Abstract
Background
As a nutritional index, preoperative serum prealbumin highly correlates with surgical complications. However, the correlation between preoperative prealbumin and postoperative complications remains unclear in liver transplantation (LT).
Methods
A total of 191 patients who underwent LT between 2015 and 2019 were included in the retrospective analysis. According to a cut-off value calculated from a receiver operating characteristic (ROC) curve, the patients were divided into normal and low preoperative prealbumin groups. Univariable and multivariable logistic regression analyses were used to identify independent risk factors for postoperative complications. In addition, patients were divided into subgroups by Model for End-stage Liver Disease (MELD) score, and the association between preoperative prealbumin and postoperative complications was also assessed in each group.
Results
A total of 111 (58.1%) patients were included in the low prealbumin group based on a cut-off value of 120 mg/L. The area under the ROC curve (AUC) was 0.754 (95% confidence interval [CI] 0.678–0.832). Low prealbumin (95% CI 1.51–12.8, P = 0.007) was identified as a predictor for postoperative complications based on multivariable regression. In the low and normal prealbumin groups, the prevalence rates of postoperative complications were 27.5% and 8.0% (P = 0.003) in the MELD score ≤ 15 subgroup and 53.3% and 20.0% (P = 0.197) in the MELD score > 15 subgroup, respectively.
Conclusions
Preoperative prealbumin was associated with postoperative complications in LT, and preoperative nutritional support benefitted postoperative recovery, especially for patients with low MELD scores.
Keywords: Liver transplantation, Preoperative prealbumin, Postoperative complications, MELD subgroups
Introduction
Liver transplantation (LT) is the best method for the radical cure of end-stage decompensated liver disease and malignancy and results in satisfactory replacement of liver function and excellent margins [1–3]. However, postoperative complications remain a concern for short- and long-term survival. Malnutrition significantly increased the risk of postoperative complications [4], while previous research found that preoperative nutritional support reduced complications significantly in abdominal surgery [5]. Patients with end-stage liver diseases frequently suffer from malnutrition with insufficient hepatic synthesis. However, the relationship between nutritional status and post-LT complications has not been thoroughly revealed.
Serum albumin is the most objective and applicable nutritional indicator [6], but it is easily affected by exogenous albumin supplements. In contrast, serum prealbumin, another measurable nutritional indicator, is also able to reflect nutritional status objectively. As a precursor for synthesizing albumin, prealbumin is barely influenced by external supplementation [7]. With a shorter half-life than serum albumin, serum prealbumin is a precise marker to evaluate the severity of liver diseases [8]. Numerous studies have incorporated prealbumin into preoperative nutritional assessments and have used it for surgical risk stratification [9, 10] As for hepatopancreatobiliary diseases, preoperative prealbumin plays a crucial role in predicting postoperative complications, such as for patients undergoing pancreaticoduodenectomy or hepatectomy [11–13]. This relationship was also demonstrated in patients undergoing total knee arthroplasty and kidney transplantation [14, 15]. Moreover, preoperative prealbumin combined with disease severity has been reported to yield better predictions in patients with liver cirrhosis [16]. However, the relationship between preoperative serum prealbumin and post-LT complications has not been demonstrated.
The present study aimed to confirmed the effect of prealbumin on the postoperative complications of LT and whether preoperative nutritional support is highly recommended for LT patients.
Methods
Patient selection
In this retrospective study, the clinical characteristics and demographic data of patients in a single-center database were reviewed and selected from the records of the Chinese Liver Transplant Registry (CLTR: http://cltr.cotr.cn). This database contains data from 191 patients who underwent LT from January 2015 to March 2019 in Southwest Hospital. Patients were diagnosed with cirrhosis based on unequivocal imaging results or liver biopsy and symptoms such as ascites and esophageal variceal bleeding. Additionally, the MELD score was used to calculate the severity of liver disease. Postoperative histopathology was used to identify malignant tumors. Perioperative data were collected from 1 to 3 days before surgery until hospital discharge. This retrospective study was conducted in accordance with the Declaration of Helsinki, and no organs from executed prisoners were used for LT.
Parameter selection
All patients’ medical records were reviewed for a series of clinical and pathological characteristics, including age, sex, body mass index (BMI), history of hepatectomy, HBV infection, American Society of Anesthesiologists (ASA) score, smoking and alcohol abuse. Blood laboratory examinations assessing liver function and nutritional status, such as total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), the international normalized ratio (INR), albumin and prealbumin, were performed within a week before the operation. The MELD score was calculated as follows: 3.78 × ln (total bilirubin, mg/dl) + 11.2 × ln (INR) + 9.57 × ln (creatinine, mg/dl) + 6.43.
The prealbumin level was based on the last measurement of the plasma concentrations of prealbumin before surgery. The cut-off value of preoperative prealbumin calculated by ROC curve divided patients into subgroups. Prealbumin was mainly synthesized by liver, having a role of transporting retinol and T4 thyroid hormone. As a negative acute-phase protein, serum level of prealbumin could be decreased in acute infections and inflammatory changes.
Postoperative assessment
Graft performance was evaluated routinely through daily laboratory examinations after transplantation until hospital discharge. Surgical complications were determined based on clinical symptoms and diagnostic examinations. The postoperative complications of all patients were documented in medical records and research software.
The Clavien-Dindo (CD) classification was used to estimate postoperative complications [17]. We defined CD grade II or greater morbidity as a postoperative complication in this study [18, 19]. The present study recorded all postoperative adverse reactions of the patients and finally documented pneumonia, renal failure, infection, biliary leakage, bleeding, pleural effusion, ascites and liver failure as complications.
Statistical analysis
Categorical and continuous baseline characteristics and perioperative variables are reported as the quantity, percentage, or median and interquartile range (IQR), as appropriate. Moreover, continuous variables were compared between two groups by Student’s t test or Mann–Whitney rank sum U tests, while categorical variables were compared by the chi-square test or Fisher's exact test. Spearman correlation analysis was used to determine the relationship between preoperative serum albumin and prealbumin. After univariable logistic regression, only variables with P < 0.1 were submitted to multivariable analysis. A receiver operating characteristic (ROC) curve and the area under the ROC curve (AUC) were used to confirm the predictive ability of preoperative prealbumin for postoperative complications. Similar to previous studies, this research used the maximum sum of sensitivity and specificity as the best cut-off value.
All tests were 2-tailed, with a P value < 0.05 indicating significance. All statistical and graphical analyses were performed using SPSS version 25.0 (IBM SPSS Inc., Chicago, IL, USA).
Results
Relationships between preoperative prealbumin and patient characteristics
A cut-off value of 120 mg/L for preoperative prealbumin calculated by ROC analysis was the optimal criterion, with a sensitivity of 84.7% and a specificity of 54.3%, as shown in Fig. 1. For predicting postoperative complications, the AUC of preoperative prealbumin was 0.754 (95% confidence interval [CI] 0.675–0.832; P < 0.001). On the basis of a cut-off value of 120 mg/L, the patients were grouped into low and normal levels of preoperative serum prealbumin. A total of 111 (58.1%) patients with low preoperative prealbumin had a significantly higher rate of postoperative complications than 80 (41.9%) patients with normal preoperative prealbumin within 3 days before surgery.
Fig. 1.
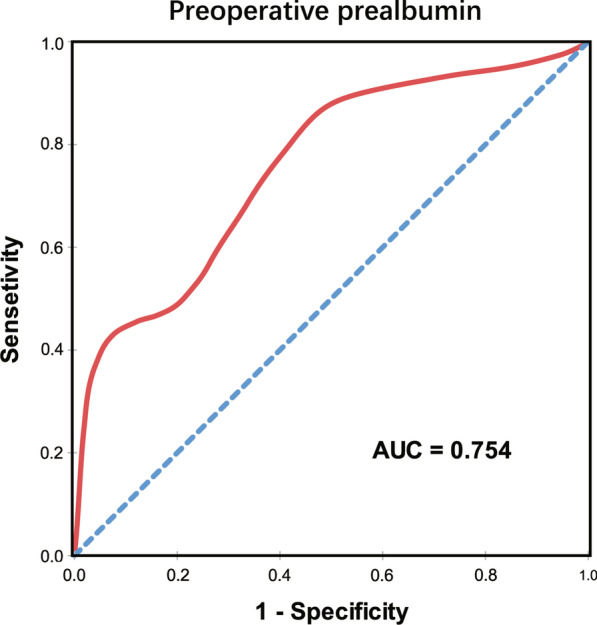
ROC curve for cut-off value of preoperative prealbumin in predicting postoperative complications. The cut-off value of preoperative serum prealbumin to predict postoperative complications was 120 mg/L, according to ROC analysis with AUC of 0.754 (95% CI 0.675–0.832; P < 0.001)
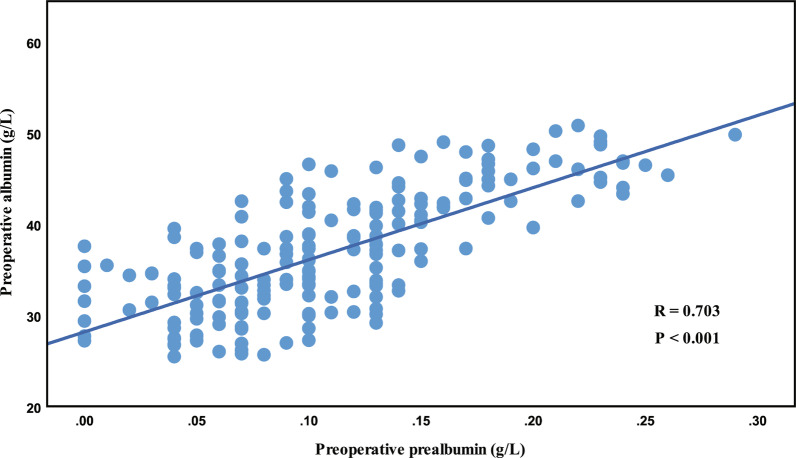
The characteristics of a total of 191 patients are shown in Table 1. Deceased donor LT was performed in all patients, and most of the patients (80.6%) had a history of HBV infection. A total of 161 (84.3%) males and 30 (15.7%) females with a median age of 49 (IQR 42–58) years and a median MELD score of 12.2 (IQR 6.2–20.0) were reviewed. As shown in Fig. 2, Spearman correlation analysis revealed a moderate relationship between preoperative albumin and prealbumin levels (R = 0.703; P < 0.001).
Table 1.
Clinical characteristics of recipients in different prealbumin levels
| N (%) | Total (N = 191) | Low prealbumin (N = 111) | Normal prealbumin (N = 80) | P value |
|---|---|---|---|---|
| Age, yearsa | 49 (42–58) | 49 (42–58) | 51 (43–58) | 0.557 |
| Smokingb | 0.300 | |||
| Yes | 92 (48.2) | 57 (51.4) | 35 (43.8) | |
| No | 99 (51.8) | 54 (48.6) | 45 (56.2) | |
| Sex | 0.301 | |||
| Male | 161 (84.3) | 91 (82.0) | 70 (87.5) | |
| Female | 30 (15.7) | 20 (18.0) | 10 (12.5) | |
| Alcohol | 0.323 | |||
| Yes | 60 (31.4) | 38 (34.2) | 22 (27.5) | |
| No | 131 (68.6) | 73 (65.8) | 58 (72.5) | |
| Hypertension | 0.651 | |||
| Yes | 17 (8.9) | 9 (8.1) | 8 (10.0) | |
| No | 174 (91.1) | 102 (91.9) | 72 (90.0) | |
| Diabetes | 0.190 | |||
| Yes | 21 (11.0) | 15 (13.5) | 6 (7.5) | |
| No | 170 (89.0) | 96 (86.5) | 74 (92.5) | |
| Previous hepatectomy | 0.001 | |||
| Yes | 26 (13.6) | 7 (6.3) | 19 (23.8) | |
| No | 165 (86.4) | 104 (93.7) | 61 (76.2) | |
| HBV infection | 0.016 | |||
| Yes | 154 (80.6) | 83 (74.8) | 71 (88.8) | |
| No | 37 (19.4) | 28 (25.2) | 9 (11.2) | |
| BMIa | 23.4 (21.0–25.6) | 23.4 (21.0–25.9) | 22.9 (21.1–25.4) | 0.217 |
| MELD score | 12.2 (6.2–20.0) | 15.2 (5.1–23.6) | 11.4 (8.2–13.6) | 0.059 |
| Preoperative albumin, g/L | 36.3 (31.6–42.1) | 33.3 (30.0–37.3) | 43.7 (40.0–46.7) | < 0.001 |
| Preoperative prealbumin, g/L | 0.10 (0.07–0.14) | 0.07 (0.05–0.10) | 0.15 (0.13–0.20) | < 0.001 |
| Preoperative ALT, IU/L | 43.2 (30.9–72.7) | 48.8 (32.2–92.4) | 35.6 (27.7–53.0) | 0.005 |
| Preoperative AST, IU/L | 53.8 (37.8–101.5) | 67.6 (47.9–127.7) | 37.9 (29.0–46.8) | < 0.001 |
| Preoperative PLT, 10^9/L | 72 (47–118) | 69 (43–110) | 76 (54–134) | 0.124 |
| Preoperative TB, umol/L | 78.0 (32.9–221.0) | 91.3 (33.0–341.0) | 68.1 (30.3–102.2) | 0.009 |
| Preoperative creatine, umol/L | 67.0 (57.0–83.0) | 65.2 (53.4–87.3) | 71.3 (58.7–82.8) | 0.134 |
| Preoperative INR | 1.23 (1.11–1.60) | 1.33 (1.17–2.16) | 1.15 (1.06–1.35) | < 0.001 |
HBV, hepatitis B virus; BMI, body mass index; MELD, Model for End-stage Liver Disease; ALT, alanine aminotransferase; AST, aspartate transaminase. PLT, platelets; TB, total bilirubin; PT, prothrombin time; INR, international normalized ratio
aValues are median (interquartile range)
bValues are count (percentage)
Fig. 2.
The scatter plot of relationship between preoperative serum albumin and prealbumin
Clinical characteristics
As shown in Table 2, clinical and pathological characteristics were included in the univariable logistic regression analysis, and preoperative MELD score, ASA score, albumin and prealbumin were found to be significantly correlated with the incidence of postoperative complications. After multivariable adjustment, MELD score, preoperative prealbumin and hypertension were shown to be independent predictive factors for postoperative complications. The odds ratios of a MELD score > 15, low levels of preoperative prealbumin and hypertension were 2.86 (95% CI 1.29–6.34), 4.40 (95% CI 1.51–12.80) and 4.09 (95% CI 1.15–14.49), respectively. However, although significant in the univariable analysis, serum albumin did not demonstrate predictive ability in the multivariable analysis.
Table 2.
Univariate and multivariate analysis for predictors of postoperative complications
| Variables | Univariable OR (95% CI) | Univariable P value | Multivariable OR (95% CI) | Multivariable P value |
|---|---|---|---|---|
| Age, years | 1.00 (0.97–1.03) | 0.943 | ||
| Male | 1.14 (0.49–2.68) | 0.764 | ||
| BMI | 1.06 (0.96–1.16) | 0.255 | ||
| Smoking | 0.62 (0.33–1.18) | 0.145 | ||
| Alcohol abuse | 1.18 (0.60–2.31) | 0.638 | ||
| Hypertension | 2.55 (0.93–7.00) | 0.070 | 4.09 (1.15–14.49) | 0.029 |
| Diabetes | 0.79 (0.28–2.29) | 0.670 | ||
| Previous hepatectomy | 0.75 (0.29–1.99) | 0.568 | ||
| HBV infection | 0.65 (0.30–1.39) | 0.266 | ||
| ASA score | 4.40 (1.12–17.22) | 0.034 | 1.95 (0.53–7.22) | 0.316 |
| Charlson score | 1.22 (0.75–2.00) | 0.421 | ||
| Preoperative MELD > 15 | 5.47 (2.76–10.81) | < 0.001 | 2.86 (1.29–6.34) | 0.010 |
| Preoperative prealbumin < 120 mg/L | 7.38 (3.12–17.49) | < 0.001 | 4.40 (1.51–12.80) | 0.007 |
| Preoperative albumin < 35 g/L | 4.21 (2.13–8.31) | < 0.001 | 1.67 (0.73–3.82) | 0.222 |
| Preoperative AST, IU/L | 1.00 (1.00–1.00) | 0.893 | ||
| Preoperative ALT, IU/L | 1.00 (1.00–1.00) | 0.220 | ||
| Preoperative A/G | 1.11 (0.61–2.04) | 0.735 | ||
| Preoperative creatinine, umol/L | 1.00 (1.00–1.01) | 0.361 | ||
| Preoperative PLT, 10^9/L | 1.00 (1.00–1.01) | 0.905 | ||
| Surgical time, hour | 1.05 (0.90–1.22) | 0.547 |
Variables were entered into multivariable logistic-regression analysis, which found significant at P < 0.1 in univariable analysis
BMI, body mass index; HBV, hepatitis B virus; ASA, American Society of Anesthesiologists; MELD, Model for End-stage Liver Disease; ALT, alanine aminotransferase; AST, aspartate transaminase; A/G, albumin to globulin ratio; OR, adds ratio; CI, confidence interval
The characteristics of the postoperative complications of the patients are also listed in Table 3, which shows that pneumonia, the number of infections, bleeding and pleural effusion were significantly different between the subgroups. Notably, all the mortality broke out in the low prealbumin group.
Table 3.
Postoperative complications between low and normal levels of preoperative prealbumin groups during hospitalization
| N (%) | Total (N = 191) | Low prealbumin (N = 111) | Normal prealbumin (N = 80) | P value |
|---|---|---|---|---|
| Morbidity | 53 (27.7) | 46 (41.4) | 7 (8.8) | < 0.001 |
| Infection | ||||
| Pneumonia | 42 (22.0) | 36 (32.4) | 6 (7.5) | < 0.001 |
| Intra-abdominal infection | 15 (7.9) | 13 (11.7) | 2 (2.5) | 0.039 |
| Effusion | ||||
| Pleural effusion | 27 (14.1) | 22 (19.8) | 5 (6.3) | 0.008 |
| Ascites | 17 (8.9) | 13 (11.7) | 4 (5.0) | 0.177 |
| Intra-abdominal bleeding | 8 (4.2) | 8 (7.2) | 0 (0) | 0.022 |
| Biliary leakage | 6 (3.1) | 5 (4.5) | 1 (1.3) | 0.394 |
| Renal failure | 9 (4.7) | 7 (6.3) | 2 (2.5) | 0.380 |
| Liver failure | 3 (1.6) | 3 (2.7) | 0 (0) | 0.266 |
| Mortality | 15 (7.9) | 15 (13.5) | 0 (0) | < 0.001 |
Clavien-Dindo grade II or greater postoperative complications were defined as morbidity
Values are count (percentage)
Stratification by MELD score and its correlation with preoperative prealbumin
To clarify whether preoperative prealbumin was meaningful in patients with different degrees of liver disease severity, patients were divided into low and high MELD subgroups by a cut-off value of 15. A total of 126 (66.0%) patients had a low MELD score, and 65 (34.0%) patients had a high MELD score. According to whether postoperative complications occurred, preoperative prealbumin levels were significantly different between the patients as shown in Fig. 3. However, the distribution of prealbumin among the patients in the high MELD subgroup seemed similar.
Fig. 3.
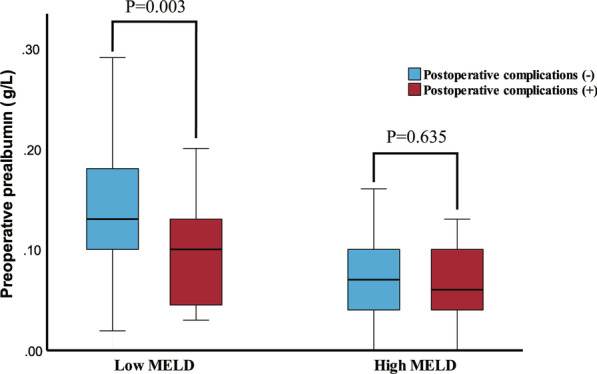
The box plot of distribution of preoperative serum prealbumin with MELD subgroups
Patients in the low MELD subgroup with low preoperative prealbumin, as shown in Table 4, had a higher prevalence of postoperative complications than those with normal preoperative prealbumin (27.5% versus 8.0%, P = 0.003). This phenomenon was not apparent in the group of patients with high MELD scores. The rate of postoperative complications among patients with low preoperative prealbumin levels was 53.3%, and although a rate of 20.0% was found among patients with normal preoperative prealbumin levels, the difference was not significant (P = 0.197).
Table 4.
Risk of postoperative complications in different MELD subgroups
| MELD ≤ 15 | N (%) | Low prealbumin | Normal prealbumin | P |
|---|---|---|---|---|
| Complications | ||||
| (+) | 14 (27.5) | 6 (8.0) | 0.003 | |
| (−) | 37 (72.5) | 69 (92.0) |
| MELD > 15 | N (%) | Low prealbumin | Normal prealbumin | P |
|---|---|---|---|---|
| Complications | ||||
| (+) | 32 (53.3) | 1 (20.0) | 0.197 | |
| (−) | 28 (46.7) | 4 (80.0) |
Discussion
As a widely used indicator, prealbumin can sensitively reflect nutritional status and hepatic synthesis. Once a patient develops an insufficient nutrient reserve or inadequate hepatic synthesis, serum prealbumin decreases obviously. In this state, patients are unable to tolerate surgery and are prone to experience postoperative complications. Therefore, prealbumin might be associated with the risk of postoperative complications. The capacity of preoperative serum prealbumin levels to predict the risk of postoperative complications was demonstrated among patients undergoing LT in this retrospective study. Compared to patients with normal preoperative prealbumin levels, patients with low preoperative prealbumin levels had a higher prevalence of postoperative complications. These results suggested that patients’ nutritional status warrants greater consideration before LT to facilitate an excellent postoperative recovery.
In this study, preoperative serum albumin was not an independent predictor, while preoperative prealbumin showed a strong ability to predict the risk of postoperative complications. Although preoperative albumin is a widely used indicator to evaluate nutritional status and liver function [20, 21], a moderate positive correlation with preoperative prealbumin was also exhibited in scatter plots based on Spearman correlation analysis (R = 0.703, P < 0.001). A remarkable finding was that patients frequently received peripheral supplemental infusion of albumin solution due to continuous deterioration of nutritional status and ascites. The level of serum albumin fluctuated with peripheral supplementation, reducing its ability to predict postoperative complications [4]. Although albumin is more widely used in clinical practice, albumin was primarily associated with colloid osmotic pressure and ascites rather than rigorous nutritional support. In addition, a long half-life (17–21 days) led to an inability of serum albumin to respond sensitively to liver damage. In contrast, serum prealbumin was less affected by peripheral supplementation due to its short half-life (2–3 days) and rapid rate of synthesis in response to nutrition supplementation, rendering this parameter a better indicator to reflect the risk of complications [22]. Preoperative prealbumin was shown to be more specific and sensitive in response to nutritional reserve not only in the context of LT but also in hepatectomy, pancreaticoduodenectomy, hemodialysis and kidney transplantation [11, 12, 14].
However, no generally accepted cut-off values are available for serum prealbumin owing to a lack of use and specificity [23]. These results might lead to the inference that the predictive capacity of preoperative prealbumin must be considered separately in different diseases. In this study, the cut-off value of 120 mg/L for preoperative prealbumin was defined by ROC analysis as shown in Fig. 1. The AUC of preoperative prealbumin was 0.754 (95% confidence interval [CI] 0.675–0.832; P < 0.001) for calculating the prevalence of postoperative complications. Moreover, low prealbumin was suggested to be an independent predictor for post-LT complications based on multivariable logistic regression analysis (95% CI 1.51–12.8, P = 0.007). Therefore, as an indicator of nutritional status and hepatic synthesis, preoperative prealbumin before LT warrants sufficient attention.
Notably, patients with liver diseases frequently suffer from malnutrition because of weakened intestinal peristalsis and anorexia, especially in end-stage liver disease. However, many clinicians prescribe a low-protein diet to prevent hepatic encephalopathy [24]. Acute esophageal variceal bleeding with prolonged fasting is also common in clinical practice. In addition, due to impaired digestive and absorptive abilities, patients are unable to tolerate multiple hospitalizations, examinations and surgical procedures. Malnutrition is closely associated with complications and is considered an independent risk factor for surgical outcomes [25] via numerous mechanisms including impaired fibroblast proliferation and collagen synthesis [26]. In addition, reduced tensile strength and angiogenesis also prolong the inflammatory phase of wound healing. These mechanisms result in delayed healing of the surgical site, intra-abdominal bleeding and a tendency for biliary leakage. Prealbumin (also called transthyretin) has thymus hormone activity and enhances immunity by promoting lymphocyte maturation. In addition to malnutrition, low prealbumin also increases the risks of intra-abdominal infection and pneumonia by impacting lymphocyte function and phagocytic activity [27]. An extremely inadequate nutritional reserve results in a decreased capacity for cell regeneration and protein synthesis. After surgery, patients are prone to developing intra-abdominal bleeding, pleural effusion, bile leakage and infection. Furthermore, the organ burden is increased, resulting in multiple organ dysfunction, such as acute kidney injury and hepatic encephalopathy [28]. Thus, these recipients suffered from high risk of graft dysfunction and even mortality.
The MELD score plays a vital role in calculating the risk of postoperative complications and the hospital length of stay. A number of studies performing subgroup analyses stratified by MELD scores achieved relatively accurate predictions [16, 29]. Extensive literature advocates pre-LT nutritional support for these patients. However, our study suggested that a low prealbumin level was associated with a high risk of complications in the low MELD subgroup. Most patients with higher MELD scores had low prealbumin levels, as shown in Fig. 3. Because of a deteriorated capacity for hepatic protein synthesis, these patients did not have sufficient metabolic activity, thereby decreasing the accuracy of prealbumin for predicting complications. For patients with high MELD scores, malnutrition was only a part of their poor general condition, and transplantation was urgently needed. Nutritional support may not be impactful, and the preoperative delay may not be long enough. In contrast, patients with low MELD scores (< 15) did not need surgery as emergently, and low prealbumin was correlated with postoperative complications (P = 0.003). For these patients, nutritional support was vital, and preoperative preparation might be sufficient.
Due to intractable symptoms and multiple organ dysfunction, devoting adequate attention to nutritional assessment and management in patients with high MELD scores is difficult for clinicians. Patients with MELD scores ≥ 15 have been reported to have an overall pretransplant mortality rate of 20 per 100 waitlist-years [3]. Moreover, patients with high MELD scores seem to have impairment of multiple organs, such as the liver, heart and kidneys [30–32]. As a result, numerous factors may increase the prevalence of postoperative complications in these patients. In patients with MELD scores ≥ 20, cardiac insufficiency had a significant influence on the prognosis of transplantation, which was not obvious in patients with MELD scores < 20 [33]. In patients with high MELD scores, other organs may have a greater influence on surgical outcomes, thus masking the role of nutritional status. Therefore, the main objective for patients with high MELD scores is not to improve nutritional status but to undergo transplantation as soon as possible.
However, nutritional supplements cannot be regarded as meaningless. In patients with low MELD scores, pretransplant mortality was lower than 10 per 100 waitlist-years. These patients had an opportunity to receive individualized nutritional management. Previous studies have demonstrated that nutritional support significantly improved serum albumin levels and decreased MELD scores, particularly in Child–Pugh B patients compared to patients with Child–Pugh A cirrhosis [34]. Additionally, with adequate nutritional support, patients’ prealbumin levels reportedly increased by 1–2 mg/dL per day [35]. The cause of low prealbumin levels was not only impaired liver function but also inadequate nutrient intake. Therefore, enteral or parenteral nutrition support was necessary if conditions permitted. Morbidity and the length of hospital stay after surgery were significantly decreased in patients receiving preoperative nutritional support compared to the same parameters in a control group that did not receive nutritional support [5]. Prealbumin was still a prognostic indicator of transplantation. This study showed that patients with normal prealbumin levels were less likely to suffer from complications after surgery, suggesting that patients should be regularly tested for prealbumin while awaiting transplantation. Once patients show low prealbumin levels, especially patients with low MELD scores, which indicate that patients may have more time to wait for a liver, surgeons can initiate measures to improve nutritional status and thus attenuate post-LT complications.
To precisely determine intraoperative risks and postoperative complication rates, preoperative assessment seems especially crucial. In addition to preoperative prealbumin, hypertension was also identified as an independent predictor of morbidity in the present study. Hypertension prior to LT increased the risk of complications, as mentioned in the European Association for the Study of the Liver (EASL) guidelines [36]. Previous studies demonstrated that postoperative complications were related to preoperative clinical markers, including albumin, total bilirubin and ALBI scores [37, 38]. The ALBI score was found to play an important role in evaluating the functional performance of the liver [39, 40]. A poor grade (ALBI grade 3) was significantly associated with postoperative complications and mortality during hospitalization [38]. It is also unclear whether prealbumin could replace albumin and increase the accuracy of these predictors.
Some limitations could not be avoided in our study. First, this was a retrospective study, and excluding all selection biases was difficult. Second, a single center may be subject to institutional problems, which impacts the external effectiveness of research. Most patients had a history of HBV infection. Whether the results of this study can be applied to patients with HCV infection is still questionable. Third, apart from liver cirrhosis, serum prealbumin can be influenced by acute inflammation, hyperthyroidism and nephritic syndrome [7], which affects the accuracy of predictions.
Conclusion
The present study demonstrated an association between preoperative serum prealbumin and the risk of postoperative complications with a cut-off value calculated from a ROC curve. A more detailed investigation was then conducted among subgroups of patients with different MELD scores. The results suggested that patients with low prealbumin had a higher risk of postoperative complications. Unfortunately, the present study was unable to determine an association between surgical outcomes and dynamic changes in prealbumin. Further studies are needed to determine whether dynamic changes in preoperative prealbumin can serve as an indicator of nutritional support or as a prognostic factor for surgery. In addition, whether prealbumin can replace albumin as a more accurate variable in the context of LT must be confirmed.
Acknowledgements
Not applicable.
Authors' contributions
Conception: YL, XL, ZX, CZ, LZ; Study design: YL, XL, YJ, WL, FH, CZ, LZ; Administrative support: CZ, LZ, PB; Data collection and acquisition: YL, XL, YJ, WL, JB, YO, YY, FH; Data analysis: YL, CZ; Manuscript preparation: All authors; Critical revision: XL, YJ, ZX, LZ, PB. All authors read and approved the final manuscript.
Funding
This work was supported by Clinical Innovation Program of Southwest Hospital (SWH2018QNLC-05), Talent Training Plan of Army Medical University (XZ-2019–505-070), the Chongqing Technology Innovation and Application Demonstration Project (cstc2018jscx-mszdX0016), New technique of laparoscope hepatobiliary surgery (2010GXJ567), and Chinese National Key Projects (201502014).
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request. Since the publication date, all the researchers in hospital or university could send email to corresponding author: zldxngd@163.com. Due to the perioperative data may be updated over time, only three years within the publication date is available.
Declarations
Ethics approval and consent to participate
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent by verbal was obtained from all participants approved by the ethics board of Southwest Hospital. The patients consented that their clinical data was enrolled in the research and their privacy information would not be disclosed. The donation and distribution of organ were registered in the CLTR (http://cltr.cotr.cn), and no organs from executed prisoners were used for LT.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuancheng Li, Xingchao Liu contributed equally to this work
Contributor Information
Chengcheng Zhang, Email: zccszcg@163.com.
Leida Zhang, Email: zldxngd@163.com.
References
- 1.Tsochatzis E, Coilly A, Nadalin S, Levistky J, Tokat Y, Ghobrial M, et al. International liver transplantation consensus statement on end-stage liver disease due to nonalcoholic steatohepatitis and liver transplantation. Transplantation. 2019;103(1):45–56. doi: 10.1097/TP.0000000000002433. [DOI] [PubMed] [Google Scholar]
- 2.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 3.Kwong A, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, et al. OPTN/SRTR 2018 annual data report: liver. Am J Transplant. 2020;20(Suppl s1):193–299. doi: 10.1111/ajt.15674. [DOI] [PubMed] [Google Scholar]
- 4.Hammad A, Kaido T, Aliyev V, Mandato C, Uemoto S. Nutritional therapy in liver transplantation. Nutrients. 2017;9(10):1126. doi: 10.3390/nu9101126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jie B, Jiang ZM, Nolan MT, Zhu SN, Yu K, Kondrup J. Impact of preoperative nutritional support on clinical outcome in abdominal surgical patients at nutritional risk. Nutrition. 2012;28:1022–1027. doi: 10.1016/j.nut.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Lin MY, Liu WY, Tolan AM, Aboulian A, Petrie BA, Stabile BE. Preoperative serum albumin but not prealbumin is an excellent predictor of postoperative complications and mortality in patients with gastrointestinal cancer. Am Surg. 2011;77:1286–1289. doi: 10.1177/000313481107701002. [DOI] [PubMed] [Google Scholar]
- 7.Delliere S, Cynober L. Is transthyretin a good marker of nutritional status? Clin Nutr. 2017;36(2):364–370. doi: 10.1016/j.clnu.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Mullen JL, Buzby GP, Waldman MT, Gertner MH, Hobbs CL, Rosato EF. Prediction of operative morbidity and mortality by preoperative nutritional assessment. Surg Forum. 1979;30:80–2. [PubMed]
- 9.Loftus TJ, Brown MP, Slish JH, Rosenthal MD. Serum levels of prealbumin and albumin for preoperative risk stratification. Nutr Clin Pract. 2019;34:340–348. doi: 10.1002/ncp.10271. [DOI] [PubMed] [Google Scholar]
- 10.Beck FK, Rosenthal TC. Prealbumin: a marker for nutritional evaluation. Am Fam Phys. 2002;65:1575. [PubMed] [Google Scholar]
- 11.Shen Z, Zhang J, Zhao S, Zhou Y, Wang W, Shen B. Preoperative biliary drainage of severely obstructive jaundiced patients decreases overall postoperative complications after pancreaticoduodenectomy: a retrospective and propensity score-matched analysis. Pancreatology. 2020;20:529–536. doi: 10.1016/j.pan.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Huang L, Li J, Yan JJ, Liu CF, Wu MC, Yan YQ. Prealbumin is predictive for postoperative liver insufficiency in patients undergoing liver resection. World J Gastroenterol. 2012;18(47):7021–7025. doi: 10.3748/wjg.v18.i47.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li JD, Xu XF, Han J, Wu H, Xing H, Li C, et al. Preoperative prealbumin level as an independent predictor of long-term prognosis after liver resection for hepatocellular carcinoma: a multi-institutional study. HPB (Oxf.) 2019;21(2):157–166. doi: 10.1016/j.hpb.2018.06.1803. [DOI] [PubMed] [Google Scholar]
- 14.Chrysostomou S, Stathakis C, Petrikkos G, Daikos G, Gompou A, Perrea D. Assessment of prealbumin in hemodialysis and renal-transplant patients. J Ren Nutr. 2010;20(1):44–51. doi: 10.1053/j.jrn.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Roche M, Law TY, Kurowicki J, Sodhi N, Rosas S, Elson L, et al. Albumin, prealbumin, and transferrin may be predictive of wound complications following total knee arthroplasty. J Knee Surg. 2018;31(10):946–951. doi: 10.1055/s-0038-1672122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu F, Cai LY, Zhong L, Chen C, Xu F, Zhao ZX, et al. Model for end-stage liver disease combined with serum prealbumin to predict the prognosis of patients with decompensated liver cirrhosis. J Dig Dis. 2010;11(6):352–357. doi: 10.1111/j.1751-2980.2010.00465.x. [DOI] [PubMed] [Google Scholar]
- 17.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann K, Hinz U, Stravodimos C, Knoblich T, Schon MR, Buchler MW, et al. Risk assessment for liver resection. Surgery. 2018;164(5):998–1005. doi: 10.1016/j.surg.2018.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T, Iida T, Ushigome H, Osaka M, Masuda K, Matsuyama T, et al. Risk factors and management for biliary complications following adult living-donor liver transplantation. Ann Transplant. 2017;22:671–676. doi: 10.12659/AOT.905485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiroi K, Matsusaki T, Kaku R, Umeda Y, Yagi T, Morimatsu H. Postoperative course of serum albumin levels and organ dysfunction after liver transplantation. Transplant Proc. 2019;51(8):2750–2754. doi: 10.1016/j.transproceed.2019.01.199. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S, Ma J, An R, Liu L, Li J, Fang Z, et al. Effect of cumulative fluid balance on acute kidney injury and patient outcomes after orthotopic liver transplantation: a retrospective cohort study. Nephrology (Carlton) 2020;25:700–707. doi: 10.1111/nep.13702. [DOI] [PubMed] [Google Scholar]
- 22.Devoto G, Gallo F, Marchello C, Racchi O, Garbarini R, Bonassi S, et al. Prealbumin serum concentrations as a useful tool in the assessment of malnutrition in hospitalized patients. Clin Chem. 2006;52(12):2281–2285. doi: 10.1373/clinchem.2006.080366. [DOI] [PubMed] [Google Scholar]
- 23.Delliere S, Neveux N, De Bandt JP, Cynober L. Transthyretin for the routine assessment of malnutrition: A clinical dilemma highlighted by an international survey of experts in the field. Clin Nutr. 2018;37(6 Pt A):2226–2229. doi: 10.1016/j.clnu.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 24.Luo L, Fu S, Zhang Y, Wang J. Early diet intervention to reduce the incidence of hepatic encephalopathy in cirrhosis patients: post-transjugular intrahepatic portosystemic shunt (TIPS) findings. Asia Pac J Clin Nutr. 2016;25:497. doi: 10.6133/apjcn.092015.14. [DOI] [PubMed] [Google Scholar]
- 25.Gunsar F, Raimondo ML, Jones S, Terreni N, Wong C, Patch D, Sabin C, et al. Nutritional status and prognosis in cirrhotic patients. Aliment Pharmacol Ther. 2006;24:563–572. doi: 10.1111/j.1365-2036.2006.03003.x. [DOI] [PubMed] [Google Scholar]
- 26.Stechmiller JK. Understanding the role of nutrition and wound healing. Nutr Clin Pract. 2010;25(1):61–68. doi: 10.1177/0884533609358997. [DOI] [PubMed] [Google Scholar]
- 27.Campos AC, Groth AK, Branco AB. Assessment and nutritional aspects of wound healing. Curr Opin Clin Nutr Metab Care. 2008;11(3):281–288. doi: 10.1097/MCO.0b013e3282fbd35a. [DOI] [PubMed] [Google Scholar]
- 28.Gines P, Sola E, Angeli P, Wong F, Nadim MK, Kamath PS. Hepatorenal syndrome. Nat Rev Dis Primers. 2018;4(1):23. doi: 10.1038/s41572-018-0022-7. [DOI] [PubMed] [Google Scholar]
- 29.Chok KSH, Chan SC, Fung JYY, Cheung TT, Chan ACY, Fan ST, et al. Survival outcomes of right-lobe living donor liver transplantation for patients with high model for end-stage liver disease scores. Hepatob Pancreat Dis Int. 2013;12(3):256–262. doi: 10.1016/S1499-3872(13)60042-9. [DOI] [PubMed] [Google Scholar]
- 30.Zardi EM, Zardi DM, Chin D, Sonnino C, Dobrina A, Abbate A. Cirrhotic cardiomyopathy in the pre- and post-liver transplantation phase. J Cardiol. 2016;67(2):125–130. doi: 10.1016/j.jjcc.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Jayakumar S, Traboulsi M, Lee SS. Cirrhotic cardiomyopathy: implications for liver transplantation. Liver Transpl. 2017;23(6):826–835. doi: 10.1002/lt.24768. [DOI] [PubMed] [Google Scholar]
- 32.Velez JCQ, Therapondos G, Juncos LA. Reappraising the spectrum of AKI and hepatorenal syndrome in patients with cirrhosis. Nat Rev Nephrol. 2020;16(3):137–155. doi: 10.1038/s41581-019-0218-4. [DOI] [PubMed] [Google Scholar]
- 33.Kwon HM, Moon YJ, Jung KW, Park YS, Kim KS, Jun IG, et al. Appraisal of cardiac ejection fraction with liver disease severity: implication in post-liver transplantation mortality. Hepatology. 2019;71:1364–1380. doi: 10.1002/hep.30913. [DOI] [PubMed] [Google Scholar]
- 34.Dag Z, Koseoglu H, Kekilli M. The use of prealbumin as a predictor of malnutrition in cirrhotic patients and the effect of nutritional support in patients with low prealbumin levels. Turk J Med Sci. 2020;50:398–404. doi: 10.3906/sag-1910-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernstein LH, Leukhardt-Fairfield CJ, Pleban W, Rudolph R. Usefulness of data on albumin and prealbumin concentrations in determining effectiveness of nutritional support. Clin Chem. 1989;35:271–274. doi: 10.1093/clinchem/35.2.271. [DOI] [PubMed] [Google Scholar]
- 36.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70(1):172–93. [DOI] [PMC free article] [PubMed]
- 37.Spiekerman AM. Nutritional assessment (protein nutriture) Anal Chem. 1995;67(12):429R–R436. doi: 10.1021/ac00108a026. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, Liu C, Tan Y, Tan L, Jiang L, Yang J, et al. Albumin-bilirubin score for predicting post-transplant complications following adult-to-adult living donor liver transplantation. Ann Transplant. 2018;23:639–646. doi: 10.12659/AOT.910824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernardi N, Chedid MF, Grezzana-Filho TJM, Chedid AD, Pinto MA, Leipnitz I, et al. Pre-transplant ALBI Grade 3 is associated with increased mortality after liver transplantation. Dig Dis Sci. 2019;64(6):1695–1704. doi: 10.1007/s10620-019-5456-6. [DOI] [PubMed] [Google Scholar]
- 40.Kornberg A, Witt U, Schernhammer M, Kornberg J, Muller K, Friess H, et al. The role of preoperative albumin-bilirubin grade for oncological risk stratification in liver transplant patients with hepatocellular carcinoma. J Surg Oncol. 2019;120(7):1126–1136. doi: 10.1002/jso.25721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request. Since the publication date, all the researchers in hospital or university could send email to corresponding author: zldxngd@163.com. Due to the perioperative data may be updated over time, only three years within the publication date is available.