REDUCE-IT was designed as a randomized, placebo-controlled, cardiovascular outcomes trial of patients treated with statins, who had controlled low-density lipoprotein cholesterol, but persistently elevated triglycerides, along with overt presence of or high risk for cardiovascular disease.1 Participants across 11 countries were randomized to receive icosapent ethyl, a highly purified form of eicosapentaenoic acid, 2 g twice daily or placebo and followed for a median of 4.9 years (maximum 6.2 years). The primary endpoint was time from randomization to first occurrence of a composite of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, coronary revascularization, or unstable angina requiring hospitalization. The key secondary endpoint was time from randomization to first occurrence of a composite of cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke.
Icosapent ethyl was associated with marked reductions in primary and key secondary endpoints with a number needed to treat of 21 for the primary composite endpoint and 28 for the key secondary endpoint.2 The relative risk reduction for the primary endpoint was 25% (P = 0.00000001) and for the key secondary endpoint was 26% (P = 0.0000006), with an absolute risk reduction of the primary endpoint of 4.8% and key secondary endpoint of 3.6%.2 There was a 32% relative reduction in total (first and recurrent) ischaemic events.3
REDUCE-IT was an event-driven trial of 8179 patients. A total of 1612 primary endpoint events were targeted to occur with a projected 90% power to detect a 15% relative risk reduction.1 The independent Data Safety and Monitoring Committee (DMC) were to perform prespecified, unblinded interim analyses1 at ∼60% of events (April 2016) with a 2.9 year median follow-up and 953 first events and at ∼80% of events (April 2017) with 3.7 year median follow-up and 1218 events. The final analysis on 6 September 2018 was at 4.9 years median study follow-up with 1606 first events.2,3
An interim statistical analysis plan guided study continuation recommendations according to a prespecified decision-making process including assessment of safety, treatment arm performance, primary composite endpoint formal analysis, and internal robustness analysis with no futility stopping boundary requirements. The DMC included two physicians, a PhD statistician, and a non-voting independent statistician. Independent statistical support was provided by Cytel, Inc. Prior to DMC interim analysis study continuation recommendations, the DMC discussed the importance of a more mature data set to support robustness of final efficacy and safety findings. The steering committee, sponsor, and clinical endpoint committee were blinded to treatment throughout the trial prior to the final database lock.
In REDUCE-IT, primary and key secondary endpoints achieved statistical significance at both interim analyses. This persisted at the final analysis, surpassing the final two-sided alpha of 0.0437 adjusted to account for interim analyses. Clarity of efficacy findings across endpoints and subgroups improved steadily with time and greater number of events. Overall safety was consistent across interim and final analyses. Stopping for overwhelming efficacy was discussed by the DMC at each interim analysis. The DMC considered historical examples of failed cardiovascular outcome studies for triglyceride lowering and mixed omega-3 therapies, reflected on potential for overestimating final demonstrated benefit using an incomplete data set, and weighed societal impacts for further safety and efficacy data sets relative to patient access.4
Considering accumulation of data at interim analyses 1 and 2 and in the final analysis, icosapent ethyl was consistently superior to placebo regarding primary and key secondary endpoints. For the primary endpoint, for interim analysis 1, the hazard ratio (HR) was 0.77 [95% confidence interval (CI) 0.68–0.87; P = 0.00005]; for interim analysis 2, the HR was 0.77 (95% CI 0.69–0.87; P = 0.0000008), and for the final analysis, the HR was 0.75 (95% CI 0.68–0.83; P = 0.00000001). For key secondary endpoints, for interim analysis 1, the HR was 0.71 (95% CI 0.60–0.83; P = 0.00005), for interim analysis 2, the HR was 0.72 (95% CI 0.62–0.83; P = 0.000009), and for the final analysis, the HR was 0.74 (95% CI 0.65–0.83; P = 0.0000006).
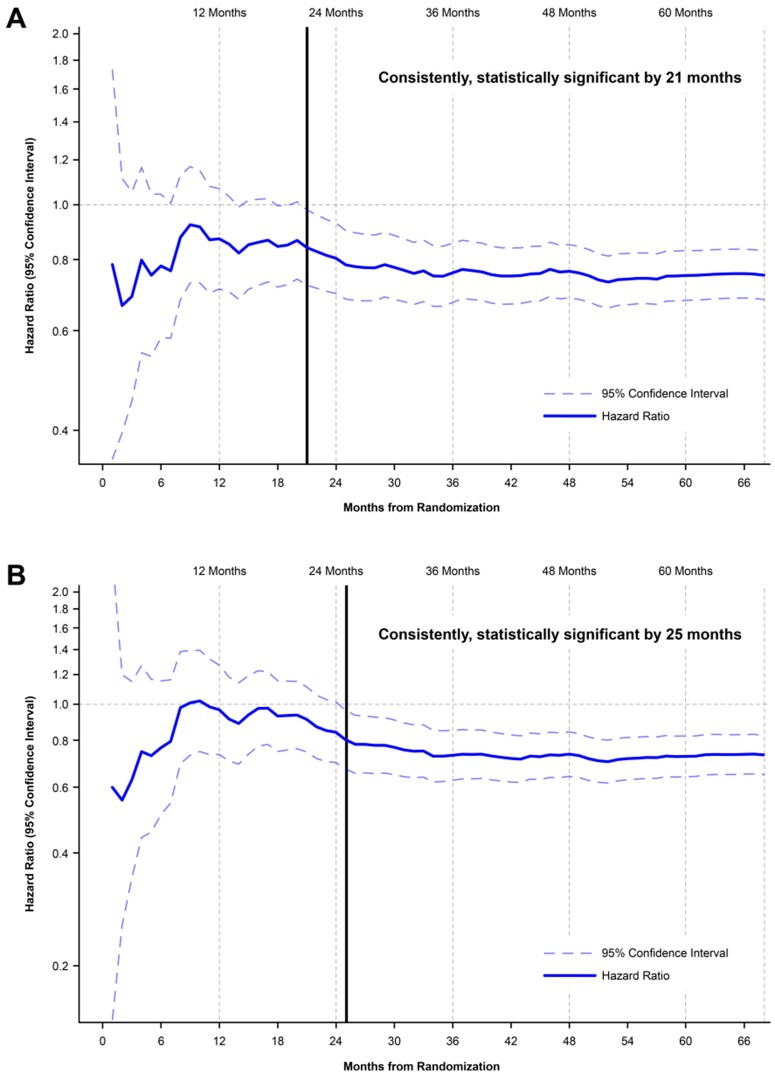
The probability that results of the first and second interim analyses would ultimately become non-significant in the final analysis was considered by the DMC to be highly unlikely. In the continuous landmark analysis of the primary composite endpoint, the HR was below unity at all timepoints, with consistent and statistically significant outcome measures starting at 21 months (Figure 1A). Similarly, a continuous landmark analysis of the key secondary endpoint showed consistent and statistically significant advantage of icosapent ethyl vs. placebo starting at 25 months (Figure 1B).
Figure 1.
(A) Rate of the primary composite endpoint over time, achieving consistent statistical significance by 21 months. (B) Rate of the key secondary composite endpoint over time, achieving consistent statistical significance by 25 months.
REDUCE-IT showed consistent and potent benefit of icosapent ethyl beginning early in the study and persisting across prespecified interim analyses through the final analysis. The final data set showed highly statistically significant reductions in primary and key secondary endpoints. Furthermore, in the final data set of the US population, total mortality was reduced with icosapent ethyl compared with placebo.5 Allowing the REDUCE-IT data set to mature fully provided physicians and patients with uncontestable, robust, consistent, and reliable efficacy and safety data upon which to base clinical decisions for use of icosapent ethyl in cardiovascular risk reduction.
Conflict of interest: B.O. served as Chair of the Data Safety Monitoring Committee (DMC) of REDUCE-IT. He is presently a consultant for Amarin, Respicardia, Sanofi Aventis, Lundbeck and serves as United States co-coordinator for GLORIA-AF sponsored by Boehringer Ingelheim. D.L.B. serves as the Chair and International Principal Investigator for REDUCE-IT, with research funding from Amarin to Brigham and Women’s Hospital. D.L.B. discloses the following relationships—Advisory Board: Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, Takeda. M.M. received consulting fees from Amarin, Pfizer, and 89bio. G.S. received research grant funding from Amarin, Bayer, Merck, Sanofi, and Servier; speaking or consulting fees from Amarin, Amgen, AstraZeneca, Bayer/Janssen, Boehringer Ingelheim, Bristol-Myers-Squibb, Idorsia, Lilly, Merck, Novartis, Novo Nordisk, Pfizer, Regeneron, Sanofi, and Servier. E.A.B. received fees as a speaker from Amarin, Amgen, Esperion, and Medicure, and consulting fees from Amarin, Amgen, Esperion, and Medicure. T.A.J. received consulting fees from Amgen, Esperion, Novartis, Regeneron, and Sanofi. S.B.K. is an employee and stockholder of Amarin Pharma, Inc. R.T.D. is an employee and stockholder of Amarin Pharma, Inc. R.A.J. is an employee and stockholder of Amarin Pharma, Inc. L.J. is an employee and stockholder of Amarin Pharma, Inc. C.G. is an employee and stockholder of Amarin Pharma, Inc. J.-C.T. received grant support from Amarin, AstraZeneca, Esperion, Ionis, and RegenXBio; grant support and consulting fees from DalCor; grant support and fees for serving on an executive committee from Sanofi; grant support and consulting fees from Servier; and holding a minor equity interest in DalCor and patents (US 9 909 178 B2) on dalcetrapib for therapeutic use and on use of colchicine after myocardial infarction. C.M. is an employee of Cytel, Incorporated. R.M. is an employee of Cytel, Incorporated. C.M.B. received consulting fees from Arrowhead, AstraZeneca, Eli Lilly, Matinas BioPharma, Merck, Boehringer Ingelheim, Novo Nordisk, Denka Seiken, and Gilead and grant support (paid to his institution) and consulting fees from Amarin, Amgen, Esperion, Novartis, Regeneron, Sanofi-Synthelabo, and Akcea. No other potential conflict of interest relevant to this article was reported. M.K.C. served on the Amarin DMC but has no relevant financial disclosures.
Trial Registration: NCT01492361.
References
- 1. Bhatt DL, Steg PG, Brinton EA, Jacobson TA, Miller M, Tardif J-C, Ketchum SB, Doyle RT Jr, Murphy SA, Soni PN, Braeckman RA, Juliano RA, Ballantyne CM; on behalf of the REDUCE-IT Investigators. Rationale and design of REDUCE-IT: reduction of cardiovascular events with Icosapent Ethyl-Intervention trial. Clin Cardiol 2017;40:138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif J-C, Ballantyne CM; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 3. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif J-C, Gregson J, Pocock SJ, Ballantyne CM; REDUCE-IT Investigators. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol 2019;73:2791–2802. [DOI] [PubMed] [Google Scholar]
- 4. Jolly SS, Gao P, Cairns JA, Yusuf S, Bhatt DL, Wyse DG, Wells GA, Dzavik V.. Risks of overinterpreting interim data: lessons from the TOTAL trial (Thrombectomy With PCI Versus PCI Alone in Patients With STEMI). Circulation 2018;137:206–209. [DOI] [PubMed] [Google Scholar]
- 5. Bhatt DL, Miller M, Brinton EA, Jacobson TA, Steg PG, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif J-C, Olshansky B, Chung MK, Gibson CM, Giugliano RP, Budoff MJ, Ballantyne CM; on behalf of the REDUCE-IT Investigators. REDUCE-IT USA: results from the 3146 patients randomized in the United States. Circulation 2020;141:367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]