Abstract
Background:
The extent to which couples change their behaviors with increasing pregnancy attempt time is not well documented.
Methods:
We examined change in selected behaviors over pregnancy attempt time in a North American preconception cohort study. Eligible females were ages 21–45 years and not using fertility treatment. Participants completed baseline and bimonthly follow-up questionnaires for up to 12 months or until pregnancy.
Results:
Among 3,339 females attempting pregnancy for 0–1 cycles at enrollment, 250 contributed 12 months of follow-up without conceiving. Comparing behaviors at 12 months versus baseline, weighted for loss-to-follow-up, we observed small-to-moderate reductions in mean caffeine intake (−19.5 mg/day, CI = −32.7, −6.37), alcohol intake (−0.85 drinks/week, CI = −1.28, −0.43), marijuana use (−3.89 percentage points, CI = −7.33, 0.46), and vigorous exercise (−0.68 hours/week, CI = −1.05, −0.31), and a large increase in activities to improve conception chances (e.g., ovulation testing) (21.7 percentage points, CI = 14.8, 28.6). There was little change in mean cigarette smoking (−0.27 percentage points, CI = −1.58, 1.04), perceived stress scale score (−0.04 units, CI = −0.77, 0.69), or other factors (e.g., sugar-sweetened soda intake, moderate exercise, intercourse frequency, and multivitamin use), but some heterogeneity within subgroups (e.g., 31% increased and 32% decreased their perceived stress scores by ≥2 units; 14% reduced their smoking but none increased their smoking by ≥5 cigarettes/day).
Conclusions:
Although many behaviors changed with increasing pregnancy attempt time, mean changes tended to be modest for most variables. The largest differences were observed for the use of caffeine, alcohol, and marijuana, and methods to improve conception chances.
Keywords: Bias, Cohort studies, Females, Fertility, Pregnancy, Prospective
About 15% of American couples experience infertility, defined as the inability to conceive after 12 months of unprotected intercourse.1 Few modifiable risk factors for infertility have been identified.2 In addition to age,3–5 behavioral factors have been associated with infertility, including smoking,6,7 obesity,8–10 nutrition,11 and exercise extremes.12,13 In September 2015, the Eunice Kennedy Shriver National Institute of Child Health and Human Development convened a workshop of scientists to discuss the available weight of evidence on fertility trends, examine research barriers, and identify ways forward for human fecundity research.2 Experts agreed that well-designed prospective studies of time-to-pregnancy are essential to advance the field.
For this purpose, a prospective study design with enrollment of couples upon discontinuation of contraception is ideal,14 but there are challenges to this approach. First, motivating pregnancy planners to enroll in a research study when they are not experiencing fertility problems can be difficult. Second, pregnancy planners do not typically publicize their intentions and are difficult to identify and enroll in studies using conventional methods. Third, prospective studies are costly because of the time and expense in maximizing follow-up. Given these challenges, the few existing preconception cohort studies have been small15–17 and have limited ability to examine rare exposures. Further, most fertility studies have been restricted to infertility clinic populations or have been retrospective and confined to already-pregnant18,19 or postpartum females.20
Prospective cohort studies of fecundity that are confined to already-infertile couples may be less generalizable because they do not include couples in the full range of the fertility spectrum.21 Moreover, measures of association from such studies may be prone to selection bias, which can occur when selection of participants (or retention of participants after enrollment) is related to both exposure and outcome.22 Finally, enrolled cohorts include individuals who at the time of enrollment have already progressed toward the outcome, and knowledge about infertility can both influence risk-factor behavior (reverse causation) and reporting of exposure status (differential exposure misclassification). The degree to which the latter biases affect measures of association in etiologic studies of fecundity has not been comprehensively evaluated. An important step in this process is to quantify the change in behaviors over pregnancy attempt time.
The New York State Prospective Pregnancy Study, a preconception cohort study, is the sole study to examine changes in smoking, caffeine, and alcohol consumption over pregnancy attempt time.23 Among 90 women attempting pregnancy immediately on discontinuing contraception, over the maximum follow-up of 12 menstrual cycles, moderately large mean reductions were observed for daily caffeinated drinks but little change was observed for alcohol or cigarette use.23
We evaluated the extent to which individual behaviors change over pregnancy attempt time in a North American preconception cohort study of pregnancy planners. Our primary goal was to describe the change in characteristics across a 12-month period among females who enrolled immediately after discontinuing contraception and who failed to conceive during 12 months of attempt time, the time at which infertility treatment is usually considered. The trajectory across 12 months would depict the behavior change among females initiating pregnancy attempts until they present to an infertility clinic 1 year later. Our secondary goal was to provide data on behavior change among the larger subset of participants completing at least one follow-up questionnaire, according to varying lengths of attempt time. Our choice of examined behaviors was informed by their prevalence in reproductive-aged females and evidence, mainly from epidemiologic studies, of their effects on fecundability.
METHODS
Study Population
Pregnancy Study Online (PRESTO) is an ongoing web-based preconception cohort study of pregnancy planners.24 Eligible females were ages 21–45 years, residing in the United States or Canada, and not using contraception or fertility treatment at enrollment. Participants completed an online baseline questionnaire containing questions on demographics, behaviors, medical history, and medication use. Participants completed follow-up questionnaires every 2 months for up to 12 months to ascertain pregnancy status and update time-varying factors. After completing the baseline questionnaire, participants were randomly assigned with 50% probability to receive a complimentary premium subscription to FertilityFriend.com, a menstrual and fertility charting application. Although participants received e-mail tutorials from FertilityFriend.com on monitoring their fertility signs, they were not provided additional incentives to use the application. The study protocol was approved by the Institutional Review Board at Boston University Medical Campus. We obtained online informed consent from all participants.
From June 2013 to September 2018, 8,773 females completed the baseline questionnaire. We excluded 102 females whose baseline date of last menstrual period (LMP) was >6 months before study entry, and 35 females with missing or implausible LMP data. We then excluded 5,297 females who had been trying to conceive for ≥2 cycles when they enrolled, so that we could study behavioral changes from the time that pregnancy attempts began. The final study population comprised 3,339 females, of whom 1,712 completed at least one questionnaire and 250 completed all six follow-up questionnaires without reporting a pregnancy.
Assessment of Behaviors
On the baseline and follow-up questionnaires, participants reported information about their current use of multivitamins or prenatal vitamins, current cigarette smoking, marijuana use in the last 2 months, alcohol consumption in the past month, caffeine intake in the past month, sugar-sweetened soda intake in the past month, vigorous, and moderate exercise in the past week,9 the 10-item version of the perceived stress scale (PSS-10) in the past month,25 intercourse frequency in the past month, and whether they were currently doing anything to improve their chances of conception (e.g., basal body temperature, cervical mucus monitoring).
Data Analysis
All participants had the opportunity to contribute the full 12 months of follow-up to the analysis. The sample contributing information for each time interval decreased with increasing follow-up (pregnancy attempt time) as participants conceived or were censored for various reasons. With contact every 2 months spanning up to 1 year, participants contributed up to seven observations to the analysis from enrollment (baseline) until pregnancy (53%) or a censoring event, including initiation of fertility treatment (5%), cessation of pregnancy attempts (5%), loss to follow-up (30%: 161 females who stopped completing questionnaires after miscarriage, 479 who stopped completing questionnaires but provided data on pregnancy status via the withdrawal form, and 349 who stopped completing questionnaires without reaching a study endpoint), or 12 months of follow-up (7%), whichever came first. The proportions who conceived, were lost to follow-up, or were still participating in the study during follow-up are shown in Figure 1. We used life-table methods to calculate the percentage of women who conceived within 12 months of follow-up.
FIGURE 1.
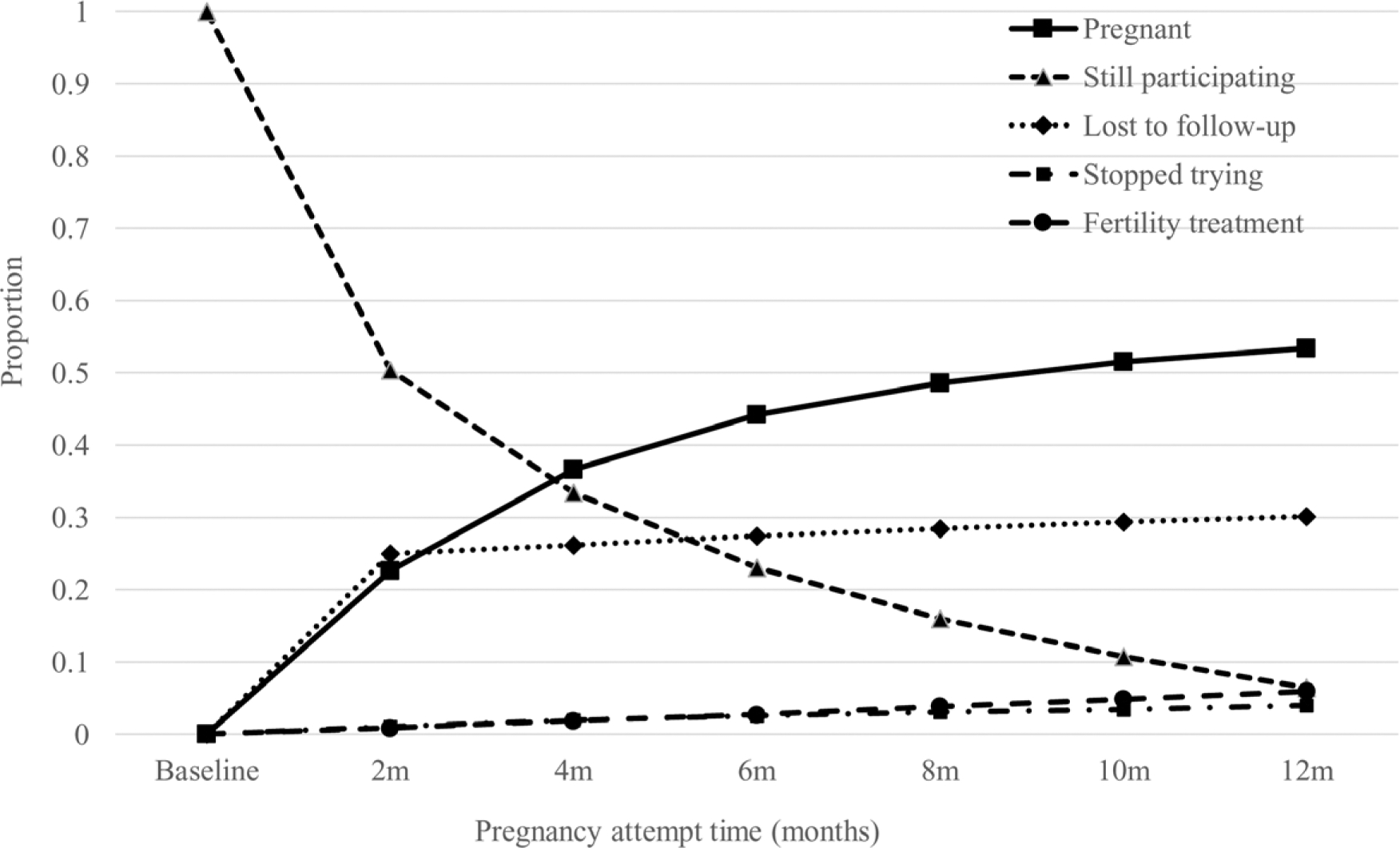
Proportion of women who conceived, were lost to follow-up, or were still participating at each follow-up interval.
We presented data on selected behaviors for the 1,712 women who completed at least one follow-up questionnaire, including the subset of 250 women who completed all follow-up questionnaires without conceiving. For continuous variables, we presented the proportion of women with meaningful change in behaviors between baseline and each follow-up interval (2 months to 12 months), and the mean change comparing the end with the beginning of each time interval. We defined meaningful change a priori as follows: caffeine = 50 mg/day; sugar-sweetened sodas = 3.5 drinks/week; alcohol = 3.5 drinks/week; smoking = 5 cigarettes/day; vigorous exercise = 2 hours/week; moderate exercise = 2 hours/week; PSS-10 score = 2 units; and intercourse frequency = 1 coital act/week. For binary variables, we presented the percentage that changed categories and the mean unit change in the prevalence of that variable, comparing the end with the beginning of each time interval (2 months to 12 months).
We stratified by age at baseline (<30 vs. ≥30 years) and gravidity (nulligravid vs. gravid) to assess whether the magnitude of behavior change differed across strata of other covariates. Older women might be more likely to have concerns about their narrowing window of childbearing and may therefore change their behaviors faster over time. Conversely, gravid women might be less likely to change their behaviors over time, given their history of proven fertility. Because some states in the United States have insurance mandates that cover infertility treatment to women ages ≥35 years after 6 months of attempt time, instead of the usual 12 months (e.g., Massachusetts), we performed secondary analyses stratifying by age at baseline (<35 vs. ≥35 years) and gravidity (nulligravid vs. gravid), and truncating follow-up time at 6 months instead of 12 months.
Because our population of primary interest was the subset of 250 women who attempted pregnancy for 12 months without conceiving during that time, we also plotted the group trajectories in selected behaviors among these women. At each 2-month interval, we plotted the mean (or percentages) of selected characteristics to assess the magnitude, direction, and timing of any behavior change.
We repeated all analyses after adjusting for bias introduced by loss to follow-up. To account for such bias, we used inverse probability weighting to create a pseudopopulation of women who, had they not been lost to follow-up, would have contributed 12 months of follow-up to the cohort. Using data from all 3,339 women, we developed logistic regression models for the probability of continuing in the study at each follow-up cycle, conditional on remaining uncensored at the previous follow-up. The model contained a set of variables, some of which were time-varying (i.e., smoking, multivitamin intake, intercourse frequency, doing something to improve chances of conception, intake of alcohol and sugar-sweetened soda, perceived stress scale score, exercise, and marijuana use), hypothesized to predict loss to follow-up (eTable 1; http://links.lww.com/EDE/B678). We fit separate logistic regression models that included only time-invariant variables as independent variables. We computed stabilized weights by dividing the predicted probability of loss to follow-up from the second model (containing time-invariant variables only) by the predicted probability of loss to follow-up from the first model (time-varying and time-invariant variables) and multiplying by stabilized weights from previous cycles. The resulting weights were inversely proportional to the probability of remaining under study at each cycle. We then applied these weights to our analyses of behavior change at each time point (i.e., follow-up cycle).
If women skipped one or more questionnaires between completed questionnaires, we assigned the value from their previous questionnaire (binary variables) or took the midpoint of values from their previous and subsequent questionnaires (continuous variables). Among those who completed at least one follow-up questionnaire, percentages of women who skipped the first, second, third, fourth, or fifth follow-up questionnaires were 4.4%, 2.8%, 1.6%, 1.2%, and 1.2%, respectively. Only 0.7% of women skipped ≥2 questionnaires. Missingness on individual questionnaires ranged from <0.1% (intercourse frequency, marijuana use, alcohol, and sugar-sweetened soda at all follow-ups) to 6.4% (vigorous exercise on follow-up 6). Analyses were performed using SAS statistical software version 9.4.26
RESULTS
At baseline, the mean age of participants was 29.8 years; 78.5% were college-educated and 85.7% self-identified as nonHispanic White (Table 1). Mean body mass index was 27.1 kg/m2 and 4.4% were current regular smokers. Participants reported on average 128.5 mg/day of caffeine intake, 1.2 sugar-sweetened sodas/week, and 3.4 alcoholic drinks/week. Mean hours/week of vigorous and moderate exercise were 2.9 and 6.1, respectively. More than 12% reported current marijuana use. Nearly 82% reported daily use of multivitamins or folic acid.
TABLE 1.
Baseline Characteristics of Participants with 0–1 Cycles of Attempt Time at Study Entry, PRESTO, 2013–2018
| Characteristic | Totala | Contributed ≥1 Interval of Follow-upb | Contributed 12 Months of Data Without Conception |
|---|---|---|---|
| Number of participants | 3,339 | 1,712 | 250 |
| Age (years, mean) | 30 | 30 | 31 |
| Education (%) | |||
| High school or less | 3 | 2 | 2 |
| Some college | 18 | 16 | 17 |
| College degree | 34 | 34 | 36 |
| Graduate school | 44 | 48 | 45 |
| Annual household income (%) | |||
| <$50,000 | 15 | 14 | 16 |
| $50,000–$99,999 | 38 | 37 | 39 |
| $100,000-$ 149,999 | 26 | 26 | 23 |
| ≥$150,000 | 18 | 20 | 21 |
| White, nonHispanic (%) | 86 | 87 | 87 |
| Body mass index (kg/m2, mean) | 27 | 27 | 27 |
| Caffeine intake (mg/day, mean) | 129 | 127 | 137 |
| Sugar-sweetened soda intake (drinks/week, mean) | 1.2 | 1.1 | 1.1 |
| Alcohol intake (drinks/week, mean) | 3.4 | 3.3 | 3.4 |
| Cigarette smoking history (%) | |||
| Never | 76 | 77 | 80 |
| Former | 17 | 16 | 14 |
| Current occasional | 3 | 3 | 2 |
| Current regular | 4 | 4 | 4 |
| Number of cigarettes/dayc, mean | 5.7 | 5.3 | 6.2 |
| Current marijuana use, % | 12 | 11 | 12 |
| Vigorous exercise (hours/week, mean) | 2.9 | 2.9 | 2.9 |
| Moderate exercise (hours/week, mean) | 6.1 | 6.1 | 5.8 |
| PSS-10 score (mean) | 16 | 15 | 15 |
| Intercourse frequency (times/week, mean) | 2.2 | 2.0 | 2.2 |
| Doing something to improve chances of pregnancy (%) | 68 | 65 | 64 |
| Daily use of multivitamin or folic acid (%) | 82 | 84 | 78 |
Sample of women used to calculate inverse probability weights.
Excludes women who conceived or were lost to follow-up within first interval (N = 1,627).
Restricted to current regular smokers at baseline.
Using life-table methods, 78.9% of participants conceived during the 12-month follow-up period. The 250 females who completed 12 months of follow-up without conceiving were similar to the remaining cohort members with respect to most baseline characteristics. They were more likely to be older, have higher education and income, self-identify as nonHispanic White, and consume higher levels of caffeine, and were less likely to engage in activities to improve their conception chances (e.g., monitoring of cervical fluid) or consume multivitamins (Table 1).
Comparing behaviors at 12 months with baseline among the 250 women with 12 months of follow-up (Tables 2 and 3), with weighting to adjust for loss to follow-up, we observed small to modest mean reductions in caffeine intake (−19.5 mg/day, 95% CI = −32.7, −6.37), alcohol intake (−0.85 drinks/week, 95% CI = −1.28, −0.43), marijuana use (−3.89 percentage points, 95% CI = −7.33, −0.46), and vigorous exercise (−0.68 hours/week, 95% CI = −1.05, −0.31), and larger increases in activities to improve the chances of conception (21.7 percentage points, 95% CI = 14.8, 28.6). Little to no mean change was seen for cigarette smoking (−0.27 percentage points, 95% CI = −1.58, 1.04), PSS-10 score (−0.04 units, 95% CI = −0.77, 0.69), intake of sugar-sweetened soda (−0.14 drinks/day, 95% CI = −0.43, 0.14), moderate exercise (−0.26 hours/week, 95% CI = −0.75, 0.24), intercourse frequency (−0.20 times/week, 95% CI = −0.39, 0.01), and multivitamin use (0.76 percentage points, 95% CI = −5.88, 7.39). The magnitude of increase in “doing something to improve the chances of pregnancy” was slightly larger when we restricted to the 55% of women not randomized to receive a FertilityFriend.com subscription (22.1 percentage points, 95% CI = 13.3, 31.0). The most commonly used methods to improve the chances of conception at baseline were charting cycles (66%), monitoring cervical fluid (36%), ovulation testing (25%), and basal body temperature (20%). Use of all methods increased on average over time, but the largest increase was observed for charting cycles (data not shown). Some behaviors showed changes in opposite directions (e.g., PSS-10 score), in relatively equal proportions (e.g., 31% increased and 32% decreased in PSS score by ≥2 units), thereby resulting in little overall mean change. Moreover, though we observed little mean change in the number of cigarettes smoked per day, 0% of women increased and 14.3% of women decreased their number of cigarettes by ≥5 per day after 12 months of attempt time.
TABLE 2.
Changes in Behavior Over Pregnancy Attempt Time Among 250 Women Who Contributed 12 Months of Follow-up (Continuous Variables)
| Increase in Behaviorb (%) | Decrease in Behaviorb (%) | Unweighted | Weighteda | |
|---|---|---|---|---|
| Mean Difference (95% CI) | Mean Difference (95% CI) | |||
| Caffeine (mg/day) | 13 | 24 | −20 (−34, −6.7) | −20 (−33, −6.4) |
| Sugar–sweetened soda (drinks/week) | 4 | 5 | −0.12 (−0.40, 0.16) | −0.14 (−0.43, 0.14) |
| Alcohol (drinks/week) | 4 | 12 | −0.85 (−1.3, −0.43) | −0.85 (−1.3, −0.43) |
| Cigarettes/day | 0 | 14 | −0.43 (−2.3, 1.5) | −0.47 (−2.3, 1.4) |
| Vigorous exercise (hours/week) | 10 | 18 | −0.67 (−1.0, −0.29) | −0.68 (−1.1, −0.31) |
| Moderate exercise (hours/week) | 21 | 24 | −0.29 (−0.79, 0.20) | −0.26 (−0.75, 0.24) |
| Perceived stress scale score | 31 | 32 | −0.08 (−0.81, 0.64) | −0.04 (−0.77, 0.69) |
| Intercourse frequency (times/week) | 19 | 24 | −0.20 (−0.39, 0.01) | −0.20 (−0.39, 0.01) |
Uses inverse probability weights to account for loss to follow-up.
Meaningful change in behavior was defined a priori as follows: caffeine = 50 mg/day; sugar-sweetened sodas = 3.5 drinks/week; alcohol = 3.5 drinks/week; smoking = 5 cigarettes/day; vigorous exercise = 2 hours/week; moderate exercise = 2 hours/week; PSS score = 2 units; and intercourse frequency = 1 coital act/week.
TABLE 3.
Changes in Behavior Over Pregnancy Attempt Time Among 250 Women Who Contributed 12 Months of Follow-up (Binary Variables)
| Unweighted Change | Weighted Changea | |||||
|---|---|---|---|---|---|---|
| Never (%) | Initiator (%) | Quitter (%) | Always (%) | Mean Difference in Percentage (95% CI) | Mean Difference in Percentage (95% CI) | |
| Cigarette smoking | 94 | 0 | 1 | 5 | −0.40 (−1.8, 0.97) | −0.27 (−1.6, 1.0) |
| Marijuana use | 86 | 2 | 6 | 6 | −3.6 (−7.0, −0.19) | −3.9 (−7.3, 0.46) |
| Doing something to improve chances of pregnancy | 8 | 29 | 7 | 57 | 22 (15, 29) | 22 (15, 29) |
| Daily multivitamin use | 8 | 14 | 14 | 64 | 0.80 (−5.8, 7.4) | 0.76 (−5.9, 7.4) |
Uses inverse probability weights to adjust for loss to follow-up.
During the 12-month follow-up period, mean behavior change was greatest for gravid women aged <30 years (sugar-sweetened soda, alcohol, smoking, number of cigarettes/day, intercourse frequency, and doing something to improve conception chances; Table 4). We observed the largest changes in caffeine and vigorous exercise for nulligravid women ≥30 years of age. We saw large reductions in marijuana use among nulligravid women in both age strata. Nulligravid women ≥30 years of age were the only subgroup for whom PSS-10 scores showed a mean increase after 12 months. Secondary analyses in which we truncated follow-up time at 6 months and stratified by age (<35 vs. ≥35 years) and gravidity showed somewhat different patterns (eTable 2; http://links.lww.com/EDE/B678). During the 6-month follow-up period, mean behavior change tended to be greatest for nulligravid women ≥35 years of age (alcohol, moderate exercise, doing something to improve chances of conception) and gravid women ≥35 years of age (caffeine, cigarettes smoked per day, intercourse frequency, multivitamin use). We saw reductions in marijuana use among all subgroups regardless of gravidity status, with slightly greater reductions among younger women.
TABLE 4.
Changes in Behavior Over Pregnancy Attempt Time Among 250 Women Who Contributed 12 Months of Follow-up, Stratified by Gravidity and Agea
| Age <30 Years | Age ≥30 Years | |||||
|---|---|---|---|---|---|---|
| Total (n = 109) | Nulligravid (n = 83) | Gravid (n = 26) | Total (n = 141) | Nulligravid (n = 84) | Gravid (n = 57) | |
| Caffeine (mg/day) | −12 (−31, 8) | −9.3 (−29, 11) | −20 (−76, 36) | −25 (−43, −7.5) | −36 (−61, −10) | −8.9 (−32, 15) |
| Sugar-sweetened soda (drinks/week) | −0.39 (−0.90, 0.11) | −0.35 (−0.94, 0.24) | −0.56 (−1.6, 0.49) | 0.04 (−0.28, 0.36) | 0.01 (−0.24, 0.26) | 0.09 (−0.64, 0.83) |
| Alcohol (drinks/week) | −1.1 (−1.7, −0.50) | −0.96 (−1.6, −0.37) | −1.7 (−3.5, 0.22) | −0.66 (−1.3, −0.07) | −1.1 (−1.9, −0.27) | 0.04 (−0.77, 0.85) |
| Smoking (%) | 0.37 (−2.0, 2.8) | 1.2 (−1.2, 3.7) | −2.8 (−9.5, 4.0) | −0.74 (−2.2, 0.69) | −1.2 (−3.6, 1.2) | 0.00 (−) |
| Cigarettes/day | −0.26 (−3.6, 3.1) | 1.1 (−0.54, 2.7) | −1.6 (−11, 7.4) | −0.74 (−2.7, 1.2) | −0.18 (−0.90, 0.55) | −1.1 (−4.6, 2.5) |
| Marijuana use (%) | −5.5 (−11, 0.21) | −5.9 (−12, 0.41) | −4.0 (−18, 9.7) | −2.7 (−7.0, 1.6) | −3.9 (−10.4, 2.6) | −0.79 (−5.4, 3.8) |
| Vigorous exercise (hours/week) | −0.68 (−1.2, −0.14) | −0.73 (−1.4, −0.08) | −0.49 (−1.5, 0.52) | −0.68 (−1.2, −0.17) | −1.1 (−1.8, −0.50) | 0.04 (−0.83, 0.91) |
| Moderate exercise (hours/week) | 0.10 (−0.65, 0.85) | 0.09 (−0.76, 0.95) | 0.11 (−1.5, 1.8) | −0.52 (−1.2, 0.14) | −0.34 (−1.2, 0.53) | −0.82 (−1.9, 0.23) |
| Perceived stress scale score | −0.19 (−1.3, 0.95) | −0.14 (−1.4, 1.2) | −0.40 (−3.0, 2.2) | 0.07 (−0.88, 1.0) | 0.60 (−0.65, 1.9) | −0.77 (−2.2, 0.70) |
| Intercourse frequency (times/week) | −0.23 (−0.54, 0.07) | −0.20 (−0.56, 0.16) | −0.35 (−0.92, 0.23) | −0.17 (−0.43, 0.10) | −0.09 (−0.38, 0.20) | −0.29 (−0.80, 0.22) |
| Doing something to improve chances of pregnancy (%) | 31 (21, 42) | 25 (13, 37) | 53 (33, 74) | 15 (5.6, 24) | 14 (2.5, 25) | 16 (0.40, 32) |
| Daily multivitamin use (%) | 3.8 (−6.8, 14) | 7.3 (−4.4, 19) | −8.6 (−34, 17) | −1.5 (−10, 7.0) | 0.28 (−11, 11) | −4.4 (−19, 10) |
Uses inverse probability weights to adjust for loss to follow-up.
Tables and graphs describing group mean changes in behaviors over pregnancy attempt time (from 0 to 12 months) showed that the magnitude of behavior change varied over time (Tables 2, 3; Figure 2A–L; eTables 3, 4; http://links.lww.com/EDE/B678). For several variables, the largest changes tended to occur in the first 2–4 months of attempt time (e.g., caffeine and alcohol intake, marijuana use, and vigorous exercise). The prevalence of cigarette smoking tended to increase within the first 2 months and then show a gradual decline thereafter (Figure 2D; eTable 4; http://links.lww.com/EDE/B678). Although there was a slight decline in cigarettes smoked per day among smokers between 2 and 4 months, it remained stable from 4 to 10 months, after which it declined more sharply (Figure 2E; eTable 3; http://links.lww.com/EDE/B678). Engaging in activities to improve conception chances increased markedly after the first 2 months of follow-up (i.e., 15 percentage points), and continued to increase, but only minimally, with increasing attempt time (Figure 2K; eTable 4; http://links.lww.com/EDE/B678). Smaller and more gradual reductions were observed for sugar-sweetened soda intake (Table 2). A small increase in frequency of intercourse in the first 2–4 months was followed by a steady decrease over time from 6 to 12 months (Table 2; Figure 2J; eTable 3; http://links.lww.com/EDE/B678). No appreciable changes were evident for moderate exercise, multivitamin use, or PSS-10 scores. Applying weights to adjust for loss to follow-up made little difference in the results (Tables 2 and 3).
FIGURE 2.
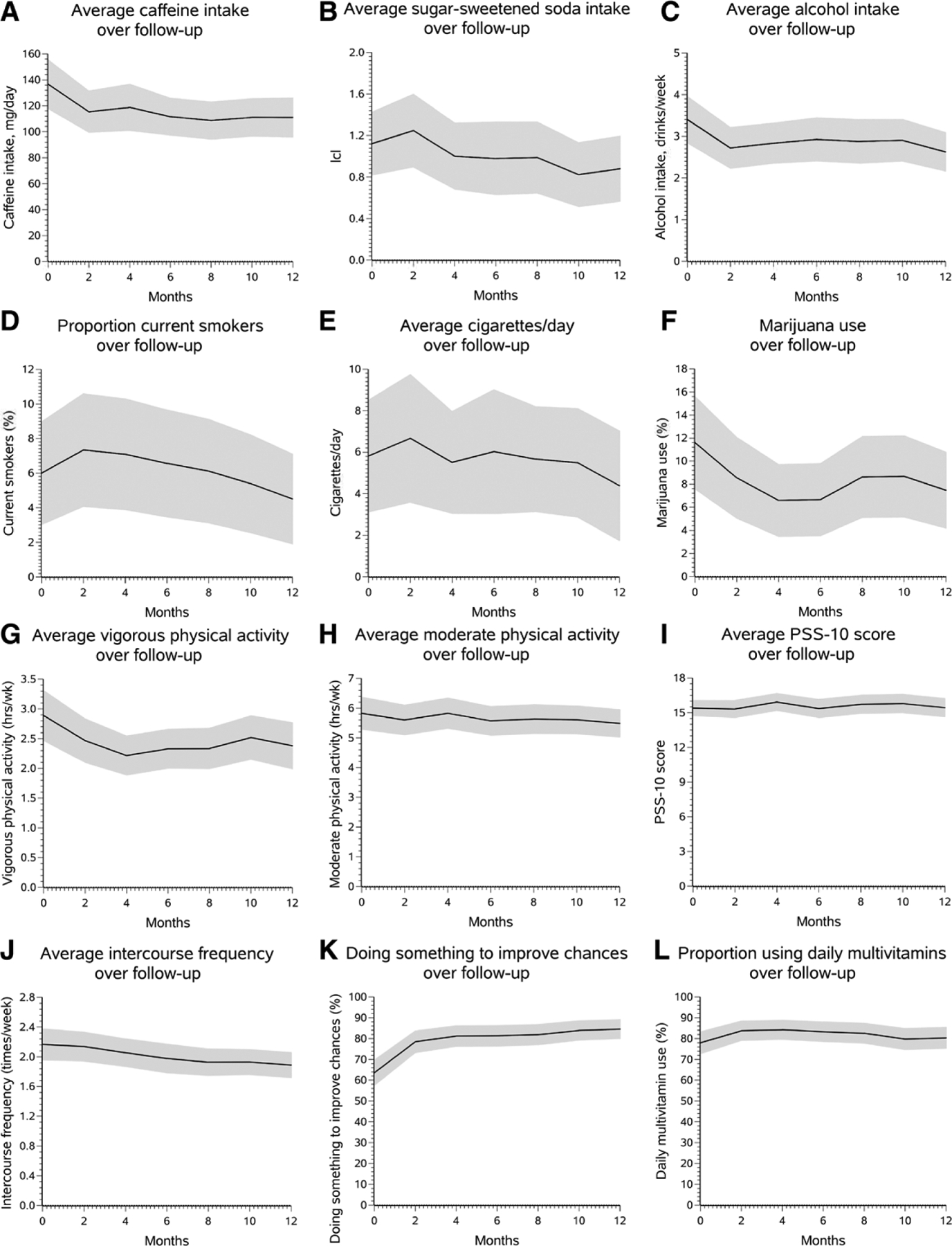
A–L, Trajectories in behavior among the 250 women who completed 12 months of follow-up. Solid line, Mean value of characteristic weighted to adjust for loss to follow-up; shaded area, 95% confidence bounds.
DISCUSSION
In this North American preconception cohort study, increasing pregnancy attempt time was associated with small-to-moderate reductions in intakes of caffeine and alcohol, use of marijuana, and vigorous exercise. Larger mean increases over pregnancy attempt time were seen for engaging in activities to improve conception chances and little change was seen for cigarette smoking overall. For many factors, the largest change tended to occur within the first 2–4 months of attempt time. The one exception was for cigarette smoking, for which the largest decrease in prevalence and number of cigarettes occurred after 10 months of attempt time. Some variables for which there was little overall mean change showed large differences in opposite directions for some women (e.g., PSS-10 score). Finally, some factors also tended to show larger differences among older women (e.g., reductions in caffeine, moderate exercise) for whom there might be greater concern about difficulties conceiving quickly, but there were exceptions (e.g., larger decreases in marijuana use among younger women).
Although intercourse frequency did not increase appreciably over pregnancy attempt time, participants were consistently more likely to engage in activities to improve their chances of conception with increasing attempt time. These observations are not entirely surprising because monitoring fertility signs (e.g., cervical mucus consistency and basal body temperature) can help women time intercourse to ovulation, which better reflects intensity of trying than intercourse frequency. Another important consideration is that 50% of participants were randomly assigned to receive a FertilityFriend.com subscription after enrollment. This fertility app incentive could thus have influenced the observed large uptake in use of menstrual charting. Nevertheless, when we restricted to those not receiving this incentive, the finding persisted.
Mean perceived stress levels did not increase consistently over pregnancy attempt time, except among nulligravid women ≥30 years of age. However, large percentages of participants reported appreciable changes in PSS-10 scores (>30% changed by ≥2 units), in opposing directions. Given that estimates from the PSS instrument are shown to be reliable for 4–8 weeks,25,27 our bimonthly PSS measurements should serve as a reliable representation of stress in each time interval. Likewise, cigarette smoking was fairly stable over increasing attempt time, with the exception of a small decline in smoking prevalence and intensity after 10 months of attempt time. Smoking is an addictive behavior that may be difficult to modify despite participants’ knowledge that it reduces fertility. After 12 months, we observed that 0% women had a meaningful increase in cigarettes smoked per day (≥5 cigarettes/day), whereas 14% had a meaningful decrease (≥5 cigarettes/day).
To our knowledge, there is only one previous study investigating changes in behavioral factors with increasing pregnancy attempt time.23 In the New York State Prospective Pregnancy Study, investigators prospectively measured daily cigarette, alcohol, and caffeine use among 90 women attempting pregnancy immediately on discontinuing contraception and followed them for up to 12 menstrual cycles.23 We observed mean reductions in caffeinated beverages (−0.52 drinks/day; 95% CI = −0.70, −0.33) when compared with baseline, but no appreciable changes for alcohol consumption.23 There was little change in cigarette smoking until cycle 8, after which smoking was reduced by approximately 1.5 cigarettes/day as compared with baseline. Thus, our results for caffeine and smoking were relatively consistent with this study.
Although the range of variables examined in the present study expands on that investigated in previous literature, we did not assess change in potentially more modifiable and important variables for fecundability, including dietary intake and body weight. We included only one assessment of diet, at baseline, given the burden a food frequency questionnaire imposed on our participants. We also reasoned that anthropometric variables like body weight would not change substantially over a short period of time, and thus did not collect updated data on body weight during the 12-month period. It is entirely possible that some women changed their diets or lost/gained nonnegligible amounts of body weight during the 12-month period. Thus, the present investigation was limited in the number and scope of variables it assessed with regard to lifestyle and behavior change.
Loss-to-follow-up in PRESTO, albeit typical for a preconception cohort study,17,24,28 could have distorted results as participants lost to follow-up may differ from those who continued in the study (e.g., smokers and marijuana users were more likely to drop out than nonusers). To address this problem, we used inverse probability weighting (IPW) to reweight the participants who remained under follow-up so that those remaining under observation were like the initial population. An important assumption of IPW is that some participants still being followed in the cohort are similar to those who dropped out. However, if there is zero overlap in the distributions of measured characteristics between those who were and were not lost to follow-up, then there will be no one in the continuing population to carry the weight of the lost participants. Moreover, if we failed to account for all important predictors of continuation in the study, selection bias is still possible. Another limitation is our assessment of average change over time. If a given behavior changed markedly in one direction for some individuals and markedly in the opposite direction for others, we would observe small average changes overall. Indeed, we observed that some behaviors in participants showed large changes in opposite directions (e.g., PSS-10 score), in relatively equal proportions, thereby canceling each other out when assessing mean change.
Our study differs from previous research in that it used web-based methods to recruit participants, included couples residing in all US states and Canadian provinces, and assessed a broader set of behavioral characteristics. Web-based recruitment would not affect the validity of study results unless the patterns of behavioral change and pregnancy attempt times differed substantially between internet users and nonusers, which seems implausible. We reduced selection bias stemming from differential participation at enrollment by restricting to women with ≤1 cycle of pregnancy attempt time. Like most prospective cohort studies, including randomized trials, participants in this study were volunteers. Earlier study by our investigative team29 and others30,31 has shown that even with large differences in characteristics between the general population and volunteers in a prospective cohort study, there is scant evidence of any bias in epidemiologic effect measures. Nevertheless, the initial characteristics of the study population influence the extent to which large changes in behaviors can be seen over time (e.g., low sugar-sweetened soda intake at baseline reduces potential to observe large reductions over time). The aim of this article was to describe changes that occur over time as women try to conceive. The results do not imply any causal effects32; however, they may be useful for understanding how cohorts that enroll infertile women differ from the general population of women attempting to conceive. Understanding such changes over time can be useful for sensitivity analyses and generalizing estimates to new populations.33
In conclusion, this study indicates that pregnancy planners change several behaviors with increasing pregnancy attempt time, but for many variables, the changes were small to moderate on average. For some behavior variables, large proportions of women changed their individual behaviors in opposite directions, thereby producing nearly no change in overall means. The extent to which such behavior changes could introduce bias in studies that recruit couples with infertility is unclear.
Supplementary Material
ACKNOWLEDGMENTS
We thank PRESTO participants and staff for their contributions. We also thank Stephen R. Cole, PhD, for his input on earlier drafts of this study, and Michael Bairos for this technical assistance with web-based questionnaire design and infrastructure.
Supported by NIH grants R01-HD086742, R21-HD072326, R01-ES028923, R01-ES029951, R03-HD094117, and R01-HD060680.
In the last 3 years, PRESTO has received in-kind donations from Swiss Precision Diagnostics, Sandstone Diagnostics, FertilityFriend.com, and Kindara.com.
Footnotes
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com). The authors would be pleased to make the analytic code available to others as needed. The participants did not provide informed consent for data sharing.
REFERENCES
- 1.Thoma ME, McLain AC, Louis JF, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99:1324–1331.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smarr MM, Sapra KJ, Gemmill A, et al. Is human fecundity changing? A discussion of research and data gaps precluding us from having an answer. Hum Reprod. 2017;32:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril. 2014;101:633–634. [DOI] [PubMed] [Google Scholar]
- 4.Eijkemans MJ, van Poppel F, Habbema DF, Smith KR, Leridon H, te Velde ER. Too old to have children? Lessons from natural fertility populations. Hum Reprod. 2014;29:1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wesselink AK, Rothman KJ, Hatch EE, Mikkelsen EM, Sørensen HT, Wise LA. Age and fecundability in a North American preconception cohort study. Am J Obstet Gynecol. 2017;217:667.e1–667.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radin RG, Hatch EE, Rothman KJ, et al. Active and passive smoking and fecundability in Danish pregnancy planners. Fertil Steril. 2014;102: 183–191.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wesselink AK, Hatch EE, Rothman KJ, Mikkelsen EM, Aschengrau A, Wise LA. Prospective study of cigarette smoking and fecundability. Hum Reprod. 2018;34:558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolúmar F, Olsen J, Rebagliato M, Sáez-Lloret I, Bisanti L. Body mass index and delayed conception: a European Multicenter Study on Infertility and Subfecundity. Am J Epidemiol. 2000;151:1072–1079. [DOI] [PubMed] [Google Scholar]
- 9.McKinnon CJ, Hatch EE, Rothman KJ, et al. Body mass index, physical activity and fecundability in a North American preconception cohort study. Fertil Steril. 2016;106:451–459. [DOI] [PubMed] [Google Scholar]
- 10.Sundaram R, Mumford SL, Buck Louis GM. Couples’ body composition and time-to-pregnancy. Hum Reprod. 2017;32:662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaskins AJ, Chavarro JE. Diet and fertility: a review. Am J Obstet Gynecol. 2018;218:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wise LA, Rothman KJ, Mikkelsen EM, Sørensen HT, Riis AH, Hatch EE. A prospective cohort study of physical activity and time to pregnancy. Fertil Steril. 2012;97:1136–42.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viru AM, Hackney AC, Välja E, Karelson K, Janson T, Viru M. Influence of prolonged continuous exercise on hormone responses to subsequent exercise in humans. Eur J Appl Physiol. 2001;85:578–585. [DOI] [PubMed] [Google Scholar]
- 14.Eijkemans MJC, Leridon H, Keiding N, Slama R. A systematic comparison of designs to study human fecundity. Epidemiology. 2019;30:120–129. [DOI] [PubMed] [Google Scholar]
- 15.Bonde JP, Hjollund NH, Jensen TK, et al. A follow-up study of environmental and biologic determinants of fertility among 430 Danish first-pregnancy planners: design and methods. Reprod Toxicol. 1998;12:19–27. [DOI] [PubMed] [Google Scholar]
- 16.Buck GM, Lynch CD, Stanford JB, et al. Prospective pregnancy study designs for assessing reproductive and developmental toxicants. Environ Health Perspect. 2004;112:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buck Louis GM, Schisterman EF, Sweeney AM, et al. Designing prospective cohort studies for assessing reproductive and developmental toxicity during sensitive windows of human reproduction and development–the LIFE Study. Paediatr Perinat Epidemiol. 2011;25:413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen J, Melbye M, Olsen SF, et al. The Danish National Birth Cohort–its background, structure and aim. Scand J Public Health. 2001;29:300–307. [DOI] [PubMed] [Google Scholar]
- 19.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C; MoBa Study Group. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol. 2006;35:1146–1150. [DOI] [PubMed] [Google Scholar]
- 20.Mutsaerts MA, Groen H, Huiting HG, et al. The influence of maternal and paternal factors on time to pregnancy–a Dutch population-based birth-cohort study: the GECKO Drenthe study. Hum Reprod. 2012;27:583–593. [DOI] [PubMed] [Google Scholar]
- 21.Baird DD, Wilcox AJ, Weinberg CR. Use of time to pregnancy to study environmental exposures. Am J Epidemiol. 1986;124:470–480. [DOI] [PubMed] [Google Scholar]
- 22.Cooney MA, Buck Louis GM, Sundaram R, McGuiness BM, Lynch CD. Validity of self-reported time to pregnancy. Epidemiology. 2009;20:56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lum KJ, Sundaram R, Buck Louis GM. Women’s lifestyle behaviors while trying to become pregnant: evidence supporting preconception guidance. Am J Obstet Gynecol. 2011;205:203.e1–203.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wise LA, Rothman KJ, Mikkelsen EM, et al. Design and conduct of an internet-based preconception cohort study in North America: pregnancy study online. Paediatr Perinat Epidemiol. 2015;29:360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 26.SAS. SAS Institute Inc. 2014. SAS/STAT® 9.4 User’s Guide. Cary, NC. Cary, NC: SAS Institute; 2014. [Google Scholar]
- 27.Cohen S, Williamson G. Perceived stress in a probability sample of the United States In: Spacapan S, Oskamp S, eds. The Social Psychology of Health: Claremont Symposium on Applied Social Psychology. Newbury Park, CA: Sage; 1988. [Google Scholar]
- 28.Lynch CD, Sundaram R, Buck Louis GM, Lum KJ, Pyper C. Are increased levels of self-reported psychosocial stress, anxiety, and depression associated with fecundity? Fertil Steril. 2012;98:453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatch EE, Hahn KA, Wise LA, et al. Evaluation of selection bias in an internet-based study of pregnancy planners. Epidemiology. 2016;27: 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nilsen RM, Vollset SE, Gjessing HK, et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23:597–608. [DOI] [PubMed] [Google Scholar]
- 31.Nohr EA, Frydenberg M, Henriksen TB, Olsen J. Does low participation in cohort studies induce bias? Epidemiology. 2006;17:413–418. [DOI] [PubMed] [Google Scholar]
- 32.Tennant PG, Arnold KF, Ellison GTH, Gilthorpe MS. Analyses of ‘change scores’ do not estimate causal effects in observational data. medRxiv. 2020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caniglia EC, Zash R, Swanson SA, et al. Methodological challenges when Studying Distance to Care as an Exposure in Health Research. Am J Epidemiol. 2019;188:1674–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


