derangement associated with prolonged resuscitation. Circulation. 2020;141:877–886. doi: 10.1161/CIRCULATIONAHA.119.042173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ringgren KB, Christensen HC, Schønau L, Lippert FK, Folke F, Christensen EF, Hendriksen OM, Nielsen PS, Hansen PA, Mikkelsen S and Torp-Pedersen C. Danish Cardiac Arrest Registry 2001–2018. (2018);22 Mar 2021:https://hjertestopregister.dk/wp-content/uploads/2019/11/Dansk-Hjertestopregister-2018-2.pdf.
- 20.de Waha S, Fuernau G, Eitel I, Desch S, Thiele H. Long-term prognosis after extracorporeal life support in refractory cardiogenic shock-results from a real-world cohort. EuroIntervention. 2016;12:414. doi: 10.4244/EIJV12I3A71. [DOI] [PubMed] [Google Scholar]
- 21.Klinzing S, Wenger U, Steiger P, Starck CT, Wilhelm M, Schuepbach RA, Maggiorini M. External validation of scores proposed for estimation of survival probability of patients with severe adult respiratory distress syndrome undergoing extracorporeal membrane oxygenation therapy: a retrospective study. Crit Care. 2015;19:142. doi: 10.1186/s13054-015-0875-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pontailler M, Demondion P, Lebreton G, Golmard JL, Leprince P. Experience with extracorporeal life support for cardiogenic shock in the older population more than 70 years of age. ASAIO J. 2017;63:279–284. doi: 10.1097/MAT.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 23.Saito S, Nakatani T, Kobayashi J, Tagusari O, Bando K, Niwaya K, Nakajima H, Miyazaki S, Yagihara T, Kitamura S. Is extracorporeal life support contraindicated in elderly patients? Ann Thorac Surg. 2007;83:140–145. doi: 10.1016/j.athoracsur.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Stub D, Nehme Z, Bernard S, Lijovic M, Kaye DM, Smith K. Exploring which patients without return of spontaneous circulation following ventricular fibrillation out-of-hospital cardiac arrest should be transported to hospital? Resuscitation. 2014;85:326–331. doi: 10.1016/j.resuscitation.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Ko RE, Ryu JA, Cho YH, Sung K, Jeon K, Suh GY, Park TK, Lee JM, Song YB, Hahn JY, Choi JH, Choi SH, Gwon HC, Carriere KC, Ahn J, Yang JH. The differential neurologic prognosis of low-flow time according to the initial rhythm in patients who undergo extracorporeal cardiopulmonary resuscitation. Resuscitation. 2020;148:121–127. doi: 10.1016/j.resuscitation.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Yannopoulos D, Bartos JA, Raveendran G, Conterato M, Frascone RJ, Trembley A, John R, Connett J, Benditt DG, Lurie KG, Wilson RF, Aufderheide TP. Coronary artery disease in patients with out-of-hospital refractory ventricular fibrillation cardiac arrest. J Am Coll Cardiol. 2017;70:1109–1117. doi: 10.1016/j.jacc.2017.06.059. [DOI] [PubMed] [Google Scholar]
- 27.Bougouin W, Dumas F, Lamhaut L, Marijon E, Carli P, Combes A, Pirracchio R, Aissaoui N, Karam N, Deye N, Sideris G, Beganton F, Jost D, Cariou A, Jouven X. Sudden Death Expertise Center I. Extracorporeal cardiopulmonary resuscitation in out-of-hospital cardiac arrest: a registry study. Eur Heart J. 2020;41:1961–1971. doi: 10.1093/eurheartj/ehz753. [DOI] [PubMed] [Google Scholar]
- 28.Debaty G, Lamhaut L, Aubert R, Nicol M, Sanchez C, Chavanon O, Bouzat P, Durand M, Vanzetto G, Hutin A, Jaeger D, Chouihed T and Labarere J. Prognostic value of signs of life throughout cardiopulmonary resuscitation for refractory out-of-hospital cardiac arrest. Resuscitation. 2021. [DOI] [PubMed]
- 29.Otani T, Sawano H, Natsukawa T, Nakashima T, Oku H, Gon C, Takahagi M, Hayashi Y. Low-flow time is associated with a favorable neurological outcome in out-of-hospital cardiac arrest patients resuscitated with extracorporeal cardiopulmonary resuscitation. J Crit Care. 2018;48:15–20. doi: 10.1016/j.jcrc.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Wengenmayer T, Rombach S, Ramshorn F, Biever P, Bode C, Duerschmied D, Staudacher DL. Influence of low-flow time on survival after extracorporeal cardiopulmonary resuscitation (eCPR) Crit Care. 2017;21:157. doi: 10.1186/s13054-017-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D'Arrigo S, Cacciola S, Dennis M, Jung C, Kagawa E, Antonelli M, Sandroni C. Predictors of favourable outcome after in-hospital cardiac arrest treated with extracorporeal cardiopulmonary resuscitation: a systematic review and meta-analysis. Resuscitation. 2017;121:62–70. doi: 10.1016/j.resuscitation.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Jung C, Bueter S, Wernly B, Masyuk M, Saeed D, Albert A, Fuernau G, Kelm M and Westenfeld R. Lactate clearance predicts good neurological outcomes in cardiac arrest patients treated with extracorporeal cardiopulmonary resuscitation. J Clin Med. 2019;8. [DOI] [PMC free article] [PubMed]
- 33.Leick J, Liebetrau C, Szardien S, Fischer-Rasokat U, Willmer M, van Linden A, Blumenstein J, Nef H, Rolf A, Arlt M, Walther T, Hamm C, Mollmann H. Door-to-implantation time of extracorporeal life support systems predicts mortality in patients with out-of-hospital cardiac arrest. Clin Res Cardiol. 2013;102:661–669. doi: 10.1007/s00392-013-0580-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1: National and regional indications for mechanical circulatory support in refractory normothermic cardiac arrest with presumed cardiac origin.
Additional file 2. Figure S1: National trend in the use of mechanical circulatory support for OHCA in Denmark.
Additional file 3. Figure S2: Kaplan-Meier survival curves of patients who had out-of-hospital cardiac arrest and received mechanical circulatory support.
Additional file 4. Figure S3: Kaplan-Meier survival curves stratified by groups.
Data Availability Statement
The data underlying this article were provided by the administrative Regions of Denmark under license from the Danish Data Protection Agency and the Danish Patient Safety Authority and cannot be shared publicly due to Danish regulations for data protection. Data are however available from the authors upon reasonable request and with permission from the five administrative Regions of Denmark.